Metallic biomaterials: types and advanced applications
Abstract:
Metallic biomaterials have found a plethora of applications as medical devices, with stainless steel, titanium and cobalt based alloys employed in most permanent metallic implants. This is due to their favourable mechanical properties, especially fracture toughness and fatigue strength. This chapter reviews the most commonly-used metallic biomaterials, such as commercially pure Ti and Ti-6Al-4 V alloys and Co-Cr alloys and stainless steel, as well as novel metallic biomaterials currently gaining increasing attention, for example bioresorbable Mg alloys and Ni-Ti shape memory materials.
5.1 Introduction
Metallic biomaterials are employed in various forms to substitute for damaged structural components and to restore lost functions within the human body (Kuhn, 2012). A favourable combination of tensile strength, fracture toughness and fatigue strength warrant their application in orthopaedics, as artificial joints, plates and screws, in orthodontics and dentistry, as braces and dental implants, and as cardiovascular and neurosurgical devices, such as artificial heart, staples, stents, wires and coils. Compared to polymer and ceramic biomaterials, metals are characterised by higher electro-conductivity, and as such have been employed to enclose electrodes in artificial electronic organs (Hsu et al., 2012).
Sherman vanadium steel was the first metallic alloy designed specifically for biomedical applications, as plates and screws to join fractured bones together (Greenhagen et al., 2011). Metals currently used for implant manufacturing include Fe, Cr, Co, Ni, Ti, Ta, Mo and W.
This chapter reviews the most commonly-used metallic biomaterials, such as commercially pure Ti and Ti-6Al-4 V alloys and Co-Cr alloys and stainless steel, as well as novel metallic biomaterials currently gaining increasing attention, for example bioresorbable Mg alloys and Ni-Ti shape memory materials.
5.2 Stainless steel
The ability of steel to resist corrosion is intimately linked to the presence of alloying elements. The first biomedical 18-8 steel alloy contained vanadium but subsequent studies indicate that V provides the alloy with insufficient corrosion resistance, and the medical use of vanadium steel has been discontinued. Modern corrosion-resistant (stainless) steel is fabricated by adding more than 12 wt% of Cr, the addition resulting in the formation of a thin oxide coating on the surface of the alloy. This film is characterised by low chemical reactivity and displays a self-healing property in contact with oxygen. In saline and chloride solutions, and in particular where dissolved oxygen can react with Cl ions, stainless steel can undergo pitting corrosion (Buhagiar et al., 2012). Elements such as Ni and Mo are also introduced to stainless steel to improve corrosion, whereas C is minimised as C tends to bind to Cr to form chromium carbide, thus minimising the protective anti-corrosion activity of the metal. The alloy containing less than 0.03 %wt of C, less than 2 %wt Mn, less than 0.025 %wt P, less than 0.01 %wt S, less than 0.75 %wt Si, 17 to 19 %wt Ni, 2.25 to 3 %wt Mo, less than 0.1 %wt N, and less than 0.5 %wt Cu, with the balance being Fe, is known as the stainless steel type 316 L and is one of the types of steel most commonly used for medical implantation. The biocompatibility of stainless steel can be varied by controlling the level of impurities, e.g. Cu (Fig. 5.1).
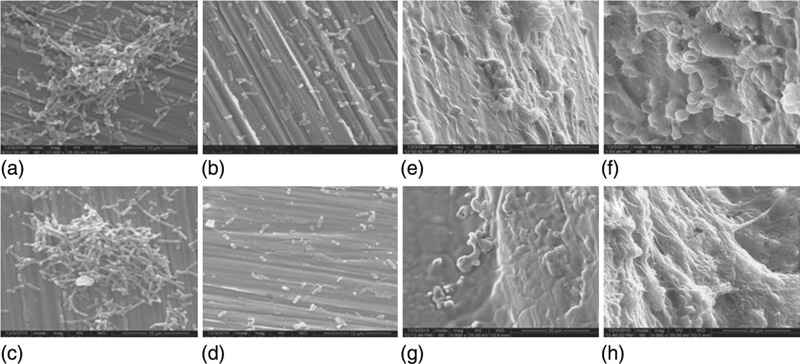
Steels can be categorised as austenitic, martensitic or ferritic stainless steel, the classification being dependent on their crystallographic structure. The microstructure of martensitic steel represents a distorted body-centred cubic obtained by rapid quenching, whereas an α-body-centred cubic structure is indicative of ferritic steel. The austenitic stainless steels, such as types 316, 316 L and 302, are characterised by a γ-faced-centred cubic structure and are the steel of choice for biomedical uses. The austenitic steels are non-magnetic, and are hardened by means of cold working rather than heat treatment. Their microstructure allows for higher corrosion stability and toughness compared to martensitic and ferritic steels (Buhagiar et al., 2012). Presence of Mo improves the resistance of 316 L steel to pitting corrosion, whereas Ni and Cr are responsible for stabilising the austenitic phase of Fe at room temperature.
A wide range of material properties can be attained, depending on the processing methodology, specifically softer steels result from heat treatment whereas cold working increases material strength and toughness. It is important to note that since austenitic steel has a propensity to harden quickly under cold working, intermediate heat treatments are frequently employed to allow the processing of steel into a device. Care should be taken during heat treatment, so as not to induce formation of chromium carbide, which is known to lower corrosion resistance of the alloy in vivo. Following the same reasoning, welding is rarely employed on steel for biomaterial assembly. Heat treatment is also known to induce the formation of a highly porous oxide surface layer, which may flake off and compromise the surface integrity of the metal, and therefore requires chemical and/or mechanical removal (polishing) prior to use.
The primary limiting factors in the clinical use of stainless steels are the reported Ni toxicity to the host organism, and vulnerability of the alloy to pit and crevice corrosion and stress-corrosion cracking (Buhagiar et al., 2012; Majid et al., 2011). It is not surprising then, that modern uses of steel in orthopaedics and other load-bearing applications are limited to temporary biomedical devices. However, an abundant supply of oxygen in some applications, such as vascular stents, slows down the rate of corrosion sufficiently to ensure the long-term performance of steel-based devices. As mentioned previously, oxygen positively contributes to the evolution of a self-healing protective layer on the surface of steel. Furthermore, surface modifications such as plasma-assisted low-temperature nitriding, carburising and carbonitriding can potentially enhance corrosion resistance of medical grade austenitic stainless steels (Buhagiar and Dong, 2012). The most important factor governing the medical use of steel stems from its relative low cost compared to Co-Cr and Ti-based alloys.
