Natural polymer biomaterials: advanced applications
Abstract:
Advanced applications of natural polymers, including chitosan, alginate, starch, collagen and gelatin, and their utilisation in the fabrication of tissue engineering matrices and drug delivery systems, are discussed. The structure, synthesis pathways and modifications of various classes of natural polymers, spanning biodegradable materials, biomaterials intended for long-term implantation, and those used for targeted drug delivery and highly specific imaging, are also considered.
2.1 Introduction
Polymers are one of the most omnipresent classes of materials in the medical industry and in our society (Fahlman, 2011). Since they occur in nature, materials of both plant and animal origin are often presumed to exhibit enhanced compatibility with human hosts, the ability to exhibit bioactivity, and to undergo biodegradation. As such, natural nanomaterials and particulate materials can exhibit these characteristics in situations where synthetic materials have not met clinical expectations (Yoo et al., 2011). In this chapter, natural polymers, including chitosan, alginate, starch, collagen and gelatin are discussed, together with their ability to be used in the fabrication of tissue engineering matrices and drug delivery systems (Sonia and Sharma, 2011).
This chapter provides an overview of the structure, synthesis pathways, ability to undergo modification, and the applications of various classes of natural polymers. The discussion spans biodegradable materials, biomaterials intended for long-term implantations, and those used for targeted drug delivery and highly specific imaging techniques. Given the vast number of available scientific reports that describe the properties and behaviour of polymeric biomaterials, together with the existence of an equally high number of comprehensive reviews in this area, only the most recent examples will be included in this chapter. Preference is also given to those studies that report the behaviour of nanostructured biomaterials and nanoparticles, since it is likely that nano- and molecular-scale materials and devices will play an increasingly important role in the advancement of medicinal and biotechnological processes. This is exemplified by the major breakthroughs that have recently taken place, together with the increases in economic activity within the tissue engineering sector. This has been growing exponentially, as indicated by an increasing number of products entering into the clinical testing phase and eventually reaching the market place (O’Brien, 2011). Sales of regenerative biomaterials have already exceeded US$240 million per annum, with further growth being expected through the support of newly available and developing technologies, the existing regulatory guidelines, and the commercial success of the private sector within the aggregate field comprised of tissue engineering, regenerative medicine and stem cell therapeutics (Lysaght et al., 2008; Vacanti, 2007).
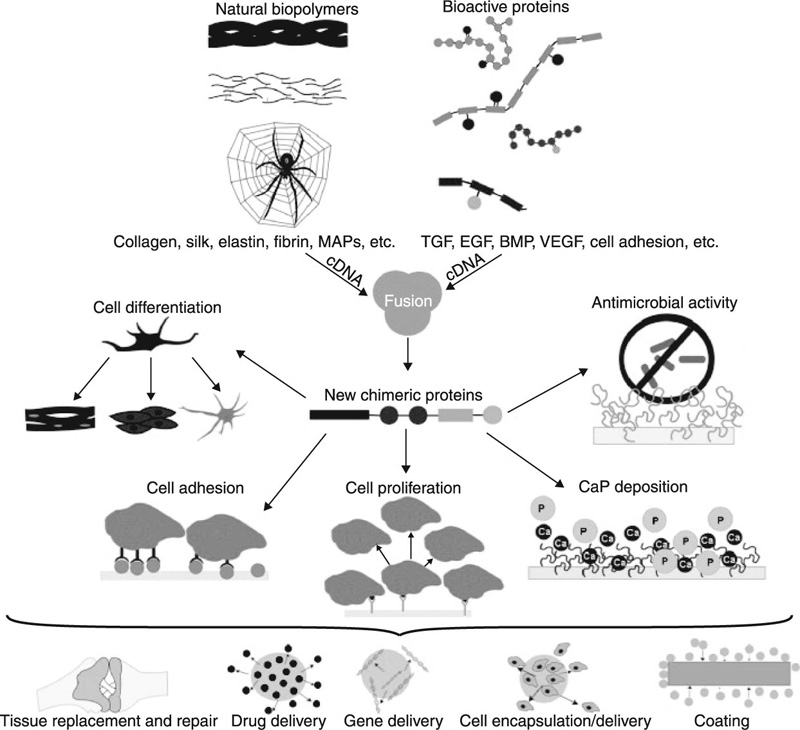
The highly specific delivery and controlled release of drugs and other biological agents is an area of immense importance for human health, since these systems can be used to substantially prolong life and provide relief for suffering patients (Jain et al., 2011a; Tabata, 2009; Timko et al., 2011). Incorporation of drugs into protective polymeric nanocarriers, such as liposomes and lipid nanoparticles, micelles, nanogels, nanospheres and nanocapsules, solid nanoparticles and nanosuspensions, block ionomer complexes, nanofibres and nanotubes (Batrakova et al., 2011), allow for new routes of drug delivery to be identified. In addition, new active agents are now able to be accessed and utilised with an unprecedented level of efficiency (Ahmad et al., 2011; Ali and Mooney, 2011). In regenerative medicine, the genetic engineering of proteins promises to overcome the limitations of traditionally used autografts and allografts, by providing a platform for the on-demand expression of biological components and highly controlled generation of new protein sequences and self-assembling peptides with tunable properties. They also allow the fusion of different bioactive domains or protein motifs (Gomes et al., 2012). Figure 2.1 depicts some of the features and applications of chimeric protein-based biomaterials expressed naturally using recombinant DNA technology.
2.2 Chitin and chitosan
Chitin is a biopolymer composed of N-acetyl-glucosamine and N-glucosamine monomers. These units can be either randomly or block distributed throughout the biopolymer chain. Depending on the N-acetyl-glucosamine to N-glucosamine ratio, the polymer is termed either chitin (number of N-acetyl-glucosamine units > 50%, poly(N-acetyl-D-glucosamine)) or chitosan (number of N-glucosamine units > 50%, poly(N-acetyl-D-glucosamine-co-D-glucosamine)) (Dash et al., 2011; Osorio-Madrazo et al., 2010). The degree of deacetylation and the molecular weight of the polymer are both known to significantly affect the material properties and the resulting performance of the chitosan and chitosan derivatives (Table 2.1). Due to the strong hydrogen bonding that exists between its chains, chitin is insoluble in most typical processing solvents, which limits its accessibility and processability. However, chitosan is a partially N-deacetylated derivative of chitin and readily dissolves in dilute acids. The broad spectrum antimicrobial and antifungal activities of chitin, chitosan and their derivatives against various microorganism groups have been comprehensively described (Du et al., 2009). The antimicrobial potency of chitosan is greater than that of chitin, owing to the cationic nature of the glucosamine component of chitosan at a pH of 6. The Positively charged under these pH conditions, the molecule is thought to interact with the cell membranes of microorganisms that are negatively charged under the same pH conditions. This results in cell membrane rupture, leakage of intercellular material, and will eventually lead to cell death. It has also been suggested that low molecular weight chitosan molecules may bind to the DNA of the pathogen, inhibiting RNA and protein synthesis, leading to cell dysfunction and death.
Table 2.1
Examples of structure–property relationships of chitosan
| Property | Degree of deacetylation | Molecular weight | References |
| Solubility | +a, b | – | (Dash et al., 2011; Lu et al., 2004; Yi et al., 2005) |
| Crystallinity | + | + | (Aranaz et al., 2009, Mecwan et al., 2011; Saboktakin et al.) |
| Biodegradability | – | – | (Freitas et al., 2011, Kofuji et al., 2005; Saboktakin et al., 2011; Yuan et al., 2011a; Zhang and Neau, 2001) |
| Thermal degradation | + | – | (Nam et al., 2010; Rong Huei and Hwa, 1996) |
| Viscosity | + | + | (Oh and Nam, 2011) |
| Biocompatibility | + | * | (Chatelet et al., 2001; Huang et al., 2004) |
| Protein binding | * | + | (Benesch and Tengvall, 2002; Gan and Wang, 2007; Kim et al., 2011a; Xu and Du, 2003; Yuan et al., 2011a) |
| Lipid binding | + | + | (Liu et al., 2008) |
| Biological mucoadhesion | + | + | (Sonia and Sharma, 2011) |
| Hypocholesterolemic effects | + | + | (Xia et al., 2011) |
| Analgesic | + | – | (Kim et al., 2002; Okamoto et al., 2002) |
| Antimicrobial | + | + | (Park et al., 2011b) |
| Antimicrobial– nanoparticle complexes | – | (Chavez de Paz et al., 2011) | |
| Antitumor activity | – | – | (Park et al., 2011a) |
| Permeation enhancing effect | + | – | (Aranaz et al., 2009; Hagesaether, 2011) |
| Antioxidant | + | – | (Je et al., 2004) |
| Haemostatic | + | * | (Hsu et al., 2011; Kang et al., 2011; Yang et al., 2008) |
| Accumulation in cells | + | + | (Yuan et al., 2011b) |
| Mechanical and thermal stability, and gelation of chitosan hydrogels and blends | + | + | (Liu et al., 2012; Tsai et al., 2011) |
| DNA and siRNA complexation, stability, transfection efficiency | *c | * | (Kiang et al., 2004b; Kim et al., 2011b; Zhou et al., 2008) |
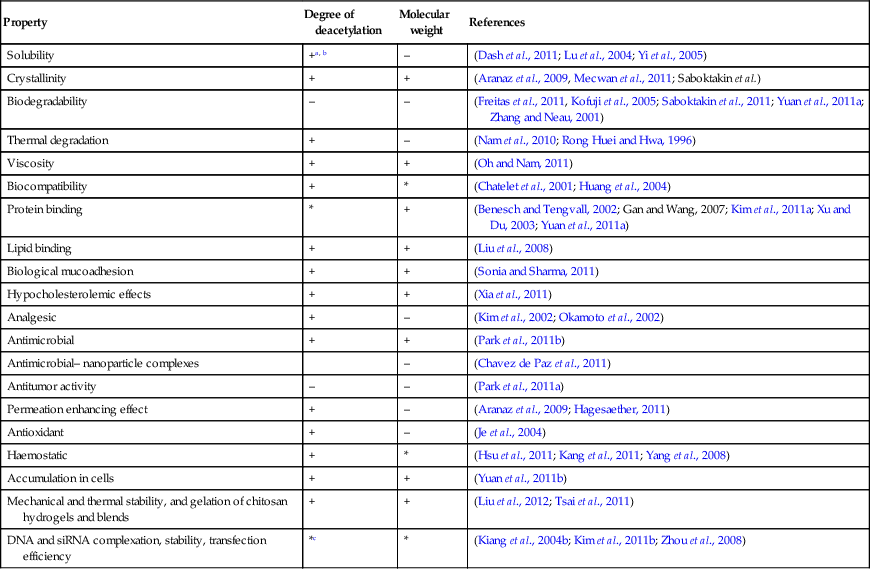
a* denotes an existing relationship between structural characteristic of chitosan and its material property or performance; however, opposing accounts exist with regard to the nature of such a relationship. Given a wide range of experimental conditions reported in the literature, it is often difficult to pinpoint a single trend underlying individual structure–property relationships.
b+ positively correlated; – negatively correlated.
cin vivo and in vitro results have been demonstrated to differ significantly, with the balance between the degree of deacetylation and the molecular weight recognised as the decisive factor.
