Cytotoxicity and biocompatibility of metallic biomaterials
Abstract:
Metallic biomaterials have found a plethora of applications as medical devices. However, the long-term performance of these materials is highly dependent on their ability to withstand synergistic effects of corrosion and wear. Loss of surface integrity and subsequent leaching of metal ions and particles into the peri-implant environment may undermine biocompatibility of metallic implants, also potentially causing untimely loss of mechanical function and device failure. This chapter reviews key issues relating to cytotoxicity and biocompatibility.
6.1 Introduction
Metallic biomaterials are employed in various forms to substitute damaged structural components and to restore lost functions within the human body (Kuhn, 2012). A favourable combination of tensile strength, fracture toughness and fatigue strength warrants their application in orthopaedics, as artificial joints, plates and screws, in orthodontics and dentistry, as braces and dental implants, cardiovascular and neurosurgical devices, and as artificial hearts, staples, stents, wires and coils, to name but a few.
Metals currently used for implant manufacturing include Fe, Cr, Co, Ni, Ti, Ta, Mo and W, and are known to be relatively non-cytotoxic in limited quantities. Furthermore, some of these elements are present naturally in the human body, and are vital for many cell functions. For instance, Mg is the second most abundant element in cellular systems, implicated in numerous metabolic pathways and intimately involved in stabilisation of DNA and chromatin structures (Hartwig, 2001). Mg is also a critical co-factor in most enzymatic systems involved in DNA processing, including nucleotide excision repair, base excision repair and mismatch repair (Rowe, 2012). In a similar manner, Fe plays an important role in cell functioning, Co is implicated in vitamin B12 synthesis, and Cu is involved in cross-linking of elastin in the aorta (Greenhagen et al., 2011). However, in large amounts, these elements can be highly toxic to the patient. As such, the biodegradation behaviour of metallic implantable materials in vivo is of significant concern, as untimely deterioration under hostile physiological environment may not only undermine the structural integrity of the implant but also have deleterious toxic effects on the peri-implant tissues.
As with most commercially-used biomaterials, metals and alloys that were widely used as components of biomedical implants were first developed for non-biomedical applications, and only later adopted by medical practitioners for their excellent mechanical properties and stability in contact with corrosive physiological fluid. Their industrial beginnings resulted in these materials having little biological activity in vivo, with consequent poor tissue integration, increased incidence of inflammation and infection, and temporal implant loosening and failure. Alloying elements at toxic levels can leach from the implants as a result of corrosion, wear and fretting arising from the mechanical load-assisted dissolution of the metallic surfaces (Mitchell and Shrotriya, 2008). The same degradation processes can lead to loss of mechanical integrity and robustness, with the implant failing to perform the intended function. The relatively high density of metals such as steel and Ti inflicts excessive stresses on the surrounding tissues, whereas relatively high rigidity of these metals contributes to stress shielding and osteolysis. This chapter reviews key aspects of toxicity and biocompatibility.
6.2 Cytotoxicity and biocompatibility of metals and alloys
The biological response to any implant debris that may be present is central to the acceptable clinical performance of these materials, particularly in complex systems such as orthopaedic devices (Hallab et al., 2012). An ageing population and joint diseases diagnosed in ever younger patients have driven an increased demand for biomaterials and devices that can restore the host function, enhance the lifestyle quality and help sustain the level of activity of the patients (Vallés et al., 2011). Orthopaedic surgery, such as total or hemi-hip replacements, are among the most common surgical procedures for management of pain and severe physical joint failure caused by osteoarthritis, as well as rheumatoid and traumatic arthritis, avascular necrosis, acetabulum defects, bone fractures, non-cancerous and malignant abnormal bone growths, to name but a few (Freemont, 2012). Recent advancements in materials engineering, especially the development of suitable metallic bulk materials, bioactive coatings and cements have been fundamental in improving implant osteo-integration, enhancing implant robustness, and positively contributing to the patient’s health and experience.
However, in spite of these numerous benefits, currently available metallic implants are still faced with the issues of limited biocompatibility, untimely biodegradation and loss of function. Upon implantation into the host, the implanted material often begins to decompose, a process that is associated with an incessant release and subsequent accumulation of wear particles and degradation debris at the implantation site and other tissues (Ma and Goodman, 2011). The process is speeded up by the inevitable and unremitting wear from the articulating motion at the bearing surfaces and micro-motion in non-articulating interfaces between implant components.
A cellular-mediated inflammatory response, which results from implant degradation, can lead to a contained bone loss in the immediate proximity to the implant, the peri-prosthetic osteolysis, with the flow on aseptic loosening and biomaterial failure (Vallés et al., 2011). In many cases, correction surgery and extended post-operative care may ensue. Furthermore, such implant loosening has been associated with an increased incidence of inflammation, complications and less successful functional performance of the implant. The extent of in vivo degradation and consequent host inflammatory response is dependent on the bulk and surface tribological and physico-chemical properties of the implantable metallic biomaterial. Adequate appreciation of the chemical and biological mechanisms that underlie the degradation by-product-induced osteolysis at the tissue and cellular levels is therefore essential for the advancement of improvements in implants and indwelling medical devices.