5.3 Co-Cr alloys
Cobalt-based alloys, of which Co-Cr based alloys are most pervasive, are superior to stainless steel in terms of their corrosion stability (Bahraminasab et al., 2012). Co-Cr alloys have evolved from aircraft industry-developed Co-Mo-W material, which was characterised by improved strength at high temperatures and lower corrosion susceptibility compared to their counterparts. Subsequent modifications of the original alloy resulted in the production of Co-Cr-Mo, Co-Ni-Cr-Mo and Co-Ni-Cr-Mo-Fe materials. The former is a casting alloy that has been extensively used in orthopaedics and dentistry, to make artificial joints and dental implants, respectively (Mitchell and Shrotriya, 2008). The Co-Ni-Cr-Mo alloy is generally worked into the device by means of hot forging and has been used extensively for fabrication of load-bearing stems for knee and hip prostheses (Saldívar-García and López, 2005). Co-Ni-Cr-Mo-Fe and Co-Cr-W-Ni are also wrought alloys, but currently their biomedical applications are limited.
The physico-chemical properties of the cast and wrought Co-Cr alloys differ significantly. The process of casting can result in larger grain sizes, pronounced boundary segregation, holes and shrinkage cavities within the bulk of the material. Wrought alloys are characterised by higher fracture toughness and fatigue strength, essential for their large load-bearing applications (Saldívar-García and López, 2005). Although both cast and wrought alloys display sound corrosion stability, the former are better at resisting wear, and pitting and crevice corrosion. The addition of Mo has been shown to decrease the size of the grain, and positively contribute to solid-solution strengthening and biodegradation resistance. Processability of cast alloys is enhanced by the presence of Ni; however, the level of the element is closely monitored due to its reported toxicity (Kurosu et al., 2010; Yoda et al., 2012). Introduction of minute amounts of C (~ 0.25% wt) notably improves castability, decreasing the melting point of the alloy by approximately 100 °C (Muterlle et al., 2010).
Carbides that form in the process of casting positively contribute to the wear resistance of the alloy, also increasing the propensity to harden when worked on (López and Saldívar-García, 2008). Therefore, low concentrations of C are desired if a material is to be forged into a particular shape. Compared to cast alloys, forged Co-Ni-Cr-Mo alloys are characterised by higher strength and improved resistance to corrosion when exposed to chloride ion containing physiological environment under applied stress. Cold working can further enhance the strength of the material; however, the process is difficult and not suitable for fabrication of large devices. Due to less than impressive frictional characteristics when in contact with itself or other materials, forged Co-Ni-Cr-Mo is not advised for the bearing surfaces in devices.
5.4 Ti and Ti-based alloys
Every year, more than 1000 tonnes of titanium-based materials and devices are implanted into patients worldwide (Addison et al., 2012). First efforts to implant Ti-based biomaterials go back to the 1930s, when it was observed that similar to stainless steel and Co-Cr-Mo, Ti was sufficiently accepted by the femoral bone tissues in felines. The biomedical utility of Ti stems from the relative lightness of the metal compared to the conventional steel and Co-Cr alloys, characterised by respective densities of 4.5, 7.9 and 8.3 g/cm3. Ti is also superior with regard to its biocompatibility, resistance to biocorrosion, specific strength and elastic modulus.
Depending on their chemical composition, commercially pure Ti is categorised into four grades, where the %wt of inclusions increases from grade I to grade IV to reach a maximum of 0.7%. The levels of O (0.18–0.4 %wt), N (0.03–0.05 %wt) and Fe (0.20–0.50 %wt) are strictly monitored, as these elements have been shown to notably affect the ductility and strength of the Ti. Allotropism in Ti means that below the allotropic transformation temperature TAT of 885 °C, the material is described by a hexagonal close-packed α-structure, whereas at higher temperatures, Ti takes on a body-centred cubic β-structure. Addition of Al, Sn, C, O or N has been demonstrated to stabilise the α-structure by increasing the TAT; however, Mo, Nb, V, Cr and Fe decrease the TAT, thus contributing to the β-structure. In contrast to the β form, α-phase materials display excellent heat and oxidation resistance, and weldability (due to single phase microstructure), but lesser workability and strength.
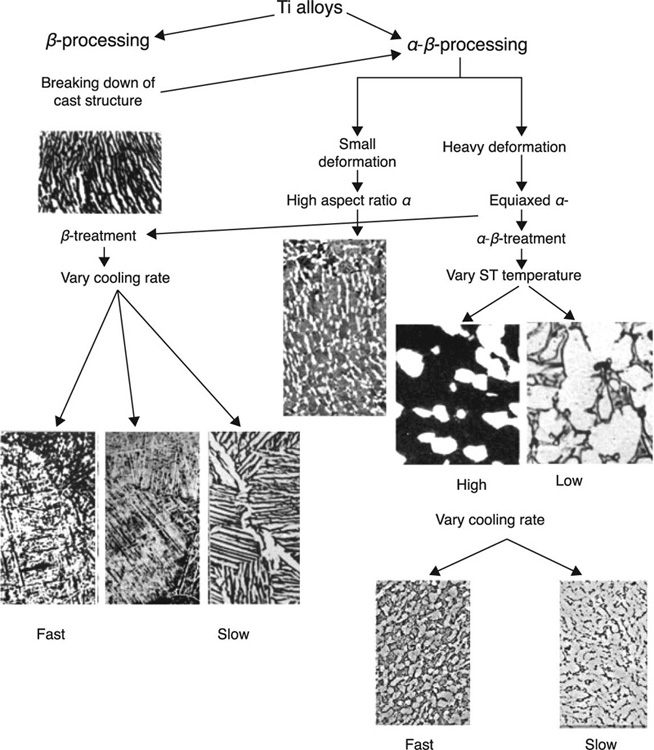
Commercially pure Ti is an α-type alloy. Hardening via heat treatment is not used for these single-phase alloys, as increased strength is generally attributed to the precipitation of one phase in the multi-phase system. Using specific amounts of β-stabilising elements, a two-phase structure comprising both α and β phases can be attained. The most popular Ti-based biomedical alloy, Ti6Al14V, is comprised of Al (5.5–6.5 %wt) and V (3.5–4.5 %wt), and is a good example of a two-phase structure where the β-phase is dispersed within the α-phase. The precipitation is achieved by means of annealing, followed by rapid cooling (quenching) and subsequent thermal ageing. The latter prompts the metastable β-phase to precipitate in a form of small particles, with the resultant structure showing improved strength compared to an α-β alloy that has been subjected to heat treatment only. In Ti-13 V-11Cr-3Al alloys, a relatively large concentration of V imparts a clearly β-type microstructure and thus annealing can significantly increase the strength of the material while reducing its ductility. Figure 5.2 shows the effect of thermomechanical processing on microstructure of Ti alloys (Geetha et al., 2009).
