Polyolefin-based polymer nanocomposites
Abstract:
This chapter describes the preparation, characterization and properties of polyolefin-based nanocomposites, including ethylene vinyl acetate, polyethylene, polypropylene, and ethylene propylene diene monomer, which are widely used. To prepare nanocomposites, layered clay minerals (sodium montmorillonite, bentonite, Cloisite, Laponite, layered double hydroxide) and carbon-based nanomaterials (graphite, carbon nanofibers, exfoliated graphite, carbon nanotubes, graphene) are used as fillers. The good mechanical and thermal properties of nanocomposites are due to interfacial interactions and homogeneous dispersion of the nanofiller in the polymer matrix. Carbon-based nanofillers have excellent electrical conductivity and electromagnetic interference shielding compared with the pure polymer. Conductive composites are used to fabricate memory devices, shielding materials, nanoelectronics, etc.
7.1 Introduction
Polymer nanocomposites are a multiphase solid material in which one of the phases has dispersed in the other phase at a nanometer level. Work on these materials was initiated by Toyota in the late 1980s, when they manufactured a nylon/clay nanocomposite for the timing belt cover for the Toyota Camry automobile.1, 2 The nanocomposite showed significantly improved physicochemical properties normally absent in pure polymers, or traditional microcomposites and macrocomposites based on conventional fillers like talc, glass fiber, calcium carbonate, aluminum (and magnesium) hydroxide, etc.3–6 Ongoing research shows that nanocomposites exhibit improved mechanical, thermal, gas-barrier, flame retardant, and swelling properties.3–7 Based on these improved physicochemical properties they have been widely used in the automobile industry, aerospace and construction engineering, electronics, biomedical applications, etc.3–13 The properties of polymer nanocomposites depend on the local chemistry, degree of curing, polymer chain mobility, and degree of crystallinity.14 Till now, extensive research has been carried out with different organic and inorganic polymers to explore these materials.15–22 Sodium montmorillonite (Na-MMT), sodium bentonite, hectorite, Laponite, etc. have been widely used as fillers in a polymer matrix.3, 4, 14 In most cases, organo-modified clay (OMC) has been used as a nanofiller to give homogeneous dispersion and a better filler/polymer interaction. With the progress in nanoscience and technology, different types of nanofillers, such as layered double hydroxide (LDH), exfoliated graphite (EG), exfoliated graphite nanoplatelets (xGnPs), metal nanoparticles, carbon black (CB), carbon nanotubes (CNTs), carbon nanofibers (CNFs), and graphene, have been used in the preparation of polymer nanocomposites.6, 23–30 The successful application of clay minerals and LDH as a nanofiller in the preparation of polymer nanocomposites is well established. In contrast, EG, xGnP, CNT, and CNF as nanofillers have been studied less often. However, the high production cost of CNTs limits their large-scale application as nanofillers.31 The recent development of graphene has added a new dimension to the field of multifunctional polymer nanocomposites.
This chapter reviews the preparation, characterization, and properties of polyolefins and polyolefin/inorganic nanocomposites. The polyolefins considered are ethylene vinyl acetate copolymer (EVA), ethylene propylene diene rubber (EPDM), polyethylene (PE), and polypropylene (PP). EVA is available in different grades, such as rubber, thermoplastic elastomer, and plastic. This copolymer has countless uses in different fields, such as for electrical cable insulation, cable jacketing and repair, waterproofing, packaging, etc.32 EPDM, a terpolymer of ethylene, propylene, and an unconjugated diene, is one of the most versatile elastomers in use today and it is an important material used in a wide variety of engineering applications.33, 34 These polymers derive their usefulness and versatility from their inherent toughness, chemical resistance, and good mechanical and electrical properties. PE and PP have the ability to elongate under stress, allowing them to maintain their integrity under differential settlement conditions without puncturing, tearing, or cracking.34, 35 Both of these polymers have a wide variety of applications including packaging, textiles (e.g., ropes, thermal underwear, and carpets), stationery, plastic parts, reusable containers of various types, laboratory equipment, loudspeakers, automotive components, and polymer banknotes.34 In order to extend these applications, the development of mechanically durable and thermally stable nanocomposite materials is required.
7.2 Preparation of polymer nanocomposites
Polymer/inorganic nanocomposites can be prepared by in situ polymerization and by blending processes, of which there are basically two types: solution blending and melt blending3, 5, 6 With in situ polymerization, the nanofillers are dispersed in the monomers and polymerization occurs in the presence of initiators. In solution blending, the nanofillers are first dispersed in the solvent and then the polymer is dissolved in another batch of the same solvent. Finally, the dispersed nanofiller solution is added to the polymer solution, stirred for the desired time and the solvent is removed at reduced pressure to produce the polymer composites. In contrast, with melt blending the nanofillers are added to a molten polymer. The distribution of the nanofiller is better with solution blending and in situ polymerization compared with melt blending. However, melt blending is regarded as a cost-effective, environmentally friendly, and industrially accepted method in comparison with the others.
7.3 Different kinds of nanofillers used in the preparation of polymer nanocomposites
Different types of inorganic fillers, e.g. clay minerals, LDH, CNT, EG, graphene, etc., have often been used in the preparation of polyolefin nanocomposites. Of these, clay and LDH are hydrophilic in nature while CNT, EG, and graphene are purely carbonaceous.15–30 In contrast, the polymeric materials are hydrophobic in nature. Therefore, all of these nanofillers will form phase-separated composites with a polymer. So, surface modification is required.
7.3.1 Surface modification of montmorillonite
The surface modification of clay minerals is a well-known method and has been used frequently to obtain organically modified clay (OMC). MMT is made organophilic by exchanging the inorganic cations (usually Na+ or Ca2+) in the interlayer space with organic cations such as alkyl or aryl ammonium (or phosphonium) ions.36 In a typical process, inorganic clay minerals are dispersed in water by stirring at 80 °C. The aliphatic amine/ammonium or phosphonium ions are dissolved in water. Then the organic amine/ammonium or phosphonium solution is added to the clay dispersion and stirred for about 2–3 hours. The incorporation of ammonium ions not only changes the hydrophilic nature of the clay but also expands the interlayer spacing, which, in turn, depends on the number of carbon atoms in the organic ammonium ions.37, 38
7.3.2 Surface modification of layered double hydroxide (LDH)
Pure LDH materials are unsuitable for intercalation by large species like polymers due to the small interlayer gallery spacing of pure LDH (approximately 0.76 nm). The small gallery spacing will not allow polymer chains to penetrate and the charged metal-hydroxide layer makes them incompatible with the non-polar species. In order to overcome these issues, different anionic surfactants like sodium dodecylbenzene sulfonate (SDBS), sodium dodecyl sulfate (SDS), aromatic carboxylate anions, aliphatic carboxylate anions, phosphonates, and polymeric anions, etc., have been used for organo modification.39, 40
7.3.3 Surface modification of carbon nanotubes (CNTs)
The surface modification of CNTs can be performed by covalent or non-covalent techniques. In non-covalent modification, small molecular weight polymers or aromatic molecules are attached to the surface of a CNT by the π/π interaction. The advantage of non-covalent attachment is that the perfect structure of the nanotube is not altered. Therefore, its physicochemical properties are not expected to change significantly. However, the disadvantage of non-covalent attachment is the weak force of attraction between the wrapping molecule and the nanotube. This may lead to poor load transfer when CNTs are used for composite applications. In contrast, a strong chemical bond is formed between the carboxyl functionality of CNT and an organic modifier. In both cases, the functionalized graphene disperses well in different organic solvents, facilitating its homogeneous dispersion in a polymer matrix.41–44
7.3.4 Surface modification of graphene
Graphene can be made water dispersible or organic-solvent dispersible by surface modification with graphene oxide. The reduction of functionalized graphene produces organo-modified graphene. Different kinds of surface-modifying agents such as organic amine, amino acids, amine-terminated ionic liquids, biomolecules, low molecular weight polymers, etc., have been used for the surface modification of graphene6, 45–48 Graphene can be prepared, with an organophilic surface, from graphite using electrochemical methods in the presence of ionic liquids.49 Very recently, organo-modified graphene has been synthesized by the sonication of flake graphite in the presence of 9-anthracene carboxylic acid.50
7.4 Characterization of polymer nanocomposites
The properties of polymer nanocomposites are highly dependent on the nature of the polymer (polar or non-polar), the nanofiller, the types of interaction between the filler and polymer, the nature of surface modification, and, certainly, on the distribution of the nanofiller in the polymer matrix. Depending on the distribution of the nanofiller, polymer inorganic nanocomposites are classified into three categories: intercalated nanocomposites, exfoliated nanocomposites, and immiscible nanocomposites. It has been reported that the improvement in physicochemical properties of exfoliated nanocomposites is much better in comparison to intercalated nanocomposites or conventional composites. The characterization and determination of the nanostructure of polymer/inorganic composites can be achieved using X-ray diffraction (XRD), transmission electron microscopy (TEM), and Fourier transform infrared spectroscopy (FT-IR).
7.4.1 X-ray diffraction (XRD) and transmission electron microscopy (TEM) analysis
The structure of a nanocomposite can be established by XRD. Due to the ease of use and its availability, XRD has been used widely to probe the structure of nanocomposites. When the layers are exfoliated and far apart, XRD will not show a peak in the overall diffraction diagram. In contrast, a small and broad peak is obtained at a lower angle compared with the pure nanofiller for intercalated or partially exfoliated nanocomposites. For immiscible composites, the original peaks of the clay layers will be retained completely in the composite. Figure 7.1(a) shows the XRD pattern of intercalated, exfoliated, and partially exfoliated nanocomposites.3 However, XRD results cannot always be taken as absolute evidence for nanolayer exfoliation, e.g. if clay/LDH layers are partially exfoliated in the polymer matrix and do not have sufficiently well-defined layered structures for XRD, then the characteristic diffractions for the basal spacing cannot be detected by bulk XRD apparatus. Moreover, if the basal spacing between layers is enlarged due to the intercalation of polymer molecules, the characteristic peak will shift to a lower angle, beyond the XRD scan range (<2°). Therefore, TEM studies have to be made before drawing any definite conclusion. TEM provides a qualitative understanding of the internal structure of a nanocomposite material. In the case of intercalated nanocomposites, the clay layers will remain parallel to each other. In contrast, for exfoliated nanocomposites, the clay layers are homogeneously and randomly dispersed in the matrix polymer. The morphology of different kinds of nanocomposite is shown in Fig. 7.1(b).3

7.1 (a) XRD patterns and (b) TEM images of three different types of nanocomposite. Reproduced from Ray and Okamoto3 by permission of Elsevier Science Ltd, UK. OMLS: Organomodified layered silicate.
