Introduction to biomaterials and implantable device design
Abstract:
Replacement and regeneration of lost function or tissue with well-matched biomaterials remains an area of active research and development, driving demand for novel and improved biomaterials. The ever-increasing complexity and high degree of integration of additives in modern biomaterials have significantly expanded the scope of biomaterial applications, but this has also introduced novel challenges. In this chapter, implantable electronic devices are used to demonstrate key issues and challenges associated with the design of complex implantable systems.
1.1 Introduction
Biomaterials play a significant role in many aspects of contemporary healthcare (Ansari and Husain 2012; Tan et al. 2011; Van Vlierberghe et al. 2011), with applications varying from coatings and delivery vehicles for pharmaceutical preparations to being used as critical constituents of extracorporeal devices, for example kidney dialysers and indwelling devices and implantable systems (Dorozhkin, 2011; Wagoner and Herschler, 2011). Their material properties and in vitro and in vivo performance are similarly diverse, with continuous advancements in technology being motivated by the changing demands of modern society (Hoppe et al. 2011; Khan and Sefton 2011; Lewis 2011; Saito et al. 2011; Shadanbaz and Dias 2012). The ageing population and demand for extended medical care being experienced in many countries provides a rapidly expanding market for biomaterials and implantable devices. Increased human life expectancy necessitates the development of longer-term performance of permanent devices and biomaterials, from orthopaedic implants to cardiovascular devices (Cardoso et al. 2011; Gioe et al. 2011; Kitao et al., 2011; Zhao et al., 2011).
Concurrently, functional materials are being developed to enable adequate tissue restoration and restructuring, thus providing a better clinical outcome for the treated individual. The interest in bioresorbable materials and constructs stems from their potential to contribute to the restoration of lost function, while overcoming the limitations often associated with the long-term use of foreign structures (Bendrea et al., 2011; Naderi et al., 2011). These limitations include the development of inflammation arising from long-term exposure to materials that exhibit limited bio- and haemo-compatibility, inadequate tissue integration and time-dependent deterioration of the biomaterial properties (Donald, 2011; Sun et al., 2011).
Materials that support fast healing and patient recovery, as well as those with the capability of limiting the incidence of implant-associated infections, are also attracting significant interest. The extensive use of systemic antibiotics and other antimicrobial agents has led to a profound increase in the number of difficult-to-treat nosocomial pathogens, the treatment of which places additional financial pressure on the healthcare system. Therefore, biomaterials and implants that minimise the length of hospital stay among the patients are highly favoured, for they not only lower the cost associated with each surgical procedure, but also reduce the chances of the patient of being infected with the aforementioned pathogens.
1.2 Biomaterials and their applications
Significant research efforts have been devoted to the development of biomaterials, biomaterial-based devices and implantable systems that are used to sustain the functions and physiological processes critical to sustaining life. Systems such as cardiovascular and neurosurgical implants, including cardiac valves, vascular grafts, pacemakers and hydrocephalus shunts, play a fundamental role in restoring and sustaining the biological processes that are essential for human life. According to Halperin et al. (2008), over 25 million US citizens are reliant on implantable medical devices to maintain life-critical functions; for example, the number of implantable cardioverter defibrillator implants has increased ten-fold between 1990 and 2002 (Stellbrink and Trappe, 2007). Biomaterials for joint replacements, fracture fixation devices and dental implants have been intensely investigated for their capacity to maintain, restore or enhance function and level of activity, thus positively contributing to the overall quality of life experienced by patients requiring these biomedical devices. According to Mota et al. (2012), in the US the number of total hip arthroplasty and total knee arthroplasty operations performed to reduce pain and restore the function and mobility of patients with severe arthritis increased by approximately 2.5 and 1.7 times, respectively, between 1993 and 2005.
The notable levels of commercial interest in elective aesthetic procedures has driven research into the development of biomaterials and implants whose primary application is to restore or improve the contour and visual appearance of a patient, hence contributing to their psychological and social well-being. Motivated by the social pressure to conform to a particular ‘look’ and the increasing affordability of elective procedures, an ever-increasing number of patients, both male and female, are undergoing plastic surgery. According to the report published by the American Society for Aesthetic Plastic Surgery, in the US, the annual number of surgical cosmetic procedures increased from 939 192 in 1997 to 1 622 290 in 2010, with breast augmentation being the leading cosmetic surgery in 2010 (ASAPS, 2010). Almost $6.6 billion were spent on cosmetic surgery in 2010 in the US alone, with a further $1.9 billion spent on injectable cosmetic procedures that included administration of calcium hydroxylapatite, collagen, hyaluronic acid, poly-L-lactic acid and other soft tissue fillers.
The number of adolescent patients that seek cosmetic surgery has also increased dramatically, guided by the changes in sociocultural representation of beauty (Larson and Gosain, 2012). Although beyond the scope of this chapter, the difference in risk-to-benefit analysis of contouring biomaterials versus those vital to the recipient’s survival should be acknowledged. Whether elective or not, surgical intervention and introduction of a foreign material into a patient’s body has significant associated health risks (Trussler and Tabbal, 2012). It is therefore not surprising that there is an ongoing debate regarding the commoditisation of medicine, where medical procedures are driven by patient demands rather than the health and safety considerations for the patient (Bismark et al., 2012).
Other important concerns include the promotion of suspect aesthetic norms and euphemistic portrayal of surgical risks and outcomes by medical practitioners, and limited understanding of the complications associated with biomaterial use in cosmetic procedures (Gilman, 1999; Raisborough, 2007). Indeed, a common perception amongst the patients is that being an elective procedure, cosmetic surgery or injection of fillers is unlikely to result in post-operative infections, inflammation, scarring, poor healing, discomfort, pain and possibly other complications, or even the death of the patient.
Over the last few decades, the role of biomaterials in the diagnosis of disease, treatment delivery and restoration of function has been changing. The use of tissue engineering for inductive tissue growth has been gaining considerable attention, with substantial advancement being achieved in our ability to understand the biomechanical and electrochemical characteristics required for these materials to succeed. Jaklenec et al. (2012) reported that from 2007 to 2011, tissue engineering and stem cell industry spending increased from $2.4 billion to $3.6 billion, with a notable increase in spending in the area of commercialisation from $1.6 billion to $2.8 billion. According to the same report, the sales for the combined tissue engineering and stem cell industry also rose by a factor of 2.7 during this period, with current annual sales approaching $3.46 billion.
