Advanced synthetic and hybrid polymer biomaterials derived from inorganic and mixed organic–inorganic sources
Abstract:
Polymeric biomaterials derived from inorganic and organo-metallic precursors promise to overcome the drawbacks associated with organic polymers. Their versatile chemistry, excellent physico-chemical and biological properties, and ability to undergo controlled biodegradation render them highly suited for a host of medical applications, from transient implants to drug and biomolecular delivery vehicles. In this chapter, inorganic, organic–inorganic hybrid and organo-metallic polymers are reviewed. Biomaterials are discussed in terms of their salient and impeding properties, and their likely role in present and future biomedical devices and treatments.
4.1 Introduction
Since their inception in the 1930s and for over 50 years, the plastics market has been dominated by polymers produced from organic monomer sources (Gleria and De Jaeger, 2005). Organic polymers were the first plastics to be developed, due to their processability and reproducibility, valuable properties, and importantly, the relative abundance of low-cost precursors that were available as a result of buoyant oil markets (Yu et al., 2006).
After many years of development in the area of organic polymer synthesis and application, however, several significant limitations of these materials have become evident. In spite of their excellent biocompatibility, processability and customisable properties, the practical value of these synthetic polymers in medical implantation and tissue engineering applications has been hampered as a result of the accumulation of acidic degradation products arising from the bulk erosion of these polymers (Deng et al., 2010a). These degradation products can also negatively influence their biocompatibility and undermine both their mechanical integrity and performance. Other significant drawbacks of organic polymers are their reduced flexibility at low temperatures, poor stability at high temperatures, propensity to swell when in contact with organic solvents, lack of electrical conductivity, and a tendency to degrade upon exposure to UV and high-energy radiation.
These limitations have encouraged scientists to search for alternative biomaterials that possess a combination of tunable material properties, cytocompatibility and controllable degradation kinetics with an increased chemical resistance, thermal stability and mechanical durability. Inorganic polymers represent a rapidly expanding area of materials science, with numerous promising applications in the electronics and biomedical industries (Deng et al., 2010a; MacKenzie et al., 2010; Rahimi and Shokrolahi, 2001; Rivard, 2011). Their potential benefits stem from their unique composition, which affords them considerable versatility in their properties. In addition, their increased durability results in a reduced extent of performance degradation from the polymer ageing. These polymers are also produced more economically and under milder mass-production conditions compared to other materials possessing similar properties, such as metals (Rivard, 2011).
In this chapter, several major classes of inorganic polymers will be reviewed in terms of their synthesis, fundamental and biological properties, and their utility in biomedical applications. The most recent advancements in the nanoscale applications of these macromolecules will be given greater attention than their more conventional medical uses. Given the abundance of original articles and high-quality review papers available in this area, only the most recent examples will be discussed.
4.2 Synthetic inorganic polymers
Inorganic polymers, like organic polymers, are comprised of a chain of repeating units; however, they do not contain carbon within their backbone. Furthermore, polymers based on a silicon and phosphorous backbone are typically characterised by a structure with wider angles, longer bonds among skeletal atoms and bonds with considerable ionic character compared to their carbon-based counterparts (Gleria and De Jaeger, 2005). Such enhanced skeletal flexibility results in a high elasticity of inorganic polymers at low temperatures. Rich in inorganic elements, these plastics can withstand higher temperatures, even in oxygen-rich environments, and are less susceptible to homolytic dissociation when compared to organic macromolecules. Stronger inorganic linkages and optical transparency over a short-wavelength region render these polymers more stable to UV and high-energy irradiation. In addition to the variability of elements that form the main polymer chain, a diversity of possible pendant side functionalities facilitates optimisation of chemical, physical and biological properties of the inorganic polymer to fit specific applications.
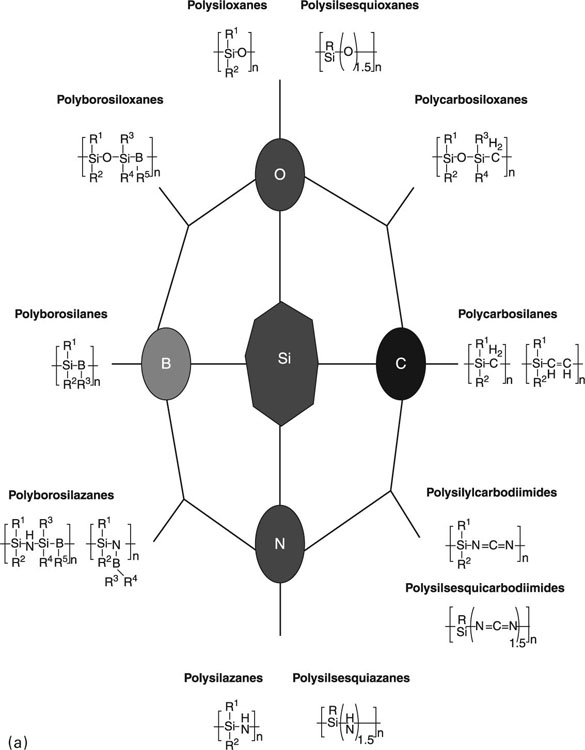
Inorganic polymers can be classified as fully inorganic, that is entirely comprised of inorganic, organic–inorganic hybrid or organo-metallic elements (Borisov et al., 2010). Currently, silicon-based materials, particularly poly(siloxane)s, are the best developed and widely utilised inorganic plastics, with applications as low-temperature elastomers, heat transfer fluids and biomaterials. Since 1946, when silicone-based elastomer was first used to repair a bile duct, these inorganic polymers have transformed into a core biomaterial. Silicon elastomers form the elements of both permanent and temporary indwelling devices, and a plethora of extracorporeal medical devices. In addition to poly(siloxane)s, other silicon-based plastics that have attracted attention from both the biomaterial scientists and industry include poly(silane)s and poly(silazane)s, and to a lesser degree, geopolymers. Inorganic macromolecules based on borazine (B3N3) and phosphazene have also been well investigated and applied in various industrial and technological fields.
