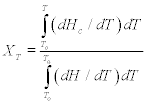Clay-containing poly(ethylene terephthalate) (PET)-based polymer nanocomposites
S. Sinha Ray, National Centre for Nanostructured Materials, Republic of South Africa and University of Johannesburg, Republic of South Africa
Abstract:
This chapter is an extensive overview of clay-containing nanocomposites of poly(ethylene terephthalate) (PET). The various techniques used to prepare clay-based PET nanocomposites, their structural and morphological characterization, their improved mechanical and material properties, their melt-state rheological and crystallization behavior, and, finally, current applications and future prospects of PET-based nanocomposite materials are discussed.
10.1 Introduction: the importance of poly(ethylene terephthalate) (PET)-based nanocomposites
Over the past few years, the mixing of nanoparticles into a polymer matrix has been an area of great research interest since such composite materials can exhibit a concurrent improvement of properties compared with the neat polymer. Such improvements include higher thermal stability and flame retardancy, mechanical properties, gas barrier properties, chemical resistance, and so on.1,2 The nanoscale dispersion of fillers or controlling the nanostructures in the composite can introduce new physical properties and novel behavior that are absent in the unfilled matrices. In general, the properties of nanocomposites depend on the following parameters: the properties of the matrix polymer and filler, the nature and the strength of the interfacial interaction, and the area of interfacial bonds. For a nanoparticle-filled composite, the area of interfacial bonds is determined by the aspect ratio (i.e., the length to thickness ratio). Therefore, the nano-level dispersion of the filler in a polymer matrix plays a key role in achieving a concurrent improvement of properties in the nanocomposites. Hence, significant research efforts are underway to control the nanostructures via innovative synthetic approaches.
Because of these improved properties, commercial applications of nanocomposites have been growing at a rapid rate. Improvements in mechanical properties have resulted in major interest in nanocomposite materials for automotive and industrial applications.3–5 Improved barrier properties make nanocomposites suitable for packaging materials.1,2,5 Coating with nanocomposite films can enhance the toughness and hardness of a material without interfering with light transmission characteristics.1 In some cases the optical transparency of a nanocomposite film is better than the neat polymer, which in turn makes nanocomposites suitable for producing new scratch- and abrasion-resistant materials.1 Furthermore, the use of nanocomposites in the next few years will include key areas such as drug delivery systems, anticorrosion barrier coatings, UV protection gels, scratch-free paints, superior strength fiber and films, etc.
Along with many other polymers, the global demand for PET, including its use as a matrix polymer in the preparation of nanocomposites, has grown rapidly over the last decade. The demand for PET is highest in Asia. Europe is the second largest consumer of PET. The demand for PET in South and Central America is also growing strongly. The majority of the world’s PET production is for synthetic fibers (in excess of 60%) with bottle production accounting for around 30% of global demand.6 PET is generally referred to as ‘polyester’ in textile applications, while ‘PET’ is used most often to refer to packaging. About 18% of the world’s polymer production comprises polyester, which ranks third after polyethylene (PE) and polypropylene (PP). The question is why are PET and PET-based nanocomposites so important from an industrial point of view?
PET is used in a variety of applications, such as fibers, bottles, films, and engineering plastics for automobiles and electronics, due to its relatively low cost and high performance.7 PET has high impact resistance and is strong and naturally colorless with high transparency. It has good gas barrier properties. For this reason, PET is the most popular material for soft drinks bottles. Sometimes polyvinyl alcohol (PVA) is sandwiched between PET films to give a further improvement in the gas barrier properties to meet the most challenging applications for sensitive beverages. A biaxially oriented PET film (known as Mylar) can be aluminized by evaporating a thin film of metal onto it to reduce its permeability and to make it reflective and opaque. This property is useful for flexible food packaging and thermal insulation, such as space blankets.
Because of its high mechanical strength (its tensile strength is 55–75 MPa), PET film is also used in tape applications, such as the carrier for magnetic tape or the backing for pressure-sensitive additive tapes.
While most thermoplastics, in principle, can be recycled, the recycling of PET bottles is more practical than recycling other thermoplastics, such as PP, polystyrene, etc. For example, recycled PET bottles can be used to prepare fleece material. Fleece is a soft, warm, comfortable fabric usually used in sweaters, jackets, gym clothes, hats, etc.
PET is also an excellent candidate for thermal disposal (incineration) since it is composed of carbon, hydrogen, and oxygen, with only trace amounts of catalyst elements (but no sulfur). PET has the energy content of soft coal.8
It is clear that PET is a very useful and important plastic from an industrial point of view. For this reason, worldwide research is being conducted on PET to make it more useful. One approach is to prepare nanocomposites of PET by using different types of nanofiller. Among the different nanofillers, such as clay, carbon nanotubes, and metal-oxide nanoparticles, the clay-based nanocomposites are very important since they often exhibit enhanced thermal and mechanical properties, heat resistance, gas permeability, and flammability characteristics.1 Hence, this chapter will focus mainly on the preparation, characterization, and properties of clay-based PET nanocomposites. The primary objective in the development of a PET/clay nanocomposite is to improve the gas barrier property of the matrix for beverage and food packaging.9 Another expectation for PET/clay nanocomposites is as an alternative to glass-fiber-reinforced PET. It is well known that the properties of a nanocomposite are directly related to the nanoscale dispersion of the clay platelets in the PET matrix. Therefore, it is a challenge for researchers to achieve good dispersion. It is necessary to understand how to control the agglomeration of dispersed nanoparticles (through the choice of nanoparticle), how processing conditions affect the properties, and, finally, the effect of nano-confinement on the properties.
10.2 Types of PET-based nanocomposite
Depending on the nanofiller, PET-based composites can be classified into three categories: clay-based PET nanocomposites, PET composites containing carbon nanotubes as a filler material, and PET composites containing metal-oxide nanoparticles. Usually most clays are environmentally friendly and possess a very high modulus and aspect ratio, which are the basic requirements for physical property improvement after nanocomposite preparation. Besides clay, the other popular nanofillers are carbon nanotubes or nanofibers. They are widely studied because of their high aspect ratio and outstanding Young’s modulus, which are favorable properties for making multifunctional polymer nanocomposites. However, significant agglomeration, difficult surface modification, and the high cost of carbon-based nanofillers restrict their widespread use in applications as advanced nanofillers.10 That is why clay is the most popular nanofiller.
10.3 Preparative methods
The methods used to prepare nanocomposite can be divided into three main groups according to the starting materials and processing techniques:1
1. Intercalation of the polymer or pre-polymer from solution. This method uses a solvent system in which the polymer or pre-polymer is soluble and the silicate layers are swellable. The clay is first swollen in a suitable solvent, such as water, chloroform, or toluene. When the polymer and clay solutions are mixed, the polymer chains intercalate and displace the solvent within the interlayers of the silicate. On solvent removal, the intercalated structure remains, resulting in a nanocomposite.
2. In situ intercalative polymerization. In this method, the clay is swollen within the liquid monomer or a monomer solution so polymer formation can occur between the intercalated sheets. Polymerization can be initiated either by heat or radiation, by the diffusion of a suitable initiator, or by an organic initiator or catalyst fixed through cation exchange inside the interlayer before the swelling step.
3. Melt intercalation. This method involves annealing, statically or under shear, a mixture of the polymer and organically modified clay at the softening point of the polymer. This method has great advantages over either in situ intercalative polymerization or polymer solution intercalation. First, this method is environmentally benign due to the absence of organic solvents. Second, it is compatible with current industrial processes, such as extrusion and injection molding. Melt intercalation can use polymers that are not suitable for in situ polymerization or solution intercalation.
Generally, layered silicate minerals are divided into three major groups: (a) the kaolinite group, (b) the smectite group, and (c) the illite or the mica group. Of these three major groups, smectite types, or more precisely montmorillonite (MMT), saponite, and hectorite, are the most commonly used layered silicates in polymer nanocomposite technology. Of these MMT is the most commonly used layered silicate for the preparation of polymer nanocomposites because it is highly abundant and inexpensive.1,2
In the primary structure of MMT, neighboring hydrophilic platelets attract each other through multiple anionic charges and exchangeable metal counter ions. As a result, there is an enormous force of ionic attraction in the layered structure of MMT, which makes it difficult to break and disperse homogeneously in hydrophobic polymer matrices.5 This problem is usually solved by modifying MMT with certain organic compounds, such as quaternary ammonium or phosphonium salts, which has proven to be an effective way of improving the compatibility between the polymer matrix and the clay. The processing temperature of PET, as well as the temperature at which this polymer is synthesized by a polycondensation reaction, is about 280 °C. This is well above the decomposition temperature of the ammonium surfactants commonly used for organic modification of pristine MMT.11 Therefore, it is a challenging job to disperse MMT particles nicely into a PET matrix.
So far researchers have used several innovative techniques to modify pristine MMT in order to enhance the compatibility between the MMT surface and matrix PET. A simple way to prepare organically modified MMT (OMMT) is as follows: stir pristine MMT in 200 ml deionized water at 70 °C for 12 h. Then slowly add a solution of 7.69 g (19.6 mequiv) N,N,N-trimethyloctadecylammonium bromide in 50 ml deionized water to the MMT solution with vigorous stirring at 70 °C. After mixing, stir the mixture at 70 °C for 12 h. After recovering the OMMT by filtration, it must be washed with 70 °C deionized water several times to remove freely existing ionic intercalants and impurities. Finally, centrifugation and vacuum drying at 70 °C for 24 h are required to obtain OMMT ready for nanocomposite preparation.7
10.3.1 Intercalation of PET from solution
Although a solvent casting method has been used to prepare a PET/clay nanocomposite, the most popular ways to prepare PET/clay nanocomposites are in situ intercalation and melt blending. Ou et al.12 modified MMT with cetylpyridinium chloride (CPC) (CPC/clay = 2/1 by equivalent) and then prepared a PET/clay nanocomposite using a phenol/chloroform mixture as the solvent. The paper contains more details of the method used to produce the nanocomposites from PET.
10.3.2 In situ intercalative polymerization
In situ polymerization to prepare PET/clay nanocomposite can be carried out in several ways. The first method is the intercalation and ring-opening polymerization of ethylene terephthalate cyclic oligomers (ETC) in pre-swelled OMMT galleries. Usually, OMMT is swelled in dichloromethane (DCM). The ETC is dissolved in DCM separately. Then the OMMT solution is added to the ETC solution under vigorous stirring followed by solvent extraction and drying resulting in an intercalated nanocomposite.7 Several sessions of extensive nitrogen purging and high vacuum treatment are necessary during polymerization. A PET/clay nanocomposite has been synthesized via the ester exchange reaction of ethylene glycol (EG) and dimethyl terephthalate (DMT) in the clay gallery in the presence of a zinc acetate catalyst followed by, finally, polycondensation in the presence of an antimony (III) oxide catalyst.2 Here, the clay was ultrasonicated with ethylene glycol and zinc acetate before the ester interchange reaction was initiated due to the addition of DMT to this mixture.