Significant progress has been made in the development of sensitive and responsive biomaterials for the detection and analysis of specific compounds, enabling rapid in situ and ex situ diagnostics and efficient disease detection. A variety of micro- and nanoparticle biomaterials, including metals, ceramics, natural and synthetic polymers, and composite systems have been studied for their potential for highly targeted spatio-temporal delivery of drugs, and chemical and biological molecules (Anitha et al., 2011; Chiu et al., 2012; El-Shabouri, 2002). In addition to treatment delivery, these particles have the capacity to enable the concomitant in vivo visualisation of cells, tissues and processes that take place within the patient’s body, thus facilitating more precise diagnostics and treatment protocols (Ai, 2011; Altinoglu et al., 2008; Giaume et al., 2008).
1.3 Biomaterial development and realisation
Development of biomaterials is a complex process that spans many traditional and emerging science disciplines, engineering and medical sciences. The ability to take a biomaterial from an initial concept to being a commercially available product for real-life clinical applications is a lengthy and costly process, which is regulated by various legislative bodies. While achieving a statistically significant improvement in performance may be adequate in terms of scientific pursuit, it may not be sufficient to attain a commercially viable product. In addition to sound in vivo performance, the biomaterial technology should meet a range of criteria, for instance, processability and reproducibility, scalability of the technology used to produce the biomaterial and the final implant, stability under typical storage conditions, the ease with which the biomaterial-based implant can be installed into the patient’s body, the skill and expertise required for such a procedure, cost and so on. The physical dimension and the weight of the implant are other important considerations that should be considered against the performance of such a device, since excessively bulky implants may restrict the day-to-day ability of the patient to readily function, and thus be unlikely to find popularity with clients.
There has been an increasing demand for novel materials that combine fundamental functions with enhanced properties to underpin the development of novel and advanced technologies. For instance, biomaterials that are typically employed to perform a defined mechanical support function are now being modified to support additional functionalities, for example, advanced biocidal and antifouling surface properties and/or improved biocompatibility to promote healing and discourage the formation of pathogenic biofilms. The complexity of implantable constructs is also increasing rapidly, with implants often being produced to support multiple functionalities. More often than ever, biomaterials are required to integrate with each other to produce the best clinical outcome for the patient. Biocompatible electronic materials are a good example of such an application, since they are capable of acting as an interface between biotic and electronic systems.
Biomaterials derived from natural sources are also receiving renewed interest through the development of various modification techniques and via successful integration of these with synthetic, metallic and ceramic components. There are clear financial and time incentives in modifying existing biomaterials as opposed to creating a novel material de novo. In addition to savings associated with time and the costs of material development and evaluation, the modification of existing materials enables the developers to preserve the favourable properties of known materials, and enhance them through the incorporation of modification process steps into an existing manufacturing method.
Whether newly developed or manufactured through a modification process, a biomaterial should address several important criteria (Fig. 1.1). Materials that illicit minimal foreign body response in the implant recipient are obviously preferred, as both acute and chronic inflammation are detrimental to the correct functioning of the implanted device; materials that elicit a rejection response also are likely to hinder healing and restoration of function. The material or the products it releases should not be toxic to surrounding cells and tissues or incite allergic reactions to the given implant or other materials. Equally, the products of material degradation should not harm the adjacent tissues or accumulate elsewhere in the body.
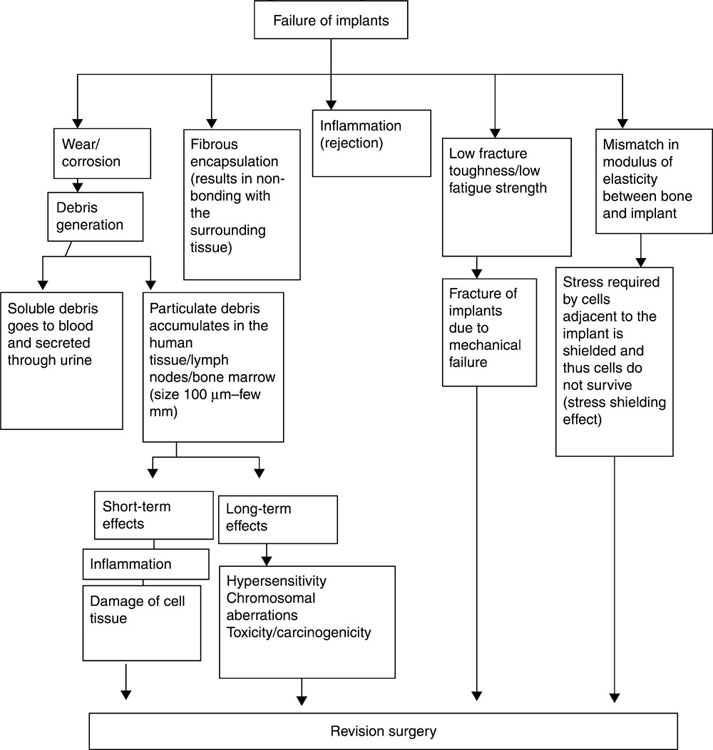
The stability under normal operation environment over the intended implantation life is also paramount to the success of the implant. Fouling by molecules and cells, leaching, biocalcification, chemical and biochemical degradation, mechanical wear and electrical malfunction are among stability indicators that will be discussed in the following chapters. The manner in which the biomaterial changes under storage conditions, both in terms of its bulk and surface characteristics, and how well it withstands sterilisation and handling prior to intended use are similarly important. The propensity of the material to be colonised by pathogenic microbial organisms will affect its clinical relevance, since infections and related inflammations are documented causes for the failure of the biomaterial and device. These are likely to necessitate the removal of the infected biomaterial, or cause limb amputation or the death of the patient. Those individuals with an inadequate innate and adaptive immunity are likely to be most at risk of developing a biomaterial-associated infection.
Designing a biomaterial that will effectively withstand bacterial adhesion and proliferation demands an in-depth knowledge regarding the forces that govern these processes, the attachment and colony formation dynamics, and the consequences for both the coloniser and the abiotic target as a result of adhesion (Bazaka et al., 2011b). The mechanism of bacterial adhesion involves initial reversible physic–chemical interactions, with subsequent intricate irreversible molecular and cellular interactions taking place. Several physical forces, including Brownian motion, van der Waals attraction forces, gravitational forces, the effect of surface electrostatic charge and hydrophobic interactions, govern the movement of the bacterial cells either towards or away from the surface (Gottenbos et al., 2002). The cells either move on their own, using such motility mechanisms as swimming, swarming and twitching, or be carried in/against the direction of the surface by flow.
