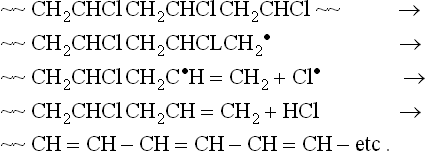Poly(vinyl chloride)(PVC)-based nanocomposites
Abstract:
This chapter first discusses the structure, processing and formulation of poly(vinyl chloride) (PVC), which are rather different from those of other thermoplastic materials. In particular, a PVC formulation typically contains a number of different additives, and exists in rigid (unplasticised PVC or uPVC) and flexible (plasticised PVC or pPVC) forms. The different nanofillers, which have been used in PVC, are then introduced individually. Next the most important properties that can be modified in PVC are considered. Finally, brief statements about the opportunities for PVC nanocomposites and future trends are provided. An extensive list of references is included.
8.1 Introduction
Poly(vinyl chloride) (PVC) exists as two distinctly different thermoplastics, rigid PVC and flexible PVC, which are used in a wide variety of applications as discussed later. Approximately two-thirds of the PVC used is the rigid form. Research has been carried out into the addition of nanofillers into both of these materials, with the earliest papers published by Wang and co-workers in 2001 and 2002.1, 2
As discussed below, there are a number of specific problems associated with introducing nanofillers into PVC, so there are no significant commercial nanocomposites.
This chapter will be divided into the following sections. Section 8.2 is concerned with the polymerisation and performance of PVC as relevant to the formation of nanocomposites. PVC is primarily made by suspension polymerisation, but also by emulsion polymerisation. The morphology and applications of these two polymerisation methods will be considered, as will their fusion to produce a final product. A key feature of PVC is poor thermal stability, which leads to degradation at relatively low temperatures. It is therefore necessary to add stabilisers in order to process PVC, so that a mixing process is required. In practice, PVC is mixed with a wide variety of additives, making it an extremely versatile polymer, with different formulations used for different applications. Typical additives will be discussed.
Section 8.3 considers the methods which have been used to produce PVC nanocomposites. Section 8.4 details the types of nanofillers that have been used, while the property changes that can be achieved are considered in Section 8.5. The remaining sections will consider opportunities, problems and future trends and provide sources of information. A comprehensive reference list is provided.
8.2 Poly(vinyl chloride) (PVC)
8.2.1 PVC polymerisation, structure and morphology
The basic repeat unit for PVC is:
All types of PVC are formed via free-radical polymerisation of vinyl chloride monomers using peroxide catalysts. Use of this type of catalyst normally produces an atactic polymer (e.g. polystyrene). However, due to the relatively large size and electronegativity of the chlorine atom, commercial PVC contains about 55 % syndiotacticity. This is located in sequences around 5–12 repeat units long; these sequences are long enough to crystallise, producing ~ 10 % crystallinity. Crystallites are small (~ 10 nm), and do not form spherulites, so PVC can be transparent. They vary considerably in size and perfection, melting over a temperature range from 110 to 240 °C.
Most PVC is produced by suspension polymerisation. Because PVC is insoluble in its own monomer, the morphology of PVC grains is complex. The grains themselves are 100–150 µm in diameter, and irregular in shape, as shown in the scanning electron micrographs in Fig. 8.1. When a grain is cut open (Fig. 8.1(b)) primary particles (∼ 1 μm in diameter) are revealed together with a considerable amount of porosity. Further examination reveals a pericellular membrane, produced from the suspending agent, which coats each grain, while the primary particles consist of smaller particles known as domains, around 10 nm in size. The crystallinity exists within these domains.
In emulsion polymerisation a latex containing spherical primary particles (usually with a size range of around 0.3−1.0 μm) is produced. This is then dried to produce aggregates of primary particles, described as secondary particles or grains, which are 30–60 μm in diameter.
Clearly, when nanofillers are added to PVC, their location with respect to the structures described is of considerable importance, as discussed later.
8.2.2 PVC fusion and processing
All rigid PVC and some flexible PVC is melt processed, mainly by extrusion, but compression moulding, injection moulding and calendering are also used. During processing, it is necessary to convert individual grains into a continuous solid structure. This process is known as fusion or gelation. The fusion, or gelation, of PVC can be defined simply as the conversion of PVC grains, which will be mixed with the required additives, to produce a product with the desired properties. In practice, the process is significantly more complex. Important work in the area was carried out by Allsopp,3 who studied morphology changes during the processing of PVC by a variety of techniques, and proposed two possible mechanisms for PVC fusion. Fusion requires both heat and shear, and the level of shear determines which mechanism occurs. In the comminution mechanism, the PVC grains are broken down into primary particles, additives are distributed, and the primary particles are then fused together to form a melt. This occurs in more aggressive high-shear equipment such as Brabender rheometers and Banbury mixers. In compression moulding, which is a very low-shear process, primary particles can be fused together within grains, but it is difficult to fuse grains together adequately, except at very high temperatures at which degradation can become a problem. For extrusion and two-roll milling, shear is higher than in compression moulding, and fusion occurs through the mechanism described by Allsopp as the compaction, elongation, densification and fusion (CDFE) mechanism.
The CDFE mechanism, as illustrated in Fig. 8.2, has the following stages:
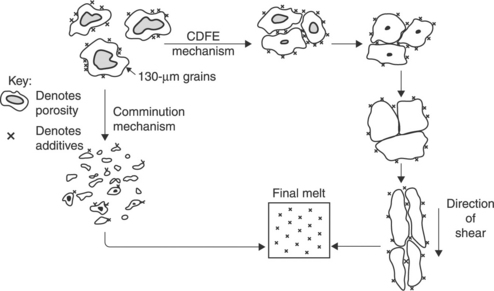
8.2 Mechanisms of PVC fusion (Fig. 8.21 in Allsopp3).
• compaction – individual grains are packed together and some porosity is removed. Additives remain distributed around the grains
• densification – further porosity is removed
• elongation – grains become elongated in the direction of shear
• fusion – grains fuse together to form a melt, and additives become distributed.
An unusual feature of PVC is the fact that it is processed below its highest melting temperature. Thermal analysis shows that melting and reformation of crystallinity have an important role in fusion. This led Covas et al.4 to develop the CDFE mechanism further to incorporate crystallisation. The results are summarised in Fig. 8.3. During processing, typically at 170 °C to 210 °C, some of the crystals will melt, whilst the remaining more perfect crystals, referred to as primary crystals, will be annealed. When a sufficient number of crystals have melted, the material will recrystallise during cooling to produce secondary crystallinity, which links the original structures to produce a strong material. Summers et al.5 also used crystallisation to explain the results obtained by a rheological method used to measure fusion.
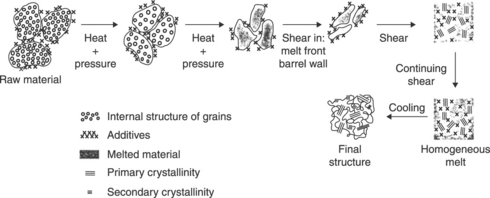
8.3 Modified CDFE mechanism.4
Although initial studies in this area related to rigid PVC, it has subsequently been shown that similar considerations apply to the fusion of flexible PVC.6 It should be noted that some flexible PVC is processed via a plastisol process using emulsion polymer. Although the process is different, the melting and crystallisation processes discussed previously still occur. When nanofillers are added, it is important that they are well dispersed in the homogeneous melt.