The clay can also be added in the polycondensation step after transesterification of EG and DMT in the presence of a manganese acetate catalyst, but the reaction temperature has to be increased.3 Instead of DMT, the combination of purified terephthalic acid (PTA), antimony (III) oxide as a catalyst, and additives, such as triethyl phosphate (TEP) as a thermal stabilizer and cobalt (II) acetate tetrahydrate (CoAc) as a color inhibitor, can also be used.13
Jung et al.14 prepared a PET/organically modified mica hybrid by transesterification of EG and DMT in the presence of isopropyl titanate. They cooled the sample to room temperature as soon as the ester interchange reaction was finished, repeatedly washed it with water and, finally, vacuum dried it in order to obtain the PET hybrid. After compression molding of the sample they extruded it through the die of a capillary rheometer.13
Since polyamide 6 (PA6) has better compatibility with clay, Li et al.15 expected that, if PA6 chains were introduced into the PET, the compatibility between PET and the clay could be improved. So they synthesized a PET/PA6 copolymer/MMT nanocomposite by transesterification of DMT and EG in the presence of a zinc acetate catalyst at 180 °C, then dissolved the PA6/MMT master batch sample with an antimony trioxide catalyst in the reaction system and increased the reaction temperature to 280 °C. The results of 1H nuclear magnetic resonance (NMR) proved that the ester amide exchange reaction had taken place and an average 3.75 repeat units of PA6 were dispersed randomly in the PET molecular chains.15
With the aim of exfoliating the clay platelets in a polyester/clay nanocomposite, Tsai et al.16 proposed a new preparative technique. They prepared three agents: Agent A contained antimony acetate dissolved in EG, Agent B contained purified clay swollen in EG, and Agent C contained sodium cocoamphohydroxypropylsulfonate dissolved in deionized water. After mixing Agents A and B, calcination resulted in modified clay intercalated with antimony oxide (Sb2O3). The Sb2O3-modified clay was added to Agent C to give Sb2O3-SB modified clay. Then the modified clays were mixed with terephthalic acid bis(2-hydroxyethylester) (BHET) at its melting point for 2 h to prepare the nanocomposite named PET/PK805/Sb-SB.16
In order to understand the effect of different in situ intercalation techniques on the dispersion of clay platelets in a PET matrix, transmission electron micrographs (TEM images) were used. Figure 10.1 shows TEM micrographs of (a) PET nanocomposite containing 2 wt% organically modified mica,14 (b) PET/PA6 copolymers/organically modified MMT nanocomposite containing 2 wt% clay loading,15 and (c) a PET nanocomposite based on organically modified clay (PET/PK805/Sb-SB composite) with antimony acetate as a catalyst.16 From these images, we can see that the dispersion of clay platelets in the PET/PA6 copolymers/OMMT nanocomposite (Fig. 10.1(b)) is better than the composite prepared via conventional in situ intercalation (Fig. 10.1(a)). The antimony-acetate-treated clay-based composite has better delaminated clay layers (Fig. 10.1(c)) compared with the PET/PA6 copolymers/OMMT nanocomposite. Note that different authors used different types of OMMT. However, the improvement in the dispersion of clay layers in the PET/PK805/Sb-SB composite may be attributed to the catalyst-treated clay, since antimony acetate catalyzes the polymerization reaction inside the clay galleries.
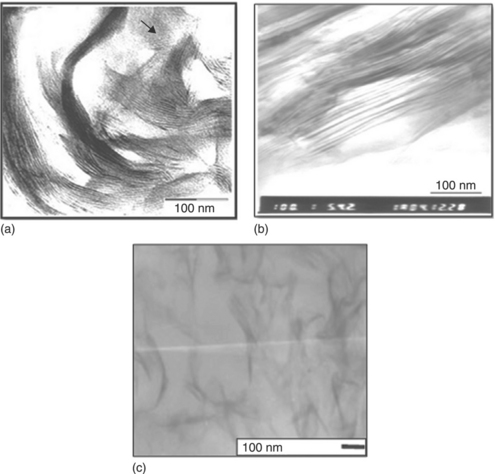
10.1 TEM micrograph of (a) PET nanocomposite containing 2 wt% organically modified mica. (b) PET/PA6 copolymers/organically modified MMT nanocomposite containing 2 wt% clay loading. (c) PET nanocomposite based on organically modified and antimony-acetate-treated clay (PET/PK805/Sb-SB composite). (a) Reproduced with permission from Jung et al.14 (b) Reproduced with permission from Li et al.15 (c) Reproduced with permission from Tsai et al.16
10.3.3 Melt intercalation
Since melt processing is compatible with current industrial processes, such as extrusion and injection molding, Costache et al.11 modified natural clays, such as MMT, hectorite, and magadiite, with the highly thermally stable surfactants hexadecyl-quinolinium (Q16) and vinylbenzyl-ammonium chloride-larnyl-acrylate copolymer (L-surfactant). They prepared PET/clay nanocomposites by melt blending in a Brabender plasticorder for 7 min at 280 °C. The authors found that, although the modified clay has better thermal stability, the nanocomposites did not show any improvement in stability, though in some cases it decreased. This can be explained from the thermal stability results of clays at the processing temperature for the processing time. The current authors believe that during processing at high temperature the clay starts to degrade and hence the thermal stability decreases.11
Processing time can be reduced significantly by preparing the nanocomposite sample in an extruder. The PET and the clay must be sufficiently dry before extrusion in order to reduce decomposition due to the moisture in the sample. So far various types of commercially available clay, such as Cloisite®Na+ (CNa),17 Cloisite®10A (C10A),17 Cloisite®15A (C15A),17,18 Cloisite®20A (C20A),19 and Cloisite®25A (C25A),20 have been used to prepare PET/clay nanocomposites via melt extrusion. The processing temperature (the temperature of different zones of the extruder to the die) of a PET/clay nanocomposite can vary between 240 and 285 °C. Giraldi et al.20 reported that a low screw speed during processing results in composites with a high Young’s modulus, low elongation at break, strength, and impact. This is because the polymer matrix is degraded in the long residence time due to the slow speed.20 Further, a nitrogen environment must be maintained in the hopper and the feeder to keep the materials dry17 and, of course, to avoid possible degradation at such a high processing temperature. Another important parameter in extrusion through a die is the draw ratio. A large draw ratio results in debonding of the organoclay and the matrix polymer and creates many nano-sized voids due to excess stretching of the fibers. These voids (bubbles) hinder energy dissipation and stress transfer under a certain strain during the measurement of mechanical properties. As a result, the modulus and strength of the material decrease. It can also affect the flexibility of the material, measured by elongation at break. N,N-dimethyl-N,N-dioctadecyl ammonium modified clay is not suitable for the preparation of PET/clay nanocomposites since degradation of this modifier makes the nanocomposite brittle. On the other hand, 1,2-dimethyl-3-N-hexadecyl imidazolium tetrafluoroborate exhibits good dispersion in the PET matrix.20
Barber et al.17 melted an extruded PET ionomer and organically modified MMT directly to prepare a nanocomposite. The preparation scheme is shown in Fig. 10.2(a). The concentration of the C10A clay in the composite was 5 wt%. The ionomer used was sulfonated poly(ethylene terephthalate).17,18
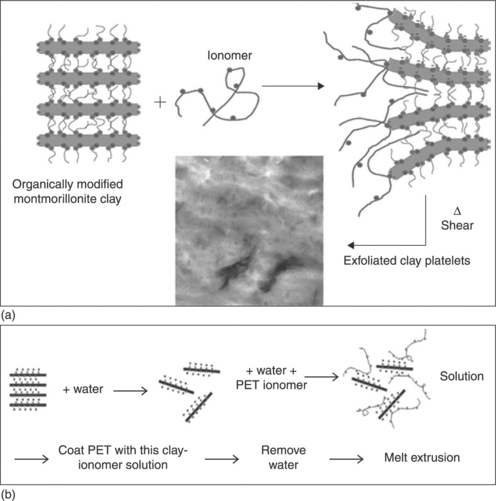
10.2 (a) Conventional melt blending. The ionomer is sulfonated poly(ethylene terephthalate). Clay concentration in the nanocomposite is 5 wt%. (b) Modified melt blending. Clay concentration in the nanocomposite is 5 wt%. (a) Reproduced with permission from Barber et al.17 (b) Reproduced with permission from Ammala et al.21
Ammala et al.21 tried to improve the compatibility between the clay and the PET matrix by incorporating the PET ionomer (AQ55S) in a different way. They compared the effect of the PET ionomer on the dispersion of C10A, a synthetic fluoromica clay (Somasif ME100) before modification, and quaternary ammonium modified synthetic fluoromica clay (Somasif MEE). In order to prepare the nanocomposite, they dispersed the clay in water with the PET ionomer, coated the suspension onto the solid PET, followed by removal of water, and then extruded. The preparation scheme is shown in Fig. 10.2(b). The concentration of clay in the composites was 5 wt%. The composite with the modified clay showed some discoloration compared with the composite with Somasif ME100. This may be due to degradation of the quaternary ammonium modifier during extrusion. Although the optical transparency improved in the PET/Somasif MEE nanocomposite compared with the PET/Somasif ME100 nanocomposite, the optical transparency attained its maximum value with the PET/C10A nanocomposite. This is due to the smaller particle size of C10A in comparison to the mica clays. Due to its small size, C10A will scatter less light and, hence, the optical transparency increased.21
Barber et al.11 and Ammala et al.21 both used 5 wt% C10A clay and a PET ionomer to prepare a nanocomposite with PET. The Barber group used conventional melt blending (Fig. 10.2(a)), whereas the Ammala group used melt blending in a different way (Fig. 10.2(b)). Now the question is which method shows better dispersion of the clay layers in the PET matrix, since the properties of the nanocomposites are directly related to the dispersion characteristics in the PET matrix. To compare the dispersion of the clay layers in the PET matrix, TEM images of the nanocomposites prepared via two different melt blending techniques are shown in Fig. 10.3. According to this figure, the dispersion of clay platelets in the PET matrix prepared by modified melt blending is more homogeneous compared with that by the conventional melt blending method.
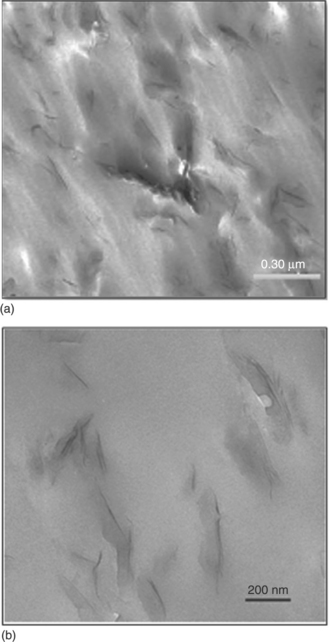
10.3 PET nanocomposite prepared via (a) conventional and (b) modified melt blending. Reproduced with permission from Barber et al.17 and Ammala et al.,21 respectively.
The dispersion of clay in a PET matrix can also be improved by equibiaxial stretching when drawing the composite through the die of the extruder. The stretching may increase the aspect ratio due to slippage of the clay platelets during stretching and preferential orientation of the clay platelets. Hence, the mechanical and barrier properties of the nanocomposites improve, which in turn is advantageous for packaging.22,23 For example, for the same stretch ratio, the oxygen permeability coefficient of neat PET (~ 2.35 cm3-mm/(m2-day-bar)) reduces after nanocomposite preparation (~ 1.82 cm3-mm/(m2-day-bar)).22
Therefore, when preparing a PET/clay nanocomposite one should consider the higher thermal stability, large d-spacing, and compatibility of the clay surface with the PET matrix. Compatibility can be determined by the polar solubility parameter using group contribution methods, such as the Fedors approach.24 The incorporation of a PET ionomer during melt blending is also effective in improving the dispersion of clay platelets in the nanocomposite.21 With in situ intercalation, it is better to treat the clay with a catalyst and then allow polymerization.16
10.4 Structural characterization
It is well known that the most efficient techniques for analyzing the structure of a clay-containing composite are wide-angle X-ray diffraction (WXRD) and transmission electron microscopy (TEM). Usually WXRD patterns start from a diffraction angle (2θ) ~ 2°. Therefore, it is hard to believe that the clay layers are exfoliated in the matrix polymer if the clay peak is absent above the 2° diffraction angle in the nanocomposite sample. For this reason, sometimes WXRD results contradict TEM results.9,14,16 The dispersion of nanoparticles can be directly visualized from TEM images. However, it is best not to use only an image of the most well-dispersed area; instead it is good research practice to use the average dispersion characteristics of low-magnification TEM images. To provide good contrast in TEM, osmium tetroxide can be used to stain the clay. To confirm the true extent of exfoliation it is necessary to perform small-angle X-ray scattering (SAXS) measurements. From a SAXS study, the probability of finding neighboring clay stacking within a certain distance as well as the electron density profile can be estimated. This type of analysis provides a much better understanding of the average dispersion characteristics.
