Solvent Cementing of Plastics
Solvent cementing (also solvent bonding or solvent welding) is a process in which thermoplastics, usually amorphous or containing low crystallinity, are softened by the application of a suitable solvent, or a mixture of solvents, and then pressed together to affect a bond. Usually the resin itself, after evaporation of the solvent, acts as the adhesive. Generally, adhesion is achieved by evaporation of the solvent, absorption of the solvent into adjacent material, and/or polymerization of the solvent cement. Many thermoplastic resins are easier to join effectively by solvent cementing than by conventional adhesive bonding. For example, solvent cementing is used to connect plastic pipes such as those made from polyvinyl chloride and chlorinated polyvinyl chloride.
Keywords
Solvent cementing; plastics; adhesion; absorption; polymerization
9.1 Introduction
Solvent cementing (also solvent bonding or solvent welding) is a process in which thermoplastics, usually amorphous or containing low crystallinity, are softened by the application of a suitable solvent, or a mixture of solvents, and then pressed together to create a bond. Usually the resin itself, after evaporation of the solvent, acts as the adhesive. Generally, adhesion is achieved by evaporation of the solvent, absorption of the solvent into adjacent material, and/or polymerization of the solvent cement. Many thermoplastic resins are easier to join effectively by solvent cementing than by conventional adhesive bonding. For example, solvent cementing is used to connect plastic pipes such as those made from polyvinyl chloride (PVC) and chlorinated polyvinyl chloride (CPVC) [1].
Often mixtures of solvents give better results than individual solvents. Frequently, small amounts of the plastic to be cemented are dissolved in the solvents to form “bodied” cements. These additions of polymer aid in gap filling and accelerate setting. They also reduce shrinkage and internal stresses. If the evaporation rates of the solvents used are too high due to excessive volatility of the solvent, crazing or blushing often results.
9.2 Background
Welding of polymers takes place when polymer chains at the surface of one component are mobile enough to entangle with chains in the other component. Usually, thermal energy is applied to raise the temperature of the polymer above the appropriate transition temperature, i.e., the glass transition temperature, Tg, for amorphous thermoplastic polymers, or the melting temperature, Tm, for semicrystalline polymers. Above these transition temperatures, polymer chains are more mobile. If two components are brought into intimate contact under these conditions, polymer chain entanglement will occur resulting in a weld.
In solvent welding, a solvent is applied which can temporarily dissolve/swell the polymer at room temperature. When this occurs, the polymer chains have significantly more freedom to move and can entangle with other similarly dissolved/swollen chains in the other component. Given sufficient time, the solvent will permeate through the polymer and out into the environment, so that the chains lose their mobility. This leaves a solid mass of entangled polymer chains, which constitutes a solvent weld.
It is possible to solvent cement different types of plastic to each other as long as the solvent must be compatible with both plastics. Usually, a mixture of fast-evaporating solvent is combined with a high-boiling solvent, often with resin addition (up to 25% by weight). Upon softening the plastic adherents, they are allowed to become tacky. At this point, they are pressed together and held under pressure until dry. As thin a coat of solvent as possible should be used. Recommended solvents and solvent mixtures will be described later for each plastic type.
Bonding should be carried out in a warm, dry situation to avoid condensation due to solvent evaporative cooling of the part. The solvents may be brushed, sprayed, or applied by dipping or with a syringe. Caution should be taken in applying the solvent, because excess may run into unwanted areas and result in damage to the appearance of the part. Heating the part is not always recommended because it can cause stress cracking as the solvent leaves the surface. Heating can also result in bubbling in the solvent layer.
Solvent cementing is the simplest and most economical method of joining thermoplastics. Solvent-cemented joints are less sensitive to thermal cycling than joints bonded with conventional adhesives and are as resistant to degrading environments as the parent plastic. This is true because the final joint consists solely of the parent plastic, with no other adhesive or solvent material present. Bond strengths in the range of 85–100% of the strength of the parent plastic can be obtained [2–5].
9.2.1 Solubility Parameter
There are a number of systems aiming to characterize solvents numerically including Kauri–Butanol number, solubility grade, aromatic character, analine cloud point, wax number, heptane number, and Hildebrand solubility parameter, among others. The Hildebrand solubility parameter is the most widely used of all the systems. A paper published by the American Institute for Conservation strives to clarify the topic of solubility parameter in a brief review [6].
Solvents used to cement plastics should be chosen with approximately the same solubility parameter (δ) as that of the plastic to be bonded. The Hildebrand solubility parameter is the square root cohesive energy density (CED) of the liquid solvent or polymer. It is defined as follows:
where ΔE is the energy of vaporization, V is the molar volume, and ΔE/V is the CED or internal pressure.
A nonpolar molecule, such as methane, evaporates readily and is a gas at ordinary temperatures. It has a low CED, and hence a low δ (~6). By contrast, a highly polar, associated (hydrogen-bonded) molecule of the same size, such as water, requires high heat input to evaporate it, and consequently has a very high δ of 23.4. Literature sources provide data for δ values of a number of plastics and resins [4,7]. A great deal of the data is shown in Tables 9.1 and 9.2. The solubility parameters help explain why polystyrene (δ=9.1) is soluble in butane (δ=9.3), but not in acetone (δ=10.0), while cellulose acetate (δ=10.9) dissolves in ethyl acetate (δ=9.1), but not in butyl acetate (δ=8.5). The concept of δ also explains why a plastic would sometimes dissolve in a mixture of two liquids, neither of which by itself is a solvent for the plastic. The classic example is the solubility of nitrocellulose (δ=11.0) in ethyl alcohol (δ=12.7, poor solvent) and diethyl ether (δ=7.4).
