Advanced bioactive and biodegradable ceramic biomaterials
Abstract:
Bioinert high-strength ceramics have been shown to be suitable for load-bearing applications. Bioactive ceramics capable of forming direct chemical bonds with hard and soft tissues have been the ceramics traditionally used for most medical implantation devices. Recently, bioresorbable ceramics that actively participate in the metabolic processes of an organism into which they are implanted are attracting greater levels of attention. This chapter discusses ceramics that can not only physically mimic the osseous tissue but also competently initiate the biological processes associated with osteogenesis. Their ability to deliver biological and chemical molecules and then safely disintegrate within the body extend their clinical utility from tissue regeneration to highly controlled drug and vaccine delivery and in vivo visualisation.
8.1 Introduction
Valued for their strength, toughness and chemical inertness, ceramic biomaterials have found a wealth of medical applications. The superior mechanical and tribological performance of materials such as alumina, zirconia and titania mean that they can withstand long-term wear under load-bearing conditions, while chemical inertness ensures that these materials do not elicit a strong inflammatory reaction in vivo.
However, in spite of their biocompatibility, inert ceramics remain a foreign entity in the human body and therefore they are unable to be fully replaced by osseous tissue. As a result, ceramic implants have the potential to elicit some degree of inflammatory response within the body. Indeed, inert ceramics are subject to such intrinsic shortcomings as limited tissue in growth and non-adherence of fibrous membrane to the implant surface.
Bioactive ceramics have been developed to address this drawback. These modified ceramics, known as second-generation ceramics, are designed to imitate the natural bone surfaces, with the ability to incite osseous tissue formation and biomineralisation in vitro and in vivo. Bioactive ceramics, typically hydroxyapatite and other calcium phosphate phases, are able to interact with the ambient physiological environment within the body. This leads to the formation of a biologically relevant apatite on the surface of these ceramics. These biological apatites are highly favourable in terms of osteoblast attachment, proliferation and mineralisation. Although biologically superior to inert ceramics, bioactive ceramics are characterised by poor mechanical properties that limit their clinical use to non-load-bearing applications. Some typical applications include small fillers, grains and coatings.
Temporary scaffolding systems represent the third generation of ceramics. These have the potential to provide adequate mechanical support and osteo-induction for the period required for the tissue to fully regenerate. As the tissue recovers, the scaffolding system is designed to be resorbed and/or biodegrade to products that can be either removed from the implantation site by natural metabolic activity, or consumed by the growing tissue. Several soluble calcium phosphate ceramics have been identified that possess the required degree of osteo-induction and bioresorption to fulfil this role; however, further in-depth characterisation and optimisation of these materials is desired. The regenerated tissue should not only be able to physically replace the lost tissue, but the replacement tissue should, in turn, be capable of performing the same natural functions as the original damaged tissue (Wang et al., 2012b).
8.2 The development of bioactive ceramics for tissue engineering
Recent developments in the area of materials engineering coupled with an improved understanding of tissue regeneration processes has seen bioactive glasses and biodegradable ceramics attracting significant interest for a wide range of applications, spanning from bone fillers to bioactive coatings and controlled drug delivery systems (Silva et al., 2012).
Complications associated with surgical repair, for example peri- and postoperative infection, wound dehiscence, implant protrusion, etc, have further highlighted the potential benefits of biologically active materials compared to inert implantable structures (Engstrand, 2012).
The ability of bioactive ceramic materials to promote host tissue chemical bonding and stimulate cell growth is particularly attractive, with significant research efforts being devoted to the development of tissue scaffolds capable of enhancing the attachment, differentiation and proliferation of bone-forming cells (Zhang et al., 2011). The scaffolds are temporary three-dimensional (3-D) templates that are designed not only to promote cell growth and the development of bone-extracellular matrix, but also to serve as a structural support throughout the process of tissue formation. With adequate design, these structures are capable of effectively replacing natural bone grafts, thus serving as an enabling synthetic platform for treatment of various bone diseases, including challenging fractures and large degenerative osseous defects.
The bioactivity of ceramic scaffolds, and thus their utility in bone restoration applications, is highly dependent on how accurately these structures imitate the natural human bone, particularly with regard to bone morphology as well as bone mechanical properties. In terms of composition, bone is a type of dense (hard) connective tissue, comprised of an organic protein matrix containing hydroxyapatite (Ca10(PO4)6(OH)2) crystals. The organic phase makes up approximately 30% of bone tissue, and is primarily composed of type I collagen (90–95%) and other non-collagenous proteins. A type of fibrous protein, collagen, is present in the form of fibres with a total weight of 95 to 102 kDa, and enables bone strength and flexibility. Depending on the orientation of the collagen fibres, there are two types of osseous tissues that form bones, namely cancellous (non-lamellar, also known as trabecular) and cortical (lamellar) bones. Cancellous tissue is relatively soft and spongy, characterised by an extensive network of interconnected pores with typical porosity of 50 to 90% and pore dimension at the order of 1 mm in diameter, whereas cortical bone is stronger and denser, with the representative porosity of 3 to 12% and a range of voids. Consequently, the characteristic apparent density values for femoral cortical bone are 1.85 ± 0.06 g/cm3, while the apparent density values for proximal tibial trabecular bone are approximately 0.30 ± 0.10 g/cm3. The highly vascular cancellous tissue is observed in vertebrae, at the ends of long bones and at fracture joints. The surrounding solid cortical bone tissue serves as a protective layer.
An inorganic mineral phase comprises close to 70% of the bone, with calcium phosphate, calcium carbonate, calcium fluoride, calcium hydroxide and citrate also present. Although principally crystalline in the form of rods and platelets, the inorganic components can also appear in amorphous forms. The dimensions of the crystals range between 8 and 15 A in thickness, 20 and 40 A in width and 200 and 400 A in length. The mineralisation of collagen tissues is initiated when minerals begin to nucleate into the holes and pores of the collagen fibrils (Barrère et al., 2006). The nucleation is a heterogeneous process driven by carboxylate and phosphated ester groups of the collagen network. In terms of chemical reactivity, hydroxyapatite crystals can undergo intercrystalline exchange or recrystallisation due to dissolution and reformation of crystals, resulting in the replacement of Ca2 + with different ions or adsorption of new ions on the surface of the crystal. For example, dentin and dental enamel are primarily comprised of carbonated calcium-deficient hydroxylapatite. In general, the extent of mineralisation differs, depending on several different factors, including the type of bone, and the age and the health status of the patient. As an example, Follet et al. (2004) reported the degree of mineralisation of 1.135 ± 0.147 g/cm3 and 1.098 ± 0.077 g/cm3 for trabecular bone from the calcaneus and the iliac crest, respectively.