In order to produce a clay-based polymer nanocomposite with improved properties, it is necessary to add ~3 to 5 wt% of organically modified clays.25 As the clay loading in a PET nanocomposite increases, the degree of delamination of the clay platelets is affected due to geometric constraints.25 Therefore, it is quite difficult to achieve full delamination of clay platelets in a PET matrix. The large clay clusters (0.5–1 μm) dispersed in a matrix can also be visualized from the images obtained by scanning electron microscopy (SEM).26 However, from an image of a fractured surface it is difficult to confirm whether the color contrasts really represent clay particles or only voids (bubbles formed during sample preparation) or both. On the other hand, atomic force microscopy (AFM) images provide a much clearer and better view of the dispersed clay layers in a polymer matrix compared with SEM.26,27 Figure 10.4 shows an AFM image of the surface of a nanocomposite. In this three-dimensional topography, the clay layers are represented by the hills and the matrix PET by the valleys.27 Using mainly TEM images, it can be inferred that most PET/clay nanocomposites possess an intercalated structure;28,29 of course, some of them have very delaminated clay layers.16 According to TEM images, the average thickness of dispersed clay layers (D) can be determined by Eq. 10.1:30
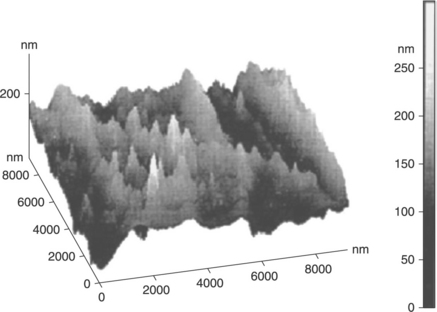
10.4 AFM image of a clay-based PET nanocomposite. Reproduced with permission from Monemian et al.27
where dn is the arithmetic mean of the thicknesses of a few hundreds of particles observed in a TEM image and 1.56 is a coefficient.
10.5 Properties of nanocomposites
10.5.1 Thermal properties and kinetics of crystallization
The most common technique for evaluating thermal properties, such as phase changes, is differential scanning calorimetry (DSC). DSC determines the amount of heat change (either absorbed or released) when a substance undergoes physical or chemical changes. Such changes alter the internal energy (U) of the substance. At a constant pressure, the internal energy is known as enthalpy (H). Any thermal transition, e.g., a phase change, can be described in terms of the change of enthalpy between the two phases. When the internal energy of a solid is increased by the application of an external energy source, the molecular vibrations in the substance increase. As a result, the substance becomes less and less ordered. Hence the enthalpy and entropy (S) of the material increase. Melting occurs when the Gibbs free energy (G = H–TS) of the liquid is lower than that of the solid since every system seeks to attain a minimum free energy state. Crystallization is the opposite of melting. During crystallization, entropy decreases because the ordering of molecules within the system is overcompensated by the thermal randomization of the surroundings. Due to the release of heat of fusion, the entropy of the universe increases. Hence, crystallization is an exothermic process. Usually crystallization occurs at a lower temperature than melting, known as supercooling. This means a crystal can be destroyed more easily than it is formed. Crystallization consists of two phenomena: nucleation and growth. Nucleation is the onset of a phase transition in a small region. The presence of foreign materials (e.g., dispersed nanoparticles in nanocomposites) can facilitate nucleation. Crystal growth is the subsequent growth of nuclei that have reached a critical cluster size.
Hamzehlou and Katbab29 described the properties of clay-based nanocomposites with bottle grade PET, and the flash PET that is left at the gate of an injection mold during preform manufacturing of PET bottles from neat PET by injection molding (which is known as recycled PET). The clay used for this purpose was MMT organically modified by methyl tallow bis(2-hydroxyethyl) ammonium salt. According to these authors, the recycled-PET-based nanocomposites showed an improvement in thermal properties. Although there was no change in melting temperature, the glass transition temperature (Tg) and crystallization temperature (Tc) shifted towards higher temperatures because they are quite thermally stable systems.29 Even for this particular case, the intrinsic viscosity remained unaltered in the nanocomposites when compared with the recycled PET. However, a change in the organic modifier of the clay to dimethyl dehydrogenated tallow ammonium salt resulted in a decrease in Tg due to clay agglomeration and may be due to degradation of the polymer matrix during processing.31 Tg for the nanocomposite may remain the same as that of the neat polymer if the composite has a homogeneous intercalated structure. In such cases, the degree of crystallinity decreases in the nanocomposite relative to the neat polymer.18 For any industrial application, it is essential to achieve high nucleation efficiency and a low degree of crystallinity in the polymer nanocomposite compared with the matrix polymer because higher nucleation efficiency allows preform injection molding, blow molding, etc., at higher temperatures. On the other hand, a reduction in the degree of crystallinity makes the material tougher.
The melting and crystallization behaviors of neat PET and PET/C20A nanocomposite samples were investigated by both conventional and temperature modulated DSC.13 The results indicate that a clay particle acts as a nucleating agent in the crystallization of the PET. To find the effect of clay on the cold crystallization of neat PET, conventional DSC and temperature-modulated DSC (TMDSC) of melt-quenched samples were also carried out. With nanocomposites, cold crystallization was accompanied by a small degree of fusion and subsequent crystallization in the reversal component during TMDSC. This may be due to the presence of short polymer chains formed by the degradation of the matrix at a high temperature in the presence of clay. These small chains undergo a small amount of fusion during crystallization of the bulk. The shift of the cold crystallization peak temperature (Tcc) of PET toward lower temperatures in nanocomposites suggested the intercalated silicate layers act as nucleating agents, so that crystallization of the matrix starts sooner. However, the decrease in enthalpy of cold crystallization (ΔHcc) of the nanocomposites confirmed that, although the clay particles act as nucleating agents, the nanocomposites lose some crystallizable moiety due to the intercalation of the polymer chains in the clay galleries. A further increase in temperature resulted in melting with re-crystallization. In the quenched state, the initial percentages of crystallinity in the nanocomposites were higher than in the neat PET.
Crystallization can simultaneously involve varying rates of nucleation, growth, aggregation breakage, re-dissolution of unstable crystal clusters, and filler concentration. The result is a complex process that is irreversible and often difficult to predict accurately – especially during scale-up or in technology transfer to alternative equipment. For polymers, however, kinetic control is the dominant factor. Polymer crystallization only occurs at a reasonable rate at temperatures well below the equilibrium melting temperature. The reasons for this behavior will emerge from the discussion of crystallization kinetics. There are several different kinds of experimental method that are commonly used to observe the time development of crystallinity (crystallization kinetics) in polymers. One method assesses the cooling rate at which the total amount of crystallinity develops from the supercooled liquid, known as non-isothermal crystallization. Another method assigns a time for crystal growth at a certain temperature near or above the supercooled phase, known as isothermal crystallization. The study of non-isothermal crystallization is more important because most current processing techniques, such as injection molding, blow molding, etc., with polymeric materials follow non-isothermal crystallization. The Avrami model32 was developed for isothermal crystallization kinetics; Ozawa33 extended the Avrami equation to non-isothermal crystallization.34 The Liu model, which is applicable for non-isothermal crystallization kinetics, is a combination of the Avrami and Ozawa models.35 Prior to the study of crystallization kinetics using these models, it is necessary to determine the relative degree of crystallinity as a function of temperature and time.
The relative degree of crystallinity, XT can be defined as:
where To (Tc, on) and Tα (Tc, fin) are, respectively, the initial and final temperatures of crystallization. For example, XT as a function of temperature for neat PET and nanocomposites with different clay loadings at different cooling rates during non-isothermal crystallization are plotted in Fig. 10.5. To see the change of the relative degree of crystallinity as a function of time (Xt), the temperature scale was transformed into a time scale using Eq. 10.3 and the equivalent time dependences of the fractional crystallinity are plotted in Fig. 10.6.
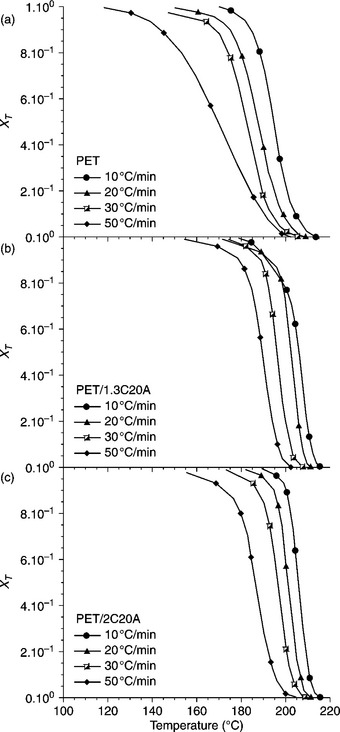
10.5 (a–c) The degree of crystallinity (XT) plotted as a function of temperature for neat PET and nanocomposites with different clay content. The cooling rates were varied during non-isothermal crystallization.
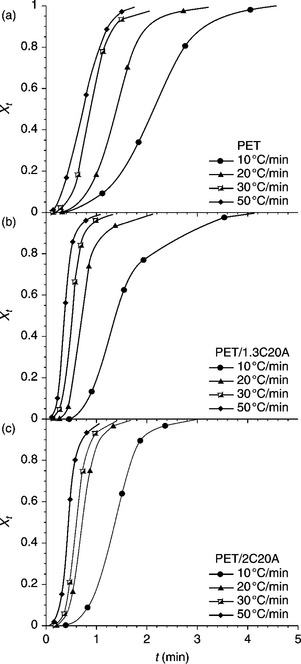
10.6 (a–c) The degree of crystallinity (Xt) plotted as a function of time for neat PET and nanocomposites with different clay content. The cooling rates were varied during non-isothermal crystallization.
where To is the onset temperature of crystallization and T is the same temperature used to determine XT. As a function of time or temperature, the relative degree of crystallinity remains the same; the suffix only denotes the abscissa.
By truncating non-isothermal crystallization into an infinitesimally small isothermal process, Ozawa extended the Avrami model for isothermal crystallization to analyze non-isothermal crystallization kinetics. According to this model, XT can be written as a function of the cooling rate:
where K(T) is the Ozawa crystallization rate constant and m is the Ozawa exponent depending on the dimension of crystal growth. Taking a double logarithm on both sides of Eq. 10.4:
Therefore, a plot of ln[–ln(1–XT)] versus ln ϕ should be a straight line if this model is valid. K(T) and m can be estimated from the antilogarithms of the y-intercept and slope, respectively.