4.3 Silicon-based inorganic polymers
Silicon-based inorganic polymers are amongst the most studied polymers, particularly with regard to their commercial applications. These include poly(silane)s, poly(siloxane)s, poly(silazane)s and their derivatives (Fig. 4.1).

4.3.1 Poly(silane)s
Poly(silane)s are composed of a linear backbone of continuous silicon atoms and organic substituent groups, synthesised most commonly through condensation reactions or ring-opening polymerisation reactions. Their material properties, such as solubility and crystallinity, will depend on the abundance and functionality of pendant groups. For example, the presence of small identical methyl functionalities along the silicon main chain will render the poly(silane) highly crystalline and insoluble, whereas the presence of larger or dissimilar functionalities will lower the crystallinity of the polymer. Poly(silane)s exhibit a variety of unique and interesting properties associated with σ-conjugation along the Si-backbone, amongst which are their high quantum efficiency photoluminescence, high hole drift mobility and ability to absorb long wavelength UV radiation (Mimura et al., 2000). As such, this polymer class holds great promise in semiconducting and optical applications, including those based on organic semiconducting materials that benefit from poly(silane)s containing metal complex functional groups. Soluble poly(silane)s modified by interrupting the Si–Si sequence of the backbone with a carbon atom resulted in pre-ceramic polymers, which can be further modified into usable silicon-carbide ceramic materials via pyrolysis or chemical vapour deposition (Borisov et al., 2010).
In the field of biomedicine, the favourable properties of poly(silane)s, such as its electro-activity and photo-degradative ability, are best exploited through the incorporation of poly(silane)s into copolymer systems, which allow for a greater processability, enhanced biocompatibility and a variety of other desirable material properties. Figure 4.2 shows an example of poly(silane) graft copolymer. Poly(silane)-containing block copolymers are commonly synthesised via pre-formed polymer chain coupling and living polymerisation techniques (Holder and Jones, 2008). The copolymer configuration also facilitates the manipulation of the macroscopic order of the resultant material via a supramolecular assembly (Stefik et al., 2009). For example, an amphiphilic multiblock copolymer comprising nearly monodisperse poly(ethylene oxide) segments and polydisperse poly(methylphenylsilane) segments has been demonstrated to form an array of well-defined, highly ordered aggregates, including vesicle, micellar rod and helix morphologies upon aggregation in water-based solvent systems (Sommerdijk et al., 2000). The composition of the solvent system was found to influence both the packing of the conjugated polymer blocks and the molecular conformation of the silicon backbone.
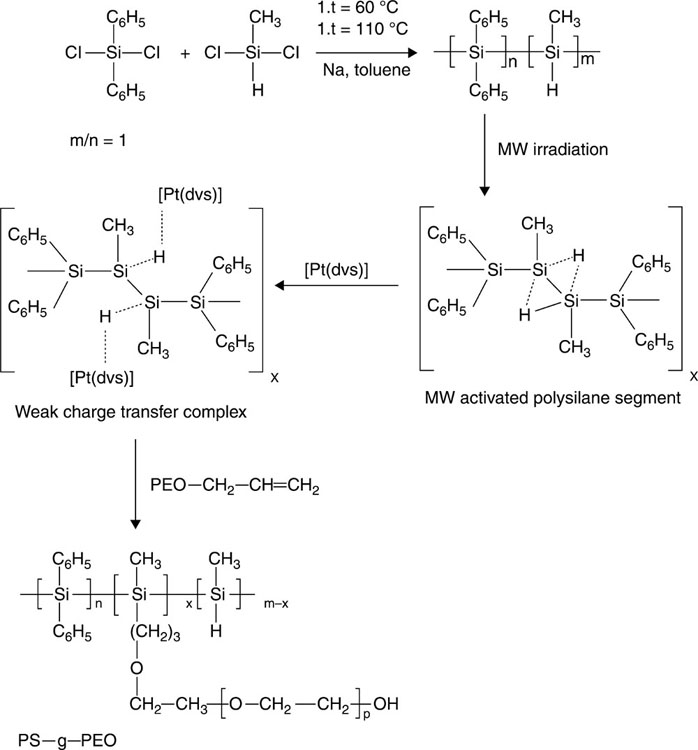
The composition of the block copolymer is designed such that the outer layers of the membrane consist of biocompatible hydrophilic poly(ethylene oxide) blocks, whereas the interior of the membrane is formed by the photolabile, hydrophobic poly(methylphenylsilane) segments (Kros et al., 2002). Shell cross-linked micelles of poly(silane) have been obtained via a cross-linking reaction of amphiphilic poly(1,1-dimethyl-2,2-dihexyldisilene)-b-poly(methacrylic acid) block with 1,10-diaza-4,7-dioxadecane and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (Sakurai, 2006). The subsequent application of a photochemical process yielded hollow spherical particles. Both types of micelles were used for the encapsulation of other molecules, with potential applications as organised systems for drug and bioactive molecule delivery. Poly(silane) shell cross-linked micelles, where the poly(silane) core is surrounded by a partially cross-linked shell of poly(methacrylic acid), can be used as a template for metal nanoparticle synthesis (Sanji et al., 2003). Large-diameter flattened round-shaped colloidal poly(silane)-gold nanoparticles, with a tendency to auto-assemble in close packed structures to form large areas over the polymer film surface, have also been reported (Sacarescu et al., 2011).