8.2.3 PVC degradation
If PVC were made up solely of (-CH2 CHCl-) repeat units, its stability would be reasonably good. However, in practice, chemical defect structures are present – labile chlorine, unsaturation, head-to-head units, and peroxide and hydroperoxide groups. The latter in particular act as sites for the initiation of degradation. The first stage of degradation is the formation of macroradicals, but these undergo a rapid ‘unzipping‘ reaction leading to the production of hydrogen chloride, which is both corrosive and acts as a catalyst for degradation, and results in the formation of conjugated unsaturation along the polymer chain, i.e.
Conjugated chains absorb UV radiation, but as the number of conjugated units increases, the conjugation starts to absorb in the visible region, and the PVC changes in appearance, producing the well-known sequence of colours white/yellow/orange/brown/black. Without a stabiliser, this degradation can start at temperatures as low as 100 °C. It will be seen later that certain nanofillers can accelerate this process, while others can act as stabilisers.
8.2.4 PVC formulation and additives
As a plastic material, PVC is unique in the extent to which it is formulated. Additives are normally introduced by high-speed mixing in which the powder is stirred at speeds of the order of 3000 rpm. Solid additives are usually added first, followed by liquid ones. Solid additives coat the PVC grains, becoming located in hollows on the grain surfaces, while liquid additives are absorbed and located in porous regions. The high speed results in shear heating to above the glass transition temperature of the PVC (~ 80 °C), and mixing is normally continued until the temperature reaches 120 °C, when the mix is discharged into a cooling chamber. The resulting powder blend may be processed directly to make a product or extruded to make a pelletised compound for subsequent processing.
When plastisol technology is used the PVC is suspended in a liquid plasticiser, and additives are introduced by mixing with the liquid state.
The main additives used in PVC formulations are summarised below.
Heat stabilisers
A variety of different compounds have been used for this purpose. Lead compounds are inexpensive and very common, and were popular in the past, but they are now being phased out in Europe, due to concerns about toxicity.7 Mixed metal soaps are widely used. At one time these contained various combinations of calcium, zinc, barium and cadmium, but cadmium has now been phased out in Europe,7 and stabilisers are based on Ca and Zn. These stabilisers are less effective, particularly during the later stages of degradation, and nanofillers that could enhance performance would provide significant benefits. The so-called organo-based stabilisers include calcium. Another group of stabilisers is based on organotin compounds. These are expensive but can be used at lower concentrations. In addition to the above there are a number of secondary stabilisers, such as epoxy compounds and hydrotalcites.
Lubricants
Both external and internal lubricants are required in rigid PVC formulations, while internal or multifunctional lubricants are used when flexible formulations are melt compounded. Although the distinction between the two types is not precise, in general external lubricants migrate to the melt surface and reduce the friction between the PVC and the surface of the processing equipment; an example of this type of lubricant would be paraffin wax. Internal lubricants reduce the friction between the PVC grains and improve particle flow. The most common internal lubricant is calcium stearate. Stabiliser/lubricant packages are frequently used in PVC formulations.
Processing aids
Processing aids are used in rigid formulations to increase melt strength, particularly for extrusion processes. The most common type of processing aid is a high molecular weight poly(methyl methacrylate), which entangles with the PVC molecules in the melt and prevents tearing when the material exits from the extruder die.
Impact modifiers
Impact modifiers are added to improve toughness for demanding applications such as pressure pipes. They are either rubber based (acrylic polymers or chlorinated polyethylene) or finely divided minerals, in particular calcium carbonate. There is considerable scope for using nanofillers for this purpose.
Fillers
Fillers have been incorporated in PVC to improve mechanical strength, hardness and stiffness, flame retardancy and electrical properties, to reduce thermal expansion and sometimes just to reduce costs. The most common filler for PVC is calcium carbonate, although various silicates (talc, kaolin and mica) have also been used. Nanofillers could replace conventional fillers for some of these purposes.
Plasticisers
Plasticisers are compatible and involatile organic liquids that reduce the glass transition temperature of the PVC to below room temperature, thus producing a flexible polymer. The more plasticiser added, the more flexible the plastic. Apart from specific applications containing fillers, the additives described above are used at levels < 10 parts per hundred of PVC resin (phr). However, typical plasticiser levels are 30–100 phr. Of the plasticisers used > 90 % are phthalates, which are good general purpose plasticisers with low cost. Because of perceived health problems associated with phthalate plasticisers, risk assessments on the commonly used phthalates have been carried out as part of the Vinyl 2010 voluntary commitment,7 and some of the lower molecular weight phthalates are no longer permitted for use in children‘s toys and childcare articles, but higher molecular weight phthalates (≥ C9) have a clean bill of health for all applications. Specialist plasticisers include phosphates for fire retardancy, polymeric plasticisers (low migration) and some newer ‘non-phthalate’ plasticisers such as citrates, benzoate esters, alkyl sulphonic phenyl esters (Mesamoll®), di-isononyl cyclohexane-1,2-dicarboxylate (Hexamoll® DINCH) and biobased plasticisers (e.g. Grindsted® Soft-n-Safe).
8.3 Manufacturing techniques
The standard addition techniques for nanofillers have all been used for PVC, namely during polymerisation, solution blending and melt blending. An additional technique exists for PVC because nanofillers can be added to plastisols. There are also a number of examples cited later in which a nanofiller is produced as a suspension in a liquid, which is then added to PVC. Specific applications of these techniques are discussed below.
8.4 Nanofillers
8.4.1 Montmorillonite (MMT)
The most widely used nanofiller for PVC is the layered clay, montmorillonite (MMT), both in its natural form as sodium montmorillonite (Na-MMT), and as organomodified (OMMT) grades. PVC/MMT nanocomposites were reviewed by Pagacz and Pielichowski in 2009.8 Organomodification of MMT was discussed in detail in this paper, which also gives a useful summary of salts used for MMT modification. Most researchers have used melt compounding to disperse nanoclays, with varying degrees of success. However, solution blending,1, 9, 10polymerisation11–17 and plastisols have also been used.18 One problem associated with these nanofillers arises because the most common organomodifiers for MMT are quaternary ammonium compounds, and the ammonium cations can actually contribute to PVC degradation.8 A variety of other modifiers have therefore been investigated.
Another of the problems associated with the formation of PVC nanocomposites is related to the morphology of PVC. This is illustrated in Fig. 8.4, which shows transmission electronic microscopy (TEM) micrographs of (a) melt compounded, (b) solution blended and (c) plastisol based nanocomposites containing Cloisite 30B OMMT.