In addition to exhibiting a bone morphological similarity, the scaffold should mimic the mechanical behaviour of the adjacent bone tissue, to ensure tissue integration and minimise bone-shielding effects. The mechanical characteristics of osseous tissues are dependent on the growth stage of the individual. The ultimate strength of the bone tissue also varies between the bone types and the status of the patient, being dependent on the amount of bone (bone mass), the spatial distribution of the bone tissue, namely bone shape and micro-architecture, and the inherent properties of the bone matrix. For instance, the elastic modulus of femoral bone tissue in a 35-year-old individual has been reported to be approximately 16.7 GPa, whereas the elastic modulus of the same cortical tissue in a 3-year-old child is significantly lower, at 7 GPa (Currey, 2004). However, once the individual is fully matured, the tensile strength and elastic modulus values of femoral cortical bone have been shown to decline by approximately 2% per decade (Karageorgiou and Kaplan, 2005). The decline in trabecular bone mass is even more profound, with the cancellous bone density declining by 45 to 56% between the ages of 20 and 90. In addition to the age of the individual, the mechanical performance of the bone will be notably influenced by the mechanical loading to which the bone is subjected and the general health status of the patient, for example metabolic diseases, hormonal balance, availability of nutrition, etc. (Fonseca et al., 2012; Nicolaije et al., 2012). The aforementioned patient-specific variations in bone properties introduce additional complexities to the design of well-matched tissue scaffolds.
Another important factor in the design of tissue scaffolds is the biocompatibility of the material chosen for the structure. In vivo, the scaffold will be directly exposed to several types of bone cells, namely osteoblasts, osteoclasts, osteocytes and bone lining cells, and a host of biological molecules. Bone lining cells are inactive cells that cover the surfaces of bones, preventing the transport of certain ions; these cells are believed to be osteoblast precursors. Osteoblasts are intimately involved in bone formation via production and mineralisation of bone matrix, whereas osteoclasts are cells responsible for the resorption of the bone tissues. Osteoblasts are mononuclear cells that secrete a collagen-rich osteoid matrix, to which calcium salts and phosphorous is bonded to mineralise the tissue. These cells also secrete alkaline phosphatase, a hydrolase enzyme that removes phosphate groups from biological molecules. Osteoblasts can be stimulated by a range of hormones and biologically active molecules, such as estrogens that have been shown to augment the amount of collagen production through increasing the number of osteoblasts present (Lerner, 2006).
During the process of bone formation, entrapped osteoblasts lose their mobility and can differentiate into osteocytes. Osteocytes are mechano-sensory cells that are the most prevalent cell type in bone, and believed to be responsible for the maintenance of the bone matrix through a complex signalling activity. They control the extracellular concentration of calcium and phosphate, and can be directly stimulated by the hormone thyrocalcitonin (also known as calcitonin) and inhibited by parathyrin. In addition to these hormones, 1,25(OH)2 vitamin D3 is also involved in maintaining the mineral homeostasis within the bone (Fortunati et al., 2010; García et al., 2013; Stancoven et al., 2013). Osteoclasts are multinuclear cells that attach to mineralised bone and secrete bone-eesorbing enzymes that digest the bone tissue. As with other bone cells, osteoclasts can be stimulated or inhibited by a range of hormones, including sex hormones, cytokines and growth factors. Among others, insulin-like growth factors, platelet-derived growth factors, fibroblast growth factors, vascular endothelial growth factors, transforming growth factors and bone morphogenetic proteins have been shown to regulate bone metabolism, function and regeneration (Biver et al., 2013; Issa et al., 2012; Kaigler et al., 2013; Karageorgiou and Kaplan, 2005; Machado et al., 2012; Vo et al., 2012; Wang et al, 2012a).
In scaffold-mediated tissue remodelling, the bone cells are transferred onto a biomaterial matrix from the adjoining living bone. The formation of the new bone tissue on the underlying implant material is termed osteo-conduction, and is commonly observed on highly biocompatible bone substitute implants. However, osteo-conductivity alone is not sufficient to initiate osteo-induction, the process of recruitment of immature bone precursor cells and activation of these cells to differentiate into pre-osteoblasts. In order to overcome this limitation, scaffolds can be loaded with specific growth factors, such as those listed above, capable of signalling recruitment and inducing transformation of recruited undifferentiated mesenchymal host cells into pre-osteoblast cells (Reichert et al., 2012; Zanetti et al., 2013). In addition to growth factor delivery, an in vitro bioreactor system approach can be applied to speed up in vivo healing. In this scenario, the mesenchymal stem cells (with the potential to transform into osteoblasts and/or pre-osteoblasts) are isolated from the host, expanded ex vivo and seeded onto the biomaterial matrix to produce an extracellular matrix (Khan et al., 2012). Once the extracellular matrix is attained, the scaffold is implanted into the host to treat the bone defect or trauma site. The pace of ex vivo cellular production can be further enhanced through use of gene therapy, whereby genetically transduced adult stem cells capable of expressing osteoinductive factors, such as bone morphogenetic proteins (BMP2, BMP4 and BMP7), core binding factor α1 (Cbfa1), vascular endothelial growth factor and noggin (NOG), are employed (Fleming et al., 2000; Khan et al., 2012; Zanetti et al., 2013).
Another important consideration for the design of scaffolds is its timely biodisintegration, which is temporally well matched to the remodelling process to facilitate cell migration within the porous network of the biomaterial scaffold. In addition to timely biodegradation kinetics, there are several other important considerations for biodegradation. The degradation process should not prematurely compromise the mechanical integrity of the scaffold. The degradation products have to be non-toxic and preferably be either used up by the cells or removed from the peri-implant space as part of normal metabolic activity. The particles and ions released in the course of degradation should illicit minimal pro-inflammatory response so as not to compromise the tissue healing process. Furthermore, these degradation products should create conditions that speed up the degradation of the main matrix. Zanetti et al., (2013) suggested that biocompatibility of an implanted scaffold is optimal if it is capable of inducing the formation of normal tissues on its surface, while concomitantly proving a contiguous interface capable of withstanding the load, which may transpire at the site of implantation.
8.3 Calcium phosphates
In spite of significant advancement in medicine, bone substitution remains a challenge. In bone tissue engineering, naturally porous, highly biocompatible calcium phosphates are an attractive candidate for the fabrication of osteo-inductive and osteo-conductive scaffolds (Barradas et al., 2012; Grandfield et al., 2012; Nie et al., 2012; Roohani-Esfahani et al., 2012). Biomedically relevant calcium phosphates can be synthetically derived, either by means of precipitation from aqueous solution or sintering, or naturally from freeze-dried bone and hydroxyapatite of marine origin (Meejoo et al., 2006). Previous studies have shown that synthetic calcium phosphates exhibit similar material properties and biological activity to that of the naturally occurring hydroxyapatite. The stability of various calcium phosphate phases is highly dependent on the ambient environment, particularly temperature and presence of solvents. At in vivo temperature conditions and low pH values (< 4.2), calcium phosphate dehydrate phase is stable, whereas at higher pH, hydroxyapatite is the stable phase. Tricalcium and tetracalcium phosphates are higher temperature phases that, under physiological conditions, can be transformed into biological-type apatite (Bauer et al., 2013).