Chitin is found in the shells of crustaceans and molluscs, in the backbone of squids and the cuticle of insects, as well as marine diatoms, in protozoa and the cell wall of several fungal species (Liu et al., 2011a). Chitosan is not generally obtained from animal or plant sources. Only a limited number of fungal strains are known to produce chitosan rather than chitin. The reactive amino and hydroxyl groups of chitosan chains are amenable to chemical modification and activation, as demonstrated in the binary immobilisation method where use of both the amino and hydroxyl groups of chitosan yielded a higher protein loading and higher activity (Dulazi and Liu, 2011). As such, chitosan holds significant potential for adsorption of metal ions, dyes and proteins, and as a potent chelating agent, which forms complexes with transition met als and heavy met als (Yu Dan et al., 2011). Chelating chitosan nano beads as a part of micro-fluidic–micro-electric trap have been demonstrated to retain lead (II) in a continuous bloodstream flow (Wang, 2011). Current and potential bio-applications for chitin and chitosan include wound dressings, haemocompatible and haemostatic films, targeted delivery systems, scaffold engineering and cell encapsulation (Jou, 2011; Nakagawa and Ito, 2011; Pozza and Millner, 2011; Singh et al., 2008; Watters et al., 2011) (Table 2.2).
Table 2.2
Examples of chitosan applications
2.2.1 Chitin and wound management
The use of chitin in wound healing dressings arises from the ability for N-acetyl-glucosamine to accelerate the rate of tissue repair, and to prevent the formation of scars and contraction of the skin (Dai et al., 2011; Kofuji et al., 2010). Originally used as a powder, chitosan and chitin are now being incorporated into a variety of materials, including films and membranes, gels and woven and non-woven dressings. The latter were determined to be particularly suited to the treatment of burns, cutaneous lesions and ulcers, and skin grafts for their good absorbance, adhesive nature and permeability to oxygen (Granville-Chapman et al., 2011; Jayakumar et al., 2011). Flexible chitosan and chitin nano fibrils have been incorporated in chitosan glycolate-based preparations, namely sprays, gels and gauzes. These have been demonstrated to be highly effective in restoring the subcutaneous architecture (Mattioli-Belmonte et al., 2007). The chitin/chitosan formulation itself was found to play a role, with sprays being most effective in dealing with superficial lesions, including extensive ones, whereas gels showed the greatest potency when repairing shallow lesions, providing the most pleasing aesthetic factor. Gauzes were found to be most promising in the treatment of slow-healing dermo-epidermal wounds. The haemostatic potential of chitosan-based gauzes has also been investigated, since the application of such gauzes results in the cross-linking of red blood cells to form a muco-adhesive barrier with chitosan (Littlejohn et al., 2011).
Fluid-absorbing chitin beads that are comprised of a chitin core encapsulated in a layer of carboxymethyl-chitin and bi-layered chitosan membranes have been proposed for the management of wound exudates. The N-carboxybutyl-chitosan derivative is characterised by the presence of advantageous water-soluble properties, the ability to form gels and its ability to be easily sterilised. Their enhanced bioactive properties have been attained by combining chitosan with a variety of other materials, including silver ions, antimicrobials, collagen and glycosaminoglycans. For instance, chitosan-collagen hydrogels containing lysostaphin were found to promote burn wound healing, whilst also displaying antimicrobial activity against methicillin-iesistant Staphylococcus aureus (Cui et al., 2011). The latter nosocomial pathogen is recognised for its increasing prevalence and often lethal nature in burn patients. Composite antibacterial fibres fabricated from non-derivatised chitosan and cellulose blends using electrospinning have also been reported (Park et al., 2011c).
2.2.2 Chitosan-based tissue scaffolds
A number of studies have reported the utilisation of chitosan in the fabrication of tissue engineering scaffolds. This is due to chitosan’s ability to be processed into a variety of morphologies, such as films and fibres, which is conducive to scaffold assembly (Wen et al., 2011). The degradation product of chitosan, N-glucosamine, is present in the extracellular matrices of eukaryotic cells, and is non-loxic. Chitosan undergoes macrophage-mediated enzymatic hydrolysis via reactions with lysozyme. The resulting oligosaccharide product is a positively charged molecule, with protonated amino groups, which can effectively interact with anionic biomolecules, including proteoglycans and glycosaminoglycans that tend to carry high numbers of cytokines and growth factors. The positive charge also makes the molecule a promising candidate for gene delivery via complexation with negatively charged DNA, also affording it protection from degradation by nucleases. The rate of chitosan degradation is dependent on the molecular weight, with lighter species showing a lower propensity for water absorption, and the degree of crystallinity, where highly deacetylated and hence more crystalline forms exhibit a relatively low degradation rate (Kim et al., 2008), factors which over time will adversely affect their mechanical stability and solubility.
Porous scaffolds can be easily synthesised via the freezing of liquid or gel chitosan samples, followed by lyophilisation to fix the matrices (Madihally and Matthew, 1999). The resultant scaffolds can feature pores of dimensions ranging from 1 to 250 μm, with varied pore orientation, which in turn will influence the distribution of low- and high-modulus regions throughout the material. Calcium carbonate and sodium acetate particles can be mixed into the chitin gel and chitosan solutions, respectively, to produce homogenous open-pore scaffolds with various degrees of porosity, and good degrees of pore interconnectivity with desirable pore sizes (Lim et al., 2011).
Post-fabrication immersion of chitosan scaffolds into a novel concentrated simulated body fluid led to the formation of calcium phosphate nucleation sites on chitosan scaffolds after six hours of treatment, with apatite particles of characteristic cauliflower-like morphology being formed after 24 hours of exposure (Aday and Gümü![]() derelio
derelio![]() lu, 2010). The resultant hydroxyapatite coated scaffolds exhibited enhanced osteoconductive behaviour, resulting in increased levels of cell proliferation and the ability to undergo cell differentiation compared to their untreated counterparts. Similarly, when coated on degradable poly(lactic) complex braid fabric, the chitosan membranes provided reactive functional groups on the surface of the scaffold, which contributed to the formation of hydroxyapatite upon exposure to the simulated body fluid (Lin et al., 2011). Crystalline vaterite disks of calcium carbonate were also formed on the silk fibroin matrix coated with the chitosan membrane, the formation of which was induced via the accumulation of hybrid CaCO3/silk fibroin nanoparticles (Wu et al., 2011b).
lu, 2010). The resultant hydroxyapatite coated scaffolds exhibited enhanced osteoconductive behaviour, resulting in increased levels of cell proliferation and the ability to undergo cell differentiation compared to their untreated counterparts. Similarly, when coated on degradable poly(lactic) complex braid fabric, the chitosan membranes provided reactive functional groups on the surface of the scaffold, which contributed to the formation of hydroxyapatite upon exposure to the simulated body fluid (Lin et al., 2011). Crystalline vaterite disks of calcium carbonate were also formed on the silk fibroin matrix coated with the chitosan membrane, the formation of which was induced via the accumulation of hybrid CaCO3/silk fibroin nanoparticles (Wu et al., 2011b).
Extensive research efforts have been made to chemically alter chitosan, with a view to further enhancing its functionality and physical performance (Mourya and Inamdar, 2009). This has been done in order to extend the current controlled release applications of chitosan towards more complex drug-eluting devices, including vascular stents, artificial skin, bone grafts and nerve guidance conduits (Chen et al., 2011b). Both chitosan and chitosan modified with anionic polymers have been shown to be excellent candidates for the repair of perforated tympanic membranes (Kim et al., 2011b) and cartilage regeneration (Jun et al., 2011). Human osteoblasts and chondrocytes, and bovine articular chondrocytes, respectively, have been shown to grow on these substrates, retaining their ability to express genotypes with regard to cell morphology and mitosis (Lahiji et al., 2000).
Chitosan was reported to assist the continued expression of type I collagen, and type II collagen and aggrecan in osteoblasts and chondrocytes, respectively. Incorporation of hyaluronic acid into chitosan scaffolds further enhanced chondrocyte adhesion and proliferation, and cartilage extracellular matrix production (Correia et al., 2011). Polyelectrolyte complex scaffolds of silk fibroin and amino polysaccharide chitosan with pore sizes in the range of 100 to 160 μm and high porosity were shown to degrade at a slower rate in lysozyme solutions compared to chitosan-only scaffolds, and were characterised by a higher compressive strength and modulus than the individual components (Bhardwaj and Kundu, 2011). However, incorporation of higher concentrations of chitosan within the blend insured an enhanced antibacterial effect, with the scaffolds supporting the growth and adhesion of feline fibroblasts.
The ability to effectively utilise chitosan scaffolds in tissue regeneration applications can be further enhanced by loading the structures with a variety of bioactive compounds whose natural function is to stimulate selective adhesion, growth and differentiation of cells. For instance, chitosan sponges that have been impregnated with dexamethasone have been produced to promote differentiation of stem cells towards the osteogenic lineage (Duarte et al., 2009). In vitro observations showed the consistent release of the drug into the ambient environment, highlighting the potential of chitosan scaffolds as a controlled drug delivery system for bone tissue regeneration. Loading of chitosan scaffolds with hydroxyapatite micro-particles not only enhanced the Young’s modulus and compressive strength of the scaffolds, with resultant values approximating those of human spongy bone, but scaffolds prepared in this way also augmented the degree of cell attachment and proliferation (Isikli et al., 2012). Porous chitosan-hydroxyapatite scaffolds with uniform and optimal pore sizes (200–500 μm), high porosity, and unidirectional interconnected network, have been suggested to have superior potential in bone engineering applications due to their excellent anisotropic mechanical properties and improved strength (Cai et al., 2011).