7.4.2 Fourier transform infra red spectroscopy (FT-IR) analysis
The presence of a nanofiller in a polymer matrix and their interaction can be evidenced by FT-IR data analysis. Acharya et al. showed that hexadecylamine-modified clay (16Me-MMT) can be successfully doped onto an EPDM matrix. FT-IR spectra of Na+-MMT, 16Me-MMT, pure EPDM and its hybrid with 4 wt.% 16Me-MMT are shown in Fig. 7.2.51 Kuila et al. showed that dodecyl-sulfate-modified LDH (DS-LDH) can be successfully grafted onto the surface of an EVA-28 matrix.52, 53 The appearance of new peaks for the EVA-28/DS-LDH nanocomposites at about 3500 cm–1 and lattice vibration bands in the region 500–800 cm–1, which are absent for EVA-28, indicated that DS-LDH was grafted onto the EVA surfaces.
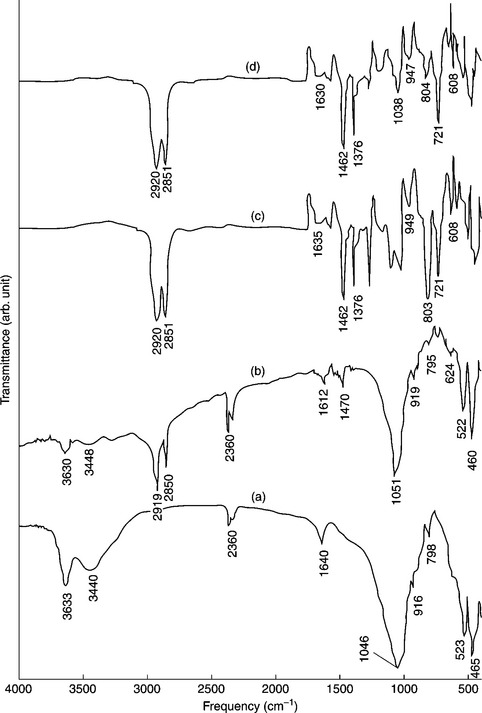
7.2 FT-IR spectra of (a) Na+-MMT, (b) 16Me-MMT, (c) EPDM, and (d) EPDM 4 wt.% 16Me-MMT Reproduced from Acharya et al.51 by permission of Wiley-VCH, Germany.
7.5 Properties of polymer nanocomposites
The important aspects of nanocomposite materials are their improved mechanical, thermal, gas-barrier, swelling, and flame-retardant properties. These improved properties make them the best type of composite and they find application in many different technological fields. However, performance during use is a key feature of any composite material and determines the real fate of the products, especially for outdoor applications. Therefore, in this section, we will discuss the significant properties of polyolefin-based nanocomposites.
7.5.1 Mechanical properties
Polymeric materials play an important role in the research and development of modern science and technology. For specific applications, such as in medical science, industrial conveyor belts, hosepipes, car bodies, component packaging, etc., special types of polymer with specific mechanical properties are required. It is necessary to know mechanical properties such as tensile strength (TS), elongation at break (EB), and tensile modulus before their application in these specific areas. Dynamic mechanical analysis (DMA) is used to study the mechanical properties of materials as they deform under periodic forces. DMA measures changes in the elastic modulus (E′), loss modulus (E′), as well as tan δ(E″/E′), with temperature. A sinusoidal mode of deformation is applied to a sample, as the temperature is scanned from well below the glass transition temperature (Tg) to the point when the sample becomes too soft to test. DMA provides the first- and second-order transitions (Tm and Tg. respectively), the degree of phase separation, crystallinity, cross-linking of the polymer, and mechanical properties such as the glassy state and rubbery plateau moduli.
Tensile properties
Pramanik et al. showed that TS increases when increasing the matrix polarity. Figure 7.3 shows that the TS of EVA/dodecyl-amine-modified montmorillonite (12Me-MMT) nanocomposites are higher in comparison to neat EVA-45.19, 54 But the EB of the nanocomposites decreases compared with pure EVA. However, for EVA-28/12Me-MMT nanocomposites, both TS and EB increase with up to 8 wt.% of filler loadings. According to Cui et al., the stress at any given strain level increases as the content of OMC increases.55 As the vinyl acetate (VA) content in EVA increases, crystallinity decreases, causing the modulus to decrease and the EB to increase. Cardenas et al. used bentonite and aluminum trihydrate (ATH) as a filler and studied the effect of filler loading on mechanical properties.56 It was noted that the EB values are higher for uncoated and fatty-acid-coated ATH fillers, while TS did not change for all the nanocomposites. In contrast, a silane-coated ATH-filler-based nanocomposite showed an important loss of deformability. This is attributed to lower compatibility between the silane modifier and the other components of the nanocomposite. Zhang et al. proved that Mg-Al LDH is an efficient filler, which can increase the EB of EVA-28 nanocomposites. But the TS of nanocomposites decreases with the addition of Mg-Al LDH.57 Kuila et al. observed that the TS and EB of EVA/organo-modified LDH (OLDH) nanocomposites increases when increasing the polarity of the EVA matrix. At lower loading (1, 3, 5 wt.%), both the TS and EB are higher than for pure EVA. However, at higher filler loading the mechanical properties were found to deteriorate due to the aggregation of OLDH in the EVA.58, 59 According to George and Bhowmick, the TS and tensile modulus at 100% elongation are always higher for EVA (EVA-40, 50, 60, and 70)/multi-walled carbon nanotube (MWCNT) (4 wt.%) nanocomposites. But the EB for all the nanocomposites was less than for pure EVA. George and Bhowmick also studied the effect of EG and CNF on the mechanical properties of EVA nanocomposites.60 They found that the reinforcing efficiency of EG and CNF is much higher than with MWCNTs. This may be attributed to the relatively poor dispersion of MWCNTs compared with the other two fillers. It was also noted that the increment is more prominent in the partially amorphous to fully amorphous transition region, which occurs beyond 50% VA content.
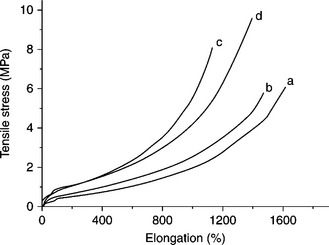
7.3 Tensile stress versus elongation curves of pure EVA (a), 12Me-MMT (2 wt.%) and EVA (b), 12Me-MMT (4 wt.%) and EVA (c), and 12Me-MMT (6 wt.%) and EVA (d). Reproduced from Pramanik et al.19 by permission of Wiley-VCH, Germany.
Acharya et al. found that the TS and EB of EPDM/clay nanocomposites increases when increasing the 16Me-MMT content up to 4 wt.%. Further addition of OMC does not influence either the TS or the EB significantly.51 Acharya et al. also studied the effect of DS-LDH on the mechanical properties of EPDM/DS-LDH nanocomposites.18 The TS and EB increase gradually when increasing DS-LDH content in the nanocomposites. Ismail et al. observed that the TS of EPDM/halloysite nanotube (HNT) composites increases gradually on increasing the HNT content.61 The TS and EB of maleic-anhydride-grafted EPDM (MAH-g-EPDM)/EPDM/HNT nanocomposites was much higher than that of EPDM/HNT nanocomposites.62 Kang et al. showed the effect of CNTs and OMC on the mechanical properties of EPDM nanocomposites. The TS increased gradually up to 50 wt.% of CNT loading in the nanocomposites. In contrast, the TS started to decrease with only 20 wt.% of CNT and OMC (mixed) loading.63
The addition of OMC can efficiently improve TS, flexural strength, and flexural modulus of PE nanocomposites.64 However, the degree of improvement is highly dependent on the nature of the organic modifier. The reinforcing efficiency of short alkyl chain modifiers is much better compared with long chain modifiers. This may be due to the plasticization effect of the long chain modifier. Organic-copolymer-modified clay is not regarded as a suitable filler because of its mechanical properties relevant for reinforcement.65, 66 A deterioration of TS and EB was noted after increasing the content of copolymer-modified clay in PE nanocomposites. However’ the moduli of the nanocomposites increased significantly with OMC loading. Similar observations were made when pristine LDH was used as a filler in the preparation of PE/LDH nanocomposites.67 The addition of OLDH was also unable to enhance the TS and EB of linear low-density polyethylene (LLDPE)/OLDH nanocomposites. However, Young’s modulus of the nanocomposites increased significantly on increasing the concentration of OLDH.68 Kanagaraj et al. studied the effect of CNT loading on the mechanical behavior of high-density polyethylene (HDPE)/CNT composites, as shown in Table 7.1.69 Note that CNT can act as a reinforcing filler to improve the mechanical properties of HDPE/CNT composites. The effects of exfoliated graphite on the mechanical properties of LLDPE and HDPE have been studied extensively.26, 70–72 For HDPE, the presence of EG slashes the ductility of the HDPE’ which showed necking without breaking in a tensile test. Kim et al. observed that the addition of xGnPs can efficiently increase the TS and Young’s modulus of LLDPE/xGnP nanocomposites, but with a deterioration of EB.72 Figure 7.4 shows stress–strain plots of LLDPE/xGnP nanocomposites with different loadings of xGnP. Kim et al. also studied the effect of paraffin-coated xGnP on the mechanical behavior of LLDPE/paraffin-coated xGnP nanocomposites.26 It was found that both the TS and Young’s modulus of the LLDPE/paraffin-coated xGnP nanocomposites increased gradually with up to 30 wt.% of filler loading. The mechanical behavior of the nanocomposites was correlated in terms of tensile fracture of the tested specimens. Figure 7.5 shows scanning electron microscopy (SEM) images of LLDPE/paraffin-coated xGnP nanocomposites for different filler loadings. Graphene has also been suggested as a reinforcing filler to improve the mechanical properties of PE-based nanocomposites. Kuila et al. showed that dodecyl amine functionalized graphene (DA-G) can enhance the TS and Young’s modulus of LLDPE/DA-G nanocomposites with up to 5 wt.% of DA-G content.35 Wang et al. found that reinforcement by graphene resulted in an increase of up to 27.0 and 92.8% in the TS and Young’s modulus of the nanocomposites, respectively.73 Young’s modulus of thermally reduced graphene oxide/LLDPE composites was also higher in comparison to neat LLDPE.
Table 7.1
Mechanical properties of HDPE/CNT nanocomposites
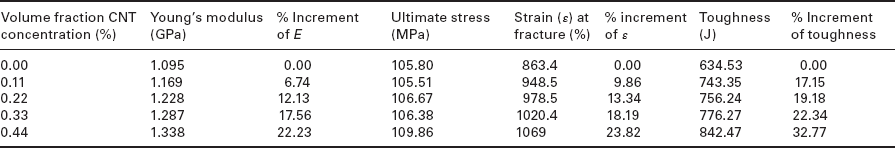
Reproduced from Kanagaraj et al.69 by permission of Elsevier Science Ltd, UK.