Debris-induced inflammation and bone cell lysis is a complex process. Deciphering the etiology and pathology of the implant-induced periprosthetic bone loss is a challenge in itself (Sundfeldt et al., 2006) (Table 6.1). Indeed, the detection and interpretation of cellular events triggered by the leaching and transfer of wear particles and their effect on the evolution of osteo-articular prosthesis failure is intricate (Ma and Goodman, 2011). The interconnected, at times snowballing nature of these cellular events and the multi-component nature of most implantable devices further complicates matters. In addition, the installation techniques and level of activity of the patient influence the lifespan of the indwelling device and amount of wear debris released.
Table 6.1
Systemic toxicity of small sized debris particles after total hip replacement using implants containing metal bearings (Polyzois et al., 2012)
| System | Action |
| Haematopoietic | Erythropoiesis impairment induced by Al Ni compounds decrease erythrocyte thermostability, deformability, and the rate of O2 release by erythrocytes Co nanoparticles showed a dose-dependent toxicity on growth and differentiation of bone marrow derived CD34+ human haematopoietic progenitor cells, with 100 and 25 ppm being too toxic for life, and 5 ppm inhibiting the formation of colonies in both systems to about 25% Co and Ni nanoparticles induced a concentration-dependent reduction of human endothelial cell number within 24 h |
| Immune | ↓CD8(+) cell levels (T-cytotoxic/suppressor) ↓leucocytes, myeloid cells, lymphocytes, CD16 cells ↔ CD3, CD4, CD8 and CD20(+)cells Alterations in spleen architecture (capsule and medulla), namely depletion of T4 and B cells Inhibition of the rapid release of reactive oxygen species required for bacterial killing by neutrophils |
| Hepatobiliary | Cr compounds cause toxicity directly resulting in hepatic malfunction, potentially severe hepatic lesions, hepatocellular necrosis and possibly disseminated intravascular coagulation Fatty degeneration of hepatocytes after intra-articular injected TiO2 nanoparticles in rats |
| Renal | Cr exerts significant renal toxicity through induction of tubular necrosis and interstitial cell damage Acute tubular necrosis in the histopathological examinations of the renal specimens in mice exposed to Cr, Cu and Ar |
| Respiratory | Metal toxicity may lead to acute chemical pneumonitis and pulmonary oedema or to acute tracheobronchitis Bronchial asthma may be caused by complex platinum (Pt) salts, Ni, Cr or Co, presumably on the basis of allergic sensitisation Intra-articular injected TiO2 nanoparticles caused toxicological effect on lung; as it was revealed follicular lymphoid hyperplasia with inflammatory cells aggregated around bronchia |
| Nervous | Long-term effects of Al include amyotrophic lateral sclerosis and Alzheimer’s disease. Retinal degeneration has been described in experimental models of Al, Co and Ni toxicity |
| Cardiovascular | Cardiotoxic effects have been described in relation to Co and are usually in the form of cardiomyopathy and impaired left ventricular function After intra-articular injected TiO2 nanoparticles in rats at different concentrations, it was revealed that the dispersed and aggregated brown particulates were observed in interstitial fascicle, cytoplasm and nucleus of vascular cells of ventricular endocardium |
| Musculoskeletal | Chronic Al exposure has been linked to osteomalacia, pathological fractures, impaired bone remodelling, impaired response to vitamin D and proximal myopathy |
| Skin | The incidence of dermal reactions and positive skin-patch testing to Co, Ni and Cr in patients with total joint replacement, with stable and loose prostheses increases by 15 and 50% respectively, above those of the general population |
| Endocrine and reproductive | Long-term oral exposure to Co may well induce goitre and myxoedema Experimental data in animals have demonstrated the capacity of metal debris to alter the production or circulation of reproductive hormones with a direct effect on reproductive cells Testicular toxicity of Cr on adult monkeys; and Cr (VI) treatment led to disrupted spermatogenesis Chronic exposure to Cr has detrimental effects on male and female fertility as a result of decreased sperm production and impaired sperm and ova quality Exposure of the unborn foetus to metals, such as Cr, Ni, Co, V and Al, has been the subject of a number of studies as translocation of metal particles can occur through the maternofoetal circulation and lactation. Metals such as Cr, Ni, Co, V and Al have potential effects on conception, foetal implantation and later teratogenicity |
| Carcinogenesis | Ionic Cr, Co, Ni, V, Al and Ti have mutagenic actions on cells in tissue culture. The genotoxic effects of the metal ions are thought to be mediated by either direct action, causing DNA breaks through attacks on free radicals or by an indirect effect by inhibiting the repair of DNA In patients at revision arthroplasty, there is evidence for mutagenic damage in bone marrow and peripheral blood lymphocytes Studies have demonstrated a higher than the general population incidence of aberrations in chromosomes of patients who have undergone joint replacement surgery The International Agency for Research on Cancer, which publishes information on the risks posed by chemicals on the development of human cancers, has classified Cr (VI) and Ni (II) as carcinogenic, metallic Ni and soluble Co as possibly carcinogenic, and metallic Cr, Cr (III) compounds and implanted orthopaedic alloys as unclassifiable Metaanalysis of the epidemiologic studies performed in the last three decades has shown a gross variation in the incidence of different cancers among patients who had metal-on-metal THA compared with the general population; suggesting that factors other than THA play a major role in the origin of cancer The incidence of bone and soft tissue sarcomas in THA patients is lower than that in general population |
Finally, there are a plethora of host-specific factors that play a significant role in the implant tolerance and wear behaviour (Malek et al., 2012). Genetic predisposition, biomaterial hypersensitivity, chronic diseases and diet are among the factors that affect implant integration and biocompatibility (Granchi et al., 2012; Greenfield et al., 2011; Tuan et al., 2008). For example, the lifespan of the metallic implant has been found to be considerably less in patients with metal sensitivity compared to that for unaffected patients (Cousen and Gawkrodger, 2012); furthermore, metal sensitivity was diagnosed in 60% of patients with metallic implant failure compared to 25% of patients with functioning implants (Frigerio et al., 2011). It has been suggested that the event of implantation can itself sensitised the host to the metal; however, testing such a hypothesis requires a large sample pool.