Table 9.1
Hildebrand Solubility Parameters for Solvents
| Solvent | Standard (cal/cm3)1/2 | SI Unit (MPa)1/2 |
| n-Pentane | (7.0) | 14.4 |
| n-Hexane | 7.24 | 14.9 |
| Freon® TF | 7.25 | |
| n-Heptane | (7.4) | 15.3 |
| Diethyl ether | 7.62 | 15.4 |
| 1,1,1-Trichloroethane | 8.57 | 15.8 |
| n-Dodecane | 16.0 | |
| White spirit | 16.1 | |
| Turpentine | 16.6 | |
| Cyclohexane | 8.18 | 16.8 |
| Amyl acetate | (8.5) | 17.1 |
| Carbon tetrachloride | 8.65 | 18.0 |
| Xylene | 8.85 | 18.2 |
| Ethyl acetate | 9.10 | 18.2 |
| Toluene | 8.91 | 18.3 |
| Tetrahydrofuran | 9.52 | 18.5 |
| Benzene | 9.15 | 18.7 |
| Chloroform | 9.21 | 18.7 |
| Trichloroethylene | 9.28 | 18.7 |
| Cellosolve® acetate | 9.60 | 19.1 |
| Methyl ethyl ketone | 9.27 | 19.3 |
| Acetone | 9.77 | 19.7 |
| Diacetone alcohol | 10.18 | 20.0 |
| Ethylene dichloride | 9.76 | 20.2 |
| Methylene chloride | 9.93 | 20.2 |
| Butyl Cellosolve® | 10.24 | 20.2 |
| Pyridine | 10.61 | 21.7 |
| Cellosolve® | 11.88 | 21.9 |
| Morpholine | 10.52 | 22.1 |
| Dimethyl formamide | 12.14 | 24.7 |
| n-Propyl alcohol | 11.97 | 24.9 |
| Ethyl alcohol | 12.92 | 26.2 |
| Dimethyl sulfoxide | 12.93 | 26.4 |
| n-Butyl alcohol | 11.30 | 28.7 |
| Methyl alcohol | 14.28 | 29.7 |
| Propylene glycol | 14.80 | 30.7 |
| Ethylene glycol | 16.30 | 34.9 |
| Glycerol | 21.10 | 36.2 |
| Water | 23.5 | 48.0 |
Standard Hildebrand values from Hansen [8].
SI Hildebrand values from Hansen [9].
Values in parentheses from Crowley et al. [10].
Table 9.2
Solubility Parameters for Polymers [11]
| Polymer | Solubility Parameter (cal/cm3)1/2 |
| Polytetrafluoroethylene | 6.2 |
| Polydimethyl siloxane | 7.3–7.6 |
| Butyl rubber | 7.7 |
| Polyethylene | 7.9–8.1 |
| Polyurethane | 10.0 |
| Polystyrene | 9.1 |
| Neoprene | 8.2–9.4 |
| Polyvinyl acetate | 9.4 |
| Polymethyl methacrylate | 9.3 |
| Polyvinyl chloride | 9.5–9.7 |
| Epoxy | 9.7–10.9 |
| Polyethylene terephthalate | 10.7 |
| Phenolic resin | 11.5 |
| Polyvinylidene chloride | 12.2 |
| Nylon 6,6 | 13.6 |
| Polybutadiene | 8.4 |
| Phenol formaldehyde | 11.5 |
9.2.2 Factors Affecting Adhesive and Solvent Bonding
9.2.2.1 Solubility
Solvent bonding is most effective with polymers having low intermolecular forces. Amorphous polymers or polymers with low crystallinity are more soluble in most solvents. Lower molecular weight polymers and polymer molecules with less cross-linking and more branching structures are more easily dissolved in solvents. Elevated temperatures increase the solubility of all polymers. Polymers dissolve most easily in solvents of the same polarity; polar polymers generally dissolve in polar solvents, and nonpolar polymers dissolve more easily in nonpolar solvents. The solubility parameter is an indication of polarity; nonpolar polymers, such as polyethylene, have a low value (7.9), while more polar materials, such as polyacrylonitrile, have higher values (15.4). Solubility of a polymer is most likely when the solubility parameter of the polymer and that of the solvent vary by <0.5. Dissimilar materials can be solvent bonded as long as the solubility parameters of both materials match that of the solvent. Solubility of either same or dissimilar thermoplastics is not assured, however, and prior testing of the solvent is recommended [12,13].
9.2.2.2 Stress Cracking
Many plastic parts exhibit stress cracking, external or internal cracks resulting from stresses that are lower than the short-term mechanical strength of the plastic. Thermosets, acetals, polyphenylene sulfide, polyolefins, polyamide, rigid PVC, and polyethylene and polybutylene terephthalate are resistant to stress cracking. Acrylics, polycarbonate, polystyrene, styrene–acrylonitrile (SAN), polysulfone, acrylonitrile–butadiene–styrene (ABS), and polyphenylene oxide are most prone to stress cracking. Esters, ketones, and aromatic hydrocarbons are the solvents most likely to cause stress cracking [14–16]. Stress cracking can be induced by strenuous or improper molding conditions, machining operations, or thermoforming at reduced temperatures. The presence of molded-in metal inserts or sharp corners in the part produces greater stress. These conditions produce small cracks in the plastic; when liquid adhesive is applied, it can penetrate the part, increasing the damage. The crack may eventually propagate through the entire part, causing part failure. Molded-in stresses can be reduced by modifying the molding cycle or annealing parts after molding. Only solvents, adhesives, and primers that are compatible with the plastic should be used. A minimum of adhesive should be used, and excess adhesive should be immediately cleaned up. Anaerobic thread-locking adhesives should not be used with plastics that are prone to stress cracking.
Stresses induced by the threads in addition to stress from uncured adhesive outside the joint produce very high stress levels in the part. Surface preparation methods to alleviate stress cracking include abrading the surface with sandpaper, cleaning with isopropyl alcohol, and assembling the parts immediately after application of alcohol [14,15,17]. The use of an incompatible adhesive for the substrates can produce stress cracks. The part surface softens and weakens, creating a crack. Joining materials with different coefficients of thermal expansion can produce stress cracking when the part is subjected to high or low temperatures.
Plastics expand at high temperatures and contract at low temperatures to different amounts, depending on their coefficients of thermal expansion. After bonding, part movement is restricted, and the two materials must expand or contract to the same extent. If the plastics have different coefficients of thermal expansion, stresses are produced, which lower the strength of the joint. Coefficients of thermal expansion can be decreased by adding fillers or reinforcements or by increasing the amount of cross-linking. If material selections cannot be modified, thicker bond lines and more flexible adhesives can help reduce problems with stress cracking; however, the adhesive film cannot restrain large relative motions of parts [14–16].