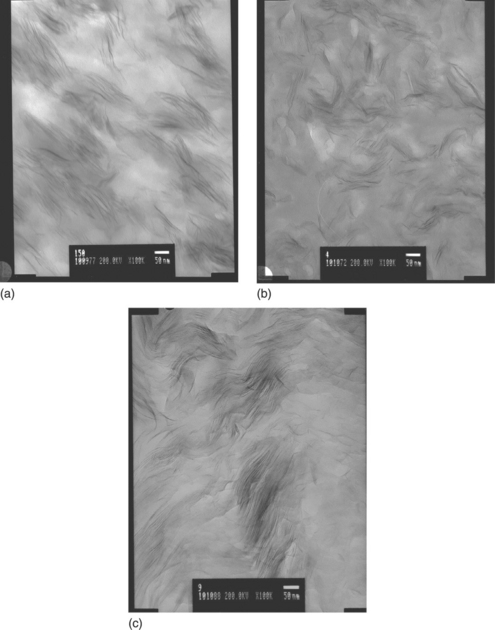
8.4 TEM micrographs of nanocomposites. (a) Melt blended in a Haake rheometer at 150 °C. (b) Melt blend dissolved in tetrahydrofuran, then cast. (c) Produced from plastisol containing 70 phr di-isodecyl phthalate.19 (Equal magnifications, scale bar equals 50 nm.)
Figure 8.4(a) shows intercalation and some exfoliation of nanoclay. Light areas without filler are PVC primary particles, which have not been destroyed. These areas could have been reduced by increasing the processing temperature, but full intercalation was not achieved because the PVC did not fully melt. When a melt-processed sample is dissolved in tetrahydrofuran, all morphology and crystallinity are destroyed, and a high level of exfoliation can be achieved (Fig. 8.4(b)). This is confirmed by the disappearance of the low-angle peaks observed by X-ray diffraction. When a nanocomposite is produced from a plastisol containing emulsion PVC, again there is some exfoliation (Fig. 8.4(c)), but less than in the solution-blended sample. In other words, the formation of PVC nanocomposites with high levels of exfoliation is difficult via melt compounding. Matuana20 addressed the problem of achieving the appropriate PVC morphology for good nanoclay dispersion, using a torque rheometer, and showed that when nanoclay is introduced at the onset of fusion, when the PVC particles are reduced in size, better dispersion and enhanced mechanical properties are obtained. Industrial applications of this approach in, for example, extrusion need to be addressed.
When nanofillers are added during polymerisation, exfoliation can be achieved much more readily.12, 13, 16, 17
8.4.2 Other clays
Other clays which have been used as nanofillers in PVC include Laponite,21 bentonite,22–25 hectorite23, 25 and kaolinite.26 Vandevyver and Eicholz24 recognised the problems associated with dispersing nanofillers in suspended PVC, so they focussed on mixing sodium bentonite with an emulsion PVC latex, taking advantage of the ability of water to cause exfoliation. The mixture produced was spray dried, and its behaviour after milling and pressing, and in plastisols, was investigated.
8.4.3 Calcium carbonate
Various types of calcium carbonate (ground calcite, ground whiting and ground limestone) with mean particle sizes ranging from 1 to 30 μm are the most common fillers for PVC. More specialist (and expensive) precipitated calcium carbonates (mean particle size < 1 μm) are also used when good mechanical properties are required, particularly for rigid formulations. It is therefore unsurprising that nano-sized calcium carbonates have received a considerable amount of attention. Nanocomposites have been produced by polymerisation,27, 28 solution blending29 and melt processing.30–39 A novel polymerisation method involved synthesising nano-porous PVC particles then reacting calcium hydroxide and carbon dioxide inside these to produce PVC/CaCO3 nanocomposites, which were finally melt blended.40
8.4.4 Silica
Silica is another type of nanofiller which has been used in PVC. Incorporation techniques include polymerisation. 17, 28 There has been a specific focus on the surface coating of silica particles to improve compatibility with the PVC matrix41–46 in melt-compounded nanocomposites.
8.4.5 Layered double hydroxides
Layered double hydroxides (LDHs), specifically hydrotalcite, although not necessarily in the form of nanoscale particles have, since 2000, found a significant application as secondary stabilisers in PVC.47, 48 They are usually found in stabiliser/lubricant packages, which are added to PVC formulations. Hydrotalcites are layered structures, but, unlike MMTs, have anions between the layers, which can be replaced by Cl– ions produced during PVC degradation, thus retarding PVC degradation. Because of the benefits of hydrotalcites, hydrotalcite nanofillers are obviously of interest. In one of the earliest papers in this area, Wang et al.49 compared nanocomposites prepared by in situ polymerisation and melt blending, and demonstrated that the former performed better in all respects. The same group also investigated nanocomposites containing various LDHs, and carried out further work on PVC/hydrotalcite nanocomposites prepared by in situ polymerisation.50, 51 LDH nanocomposites have also been prepared by melt processing,52 solution intercalation53 and using a novel method in which the filler was delaminated by lauryl ether phosphate in tetrahydrofuran and then the suspension produced was added to PVC.54 LDH nanofillers have also been modified by grafting with toluene diisocyanate to improve dispersion.55
8.4.6 Carbon nanotubes
There are some reports of the use of carbon nanotubes (CNTs) in PVC, although the addition of a costly filler to an inexpensive commodity thermoplastic polymer can only be justified for very specialist applications, as discussed later. Solution blending has been used for the preparation of nanocomposites.56
8.4.7 Other nanofillers
The use of a variety of other nanofillers in PVC has been reported. These include antimony trioxide,57 polyhedral oligomeric silsesquioxane (POSS),58, 59 calcium sulphate,60, 61 calcium phosphate62 and zinc oxide.63 Finally, combinations of two different fillers have been used on a number of occasions. These include CNTs with wood-flour, to improve the performance of commercially available wood-filled PVC,64 and nano-calcium carbonate and wood-flour.65 Nanoclays have been used with metallic oxides, which affect combustion and smoke suppressant properties,66 and with zeolite to improve thermal stability.67
8.5 Effects of nanofillers
8.5.1 Thermal stability
The thermal stability of PVC compounds may be improved or reduced by nanofillers. Frequently thermal stability has been assessed by thermogravimetric analysis,8 but in practice tests routinely used to assess the thermal stability of PVC compounds (e.g. the Congo red test, oven ageing and torque rheometry) are more important. Unsurprisingly, different nanofillers have different effects. Generally, MMTs reduce thermal stability, calcium carbonate and other clays can improve performance, but the effect of hydrotalcite is the most positive.
Commercially available organically modified MMTs tend to have adverse effects on PVC thermal stability,8 although effects can be reduced to some extent by changing the stabilisers present in the PVC formulation.68 Wan et al.69 suggested that discolouration rather than degradation occurred when OMMT was used in PVC, but this is not a widely held view. Attempts have been made to avoid the problem of poor stability by developing alternative modifiers. It has been shown that the incorporation of silica into a modifier improves thermal stability67, 70 Sterky et al.71 showed that, unlike normal cationic modifiers, non-ionic intercalants did not affect thermal stability. Another approach is the use of chelating agents to modify surface-treated MMT.72 Added metallic oxides also produced favourable results.66 Other modifications have been discussed in the literature.8
Nano-calcium carbonate,35, 73 kaolinite26 and sodium bentonite24 also appear to improve the thermal stability of PVC compounds to some extent.
