Polymer nanocomposite coatings
Abstract:
This chapter discusses the properties of coatings made of polymer nanocomposites based on non-conductive polymers, electroactive polymers and conductive polymers. Anticorrosion resistance and tribology can be efficiently improved when nanoclay or nanoparticles are embedded in the polymer. Polymer nanocomposites made with carbon nanotubes have electrical properties and act as electromagnetic interference shielding. Some coating technologies suitable for polymer nanocomposites are discussed.
18.1 Introduction
Polymer nanocomposites or nanofilled polymers1 are polymer matrices containing organic or inorganic fillers with a homogeneous nanoscale distribution (normally from 10 to 100 nm in at least one dimension), which are prepared by physical blending or chemical polymerizing technologies. The fillers can be particles, layered materials, fibres or clusters embedded in a wide variety of natural or synthetic polymers. Their distinctive physical and chemical properties, which enhance the performance of the composites, attract much interest even after decades of study. These outstanding properties, due to the fillers, give these polymers a high potential for use in aeronautics, the automotive industry, electronics, medical equipment and consumer goods. Different types of nanofilled polymer, such as powders, bulk and functional thin films, are used widely in industry and academia. Polymer nanocomposite coatings are especially significant because they improve the surface characteristics of substrates for specific purposes. For instance, a polymer nanocomposite, with an inorganic layered filler, coated onto the surface of steel is able to slow corrosion considerably. This protection mechanism can also be used to construct a gas barrier coating,2, 3 since the inorganic layered filler lengthens the penetration pathway for the gas. Other coatings for special functions, such as self-cleaning, temperature resistance, wear resistance and special optics, have appeared in many commercial products. Recently, electroactive coatings based on polymer nanocomposites have been shown to have a much lower resistivity than traditional coatings, leading to new applications as electrochemical sensors,4 materials with a high dielectric constant,5 functional membranes6 and electrochromic materials.7 Other than their intrinsic material behaviour, the key parameter for defining successful polymer nanocomposite coatings is how easily and efficiently they can be deposited onto substrates.
18.2 Coating technologies for polymer nanocomposites
A successful coating requires not only the intrinsic properties of the polymer nanocomposite but also workable technologies for depositing the material on different kinds of substrate. 'Workable', in the sense used here, implies conforming to several conditions to ensure good coating quality characteristics such as surface uniformity, adhesion between interphases, thickness control and the non-toxicity of the material. Production capacity is another significant consideration for large-scale manufacturing. Many different coating technologies have been used in research and production. However, not all of them are practical for nanofilled polymers. Polymer chains can be easily damaged by vaporizing processes, which are normally performed at extremely high temperatures or energies. Hence, a low deposition temperature is usually a basic requirement of a suitable coating technique for polymer nanocomposites. In general, suitable coating techniques for polymer nanocomposites can be classified into four main groups: (1) physical vapour deposition (PVD),8, 9 (2) chemical vapour deposition (CVD),10–13 (3) chemical and electrochemical deposition14–16 and (4) roll-to-roll (R2R) casting deposition.17–19 We provide a brief introduction to each deposition technique in this chapter.
18.2.1 Physical vapour deposition
PVD first involves the generation of a vapour of the target substance, e.g. a gas mixture plasma, via a high-energy source. Next, the vaporized material is condensed onto the surface of the substrate in a very low-pressure chamber (0.1–10 Pa) to gradually form a thin film on the substrate. The creation of a high-density plasma using a low-pressure gas requires a static magnetic field of ~0.03 T. The field is formed by a configuration of equivalent (or balanced) magnets within the region in front of the sputtering target and traps electrons close to the surface. The magnetic field lines are predominantly concentrated behind the target, while the plasma density near the substrate is about ηe (~1010 cm−3). Magnetron sputtering or direct current magnetron sputtering involves the application of a direct potential to the substrate to induce and sustain the plasma. A related technique, unbalanced magnetron sputtering (UMS), was developed to deposit alloys and their nitrides and carbides. However, this type of coating technology still relies on relatively high energies and is rarely utilized for nanofilled polymers due to the hazardous conditions.
18.2.2 Chemical vapour deposition
CVD is based on the production of a vapour from the solid target material via a heating process and chemical reactions in the vapour phase. Hence, this deposition involves a homogeneous gas phase or heterogeneous chemical reactions, which occur on or in the vicinity of the heated surface, leading to the formation of powders or films, respectively.20 CVD is typically performed at very high temperatures, up to 1000 °C, in order to activate the chemical reactions in the vapour phase. These extremely high temperatures lead to a breakdown of the chemical structure of the solid targets, resulting in different properties compared with the original material. Therefore, low-temperature CVD at 350–700 °C has been carried out using inorganic and metallo-organic precursors. Even lower activation temperatures of 200–400 °C have been achieved using a plasma, as described below. The two most widely used CVD devices for providing a high-temperature stage are hot-wall CVD (HWCVD) and hot-filament CVD (HFCVD) reactors, due to their low cost compared with other coating techniques. However, HWCVD is more appropriate for the deposition of polymer nanocomposites because it does not require the excessively high filament temperatures of HFCVD. Even so, these coating technologies are rarely used for polymer nanocomposites, except for very special cases like the deposition of a nanodiamond.
Plasma-assisted CVD is an efficient way to reduce the temperature in CVD. It uses a microwave plasma at an extremely low pressure (10–100 Pa), instead of at high temperature, to activate the chemical reactions. The method requires only a low environmental temperature and uses a 2.45-GHz microwave plasma exciting source. This deposition method has been used to deposit silicon dioxide, carbon nitride and cubic boron nitride. Polymers containing fluorine, such as polyfluorohydrocarbon and polyperfluorocarbon,21, 22 have also been deposited on specific surfaces.23
18.2.3 Chemical and electrochemical deposition
Chemical and electrochemical deposition is an area of considerable interest, both from a fundamental and an applied standpoint; these methods can be used for electroplating and solution analysis. The low ionizing temperature (in most cases, the temperature can be ambient) should have a negligible hazardous effect on the coating targets. The key objective of chemical and electrochemical deposition is to reduce the precursors to active species through either reducing agents or an input voltage applied to the polyelectrolyte matrix. Based on this prerequisite, conductive polymers such as polyaniline (PANI), polythiophene (PTh), polypyrrole and poly(3,4-ethylenedioxythiophene) (PEDOT) have been the most commonly used for the conductive polymer matrix in nanocomposites. The mechanism for this coating technology is rather simple and normally includes two steps: (1) the target precursors are driven into becoming active monomers, including cathodic or free radicals, and (2) the active monomers diffuse to the cathode (the working electron source and target substrate) and gradually accumulate on the surface of the substrate. However, in the electrochemical method, reduction requires an external current and the sites for anodic and cathodic reactions are separate. For chemical deposition, reduction is induced by a reducing agent and the anodic and cathodic reactions occur together on the workpiece.24 In addition, these reactions only proceed on catalytically active surfaces, i.e. newly coated metallic surfaces should be catalytically active enough to promote redox reactions. Electrochemical deposition has been widely used with conductive polymer nanocomposites to immobilize nanofillers such as heavy metal colloids or specific enzymes, which are inside the conducting polymer matrix. In addition, bioactive thin films may be able to act as biotransistors to convert analogue biosignals into electronic signals.
18.2.4 Roll-to-roll processing deposition
R2R (Fig. 18.1) processing deposition is a high production capacity and low-cost industrial coating technology, which has been employed in the manufacture of flat devices such as organic light-emitting diodes (OLEDs), photovoltaics (PV) and electrophoresis displays (EPDs). A simple roll coating is defined by Coyle et al. as follows: the fluid flows into the space between a pair of rotating rollers that control both the thickness and the uniformity of the coated film. There are some variations of this definition, like reverse-roll coating, 25 gravure coating, 26 knife-over-roll coating (gap coating) and slot die coating, which have been used industrially for specific purposes. R2R is a 'non-stop' coating process and can be combined with most other deposition techniques. Many practical techniques, for instance R2R casting, R2R sputtering and R2R plasma-assisted CVD, have been used in the manufacture of nanocomposites for photoconversion thin films.
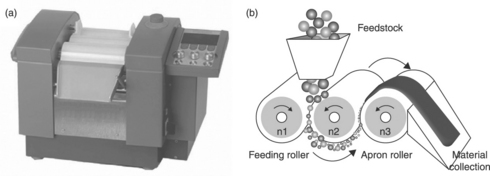
18.1 (a) Calendering (or three-roll milling) machine used for dispersing particles into a polymer matrix. (b) General configuration and mechanism. After Ma et al.19
18.3 Key properties of polymer nanocomposite coatings
18.3.1 Anticorrosion properties
Organic coatings have long been used to protect metals against corrosive environments in both academic and industrial settings. The primary objective of organic coatings is to act as a physical barrier against aggressive species, such as O2 and H2O. In the past decade, scientists have focused on a new type of coating: hybrid organic–inorganic coatings. These coatings combine the flexibility and ease of processing of polymers with the hardness of inorganic materials and have been successfully applied to various substrates. For example, Yeh and co-workers demonstrated that the incorporation of organophilic clay platelets into a polymer matrix, in the form of organic-based coatings, may effectively enhance the corrosion protection of polymers on metallic surfaces.27–38 This is because of the good dispersion (intercalation and exfoliation, Fig. 18.2) of the plate-like clay platelets in the polymer matrix, which effectively increase the length of the diffusion pathways for oxygen and water. In addition, the dispersion may also decrease the permeability of the coatings.