Recently, Bjelland et al. (2012) reported quorum sensing to be a cell-to-cell communication system capable of regulating motility, adhesion, cell aggregation and biofilm formation, as well as virulence and metabolic activity in several bacterial species. Directed cell motility has been shown to play a significant role in the attachment of microorganisms and the subsequent formation of biofilms, since it is directly affected by chemotaxis functions (Merritt et al., 2007). Chemotactic sensing has been demonstrated to occur in numerous bacterial species. It contributes to bacterial colonisation of surfaces by regulating the expression of a number of cellular adhesion components and cell–cell and cell–surface interactions (Jenal et al., 2005; Kirov, 2003; Velasco-Casal et al., 2008).
Another mechanism implicated in the attachment of microorganisms and establishment of a biofilm is haptotaxis. It appropriates the cell movement behaviour, specifically speed and random turning, to the magnitude of the adhesion ligands on the surface of the target, thus relating the net direction of the cell movement to the gradient of adhesion (Dickinson and Tranquillo, 1993; Pavithra and Mukesh, 2008). More information regarding the role of environmental signals and signalling pathways in regulation of biofilm establishment, matrix composition and biofilm dispersal can be found in a review by Karatan and Watnick (2009).
The nonspecific interactions that take place at pathogen–surface/other cell distances above 50 nm are heavily influenced by the attributes of the surfaces, especially the free energy characteristics and the distance between them (Gottenbos et al., 2002). When designing a biomaterial, it is important to ensure that these forces are repulsive for applications where bacterial attachment is undesirable, or attractive for when bacterial attachment is favoured. These forces will also predetermine the likelihood of the microorganism entering the second stage of attachment, namely the molecular or cellular phase of adhesion. In addition to surface chemistry, surface topology and roughness have been shown to notably influence the adhesion and settlement of microorganisms. The exact mechanism of how roughness affects the ability of bacterial cells to adhere and remain on the surface remains a subject of debate (Anselme et al., 2010).
A number of studies have shown that heightened bacterial adhesion and retention on surfaces occurs as the surface roughness is increased, and this can be attributed to the presence of a greater surface area for colonisation. In addition, the morphological features of the surface can provide a protective habitat against shear forces (Donlan, 2002). Others have shown that surface topography is a comparatively insignificant factor in the colonisation of bacteria, with little preference being shown by bacterial cells for topographical cues (An et al., 1995; Bos et al., 1999; Scheuerman et al., 1998b). Similarly, there is little consensus in the literature regarding the length scale over which the influence of roughness is most pronounced (An et al., 1995; Boulangé-Petermann et al., 1997; Medilanski et al. 2002; Whitehead et al., 2005, 2006).
Our investigations into the role that surface properties play in bacterial cell adhesion, proliferation and biofilm development indicate that both the level of surface roughness and the unique topographical peculiarities of that surface are important (Ivanova et al., 2008; Mitik-Dineva et al., 2008, 2009). As such, the design of biomaterial surfaces should consider both chemical and morphological aspects. At cell-surface distances of below 5 nm, chemical interactions such as hydrogen bonding, ionic and dipole interactions, hydration and/or hydrophobic interactions lead to a more stable attachment of the cell to the abiotic surface (Mayer et al., 1999). At this stage, molecular structures present on the surface of bacteria, for example capsules, fimbriae, pili and slime, will enter into the molecular specific irreversible reactions with the chemical features available at the biomaterial surface (Bazaka et al., 2011b).
It may be tempting to configure the surface of the biomaterial so that it non-selectively repels bacterial cells at the initial stage of colonisation, for example by creating a super-hydrophobic surface; however, this material may have limited suitability for applications that require host tissue growth on the implant surface. Indeed, the physiological conditions required to sustain adequate cell/tissue recovery and growth are quite specific, and include factors that enable site-specific cell differentiation, protein fouling and formation of tissue matrix. Importantly, attachment of host cells to the surface of the implant does not guarantee tissue growth in the form that can effectively support lost function. For instance, a biomaterial designed to support bone regeneration may provide sufficient mechanical support to enable cell attachment and proliferation; however, the newly-formed tissue may not develop the three-dimensional (3-D) structure, vascularisation or calcification.
1.4 Implantable systems design
Considering the high degree of integration being used with modern biomaterials, it is important to understand how various components of these devices may affect each other. For instance, using a ceramic cup against a metallic femoral head has been shown to produce notably less wear-induced damage, such as particulate debris, compared to a polymer cup used under similar conditions. Similarly, a metal head-bearing hip implant may be supplemented with an electronic component capable of monitoring the temperature within the implant and wirelessly transmitting the detected data to the external receiver (Bergmann et al., 2012). The temperature telemetry is achieved using a thermistor, electronic circuit and power/data coil fitted inside the neck of the implant, and the data can be used to infer whether the peri-implant sites are likely to be inflamed. In many implantable sensor designs, suitable electrical performance should be balanced against biocompatibility and in vivo stability. For example, Koo et al. (2012) recently developed active, flexible devices for use in cardiac electro-physiological mapping activities. The circuits were designed by electrical engineers to perform in a slightly bent state to enable good contact with the naturally curved surfaces of internal organs and support effective movement.
The multidisciplinary nature of the development process can be of great benefit to the advancement of biomaterials, since it may facilitate the employment of ‘unconventional’ methodologies adopted from other areas of science and engineering. In fact, many of the biomaterials currently being used were adopted from non-medical industry applications. Cross-disciplinary communication can also prevent the biomaterial researchers and developers heading in the wrong direction, something that can be minimised by employing the expertise of clinical practitioners, chemists and microbiologists, to name but a few. At the same time, cross-disciplinary and user-developer communication can be an obstacle, especially in the cases where the developer has limited experience in the biomedical area, or where the requirements or capabilities are miscommunicated to the other party. Although a detailed discussion of the individual implantable systems currently available is beyond the scope of this book, it is important to understand the complexities associated with the design, implementation and operation of such systems. The following section gives an example of some considerations that should be taken into account when designing a complex implantable system.