Nanoparticle sensors based on core-shell silica and fluorescein dye covalently incorporated into the structure via coupling to a reactive silane are ideally suited for functional tomographic imaging via confocal fluorescence microscopy (Hidalgo et al., 2009). In addition to probing bacterial communities attached to substrates (biofilms), specifically the morphology and temporal evolution of pH micro-environments in single and mixed-culture biofilms, highly fluorescent silica nanoparticles can be used as in vivo nanoprobes in eukaryotic cells and organisms due to their high sensitivity, biocompatibility and biostability (Larson et al., 2008). Furthermore, a neutral organic coating is designed to prevent adsorption of serum proteins, hence facilitating efficient biodistribution and transport across biological barriers, and timely clearance profiles via urinary excretion (Burns et al., 2008).
Patterned thin films generated from poly(silane)-based block copolymers, subsequently engaged as templates for the directed growth of cell cultures, has also received a great deal of attention. Amphiphilic poly(silane)-based block copolymers, namely poly(methylphenylsilane)-b-poly[oligo(ethyleneglycol)methacrylate] and poly(methylphenylsilane)-b-poly(2-hydroxyethylmethaciylate), were employed in biomaterial scaffold micro-patterning for attachment and growth of aligned muscle cell monolayers. Since poly(silane)s display photosensitivity, that is they undergo photodecomposition by UV-light irradiation via scission of Si-Si bonds in the backbone and formation of Si-OH, Si-H and Si-O-Si bonds, they can be easily patterned by shining UV light through a mask. As such, selective degradation of poly(silane)-poly(methacrylate) derived block copolymers was achieved via application of the mask, resulting in topography where one component can foster attachment, polarised spreading and growth of cells, while another segment inhibits these responses. These structures can serve as a platform for studying cell responses to specific mechanical and morphological cues. These will also contribute to the development of materials for controlling cell orientation, fusion and subsequent cell alignment, which is essential for muscle development.
Hybrid silane materials have been suggested as potential candidates for the development of biocompatible protective coatings for implantable devices using sol–gel technology. The important role of sol–gel technology for the fabrication of bioactive materials stems from the versatility of sol–gel chemistry, which allows for fine-tuning of materials characteristics to a desired application (Radha and Ashok, 2008; Inada et al., 2008). As such, sol–gel derived biomaterials have been used for film deposition, including fabrication of nitric-oxide-releasing antibacterial coatings for orthopaedic implants, and assembly of super-paramagnetic nanoparticles and implants, to name but a few. Materials based on organically-modified silanes are flexible and bioactive, and can find use in soft and hard tissue engineering. These can also be used to encapsulate implantable electronic devices. Tetraethylorthosilicate-based materials have been used as biocompatible encapsulating coatings for implantable glucose sensors, designed to mitigate adsorption of biological molecules, and subsequently, the kinetics and specificity of in vivo cellular adhesion (Kros et al., 2002). It was found that the protein adsorption (and rate of cell proliferation) differed between composites containing heparin, nafion, poly(ethylene glycol) and poly(styrene sulfonate), dextran sulphate, with the latter showing most promise, both in terms of its in vitro and in vivo compatibility and stability when in contact with glucose.
Poly(silane)-based block copolymers show excellent potential for tissue engineering and other biomedical applications due to their biocompatibility and capacity to accomplish numerous bio-related functions, including topographical cell guidance, controlled drug delivery and release, mechanical stimulation and electrical stimulation (Gelmi et al., 2010; Hangarter et al., 2010). This is particularly so where the regeneration of electrically-responsive cells, such as nerves and muscles, is required (Ghasemi-Mobarakeh et al., 2011; Lundin et al., 2011).
