9
Pesticides
So naturalists observe, a flea has smaller fleas that on him prey; And these have smaller still to bite ‘em, And so proceed ad infinitum.
Jonathan Swift
Introduction
The term pesticides refers to a large body of diverse chemicals that includes insecticides, herbicides, fungicides, rodenticides, and fumigants employed to control one or more species deemed to be undesirable from the human viewpoint. Pesticides are of environmental concern for two main reasons. Although considerable progress has been made with respect to their selective toxicity, many still possess significant toxicity for humans, and many are persistent poisons, so that their long biological T1/2 allows bioaccumulation and biomagnification up the food chain (see Chapter 3). There is, thus, the possibility that they may enter human food supplies as well as constituting an ecological hazard. By their very nature, pesticides must have an impact on any ecosystem since they are designed to modify it by their selective elimination of certain species. As is always the case in considering chemicals used in the service of humankind, there is a complex risk–benefit equation that must be taken into account in making decisions regarding the use of pesticides. There is no question that they have increased agricultural production when used properly, and they have, in the past, been highly effective in controlling the insect vectors of human diseases like malaria and yellow fever spread by mosquitoes, and African sleeping sickness, which affects both humans and animals and which is spread by the tsetse fly. As shall be seen, however, these gains have not been without their problems.
Efforts to control agricultural pests probably evolved in parallel with cultivation techniques. Early methods included manual removal of weeds and insects, rigorous hoeing to prevent weed growth, and the use of traps for animal and insect pests. The first chemical controls to be used against agricultural pests were the arsenical compounds. In 1910, Erlich discovered that arsphenamine was an effective treatment for syphilis. This was the first chemotherapeutic agent for a bacterial infection and the first example of a structure–activity relationship. It opened the door on the entire field of chemical control of both infections and of pests. Paracelsus had introduced the use of inorganic arsenicals, notably arsenic trioxide (As2O3, white arsenic) into medicine in the sixteenth century, but its use was limited by its extreme toxicity. Ehrlich’s discovery revived interest in these compounds and in 1824 the Colorado potato beetle was discovered east of the Rockies and its eastward spread accelerated the search for an effective control. As3O3 was found to be effective and came into widespread use. Other arsenicals were developed, including Paris green (copper arsenite) which is still used as slug bait. Being a heavy metal, arsenic is persistent in the environment, the significance of which was not appreciated when it was being widely used.
Natural-source insecticides also evolved fairly early on. Certain plants have been employed as fish poisons in Southeast Asia and in South America for centuries, and in 1848 a decoction of derris root was used to control an insect infestation in a nutmeg plantation in Singapore. By 1920, large amounts were being imported into North America. The active ingredient is rotenone and it has the advantages of low mammalian toxicity and short T1/2 in nature. Pyrethrum flowers (chrysanthemums) have been known for their insecticidal properties for centuries. Commercial manufacture began in 1828. In 1945, the United States imported 13.5 million lb. By 1954 this had fallen to 6.5 million because of the widespread use of DDT, the banning of which has led to a resurgence of use of pyrethrin compounds. Nicotine sulfate (Blackleaf 40 is a 40% solution) from tobacco is used to control aphids and other insects. It has a short biological T1/2 but significant mammalian toxicity.
The mechanization of farming led to a second agricultural revolution by making possible the planting and cultivation of vast tracts of land. Pest control techniques also changed from the small-scale operations of the past to include mechanized spraying from the ground and the air (see also Chapter 4). This involved a marked increase in the use of pesticides and it coincided with the introduction of the first, modern, synthetic insecticide, DDT.
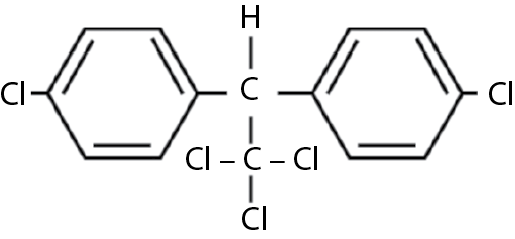
Dichlorodiphenyltrichloroethane, or DDT, was first synthesized in 1874, but its insecticidal properties were not recognized until 1939. Its structural formula is shown in Figure 9.1. Its first major use occurred in Sicily in 1943, where it was used to halt an epidemic of tick-borne typhus.