6.3 Effect of load and wear on implant degradation
Wear-induced deterioration is hardly unexpected when metallic biomaterials are used as load-bearing articulating surfaces of artificial joints. Mechanical load-assisted dissolution is regarded as one of the primary processes that cause material removal in fretting and crevice corrosion of metallic biomaterials (Mitchell and Shrotriya, 2008). The peri-implantable loosening of orthopaedic implants historically was associated primarily with the degradation fragments from bone cements, such as debris of poly (methyl methacrylate), and polymer cup materials, such as ultra-high molecular-weight polyethylene (Pal et al., 2011; Vallés et al., 2011). Indeed, the rotational movement of the cup against the femoral head produced a large amount of polyethylene particles, with the degree of polymer wear significantly higher for polyethylene cup-metal head combinations compared to polymer cup-ceramic head design. Once released, the particles come into close contact with the tissues within the implantation site, driven by the pressure of the joint fluid. As a result of this contact, the cells activate causing bone erosion, which in turn facilitates penetration of joint fluid and degradation debris further into the interface, leading to osteolytic implant loosening (Hallab et al., 2012). However, unfilled screw openings and areas of poor osseogenicity within the acetabular space contribute to degradation fragments accessing the tissue-device interface. The use of highly cross-linked polymers for articulating bearing surfaces notably reduced the overall wear of the implant, as did cementless metal-on-metal and ceramic-on-ceramic configurations. Yet, the issue of the aseptic loss of bone tissue in the immediate proximity of the implant remained.
As with polymer particles, the presence of metallic debris has been linked to activation of macrophages and giant cells in the peri-implant area, contributing to the implant-associated bone loss and third-body accelerated degradation of the implant (Huber et al., 2009). Metallic particles were smaller, more uniformly sized and more abundant compared to the polymer fragments. Where larger, irregular ultra-high-molecular-weight polyethylene debris tended to accumulate in tissues close to the implant site, their nano-scale dimensions allowed for a higher mobility of the metallic wear fragments. This facilitated their transfer from the peri-implant space to distant tissues and organs, where they were implicated in host immune cells activation and triggered an implant-associated inflammatory reaction in the host.
The high surface area to volume ratio lead to the elevated reactivity of these metallic nanoparticles, with characteristic release of ions and corrosion taking place as a result of the interaction between the nanoparticles and the physiological fluids present. A comparative in vitro investigation of the inflammatory response of the macrophage cell to metallic and ceramic titanium-based particles demonstrated the relative inertness of the ceramic TiO2 versus Ti particles (Vallés et al., 2006). Whilst found to be notably less reactive than the metallic degradation fragments, ceramic particles, such as alumina, have been demonstrated to instigate an end-stage inflammatory response and subsequent bone resorption in some individuals (Goodman et al., 2006; Hatton et al., 2002; Kamath et al., 2011). Encapsulation of the metallic femoral head component of the prosthetic device with a suitable coating has been shown to lower the occurrence of implant-induced bone resorption by preventing the wear debris from entering the tissue-biomaterial interface.
Minimal load-bearing metallic implants, such as those used in dental implants and cranial anchorage devices, have also been shown to undergo deterioration in vivo. These devices are produced using commercially-pure titanium grades II to IV, that differ significantly in terms of their reactivity from titanium grade V alloys typically used for orthopaedic implants. Loss of surface integrity can lead to the liberation of metal ions, with particulate debris arising from both normal wear and surface corrosion (Cadosch et al., 2010). For instance, anchorage devices composed of commercially-pure Ti have been shown to release Ti debris and ions into the surrounding soft tissue (Addison et al., 2012). Well-fixed cemented Ti hip replacements have been reported to undergo gross corrosion of the intra-medullary stem, causing cortical hypertrophy and obstinate pain in patients, and prosthesis replacement (Hallam et al., 2004). Post-removal examination revealed the highly acidic pH level of the tip of the stem, with macroscopic signs of crevice corrosion as evident by the multiple layers of TiO2.
Corrosive degradation has also been reported in Ti intra-medullary fixation implants subjected to minimal wear, with elevated serum Ti levels over extended time periods (Nuevo-Ordóñez et al., 2011). It has been suggested that in tight crevices, such as at the interface between metallic components of the devices, moisture and relative micro-motion of component surfaces, may lead to the ionic lattice break-up and salvation of metallic ions, particle release, local acidification and the deterioration of the oxide surface layer, making the underlying bulk material vulnerable to penetration by corrosive physiological fluids (Addison et al., 2012; Baldwin and Hunt, 2006). Following this reasoning, any multi-component metallic device can undergo crevice corrosion, independent of the degree of load-bearing and wear.