4.3.2 Poly(siloxane)s
Poly(siloxane)s feature a backbone of repeating Si–O units and, depending on the functionality of the pendant groups, these inorganic polymers can exist as linear chains or cross-linked networks, and as such their physical state can vary from fluids to gels to elastomers to resins (Borisov et al., 2010). Poly(siloxane)s owe their backbone flexibility to several structural features, namely the greater length of Si–O bond and increased Si–O–Si bond angles compared to C-C bonds of organic polymers. As a result of such dynamic flexibility of the chains, poly(siloxane)s can be highly permeable to gasses and can retain their elasticity, even at low temperatures that can render other polymers brittle (Orme and Stewart, 2011). For example, the glass transition temperatures of poly(dimethyl siloxane) and poly(methyl hydrosiloxane) are −123 and −137 °C, respectively. Relatively high Si–O bond strengths also contribute to the thermal, chemical and UV stability of poly(siloxane)s (Vezir Kahraman et al., 2006).
Material properties, and hence potential applications of poly(siloxane)s and their derivatives, are highly varied, and depend on functionality and abundance of side-chain structures, and the processing that materials undergo, including curing, reinforcing and copolymerisation (Alexandra et al., 2011). Biomedical applications of poly(siloxane)s take advantage of their high hydrophilicity, excellent biocompatibility, permeability, stability and inertness. As such, poly(siloxane)s-based materials can be found in soft contact lenses, artificial skin, organs and tissues, as components of various prosthetic and cardiovascular devices, drug delivery systems and denture liners (Lepley et al., 2011), to name but a few. A comprehensive review with regard to surface and bulk modifications of poly(siloxane)s, specifically in light of their biomedical applications, can be found in Abbasi et al. (2001).
Surface encapsulation of various nanoparticles, with a functional poly(siloxane) shell as a means of particle functionalisation for subsequent applications as biological probes for in vitro or in vivo experiments, has been reported. The encapsulation is performed to improve the particle reactivity with biomolecules of interest while reducing non-specific binding, prolonging the in vivo circulation time and increasing the aqueous solubility, such as in the case where amine or poly(ethyleneoxy)-bearing poly(siloxane) coatings of 3-aminopropyltrimethoxy-silane and 2-[methoxy(polyethyleneoxy)propyl]trimethoxysilane were used to encapsulate synthetic anti-ferromagnetic nanoparticles (Zhang et al., 2010). It has been demonstrated that a protein, such as plasma fibronectin, may be either denatured or stabilised on the surface of the poly(siloxane)-based material, depending on whether hydrophilic or hydrophobic interactions dominate (Giamblanco et al., 2010). Luminescent rare earth doped oxide inorganic nanoparticles have also been encapsulated by first adsorbing a primary layer of silicate ions for subsequent polymerisation of either amino-propyltriethoxysilane or glycidoxypropyltrimethoxysilane (Giaume et al., 2008). The amino- or epoxy-functions born by the silane facilitate versatile coupling of the particles with various bioorganic species, such as α-bungarotoxins. Planar GaN substrates and individual GaN nanowires intended as patterned devices for label-free bio-sensing have also been coated with a functional amino-propyltrimethoxysilane layer, to which amine-terminated, fluorescently labelled DNA was then attached (Simpkins et al., 2007).
Blending of polymers is an effective way of fabricating materials possessing enhanced bulk and surface properties. Numerous organic–inorganic hybrids have been synthesised by reacting organic polymers with tetraethoxy silane or tetramethoxy silane. Transparent free-standing hybrid films of poly(vinyl alcohol) with oxysilane inorganic phase modified with calcium and phosphate compounds showed cross-link density and reactivity to vary with, among others, the concentrations of the inorganic component and processing temperature (Pereira et al., 2000). Haemocompatible poly(siloxane)/liquid crystal composite membranes were preparedby blending and cross-linking of poly(dimethyl-methylhydrosiloxane) and poly(dimethyl-methylethylenesilosiane) with cholesteryl oleyl carbonate (Li et al., 2001). Amino-propyltriethoxysilan-based poly(L-lactic acid)/calcium carbonates produce a hybrid membrane with the ability to form a Si-containing hydroxycarbonate apatite layer when soaked in simulated body fluid. These were developed for use in biodegradable bone-guided regeneration processes (Maeda et al., 2006). The membrane coated with silicon-containing hydroxycarbonate apatite displayed no toxicity and had the ability to proliferate high levels of osteoblast-like cells. Glucosamide-grafted amphiphilic glycopolysiloxanes synthesised from aminopropyl functional poly(siloxane)s showed higher surface activity in aqueous solution compared to conventional carbon–carbon chain glycopolymers and as a result, self-assembled into spherical micelles (Du et al., 2011). Biodegradable glycopolymers possessing high surface activity are becoming increasingly important in the field of biosciences, due to their recognition properties (Slavin et al., 2011).
Microwave assisted curing was suggested as a means to accelerate the rate of reaction observed under conventional heating (Sosnik et al., 2011). The extent of condensation resulting from several hours of conventional heating of tetraethoxy silane and triethoxysilane-terminated poly(ethylene)-b-poly(ethylene glycol) hybrid materials was found to be similar to that obtained via one minute of microwave treatment (Geppi et al., 2007). Although the heating method had very subtle effect on the overall extent of cross-linking in the inorganic network, significantly different distributions of silicon sites and different hydrogen bond interactions were obtained under different curing conditions.