Although the Ozawa model is designed to analyze non-isothermal crystallization kinetics, this model is not valid for PET/C20A nanocomposites since this model does not take into account the secondary crystallization process (impingement of crystals). Therefore, the Ozawa model fails to describe non-isothermal crystallization kinetics with secondary crystallization. As an alternative approach, the Avrami model can be used to explain crystallization kinetics for such systems.34,36
According to the Avrami model, the equivalent time-dependent crystallinity can be expressed as:
where Zt is a composite rate constant involving both nucleation and growth rate parameters and the Avrami exponent, n, is a constant depending on the type of nucleation and the growth process. Taking a double logarithm on both sides of Eq. 10.6:
Equation 10.7 should be a straight line if this model is valid, and Zt and n can be determined from the antilogarithms of the y-intercept and slope, respectively. Note that, during analysis of non-isothermal crystallization by this model, Zt and n do not possess the same physical meaning as in the original Avrami analysis for isothermal crystallization because the temperature changes instantaneously in the non-isothermal process. Here they are adjustable parameters to fit the experimental results and they help to analyze the crystallization kinetics. However, the variation of the Avrami exponent with cooling rate implies that crystallization of the sample has occurred in various growth forms.37 According to this model, the primary and secondary crystallization of PET produce almost the same size of crystallites. Hence, there is almost no deviation from straight lines in the Avrami plot as shown in Fig. 10.7(a). In nanocomposites, although crystallization starts by nucleation in the presence of foreign materials, the dispersed clay layers hinder impingement and, as a result, the growth of small crystallites occurs due to secondary crystallization. Actually, the different size distribution of crystallites is responsible for the deviation of the linear portion of the Avrami plot as shown in Fig. 10.7(b) and Fig. 10.7(c).
Jeziorny suggested the parameter Zt should be modified when Avrami analysis is applied to non-isothermal crystallization kinetics. Assuming a constant or almost constant cooling rate, the final form of this parameter as suggested by Jeziorny is:38
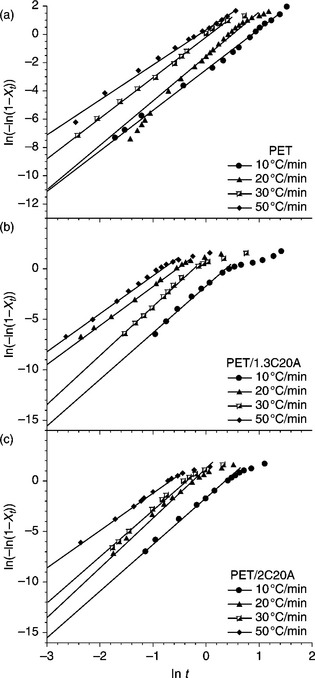
The combined Avrami and Ozawa model, i.e., the Liu model, is given by Eqs 10.9 and 10.10, and is also valid for the current system as shown in Fig. 10.8.
By rearranging Eq. 10.9, the final form of the Liu model is:35,39
where F(T) = [K(T)/Zt]1/m is for the cooling rate to reach a defined degree of crystallinity and a is the ratio of the Avrami exponent to the Ozawa exponent, i.e., a = n/m. For a given degree of crystallinity, F(T) and a can be determined from the y-intercept and slope of the straight lines defined by Eq. 10.10.
The different kinetic parameters determined from these models proved that, in nanocomposites, organoclay is efficient at initiating crystallization earlier by nucleation, but crystal growth will decrease in nanocomposites due to intercalation of the polymer chains in the silicate galleries. Wang et al.40 also observed similar non-isothermal crystallization kinetics as for PET/C20A nanocomposites in their systems. If there is a strong interfacial interaction between the clay and the matrix PET, the polymer segmental motion reduces and hence the crystal growth rate decreases either because the crystals form more slowly or because there is less overall crystallization, i.e., the n value reduces.27
Similar crystal growth with secondary crystallization has also been observed during isothermal crystallization.30 For neat PET, the average value of the Avrami exponent, n, is 3. This implies that crystal growth is three dimensional (spherulitic). This value changes in nanocomposites depending on secondary crystallization.41,42 Fourier transform infrared spectroscopy (FTIR) can be used to determine crystalline structure and perfection. In some nanocomposites, the crystal lamellar thickness and perfection of the crystal is reduced, since the ethylene glycol segments are more likely to sit in the polymer/clay interface.41
From a technical point of view, the study of non-isothermal cold crystallization kinetics is important since it is frequently encountered in processing methods, such as the reheat stretch blow molding of bottles, heat setting, production of films and fibers, etc. The physical and mechanical properties of such products are directly or indirectly controlled by the crystallization processes.
In order to study non-isothermal cold crystallization kinetics, it is necessary to quench samples from the molten state to a temperature below Tg, and then heat them at different rates.43
Crystal growth can be directly visualized using polarized optical microscopy (POM) images taken at the same isothermal or non-isothermal crystallization conditions as used in DSC.34,41,42,44,45
The activation energy (ΔE) for non-isothermal crystal growth can be determined using the Augis–Bennett, Kissinger, and Takhor methods represented by the following equations.
Augis–Bennett method:46
Kissinger method:47
Takhor method:48
The reduction of the activation energy in nanocomposites compared with the neat polymer leads to the conclusion that the nanoparticles in the nanocomposite facilitate crystal growth.34
The determination of the activation energy by the Kissinger method is more reliable than that determined by the Augis–Bennett method since the Kissinger method is model free. On the other hand, there are two assumptions in the Augis–Bennett method: first, ΔE > > RT and second, (T1/2)1. (T1/2)2 ≈ Tc2;49 where (T1/2)1 and (T1/2)2 are the temperatures at the half maximum before and after the crystallization peak, respectively. Determination of the activation energy by the Augis–Bennett method is really fruitful if the reaction obeys the Avrami law.49
10.5.2 Thermal stability
Although some surfactants used to modify pristine clays are very thermally stable, there is a chance of degradation of the surfactant during nanocomposite preparation due to the high processing temperature of PET.19,28 Therefore, to reduce the possibility of organoclay degradation, it is better to dry the clay overnight under vacuum before processing and to minimize the processing time. The processing time can be reduced significantly by preparing the sample in an extruder. The processing time can be estimated from a study of isothermal degradation of the organoclay at the processing temperature.19 Further, under oxidative conditions a PET/C20A nanocomposite sample exhibits a two-step decomposition. In the first half of the degradation process, the nanocomposite exhibits less onset thermal stability than neat PET. This is due to the degradation of the surfactant used for the modification of MMT,50,51 as alkyl ammonium modifiers are known to undergo Hoffman degradation at around 200 °C.52 As for the oxidative condition, under an inert atmosphere the nanocomposite samples also exhibit less onset thermal stability (at 10% weight loss) than neat PET. However, the main degradation temperature for the nanocomposite samples increased in air compared with a nitrogen atmosphere. It is possible that the different types of char formation mechanism under an oxidative environment actually slow down oxygen diffusion, thus hindering oxidation under thermo-oxidative conditions. This observation suggests that there is improved flame retardancy for nanocomposites.13 The same behavior was also obtained for PET/C30B nanocomposites.28 On the other hand, phosphonium-modified MMT with low phosphonium content exhibits an improvement in thermal stability of the PET/clay nanocomposite when compared with the neat PET.28,53,54 Different phosphonium salts, such as (4-carboxybutyl) triphenylphosphonium bromide53 and dodecyltriphenyl phosphonium chloride,54,55 can be used to modify the natural MMT. The thermal stability of the PET/ammonium-salt-modified MMT nanocomposites can be improved by using recycled PET instead of neat PET.29 An imidazolium-surfactant-modified MMT also enhances the thermal stability of PET/clay nanocomposites.56,57 Aminosilane- and imidosilane-modified palygorskite clay-based PET nanocomposites also show an improvement in thermal stability compared with the neat PET resin.58 However, it has already been proved, at the same level of loading, that clay-based PET composites exhibit much more thermal stability compared with composites based on silica nanoparticles.59 Because of its high aspect ratio, the clay has a much higher surface area for polymer–filler interactions, which in turn allows better dispersion of the clay layers in the polymer matrix than spherical nanoparticles. Due to this better dispersion, the tortuous path decelerates the permeation of oxygen gas and enhances the thermal stability of the PET/clay nanocomposite.
The main factors for polymer degradation in clay-based nanocomposites are the number of hydroxyl groups on the edge of the clay platelets and the ammonium linkage on the clay.60 For example, acid-treated sodium MMT (H-MMT) has a reduced thermal stability of the PET matrix due to the larger number of Brensted acid sites generated by the acid treatment (Fig. 10.9). On the other hand, silane-modified MMT (S-MMT) shows less degradation of the PET matrix during preparation of the nanocomposite. After silane modification, the gallery spacing of the clay remains unaltered since the silane coupling agent was grafted onto the sides of the clay layers as shown in Fig. 10.9. Therefore, ammonium modification is necessary. However, the preparation of ammonium-modified clay (A-MMT) further accelerates the degradation of the PET since the ammonium modifiers take part in the Hoffman elimination reaction, which produces more Brønsted acid sites. The effect of ammonium modification on the degradation of PET can be lowered by washing the modified clay with ethanol (W-A-MMT, Fig. 10.9) or adding a silane grafting agent (S-A-MMT in Fig. 10.9).
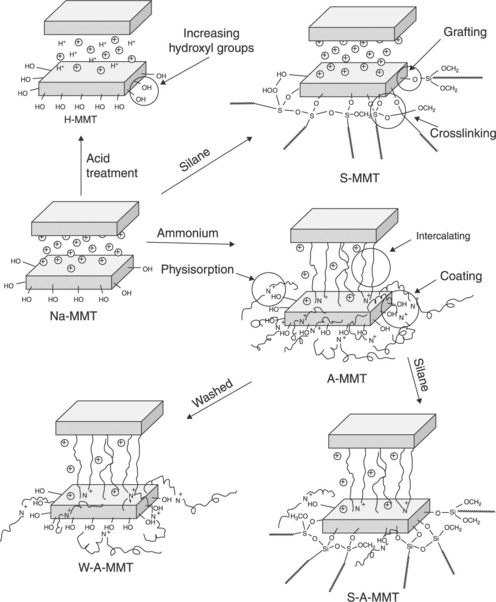
10.9 Preparation of clay with different contents of hydroxyl groups on the edge of the clay platelets and ammonium linkage on clay. Reproduced with permission from Xu et al.60
We have discussed the thermal stability of clay-based PET nanocomposites. What happens if the clay has fibrous rod-like structures instead of layered ones? Yuan et al.61 described the thermal stability of organically modified fibrous silicate (palygorskite) (modified by water-soluble polyvinylpyrrolidone)/PET nanocomposites. According to these researchers, the thermal stability of a nanocomposite containing 3 wt% of clay exhibited an improvement in the onset degradation temperature for all heating rates under air, as shown in Table 10.1. On the other hand, under a nitrogen atmosphere the nanocomposite exhibited a reduction of the onset degradation temperature for slower heating rates; however, stability increased at higher heating rates. Due to the coexistence of the barrier effect and catalytic decomposition, such nanocomposites exhibit a complex degradation mechanism. For both atmospheres, the catalytic decomposition reaction relied on the metal cations dissociating from the chemical constitution of the clay crystal structure, which mainly exists in the inner part of the matrix. Hence, the thermal degradation reaction should principally originate from the inner part of the matrix. Regardless of the atmospheres examined, the well-dispersed silicate layers in the PET matrix hindered thermal transport from the surface to the inner matrix and weakened the thermal transduction rate. Furthermore, the barrier effect of the nanocomposite was also ascribed as being due to blocking by the thermal degradation products volatized from the inner matrix to the external environment. However, compared with nitrogen, in air there was an additional path for oxygen from the outside to the interior of the nanocomposite, as shown in Fig. 10.10 by the black solid arrows. The delaminated clay also acted as an obstacle in the transfer of oxygen gas, which enhanced the barrier effect of the nanocomposite compared with the nitrogen environment. The additive barrier effect of the nanocomposite is the primary reason for the increasing thermal oxidative stability of the nanocomposite at the first stage for all heating rates in air compared with nitrogen.