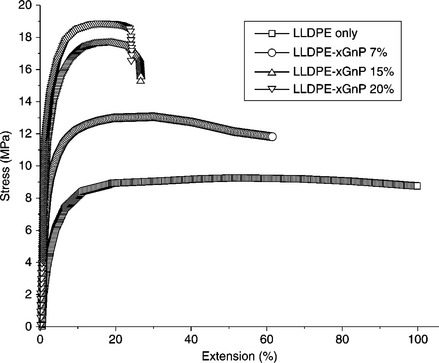
7.4 Stress–strain curves of LLDPE/xGnP nanocomposites produced by solution mixing against xGnP loading. Reproduced from Kim et al.71 by permission of Wiley-VCH, Germany.

7.5 SEM images of the fracture surface of LLDPE/paraffin/xGnP nanocomposites with (a) 10 wt.%, (b) 20 wt.%, and (c, d) 30 wt.% of xGnP (5 wt.%)/paraffin loading. Reproduced from Kim and Drzal26 by permission of Wiley-VCH, Germany.
Cui et al. and Kim et al. noticed that the TS of PP/OMC and maleic-anhydride-grafted polypropylene (PP-g-MA)/OMC nanocomposites decreased compared with pure PP.74, 75 However, in both cases Young’s modulus of the nanocomposites was significantly higher than that of the pure PP. Sharma and Nayak found that the TS of PE/clay composites was higher than that of pure PP. At higher loading (7 wt.%) of nanoclay, the TS of the nanocomposites started to decrease but remained higher than that of the pure PP.76 Kotsilkova et al. showed that the TS of PP/CNT nanocomposites reached a maximum with only 0.5 wt.% of CNT. Beyond this concentration, the TS started to decrease and fell to the level of pure PP.77 In contrast, the EB of the nanocomposites decreased gradually with the addition of CNTs.
Dynamic mechanical analysis
Pramanik et al. reported DMA results for EVA-45/12Me-MMT nanocomposites.19 They showed that, with the addition of clay, the storage modulus (E′) of the nanocomposites increased but the glass transition temperature (Tg) decreased. The clay restricted the chain mobility of the polymer matrix, which resulted in the enhancement of E′ of the nanocomposites. The plasticization effect of modified clay is mainly responsible for the decrease in Tg It was also noted that the height of the tan δ peak (β relaxation) for the nanocomposites decreased compared with neat EVA-45. This was attributed to the restricted mobility of the polymer chains between the clay layers. Zhang et al. measured the dynamic mechanical properties of different EVA and EVA/OMC nanocomposites.78 They showed that E for EVA-28/SIOM nanocomposites was higher but for EVA-28/unmodified clay composites it was lower than that of pure EVA-28. (SIOM is CH3(CH2)17N+(CH3)2Br–.) E′ for EVA-28/SIOM (5 wt.%) is 1381 MPa, which is approximately 50% higher than that of pure EVA-28. This can be interpreted as due to the interaction of the clay layers with the EVA-28 chains, strengthening E′ for EVA-28. However, Tg. for all the nanocomposites and pure EVA did not shift to a higher temperature. E′ for the EVA-80/SIOM (5 wt.%) was 3053 MPa at −50 °C, which is about 62% higher than that of pure EVA-80. Note that the degree of enhancement in E and Tg. for EVA-80/SIOM nanocomposites is a little more than that of EVA-28/SIOM. This was attributed to the better dispersion of the filler in the highly polar EVA-80 matrix.78
The storage modulus (E′) of LDPE/Cloisite nanocomposites is much higher than that of pure LDPE.79 Exfoliated graphite is regarded as a very effective filler for the reinforcement of the dynamic mechanical properties of HDPE/EG nanocomposites. Almost a twofold increase in E′ was noted with 10 wt.% of EG. At 5 wt.% loading, the increase in E′ was estimated to be 50%.70 Kim et al. studied the effect of xGnP on the dynamic mechanical behavior of LLDPE nanocomposites.72 Figure 7.6 shows that, with the addition of only 7 wt.% of xGnP, E′ for the composite was 2.5 times higher than the control LLDPE.72
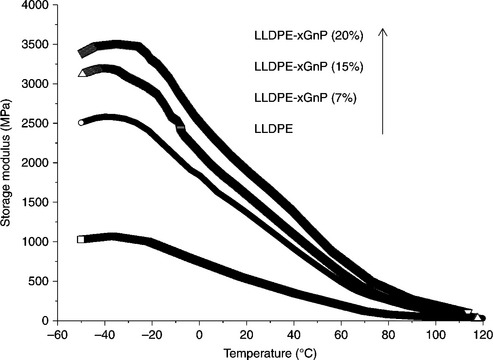
7.6 The storage modulus (E") of LLDPE/xGnP nanocomposites prepared by solution mixing and injection molded against xGnP loading. Reproduced from Kim et al.72 by permission of Wiley-VCH, Germany.
E′ for neat PP at 20 °C is 1450 MPa. E′ for specimens infused with 3 and 5 wt.% was 1750 and 1500 MPa, respectively.80 E′ for the nanocomposites gradually increased with the addition of OMC into PP-g-MA. E′ for nanocomposites containing 1 wt.% of MWCNT was similar to that of the pure matrix PP. However, an improvement of about 30% of the E′ in the glassy region was observed for 3 wt.% MWCNT composites compared with pure PP.77
7.5.2 Thermal properties
Thermal analysis is a well-established technique for obtaining qualitative and quantitative information about the effects of various heat treatments on materials. The heating is performed under strictly controlled conditions and can reveal changes in the structure and other important properties of the materials under investigation, thereby determining the practical importance of the material. Polymer nanocomposites constitute an important class of material due to their unusual improved properties, which are normally unavailable in the pure forms or in conventional composites. It is therefore necessary to evaluate their thermal stability, the effect of heat and any phase changes. In this regard, thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) have proved to be very useful.
Thermogravimetric analysis
Zhang et al. studied the effect of different types of OMC on the thermal degradation of EVA-28 in a nitrogen and air atmosphere.78 It was found that the thermal degradation of EVA-28/SIOM, EVA-28/DIOM, and EVA-28/TRIOM (in N2) nanocomposites is much faster than pure EVA-28. (DIOM is (CH3(CH2)17)2N+(CH3)2Br– and TRIOM is (CH3(CH2)17)3N+(CH3)2Br–.) Above 460 °C, the nanocomposites have a higher thermal decomposition temperature. In contrast, the EVA-28/Na-MMT microcomposite has better thermal stability than pure EVA-28. The thermal degradation of all the nanocomposites was obviously different when in nitrogen. At 30% weight loss, the thermal degradation temperature of EVA-28/SIOM nanocomposites is about 60 °C higher compared with pure EVA-28. TGA thermograms of EVA and EVA/rectorite nanocomposites under nitrogen and air were produced by Fang and co-workers.81 The mass loss of EVA nanocomposites at the beginning of combustion was higher than that of pure EVA in air, which indicates that the deacylation reaction of EVA nanocomposites was accelerated. This is probably due to catalysis by strongly acidic sites of the layered silicates deriving from thermal decomposition of the organic alkylammonium cations. The maximum weight loss temperature of the nanocomposites with 7.5 wt.% of rectorite was 468 °C, whereas for pure EVA it was 429 °C. This increase in thermal stability is mainly due to the strong barrier effect of layered silicates in the EVA matrix.
Acharya et al. studied the effect of dodecyl-sulfate-modified MgAl LDH on the thermal stability of EPDM nanocomposites. It was found that the thermal stability of the EPDM nanocomposites containing 3 wt.% of LDH was about 40 °C higher than pure EPDM (at 10% weight loss). The enhanced thermal stability of the nanocomposites was attributed to the lower permeability of oxygen and the diffusibility of the degradation products from the bulk of the polymer caused by the partially exfoliated LDH in the composites.18
Polyethylene/clay nanocomposites have better thermal properties compared with the original PE due to the special structure of MMT.82 During the first stage of degradation (below 400 °C) (the Hofmann elimination reaction and clay catalyzed degradation), PE/clay nanocomposites degrade faster than pure PE.64 At higher temperatures (>400 °C), PE/clay nanocomposites are more stable than pure PE. This is attributed to the formation of a charred layer on the surface of the nanocomposites, which disrupts the oxygen supply and prevents the emission of thermally degraded small gaseous molecules. Figure 7.7 shows that the thermal stability of LDPE/LDH nanocomposites is significantly higher than that of pure LDPE.83 It is evident that both the onset degradation and final degradation temperature have increased with the addition of LDH into the nanocomposites. The enhanced thermal degradation temperature of the nanocomposites was attributed to the endothermic degradation of LDH, which produces smoke and water vapor and disrupts the oxygen supply from the surface to the bulk of the specimen. A charred layer of metal oxide forms a protective coating on the surface of the nanocomposite and hinders the emission of thermally degraded small gaseous molecules. By increasing the content of LDH in the nanocomposites, the size of the charred layer increased, as is evident in Fig. 7.8.83 Chen et al. also found a similar effect while using dodecyl-sulfate-modified ZnAl LDH as a filler in an LLDPE matrix.68 The thermal stability of CNT-filled PE nanocomposites was found to be higher than that of pure PE. The onset and final degradation temperatures of PE nanocomposites containing 0.5 wt.% of CNT were ~ 50 °C higher than pure PE.84 Kuila et al. found that the onset degradation temperature for a nanocomposite with 3 wt.% DA-G was ~ 40 °C higher than that of neat LLDPE.35 Thermal stability decreased at higher loadings of DA-G (5 and 8 wt.%). This was due to the presence of a larger number of DA-G layers in the nanocomposite, which act as a heat source domain inducing thermal degradation of the composite. In addition, the larger amount of DA-G produces a less stable charred layer during thermal decomposition.
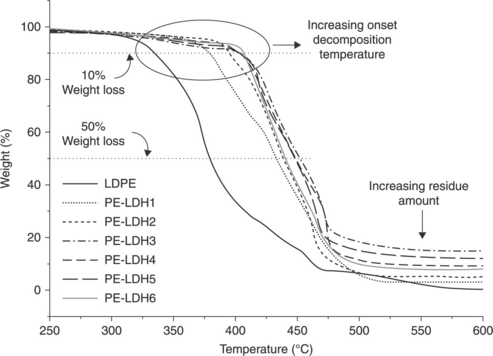
7.7 TGA plots of LDPE/LDH nanocomposites prepared by melt mixing in an extruder. Reproduced from Costa et al.83 by permission of Elsevier Science Ltd, UK.