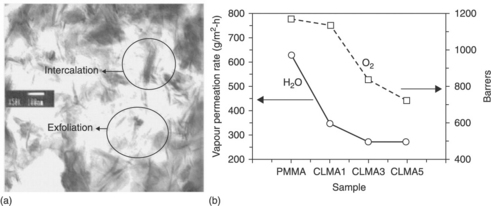
18.2 (a) Transmission electron micrograph of PMMA/clay nanocomposites. (b) Permeability of H2O and O2 as a function of clay content in PMMA/clay nanocomposites.2
Apart from clay, another important material with this advantage is SiO2. The incorporation of low-cost SiO2 nanoparticles into polymers can significantly improve thermal properties,39, 40 mechanical properties,41, 42 anticorrosion,43, 44 wear resistance,45, 46 barrier properties46, 47 and the customization of electronic packaging properties.48 Nanoparticles can significantly alter the mechanical properties of the polymer close to the particle surface due to changes in polymer chain mobility.46
Graphene, a monolayer of sp2-hybridized carbon atoms arranged in a two-dimensional lattice, has also attracted tremendous attention as a nanofiller in recent years due to its exceptional thermal,49–52 mechanical53–61 and barrier properties.62–66 The incorporation of graphene fillers can significantly reduce gas permeation through a polymer composite relative to the neat polymer matrix. A percolating network of platelets can provide a 'tortuous path', which inhibits molecular diffusion through the matrix, thus resulting in significantly reduced permeability (Fig. 18.3). Nguyen et al. demonstrated that the incorporation of graphene nanosheets into a polymer matrix improves the barrier capability. At low concentrations (below 0.05 vol%), crumpled graphene sheets are as effective as clay-based nanofillers with loadings that are approximately 25–130 times higher.63
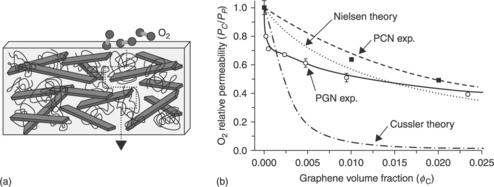
18.3 (a) A ‘tortuous path’ of platelets inhibits the diffusion of gases through a polymer composite (Nielsen model). (b) Oxygen permeability of polystyrene/graphene (PGN) and polystyrene/clay (PCN) composites as a function of filler loading, compared with two theoretical models of composite permeability.63
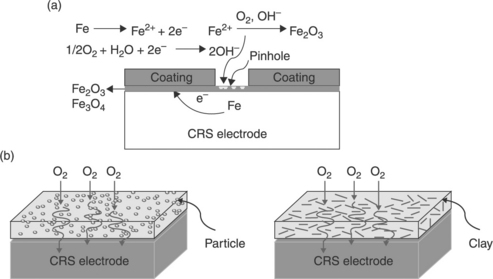
Nanofillers can also be loaded into conductive polymers (in addition to non-conductive polymers) to form coating materials, while retaining their barrier capabilities. Conductive polymers such as PANI, polypyrrole, and PTh have also been applied as anticorrosion coatings. PANI in particular is one of the most promising electrode materials due to its simple synthesis and environmental stability.67 PANI and its derivatives have been extensively studied as anticorrosive coatings for various metals.68–70 For instance, Ahmad and MacDiarmid71 and Wei et al.72 evaluated the corrosion protection of PANI by performing a series of electrochemical measurements. Wessling and co-workers reported that conducting polymers form a complex with Fe (passivation oxide layers, Fig 18.4), which improved corrosion protection.73–75 A possible passivation oxide layer mechanism is shown in Fig. 18.5. The chemical composition of the passivation oxide layers was determined by X-ray photoelectron spectroscopy (XPS). Binding energy plots versus intensity for iron oxide layers on the exterior of the sample and for the oxides right up against the iron surface are shown in Fig. 18.6. These plots indicate that the passive oxide layer is predominately composed of Fe2O3 on the outside with an Fe3O4 layer sandwiched between this layer and the nascent steel.
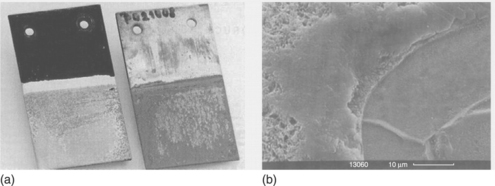
18.4 (a) An untreated iron plate (right) exhibits rust after a short time in salt water or after one anodic corrosion current measurement. A passivated plate (left, passivation was performed only on the lower half of the plate and the polyaniline layer was removed after passivation) exhibits no rust even after a corrosion current density measurement. (b) Scanning electron microscopy (SEM) image showing that passivation is achieved with an oxide layer several micrometres thick coating the whole metal surface.
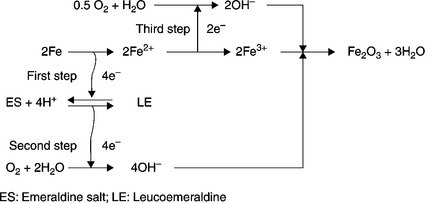
18.5 Catalytic passivation oxide layer of iron by PANI.74
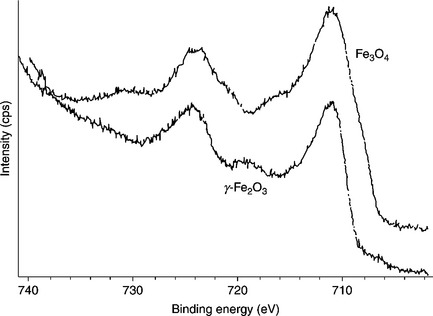
18.6 XPS analysis of passive iron oxide layers formed in the presence of doped polyaniline.73
Although PANI has outstanding anticorrosion properties, the adhesion, mechanical properties and barrier effect of the PANI coating can still be improved to enhance the efficiency of corrosion protection. For example, different nanofillers, such as inorganic nanoparticles,76 graphite,77 carbon nanotubes78, 79 and nanoclays (layered silicates),36, 37 have been used as additives to strengthen the mechanical and barrier performance of polymers.80, 81 Shabani-Nooshabadi et al.82 synthesized PANI/montmorillonite nanocomposites and coated them on aluminium substrates. The corrosion rate was found to be 190 times lower than that observed for uncoated Al. The redox catalytic and barrier properties affected corrosion performance. Yeh and co-workers36 synthesized a PANI/clay nanocomposite and coated it onto cold-rolled steel (CRS). The results indicated that a PANI coating containing 1 wt% clay enhanced the corrosion protection of the CRS electrode.
Nanoparticles have been extensively used as stabilizers in the preparation of PANI dispersions and colloids,83, 84 and various hybrid systems consisting of PANI and silica particles have been reported.85–87 Currently, nanoparticle/PANI hybrid systems have mostly been constructed through the chemical polymerization of aniline in the presence of nanoparticles, or by modifying the nanoparticles with PANI prepared in advance. Radhakrishnan et al. used nano-TiO2 as a metal oxide additive in the composite, which gave better dispersion of the formulation as well as better barrier properties in the coatings.88 Luo et al. prepared a SiO2 particle/PANI hybrid system (the SiO2 particles were covered with a PANI nanofilm) on a glassy carbon (GC) electrode surface through a simple electrochemical method for use in biosensor applications.89
Nanoparticles (SiO2, TiO2) and nanoclays used as fillers to synthesize PANI nanocomposites can usually improve thermal, mechanical and anticorrosion properties. However, the peak current of the PANI nanocomposite is reduced as a result.89 This phenomenon may be due to the presence of the non-conductive nanofiller, which partially blocks the electrode surface (Fig. 18.7). A reduction of the electrical conductivity of the PANI/clay nanocomposite was observed in comparison to neat PANI. This is expected because the clay component is not electrically conductive and because incorporating the clay into the PANI matrix lowers the molecular weight and decreases electrical conductivity (Fig. 18.8).
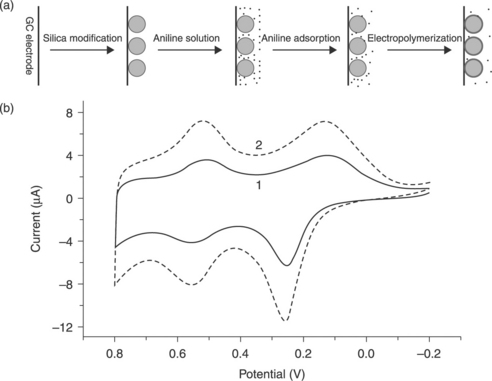
18.7 (a) Preparation of PANI-covered silica particles. (b) Cyclic voltammograms of (1) PANI/SiO2 /GC and (2) PANI/GC electrodes in 1.0 M HCl solution.89
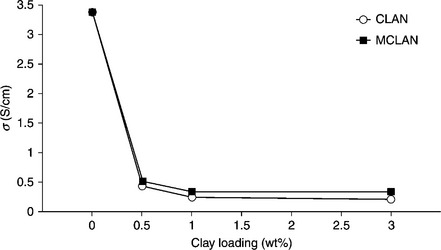
18.8 Electrical conductivity versus clay loading obtained from four-probe technique measurements.36
18.3.2 Electrical properties of polymer/carbon nanotube(CNT) materials
Another widely used nanofiller, namely, the carbon nanotube (CNT), has clearly demonstrated its capability as a filler in diverse multifunctional nanocomposites. An enhancement of electrical conductivity by several orders of magnitude at very low percolation thresholds (<0.1 wt%) has been observed for CNTs in polymer matrices. Electrically conducting composites with a volume conductivity higher than 10–10 S/cm are considered to be an important group of relatively inexpensive materials for many engineering applications (Fig. 18.9),19, 90, 91 such as electrically conducting adhesives, antistatic coatings92 and electromagnetic interference (EMI) shielding for electronic devices.
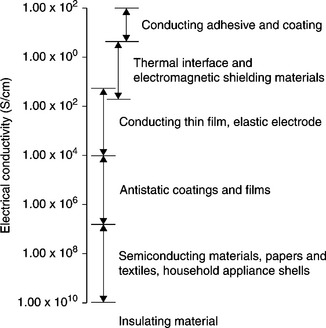
18.9 Typical applications of conducting composites19
Ajayan and co-workers93 first reported the electrochemical oxidation of aniline and the behaviour of PANI films formed on multiwalled CNT (MWNT) electrodes. The results suggest that PANI films on nanotubes are oxidized more readily and produce a higher current density during anodic oxidation compared with conventional electrodes such as Pt. This is attributed to the unusual surface topology and relatively large surface area of the nanotube electrodes. The morphology of PANI films formed on nanotube surfaces is granular and nanostructural.
Zengin et al.94 reported that PANI could be doped with CNTs to construct a donor-acceptor (DA) nanofilled thin film. CNTs are relatively good electron acceptors, whereas PANI is a good electron donor. CNTs dispersed well in PANI may serve as electron acceptors and conducting bridges. Conductivity increases from 3.36 S cm–1 for HCl-doped PANI to 33.374 S cm–1 for doped PANI/CNTs (10 wt% CNT). The Fourier transform infrared (FTIR) spectrum of PANI/CNT composites illustrates several differences from the spectrum of neat PANI (Fig. 18.10). The composite spectrum exhibits an increased quinoid (Q) to benzenoid (B) band (1600–1500 cm–1) intensity ratio compared with neat PANI. These data reveal that PANI/CNT is richer in quinoid units than pure PANI. This fact may suggest that PANI/CNT interactions promote or stabilize the quinoid ring structure. The π-bonded surface of the CNTs might interact strongly with the conjugated structure of PANI, especially through the quinoid ring. The band at 1150 cm–1 was described by Macdiarmid and co-workers95 as an electronic-like band, and it is considered to be a measure of the degree of delocalization of the electrons, and, thus, it is a characteristic peak of the conductivity of PANI. In PANI/CNT composites, the intensity of the signal at 1143 cm−1 increased and shifted to 1123 cm−1. This increase of the electronic-like absorption peak indicates increased electron delocalization in the composite compared with pure PANI and agrees well with the increased conductivity measurements.