Sometimes called the grandfather of all chlorinated aromatic hydrocarbons, DDT was the first of such agents to arouse environmental concern. Rachel Carson’s Silent Spring called attention to the ecological damage caused by DDT and led to its banning in the United States and Canada in 1972. Prior to that, however, its use had led to the eradication of malaria in 37 countries and dramatically reduced its incidence in a further 80, providing relief to 1.5 billion people. Its effectiveness in controlling agricultural pests, coupled with its low mammalian toxicity (oral LD50 113 mg/kg, dermal LD50 2.5 gm/kg), resulted in extensive use in North America. U.S. production reached 50,000 metric tons annually. The availability of cheap, surplus aircraft after World War II resulted in the spraying of huge areas to control not only agricultural pests but human ones as well. Organochlorines, including the cyclodienes, dominated the insecticide field until the early 1960s, when organophosphorus insecticides (organophosphates) and carbamates were developed. These, plus the development of more disease-resistant hybrid crops, led to the “Green Revolution” of the 1960s, with dramatic increases in food production.
Classes of Insecticides
Organochlorines (Chlorinated Hydrocarbons)
As already discussed, the parent compound of this group is DDT. Its human toxicity is extremely low. In one rather heroic experiment, volunteers were fed 35 mg/day for up to 25 months without obvious ill effects. Another study of 35 male workers who had DDT levels in fat and liver 80 times the American average, and who had worked in a manufacturing plant for up to 19 years, showed no ill effects. DDT is, however, a potent inducer of cytochrome P450 hepatic microsomal enzymes and may thus affect the rate of biotransformation of other chemicals and drugs. Extremely high doses cause neurological signs and symptoms including numbness of the tongue, lips and face, dizziness, hyperexcitability, tremor, and convulsions.
DDT has very high lipid solubility and it is sequestered in body fat. Virtually everyone who was alive after 1940 has DDT in body fat. In the 1960s, significant amounts were found in people all over the world from Sri Lanka to North America. In 1970, the mean concentration in human fat was 7.88 ppm. After the ban, it fell to 4.99 in 1975. There is no evidence that chronic exposure to DDT has resulted in any health problems. In insects, DDT opens up ion channels to prevent normal axonal repolarization. Disorganized neuronal function leads to death.
Other life forms are not as resistant as humans are. Fish are extremely vulnerable, and die-offs occurred after heavy rains washed DDT into streams and lakes. Deformities also occur. Predatory birds at the top of the food chain are very vulnerable as well. Reproduction is disturbed in a number of ways. DDT induces cytochrome P450 to increase estrogen metabolism and DDT itself has estrogenic activity that affects fertility. Ca2+-ATPase is inhibited as is calcium deposition in eggshells. This effect is largely due to stable metabolites, notably DDE (dichlorodiphenyldichloroethane). Some bird species are only now recovering. The limited use of DDT against the tussock moth was reapproved in the United States in 1974 and its use in malarial areas has continued without interruption, so that DDT exposure on a worldwide basis still occurs.
The cyclodienes are a subgroup of the organochlorines. This group includes aldrin, dieldrin, heptachlor, and chlordane. Their mechanism of insecticidal action is the same as for DDT, but their toxicity for humans is much greater because of more efficient transdermal absorption. Signs of excessive CNS excitation and convulsions occur before less serious signs appear. Several deaths, mostly in those who handle the pesticide, have occurred. These agents too, are persistent in the environment. There is concern about their potential for carcinogenicity since this has been shown in some animals. However, Ribbens reported on a study of 232 male workers who had been exposed to high levels of cyclodienes in a manufacturing plant in Holland for up to 24 years (mean 11 years). Mortality and cancer incidence were compared to the means for the Dutch male population of the same age group. The observed mortality in the group was 25, which was significantly lower than the expected mortality of 38. Nine of the deaths were from cancer, as opposed to an expected incidence of 12. These workers had been exposed to very high levels of cyclodienes in the early days of manufacture, with recorded dieldrin blood levels of up to 69 μg/L at sometime in their history.