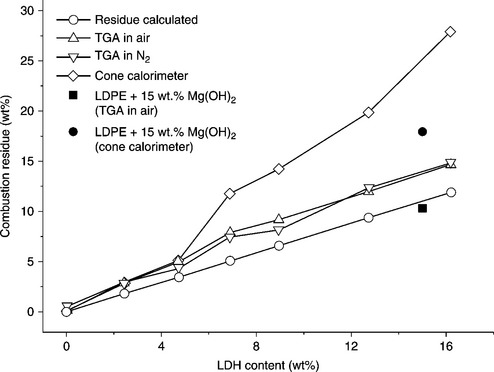
7.8 Influence of LDH concentration on the amount of residue obtained from TGA (heated to 750 °C), from cone calorimeter experiments and theoretically calculated. Reproduced from Costa et al.83 by permission of Elsevier Science Ltd, UK.
The thermal degradation behavior of pure PP and its nanocomposites with OMC is shown in Fig. 7.9 and the data derived from this is shown in Table 7.2.76 There was only 10 °C of improvement in thermal stability for the PP/unmodified clay nanocomposites compared with unfilled PP. In contrast, the thermal stabilities of PP/OMC nanocomposites are 35–50 °C higher compared with pure PP. In comparison to unmodified clay, the OMC disperses homogeneously in the nanocomposites and can act as a barrier, preventing the emission of volatile products from the bulk matrix. Fereidoon et al. showed that the thermal stability of PP/single-walled carbon nanotube (SWCNT) nanocomposites increased due to: (1) the higher thermal stability of the SWCNTs than that of the PP matrix, (2) the thermal conductivity of the SWCNTs, and (3) the interaction between the matrix and the SWCNTs, which, if it were better, would cause a more homogeneous spread and conduction of heat.85
Table 7.2
TGA values of PP and PP nanocomposites
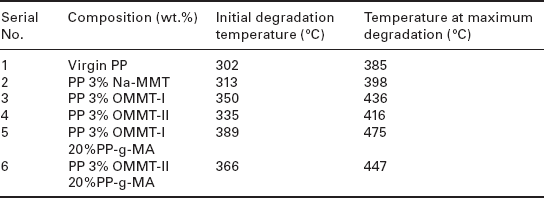
Source: Reproduced from Sharma and Nayak76 by permission of Elsevier Science Ltd, UK.
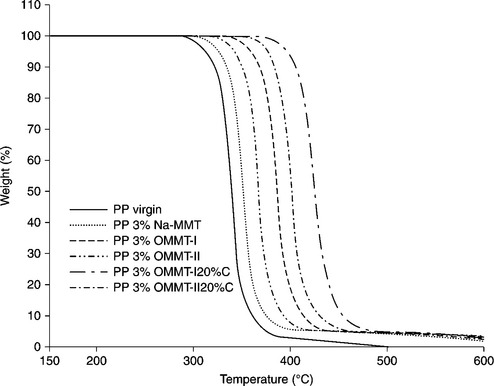
7.9 TGA thermograms of PP and PP nanocomposites. Reproduced from Sharma and Nayak76 by permission of Elsevier Science Ltd, UK.
Differential scanning calorimetric analysis
Figure 7.10 shows the DSC cooling scan curve of PE/OMC nanocomposites. As seen from this figure, the crystallization temperatures (Tc) of pure PE and its nanocomposites with 1 wt.% OMC are 117.3 and 119.3 °C, respectively. Further addition of clay has a slight influence on the Tc of the composites. This can be explained by the assumption that the silicate layers act as efficient nucleating agents for crystallization.82 Yang et al. showed that the addition of CNT has a similar effect on the Tc and Tm of the HDPE matrix.86
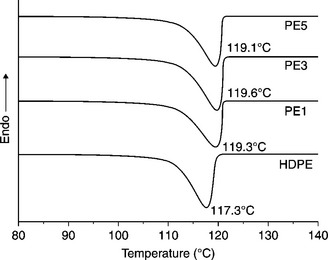
7.10 DSC curve of PE/OMC nanocomposites. Reproduced from Zhai et al.82 by permission of Elsevier Science Ltd, UK.
The melting point of pure PP has been determined by DSC analysis to be 155 °C.87 As the clay content increases, the melting point has been observed to increase. Ko et al. showed that the glass transition temperatures (Tg) of PP hybrids are virtually constant for CNT loadings in the range 0.5–3.0 wt.%.88 Thus, the effects of small amounts of dispersed CNTs on the free volume of PP are not significant, and the Tg of PP hybrids is not affected by increasing the CNT loading. The melting transition temperatures (Tm) of hybrids increase with an increase in CNT content up to 0.5 wt.%, and then remain constant with further increases in CNT loading.
7.5.3 Flame-retardant properties
The flame retardancy of polyolefinic nanocomposites has been measured by limiting oxygen index (LOI), cone calorimetry, and Underwriters Laboratories (UL94) testing. Shi et al. prepared EVA/layered silicate nanocomposites using melt mixing and observed that the heat release capacity (HRC) and total heat release (THR) are reduced by 21–24% and 16%, respectively.89 The heat release rate of EVA nanocomposites containing 5 wt.% clay was reduced by 80% and the mass loss rate (MLR) plot was spread over a much longer period of time. Beyer synthesized flame-retardant nanocomposites by melt blending ethylene vinyl acetate copolymers (EVA) with modified layered silicates.90 A roughly 47% decrease in peak heat release rate (PHRR) as well as a shift towards longer times for burning were detected for a nanocomposite containing 5 wt.% of nanofiller. No significant decrease in PHRR was observed for further addition of nanofiller up to 10 wt.%. It was found that the limiting oxygen index values of the EVA/LDH composites gradually increased on increasing the hydrotalcite content in the EVA. Cone calorimeter characterization showed that pure EVA has a very sharp peak with a PHRR of 1813 kW/m2. and an ignition time of 92 s. However, the peaks of all the samples were relatively smooth with PHRR below 597 kW/m2 and the ignition times were longer. PHRR decreased when the hydrotalcite content was increased.91 Gao et al. found that CNT plays an important role in the reduction of PHRR by forming low permeability char containing graphitic carbon.92 Peeterbroeck et al. showed that the time to ignition (TTI) for EVA/MWCNT nanocomposites was delayed in comparison to unfilled EVA.93 The decrease of the TTI for composites based on MWCNTs in comparison with the EVA matrix is probably due to the presence of acidic functions that have formed on the surface of the MWCNTs during purification. Ye et al. showed that MWCNTs can considerably decrease the heat release rate (HRR) and MLR by about 50–60%; they can almost double the combustion time and increase LOI values by 5% when 2 wt.% MWCNTs substitute for magnesium hydroxide (MH) in EVA/MH/MWCNT samples.94
Barbosa et al. prepared polyethylene/Brazilian clay nanocomposites and PE/commercial flame-retardant systems by direct melt compounding.95 The PE/flame-retardant systems had a lower rate of combustion in comparison to the pure polymer and also a lower tendency for dripping. The burning rate of the nanocomposites was significantly reduced by the addition of 3 wt.% of clay compared with a neat PE matrix. The result demonstrates that the flammability resistance of PE/clay nanocomposites is much better compared with PE/commercial flame-retardant composite systems. This is due to the significant contribution of the OMC in decreasing the flammability of the systems, suggesting the barrier properties of the clay minerals. Zhao and co-workers prepared polyethylene nanocomposites with different kinds of OMC. In comparison to pure PE, the PHRR of PE/OMC nanocomposites was significantly reduced and the value decreased on increasing the clay content (Fig. 7.11).64 The presence of 5% of ZnAl hydrotalcite (HTlc) caused a 55% reduction in PHRR and a delay of about 50 s.22 The fire performance index of the nanocomposites decreased to 37.6 kW/m2s from 70.5 kW/m2s for the pure polymeric matrix. The formation of a protective layer on the specimen surface acted as a good barrier during combustion in the cone calorimeter test. Lu et al. prepared96 flame-retardant maleated PE/magnesium hydroxide sulfate hydrate (MHSH) whisker composites containing OMC by direct melt intercalation. Cone calorimetry results indicated that a synergistic flame-retardant effect on reducing HRR occurred when MHSH and OMC were present in the nanocomposites.
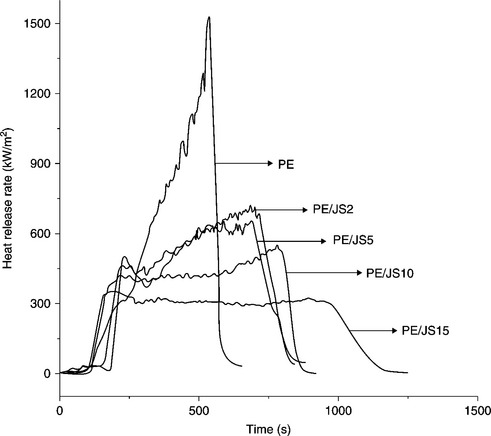
7.11 Heat release rate for PE and PE/clay nanocomposites. Reproduced from Zhao et al.64 by permission of Elsevier Science Ltd, UK.
Wagenknecht etal. showed that the burning behavior of PP/clay nanocomposites can be decreased by increasing the nanofiller content in the polymer matrix.97 Tang and co-workers prepared PP/clay nanocomposites starting from pure clay and the reactive compatibilizer hexadecyltrimethylammonium bromide (C16)/98The PHRR of PP/MMT hybrids containing 4 wt.% MMT and a mixture of MMT (4 wt.%) and C16 (2 wt.%) was reduced by about 37% and 27%, respectively. Song et al. investigated the effect of OMC on the fire retardancy of PP/OMC nanocomposites.99 It was proposed that the better dispersion of OMC in the matrix is useful at reducing the flame retardancy of PP/OMC nanocomposites. Zhang et al. studied the effect of OLDH on the flame-retardant behavior of PP/intumescent-flame-retardant (IFR) nanocomposites. Cone calorimeter tests showed that pure PP burns very quickly (about 300 s) after ignition. 100 A very sharp HRR curve appears in the range 50–300 s with a PHRR of 1275 kW/m2. In contrast, the samples with IFR show a dramatic decline in the HRR curves and the prolongation of the combustion time. Of all the flame-retardant samples, the PP/IFR/ZnAl-LDH sample had the lowest PHRR of 318 kW/m2. Pure PP has a PHRR of 1071 kW/m2 whilst in the nanocomposites with PP-g-MA (6 phr) and OLDH (6 phr) this value is only 754 kW/m2.101 This is more than a 25% reduction, and, even after taking into account the dilution effect of the filler, it can be considered a significant improvement in flame resistance. Total heat release, which is calculated as the area under a HRR curve, is another important parameter for fire-hazard evaluation. The nanocomposites had THR values of 37.7, 37.4, and 32.2 kJ/g, respectively, corresponding to the increasing OLDH content. In contrast, 38.8 kJ/g was found for neat PP. Pristine CNTs and hydroxylated CNTs (CNT-OHs) have been employed to enhance the thermal stability and flame retardancy of PP/wood flour composites compatibilized by PP-g-MA. 102 Kashiwagi and co-workers measured the flammability properties of PP/MWCNT nanocomposites as the MWCNT content varied from 0.5 to 4% by weight.103, 104 The lowest HRR was observed with a PP/MWCNT (1%) sample due to the balance between the effect of thermal conductivity and the shielding performance of the external radiant flux (and heat feedback from the flame), depending on the concentration of MWCNT in the sample.