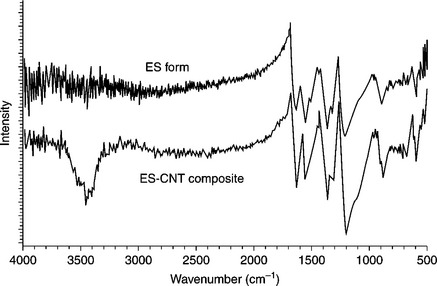
18.10 FTIR spectra of PANI emeraldine salt (ES) and PANI/CNT ES Composites.94
Several methods to modify CNTs have been reported in the literature;96–101 for instance, physical treatments such as ultrasonication and harsh chemical treatments in strong acids such as sulphuric acid, nitric acid or their mixtures. These treatments work well to improve dispersibility. Unfortunately, these approaches often result in significant damage to the CNT framework, which involves sidewall opening, breaking, and conversion into amorphous carbon. 102, 103 The damage to the CNTs definitely diminishes their original outstanding electrical, thermal and physical properties. Recently, the direct Friedel–Crafts acylation of nanofibres and nanotubes has been developed by Baek and co-workers. 104–109 The reaction condition in this approach is known to be a less destructive chemical modification. The CNTs were functionalized with 4-aminobenzoic acid via direct Friedel-Crafts acylation in a mild polyphosphoric acid (PPA)/phosphorous pentoxide (P2O5) medium. The 4-aminobenzoyl-functionalized CNTs should improve the electrochemical properties of PANI/CNT nanocomposites. Cyclic voltammetry (CV) is generally used to investigate the electrochemical properties of conductive materials. The output current of PANI/CNT nanocomposites was observed to be much larger than that of pure PANI. The results suggest that ion inclusion and exclusion in PANI/CNT nanocomposites were much more effective during the redox process (Fig. 18.11). The results are promising for the possible deposition of high-quality PANI films on nanotubes for future applications in, for example, devices, sensors110 (in which high currents would improve performance) and nanocomposites for electromagnetic shielding.64, 111
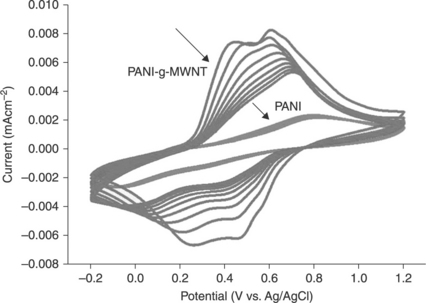
18.11 Cyclic voltammograms of PANI and PANI-g-MWNT in 1.0 M H2SO4 aqueous solution. Scan rate is 10 mV s-1.105
EMI, also called radio-frequency interference, is a serious issue due to the rapid proliferation of electronics and wireless systems in fields such as navigation and space technology.112 EMI not only affects the performance of electronic devices but may also be harmful to life forms, including humans. Therefore, some kind of shielding material must be employed to prevent electromagnetic noise or pollution. Various types of materials have been used for EMI shielding, including metals, carbon materials and conducting polymers. Recently, the EMI shielding and microwave absorption properties of conducting polymers have attracted increased attention due to their good electrical conductivity and processability.113 The EMI shielding efficiency (SE) of a composite material depends on many factors, including the intrinsic conductivity of the filler, dielectric constant and aspect ratio.114, 115 The small diameter, high aspect ratio, high conductivity and mechanical strength of CNTs, including single-walled CNTs (SWNTs) and MWNTs, make them an excellent option for creating conductive composites for high-performance EMI shielding materials at low filling rates. For example, MWNTs have been added to polymer matrices for EMI shielding materials and tested in the frequency range 8.2–12.4 GHz (X band) with 20 dB for 7% MWNT in polystyrene (PS).116, 117 Joo and co-workers studied the electrical conductivity and EMI properties of MWNTs in poly(methyl methacrylate) (PMMA) containing Fe. They achieved 27 dB for a 40% MWNT loading. 118 The microwave absorption properties of MWNT composites with encapsulated Fe, with different phases and shapes of the included Fe, have also been studied.119 Recently, Xiang et al. synthesized MWNT composites with silica and studied their microwave attenuation in the X band.120 Grimes et al.121 reported that polymer/SWNT composites possess a high real permittivity component (polarisation, ε') as well as an imaginary permittivity component (absorption or electric loss, ε") in the 0.5–2 GHz range. They also found that the permittivity decreased rapidly with increasing frequency.
Eklund and co-workers122 demonstrated that epoxy/SWNT nanocomposites can be used as effective lightweight EMI shielding materials. The highest EMI shielding effectiveness for epoxy/SWNT composites was found for materials with 15 wt% SWNT. The SE was found to be ~49 dB at 10 MHz, and the shielding effectiveness is around 15–20 dB in the 500 MHz to 1.5 GHz range.
Conducting polymers can be combined with other nanocomponents to enhance EMI shielding. The microwave absorption properties of CNT/conducting-polymer nanocomposites with a core-sheath nanostructure prepared by in situ polymerization have been measured.123 The conductivity of the PANI/CNT nanocomposites was higher than pure PANI and also pure CNTs -an example of the synergistic effect of the two components. PANI/CNT nanocomposites can be used for shielding in the Ku band (12.4–18.0 GHz) as their total shielding effectiveness is in the range of −27.5 to 39.2 dB. The microwave absorption properties of one-dimensional PANI/hexanoic acid (HA)/TiOi nanocomposites have also been studied.124 The results showed that the conductivity of the composite significantly decreased after addition of TiO2 and that the PANI/HA/TiO2 nanocomposites synthesized at 0 °C had a maximum reflection loss (RL) of 31 dB (>99.9% power absorption) at 10 GHz. To increase the conductivity and microwave absorption of the above system, SWNTs were also incorporated.125
When the SWNT content was 20%, the PANI/HA/TiO-/CNT nanocomposite exhibited an RL of less than −15 dB with a broad bandwidth (4 GHz), while an RL less than −20 dB with a narrow bandwidth (1 GHz) was observed when the SWNT content reached 60%. Although only a few reports on the use of one-dimensional nanostructured conducting polymers for microwave absorption and EMI shielding are available, it is believed that one-dimensional nanocomposites could be important in this field.64
18.3.3 Electroactive polymer coatings
Although PANI nanocomposites have excellent anticorrosion properties, the poor solubility of PANI in common organic solvents has limited its practical application in many fields. Recently, electroactive polymers incorporating an aniline oligomer have attracted immense research interest because of their advanced properties such as good solubility, mechanical strength and film-forming ability.126
A novel route for oxidative coupling polymerization has been developed by Wei, Zhang and co-workers.127–131 The fabricated copolymers not only contained well-defined conjugated segments, but also provided a better understanding of the structure–property relations and the conducting mechanism of conjugated polymers; knowledge which is limited by the complexity of their molecular structure and their poor solubility in organic solvents. Zagorska and co-workers132, 133 prepared new electroactive polymers combining the electrochromic properties of PTh and oligoaniline. Both the oligoaniline side chains and the PTh main chain could be doped in different ways. Wang and co-workers134–136 prepared electroactive polymers by including the aniline oligomer in the main and side chains, which exhibited electrochemical properties, a high dielectric constant and electrochromic behaviour.
Albertsson and co-workers137–140 incorporated the electroactive aniline oligomer into a polymer to obtain biodegradable and electroactive copolymers. The aniline oligomer has a well-defined electroactive structure, good processing properties and high degradability. Wei and co-workers 141, 142 incorporated aniline pentamer (AP) into polylactide and obtained copolymers that showed good electroactivity, biodegradability and processing properties. Electroactive polyimide (EPI) and electroactive epoxy (EE) have been extensively studied as electrochemical sensors,4 functional membranes,6 electrochromic materials 143 and anticorrosion coatings.144, 145
EPIs derived from bis-amino-terminated aniline trimer units were prepared by Wei and co-workers.146 Yeh and co-workers presented the first evaluations of the effect of an amine-capped aniline trimer (AT) on the corrosion protection efficiency of as-prepared EPI.145 A possible mechanism for the enhanced corrosion protection of EPI coatings on CRS electrodes is: (1) the EPI coatings may act as a physical barrier and (2) the redox catalytic properties of the aniline pentamer units in EPI may induce the formation of a passive metal oxide layer on the CRS electrode, as evidenced by scanning electron microscopy (SEM) and electron spectroscopy for chemical analysis (ESCA) studies (Fig. 18.12). The electrochromic performance of EPI was investigated by studying electrochromic photographs and measuring UV absorption spectra. Across different voltages, the colour of the EPI thin film was found to change from grey (at 0.0 V), to green (at 0.4 V), to blue (at 0.6 V) and to metallic blue (at 1.0 V), as shown in Fig. 18.13.143
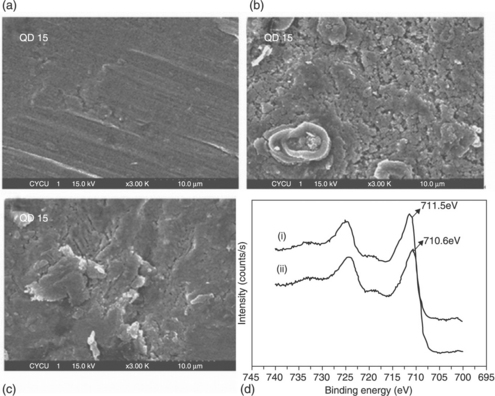
18.12 SEM images for (a) polished, (b) electroactive copolyimidecoated and (c) electroactive polyimide-coated CRS surfaces. (d) ESCA Fe 2p core level spectra of (i) electroactive copolyimide and (ii) electroactive polyimide.145
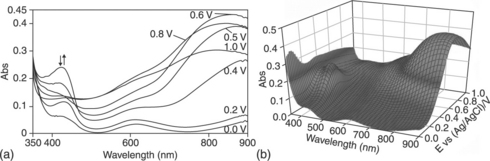
18.13 (a) Electrochromic behaviour of EPI thin film on glass electrodes coated with indium tin oxide (ITO) at different oxidation potentials. (b) Three-dimensional spectroelectrochemical behaviour of the EPI thin film on an ITO-coated glass electrode between 0.00 and 1.00 V (vs. Ag/AgCl).143
An electroactive epoxy thermoset (EET) coating, an electroactive polymer, has been studied.144 A higher concentration of conjugated amino-capped aniline trimer (ACAT) units existed in the as-prepared EET samples, and a higher redox current was observed in the CV studies. Electrochemical corrosion experiments showed that a higher concentration of the ACAT segment in the as-prepared EET led to better corrosion protection of the coated CRS electrode. The mechanism for enhanced corrosion protection is similar to that for the EPI material. Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC) and dynamic mechanical analysis (DMA) showed that incorporating a higher percentage of ACAT with a rigid aromatic structure in as-prepared EET led to a significant increase in thermal and mechanical properties (Fig 18.14).
