Layered double hydroxides (LDHs) as functional fillers in polymer nanocomposites
Abstract:
Layered double hydroxides (LDHs) are lamellar inorganic solids with a brucite-like structure similar to hydrotalcite, where the partial substitution of trivalent for divalent cations results in a positive sheet charge, compensated by anions situated in the interlayer galleries. Their layered structure enables the intercalation of polymer macromolecules, in some cases resulting in the exfoliation of their sheets, and hence in the formation of polymer nanocomposites. This chapter reviews the state-of-the-art preparation of hybrid LDHs and incorporation into polymers, the structure of these nanocomposites and the resulting properties: rheological, mechanical, and thermal. Particular attention is given to their possible applications and future trends.
4.1 Introduction: the role of layered double hydroxides (LDHs) as reinforcements
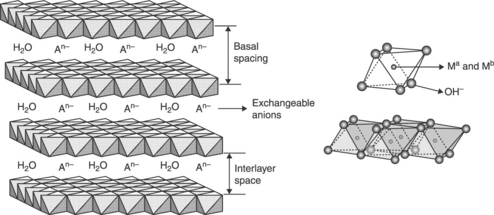
Layered double hydroxides (LDHs) are a family of lamellar compounds containing exchangeable anions in the interlayer space. For this reason, they are also known as anionic clays. Their structure consists of brucite-like sheets with a typical thickness of 0.5 nm, in which partial substitution of trivalent for divalent metallic ions results in a positive charge, compensated by anions situated within the interlayer space (see Fig. 4.1). The general formula of LDHs is (M2 +1-x M3 +x (OH)2)(An−x/n•mH2O), where M2 + and M3 + are divalent and trivalent metal cations, respectively, which occupy octahedral positions in the hydroxide layers, and An− is an interlayer anion. The layers are based on octahedral M(OH)6 units, where the metal (M) is coordinated by six hydroxyl groups (OH), hence forming M(OH)2 brucite-like sheets. As shown in Fig. 4.1, the centers of the octahedral units are occupied by divalent and trivalent metal cations and the vertices by the hydroxyl anions (Rives, 2001, 2002).
Typically, the metal ions have ionic radii similar to Mg2 + (0.65 Ǻ). Combinations of divalent cations like Mg2 +, Ni2 +, Zn2 +, Co2 +, Cu2 +, or Cd2 +, and trivalent cations such as Al3 +, Cr*+, Fe3 +, Mn3 +, or Ga3 +, are usual. The most common interlayer anions are inorganic in nature, such as carbonates, chlorides, nitrates, and sulfates. The most used LDH is hydrotalcite, where the constituents of the layers are magnesium and aluminum and the interlayer anions are mainly carbonates. Its general formula is (Mg2 +1−x Al3 +x (OH)2)(CO32−x/n •mH2O) (0.2 < x < 0.33) (Rives, 2002).
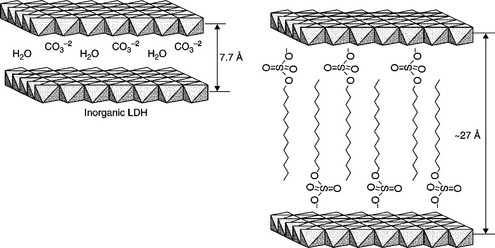
In order to prepare hybrid LDHs, the interlayer anions are exchanged by organic ones with a larger volume. In the literature, several works have considered the preparation of a great variety of LDH hybrids. Generally speaking, hydrocarbonated sulfate and sulfonate anions lead to a high expanded crystalline structure along the c axis, i.e., with a higher interlayer space, as illustrated in Fig. 4.2. Specifically, LDH hybrids have been made with expanded interlayer spaces from 21.1 Å (C8) to 32.6 Å (C18) (Newman and Jones, 1998; Trujillano et al., 2002).
The role of LDHs as functional or structural fillers in polymers lies in combining, in an adequate manner, different aspects of their composition, dispersion efficiency, distribution, and orientation.
As expected when preparing a nanocomposite, LDH concentrations should be rather low. Typical LDH weight concentrations are below 10 wt.%, of which an important part (around 50 %) corresponds to the organic part of the prepared hybrid.
Concerning the nature of the metal cations, although most of the studies use LDHs containing MgAl or ZnAl, other cations can promote interesting properties. For instance, Manzi-Nshuti et al. (2008b) studied the effect of NiAl–LDH ZnAl and CoAl–LDH on the mechanical properties and flame retardancy of poly(methyl methacrylate) (PMMA), and found high efficiency with these LDHs; Peng et al. (2009) used CoAl–LDH in polyamide 6 (PA6) to prepare PA6 nanocomposites. Wang et al. (2008) found that cobalt chelate or the respective compound showed better flame retardancy on polyethylene than other types of metal chelates or metal chelate compounds.
The interlayer anions play a double role. On one hand, they contribute in expanding the layered structure of the LDHs, increasing their interlayer distance; on the other hand, they promote compatibilization with the polymer matrix. A careful selection of the interlayer anion may allow the preparation of hybrids, which, depending on the nature of the selected anion, may add functionality to the nanocomposite.
The dispersion of the LDH nanoparticles in the polymer matrix is mainly done through dissolution or melt-blending. In order to achieve a high dispersion and hence a high efficiency of the LDH nanoparticles, the local strains that have to be applied during this step must exceed the cohesive forces that keep the hybrid platelets together. Of these two methods, melt-blending is the less effective due to the high viscosity of the matrix at typical processing temperatures. High dispersive mixing devices are required, although the degree of dispersion is not optimal. Generally speaking, the morphology is a combination of crystalline aggregates (known as tactoids), groups of a couple of platelets, and individual platelets. Due to the expanded interlayer structure of hybrid LDHs, as well as its compatibility, polymer molecular intercalation is favored, although full exfoliation is rather difficult to attain. LDH layer morphology induces a high anisotropy in the prepared nanocomposites. During molding, the platelets or aggregates tend to orientate preferentially along the flow direction. Likewise, a certain inhomogeneity is commonly observed in terms of particle distribution.
The combination of all these factors plays a key role in the use of LDHs as functional or structural fillers in polymer nanocomposites.
4.2 Preparation of hybrid LDHs for polymer nanocomposites
The preparation of hybrid LDHs is commonly made by one of the following three routes (Newman and Jones, 1998): direct synthesis, anionic exchange, and reconstitution (rehydration).
4.2.1 Direct synthesis
Direct synthesis by co-precipitation is the most common method used to prepare LDHs with inorganic anions and it can be used to synthesize hybrid LDHs. This method consists of the addition of the desired anion to a solution containing metal salts of the ions that will form the layers (usually chloride or nitrate salts). Control of the pH prevents co-precipitation of other phases, such as oxide impurities from the metals. To avoid the incorporation of metal salt anions used in the synthesis, it is important that the organic anions have a good affinity for the hydroxide layers. The main advantages of this method are the control of the charge density of the layers (M2 + to M3 + ratio) and the high purity of the synthesized LDHs.
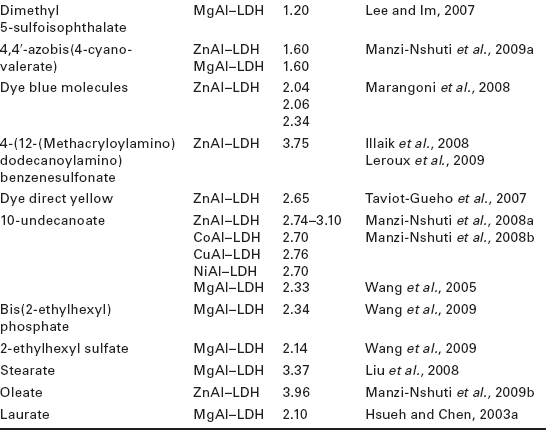
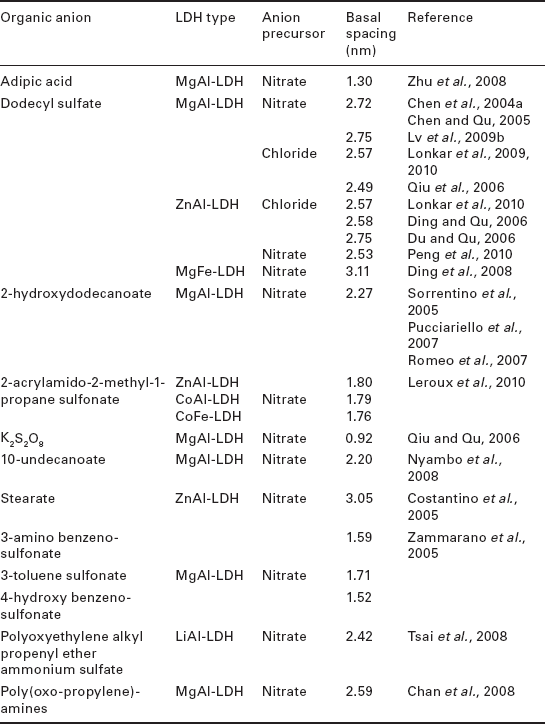
As can be seen from Table 4.1, a wide variety of surfactant anions have been used in this method, leading to variable results in terms of structural modification efficiency. For instance, in the case of MgAl–LDHs, the best results concerning the interlayer distance have been found for hybrids with stearate anions (Liu et al., 2008) (3.37 nm interlayer distance), dodecylbenzenesulfonate (Zammarano et al., 2006; Wang et al., 2009) (interlayer distances from 2.76 to 3.15 nm) and dodecyl sulfate (Du et al., 2007; Kuila et al., 2007; Zhang et al., 2007; Martínez-Gallegos et al., 2008; Bao et al, 2006, 2008b; Liu et al, 2007, 2008; Kotal et al., 2009) (interlayer distances between 2.59 and 2.75 nm). In contrast, no significant effects were observed when using borate-like anions, dimethyl 5-sulfoisophthalate, benzoate, or adipic acid, with interlayer distances below 1.6 nm.
With ZnAl–LDH, significant increases of the interlayer distance were observed using oleate (Manzi-Nshuti et al., 2009b) (3.96 nm), 4-(12-(methacryloylamino) dodecanoylamino) benzenesulfonate (Illaik et al., 2008; Leroux et al., 2009) (3.75 nm), and dodecyl sulfate (Zhang et al., 2008a; Chen et al., 2004b) (between 2.54 and 2.85 nm). Borate (Nyambo et al., 2009a) and benzoate (He et al., 2006) anions produced the least remarkable results of all works presented in Table 4.1.
4.2.2 Anionic exchange
This procedure is very simple and consists of the dispersion of an LDH precursor in a solution containing an excess of the organic anions to be incorporated. Prior to choosing the LDH precursor, it is important to take into account the affinity of the anions with the LDH structure. Hence, the biggest difficulty lies in the carbonate anions, due to the high affinity of the layers for small divalent anions, followed by sulfate anions. On the other hand, among the most frequent monovalent anions present in LDHs, hydroxide shows the highest interchange difficulty, followed by chloride, fluoride, bromine, nitrate, and iodide (Miyata and Kumura, 1973).
This procedure was first reported by Miyata and Kumura (1973) and used to prepare an LDH hybrid with acetate anions. Meyn et al. (1990) also used ionic exchange to prepare hybrids using different organic anions.
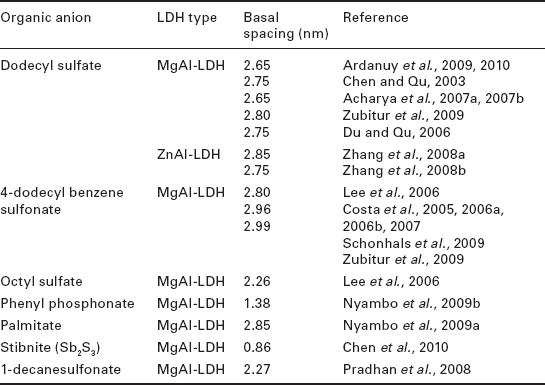
Since then, many researchers have prepared hybrids with a wide variety of organic molecules when making LDH polymer nanocomposites, as shown in Table 4.2.
4.2.3 Hydration and reconstitution from oxides
Miyata (1980) reported that layered double hydroxides, after being transformed to oxides by calcination at temperatures between 500 and 800 °C, are able to rehydrate to their original form in the presence of anions and water, i.e., that LDHs are able to regenerate after calcination. This ability, known as a ‘memory effect,’ may be used to prepare hybrids using different anions in a rather easy way.
A wide variety of LDHs have been prepared with inorganic anions such as carbonates (Hibino and Tsunashima, 1998), organic-like naphthalene carboxylates (Tagaya et al., 1993), or carboxylates (Dimotakis and Pinnavaia, 1990) using this method. Regardless of the preparation method, the main problem when preparing high-purity hybrids lies in their high affinity for carbonates, which requires specific conditions in order to avoid the presence of this anion. For this reason, it is essential to use water free of carbonates (bi-distilled water is normally used) and an inert atmosphere (nitrogen or other inert gases are conventionally used).
As shown in Table 4.3, this method has been also used for the preparation of hybrids with a wide variety of organic molecules.