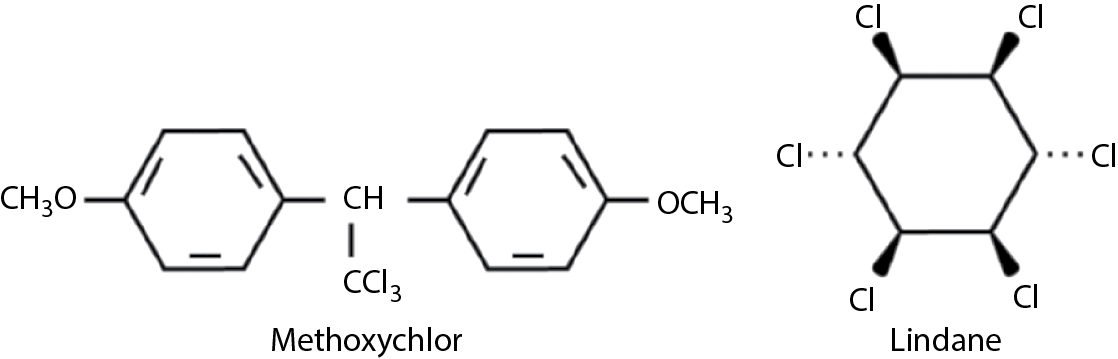
Other organochlorines include methoxychlor, lindane, toxaphene, mirex, and chlordecone (kepone). Mirex and kepone are extremely persistent, toxic to mammals (CNS toxicity), and carcinogenic in animals. They also induce cytochrome P450. They are no longer used in North America. Lindane shares the same toxicity but is much less persistent and it is used to treat head lice. Lindane (chemically 1,2,3,4,5,6-hexachlorocyclohexane) is the active isomer of benzene hexachloride. Toxaphene induces liver tumors in mice and is fairly toxic and its use is declining. Methoxychlor is similar to DDT but it is much less persistent and less toxic to mammals, which can metabolize it. It also is stored in fat to a much lesser degree. Its formula, along with that of lindane, is shown in Figure 9.2.
Organophosphorus Insecticides
These insecticides, often referred to as organophosphates, are the most frequent cause of human poisonings. The group includes parathion, dichlorvos (present in Vapona strips), and diazinon. They all act as irreversible inhibitors of acetylcholinesterase, so that the neurotransmitter acetylcholine is not inactivated following its release from the nerve terminal. Signs and symptoms are those of a massive cholinergic discharge and include dizziness and disorientation, profuse sweating, profuse diarrhea, constricted pupils, and bradycardia (slowing of the heart) possibly with arrhythmias. Parathion has a dermal LD50 of 21 mg/kg and an oral LD50 of 13 mg/kg in male rats but the NOEL in both rats and humans is only 0.05 mg/kg. Parathion itself is not toxic but it is transformed in the liver to para-oxone, its oxygen analog (see Chapter 1, Figure 1.3).
The following is a typical case history of organophosphorus poisoning:
A 52 year old white, male farmer was admitted to a hospital emergency department following a highway accident in which his tractor collided with the rear of a motor vehicle about to make a turn. He incurred numerous lacerations and contusions and a fractured right humerus. He was restless, incoherent, and required physical restraint. His pupils were bilaterally constricted, his heart rate was 55 beats/min and he was sweating profusely. His clothing had a strong, chemical odor. His wife volunteered that he had several episodes of visual difficulty over the preceding 2 weeks. Further questioning revealed that he had been spraying organophosphorus insecticides during this period (organophosphorus poisoning is frequently delayed). Atropine was given intravenously in repeated small doses until the signs of cholinergic discharge abated. Another drug that can be used is pralidoxime, which complexes with the phosphate component of the organophosphorus and releases the cholinesterase. The principal advantage of the organophosphates is their short life in the environment. The sites of action of organophosphates, atropine, and pralidoxime are shown in Figure 9.3.