Consistently with the use of layered double hydroxides, such as hydrotalcite, as secondary stabilisers, these compounds appear to be the most effective nanofillers for enhancing PVC stability.49, 51, 53, 55, 72–76
8.5.2 Mechanical properties
One of the most common benefits sought by the incorporation of nanofillers in a polymer is an improvement in mechanical properties. It should be remembered that rigid PVC itself has good strength and stiffness compared with other commodity thermoplastics (tensile strength 60 MPa and Young‘s modulus 2.8 GPa), providing that it is processed correctly. Toughness is typically improved by the addition of impact modifiers, as discussed in Section 8.2.4. The mechanical properties of PVC nanocomposites have been reported extensively. As with any filler, the modulus normally increases. Improvements in tensile properties can be achieved, but, unless dispersion is good, toughness and elongation can often decrease.
The mechanical properties of MMT/PVC nanocomposites have been discussed previously.8 It is generally concluded that improvements can be achieved at low levels of MMT, but they are relatively modest compared with improvements seen for other polymers. In recent work the use of OMMT with a silane coupling agent has produced impact strengths higher than that of PVC alone when the clay content was < 7 phr.77
Nano-calcium carbonate was found to be effective in increasing toughness in nanocomposites.37 In order to enhance toughness, specific filler treatments have been used on a number of occasions. Examples include grafted nanotubes78 and nano-calcium carbonate coated with silicone rubber.79
8.5.3 Flammability and smoke suppression
Due to the presence of chlorine, rigid PVC does not burn readily but does produce smoke on burning. Flexible PVC burns more readily due to the presence of relatively large quantities of plasticiser (Section 8.2.4). Nanofillers that reduce the amount of smoke produced or make flexible PVC, particularly in applications like cables, more difficult to burn are of significant interest. A number of workers have investigated the use of MMTs to improve the fire performance of PVC.8 A considerable amount of research has been carried out by Beyer and co-workers25, 80–82 but results remain inconclusive, and, as observed previously, significant discolouration (dehydrochlorination) of the PVC occurs. However, modified flexible PVC nanocomposites also containing halloysite, an aluminosilicate (nanotube-based) filler, were found to exhibit enhanced flame retardancy.83 It has also been shown using cone calorimetry that flame retardancy and smoke suppression were significantly improved by the addition of OMMT to PVC/wood-flour composites.84
Modified OMMTs in which sodium ions were replaced by Cu2 + have been shown to have improved flame retardancy.85, 86 The Cu2 + ions were also found to be effective in smoke suppression. Fe-modified OMMTs have also shown improved flame retardant properties.87 A detailed study of OMMT with added zinc, copper and molybdenum oxides has been carried out, and has shown that the metal oxides have a more significant effect than OMMT in improving fire and smoke suppression.88
The beneficial effects of hydrotalcite on the thermal stability of PVC have already been discussed. It also appears to improve, very often in a modified form, fire performance. Nanofillers that appear promising include LDH/ZnO combinations, which reduced smoke density and increased the limiting oxygen index.89, 90 Phosphate-modified hydrotalcite has been shown to reduce smoke emissions.91
Zinc hydroxystannate (ZnSn(OH)6) is well known as a flame-retardant/smoke suppressant for PVC, and nano-(ZnSn(OH)6) has been shown to provide even better performance.92
8.5.4 Permeability
Nanofillers have been used to reduce gas and liquid permeation through a polymer by increasing the tortuosity of the diffusion path. Hectorite and bentonite clays have been shown to decrease oxygen permeation in flexible PVC by up to 77 %. A fivefold improvement in oxygen barrier properties by the addition of OMMT has been reported.93 PMMA-grafted silica has also been used to reduce oxygen and water permeation.43 Recent work has investigated the use of OMMTs to reduce plasticiser migration in flexible PVC.94 It was found that the nanoclay reduced plasticiser migration slightly when compounds were melt compounded, but the effect was considerably greater after dissolving the nanocomposite in tetrahydrofuran, and producing a cast film, because clay dispersion was much better, as discussed in Section 8.4. A novel application of nanofillers is the coextrusion of a carbon nanotube coating to reduce the ingress of moisture into wood-filled composites.95
8.5.5 Transition temperatures
PVC has three transition temperatures. The broad melting temperature discussed in Section 8.2.1 is generally unaffected by additives, being controlled by the syndiotacticity of the PVC and the processing conditions. The glass transition temperature of rigid PVC is approximately 80 °C, depending on the precise formulation; this reduces to temperatures from below ambient down to temperatures as low as − 50 °C as plasticiser concentration increases. PVC also has a β transition, detected by dynamic mechanical analysis. This is split into two, with maxima at − 50 °C and 0 °C (using a frequency of 110 Hz), attributed to the movement of small chain segments in the amorphous and crystalline regions, respectively.
The effect of nanofillers on PVC transition temperatures has been reported extensively in the literature, with research mainly focussing on the glass transition temperature in rigid PVC. One paper96 commented on the effects of MMT on the β transition in a lightly plasticised PVC, which was found to become broader with a similar peak value as Na-MMT but moved to a lower temperature; this transition also broadened in the presence of OMMT. Reasons for this are unclear because the glass transition temperature was virtually unchanged in these compounds. Mkhabela et al.97 suggested that the PVC melting temperature was increased in carbon nanotube/PVC composites, but the differential scanning calorimetry traces reported do not illustrate PVC melting.
Nanofillers have been reported as increasing, 13, 14, 22, 27, 30, 33, 36, 50, 77, 97–99 decreasing16, 54, 100 and having no significant effect99–101 on the glass transition temperature of rigid PVC, but where changes are reported they generally seem to be no more than 2–3 °C, so probably the latter is nearer the truth in most cases.
Benderly et al.23 observed no change in the glass transition temperature of pPVC with the addition of hectorite and bentonite clays.
The Vicat softening point is a penetrometer technique, and measures the temperature at which a loaded probe penetrates a sample by a specific amount. It is a technological measure of transition temperature and, in an unfilled largely amorphous polymer such as PVC, it would be expected to approximate to the glass transition temperature. In filled polymers, the fillers themselves restrict penetration of the probe, such that Vicat softening points of filled polymers are often much higher than conventional glass transition temperatures. The Tg of rigid PVC is relatively low at 80 °C, so additives that increase this are beneficial. There are significant increases in the Vicat softening point with the addition of Na-MMT13, 102 and nanoscale calcium carbonate.30
8.5.6 Electrical properties
Multi- and single-walled carbon nanotubes have been used to reduce electrical resistance.56 Increased electrical conductivity with a very low percolation threshold has been achieved by the use of multi-walled carbon nanotubes. 103, 104 PVC/graphite nanosheet/nickel nanocomposites have been developed for electrostatic charge dissipation.105
8.5.7 Other properties
PVC nanocomposites have been developed to enhance various other properties. Magnetite106–108 has been used to impart magnetic properties. UV resistance was achieved by using phthalocyanine-modified Laponite clay.21
Various commercially available nanofillers have been used to improve specific compressive strength, specific flexural modulus and the density of PVC foams.109 It has been shown that nanoparticles can increase the orientation of crystalline and amorphous phases in oriented PVC films110 and modify plastisol rheology.24 Nanofillers can also be effective during recycling. The properties of recycled PVC were shown to be improved by the addition of nanoclay,111 and surface-modified nano-calcium carbonate can improve compatibility with polypropylene in mixed plastic waste so that material containing 10–20 % polypropylene can be produced.