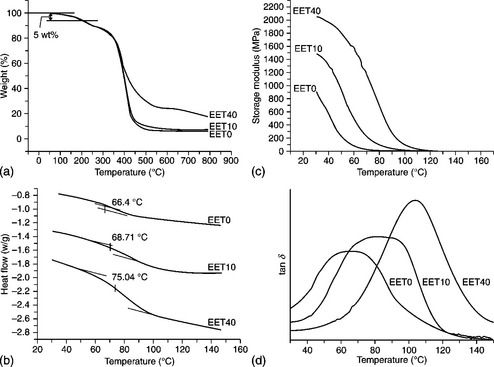
18.14 (a) TGA curves, (b) DSC curves, (c) storage modulus and (d) tan δ for EET40, EET10 and EET0 membranes.144
A superhydrophobic electroactive epoxy (SEE) from the surface structure of fresh Xanthosoma sagittifolium leaves was prepared and coated on the surface of a CRS electrode using a nanocasting technique for corrosion protection. 147 The electrode was found to have a water contact angle (CA) of 153°, which was significantly higher than smooth EE coated on CRS by spin coating (CA = 97°), as shown in Fig. 18.15.
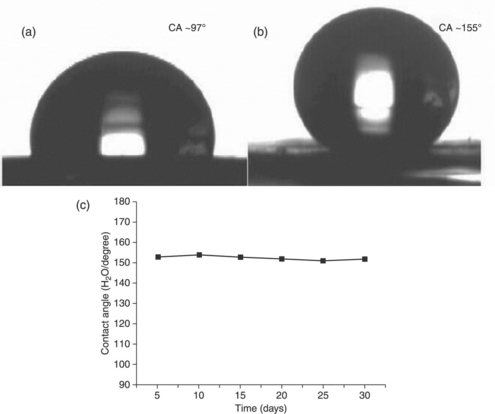
18.15 CCD images of water droplets: (a) EE with CA of 97° and (b) SEE with CA of 155°. (c) Change in water contact angle with time for SEE.147
Nanofillers such as clay, SiO2 and TiO2 also can be included in EPI and EE to capitalize on the properties of these nanoparticles.148–150 The good dispersion of these nanofillers in the polymer matrix leads to an effective increase in the length of the diffusion pathways for oxygen gas and water vapour and to a decrease in the gas permeability of the coatings, as shown in Fig. 18.16.
18.3.4 Friction and wear properties
Both friction and wear belong to the discipline of tribology and surface engineering; friction is the force between two surfaces in contact (the force resists the relative motion of the two objects), and wear is the erosion of material from a solid surface by the action of another solid. Scientists and engineers consider that surface engineering is one of the most crucial branches of technology in present society. Surface engineering is defined as the design of a surface/substrate composite system to achieve performance that could not be achieved by either the surface composition or the substrate alone, through engineering the substrate surface to improve the appearance, to provide protection from environmental damage or to enhance the mechanical or physical performance of the surface.45 Damage, such as corrosion, friction, wear, heat, radiation, weathering and the like, occurs on the surface of a component or is transferred via the surface into the component. Therefore, surface protection is of considerable significance to modern materials technology, and a wide variety of coatings can improve the wear resistance of a substrate. The addition of a second phase is one of the methods used to improve the tribological properties (such as the coefficient of friction and wear rate) of polymer materials. Factors that have an influence on the friction and wear characteristics of polymer composites are particle size, morphology and the concentration of the filler.
An epoxy/SiO2 composite was prepared by blending and the subsequent addition of a curing agent, and the wear properties were studied. The mixtures obtained were then gently transferred onto a clean, flat, smooth poly(tetrafluoroethylene) (PTFE) sheet, and an aluminium disc was placed on top of the epoxy composite. The PTFE/composite/aluminium sandwich was cured at 23 °C for 168 h and then the thickness of the cured composite was measured. The results showed that spherical silica particles improved the wear resistance of the epoxy matrix even though the filler concentration was relatively low (0.5–4.0 wt%), as shown in Fig. 18.17.151
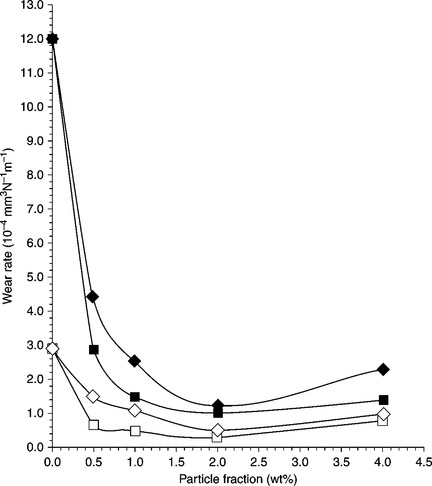
18.17 Wear rate of epoxy matrix composites filled with silica particles.![]() : 120 nm, 1 N; ◊: 510 nm, 1 N;
: 120 nm, 1 N; ◊: 510 nm, 1 N; ![]() : 120 nm, 2 N;
: 120 nm, 2 N; ![]() : 510 nm, 2 N.151
: 510 nm, 2 N.151
The wear mechanisms of epoxy and particulate-filled epoxy matrix composites under the pin-on-disc condition were studied by Durand et al.152 During wear testing, the unfilled epoxy exhibited brittle behaviour and cracks formed perpendicular to the sliding direction. Therefore, material waves were created and wear debris was produced (Fig. 18.18(a)). With the incorporation of uniformly sized submicron spherical silica particles in the epoxy matrix, the propagation of cracks into the epoxy matrix was hindered by particles on or near the surface layer. This resulted in the formation of finer material waves and, consequently, less debris (Fig. 18.18(b)). Furthermore, hard filler particles in a polymer matrix can reduce the wear rate when the applied stress is less than the critical value.153 Both of these factors could contribute to a reduction in the wear rate of an epoxy matrix.
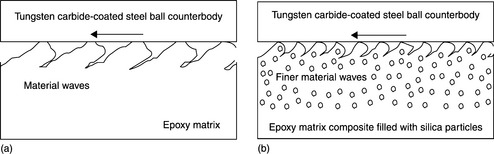
18.18 Wear mechanisms. (a) Cracks and material waves on the worn surface of an epoxy matrix. (b) Finer cracks and material waves on the worn surface of an epoxy matrix composite filled with silica particles151–153
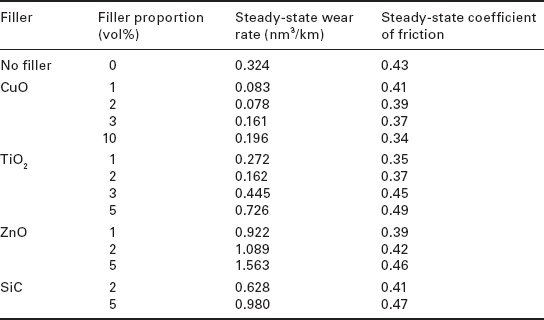
Wang et al.154–157 incorporated nano-Si3N4, nano-SiO2, nano-SiC and nano-ZrO2 into poly(ether ether ketone) (PEEK) and the testing results indicated that these nanometre-sized particles could effectively reduce the wear rate of PEEK, which was attributed to a thin, uniform and tenacious transfer film that formed on the surface of the counterpart. Bahadur and co-workers 158,159 investigated the tribological properties of poly(phenylene sulphide) (PPS) filled with nanometre TiO2 i ZnO, CuO or SiC, as shown in Table 18.1. An increase in wear resistance and a decrease in the coefficient of friction were observed under specific conditions.
Table 18.1
Steady-state wear rates and the coefficients of friction of PPS and its composites filled with different proportions of nanoparticles as filler materials159
Li et al.160 reported that PTFE filled with nano-ZnO could greatly reduce the wear rate of this polymer. The nano-ZnO antiwear mechanism works by preventing the destruction of the PTFE's banded structure during the friction process. Sawyer et al.161 explored the friction and wear behaviour of PTFE composites filled with 40-nm Al2O3, and they found that the friction coefficient of the composites increased slightly compared with an unfilled sample and that the wear resistance increased monotonically with increasing filler concentration. Lai et al.162–164 used nanometre attapulgite and ultrafine diamond to improve the tribological performance of PTFE and analysed the mechanism of the filler action in reducing the wear rate of the PTFE polymer through DSC and SEM. Yu et al.165 compared the friction and wear behaviour of poly(oxymethylene) (POM) filled with micrometre and submicrometre copper particles. The submicrometre copper particles were more effective in lowering the wear and coefficient of friction of the composites. Ng et al.166 dispersed TiO2 nanoparticles in epoxy, and the resultant composites not only appeared to be tougher than the traditional microparticle-filled epoxy but also had a higher scratch resistance. Cai et al.167, 168 researched the tribological properties of polyimide (PI) filled with Al2O3 and CNTs, and they reported that the fillers could effectively enhance the friction resistance and antiwear capacity of the composites. Lai et al.169 showed also that nano-attapulgite particles could improve the tribological behaviour of PI.
Li and co-workers 170 studied the effect of SiO2 particle size on the friction and wear behaviour of PI/SiO2 hybrids synthesized through a sol–gel method. Nanosilica could simultaneously provide PI with strength, toughness, friction and wear resistance. The friction coefficient of the hybrid with 100-nm SiO2 particles was the lowest and approximately 20% lower than that of pure PI. However, the lowest wear rate was recorded for the hybrid with 300-nm SiO2 particles and was approximately 20% lower than that of neat PI. The strength and toughness of PI/SiO2 hybrids are enhanced by approximately 50 and 200%, respectively, compared with pure PI when the size of the silica is less than 300 nm. These results are shown in Fig 18.19.
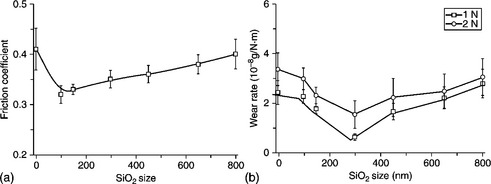
18.19 (a) Average friction coefficient for samples as a function of the size of the silica in the hybrid films (load: 2 N). (b) Variation of wear rate of PI hybrids with the size of the silica at various applied loads170
Gu and co-workers171 studied the indentation and scratch behaviour of a SiO2/polycarbonate (PC) composite coating at the microscale and nanoscale. The experimental results showed that hardness and stiffness increased after the addition of nano-SiO2. The scratch tests indicated that the nano-SiO2/PC coating exhibits a smaller scratch depth (Fig. 18.20) and a lower frictional coefficient. The addition of nano-SiO2 particles was reported as increasing the hardness and the modulus of the PC film. The elastic recovery of the nanocomposite was also improved. It was suggested that the improvement of these mechanical properties could be ascribed to the excellent performance of the nanoparticles and the increased crystallinity of the nanocomposites.172–174
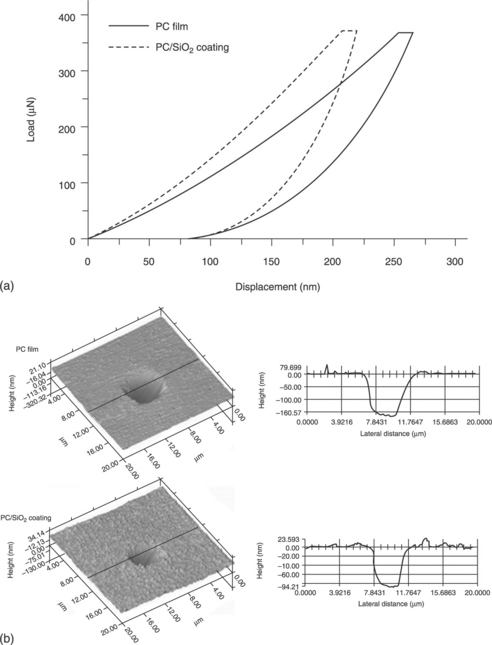
18.20 (a) Load–displacement curves of PC film and PC/SiO2 coating. (b) Three-dimensional images of the indentations171
The friction and wear properties of polyester (PE)/clay nanocomposites prepared by a mechanical stirrer was studied by Balasubramanian and co-workers.175 PE/clay nanocomposites have a slightly higher hardness than pristine PE. The lowest specific wear rate and friction coefficient were obtained for PE containing 3 wt% clay, as shown in Fig. 18.21.