Typically, scaffold constructs are fabricated using indirect rapid prototyping (Schumacher et al., 2010; Wilson et al., 2011; Xu et al., 2012), phase mixing (Dorozhkin, 2012; Kunjalukkal Padmanabhan et al., 2013; Lim et al., 2012; Lode et al., 2012), sintering (Deng et al., 2012; Schlosser and Kleebe, 2012), shape replication (Jokic et al., 2012; Sung et al., 2012; Tripathi and Basu, 2012), gas foaming (Chatterjee et al., 2012), salt leaching (Kim et al., 2012), freeze casting (Jafarkhani et al., 2012; Qi et al., 2012), to name but a few (Zhou et al., 2012). Three-dimensional printing has also been investigated for fabrication of 3-D calcium phosphate scaffolds with tailored architecture and porosity at macro- and micro-scales (Butscher et al., 2012, 2013; Lewis et al., 2006; Lode et al., 2012; Michna et al., 2005). In contrast to highly crystalline scaffolds fabricated via high temperature sintering, scaffold structures obtained via low temperature methodologies are less crystalline, with enhanced resorption profile and reduced shrinkage. Bioresorbable structures of low crystalline monetite, brushite, hydroxyapatite, tricalcium phosphate, calcium pyrophosphate and tetracalcium phosphate can be fabricated (Suwanprateeb et al., 2010). Microwave sintering has also been shown to enhance the mechanical strength of the calcium phosphate scaffolds fabricated using direct 3-D printing (Tarafder et al., 2012). Calcium phosphate particles and coatings can also be introduced onto polymeric hydrogels and metallic scaffolds to enhance their tissue repairing capacity (Heinemann et al., 2013).
Calcium phosphate-based injectable bone cements have several advantages; specifically these cements can be introduced into the site of the osseous defect without invasive surgical intervention and set as a result of exposure to normal physiological conditions (Driessens et al., 2002). Highly workable, these cements can easily conform to different geometries, making them suitable for both infilling the osseous cavities and enhancement of fracture repair. The versatility in mechanical properties, biological activity and in vivo disintegration kinetics can be attained by controlling material composition, porosity and solubility during material synthesis. From the design point of view, the transformation from injectable to solid or gel phase remains challenging, since it ought to occur in a timely manner without inflicting harm onto the surrounding tissues, and potentially while carrying living cells and degradation-sensitive active molecules (Rahman et al., 2011). Calcium phosphate-containing resorbable glasses have tunable solubility and in vitro and in vivo biodegradation activity (Sanzana et al., 2008).
In terms of biomaterial introduction into the host body, glasses are typically implanted as granules or a mixture of granules dispersed in a carrier fluid. The calcium oxide and sodium oxide proportion will directly affect the pace of solubility, and thus the rate of dissolution. Increasing the sodium oxide content has been demonstrated to enhance the solubility propensity of the material, whereas increasing calcium oxide content has been shown to positively contribute to the stability of the glass. Introduction of Fe2O3, ZnO and/or TiO2 into the calcium metaphosphate glasses has also been reported to improve the chemical durability of these materials, with the presence of Fe2O3 and TiO2 considerably lowering the solubility, whereas high levels of ZnO notably increased the dissolution of the glasses (Clement et al., 2000). Materials containing iron oxide displayed a higher Young modulus and fracture toughness compared to those incorporating titanium dioxide, whereas zinc oxide-containing glasses were characterised by reduced mechanical properties.
Excellent biocompatibility and tunable stoichiometry, surface charge density, functionality and dissolution properties, render calcium phosphates suitable for drug and growth factor delivery (Bose and Tarafder, 2012). For osseous tissue regeneration, calcium phosphate implantable structures can be loaded with various growth factors, biological molecules and chemicals to promote cell migration, differentiation, organic network formation and mineralisation, as well as prevent bacterial attachment and suppress any inflammatory response. Calcium phosphate biomaterials can also serve as vehicles for local treatment and systemic drug delivery. Locally, sustained drug delivery can be employed to better the postoperative care for bone tumour patients and can be effective in treatment of various musculoskeletal diseases (Chen et al., 2012).
Systemically, calcium phosphates can be used for targeted delivery of drugs, sensors, antibiotics and vaccines, which allows for the reduction in the amount of drug required to produce a positive effect and minimisation of side effects (Alkhraisat et al., 2010; Chiu et al., 2012). For example, clinical applications of nucleic acid drugs are limited due to their poor intracellular bioavailability and rapid degradation (Oyane et al., 2012). Indeed, the effective transfection of polyanionic high molecular weight nucleic acids across the negatively charged plasma membrane is difficult (Giger et al., 2011). Intracellular transport from the membrane to the nucleus is difficult, since the molecules may be digested prior to reaching their destination. In order to fully capitalise on their strong targeting potential and concomitant lower off-target effects in comparison with conventional drug agents, the entrapment of genetic material into calcium phosphate nanovehicles has been investigated (Giger et al., 2011). Stimulus responsive calcium phosphate nanocomposites have been investigated for concurrent sensing and drug delivery, where the release and fluorescent response was triggered by changes in pH conditions (Banerjee et al., 2011). As such, these nanoparticles can be used for in vivo visualisation and treatment of cancerous tumours (Altinoglu et al., 2008; Kester et al., 2008; Morgan et al., 2008; Muddana et al., 2009). The nanocrystalline powders of β-tricalcium phosphate and pure zinc and magnesium doped hydroxyapatite were shown as suitable active centres in sunscreens suitable for the prevention of skin cancer while permitting gradual tanning (de Araujo et al., 2010).
In addition to applications as tissue scaffolds, bioactive cements and biocompatible coatings for permanent implantable materials, for example commercially pure titanium, calcium phosphates are being investigated as coatings to control biodegradation and enhance osteogenicity of biodegradable implants (Yang et al., 2012a; Yuan et al., 2011). For instance, magnesium is a promising implantable metal with excellent biocompatibility and mechanical properties close to those of human bone. Notably, magnesium implants degrade under normal physiological conditions to non-toxic by-products, with magnesium ions demonstrated to be intimately linked to bone regeneration and bone health. However, the major clinical limitation of magnesium and magnesium alloys-based biomaterials lie in their propensity to degrade too quickly, which leads to the untimely loss of mechanical strength and spatial conformation, release of relatively large degradation fragments into the peri-implant space, and localised rapid evolution of hydrogen gas. To overcome untimely degradation and decrease wear particle production, encapsulation of solid and porous magnesium structures with a calcium phosphate layer has been proposed. Bone, teeth and other biological tissues contain calcium phosphates that are similar to bioactive calcium phosphates used for biomedical application (Yuan et al., 2011). As these materials are naturally porous and mechanically relatively weak, they are not used for replacement of bone tissue where load-bearing function is required (Wagoner Johnson and Herschler, 2011). In addition to having attractive biological properties, the synthesis methodologies often used for deposition of calcium phosphates are compatible with the highly reactive property and low melting point of magnesium.
In addition to good cell and tissue compatibility, calcium phosphate bone substitute materials have displayed a minimal inflammatory response in vitro and in vivo (Fig. 8.1). Human acute monocytic leukemia cell line (THP-1) incubated in the presence of octacalcium phosphate, hydroxyapatite, β-tricalcium phosphate and dicalcium phosphate dehydrate, did not produce detectable amounts of inflammatory cytokine TNF-α (Shinji et al., 2012). Implantation of fluorapatite, hydroxyapatite and β-tricalcium phosphate microparticles into mouse peritoneal cavity for up to 24 weeks resulted in a short, mild inflammatory reaction due to the rapid aggregation of the particles in vivo and consequent formation of foreign body granulomas within 2 weeks (van der Meulen and Koerten, 1994). The degradation products of all ceramics were found in both intracellular and extracellular space. When implanted subcutaneously in Wistar rats for up to 30 days, granules of hydroxyapatite, β-tricalcium phosphate and hydroxyapatite/β-tricalcium phosphate resulted in multi-nucleated giant cell formations (Shahram et al., 2012). Compared to hydroxyapatite, β-tricalcium phosphate resulted in a higher number of multi-nucleated giant cells and vascularisation of the implantation cavity within the first 2 weeks of insertion. After 15 days, the extent of inflammatory reaction was similar across the different calcium phosphates. Nanoscale modification of metallic implants, such as titanium, with calcium phosphate elicited a gene expression profile with discernible down-regulation of several pro-i nflammatory cytokines and chemokines (Hamlet and Ivanovski, 2011).