4.3.3 Poly(silazane)s
Poly(silazane)s are inorganic polymers characterised as low molecular weight polymers with a linear chain backbone of alternating Si and N atoms. Dehydrocoupling of oligomers can be used to generate poly(silazane) species of significantly higher molecular weights. One of the major applications of poly(silazane)s is as a ceramic precursor resin that undergoes thermal solidification, the temperature at which this occurs being dependent on the addition of free radical initiators. Another way to solidify poly(silazane) is via the addition of UV sensitisers followed by UV radiation exposure. Silicon nitride and silicon carbide fibres for ceramic and metal matrix composites and ceramic nanocomposites can be produced from solidified poly(silazane) and poly(urea silazane) materials via heat treatment (Wan et al., 2005). Poly(silazane)-based coatings demonstrate excellent adhesion to a broad array of substrates, including metals, glass, ceramics and plastics, due to the reaction between the Si–N of poly(silazane)s and the hydroxyl functionality present on the substrate surface. Both organopolysilazanes and perhydropolysilazanes have been employed as mechanically and chemically stable anti-corrosion coatings. Under ambient conditions, perhydropolysilazanes can be cured to form a carbon-free amorphous coating, characterised by sound vapour barrier properties and a low electrical conductivity.
Poly(silazane)s can be reacted with isocyanate and epoxy functional groups to form hybrid polymers characterised by processability, high mechanical strength and chemical and thermal resistance. These can be effectively used as ceramic-forming precursors which, when added to templating agents such as self-assembled surfactants or organic block copolymers, can form ordered nanostructured non-oxide ceramics (Malenfant et al., 2007; Nghiem et al., 2007). For example, ceramic nanoparticles of highly defined shape and size have been produced from poly(ureamethylvinyl)silazane using amorphous poly(isoprene)-b-poly(dimethylaminoethyl methacrylate) or semi-crystalline poly(isoprene-b-ethylene oxide) block copolymers as structure directing agents (Kamperman et al., 2008). The composition and morphology of the resultant mesostructured high-temperature ceramics could be controlled by varying the inorganic-to-organic ratio or by changing the molecular weight of the block copolymer (Kamperman et al., 2007). Continuous core-shell nanofibres possessing ordered morphologies, which may lead to the production of highly functional and porous fibres, were also manufactured using the aforementioned co-assembly with rigid poly(acrylonitrile) shells used to impose cylindrical confinement (Kamperman et al., 2010). Well-ordered mesoporous SiC and SiCN ceramic nanostructures with a high surface area were synthesised from poly(carbosilane)-b-poly(styrene) and poly(vinyl)silazane-b-poly(styrene) copolymers, respectively, via living polymerisation (Nghiem and Kim, 2008). The ceramic precursors poly(butadiene)-b-poly(ethylene oxide) with poly(silazane) were also explored for building nano-order in a Si–C–N system (Wan et al., 2007).
4.4 Poly(phosphazene)s
Poly(phosphazene)s are linear high molecular weight polymers with an inorganic backbone consisting of phosphorous and nitrogen atoms linked by alternating single and double bonds, with two organic side groups attached to each phosphorous atom (Deng et al., 2010a). Common pendant functionalities include alkoxy, aryloxy and amino acid groups as individual moieties or in combination with other functionalities. As with other Si-containing backbone polymers, the torsional and angular freedom within a phosphorus-nitrogen backbone facilitates both an enhanced elasticity and high molecular weight of the poly(phosphazene)s. The general synthesis routes for poly(dichlorophosphazene) include the cationic polymerisation of phosphoroanimine at ambient temperature, thermal ring-opening polymerisation of hexachlorocyclotriphosphazene and thermal condensation of N-(dichlorophosphinoyl)phosphorimidic trichloride (Borisov et al., 2010). The subsequent substitution of the chlorine atoms of poly(dichlorophosphazene) by various nucleophiles, such as alkoxy, aryloxy or amino acid functionalities, results in the production of derivatives with desirable material and biological properties as shown in Fig. 4.3. Sequential substitution can be used to link groups of different molecular weight, with the larger functionalities being first to take part in reactions.