Chemical attack of uncured UV-curable adhesives produced stress cracking in medical grade polycarbonate. Stress cracking was more prevalent in low-molecular-weight polycarbonates; increasing polycarbonate molecular weight from 22,000 to 26,000 increased the bond strength by 15%. In solvent bonding of hollow articles, stress cracking can result if enclosed areas are not vented [18,19]. In solvent bonding, plasticizer migration from a less rigid substrate, such as flexible PVC, to a more rigid substrate can result in stress cracking and crazing, depending on the solvents and combination of substrates used in bonding. Plasticizer migration will occur after a plasticizer-free surface has been obtained. The most satisfactory adhesives for PVC are the acrylates, epoxies, urethanes, and hot melts [17].
The mixture of poor solvents, one with a δ too high and the other with a δ too low can have a net δ within the required range of solubility. For example, acetone (δ=10.0) mixed with a small percentage of the poor solvent ethanol (δ=12.7) is a better solvent for cellulose acetate (δ=10.0) than acetone alone. Generally, the more polar plastics require more polar solvents. Figure 9.1 shows good correlation between the solubility parameter and the critical surface tension for polymers at the low end of both scales. With increasing values, however, anomalies become apparent. The discrepancy can be attributed, at least in part, to differences in crystallinity, the presence of compounding ingredients, and differences in chemical composition of the bulk polymer from the surface. Surface treatment of polyethylene may have a strong effect in that polymer. Where solubility parameters and contact-angle measurements disagree, the latter provides the better direction for choosing adhesives, provided they have been carried out on the materials as they are actually prepared for bonding [4,7].
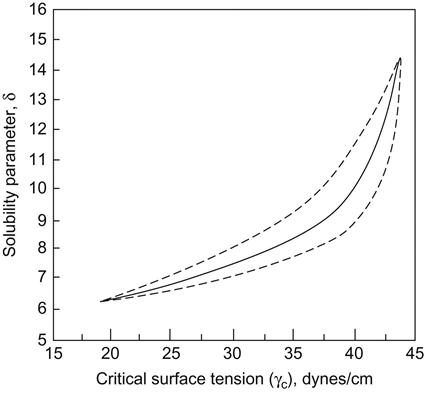
A good source for solubility parameters of a wide range of materials has been published by CRC Press [20].
9.3 Solvents for Specific Polymers
Table 9.3 shows some of the suitable solvents for cementing/welding a few common plastics.
Table 9.3
Suitable Solvents for Solvent Welding Various Plastics [21]a
| Plastic | Solvent | ||||||||||||||
| Acetone | Cyclohexanone | N,N-Dimethyl Formamide | Ethyl Acetate | Dichloroethane | Dichloromethane | Glacial Acetic Acid | Methyl Ethyl Ketone | 2-Methoxy Ethanol | N-Methyl Pyrrolidone | O-Dichlorobenzol | Tetrachloroethylene | Tetrahydrofuran | Toluene | Xylene | |
| ABS | |||||||||||||||
| Acrylic | |||||||||||||||
| Cellulose acetate | |||||||||||||||
| Polyaryl ether | |||||||||||||||
| Polyaryl sulfone | |||||||||||||||
| Polycarbonate | |||||||||||||||
| Polystyrene | |||||||||||||||
| Polysulfone | |||||||||||||||
| PVC | |||||||||||||||
| PPO | |||||||||||||||
| Styrene–acrylonitrile | |||||||||||||||
| Vinylidene chloride | |||||||||||||||
| Polyamide | Formic acid, phenol, resorcinol or cresol in aqueous or alcoholic solutions, calcium chloride in alcoholic solution | ||||||||||||||
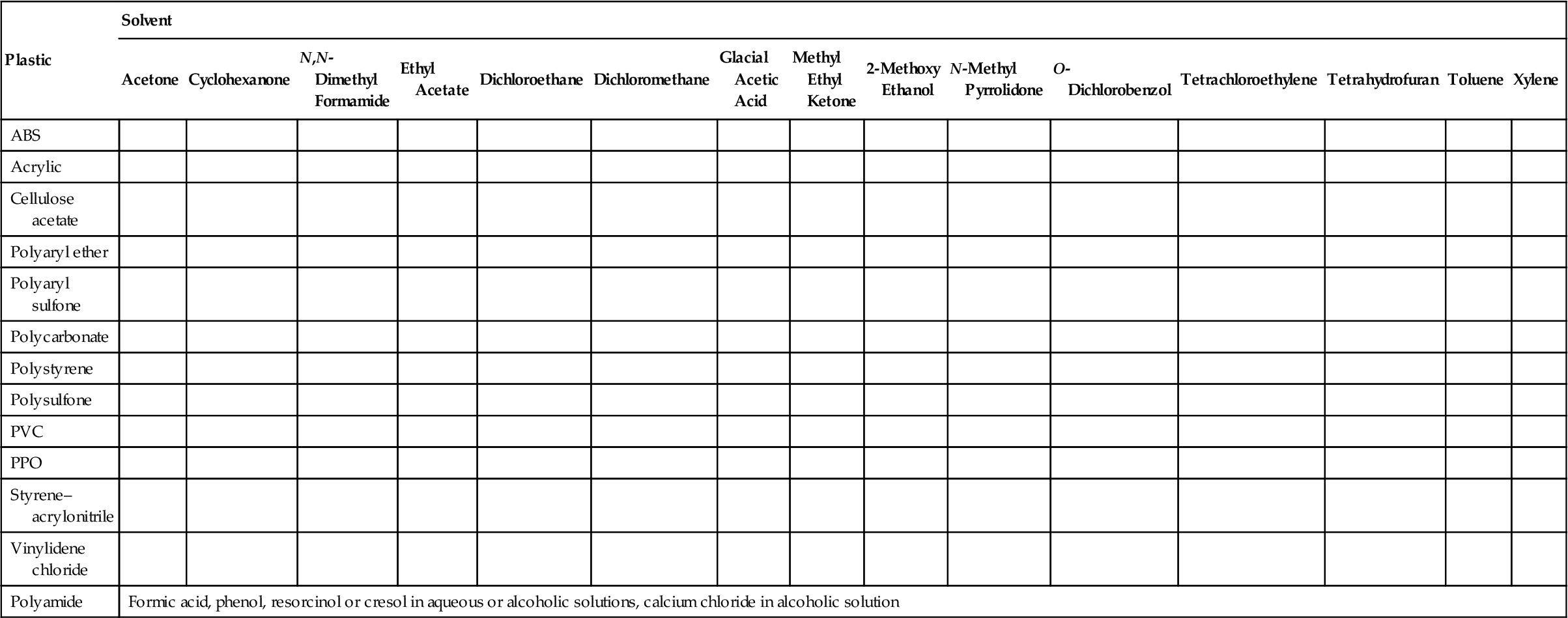
aOriginal source [22].