1.4.1 Implantable electronics and their applications
Implantable electronic systems and devices have undergone a significant transformation over the last 60 years, to become a valuable biomedical tool for monitoring, measuring and soliciting physiological responses in vivo using wireless communication (Fig. 1.2). The discovery and subsequent progression of these devices have relied heavily on the growing knowledge regarding various aspects of the human neuro-motor system, and the development of biomaterials and electronics technologies capable of interfacing with living tissues and organs at micro-and nano-scale. Improved in vivo stability, miniaturisation and the lower energy requirement of modern electronics has resulted in the development of a multitude of miniature wireless electronic devices, such as sensors, intelligent gastric and cardiac pacemakers, cochlear implants, implantable cardioverter defibrillators, and deep brain, nerve and bone stimulators that are currently being used in patients worldwide (Bolz, 2012; Boveda et al., 2013; Cheng and Tereshchenko, 2011; Majerus et al., 2012). Significant advances in semiconductor technology, particular in the area of micro-electro-mechanical systems (MEMS) and microfluidic lab-on-chip biomedical systems, have facilitated the development of modules for rapid diagnostics, and precisely controlled pulsatile, rapid or sustained delivery of drugs and biomolecules and complex therapeutics (Chirra and Desai, 2012; Lee et al., 2012; Lowrie et al., 2009; Pararas et al., 2012; Stevenson et al., 2012). These systems have also been used to create electrically inductive tissue engineering platforms and in regenerative medicine applications, where electrical and mechanical stimulations are essential for appropriate muscular and nervous tissues development (Godin et al., 2012; Millet et al., 2012).
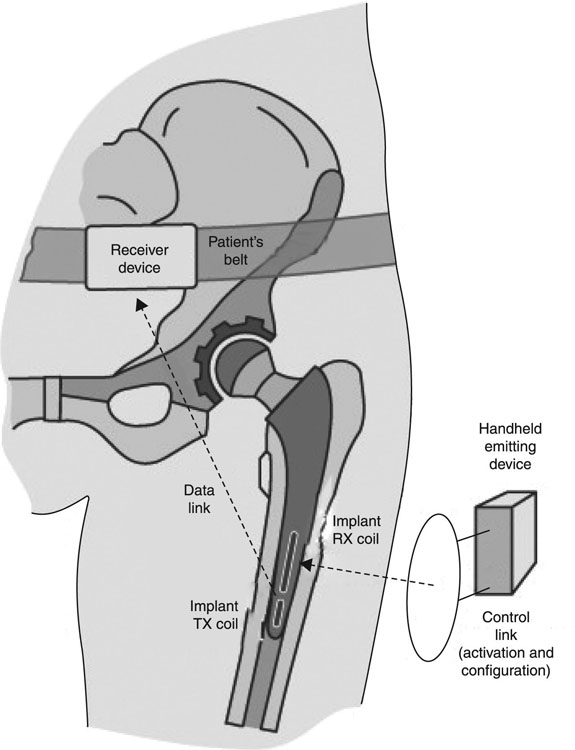
In addition to considerably improving the rate of survival and the quality of life of patients suffering from life-threatening and debilitating illnesses, implantable electronic systems have contributed significantly to our understanding of the biological processes taking place in vivo, particularly with regard to the complex mechanisms of neural communication and control. They have also allowed a greatly enhanced understanding of how these mechanisms are affected by various diseases and treatments. MEMS and dielectric elastomer actuators have been employed to investigate the manner in which eukaryotic and prokaryotic cells modulate their behaviour ex vivo, express genes, proliferate or differentiate in response to mechanical and electrical stimuli, information essential to enable effective tissue engineering (Akbari and Shea, 2012; Soon et al., 2013; Ting and Sniadecki, 2011).
In addition to playing a profound role in the advancement of restorative medicine and biomedical sciences, implantable information and communication technologies have driven notable changes in the social and cultural attitudes of people towards technology (Gasson et al., 2012), taking implantation beyond the medical context to being a means to enhance the abilities and experiences of healthy individuals. One example of a non-medical use of implants is the voluntary subcutaneous implantation of radio-frequency (RF) identification tags into humans to potentially prevent identity theft, help identify disaster victims and enable appropriate medical care to be given to people suffering from various illnesses, such as heart conditions or allergies to medication.
1.4.2 Device development and system requirements
Implantable electrical systems are used to collect biological data (sensing function), to induce a specific response (stimulation function), or to perform both functions in the form of a closed loop control. Fundamentally, these systems are comprised of two components: an external and an indwelling module, the latter of which can reside intracavity, for example within the intestinal, oral or urinary systems. They can be implanted subcutaneously or deep within tissue, or be located on the external surfaces of the body (de Haas et al., 2012; Fedele et al., 2008; Stanslaski et al., 2012). The external module receives data from the indwelling module. It can also transmit commands and/or power to the indwelling module. The implanted module can be fully electronic, or contain other chemical, biological or mechanical elements. When employed for sensing, the indwelling module (the sensor) detects, collects and translates the specific biological and physiological parameters into electrical signals. The interface electronics then modulate these signals so that they can be transferred via a coupling link to the external (receiver) module. For instance, a micro-accelerometer can be introduced directly onto the surface of the heart of a patient who has just undergone coronary artery bypass graft surgery to measure the heart wall motion. This assists in the early detection of surgery complications (Lowrie et al., 2009).
For stimulation applications, the external module wirelessly transmits commands to the indwelling module, where the interface electronic circuitry interprets the signals received to induce a range of electrical currents, which can then be delivered to various tissues and nervous structures via electrodes. For instance, diabetic gastroparesis can be treated by applying electrical stimulation to the antrum via two indwelling unipolar intramuscular leads and a neurostimulator, with the stimulation parameters able to be adjusted in a non-invasive manner (Guerci et al., 2012). In a closed loop system, sensing, stimulation, information transfer and information processing occurs internally, although external modules are still able to be employed for device interrogation and data collection. Such systems are used to maintain a specific level of function within the body, such as cardiac resynchronisation, to facilitate the automated provision of medical care and prevention of critical incidents, such as sudden cardiac death (Costa et al., 2010). Rate-responsive pacing is achieved through hemodynamic sensors that are integrated into implantable pacemakers, with the latter functioning on both sensed and paced ventricular beats, thus overcoming the need for permanent ventricular pacing (Occhetta et al., 2011). In neuromodulation, application of simultaneous sensing and stimulation enables the enhanced therapeutic treatment of neurological diseases by providing information on the instantaneous response of the neural system to stimuli (Stanslaski et al., 2012).