6.3.1 Direct and indirect effects of wear particles
The evolution of an inflammatory event in vivo is driven by both resident and recruited cells, including macrophages and giant cells, osteoblasts, osteoclasts, fibroblasts and lymphocytes, and a host of cell-activated aggressive mediators, such as chemokines, growth factors, pro- and anti-inflammatory cytokines, eicosanoids, degradative enzymes and reactive oxygen radicals (Mandelin et al., 2005; Tuan et al., 2008). These soluble chemical factors expressed by the cells directly in contact with the wear debris have the capacity to further enhance the recruitment and stimulation of cells implicated in inflammation-induced bone tissue loss and provoke the development of fibrous pseudo-membrane that develops at the bone–biomaterial interface. Fibrosis of the tissues surrounding the implant further undermines biomaterial integration and positively contributes to the inflammation process.
6.4 Macrophage-mediated inflammatory events
The role of macrophages in the implant-induced inflammatory response has been well acknowledged. The macrophages are recognised as primary target cells for metallic debris, in part owing to their ability to move to the areas of high particle concentration. Local immune reactivity is contingent on such degradation factors as the abundance of particles produced, specifically the concentration of phagocytable particles per tissue volume, the debris size, shape and their chemical reactivity (Abdelhalim, 2012; Hallab, 2009). For instance, spherical degradation fragments have been reported to illicit less of an inflammatory response compared to fibre-like particles. Smaller wear debris particles with dimensions below 150 nm undergo endocytotic or pinocytotic uptake, whereas larger debris particles (150 nm–10 μm) are internalised via phagocytosis by osteoblasts, fibroblasts, endothelial cells and macrophages (Hallab and Jacobs, 2009; Shukla et al., 2005). Above 10 μm, the debris particles are too large to be engulfed by a single macrophage cell; in order to mitigate such a fragment, macrophages fuse into a multinuclear foreign body giant cell (Freemont, 2012).
Limited contact with relatively small quantities of metallic debris has been shown to influence mice monocyte and macrophage survival, explaining cellular events prior to the onset of the osteolytic progression. Temporary reduction in macrophages has been demonstrated to thwart the polymer debris-generated inflammatory processes. Once activated, macrophages have the ability to intensify the inflammatory response via the production of a range of soluble chemical triggers and cell-to-cell contact with monocytes and inflammatory cells (Fig. 6.1).

Recently, in vitro studies have shown that chemical triggers released by the activated macrophages and the events of phagocytosis of degradation debris are responsible for macrophage specialisation into osteoclastic bone resorbing cells. Engulfing the debris fragments damages the macrophage cell, with consequent liberation of a range of chemical triggers, such as resorption stimulating factors TNF-α, IL-1β, IL-6, PGE2 and GM-CSF, which advance the development of osteoclasts and hinder ossification. In addition, soluble and particulate products of Co-Cr-Mo alloy implant degradation were implicated in the activation of inflammasome danger signalling in human macrophages (Caicedo et al., 2009; Hallab and Jacobs, 2009; Malik et al., 2011).
Other effects of internalisation of metallic debris that require consideration include cytotoxicity and DNA damage (Hallab et al., 2005; Keegan et al., 2007; Møller et al., 2010; Wang et al., 2010b). When challenged by Al+ 3, Co+ 2, Cr+ 3, Fe+ 3, Mo+ 5, Ni+ 2 and V+ 3 chloride solutions (and Na+ 2 as a control) over a wide range of concentrations (0.01–10.0 mM), peri-implant cells, specifically osteoblasts, fibroblasts and lymphocytes, have been shown to react differently, with the effect being a function of the composition and concentration of metal challenge more so than the cell type (Hallab et al., 2005). The study found that below 0.01 mM, no cytotoxicity was observed. At challenge concentrations of less than 1 mM, Co, Ni and V hindered proliferation, limited viability and affected cell morphology of tested cells by less than 50%. Concentrations of above 5 mM were required for Al, Cr, Fe and Mo to exert a similar influence, with loss of filopodia or lamellipodia, and changes in cell shape were observed at challenge concentrations of less than 1 mM. These results suggest that soluble Co and V degradation by-products of Co- and Ti-based alloys, respectively, are the most probable to mediate cell toxicity in the peri-implant space. A subsequent study showed that it was possible to mitigate metal ion-induced lymphocyte activation by blocking CD80/CD86 or by addition of the natural IL-1β receptor antagonist (Caicedo et al., 2010). The comparative importance of the soluble metal ions to the particulate metallic debris in provoking implant-related metal allergy via stimulation of macrophage secretion of IL-1β, IL-6, TNFα and/or upregulation of costimulatory molecules CD80, CD86 and ICAM-1 was also demonstrated.