8.6 Opportunities and problems
Mixing PVC with additives, together with an industry very familiar with the issue of formulation, provides an excellent opportunity for introducing nanofillers. However, their dispersion is hampered by the complex morphology of PVC. The areas in which nanofillers are most likely to offer benefits are for impact modification, stabilisation (thermal and UV), smoke suppression and electrical and thermal applications.
8.7 Future trends
At present only one commercial PVC compound containing nanofillers has been made available. This product, a paste PVC grade called NanoVin, contained bentonite, and was launched some years ago by Solvin. Unfortunately, despite potential benefits,24 the market for this product did not develop, but it still remains on standby.
The areas in which PVC nanocomposites are likely to be of most interest in the future are in foams, 112 as smoke suppressants and flame retardants, to improve thermal stability (particularly nanohydrotalcites) and to aid recycling.
8.8 Sources of further information and advice
This article provides a thorough coverage of the literature available on PVC nanocomposites. Due to the lack of commercial materials, it is difficult to identify further sources of information at the present time.
8.9 References
1. Wang, D.Y., Parlow, D., Yao, Q., Wilkie, C.A. PVC-clay nanocomposites: Preparation, thermal and mechanical properties. J. Vinyl and Additive Tech.. 2001; 7:203–213.
2. Wang, D.Y., Parlow, D., Yao, Q., Wilkie, C.A. PVC-clay nanocomposites: Preparation, thermal and mechanical properties. J. Vinyl and Additive Tech.. 2002; 8:139–150.
3. Allsopp, M.W. Mechanism of gelation of rigid PVC. In: Burgess R.H., ed. Manufacture and Processing of PVC. London: Applied Science Publishers, 1982.
4. Covas, J.A., Gilbert, M., Marshall, D.E. Twin screw extrusion of a rigid PVC compound – effect on fusion and properties. Plast. & Rubb. Process. Proc. and Appl.. 1988; 9:107.
5. Summers, J.W., Rabinovitch, E.B., Booth, P.C. Measurement of PVC fusion (gelation). J. Vinyl Tech.. 1986; 8:6.
6. Patel, S.V., Gilbert, M. Effect of processing on the fusion of plasticised PVC. Plast. and Rubb. Proc. and Appl.. 1985; 5:85.
7. The European PVC Industry’s Sustainable Development Programme. Vinyl 2010, 2010. [Progress report].
8. Pagacz, J., Pielichowski, K. Preparation and characterization of PVC/ montmorillonite nanocomposites – A review. J. Vinyl and Additive Tech.. 2009; 15:61–76.
9. Wang, D.Y., Wilkie, C.A. Preparation of PVC-clay nanocomposites by solution blending. J. Vinyland Additive Tech.. 2002; 8:238–245.
10. Magdaleno, L., Schjodt-Thomsen, J., Pinto, J.C. Morphology, thermal and mechanical properties of PVC/MMT nanocomposites prepared by solution blending and solution blending plus melt compounding. Composites Sci. and Tech.. 2010; 70:804–814.
11. Aguilar-Solis, C., Xu, Y., Brittain, W. Polymer-layered silicate nanocomposites by suspension and emulsion polymerizations: PVC-MMT nanocomposites. Polym. Prepr. (ACMS. Div. Polym. Chem). 2002; 43:1019.
12. Gong, F.I., Feng, M., Zhao, C.G., Zhang, S.M., Yang, M.S. Particle configuration and mechanical properties of poly(vinyl chloride)/montmorillonite nanocomposites via in situ suspension polymerization. Polymer Testing. 2004; 23:847–853.
13. Pan, M.W., Shi, X.D., Li, M.C., Hu, H.Y., Zhang, L.C. Morphology and properties of PVC/clay nanocomposites via in situ emulsion polymerisation. J. Appl. Polym. Sci.. 2004; 94:277–286.
14. Gong, F.I., Feng, M., Zhao, C.G., Zhang, S.M., Yang, M.S. Thermal properties of poly(vinyl chloride)/montmorillonite nanocomposites. Polym. Deg. and Stab.. 2004; 84:289–294.
15. Hu, H.Y., Pan, M.W., Li, X.C., Shi, X.D., Zhang, L.C. Preparation and characterization of poly(vinyl chloride)/organoclay by in situ intercalation. Polymer International. 2004; 53:225–231.
16. Yang, D.Y., Liu, Q.X., Xie, X.L., Zeng, F.D. Structure and thermal properties of exfoliated PVC/layered silicate nanocomposites via in situ polymerisation. J Thermal Analysis and Calorimetry. 2006; 84:355–359.
17. Obloj-Muzaj, M., Zielecka, M., Kozakiewicz, J., Abramowicz, A., Szulc, A., et al. Polymerization of vinyl chloride in the presence of nanofillers – effects on the shape and morphology of PVC grains. Polimery. 2006; 51:133–137.
18. Peprnicek, T., Kalendova, A., Pavlova, E., Simonik, J., Duchet, J., et al. Poly(vinyl chloride-paste/clay nanocomposites: Investigation of thermal and morphological characteristics. Polym. Deg. and Stab.. 2006; 91:3322–3329.
19. Zheng, X.Development of plasticised PVC/clay nanocomposites. Loughborough University, 2009. [PhD thesis].
20. Matuana, L.M. Rigid PVC/(layered silicate) nanocomposites produced through a novel melt-blending approach. J. Vinyl and Additive Tech.. 2009; 15:77–86.
21. Essawy, H.A., El-Wahab, N.A.A., El-Ghaffar, M.A.A. PVC-Laponite nanocomposites: Enhanced resistance to UV radiation. Polym. Deg. and Stab.. 2008; 93:1472–1478.
22. Romero-Guzman, M.E., Romo-Uribe, A., Ovalle-Garcia, E., Olayo, R., Cruz-Ramos, C.A. Microstructure and dynamic mechanical analysis of extruded layered silicate PVC nanocomposites. Polymers for Advanced Technologies. 2008; 19:1168–1176.
23. Benderly, D., Osorio, F., Ijdo, W.L. PVC nanocomposites-nanoclay chemistry and performance. J. Vinyl and Additive Tech.. 2008; 14:155–162.
24. Vandevyver, E., Eicholz, E., Latest advancements in PVC/clay composites: Potential applications in plastisols. 10th International PVC Conference, Brighton, England. 2008.
25. Awad, W.H., Beyer, G., Benderly, D., Ijdo, W.L., Songtipya, P., et al. Material properties of nanoclay PVC composites. Polymer. 2009; 50:1857–1867.
26. Turhan, Y., Dogan, M., Alkan, M. Poly(vinyl chloride)/kaolinite nanocomposites: Characterization and thermal and optical properties. Ind. and Eng. Chem. Res.. 2010; 49:1503–1513.