There are a few publications that compare the procedures described previously or in which a combination of more than one method is used to prepare a specific hybrid. One of the groundbreaking studies was Ulibarri et al. (1994), which compared anionic exchange and reconstitution after calcination for preparing LDH hybrids using vanadate anions. Trujillano et al. (2002) compared anionic exchange and direct synthesis for preparing nickel and aluminum hybrids with alkyl sulfate or sulfonate anions. Finally, a recent work (Dupin et al., 2004) compared the three methods previously mentioned (anionic exchange, direct synthesis, and reconstitution after calcination) in the preparation of magnesium and aluminum LDHs using dichlorophenyl amino phenyl acetate.
4.3 Nanocomposite preparation routes
The successful preparation of nanocomposites with lamellar nano-sized particles depends on the rupture of their primary structure (exfoliation of the layers) or the intercalation of polymer molecules between the platelets (intercalation of the layers), as well as on their homogeneous dispersion in the polymer matrix. Moreover, it is important to achieve good interaction (compatibilization) between the polymer matrix and the layers (Pinnavaia and Beall, 2001).
Several strategies may be used to prepare polymer-layered double hydroxide nanocomposites. The most commonly used are in situ polymerization, melt-mixing, and solution blending (Alexandre, 2002).
4.3.1 In situ polymerization
With in situ polymerization, the LDH hybrid is first mixed with a solution of the monomer or with the liquid monomer to allow intercalation into the interlayer gallery. Second, a catalyst is added to the solution and the monomer is polymerized in situ. The polymerization of the monomer in the interlayer gallery leads to rupture of the lamellar structure and promotes the homogeneous dispersion of the layers (see Fig. 4.3).
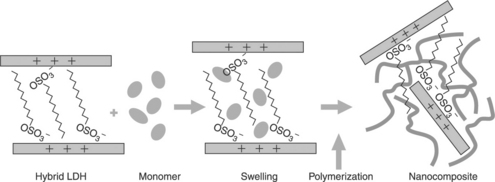
4.3 In situ polymerization (adapted from Kornmann, 2001)
This method was the first reported in the literature to prepare a polymer-clay nanocomposite (Okada et al., 1990). It is conventionally used to prepare nanocomposites based on polar polymers or thermoset resins. In the latter, the resins are cured in the presence of the layered particles. Nonetheless, it is not the usual method for preparing nanocomposites based on commodity thermoplastics such as polyolefins.
The main advantage of this method is that it promotes high exfoliation of the layered particles. However, it also has drawbacks, as it is a somewhat complex process and is rather industrially unfeasible for commodity polymers.
This method has been used successfully to prepare LDH nanocomposites based on PA6.6 (Herrero et al., 2010; Zhu et al., 2008), poly(ethylene terephthalate) (Lee and Im, 2007; Martínez-Gallegos et al., 2008), polystyrene (PS) (Leroux et al., 2005, 2010; Manzi-Nshuti et al., 2008b, 2009b; Qiu and Qu, 2006; Ding and Qu, 2006; Marangoni et al., 2008; Illaik et al., 2008; Taviot-Gueho et al., 2007; Matusinovic et al., 2009), poly(methyl methacrylate) (Nyambo et al., 2008; Wang et al., 2005, 2006, 2009; Chen et al., 2004a; Chen and Qu, 2005; Ding et al., 2008), poly(vinyl chloride) (Bao et al., 2006, 2008b), epoxy (Hsueh and Chen, 2003a; Zammarano et al., 2005; Tseng et al., 2007; Tsai et al., 2008; Chan et al., 2008; Lv et al., 2009a), and polyimide (Hsueh and Chen, 2003b), among others.
4.3.2 Melt-mixing
The melt-mixing preparation of polymer-LDH nanocomposites consists of the dispersion of a LDH hybrid in a polymer matrix by applying high local shear stresses and using rather high temperatures in a melt-mixer dispositive, such as a twin-screw extruder or internal mixer (Fig. 4.4).
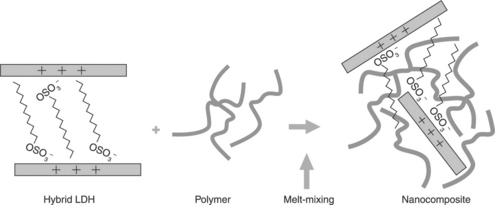
4.4 Melt-mixing (adapted from Kornmann, 2001)
With this procedure it is possible to prepare a wide range of nanocomposites with intercalated or partially exfoliated structures, depending on the degree of intercalation of the polymer molecules in the interlayer gallery of the layered particles. It also has important advantages over other methods. First of all, from an environmental point of view, it does not require the use of organic solvents, and second, it is compatible with common plastic-processing technologies such as extrusion or injection.
The first report of this method to prepare polymer nanocomposites with cationic clays was by Giannelis (1996). Since then, it has been widely used due to its versatility. Gopakumar et al. (2002) suggest that, in the case of non-polar polymers, in order to attain good dispersion and partial exfoliation of the platelets, it is necessary, besides prior organophilization of the layered particles, to add a third component to act as a compatibilizer between the polymer and the particles. This compatibilizer needs polar groups, which should interact with the surface of the inorganic particles, promoting, alongside the high shear stresses generated during mixing, their exfoliation. In the case of polypropylene (PP), the most commonly used compatibilizer is a polypropylene copolymer with grafted groups of maleic anhydride (PP-g-MAH).
One of the first studies published describing melt-mixing was the work of Costa et al. (2005), which described the preparation and characterization of nanocomposites of low-density polyethylene (LDPE) modified with a polyethylene copolymer with methacrylic acid and hydrotalcite hybrids, prepared using dodecylbenzene sulfonate anions. Though total exfoliation of the LDH layers in the polymer was not achieved, significant changes were observed in its viscoelastic response compared with the pure polymer, even at very low LDH concentrations. It is the most widely used method nowadays. Alongside the polymers used to prepare LDH nanocomposites by means of in situ polymerization, melt-mixing has been employed with different results for many more polymers. For instance, different nanocomposites have been prepared with PA6 (Du et al., 2007; Zammarano et al.. 2006), ethylene vinyl acetate (EVA) copolymer (Du et al., 2006; Du and Qu, 2007; Zhang et al., 2007, 2008a; Nyambo and Wilkie, 2009; Nyambo et al., 2009b), poly(ε-caprolactone) (Pucciariello et al., 2007), poly(ethylene terephthalate) (Lee et al., 2006), poly(methyl methacrylate) (Manzi-Nshuti et al., 2008b; Nyambo et al., 2008, 2009a; Wang et al., 2009), poly(vinyl chloride) (Bao et al.. 2006; Chen et al.. 2010; Xu et al.. 2006), polypropylene (Ardanuy et al., 2008, 2010; Lonkar et al., 2009; Zhang et al., 2008b; Wang et al., 2010; Ding and Qu, 2006; Shi et al.. 2010), polyethylene (Manzi-Nshuti et al., 2009b; Costa et al., 2005, 2006a, 2006b, 2007; Schonhals et al., 2009; Ardanuy et al., 2009; Costantino et al., 2005; Du and Qu, 2006), among others.
4.3.3 Solution blending
With solution, the pre-expanded layered particles are dispersed in a polymer solution to promote the entry of the polymer molecules into the interlayer gallery. The remaining solvent is evaporated afterwards, resulting in the precipitation of the polymer incorporated between the inorganic composite platelets (see Fig. 4.5).
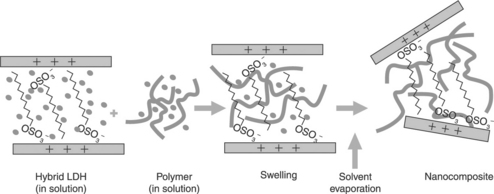
4.5 Solution blending (adapted from Kornmann, 2001)
Though this procedure is quite simple, in practice it is rather complicated to find a solvent that is able to completely dissolve the polymer and fully disperse the layered particles. This method is suitable for the intercalation of polymers with a low or null polarity, facilitating the preparation of thin films with a high orientation of the inorganic particles. However, from an environmental point of view, this procedure has the inconvenience of using organic solvents, which may also be quite expensive.
In the literature, this technique has been described in the preparation of nanocomposites from water-soluble polymers such as poly(ethylene oxide) or polyvinyl alcohol (Ramaraj et al., 2010), as well as the preparation of nanocomposites in non-polar solvents like toluene (EVA: Kuila et al., 2007; PS: He et al., 2006), xylene (EVA: Ramaraj and Yoon, 2008; Zhang et al., 2008a; Kuila et al., 2008; PS: Qiu et al., 2005), linear low-density polyethylene (LLDPE): Qiu et al., 2006; Chen and Qu, 2003; Chen et al., 2004b), tetrahydrofuran (poly(vinyl chloride) (PVC): (Liu et al., 2007, 2008), or dimethylformamide (poly(vinylidene fluoride): Bao et al., 2008a), among others.
4.3.4 Others
A new procedure for preparing poly(ε-caprolactone)–LDH nanocomposites, high-energy ball milling (HEBM), was proposed by Sorrentino et al. (2005) and successfully used by other authors like Bugatti et al. (2010) and Romeo et al. (2007). This method consists in mixing the LDH particles (in this specific case a hydroxydecanoate-hybrid) with the polymer, both in powder form. Through an intensive mixing it is possible to break down the powder particles, causing the diffusion of the atoms and creating an intimate mix that results in the exfoliation of the LDH particles in the polymer. In this work, the dynamic-mechanical-thermal properties of the nanocomposites were better over the whole temperature range, with an increase of the storage modulus and tan δ decrease.
4.4 Structure of polymer-LDH nanocomposites
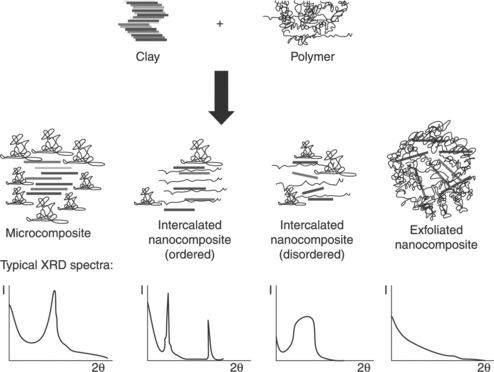
In order to obtain a higher efficiency of LDH, the process used to prepare the nanocomposites should ideally result in the rupture of the pilled structure of the particles, leading to a good dispersion of the layers (Fig. 4.6). Thus, there will be an increase in the contact surface with the matrix, improving the possible mechanical reinforcement efficiency of the particles by going from micro-sized particles to nano-sized ones. Nonetheless, it is not always possible to achieve full exfoliation of the particles in the polymer matrix. A microcomposite morphology such as the one displayed in Fig. 4.6 could occur, in which the layered particles keep their primary structure while mixed with the polymer; also possible is an intercalated morphology, in which the polymer molecules are between the platelets, thus increasing the contact surface between the particles and the matrix; finally, it is possible to obtain hybrid structures where fully exfoliated particle layers coexist with intercalated polymer-particle structures.
Among the several techniques used to elucidate the structure of silicate layered polymer nanocomposites, X-ray diffraction (XRD) and transmission electron microscopy (TEM) are the most extensive for evaluating the degree of exfoliation (Morgan and Gilman, 2003). XRD is used to determine the position, shape, and intensity of the peaks corresponding to the diffraction planes of the layered particles. More specifically, the first diffraction peak is commonly taken as a reference, due to its high intensity and to the fact that it is the one used to determine the interlayer distance. In the case where the primary structure of the layered composite is unaltered (immiscible mixtures, leading to the formation of microcomposites), so does the first diffraction peak (having the same position as in the pure particle) (see Fig. 4.6). For intercalated structures, the peak corresponding to the first diffraction plane appears at a lower angle because of an increment of the interlayer distance as a consequence of the presence of polymer molecules in the interlayer gallery. Last but not least, the total exfoliation of the particles leads to the disappearance of this diffraction peak (Vaia, 2001). However, XRD only detects a periodic order of the layers; thus it is important to complement the XRD measurements with TEM observations, which give the distribution of the particles in the matrix.
The exfoliation of the LDH particles in the matrix depends on several factors, such as the chemical nature and concentration of the cations in the inorganic layers, the interlayer distance between the layers, as well as the nature of the interlayer anions, the particle concentration in the composites, the preparation method, the nature of the polymer matrix, and the use of compatibilizing agents, among others.
The ionic exchange capacity of the layered particles is determined by the density charge of its layers. Comparing the residual charge of layered double hydroxides with that of cationic clays such as montmorillonite, the first have a much higher ionic interchange capacity and hence a higher density charge per unit area. Thus, using an LDH with a lower ionic exchange capacity should promote the formation of a nanocomposite, since particle exfoliation should be promoted due to lower attraction between the layers. For this reason, Zammarano et al. (2006) analyzed the effect of the density charge of LDHs in composites with a 5 wt.% of MgAl–LDH hybrids prepared with two different Mg/Al ratios (3:1 and 6:1). These authors found that, for complete exfoliation of the particles, it was necessary to use low anionic-exchange-capacity (AEC) LDHs combined with a mixing system with a minimum applied shear stress. Exfoliated PA6−LDH nanocomposites can be prepared by melt-mixing when suitable modified LDHs with appropriate AEC values have been synthesized. Shear, together with the inner ionic exchange capacity of LDHs, seems to be a key factor for the delamination of LDH in PA6.