7.5.4 Thermomechanical analysis
Kim et al. measured the thermomechanical properties of LLDPE/xGnP nanocomposites to determine the coefficient of thermal expansion (CTE). An obvious change in the slope in the range 80–85 °C for the xGnP-loaded composites was detected. The CTE was calculated for two temperature ranges, 45–80 °C and 85–100 °C. The CTE of the control LLDPE was 299.8 × 10–68 °C below 80 °C and 365.3 × 10–68 °C above 85 °C. Figure 7.12 shows that the CTE for LLDPE/xGnP composites in the range 45–80 °C is much lower than that of pure LLDPE.72 However, there was no obvious change in CTE with the addition of xGnP. Kalaitzidou et al. measured the CTE of PP/xGnP composites along each direction for two temperature regimes.105 A decrease in CTE along the longitudinal direction was observed for all the fillers (xGnP-1, xGnP-2, xGnP-15, CB, carbon fiber, and clay) at both below and above Tg. In particular, for a temperature below Tg xGnP-1 exhibited a similar reduction in CTE as the carbon fibers, i.e., a reduction of CTE by ~25%. At higher Tg, the effect of carbon fibers on the CTE was more than that of xGnP. This is attributed to the high degree of alignment of the carbon fibers along the flow direction and the fact that these fibers are stiffer compared with the other fillers.
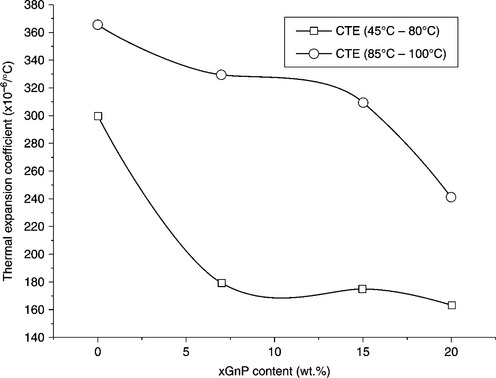
7.12 Coefficient of thermal expansion of LLDPE/xGnP nanocomposites prepared by solution mixing and injection molded against xGnP content in the ranges 45–80 °C and 85–100 °C. Reproduced from Kim et al.72 by permission of Wiley-VCH, Germany.
7.5.5 Gas-barrier properties
Although extensive research has been carried out on polymer/inorganic nanocomposites, the gas-barrier properties of polyolefin nanocomposites have been less well studied. Mittal studied the effect of organically modified MMT (OMMT) on the oxygen permeability of PP/clay nanocomposites. 106 The relative oxygen permeability of the nanocomposites decreased gradually with the addition of OMC. Figure 7.13 shows that the relative oxygen permeability of the nanocomposites with 4 vol.% of filler was half that of pure PP. The much better oxygen permeation behavior for OMC can be attributed to its better thermal stability. Permeation properties are sensitive to interfacial interactions; therefore, the improved permeation behavior can be attributed to the thermal stability of the nanocomposites. Kalaitzidou et al. studied the effect of xGnP and other fillers (CB, carbon fiber, and clay) on the O2 permeability of PP composites with 3 vol.% of nanofiller.105 They found that CB did not improve the O2 barrier of PP due to the irregular shape of the CB agglomerates. In contrast, both xGnP-1 and carbon fiber caused a similar decrease in O2 permeability, i.e., 10% at a reinforcement loading of 3 vol.%. For xGnP-15, a 20% improvement in O2 permeability was observed with 3 vol.% loading.
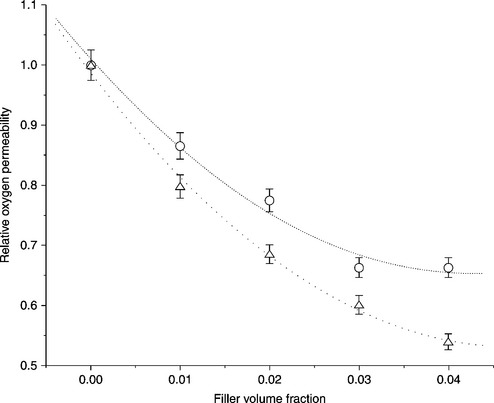
7.13 Relative oxygen permeability of imidazolium OMMT/PP composites as a function of filler volume fraction compared with 2C18 ammonium OMMT/PP composites. O: 2C18 ammonium OMMT composites; Δ: imidazolium OMMT composites. The dotted lines serve only as guides. Reproduced from Mittal106 by permission of Elsevier Science Ltd, UK.
7.5.6 Electrical conductivity
EVA, PE, EPDM, and PP are insulating in nature. The electrical conductivity of these polymers lies in the range 10–14 to 10−16 S/m. Therefore, clay minerals and LDH are not suitable fillers for conductive polymer composites, due to their insulating nature. Carbon-based nanofillers such as EG, CNT, CNF, and graphene are very effective in this respect. Das et al. showed that the percolation in electrical conductivity of CNT-filled EVA composites was achieved with 5 wt.% of CNTs.107 The electrical conductivity of nanocomposites with 30 wt.% of CNTs was 1 S/m at room temperature. Ciselli et al. studied the DC conductivity of ultra-high molecular weight PE/MWCNT composite films as a function of MWCNT weight fraction.108 They observed that the percolation in electrical conductivity was achieved with only 3 wt.% of MWCNTs. The electrical conductivity of nanocomposites with 5 wt.% of MWCNTs increased to 0.1 S/m, indicating the formation of conductive composites. The percolation in electrical conductivity occurred with 3 wt.% for EG-filled PE nanocomposites.70 The electrical conductivity of PE/EG composites was significantly higher than that of PE/graphite composites. This was attributed to the large surface area of EG compared with graphite. Kim and co-workers studied the effect of paraffin-oil-coated xGnP on the electrical conductivity of LLDPE nanocomposites.3 Conductivity increased gradually on increasing the concentration of xGnP in the nanocomposites. The electrical conductivity of the nanocomposites reached a maximum when the weight ratio of xGnP, paraffin, and LLDPE was 1:1:8. Kuila et al. showed that the percolation in electrical conductivity occurred with 3 wt.% of DA-G in LLDPE/DA-G nanocomposites.35 The conductivity of the sample reached 10− 4 S/m with 8 wt.% DA-G. Kim et al. studied the effect of thermally reduced graphene oxide (TRG) on the electrical conductivity of LLDPE/TRG nanocomposites prepared using melt mixing.109 Surprisingly, the percolation in electrical conductivity was achieved with only 0.4 vol.% TRG in the LLDPE matrix. The conductivity of LLDPE changed sharply even below 0.4 vol.% TRG. Kalaitzidou et al. studied the effect of compounding on the electrical conductivity of PP/xGnP nanocomposites.110 It was evident that the combination of coating and compression molding yielded composites with a lower percolation threshold and higher conductivity. The coated and compression molded PP composites had a percolation threshold at ~0.3 vol.% xGnP−15 (Fig. 7.14), whereas the coated and injection molded composites had a percolation threshold of ~5 vol.% (Fig. 7.15). Chen et al. showed that the percolation threshold for PP/EG composites is 0.67 vol.%.111 The electrical conductivity reached 0.1 S/m when the EG concentration was 3.90 vol.%.
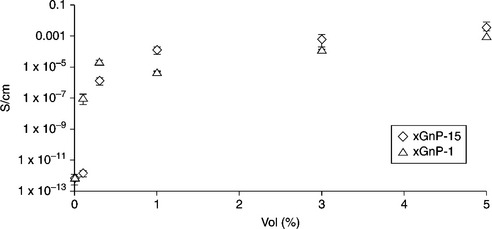
7.14 Effect of xGnP aspect ratio on the percolation threshold and electrical conductivity of PP/xGnP made by coating and compression molding. Reproduced from Kalaitzidou et al.110 by permission of Elsevier Science Ltd, UK.
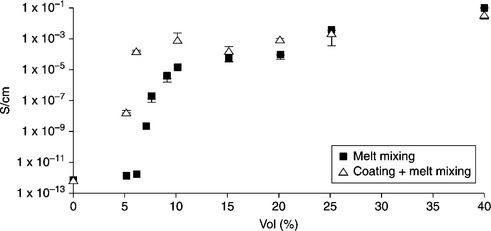
7.15 Effect of compounding on the percolation threshold and conductivity of PP/xGnP-15 made by injection molding. Reproduced from Kalaitzidou et al.110 by permission of Elsevier Science Ltd, UK.
7.5.7 Electromagnetic shielding
Electromagnetic interference (EMI) shielding refers to the reflection or absorption of electromagnetic radiation by a material which acts as a shield against the penetration of the radiation.112 When plastic materials are used as shielding, noise signals are produced due to their very poor electrical conductivity. Carbon-based conductive fillers such as CB, graphite, EG, CNT, CNF, and graphene are very good at improving the EMI shielding efficiency (SE) of plastics. Das et al. showed that CB and short carbon fibers (SCFs) are very effective in improving the EMI SE of EVA composites. The target value of the EMI SE needed for commercial applications is around 20 dB, which is only obtained with 20 wt.% of CB/SCF loading. The EMI SE of the composites reached 50 dB with the addition of 50 wt.% filler.113 Das et al. showed that conductive polyaniline is a very effective filler in achieving the target value for EVA-based composites.114 Das et al. also studied the effect of CNT loading on the EMI SE of EVA/CNT composites.107 The EMI SE of a composite with 15 wt.% SWCNTs was ~22–23 dB in the gigahertz frequency range, showing promise for its commercial use as an EMI shielding material for different electronic devices. Due to its very high electrical conductivity, CNF has been used in the preparation of PE/CNF composites for EMI shielding.115 Panwar and Mehra studied the effect of graphite loading on the EMI SE of PE/graphite composites.116 All the composites showed good reproducibility and EMI SE up to 90 °C. Ghosh and Chakrabarti developed EPDM/CB composites for EMI shielding applications.117 Mahapatra et al. showed that the EMI SE of EPDM/Vulcan XC 72 composites increased on increasing the content of Vulcan XC 72. It was also found that there was an initial increase in shielding with an increase in frequency, with a peak at 9.5 GHz, before showing a decreasing trend.118
7.5.8 Other properties
Polymer/inorganic nanocomposites have improved thermal conductivity, rheological properties, solvent resistance properties, etc. The thermal conductivity of EVA, PE, and PP can be improved by using CNTs, CNFs, EG, or graphene as a nanofiller due to their inherent high thermal conductivity values. The thermal conductivity of CNTs, CNFs, EG, and graphene has been found to be 3500, 2000, 380, (4.84 ± 0.44) to (5.30 ± 0.48)×103W/mK, respectively.6, 119 The thermal conductivity of EVA-50 can be improved by 70 and 188% with the incorporation of 1 and 4 wt.% CNF, respectively.120 Ghose et al. showed that CNTs can enhance the thermal conductivity of an EVA sample. 121 The thermal conductivity of PP/xGnP composites has been measured as a function of xGnP aspect ratio and concentration.105 The high aspect ratio of xGnPs enhances the thermal conductivity of a pure PP matrix. Melt rheological analysis shows that the incorporation of a nanofiller in a polymer matrix effectively increases the storage modulus (G′) and loss modulus (G″) of the nanocomposites.122 It was also found that, generally, dynamic complex viscosity increased substantially on increasing the nanoclay content. This was attributed to the interaction and dispersion of the nanoclay in the polymer matrix. However, in some cases the viscosity may decrease due to the plasticization effect of the nanoclay.