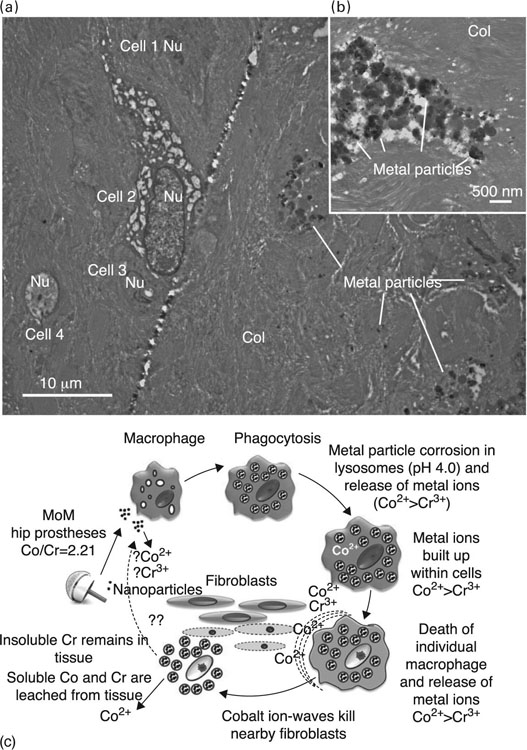
A series of works reported that Co2 + and Cr3 + ions induce macrophage toxicity and apoptosis with the implication of a caspase-3 pathway (Catelas et al., 2003, 2005; Petit et al., 2004). Furthermore, Co2 + and Cr3 + ions were demonstrated to instigate protein nitration in macrophages in vitro (Fig. 6.2), resulting in the formation of nitrotyrosine, a molecule previously linked to cell injury and the induction of apoptosis in a number of cell types (Petit et al., 2006). Nitric oxide is a small, highly reactive lipid- and water-soluble gas molecule that mammalian cells, including macrophages and T cells, produce to mediate servo-regulatory and cytotoxic functions (Nathan, 1992). Nitric oxide has been shown to react with oxygen and reactive oxygen species to produce a host of reactive nitrogen species. Together, reactive oxygen and nitrogen species have been demonstrated to induce the nitration of cytoplasmic proteins in human U937 macrophages, indicating that the metal ions produced by degrading metallic implants have the potential to alter the protein function in the peri-prosthetic environment and in circulating cells (Petit et al., 2006).
Oxidative stress plays an important role in debris-induced inflammatory response (Kinov et al., 2006). Studies have demonstrated that macrophages can be activated without phagocytosis, but rather through signalling events involving free radicals, sphingomyelinase, NFkB and TNF α (Soloviev et al., 2005). Free radicals produced by titanium particles were shown to induce plasma membrane peroxidation of linoleic acid, producing malondialdehyde, and to stimulate NSmase activation, hydrolysing sphingomyelin. The latter resulted in activation of the NFkB signalling pathway and induction of responsive genes, including TNFα.
Macrophages are not the only cells susceptible to metallic particle-induced oxidative stress. In a recent study, rats treated with gold nanoparticle-containing aerosols were demonstrated to suffer from interstitial pneumonia, fibrosis, chronic inflammatory cell infiltrates of small lymphocytes, congested and dilated blood vessels, scattered dense extravasation of red blood cells, and foci of hemosiderin granules (Abdelhalim, 2012). The degree of histological lung tissue alterations was found to be exposure time-and particle size-dependent, with 10 to 20 nm particles eliciting a significantly more pronounced response compared to 50 nm particles. The nature of the metabolic and structural disturbances to the lung tissue indicates that gold nanoparticles come into contact with proteins and enzymes, impeding the antioxidant defence mechanism of these cells. This led to free radical and reactive oxygen species generation and lipid peroxidation.
Macrophages are unable to fully digest the metallic debris particles, which are released back into the extracellular space once the macrophage breaks down, where the particles can potentially activate other cells to secrete cytokines. However, a study on the activation ability of untreated titanium particles and those undergoing phagocytosis by macrophages reported that while being of the same shape and size as untreated particles, phagocytosis by macrophages depleted the ability of the particles to stimulate TNF-α secretion by macrophages (Xing et al., 2008). The lack of physical degradation of the particles indicates that the observed effect is achieved by altering the surface chemical reactivity of the titanium debris. It is important to note that given the ongoing high-volume delivery of wear and degradation particles, the impact of such macrophage-mediated deactivation will be limited.
6.5 Role of bacterial endotoxins in triggering a particle-induced inflammatory response
The inflammation inciting property of metallic particulate debris has been linked to the surface characteristics of these particles, in particular to the presence of bacterial fragments and endogenous alarms or danger signals released by activated or dying cells on the surface of the metallic debris. Indeed, a number of authors have suggested that the wear particles should be viewed as none other than particles with a large surface area, or a foreign body platform that is available for the attachment by bacterial remnants, such as extracellular polysaccharides, and living bacterial cells that may form a biofilm (Nelson et al., 2005). There is a growing body of evidence that these bacterial fragments can be detected by immune cells, leading to their activation and triggering the release of pro-inflammatory cytokines in the apparent absence of clinical signs of infection (Beidelschies et al., 2008; Greenfield et al., 2005; Nelson et al., 2005; Sundfeldt et al., 2006). In vitro and in vivo experiments using untreated Ti particles and those from which bacterial endotoxins were removed, showed a significant decrease in the inflammatory response to the latter by both murine marrow cells and human peripheral blood monocytes, and 50 to 70% reduction in particle-induced osteolysis (Bi et al., 2001). Re-introduction of bacterial LPS to such particles re-instated their capacity to provoke cytokine production and osteoclast differentiation in vitro. Furthermore, inactivation of LPS using polymyxin B was shown to suppress the activity of LPS-coated titanium particles (Greenfield et al., 2010).