On the other hand, it is also possible to regulate exfoliation through the type of cations present in the layers. For instance, Lonkar et al. (2010) have compared different PP composites with a concentration of 5 and 10 wt.% of LDHs with layers formed by combinations of MgAl, MgZnAl, and ZnAl cations, and DS as the interlayer anion. The PP−LDH composites were prepared in two steps using a co-rotating twin-screw extruder and PP-g-MAH was used as compatibilizer. The authors found that partial exfoliation was achieved for all combinations, though the rates of oxidation of the composites were influenced by the composition of the layers.
Another way to facilitate and promote the exfoliation of the particles without modifying the density charge of the layers consists in separating them until the attraction forces decrease through the incorporation of a highly voluminous organic anion. For example, Romeo et al. (2007) reported that for MgAl/LDH–poly(ε-caprolactone) composites a partial exfoliation is only reached when using an LDH hybrid with 12-hydroxydecanoate. Tseng et al. (2007) compared the structure of epoxy nanocomposites with LDH–amino benzoate and LDH–carbonate particles. In the former, no diffraction peak was observed for contents between 1 and 7 wt.%. This is because the LDHs nanolayers can be sufficiently exfoliated in the epoxy matrix to form exfoliated nanocomposites. In contrast, characteristic diffraction peaks were observed for all LDH−carbonate/epoxy nanocomposites. For these reasons, LDH−amino benzoate/epoxy nanocomposites exhibit important thermal and mechanical improvements compared with the pristine epoxy resin. In contrast, LDH−carbonate/epoxy nanocomposites show only a small enhancement because of their intercalated morphology.
On the other hand, it is also important to take into account the type of organic anion used. Bugatti et al. (2010) compared poly(ε-caprolactone) composites with ZnAl–LDHs prepared using different anions (benzoate (Bz), 2,4-dichlorobenzoate (BzDC), para-hydroxybenzoate (p-BzOH), and ortho-hydroxybenzoate (o-BzOH) anions) by the high-energy ball milling. They found that both the nature and position of the aromatic ring substituent affect the value of the interlayer distance and the hydrogen bonds of the hybrids. X-ray diffraction analysis of all the composites indicated that LDHs containing the BzDC anion were mainly exfoliated in the polymer matrix, whereas those containing p-BzOH remained almost unchanged, resulting in microcomposites. A partially exfoliated/partially intercalated structure was found for LDH modified with Bz and o-BzOH anions.
Lee et al. (2006) compared the structure of polyethylene terephthalate (PET) composites with LDHs modified using different anions, dodecyl sulfate (DS), dodecylbenzene sulfonate (DBS), and octyl sulfate (OS), prepared by melt-mixing. XRD analysis combined with TEM observations showed exfoliation for all cases, though to a higher extent in the MgAl hybrid prepared with DS. This composite also displayed better thermal and mechanical properties, thus supporting the higher exfoliation degree and interaction of the particles. Moreover, these authors (Lee and Im, 2007) used dimethyl 5-sulfoisophthalate (DMSI) anions for in situ polymerized LDH nanocomposites (LDH−DMSI), in order to enhance the compatibility between the PET matrix and LDH, resulting in full exfoliation of the LDH particles. The morphology of the nanocomposites was studied by TEM and XRD, clearly showing that LDH−DMSI was exfoliated in the PET matrix.
Nyambo et al. (2008) compared the structure of PMMA composites prepared with modified MgAl LDHs using decanoate (MgAl–C10), undecanoate (MgAl–C11), laurate (MgAl–C12), myristate (MgAl–C14), palmitate (MgAl–C16), stearate (MgAl–C18), and behenate (C22) as anions. From these, C10 produced an exfoliated morphology, C12 and C14 in mixed intercalated-exfoliated, and C18 and C22 in intercalated.
Shi et al. (2010) compared the structure of PP composites with LDHs modified with sodium dodecyl sulfate (SDS), itaconic acid (IA), or a combination of both, prepared by melt-mixing. The highest degree of exfoliation was obtained when combining both anions.
Another factor that affects the formation of exfoliated particle morphology is the concentration of particles in the polymer matrix. For instance, Herrero et al. (2010) and Zhu et al. (2008) showed that, for in situ polymerized PA6.6 composites prepared with hybrid LDHs, the best dispersion was achieved for the nanocomposites with a low LDH concentration (with an exfoliated structure for LDH concentrations below 0.5 wt.%). Similarly, Du et al. (2007) observed an exfoliated structure in PA6 nanocomposites with SDS-modified LDH particles prepared by melt-mixing for LDH concentrations below 10 wt.%, while Zhang et al. (2007) observed this exfoliated morphology in EVA for SDS-modified LDHs concentrations < 5 wt.%.
Martínez-Gallegos et al. (2008) demonstrated that, for in situ polymerized PET composites, exfoliation and dispersion seems to be complete for LDH contents up to 5 wt.%. Higher concentrations lead to the formation of LDH aggregates in the composite.
Kuila et al. (2008) found that, for EVA/LDPE/DS–LDH composites prepared by solution blending, there was full delamination of the DS–LDH layers at low DS–LDH contents, whereas partially exfoliated structures were observed for higher filler loadings. Similarly, Zhang et al. (2008a) compared the structure of EVA composites prepared by melt-mixing and solution blending and found an exfoliated particle morphology for particle contents < 10 wt.%.
Regarding preparation conditions, Qiu et al. (2005) showed that the exfoliation of PS/ZnAl–LDH nanocomposites prepared by solution blending involved two steps during refluxing: solvent swelling and layer breaking. Chen et al. (2004b) described a method, based on refluxing the LDH particles in a non-polar xylene solution of the base polymer, which allowed the preparation of LDH nanocomposites with a high degree of exfoliation even for high concentrations.
Another important factor in achieving exfoliated nanocomposite morphology is the nature of the polymer matrix. Hence, Wang et al. (2009) compared different anions, to give different LDHs (2-ethylhexyl sulfate (SEHS), bis(2-ethylhexyl) phosphate (HDEHP), and dodecylbenzenesulfonate (SDBS)), and also different preparation methods (melt-mixing and in situ polymerization) and different polymers (PMMA and PS). They found that it was much easier to disperse the particles in PMMA than in PS, although SEHS did not disperse well in either polymer.
4.5 Properties
4.5.1 Rheological properties
To understand the role of the LDH particles as fillers, rheological measurements are used to reveal possible interactions and relate them with the degree of dispersion of LDH in the polymer. The linear viscoelastic regime provides important information on LDH dispersion, as well as possible particle/particle and particle/polymer interactions and, therefore, may be used to relate structure and properties.
The rheological properties of PET nanocomposites have been studied by Lee et al. (2006). PET nanocomposites containing 2.0 wt.% of MgAl–LDH with carbonate (MgAlCO3), dodecylbenzene sulfonate (MgAlDBS), and octyl sulfate (MgAlOS) showed similar G′ vs. G″ curves, obtained from dynamic parallel plate rheometry measurements. All these nanocomposites displayed lower slopes than pure PET (1.27 vs. 2), with higher values of the storage modulus at a low loss modulus, indicative that the system was heterogeneous in terms of filler/filler interactions. However, PET nanocomposites with 2.0 wt.% dodecyl sulfate (MgAlDS) displayed a shallower slope (around 0.87) because of network structures with filler/filler and filler/matrix interactions due to the increased hydrophobic nature and exfoliation of the MgAlDS system. However, for G″ > 103, the G′ vs. G″ slopes were steeper for all nanocomposites and approached that of the PET homopolymer. The authors argued that these results reflected the fact that some network structures in filler/filler or filler/matrix interactions were ruptured by the applied shear force, the system becoming isotropic and homogeneous.
The viscosity vs. frequency curve of PET/MgAlCO3 indicated a drastic shear-thinning behavior due to slip between the polymer and filler caused by a low filler/matrix interaction. Additionally, although the viscosity of PET with MgAlDBS, MgAlDS, and MgAlOS has shear thinning in a low-frequency range, PET with MgAlDBS and MgAlOS showed continuous-like shear-thinning behavior, due to the breakdown of the network structure and slip between PET and the filler. PET/MgAlDS retained the viscosity at high frequencies, much like the PET homopolymer, due to improved filler/matrix interactions.
The rheological behavior of PS nanocomposites with a Zn2Al–LDH dye (Chicago sky blue (CB), Evans blue (EB), and Niagara blue (NB)) hybrid filler was studied by Marangoni et al. (2008). The linear viscoelastic properties were studied under dynamic oscillatory shearing. The elastic G′(ω) and loss G″(ω) moduli of the unfilled PS showed in the low-ω region the usual Newtonian behavior with the expected relaxation exponent G′ ∝ ω1.92 and G″ ∝ ω0.98, close to the ideal slopes G′∝ ω)2 and G″∝ ω1, characteristic of the frequency-dependent liquid-like flow behavior (with a plateau in the complex viscosity master curve). For a PS nanocomposite containing either Zn2 Al/CB or Zn2 Al/EB, both the curvature of G′(ω) and G″(ω) and the relaxation parameters in the low-ω region remained similar to PS. In contrast, PS–Zn2Al/NB displayed a change in slope of both moduli in the terminal zone.
This was even more pronounced in the |η*|(ω) curve, where a plateau was not observed (see Table 4.4). This effect was considered by the authors to be an incomplete relaxation of PS chains. Although this is consistent with the shift of Tg it was, however, reported as unusual for an immiscible PS nanocomposite structure. The pseudosolid-like behavior of PS–Zn2,Al/NB was explained by attrition phenomena between PS and the filler. The changes occurring in Tg and the modulus in the low-ω domain were explained by interfacial interactions. The pseudosolid behavior of PS−Zn2 Al/NB was considered to be a rupture of the filler's lamellar structure, in strong contradiction to the trend usually observed, where solid-like behavior is seen in intercalated or exfoliated nanocomposite structures. The authors surmised that the attrition phenomena largely developed at the interface of Zn2Al/NB platelets and the PS chain should be attributed to the molecular arrangement of NB at the surface of the platelets.
Table 4.4
Rheological properties obtained under dynamic oscillatory shearing of PS nanocomposites with Zn2Al−LDH modified with different dyes
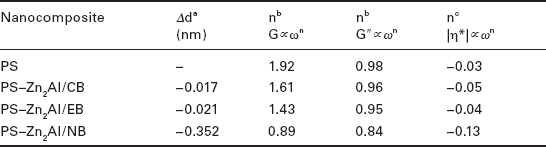
aChange in the basal spacing compared with the blue LDH filler before their dispersion into styrene. A negative value is interpreted as a reduction.
bRelaxation parameters in the terminal zone.
cShear-thinning parameters in the terminal zone.
Source: Adapted from Marangoni et al., 2008
Finally, it was noted that the molecular similarity between EB and CB was the reason for the super imposable rheological behavior, while the difference from NB was easily seen in the |η*|(ω) curve.
Leroux et al. (2009) proved that the shear-thinning exponent did not allow a quantitative discrimination between intercalated and exfoliated nanocomposite structures. The change in the shear-thinning parameter, directly correlated to the relaxation parameter, was interpreted as a modification in the interfacial interaction between the LDH filler and PS chains. In order to see if this effect a rose from the alkyl chain alone, a PS–Zn2Al/DBS nanocomposite was studied. As with the Zn2Al/MADABS hybrid phase, Zn2,Al/DBS presented a well-defined lamellar structure, with several basal peaks. When incorporated into PS, the basal spacing distance remained unaltered (2.93 nm), hence defining a non-miscible PS nanocomposite structure, apparently comparable to PS-Zn2,Al/MADABS. The glass transition temperature was similar to that of unfilled PS, as were the relaxation and shear-thinning exponents. Indeed, the presence of Zn2 Al/DBS strongly decreased the molecular weight of PS. Also, the amplitude of the elastic modulus G′ was drastically reduced in the low-ω region. Consequently, the attrition phenomena cannot be explained solely by the length of the surfactant molecule.
The rheological behavior of hot-pressed films of LDH–PS nanocomposites was also studied. A filler percentage of 5 wt.% was used. The glass transition temperature shifted in comparison to unfilled PS, although not for the same magnitude as for a 10 wt.% content sample. PS films with 5 wt.% had intermediate rheological behavior, i.e. incomplete PS chain relaxation in the low-ω region with a shear-thinning factor different from zero. Once again, the lowering observed in the relaxation can be considered to be tethering of a polymer chain on a particle's surface and particle–particle interactions. The authors concluded that, after checking the consistency between the rheological behavior of LDH–PS nanocomposites and the state of dispersion observed by TEM, one can infer that XRD is not the most appropriate technique for elucidating the structure of polymer nanocomposites, as their morphology is much more complex than previously thought. The general trend can be explained by the existence of an LDH-percolated network. The use of lower filler loadings should induce a smaller interface, which would cause smaller attrition phenomena. These observations are to some extent similar to those for cationic clays, which form three-dimensional deformable networks in PS. Nonetheless, the effect is much more pronounced in the case of the present filler, hence the interest in the use of LDH-based materials as inorganic platelet-like fillers. Filler preparation was also reported to be important, since polymerization of the compatibilizer at the platelets' surface may give rise to aggregation when performed concomitantly with styrene bulk polymerization. Bulk polymerization acts as mechanical grinding when the platelets are pushed together in advance after a thermal pre-treatment, thus leading to a concatenated filler structure with solid-like rheological behavior.