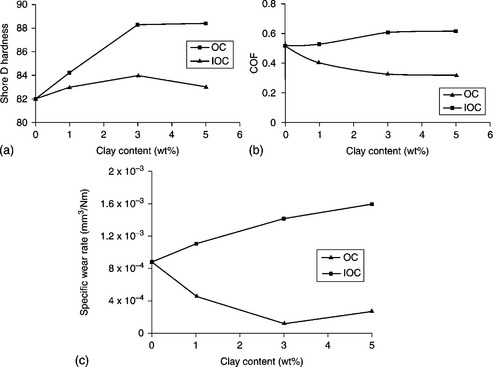
18.21 (a) Hardness. (b) Coefficient of friction. (c) Specific wear rate. (OC: organo-modified clay; IOC: inorganic clay).175
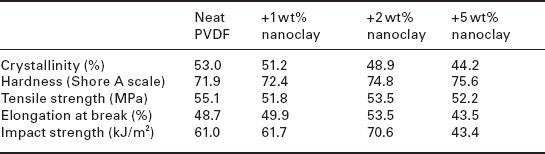
The tribological properties of poly(vinylidene fluoride) (PVDF)/clay nanocomposites were studied by Li and co-workers.176 Table 18.2 and Fig 18.22 show the crystallinity and mechanical properties of the nanocomposites. The data shows that adding 1–2 wt% clay to PVDF was effective in improving the mechanical and tribological properties of neat PVDF. The nanoclay acts as a reinforcing element for the load bore and thus decreased plastic deformation, which in turn reduced the friction coefficient, while the increased polarity of the material caused by crystal transformation may be the main reason for the lower wear. The PVDF/clay nanocomposite containing 5 wt% nanoclay had the highest wear rate. Weak compatibility between the nanoclay and PVDF, and also decreased crystallinity, may be responsible for the higher wear rate.
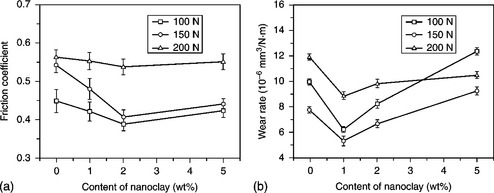
18.22 Effect of load on (a) the friction coefficient and (b) the wear rate of PVDF and PVDF nanocomposites with different contents of nanoclay176
18.4 Future trends
Conductive polymers and non-conductive polymers had been used in many fields in applications such as antistatic coatings, EMI shielding, anticorrosion coatings, wear-resistant coatings etc. Recently, synthetic electroactive polymers have been demonstrated to undergo redox reactions, similarly to conductive polymers, and were also shown to have good solubility and film formation. Because of these advantageous properties, electroactive polymers can be exploited for use in electrochemical sensors, functional membranes, electrochromic materials, anticorrosion coatings and biomaterials. However, the mechanical, thermal and electrical properties have not been studied in much depth since these materials are still a new field of exploration. Their reported behaviour is promising enough for further research to be undertaken in the hope of exploiting their valuable properties.
18.5 Acknowledgements
We thank all the publishers (Elsevier Ltd, John Wiley & Sons, Inc. and the American Chemical Society) who give us copyright permission for the figures and tables in this chapter. This work was supported by the National Sciences Council of the Republic of China under Grant number NSC 98-2113-M-033-001-MY3 and NSC 100-2811-M-033-012.
18.6 References
1. Manias, E. Nanocomposites: Stiffer by design. Nat. Mater.. 2007; 6:9–11.
2. Yeh, J.M., Liou, S.J., Lin, C.Y., Cheng, C.Y., Chang, Y.W., et al. Anticorrosively enhanced PMMA/clay nanocomposite materials with quaternary alkylphosphonium salt as an intercalating agent. Chem. Mater.. 2002; 14:154–161.
3. Yeh, J.M., Liou, S.J., Lai, C.Y., Wu, P.C., Tsai, T.Y. Enhancement of corrosion protection effect in polyaniline via the formation of polyaniline-clay nanocomposite materials. Chem. Mater.. 2001; 13:1131–1136.
4. Weng, C.J., Jhuo, Y.S., Chen, Y.L., Feng, C.F., Chang, C.H., et al. Intrinsically electroactive polyimide microspheres fabricated by electrospraying technology for ascorbic acid detection. J. Mater. Chem.. 2011; 21:15666–15672.
5. Chao, D., Jia, X., Liu, H., He, L., Cui, L., et al. Novelelectroactivepoly(arylene ether sulfone) copolymers containing pendant oligoaniline groups: Synthesis and properties. J. Polym. Sci., Part A: Polym. Chem.. 2011; 49:1605–1614.
6. Weng, C.J., Jhuo, Y.S., Huang, K.Y., Feng, C.F., Yeh, J.M., et al. Mechanically and thermally enhanced intrinsically dopable polyimide membrane with advanced gas separation capabilities. Macromolecules. 2011; 44:6067–6076.
7. Jia, X., Chao, D., Liu, H., He, L., Zheng, T., et al. Synthesis and properties of novel electroactive poly(amic acid) and polyimide copolymers bearing pendant oligoaniline groups. Polym. Chem.. 2011; 2:1300–1306.
8. Jäger, S., Böttcher, H. 'Dye composite layers by physical vapor deposition'. Adv. Mater. 1996; 8:93–97.
9. Hauert, R., Patscheider, J., Knoblauch, L., Diserens, M. New coatings by nanostructuring. Adv. Mater.. 1999; 11:175–177.
10. Awad, Y., El Khakani, M.A., Brassard, D., Smirani, R., Camiré, N., et al. Effect of thermal annealing on the structural and mechanical properties of amorphous silicon carbide films prepared by polymer-source chemical vapor deposition. Thin Solid Films. 2010; 518:2738–2744.
11. Wardle, B.L., Saito, D.S., Garcia, E.J., Hart, A.J., de Villoria, R.G., et al. Fabrication and characterization of ultrahigh-volume-fraction aligned carbon nanotube-polymer composites. Adv. Mater.. 2008; 20:2707–2714.
12. Hart, A.J., Slocum, A.H. Rapid growth and flow-mediated nucleation of millimeter-scale aligned carbon nanotube structures from a thin-film catalyst. J. Phys. Chem. B. 2006; 110:8250–8257.
13. Polini, R., Amar, M., Ahmed, W., Kumashiro, S., Sein, H., et al. A study of diamond synthesis by hot filament chemical vapour deposition on nanocomposite coatings. Thin Solid Films. 2005; 489:116–121.
14. Ishizawa, H., Ogino, M. Thin hydroxyapatite layers formed on porous titanium using electrochemical and hydrothermal reaction. J. Mater. Sci.. 1996; 31:6279–6284.
15. Helen Annal Therese, G., Vishnu Kamath, P., Subbanna, G.N. Novel electrosynthetic route to calcium phosphate coatings. J. Mater. Chem.. 1998; 8:405–408.
16. Peng, P., Kumar, S., Voelcker, N.H., Szili, E., Smart, R.S.C., et al. Thin calcium phosphate coatings on titanium by electrochemical deposition in modified simulated body fluid. J. Biomed. Mater. Res.. 2006; 76A:347–355.
17. Park, H.J., Kang, M.G., Ahn, S.H., Guo, L.J. A facile route to polymer solar cells with optimum morphology readily applicable to a roll-to-roll process without sacrificing high device performances. Adv. Mater.. 2010; 22:E247–E253.
18. Ahn, S.H., Guo, L.J. High-speed roll-to-roll nanoimprint lithography on flexible plastic substrates. Adv. Mater.. 2008; 20:2044–2049.
19. Ma, P.C., Siddiqui, N.A., Marom, G., Kim, J.K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Composites Part A. 2010; 41:1345–1367.
20. Choy, K.L. Chemical vapour deposition of coatings. Prog. Mater. Sci.. 2003; 48:57–170.
21. Yasuda, H., Hsu, T. Some aspects of plasma polymerization investigated by pulsed r.F. Discharge. J. Polym. Sci. Polym. Chem.. 1977; 15:81–97.
22. Yasuda, H., Lamaze, C.E. Polymerization in an electrodeless glow discharge. Ii. Olefinic monomers. J. Appl. Polym. Sci.. 1973; 17:1519–1531.
23. Laimer, J., Matsumoto, S. Pulsed microwave plasma-assisted chemical vapour deposition of diamond. Int. J. Refract. Met. Hard Mater.. 1996; 14:179–184.
24. Rao, C.R.K., Trivedi, D.C. Chemical and electrochemical depositions of platinum group metals and their applications. Coord. Chem. Rev.. 2005; 249:613–631.
25. Coyle, D.J., Macosko, C.W., Scriven, L.E. The fluid dynamics ofreverse roll coating. AlChE J.. 1990; 36:161–174.
26. Iwasaki, T. Gravure Coating Device and Method, 1990. [United States patent, 4,948,635, 14 August 1990].
27. Yeh, J.M., Liou, S.J., Lai, M.C., Chang, Y.W., Huang, C.Y., et al. Comparative studies of the properties of poly(methyl methacrylate)-clay nanocomposite materials prepared by in situ emulsion polymerization and solution dispersion. J. Appl. Polym. Sci.. 2004; 94:1936–1946.
28. Yeh, J.M., Chen, C.L., Chen, Y.C., Ma, C.Y., Huang, H.Y., et al. Enhanced corrosion prevention effect of polysulfone-clay nanocomposite materials prepared by solution dispersion. J. Appl. Polym. Sci.. 2004; 92:631–637.
29. Yeh, J.M., Huang, H.Y., Chen, C.L., Su, W.F., Yu, Y.H. Siloxane-modified epoxy resin-clay nanocomposite coatings with advanced anticorrosive properties prepared by a solution dispersion approach. Surf. Coat. Technol.. 2006; 200:2753–2763.
30. Yeh, J.M., Chen, C.L., Kuo, T.H., Su, W.F., Huang, H.Y., et al. Preparation and properties of (BATB-ODPA) polyimide-clay nanocomposite materials. J. Appl. Polym. Sci.. 2004; 92:1072–1079.
31. Yu, Y.H., Yeh, J.M., Liou, S.J., Chen, C.L., Liaw, D.J., et al. Preparation and properties of polyimide-clay nanocomposite materials for anticorrosion application. J. Appl. Polym. Sci.. 2004; 92:3573–3582.