An optimal implantable electronic system exhibits a number of characteristics, namely low power consumption, good reliability, high data rate and data latency, minimal size and weight, high biocompatibility and minimal toxicity. Although the former three criteria lie in the domain of electronics engineering, the latter four rely on the ability to develop biomaterials possessing appropriate properties.
1.4.3 Device encapsulation
Bio-inert or biocompatible hard shell packaging is often used to protect the electronic circuitry contained within an implant from the harsh environment in which it will reside, while the remainder of the indwelling assembly is typically encapsulated into a soft protective layer (Vanhoestenberghe, 2009). The hermetic protective casing ensures the in vivo integrity and reliability of electronic performance of the devices over the life time of the implant under specific physiological conditions. The casing protects the device elements from the highly corrosive environment and limits the extent of current leakage flowing through the electrodes. Indeed, device reliability is paramount, as device malfunction may not only result in discomfort, pain, or local damage to the peri-implant space, but may in some cases result in the irreversible damage of adjacent tissues or even the death of the patient. Many implants are introduced deep into the tissues and cavities of the body, and hence device maintenance is inherently complicated and surgical intervention to correct any malfunctioning implant has its own risks. The encapsulation layer performs a biocompatibility function, protecting the host tissues from the potentially harmful elements of the device. Mechanically, the hard casing may provide physical support to devices that are submitted to a considerable load or strain during extension/flexion and wear, whereas the soft encapsulant may act as a low-iriction conditioning layer to enable a smooth integration within host tissues.
While a thicker protective layer may provide better device protection from the physiological environment and minimise the likelihood of undesirable and potentially dangerous contact between living tissues and the electronics, any soft and hard protective layer that is excessive in size and weight is not desirable (Merrill et al., 2005; Paralikar et al., 2011). Indeed, the encapsulation and power supply (battery) are the major contributors to the overall size and weight of the implantable electronic devices, with the dimensions of the electric circuitry components decreasing considerably with the advancements in MEMS and nanotechnology.
In a modern consumer-driven society, the design of an implantable device is not only driven by the medical requirements, but is also heavily influenced by the demands and preferences of the potential customers (e.g. surgeons, patients). From a surgical perspective, smaller and lighter devices are likely to be less invasive to the body of the patient during and after implantation, potentially leading to less pain and discomfort and faster healing to the host. Conversely, implants of excessive size and weight may adversely affect the healing process by placing excessive pressure on the tissues adjacent to the implant, tissues that would likely have already undergone damage as a result of surgery, and as such contribute to the inflammatory processes within the peri-implant space. From the patient’s perspective, smaller and lighter devices are less restrictive with regard to the normal level of human activity, and thus afford the potential for a better quality of life to the patients.
1.4.4 Electrode material
In addition to the encapsulation component of implants used for stimulation applications, great care should be taken in selecting the electrode material and structure being used in the device. Surgical placement, orientation and extraction of the electrodes are intricate processes, particularly where the neural system is concerned, making the revision of electrodes difficult (Butson and McIntyre, 2008; Trohman et al., 2004). If the electrodes are intended to be in close proximity to living tissue, restrictions are placed on the amount of power dissipation that occurs, since extensive dissipation may inflict damage onto these soft tissues (Merrill, 2011). In addition to thermally-induced damage (Opie et al., 2012), electrical stimulation-induced tissue injury (over-stimulation) and damage due to the electro-chemical products released into physiological medium as a result of electrode corrosion, are factors that require great attention (Merrill et al., 2005). Indeed, electrodes in these devices should be designed so that they inject a charge sufficient to elicit the desired response while minimising the level of products from irreversible Faradaic reactions.
These products can not only damage the surrounding tissue but also be detrimental to the electrode itself (Merrill et al., 2005; Merrill, 2011). For sensing applications, the difference in impedance that exists between the electrodes and the surrounding tissue reduces the ability of the device to detect neural signals, reducing the quantity and quality of the information being sensed. Micro-electrode impedance has been shown to play a key role in the monitoring of low amplitude and high-resolution extracellular neural signals, with changes in the impedance of the electrical interface being an indicator of long-term viability of the electrode (Prasad and Sanchez, 2012). The difference in impedance between the electrode and the adjacent tissue is not static; some studies have demonstrated that it increases with the length of implantation of the device. Thus, even those electrode configurations that perform adequately in acute testing may not necessarily show the same level and consistency of signal detection and capture under chronic implantation conditions (Polikov et al., 2005).
Recently, Prasad et al. (2012) reported the results of an in vivo study involving the implantation of polyimide insulated tungsten microwire arrays into the neural tissue of rats (Prasad and Sanchez, 2012). These authors determined that the first 2 to 3 weeks post-implantation represented the most dynamic stage in the chronic electrode life time, characterised by greater variations in the electrode impedance, functional electrode performance, and the structural changes occurring at the electrode recording tips. Extended periods of implantation were associated with further electrode recording site deterioration, insulation damage and recession of the recording surface. Similar outcomes were reported for intracortical micro-electrode arrays implanted into the pericruciate gyrus of cats, where the electrode–tissue interface changed daily over the first 1–2 weeks, then weekly for 1 to 2 months, after which time it stabilised (Liu et al., 1999).
In addition to the possibility that chemical and/or heating damage may occur to the tissues being stimulated or sensed, the mechanical tissue damage during the surgical insertion (acute trauma), long-ierm contact of micro-electrodes with electrically excitable tissues and micro-movements associated with electrode anchoring (chronic disturbance), provoke activation of cells associated with foreign body response (Freire et al., 2011) (Fig. 1.3). The mechanical mismatch that exists between the electrode material and living tissues has been shown to stimulate an inflammatory response, with factors such as proteoglycans and intermediate filaments involved in modulating the response to the compliant electrode material (Harris et al., 2011). In an effort to eliminate the foreign body, these cells liberate a host of chemical and biological factors into the peri-implant space, some of which are cytotoxic and neurotoxic factors that contribute to localised neuronal degeneration and cell death (Potter et al., 2012). Since these cells are not able to enzymatically break down the implanted electrode, the body initiates the formation of a thin layer of reactive glial tissue around the implant to segregate the foreign matter from the adjoining tissues (Hashemi et al., 2011; Turner et al., 1999). Such encapsulation limits the capacity of the electrode to capture signals, as it alters the diffusion properties of nervous tissue (making it less permissive) and increases impedance (Roitbak and Syková, 1999; Prasad and Sanchez, 2012), widens the distance between the electrode and its nearest target neurons (Liu et al., 1999), and generates an inhibitory environment for neurite extension, thus steering neural regeneration away from the electrodes (Bovolenta and Fernaud-Espinosa, 2000; Polikov et al., 2005).