The medical use of Ti-12.5Mo, Ti-8Al-7Nb, Ti-13Nb-13Zr, Ti-29Nb-13Ta-4.6Zr and Ti-121Mo-6Zr-2Fe has also been suggested (Cvijovi![]() -Alagi
-Alagi![]() et al., 2011; Diomidis et al., 2012; More et al., 2011). Excellent mechanical properties, anticorrosion ability, cytocompatibility and biocompatibility of Ti15Nb4Ta4Zr alloys make them suitable for orthopaedic implants (Nakada et al., 2008). Compared to Ti6Al4V, this alloy demonstrated improved new bone formation and bone mineral density, which was equivalent to, or higher than that of Ti6Al4V (Choe et al., 2009; Nakada et al., 2008). Increasing the content of Nb, particularly via an oxidation treatment, has been reported to enhance wear resistance of the alloy due to hardness and lubricity of the Nb2O5 layer (Li et al., 2004). As Ti-Nb-Ta-Zr alloys are unable to form apatite on their surface under conventional chemical and heat-treatment processes, then another surface modification method is required (Fukuda et al., 2011; Niinomi, 2008b).
et al., 2011; Diomidis et al., 2012; More et al., 2011). Excellent mechanical properties, anticorrosion ability, cytocompatibility and biocompatibility of Ti15Nb4Ta4Zr alloys make them suitable for orthopaedic implants (Nakada et al., 2008). Compared to Ti6Al4V, this alloy demonstrated improved new bone formation and bone mineral density, which was equivalent to, or higher than that of Ti6Al4V (Choe et al., 2009; Nakada et al., 2008). Increasing the content of Nb, particularly via an oxidation treatment, has been reported to enhance wear resistance of the alloy due to hardness and lubricity of the Nb2O5 layer (Li et al., 2004). As Ti-Nb-Ta-Zr alloys are unable to form apatite on their surface under conventional chemical and heat-treatment processes, then another surface modification method is required (Fukuda et al., 2011; Niinomi, 2008b).
As is the case with other metallic biomaterials, the mechanical properties of Ti and Ti-based alloys vary with the type and %wt of alloying impurities, and the processing methodology. At 100 GPa, the elastic modulus of Ti-based materials is considerably lower compared to steel and Co-Cr alloys, whereas the strength profile is similar between these materials (Niinomi and Hattori, 2010). A biomaterial with a lower Young’s modulus can homogeneously transfer stress between itself and the bone; however, as the modulus approaches that of a bone, the possibility of failure under high shear deformation in vivo will increase (Minagar et al., 2012). It is difficult to predict exactly how the material will behave under stress once it has been implanted, as fatigue testing that adequately replicate in vivo conditions is complex (Niinomi, 2008a).
Standardised in vitro fatigue testing involves tension/compression, bending, torsion and rotating bending fatigue studies, with Ti-6Al-4 V alloy employed as a standard material against which the results are compared (Long and Rack, 1998). When compared in terms of their specific strength, Ti-based materials are superior to other implantable metals. For commercially pure Ti, the tensile strength values of 240 to 550 MPa yield strength of 170 to 485 MPa, elongation of 15 to 24% and reduction of area of 25 to 30% are expected. The tensile strength of Ti-6Al-4 V alloys is approximately 860 MPa, independent of whether the alloy is cast or wrought. Other parameters differ, with yield strengths of 758 and 795 MPa, minimum elongation of 8 and 10%, and the minimum reduction of area of 14 and 20% for the cast and wrought alloys, respectively. The sheer strength of Ti biomaterials, that is the strength against a force capable of producing sliding failures on a material parallel to the direction of the force, is relatively low.
In addition, Ti materials are susceptible to tribocorrosion in the applications that entail a sliding contact between the device components in physiological fluids, such as between the femoral and the tibial or acetabular elements of the hip joint replacement implant (Diomidis et al., 2012; Manhabosco et al., 2011). Tribocorrosion is influenced by the electro-chemical and mechanical conditions of the contact, and generally results in the increased rate of biomaterial degradation (Cvijovi![]() -Alagi
-Alagi![]() et al., 2011). Certain Ti alloys, such as Ti-29Nb-13Ta-4.6Zr, have been demonstrated to recover their passive surface configurations under both sliding and fretting contacts (Diomidis et al., 2012; More et al., 2011). Wear and corrosion resistance of martensitic Ti 6Al 4 V ELI alloys was significantly better compared to Ti-6Al-4 V ELI alloys with an α-β microstructure (Cvijovi
et al., 2011). Certain Ti alloys, such as Ti-29Nb-13Ta-4.6Zr, have been demonstrated to recover their passive surface configurations under both sliding and fretting contacts (Diomidis et al., 2012; More et al., 2011). Wear and corrosion resistance of martensitic Ti 6Al 4 V ELI alloys was significantly better compared to Ti-6Al-4 V ELI alloys with an α-β microstructure (Cvijovi![]() -Alagi
-Alagi![]() et al., 2011). Other studies have suggested the relationship between the microstructure of the material and the rate of its wear is not straightforward (Majumdar et al., 2011). Plasma nitriding of the Ti surfaces has also been shown to improve the wear properties of the material through the formation of a hard compound layer of TiN and Ti2N (Manhabosco et al., 2011; Fernandes et al., 2006). Plasma assisted chemical vapour deposition of hydrogenated amorphous carbon (a-C:H) onto the surface of the Ti-6Al-4 V alloy was also suggested as a method to improve corrosion and wear resistance of the material (Martini et al., 2011). Although beneficial at low applied load, the coatings failed prematurely under higher load.
et al., 2011). Other studies have suggested the relationship between the microstructure of the material and the rate of its wear is not straightforward (Majumdar et al., 2011). Plasma nitriding of the Ti surfaces has also been shown to improve the wear properties of the material through the formation of a hard compound layer of TiN and Ti2N (Manhabosco et al., 2011; Fernandes et al., 2006). Plasma assisted chemical vapour deposition of hydrogenated amorphous carbon (a-C:H) onto the surface of the Ti-6Al-4 V alloy was also suggested as a method to improve corrosion and wear resistance of the material (Martini et al., 2011). Although beneficial at low applied load, the coatings failed prematurely under higher load.
The biocompatibility and corrosion resistance in vivo of otherwise highly reactive Ti and Ti-based alloys arise from the presence of a robust passive oxide film of TiO2 on their surfaces (Addison et al., 2012). Generally, the corrosion process results in the rapid formation of a thin reaction film on the surface of all metals, from reactive Ti to noble Au. Under certain environmental conditions, for example low solubility and absence of defects, such a reaction film will be characterised by strong adhesion to the substrate and will protect the underlying bulk material from degradation. Typically, these oxide passive layers are 1 to 5 nm in thickness, optically transparent and amorphous in nature. The amorphous structure of the layer with minimal grain boundary and the self-repairing property of the film ensure low susceptibility to corrosion. In the case of Ti, the oxide layer has been shown to consist of amorphous and slightly crystalline TiO2, with Ti2O3 and TiO also detected (Lausmaa, 1996). The passive film formed on the surface of Ti-6Al-4 V alloy was similar in chemical composition to that of commercially pure Ti, except for a minute quantity of Al2O3 and hydroxyl moieties detected in the alloy. Similarly, a primarily titanium dioxide film was formed on the surface of Ti-Ni alloy, with limited quantities of NiO, metallic Ni and –OH functionalities. In contrast, the surface oxide layer on the surface of Ti-Zr alloy has been shown to consist of titanium and zircon oxides in the proportion that reflected the relative concentration ratio of Ti to Zr in the bulk material. Higher concentrations of Zr resulted in a thicker, more stable protective coating.