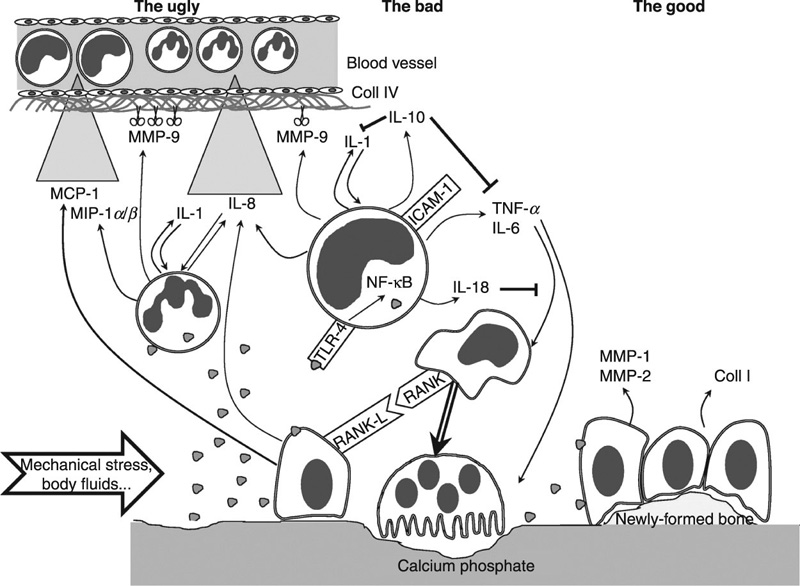
 to promote bone synthesis by osteoblasts
to promote bone synthesis by osteoblasts  , which ensures homeostasis between bone matrix degradation and synthesis. The bad: due to several physico-chemical factors (e.g. mechanical stress, body fluid shear stress), ceramics can be fragmented and the particles
, which ensures homeostasis between bone matrix degradation and synthesis. The bad: due to several physico-chemical factors (e.g. mechanical stress, body fluid shear stress), ceramics can be fragmented and the particles  may interact with inflammatory cells, such as monocytes
may interact with inflammatory cells, such as monocytes  and polymorphonuclear cells
and polymorphonuclear cells  . This leads to the production of inflammatory mediators, such as cytokines, chemokines and proteases
. This leads to the production of inflammatory mediators, such as cytokines, chemokines and proteases 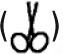 . In such an environment, the osteoblasts trigger the monocytes/macrophages
. In such an environment, the osteoblasts trigger the monocytes/macrophages  , which fuse and form more osteoclasts. The ugly: when uncontrolled, the production of inflammatory mediators may lead to extracellular matrix damage and to type IV collagen degradation in the vicinity of blood vessels, coupled with chemokine gradients
, which fuse and form more osteoclasts. The ugly: when uncontrolled, the production of inflammatory mediators may lead to extracellular matrix damage and to type IV collagen degradation in the vicinity of blood vessels, coupled with chemokine gradients  with the recruitment of numerous new inflammatory cells. MCP: macrophage chemo-attractant protein; MIP: macrophage inflammatory protein; IL: interleukin; MMP: matrix metalloproteinase; Coll: collagen; TNF: tumour necrosis factor; TLR: toll-like receptor; ICAM: intercellular adhesion molecule; NF-κB: nuclear factor κ-B; RANK: receptor activator of NF-κB; RANK-L: RANK-ligand (Velard et al., 2013).
with the recruitment of numerous new inflammatory cells. MCP: macrophage chemo-attractant protein; MIP: macrophage inflammatory protein; IL: interleukin; MMP: matrix metalloproteinase; Coll: collagen; TNF: tumour necrosis factor; TLR: toll-like receptor; ICAM: intercellular adhesion molecule; NF-κB: nuclear factor κ-B; RANK: receptor activator of NF-κB; RANK-L: RANK-ligand (Velard et al., 2013).There are numerous approaches for the production of calcium phosphate coatings, including solution-based processing, thermal spraying and laser-based processing, the latter being successfully used to coat titanium-based implants. For example, compositionally graded hydroxyapatite coatings were fabricated on titanium by combining laser engineering net shaping and radio frequency induction plasma spraying processes (Roy et al., 2011). Laser engineered net shaping has also been used to prepare tricalcium phosphate coatings with thicknesses ranging from 200 to 400 μm (Roy et al., 2008). Plasma spraying was also used to produce hydroxyapatite/tricalcium phosphate coatings on plasma sprayed titanium alloy implants (Stewart et al., 2004). Other laser-based fabrication methods under investigation include pulsed laser deposition, laser irradiation and laser cladding.
8.3.1 Calcium phosphate dehydrate
Depending on the synthesis methodology and the phase, calcium phosphate ceramics have been demonstrated to differ in terms of in vitro and in vivo degradability, cytocompatibility, and osteoconductive and osteo-inductive behaviour. For instance, calcium phosphate dehydrate phase (CaHPO4 · 2H2O), also known as brushite, is characterised by increased solubility compared to the majority of biologically relevant calcium phases. Brushite is a monoclinic crystal system with a Ca/P ratio of 1. The synthesis of the calcium phosphate dehydrate phase is comparatively simple and economical, and can be effectively used to encapsulate different types of implantable metallic implants. In vivo, the dehydrate phase coatings can be employed to locally increase phosphate and calcium ions in peri-implant space in order to promote bone tissue regeneration. Brushite can also be used for hydroxyapatite synthesis and as a constituent of bone cements. Employment of brushite as a precursor phase allows for a higher degree of control over the size and packing of the calcium phosphate crystals, compared to direct precipitation of hydroxyapatite crystals where the control over the size of the crystal is more complicated. The transformation from the dehydrate phase to hydroxyapatite takes place upon exposure to physiological environment with a pH of more than 6 to 7. The biocompatibility of brushite has been reported in vitro for a number of cell types, such as murine pre-osteoblastic macrophage and fibroblastic cells, and in vivo, such as in cranial defect treatment in sheep, post-anterior cruciate ligament reconstruction in rabbits, and as a cement for pterional craniotomy in humans. In all in vivo studies, osseous tissue formation took place with negligible occurrences of inflammation or infection.