Gliosis and enhanced formation of associated extracellular matrix molecules have been reported to influence molecule diffusion, and as such, neuronglia communication, ‘cross-talk’ between synapses, extrasynaptic volume transmission and tissue regeneration (Roitbak and Syková, 1999; Zamecnik et al., 2012). Even comparatively minute increases in the distance between the surface of the electrode and the tissue may greatly hinder the ability of the former to detect a signal. Potter et al. (2012) reported that for adequate sensing of the neuronal spikes and local field potentials, a distance of less than 50 μm between the neuronal ensembles and the target neurons is required. Local field potentials hold key information regarding functional behaviour of neural networks; their changes can be correlated to disease symptoms, thus establishing the role of local field potentials as biomarkers for disease detection (Stanslaski et al., 2012).
Various electrode treatments have been examined in order to improve the electrode-tissue integration and maintain the sensing and stimulation ability of the electrodes under chronic implantation conditions. For instance, Han et al. (2012) used mechanical shaping and deep reactive ion etching to reduce the insertion force of silicon-based multi-electrode arrays used for neural stimulation. The sensing and recording performance of the modified arrays were observed over time in vivo, with neuronal spike activity recorded up to 566 days after implantation. The prolonged implantation was reported to have minimal impact on the tissue architecture, as indicated by histopathology evaluation of neurons and astrocytes.
1.4.5 Power supply
Although much engineering effort has been devoted to the development and implementation of ultra-low power consuming implantable electronic devices, the power supply remains a major contributor to the overall weight and dimension of the indwelling systems. The in vivo life time of the device fitted with a single-use non-rechargeable battery is determined by the longevity and reliability of such a battery. The ability of the battery to supply power also influences the type of activities this device can support. Implants containing rechargeable batteries and battery-less devices can be powered wirelessly using an external power source. The non-rechargeable batteries are used in cardiac pacemakers and deep brain stimulators, and examples of the latter include cochlear implants and RFID tags, respectively (Schuettler and Stieglitz, 2012).
While wireless supply of power is an attractive option in terms of device miniaturisation ability, extending device longevity and supporting extended functionalities, it may not offer the same degree of reliability required to support life functions, as it relies on the availability of an external power source to operate. Only a portion of the magnetic field generated by the external module is able to reach the indwelling part of the system (Hannan et al., 2011; Schuettler and Stieglitz, 2012), with low wireless power transfer efficiency resulting in heating of the tissues between the external and internal modules, interference with other devices, and requiring a larger energy source to deliver sufficient power to the internal device (Gosselin, 2011; Jow and Ghovanloo, 2009; Ramrakhyani et al., 2011; Yakovlev et al., 2012). The efficiency of the power transfer has been demonstrated to depend on, among other factors, the addition of intermediate physical barriers, such as an encapsulation layer. These layers further attenuate the strength of the magnetic field reaching the indwelling component, which has already been reduced due to Foucault currents generated within the tissues (Pethig, 1987). Encapsulant conductivity and thickness have been demonstrated to be principle parameters that influence encapsulant-related field attenuation. However, devices utilising single-use batteries require surgical removal to replace those, which is not desirable.
Recent times have seen the development of alternative energy harvesting technologies embedded into the indwelling module itself and capable of charging internally from the energy produced by the physiological environment or natural body motion (Justin et al., 2004; Rapoport et al., 2012). Highly dense electro-active conjugated polymer brushes of poly(thiophene) and poly(phenylene) for in vivo power generation were developed by Sontag et al. (2009) using a surface-initiated Kumada-type polycondensation reaction. A power source for an anatomically sized, ultra-low quiescent-power energy harvester chip integrated with a wireless sensor capable of monitoring the ear electro-chemical gradient was reported by Mercier et al. (2012). The authors showed that energy extraction arose from the biologic battery in the inner ear, whereby the electro-chemical gradient within the ear was utilised to power the chip. In vivo study using a guinea pig model showed that the chip was capable of generating a minimum of 1.12 nW for up to 5 h, enabling a 2.4 GHz radio to transmit measurement of the electrochemical potential every 40 to 360 s.
Several research groups have investigated implantable fuel cells that generate energy via glucose oxidation. For instance, Rapoport et al. (2012) reported the development of a cell with the capacity to produce 3.4 μW cm−2 and up to 180 μW cm−2 steady-state power and peak power, respectively. Glucose fuel cells capable of simultaneous and independent oxidation and reduction have also been reported. Here, the mechanism of power generation involves glucose oxidation at the nanostructured surface of an activated platinum anode, and concomitant oxygen reduction to water at the surface of a self-assembled network of single-walled carbon nanotubes embedded in film that forms the cathode (Cinquin et al., 2010; Kerzenmacher et al., 2008). The reported half-opened geometry enabled the authors to minimise the potential for electro-chemical short circuits.
Although the currently available fuel cells may not yet be at the stage to fully support the power needs of brain–machine interfaces, the ongoing lowering of the energy consumption of the implantable electronics may all be facilitated in the future. Theoretical investigations have shown that glucose can be harvested from the cerebrospinal fluid to an energy level of more than 1 mW without negative physiological consequences. In addition to increasing the generated power density, the longevity and reliability of power generation are also essential for the utilisation of these biofuel cells in devices intended for long-term implantation. Zebda et al. (2011) recently reported biofuel cells with glucose oxidase and laccase enzymes mechanically integrated into a conductive pure carbon nanotube matrix (Fig. 1.4). The cells were able to generate high power density of up to 1.3 mW cm−2 and an open circuit voltage of 0.95 V, bringing the technology closer to the outputs required by low-power implantable electronic circuits.