5.5 Noble metal alloys
As a result of their high durability, stability and excellent corrosion resistance, noble materials and their alloys are widely used in restorative dentistry (Mehl et al., 2011; Ucar et al., 2011b). Gold fillings can be produced by either casting or malleting, with Au alloys favoured over pure Au for the casting method. The mechanical performance of Au is inferior when compared to the alloyed materials, with impurities such as Cu and Pt (< 4%) known to strengthen the Au-based alloys. However, high concentration of noble materials (> 75%) ensure their anti-corrosion performance. If the level of Au exceeds 83%, the alloy becomes too soft to be employed in stress-bearing applications, such as cups and crowns. Pure gold foil is employed for malleted restorations, where soft layers are assembled in the cavity and joined by means of thermal diffusion of atoms between layers under applied pressure. Elemental Ag is introduced into the alloy to improve the colour of the resultant product. Higher concentrations of Pt (> 4%) have been shown to increase the melting point of the alloy, thus making the processing more complex, whereas addition of minute quantities of Zn has been demonstrated to lower the melting point. The surfaces of Au alloys, such as Ag-Cu-Au, Pd-Ag-In-Sn and Ag-Pd-Cu-Au, are encapsulated by Cu and Ag oxides (Guo et al., 2003). There were no significant differences in mechanical yield strength between Au-Pd, Pd-Ag, Pd-Ag-Au and Au-Ag-Pd alloys; however, their percentage elongation varied extensively, with the Pd-Ag and Pd-Ag-Au alloys characterised by the highest elongation values (Ucar et al., 2011a).
Silver-based amalgam is a mercury-containing alloy that has been widely used as a tooth filling material. Its dental utility stems from the unique property of elemental Hg to remain in a liquid phase at room temperature and to react with other metals, such as Ag and Sn, to produce a plastic substance which can be easily deformed. The use of amalgam is preferable to the use of composite in large and complex restorations, with margins located in dentine or cement, where isolation is deficient (Soares and Cavalheiro, 2010). In practice, dry Ag-Sn alloy is mixed with Hg, resulting in the reaction: Ag3Sn+Hg ↔ Ag3Sn+Ag2Hg3 + Sn7Hg. Typical dry alloys consist of more than 65%wt Ag, less than 29%wt Sn, less than 6%wt Cu, less than 2%wt An and less than 3%wt Hg. The plasticity facilitates packing of the alloy into the tooth cavity, which is then hardened over time. Generally, the alloy is expected to reach 2% of the final strength after 60 min, and nearly all of its final strength after 24 h of curing. Once fully hardened, the alloy should contain 45 to 55% Hg, 35 to 45% Ag and 15% Sn. Tin oxide forms the protective oxide layer at the surface of the material.
Dental amalgams have been employed for dental restoration for over 150 years owing to their malleability, durability and affordability compared to gold or composite dental materials and also in terms of minimal technological requirements for amalgam installation (Ye et al., 2009). However, there has been much debate over the potential toxicity of these materials in vivo. Indeed, even at minute levels, Hg0 is thought to be neurotoxic and nephrotoxic (Clarkson and Magos, 2006). It is therefore possible that Hg0 can leach out of the amalgam, and thus subjecting the patient’s body to the increased burden of mercury (Counter and Buchanan, 2004; Scholtanus et al., 2009). Over the years, amalgams were alleged to contribute to a multitude of diseases, from multiple sclerosis to chronic fatigue, Alzheimer’s or Parkinson’s diseases (Bates, 2006). Yet, only a few relevant epidemiologic studies were conducted, and even then, the data from dental exposure were compared to occupational hazardous exposures to mercury. Full-scale clinical studies of the amalgam-exposed population are complicated by the inadequate longitudinal exposure assessment and negative confounding since higher socio-economic groups would be able to access restorative dental care. Several recent clinical trials found that neurobehavioural and neuropsychological performance did not differ significantly between children with and without amalgam fillings (Bellinger et al., 2006, 2008; Gottwald et al., 2001). All studies reported elevated urinary total mercury levels, higher mean urinary concentration of albumin, and increased micro-albuminuria in patients with amalgam.
5.6 Shape memory alloys
The significance of shape memory alloys as materials for implantable devices has been increasing in recent times due to a favourable combination of their unique mechanical and functional properties, namely ‘the shape memory effect’, and their pseudo-elasticity (Biesiekierski et al., 2012). Pseudo-elasticity refers to the material’s ability to recover its original shape after a mechanical load has induced large deformations, whereas the shape memory effect describes the ability of such a material to be plastically deformed below its transformation temperature, and recover its original shape once the temperature is increased. This effect can be explained by studying the crystallography and thermodynamics of shape memory alloys. These materials are characterised by two solid phases: the austenitic parent phase and the martensitic phase. The austenitic phase is stable at high temperatures and characterised by high symmetry, whereas the martensitic phase is stable at low temperatures and of low symmetry (Petrini and Migliavacca, 2011). The latter phase can exist in two configurations, either as stress-free or stress-induced martensite, the latter the result of macroscopic deformation. The shape memory effect can therefore be generally related to a stress–temperature induced diffusionless martensitic phase transformation that is also thermo-elastic in nature, the thermo-elasticity being attributed to ordering in the austenitic and martensitic phases.
Martensitic transformation can be triggered by lowering the temperature below the martensite transformation temperature (T<TMs), and reversed by heating the material to T>TAs, TAs being the temperature at which austenitic transformation is initiated. Subjecting the material to mechanical deformation at temperature T>TMs can also instigate the martensite transformation, since both TA and TM can be raised through application of stress below the yield point. Significant residual strains of up to 10% can be recovered in this way, with the process termed ‘free recovery’. Subjecting these alloys to a series of thermo mechanical treatments can be used to imprint characteristic shapes to which they can revert under the martensitic and austenitic phases. However, achieving this effect can be complicated by the memory loss resulting from a growing number of temperature cycles.
If the shape recovery is obstructed by the presence of a physical restriction before the austenite finish transformation temperature TAf is attained, high stresses of up to 800 MPa can be achieved in shape memory alloy elements (Videnic et al., 2008). This process, termed ‘constrained recovery’, is often used for generating forces in fasteners, seals, clamps and connectors (Bujoreanu et al., 2008; Fei et al., 2009). The events of constrained recovery only take place in the case of the orientated (stress-induced) martensite, and not the multi-variant (stress-free) martensite obtained under thermal regime. The pseudo-elastic effect is evident at constant high temperature T>TAf, where highly nonlinear large deformations induced by the mechanical loading-unloading cycle results in no permanent deformations at the end of the cycle. Material in its austenite state is more difficult to deform due to higher elastic modulus compared to that of the martensite, EA>EM.