Taviot-Gueho et al. (2007) also studied the rheological properties of several LDH–PS nanocomposites. The linear viscoelastic dynamic storage and loss modulus (G′ and G″, respectively) for PS–Zn2Al/yellow were good matches with those of PS, whereas for PS–Zn2Al/DBS there was a large decrease over the entire frequency domain, considerably more so for G′ than for G″, hence explaining the increase in tan δ. The increase in tan δ has to be explained by greater molecular mobility. It appears that the presence of Zn2Al/yellow in PS, even as an intercalated structure and in contrast to Zn2Al/DBS, does not result in worse rheological properties. The rheological behavior, expressed as η″ vs. η′, was found to be different for the three samples. At 200 °C, PS and PS–Zn2Al/yellow curves matched well. The viscosity at zero shear (η0), similar for the two systems, suggested similar molecular weights, which were confirmed by gel permeation chromatography (GPC). For PS–Zn2Al/DBS, the intercept was at a much lower value, once again consistent with the GPC results. Finally, the size of the hybrid LDH aggregates was argued as a possible disadvantage during bulk polymerization. Both fillers had an intercalated structure, though it was ill-defined for PS–Zn2Al/yellow and well-defined for PS–Zn2Al/DBS. The presence of large Zn2Al/DBS filler particles prevented the formation of extended polymer chains and consequently impoverished the rheological properties.
The rheological properties of LDH–polyethylene nanocomposites were studied by Costa et al. (2006a). The evolution of the complex viscosity of LDH–LDPE nanocomposites at low frequency was studied from 180 to 260 °C. The dependence of the rheological properties on temperature was weaker for the nanocomposites with an unfilled polymer, especially beyond a critical LDH content, representing a deviation of the liquid-like low-frequency flow behavior towards a pseudosolid one.
On the other hand, HDPE-g-MAH nanocomposites (HDPE is high-density polyethylene) showed the shifting behavior at much lower LDH concentrations (around 5 phr) compared with the LDPE/compatibilizer composites, where changes in viscosity with temperature were still observed at a 10 phr LDH concentration. This means that the nature of the polymer matrix strongly influences the effect of increasing the amount of LDH in terms of the rheological behavior of LDH nanocomposites. With increasing LDH nanoparticle concentration, the low-frequency response of the complex viscosity vs. frequency shifted to a shear-thinning mode, with similar behavior for both nanocomposites. Two factors could explain this situation: first of all, the polarity difference between the two matrices, and second, the presence of a low molecular weight compatibilizer in one of the composites.
It is well known that both unfilled and particulate polyolefin microcomposites conventionally display a Newtonian liquid-like behavior at low frequency. Nevertheless, LDH–LDPE nanocomposites display shear-thinning behavior with a higher shear-thinning exponent and lower relaxation exponent. Both exponents become almost independent of LDH concentration at higher contents. The shear-thinning exponent has been used as a semi-quantitative parameter of the degree of clay delamination in polymer nanocomposites (Wagner and Reisinger, 2003). Although higher negative values of this exponent are normally considered to be due to higher clay exfoliation, the higher shear-thinning exponent values of the LDH−LDPE nanocomposites were due to polymer molecule confinement, delaying the relaxation process. Higher LDH loadings enhanced this effect, with G′ increasing and being less frequency dependent. Additionally, as the average distance between the dispersed particles decreased, the tendency to interlock, which acts as an energy barrier for molecular relaxation, increased.
4.5.2 Mechanical properties
Taking into account the large surface area of layered double hydroxides, in some cases reaching 800 m2/g, a large interaction with polymer molecules is possible, and thus a large mechanical reinforcement effect is to be expected. This situation could partly explain why these layered materials may dramatically improve the modulus of polymers even when present in small amounts.
Most research into the mechanical properties of polymer–LDH nanocomposites has analyzed their tensile properties, particularly the modulus and tensile strength, as a function of LDH content. Generally speaking, the addition of low amounts of LDH increases the Young's modulus and tensile strength, mainly due to the ease of attaining better particle dispersion in the polymer matrix at low LDH contents. Higher LDH concentrations increase the probability of particle cluster formation, leading to deterioration of the mechanical properties.
Zhang et al. (2008a) reported a gradual increase in Young's modulus from 42.8 to 72.1 MPa for EVA composites containing up to 20 wt.% of ZnAl–LDH particles and an increase in tensile strength up to 5 wt.% LDH, decreasing for higher loadings. Another study (Sorrentino et al., 2005) showed how small amounts of LDH particles (up to 2.8 wt.%) significantly improved the mechanical properties of composites, with an increase of both Young's modulus and yield stress of about 100 %. However, for concentrations up to 6 wt.%, the mechanical properties, although still higher than for the pure polymer, decreased. A similar phenomenon was observed in PU/DS–LDH nanocomposites (PU is polyurethane) (Kotal et al., 2009). Kotal et al. studied the mechanical properties of nanocomposites containing 1, 3, 5, and 8 wt.%, finding that the maximum enhancement of tensile strength and elongation at break corresponded to the nanocomposite containing 3 wt.% DS– LDH. These authors suggested that the decrease observed beyond this DS–LDH content was probably due to the increasing tendency of DS–LDH to aggregate in the PU matrix at high loadings. Du et al. (2006) also studied the effect of DS/MgAl–LDH particle content (5 and 10 wt.% loadings were used) in poly(propylene carbonate) (PPC). They found that the nanocomposites displayed enhanced tensile strengths and Young's moduli compared with pure PPC. The nanocomposite containing 5 wt.% showed the highest tensile strength and Young's modulus (72 % and 57 % higher than those of pure PPC, respectively). This improvement was due to the structure found in the nanocomposites: exfoliated for the 5 wt.% LDH nanocomposite and partly exfoliated for the 10 wt.% one. A similar phenomenon was observed in aminobenzoate/MgAl–LDH/polyimide nanocomposites (Hsueh and Chen, 2003b). When LDH particle contents exceeded 5 wt.%, a decrease in the tensile strength at break was found (although still higher than for pure polyimide). Once again, this was due to the aggregation of the LDH layers.
The results of tensile tests performed by the same authors (Hsueh and Chen, 2003a) on laurate-modified MgAl–LDH epoxy nanocomposites showed how both the tensile strength and modulus increased gradually when the amount of LDH was increased up to 7 wt.%, the maximum concentration analyzed. However, these authors also found that elongation at break decreased slightly for LDH contents higher than 3 wt.%. Wang et al. (2006) also found interesting results for PMMA nanocomposites reinforced with different amounts of undecanoate-modified LDH (LDH-U). The authors found that the tensile modulus was enhanced with increasing LDH-U content (38 % and around 80 %, respectively, for LDH contents of 3 and 5 wt.%). This was due to good dispersion of the LDH layers and a strong interfacial adhesion between the layers and the PMMA matrix. A similar trend was observed for tensile strength. Nevertheless, a slight decrease was noticed for the elongation at break with increasing LDH content, due to the high resistance of the LDH layers to deformation of PMMA.
Acharya et al. (2007b) also reported an increase of both the tensile modulus and strength when increasing the amount of LDH up to 8 wt.% in EPDM/DS–LDH nanocomposites.
Other studies reported the effect of the interlamellar anion and the composition of the layers in the mechanical properties of LDH nanocomposites. For example, Manzi-Nshuti et al. (2008b) studied the effect in PMMA systems of LDH particles prepared with different combinations of NiAl, CoAl, and ZnAl layers, as well as different Zn/Al ratios (2:1 and 3:1). The authors did not find significant effects of the LDH particles on the mechanical properties, regardless of the composition and ratios of the divalent to trivalent metal cations. In another study, Bugatti et al. (2010) analyzed the effect of LDH hybrids prepared using different interlayer anions (benzoate, dichlorobenzoate, p-hydroxybenzoate, and o-hydroxybenzoate), as well as their concentration on the mechanical properties of polycaprolactone (PCL) films. Slight improvements were observed for all mechanical parameters, with some interesting differences between the differently dispersed nanohybrids. These authors showed that for a given LDH concentration, for instance 3 wt.%, the tensile modulus may vary between 218 and 300 MPa, depending on the type of interlayer anion (in this case the higher value corresponded to dichlorobenzoate-modified LDH). Wang et al. (2009) also analyzed the effect of LDH concentration and the use of different interlayer anions on the mechanical properties of PMMA and PS nanocomposites. No significant differences were found when varying the type of interlayer anion.
An interesting study was conducted by Bao et al. (2006), which analyzed the effect of LDH particles on the tensile strength and Young's modulus of PVC composites prepared by both melt-mixing and in situ polymerization. The authors found that, although the tensile strength and Young's modulus increased for both PVC/LDH–DS composites when increasing the amount of LDH–DS, this increase was higher for the ones prepared by in situ polymerization for a given LDH concentration. On the other hand, Pradhan et al. (2008) showed that, when well dispersed, LDH acted as a mechanical reinforcement in both ethylene propylene diene monomer (EPDM) and acrylonitrile butadiene carboxy monomer (XNBR) elastomers. In particular, the tensile strength increased from 1.54 and 1.94 MPa to values as high as 3.25 and 17.83 MPa for a 10 wt.% LDH content, respectively, for the EPDM and XNBR elastomers, clearly demonstrating the mechanical reinforcement effect, especially in the case of the XNBR elastomer.
Regarding some of our previous results, we studied the mechanical properties of PS and styrene-acrylonitrile resin (SAN) composites with 5 and 10 wt.% of dodecyl-sulfate-modified MgAl layered double hydroxides (o-HT) (Realinho et al., 2009). The tensile modulus and strength of PS remained almost unaltered when incorporating the o-HT particles, with only a slight increase being observed for Young's modulus. In contrast, the addition of o-HT to SAN, although decreasing its tensile strength, increased the stiffness to a higher extent than in PS and noticeably increased the ductility of the material in terms of strain at break. These results were due to better o-HT dispersion in SAN compared with PS. The decrease in tensile strength observed when incorporating o-HT into SAN was explained by the combination of a relatively weak particle–matrix interface adhesion and inherent high deformability of SAN, which promoted o-HT particle debonding at low stress levels and subsequent plastic flow.
For semi-crystalline polypropylene (Ardanuy et al., 2010), we showed that the incorporation of 10 wt.% of unmodified (HT) and dodecyl-sulfate-modified hydrotalcite (HTDS) to a PP matrix with and without PP-g-MAH and PP-g-SEBS compatibilizers resulted in nanocomposites with slightly higher Young's moduli. However, this increase was considerably lower than that observed for similar PP–montmorillonite (PP-MMT) nanocomposites, due to a higher inherent flexibility of HT layers compared with cationic clays such as MMT. Comparing HT- and HTDS-reinforced nanocomposites, the latter had higher values of Young's modulus, probably due to a higher exfoliation of the HTDS particles in PP, especially when using the PP-g-MAH compatibilizer (there were more interactions between the polymer and HT platelets). A low degree of adhesion was considered to be the reason for the slight decrease observed regarding the tensile strength for both the HT- and HTDS-reinforced PP nanocomposites, compared with the higher values obtained for the nanocomposites with compatibilizer.
4.5.3 Thermal stability
Generally speaking, the incorporation of layered particles into polymer matrices results in an enhancement of their thermal stability (Pavlidoua and Papaspyrides, 2008). The increase in thermal stability may be due to the layers hindering the diffusion of oxygen and volatile products throughout the polymer, as well as to the formation of a char layer after thermal decomposition of the organic matrix. Additionally, endothermic decomposition of the host metal hydroxide layer can provide a cooling effect, which could delay the combustion process of the organic species (surfactant anion, polymer chain segments, etc.) constrained within the interlayer gallery of the LDH particles.
As can be seen in Table 4.5, except for some specific polymer matrices like polyamide 6 (PA6) or PCL, the addition of LDH hybrids to different polymers results in an increase in the decomposition temperatures corresponding to a 50 % weight loss.
Table 4.5
Decomposition temperatures for a 50 wt.% loss (T0.5) of various polymernanocomposites as a function of LDH content
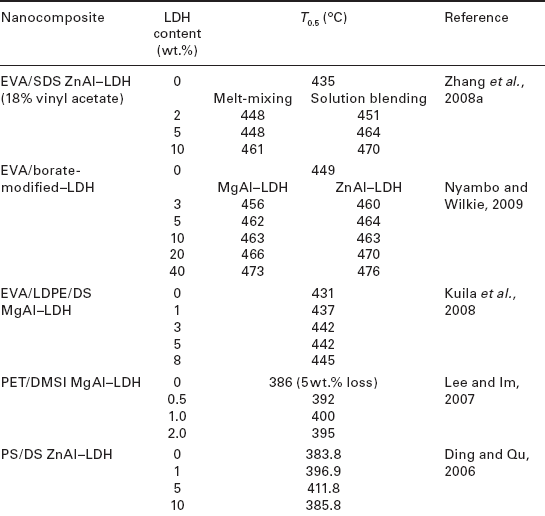
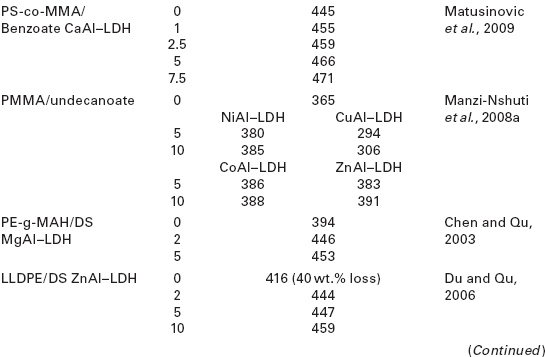
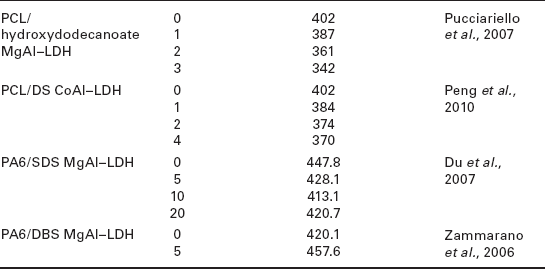
Zhang et al. (2008a) studied the thermal stability of DS/ZnAl–LDH/EVA nanocomposites as a function of LDH concentration and preparation procedure. These authors observed that the thermal degradation temperatures of the nanocomposites were between 13 and 35 °C higher than that of pure EVA, increasing with increasing LDH concentration. The authors explained this effect based on the fact that at a higher loading the LDH layers favored the formation of an obstructive char layer at the surface of the polymer, increasing the thermal stability of the nanocomposites. Moreover, samples prepared by solution blending displayed higher degradation temperatures than those prepared via melt-mixing for identical LDH contents. The authors attributed this effect to a better dispersion of the LDH particles in the nanocomposites prepared by solution blending.