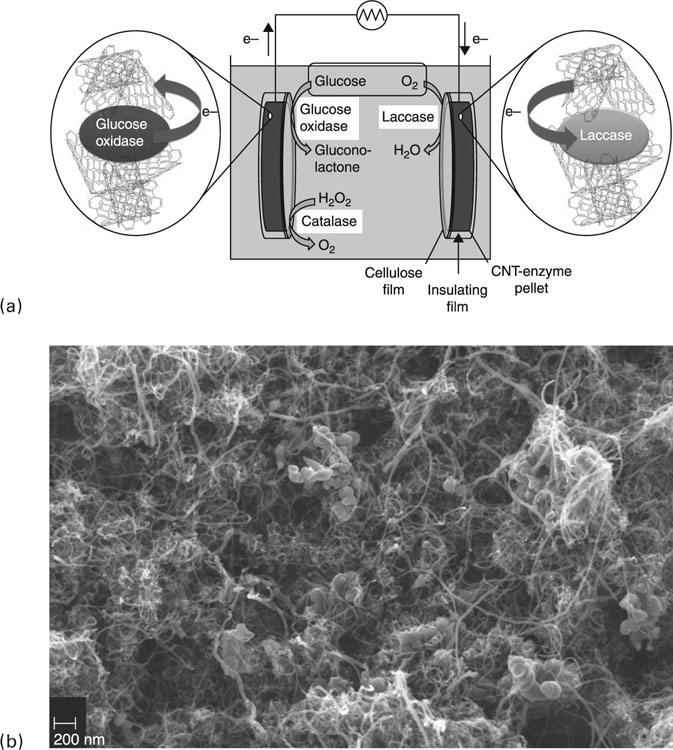
In vitro studies showed that under a physiological environment of 5 × 10−3 mol L−1 glucose and pH 7, the devices were able to reliably produce 1 mW cm−2 power density for 1 month. The authors further substantiated the potential of these devices to power implantable electronics by connecting two of these cells in series. Using this set-up, an open circuit voltage of 1.8 V with a maximum power of 3.25 mW at 1.2 V was attained. For comparison, most implanted electronics typically demand a minimum operating voltage of 0.5 to 0.6 V, while a cytochrome P450-based molecular biosensor used for drug sensing with temperature and pH monitoring was reported to require 48 μW, where 32 μW expended on the molecular detection, 2.5 μW on the pH measurement, 1.4 μW on the control over the temperature sensor, and 12 μW on the multiplexing and measurement reading (Carrara et al., 2010).
Another impediment for implantable electronics systems in general, and for alternative energy sources in particular, is the ability to achieve a similar level of performance in vivo to that obtained under i n vitro experimental conditions. Indeed, the in vivo performance of most enzymatic biofuel cells has been found to be considerably lower. As an example, under physiological conditions of 4.7 × 10−3 mol L−1 glucose and pH 7.2, an intravenous implantable glucose/dioxygen hybrid enzyme-Pt micro-biofuel cell was demonstrated to have high electrocatalytic performance, characterised by an open circuit voltage of 0.4 V and a maximum output power of 0.2 mW cm−2 at 0.25 V (Ferreira et al., 2013). However, upon implantation into the jugular vein of a living rat, the device was only able to achieve an open circuit voltage of 125 mV at a maximum power density of 100 μW cm−2 at 80 mV Moreover, use of enzymes for power generation potentially limits the length of in vivo performance, with enzyme degradation leading to loss in the power generated with time (Olivo et al., 2011).
1.5 Device-associated infections
In addition to biocompatibility, the reliability of implantable electronic systems is influenced by the tendency of the implanted constructs to become infected over time. The surgical introduction of the implant, often deep into tissue, may deliver infection agents into the peri-implant area. Since some of the implant recipients may have other medical conditions or weakened immunity, the immune response to these infection agents may not be sufficient to eliminate them from the site and hinder the development of implant-associated infections (Nagpal et al., 2012). Patient-specific factors, including diabetes mellitus and long-term anti-inflammatory medication of the patient using corticosteroids and other immunosuppressive drugs, may slow down surgical site healing and patient recovery, making the host more susceptible to developing an infection (Dababneh and Sohail, 2011). Although peri- and post-operative contaminations are amongst the most common causes for biomaterial-associated infections, the pathogens can also originate elsewhere in the body, spreading to the implant site via blood to initiate late haemotogenous infection (Busscher et al., 2009; Subbiahdoss et al., 2009).
Potential sources of infectious agents include the implanted central venous catheter used for haemodialysis or other long-term access, a distant focus of primary infection, for example pneumonia, skin and soft tissue infections, and invasive procedures unrelated to the implanted device, such as dental work (Bloom et al., 2006; Le et al., 2011). This route of infection is particularly relevant to the implants that are exposed to the blood stream (Hanssen, 2002; Montanaro et al., 2007). The complications of biomaterial-related infections may vary from being painful and requiring localised antibiotic therapy to those where complete removal of the infected device and systemic antimicrobial therapy are required (Trohman et al., 2004). If not addressed, these infections may lead to septicemia, a potentially lethal complication that often leads to further organ infections.
Trohman et al. (2004) reported that around 1 to 19% of cardiac pacemaker implants can become infected, with laboratory handling and surgery-related contaminations accounting for 7 to 8% of those incidences. In terms of infectious agents, approximately 68% of the pathogens implicated in infections of anti-arrhythmic devices were coagulase-negative staphylococci, with an additional 23% being Staphylococcus aureus, and 13% comprising multipathogen infections (Chua et al., 2000; Deresinski, 2010; Rohacek et al., 2010). Local pain and pocket erythema were the typical symptoms associated with infected anti-arrhythmic devices; however, it is not uncommon for the infections associated with implantable cardiac devices to remain undetected for extended periods of time, or even for the duration of the implantation (Deresinski, 2010).
Late discovery and removal of the contaminated implanted and/or external modules may be of considerable detriment to patient recovery (Deharo et al., 2012). Furthermore, in order to re-instate the implant, the patient may have to undergo additional treatment to control the infected and/or inflamed peri-implant space prior to re-implantation (Gandhi et al., 2012). Even after successful treatment, an alternative site for re-implantation may need to be sought as the status of the previous tissue may not be satisfactory to attain successful healing. Continual infections, especially originating from non-retrieval of infected modules from the patient’s body, are associated with a significant morbidity rate of over 60% (Pavia and Wilkoff, 2001; Sohail et al., 2007).