32. Yu, Y.H., Yeh, J.M., Liou, S.J., Chang, Y.P. Organo-soluble polyimide (TBAPP-OPDA)/clay nanocomposite materials with advanced anticorrosive properties prepared from solution dispersion technique. Acta Mater.. 2004; 52:475–486.
33. Huang, T.C., Hsieh, C.F., Yeh, T.C., Lai, C.L., Tsai, M.H., et al. Comparative studies on corrosion protection properties of polyimide-silica and polyimide-clay composite materials. J. Appl. Polym. Sci.. 2011; 119:548–557.
34. Yeh, J.M., Chen, C.L., Chen, Y.C., Ma, C.Y., Lee, K.R., et al. Enhancement of corrosion protection effect of poly(o-ethoxyaniline) via the formation of poly(o-ethoxyaniline)-clay nanocomposite materials. Polymer. 2002; 43:2729–2736.
35. Yeh, J.M., Chin, C.P. Structure and properties of poly(o-methoxyaniline)–clay nanocomposite materials. J. Appl. Polym. Sci.. 2003; 88:1072–1080.
36. Chang, K.C., Lai, M.C., Peng, C.W., Chen, Y.T., Yeh, J.M., et al. Comparative studies on the corrosion protection effect of DBSA-doped polyaniline prepared from in situ emulsion polymerization in the presence of hydrophilic Na+-MMT and organophilic organo-MMT clay platelets. Electrochim. Acta. 2006; 51:5645–5653.
37. Chang, K.C., Jang, G.W., Peng, C.W., Lin, C.Y., Shieh, J.C., et al. Comparatively electrochemical studies at different operational temperatures for the effect of nanoclay platelets on the anticorrosion efficiency ofDBSA-doped polyaniline/Na+-MMT clay nanocomposite coatings. Electrochim. Acta. 2007; 52:5191–5200.
38. Yeh, J.M., Chin, C.P., Chang, S. Enhanced corrosion protection coatings prepared from soluble electronically conductive polypyrrole-clay nanocomposite materials. J. Appl. Polym. Sci.. 2003; 88:3264–3272.
39. Chen, C., Morgan, A.B. Mild processing and characterization of silica epoxy hybrid nanocomposite. Polymer. 2009; 50:6265–6273.
40. Cardiano, P., Mineo, P., Sergi, S., Ponterio, R.C., Triscari, M., et al. Epoxy-silica polymers as restoration materials. Part II. Polymer. 2003; 44:4435–4441.
41. Ochi, M., Takahashi, R., Terauchi, A. Phase structure and mechanical and adhesion properties of epoxy/silica hybrids. Polymer. 2001; 42:5151–5158.
42. Ragosta, G., Abbate, M., Musto, P., Scarinzi, G., Mascia, L. Epoxy-silica particulate nanocomposites: Chemical interactions, reinforcement and fracture toughness. Polymer. 2005; 46:10506–10516.
43. Huang, K.Y., Weng, C.J., Lin, S.Y., Yu, Y.H., et al. Preparation and anticorrosive properties of hybrid coatings based on epoxy-silica hybrid materials. J. Appl. Polym. Sci.. 2009; 112:1933–1942.
44. Chang, K.C., Lin, H.F., Lin, C.Y., Kuo, T.H., Huang, H.H., et al. Effect of amino-modified silica nanoparticles on the corrosion protection properties of epoxy resin-silica hybrid materials. J. Nanosci. Nanotechnol.. 2008; 8:3040–3049.
45. Li, Y., Ma, Y., Xie, B., Cao, S., Wu, Z. Dry friction and wear behavior of flame-sprayed polyamide1010/n-Si02 composite coatings. Wear. 2007; 262:1232–1238.
46. Zou, H., Wu, S., Shen, J. Polymer/silica nanocomposites: Preparation, characterization, properties, and applications. Chem. Rev.. 2008; 108:3893–3957.
47. Petrovicova, E., Knight, R., Schadler, L.S., Twardowski, T.E. Nylon 11/silica nanocomposite coatings applied by the HVOF process. Ii. Mechanical and barrier properties. J. Appl. Polym. Sci.. 2000; 78:2272–2289.
48. Sun, Y., Zhang, Z., Wong, C.P. Influence of interphase and moisture on the dielectric spectroscopy of epoxy/silica composites. Polymer. 2005; 46:2297–2305.
49. Ramanathan, T., Abdala, A.A., Stankovich, S., Dikin, D.A., HerreraAlonso, M., et al. Functionalized graphene sheets for polymer nanocomposites. Nat. Nano.. 2008; 3:327–331.
50. Verdejo, R., Barroso Bujans, F., Rodriguez Perez, M.A., Antonio de Saja, J., Lopez Manchado, M.A. Functionalized graphene sheet filled silicone foam nanocomposites. J. Mater. Chem.. 2008; 18:2221–2226.
51. Verdejo, R., Saiz Arroyo, C., Carretero Gonzalez, J., Barroso Bujans, F., Rodriguez Perez, M.A., et al. Physical properties of silicone foams filled with carbon nanotubes and functionalized graphene sheets. Eur. Polym. J.. 2008; 44:2790–2797.
52. Singh, V., Joung, D., Zhai, L., Das, S., Khondaker, S.I., et al. Graphene based materials: Past, present and future. Prog. Mater Sci.. 2011; 56:1178–1271.
53. Ansari, S., Giannelis, E.P. Functionalized graphene sheet-poly(vinylidene fluoride) conductive nanocomposites. J. Polym. Sci., PartB: Polym. Phys.. 2009; 47:888–897.
54. Jang, J.Y., Kim, M.S., Jeong, H.M., Shin, C.M. Graphite oxide/poly(methyl methacrylate) nanocomposites prepared by a novel method utilizing macroazoinitiator. Compos. Sci. Technol.. 2009; 69:186–191.
55. Liang, J., Huang, Y., Zhang, L., Wang, Y., Ma, Y., et al. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater.. 2009; 19:2297–2302.
56. Liang, J., Xu, Y., Huang, Y., Zhang, L., Wang, Y., et al. Infrared-triggered actuators from graphene-based nanocomposites. J. Phys. Chem. C. 2009; 113:9921–9927.
57. Nguyen, D.A., Lee, Y.R., Raghu, A.V., Jeong, H.M., Shin, C.M., et al. Morphological and physical properties of a thermoplastic polyurethane reinforced with functionalized graphene sheet. Polym. Int.. 2009; 58:412–417.
58. Rafiee, M.A., Rafiee, J., Srivastava, I., Wang, Z., Song, H., et al. Fracture and fatigue in graphene nanocomposites. Small. 2010; 6:179–183.
59. Verdejo, R., Bernal, M.M., Romasanta, L.J., Lopez-Manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem.. 2011; 21:3301–3310.
60. Stankovich, S., Dikin, D.A., Dommett, G.H.B., Kohlhaas, K.M., Zimney, E.J., et al. Graphene-based composite materials. Nature. 2006; 442:282–286.
61. Rafiee, M.A., Rafiee, J., Wang, Z., Song, H., Yu, Z.Z., et al. Enhanced mechanical properties of nanocomposites at low graphene content. ACS Nano. 2009; 3:3884–3890.
62. Zhu, Y., Murali, S., Cai, W., Li, X., Suk, J.W., Potts, J.R., et al. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater.. 2010; 22:3906–3924.
63. Compton, O.C., Kim, S., Pierre, C., Torkelson, J.M., Nguyen, S.T. Crumpled graphene nanosheets as highly effective barrier property enhancers. Adv. Mater.. 2010; 22:4759–4763.
64. Kim, H., Miura, Y., Macosko, C.W. Graphene/polyurethane nanocomposites for improved gas barrier and electrical conductivity. Chem. Mater.. 2010; 22:3441–3450.
65. Kim, H., Abdala, A.A., Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules. 2010; 43:6515–6530.
66. Potts, J.R., Dreyer, D.R., Bielawski, C.W., Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer. 2011; 52:5–25.
67. Lu, X., Zhang, W., Wang, C., Wen, T.C., Wei, Y. One-dimensional conducting polymer nanocomposites: Synthesis, properties and applications. Prog. Polym. Sci.. 2011; 36:671–712.
68. Martins, N.C.T., Moura e Silva, T., Montemor, M.F., Fernandes, J.C.S., Ferreira, M.G.S. Polyaniline coatings on aluminium alloy 6061-T6: Electrosynthesis and characterization. Electrochim. Acta. 2010; 55:3580–3588.
69. Bagherzadeh, M.R., Mahdavi, F., Ghasemi, M., Shariatpanahi, H., Faridi, H.R. Using nanoemeraldine salt-polyaniline for preparation of a new anticorrosive water-based epoxy coating. Prog. Org. Coat.. 2010; 68:319–322.
70. Sakhri, A., Perrin, F.X., Aragon, E., Lamouric, S., Benaboura, A. Chlorinated rubber paints for corrosion prevention of mild steel: A comparison between zinc phosphate and polyaniline pigments. Corros. Sci.. 2010; 52:901–909.
71. Ahmad, N., MacDiarmid, A.G. Inhibition of corrosion of steels with the exploitation of conducting polymers. Synth. Met.. 1996; 78:103–110.
72. Wei, Y., Wang, J., Jia, X., Yeh, J.M., Spellane, P. Polyaniline as corrosion protection coatings on cold rolled steel. Polymer. 1995; 36:4535–4537.
73. Lu, W.K., Elsenbaumer, R.L., Wessling, B. Corrosion protection of mild steel by coatings containing polyaniline. Synth. Met.. 1995; 71:2163–2166.
74. Wessling, B. Scientific and commercial breakthrough for organic metals. Synth. Met. 1997; 85:1313–1318.
75. Wessling, B. Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes. Adv. Mater.. 1994; 6:226–228.
76. Yongjun, H. Synthesis of polyaniline/nano-CeO2 composite microspheres via a solid-stabilized emulsion route. Mater. Chem. Phys.. 2005; 92:134–137.
77. Li, J., Vaisman, L., Marom, G., Kim, J.K. Br treated graphite nanoplatelets for improved electrical conductivity of polymer composites. Carbon. 2007; 45:744–750.
78. Cong, H., Zhang, J., Radosz, M., Shen, Y. Carbon nanotube composite membranes of brominated poly(2,6-diphenyl-1,4-phenylene oxide) for gas separation. J. Membr. Sci.. 2007; 294:178–185.
79. Geblinger, N., Thiruvengadathan, R., Regev, 0. Preparation and characterization of a double filler polymeric nanocomposite. Compos. Sci. Technol.. 2007; 67:895–899.
80. Malinauskas, A., Malinauskiene, J., Ramanavičius, A. Conducting polymer-based nanostructurized materials: Electrochemical aspects. Nanotechnology. 2005; 16:R51.
81. Hackman, I., Hollaway, L. Epoxy-layered silicate nanocomposites in civil engineering. Composites Part A. 2006; 37:1161–1170.