Impregnation of chitosan-based scaffolds with bactericidal agents for the prevention of biomaterial associated bacterial infections has been reported. A berberine-loaded chitosan coating applied to a nano-hydroxyapatite-poly(amide) system was found to have improved the mechanical performance of the scaffold, ensuring the continuous release of the antimicrobial agent for over 150 hours (Huang et al., 2011a). This combined effect is attractive, since it fulfils the requirements of orthopaedic surgery, in that it suppresses the extent of bacterial growth, concomitantly promoting osteoblast-like cell adhesion, crawl, growth and proliferation. Scaffolds comprised of chitosan/nano-hydroxyapatite/nano-silver particles have been demonstrated to show activity against Gram-positive and Gram-negative bacterial strains, while being non-toxic to rat osteoprogenitor cells and human osteosarcoma cell lines (Saravanan et al., 2011).
2.2.3 Chitin derivatives and their potential as vehicles for targeted drug delivery
Chitosan forms colloidal particles and is able to encapsulate bioactive molecules, including proteins, genetic drugs and chemical synthetic agents (Lakshmanan et al., 2011). This occurs via a variety of mechanisms, such as chemical cross-linking, ionic cross-linking and ionic complexation (Prabaharan and Mano, 2004; Grenha et al., 2007; El-Shabouri, 2002; Wu et al., 2011a). A wide range of chitosan-based amphiphilic copolymers containing hydrophobic and hydrophilic segments have been developed, because they facilitate the spontaneous self-assembly of diverse functional compartment structures such as micelles, vesicles and gels, with promising prospects in biotechnology and pharmaceutics applications (Aranaz et al., 2010). These nanoparticles can then be administered via oral, nasal, intravenous and ocular routes (Chaudhury and Das, 2011; Meng et al. 2011; Wang et al., 2011b). Its high affinity for cell membranes renders chitosan a suitable encapsulating agent for liposome formulations (Naberezhnykh et al., 2011). Materials derived from chitosan have also been trialled as transfection agents for non-viral gene delivery (Kim et al., 2011b; Morris et al., 2011).
Chitosan has also been studied as an ocular drug delivery vehicle for topically applied vancomycin in rabbit, with promising results (Khangtragool et al., 2011). It was found that the drug bioavailability in chitosan-containing solutions was of a similar order to that of commercially available products, identifying the material as a cost-effective alternative for vancomycin ocular drug delivery. A similar study, using econazole nitrate as the model drug molecule, was able to demonstrate that spherical chitosan-sulfobutylether-β-cyclodextrin nanoparticles displayed potential as drug carriers in albino rabbits (Mahmoud et al., 2011). Adjustment of process variables, such as the chitosan molecular weight and the concentration of the two ionic agents, allowed for the modification of particle size, polydispersity index, zeta potential, drug content, in vitro release and muco-adhesive properties of the resultant nanocomposite.