8.3.2 Anhydrous calcium phosphate
As with brushite, anhydrous calcium phosphate phase (CaHPO4), also known as monetite, has a Ca/P ratio of 1. Monetite is a triclinic crystal system. This calcium phosphate phase displays better thermal stability, and like brushite, it can transform to a more stable hydroxyapatite phase upon exposure to environmental conditions with pH of above 6 to 7. Given that its solubility is lower compared to the brushite phase, monetite is less effective when applied as calcium and phosphate releasing coatings for peri-implant space ion enrichment. In terms of synthesis, control over the crystal size and packing in anhydrous calcium phosphate phase can be attained by varying respective concentrations of Ca2 +, HPO42 − and H2PO4−. In vitro studies employing human osteoblast cells (h-OBs) seeded onto 3-D printed monetite coatings (85% CaSO4 · 0.5H2O) showed good cell adherence, healthy cell morphology and mineralisation after 21 days of observation (Suwanprateeb et al., 2010). In vivo investigations also confirmed the biocompatibility of the monetite. Khairoun et al. (2002) reported that calcium phosphate bone cements injected into the osseous defect site at the distal end of rabbit femora displayed direct bone/cement interaction without soft tissue interposition after 3 weeks of observation, indicating high biocompatibility, bioactivity and osteo-conductivity of these materials. Ooms et al. (2003) reported the development of a soft tissue capsule around the calcium phosphate cements injected into the vertebrae of goats with minimal inflammation or cement disintegration at 8 weeks post-operation, confirming the soft tissue biocompatibility of this calcium phosphate phase.
Tamimi et al. (2010) implanted bovine hydroxyapatite and dicalcium phosphate anhydrous porous granules into the post-extraction alveolar sockets of human patients to compare the bioperformance of these materials (Fig. 8.2). At 6 months, a biopsy of the implantation site demonstrated a significantly higher degree of bone regeneration and implant resorption in the case of monetite, at 59.5 ± 13% and 25.8 ± 14.3, respectively, compared to bovine hydroxyapatite graft, at 33.1% ± 4.9 and 37.8 ± 6.1, respectively. The enhanced bone formation of monetite can be potentially explained by the higher resorbability of this material compared to bovine hydroxyapatite, which is practically unresorbable in vivo. As monetite is disintegrated over time, it provides both the space and the Ca2 + and PO43 − ions that enable new osseous growth. Compared to animal studies, the extent of new bone growth was lower in humans, attributed to the age, metabolic problems and other pre-existing conditions of the patients used in the study. The degree of resorption was also lower in human cases compared to rabbits, due to the inverse relationship that exists in mammals between metabolic activity/water intake and body weight.
8.3.3 Octacalcium phosphate
As monetite, octacalcium phosphate (Ca8H2(PO4)6 · 5H2O, Ca/P molar ratio = 1.33) is a triclinic crystal system with Van der Walls and hydrogen bond-linked alternating hydrated and apatite-like layers. The latter is characterised by a relative arrangement of Ca2 + and PO43 − groups similar to that of apatite, whereas the former contains cations and anions that are more broadly distributed because of the presence of the interdispersed structural water molecules (Mahmud et al., 2010). Since the hydroxyapatite phase cannot be formed directly from solution, both octacalcium phosphate and dicalcium phosphate have been suggested as a primary mineral phase from which mature bone apatite is formed in vivo (Shadanbaz and Dias, 2012; Suzuki et al., 2008). Therefore, the octacalcium phosphate phase can be used as a precursor in hydroxyapatite synthesis. Notably, the association between these two phases potentially elucidates the hydrolytic inclusion of impurities, especially ions of magnesium, sodium and carbonate into the precipitated apatites (Barrère et al., 2006).
As with other calcium phosphate phases, octacalcium phosphate is biocompatible, biodegradable and conducive to osseous tissue formation. Under normal physiological conditions (pH, temperature), the octacalcium phosphate phase is stable, whereas under more acidic conditions it transforms into a more thermodynamically favourable hydroxyapatite state (Habibovic et al., 2004). The latter propensity can be effectively used in implantation applications, where an inflammatory reaction and a subsequent acidification of the peri-implant space are expected. In vivo (rat model) and in vitro testing of biomimetic carbonate apatite and octacalcium phosphate coatings fabricated onto Ti6Al4V substrates showed the dissolution-precipitation driven formation of carbonate apatite on the coated surfaces over time (Barrère et al., 2003). Interestingly, while ex vivo both coating types dissolved over time, in vivo only octacalcium phosphate underwent partial dissolution at one week of observation, whereas biomimetic carbonate apatite calcified. After one week, the octacalcium phosphate coating remained stable.
In addition to their demonstrated in vitro cytocompatibility with human mature primary osteoblast and foetal osteoblast-like cells, octacalcium phosphate biomaterials have been shown to be biocompatible with other cell types, including rat bone marrow cells (Fig. 8.3), murine fibroblasts, polymorphonuclear neutrophils and peripheral blood mononuclear cells (Imaizumi et al., 2006; Kim et al., 2011; Liu et al., 2007). Tanuma et al. (2012) demonstrated an enhancement in the osteo-conductivity of octacalcium phosphate-collagen composites by controlling the crystal size of the inorganic octacalcium phosphate phase. The grain size of octacalcium phosphate was varied from approximately 50 to 1 000 μm in diameter; however, the pore size within the composite remained constant at approximately 30 μm. When implanted into the critical-sized calvaria defects in Wistar rats for 4, 8 and 12 weeks, the inorganic phase demonstrated to undergo conversion to hydroxyapatite, with the smallest granules showing superior bone regeneration and biodegradation via tartrate-resistant acid phosphatase-positive osteoclastic cellular resorption (Tanuma et al., 2012).
Loading of an octacalcium phosphate coating with a bisphosphonate calcium-regulating agent, such as alendronate, has also been investigated as a strategy to further improve the osteo-inductive properties of this calcium phosphate phase by inhibiting the extent of bone resorption. In vitro investigations, using cultures of osteoclasts, osteoblasts and their co-cultures, showed that addition of alendronate stimulated osteoblast differentiation, matrix deposition and mineralisation, while concomitantly reducing osteoclast differentiation and proliferation, and increasing the caspase 3 protease associated with cell apoptosis (Boanini et al., 2012). The study also confirmed previously reported observations that octacalcium phosphate is more effective in inducing stromal ST-2 cells differentiation into osteoblasts compared to hydroxyapatite (Anada et al., 2008; Boanini et al., 2012). Comparing tissue plates coated with varied amounts of synthetic octacalcium phosphate and synthetic sintered ceramic hydroxyapatite, Anada et al. (2008) found that increasing the octacalcium content resulted in a substantial decrease in the Ca2 + content and a concomitant increase in the concentration of the inorganic phosphate attributed to the phase conversion into a stable apatite. Although the attachment and initial proliferation significantly decreased for coatings that were high in octacalcium phosphate, the expression of osteogenic markers, including type I collagen, alkaline phosphatase and osterix, was notably elevated. In contrast, varying the amount of hydroxyapatite had no significant influence on the expression of the aforementioned markers.
Fluoride-containing apatitic calcium phosphates, obtained through hydrolysis and direct precipitation of octacalcium phosphate in the presence of fluoride, were also investigated for biomaterial applications (Shiwaku et al., 2012). The study found that increasing the fluoride concentration led to the production of increasingly apatitic calcium phosphates, with co-precipitated products being characterised by the octacalcium phosphate properties, specifically good solubility and hydrolysis products presenting the properties of fluoridated apatite. The crystal structure of the former products was rod-like and the latter products were small and irregular crystals, as opposed to the plate-like structure of the original octacalcium phosphate. The osteoblastic activity of murine stromal cells was higher on co-precipitated compared to hydrolysis products, whereas bovine serum albumin adsorption was increased on the latter products.