Activation of a toll-like receptor (TLR) signalling pathway has been reported for relatively inert wear particles that have been opsonised by bacterial remnants and subclinical biofilms residing on the surface of the implant. Such microbial pathogen-associated molecular patterns (PAMPs) were recognised by pattern-recognition receptors of the innate immune system, specifically TLRs such as TLR2 and TLR4, increasing the biological activity of the wear debris in vitro and resulting in the considerable macrophage infiltration to the peri-implant tissues in animal model (Islam et al., 2011). TLRs are germ-line encoded transmembrane proteins that facilitate recognition of many evolutionary well-conserved molecular structures of viral, bacterial and fungal origin (Lähdeoja et al., 2010). TLR4 is the principal receptor present on the surface of mammalian cells responsible for responding to ligands of microbial components, specifically lipopolysaccharides (LPS) from Gram-negative bacteria, whereas TLR2 responds to lipoteichoic acid (LTA) and peptidoglycan secreted by Gram-positive microorganisms. Recognition of PAMPs by the appropriate TLRs triggers macrophage activation and subsequent cytokine production, initiating several inflammatory pathways. Both TLR4/LPS and TLR2/LTA were detected in the patients diagnosed with aseptic loosening (Greenfield et al., 2010; Takagi et al., 2007). Further studies have indicated that in addition to molecules of bacterial origin, the PAMPs, TLRs respond to endogenous alarms or danger signals released from necrotic and activated cells. Indeed, both aseptic and septic peri-implant tissues were abundant in inflammatory cells equipped with TLRs specific to endogenous and exogenous ligands (Pajarinen et al., 2010; Tamaki et al., 2009).
There are several routes by which the bacterial molecular fragments can appear on the surface of the metallic debris. Many implantable materials will be colonised by bacterial cells, most likely being Gram-positive organisms (Greenfield et al., 2005). These cells will secrete a range of extracellular polymeric substances, forming a clinical or subclinical biofilm. Minor infections elsewhere in the body, normal flora and medical procedures can introduce bacterial endotoxins into the host system, where they can attach to the metallic wear debris. If left untreated, subclinical biofilms as well as minor chronic infections can provide a steady supply of bacterial endotoxins, exacerbating the inflammatory response to seemingly inert wear particles.
Titanium particles cleared of all endotoxins and introduced on murine calvaria were subject to time-dependent attachment of systemically derived endotoxins (Tatro et al., 2007). Owing to surface energy effects, bacterial endotoxins have strong affinity for metallic biomaterials, with conventional depyrogenation methods often failing to fully remove the endotoxins attached to the surfaces during implant manufacturing (Ragab et al., 1999; Tarafa et al., 2011). LPS from Porphyromonas gingivalis and Escherichia coli were demonstrated to attach strongly to titanium biomaterial surfaces with varied surface chemistry and morphology, with little LPS elution over time (Nelson et al., 1997). Strenuous cleaning of the surface itself may become a source of other contaminants, for example material by-products, capable of eliciting the prostanoid response in macrophages (Schwab et al., 2011).
6.6 Osteoclast-mediated bone resorption
In addition to their ability to incite the inflammatory response and stimulate bone resorption via the recruitment, differentiation and activity of osteoclasts, metallic degradation debris particles have been shown to negatively affect the differentiation, functionality and activity of bone forming osteoblasts (Fig. 6.3). Together, these processes undermine both the initial osseo-integration of implants and the ongoing regeneration of the peri-prosthetic bed, which is essential for the long-term performance of the implanted metallic device (Goodman et al., 2006). Particles of commonly-used metallic biomaterials have been reported to negatively affect mesenchymal stem-cell differentiation into functional osteoblasts, significantly suppressing pro-collagen gene expression, reducing cell proliferation (in a dose-dependent manner) and inducing apoptosis of osteoblasts (Saldaña et al., 2011; Vermes et al., 2001). Debris from Ti and Ti alloys have been shown to impact on the attachment apparatus of the osteoblasts via alterations to cytoskeletal structures, such as reduced ventral stress fibres and a disorderly arrangement of β-tubulin and acetylated α-tubulin fibres (Saldaña and Vilaboa, 2010). The degree of susceptibility of osteoblasts varied with the stage of maturation, with less mature cells displaying most vulnerability (Pioletti et al., 2002). In this study, particles were found to initiate a caspase-dependent apoptosis and greatly affect the genes that code for inflammatory cytokines and nuclear architecture. Furthermore, the size of the particles was found to affect the mechanism of debris-induced osteolysis, with exposure to larger Ti particles reported to enhance the activity of matrix metallo-proteinases (MMP) 2 and 9 (Choi et al., 2005). The results highlighted the importance of suppressed bone formation in addition to enhanced bone resorption in the development of peri-implant osteolysis.