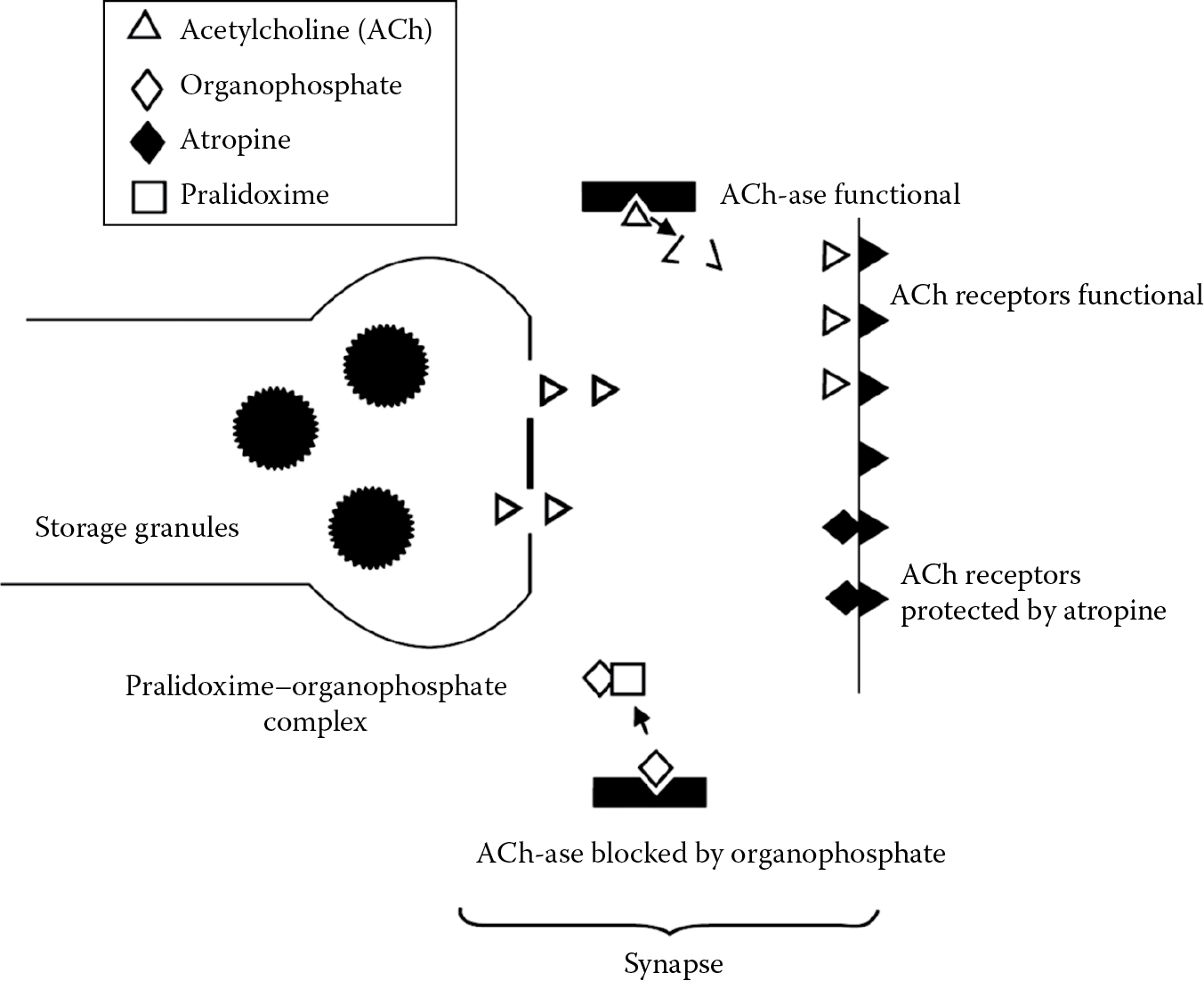
Sites of action of organophosphorus insecticides, atropine, and pralidoxime. Although the neurotransmitter site is labeled a synapse, muscarine is primarily a muscarinic receptor-blocking agent, acting at parasympathetic neuroeffector junctions. Acetylcholine is present there, as well as in all ganglia, at the neuromuscular junction, the brain, and the adrenal gland.
Carbamate Insecticides
Carbamates (e.g., Sevin) are also inhibitors of acetylcholinesterase, but they do not require metabolic activation and they are reversible. They are not persistent in the environment. Because they lack the phosphate group, pralidoxime cannot be used for treatment of poisoning. In fact, it is contraindicated because it may tie up more reactive sites on the enzyme and increase the degree of inhibition. This group includes aldicarb (Temik), carbaryl, and Baygon. The dermal LD50 for aldicarb in male rats is 3.0 mg/kg. It is also fairly toxic for humans. Although these agents are generally not persistent in the environment, aldicarb may be an exception. Under certain conditions (sandy soil over aquifers) it may reach water supplies and persist for a considerable time. In Long Island New York it has been estimated that the levels of 6 ppb may persist for up to 20 years.
Botanical Insecticides
The more common botanical insecticides were discussed briefly earlier. While it is commonly felt that natural-source insecticides are safer than synthetic ones (another example of the “nature knows best” syndrome), this is not necessarily so. Pyrethrins and rotenone have oral LD50s of about 600–900 and 100–300 mg/kg, respectively. Nicotine is quite toxic, with an oral LD50 of 10–60 mg/kg. The main problem with pyrethrins has been the rapidity with which they are destroyed in the environment. Newer ones have been isolated with longer T1/2s to permit more effective kills.