Chitosan derivatives such as chitosan-EDTA conjugates (González-Rodríguez and Rabasco, 2011), N-trimethylated chitosan (Bal et al., 2011; Martins et al., 2011; Verheul et al., 2011), mono-N-carboxymethyl chitosan, N-sulfo-chitosan (Werle and Bernkop-Schnürch, 2008) and thiolated chitosan have also been studied (Table 2.3). These materials offer the additional advantageous properties that can further extend the utilisation of chitosan in non-invasive drug delivery systems (Sarti and Bernkop-Schnürch, 2011). For instance, immobilisation of thiol groups resulted in the formation of polymers with enhanced permeation and muco-adhesion properties (Verheul et al., 2010), with covalent bonds being formed between the functional moieties of chitosan and cysteine-rich sub-domains of mucus glycoproteins, efflux pump inhibition and in situ gelling capacity compared to the unmodified chitosan (Saboktakin et al., 2011a,b). Muco-adhesion can be further enhanced via hydrophilic modification (Sajeesh and Sharma, 2011).
Table 2.3
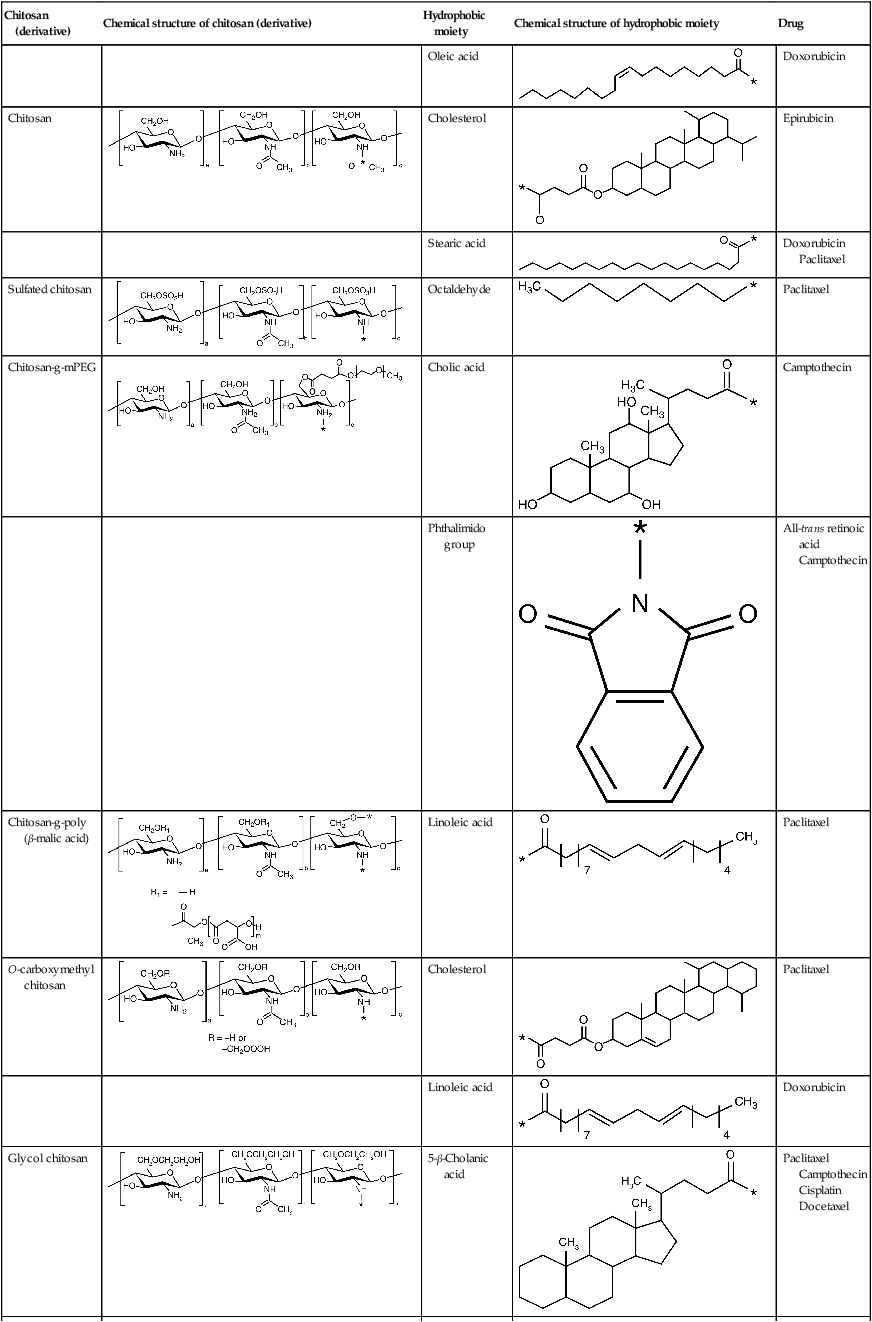
Amphiphilic chitosan derivatives for drug delivery (Park et al., 2010)
| Chitosan (derivative) | Chemical structure of chitosan (derivative) | Hydrophobic moiety | Chemical structure of hydrophobic moiety | Drug |
| Oleic acid |  |
Doxorubicin | ||
| Chitosan | 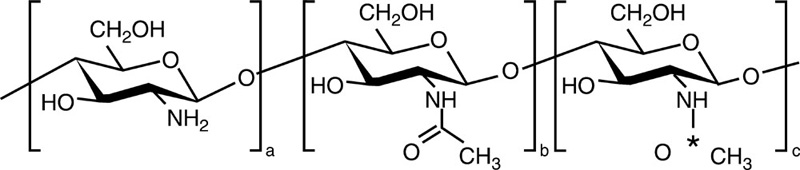 |
Cholesterol | 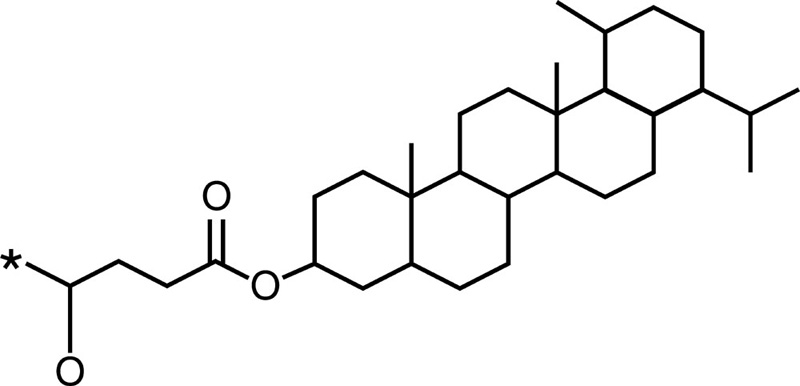 |
Epirubicin |
| Stearic acid | 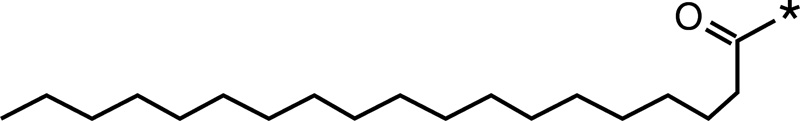 |
Doxorubicin Paclitaxel | ||
| Sulfated chitosan |  |
Octaldehyde |  |
Paclitaxel |
| Chitosan-g-mPEG |  |
Cholic acid | 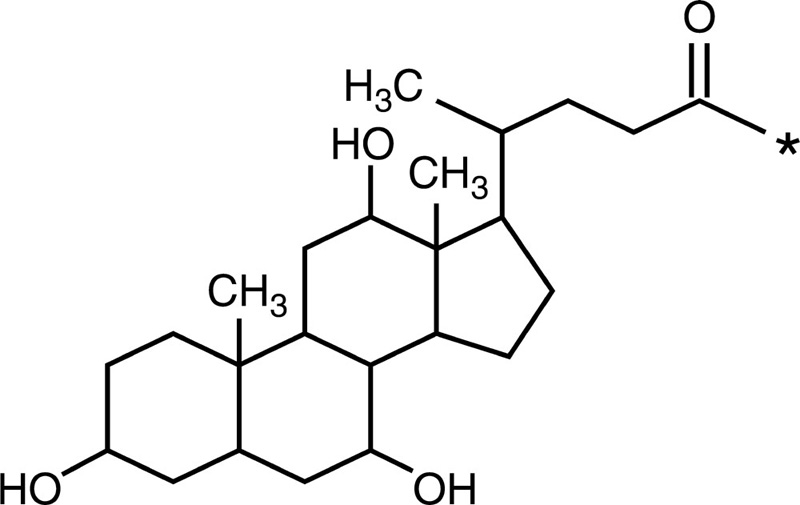 |
Camptothecin |
| Phthalimido group | 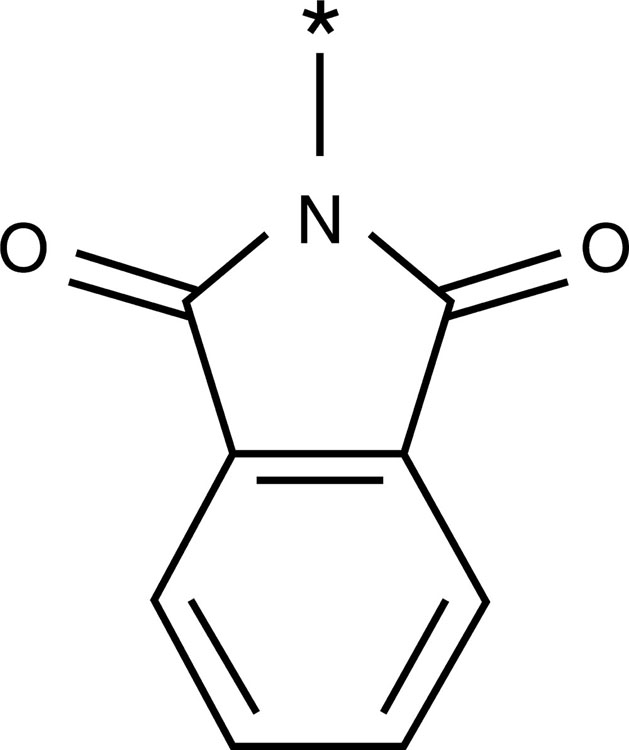 |
All-trans retinoic acid Camptothecin | ||
| Chitosan-g-poly (β-malic acid) | 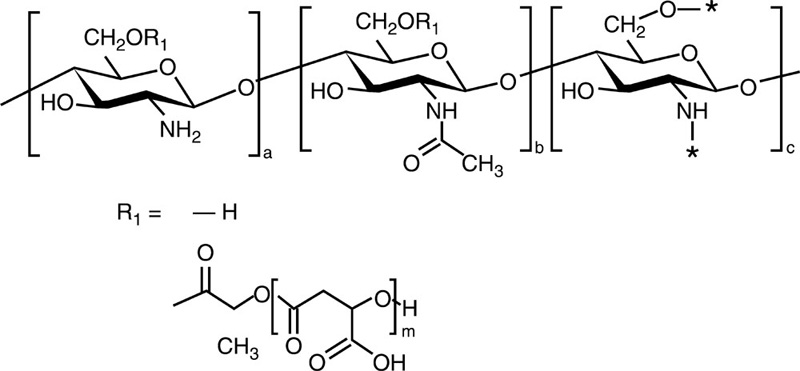 |
Linoleic acid | 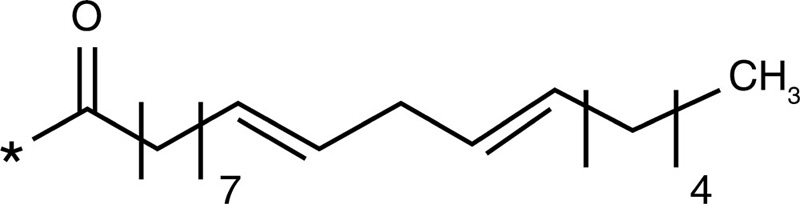 |
Paclitaxel |
| O-carboxymethyl chitosan | 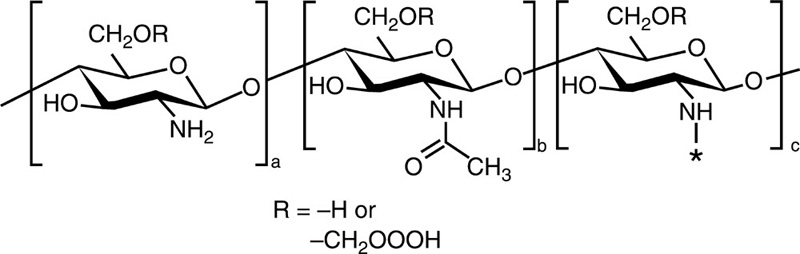 |
Cholesterol | 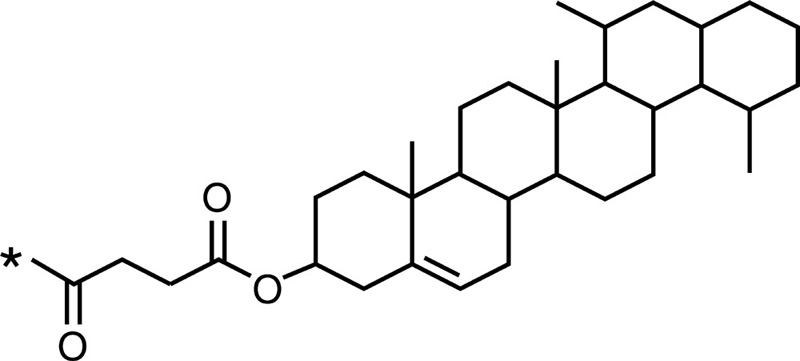 |
Paclitaxel |
| Linoleic acid | 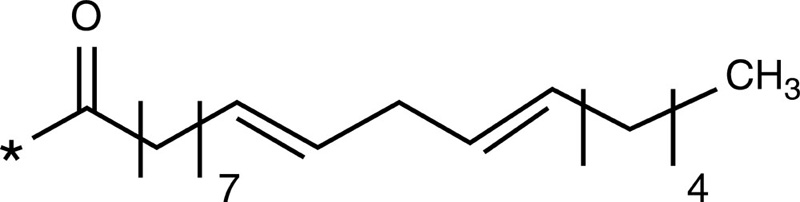 |
Doxorubicin | ||
| Glycol chitosan | 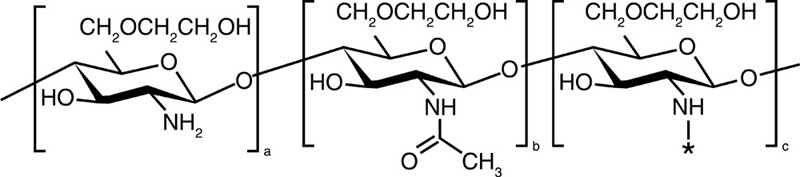 |
5-β-Cholanic acid | 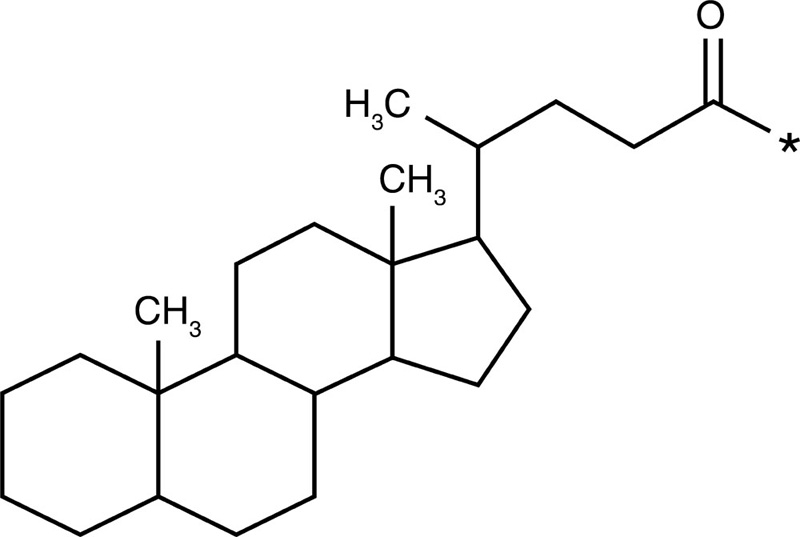 |
Paclitaxel Camptothecin Cisplatin Docetaxel |
| Deoxycholic acid | 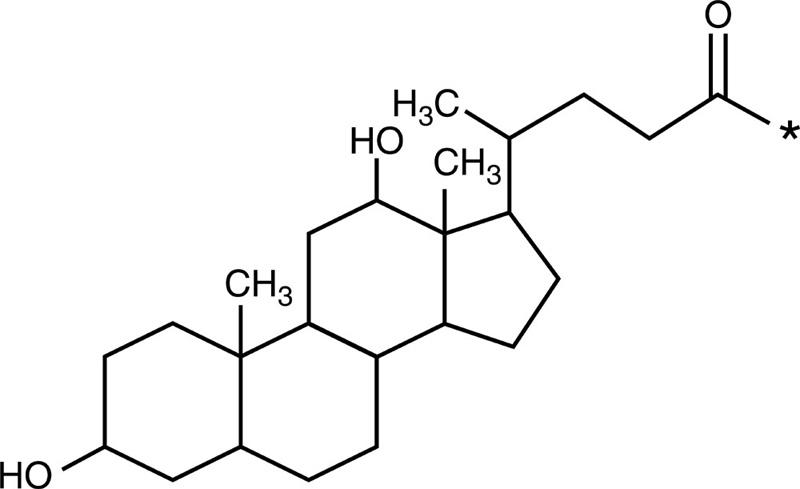 |
Doxorubicin |
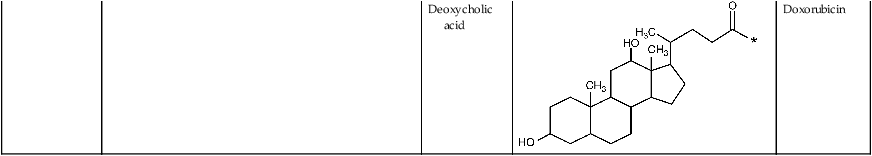
Chemical derivatives of chitosan can be designed to control the type of molecules with which they can associate, therefore altering the chitosan degradation rate and the biomolecule release profile (Suknuntha et al., 2011; Yadav et al., 2011) (Table 2.3). For instance, the release of paclitaxel and rutin model drugs from biodegradable chitosan-poly(ε-caprolactone)-poly(ethylene glycol) graft copolymers self-assembled into spherical micelles in aqueous media was prolonged by increasing the cross-linking density via glutaraldehyde treatment (Chen et al., 2011a). In addition, this treatment rendered the micelles self-luminescent. Addition of folate and hyaluronic acid to 5-fluorouracil-bearing chitosan nanoparticles allowed for better control over the rate of drug release (Li et al., 2011a) and, in the case of hyaluronic acid, resulted in a significantly higher uptake of the drug by cancer cells compared to that obtained using chitosan-only nanoparticles (Jain and Jain, 2008). Hyaluronic acid-coupled chitosan nanoparticles containing oxaliplatin demonstrated a similar degree of effective delivery of a high local drug concentration to colon tumours, indicating the potential of the hyaluronic acid-coupled chitosan system in enhancing antitumor efficacy with low systemic toxicity (Jain et al., 2010).