ABS, acrylonitrile–butadiene–styrene; PVC, polyvinyl chloride; PPO, polyphenylene oxide.
9.3.1 Acetal Copolymer
A room temperature solvent, hexafluoroacetone sesquihydrate, when used at full strength, is a very effective bonding agent for bonding acetal copolymer to itself, to nylon, and to ABS. Bond strengths (in shear mode) for acetal copolymer binding to itself and acetal copolymer to nylon >5.86 MPa have been obtained on Celcon® to ABS. Hexafluoroacetone sesquihydrate is a severe eye and skin irritant and care should be taken in its use [23].
9.3.2 Acetal Homopolymer
Because of the high solvent resistance of acetal homopolymers (e.g., Delrin®), molded surfaces cannot be joined by the use of cements unless they have been specially roughened [24,25].
9.3.3 Acrylonitrile–Butadiene–Styrene
See Section 9.3.1 for a discussion of the use of hexafluoroacetone sesquihydrate in bonding ABS to acetal copolymer. Solvents used in cementing ABS should be quick drying to prevent moisture absorption, yet slow enough to allow assemblage of parts. The recommended cure time is 12–24 h at room temperature, 23°C. The required time can be reduced by setting at 55–70°C. The solvents recommended include methyl ethyl ketone, methyl isobutyl ketone, tetrahydrofuran, and methylene chloride. These solvents can be “bodied” with up to 25% ABS resin [15]. ASTM D2235, a specification on solvent cement for ABS pipe and fittings, calls for a solution of ABS (min. 15%) in methyl ethyl ketone [26]. ASTM D3138 covers solvent cements for ABS-PVC transition joints for plastic pipe. A minimum of 10% PVC must be used in tetrahydrofuran in combination with cyclohexanone, or methyl ethyl ketone, or both [27].
9.3.4 Cellulosics
These plastics include cellulose acetate, cellulose acetate butyrate (CAB), cellulose nitrate, cellulose propionate, and ethyl cellulose. These materials are most commonly bonded by solvent cementing. Adhesive bonding is used to a minor extent.
9.3.4.1 Cellulose Acetate
The solvents listed in Table 9.4 may be used alone in cementing cellulose acetate [28].
Table 9.4
Specific Solvents for Cellulose Acetate [28]
| Acetone | Dioxane | Ethyl lactate |
| Methyl acetate | Nitromethane | Cellosolve acetate |
| Ethyl acetate | Methyl Cellosolvea | Diacetone alcohol |
| Methyl ethyl ketone | Methyl Cellosolve acetate |
aCellosolve is an ethylene glycol monoethyl ether (2-ethoxy ethanol) made by Union Carbide Corporation.
Solvent mixtures recommended include:
– acetone/ethyl lactate (70/30)
– ethyl acetate/acetone/ethyl lactate (30/40/30)
– dope-type formulations: these may be made by including a 10–20% solution of cellulose acetate in any of the appropriate solvent mixtures shown above. A typical example is cellulose acetate/acetone/methyl Cellosolve/methyl Cellosolve acetate (18/55/20/7).
9.3.4.2 Cellulose Acetate Butyrate
CAB may be cemented with the solvents listed for cellulose acetate above in addition to those listed in Table 9.5 [28].
Table 9.5
Specific Solvents for CAB [28]
| Methylene chloride | Isopropyl alcohol | Cyclohexanone |
| Chloroform | Nitromethane | Butyl lactate |
| Ethylene dichloride | Butyl acetate |
Solvent mixtures suitable for CAB and CAB to cellulose propionate are:
– acetone/Ektasolve EM acetate (70/30)
– acetone/methyl Cellosolve acetate (70/30)
– butyl acetate/butyl lactate (80/20)
– acetone/butyl acetate/Ektasolve EM acetate (30/50/20)
– acetone/butyl acetate/methyl Cellosolve acetate (30/50/20)
– acetone/ethyl lactate (90/10)
– acetone/methoxyethyl acetate (80/20)
– butyl acetate/acetone/methyl acetate (50/30/20)
(Ektasolve is a solvent series of glycol ethers and glycol-ether esters available from Eastman Chemical Products, Inc., Kingsport, TN.)
Formulas for dope of “bodied” cements are as follows:
– CAB/acetone/ethyl acetate (20/40/40)
– cellulose propionate/acetone/methyl Cellosolve/methyl Cellosolve acetate (18/55/20/7).
CAB should not be solvent cemented to cellulose acetate. A nitrocellulose-based adhesive should be used for such joints. ASTM D2560, a specification of CAB pipe, tubing, and fittings, calls for a solution of CAB in one of the three thinners as follows [29]:
– thinner A: acetone/butyl acetate/methyl Cellosolve acetate (30/50/20)
– thinner C: acetone/toluene/methyl Cellosolve acetate (equal parts).
9.3.4.3 Cellulose Nitrate
This material may be readily joined to itself with acetone. To obtain high optical clarity, use medium-boiling ketones and esters, or mesityl oxide (isopropylidene acetone). Ethyl acetate, methyl acetate, butyl acetate, ethyl lactate, diacetone alcohol, and methyl ethyl ketone are also used. A dope-type cement made with 10% by weight of cellulose nitrate and 90% by weight of diacetone alcohol has also been suggested [28].
9.3.4.4 Cellulose Propionate
Cellulose propionate may be cemented with the solvents listed for cellulose acetate, and in addition, with those listed in Table 9.6 [29].