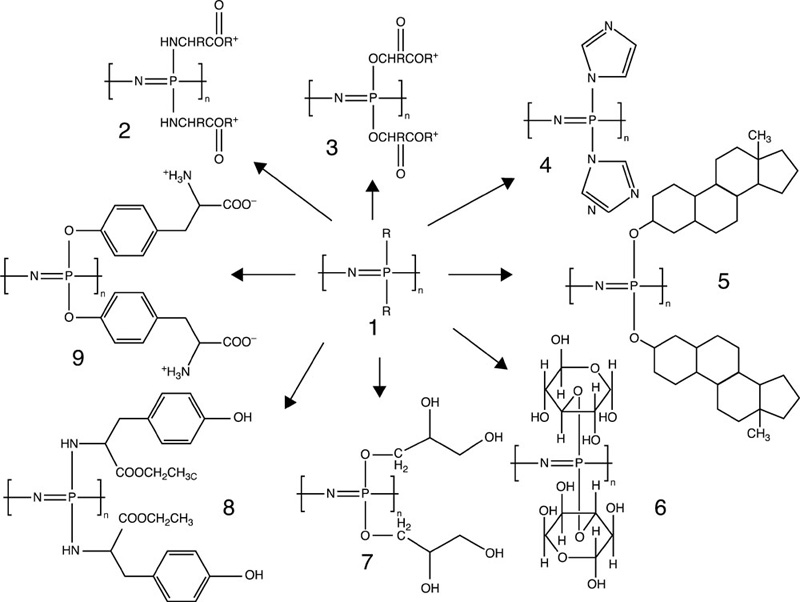
This synthetic flexibility of poly(phosphazene)s makes them suitable for a wide range of applications, including artificial bone grafts, soft tissue prostheses, chemotherapeutic models, drug delivery systems, electrical and optical devices, and membranes (Rahimi and Shokrolahi, 2001). Several non-degradable fluorinated poly(organophosphazene)s were demonstrated to possess high levels of tissue compatibility. With regard to tissue engineering and controlled delivery and release systems applications, biodegradable poly(phosphazene)s are particularly attractive as they undergo hydrolytic degradation to non-toxic and pH neutral products, due to the buffering capacity of the phosphates and ammonia that are simultaneously released in the course of material degradation (Deng et al., 2010a). Their physico-chemical properties can be effectively controlled by the nature, composition and abundance of functional groups.
For example, the sensitivity of poly(phosphazene)s to hydrolytic dissociation can be achieved by appending amino acid ethyl esters or imidazolyls, whereas substitution of hydrophobic methylphenoxy groups resulted in the production of more stable materials. Electrospinning of amino-acid substituted poly-(phosphazene)s with gelatine was reported to further enhance the hydrophilicity of the polymer. Poly(dichlorophosphazene) can be reacted with both glycine ethyl ester and methylparaben to produce a mix-substituted biodegradable polymer. The ratio of methylparaben to glycine ethyl ester used in this process was found to influence the extent of degradability of the polymer (Huang et al., 2011). Alanyl-glycine ethyl ester, valinyl-glycine ethyl ester and phenylalanyl-glycine ethyl ester dipeptides were used to functionalise poly(dichlorophosphazene), with the N-terminus used as a reactive site and the C-terminus protected with an ethyl ester to avert side reactions and cross-linking (Weikel et al., 2009). The hydrolytic behaviour of the resultant polymers varied with pH, with structures undergoing rapid hydrolysis under acidic conditions, but exhibiting less sensitivity to hydrolysis under neutral and basic conditions. Phosphazenes with reversible cross-linking groups were prepared by the addition of cysteine and methionine amino acid side groups. Given their controlled mechanical stability and extent of hydrolysis, these polymers were suggested as suitable candidates for reversible cross-linking in drug delivery systems and for achieving cross-linked stabilisation of tissue engineering scaffolds (Weikel et al., 2010).
A highly versatile platform of poly(phosphazene)-poly(ester) blends with strong hydrogen bonding capacity was fabricated by co-substituting a poly(phosphazene) backbone with both a hydrophilic glycylglycine dipeptide and hydrophobic 4-phenylphenoxy group (Deng et al., 2010b). Poly(ester) segment hydrolysis is used as an erosion tool to transform a solid coherent film into an assemblage of microspheres with an interconnected three-dimensional (3-D) porous structure. The structure holds great potential as agents for robust cell infiltration and collagen tissue in-growth between microspheres based on time-controlled degradation, as observed through in vitro and in vivo studies.
Thermo-sensitive and photo-cross-linkable poly(organophosphazene) gel comprising varied proportion of isoleucine ethyl ester, poly(ethylene glycol), aminoethyl methacrylate and depsipeptid were identified for applications where the gel strength and degradation rate need to be carefully controlled (Potta et al., 2011). The degradation rate of the injectable and dual cross-linkable gels can be tuned to the desired extent by varying the degree of photo-cross-linking, and by controlling the depsipeptide and poly(ethylene glycol) chain lengths within the polymer network. Bulkier groups such as phenylalanine have been shown to afford poly(phosphazene)s a higher transitional glass temperature compared to other amino acid ethyl ester substitutes. Poly(phosphazene)s containing L1, E and B6 vitamin substituents were also synthesised for their potential in biocompatible hard tissue engineering scaffolds (Morozowich et al., 2011). This study was prompted by the need to enhance the mechanical performance of poly(phosphazene)s. Co-substitutions with either glycine ethyl ester or sodium ethoxide, and with phenylalanine ethyl ester were used to adjust for steric hindrance generated by vitamin E and to favour biocompatibility in vitamin B6 compounds, respectively. The polymers were found to be biodegradable over a wide pH range, undergoing between 10 and 100% weight loss over 6 weeks via polymer backbone cleavage and/or solubilisation.