Nyambo and Wilkie (2009) reported similar results for EVA-based polymer nanocomposites reinforced with borate-modified LDHs with a different composition of the metal cations. The authors found that the addition of MgAl or ZnAl-borate LDHs improved the thermal stability of EVA, more markedly with increasing LDH concentration. In particular, the best thermal stability effect was obtained for a 40 wt.% LDH loading regardless of the nature of the layer metal cations. Using a similar approach, Kuila et al. (2008) studied the thermal decomposition of EVA/LDPE-based LDH nanocomposites. They found that, although the initial weight loss for the nanocomposites was accelerated due to the early degradation of DS molecules, the thermal decomposition temperatures corresponding to a 50 % weight loss for nanocomposites with 1, 3, 5, and 8 wt.% DS-LDH were higher than for pure EVA/LDPE, increasing with increasing LDH content. This increase in thermal stability was attributed to the homogeneous dispersion of DS–LDH in the EVA/LDPE matrix. In another study, Qiu et al. (2005) analyzed the effects of LDH concentration on the thermal decomposition of PS nanocomposites containing 5, 10, and 20 wt.% ZnAlDS–LDH. They showed that the thermal decomposition temperature of the nanocomposites was between 16 and 39 °C higher than that of pure PS, with optimal concentration being 10 wt.%. Higher LDH loadings (20 wt.%) resulted in nanocomposites with lower thermal stability. These authors also analyzed the thermal behavior of the same nanocomposite systems prepared using different methods. They found that the samples prepared by rapid precipitation and solvent evaporation at 140 °C, although displaying similar thermal behavior when varying the amount of LDH, showed better thermal stability compared with samples prepared by solvent evaporation at 60 °C. This was explained by the different proportions of exfoliated and intercalated structures in these nanocomposites.
Different effects of LDH content on the thermal stability of polyolefin-based nanocomposites have been reported in the literature. For example, for PP–LDH systems, Shi et al. (2010) reported that the thermal decomposition temperatures of a series of PP-LDH nanocomposites with various LDH concentrations were higher than for pure PP (from 7 to 30 °C). Moreover, the authors found that the nanocomposites containing organo-modified MgAl–LDH particles exhibited higher decomposition temperatures than the nanocomposites containing unmodified MgAl–LDH particles. In the same way, Ardanuy and Velasco (2011) reported that the decomposition temperature of pure PP increased with organophilized LDH particles, while the thermal stability decreased when using unmodified LDH particles. These authors also found that the increase of thermal stability was more noticeable when PP-g-MAH was used as a compatibilizer. They noted that the exfoliated layers of organophilized LDH hindered the diffusion of oxygen after combustion ignition to a greater extent. Nevertheless, Wang et al. (2010) did not find any differences between the thermal decomposition of pure PP and nanocomposites with organic modified CaAl–LDH for particle loadings between 1 and 6 wt.%. These authors concluded that the particles did not improve the thermal stability of PP.
Concerning polyethylene–LDH nanocomposites, Qiu et al. (2006) compared the thermal stability of LLDPE systems with different MgAl, ZnAl–LDH, and MMT contents. They showed that the thermal stability of the nanocomposites was enhanced compared with virgin LLDPE, although the enhancement was quite different depending on the composition of the layered particles. The T0.2 values (decomposition temperature corresponding to a 20 wt.% loss) for the LLDPE/MMT and LLDPE/MgAl–LDH samples increased gradually to 399 and 429 °C, respectively, with increasing MMT and Mg3 Al(DS) contents from 0 to 10 wt%. However, LLDPE/ZnAl–LDH with only 2.5 wt% of Zn3Al(DS) showed important improvements, T0.2 increasing till 428 °C. It reached a maximum value of 434 °C for 5 wt.% Zn3Al(DS), and decreased to 420 °C for 10 wt.% Zn3 Al(DS). The possible reason behind these differences could be the relative extent of exfoliation in the different nanocomposites.
Costa et al. (2007) also studied LDPE–LDH nanocomposites, reporting that the onset of the decomposition temperature was delayed significantly with the addition of only 2.4 wt.% of LDH particles. Once again, the decomposition temperature increased with increasing LDH concentration, with a maximum increase of 70 °C compared with pure LDPE for a 9 wt.% LDH content. Ardanuy et al. (2009) reported similar results for HDPE nanocomposites. The onset of decomposition of HDPE was significantly delayed by the effect of the organo-modified LDH particles and, similarly, T0.5 increased because of these particles.
However, there are results in the literature in which the incorporation of LDH particles resulted in a decrease in the decomposition temperature of the polymer. For example, Du et al. (2007) reported that the thermal degradation temperature of PA6/DS MgAl–LDH nanocomposites was lower than that of pure PA6. This effect was highlighted with increasing LDH content. These authors considered that this effect was due to a catalytic degradation phenomenon caused by the organically modified LDH layers. Herrero et al. (2010) reported similar results for PA6.6–LDH systems with filler contents higher than 1 wt.%. This behavior is because the high content of Ad-LDH could catalyze the alkaline degradation of PA6.6. Zammarano et al. (2006) also reported a reduction in the onset of the thermal decomposition temperature for LDH−PA6 nano-composites. Again, it was suggested that this effect was due to a nucleophilic attack mechanism.
4.5.4 Other properties
Besides the few works that analyze properties such as arc resistance and dielectric strength (Ramaraj and Yoon, 2008), barrier properties (Bugatti et al., 2010; Sorrentino et al., 2005), dielectric properties (Leroux et al., 2010; Schonhals et al., 2009), and optical properties (Marangoni et al., 2008; Zhang et al., 2008b; Acharya et al., 2007b), one of the most extensively studied properties in polymer-layered double hydroxide nanocomposites is fire resistance, mainly using cone calorimetry and limiting oxygen index (LOI) determination.
For instance, Du et al. (2007) reported the combustion characterization of PA6/MgAl–LDH nanocomposites. They found that the heat release rate (HRR) decreased considerably when increasing the amount of LDH in the polymer matrix. Moreover, they found that the ignition time was delayed compared with pure PA6 for low LDH contents. However, ignition times increased when the content of MgAl(H-DS) exceeded 10 wt.%. The authors suggested that this phenomenon could be due to a catalytic degradation effect of PA6 promoted by the organo-modified particles, which decreased the molecular weight of the polymer and thus caused the material to burn more easily.
Ramaraj and Yoon (2008) reported the LOI estimation and flammability rating of EVA–LDH nanocomposite films. Results showed a significant improvement in the flame retardancy of EVA nanocomposites due to the LDH particles. They showed that the minimum oxygen concentration required for a flame to appear increased from 19 to 26 % and the flammability rating improved from no rating to V1 rating with increasing LDH concentration. They reported that the flame-retardant characteristics of LDH were due to their Mg(OH)2-like behavior, which involved endothermic decompositions with the formation of water vapor and a metal oxide residue. This residue prevented the burning process from proceeding by reducing the oxygen supply to the underlying combustible polymer.
Another study by Manzi-Nshuti et al. (2009a) gave the cone calorimetric results of PS-LDH systems. The best reduction in peak heat release rate (PHRR) (35 %) was obtained for PS filled with 10 wt.% of an organo-modified ZnAl-MgAl–LDH. Lower LDH contents did not give significant fire retardancy improvements. Nyambo et al. (2009a) also studied the fire behavior of PMMA/MgAl–LDH nanocomposites using cone calorimetry. A significant reduction was observed regarding PHRR, due to improved physical interaction and good nano-dispersion of the organically modified MgAl–LDH particles in the PMMA matrix. A lower reduction was observed when incorporating unmodified MgAl–LDH particles.
Similar improvements in fire resistance with LDH particles were found by other researchers, such as Costa et al. (2007). These authors found that the incorporation of MgAl–LDH particles resulted in a very efficient reduction of the heat release rate and total heat released during combustion. MgAl–LDH particles were also found to facilitate the formation of a carbonaceous char on the burning surface, causing a lower emission of carbon monoxide during the initial phase of burning. For LDH concentrations above 10 wt.%, the nanocomposites not only burnt at extremely slow rates, but also showed low dripping tendencies. However, they reported that LDH by itself, even at these concentrations, was not enough to obtain a high LOI value or V0 burning rate.
All of these results prove the effectiveness of LDH particles as flame retardants in polymer systems.
4.6 Applications and future trends
LDH nanocomposites are promising functional materials, as they combine the characteristics and properties of both a polymer and an inorganic phase. This is a particularly interesting field for academics, since it offers the possibility of merging different scientific fields such as organic, macromolecular or inorganic chemistries, as well as for materials engineers, with potential applications in different industrial sectors such as energy production and storage (photovoltaics, batteries, fuel cells,…), medicine (drug delivery, biomaterials, imaging,…), functional coatings, environment and safety (membranes, fire resistance,…), nano- and microelectronics, optics, etc.
The use of LDH in polymer nanocomposites is an emerging research field with several potential applications. LDH–polymer nanocomposites have advantages compared with montmorillonite-based ones, due to the versatility of LDHs regarding chemical composition, as well as tunable charge density, allowing multiple interactions with the polymer. This makes them attractive for enhancing thermal stability and improving fire resistance in different polymeric materials (Camino et al., 2001; Nyambo et al., 2009b; Nyambo and Wilkie, 2009; Manzi-Nshuti et al., 2009b). In particular, for flame-retardant polyolefins, different products such as cables and construction panels can be produced using LDH nanocomposites to meet the increasingly more stringent ratings of the low-smoke zero-halogen (LSZH) classification with reduced contents of mineral flame retardants such as aluminum trihydroxide or magnesium dihydroxide.
The fire behavior of LDH–polymer nanocomposites has been investigated by microcombustion calorimetry (Wang et al., 2010), which revealed O-CoAl–LDH as a very efficient system in reducing both HRR and PHRR during burning. The heat release capacity and total heat released during burning were also reduced significantly with increasing nanofiller concentration. These results emphasize the fact that organo-LDH's flame-retardancy mechanisms are different from those of organo-layered silicates.
Nanofire products, developed by CIMTECLAB (Italy), based on LDH nanocomposites, have been reported as a solution for considerably reducing the content of toxic substances in polymeric formulations whilst maintaining excellent fire-retardant properties. Nanofire additives, applied to thermoplastic polymers, combine nanotechnology and a synergistic effect with a liquid plasticizer in various thermoplastic materials, such as PVC, polyamides, and polycarbonate/acrylonitrile–butadiene–styrene copolymer blends, hence enabling the reduction of toxic additives such as antimony and halogen without altering fire performance.
Other applications of polyolefin–LDH nanocomposites include water-storage systems, automotive injection-molded parts, and even foams. Moreover, in polyolefin packaging, films, bottles, and cups can be produced with improved mechanical, barrier, and rheological properties, resulting in better thermoforming properties.
Over the past decade, significant interest has been given to the synthesis of LDHs with new compositions, which would allow them to be used in different application areas. Das et al. (2011) prepared and characterized transparent rubber nanocomposites using LDHs, which are a more environmentally friendly rubber composite vulcanized without the use of ZnO. In this material, LDH nanoparticles deliver zinc ions during vulcanization as accelerators and stearate anions as activators, while the mineral platelets act as a nanofiller reinforcement.
Martínez et al. (2011) prepared and characterized microcellular foams from nanocomposites of PS, SAN, and PMMA with LDH, displaying fine uniform cellular structures with sub-micrometric cells. The thermo-mechanical properties and gas impermeability of these foamed nanocomposites were enhanced compared with pure polymer foams.
Kotal et al. (2011) produced thermoplastic polyurethane (TPU) nanocomposites with improved mechanical strength and ductility due to stearate-intercalated LDH particles. These nanocomposites showed maximum improvements in tensile strength (45 %) and elongation at break (53 %) for 1 and 3 wt.% LDH contents. Maximum improvements in storage and loss moduli (20 %), with a shift of the glass transition temperature (15 °C) and an increase in thermal stability (32 °C) at 50 % weight loss, were observed for an 8 wt.% LDH loading.