7.6 Application of polyolefin nanocomposites
Polyolefin/inorganic hybrids or nanocomposites always exhibit improved properties over their pure form. Therefore, these nanocomposites should exhibit better performance compared with pure polymer when used for the same applications. This makes nanocomposite materials potentially useful in numerous automotive, general and industrial applications, including mirror housings for various vehicle types, door handles, engine covers, intake manifolds, timing belt covers, and many more. More general applications currently being considered include use in impellers and blades for vacuum cleaners, power-tool housings, aerospace, mower hoods, and covers for portable electronic equipment such as mobile phones, pagers, etc.5, 9–13, 123
The ability of clay, LDH, hydrotalcites, and CNTs to reduce the flammability of polymeric materials is a major point of interest to many researchers. Research has demonstrated the extent to which flammability can be restricted in polymers such as polyolefins with as little as 2% filler loading. In particular, heat release rates, as obtained from cone calorimetry experiments, have been found to diminish substantially with the incorporation of a nanofiller. Although the incorporation of conventional microparticles, together with flame-retardant and intumescent agents, would also minimize flammability, it is usually accompanied by reductions in various other important properties. With LDH, a reduction in flammability is usually achieved whilst maintaining or enhancing other properties and characteristics.
Carbon-based conductive fillers can convert insulating plastic materials to electrically conductive materials. Near the percolation threshold, high values of conductivity and the dielectric constant have been achieved with these composites, which means they can be used in charge-storage devices. The observed high dissipation factor makes them suitable for decoupling capacitor applications. This type of composite could be utilized in self-controlled heaters, current-limiters, etc.
7.7 Conclusions and future trends
This chapter has reviewed in detail nanocomposites made with polyolefins (EVA, EPDM, PE, and PP). Different kinds of nanofillers including layered clay (Na-MMT, bentonite, Cloisite, Laponite), ATH, LDH, nano-Mg(OH)2, graphite, CNFs, EG, CNTs, graphene, etc., have been used to prepare polyolefin-based nanocomposites. The nanostructure of the composites has been established by XRD, FT-IR, and TEM. Finally, the properties of the nanocomposites have been discussed in detail in terms of their mechanical behavior, thermal stability, thermomechanical stability, flammability, gas-barrier capability, and electrical conductivity. Unmodified fillers are not suitable for enhancing mechanical properties. In this context, OMC, LDH, CNT, and graphene are very effective fillers. The thermal stability of the nanocomposites has also been found to be better compared with their pure counterparts. However, the thermal stability of EVA-based nanocomposites is almost comparable with pure EVA. This is due to the catalytic effect of the nanofiller and the presence of labile acetate groups of EVA. The flame retardancy of clay minerals, LDH, and ATH is significantly higher than for carbon-based nanofillers. The synergistic effect of CNTs with LDH or ATH on the flame retardancy of the nanocomposites has attracted extensive interest from researchers. The thermomechanical properties of nanocomposites are improved with the addition of EG as a nanofiller. Carbon-based nanofillers can be used to convert insulating polyolefins into conductive composites, which are very useful in applications.
Graphene-based composite materials have been studied less well in comparison to polymeric composites based on clay minerals, LDH, EG, and CNTs. There is a possibility of employing graphene as a conductive filler in the preparation of polymer composites for electronic and electrochemical devices. The addition of a very small amount of graphene (0.5 vol.%) can increase the electrical conductivity of polystyrene from 10–16 S/m to 0.01 S/m.124 Graphene also has the ability to enhance the EMI SE of polyolefins at much lower loading. The very high aspect ratio and presence of different polar groups on the surface of graphene also help to improve the mechanical properties of composite materials. Flammability characteristics can also be increased with the addition of graphene. The synergistic effect of graphene and other flame-retardant fillers (ATH, LDH, etc.) on the flame-retardant properties of composites has not been studied. Therefore, much work is needed on the preparation, characterization, and application of graphene/polyolefin composite materials.
7.8 References
1. Kojima, Y., Usuki, A., Kawasumi, M., Okada, A., Fukushima, Y., et al. Mechanical properties of nylon 6-clay hybrid. J. Mater. Res.. 1993; 8:1185–1189.
2. Kojima, Y., Usuki, A., Kawasumi, M., Okada, A., Fukushima, Y., et al. Sorption of water in nylon 6-clay hybrid. J. Appl. Polym. Sci.. 1993; 49:1259–1264.
3. Ray, S.S., Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci.. 2003; 28:1539–1641.
4. Pavlidou, S., Papaspyrides, C.D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci.. 2008; 33:1119–1198.
5. Hussain, F., Hojjati, M., Okamoto, M., Gorga, R.E.J. Polymer-matrix nanocomposites, processing, manufacturing, and application: An overview. Compos. Mater.. 2006; 40:1511–1575.
6. Kuila, T., Bhadra, S., Yao, D., Kim, N.H., Bose, S., et al. Recent advances in graphene based polymer composites. Prog. Polym. Sci.. 2010; 35:1350–1375.
7. Gao, F. Clay/polymer composites: The story. Mater. Today. 2004; 7:50–55.
8. Majumdar, D., Blanton, T.N., Schwark, D. Clay-polymer nanocomposite coatings for imaging application. Appl. Clay Sci.. 2003; 23:265–273.
9. Cox, H., Dearlove, T., Rodgers, W., Verbrugge, M., Wang, C.S., Nanocomposite systems for automotive applications4th World Congress in Nanocomposites. San Francisco: EMC, 1–3 September 2004.
10. , Transparent nanocomposites for aerospace applications. Advanced Composites Bulletin. Feb 2004.
11. Hule, R.A., Pochan, D.J. Polymer nanocomposites for biomedical application. MRS Bulletin. 2007; 32:354–358.
12. Boccaccini, A.R., Erol, M., Stark, W.J., Mohn, D., Hong, Z., et al. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Compos. Sci. Technol.. 2010; 70:1764–1776.
13. Godovsky, D.Y. Device applications of polymer-nanocomposites. Adv. Polym. Sci.. 2000; 153:163–205.
14. Kornmann, X. Synthesis and characterization of thermoset-layered silicate nanocomposites. Sweden: Lulea Tekniska Universitet; 2001. [PhD thesis].
15. Mu, O., Feng, S. Thermal conductivity ofgraphite/silicone rubber prepared by solution intercalation. Thermochim. Acta. 2007; 462:70–75.
16. Dong, W., Zhang, X., Liu, Y., Wang, Q., Guia, H., et al. Flame retardant nanocomposites of polyamide 6/clay/silicone rubber with high toughness and good flowability. Polymer. 2006; 47:6874–6879.
17. Okutan, E., Aydin, G.O., Hacrivelioglu, F., Lilica, A. Synthesis and characterization of soluble multi-walled carbon nanotube/poly(organophosphazene) composites. Polymer. 2011; 52:1241–1248.
18. Acharya, H., Srivastava, S.K., Bhowmick, A.K. Synthesis of partially exfoliated EPDM/LDH nanocomposites by solution intercalation: Structural characterization and properties. Compos. Sci. Technol.. 2007; 67:2807–2816.
19. Pramanik, M., Srivastava, S.K., Samantaray, B.K., Bhowmick, A.K. Rubberclay nanocomposites by solution blending. J. Appl. Polym. Sci.. 2003; 87:2216–2220.
20. Wang, G.A., Wang, C.C., Chen, C.Y. The disorderly exfoliated LDHs/PMMA nanocomposite synthesized by in situ bulk polymerization. Polymer. 2005; 46:5065–5074.
21. Lee, W.D., Im, S.S. Thermomechanical properties and crystallization behavior of layered double hydroxide/poly(ethylene terephthalate) nanocomposites prepared by in situ polymerization. J. Polym. Sci. PartB: Polym. Phys.. 2007; 45:28–40.
22. Costantino, U., Gallipoli, A., Noccheti, M., Camino, G., Bellucci, F., Frache, A. New nanocomposites constituted of polyethylene and organically modified ZnAl-hydrotalcite. Polym. Degrad. Stabil.. 2005; 90:586–590.
23. Costa, F.R., Saphiannikova, M., Wagenknecht, U., Heinrich, G. Layered double hydroxide based polymer nanocomposites. Adv. Polym. Sci.. 2008; 210:101–168.
24. Brindley, G.W., Kikkawa, S. Thermal behavior of hydrotalcite and of anion-exchanged forms of hydrotalcite. Clays Clay Miner.. 1980; 28:87–91.
25. Sengupta, R., Bhattacharya, M., Bandyopadhyay, S., Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified graphite reinforced polymer composites. Prog. Polym. Sci.. 2011; 36:638–670.
26. Kim, S., Drzal, L.T. Improvement of electric conductivity of LLDPE based nanocomposites by paraffin coating on exfoliated graphite nanoplatelets. Compos Part A: Appl. S.. 2010; 41:581–587.
27. Spitalsky, Z., Tasis, D., Papagelis, K., Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci.. 2010; 35:357–401.
28. Li, Q., Park, O.K., Lee, J.H. Positive temperature coefficient behavior of HDPE/EVA blends filled with carbon black. Adv. Mater. Res.. 2009; 79:2267–2270.
29. Jeevananda, T., Kim, N.H., Lee, J.H., Basavarajaiah, S., Urs, M.V.D., Ranganathaiah, C. Investigation of multi-walled carbon nanotube reinforced high-density polyethylene/carbon black nanocomposites using electrical, DSC and positron lifetime spectroscopy techniques. Polym. Int.. 2009; 58:755–780.
30. Tibbetts, G.G., Lake, M.L., Strong, K.L., Rice, B.P. A review of the fabrication and properties of vapor-grown carbon nanofiber/polymer composites. Compos. Sci. Technol.. 2007; 67:1709–1718.