Table 10.1
Heating rate dependency of the onset degradation temperatures of neat PET and a palygorskite-based PET nanocomposite under air and a nitrogen atmosphere
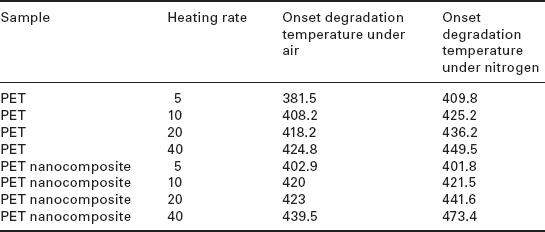
10.10 Degradation of nanocomposites in nitrogen and air. Reproduced with permission from Yuan et al.61
In order to avoid decomposition of the organic modifier of organoclay at the processing temperature of PET, Wang et al.62 first prepared a master batch of PET and sodium MMT (PET:MMT = 50:50) by solid-state shear milling. Then they prepared the nanocomposites by extruding the neat PET with a varying proportion of the master batch. Although the nanocomposites had intercalated structures, the thermal stability of the nanocomposites improved compared with the neat PET resin.62 They also compared the thermal stability of PET/sodium-MMT nanocomposites prepared by simple extrusion (PETCNx where x is the clay content) with nanocomposites prepared by extrusion of the PET/MMT master batch (PETSNx). PETSNx showed better thermal stability compared with PETCN with the same amount of clay loading.
Therefore, to prepare a PET/organically modified MMT nanocomposite one must choose a surfactant (used for organic modification) with a high thermal stability. Otherwise, degradation of the surfactant will facilitate the collapse of the clay layers. As a result, intercalation of the polymer chains in the clay gallery will be difficult. This will result in an intercalated nanocomposite, possibly even a phase-separated composite material. The collapse of the clay structures results in much better heat transfer between the polymer and the clay. This accelerates the degradation of the matrix polymer during processing.
10.5.3 Mechanical properties
Generally, the mechanical properties of nanocomposites depend on the extent of delamination of the nanoparticles (particularly nanoclay) in the polymer matrix, which actually depends on the processing conditions and the chemical treatment and organic modification of the clay surface.63 Better dispersion and orientation of the nanoclay in the direction of application of the mechanical force promotes better stress transfer from one end to the other of the sample.64 As a result, the nanocomposites exhibit enhanced mechanical properties when compared with the neat polymer.
The tensile property of a phosphonium-modified MMT-containing PET nanocomposite exhibited a significant improvement in modulus, but on the other hand the strength and elongation at break decreased a lot. Usually, clay has a very high modulus compared with polymers. Therefore, it is expected that the incorporation of clay in a polymer matrix will result in an improved modulus for the nanocomposite. If the clay layers are not delaminated homogeneously, the energy-dissipation mechanism will be hindered, which in turn will be responsible for a reduction of the elongation at break.
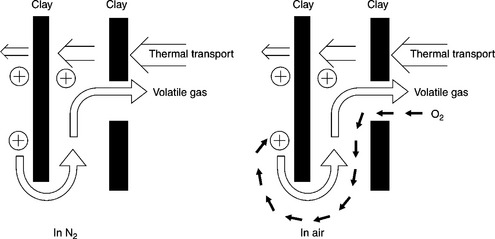
The tensile strength can be improved by preparing the nanocomposite with recycled PET instead of neat PET (Fig. 10.11(a)).29 In this figure neat PET is labeled as FPET and recycled PET as RPET. The tensile strength is the ultimate capacity of the material to resist a tensile load regardless of deflection. Figure 10.11(a) shows that the highest value of the tensile strength can be achieved with 3 wt% clay loading. The RPET-based nanocomposite containing 3 wt% clay loading has the maximum tensile strength. According to the current authors, the probable reason for this improvement can be explained as follows. During injection molding or melt stretch molding, the polymer chains become disentangled or partially oriented. This disentangled structure is responsible for a better dispersion of the clay platelets in the recycled PET matrix. However, according to XRD results, the nanocomposites still possess intercalated structures. As a result, the energy dissipation mechanism improves slightly and hence the tensile strength. However, as shown in Fig. 10.11(b), the tensile modulus is lower for a RPET-based composite compared with a FPET-based composite when the clay loading is below 5 wt%. The tensile modulus is a measure of the stiffness of an isotropic elastic material. It is defined as the ratio of the uniaxial stress over the uniaxial strain. It is determined from the slope of the stress-strain curve traced during tensile tests conducted on a sample of the material. Now it is interesting to note that the tensile strength decreases above 3 wt% clay loading but the modulus of the composites still increases above this value. Although the authors only reported the TEM image of the composite containing the 3 wt% clay loading, the dispersion of the clay in the composite containing a higher clay loading can be inferred from the results of tensile property measurements. The improvement of tensile strength up to 3 wt% OMMT loading indicates that the intercalation of PET chains in the clay gallery reaches a maximum for this specific composite. After that the stacking of clay layers increases. This hinders the energy dissipation mechanism during tensile testing. As a result, the tensile strength decreases with higher clay loadings beyond 3 wt%. On the other hand, the modulus increases as the clay loading increases above 3 wt% due to the high modulus of clay itself.
10.11 (a) Tensile strength and (b) tensile modulus of neat PET (FPET) and RPET-based nanocomposites as a function of clay loading. Reproduced with permission from Hamzehlou and Katbab.29
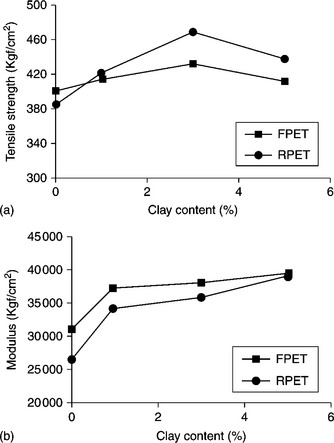
PET/silane-modified organoclay composites exhibit improved tensile properties, which can be useful for fiber and film applications.65 Nanocomposites prepared via solid-state shear milling (PETSNx, as mentioned above) can exhibit increased strength compared with nanocomposites prepared via simple extrusion (PETCNx) (Fig. 10.12(a)). However, tensile strength increases systematically with an increase in clay loading in both samples. Since, according to the TEM images, the dispersion of PETSNx is better than PETCNx, it is expected that the modulus and strength of the PETSNx will be higher in this case due to the high modulus of the dispersed silicate layers. However, there is no significant change in elongation at break (Fig. 10.12(b)) between the composites prepared via the two different techniques.54 For the PET/C20A nanocomposite such improvements can be achieved with 1 wt% clay loading. The reason is that the enhanced amorphous orientation of the intercalated clay layers leads to improvements in modulus and strength.66
10.12 (a) Tensile strength and (b) elongation at break of PET nanocomposite samples prepared via solid-state shear milling and via extrusion as a function of clay content. Reproduced with permission from Kráčalík et al.65
As well as the tensile properties, the thermomechanical properties of clay-based PET nanocomposites were studied by dynamic mechanical analysis (DMA). The variation of storage and loss moduli as a function of temperature explains how the rigidity of the nanocomposite material varies with clay content and how clay concentration affects the glass transition temperature of the matrix.67
10.5.4 Rheological properties
Rheological studies on PET nanocomposites are scarce, but exhibit very interesting features.55 A rheological analysis of a polymer/clay nanocomposite is a powerful and complementary technique for characterizing the state of clay dispersion due to the sensitivity of the rheological response to the structure and surface characteristics of the dispersed phase.60 The advantages of rheological characterization over electron microscopy and X-ray scattering reside in the fact that measurements are performed of the molten state and that rheological methods can be used under both linear and non-linear deformation. A disadvantage of rheological methods is that they only provide indirect information on the structures of nanocomposites.68
Vidotti et al.68 studied the rheological properties of C20A (o-MMT)-based PET nanocomposites in the presence of a polyester ionomer (PETi). The melt-state rheological properties were measured by a stress-controlled rheometer with parallel plate geometry (25 mm diameter) at a temperature of 280 °C. Figure 10.13 show the complex viscosity (η*) as a function of angular frequency (ω) for the neat and extruded PET and PET/PETi/o-MMT nanocomposites containing 1, 3, and 5 wt% of o-MMT. Figure 10.13(a) shows that both the neat and extruded PET exhibited a Newtonian behavior (η* being nearly constant) over the whole frequency range studied. The complex viscosity of all the PET/PETi/o-MMT nanocomposites containing 1 wt% of clay was smaller than that of the neat PET, but similar to or higher than that of the extruded PET. Moreover, the complex viscosity increased when increasing the amount of PETi in the nanocomposite. This observation indicates that an increase in the amount of PETi in a nanocomposite improves the state of dispersion of the clay particles in the PET matrix. A decrease in viscosity of the extruded PET compared with that of the neat PET indicates that the PET had undergone degradation during extrusion.63,68 Parts (b) and (c) of Fig. 10.13 show that at very low frequencies the complex viscosity of the PET nanocomposites was higher than that of the neat PET and much higher than that of the extruded PET. As shown in Fig. 10.14, the elastic modulus (G′) at low frequencies for the nanocomposites is almost independent of frequency. This is typical of solid-like behavior and indicates the formation of a percolated network, which in the case of nanocomposites implies good dispersion of the organoclay.