For biomaterial applications, it is important to attain the desired mechanical characteristics, shape memory and pseudo-elastic behaviour at temperatures appropriate to the living systems, for example the austenite finishing temperature should be below the body temperature of 37 °C (Kim et al., 2012; Biesiekierski et al., 2012). The shape memory and pseudo-elastic performance of the alloys can be effectively controlled by altering their chemical composition, which will influence the respective temperatures at which phase transitions initiate and finish, the maximum strain that can be thermally recovered, and the hysteresis characteristics. For instance, the martensitic transformation temperature has been demonstrated to be highly sensitive to a variation in the stoichiometry or introduction of new alloying elements (Zhao et al., 2005). However, this may introduce new challenges, such as decreased biocompatibility, element toxicity and altered biodegradation profile.
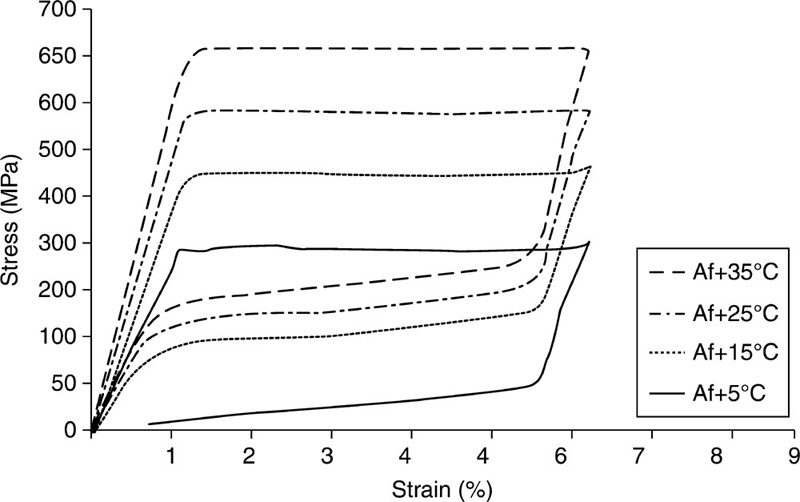
Amongst the shape memory alloys that include AgCd, AuCd, CuAlNi, CuAlBe, CuSn, CuZn, InTl, NiAl, FePt, FePd, MnCu and FeMnSi, Ti-based materials have been regarded as the most promising (Bartolo et al., 2012; Bujoreanu et al., 2008; Petrini and Migliavacca, 2011, Zhao et al., 2005). Nitinol is a Ti-based thermo-elastic biomaterial with approximately 50% atomic Ni content (Fig. 5.3). It rose to prominence in the 1960s, owing to its highly desirable shape memory behaviour. The commercial attractiveness of Nitinol stems from a combination of sound mechanical stability, lower stiffness, nonlinear mechanical behaviour, thermo-elasticity and biodegradation corrosion resistance, making it a suitable replacement for stainless steel implants (Tozzi, 2007). The plateau stress of Nitinol is analogous to the yield strength of other metallic biomaterials, whereas at ultimate conditions, NiTi stress is higher but strain is considerably lower compared to other biometals, attributed to strong work hardening of Nitinol, even post-annealing (Petrini and Migliavacca, 2011). Sound magnetic resonance and computer tomography compatibility (Bell et al., 2012; Bunck et al., 2012), and enhanced bio-integration capability compared to commonly used stainless steel 316 L and Cr-Co biomaterials, make Nitinol an alloy of choice for most shape memory applications in vivo.
Utilisation of Nitinol as a medical material started in the 1970s, when the pseudo-elastic characteristic of the material was used in an orthodontic device (Andreasen and Barrett, 1973). Table 5.1 provides some of the key biomedical applications of Nitinol. Wires of Nitinol have been commonly used in fixed orthodontic treatments, specifically to inflict constant pressure for wide dental movements (Huang et al., 2013). The wires designed to have TA of the buccal cavity undergo stress-induced martensitic transformation during insertion. However, once installed, the wires attempt to regain their austenitic conformation. When the recovery is constrained, constant forces are applied to the teeth, facilitating their movement into the correct position in the mandible. The force applied by steel palatal arches with Nitinol components is lighter compared to steel-only arches; this is also the case for orthodontic distracters used to mitigate teeth overcrowding via expansion of the mandible (Gil et al., 2012). Nitinol has also replaced steel in files used to shape and clean root canals prior to filling, where pseudo-elastic property gives the device good flexibility and strain recovery (Gutmann and Gao, 2012). Furthermore, limitation of the force that can be applied to the canal using a Nitinol file facilitates motorisation of the process.
Table 5.1
Key applications for Nitinol according to its shape memory alloy characteristics
| Medical field | Effect: pseudoelasticity | Effect: shape memory |
| Specific properties: mechanical shape recovery, wide plateau, constrained recovery | Specific properties: heat-induced shape recovery/constrained recovery | |
| Orthodontic | Wires, palatal arches, distracters, endodontic files | Wires |
| Orthopaedic | Intraspinal implants, intramedullary nails | Staples or plates, devices for correcting scoliosis, spinal vertebrae spacer, intramedullary nails, devices for physiotherapy |
| Vascular | Venous filters, devices for closing ventricular septal defects, self-expandable vascular stents, stent-graft, percutaneous devices to treat valvular diseases | Venous filters, devices for closing ventricular septal defects |
| Neurosurgical | Coils, stents, microguidewires | |
| Surgical | Mini-invasive surgical instruments |
adapted from Petrini and Migliavacca (2011)
In orthopaedics, the restricted shape recovery property of Nitinol is used for the treatment of fractures (Zhao et al., 2012b). Plates or staples of Nitinol are installed while they are in their deformed martensitic phase, and then they regain their austenitic shape upon reaching body temperature. Since the recovery is restricted, the implant applies a stable pressure, thus connecting the fractured bone fragments together. This stable force can also be utilised to rectify scoliosis, by adjusting the relative position of the vertebrae in the spine column (Anand and Baron, 2011; Wang et al., 2011). Nitinol spacers have been employed to replace affected intervertebral discs; low elastic modulus of the martensitic alloy facilitates high deformability, allowing the insertion of the spacer between the vertebrae (Marcolongo et al., 2011). Shape memory alloys have also been suggested for use as intraspinal implants for stabilising spinous processes in the case of vertebral discs and as intramedullary nails in fractured elongate bones (Petrini and Migliavacca, 2011). Recently, porous shape memory alloy scaffolds have attracted notable attention for their low elastic modulus combined with new bone tissue ingrowth ability and vascularisation (Barras and Myers, 2000; Wen et al., 2010). These materials are also characterised by low-weight, high-strength, high-energy dissipation and biocompatibility, making them ideally suited as bone scaffold materials (Bernard et al., 2012; Kim et al. (in press)).