82. Shabani-Nooshabadi, M., Ghoreishi, S.M., Behpour, M. Direct electrosynthesis of polyaniline-montmorillonite nanocomposite coatings on aluminum alloy 3004 and their corrosion protection performance. Corros. Sci.. 2011; 53:3035–3042.
83. Riede, A., Helmstedt, M., Riede, V., Stejskal, J. Polyaniline dispersions. 9. Dynamic light scattering study of particle formation using different stabilizers. Langmuir. 1998; 14:6767–6771.
84. Stejskal, J., Kratochvil, P., Armes, S.P., Lascelles, S.F., Riede, A., et al. Polyaniline dispersions. 6. Stabilization by colloidal silica particles. Macromolecules. 1996; 29:6814–6819.
85. Liu, P., Liu, W., Xue, Q. In situ chemical oxidative graft polymerization of aniline from silica nanoparticles. Mater. Chem. Phys.. 2004; 87:109–113.
86. Niu, Z., Yang, Z., Hu, Z., Lu, Y., Han, C.C. Polyaniline-silica composite conductive capsules and hollow spheres. Adv. Funct. Mater.. 2003; 13:949–954.
87. Riede, A., Helmstedt, M., Riede, V., Zemek, J., Stejskal, J. In situ polymerized polyaniline films. 2. Dispersion polymerization of aniline in the presence of colloidal silica. Langmuir. 2000; 16:6240–6244.
88. Radhakrishnan, S., Siju, C.R., Mahanta, D., Patil, S., Madras, G. Conducting polyaniline-nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta. 2009; 54:1249–1254.
89. Luo, X., Killard, A.J., Morrin, A., Smyth, M.R. In situ electropolymerised silica-polyaniline core-shell structures: Electrode modification and enzyme biosensor enhancement. Electrochim. Acta. 2007; 52:1865–1870.
90. Grossiord, N., Loos, J., Regev, O., Koning, C.E. Toolbox for dispersing carbon nanotubes into polymers to get conductive nanocomposites. Chem. Mater.. 2006; 18:1089–1099.
91. Zhang, W., Dehghani Sanij, A., Blackburn, R. Carbon based conductive polymer composites. J. Mater. Sci.. 2007; 42:3408–3418.
92. Lou, F.L., Sui, Z.J., Sun, J.T., Li, P., Chen, D., et al. Synthesis of carbon nanofibers/mica hybrids for antistatic coatings. Mater. Lett.. 2010; 64:711–714.
93. Downs, C., Nugent, J., Ajayan, P.M., Duquette, D.J., Santhanam, K.S.V. Efficient polymerization ofaniline at carbon nanotube electrodes. Adv. Mater.. 1999; 11:1028–1031.
94. Zengin, H., Zhou, W., Jin, J., Czerw, R., Smith, D.W., Echegoyen, L., et al. Carbon nanotube doped polyaniline. Adv. Mater.. 2002; 14:1480–1483.
95. Quillard, S., Louarn, G., Lefrant, S., Macdiarmid, A.G. Vibrational analysis of polyaniline: A comparative study of leucoemeraldine, emeraldine, and pernigraniline bases. Phys. Rev. B. 1994; 50:12496.
96. Bahr, J.L., Tour, J.M. Covalent chemistry of single-wall carbon nanotubes. J. Mater. Chem.. 2002; 12:1952–1958.
97. Gohier, A., Nekelson, F., Helezen, M., Jegou, P., Deniau, G., et al. Tunable grafting of functional polymers onto carbon nanotubes using diazonium chemistry in aqueous media. J. Mater. Chem.. 2011; 21:4615–4622.
98. Hamon, M.A., Chen, J., Hu, H., Chen, Y., Itkis, M.E., et al. Dissolution of single-walled carbon nanotubes. Adv. Mater.. 1999; 11:834–840.
99. Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Ed.. 2002; 41:1853–1859.
100. Tsang, S.C., Chen, Y.K., Harris, P.J.F., Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature. 1994; 372:159–162.
101. Liu, J., Rinzler, A.G., Dai, H., Hafner, J.H., Bradley, R.K., et al. Fullerene pipes. Science. 1998; 280:1253–1256.
102. Kuznetsova, A., Popova, I., Yates, J.T., Bronikowski, M.J., Huffman, C.B., et al. Oxygen-containing functional groups on single-wall carbon nanotubes: NEXAFS and vibrational spectroscopic studies. J. Am. Chem. Soc.. 2001; 123:10699–10704.
103. Salzmann, C.G., Llewellyn, S.A., Tobias, G., Ward, M.A.H., Huh, Y., et al. The role of carboxylated carbonaceous fragments in the functionalization and spectroscopy of a single-walled carbon-nanotube material. Adv. Mater.. 2007; 19:883–887.
104. Kumar, N.A., Jeon, I.Y., Sohn, G.J., Jain, R., Kumar, S., et al. Highly conducting and flexible few-walled carbon nanotube thin film. ACS Nano. 2011; 5:2324–2331.
105. Jeon, I.Y., Kang, S.W., Tan, L.S., Baek, J.B. Grafting of polyaniline onto the surface of 4-aminobenzoyl-functionalized multiwalled carbon nanotube and its electrochemical properties. J. Polym. Sci., PartA: Polym. Chem.. 2010; 48:3103–3112.
106. Choi, E.K., Jeon, I.Y., Bae, S.Y., Lee, H.J., Shin, H.S., et al. High-yield exfoliation of three-dimensional graphite into two-dimensional graphene-like sheets. Chem. Commun.. 2010; 46:6320–6322.
107. Choi, H.J., Jeon, I.Y., Chang, D.W., Yu, D., Dai, L., et al. Preparation and electrocatalytic activity of gold nanoparticles immobilized on the surface of 4-mercaptobenzoyl-functionalized multiwalled carbon nanotubes. J. Phys. Chem. C. 2011; 115:1746–1751.
108. Han, S.W., Oh, S.J., Tan, L.S., Baek, J.B. One-pot purification and functionalization of single-walled carbon nanotubes in less-corrosive poly(phosphoric acid). Carbon. 2008; 46:1841–1849.
109. Lee, H.J., Han, S.W., Kwon, Y.D., Tan, L.S., Baek, J.B. Functionalization of multi-walled carbon nanotubes with various 4-substituted benzoic acids in mild polyphosphoric acid/phosphorous pentoxide. Carbon. 2008; 46:1850–1859.
110. Liao, Y., Zhang, C., Zhang, Y., Strong, V., Tang, J., et al. Carbon nanotube/polyaniline composite nanofibers: Facile synthesis and chemosensors. Nano Lett.. 2011; 11:954–959.
111. Spitalsky, Z., Tasis, D., Papagelis, K., Galiotis, C. Carbon nanotube-polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci.. 2010; 35:357–401.
112. Geetha, S., Satheesh Kumar, K.K., Trivedi, D.C. Polyaniline reinforced conducting e-glass fabric using 4-chloro-3-methyl phenol as secondary dopant for the control of electromagnetic radiations. Compos. Sci. Technol.. 2005; 65:973–980.
113. Stafström, S., Brédas, J.L., Epstein, A.J., Woo, H.S., Tanner, D.B., et al. Polaron lattice in highly conducting polyaniline: Theoretical and optical studies. Phys. Rev. Lett.. 1987; 59:1464–1467.
114. Joo, J., Lee, C.Y. High frequency electromagnetic interference shielding response of mixtures and multilayer films based on conducting polymers. J. Appl. Phys.. 2000; 88:513–518.
115. Chung, D.D.L. Electromagnetic interference shielding effectiveness of carbon materials. Carbon. 2001; 39:279–285.
116. Yang, Y., Gupta, M.C., Dudley, K.L., Lawrence, R.W. Novel carbon nanotube-polystyrene foam composites for electromagnetic interference shielding. Nano Lett.. 2005; 5:2131–2134.
117. Yang, Y., Gupta, M.C., Dudley, K.L., Lawrence, R.W. A comparative study of EMI shielding properties of carbon nanofiber and multi-walled carbon nanotube filled polymer composites. J. Nanosci. Nanotechnol.. 2005; 5:927–931.
118. Kim, H., Lee, C., Joo, J., Cho, S., Yoon, H., et al. Electrical conductivity and electromagnetic interference shielding of multiwalled carbon nanotube composites containing Fe catalyst. Appl. Phys. Lett.. 2004; 84:589–591.
119. Che, R.C., Peng, L.M., Duan, X.F., Chen, Q., Liang, X.L. Microwave absorption enhancement and complex permittivity and permeability of Fe encapsulated within carbon nanotubes. Adv. Mater.. 2004; 16:401–405.
120. Xiang, C., Pan, Y., Liu, X., Sun, X., Shi, X., et al. Microwave attenuation of multiwalled carbon nanotube-fused silica composites. Appl. Phys. Lett.. 2005; 87:123103–123105.
121. Grimes, C.A., Mungle, C., Kouzoudis, D., Fang, S., Eklund, P.C. The 500 MHz to 5.50 GHz complex permittivity spectra of single-wall carbon nanotube-loaded polymer composites. Chem. Phys. Lett.. 2000; 319:460–464.
122. Li, N., Huang, Y., Du, F., He, X., Lin, X., et al. Electromagnetic interference (EMI) shielding of single-walled carbon nanotube epoxy composites. Nano Lett.. 2006; 6:1141–1145.
123. Saini, P., Choudhary, V., Singh, B.P., Mathur, R.B., Dhawan, S.K. Polyaniline-MWCNT nanocomposites for microwave absorption and EMI shielding. Mater. Chem. Phys.. 2009; 113:919–926.
124. Phang, S.W., Tadokoro, M., Watanabe, J., Kuramoto, N. Microwave absorption behaviors of polyaniline nanocomposites containing TiO2 nanoparticles. Curr. Appl Phys.. 2008; 8:391–394.
125. Phang, S.W., Tadokoro, M., Watanabe, J., Kuramoto, N. Synthesis, characterization and microwave absorption property of doped polyaniline nanocomposites containing TiO2 nanoparticles and carbon nanotubes. Synth. Met.. 2008; 158:251–258.
126. Udeh, C.U., Fey, N., Faul, C.F.J., Functional block-like structures from electroactive tetra(aniline) oligomers. J. Mater. Chem. 2011;, doi: 10.1039/c1jm12557e.
127. Chao, D., Lu, X., Chen, J., Zhao, X., Wang, L., et al. New method of synthesis of electroactive polyamide with amine-capped aniline pentamer in the main chain. J. Polym. Sci., Part A: Polym. Chem.. 2006; 44:477–482.
128. Chao, D., Cui, L., Lu, X., Mao, H., Zhang, W., et al. Electroactive polyimide with oligoaniline in the main chain via oxidative coupling polymerization 2. Eur. Polym. J.. 2007; 43:2641–2647.
129. Chao, D., Lu, X., Chen, J., Liu, X., Zhang, W., et al. Synthesis and characterization of electroactive polyamide with amine-capped aniline pentamer and ferrocene in the main chain by oxidative coupling polymerization. Polymer. 2006; 47:2643–2648.