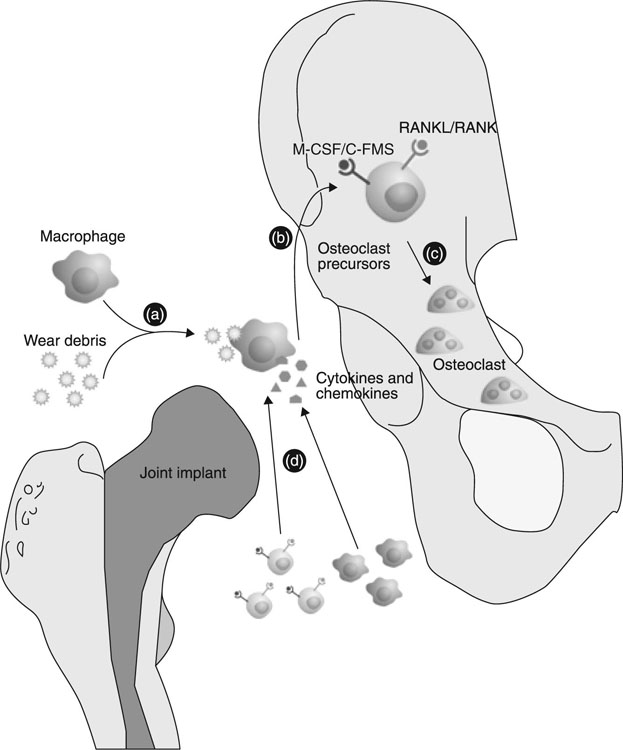
It is clear that the process of implantation changes the physico-chemical and mechanical environment, with both macrophages and osteoblasts reacting by producing a range of soluble messengers, which participate in paracrine and autocrine signalling (Vallés et al., 2011). Several studies demonstrated that osteoblasts were able to modulate the inflammatory response initiated by metallic wear debris activated macrophages (Vallés et al., 2008; St Pierre et al., 2010). The in vitro experiment involved co-culturing of osteoblasts and macrophages in the presence of metallic particles to simulate in vivo peri-implant conditions and allow for exchange of soluble messengers between the cell types without direct cell contact (Horowitz and Gonzales, 1996; Rodrigo et al., 2006; Vallés et al., 2008). An appreciation of the many co-existing cells and chemical messengers susceptible to feedback loops is essential for adequate interpretation of metallic debris induced inflammatory events (Vallés et al., 2011).
6.7 Osteolysis as a function of implant-associated mechano-transduction
Several researchers have suggested that particles may not be the only reason for peri-implant inflammation and osteolysis. In addition to cell–particle interactions, it has been demonstrated that the physical peri-implant environment can impact on both bone formation and resorption, such as the reports of pressure-induced bone resorption. In bone, osteocytes are in constant direct contact with the surrounding bone and neighbouring osteocytes via long slender cell processes, located in canaliculi, which are filled with pericellular interstitial fluid (Tan et al., 2007). Osteoblasts, osteoclasts and other cells lining the bone surface are also affected by these processes, resulting in a three-dimensional network of interconnected cells. This network coupled with lacuno-canalicular porosity is regarded as the site of mechano-sensing in bone. The changes in canalicular flow are translated by osteocytes into cell signals, with such mechano-transduction resulting in the secretion of a range of signalling molecules by the activated osteocytes (Fig. 6.4). These signalling molecules, such as nitric oxide, facilitate the recruitment of osteoclasts and osteoblasts to the site.
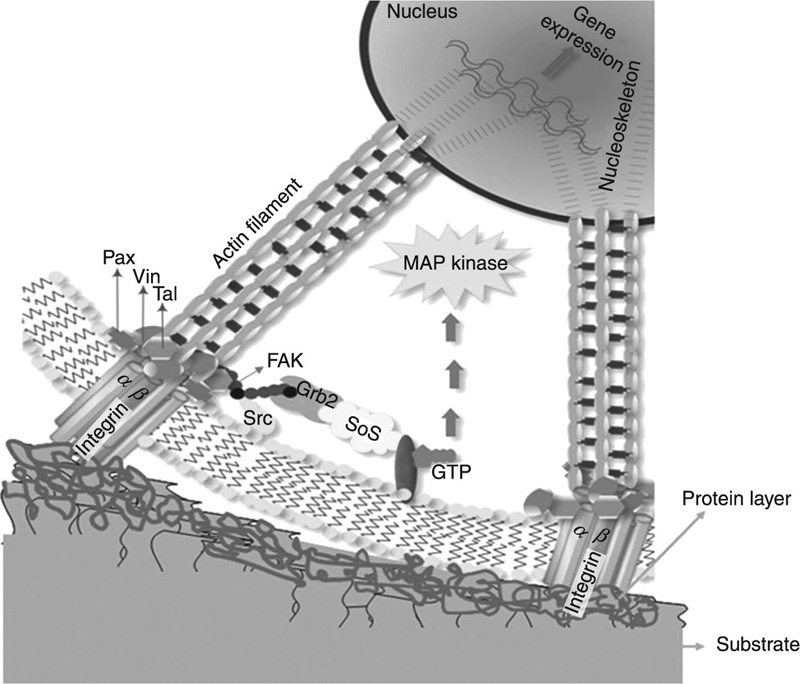
Implant migration and ensuing instability has been shown to create a means for joint fluid to enter the implant–bone interface as a result of normal joint motion, increasing the pressure in the peri-implant space. This oscillating fluid pressure has been shown to modulate osteoclast formation and activity via soluble chemical factors, contributing to bone resorption (Tan et al., 2007). The osteolysis at a titanium–bone interface in an animal model has been attributed to this process, with debris particle activation suggested to be a secondary mechanism in terms of both the timing and the magnitude of the impact (Aspenberg and van der Vis, 1998). Subsequent studies have demonstrated the synergistic effect of particles and cyclic pressure on human monocyte and macrophages activation and cytokine production (Evans et al., 2006; Mevoy et al., 2002).