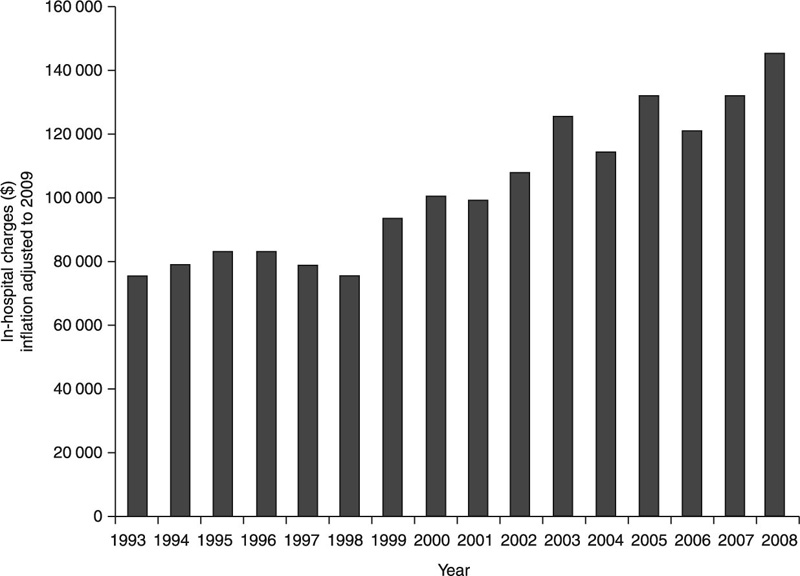
The cost attributed to medical and surgical treatments of an infection around the implantable cardiac electronic device varies from $25 000 for permanent pacemakers to $50 000 for implantable cardioverter-defibrillators (Dababneh and Sohail, 2011) (Fig. 1.5). Being abiotic in nature, the implant surface is unable to act in response to being colonised: it can neither kill pathogen organisms nor signal the adjacent tissues of the forthcoming threat. Since attributes of most implanted surfaces leave them susceptible to cell colonisation, designing an encapsulation material with the ability to hinder initial stages of bacterial attachment may be used to minimise the rate of implant-associated infections. Other encapsulants have been designed to not only prevent bacterial cell attachment but to address those organisms that manage to adhere to the surface of the implant. Many pathogenic organisms are known to pre-condition an unfavourable colonisation substrate by producing a wide range of extracellular substances. In addition to helping the bacterial cells to attach, these extracellular substances are involved in the formation of 3-D polymer frameworks, a fundamental structure of the biofilm. The biofilm acts as a protective habitat for the microorganisms, providing a physical barrier against mechanical detachment and predation and acting as a filter to control chemical and biological agents that can reach cells. Not surprisingly then, bacterial cells residing in a biofilm are less susceptible to drug treatments or host immune activity.
Encapsulation materials that combine bacterial cell-repelling properties with the capacity to eradicate those pathogens that do attach are highly desired. Responsive elution of drugs or any other type of antimicrobial activity is preferred, since it minimises the unnecessary exposure of the adjacent tissues to the treatment. Surface functionalisation, both in terms of chemico-physical modification and fabrication of physical structure for drug or molecule loading, are frequently considered, since they provide an avenue for use of existing commercially available, clinically tested biomaterials (Bazaka et al., 2010, 2011a). Amongst the antimicrobial agents, traditional and novel antibiotics, as well as a wide host of alternative antimicrobial agents, for example silver ions, nitric oxide, bioactive antibodies and other bactericidal compounds, have been reported. Clinically, enhanced hygiene during operative and post-operative procedures and administration of prophylactic antibiotic supplemented with the use of an AIGISRx antibacterial envelope (TYRX Pharma, Inc., Monmouth Junction, NJ) resulted in improved implantation outcomes. The envelope comprises a polypropylene mesh impregnated with minocycline and rifampin, and upon implantation along with a cardiac electronic device, has been reported to progressively release these agents into the generator pocket (Jordan and Bloom, 2010). Externally, systems such as arglaes wound dressing (Medline Industries, Inc., Mundelein, IL), Silverlon CA (Argentum Medical, Chicago, IL), Aquacel Ag (Conva Tec USA, Skillman, NJ) and Silvercel (Systagenix, Quincy, MA) have been used to improve healing at the site of surgical incision. These dressings prevent bacterial site infections via a continuous release of silver ions into the wound space.
1.6 Current trends in biomaterials design and fabrication
Replacement of lost function or tissue with well-matched biomaterials remains an area of active research and development; however, recent times have seen a heightened interest in areas related to tissue and function regeneration. The following chapters will discuss a range of polymer, ceramic and metals that are intended to enable and support adequate cell, tissue and organ regeneration and restoration of function. These materials have a pre-defined lifespan in vivo and a highly controlled degradation profile, which allows them to provide the necessary support for cell matrix development, and then safely disintegrate into the peri-implant space, freeing up the space for the growing tissue. Furthermore, temporal control over the in vivo material degradation allows limited release of by-products into the host systems, thus limiting potentially harmful consequences of biomaterial breakdown. Equally important, such biomaterials can be impregnated with various chemical and biological molecules that induce, promote and support tissue regeneration, suppress pro-inflammatory response or bacterial colonisation, or provide building blocks for the growing tissue.
The interest in temporary biodegradable structures is not restricted to biomaterials. As was the case with most biomaterials, for many years engineers and scientists worked towards enhancing the stability and long-term performance of all implantable electronic devices when operating in vivo. Recently, however, there has been a strong interest in the development of fully resorbable electronic systems. These systems are specifically designed to remain stable for a pre-defined length of time, during which the implanted device will perform its sensing or stimulation function. Resorbable devices are particularly useful for providing a temporary physical framework and stimulation to enable tissue restoration, particularly those tissue types that require electrical stimulation to induce correct cell differentiation, medical diagnostics, and accurate spacio-temporal delivery of drugs and other molecules. Once the task is completed, the implantable device will break down under the influence of the physiological environment in which the implant resides. The key competencies this technology aims to achieve include sound performance of the electronics and device reliability over the intended time of operation, precisely controlled degradation onset and kinetics, and cyto- and tissue-compatibility of the degradation of by-products.
This is not an easy task, since most of the materials used in conventional electronic devices may not be biodegradable or may break down into toxic or irritant agents. However, the use of biocompatible and biodegradable materials may not deliver an adequate electronic performance. Recently, Hwang et al. (2012) reported fabrication of transient silicon-based electronic devices on biodegradable silk substrates; the devices were reported to have tunable electrical properties and a controlled degradation profile in vitro. The devices were proposed as a bioresorbable tool for non-antibiotic thermal therapy to control surgical site infection and were fabricated using Mg-based inductive coils, resistive doped Si NMs microheaters and a silk-based substrate and packaging.