8.3.4 Tricalcium phosphate
Tricalcium phosphate (Ca3(PO4)2, Ca/P ratio of 1.5), also known as whitlockite, typically exists in two phases, α-and β-tricalcium phosphates. The crystals in α-tricalcium phosphates belong to a monoclinic space group (180 Å), whereas β-tricalcium phosphates crystals conform to a rhombohedral space group (168 Å) (Sureshbabu et al., 2012). Typically, both phases can be attained during sintering, with higher temperatures (> 1250 °C) resulting in α-tricalcium phosphates, while β-tricalcium phosphates occur at temperatures between 900 and 1100 °C (Boilet et al., 2013) (Fig. 8.4). Under physiological conditions, the α-phase is more soluble compared to the β-phase, due to the aforementioned differences in the crystalline structure, and thus the internal energy and cohesion within the lattice. Although α-tricalcium phosphates have been reported to have biocompatibility in vivo and in vitro superior to that of β-tricalcium phosphates (Rojbani et al., 2011; Kamitakahara et al., 2008; Yamada et al., 2007; Merten et al., 2001), the latter phase is more commonly used for the fabrication of clinically relevant biphasic hydroxyapatite/tricalcium phosphate ceramics. One of the cited reasons is the relative complexity of material synthesis, specifically the β to α conversion occurring at above 1160 °C, with sintering at 1200 °C typically used for fabrication of the α-tricalcium phosphate polymorphic structures. Such high temperature treatment has several detrimental effects, namely decomposition of hydroxyapatite, large particle size of calcium phosphate powders, and heterogeneous dispersion of the different phases within the bulk material with consequent effect on the biological and material properties (Li et al., 2009). It is therefore desirable to lower the temperatures at which biphasic calcium phosphate powders containing α-phase can be attained. Heat treatment of amorphous carbonated calcium phosphate precursors, with different structures at 800 °C under controlled ageing time and pH conditions, has been demonstrated to yield α/β-tricalcium phosphate and α-tricalcium phosphate/hydroxyapatite materials (Dorozhkin, 2012; Li et al., 2007, 2009; Lopez-Heredia et al., 2012). By controlling the ratio between α- and β-phases, a range of materials with tailored biological, mechanical and degradation properties can be attained (Zou et al., 2011). Microwave assisted synthesis can be effectively used to reduce the time required for synthesis of biphasic calcium phosphate bioceramics, with the added benefit of an improved homogeneity within the structure (Farzadi et al., 2011).
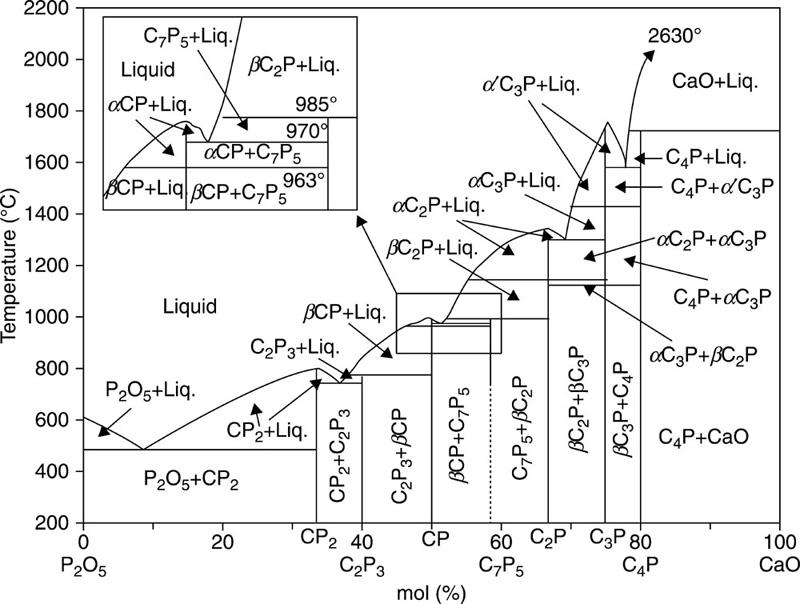
β-tricalcium phosphate is the dominant phase used for biomedical applications, typically used in a form of particles, porous 3-D structures, and frequently combined with other ceramic materials, such as calcium silicate (Fei et al., 2012), polymers, such as poly(trimethylene carbonate) (van Leeuwen et al., 2012), carboxymethyl-chitin (Taniyama et al., 2013), poly(lactic-co-glycolic acid) (Gala-García et al., 2012), poly-(D,L-lactide-glycolide) (Wang et al., 2013), poly(urethane) (Yoshii et al., 2012), chitosan (Tai et al., 2012) and proteins, such as collagen (Bian et al., 2012), recombinant human protein (Matsumoto et al., 2012), etc. Tricalcium phosphate in the β-phase is frequently paired with hydroxyapatite in a biphasic structure, to attain materials with tunable resorption characteristics and enhanced tissue compatibility. Yamada et al. (1997) reported that ceramics with higher β-tricalcium phosphate to hydroxyapatite (75:25) underwent osteoclastic resorption, producing lacunae resembling those ordinarily formed by osteoclasts on natural mineralised organic tissues. Although a more soluble material, pure β-tricalcium phosphate underwent resorption to a lesser extent, the lower amount being attributed to the significant number of calcium ions released by the material in acidic environment. The intensity of the calcium ion release was sufficient to shift the functional phase of the osteoclast resorption cycle towards migration prematurely. It has been suggested that in addition to solubility, porosity of the structures may determine the osteoclastic resorption of the calcium phosphate ceramics (Li et al., 2011).
Substitution of Ca with Co has been demonstrated to stabilise the β-tricalcium phosphate phase and increase the density of the sintered ceramics, similar to Mg and Zn, while maintaining the cytocompatibility of the material similar to that of pure tricalcium phosphate (Zhang et al., 2012). The increase in the phase transformation temperature was primarily attributed to two factors. Since ions of Co are smaller than those of Ca in terms of ionic radius, the replacement of calcium with cobalt leads to the decrease in the interplanar distance and crystal unit volume, which in turn hinders the transport of atoms and thus curbs the transition from β to α. The improvement in octahedral geometry of the crystal further stabilises the phase, by reducing the degree of distortion within the structure. Given their small ionic radius, Co ions may preferentially occupy the Ca(5) site, similar to behaviour reported for Mg and Zn, with the Co–O bond being shorter compared to the Ca–O bond, at 2.093 Å and 2.238 to 2.287 Å, respectively (Enderle et al., 2005; Velard et al., 2013). Introduction of a silicon dioxide doping agent was also demonstrated to increase the transition temperature of the material, significantly improve the compressive strength and enhance proliferation of human foetal osteoblast cells (Fielding et al., 2012). Addition of strontium ions to β-tricalcium phosphate has also been investigated (Aina et al., 2012; Roy and Bose, 2012). An improvement in the mechanical properties of β-tricalcium phosphate has also been achieved by treating the sintered material with hot isostatic pressing (Mihaela et al., 2012). This approach resulted in the production of a material with high density (> 99.9%), fine microstructure and transparency, without compromising their static and dynamic in vitro biocompatibility.