Indeed, highly toxic and poorly water soluble antitumour agents, such as camptothecin, can be effectively encapsulated into a chitosan-based degradable system for local administration, potentially decreasing the concentration required to cause cancer cell death (Li et al., 2011b).
2.2.4 Complex carrier structures
Further control over time- and location-specific degradation can be achieved by producing layered thin coatings comprised of various chemical composition compounds, with the functionality and degradation behaviour designed to attain complex device morphologies (Fig. 2.2). Chitosan intercalated in an expandable layered aluminosilicate coating was characterised by improved controlled and extended release compared to that obtained using pure chitosan, with the release occurring through the degradation of the carrier into its individual components or nanostructures with a different composition (Yuan et al., 2010). The enhanced encapsulation efficiency and the decreased burst release of biodegradable hollow capsules for protein drugs were achieved via a layer-by-layer assembly of water-soluble chitosan and dextran sulphate on protein-entrapping amino-functionalised silica particles, followed by the removal of the silica (Shu et al., 2010). The muco-adhesion capacity of chitosan-coated poly(isobutyl cyanoacrylates) nanoparticles was improved via the incorporation of a high concentration of thiol groups into the chitosan membrane, which led to formation of covalent bonds with the cysteine residues of the mucus glycoproteins (Bravo-Osuna et al., 2007). Introduction of highly pH sensitive components, such as poly(propyl acrylic acid) into a chitosan-based system-carrying DNA complex was demonstrated to improve the efficiency of gene transfection (Kiang et al., 2004a). Incorporation of poly(N-isopropylacrylamide) into the formulation rendered the resultant chitosan nanoparticle both pH- and thermo-responsive (Tourrette et al., 2009).
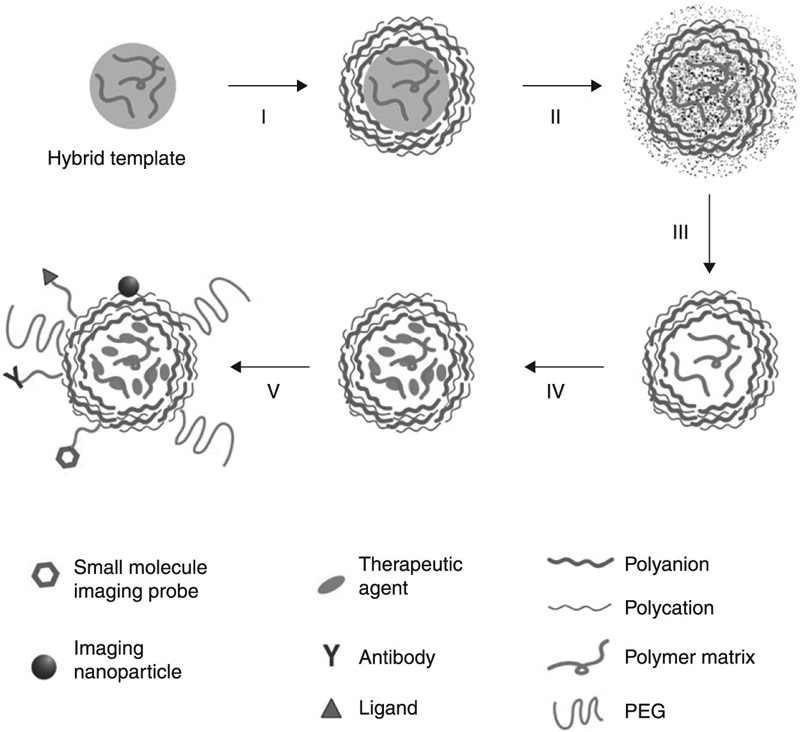
In addition to chitosan, several other biodegradable polyelectrolytes have found applications in biotechnology and pharmacology, including the positively-charged protomine sulfate, dextran amine, gelatin A, and negatively-charged sodium alginate, dextran sulphate, chondroitin sulphate, hyaluronic acid, heparin and gelatin B (Ariga et al., 2011). Complex carrier structures assembled in a component-by-component and/or layer-by-layer manner from the aforementioned materials have been found to be more suited for lower-dose and highly specific drug delivery applications (Mu et al., 2011). For instance, artemisinin drug crystals have been encapsulated with chitosan, gelatin and alginates using this layer-by-layer technique for the purpose of controlled release (Chen et al., 2009). The size distribution, zeta potential and swelling property of artemisinin nanocapsules, as well as their drug release properties, were found to be dependent on the properties of the polyelectrolyte solutions, including polyelectrolyte type, number of polyelectrolyte multilayers, sodium chloride and ethanol concentrations. Overall, artemisinin nanocapsules were found to demonstrate improved hydrophilicity and dispersibility in aqueous solutions.
2.3 Alginate
Alginate, also known as alginic acid, is an anionic polysaccharide found in cell walls of algae and is produced by two bacterial genera, Pseudomonas and Azotobacter (Robitzer et al., 2011). Structurally, alginates are linear unbranched co-polymers comprising covalently linked blocks of β-(1→4)-linked D-mannuronic acid and α-(1→4)-linked L-guluronic acid residues (Fig. 2.3). L-guluronic acid residues are epimers of D-mannuronic acid residues, with the latter being enzymatically converted to the former after polymerisation, and differ at C5. Despite being structurally similar, these homopolymers are characterised by significantly different conformations; D-mannuronic acid being 4C1 with di-equatorial links between the units and L-guluronic acid being 1C4 with di-axial links between the units. Furthermore, the D-mannuronic acid residues of alginates derived from bacteria are additionally O-acetylated on the second and third positions.
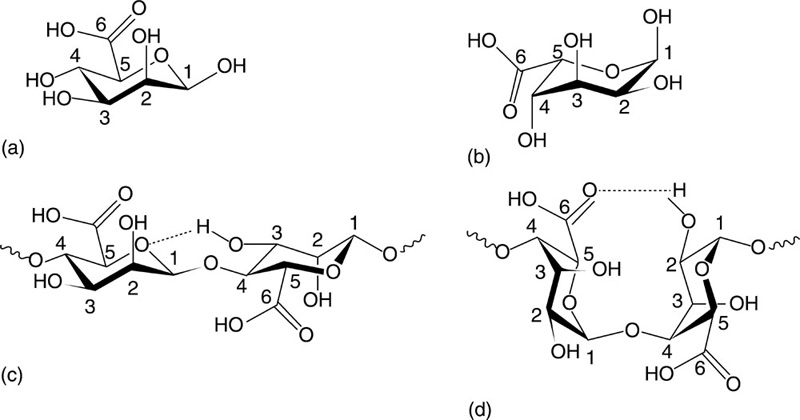
As such, alginate poly(β-(1→4)-linked D-mannuronate) tends to form a threefold left-handed helix characterised by a weak intra-molecular hydrogen bonding between the hydroxy moiety in the third position and the subsequent ring oxygen. However, poly α-(1→4)-linked L-guluronate conforms to a two-fold screw helical chain, favouring intra-molecular hydrogen bonding between the carboxyl moiety and the hydroxy group in the second position of the prior residues and the hydroxy moiety in the third position of the subsequent residues. As the di-axial links allow less flexibility, this conformation is stiffer, with the polymer being more stable in acids. Poly(α-(1→4)-linked L-guluronate-b-β-(1→4)-linked D-mannuronate) features both equatorial-axial and axial-equatorial links. Although the hydrogen bonding between the carboxyl group on the mannuronate and hydroxy moieties in the second and third positions of the subsequent guluronate is present, differing degrees of freedom between two types of units allow for greater overall flexibility of the structure.
The material properties and functionality of alginates are greatly influenced by the ratio between mannuronate and guluronate and block configuration (Smith and Miri, 2011). Furthermore, the carboxyl groups of the residues can undergo chemical reaction with several kinds of cations, giving rise to alginates with significantly different properties and functionality (Frampton et al., 2011). For instance, sodium alginate, NaC6H7O6, potassium alginate, KC6H7O6, and ammonium alginate, (C6H11NO6)n, are soluble, whereas alginic acid and calcium alginates are insoluble. Functionalisation with covalently bound proteins and peptide epitopes can provide sites for targeted cell attachment within a three-dimensional (3-D) alginate cell scaffold (Jeon et al., 2011), such as those used to entrap and investigate a variety of neural cell types including astroglioma cells, astrocytes, microglia and neurons (Frampton et al., 2011). The molecular weight of alginate affects both the viscosity and the physical stability of the gel; however, high viscosity may adversely affect the ability for the alginate to be processed (Lee and Mooney, 2012). The viscosity of alginate is also pH dependent, with maximum viscosity occurring at pH 3, at which point the carboxylate groups within the polymer main chain become protonated, forming hydrogen bonds. The optimum properties of alginate can be attained by combining low and high molecular weight alginate species.