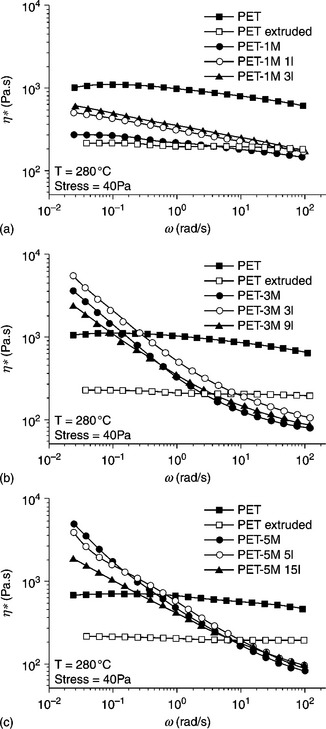
10.13 Complex viscosity of the PET/PETi/o-MMT nanocomposites containing (a) 1 wt%, (b) 3 wt%, and (c) 5 wt% of o-MMT and different PETi concentrations. Reproduced with permission from Vidotti et al.68
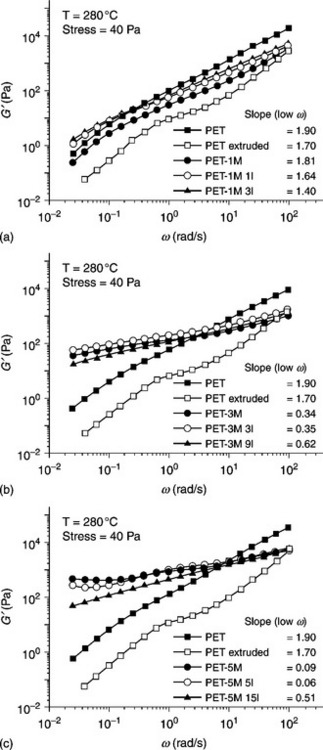
10.14 Storage modulus (G′) of the PET/PETi/o-MMT nanocomposites containing (a) 1 wt%, (b) 3 wt%, and (c) 5 wt% of o-MMT and different PETi concentrations. Reproduced with permission from Vidotti et al.68
Giraldi et al.20 described how the intrinsic viscosity of recycled PET changes with the screw speed in the extruder and also in the presence or absence of an antioxidant (Table 10.2). Higher screw speeds lead to higher viscosity. Moreover, the viscosity increases in the presence of an antioxidant for both screw speeds. However, they did not report the same result for nanocomposites.20
Table 10.2
Change of intrinsic viscosity of recycled PET for two screw speeds and with or without an antioxidant

Usually a homopolymer exhibits Newtonian behavior in a complex viscosity versus frequency plot. In comparison, the complex viscosity of nanocomposites increases significantly in the low frequency region. This shear thinning phenomenon observed for nanocomposites in the low frequency region is initially due to the disruption of the network structure and then in a later stage by the orientation of filler particles in the flow direction.65 For a silane-modified commercially available organoclay, the complex viscosity either increases or decreases depending on the silane coupling agent. However, if the complex viscosity of a nanocomposite exceeds the complex viscosity of the neat polymer, then it is expected that this is because the degradation of the matrix was significantly reduced during processing and rheological property measurements.65 An inert (e.g., nitrogen) atmosphere also plays an important role in achieving this. However, during silanization, particularly in the case of C10A and C30B, the retention of water within silicate layers via a chemical reaction between the organic groups of the organoclay and the silane modifier can reduce the melt strength of the composite material.65
It has been reported that clay nanoparticles suppress crystallization and orientation of the PET in high-speed melt spinning. On the other hand, polyhedral oligomeric silsesquioxane (POSS) nanoparticles slightly promote the crystallization of PET even in high-speed melt spinning. Hence, Lee et al.69 studied the shear-induced crystallization behavior of PET/clay nanocomposites and PET/POSS nanocomposites. They melted samples at 280 °C for 5 min in the 1 mm gap of parallel plates (diameter of the plates was 25 mm) and then performed time sweep experiments at 220, 210, and 200 °C over an angular frequency range 0.5 to 1 rad/s. A comparison of the shear-induced crystallization behavior of PET/clay nanocomposites and PET/POSS nanocomposites is shown in Fig. 10.15. According to this figure, the increase of G′ at the early stage of crystallization is almost negligible. An abrupt increase of G′ follows in a few minutes because the homogeneous melt becomes a heterogeneous system with the formation and growth of crystallites. Hence G′ increases with time. In addition, the clay nanoparticles are more effective in the crystallization process than POSS nanoparticles.69
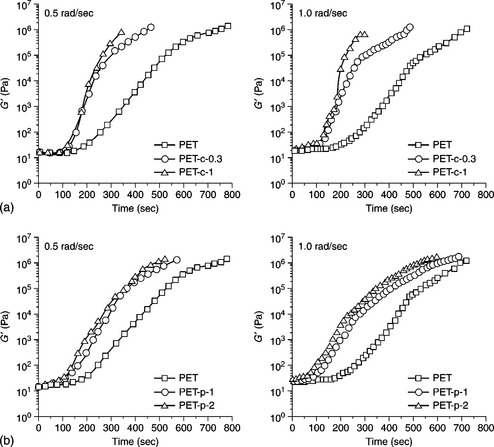
10.15 Variation of storage modulus (G′) with time at 0.5 rad/s (left) and 1.0 rad/s (right) at 220 °C for PET and PET nanocomposites; (a) PET/clay nanocomposites, (b) PET/POSS nanocomposites. Reproduced with permission from Lee et al.69
10.5.5 Flame retardancy
The wide application of PET is sometimes hindered due to its combustibility, and the increasing uses of PET also place emphasis on improving flame retardancy. Incorporating a chemically reactive phosphorus-containing monomer into the polymer chain is one of the most efficient methods of improving the flame retardancy of polyesters.70
On the other hand, the study of polymer/clay nanocomposites has become even more attractive due to the demonstrations of their flame-retardant properties, which mainly demonstrate a significant decrease in the peak heat release rate (PHRR), a change in char structure, and a decrease in the rate of mass loss during combustion in a cone calorimeter.70 Not only was reduced flammability obtained at very low organoclay contents (2–5 wt%) without increasing carbon monoxide or smoke yields, but also the physical properties of the polymers improved simultaneously. Moreover, the incorporation of nanoclay particles with other flame retardants allows a significant portion of a conventional flame retardant to be removed from the formulated polymer products while maintaining or improving flammability performance and enhancing the physical properties. So, the preparation of nanocomposites is another tool for chemists who are engaged in the research and development of flame-retardant materials.70
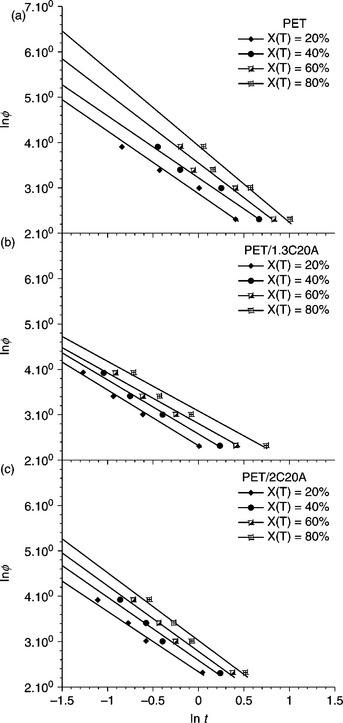
A novel phosphorus-containing flame-retardant copolyester/MMT nanocomposite (PET-co-HPPPA/O-MMT) was synthesized by in situ intercalation polycondensation of terephthalic acid, ethylene glycol, and 2-carboxyethyl(phenyl phosphinic) acid (HPPPA) with OMMT.70 The flame retardancy of the samples was characterized by the limiting oxygen index test (LOI) and the UL-94 vertical test. The LOI is defined as the minimum fraction of oxygen (O2) in a mixture of O2 and nitrogen (N2) that will just support flaming combustion. It is an important and representative parameter for describing the flame-retardant properties of a material. UL-94 ratings are used to describe the ease with which a polymer can be burned or extinguished. LOI and UL-94 test results are shown in Fig. 10.16 and Table 10.3, respectively. It can be seen that PET-co-HPPPA/O-MMT nanocomposites exhibit much better flame retardancy than PET-co-HPPPA. It is interesting that there is a considerable increase in LOI (from 31.4 to 34.0) at very low OMMT content (1 wt%), while for a further increase in OMMT content (from 1 wt% to 3 wt%) no further increase in LOI was observed. Furthermore, the UL-94 vertical test results show that the after-flame time of each sample was 0 s. When the OMMT content is lower than 2 wt%, the samples cannot reach a rating of V-0 because the surgical cotton ignites. However, when the amount of OMMT increases to 2 wt%, the sample (PET5-2) can achieve the rating of V-0 in the UL-94 test, which also indicates that the incorporation of nanoclay particles with HPPPA improves flame retardancy. The higher loadings of MMT are not necessary for improving the LOI, but, by increasing the viscosity of the burning polymer, they reduce its tendency to drip with fire. This phenomenon can be explained by the following flame-retardant mechanism: the carbonaceous–silicate char builds up on the surface during burning, and insulates the underlying material, reducing the mass loss rate of the decomposition products.70
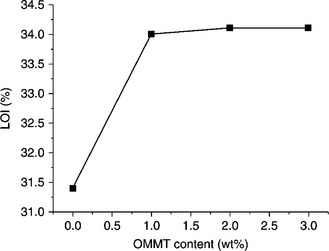
10.16 LOI versus OMMT content for PET-co-HPPPA/O-MMT nanocomposites. Reproduced with permission from Ge et al.70
10.5.6 Gas barrier properties
PET is a widely used packaging material for beverages and drugs. However, its application could be broadened if packaging materials could be made more sensitive to oxygen and carbon dioxide by improving the barrier property. One approach for achieving this is in the preparation of PET/clay nanocomposites. If the clay layers are dispersed nicely in the polymer matrix, a tortuous path will be created by the homogeneously dispersed clay layers in the nanocomposite, as shown in Fig. 10.17,1 which actually reduces the permeability of oxygen and carbon dioxide. For example, the Nanolin DK2 clay-based PET nanocomposite exhibits an improved gas barrier property compared with a C15A-based nanocomposite.18 Tsai et al.16 showed that the barrier property against carbon dioxide gas can be improved by in situ polymerization of a monomer/oligomer in the clay gallery in the presence of an antimony acetate catalyst. According to Fig. 10.18, it is clear that the addition of a few wt% OMMT in the PET matrix can effectively reduce the oxygen (O2) gas permeability of a PET film. When the content of the OMMT reached 3 wt%, the permeation of O2 was reduced to half of the neat PET film.71 Equibiaxial stretching sometimes promotes the gas barrier property since the effective length of the clay tactoids increases due to stretching.22
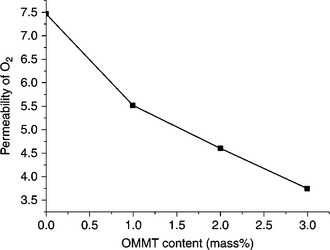
10.18 Oxygen permeability of a PET nanocomposite. Reproduced with permission from Tsai et al.16
On the other hand, recycled PET-based nanocomposites show some property improvement, but the oxygen gas permeability decreases significantly for such composites.29
10.6 Applications of PET-based nanocomposites
PET is commonly used for textile applications and has the largest percentage of synthetic fibers in the market. It is used as a neat material or mixed with cellulose. PET fiber has the most compact and crystalline structure, and is markedly hydrophobic. Its hydrophobic nature, which accounts for its inability to wick moisture away from the body and poor launderability, can be a disadvantage in the underlying applications.7,9,10 To change this passive and lazy role of PET, surface modification of PET fiber without loss of bulk properties has been an oft-sought goal in the fiber industry. Alkaline hydrolysis of PET fibers is often used to improve the soil release property and moisture regain. Recently, PET/SiO2 nanocomposites have been prepared via in situ polymerization and melt spun to fibers. Such nanocomposites exhibit tougher superfine structures (e.g., cracks, craters, and cavities), which facilitate certain applications like deep dyeing.72 In order to improve the mechanical properties of a PET/cotton fabric, different mixtures of resin/clay were deposited with different clay percentages on a PET/cotton fabric sheet. Finally, different mechanical tests, such as tensile, breakout, abrasion, and tear tests, were performed. Furthermore, it appears that a fabric’s mechanical performance globally increases versus the amount of clay for all resins except polyvinyl acetate, which showed a decrease in fabric tear resistance when using more than 20 wt% clay. It was also noticed that the mechanical characteristics of the coated fabric without clay decreased significantly in comparison with the reference, but became better when clay was added to the resin. Consequently, it is obvious that the type of resin is important, because the results differ from one resin to another.73
In addition to textiles, the other important application of PET is packaging. The shelf life of any product depends on the gas barrier properties of the packaging material. This property can be improved by incorporation of clay in the PET matrix as discussed in the previous section. A thin layer of hybrid organic/inorganic (creamer) dramatically improves the oxygen barrier properties of PET film. The apparent gas permeability of a multilayer film can be reduced by three orders of magnitude compared with neat PET. The pure coating properties are calculated taking into account the effect of the different diffusion resistances in the various layers. The effect of aging has also been investigated by comparing oxygen permeability before and after immersion in water for films that are suspected of being degraded by water.74
Smart materials are defined as materials that sense and react to environmental conditions or stimuli (e.g., mechanical, chemical, electrical, or magnetic signals). In the last decade, a wide range of novel smart materials has been produced for aerospace, transportation, telecommunications, and domestic applications. Based on their advantages over other forms of material, such as their high specific area and superior mechanical and handling properties, fibers are being used more and more in applications in different areas. Preliminary results for PET and poly(methacrylic acid) or poly(2-acrylamido-2-methyl-1-propanesulfonic acid) showed that the selective transport ofwatervapor over dimethyl methylphosphonate vapor increased 12 times for both nanocomposites in comparison to a PET dense membrane.75 It is well known that liquid crystal polymers (LCPs) possess some excellent properties, such as high-strength and high-stiffness fibers, lower melt viscosity, and highly resistant chemical and thermal properties. For this reason, it is expected that, depending on the interaction between the LCP, the thermoplastic polymer, and the organoclay, LCPs can facilitate the dispersion of clay in a thermoplastic matrix or induce a particular orientation in the dispersed clay layers of polymer/clay nanocomposites. Further, it has already been proved that LCP molecules align themselves according to the direction of an applied mechanical, electric or magnetic field. So, when polymer/clay/LCP nanocomposites are exposed to different fields, the LCP molecules will tend to align themselves in accordance with the field direction and will induce further orientation of the clay particles. In a recent study, small-angle X-ray scattering showed that the degree of anisotropy and mean orientation angles of clay platelets in a PET/LCP blend matrix were altered significantly after solid-state rheological property measurements (frequency and temperature sweep tests) were carried out in bending mode.76
In order to make a polymer surface resistant to calcification, multiblock copolymers containing PET (30 wt%) as a hard segment and dilinoleic acid (DLA) as a soft segment were prepared in the presence of two concentrations of TiO2 particles (23 nm diameter) as a nanofiller (0.2 and 0.4 wt%) via in situ polycondensation. Changes in the thermal properties as well as investigations of the topography of these nanocomposites suggest that the addition of a small number of TiO2 nanoparticles resulted in higher crystallinity and roughness of the copolymer surface. Incubation in a simulated body fluid (SBF) increased the roughness of all copolymers, as characterized by root mean square (rms) values. However, no hydroxyapatite layer formed on the sample surface. The highest difference of rms values was found for the neat PET/DLA material. The addition of nanocrystalline TiO2 seems to protect the material surface from calcification, which represents a positive effect when a nanocomposite is intended for medical applications, especially when it will come into contact with soft tissue.77
An inorganic electroluminescent (EL) device on a flexible PET substrate was fabricated by Kim et al.78 It was found that the brightness of the inorganic EL device was strongly dependent on the quality of the carbon nanotube (CNT) composite films. After treatment of the PET substrate with 3-aminopropyltriethoxysilane, the CNTs were uniformly dispersed and showed good adhesion to the substrate, and the resulting EL device showed better performance. The flexible EL device had a brightness of 96.8 cd/m2 at 28 kHz and 50 V
10.7 Future trends
The main downstream industries based on PET are the production of polyester fibers, accounting for around 65% of global consumption, and PET bottle resins consuming around 30%. Other applications are for polyester film and polyester engineering resins. Although much research has been carried out to improve the mechanical, barrier, and thermal properties of PET resin, it is still necessary to improve these properties to meet the high demands on this resin. The enhanced barrier properties of PET nanocomposites are not only useful in the bottle industry; they are also important for sports shoes, which have gas-filled bladders for shock damping, and tennis balls, which are claimed to maintain their internal pressure for a longer time. Other commercial products dependent on the barrier properties of nanocomposites include fuel hoses, where environmental regulations require low levels of leakage by diffusion through the hose.