In vitro investigations have demonstrated tricalcium phosphate cytocompatibility to human and murine osteoblast cells, and bone marrow stromal cells (Shadanbaz and Dias, 2012). A comparative study using unloaded cylindrical grit-blasted titanium (Ti–6Al–4 V) implants, coated with either hydroxyapatite or tricalcium phosphate and inserted into the proximal humerus of dogs, showed significantly higher bone ongrowth and 10-fold stronger fixation in hydroxyapatite coated samples compared to those with tricalcium phosphate coating (Lind et al., 1999). In a rabbit model, a hydroxyapatite/tricalcium phosphate coating plasma sprayed onto titanium alloy showed improved bone apposition and accelerated the establishment of a stable prosthesis–bone interface (Stewart et al., 2004). The gap bone volume was slightly lower in the former case, with little resorption at 6 weeks. In human patients, addition of hydroxyapatite/tricalcium phosphate coating onto cementless femoral stem resulted in no statistically significant improvement from the clinical point of view after 127 months of observation (Yoon et al., 2007). An addition of 70% hydroxyapatite and 30% tricalcium phosphate ceramic coating to the porous press-fit cups did not affect the median annual proximal wear for the implant type (Thanner et al., 2000).
8.3.5 Hydroxyapatite
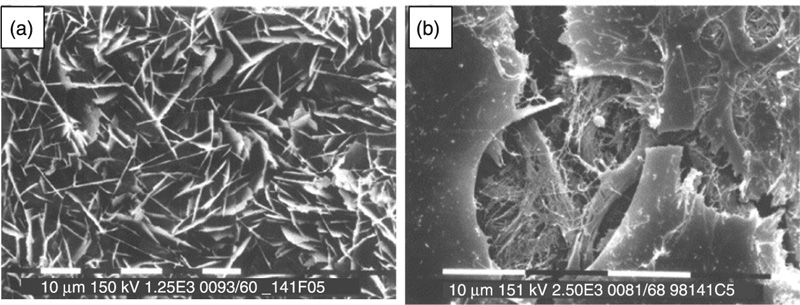
The most prevalent calcium apatite in bone tissues, hydroxyapatite (Ca10(PO4)6(OH)2, Ca/P ratio of 1.67) is a naturally occurring mineral that crystallises in the hexagonal crystal system. Figure 8.5 shows examples of some of the morphologies of hydroxyapatite. The hydroxyl group of the hydroxyapatite can be substituted with fluoride, chloride or carbonate to yield non-stoichiometric minerals, fluorapatite or chlorapatite. Substitutions for Ca2 + and PO43 − ions are also common (Shpak et al., 2004). As a hydrated calcium phosphate, hydroxyapatite commences dehydroxylation at approximately 800 °C to form oxyhydroxyapatite (Ca10(PO4)6(OH)2–2xOxϒx, where ϒ is a vacancy). Under typical sintering conditions, hydroxyapatite remains thermally stable up to approximately 1050 to 1100 °C, although some apatites have been reported to be thermally stable up to 1200 to 1300 °C. Upon heating, hydroxyapatite breaks down to form β-tricalcium phosphate, tetracalcium phosphate (Ca4P2O9), and water. Exposure to even higher temperatures initiates transition of tricalcium phosphate from the β- to α-phase. Changing the conditions under which sintering is performed will affect the decomposition initiation temperature; for example, sintering under vacuum may lower hydroxyapatite degradation, while the presence of water vapours may postpone the decomposition. The thermal stability of hydroxyapatite will also be influenced by the type and extent of substitutions within its structure. For instance, sufficient fluorine substitution has been shown to markedly improve thermal stability and corrosion resistance of the ceramics compared to pure hydroxyapatite (Chen and Miao, 2005).
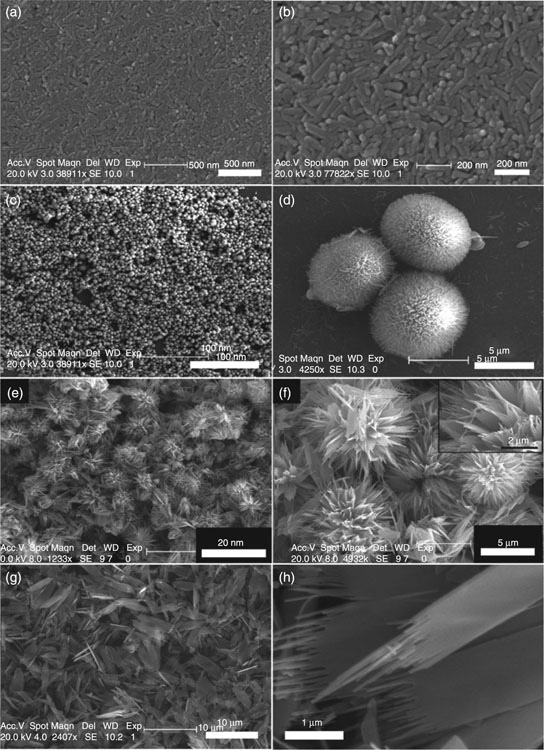
The biological activity of hydroxyapatite in vitro and in vivo is well documented, with hydroxyapatite being the most abundant material used in modern bone substitution (Barrère et al., 2006; Kim, 2003). In vitro, hydroxyapatite materials have been demonstrated to be compatible with mesenchymal stem cells, stimulating their adhesion and differentiation into osteoblastic cell lines, primary human osteoblast cells, human embryonic kidney cell lines and human keratinocyte cell lines (Douglas et al., 2009; Guo et al., 2009; Negroiu et al., 2008; Sandeman et al., 2009; Shadanbaz and Dias, 2012; Zhang et al., 2009b). Superior osteo-conductivity of hydroxyapatite was confirmed in vivo using several animal models, including rabbit (Patel et al., 2002; Tamai et al., 2002), pig (Chu et al., 2002; Ramselaar et al., 1991) and dog (Kikuchi et al., 2001). In human patients, the use of hydroxyapatite-coated pins for external fixation of unstable wrist fractures indicated potentially superior clinical performance; however, the difference in healing outcomes in this study was not statistically significant (Pieske et al., 2010). In addition to coatings, clinical applications of hydroxyapatite include sintered macroporous granules and cements in nonload-bearing applications. Recently, Johansson et al. (2012) used a spherical, hollow and perforated hydroxyapatite space-maintaining device to successfully establish a void space where new bone can form within the sinus cavity.