27. Xie, X.L., Liu, Q.X., Li, R.X.Y., Zhou, X.P., Zhang, Q.X., et al. Rheological and mechanical properties of PVC/CaCO3 nanocomposites prepared by in situ polymerization. Polymer. 2004; 45:6665–6673.
28. Georgiadou, S., Thomas, N.L., Gilbert, M., Brooks, B.W. Suspension polymerisation of vinyl chloride in presence of ultra fine filler particles. Plastics, Rubber and Composites. 2008; 37:431–435.
29. Liu, P., Zhao, M., Guo, J. Thermal stabilities ofpoly(vinyl chloride)/calcium carbonate (PVC/CaCO3) composites. J.Macromol. Sci. Part B: Phys.. 2006; 45:1135–1140.
30. Chen, N., Wan, C.Y., Chang, Y., Zhang, Y.X. Effect of nano-CaCO3 on mechanical properties of PVC and PVC/blendex blend. Polym. Test.. 2004; 23:169–174.
31. Wu, D.Z., Wang, X.D., Song, Y.Z., Jin, R.G. Nanocomposites ofpoly(vinyl chloride) and nanometric calcium carbonate particles: Effects of chlorinated polyethylene on mechanical properties, morphology, and rheology. J. Appl. Polym. Sci.. 2004; 92:2714–2723.
32. Chen, N., Wan, C.Y., Zhang, Y., Zang, Y.X., Zhang, C.M. Fracture behavior of PVC/blendex/nano-CaCO3 composites. J. Appl. Polym. Sci.. 2005; 95:953–961.
33. Chen, C.H., Teng, C.C., Su, S.F., Wu, W.C., Yang, C.H. Effects of microscale calcium carbonate and nanoscale calcium carbonate on the fusion, thermal, and mechanical characterizations of rigid poly(vinyl chloride)/calcium carbonate composites. J. Polym. Sci. Part B: Polym. Phys.. 2006; 44:451–460.
34. Sun, S.S., Li, C.Z., Zhang, L., Du, H.L., Burnell-Gray, J.S. Interfacial structures and mechanical properties of PVC composites reinforced by CaCO3 with different particle sizes and surface treatments. Polym. Int.. 2006; 55:158–164.
35. Zheng, X.F., Wang, W.Y., Wang, G.Q., Chen, J.F. Influence ofthe diameter of CaCO3 particles on the mechanical and rheological properties of PVC composites. J. Mat. Sci.. 2007; 43:3505–3509.
36. Patil, C.B., Kapadi, U.R., Hundiwale, D.G., Mahulikar, P.P. Preparation and characterization of poly(vinyl chloride) calcium carbonate nanocomposites via melt intercalation. J. Mat. Sci.. 2009; 44:3118.
37. Kemal, I., Whittle, A., Burford, R., Vodenitcharova, T. Toughening of unmodified polyvinylchloride through the addition of nanoparticulate calcium carbonate. Polymer. 2009; 50:4066–4079.
38. Shimpi, N.G., Verma, J., Mishra, S. Dispersion of nano CaCO3 on PVC and its influence on mechanical and thermal properties. J. Comp. Mat.. 2010; 44:211–219.
39. Zhang, L., Luo, M.F., Sun, S.S., Ma, J., Li, C. Effect of surface structure of Nano-CaCO3 particles on mechanical and rheological properties of PVC composites. J. Macromol. Sci. Part B: Phys.. 2010; 49:970–982.
40. Xiong, C.X., Liu, S.J., Wang, D.Y., Dong, L.J., Jiang, D.D., et al. Microporous polyvinyl chloride: Novel reactor for PVC/CaCO3 nanocomposites. Nanotechnology. 2005; 16:1787–1792.
41. Sun, S.S., Li, C., Zhang, L., Du, H.L., Burnell-Gray, J.S. Effects of surface modification of fumed silica on interfacial structures and mechanical properties of poly(vinyl chloride) composites. Europ. Polym. J.. 2006; 42:1643–1652.
42. Guo, Y.K., Wang, M.Y., Zhang, H.Q., Qu, H. The surface modification of nanosilica, preparation of nanosilica/acrylic core-shell composite latex, and its application in toughening PVC matrix. Polym. Eng. Sci.. 2008; 107:2671–2680.
43. Zhu, A.P., Cai, A.Y., Zhang, J., Jia, H.W., Wang, J.Q. PMMA-grafted-silica/ PVC nanocomposites: Mechanical performance and barrier properties. J. Appl. Polym. Sci.. 2008; 108:2189–2196.
44. Zhu, A.P., Cai, A.Y., Zhou, W.D., Shi, Z.H. Effect of flexibility of grafted polymer on the morphology and property of nanosilica/PVC composites. Appl. Surf. Sci.. 2008; 254:3745–3752.
45. Ziong, Y., Chen, G.S., Guo, S.Y. Solid mechanochemical preparation of core-shell SiO2 particles and their improvement on the mechanical properties of PVC composites. J. Polym. Sci. Part B: Polym. Phys.. 2008; 46:938–948.
46. Zhu, A.P., Shi, Z.H., Liao, T.Q., Zhao, F., Cai, A.Y. Synthesis of core-shell PMMA-SiO2 nanoparticles with suspension-dispersion-polymerization in an aqueous system and its effect on mechanical properties of PVC composites. Polym. Test. 2008; 27:540–547.
47. Van der Ven, L., Van Gemert, M.L.M., Batenburg, L.F., Keern, J.J., Gielgens, L.H., et al. On the action of hydrotalcite-like clay minerals as stabilizers in poly(vinyl chloride). Appl. Clay Sci.. 2000; 17:25–34.
48. Grossman, R.F. Acid absorbers as PVC costabilizers. J Vinyl and Additive Tech.. 2000; 6:4–6.
49. Wang, H., Bao, Y.Z., Huang, Z.M., Weng, Z.X. Morphology andmechanical properties of poly(vinylchloride)/nano-hydrotalcite composites. Acta Polymerica Sinica. 2006; 44:451–461.
50. Bao, Y.Z., Huang, Z.M., Weng, Z.X. Preparation and characterization of poly(vinyl chloride)/layered double hydroxides nanocomposite via in situ suspension polymerization. J. Appl. Polym. Sci.. 2006; 102:1471–1477.
51. Bao, Y.Z., Huang, Z.M., Weng, Z.X. Thermal stability, smoke emission and mechanical properties of poly(vinyl chloride)/hydrotalcite nanocomposites. Polym. Degrad. and Stab.. 2008; 93:448–455.
52. Chen, G.M., Chen, G. Preparation of a poly(vinyl chloride)/layered double hydroxide nanocomposite with a reduced heavy-metal thermal stabilizer. J. Appl. Polym. Sci.. 2007; 106:817–820.
53. Liu, J., Chen, G., Yang, J. Preparation and characterization of poly(vinyl chloride)/layered double hydroxide nanocomposites with enhanced thermal stability. Polymer. 2008; 49:3923–3927.
54. Huang, N.H., Wang, J.Q. A new route to prepare nanocomposites based on polyvinyl chloride and MgAl layered double hydroxide intercalated with laurylether phosphate. Express Polym. Lett.. 2009; 3:595–604.