A current problem in the commercialization of polymer nanocomposites is the homogeneous dispersion of clay layers in the polymer matrix. Particle aggregation reduces the effective particle aspect ratio and limits the surface area. This prevents the improvement of properties of the resultant nanocomposites.79 The commercial technique most preferred for preparing nanocomposites is melt blending. For PET nanocomposites, in situ intercalation is also very important. During the preparation of a PET nanocomposite, via either melt blending or in situ intercalation, a high processing temperature is required. Hence it is necessary to choose clay with a surfactant with high thermal stability. Among such thermally stable surfactants, the most interesting are alkyl chain imidazolium and phosphonium halides. However, the dispersion characteristics obtained are not sufficient for commercialization. So far, most PET/clay nanocomposites exhibit higher strength and stiffness, but not higher elongation at break (or tenacity or toughness). Therefore, there is still a need to improve the toughness of these materials for defense applications.
It should always be kept in mind that the improvement of properties of a polymer nanocomposite is directly related to the dispersion characteristics of the nanofillers in the polymer matrix. So far the best dispersion of clay platelets in a polymer matrix has been achieved by in situ intercalation16 and melt blending.21 Those procedures are mentioned in the preparative methods section of this chapter. In both references the authors described the preparation and structural characterization of the clay-based PET nanocomposites. But in our opinion, on the basis of one TEM result for a specific region, and XRD, it is difficult to get an impression of the overall dispersion of clay layers in a PET matrix. It is therefore important to study the dispersion of silicate particles in a PET matrix by SAXS in detail. As a complementary technique, the three-dimensional reconstruction of images obtained from focused ion beam SEM is also very useful. It is necessary to explore the properties of such composites in detail to move PET nanocomposites one step closer to commercialization.
10.8 Acknowledgements
The authors would like to thank the Department of Science and Technology and the Council for Scientific and Industrial Research in South Africa for financial support.
10.9 References
1. Sinha Ray, S., Okamoto, M. Polymer/clay nanocomposites: A review from preparation to processing. Prog Polym Sci. 2003; 28:1539–1641.
2. Sinha Ray, S., Bousmina, M. Biodegradable polymers/layered silicate nanocomposites: in greening the 21st century materials science. Prog Polym Sci. 2003; 50:962–1035.
3. Bogue, R. Nanocomposites: A review of technology and application. Assembly Automation. 2011; 31:106–112.
4. Camargo, P.H.C., Satyanarayana, K.G., Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mat Res. 12, 2009. [Print version ISSN 1516–1439].
5. Yin, M., Li, C., Guan, G., Yuan, X., Zhang, D., Xiao, Y. In-situ synthesis of poly(ethylene terephthalate)/clay nanocomposites using TiO2/SiO2 sol-intercalated montmorillonite as polycondensation catalyst. Polym Eng Sci. 2009; 1562–1572.
6. www.worldofplastic.net/PolyethyleneTerephthalate.htm, Types of Plastic – Worldofplastic.net, downloaded on 24 October 2011.
7. Lee, S.-S., Ma, Y.T., Rhee, H.-W., Kim, J. Exfoliation of clay facilitated by ring-opening reaction of cyclic oligomers in PET-clay nanocomposites. Polymer. 2005; 46:2201–2210.
8. www.petmachine.in/advantages_of_pet.htm’ Advantages of PET – Plastic PET, downloaded on 24 October 2011.
9. Hwang, S.Y., Lee, W.D., Lim, J.S., Park, K.H., Im, S.S. Dispersibility of clay and crystallization kinetics for in situ polymerized PET/pristine and modified montmorillonite nanocomposites. J Polym Sci: Part B: Polym Phys. 2008; 46:1022–1035.
10. Yuan, X., Li, C., Guan, G., Liu, X., Xiao, Y., Zhang, D. Synthesis and characterization of poly(ethylene terephthalate)/attapulgite nanocomposites. J Appl Polym Sci. 2007; 103:1279–1286.
11. Costache, M.C., Heidecker, M.J., Manias, E., Wilkie, C.A. Preparation and characterization of poly(ethylene terephthalate)/clay nanocomposites by melt blending using thermally stable surfactants. Polym Adv Techno. 2006; 17:764–771.
12. Ou, C.F., Ho, M.T., Lin, J.R. Synthesis and characterization of poly(ethylene terephthalate) nanocomposites with organoclay. J Appl Polym Sci. 2004; 91:140–145.
13. Kim, S.-H., Park, S.-H., Kim, S.-C. Novel clay treatment and preparation of poly(ethylene terephthalate)/clay nanocomposite by in situ polymerization. Polym Bull. 2005; 53:285–292.
14. Jung, M.-H., Chang, J.-H., Kim, J.-C. Poly(ethylene terephthalate) nanocomposite fibers with organomica via in situ intercalation. Polym Eng Sci. 2007; 1820–1826.
15. Li, C., Xiao, Y., Guan, G., Liu, X., Zhang, D. Preparation and properties of PET/PA6 copolymer/montmorillonite hybrid nanocomposite. J Appl Polym Sci. 2006; 101:2512–2517.
16. Tsai, T.-Y., Li, C.-H., Chang, C.-H., Cheng, W.-H., Hwang, C.-L., Wu, R.-J. Preparation of exfoliated polyester/clay nanocomposites. Adv Mater. 2005; 17:1769–1773.
17. Barber, G.D., Calhoun, B.H., Moore, R.B. Polyethylene terephthalate) ionomer based clay nanocomposites produced via melt extrusion. Polymer. 2005; 46:6706–6714.
18. Frounchi, M., Dourbash, A. Oxygen barrier properties of poly(ethylene terephthalate) nanocomposite films. Macromol Mater Eng. 2009; 294:68–74.
19. Bandypadhyay, J., Sinha Ray, S., Bousmina, M. Thermal and thermo-mechanical properties of poly(ethylene terephthalate) nanocomposites. J Ind Eng Chem. 2007; 13:614–623.
20. Giraldi ALF de, M., Bizarria, M.T.M., Silva, A.A., Velasco, J.I., d’Ávila, M.A., Mei, L.H.I. Effects of extrusion conditions on the properties of recycled poly(ethylene terephthalate)/nanoclay nanocomposites prepared by a twin-screw extruder. J Appl Polym Sci. 2008; 108:2252–2259.
21. Ammala, A., Bell, C., Dean, K. Poly(ethylene terephthalate) clay nanocomposites: improved dispersion based on an aqueous ionomer. Compo Sci Tech. 2008; 68:1328–1337.
22. Rajeev, R.S., Soon, K., McNally, T., Menary, G., Armstrong, C.G., Martin, P.J. Studies on the effect of equi-biaxial stretching on the exfoliation of nanoclays in poly(ethylene terephthalate). Euro Polym J. 2009; 45:332–340.
23. Rajeev, R.S., Harkin-Jones, E., Soon, K., McNally, T., Menary, G., et al. A method to study the dispersion and orientation of nanoclay tactoids in PET matrix-focused ion beam milling combined with electron microscopy. Mater Lett. 2008; 62:4118–4120.
24. Krevelen, D.W.V.Properties of Polymers. Amsterdam, the Netherlands: Elsevier, 1990.
25. Bandypadhyay, J., Maity, A., Khatua, B.B., Sinha Ray, S. Thermal and rheological properties of biodegradable poly[(butylene succinate)-co-adipate] nanocomposites. J Nanosci Nanotechnol. 2010; 10:4184–4195.
26. Kim, S.H., Kim, S.C. Synthesis and properties of poly(ethylene terephthalate)/clay nanocomposites by in situ polymerization. J Appl Polym Sci. 2007; 103:1262–1271.
27. Monemian, S.A., Goodarzi, V., Zahedi, P., Angaji, M.T. PET/imidazolium based nanocomposites via in situ polymerization: Morphological, thermal, and non-isothermal crystallization studies. Adv Polym Technol. 2007; 26:247–257.
28. Patro, T.U., Khakhar, D.V., Misra, A. Phosphonium-based clay-poly(ethylene terephthalate) nanocomposites: Stability, thermal and mechanical properties. J Appl Polym Sci. 2009; 113:1720–1732.
29. Hamzehlou, Sh., Katbab, A.A. Bottle-to-bottle recycling of PET via nanostructure formation by melt intercalation in twin screw compounder: Improved thermal, barrier, and microbiological properties. J Appl Polym Sci. 2007; 106:1375–1382.