A highly organised single polymer scaffold of high porosity and a regular interconnected porous structure in the scale of 250 μm was fabricated from alginate using a microfluidic device (Wang et al., 2011a). These structures were found to be effective in chondrocyte cultures, indicating good cell viability, non-toxicity, cell proliferation and the gene expression of aggrecan and type II collagen. Furthermore, cells were found to be phenotypically normal, with the scaffold structure being conducive to the production of high amounts of extracellular polymeric structures associated with matrix assembly, such as glycosaminoglycans. As such, alginate scaffolds are an economical and promising solution for cartilage regeneration processes designed to combat osteoarthritis, and as a material for total joint arthroplasty. Furthermore, the feasibility of fabricating stratified cartilage tissues through the layering of bovine chondrocyte-seeded alginate scaffolds has been demonstrated (Lee et al., 2007). Alginate is a promising biomaterial for the delivery of chondrocytes designed to treat cartilage lesions. It can also be used as a 3-D culture model to investigate the extent of cellular metabolism, ultrastructure and gene expression of cells that would otherwise lose their phenotype in the course of monolayer expansion. For instance, alginate beads were used to encapsulate human chondrocytes, the latter sustaining their phenotype in vitro (Andrade et al., 2011).
Sodium alginate polymers containing chitosan and calcium and impregnated with tetracycline hydrochloride, chloramphenicol and rifampicin were applied over bamboo yarn to enhance the antibacterial and wound healing property of the bandage (Shanmugasundaram and Mahendra Gowda, 2011). The results showed a steady drug release over the 4 days of the study and an excellent cidal activity against the selected pathogens, namely Staphylococcus aureus and Proteus bacteria. Alginate-chitosan composite 3-D porous scaffolds have been demonstrated as suitable for stem-cell based tissue engineering to treat cartilage lesions, with synovium-derived mesenchymal stem cells serving as a cell precursor for cartilage repair (Jun et al., 2011). The scaffold was found to provide a favourable micro-environment for supporting cell proliferation and chondrogenic cell differentiation.
Alginate microcapsules coated with chitosan and loaded with Bifidobacterium breve have been investigated with the intention of improving the gastro-intestinal viability of this probiotic (Cook et al., 2011). The selection of drying method was reported to influence both the properties of the microcapsules and the viability of the encapsulated bacteria. Chitosan was demonstrated to stabilise alginate matrices for acidic pH levels, similar to those encountered in the gastro-intestinal tract, also leading to an enhanced survival and slower probiotic release when exposed to simulated gastric fluids. A novel formulation for the triggered delivery of insulin, consisting of magnetic particles dispersed in alginate spheres, was reported (Finotelli et al., 2010). Figure 2.4 shows the SEM images of alginate beads containing iron oxide nanoparticles. The magnetically responsive alginate spheres, which release insulin upon exposure to magnetic field, were found to facilitate the rhythmic delivery of peptides (Saslawski et al., 1988).

2.4 Collagen
Collagen is the most abundant protein class in the body. It is widely applied for in vitro and in vivo tissue engineering applications. Although there are a number of types of collagen that can be accessed from a wide range of sources, collagen types I, II and III comprise more than 80% of all collagens within the body. For instance, type II collagen comprises 90 to 95% of the collagen found in articular cartilage, and is characterised by a high amount of bound carbohydrate groups, which makes this type of collagen highly water interactive (Little et al., 2011). Its biological role is to form a mesh of fibrils which provides tensile strength, facilitating physical entrapment of other macromolecules (Duda et al., 2011). However, the role of type IX and XI collagens found in articular cartilage is not well understood, with type IX suspected of working towards stabilising the mesh.
Collagen medical applicability stems from the inherent non-immunogenicity of collagen proteins and the ability to provide adequate structural support and chemical environment for tissue restoration. An introduction of a stable collagen layer incorporated with a basic fibroblast growth factor onto poly(L-lactic acid) porous scaffolds was shown to significantly improve the spreading and growth of chondrocyte cells (Ma et al., 2005). Nanofibrous collagen scaffolds that closely resemble the collagen structures in vivo can be pre-formed in vitro using electrospun collagen fibres. Both cross-linked and non-cross-linked electrospun scaffolds have been prepared, with the degree of cross-linking determined to increase the diameter of collagen nanofibres and the thickness of the resultant scaffolds (Bhardwaj and Kundu, 2010). These pre-formed structures can then be effectively applied to wounds and in preliminary vascular tissue repair. Clinical studies on 13 patients treated with biomimetic osteochondral scaffolds fabricated by nucleating collagen fibrils with hydroxyapatite nanoparticles showed sound cartilage repair, with a completely attached graft and repair tissue found in 13 of 15 lesions (Kon et al., 2010). Furthermore, in 10 lesions, complete filling of the cartilage defect and congruency of the articular surface were observed at 6 months, indicating high prospective for this osteochondral scaffold in cartilage tissue repair.
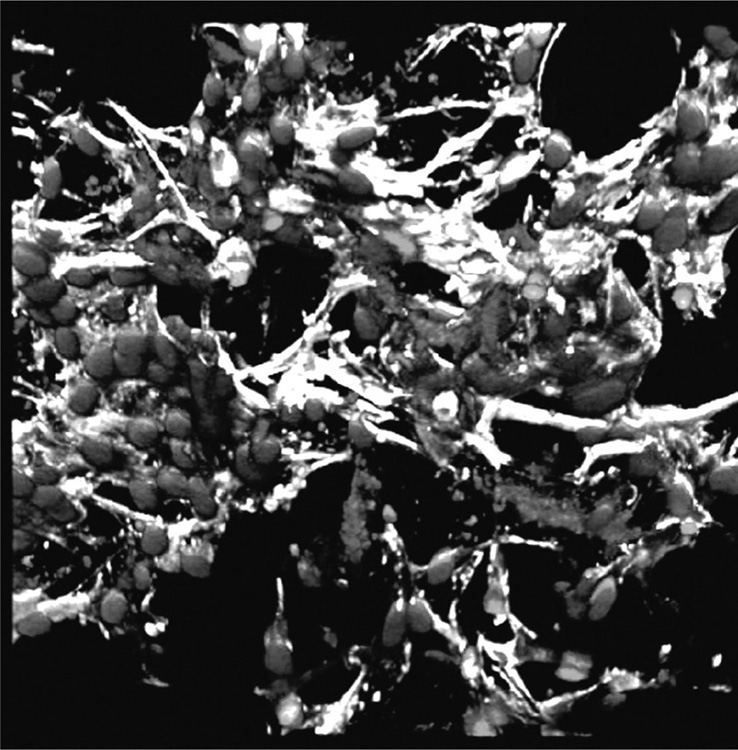
Collagen and silk-fibroin based scaffolds have also been used for engineering micro-vasculature by cells in the scaffolds prior to implantation (Fuchs et al., 2009; O’Brien, 2011; Unger et al., 2010). Vascular tissue engineering is challenging due to lack of vascularity in scaffolds and tissue engineered constructs, graft failure or inferior mechanical and antithrombotic properties (Bordenave et al., 2008, Tsigkou et al., 2010). Pre-vascularisation of scaffolds in vitro by seeding them with cells harvested from a patient or donor may allow for engineering of a desired tissue structure prior to implantation, thus ensuring the success of the implant (Fig. 2.5). However, the methodology is questionable with regard to clinical efficacy and commercial viability. In addition, an extended time is required to effectively treat the patient. The approach is promising for those tissues that are unable to regenerate in vivo, such as cartilage tissue.
2.5 Gelatin
Gelatin is a denatured collagen, generally obtained by the controlled hydrolysis of collagen extracted from animal tissues, such as skin and bovine and porcine bone. The resultant product is a mixture of polypeptides dispersed according to size and chemical reactivity, with its properties being dependent on both the collagen from which the gelatin is extracted and the method of conversion, including acid, base and enzymatic isolation. Recently, production of hydroxylated human gelatin via a microbial expression system (Pichia pastoris KM71) has been reported (Duan et al., 2011). The methodology promises to address several significant drawbacks of animal-source gelatin, including product inconsistency, the presence of agents that are potentially infectious to humans, such as bovine spongiform encephalopathy, and immune hypersensitivity in humans. Importantly, the aforementioned technique will facilitate the production of gelatin of desirable molecular size and charge.
2.5.1 Gelatin-based drug delivery vehicles
Gelatin is broadly applied in the pharmaceuticals industry for drug delivery, with many orally delivered capsules being based on gelatin. Recently, gelatin and other natural polymers have been studied in vivo and in vitro for their potential in pulmonary drug delivery and sustained release (Sheth and Myrdal, 2011). The mannosylated gelatin nanoparticles have been demonstrated as suitable carriers for the selective delivery of an antitubercular drug, isoniazid, to the alveolar macrophages (Saraogi et al., 2011). The organ distribution studies using these nanoparticles demonstrated adequate efficiency for spatial delivery of the drug to alveolar tissues through intravenous administration. Active targeting of drug molecules led to reduction in the hepatotoxicity of the drug and enhanced antibacterial efficacy compared to free drug. Furthermore, the therapeutic concentration was successfully maintained for an extended period of time, even at a reduced clinical dose (Tiwari et al., 2011). Decoration of gelatin nanoparticles with mannose ligands improved particle stability in broncheo-alveolar lavage fluid.
2.5.2 Gelatin tissue scaffolds and hydrogels
As a result of its strong hydrogen-bonding characteristics, polyelectrolytic behaviour and the ability to congeal at lower temperatures, conventional spinning of gelatin to produce micro- and nano-fibres is not common (Bhardwaj and Kundu, 2010). Numerous composite materials containing gelatin in combination with other organic and synthetic materials have been developed to enhance its tissue engineering potential. Composite scaffolds prepared from gelatin, chitosan and hydroxyapatite of varied physical, chemical and mechanical properties showed the highest osteoconductive affinity towards Saos-2 cells for sintered hydroxyapatite (Isikli et al., 2012). Gelatin/montmorillonite-chitosan nanocomposite scaffolds prepared via an intercalation process and a freeze-drying technique with ice particulates as the porogen material showed sound mechanical properties and a controllable degradation rate (Zheng et al., 2007). Ferrogels containing 3% Fe3O4 magnetic nanoparticles that were prepared through cross-linking gelatin with genipin and reinforced by chitosan demonstrated controlled highly-responsive pulsatile drug release by a high-frequency magnetic field (Hu et al., 2007).