Table 9.6
Specific Solvents for Cellulose Propionate [29]
| Methylene chloride | Butyl acetate |
| Chloroform | Mesityl oxide (isopropylidene acetate) |
| Ethylene dichloride | Cellosolve |
| Isopropyl acetate | Cyclohexanone |
| Nitromethane | Butyl lactate |
9.3.4.5 Ethyl Cellulose
Solvents recommended are ethylene dichloride, butyl alcohol, and ethyl acetate. Mixtures suggested are [28]:
– toluol/ethanol (80/20) or (90/10)
– benzol (highly toxic) methanol (2:1) (67/33)
– ethyl acetate/ethanol (60/40) or (80/20)
– butyl acetate/toluol/ethanol (equal parts)
For a bodied cement, a suggested formulation is to use solvent solutions of the polymer ethyl cellulose in ethyl acetate/ethyl cellulose (80/20).
9.3.5 Nylons (Polyamides)
At room temperature, conventional solvents will not provide effective bonds on nylon. Generally, conventional adhesives are used. Three nonconventional solvent cements are sometimes used. These are the following:
Bonds produced with these cements are nonembrittling, tough, and quick-setting. Detailed directions for their preparation have been documented [30].
• Aqueous phenol: This cement, containing 10–15% water, is useful in bonding nylon 6,6 to itself. It can be purchased in this form from chemical supply houses.
• Resorcinol-ethanol: Equal parts of resorcinol and ethanol are stirred together or shaken together at room temperature for 15–20 min to dissolve the resorcinol. The concentration is not critical.
• Nylon-bodied calcium chloride-ethanol: 10 parts Zytel 101 NC-10, 22.5 parts calcium chloride, 67.5 parts ethanol.
For nylon 6, 15% by weight of nylon 6 is mixed with 85% formic acid (20 g nylon 6/100 ml formic acid). This mixture is brushed onto both surfaces to be joined. A 1-min period should be used to permit some of the formic acid to evaporate, leaving a tacky film. The surfaces should then be joined and allowed to set for 5 min at 0.69 MPa and 93°C. The higher plastic temperature will double the peel strength. Shear strengths of 8.28–17.2 MPa are obtained with this cement. Unfortunately, the cement is very toxic and highly corrosive because of the formic acid. Rubber gloves and proper ventilation are required when working with this material [28].
9.3.6 Polycarbonate
Solvent cementing is the most common method of bonding polycarbonate. It can be carried out with specific solvents (Table 9.7), mixtures of solvents, and mixtures of polycarbonate and solvents.
Table 9.7
Specific Solvents for Polycarbonate
| Methylene chloride | Tetrachloroethane |
| Ethylene dichloride | 1,1,2-Trichloroethane |
| Methyl methacrylate monomer (used with methylene chloride) | |
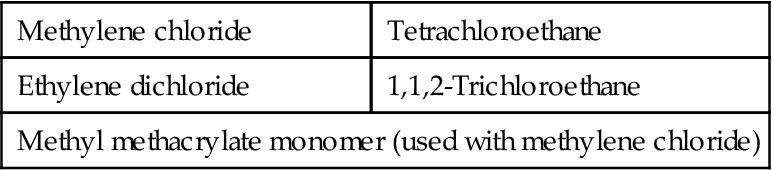
Methylene chloride when used alone has an extremely fast evaporation rate. This solvent is recommended for most temperate climate zones and small areas. A solution of 1–5% of polycarbonate in methylene chloride can be used in extreme cases where perfectly mated bonding areas are impossible to obtain. A mixture of methylene chloride with a maximum of 40% ethylene dichloride may be used, where it is difficult to join parts quickly enough to prevent complete evaporation of methylene chloride. The evaporation rate of methylene chloride is 6.7 times faster than that of ethylene dichloride. Bonds made with the mixture have strengths of 62.1–69.0 MPa. Tensile shear strengths of 31.0–44.8 MPa have been obtained by SaBIC Corp after 48 h of setting at room temperature for both methylene chloride and ethylene chloride solvent bonds. This is superior to conventional adhesive bond tensile shear strengths of 2.41–20.0 MPa [28].
9.3.7 Polystyrene
Polystyrene may be bonded to itself by solvent cementing; conventional adhesive bonding; thermal, spin, and ultrasonic welding; or electromagnetic bonding. However, solvent cementing is the most effective approach in many applications—can be used to bond polystyrene to a variety of dissimilar materials. A wide variety of solvent types are available, and the selection of the specific solvent to be used is determined by the time required to set the joint, which, in turn, is governed by the evaporation rate of the solvent. Fast evaporation rates result in a quick-setting joint, usually with crazing. Slow-drying solvents are often mixed with fast-drying cements for optimum results. A 50:50 mixture of ethyl acetate and toluol bodied with polystyrene is an excellent general-purpose adhesive. Perchloroethylene can be added to reduce flammability. Bond strengths up to 100% of the strength of the parent material are common [2].
Table 9.8 gives a list of some of the solvents recommended for polystyrene, along with notations on crazing and joint strength. As noted, the fast-drying solvents tend to cause crazing in the relatively low-elongation polystyrene. The less soluble impact grades contain polybutadiene. Solvents attack this additive and cause subsequent stress cracking. Impact-grade polystyrene should be bonded with medium- to slow-drying solvents. Quantities of polystyrene from 5% to 15% by weight are often added to provide gap filling [28]. Polystyrene resin ground to a powder and dissolved in an appropriate solvent provides excellent gap filling when poor-fitting parts are to be joined. One formula suggested is 90% toluene and 10% polystyrene. A polystyrene cement recommended for transparent joints consists of the following mixture [30]:
High-boiling solvent (boiling above 200°C)
(The dissolved polystyrene increased to 15% for airtight or watertight seals).