One of the most commercially successful examples of the utilisation of Nitinol is in the production of expandable stents that are used to restore the blood flow obstructed by atherosclerotic deposits in arteries, including coronary, carotid, femoral and peripheral arteries (Huang et al., 2013). Similar to stainless steel and Cr-Co structures, Nitinol stents are delivered to the stenotic region of the vessel using a catheter via a small incision. Once inserted, an inflatable balloon is used to open up the steel and Cr-Co stents, pushing them against the walls of the artery, which can potentially inflict further damage on the vascular tissue. In the case of Nitinol stents, the opening is driven by the shape memory transformation from the martensite phase (TM<Tbody) to austenite (TA=Tbody) upon removal of a protective sheath. Austenite is a stable configuration, and therefore does not necessitate an over-expansion to counteract the elastic recoil observed in the stainless steel stents. Furthermore, the pressure exerted on the walls is constant, which is particularly significant in the peripheral arteries where squeezing due to muscle contractions can occur. Biomimetic modifications of the surface of cardiovascular metallic materials via endothelialisation or by mimicking the endothelium can further improve in vivo utility of Nitinol stents by limiting material-induced thrombosis and restenosis (Weng et al., 2012a).
One of the most notable drawbacks of using Nitinol stents lies in its fatigue behaviour, which is most prominent in devices used to treat peripheral arterial diseases (Robertson and Ritchie, 2007). This is particularly the case in stents residing in superficial femoral and femoropopliteal arteries, where pulsatile forces, due to systolic/diastolic pressure cycles, and nonpulsatile forces resulting from normal leg movements, contribute to fracturing. However, the ability to adequately describe the fatigue life of the alloy is complicated by the nonlinear pseudo-elastic characteristic of the biomaterial, which is also dependent on the elemental composition, production and subsequent treatments of the material (Elahinia et al., 2012). Tozzi (2007) investigated the fatigue growth rate of Nitinol compared to other materials used for fabrication of cardiac valve prostheses, namely stainless steel, commercially pure Ti, Ti-based alloy Ti6Al4V and Co-Cr alloy. Although these investigations were conducted in air and not under the physiological environment such as in simulated body fluid, the fatigue tolerance was found to be lowest, with cracks appearing fastest, in Nitinol compared to the other tested metals. However, some of these alloys display a notable reduction in their stability when exposed to corrosive conditions, whereas Nitinol has been demonstrated to resist these conditions. Irrespective of these comparisons, the possibility of cracking should be carefully considered when designing endovascular devices that have a very fine architecture, specifically to hinder crack propagation throughout the stent, otherwise metal fracture, fabric erosion, suture breakage, and ultimately device failure, can result (Kleinstreuer et al., 2008).
Venous filters are deployed into the vessel to prevent emboli in a similar manner to arterial stents. As with stents, the filters are fabricated so their open austenitic configuration is attained at the body temperature, whereas a tightly closed martensitic configuration facilitates easy insertion into the veins via a catheter. Instead of a protective sheath, a cooled saline solution is used to keep the filter in its martensite phase. Once inserted into position, the flow of the saline solution is discontinued, and the resultant temperature rise drives the transformation of the filter to its open form. A similar approach is used to mitigate ventricular septal defects in the heart, where a device made of shape memory wires and impermeable polymer membrane is used to close the atrial hole, to treat abdominal aortic aneurisms using stent-grafts, and to treat mitral, pulmonary and aortic valvular diseases using stent-valve devices, which require minimal invasion for deployment.
Nitinol-based neurosurgical devices, including coils, self-expandable stents and micro-guidewires are also widely used. Coils are commonly employed to mitigate cerebral aneurism and resultant possible cerebral haemorrhage by preventing the rupture of locally dilated, but unruptured intracranial arteries. The coil is capable of stimulating clotting and thrombosis to minimise the possibility of rupture. In the event of ruptured aneurisms, coiling is carried out shortly after rupture as the incidence of bleeding within the first weeks after initial rupture is high. Endovascular coiling involves insertion of a guiding catheter through the femoral artery, which then advances towards the site of the aneurism with the help of fluoroscopic imaging. Once the aneurism is located, a micro-catheter with a coil is deployed. A series of progressively smaller detachable coils is then inserted into the aneurismal sac until it is filled. Compared to platinum-only coils, platinum-Nitinol coils are thought to produce less stretching and are characterised by improved resistance to compaction (Sfyroeras et al., 2012). In cases where the neck of the aneurism is large, a stent treatment may be required; the stent can be used to hold the coil ball in the correct space. Nitinol micro-guidewires can be employed to aid positioning of the stent. In addition to the aforementioned vascular and neurosurgical applications, Nitinol stents are employed to remedy urethral, esophageal, rectosigmoidal, prostatic constrictions, biliary obstruction and tracheal stenosis (Petrini and Migliavacca, 2011).
Nitinol-based materials have found numerous applications as tools for surgery, particularly for minimally-invasive surgical procedures, and as sutures and self-closure clips that allow for quick wound closure. In addition to guidewires, Nitinol is used to fabricate baskets for removal of kidney and bladder stones, catheters, snare loops for manipulation and challenging or unplanned foreign body retrieval, compression rings, and in laparoscopic surgery, where Nitinol graspers and dissectors are introduced straight, and once in the peritoneal cavity form a curve that is then used to retract intra-abdominal organs. The SMart prosthesis that comprises a Nitinol-based Shepard hook and a Telfon™-based piston are used for the treatment of otosclerosis. The piston wire has the ability to self fasten around the incus, providing an extra secure fit between the wire and the incus and thus improving the transmission of sound (Randhawa et al., 2012).
As mentioned previously, the chemical composition of the alloy and processing methodology will have a significant impact on the resultant properties of the biomaterial, and therefore should be fine tuned depending on the desired use (Chan et al., 2012). Addition of Fe, Pt, Au, Al, Cu, Zr and Hf to form alloys has been trialled to enhance the shape memory effect of Nitinol (Bozzolo et al., 2005; Young et al., 2012; Zarnetta et al., 2012). Hafnium ion implantation has also been suggested as a modification to modulate the wear resistance and surface integrity of Nitinol alloys (Zhao et al., 2012a). The formation of a thicker TiO2/HfO2 nanofilm on the surface of the alloy, coupled with a reduced nano-hardness of the material was found to enhance the wear resistance, and provide improved surface integrity and increased pseudo-elastic recovery strain. Improved functional fatigue properties for (Ti,Hf)-rich alloy Ti 40.0Ni 47.5Hf 12.5 thin films was also reported (König et al., 2011). Addition of pure Ag has been suggested to impart antibacterial activity, significantly reducing the attachment of several pathogenic bacterial strains, including S. aureus, S. epidermidis and P. gingivalis compared to Nitinol (Zheng et al., 2011). Introduction of Ag was found to slightly increase the tensile strength and elongation of the alloy compared to the binary counterpart, with a maximum shape recovery of 6.4%. The corrosion resistance in stimulated body fluid was also improved in the case of TiNiAg compared to commercially pure Ti and NiTi.
It has been suggested that the biocompatibility and biocorrosion resistance of the Ni-Ti based alloy can be improved by introducing molybdenum into the binary alloy. Furthermore, the medical utility of Ti-Ni-Mo alloys would therefore be improved, since an increase in the Mo content notably decreases the martensitic transformation temperature TM in these materials (Skorentsev and Demidenko, 1995). Porous Ti50Ni49.9Mo0.1 and Ti50Ni49.7Mo0.3 shape memory alloys were characterised by TAf of 40.4 and 34.4 °C and recovered strain of 1.5 and 2.0%, respectively (Kim et al.).