8.4 Bioactive glasses
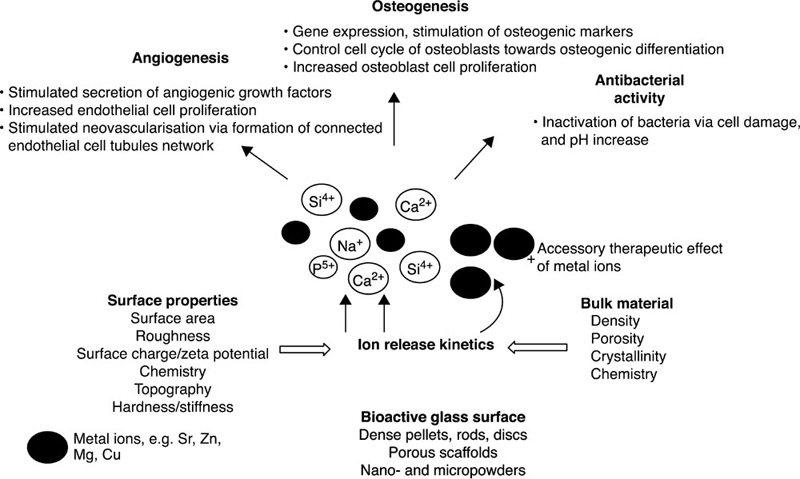
Bioactive glasses are a group of bioresorbable inorganic materials with good osteo-inductive properties and the ability to form permanent bonds with soft tissues in vivo (Hoppe et al., 2011). They are typically produced from phosphate or silicate precursors. The carbonated hydroxyapatite layer that is formed on the surface of bioactive glasses under physiological conditions is highly favourable in terms of osseous tissue formation and mineralisation. Furthermore, the ionic products of their degradation are the same ions inherently involved in natural bone formation, and have been demonstrated to stimulate cells within osteoblastic lineage to express genes that favour bone formation and promote neovascularisation of the newly-formed tissues (Day, 2005; Gorustovich et al., 2010; Leu and Leach, 2008). Their antibacterial and anti-inflammatory potential has also been investigated, with some promising results (Allan et al., 2001; Day and Boccaccini, 2005; Gorriti et al., 2009; Hu et al., 2009; Jones et al., 2006; Leppäranta et al., 2008; Munukka et al., 2008).
Bioactive glasses based on phosphate (CaO-Na2O-P2O5) are frequently employed for the fabrication of bone fillers and scaffolds due to their high solubility, tunable material properties and degradation kinetics, and chemical resemblance to inorganic componsent of osseous tissues. These properties can be effectively controlled by adjusting the glass composition. In vivo and in vitro, phosphate-based bioactive glasses have been demonstrated to be biocompatible with several cell types, are osteo-inductive and illicit minimal inflammatory response due to low macrophage activation (Gough et al., 2002). Their osteoinductive and osteo-inductive properties have been shown to vary with composition. For instance, decreasing CaO content resulted in reduction in dissolution rate of the glass, and concomitant stimulation of osteoblast (MG63 and HOS) proliferation and expression of genes related to bone sialoprotein, osteonectin and fibronectin, proteins that have an essential role in ensuring the integrity and function of hard connective tissue (Salih et al., 2000). In contrast, increasing the CaO content and glass solubility adversely affected osseous tissue formation, down-regulating the expression of the bone associated proteins.
Phosphate bioactive glasses containing at least 46 mol% CaO were demonstrated to be cytocompatible with both osteoblasts and fibroblasts, indicating the suitability of such glasses for hard/soft tissue interface engineering (Bitar et al., 2004). However, a different study reported ternary phosphate-based glass to impede in vitro proliferation, differentiation and death of adult human bone marrow stromal cells and human foetal osteoblast when compared to calcium phosphate controls (Skelton et al., 2007). In order to control the solubility and biodegradation profile of the bioactive glasses, a range of oxides have been introduced into the glass matrix. These include the oxides of titanium, aluminium, boron, zinc, magnesium, strontium and iron (Hoppe et al., 2011).
Another important class of bioactive glasses are fabricated from silicate, with the general formula SiO2-CaO-P2O5. One of the best studied bioactive glasses, 45S5 Bioglass (wt%: 45SiO2-25CaO-25Na2O-6P2O5) is characterised by highly favourable osteo-inductive properties and sound biocompatibility, which makes this material an excellent candidate for bone tissue scaffolds. The ionic products leached from the silicon-based glasses upon exposure to physiological medium have been demonstrated to affect cell metabolism by changing the ionic composition of their extracellular environment. For example, in vitro degradation of 45S5 Bioglass led to extra- and intracellular alkalinisation, a rise in [Ca2 +]i and [K+]i, a small plasma membrane hyperpolarisation and an increase in lactate production in murine osteoblasts (Silver et al., 2001).
In contrast, bioactive glasses 58S and 77S were not found to alter either the ion composition or metabolic activity of the cells. It was suggested that beneficial effect of 45S5 in vivo osseous tissue growth may at least to some degree be associated with alkalinisation, and subsequent increase in collagen synthesis and cross-linking, and hydroxyapatite formation. A paper by Laquerriere et al. (2003) reported an increased cellular concentration of phosphorous and sulphur in response to a different bioactive glass, described by mol%: 50SiO2-16CaO-6P2O5-5K2O-2Al2O3-1MgO. Several studies have shown favourable up-regulation of osteogenic markers and genes pertaining to bone formation in cells exposed to silicon-based bioactive glasses (Jell et al., 2008; Kaufmann et al., 2000; Xynos et al., 2001). Bioactive glasses fabricated using the sol–gel approach were demonstrated to illicit similar osteo-inductive response, stimulating osteogenic differentiation of bone marrow stromal cells into osteoblast-like cells and encouraging cell mineralisation (Bosetti and Cannas, 2005; Jell and Stevens, 2006).
In addition to varying the content of key elements within silicon-based bioactive glasses, property tuning can be achieved through introduction of metallic ions, including cobalt, zinc, copper, strontium, magnesium, silver, cerium, boron and fluoride (Hoppe et al., 2011) (Fig. 8.6). Biologically active glasses fabricated from CaO-MgO-SiO2 with the inclusion of B2O3, CaF2, Na2O and P2O5 have been demonstrated to have acceptable level of bioactivity, with phosphate-enriched glasses supporting the formation of biological apatites on the glass surface, whereas higher proportion of CaO and SiO2 hinders apatite formation. Doping of 45S5 bioactive glass with titanium resulted in an increased osteoblast proliferation and expression, while B, Fe and F additions resulted in inferior osteoblast activity compared to control (Vrouwenvelder et al., 1994). Introduction of zinc and magnesium into the network of cobalt-doped glasses was investigated to develop a potentially vascularisation-promoting or suppressing material. Presence of cobalt, zinc and magnesium oxides slowed down the deposition of the hydroxyapatite layer, which may be a favourable outcome for soft tissue engineering uses (Azevedo et al., 2010).
8.5 Conclusion
From the literature reviewed, it is evident that ceramics play an important role in many areas of medicine. First-generation, inert ceramics exhibit excellent mechanical strength, corrosion and wear resistance, and as such these materials have become an integral element of many load-bearing implants. However, these materials are brittle and suffer from poor integration with soft and hard tissues in vivo. Less mechanically robust, the second-generation ceramics have been developed to interact with living tissues at the molecular level, stimulating cellular attachment, proliferation and healthy tissue formation. These materials are capable of influencing the gene expression and metabolic activity of cells in vitro and in vivo, and are excellent candidates to support tissue re-growth. Third-generation ceramics have been designed to not only physically mimic the osseous tissue but to competently initiate the biological processes associated with tissue formation. Their ability to deliver various biological and chemical molecules to the site of implantation, or to specific organs and tissues, and then safely disintegrate as part of normal metabolic activity will continue to attract significant attention for highly controlled drug and vaccine delivery and in vivo visualisation. However, with a plethora of bioresorbable ceramic materials being developed annually, there is a strong need for comprehensive mechanisms for evaluation of their properties in vitro and in vivo, to ensure we can take full benefit from these discoveries.