55. Liu, J., Chen, G.M., Yang, J.P., Ding, L.P. Improved thermal stability of poly(vinyl chloride) by nanoscale layered double hydroxide particles grafted with toluene-2,4-di-isocyanate. Mat. Chem. Phys.. 2009; 118:405–409.
56. Broza, G., Piszczek, K., Styerzynski, T. Nanocomposites of poly(vinyl chloride) with carbon nanotubes (CNT). Compos. Sci. Tech.. 2005; 67:890–894.
57. Xie, X.L., Li, R.K., Liu, X., Mai, Y.W. Structure-property relationships of 5n situ PMMA modified nano-sized antimony trioxide filled poly(vinyl chloride) nanocomposites. Polymer. 2004; 45:2793–2802.
58. Soong, S.Y., Cohen, R.E., Boyce, M.C., Mulliken, A.D. Rate-dependent deformation behavior of POSS-filled and plasticized poly(vinyl chloride). Macromolecules. 2006; 39:2900–2908.
59. Gao, J., Du, Y., Dong, C. Rheological behavior and mechanical properties of blends of poly(vinylchloride) with CP-POSS. Int. J. Polymeric Mat.. 2010; 39:15–24.
60. Shimpi, N.G., Verma, J., Mishra, S. Preparation, characterization and properties of poly(vinylchloride)/CaSO4 nanocomposites. Polym.-Plast. Tech. Eng.. 2009; 48:997–1001.
61. Patil, C.B., Shisode, P.S., Kapadi, U.R., Hundiwale, D.G., Mahulikar, P.P. Effect of calcium sulphate nanoparticles on fusion, mechanical and thermal behaviour of polyvinyl chloride (PVC). Int. J. Modern Phys.. 2010; 24:64–75.
62. Patil, C.B., Shisode, P.S., Kapadi, U.R., Hundiwale, D.G., Mahulikar, P.P. Preparation and characterization of poly(vinyl chloride) calcium phosphate nanocomposites. Mat. Sci. Eng. B – Advanced Functional Solid State Mat.. 2010; 168:231–236.
63. Li, X., Chen, W., Xing, Y., Zhang, P. Effect of ZnO nanoparticles on the UV light fastness and climate resistance of PVC film. Manufacturing Sci Eng.. 2010; 97–101:2197–2200.
64. Faruk, O., Matuana, L.M. Reinforcement of rigid PVC/wood-flour composites with multi-walled carbon nanotubes. J. Vinyl Additive Tech.. 2008; 19:60–64.
65. Jia, M.Y., Xue, P., Zhao, Y.S., Wang, K.J. Creep behaviour of wood flour/ poly(vinyl chloride) composites. J. Wuhan University of Tech.-Materials. 2009; 24:440–447.
66. Rodolfo, A., Innocentini-Mei, L.H. Poly(vinyl chloride)/metallic oxides/ organically modified montmorillonite nanocomposites: Preparation, morphological characterization, and modeling of the mechanical properties. J. Appl. Polym. Sci.. 2010; 116:422–432.
67. Thongpin, C., Juntum, J., Sa-Nguan-Moo, R., Siksa-Ard, A., Sombatsompop, N. Thermal stability of PVC with gamma-APS-g-MMT and zeolite stabilizers by TGA technique. J. Thermoplast. Comp. Mat.. 2010; 23:435–445.
68. Zheng, X., Gilbert, M. An investigation into the thermal stability of PVC/ MMT composites. J. Vinyl Additive Tech.. 2011; 17:77–84.
69. Wan, C.Y., Tian, G.H., Cui, N., Zhang, Y.X., Zhang, Y. Processing thermal stability and degradation kinetics of poly(vinylchloride)/montmorillonite composites. J. Appl. Polym. Sci.. 2004; 92:1521–1526.
70. Wu, B., Qi, S.H., Wang, X. Thermal behaviour of poly(vinyl chloride) treated montmorillonite-silica-3-triethoxysilyl-1-propanamine (K-Si-MMT) nanocomposites. Polym. Test. 2010; 29:717–722.
71. Sterky, K., Hjertberg, T., Jacobsen, H. Effect of montmorillonite treatment on the thermal stability of poly(vinyl chloride) nanocomposites. Polym. Deg. Stab.. 2009; 94:1564–1570.
72. Yarahmadi, N., Jakubowicz, I., Hjertberg, T. Development of poly(vinyl chloride)/montmorillonite nanocomposites using chelating agents. Polym. Deg. Stab.. 2010; 95:132–137.
73. Chen, T.Y., Li, W.F., Jun, J.D., Pen, J.H., Chao, J.X. Modification of nanometre calcium carbonate and its application on PVC composites in situ suspension polymerisation. Mat. Sci. Tech.. 2010; 26:871–874.
74. Gu, Z., Liu, W., Dou, W., Tang, F. Preparation of a novel heat stabilizer for poly(vinyl chloride)-Zn, Mg, Al-layered double hydroxide. Polym. Comp.. 2010; 31:928–932.
75. Liu, J., Chen, G.M., Yang, J., Ding, L. Thermal stability of poly (vinyl chloride)/ layered double hydroxide nanocomposites. J. Appl. Polym. Sci.. 2010; 116:2058–2064.
76. Yarahmadi, N., Jakubowicz, I., Hjertberg, T. Development of poly(vinyl chloride)/montmorillonite nanocomposites using chelating agents. Polym. Deg. Stab.. 2010; 95:132–137.
77. Ge, M.L., Jia, D.M. Influence of organoclay prepared by solid state method on the morphology and properties of polyvinyl chloride/organoclay nanocomposites. J. Elast. Plast.. 2008; 40:223–235.
78. Shi, J.H., Yang, B.X., Pramoda, K.P., Goh, S.H. Enhancement of the mechanical performance of poly(vinyl chloride) using poly(n-butyl methacrylate)- grafted multi-walled carbon nanotubes. Nanotechnology. 2007; 18:19.
79. Yang, L., Hu, Y., Guo, H., Song, L., Chen, Z.Y., et al. Toughening and reinforcement of rigid PVC with silicone rubber/nano-CaCO3 shell-core structured fillers. J. Appl. Polym. Sci.. 2006; 102:2560–2567.
80. Beyer, G. Flame retardancy of thermoplastic polyurethane and polyvinyl chloride by organoclays. J Fire Sciences. 2007; 25:65–78.
81. Beyer, G. Organoclays as flame retardants for PVC. Polymers for Adv. Tech.. 2008; 19:485–488.
82. Beyer, G. Organoclays as flame retardants for PVC and new application of nanocomposites for simplifying flame retardant cable designs. PMSE Preprints. 2008; 98:822–823.
83. Beyer, G., PVC nanocomposites and new nanostructured flame retardants. Proc. 13th Int. Plastics and Additives and Compounding Conf. Addcon World, Frankfurt, Germany, 2007. [Paper 19].
84. Zhao, Y., Wang, K., Zhu, F., Xue, P., Jia, M. Properties of poly(vinylchloride)/ wood flour/montmorillonite composites: Effects of coupling agents and layered silicate. Polym. Deg. Stab.. 2006; 91:2874–2883.