Although widely used in numerous medical applications, the potential toxicity of Nitinol in vivo remains a subject of debate. There are many conflicting reports regarding the ability of Nitinol to withstand corrosion, especially under dynamic loading conditions. The corrosion stability of Nitinol is generally attributed to the formation of a protective Ti oxide, which precludes Ni from leaching out into the peri-implant space. However, recent studies questioned the stability of the oxide, suggesting replacing toxic Ni with a biocompatible oxide forming niobium (McMahon et al., 2012). In this study, TiNb (26 at.% Nb) shape memory alloy was compared to Nitinol (49.2 at.% Ti), finding the former more cytocompatible under static culture. The improved cytocompatibility of TiNb was attributed to the combined effect of decrease in the ion release and enhanced corrosion resistance. Furthermore, calcium phosphate deposits detected within the Nitinol oxide layer were not present on the surface of TiNb. Several surface modification techniques have also been reported to enhance surface integrity, limit biocorrosion and improve tissue integration of Nitinol in vitro and in vivo. These include plasma immersion ion implantation, laser surface melting and laser gas nitriding, passivation, coating and reduction annealing (Chu et al., 2009; Cui et al., 2003; Gu et al., 2005; Neelakantan et al., 2009; Shen et al., 2012; Shevchenko et al., 2004; Yuan et al., 2009, 2011).
Overall, the exceptional shape memory effect property of the aforementioned materials offers alternative solutions to numerous biomedical applications, such as minimally-invasive surgery, requirements for which may be problematic to meet using conventional biomaterials and mechanisms (Huang et al., 2013). Further characterisations, especially with regard to the biodegradation of these materials, are required to fully utilise these unique properties in vivo.
5.7 Biodegradable metals
Since the invention of stainless steel almost 100 years ago, metal implants have undergone an immense transformation in terms of their material development and in vitro and in vivo applications. A wide range of materials with excellent corrosion resistance has been developed and clinically applied, including 316 L stainless steels, Ti and its alloys and Co-Cr alloys (Xin et al., 2011). The novel generation of metallic biomaterials and processing methodologies have been developed to ensure these devices would maintain their physico-chemical properties for the duration of implantation. In the implanted environment, they would remain in a primarily neutral state, and in the case of fixation of large fractures, these implants would require subsequent surgery for removal. Recently implantable materials that can undergo controlled degradation in vivo have been attracting a considerable amount of attention (Hermawan, 2012).
Biodegradable metals, such as magnesium, hold great promise in applications that support tissue regeneration and healing, particularly where a load-bearing function is required (Shimizu et al., 2010). Magnesium is highly biocompatible and nontoxic, with Mg ions being essential to human metabolism (Xin et al., 2011). It is highly suited for the fabrication of fully resorbable intravascular stents for the treatment of arterial disease, minimising the risk of chronic inflammation and late thrombosis associated with the implantation of a permanent metallic stent. For osteosynthesis, Mg and its alloys offer high primary stability, high tensile strength and resistance to fracture. It is also lightweight, with a density of 1.74 g/cm3. which is 1.6 and 4.5 times less dense than aluminium and steel, respectively (Staiger et al., 2006). The specific gravity and elastic modulus of Mg is very close to those of human cortical bone, decreasing the stress-shielding effects in bone tissue associated with implant integration. Furthermore, Mg bivalent ions are intimately involved in the formation of biological apatites and thus determine the extent of bone fragility, bone healing and regeneration (Rude et al., 2009; Witte, 2010).
In spite of its favourable mechanical and biological properties, the clinical applications of Mg are limited by its rapid corrosion rate in vivo (Fig. 5.4), especially in a physiological environment with pH values between 7.4 and 7.6 and biological fluid being present with chloride ions at levels of 150 mmol/L (Li et al., 2012). Such rapid degradation may lead to the release of large amounts of Mg2 +, localised hydrogen gas (H2) accumulation and alkalisation, and to an untimely loss of mechanical strength of the implanted material (Witte, 2010). For instance, intravascular ultrasound imaging of absorbable Mg stents in human coronary arteries indicated the loss of the radial force and consequent early recoil as a main contributor for restenosis at 4 months (Waksman et al., 2009). Thus, to meet clinical requirements, precise understanding of degradation kinetics and control over in vivo degradation of implants based on biodegradable metals and alloys are essential, especially at the early stages of implantation where degradation may be most pronounced.
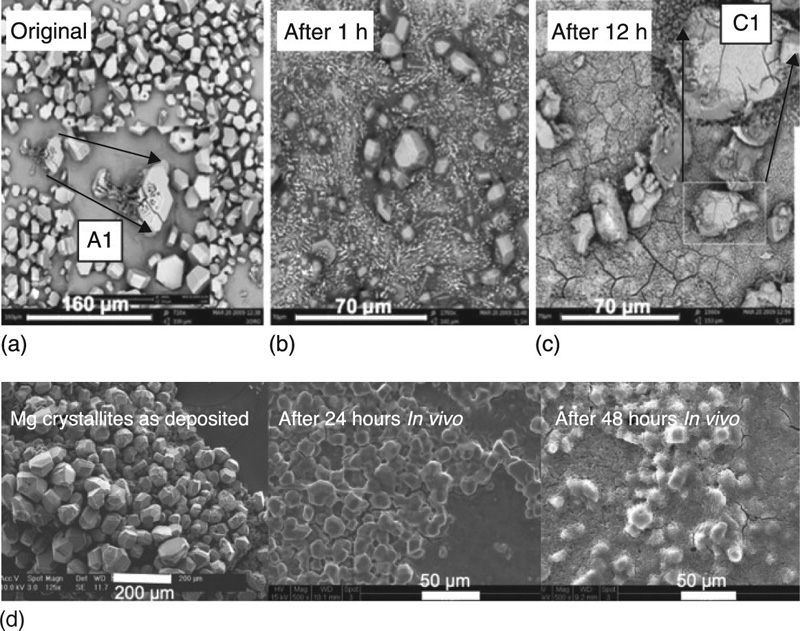
It is important to understand that the environment largely influences the biodegradation behaviour of resorbable material. By means of different physicochemical parameters (e.g. pH, ion concentrations, oxygen), the biological environment directly affects the properties and the behaviour of the implant material. Concomitantly, the implant, as an introduced foreign body, incites an immunological response and influences the surrounding tissues due to the direct and intimate contact. For example, the biodegradation of Mg alloy (AZ31) screws implanted into a sheep hip bone differed in terms of the corrosion morphology and dynamics, with the screw threads inside the bone displaying significantly less corrosion compared to the screw head in contact with overlying muscle/connective tissue (Willbold et al., 2011). Similarly, Mg wires were demonstrated to undergo extensive biocorrosion when placed in the rat arterial wall, whereas little corrosion was observed for those Mg wires exposed to blood in the arterial lumen for 3 weeks (Pierson et al., 2012). Therefore, in order to adequately predict the long-term behaviour of biodegradable metals such as Mg and Mg alloys, an in vitro evaluation needs to be supplemented with an assessment of the extent of biocorrosion taking place under complementary in vivo conditions.