130. Zhang, J., Chao, D., Cui, L., Liu, X., Zhang, W. Electroactive polymer with oligoanilines in the main chain: Synthesis, characterization and dielectric properties. Macromol. Chem. Phys.. 2009; 210:1739–1745.
131. Chao, D., Ma, X., Liu, Q., Lu, X., Chen, J., et al. Synthesis and characterization of electroactive copolymer with phenyl-capped aniline tetramer in the main chain by oxidative coupling polymerization. Eur. Polym. J.. 2006; 42:3078–3084.
132. Buga, K., Majkowska, A., Pokrop, R., Zagorska, M., Djurado, D., et al. Postpolymerization grafting of aniline tetramer on polythiophene chain: Structural organization of the product and its electrochemical and spectroelectrochemical properties. Chem. Mater.. 2005; 17:5754–5762.
133. Dufour, B., Rannou, P., Travers, J.P., Pron, A., Zagórska, M., et al. Spectroscopic and spectroelectrochemical properties of a poly(alkylthiophene)-oligoaniline hybrid polymer. Macromolecules. 2002; 35:6112–6120.
134. Chao, D., Zhang, J., Liu, X., Lu, X., Wang, C., et al. Synthesis of novel poly(amic acid) and polyimide with oligoaniline in the main chain and their thermal, electrochemical, and dielectric properties. Polymer. 2010; 51:4518–4524.
135. Cui, L., Chao, D., Zhang, J., Mao, H., Li, Y., et al. Synthesis and properties of novel electroactive polyamide containing crown ether in the main chain. Synth. Met.. 2010; 160:400–404.
136. He, L., Chao, D., Jia, X., Liu, H., Yao, L., et al. Electroactive polymer with oligoanilines in the main chain and azo chromophores in the side chain: Synthesis, characterization and dielectric properties. J. Mater. Chem.. 2011; 21:1852–1858.
137. Guo, B., Finne Wistrand, A., Albertsson, A.C. Universal two-step approach to degradable and electroactive block copolymers and networks from combined ring-opening polymerization and post-functionalization via oxidative coupling reactions. Macromolecules. 2011; 44:5227–5236.
138. Guo, B., Finne Wistrand, A., Albertsson, A.C. Enhanced electrical conductivity by macromolecular architecture: Hyperbranched electroactive and degradable block copolymers based on poly(e-caprolactone) and aniline pentamer. Macromolecules. 2010; 43:4472–4480.
139. Guo, B., Finne Wistrand, A., Albertsson, A.C. Degradable and electroactive hydrogels with tunable electrical conductivity and swelling behavior. Chem. Mater. 2011; 23:1254–1262.
140. Guo, B., Finne Wistrand, A., Albertsson, A.C. Molecular architecture of electroactive and biodegradable copolymers composed of polylactide and carboxyl-capped aniline trimer. Biomacromolecules. 2010; 11:855–863.
141. Huang, L., Hu, J., Lang, L., Wang, X., Zhang, P., et al. Synthesis and characterization of electroactive and biodegradable ABA block copolymer of polylactide and aniline pentamer. Biomaterials. 2007; 28:1741–1751.
142. Huang, L., Zhuang, X., Hu, J., Lang, L., Zhang, P., et al. Synthesis of biodegradable and electroactive multiblock polylactide and aniline pentamer copolymer for tissue engineering applications. Biomacromolecules. 2008; 9:850–858.
143. Huang, T.C., Yeh, T.C., Huang, H.Y., Ji, W.F., Chou, Y.C., et al. Electrochemical studies on aniline-pentamer-based electroactive polyimide coating: Corrosion protection and electrochromic properties. Electrochim. Acta. 2011; 56:10151–10158.
144. Huang, K.Y., Shiu, C.L., Wu, P.S., Wei, Y., Yeh, J.M., et al. Effect of amino-capped aniline trimer on corrosion protection and physical properties for electroactive epoxy thermosets. Electrochim. Acta. 2009; 54:5400–5407.
145. Huang, K.Y., Jhuo, Y.S., Wu, P.S., Lin, C.H., Yu, Y.H., et al. Electrochemical studies for the electroactivity of amine-capped aniline trimer on the anticorrosion effect of as-prepared polyimide coatings. Eur. Polym. J.. 2009; 45:485–493.
146. Wang, Z.Y., Yang, C., Gao, J.P., Lin, J., Meng, X.S., et al. Electroactive polyimides derived from amino-terminated aniline trimer. Macromolecules. 1998; 31:2702–2704.
147. Weng, C.J., Chang, C.H., Peng, C.W., Chen, S.W., Yeh, J.M., et al. Advanced anticorrosive coatings prepared from the mimicked Xanthosoma sagittifolium-leaf-like electroactive epoxy with synergistic effects of superhydrophobicity and redox catalytic capability. Chem. Mater.. 2011; 23:2075–2083.
148. Huang, T.C., Su, Y.A., Yeh, T.C., Huang, H.Y., Wu, C.P., et al. Advanced anticorrosive coatings prepared from electroactive epoxy-SiO2 hybrid nanocomposite materials. Electrochim. Acta. 2011; 56:6142–6149.
149. Huang, H.Y., Huang, T.C., Yeh, T.C., Tsai, C.Y., Lai, C.L., et al. Advanced anticorrosive materials prepared from amine-capped aniline trimer-based electroactive polyimide-clay nanocomposite materials with synergistic effects of redox catalytic capability and gas barrier properties. Polymer. 2011; 52:2391–2400.
150. Weng, C.J., Huang, J.Y., Huang, K.Y., Jhuo, Y.S., Tsai, M.H., et al. Advanced anticorrosive coatings prepared from electroactive polyimide-TiO2 hybrid nanocomposite materials. Electrochim. Acta. 2010; 55:8430–8438.
151. Xing, X.S., Li, R.K.Y. Wear behavior of epoxy matrix composites filled with uniform sized sub-micron spherical silica particles. Wear. 2004; 256:21–26.
152. Durand, J.M., Vardavoulias, M., Jeandin, M. Role of reinforcing ceramic particles in the wear behaviour of polymer-based model composites. Wear. 1995; 181–183. [833–839].
153. Shi, M.M. Solid lubricating materials. Beijing: Chemical Industry Press; 2000.
154. Wang, Q., Xu, J., Shen, W., Liu, W. An investigation of the friction and wear properties of nanometer Si3N4 filled PEEK. Wear. 1996; 196:82–86.
155. Wang, Q., Xue, Q., Shen, W. The friction and wear properties of nanometre SiO2 filled polyetheretherketone. Tribol. Int.. 1997; 30:193–197.
156. Wang, Q.-H., Xu, J., Shen, W., Xue, Q. The effect of nanometer SiC filler on the tribological behavior of PEEK. Wear. 1997; 209:316–321.
157. Wang, Q., Xue, Q., Shen, W., Zhang, J. The friction and wear properties of nanometer ZrO2-filled polyetheretherketone. J. Appl. Polym. Sci.. 1998; 69:135–141.
158. Schwartz, C.J., Bahadur, S. Studies on the tribological behavior and transfer film-counterface bond strength for polyphenylene sulfide filled with nanoscale alumina particles. Wear. 2000; 237:261–273.
159. Bahadur, S., Sunkara, C. Effect of transfer film structure, composition and bonding on the tribological behavior of polyphenylene sulfide filled with nano particles of TiO2, ZnO, CuO and SiC. Wear. 2005; 258:1411–1421.
160. Li, F., Hu, K.A., Li, J.L., Zhao, B.Y. The friction and wear characteristics of nanometer ZnO filled polytetrafluoroethylene. Wear. 2001; 249:877–882.
161. Sawyer, W.G., Freudenberg, K.D., Bhimaraj, P., Schadler, L.S. A study on the friction and wear behavior of PTFE filled with alumina nanoparticles. Wear. 2003; 254:573–580.
162. Lai, S.Q., Li, T.S., Liu, X.J., Lv, R.G. A study on the friction and wear behavior of PTFE filled with acid treated nano-attapulgite. Macromol. Mater. Eng.. 2004; 289:916–922.
163. Lai, S.Q., Li, T.S., Liu, X.J., Lv, R.G., Yue, L. The tribological properties of PTFE filled with thermally treated nano-attapulgite. Tribol. Int.. 2006; 39:541–547.
164. Lai, S.Q., Yue, L., Li, T.S., Hu, Z.M. The friction and wear properties of polytetrafluoroethylene filled with ultrafine diamond. Wear. 2006; 260:462–468.
165. Yu, L., Yang, S., Wang, H., Xue, Q. An investigation of the friction and wear behaviors of micrometer copper particle-and nanometer copper particle-filled polyoxymethylene composites. J. Appl. Polym. Sci.. 2000; 77:2404–2410.
166. Ng, C.B., Schadler, L.S., Siegel, R.W. Synthesis and mechanical properties of TiO2-epoxy nanocomposites. Nanostruct. Mater.. 1999; 12:507.
167. Cai, H., Yan, F., Xue, Q. Investigation of tribological properties of polyimide/carbon nanotube nanocomposites. Mater. Sci. Eng., A. 2004; 364:94–100.
168. Cai, H., Yan, F., Xue, Q., Liu, W. Investigation of tribological properties ofAl2O3-polyimide nanocomposites. Polym. Test.. 2003; 22:875–882.
169. Lai, S.Q., Yue, L., Li, T.S., Liu, X.J., Lv, R.G. An investigation of friction and wear behaviors of polyimide/attapulgite hybrid materials. Macromol. Mater. Eng.. 2005; 290:195–201.
170. Lai, S.Q., Li, T.S., Wang, F.D., Li, X.J., Yue, L. The effect of silica size on the friction and wear behaviors of polyimide/silica hybrids by sol–gel processing. Wear. 2007; 262:1048–1055.
171. Wang, Z.Z., Gu, P., Zhang, Z. Indentation and scratch behavior of nano-SiO2/polycarbonate composite coating at the micro/nano-scale. Wear. 2010; 269:21–25.
172. Omrani, A., Simon, L.C., Rostami, A.A. The effects of alumina nanoparticle on the properties of an epoxy resin system. Mater. Chem. Phys.. 2009; 114:145–150.
173. Liu, L., Barber, A.H., Nuriel, S., Wagner, H.D. Mechanical properties of functionalized single-walled carbon-nanotube/poly(vinyl alcohol) nanocomposites. Adv. Funct. Mater. 2005; 15:975–980.
174. Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng., R. 2006; 53:73–197.
175. Jawahar, P., Gnanamoorthy, R., Balasubramanian, M. Tribological behaviour of clay-thermoset polyester nanocomposites. Wear. 2006; 261:835–840.
176. Peng, Q.Y., Cong, P.H., Liu, X.J., Liu, T.X., Huang, S., et al. Thepreparation of PVDF/clay nanocomposites and the investigation of their tribological properties. Wear. 2009; 266:713–720.