Guo et al. (2011) reported a slower thermo-oxidative rate in polyurethane/CoAl–LDH nanocomposites compared with neat PU, from 160 to 340 °C, probably due to the barrier effect of the exfoliated LDH layers. These results suggest potential applications of CoAl–LDH as flame retardants in PU.
Zhao et al. (2011) described transparent ethylene–vinyl alcohol copolymer (EVOH) nanocomposite films containing partially exfoliated LDHs intercalated with UV absorbers, prepared using DMSO as a solvent. The composite film obtained had a visible light transmittance of 90 %, comparable to that of the pure matrix, was flexible and exhibited excellent UV-shielding capability, as well as improved thermal stability.
Bugatti et al. (2011) prepared films of microcomposites and exfoliated composites of PCL containing sodium 2,4-dichlorobenzoate or p-hydroxybenzoate simply dispersed in the polymer, which released antimicrobial species when in contact with physiologic solutions. The release of antimicrobial moieties ‘freely dispersed’ in the polymer happened much faster than for molecular anions bonded to the inorganic compound, and occurred in one step. In contrast, the release from the nanohybrids occurred in two stages: the first, a fast ‘burst,’ occurring during the first few days; and the second, very slow, extending over many months. The diffusion of the active molecules out of the microcomposite seemed to be slower than for the exfoliated nanohybrid. Moreover, the composite showed a chemical absorption hysteresis; hence this parameter has to be taken into account when tuning a release.
Lv et al. (2009b) developed UV-cured coatings consisting of urethane acrylates as oligomers and a diacrylate monomer reinforced with layered double hydroxides, resulting in coatings with enhanced thermal and mechanical properties. Also, a novel UV-cured polymer–LDH nanocomposite was prepared (Yuan and Shi, 2011) by modifying LDH with sodium dodecyl sulfate and (3-(methyl-acroloxy) propyl)trimethoxysilane (KH570), followed by UV irradiation after blending with the acrylate system. The storage modulus and glass transition temperature of the nanocomposite containing 5 wt.% LDH–KH increased to 47.5 MPa and 67.8 °C, respectively, compared with 39.7 MPa and 66 °C for the pure polymer. The tensile strength and Persoz hardness increased to 10.6 MPa and 111 s, respectively, from the 7.7 MPa and 85 s for the pure polymer. Similarly, Hu et al. (2011) prepared exfoliated polymer–LDH nanocomposites by UV-initiated photo-polymerization of acrylate systems using 5 wt.% of an Irgacure 2959-modified LDH precursor (LDH-2959) as a photo-initiator complex. The glass transition temperature of the UV-cured exfoliated nanocomposites increased from the 55 °C of the pure polymer to 64 °C. The tensile strength increased from 10.1 MPa to 25.2 MPa; the Persoz hardness also increased, while the elongation at break remained at an acceptable level.
Marangoni et al. (2011) showed the possibilities for a new range of applications for layered double hydroxides intercalated with dyes in the preparation of polymer composite multifunctional materials. They prepared transparent, homogeneous, and colored nanocomposite films by casting after dispersing dye-intercalated LDHs (pigments) into commercial polyvinyl alcohol (PVA). They used ZnAl–LDH intercalated with anions of the dyes orange G, orange II, and methyl orange.
Jaymand (2011) synthesized and characterized an exfoliated modified syndiotactic polystyrene/MgAl-layered double-hydroxide nanocomposite. Compared with pure sPS-g-(PS-b-PMS), the nanocomposite showed a much higher decomposition temperature and higher glass transition temperature.
Intercalation, an important step for pillaring, means that layered double hydroxides can be used as carrier systems. The micro-porous properties can be used for controlled drug delivery. Cao et al. (2011) evaluated the potential use of an LDH−polymer nanocomposite as a drug delivery system for ocular delivery. Diclofenac was successfully intercalated into ZnAlNO3–LDH using co-precipitation. In vivo pre-corneal retention studies were conducted with diclofenac sodium saline, diclofenac-LDH nanocomposite dispersion, 2 % poly(vinyl pyrrolidone) (PVP) K30-diclofenac-LDH nanohybrid dispersion, and 10 % PVP K30-diclofenac–LDH nanohybrid dispersion, separately. Compared with diclofenac sodium saline, all the dispersions extended the detectable time from 3 to 6 h; Cmax and AUC0-t of diclofenac–LDH nanocomposite dispersion showed 3.1-fold and 4.0-fold increases, respectively; Cmax and AUC0-t of 2 % PVP K30–LDH nanohybrid dispersion were enhanced by about 5.3 and 6.0-fold, respectively. Additionally, no eye irritation was demonstrated in rabbits after single and repeated administration. These results show that this novel ocular drug delivery system is promising for improving the bioavailability of drugs in ophthalmic applications.
Wang et al. (2011) studied the combustion behavior via microcombustion calorimetry of polypropylene/organo-modified MgAl–LDH (PP/o-MgAl–LDH) composites prepared by melt-mixing. The results showed that the specific heat release rate (HRR), the heat release capacity (HRC), and the total heat release (THR) were reduced compared with the pure polymer. Further enhancements were observed by increasing the concentration of MgAl–LDH.
Kakati et al. (2011) prepared PP/NiAl–LDH nanocomposites with enhanced thermal stability due to a barrier effect induced by the LDH lamellar layers. For 10 wt.% loss, the decomposition temperature of a PP–LDH (5 wt.%) nanocomposite was 15 °C higher than that of neat PP. The thermal stability of the nanocomposites also increased with increasing LDH loading.
Hintze-Bruening et al. (2011) described an economically scalable approach to realize impact-resistant coatings built on substantially ordered platelets. Unlike other artificial structured nanocomposites, the parallel alignment of the LDH particles is obtainable with a single coating process. The alignment originated from an aqueous intermediate formed by a lyotropic liquid-crystal phase of polymer-stabilized LDH particles.
4.7 Conclusions
In conclusion, LDH−polymer nanocomposites are quite promising materials in several application areas. Although advances have been made in the development of LDHs for novel applications, further research is still necessary in order to develop feasible synthetic procedures for mass production to be used in full-scale anion exchange applications, as well as to improve selective absorption from multi-anionic systems. The great versatility of LDHs, that is the ability to modify their structures, provides many possibilities in using them as multifunctional materials for applications in materials science.
4.8 Sources of further information and advice
Patents
Although many patents discuss LDH–polymer nanocomposites, most of them include these materials merely as a particular case of clay-polymer nanocomposites. Therefore, not many patents are specifically about LDH–polymer nanocomposites. Some of the most recent ones consider the application of LDH nanocomposites in preventing hair loss.
Choy et al. (2010a, 2010b, 2010c) described an organic/inorganic nanohybrid complex containing eicosapentaenoic acid (EPA), vitamin C, or an indole-3-acetic acid, respectively, to maximize hair growth, having stability in heat, light, air, and oxygen. The nanohybrid complex for promoting hair growth and preventing hair loss was inserted between the LDH with the structural formula (M2 +(1 − 3)N3+x(OH)2)Axy H2O. A specific composition for preventing hair loss and promoting hair growth contained 0.01 to 20 wt.% of nanocomposite with EPA. Another composition used the nanohybrid complex as an active ingredient in which vitamin C was inserted between LDH layers. Other compositions used indole-3-acetic acid or a nanohybrid complex as the active ingredient and were applied as a lotion, emulsion, cream, essence, or spray.
Gook et al. (2008) patented a polyelectrolyte nanocomposite and a manufacturing method capable of improving the tensile modulus and ion conductance to obtain a high photoelectric effect and to improve productivity while maintaining uniform physical properties. The polyelectrolyte nanocomposite consisted of LDH dispersed in a polyethylene glycol acrylate (PEDGA) polymer matrix.
Ferrara et al. (2010) prepared a polyolefin nanocomposite material based on a crystalline or semi-crystalline polyolefin matrix and 0.02 to 6 wt.% of modified hydrotalcite. In particular, these authors reported the development of fibers, films, blisters, thermoformed, blow-molded, and injection-molded products based on these materials all having improved properties (good barrier properties, good stiffness and optical properties and, in some cases, improved thermo-mechanical and processing properties) compared to similar systems reinforced with conventional clays.
Yongzhong et al. (2005) described a method for preparing poly(vinyl chloride)/hydrotalcite nanocomposites including steps of reacting the hydrotalcite with fatty acids or anion surface activators, and obtained modified hydrotalcites through filtering and drying. The addition of these modified hydrotalcites, the initiator, and the dispersing agent to vinyl chloride and deionized water inside a reactor at room temperature produced PVC composites by increasing the temperature to between 35 and 65 °C.
LDH producers
• Süd-Chemie (www.sud-chemie.com/scmcms/web/page_en_5375.htm) produces the hydrotalcite grades SORBACID and HYCITE.
• Sasol (www.sasolgermany.de/index.php?id=47) produces boehmite, high-purity alumina, and hydrotalcite (PURAL MG grades).
• INEOS Silicas (www.ineossilicas.com) produces hydrotalcite grades MACROSORB.
• AkzoNobel (www.akzonobel.com/brands_products) produces aluminum magnesium layered double hydroxide modified with hydrogenated fatty acids (PERKALITE grades).
• CIMTECLAB (www.cimteclab.net) produces modified hydrotalcite for fire-retardancy applications (Nanofire grades).
4.9 References
Acharya, H., Srivastava, S.K., Bhowmick, A.K. A solution blending route to ethylene propylene diene terpolymer/layered double hydroxide nanocomposites. Nanoscale Res Lett. 2007; 2:1–5.
Acharya, H., Srivastava, S.K., Bhowmick, A.K. Synthesis of partially exfoliated EPDM/LDH nanocomposites by solution intercalation: Structural characterization and properties. Compos Sci Tech. 2007; 67:2807–2816.
Alexandre, M., Dubois, P., Sun, T., Garces, J.M., Jérome, R. Polyethylene-layered silicate nanocomposites prepared by the polymerization-filling technique: synthesis and mechanical properties. Polymer. 2002; 43:2123–2132.
Ardanuy, M., Velasco, J.I. Mg–Al Layered double hydroxide nanoparticles. Evaluation of the thermal stability in polypropylene matrix. Appl Clay Sci. 2011; 51:341–347.
Ardanuy, M., Velasco, J.I., Antunes, M., Rodriguez-Perez, M.A., de Saja, J.A. Structure and properties of polypropylene/hydrotalcite nanocomposites. Polym Compos. 2010; 870–878.
Ardanuy, M., Velasco, J.I., Maspoch, M.L., Haurie, L., Fernández, A.I. Influence of EMAA compatibilizer on the structure and properties of HDPE/hydrotalcite nanocomposites prepared by melt mixing. J Appl Polym Sci. 2009; 113:950–958.
Ardanuy, M., Velasco, J.I., Realinho, V., Arencón, D., Martínez, A.B. Non-isothermal crystallization kinetics and activity of filler in polypropylene/Mg-Al layered double hydroxide nanocomposites. Thermochim Acta. 2008; 479:45–52.
Bao, Y.Z., Cong, L.F., Huang, Z.M., Weng, Z.X. Preparation and proton conductivity of poly(vinylidene fluoride)-layered double hydroxide nanocomposite gel electrolytes. J Mater Sci. 2008; 43:390–394.
Bao, Y.Z., Huang, Z.M., Li, S.X., Weng, Z.X. Thermal stability, smoke emission and mechanical properties of poly(vinyl chloride)/hydrotalcite nanocomposites. Polym Degrad Stab. 2008; 93:448–455.
Bao, Y.Z., Huang, Z.M., Weng, Z.X. Preparation and characterization of poly(vinyl chloride)/layered double hydroxides nanocomposite via in situ suspension polymerization. J Appl Polym Sci. 2006; 102:1471–1477.
Bugatti, V., Costantino, U., Gorrasi, G., Nocchetti, M., Tammaro, L., Vittoria, V. Nano-hybrids incorporation into poly(ε-caprolactone) for multifunctional applications: Mechanical and barrier properties. Eur Polym J. 2010; 46:418–427.
Bugatti, V., Gorrasi, G., Montanari, F., Nocchetti, M., Tammaro, L., Vittoria, V. Modified layered double hydroxides in polycaprolactone as a tunable delivery system: In vitro release of antimicrobial benzoate derivatives. App. Clay Sci. 2011; 52(1–2):34–40.
Camino, G., Maffezzoli, A., Braglia, M., de Lazzaro, M., Zammarano, M. Effect of hydroxides and hydroxycarbonate structure on fire retardant effectiveness and mechanical properties in ethylene-vinyl acetate copolymer. Polym Degrad Stab. 2001; 74:457–464.
Cao, F., Wang, Y., Ping, Q., Liao, Z. Zn-Al-NO3-layered double hydroxides with intercalated diclofenac for ocular delivery. Int J Pharma. 2011; 404(1–2):250–256.
Chan, Y.N., Juang, T.Y., Liao, Y.L., Dai, S.A., Lin, J.J. Preparation of clay/epoxy nanocomposites by layered-double-hydroxide initiated self-polymerization. Polymer. 2008; 49:4796–4801.
Chen, W., Feng, L., Qu, B. In situ synthesis of poly(methyl methacrylate)/MgAl layered double hydroxide nanocomposite with high transparency and enhanced thermal properties. Solid State Communications. 2004; 130:259–263.