31. Kotov, N.A. Carbon sheet solutions. Nature. 2006; 442:254–255.
32. Kuila, T. Preparation, characterization and properties of ethylene vinyl acetate copolymer/Mg-Al layered double hydroxide nanocomposites. India: IIT Kharagpur; 2009. [PhD thesis].
33. Acharya, H. Synthesis, characterization and properties of polyolefinic elastomer nanocomposites. India: IIT Kharagpur; 2007. [PhD thesis].
34. Bhowmick, A. K.; Stephens, H. Handbook of elastomers. Second edition, CRC Press, USA
35. Kuila, T., Bose, S., Hong, C.E., Uddin, M.E., Khanra, P., et al. Preparation of functionalized graphene/linear low density polyethylene composites by a solution mixing method. Carbon. 2011; 49:1033–1037.
36. Pramanik, M. Studies on layered materials and polymer nanocomposites. India: IIT Kharagpur; 2004. [PhD thesis].
37. Huang, J.C., Zhu, Z.K., Ma, X.D., Qian, X.F., Yin, J. Preparation and properties of montmorillonite/organo-soluble polyimide hybrid materials prepared by a one-step approach. J. Mater. Sci.. 2001; 36:871–877.
38. Bala, P., Samantaray, B.K., Srivastava, S.K. Synthesis and characterization of Na-montmorillonite-alkylammonium intercalation compounds. Mater. Res. Bull.. 2000; 35:1717–1724.
39. Newman, S.P., Jones, W. Synthesis, characterization and applications of layered double hydroxides containing organic guests. New J. Chem. 1998; 105–115.
40. Vucelic, M., Moggridge, G.D., Jones, W. Thermal properties of terephthalate- and benzoate-intercalated LDH. J. Phys. Chem.. 1995; 99:8328–8337.
41. Banerjee, S., Benny, T.H., Wong, S.S. Covalent surface chemistry of single walled carbon nanotubes. Adv. Mater.. 2005; 17:17–29.
42. Chen, J., Hamon, M.A., Hu, H., Chen, Y.S., Rao, A.M., et al. Solution properties of single-walled carbon nanotubes. Science. 1998; 282:95–98.
43. Mickelson, E.T., Huffman, C.B., Rinzler, A.G., Smalley, R.E., Hauge, R.H., et al. Fluorination of single-wall carbon nanotubes. Chem. Phys. Lett.. 1998; 296:188–194.
44. Dyke, C.A., Tour, J.M. Solvent-free functionalization of carbon nanotubes. J. Am. Chem. Soc.. 2003; 125:1156–1157.
45. Bekyarova, E., Itkis, M.E., Ramesh, P., Berger, C., Sprinkle, M., et al. Chemical modification of epitaxial graphene: Spontaneous grafting of aryl groups. J. Am. Chem. Soc.. 2009; 131:1336–1337.
46. Shan, C., Yang, H., Han, D., Zhang, Q., Ivaska, A., et al. Water soluble graphene covalently functionalized by biocompatible poly-L-lysine. Langmuir. 2009; 25:12030–12033.
47. Bai, H., Xu, Y., Zhao, L., Li, C., Shi, G. Non-covalent functionalization of graphene sheets by sulfonated polyaniline. Chem. Commun.. 2009; 1667–1669.
48. Salavagione, H.J., Gomez, M.A., Martinez, G. Polymeric modification of graphene through esterification of graphite oxide and poly(vinyl alcohol). Macromolecules. 2009; 42:6331–6334.
49. Liu, N., Luo, F., Wu, H., Liu, Y., Zhang, C., et al. One step ionic-liquid-assisted electrochemical synthesis of ionic-liquid-functionalized graphene sheets directly from graphene. Adv. Func. Mater.. 2008; 18:1518–1525.
50. An, X., Simmons, T., Shah, R., Wolfe, C., Lewis, K.M., et al. Stable aqueous dispersions of noncovalently functionalized graphene from graphite and their multifunctional high-performance applications. Nano Lett.. 2010; 10:4295–4301.
51. Acharya, H., Pramanik, M., Srivastava, S.K., Bhowmick, A.K. Synthesis and evaluation of high-performance ethylene-propylene-dene terpolymer/organoclay nanoscale composites. J. Appl. Polym. Sci.. 2004; 93:2429–2436.
52. Kuila, T., Acharya, H., Srivastava, S.K., Bhowmick, A.K. Synthesis and characterization of ethylene vinyl acetate/Mg-Al layered double hydroxide nanocomposites. J. Appl. Polym. Sci.. 2007; 104:1845–1851.
53. Kuila, T., Acharya, H., Srivastava, S.K., Bhowmick, A.K. Effect of vinyl acetate content on the mechanical and thermal properties of ethylene vinyl acetate/MgAl layered double hydroxide nanocomposites. J. Appl. Polym. Sci.. 2008; 108:1329–1335.
54. Pramanik, M., Srivastava, S.K., Samantaray, B.K., Bhowmick, A.K. Synthesis and characterization of organosoluble, thermoplastic elastomer/clay nanocomposite. J. Polym. Sci. PartB: Polym. Phys.. 2002; 40:2065–2072.
55. Cui, L., Ma, X., Paul, D.R. Morphology and properties of nanocomposites formed from ethylene-vinyl acetate copolymers and organoclays. Polymer. 2007; 48:6325–6339.
56. Cardenas, M.A., Lopez, D.G., Mitre, G., Merino, J.C., Pastor, J.M., et al. Mechanical and fire retardant properties of EVA/clay/ATH nanocomposites – effect of particle size and surface treatment of ATH filler. Polym. Degrad. Stab.. 2008; 93:2032–2037.
57. Zhang, G., Ding, P., Zhang, M., Qu, B. Synergistic effects of layered double hydroxide with hyperfine magnesium hydroxide in halogen-free flame retardant EVA/HFMH/LDH nanocomposites. Polym. Degrad. Stab.. 2007; 92:1715–1720.
58. Kuila, T., Srivastava, S.K., Bhowmick, A.K. Rubber/LDH nanocomposites by solution blending. J. Appl. Polym. Sci.. 2009; 111:635–641.
59. Kuila, T., Acharya, H., Srivastava, S.K., Bhowmick, A.K. Ethylene vinyl acetate/Mg-Al LDH nanocomposites by solution blending. Polym. Compos.. 2009; 30:497–502.
60. George, J.J., Bhowmick, A.K. Ethylene vinyl acetate/expanded graphite nanocomposites by solution intercalation: Preparation, characterization and properties. J. Mater. Sci.. 2008; 43:702–708.
61. Ismail, H., Pasbakhsh, P., Fauzi, M.N.A., Bakar, A.A. Morphological, thermal and tensile properties of halloysite nanotubes filled ethylene propylene diene monomer (EPDM) nanocomposites. Polym. Test.. 2008; 27:841–850.
62. Pasbakhsh, P., Ismail, H., Fauzi, M.N.A., Bakar, A.A. Influence ofmaleic anhydride grafted ethylene propylene diene monomer (MAH-g-EPDM) on the properties of EPDM nanocomposites reinforced by halloysite nanotubes. Polym. Test.. 2009; 28:548–559.
63. Kang, I., Khaleque, M.A., Yoo, Y., Yoon, P.J., Kim, S.Y., Lim, K.T. Preparation and properties of ethylene propylene diene rubber/multi walled carbon nanotube composites for strain sensitive materials. Compos. Part A: Appl. S.. 2011; 42:623–630.
64. Zhao, C., Qin, H., Gong, F., Feng, M., Zhang, S., et al. Mechanical, thermal and flammability properties of polyethylene/clay nanocomposites. Polym. Degrad. Stab.. 2005; 87:183–189.
65. Zhang, J., Jiang, D.D., Wilkie, C.A. Thermal and flame properties of polyethylene and polypropylene nanocomposites based on an oligomerically-modified clay. Polym. Degrad. Stab.. 2006; 91:298–304.
66. Zhang, J., Jiang, D.D., Wilkie, C.A. Polyethylene and polypropylene nanocomposites based upon an oligomerically modified clay. Thermochim. Acta. 2005; 430:107–113.
67. Costa, F.R., Satapathy, B.K., Wagenknecht, U., Weidisch, R., Heinrich, G. Morphology and fracture behaviour of polyethylene/Mg-Al layered double hydroxide (LDH) nanocomposites. Eur. Polym. J.. 2006; 42:2140–2152.
68. Chen, W., Qu, B. LLDPE/ZnAlLDH-exfoliated nanocomposites: Effects of nano layers on thermal and mechanical properties. J. Mater. Chem.. 2004; 14:1705–1710.
69. Kanagaraj, S., Varanda, F.R., Zhil’tsova, T.V., Oliveira, M.S.A., Simoes, J.A.O. Mechanical properties of high density polyethylene/carbon nanotube composites. Compos. Sci. Technol.. 2007; 67:3071–3077.
70. Zheng, W., Lu, X., Wong, S.C. Electrical and mechanical properties of expanded graphite-reinforced high-density polyethylene. J. Appl. Polym. Sci.. 2004; 91:2781–2788.
71. Kim, S., Do, I., Drzal, L.T. Multifunctional xGnP/LLDPE nanocomposites prepared by solution compounding using various screw rotating systems. Macromol. Mater. Eng.. 2009; 294:196–205.
72. Kim, S., Do, I., Drzal, L.T. Thermal stability and dynamic mechanical behavior of exfoliated graphite nanoplatelets-LLDPE nanocomposites. Polym. Compos.. 2010; 31:755–761.
73. Wang, J., Xu, C., Hu, H., Wan, L., Chen, R., Zheng, H., et al. Synthesis, mechanical, and barrier properties of LDPE/graphene nanocomposites using vinyl triethoxysilane as a coupling agent. J. Nanopart. Res.. 2011; 13:869–878.
74. Cui, L., Hunter, D.L., Yoon, P.J., Paul, D.R. Effect of organoclay purity and degradation on nanocomposite performance, Part 2: Morphology and properties of nanocomposites. Polymer. 2008; 49:3762–3769.
75. Kim, D.H., Fasulo, P.D., Rodgers, W.R., Paul, D.R. Structure and properties of polypropylene-based nanocomposites: Effect of PP-g-MA to organoclay ratio. Polymer. 2007; 48:5308–5323.
76. Sharma, S.K., Nayak, S.K. Surfacemodifiedclay/polypropylene(PP)nanocomposites: Effect on physico-mechanical, thermal and morphological properties. Polym. Degrad. Stab.. 2009; 94:132–138.
77. Kotsilkova, R., Ivanov, E., Krusteva, E., Silvestre, C., Cimmino, S., et al. Isotactic polypropylene composites reinforced with multiwall carbon nanotubes, Part 2: Thermal and mechanical properties related to the structure. J. Appl. Polym. Sci.. 2010; 115:3576–3585.