Herbicides
Chlorphenoxy Compounds
These agents, characterized by 2,4-D and 2,4,5-T, act as growth hormones, forcing plant growth to outstrip the ability to provide nutrients. They are employed as a variety of salts and esters. The acute toxicity of these agents is relatively low, with LD50s of 300–>1000 mg/kg reported for several species of mammals. The dog may be more sensitive (LD50 100 mg/kg). Ventricular fibrillation appears to be the immediate cause of death. Acute toxicity in humans is manifested largely as chloracne.
The main concern about 2,4-D and 2,4,5-T is the likelihood of their contamination with dioxin (TCDD). This subject is dealt with in Chapters 2 and 4. The chemical structures of these compounds are shown in Figure 9.4.
Dinitrophenols
Several substituted dinitrophenols are used as herbicides, the most common probably being Dinoseb (see Figure 9.5). It has been reported to have an LD50 of 20–50 mg/kg in rats. Dinoseb, first registered in 1947, is out of favor because handlers may be at considerable risk for teratogenic effects, cataracts, and male reproductive disturbances, even when protective clothing is worn. The U.S. EPA suspended all use in 1987.
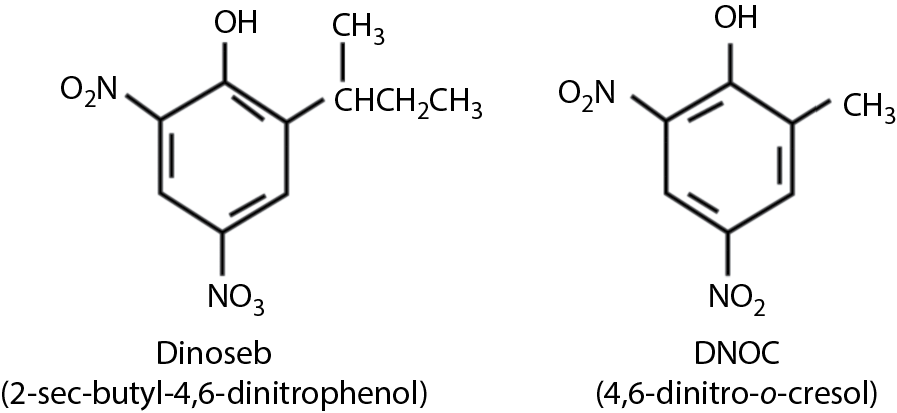
Chemical structures of Dinoseb (2-sec-butyl-4,6-dinitrophenol) and DNOC (4,6-dinitro-o -cresol).
4,6-dinitro-o-cresol (DNOC, Figure 9.5) has caused acute poisoning in humans with signs and symptoms including nausea, vomiting, restlessness, and flushing of the skin, progressing to collapse and coma. Hyperthermia may occur. Death may ensue in 24–48 h. Uncoupling of oxidative phosphorylation is probably the mechanism of toxicity. Atropine is contraindicated in DNOC poisoning because there is no anticholinesterase activity and the CNS effects of atropine may complicate the outcome. Treatment is symptomatic and includes ice baths to reduce fever, fluids intravenously, and the administration of O2.
Bipyridyls
Paraquat and diquat are the most familiar members of this group (see Figure 9.6). Both are toxic but their toxicity differs. The principal organ of toxicity for paraquat is the lungs, although liver and kidney also may be damaged. Respiratory failure may be delayed for several days after the ingestion of paraquat. It appears to be selectively concentrated in the lungs by an energy-dependent system. Paraquat is believed to undergo conversion to superoxide radical (O2•–), which causes the formation of unstable lipid hydroperoxides in cell membranes. Widespread fibroblast formation occurs, and O2 transfer to capillary blood is impaired. Treatment consists of attempts to remove or neutralize any paraquat remaining in the gastrointestinal tract by gastric lavage, cathartics, and Fuller’s earth as an adsorbant. In complete lung failure, double lung organ transplant offers the only hope for recovery.
In contrast, diquat toxicity is centered on the liver, kidney, and gastrointestinal tract. Superoxide anion formation is believed to play a role also in these organs. Poisoning with paraquat is far more common and it has been used as an instrument of suicide on numerous occasions.
Carbamate Herbicides
Unlike the insecticide carbamates, the herbicides do not possess anticholinesterase activity. They have low acute toxicity. Dithiocarbamates are used as fungicides and have similar low acute toxicity; LD50s for these agents are in the gm/kg range for rodents.