Chen, W., Feng, L., Qu, B. Preparation of nanocomposites by exfoliation of ZnAl layered double hydroxides in nonpolar LLDPE solution. Chem Mater. 2004; 16:368–370.
Chen, W., Qu, B. Structural characteristics and thermal properties of PE-g-MA/MgAl–LDH exfoliation nanocomposites synthesized by solution intercalation. Chem Mater. 2003; 15:3208–3213.
Chen, W., Qu, B. Enhanced thermal and mechanical properties of poly(methyl acrylate)/ZnAl layered double hydroxide nanocomposites formed by in situ polymerization. Polym Degrad Stab. 2005; 90:162–166.
Chen, X.G., Wu, D.D., Lv, S.S., Zhang, L., Ye, Y., Cheng, J.P. Layered double hydroxide/NaSb(OH)6-poly(vinyl chloride) nanocomposites: Preparation, characterization, and thermal stability. J Appl Polym Sci. 2010; 116:1977–1984.
Choy, J.H., Choul, K.B., Hwan, P.D., Cnpharm Co Ltd KR20090005980 20090123. Nanocomposite comprising eicosapentaenoic acid for preventing hair loss and enhancing hair restoration, composition comprising the nanocomposite. 2010.
Choy, J.H., Choul, K.B., Hwan, P.D., Univ EWHA Ind Collaboration (KR); Cnpharm Co Ltd (KR). Nanocomposite comprising vitamin C for preventing hair loss and enhancing hair restoration, composition comprising the nanocomposite (KR 20100086560-A), 2010. [Application number: KR20090005825 20090123, Priority number: KR20090005825 2009012].
Choy, J.H., Choul, K.B., Hwan, P.D., Univ EWHA Ind Collaboration (KR). Indole-3-acetic acid for preventing hair loss and enhancing hair restoration, nanocomposite comprising the compound, and composition comprising the compound or the nanocomposite (KR 20100086122-A), 2010. [Application number: KR20090005317 20090122; Priority number: KR20090005317 20090122].
Costa, F.R., Abdel-Goad, M., Wagenknecht, U., Heinrich, G. Nanocomposites based on polyethylene and Mg-Al layered double hydroxide. I. Synthesis and characterization. Polymer. 2005; 46:4447–4453.
Costa, F.R., Satapathy, B.K., Wagenknecht, U., Weidisch, R., Heinrich, G. Morphology and fracture behaviour of polyethylene/Mg–Al layered double hydroxide (LDH) nanocomposites. Eur Polym J. 2006; 42:2140–2152.
Costa, F.R., Wagenknecht, U., Heinrich, G. LDPE/MgeAl layered double hydroxide nanocomposite: Thermal and flammability properties. Polym Degrad Stab. 2007; 92:1813–1823.
Costa, F.R., Wagenknecht, U., Jehnichen, D., Goad, M.A., Heinrich, G. Nanocomposites based on polyethylene and Mg-Al layered double hydroxide. Part II. Rheological characterization. Polymer. 2006; 47:1649–1660.
Costantino, U., Gallipoli, A., Nocchetti, M., Camino, G., Bellucci, F., Frache, A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcites. Polym Degrad Stab. 2005; 90:586–590.
Das, A., Wang, D.Y., Leuteritz, A., Subramaniam, K., Greenwell, H.C., et al. Preparation of zinc oxide free, transparent rubber nanocomposites using a layered double hydroxide filler. J Mater Chem. 2011; 21(20):7194–7200.
Dimotakis, E.D., Pinnavaia, T.J. New route to layered double hydroxides intercalated by organic anions: precursors to polyoxometalate-pillared derivates. Inorg Chem. 1990; 29(13):2393–2394.
Ding, P., Qu, B. Synthesis of exfoliated PP/LDH nanocomposites via melt-intercalation: structure, thermal properties, and photo-oxidative behavior in comparison with PP/MMT nanocomposites. Polym Eng Sci. 2006; 14:1153–1159.
Ding, Y., Gui, Z., Zhu, J., Hu, Y., Wang, Z. Exfoliated poly(methyl methacrylate)/MgFe-layered double hydroxide nanocomposites with small inorganic loading and enhanced properties. Mater Res Bulletin. 2008; 43:3212–3220.
Du, L., Qu, B. Structural characterization and thermal oxidation properties of LLDPE/MgAl–LDH nanocomposites. J Mater Chem. 2006; 16:1549–1554.
Du, L., Qu, B. Effects of synthesis conditions on crystal morphological structures and thermal degradation behavior of hydrotalcites and flame retardant and mechanical properties of EVA/hydrotalcite blends. Polym Compos. 2007; 131–138.
Du, L., Qu, B., Xu, Z. Flammability characteristics and synergistic effect of hydrotalcite with microencapsulated red phosphorus in halogen-free flame retardant EVA composite. Polym Degrad Stab. 2006; 91:995–1001.
Du, L., Qu, B., Zhang, M. Thermal properties and combustion characterization of nylon 6/MgAl–LDH nanocomposites via organic modification and melt intercalation. Polym Degrad Stab. 2007; 92:497–502.
Dupin, J.C., Martínez, H., Guimon, C., Dumitriu, E., Fechete, I. Intercalation compounds of Mg-Al layered double hydroxides with dichlophenac: different methods of preparation and physico-chemical characterization. Appl Clay Sci. 2004; 27:95–106.
Ferrara, G., Costantini, E., Consalvi, M., Basell Poliolefine SRL. Polyolefin nanocomposites materials, 2010. [(US 2010304068-A1) Application number: US20080734858 20081015; priority number: US20080734858 20081015; EP20070121629 20071127; US20070005731P 20071207; W02008EP63819 20081015].
Giannelis, E.P. Polymer layered silicate nanocomposites. Adv Mater. 1996; 8:29–35.
Gook, S.K., Kwan, L.Y., Suk, C.M., Hee, P.S., Univ Kyung Hee Univ Ind Coop (KR); Gyeonggi Do (KR). Polymer electrolyte comprising nanocomposite and preparing method thereof(KR 20080105587-A), 2008. [Application number: KR20070053355 20070531; priority number: KR20070053355 20070531].
Gopakumar, T.G., Lee, J.A., Kontopoulou, M., Parent, J.S. Influence of clay exfoliation on the physical properties of montmorillonite/polyethylene composites. Polymer. 2002; 43:5483–5491.
Guo, S., Zhang, C., Peng, H., Wang, W., Liu, T. Structural characterization, thermal and mechanical properties of polyurethane/CoAl layered double hydroxide nanocomposites prepared via in situ polymerization. Compos Sci Tech. 2011; 71:791–796.
He, F.A., Zhang, L.M., Yang, F., Chen, L.S., Wu, Q. New nanocomposites based on syndiotactic polystyrene and organo-modified ZnAl layered double hydroxide. J Polym Res. 2006; 13:483–493.
Herrero, M., Benito, P., Labajos, F.M., Rives, V., Zhu, Y.D., et al. Structural characterization and thermal properties of polyamide 6.6/Mg, Al/adipate-LDH nanocomposites obtained by solid state polymerization). J Solid State Chem. 2010; 183:1645–1651.
Hibino, T., Tsunashima, A. Characterization of repeatedly reconstructed Mg-Al hydrotalcite-like compounds: gradual segregation of aluminium from the structure. Chem Mater. 1998; 10:4055–4061.
Hintze-Bruening, H., Troutier, A.L., Leroux, F. Layered particle based polymer composites for coatings: Part III – Textured coatings obtained via lyotropic liquid crystals. Prog Org Coat. 2011; 70(4):240–244.
Hsueh, H.B., Chen, C.Y. Preparation and properties of LDHs/epoxy nanocomposites. Polymer. 2003; 44:5275–5283.
Hsueh, H.B., Chen, C.Y. Preparation and properties of LDHs/polyimide nanocomposites. Polymer. 2003; 44:1151–1161.
Hu, L., Yuan, Y., Shi, W. Preparation of polymer/LDH nanocomposite by UV-initiated photopolymerization of acrylate through photoinitiator-modified LDH precursor. Mater Res Bulletin. 2011; 46(2):244–251.
Illaik, A., Taviot-Gueho, C., Lavis, J., Commereuc, S., Verney, V., Leroux, F. Unusual polystyrene nanocomposite structure using emulsifier-modified layered double hydroxide as nanofiller. Chem Mater. 2008; 20:4854–4860.
Jaymand, M. Synthesis and characterization of an exfoliated modified syndiotactic polystyrene/Mg-Al-layered double-hydroxide nanocomposite. Polym J. 2011; 43(2):186–193.
Kakati, K., Prakash, A., Pugazhenthi, G. Thermal properties and thermal degradation kinetics of polypropylene/organomodified Ni-Al layered double hydroxide (LDH) nanocomposites prepared by melt intercalation technique. Key Eng Mater. 2011; 471–472:209–214.
Kornmann, X.Synthesis and characterisation of thermoset-clay nanocomposites. Lulea, Sweden: Lulea University of Technology, 2001. [PhD dissertation, Division of Polymer Engineering].
Kotal, M., Kuila, T., Srivastava, S.K., Bhowmick, A.K. Synthesis and characterization of polyurethane/Mg-Al layered double hydroxide nanocomposites. J Appl Polym Sci. 2009; 114:2691–2699.
Kotal, M., Srivastava, S.K., Bhowmick, A.K., Chakraborty, S.K. Morphology and properties of stearate-intercalated layered double hydroxide nanoplatelet-reinforced thermoplastic polyurethane. Polym Int. 2011; 60(5):772–780.
Kuila, T., Acharya, H., Srivastava, S.K., Bhowmick, A.K. Synthesis and characterization of ethylene vinyl acetate/Mg-Al layered double hydroxide nanocomposites. J Appl Polym Sci. 2007; 104:1845–1851.
Kuila, T., Srivastava, S.K., Bhowmick, A.K., Saxena, A.K. Thermoplastic polyolefin based polymer – blend-layered double hydroxide nanocomposites. Compos Sci Tech. 2008; 68:3234–3239.
Lee, W.D., Im, S.S. Thermomechanical properties and crystallization behavior of layered double hydroxide/poly(ethylene terephthalate) nanocomposites prepared by in situ polymerization. JPolym Sci B: Polym Phys. 2007; 45:28–40.
Lee, W.D., Im, S.S., Lim, H.M., Kim, K.J. Preparation and properties of layered double hydroxide/poly(ethyleneterephthalate) nanocomposites by direct melt compounding. Polymer. 2006; 47:1364–1371.
Leroux, F., Illaik, A., Stimpfling, T., Troutier-Thuilliez, A.L., Fleutot, S., et al. Percolation network of organo-modified layered double hydroxide platelets into polystyrene showing enhanced rheological and dielectric behavior. J Mater Chem. 2010; 20:9484–9494.
Leroux, F., Illaik, A., Verney, V. A comprehensive study of an unusual jammed nanocomposite structure using hybrid layered double hydroxide filler. J Coll Inter Sci. 2009; 332:327–335.
Leroux, F., Meddar, L., Mailhot, B., Morlat-Therias, S., Gardette, J.L. Characterization and photooxidative behaviour of nanocomposites formed with polystyrene and LDHs organo-modified by monomer surfactant. Polymer. 2005; 46:3571–3578.
Liu, J., Chen, G., Yang, J. Preparation and characterization of poly(vinyl chloride)/layered double hydroxide nanocomposites with enhanced thermal stability. Polymer. 2008; 49:3923–3927.
Liu, J., Chen, G., Yang, J., Ma, Y. New facile preparation of a poly(vinyl chloride)/layered double hydroxide nanocomposite via solution intercalation. Chem Lett. 2007; 36(12):1454–1455.
Lonkar, S.P., Morlat-Therias, S., Caperaa, N., Leroux, F., Gardette, J.L., Singh, R.P. Preparation and nonisothermal crystallization behavior of polypropylene/layered double hydroxide nanocomposites. Polymer. 2009; 50:1505–1515.
Lonkar, S.P., Therias, S., Capera, N., Leroux, F., Gardette, J.L. Photooxidation of polypropylene/layered double hydroxide nanocomposites: Influence of intralamellar cations. Eur Polym J. 2010; 46:1456–1464.
Lv, S., Yuan, Y., Shi, W. Strengthening and toughening effects of layered double hydroxide and hyperbranched polymer on epoxy resin. Prog Org Coat. 2009; 65:425–430.
Lv, S., Zhou, W., Miao, H., Shi, W. Preparation and properties of polymer/LDH nanocomposite used for UV curing coatings. Prog Org Coat. 2009; 65:450–456.
Manzi-Nshuti, C., Chen, D., Su, S., Wilkie, C.A. Structure-property relationships of new polystyrene nanocomposites prepared from initiator-containing layered double hydroxides of zinc aluminum and magnesium aluminum. Polym Degrad Stab. 2009; 94:1290–1297.
Manzi-Nshuti, C., Hossenlopp, J.M., Wilkie, C.A. Fire retardancy of melamine and zinc aluminum layered double hydroxide in poly(methyl methacrylate). Polym Degrad Stab. 2008; 93:1855–1863.
Manzi-Nshuti, C., Hossenlopp, J.M., Wilkie, C.A. Comparative study on the flammability of polyethylene modified with commercial fire retardants and a zinc aluminum oleate layered double hydroxide. Polym Degrad Stab. 2009; 94:782–788.