85. Yang, Z., Li, B., Tang, F. Influence of Cu2+ -organic montmorillonites on thermal decomposition and smoke emission of poly(vinyl chloride) by cone calorimetric study. J. Vinyl Addit. Tech.. 2007; 13:31–39.
86. Li, B., Yang, Z. An Investigation of the flammability, morphology and torque rheology of poly(vinyl chloride) with silanes and Cu2+ modified montmorillonites. Polym. and Polym. Comp.. 2009; 17:291–301.
87. Kong, Q., Zhang, J., Ma, J., Li, F., Liu, H., et al. Flame retardant and smoke suppressant of Fe-organophilic montmorillonite in polyvinyl chloride nanocomposites. Chinese J Chem.. 2008; 26:2278–2284.
88. Rodolfo, A., Innocenti-Mei, L.H. Poly(vinyl chloride)/metallic oxides/ organically modified montmorillonite nanocomposites: Fire and smoke behaviour. J. Appl. Polym. Sci.. 2010; 116:946–958.
89. Zhang, Z., Zhu, M., Sun, B., Zhang, Q., Yan, C., et al. The effect of hydrotalcite and zinc oxide on smoke suppression of commercial rigid PVC. J. Macromol. Sci., Pt. A: Pure and Appl. Chem.. 2006; 43:1807–1814.
90. Yan, C., Zhang, Z., He, L., He, Z., Zhang, Z. The effect of nano-hydrotalcite composites on smoke suppression and flame retardant of flexible PVC. Suliao. 2007; 36:8–11. [93].
91. Bao, Y., Huang, Z., Li, S., Weng, Z. Thermal stability, smoke emission and mechanical properties of poly(vinyl chloride)/hydrotalcite nanocomposites. Polym. Deg. Stab.. 2008; 93:448–455.
92. Zhang, Y., Li, B., Xu, X., Li, Y., Wu, Z., et al. Influences of (ZnSn(OH)f) on flame retardancy and smoke suppression of flexible poly(vinyl chloride). Yingyong Huaxue. 2007; 24:286–290.
93. Francis, N., Schmidt, D.F., PVC/layered silicate nanocomposites: Preparation, characterization, and properties. SPE ANTEC 2007, Proc. 65th SPE Annual Conference. Cincinnati, 2007.
94. Zheng, X., Gilbert, M. The effect of processing on the structure of PVC/ montmorillonite composites. Melt and solution process. J. Vinyl Addit. Tech.. 2011; 17:231–238.
95. Jin, S., Matuana, L.M. Wood/plastic composites co-extruded with multi-walled carbon nanotube-filled rigid poly(vinyl chloride) cap layer. Polym. Int.. 2010; 59:648–657.
96. Wan, C.Y., Qiao, X.Y., Zhang, Y., Zhang, Y.X. Effect of different clay treatment on morphology and mechanical properties of PVC-clay nanocomposites. Polymer Testing. 2003; 22:453–461.
97. Mkhabela, V.J., Mishra, A.K., Mbianda, X.Y., Mkhabela, V.J., Mishra, A.K., et al. Thermal and mechanical properties of phosphorylated multiwalled carbon nanotube/polyvinyl chloride composites. Carbon. 2011; 49:610–617.
98. Elashmawi, I.S., Hakeem, N.A., Marei, L.K., Hanna, F.F. Structure and performance of ZnO/PVC nanocomposites ". Physica B: Condensed Matter. 2010; 405:4163–4169.
99. Sterzynskia, T., Tomaszewska, J., Piszczek, K., Skorczewska, K. The influence of carbon nanotubes on the PVC glass transition temperature. Composite Science and Technology. 2010; 70:966–969.
100. Xu, W.B., Zhou, Z.F., Ge, M.L., Pan, W.P. Polyvinyl chloride/ montmorillonite nanocomposites – Glass transition temperature and mechanical properties. J. Thermal Anal. & Calorimetry. 2004; 78:91–99.
101. Ren, T.B., Yang, J., Huang, Y.X., Ren, J., Liu, Y. Preparation, characterization, and properties of poly(vinylchloride)/organophilic-montmorillonite nanocomposites. Polymer Composites. 2006; 27:55–64.
102. Shi, X.D., Pan, M.W., Li, X.C., Zhang, L.C., Ding, H.L. Studies on the morphology and properties of PVC/Na + − MMT nanocomposites prepared by in situ emulsion polymerization. Acta Polymerica Sinica. 2004; 1:149–1520.
103. Mamunya, Y., Bodenne, A., Lebovka, N., Ibos, L., Candeau, Y., et al. Electrical and thermophysical behaviour of PVC-MWCNT nanocomposites. Comp. Sci. Tech.. 2008; 68:1981–1988.
104. Mamunya, Y.P., Levchenko, V.V., Rybak, A., Boiteux, G., Lebedev, E.V., et al. Electrical and thermomechanical properties of segregated nanocomposites based on PVC and multiwalled carbon nanotubes. J. Non-crystalline Sol.. 2010; 356:635–641.
105. Al-Ghamdi, A.A., El-Tantawy, F., Aal, N.A., Mossalamy, E.H., Mahmoud, W.E. Stability of new electrostatic discharge protection and electromagnetic wave shielding effectiveness from poly(vinyl chloride )/graphite/nickel nanoconducting composites. Polym. Degrad. Stab.. 2009; 94:980–986.
106. Yanez-Flores, I.G., Betancort-Galindo, R., Aquino, J.A.M., Rodriguez-Fernandez, O.S. Preparation and characterization of magnetic PVC nanocomposites. J. Non-Cryst. Sol.. 2007; 353:799–801.
107. Rodriguez-Fernandez, O.S., Rodriguez-Calzadiaz, C.A., Yanez-Flores, I.G., Montemayor, S.M. Preparation and characterization of a magneto-polymeric nanocomposite: Fe3O4 nanoparticles in a grafted, cross-linked and plasticized poly(vinyl chloride) matrix. J. Mag. Mag. Mat.. 2008; 320:E81–E84.
108. Servin-Hernandez, E., Rodriguex-Fernandez, O.S., Garcia-Cerda, L.A. Synthesis of plasticizer-based ferrofluid and its use in the preparation of magnetic PVC nanocomposites. Adv Electron Microscopy and Nanomaterials. 2010; 644:13–16.
109. Alian, A.M., Abu-Zahra, N.H. Mechanical Properties of Rigid Foam PVC-Clay Nanocomposites. Polym. Plast. Tech. Eng.. 2009; 48:1014–1019.
110. Yalcin, B., Cakmak, M. Molecular orientation behavior of poly(vinyl chloride) as influenced by the nanoparticles and plasticizer during uniaxial film stretching in the rubbery stage. J. Polym. Sci. Part B: Polym. Phys.. 2005; 43:724–742.
111. Yoo, Y., Kim, S.S., Won, J.C., Choi, K.Y., Lee, J.H. Enhancement of the thermal stability, mechanical properties and morphologies of recycled PVC/clay nanocomposites. Polym. Bull.. 2004; 52:373–380.
112. Thomas, N.L. Cellular PVC-U: Current technology and future challenges. J. Cell. Plast.. 2007; 43:237–255.