Table 9.8
Solvents Recommended for Cementing Polystyrene [31–33]
| Solvent | Boiling Point (°C) | Crazing | Tensile Strength of Joint (MPa) |
| Fast-drying (20 s or less) | |||
| Methylene chloride | 104 | Yes | 12.4 |
| Ethyl acetate | 171 | Yes | 10.3 |
| Methyl ethyl ketone | 175 | Yes | 11.0 |
| Ethylene dichloride | 182 | Slight | 12.4 |
| Trichloroethylene | 187 | Slight | 12.4 |
| Medium-drying | |||
| Toluene (toluol) | 111 | Slight | 11.7 |
| Perchloroethylene (tetrachloroethylene) | 120.6 | Very slight | 11.7 |
| Xylene (xylol) | 133–143 | Very slight | 10.7 |
| Diethyl benzene | 185 | Very slight | 9.7 |
| Slow-drying | |||
| Monoamyl benzene | 202.2 | Very slight | 9.0 |
| Ethyl naphthalene | 257.2 | Very slight | 9.0 |
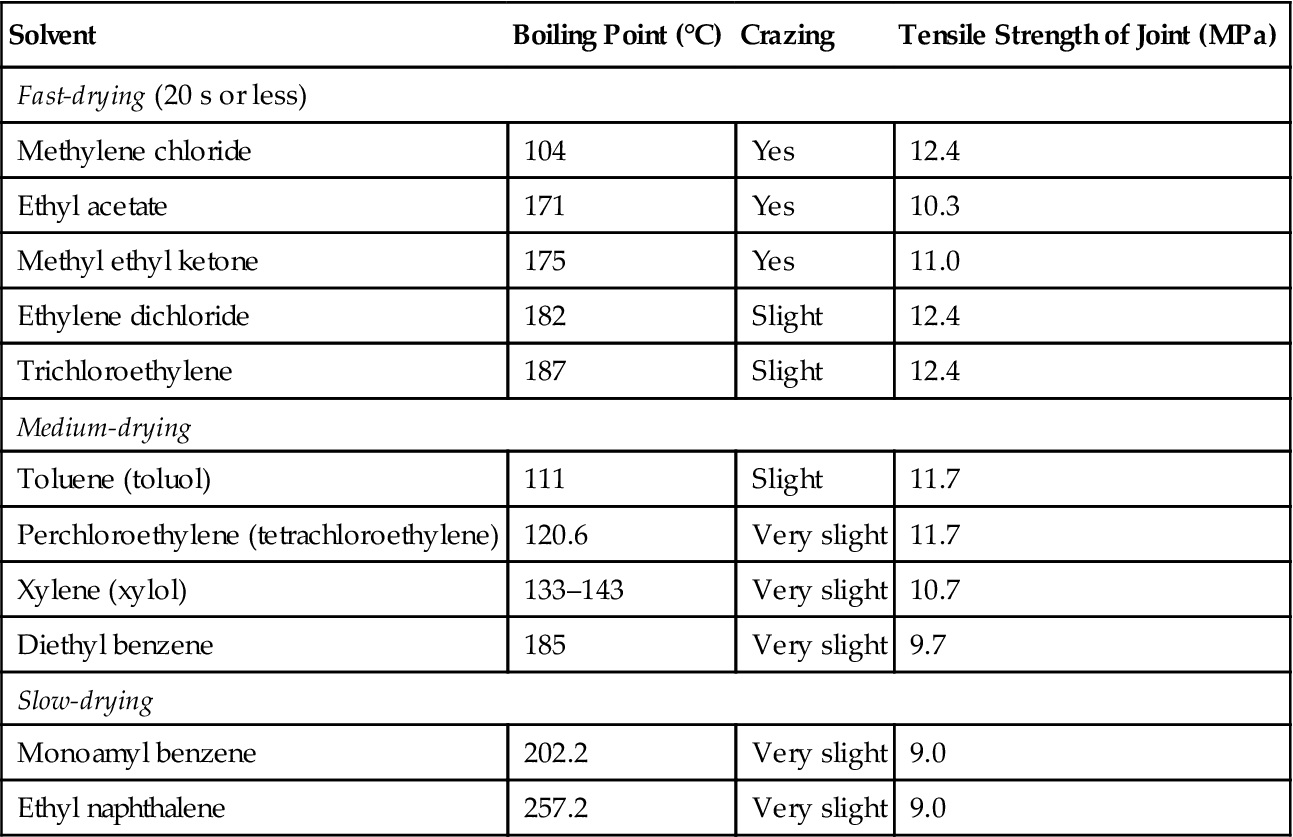
In general, where optical clarity or maximum mechanical properties are not mandatory, solvents in the range of 77–121°C provide satisfactory drying time, good sealing, and high bond strength [34].
9.3.8 Styrene–Acrylonitrile
The techniques used for solvent cementing polystyrene are applicable to SAN, but the list of solvents is more restricted. Solvent cements recommended are given in Table 9.9.
Table 9.9
| Acetone | Methylene chloride |
| Methyl ethyl ketone | Ethylene dichloride |
| Tetrahydrofuran |
Solutions of approximately 5% SAN in methyl ethyl ketone may be used effectively as bodied cements [32].
9.3.9 Polysulfone
Solvent cementing of polysulfone can be carried out with chlorinated hydrocarbons. A solution of 5% polysulfone resin in methylene chloride can be used to bond polysulfone to itself. High pressures (3.45 MPa) for 5 min are required. A minimum amount of solvent should be applied to the mating surfaces. The strength of a properly prepared joint will exceed the strength of the polysulfone parts. Polysulfone can be solvent cemented to other plastics using a solvent compatible with both plastics [28,32].
9.3.10 Polybutylene Terephthalate (Valox®)
Solvents recommended for this solvent-resistant plastic are hexafluoroisopropanol and hexafluoroacetone sesquihydrate, used separately or in combination. The solvent is brushed on the mating surface and dried under pressure. These solvents are toxic and should be applied only in areas of positive ventilation [35]. A recent design guide by the manufacturer (SABIC Corp) omits any mention of solvent cementing [35].
9.3.11 Polymethyl Methacrylate
Acrylics such as polymethyl methacrylate should be annealed before solvent cementing to minimize the formation of internal stresses that can cause crazing. Acrylic sheets can be annealed by heating in a forced-air oven at about 5°C below the temperature which will cause the part to distort (heat distortion temperature). Thin sections of acrylics are ordinarily heated for 2 h at 60°C for easy-flow formulations, while hard flows will require temperatures of 77°C. Thicker sections will require much longer periods.
Solvent cements recommended for acrylics include ethylene dichloride, methylene chloride, and methylene chloride/diacetone alcohol (90/10) for medium joint strength, and a blend of methylene chloride/methylmethacrylate monomer (60/40) with 0.2 parts of benzoyl perioxide catalyst and sufficient acrylic resin for body for high joint strength. This type of monomer–polymer cement sets by conversion of the liquid monomer into solid polymer and have the advantage of fast initial set, the cemented joints being usually sufficiently hard and strong for machining within 4 h after assembly. The pot life of monomer–polymer mixtures is very short (1 h), however, because of the nonreversible polymerization reaction. Joint strengths of these mixtures are excellent and weathering resistance is very good [36].