While amino acid esters and other hydrophilic functionalities contribute to the hydrolytic dissociation of poly(phosphazene) polymers, hydrophobic pendant chains, including p-methylphenoxy, p-phenylphenoxy, tyrosine, pyrrolidone, depsipeptide, and organic groups with –COOH functionalities, can be introduced to enhance polymer backbone rigidity and improve their mechanical properties. In order to achieve a balance between mechanical durability and timely degradation, different ratios of hydrophobic and hydrophilic moieties are introduced into the poly(phosphazene) structure. Special attention is also paid to the functional site on which the group is attached to the backbone. For example, amino-attachment of tyrosine renders poly(phosphazene) biodegradable, whereas phenolic attachment renders the polymer biostable. When methylamine, glucosyl, glyceryl, sulfonic acid or another highly polar functionality is employed for poly(phosphazene) synthesis, water solubility can be attained. Stimuli-responsive poly(phosphazene) materials, such as those that undergo pH dependent solubility, swelling, cross-linking and degradation, have also been reported for polymers containing –OH, –COOH, –SO3H and –COONa polar functionalities.
4.5 Organic–inorganic hybrid polymers
4.5.1 Synthetic organic polymeric materials
Numerous other materials of biomedical significance have been investigated. Aligned poly(acrylonitrile)-methylacrylate fibres and films have been used to elucidate the effect of topographical features on neurite outgrowth and Schwann cell migration, with aligned fibres influencing fibronectin distribution, and hence promoting aligned fibronectin network formation (Mukhatyar et al., 2011). An understanding of the dynamics of the Schwann cell-generated fibronectin matrix organisation may have significant implications for scaffold designs to bridge long peripheral nerve gaps. Poly(vinyl pyrollidone) coatings over poly (urethane) significantly enhanced hydrophilicity and lubricity of the biomaterial and reduced the adherence of hydrophobic Enterococcus faecalis isolate, whereas the attachment of a hydrophilic Escherichia coli isolate was similar between two test materials (Tunney and Gorman, 2002). The aforementioned isolates are responsible for biofilm formation in urethral catheters and ureteral stents. Struvite and hydroxyapatite encrustation, frequently responsible for obstruction and blockage of catheters, was significantly reduced on the coated devices, indicating the coating’s potential in preserving the efficient functioning of urinary tract implants.
Inherently conducting polymers, such as polypyrroles, polythiophenes and polyanilines, are attracting attention for their ability to electronically control a range of physical and chemical properties, with the potential for these materials to bridge the bionic interface (Wallace and Spinks, 2007). The potential for such conductive polymers spans biosensors, tissue engineering and neural probes (Guimard et al., 2007). Their prospective is particularly interesting in the area of neural tissue engineering, where these materials can not only provide growing neurites with mechanical support and mediate fibrous scar tissue ingrowth, but also generate appropriate biological signals to direct the axonal growth cone to the distal stump (Ghasemi-Mobarakeh et al., 2011). Electrical stimulation has been demonstrated to effectively and selectively promote either the proliferation or differentiation of various cell types, including neurons (Yu et al., 2008; Zhang et al., 2007), bone marrow-derived mesenchymal stem cells (Sun et al., 2006), fibroblasts (Shi et al., 2008), myoblasts (Jeong et al., 2008) and keratinocytes (Ateh et al., 2006). The biocompatibility of the electrically-conducting polymers can be enhanced to modulate survival and maintenance of the targeted cells (Lundin et al., 2011).
4.5.2 Metal-containing inorganic polymers
Metal-containing inorganic polymers possess a variety of main group metals, transition metals or rare earth metals in the repeating unit, either incorporated directly in the backbone, or covalently bonded to the backbone (Borisov et al., 2010). As such, these unique materials can benefit from favourable properties commonly associated with conventional polymer materials, such as biocompatibility, with electrical and redox properties of metals, such as electrolytic activity (Hamciuc et al., 2007). Among the poly(metallocene)s, which are composed of repeating units of metals with two η5-bound cyclopentadienyl ligands, the iron-containing polymers (poly(ferrocene)s) have attracted the most attention.
Macromolecular systems containing ferrocenyl moiety attached to highly flexible dimethyl siloxane and organic or silane sequences, such as poly(ferrocenyl silane)s, have been extensively investigated for their controlled response to a redox stimulus, ability to form crystalline, self-assembled materials, and their potential as precursors to nanostructured magnetic ceramics and carbon nanotube growth (Patra et al., 2010). Fabrication of degradable ferrocenyl-siloxane copolymers having ester, amide and silyl ester internal functions via polycondensation procedure have been reported (Cazacu et al., 2009). Presence of the silyl ester groups in the chain of the latter copolymer rendered the material hydrolytically degradable, whilst poly(amine)s and poly(amide)s containing ferrocene have been reported as potential stimulants of antibody formation (Cazacu et al., 2006). The ferrocene-ferricinium redox systems have also been explored for applications in tumour and cancer treatments (Milaeva et al., 2010; Osella et al., 2000). Ferrocene-based organophosphorus materials, such as poly(ferrocenyl phosphine)s, have also been the focus of several investigations as a result of their potential application in sensors and biomaterials. The micellisation of the poly(ferrocenyl phosphine) s-containing block copolymers facilitates the preparation of polymer-metal hybrid nanomaterials for use as precursors to magnetic nanostructures.