As with many other metals, the physico-chemical properties of Mg can be tuned by introducing other elements into the alloy (Hort et al., 2010; Seitz et al., 2012). The changes in chemical composition as a result of the addition of ligands, coupled with a chosen processing methodology, influence the resulting microstructure of the alloy, potentially improving the mechanical and corrosion behaviour of the material. Rare earth metals, such as Gadolinium, added to the alloy in small quantities, have been reported to have the most profound impact on the corrosion susceptibility of Mg alloys (Staiger et al., 2006). A significant reduction in the rate of bio-corrosion has been reported for Mg alloys containing 0.40 to 4.0 wt% rare metals (Nd or Y), 0.05 to 1.2 wt% Cd, 0.05 to 1.0 wt% Ca or Al, 0.05 to 1.0 wt% Mn, less than 0.8 wt% Ag, less than 0.8 wt% Zr and less than 0.3 wt% Si (Stroganov, 1972). The authors reported that pins of 3 and 8 mm in diameter remained intact for 5 and 11 months in vivo, respectively, although no discussion was provided with regard to the potential toxicity of the alloying components.
In a different study, two combinations of rare earth elements were used at a maximum total concentration of below 10 wt%: WE43 comprising of 71 wt% Nd, 8 wt% Ce, 8 wt% Dy and 6 wt% La; and LAE442 consisting of 51 wt% Ce, 22 wt% La, 16 wt% Nd and 8 wt% Pr (Witte et al., 2005). Polylactide, Mg-Al (AZ31) and Mg-Zn (AZ91) alloys were also investigated. Rods of 1.5 mm in diameter were then implanted into the femora of guinea pigs, and then removed at 6 and 18 weeks. Typically, addition of elements such as Al and Zn is thought to increase the oxidation rate of the alloy, whereas rare earth elements are believed to slow the rate of oxidation. When alloyed with Mg, Al has been proposed to reduce the corrosion rate by stabilising hydroxides in chloride conditions (Makar and Kruger, 1993). Increased osteoblastic activity in the bone tissue in the proximity of the Mg-Al alloy indicated that small quantities of Al leached from the degrading implant may be well tolerated by the surrounding tissue. The introduction of rare earth elements has been demonstrated to lower the threshold on the corrosion protective action of Al (Nordlien et al., 1997). Addition of Li can further contribute to reducing the rate of corrosion, by alkalising the surface layer and stabilising the Mg hydroxides within it. In the case of all four Mg alloys, biological amorphous calcium phosphates together with Mg oxides and Mg hydroxides formed an intricate corrosion layer believed to slow down the corrosion and enhance ossification (Witte et al., 2005). The corrosion rate was most reduced in the case of LAE442, whereas AZ31, AZ91 and WE43 alloys were characterised by similar corrosion rates.
While they have been used to enhance the mechanical and anticorrosive characteristics of Mg alloys, many rare earth elements are yet to be properly described with regard to their toxicity, cell and tissue compatibility, and systemic biocompatibility (Yuen and Ip, 2010). In a recent study, in vitro cytotoxicity of elemental Y, Nd, Dy, Pr, Gd, La, Ce, Eu, Li and Zr was studied by incubation with the chlorides (Feyerabend et al., 2010). Amongst the elements tested, La and Ce showed the highest cytotoxicity; among elements with high solubility in the alloy, Gd and Dy were determined to be more suited to biomaterial application compared to Y. Given their potential toxicity, Mg alloys with reduced rare earth content or containing no rare earth elements may be preferred. For instance, the novel alloys ZEK100 and AX30 are promising with regard to their in vitro cytocompatibility. ZEK100 is composed of Mg with 1 wt% of Zn, less than 1 wt% of Zr and less than 1 wt% of rare earths; AX30 is comprised of Mg with 3 wt% of Al and less than 1 wt% of Ca (Huehnerschulte et al., 2012). In vivo studies using a rabbit model showed that in the first 3 months, ZEK100 underwent a faster corrosion compared to that of AX30; however, after 6 months the difference was found to be absent. Both alloys were demonstrated to stimulate adverse host reactions and increased numbers of osteoclasts in the peri-implant bone tissues.
Application of an encapsulation protective coating has been suggested as a way to control Mg alloy degradation under physiological conditions. Hydrothermal treatment of an AZ31 magnesium alloy using deionised water resulted in the formation of a uniform, compact hexagonal magnesium hydroxide Mg(OH)2 protective coating with a spot of monoclinic aluminium magnesium hydroxide Mg2Al(OH)7 (Zhu et al., 2012). A recent study showed that plasma enhanced chemical vapour deposition was used to apply a thin amorphous layer of SiC onto WE43 alloys (Li et al., 2012). The polymer-coated materials exhibited significantly lower corrosion rates and improved haemocompatibility compared to that of the unmodified WE43 alloys, with attached platelets being seen to be slightly activated. It has been suggested that the semiconducting characteristics of SiC are responsible for the material’s anti-thrombogenic activity, making the material an appropriate choice for vascular and coronary stent applications (Mani et al., 2007; Monnink et al., 1999).
Cell viability was also improved for SiC-coated WE43 alloys compared to their uncoated counterparts. Organic coatings have also been suggested as being suitable, although there are several limiting factors that hinder their utilisation, including poor coating adhesion, presence of defects within the polymer film, and adverse effects resulting from the solvents used in many of the polymer precursors (Hu et al., 2012). Plasma assisted depositions and sol–gel polymerisation have been shown to produce coatings that result in significantly enhanced adhesion and minimal pre-treatment requirements (Bazaka et al., 2011; Lamaka et al., 2009; Wang et al., 2010a). Organic coatings produced via plasma polymerisation are also characterised by smooth, defect-free surfaces, with minimal pores and defects; this minimises the number of layers that have to be coated onto the substrate to achieve appropriate corrosion protection.
The aforementioned results clearly support the viability of Mg-based biomaterials as biologically compatible, fully degradable, lightweight and potentially osteo-inductive materials. However, to truly facilitate the clinical implementation of Mg-based bioresorbable devices, more comprehensive in vitro and in vivo investigations are required to definitively confirm the safety of such devices (Staiger et al., 2006).
5.8 Conclusion
Metallic materials have found many important medical applications, as orthopaedic implants, dental materials and cardiovascular devices. These are likely to remain a biomaterial of choice for applications that require high tensile and fatigue strength and fracture toughness. Current trends in biomaterials design include the development of materials that are lighter, stronger, smaller and more complex, with an enhanced bioactivity profile and highly controlled biodegradation kinetics. Biofunctionalisation of these materials via surface modification has been identified as a low-cost and relatively short development time approach to attain an optimal range of biofunctions. These issues are discussed in more detail in Chapter 6.