There are commercial acrylic cements available [37], which consist of solutions of acrylic resins in solvents. Parts to be joined should be clean, and fited without forcing. Apply cement with syringe, eyedropper, or brush. Assemble while parts are still wet. If cement is applied to one surface, let the two surfaces be in gentle contact for a few seconds to allow the cement to soften the dry surfaces, then press parts together in firm contact.
For the capillary method, parts are placed lightly together and cement is applied to the edge of the joint via syringe or eyedropper. By capillary action, the cement will flow a considerable distance (approximately 6 mm) between two such surfaces. Allow a few seconds for the cement to soften the surfaces. Press parts firmly together.
For the soak method, vertically dip surfaces until softened (approx. 2–5 min); then join the pieces firmly together. Initial bonds form very quickly. Bond strength continues to develop very rapidly, reaching high levels within 24 to 48 h. Thereafter, strength will continue to increase gradually for some weeks.
9.3.12 Phenylene Oxide-Based Resins (Noryl®)
This material may be solvent cemented to itself or to certain dissimilar plastics, using a number of commercially available solvents, solvent mixtures, and solvent solutions containing 1–7% of the resin. The addition of 5–20% of the resin will reduce the evaporation rate and fill minor imperfections on the surface of the bonded joints. Recommended solvents are shown in Table 9.10. The solvents and solvent combinations shown for cementing phenylene oxide-based resins to themselves are especially designed to control the evaporation rate [38].
Table 9.10
Solvent Combinations for Cementing Phenylene Oxide-Based Resins (Noryl) [38]
Noryl to Noryl
For surface areas <0.1 m2 and/or open time <60 s
Trichloroethylene/methylene chloride (1/1)a
Trichloroethylene/1,2-dichloroethylene (1/1) (15 s)
Trichloroethylene (30 s)
Trichloroethylene/monochlorobenzene (4/1)b (45 s)
For surface areas >0.1 m2 and/or open time >60 s
Trichloroethylene/monochlorobenzene (1/1)
Trichloroethylene/toluene (1/1)
Trichloroethylene/monochlorobenzene (4/1)+5–25%
Noryl weight/vol. If more open time is needed, increase monochlorobenzene by about 10 parts at a time up to a maximum of 60 parts
Noryl to ABS/PVC alloy
Trichloroethylene/monochlorobenzene/tetrahydrofuran (1/12)
Noryl to ABS
Trichloroethylene/methyl ethyl ketone (4/1)
Trichloroethylene/xylene (1/1)
Noryl to PVC or CPVC
Xylene/methyl ethyl ketone (1/1)
Tetrahydrofuran
Tetrahydrofuran/trichloroethylene (1/1)
aSignifies equal parts on a volume basis.
bSignifies four parts to one part, respectively, on a volume basis.
To attain maximum bond strength with solvent-cemented Noryl joints, the manufacturer of the resin recommends the following steps [38]:
1. Remove all surface contaminants, such as grease, oil, and dust with an isopropyl alcohol wipe. Avoid use of mold release agents if possible, either directly or in the vicinity of the molding or extrusion operations.
2. Abrade the surface lightly with fine sandpaper or treat with chromic acid etchant (E-20 etchant, Marbon Co.). When etching, best results are obtained by immersing the areas to be bonded in an 80°C (176°F) bath for 50–60 s.
3. Wipe the bond surfaces again with a cloth dampened in isopropyl alcohol.
4. Apply the solvent to be used for cementing to both surfaces and quickly join the two parts. Rapid connection of the bonding surfaces will prevent excessive solvent evaporation.
5. Clamp the parts together as soon as they are joined. The amount of pressure required will generally depend on the part geometry. Moderate pressure will usually suffice. Clamping pressure should be sufficient to insure good interfacial contact, but not so high that the parts are deformed, or that the solvent is forced from the joint.
6. Maintain uniform clamping pressure for 30–50 s, or as long as the particular part requires. Bonded parts may be handled safely after the original hold time, although maximum bond strength is usually reached at a later time (Table 9.10).
9.3.13 Polyvinyl Chloride
The homopolymer of PVC is not readily soluble and is therefore difficult to bond by solvent-cementing techniques, although a number of solvents and solvent mixtures have been used with varying degrees of success [30,34]. A large number of solvents have been suggested for solvent cementing PVC (given in Table 9.11 [28]).
Table 9.11
Specific Solvents for PVC [28]
| Ketones | Alcohols |
| Acetone | Methanol |
| Methyl ethyl ketone | Ethanol |
| Methyl isobutyl ketone | Isopropanol |
| Isophorone | |
| Cyclohexanone | |
| Other solvents | |
| Propylene oxide | Trichloroethylene |
| Toluol | Petroleum ether (low-boiling fraction) |
| Xylol | Methylene chloride |
| Tetrahydrofuran | Ethyl acetate |
| Dimethyl formamide | Dichlorobenzene |
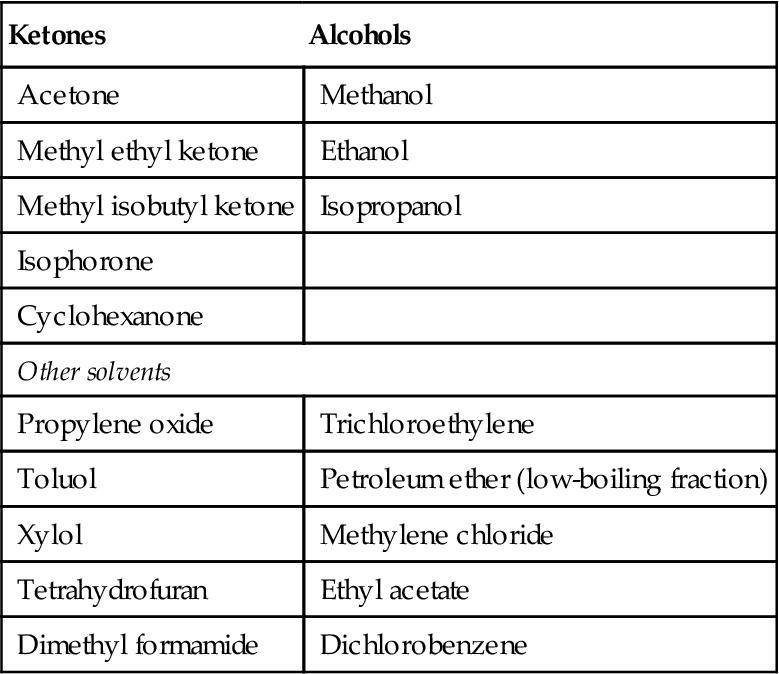
When smooth, rigid PVC surfaces are to be joined, the preferred method of using solvent cements is to apply the cement to the two edges of the pieces while they are clamped closely together, thus permitting the solvent to flow between them by capillary action [11]. Ketones are often used and propylene oxide (boiling point 35°C) is usually included as an ingredient, as it contributes to very rapid attack on the plastic. The propylene oxide should be blended with high-boiling ketones, such as methyl ethyl ketone and methyl isobutyl ketone. A moderate percentage of an aromatic hydrocarbon is sometimes used to hasten softening of the PVC. Methyl ethyl ketone and methyl isobutyl ketone are better solvents for the low- and medium-molecular-weight copolymers, and the homopolymers usually require the more powerful cyclohexanone, or 5% dioctylphthalate may be added to improve the flexibility at the joint and to reduce stresses. Acetic acid is sometimes added to increase the “bite” of the cement. A mixture of solvents and nonsolvents is frequently used, as given in Table 9.12 [28].