Polymeric ferrocenes have also been suggested as suitable mediators in amperometric biosensors (Losada et al., 1997), with implications for the design of stable biosensors and bioelectric devices involving electron transfer from oxido-reductases to electrode surfaces (Hendry et al., 1993). The high flexibility of the siloxane polymer backbone arising from the combined effect of reduced steric hindrance and intramolecular congestion (Si-O bond being longer compared to C-C bond of organic polymers), unencumbered skeletal oxygen atoms and increased torsional rotation due to wider bond angle, facilitates adequate proximity between the redox complexes of the polymeric system and the enzyme. Such proximity allows for an efficient charge transfer, with significant clinical value of implantable sensors comprising of such systems. In addition to their application for chemical modification of electrodes and in electro-chemical sensors, the ferrocene-containing macromolecules are of interest for their magnetic properties. The self-assembling property of poly(ferrocene) block copolymers in bulk or solution facilitates fabrication of a variety of functional nanomaterials that can be used as drug delivery carriers or templates for the fabrication of one-dimensional (1-D) nanostructures (Cazacu et al., 2009).
4.6 Geopolymers
Geopolymers are amorphous to semi-crystalline aluminosilicate materials with a structure of a random 3-D array of tetrahedrally-coordinated AlO4 and SiO4 units in a random arrangement, charge-stabilised by the hydrated alkali metal cations (MacKenzie et al., 2007). Geopolymers are generally synthesised at near-ambient temperature with chemical reaction of aluminosilicate oxides (Al3 + in IV-fold coordination) with alkali poly(silicate)s yielding polymeric Si-O-Al bonds (Davidovits, 1991). The aluminosilicate itself is based on a clay mineral such as kaolinite in which the structure has been changed upon thermal removal of structural water or by high-energy grinding (MacKenzie et al., 2010). These inorganic polymers are strong and durable, with high thermal stability of conventional ceramic materials. Depending on their structure, geopolymers can be classed as poly(sialate) [-SiO-Al-O-], poly(sialate-siloxo) [-Si-O-Al-O-Si-O-] and poly(sialate-disiloxo) [-Si-O-Al-O-Si-O-Si-O-] types. Aluminosilicates can also be organically modified and then used in conjunction with poly(isoprene-b-ethyleneoxide) block copolymers for the preparation of various silica-type mesostructures (Templin et al., 1997).
In addition to their conventional applications, geopolymers have been investigated as potentially bioactive materials capable of forming bone-like minerals by interaction with blood plasma. In one study, heat treatment was used to fix both the leachable alkali and aluminium, and afford a calcium-free potassium geopolymer sufficiently porous for the permeation of bone-forming fluids. Highly alkaline characteristics of the material can lead to cell death, while leaching of large amounts of aluminium may be toxic for the host. Interestingly, exposure to small concentrations of aluminium may be beneficial, as it was found to stimulate osteoblast proliferation and subsequent bone formation. Although the resultant geopolymer-based material was stable in vitro and in vivo, there was no evidence of hydroxycarbonate apatite or any other bioactive phase formation, indicating lack of bioactivity.
High-silica potassium geopolymers containing tricalcium phosphate and hydroxyapatite have also undergone heat treatment to render it porous and decrease alkalinity. In vivo studies using a rabbit model demonstrated non-toxicity (over one month of implantation) and improved bonding between the bone and the material (Martin et al., 2005; Simon et al., 2008). The evaluation also indicated chemical stability of composites, with negligible leaching of aluminium into the blood stream for up to 750 hours of implantation. A high-quality bio-integration and bioconsolidation between composites and bony matrix, and the total absence of inflammation or fibrous tissues, was detected at the bone–implant interface (Oudadesse et al., 2007a,b). Samples of approximately 65% porosity and compressive strengths of about 5 MPa were determined to be most favourable for stability and growth (Oudadesse et al., 2007).
4.7 Conclusion
While most of the polymer biomaterials currently on the market are based on synthetic organic polymers and natural polymers such as those derived from collagen, the last three decades have seen significant research effort devoted to the advancement of synthetic inorganic chemistry and novel bioprocesses. Inorganic and organometallic macromolecules are attracting a great deal of attention for their promise to overcome the drawbacks associated with organic plastics, and to extend the utility of plastics to organic polymers. Their versatile chemistry, excellent physico-chemical and biological properties, and controlled biodegradation render them highly suited for a host of medical applications, from transient implants to drug and biomolecule delivery vehicles. Nanoparticles based on well-defined, highly ordered inorganic polymer systems can be utilised for highly specific molecule delivery applications and are ideally suited for functional tomographic imaging. In tissue engineering, the future success of inorganic biopolymers lies in our ability to achieve structures that accurately mimic the mechanical, chemical and biological properties of natural bone to effectively elicit favourable biological responses. A comparison of the properties of currently available scaffold materials with native tissues reveals a significant scope for improvement. The ongoing development of novel polymers, modification techniques and bioprocesses will support the necessary advancement of inorganic polymer biomaterials.