6.8 Surface modification as a means of enhancing biocompatibility and corrosion resistance
As mentioned previously, passive oxide layers form spontaneously on the surfaces of metals and alloys in the presence of oxygen. These layers remain macroscopically stable, even though their chemical composition undergoes constant transformation. Through various degradation pathways, such as corrosion by electrolytes or mechanical wear, the layer undergoes partial dissolution and regeneration, providing an ongoing barrier for the underlying bulk material. The properties of the regenerated layer may notably differ from those of an oxide layer formed under controlled conditions prior to in vivo integration. For example, a titanium oxide layer formed in a stimulated body fluid contains Ca, P and S species that are absent from a film grown in an oxygen atmosphere. Similarly, calcium phosphate was generated on the surfaces of Ti-6Al-4 V and Ti-Ni alloys and stainless steel, but not on Ti-Zr alloys. Exposure to physiological fluid also altered the oxide layer of Cr-Co-Mo, changing it to primarily Cr oxide with limited amount of Mo oxide. Addition of albumin to the solution yielded the formation of porous, non-uniform albumin containing a hydroxyapatite layer on Ti surface. Clearly, the aforementioned changes in the chemistry and morphology of the passive layer would influence the dynamics between the surface and the living system.
To ensure consistent performance, optimal stability and maximum bioactivity when interacting with natural tissues, a number of surface modification approaches have been proposed. These include encapsulation of the metals and alloys with materials that display the desired characteristics, etching of the undesirable substances from the surface, changing material properties in the top layer and functionalisation of the surface. Surface treatments can be broadly divided into mechanical, physical, chemical and biochemical surface modifications. Mechanical surface modifications include machining, grinding, polishing and blasting. Physical surface treatments contain thermal spray, physical vapour deposition, ion implantation and deposition, and glow discharge plasma techniques. Chemical surface modifications include chemical treatment (acid, hydrogen peroxide, alkaline), anodic oxidation and sol–gel and chemical vapour deposition. Photochemistry, self-assembled monolayers, protein resistance and protein immobilisation, and silanised titania have also been considered for modification of Ti and its alloys (Duan and Liu et al., 2004; Wang, 2006).
Nanoporous oxide layers have been recently investigated for their ability to enhance the biological activity of metallic implants. Electro-chemical anodic oxidation has been employed to grow thick uniform oxide layers on metals for many years, with the modified materials displaying enhanced levels of biocompatibility (Lausmaa, 1996). By changing the type of electrolyte, current density, electrolyte concentration, temperature and other factors, an ordered oxide layer can be attained (Roy et al., 2011; Sul et al., 2001). Nanoporous oxide layer formation on Al (Masuda et al., 1997), Ti (Zwilling et al., 1999), Nb (Sieber et al., 2005a), Ta (Sieber et al., 2005b) and Zr (Tsuchiya and Schmuki, 2004) has been reported. A surface layer of TiO2. nanotubes has been associated with enhanced osseo-integration, the phenomenon that has been attributed to the improved hydroxyapatite adhesion onto the surface of the oxide. The mechanical interlocking between the hydroxyapatite coating and the nanotube surface layer enhanced osteoblast attachment by up to 400% (Oh et al., 2006).
Immobilisation of biofunctional polymers on the surfaces of implantable metals has also been suggested as an effective means of controlling the adsorption of proteins, peptides, antibodies, and DNA and platelet adhesion (Hanawa, 2010). For example, the availability of poly(ethylene glycol) on the surface is known to limit the amount of adsorption of proteins in a physiological environment, whereas grafting of the surface with RGD peptides has been shown to enhance adhesion, spreading and focal contact formation of primary bone-derived cells sequence (Rezania et al., 1997; Schliephake et al., 2002). RGD is a peptide containing an Arg-Gly-Asp sequence, the latter also being a sequence that is part of the cell attachment domain of fibronectin responsible for cell binding (Oya et al., 2009; Pierschbacher and Ruoslahti, 1984).
Other functional molecules known to modify the attachment of cells onto the surfaces of implants include ethane-1,1,2-triphosphonic acid, methylene-diphosphonic acid, morphogenetic protein-4, polydopamine, selenocystamine and cell-adhesive peptide Arg-Gly-Asp-Cys (Lee et al., 2007; Puleo et al., 2002; Viornery et al., 2002; Weng et al., 2011, 2012b; Xiao et al., 1997). Functional molecules can also serve as a platform for subsequent grafting of other biologically-active molecules and structures to induce specific attachment of cells onto the surface. For instance, the –COOH functionality of PEG has been used to immobilise the RGD peptide to induce the attachment of fibroblasts and osteoblasts and promote osteo-integration of the Ti implant (Hanawa, 2010; Oya et al., 2009).
6.9 Conclusion
Metallic materials have found many important medical applications, as orthopaedic implants, dental materials and cardiovascular devices. These are likely to remain a biomaterial of choice for applications that require high tensile and fatigue strength and fracture toughness. However, their future success is reliant on their ability to interact with their biological environment in a biocompatible, non-toxic and highly controlled manner. As such, it is imperative to gain an appreciation of the specific interactions between these materials and living tissues in vivo. This understanding is also vital for the advancement of current biomaterials, and the development of novel new biomaterials. It is clear that materials with a minimum content of potentially toxic elements are highly preferred. Yet there is a lack of evidence with regard to the toxicity of a large number of metallic elements, their ions and compounds, and designing appropriate systematic in vitro testing protocols to reflect the actual in vivo conditions is difficult. The events implicated in the biodeterioration of implantable materials and their effect on tissue integration and local and systemic inflammatory response are also poorly described and lack in-depth interpretation.
Current trends in biomaterials design include the development of materials that are lighter, stronger, smaller and more complex, with an enhanced bioactivity profile and highly controlled biodegradation kinetics. Biofunctionalisation of these materials via surface modification has been identified as a low-cost and relatively short development time approach to attain an optimal range of biofunctions.