Composite hydrogel systems consisting of alginate, gelatin and biphasic calcium phosphate were studied for their potential in bone regeneration applications (Nguyen and Lee, 2012). The study demonstrated that oxidation of alginate can be an effective tool for tuning the material properties and rate of degradation of the hydrogel. Increasing the degree of cross-linking between the -CHO moieties of the oxidised alginate and the -NH2 groups of gelatin enhanced both the extent of water uptake and the compressive strength of the hydrogel, while reducing its porosity, gelation time and the rate of biodegradation. Furthermore, unique channel zed morphology was attained, characterised by an extensive branching of the channels divided into multiple segments by thin separators. Thermoplastic gelatine-poly (ε-caprolactone) scaffolds with a pre-defined porous network have been manufactured by a melt-mixing process, followed by selective extraction of one of the polymers and gas foaming (Salerno et al., 2009). Variations in solvent and extraction parameters resulted in structures with multi-scaled and highly interconnected porosities of desirable configuration. Heat treatment was also used to vary interpore connectivity of 3-D gelatin scaffolds (Fig. 2.6).
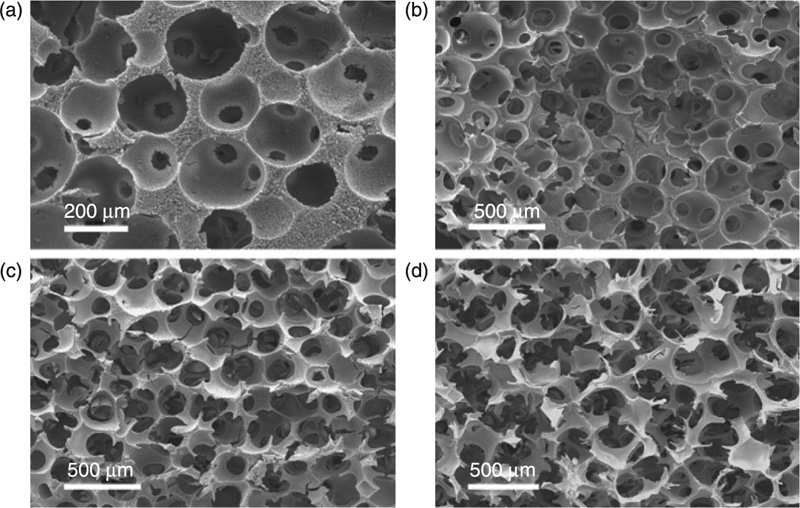
Cross-linking is frequently used to facilitate the tissue engineering applications of gelatine scaffolds by enhancing the mechanical properties and stability of the material. Gelatin scaffolds of high porosity and orientated microtubule pores of tunable size were fabricated using unidirectional freeze-drying technology, with the resultant properties being a function of the gelatin concentration and cross-linking treatment used (Wu et al., 2010). In vitro studies demonstrated that scaffolds underwent enzymatic degradation, with the absolute weight loss increasing with gelatin concentration used to fabricate scaffolds. Another 3-D polymer composite system of polyvinyl alcohol and gelatin was obtained using electrospinning followed by methanol-initiated cross-linking (Linh and Lee, 2012). The latter treatment was demonstrated to increase density, hardness, water stability and aggregation tendency of the system. The biocompatibility was also enhanced as a result of the treatment, with osteoblast-like cells firmly attaching to the surfaces via expression of philopodial extensions and proliferating.
In vivo cartilage repair study using a pig model demonstrated that gelatin/chondoitin-6-sulfate/hyaluronan tri-copolymer engineered scaffold was suitable for the treatment of full thickness and osteochondral articular defects (Chang et al., 2006). The study also demonstrated that autogenous osteochondral transplantation was more successful compared to allogenous transplantation, with the repair tissue being hyaline cartilage and/or fibrocartilage; yet, the integration into host cartilage was poor in both cases. Furthermore, these scaffolds were not effective in restoring the subchondral bone plate associated with osteochondral defects.
2.6 Hyaluronic acid
Hyaluronic acid is a linear polysaccharide, with the structure of glucuronic acid and N-acetylglucosamine repeating units linked via alternating β-1,4 and β-1,3 glycosidic bonds. It is one of the key elements of the extracellular matrix of connective tissues, with significant biological functions including shock absorption, molecular filtering and collagen fibril support. Similar to other natural polyelectrolytes, the biocompatibility and biodegradability of hyaluronic acid insures its position as one of the most broadly applied carbohydrate-based natural polymers for tissue engineering. Their natural function of providing support to collagen fibrils makes hyaluronic acid materials ideally suited for utilisation in wound and arthritis treatment, as tissue scaffolds, for drug delivery and as components of implantable devices.
Fabrication of uniform hyaluronan nanofibres via electrospinning has been hindered by the high viscosity, surface tension and strong water retention ability of the hyaluronan solution. The water retention ability in particular was responsible for the fusion of spun fibres. However, the introduction of blowing-assisted electrospinning coupled with an appropriate choice of solvents and a modified rate and temperature of air flow allowed the fabrication of nanofibrous nonwoven hyaluronan membranes with desirable properties (Bhardwaj and Kundu, 2010). Mixing small amounts of extracellular matrix molecules, including proteins (collagen and elastin) and polysaccharides (hyaluronic acid and condroitin sulphate), into porous polyurethanes have been demonstrated to reduce the porosity of these biodegradable scaffolds, which enhanced their surface properties and biocompatibility (Popescu et al., 2010). Incorporation of hyaluronic acid into chitosan scaffolds further exhibited enhanced chondrocyte adhesion and proliferation, and cartilage extracellular matrix production (Correia et al., 2011). Addition of hyaluronic acid to anticancer drug-bearing chitosan nanoparticles enhanced the control over the rate of drug release (Li et al., 2011a), and significantly increased the uptake of the drug by cancer cells as compared to chitosan-only nanoparticles (Jain and Jain, 2008). Hyaluronic acid-coupled chitosan nanoparticles bearing oxaliplatin demonstrated a similar level of effective delivery to colon tumours, with a high local drug concentration, demonstrating the potential of the hyaluronic acid-coupled chitosan system in enhancing antitumor efficacy with low systemic toxicity (Jain et al., 2010).
2.7 Fibrinogen
Fibrinogen is a soluble plasma glycoprotein, which is synthesised by the liver. It plays an important role in blood coagulation, whereby fibrinogen is converted by thrombin into fibrin. The inherent properties of fibrinogen, such as the ability to induce cellular interactions and act as a provisional matrix for tissue repair, render the material a natural choice for engineering of tissue scaffolds, haemostatic and wound dressings. The biodegradable nature of fibrinogen-derived biomaterials, coupled with their non-immunogenicity and the inherent aptitude to promote cell migration, has made fibrinogen a frequently used material for the fabrication of electrospun nanofibres and scaffolds. As with other natural polyelectrolytes, nanofibrous conformation ensures high surface area to volume ratio and provides ample number of functionalities available at the surface of the material for wound interaction and clot formation. In this state, fibrinogen shows high structural integrity, which positively affects the processability of this biomaterial. Electrospun nanofibrous fibrinogen mats are rapidly hydrated, with the ability to remain in this hydrated state for at least 48 hours without losing their structural integrity in normal saline. In addition to its use as a pre-formed implantable scaffold, fibrinogen has been combined with thrombin to produce a degradable fibrin mesh which can be loaded with cells, such as chondrocytes, and then injected into the host tissues. Upon injection, the polymer composite undergoes a cross-linking reaction, resulting in a scaffold for new tissue formation and growth. This technique is favourable where an operation to implant a pre-formed scaffold is to be avoided.
Hybrid fibrous scaffolds of synthetic poly(L-lactic acid)-b-poly(ε-caprolactone) elastomer and fibrinogen showed higher in vitro attachment and proliferation of L929 cells than that observed on scaffolds from individual materials (He et al., 2011). Fibre diameter and shape were found to depend on the ratio of elastomers to fibrinogen, with the former increasing with elastomer content. Degradation behaviour, physical and thermal properties of the hybrid scaffold were also found to differ significantly from those of individual components, owing to two-phase structure of the hybrid, one being pure fibrinogen and the other composed of a fibrinogen-elastomer mixture. The amino functionalities introduced by fibrinogen enhanced the hydrophilicity of the blended scaffolds, with corresponding increase in viability and proliferation of human umbilical vein endothelial cells (Fang et al., 2011).
Cross-linked poly(ethylene glycol)-fibrinogen hydrogel scaffolds have been incorporated with nanoscale structural features in the form of difunctional block copolymer surfactant Pluronic F127, in an attempt to enhance control over cellular morphogenesis through defined cell–matrix interactions (Frisman et al., 2011a). The study showed that the introduction of imperfections into the hydrogel network structure provided cells with a more favourable environment, reflected in enhanced cell spreading within the dense hydrogel. Furthermore, the temperature at which the cross-linking was initiated profoundly affected the mechanical, chemical and structural properties, as well as the thermo-responsive behaviour of the resultant hydrogel (Frisman et al., 2011b). Although conjugation of natural proteins with poly(ethylene glycol) has been successfully used for enhancing bioactivity and structural versatility of hydrogels, the biophysical characteristics of the resultant structure may be negatively affected by such a treatment. Compared to thrombin cross-linking of fibrin hydrogels, poly(ethylene glycol) conjugation led to a slight reduction in actin and matrix met allo-proteinase expression, and spindled morphology of neonatal human foreskin fibroblasts, indicating a small reduction in biocompatibility of the hydrogel (Gonen-Wadmany et al., 2011). Nonetheless, the overall performance indicated retention of most of the inherent biofunctionality of the fibrin precursor.
Cross-linked fibrinogen-based microspheres were shown to be suitable candidates for drug and biomolecule delivery, with a potential to transport chemokines, cytokines, drugs and other peptides or proteins (Rajangam et al., 2011). The cross-iinked nature of fibrinogen microspheres ensured solvent and protease resistance, with their mechanical properties dependent on 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide concentration used during the fabrication process. Fibrin glue of different fibrinogen concentrations has been applied in clinical and laboratory settings for targeted and sustained release of factors pertaining to successful tissue engineering, including therapeutic gene delivery (Lee et al., 2011). Fibrin scaffolds with multiple 10 to 250 μm diameter conduits were also suggested as biomaterial nerve cuffs for repair of segmental peripheral nerve defects (Scott et al., 2011). The highly aligned nature of the pores within the scaffold was conducive to axon migration, with the rate of migration being independent of conduit diameter.