Manzi-Nshuti, C., Wang, D., Hossenlopp, J.M., Wilkie, C.A. Aluminum-containing layered double hydroxides: the thermal, mechanical, and fire properties of (nano) composites of poly(methyl methacrylate). J Mater Chem. 2008; 18:3091–3102.
Marangoni, R., da Costa-Gardolinski, J.E.F., Mikowski, A., Wypych, F. PVA nanocomposites reinforced with Zn2Al LDHs, intercalated with orange dyes. J Solid State Electrochem. 2011; 15(2):303–311.
Marangoni, R., Taviot-Gueho, C., Illaik, A., Wypych, F., Leroux, F. Organic inorganic dye filler for polymer: Blue-coloured layered double hydroxides into polystyrene. J Coll Inter Sci. 2008; 326:366–373.
Martínez, A.B., Realinho, V., Antunes, M., Maspoch, M.L., Velasco, J.I. Microcellular foaming of layered double hydroxide-polymer nanocomposites. Ind Eng Chem Res. 2011; 50(9):5239–5247.
Martínez-Gallegos, S., Herrero, M., Rives, V. In situ microwave-assisted polymerization of polyethylene terephtalate in layered double hydroxides. J Appl Polym Sci. 2008; 109:1388–1394.
Matusinovic, Z., Rogosic, M., Sipusic, J. Synthesis and characterization of poly(styrene-co-methyl methacrylate)/layered double hydroxide nanocomposites via in situ polymerization. Polym Degrad Stab. 2009; 94:95–101.
Meyn, M., Beneke, K., Lagaly, G. Anion-exchange reactions of layered double hydroxides. Inorg Chem. 1990; 29:5201–5207.
Miyata, S. Physico-chemical properties of synthetic hydrotalcites in relation to compositions. Clay Miner. 1980; 28:50–56.
Miyata, S., Kumura, T. Synthesis of new hydro talcite-like compounds and their physico-chemical properties. Chem Lett. 1973; 843–848.
Morgan, A.B., Gilman, J.W. Characterization of polymer-layered silicate (clay) nanocomposites by transmission electron microscopy and X-ray diffraction: A comparative study. J Appl Polym Sci. 2003; 87:1329–1338.
Newman, S.P., Jones, W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J Chem. 1998; 105–115.
Nyambo, C., Wilkie, C.A. Layered double hydroxides intercalated with borate anions: Fire and thermal properties in ethylene vinyl acetate copolymer. Polym Degrad Stab. 2009; 94:506–512.
Nyambo, C., Chen, D., Su, S., Wilkie, C.A. Does organic modification oflayered double hydroxides improve the fire performance of PMMA? Polym Degrad Stab. 2009; 94:1298–1306.
Nyambo, C., Kandare, E., Wilkie, C.A. Thermal stability and flammability characteristics of ethylene vinyl acetate (EVA) composites blended with a phenyl phosphonate-intercalated layered double hydroxide (LDH), melamine polyphosphate and/or boric acid. Polym Degrad Stab. 2009; 94:513–520.
Nyambo, C., Songtipya, P., Manias, E., Jimenez-Gasco, M.M., Wilkie, C.A. Effect of MgAl-layered double hydroxide exchanged with linear alkyl carboxylates on fire-retardancy of PMMA and PS. J Mater Chem. 2008; 18:4827–4838.
Okada, A., Kawasumi, M., Usuki, A., Kojima, Y., Kurauchi, T., Kamigaito, O. Synthesis and properties of nylon-6/clay hybrids. In: Schaefer D.W., Mark J.E., eds. Polymer based molecular composites. Pittsburgh: MRS Symposium Proceedings; 1990:45–50. [171].
Pavlidoua, S., Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Progr Polym Sci. 2008; 33:1119–1198.
Peng, H., Han, Y., Liu, T., Tjiu, W.C., He, C. Morphology and thermal degradation behavior of highly exfoliated CoAl-layered double hydroxide/polycaprolactone nanocomposites prepared by simple solution intercalation. Thermochim Acta. 2010; 502:1–7.
Peng, H.D., Tjiu, W.C., Shun, L., Huang, S., He, C.B., Liu, T.X. Preparation and mechanical properties of exfoliated CoAl layered double hydroxide (LDH)/polyamide 6 nanocomposites by in situ polymerization. Compos Sci Tech. 2009; 69:991–996.
Pinnavaia, T.J., Beall, G.W.Polymer-clay Nanocomposites. England: John Wiley & Sons, 2001.
Pradhan, S., Costa, F.R., Wagenknecht, U., Jehnichen, D., Bhowmick, A.K., Heinrich, G. Elastomer/LDH nanocomposites: Synthesis and studies on nanoparticle dispersion, mechanical properties and interfacial adhesion. Eur Polym J. 2008; 44:3122–3132.
Pucciariello, R., Tammaro, L., Villani, V., Vittoria, V. New nanohybrids of poly(ε-caprolactone) and a modified Mg/Al hydrotalcite: mechanical and thermal properties. J Polym Sci B: Polym Phys. 2007; 45:945–954.
Qiu, L., Chen, W., Qu, B. Structural characterisation and thermal properties of exfoliated polystyrene/ZnAl layered double hydroxide nanocomposites prepared via solution intercalation. Polym Degrad Stab. 2005; 87:433–440.
Qiu, L., Chen, W., Qu, B. Morphology and thermal stabilization mechanism of LLDPE/MMT and LLDPE/LDH nanocomposites. Polymer. 2006; 47:922–930.
Qiu, L., Qu, B. Preparation and characterization of surfactant-free polystyrene/layered double hydroxide exfoliated nanocomposite via soap-free emulsion polymerization. J Coll Inter Sci. 2006; 301:347–351.
Ramaraj, B., Nayak, S.K., Yoon, K.R. Poly(vinyl alcohol) and layered double hydroxide composites: Thermal and mechanical properties. J Appl Polym Sci. 2010; 116:1671–1677.
Ramaraj, B., Yoon, K.R. Thermal and phy sicomechanical properties of ethylenevinyl acetate copolymer and layered double hydroxide composites. J Appl Polym Sci. 2008; 108:4090–4095.
Realinho, V., Antunes, M., Arencón, D., Fernandez, A.I., Velasco, J.I. Effect of a dodecylsulfate-modified magnesium-aluminum layered double hydroxide on the morphology and fracture of polystyrene and poly(styrene-coacrylonitrile) composites. J Appl Polym Sci. 2009; 111:2574–2583.
Rives, V.Layered Double Hydroxides: Present and Future. New York: Nova Science Publishers, 2001.
Rives, V. Characterisation of layered double hydroxides and their decomposition products. Mater Chem Phys. 2002; 75:19–25.
Romeo, V., Gorrasi, G., Vittoria, V. Encapsulation and exfoliation of inorganic lamellar fillers into polycaprolactone by electrospinning. Biomacromol. 2007; 8(10):3147–3152.
Schonhals, A., Goering, H., Costa, F.R., Wagenknecht, U., Heinrich, G. Dielectric properties of nanocomposites based on polyethylene and layered double hydroxide. Macromol. 2009; 42:4165–4174.
Shi, Y., Chen, F., Yang, J., Zhong, M. Crystallinity and thermal stability of LDH/polypropylene nanocomposites. Appl Clay Sci. 2010; 50:87–91.
Sorrentino, A., Gorasi, G., Tortora, M., Vittoria, V., Constantino, U., et al. Incorporation of Mg-Al hydrotalcite into biodegradable poly(ε-caprolactone) by high energy ball milling. Polymer. 2005; 46:1601–1608.
Tagaya, H., Sato, S., Morioka, H., Kadokawa, J., Karasu, M., Chiba, K. Preferential intercalation of isomers of naphthalenecarboxylate ions into the interlayer of layered double hydroxides. Chem Mater. 1993; 5:1431–1433.
Taviot-Gueho, C., Illaik, A., Vuillermoz, C., Commereuc, S., Verney, V., Leroux, F. LDH−dye hybrid material as coloured filler into polystyrene: Structural characterization and rheological properties. J Phys Chem Solids. 2007; 68:1140–1146.
Trujillano, R., Holgado, M.J., Rives, V. Alternative synthetic routes for NiAl layered double hydroxides with alkyl and alkylbenzene sulfonates. In: Aiello R., Giordano G., Testa F., eds. Studies in Surface Science and Catalysis. Elsevier Science; 2002:142.
Tsai, T.Y., Lu, S.W., Li, F.S. Preparation and characterization of epoxy/layered double hydroxides nanocomposites. J Phys Chem Solids. 2008; 69:1386–1390.
Tseng, C.H., Hsueh, H.B., Chen, C.Y. Effect of reactive layered double hydroxides on the thermal and mechanical properties of LDHs/epoxy nanocomposites. Compos Sci Tech. 2007; 67:2350–2362.
Ulibarri, M.A., Labajos, F.M., Rives, V., Trujillano, R., Kagunya, W., Jones, W. Comparative study of the synthesis and properties of vanadate-exchanged layered double hydroxides. Inorg Chem. 1994; 33:2592–2599.
Vaia, R.A., Structural characterization of polymer-layered silicate nanocompositesPinnnavaia T.J., Beall G.W., eds. Polymer-Clay Nanocomposites, Wiley Series in Polymer Science. 2001:229–263.
Wagner, R., Reisinger, T.J.G. A rheological method to compare the degree of exfoliation of nanocomposites. Polymer. 2003; 44:7513–7518.
Wang, D.Y.W., Das, A., Costa, F.R., Leuteritz, A., Wang, Y.Z., et al. Synthesis oforgano cobalt-aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame retardant nanofiller in PP. Langmuir. 2010; 26(17):14162–14169.
Wang, D.Y.W., Leuteritz, A., Kutlu, B., Landwehr, M.A.D., Jehnichen, D., et al. Preparation and investigation of the combustion behavior of polypropylene/organomodified MgAl–LDH micro-nanocomposite. J Alloys and Compounds. 2011; 509(8):3497–3501.
Wang, D.Y., Liu, L., Wang, Y.Z., Stec, A.A., Hull, T.R., Price, D. Effect of metal chelates on the ignition and early flaming behaviour of intumescent fire-retarded polyethylene systems. Polym Degrad Stab. 2008; 93:1024–1030.
Wang, L., Su, S., Chen, D., Wilkie, C.A. Variation of anions in layered double hydroxides: Effects on dispersion and fire properties. Polym Degrad Stab. 2009; 94:770–781.
Wang, G.A., Wang, C.C., Chen, C.Y. The disorderly exfoliated LDHs/PMMA nanocomposite synthesized by in situ bulk polymerization. Polymer. 2005; 46:5065–5074.
Wang, G.A., Wang, C.C., Chen, C.Y. The disorderly exfoliated LDHs/PMMA nanocomposites synthesized by in situ bulk polymerization: The effects of LDH-U on thermal and mechanical properties. Polym Degrad Stab. 2006; 91:2443–2450.
Xu, Z.P., Saha, S.K., Braterman, P.S., d’Souza, N. The effect of Zn, Al layered double hydroxide on thermal decomposition of poly(vinyl chloride). Polym Degrad Stab. 2006; 91:3237–3244.
Yongzhong, Y.B., Zhiming, H., Zhixue, W., Univ Zhejiang. Nano composite resin ofpoly(vinyl chloride)/hydrotalcite, 2005. [(CN 1563179-AApplicant number: CN20041017944 20040421; priority number: CN20041017944 20040421].
Yuan, Y., Shi, W. Preparation and properties of UV-cured acrylated silane intercalated polymer/LDH nanocomposite. Mater Res Bul. 2011; 46(1):124–129.
Zammarano, M., Bellayer, S., Gilman, J.W., Franceschi, M., Beyer, F.L., Harris, R.H., Meriani, S. Delamination of organo-modified layered double hydroxides in polyamide 6 by melt processing. Polymer. 2006; 47:652–662.
Zammarano, M., Franceschi, M., Bellayer, S., Gilman, J.W., Meriani, S. Preparation and flame resistance properties ofrevolutionary self-extinguishing epoxy nanocomposites based on layered double hydroxides. Polymer. 2005; 46:9314–9328.
Zhang, M., Ding, P., Du, L., Qu, B. Structural characterization and related properties of EVA/ZnAl–LDH nanocomposites prepared by melt and solution intercalation. Mater Chem Phys. 2008; 109:206–211.
Zhang, M., Ding, P., Qu, B., Guan, A. A new method to prepare flame retardant polymer composites. J Mater Proces Tech. 2008; 208(1–3):342–347.
Zhang, G., Ding, P., Zhang, M., Qu, B. Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym Degrad Stab. 2007; 92:1715–1720.
Zhao, Y., Yang, W., Xue, Y., Wang, X., Lin, T. Partial exfoliation of layered double hydroxides in DMSO: A route to transparent polymer nanocomposites. J Mater Chem. 2011; 21(13):4869–4874.
Zhu, Y.D., Allen, G.C., Adams, J.M., Gittins, D., Herrero, M., et al. Dispersion characterization in layered double hydroxide/nylon 66 nanocomposites using FIB imaging. J Appl Polym Sci. 2008; 108:4108–4113.
Zubitur, M., Gómez, M.A., Cortazar, M. Structural characterization and thermal decomposition of layered double hydroxide/poly(p-dioxanone) nanocomposites. Polym Degrad Stab. 2009; 94:804–809.