78. Zhang, W., Chen, D., Zhao, Q., Fang, Y. Effects of different kinds of clay and different vinyl acetate content on the morphology and properties of EVA/clay nanocomposites. Polymer. 2003; 44:7953–7961.
79. Malucelli, G., Ronchetti, S., Lak, N., Priola, A., Dintcheva, N.D., et al. Intercalation effects in LDPE/o-montmorillonites nanocomposites. Eur. Polym. J.. 2007; 43:328–335.
80. Kanny, K., Jawahar, P., Moodley, V.K. Mechanical and tribological behavior of clay-polypropylene nanocomposites. J. Mater. Sci.. 2008; 43:7230–7238.
81. Fang, P., Chen, Z., Zhang, S., Wang, S., Wang, L., et al. Micro structure and thermal properties of ethylene-(vinyl acetate) copolymer/rectorite nanocomposites. Polym. Int.. 2006; 55:312–318.
82. Zhai, H., Xu, W., Guo, H., Zhou, Z., Shen, S., et al. Preparation and characterization of PE and PE-g-MAH/montmorillonite nanocomposites. Eur. Polym. J.. 2004; 40:2539–2545.
83. Costa, F.R., Wagenknecht, U., Heinrich, G. LDPE/MgAl layered double hydroxide nanocomposite: Thermal and flammability properties. Polym. Degrad. Stab.. 2007; 92:1813–1823.
84. Barus, S., Zanetti, M., Bracco, P., Musso, S., Chiodoni, A., et al. Influence of MWCNT morphology on dispersion and thermal properties of polyethylene nanocomposites. Polym. Degrad. Stab.. 2010; 95:756–762.
85. Fereidoon, A., Ahangari, M.G., Saedodin, S. Thermal and structural behaviors of polypropylene nanocomposites reinforced with single-walled carbon nanotubes by melt processing method. J. Macromol. Sci. Part: B. 2008; 48:196–211.
86. Yang, J., Wang, K., Deng, H., Chen, F., Fu, Q. Hierarchical structure of injection-molded bars of HDPE/MWCNTs composites with novel nanohybrid shish-kebab. Polymer. 2010; 51:774–782.
87. Isci, S., Unlu, C.H., Atici, O. Polypropylene nanocomposites prepared with natural and ODTABr-modified montmorillonite. J. Appl. Polym. Sci.. 2009; 113:367–374.
88. Ko, J.H., Yoon, C.S., Chang, J.H. Polypropylene nanocomposites with various functionalized-multiwalled nanotubes: Thermomechanical properties, morphology, gas permeation, and optical transparency. J. Polym. Sci. Part: B. 2011; 49:244–254.
89. Shi, Y., Kashiwagi, Y., Walters, R.N., Gilman, J.W., Lyon, R.E., et al. Ethylene vinyl acetate/layered silicate nanocomposites prepared by a surfactant free method: Enhanced flame retardant and mechanical properties. Polymer. 2009; 50:3478–3487.
90. Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater.. 2001; 25:193–197.
91. Jiao, C.M., Wang, Z.Z., Ye, Z., Hu, Y., Fan, W.C. Flame retardation of ethylene vinyl acetate copolymer using nano magnesium hydroxide and nano hydrotalcite. J. Fire Sci.. 2006; 24:47–63.
92. Gao, F., Beyer, B., Yuana, O. A mechanistic study of fire retardancy of carbon nanotube/ethylene vinyl acetate copolymers and their clay composites. Polym. Degrad. Stab.. 2005; 89:559–564.
93. Peeterbroeck, S., Laoutid, F., Swoboda, B., Lopez-Cuesta, J.M., Moreau, N., et al. How carbon nanotube crushing can improve flame retardant behaviour in polymer nanocomposites? Macromol. Rapid Commun.. 2007; 28:260–264.
94. Ye, L., Wu, Q., Qu, B. Synergistic effects and mechanism of multiwalled carbon nanotubes with magnesium hydroxide in halogen-free flame retardant EVA/MH/MWNT nanocomposites. Polym. Degrad. Stab.. 2009; 94:751–756.
95. Barbosa, R., Araujo, E.M., Melo, T.J.A., Ito, E.N. Comparison of flammability behavior of polyethylene/Brazilian clay nanocomposites and polyethylene/flame retardants. Mater. Lett.. 2007; 61:2575–2578.
96. Lu, H., Hu, Y., Xiao, J., Wang, Z. Magnesium hydroxide sulfate hydrate whisker flame retardant polyethylene/montmorillonite nanocomposites. J. Mater. Sci.. 2006; 41:363–367.
97. Wagenknecht, U., Kretzschmar, B., Reinhardt, G. Investigation of fire retardant properties of polypropylene-clay-nanocomposite. Macromol. Symp.. 2003; 194:207–212.
98. Tang, Y., Hu, Y., Li, B., Liu, L., Wang, Z., et al. Polypropylene/montmorillonite nanocomposites and intumescent, flame retardant montmorillonite synergism in polypropylene nanocomposites. J. Polym. Sci: Part A. 2004; 42:6163–6173.
99. Song, R., Wang, Z., Meng, X., Zhang, B., Tang, T. Influences of catalysis and dispersion of organically modified montmorillonite on flame retardancy of polypropylene nanocomposites. J. Appl. Polym. Sci.. 2007; 106:3488–3494.
100. Zhang, M., Ding, P., Qu, B. Flammable, thermal, and mechanical properties of intumescent flame retardant PP/LDH nanocomposites with different divalent cations. Polym. Compos.. 2009; 1000–1006.
101. Wang, D.Y., Das, A., Costa, F.R., Leuteritz, A., Wang, Y.Z., et al. Synthesis of organo cobalt-aluminum layered double hydroxide via a novel single-step self-assembling method and its use as flame retardant nanofiller in PP. Langmuir. 2010; 26:14162–14169.
102. Fu, S., Song, P., Yang, H., Jin, Y., Lu, F., et al. Effects of carbon nanotubes and its functionalization on the thermal and flammability properties of polypropylene/wood flour composites. J. Mater. Sci.. 2010; 45:3520–3528.
103. Kashiwagi, T., Grulke, E., Hilding, J., Harris, R., Awad, W., et al. Thermal degradation and flammability properties of poly(propylene)/carbon nanotube composites. Macromol. Rapid Commun.. 2002; 23:761–765.
104. Kashiwagi, T., Grulke, E., Hilding, J., Groth, K., Harris, R., et al. Thermal and flammability properties of polypropylene/carbon nanotube nanocomposites. Polymer. 2004; 45:4227–4239.
105. Kalaitzidou, K., Fukushima, H., Drzal, L.T. Multifunctional polypropylene composites produced by incorporation of exfoliated graphite nanoplatelets. Carbon. 2007; 45:1446–1452.
106. Mittal, V. Gas permeation and mechanical properties of polypropylene nanocomposites with thermally-stable imidazolium modified clay. Eur. Polym. J.. 2007; 43:3727–3736.
107. Das, N.C., Maiti, S. Electromagnetic interference shielding of carbon nanotube/ethylene vinyl acetate composites. J. Mater. Sci.. 2008; 43:1920–1925.
108. Ciselli, P., Zhang, R., Wang, Z., Reynolds, C.T., Baxendale, M., et al. Oriented UHMW-PE/CNT composite tapes by a solution casting-drawing process using mixed-solvents. Eur. Polym. J.. 2009; 45:2741–2748.
109. Kim, H., Kobayashi, S., AbdurRahim, M.A., Zhang, M.J., Khusainova, A., et al. Graphene/polyethylene nanocomposites: Effect of polyethylene functionalization and blending methods. Polymer. 2011; 52:1837–1846.
110. Kalaitzidou, K., Fukushima, H., Drzal, L.T. A new compounding method for exfoliated graphite-polypropylene nanocomposites with enhanced flexural properties and lower percolation threshold. Compos. Sci. Technol.. 2007; 67:2045–2051.
111. Chen, X.M., Shen, J.W., Huang, W.Y. Novel electrically conductive polypropylene/graphite nanocomposites. J. Mater. Sci. Lett.. 2002; 21:213–214.
112. Chung, D.D.L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon. 2001; 39:279–285.
113. Das, N.C., Chaki, T.K., Khastgir, D., Chakraborty, A. Electromagnetic interference shielding effectiveness of ethylene vinyl acetate based conductive composites containing carbon fillers. J. Appl. Polym. Sci.. 2001; 80:1601–1608.
114. Das, N.C., Yamazaki, S., Hikosaka, M., Chaki, T.K., Khastgir, D., et al. Electrical conductivity and electromagnetic interference shielding effectiveness of polyaniline-ethylene vinyl acetate composites. Polym. Int.. 2005; 54:256–259.
115. Al-Saleh, M.H., Sundararaj, U. Electrically conductive carbon nanofiber/polyethylene composite: Effect of melt mixing conditions. Polym. Adv. Technol.. 2011; 22:246–253.
116. Panwar, V., Mehra, R.M. Analysis of electrical, dielectric, and electromagnetic interference shielding behavior of graphite filled high density polyethylene composites. Polym. Eng. Sci.. 2008; 48:2178–2187.
117. Ghosh, P., Chakrabarti, A. Conducting carbon black filled EPDM vulcanizates: Assessment of dependence of physical and mechanical properties and conducting character on variation of filler loading. Eur. Polym. J.. 2000; 36:1043–1054.
118. Mahapatra, S.P., Sridhar, V., Tripathy, D.K. Impedance analysis and electromagnetic interference shielding effectiveness of conductive carbon black reinforced microcellular EPDM rubber vulcanizates. Polym. Compos.. 2008; 29:465–472.
119. Xiang, J., Drzal, L.T., Thermal conductivity of a monolayer of exfoliated graphite nanoplatelets prepared by liquid-liquid interfacial self-assembly Article ID 481753. J. Nanomater 2010;
120. George, J.J., Bhowmick, A.K. Influence of matrix polarity on the properties of ethylene vinyl acetate-carbon nanofiller nanocomposites. Nanoscale Res. Lett.. 2009; 4:655–664.
121. Ghose, S., Watson, K.A., Working, D.C., Connell, J.W., Smith, J.G., Jr., et al. Thermal conductivity of ethylene vinyl acetate copolymer/nanofiller blends. Compos. Sci. Technol.. 2008; 68:1843–1853.
122. Chafidz, A., Ali, M.A., Elleithy, R. Morphological, thermal, rheological, and mechanical properties of polypropylene-nanoclay composites prepared from masterbatch in a twin screw extruder. J. Mater. Sci.. 2011; 46:6075–6086.
123. Njuguna, J., Pielichowski, K. Polymer nanocomposites for aerospace applications: Properties. Adv. Eng. Mater.. 2003; 5:769–778.
124. Stankovich, S., Dikin, D.A., Dommett, G.H.B., Kohlhaas, K.V., Zimney, E.J., et al. Graphene-based composite materials. Nature. 2006; 442:282–286.