Table 9.12
Composition of Mixture for PVC Cementing [28]
| Components | Part by Weight |
| Dioxane | 20 |
| Methanol | 12 |
| Methyl ethyl ketone | 60 |
| Dioctylphthalate | 3 |
| Glacial acetic acid | 2 |
| iso-Phorone | 3 |
Dissolved chips or shavings of PVC will increase the viscosity of the solution and make the solvent more effective in joining mating surfaces that are not perfectly smooth. Another formulation that will work with either flexible or rigid PVC is listed in Table 9.13 [28].
Table 9.13
Composition of High Viscosity Mixture for PVC Cementing [28]
| Components | Part by Weight |
| PVC resin, med. mol. wt. (22.4% by wt.) | 100 |
| Tetrahydrofuran | 100 |
| Methyl ethyl ketone | 200 |
| Methyl isobutyl ketone | 25 |
| Dioctylphthalate | 20 |
| Organic tin stabilizer | 1.5 |
Care must be used in handling this formulation because of the slightly toxic nature of the tetrahydrofuran. Good ventilation is required [28].
ASTM D2564 specifies a solvent cement used for PVC pipe and fittings. No particular solvent is recommended, but a minimum of 10% PVC resin must be used for bodying. Solvent systems consisting of blends of tetrahydrofuran and cyclohexanone are suggested [39].
9.3.14 Chlorinated Polyvinyl Chloride
An ASTM specification, ASTM F493, covers solvent cements for CPVC pipes and fittings. No particular solvent system is specified, but solvent systems consisting of blends of tetrahydrofuran and cyclohexanone are suggested. A minimum of 10% CPVC resin is required for bodying [40].
9.3.15 Polyetherimide (Ultem®)
Methylene chloride, with or without a 1–5% solution of Ultem® resin, is recommended by SABIC Corp., the manufacturer. Moderate pressures of 6.89–41.3 MPa for 5 min are required [41].
9.4 Solvent Cementing—A Commercial Perspective
Solvent-cemented connection in thermoplastic pipes and fittings is the last vital link in a plastic pipe installation. It can mean the success or failure of the system as a whole. Accordingly, it requires the same professional care and attention that are given to other components of the system. There are many published solvent cementing techniques that cover step-by-step procedures on making solvent-cemented joints. However, explanation of basic principles is required for a better understanding of the techniques including temperature and variations in size and fits of pipe and fittings [42].
Of paramount significance are good safety practices. Solvent cements for pipe and fittings are flammable, requiring removal of heat or flame in working or storage areas. Work areas must be well ventilated and unnecessary skin contact with all solvents must be avoided. Safety issues should be consulted prior to starting any work.
To make good joints consistently the following items should be considered [42]:
1. The joining surfaces must be softened and made semifluid.
These areas must be softened and penetrated. This can be achieved by the cement itself using a suitable primer or both primer and cement. An effective primer will usually penetrate and soften the surfaces more quickly than the cement by itself.
2. Sufficient cement must be applied to fill the gap between pipe and fitting.
Excess quantity of cement to fill the gap in the loose part of the joint must be applied. In addition to filling the gap, adequate cement layers will penetrate the joining surfaces and remain fluid until the joint is assembled.
3. Assembly of pipe and fittings must be made while the surfaces are still wet and fluid.
If the cement coatings on the pipe and fittings are wet and fluid when assembly takes place, they will tend to flow together and become one layer. Also, if the cement is wet the surfaces beneath them will still be soft, and these softened surfaces in the tight part of the joint will tend to fuse together.
4. Joint strength develops as the cement dries. In the tight part of the joint the surfaces will tend to fuse together; in the loose part the cement will bond to both surfaces.
As the solvent dissipates, the cement layer and the softened surfaces will harden with a corresponding increase in joint strength. A good joint will take the required working pressure long before the joint is fully dry and final strength is obtained. In the tight (fused) part of the joint, strength will develop more quickly than in the looser (bonded) part of the joint. Information about the development of bond strength of solvent-cemented joints is available.
Solvent Cementing in High Temperatures [42]:
1. Store solvent cements in a cool or shaded area prior to use.
2. If possible, store the fittings and pipe, or at least the ends to be solvent welded, in a shady area before cementing.
3. Cool surfaces to be joined by wiping with a damp rag. Be sure that surfaces are dry prior to applying solvent cement.
4. Try to do the solvent cementing in cooler morning hours.
5. Make sure that both surfaces to be joined are still wet with cement when putting them together.
Solvent Cementing in Cold Temperatures [42]:
1. Prefabricate as much of the system as possible in a heated work area.
2. Store cements in a warmer area when not in use and make sure they remain fluid.
3. Take special care to remove moisture, including ice and snow.
4. Use special care to ensure joining surfaces are adequately softened; more than one application may be necessary.