2.8 Silk fibroin
Silk is a natural fibre composed of hydrophobic fibroin and hydrophilic sericin. Silk can be attained from a range of sources, such as cocoons of Antheraea mylitta and Bombyx mori silkworms, dragline silk of Nephila clavipes spider and recombinant hybrid silk-iike polymers with fibronectin functionality (Bhardwaj and Kundu, 2010). Silk fibroin is characterised by excellent mechanical properties and stability, biocompatibility and biodegradability, and it generates a minimal inflammatory response by the host (Table 2.4). These notable advantages led to silk fibroin being considered for numerous biomaterial applications, including tissue engineering, wound repair and controlled drug delivery and release (Pritchard and Kaplan, 2011). Furthermore, silk micro- and nano-fibres can be easily attained via electrospinning of raw silk material with an appropriate selection of solvents, such as hexafluoro-2-propanol, and processing conditions, which allow for a controlled conformational transition of fibroin into nano-dimensional fibres of desired size and morphology. In addition to the aforementioned biocompatibility and biodegradability of silk fibroins, electrospun silk nanofibres are characterised by high specific surface area, enhanced mechanical strength and surface energy, and improved thermal and electrical properties.
Table 2.4
Tensile properties of silk polymeric fibres (Kundu et al., 2013)
| Source organisms | Tensile strength (g/den) | Tensile modulus (g/den) | Breaking strain (%) |
| Bombyx mori | 4.3–5.2 | 84–121 | 10.0–23.4 |
| Antheraea mylitta | 2.5–4.5 | 66–70 | 26–39 |
| Philosamia cynthia ricini | 1.9–3.5 | 29–31 | 28.0–24.0 |
| Coscinocera hercules | 5 ± 1.2 | 87 ± 17 | 12.1 ± 5.1 |
| Hyalophora euryalus | 2.7 ± 0.9 | 59 ± 18 | 11.1 ± 5.8 |
| Rothschildia hesperis | 3.3 ± 0.8 | 71 ± 16 | 9.5 ± 4.4 |
| Eupackardia calleta | 2.8 ± 0.7 | 58 ± 18 | 11.8 ± 5.5 |
| Rothschildia lebeau | 3.1 ± 0.8 | 54 ± 14 | 15.5 ± 6.7 |
| Antheraea oculea | 3.1 ± 0.8 | 57 ± 15 | 14.5 ± 6.6 |
| Hyalophora gloveri | 2.8 ± 0.4 | 48 ± 13 | 19.3 ± 6.9 |
| Copaxa multifenestrata | 0.9 ± 0.2 | 39 ± 6 | 4.1 ± 2.7 |
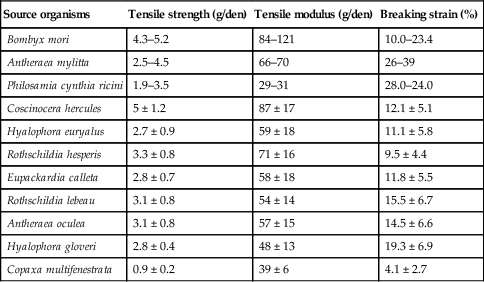
Nanomaterials derived from pure silk and silk blends with other natural and synthetic materials have been designed and characterised. Nanofibrous silk fibroin chondrogenic scaffolds have been demonstrated to have excellent potential in cartilage tissue engineering. The surface modification of these scaffolds, such as microwave-induced argon plasma treatment, induced the enhanced attachment and proliferation of human articular chondrocytes, with a concomitant increase in the glycosaminoglycan synthesis (Baek et al., 2008). Silk fibroin was introduced into amino polysaccharide chitosan complex scaffolds to delay matrix degradation when in contact with lysozyme solution (Bhardwaj and Kundu, 2011). The resultant complex structure also possessed a higher compressive strength and modulus compared to respective parameters of the individual scaffolds, and was suitable for the attachment and propagation of feline fibroblasts. Regenerated silk fibroin matrices have also been coated with a chitosan membrane to promote formation of crystalline vaterite disks of calcium carbonate on their surface (Wu et al., 2011b).
Heparin-bearing native silk fibroin powders blended with biomedical polyurethane were fabricated into composite membranes intended for the controlled release of heparin (Yang et al., 2011a,b). In addition to exhibiting excellent hydrophilicity, water vapour permeability and water absorption, the heparin release rate could be controlled by altering silk fibroin to polyurethane ratio, heparin content and membrane thickness. The delivered heparin was characterised by excellent bioactivity, with the system exhibiting potential for enhancement of polyurethane biocompatibility and haemocompatibility (Liu et al., 2011b). Osteo-inductive and biodegradable composite biomaterials for bone regeneration were also fabricated from silk fibroin incorporated with silica particles (Mieszawska et al., 2010). Human mesenchymal stem cells were found to successfully adhere, proliferate and undergo osteogenic differentiation on silk/silica films, with evidence of early bone formation. Introduction of silica nanoparticles enhanced gene expression of bone sialoprotein and collagen type Iosteogenic markers. Leaching of small-sized silica particles from silk fibroin matrix further promoted formation of apatite deposits and enhanced collagen content.
2.9 Viral particles and bacteriophage capsids for drug delivery
Plant virus and bacteriophage capsids are naturally-prevalent protein nanoparticles designed to carry genetic cargo to their target cells. They effectively penetrate cell membranes, and release their cargo in a timely manner (Algar et al., 2011). It is therefore not surprising that viral capsids are investigated for their potential as vehicles for targeted drug and biomolecule delivery, and for transport of contrast agents for various visualisation techniques. Several issues concerning the use of these nanoparticles need to be considered in order to fully appreciate their true clinical utility. These include their carrier stability over the transfer time, biocompatibility and the non-toxicity of the vehicle and its degradation products, colloidal stability, uptake by the reticulo-endothelial system and timely clearance from the body (Franzen and Lommel, 2009). In light of these issues, biodegradable nanoparticles derived from plant viruses are considered to have the most promise owing to their structural uniformity, cargo capacity, responsive behaviour and ease of manufacture. Inherently biocompatible and biodegradable, viral nanoparticles can be designed and engineered using both genetic and chemical protocols, with numerous functionalisation strategies developed over the last 20 years (Steinmetz, 2010). Depending on their therapeutic or imaging applications, nanoparticles can be functionalised with imaging reagents, targeting ligands and therapeutic molecules.
However, the main advantage of viral nanoparticles lies in their size and morphological consistency, as opposed to synthetic counterparts often characterised by high polydispersity. Comprised of identical protein subunits, viral capsids allow effective chemical modification and bioconjugation via a well-defined and regular array of amino acid residues available at both their interior and exterior surfaces. Site-specific placement of unique functionalities, such as cysteine-thiols, insertion, removal or replacement of specific amino acids can be attained via recombinant modification in genetic engineering. In addition to targeted delivery, viral nanoparticles find their applications as protein cages, scaffolds and high-precision templates for biohybrid nanostructured materials and crystal growth (Soto and Ratna, 2010). MS2 bacteriophage and cowpea mosaic viral capsids of approximately 27 and 28 nm in diameter have been identified as highly prominent nanoparticle precursors (Hooker et al., 2004; Steinmetz et al., 2006), with other rod-like and spherical viruses including tobacco mosaic virus, M13 bacteriophage, and Cowpea Chlorotic Mottle virus also being intensely investigated.
2.10 Immunocytes as ‘Trojan horses’ for molecule delivery
Drug-delivery mediated by immunocytes, including mononuclear phagocytes, such as dendritic cells, monocytes and macrophages, and neutrophils and lymphocytes, is attracting significant attention (Batrakova et al., 2011). Owing to their high mobility, ability to migrate across impermeable barriers and site-specific release, these immune cells can be employed for treatment of infections, inflammations and cancer. They are also used to promote tissue repair in a highly precise, non-toxic manner.
Monocytes containing therapeutic nanoparticles have been proposed to transport drugs to hypoxic regions within tumours, which are virtually inaccessible to conventional cancer therapies, but possible via monocyte-mediated therapy due to the tumour’s recruitment of monocytes into these regions (Choi et al., 2007). Antitumor macrophages obtained by the culture of human mononuclear cells in hydrophobic bags were used for adoptive treatment in metastatic cancer patient with no dose-limiting toxicity (Chokri et al., 1992).
A bone-marrow-derived macrophage system has been suggested for selective delivery of antioxidants to the substantia nigra pars compacta in patients with Parkinson’s disease. This system has the potential to attenuate oxidative stress and as such increase survival of dopaminergic neurons (Batrakova et al., 2007). Erythrocytes carrying 5-fluorouracil were synthesised with a hyperosmotic technique and their potency of antitumour activity in mice bearing malignant ascites was investigated (Wang et al., 2010). The study found a significant regression in the quantity of malignant ascites and the prolonged survival time. Neural stem cells have been proposed as vehicles for gene therapy due to their high migratory nature and as they have been observed to be attracted to areas of brain pathology (Muller et al., 2006).
2.11 Future trends
This chapter detailed some of the most recent and exciting applications of natural biomaterials for use as scaffolds and delivery vehicles. It aimed to provide an overview of the types of natural materials that are currently available, their sources and derivatives, and the unique properties which make such materials perfect candidates for numerous bio-related applications. For centuries, their inherent biocompatibility and healing properties have attracted the interest of medical practitioners and scientists, who endeavoured to repair, restore or enhance the human body. Their availability, ease of processing and relatively low cost ensured their prevalence in many biomedical fields, from burn treatments to the design of drug capsules, to tissue replacement. With the advent of synthetic polymers that promised to overcome every disadvantage associated with natural materials, from synthetic versatility to property tunability, the future prospects of naturally-derived biomaterials seemed bleak. However, recent technological advancements have brought the spotlight back onto natural biomaterial, as they provide scientists with the tools to fully characterise these complex structures and more fully explore their potential. Our enhanced appreciation of the fundamental properties and in vitro and in vivo behaviour of these interesting biomaterials, coupled with growing knowledge in the areas of biochemistry, molecular biology and bioengineering, will facilitate the development of the methods to optimise their performance and their clinical utility.