30. Ke, Y.-C., Yang, Z.-B., Zhu, C.-F. Investigation of properties, nanostructure, and distribution in controlled polyester polymerization with clay. J Appl Polym Sci. 2002; 85:2677–2691.
31. Bizarria, M.T.M., Giraldi ALF de, M., de Carvalho, C.M., Velasco, J.I. Morphology and thermomechanical properties of recycled PET-organoclay nanocomposites. J Appl Polym Sci. 2007; 104:1839–1844.
32. Avrami, M.J. Kinetics of phase change. II Transformation-time relations for random distribution of nuclei. Chem Phys. 1940; 8:212–225.
33. Ozawa, T. Kinetics of non-isothermal crystallization. Polym. 1971; 12:150–158.
34. Bandypadhyay, J., Sinha Ray, S., Bousmina, M. Nonisothermal crystallization kinetics of polyethylene terephthalate nanocomposites. J Nanosci Nanotechnol. 2007; 8:1–11.
35. Liu, T.X., Mo, Z.S., Wang, S.E., Zhang, H.F. Nonisothermal melt and cold crystallization kinetics of poly(Ary1 Ether Ether Ketone Ketone). Poly Eng Sci. 1997; 37:568–575.
36. Chen, G.-X., Yoon, J.-S. Nonisothermal crystallization kinetics of poly(butylene succinate) composites with a twice functionalized organoclay. J Polym Sci: Part B: Polym Phys. 2005; 43:817–826.
37. Durmus, A., Ercan, N., Soyubol, G., Deligöz, H., Kaşgöz, A., Nonisothermal crystallization kinetics of poly(ethylene terephthalate)/clay nanocomposites prepared by melt processing. Polym compos 2010; 1056–1066, doi: 10.1002/pc.20892.
38. Jeziorny, A. Parameters characterizing the kinetics of the nonisothermal crystallization of poly(ethylene terephthalate) determined by d.s.c. Polymer. 1978; 19:1142–1144.
39. Yao, X., Tian, X., Zheng, K., Zhang, X., Zheng, J., Wang, R., Liu, C., Li, Y., Cui, P. Non-isothermal crystallization kinetics of poly(butylene terephthalate)/silica nanocomposites. J Macromol Sci. 2009; 48:537–549.
40. Wang, Y., Shen, C., Li, H., Chen, J. Nonisothermal melt crystallization kinetics of poly(ethylene terephthalate)/clay nanocomposites. J Appl Polym Sci. 2004; 91:308–314.
41. Wan, T., Chen, L., Chua, Y.C., Lu, X. Crystalline morphology and isothermal crystallization kinetics of poly(ethylene terephthalate)/clay nanocomposites. J Appl Polym Sci. 2004; 94:1381–1388.
42. Guan, G., Li, C., Yuan, X., Xiao, Y., Liu, X., Zhang, D. New insight into the crystallization behavior of poly(ethylene terephthalate)/clay nanocomposites. J Polym Sci: Part B: Polym Phys. 2008; 46:2380–2394.
43. Wang, Y., Shen, C., Chen, J. Nonisothermal cold crystallization kinetics of poly(ethylene terephthalate)/clay nanocomposites. Polym J. 2003; 35:884–889.
44. Calcagno, C.I.W., Mariani, C.M., Teixeira, S.R., Mauler, R.S. The effect of organic modifier of the clay on morphology and crystallization properties of PET nanocomposites. Polymer. 2007; 48:966–974.
45. Stoeffler, K., Lafleur, P.G., Denault, J. Thermal decomposition of various alkyl onium organoclays: effect on poly(ethylene terephthalate) nanocomposites’ properties. Polym Degrad Stabil. 2008; 93:132–1350.
46. Augis, J.A., Bennett, J.E. Calculation of the Avrami parameters for heterogeneous solid state reactions using a modification of the Kissinger method. J Therm Anal Cal. 1978; 13:283–292.
47. Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal Chem. 1957; 29:1702–1706.
48. Takhor, R.L., Advances in Nucleation and Crystallization of Glasses. American Chemical Society, Columbus, OH, 1971:166–172.
49. Bandypadhyay, J., Sinha Ray, S., Bousmina, M. Effect of nanoclay incorporation on the thermal properties of poly(ethylene terephthalate)/liquid crystal polymer blends. Macromol Mater Eng. 2010; 295:822–837.
50. Xie, W., Gao, Z., Pan, W.-P., Hunter, D., Singh, A., Vaia, R. Thermal degradation chemistry of alkyl quaternary ammonium montmorillonite. Chem Mater. 2001; 13:2979–2990.
51. Xie, W., Gao, Z., Liu, K.-L., Pan, W.-P., Vaia, R., et al. Thermal characterization of organically modified montmorillonite. Thermochim Acta. 2001; 367:339–350.
52. Sinha Ray, S., Bousmina, M., Okamoto, K. Structure and properties of nanocomposites based on poly(butylene succinate-co-adipate) and organically modified montmorillonite. Macromol Mater Eng. 2005; 290:759–768.
53. Lai, M.-C., Chang, K.-C., Huang, W.-C., Hsu, S.-C., Yeh, J.-M. Effect of swelling agent on the physical properties of PET-clay nanocomposite materials prepared from melt intercalation approach. J Phys Chem Solids. 2008; 69:1371–1374.
54. Chang, J.-H., Mun, M.K. Nanocomposite fibers ofpoly(ethylene terephthalate) with montmorillonite and mica: thermomechanical properties and morphology. Polym Int. 2007; 56:57–66.
55. Chang, J.-H., Mun, M.K., Lee, I.C. Poly(ethylene terephthalate) nanocomposite fibers by in situ polymerization: The thermomechanical properties and morphology. J Appl Polym Sci. 2005; 98:2009–2016.
56. Guan, G., Li, C., Zhang, D., Jin, Y. The effects of metallic derivatives released from montmorillonite on the thermal stability of poly(ethylene terephthalate)/montmorillonite nanocomposites. J Appl Polym Sci. 2006; 101:1692–1699.
57. Kráčalík, M., Studenovský, M., Mikešová, J., Sikora, A., Thomann, R., Friedrich, C. Recycled PET-organoclay nanocomposites with enhanced processing properties and thermal stability. J Appl Polym Sci. 2007; 106:2092–2100.
58. Yuan, X., Li, C., Guan, G., Xiao, Y., Zhang, D. Thermal stability of surfactants with amino and imido groups in poly(ethylene terephthalate)/clay nanocomposites. J Appl Polym Sci. 2008; 109:4112–4120.
59. Vassiliou, A.A., Chrissafis, K., Bikiaris, D.N. In situ prepared PET nanocomposites: Effect of organically modified montmorillonite and fumed silica nanoparticles on PET physical properties and thermal degradation kinetics. Thermochimica Acta. 2010; 500:21–29.
60. Xu, X., Ding, Y., Qian, Z., Wang, F., Wen, B., Zhou, H. Degradation of poly(ethylene terephthalate)/clay nanocomposites during melt extrusion: effect of clay catalysis and chain extension. Polym Degrad Stabil. 2009; 94:113–123.
61. Yuan, X., Li, C., Guan, G., Xiao, Y., Zhang, D. Thermal degradation investigation of poly(ethylene terephthalate)/fibrous silicate nanocomposites. Polym Degrad Stabil. 2008; 93:466–475.
62. Wang, G., Chen, Y., Wang, Q. Structure and properties of poly(ethylene terephthalate)/Na+-montmorillonite nanocomposites prepared by solid state shear milling (S3M) method. J Polym Sci: Part B: Polym Phys. 2008; 46:807–817.
63. Sánchez-Solís, A., Romero-Ibarra, I., Estrada, M.R., Calderas, F., Manero, O. Mechanical and rheological studies on poly(ethylene terephthalate)/montmorillonite nanocomposites. Polym Eng Sci. 2004; 44:1094–1102.
64. Bandypadhyay, J., Sinha Ray, S. Mechanism of enhanced tenacity in a polymer nanocomposite studied by small-angle X-ray scattering and electron microscopy. Polymer. 2010; 51:4860–4866.
65. Kráčalík, M., Studenovský, M., Mikešová, J., Sikora, A., Thomann, R., et al. Recycled PET nanocomposites improved by silanization of organoclay. J Appl Polym Sci. 2007; 106:926–937.
66. Litchfield, D.W., Baird, D.G. The role of nanoclay in the generation of poly(ethylene terephthalate) fibers with improved modulus and tenacity. Polymer. 2008; 49:5027–5036.
67. Hao, J., Lu, X., Liu, S., Lau, S.K., Chua, Y.C. Synthesis of poly(ethylene terephthalate)/clay nanocomposites using aminododecanoic acid-modified clay and a bifunctional compatibilizer. J Appl Polym Sci. 2006; 101:1057–1064.
68. Vidotti, S.E., Chinellato, A.C., Hu, G.-H., Pessan, L.A. Preparation of poly(ethylene terephthalate)/organoclay nanocomposites using a polyester ionomer as a compatibilizer. J Polym Sci: Part B: Polym Phys. 2007; 45:3084–3091.
69. Lee, S.J., Hahm, W.G., Kikutani, T., Kim, B.C., Effects of clay and POSS nanoparticles on the quiescent and shear-induced crystallization behavior of high molecular weight poly(ethylene terephthalate). Polym Eng Sci 2009; 317–323, doi: 10.1002/pen.21262.
70. Ge, X.-G., Wang, D.-Y., Wang, C., Qu, M.-H., Wang, J.-S., et al. A novel phosphorous containing copolyester/montmorillonite nanocomposites with improved flame retardancy. Euro Polym J. 2007; 43:2882–2890.
71. Ke, Z., Yongping, B. Improve the gas barrier property of the PET film with montmorillonite by in situ interlayer polymerization. Mater Lett. 2005; 59:3348–3351.
72. Yang, Y., Gu, H. Superfine structure, physical properties, and dyeability of alkaline hydro lyzed poly (ethylene terephthalate)/silica nanocomposite fibers prepared by in situ polymerization. J Appl Polym Sci. 2006; 102:3691–3697.
73. Abid, K., Dhouib, S., Sakli, F. Addition effect of nanoparticles on the mechanical properties of coated fabric. J Textile Institute. 2010; 101:443–451.
74. Minelli, M., Rocchetti, M., De Angelis, M.G., Montenero, A., Doghieri, F. Gas barrier properties of polymeric films with hybrid organic/inorganic coatings for food packing applications. AIChE Annual Meeting, San Francisco. 2006.
75. Chen, H. Smart nanotechnology in biomaterials, sensors, actuators and textiles. AIChE Annual Meeting and Fall Showcase, Cincinnati. 2005.
76. Bandyopadhyay, J., Sinha Ray, S. Determination of structural changes of dispersed clay platelets in a polymer blend during solid-state rheological property measurement by small-angle X-ray scattering. Polymer. 2011. [submitted].
77. Piegat, A., El Fray, M., Jawad, H., Qz, Chen, Boccaccini, A.R. Inhibition of calcification of polymer-ceramic composites incorporating nanocrystalline TiO2. Adv Appl Ceram. 2008; 107:287–292.
78. Kim, M.J., Shin, D.W., Kim, J.-Y., Park, S.H., Han, I., Yoo, J.B. The production of a flexible electroluminescent device on polyethylene terephthalate films using transparent conducting carbon nanotube electrode. Carbon. 2009; 47:3461–3465.
79. Litchfield, D.W., Baird, D.G., Rim, P.B., Chen, C., Improved mechanical properties of poly(ethylene terephthalate) nanocomposite fibers. Polym Eng Sci 2010;, doi: 10.1002/pen.21758.