Triazines
This group, typified by Atrazine, also is characterized by low acute toxicity. Amitrole is a herbicide somewhat related to the triazines. It has similar low acute toxicity, but it has peroxidase-inhibiting activity and it has been associated with tumor formation in the thyroid in rats fed the chemical for 2 years.
Fungicides
A wide variety of agents has been used for their fungicidal properties, some of them quite toxic. Seed grains treated with mercurials have sometimes entered the human food supply with disastrous results (see Chapter 6). Pentachlorophenol and hexachlorobenzene are halogenated hydrocarbons with the toxicity typical of that group (see Chapter 4). Thiabendazole is a fungicide of low toxicity as evidenced by the fact that it is also used as an anthelmintic in domestic animals and humans for the eradication of roundworms.
Dicarboximides
Captan and Folpet are agents of some concern. Structurally similar to thalidomide, they have been shown to possess similar teratogenic properties in the chick embryo. Captan has been shown to be mutagenic, carcinogenic, and immunotoxic in animals. The EPA has judged Folpet to be a probable human carcinogen with a lifetime risk of cancer of 2 per million for lettuce and small fruits and a total of 5.5 per million when all food sources are combined.
Newer Biological Control Methods
The earliest form of biological control no doubt was the development of strains of plants and animals with a high degree of resistance to disease, through selective breeding. Observant farmers probably began this process soon after the domestication process began, and it continues today. Over 40 years ago, as a high school student, the author worked with Professor Waddell who developed, at the Ontario Agricultural College, the first strains of wheat to be resistant to wheat rust, a fungal infestation. Recently, a strain of American elms with a high degree of resistance to Dutch elm disease has been developed. Ladybugs have been bred and released to control the cottony cushion scale on oranges in California, and Bacillus thuringiensis var. kurstaki (Btk) is used to control forest pests (see also Chapter 4 regarding aerial spraying of Btk).
One of the earliest “high-tech” biological controls was developed in the 1950s and involves sterilization by radiation of millions of male insects that are then released to mate with the females. In species in which the female only mates once, this results in a high frequency of infertile unions with a resulting decline in the insect population. This method was first used successfully to control the screwworm fly in the southern United States. This fly lays its eggs in wounds in the skin of cattle and other livestock. The larvae then live on the flesh of the unwilling host. By 1966 the screwworm had been successfully eradicated in the United States and northern Mexico. It resurfaced in Libya, creating a political dilemma for the United States. Withholding technological assistance could result in massive infestations throughout Africa (the fly will also lay its eggs in wounds on humans), but the alternative at the time was to offer help to Quaddafi. Humanitarian considerations prevailed. This form of biological control has also been used more recently to control the Mediterranean fruit fly in California.
Analogs of insect hormones have been developed that are highly specific to a given species. These hormones trigger the molting metamorphosis in the larval stage so that the larva cannot develop normally and dies. Others, such as the pheromone for the light brown apple moth (see also Chapter 4), can be released to flood an area with this mating hormone so that male moths cannot home in on the females.
Pathogenic bacteria exist that can be cultured in commercial quantities and released to control specific pests. Some agents have been genetically modified for this purpose, but public concerns about “superbugs” have blocked approval of all but a few of these. Given that there are no, known bacteria that are infectious for both insects and mammals (as opposed to insects being vectors for infection), this fear seems unjustified. A more legitimate concern is that beneficial or harmless species may also be attacked by the organisms (see also Chapters 4 and 13).
Government Regulation of Pesticides
Most governments have regulations regulating the use of pesticides. The Canadian regulations are fairly typical of those in place in industrialized countries. The Pest Control Products Act, administered by Agriculture Canada, regulates the introduction of new pesticides. The risk–benefit principle is applied to decisions, that is, the degree of risk must be acceptable in light of the potential benefit to be derived from pest control with the new agent. Its relative safety and effectiveness compared to existing pesticides will influence the decision.
Table 9.1 compares the rodent LD50 values and the estimated lethal doses for humans of a number of pesticides. This is just a small sample of the hundreds listed on the Pennsylvania State website. It is evident that the cholinesterase-inhibiting insecticides, the carbamates and organophosphorus compounds, are the most toxic agents on the list. The herbicide paraquat is also very toxic.
Oral LD50 (Rodent) Values and Estimated Lethal Doses for Some Pesticides for a 70–155 kg Human
|
Class and Chemical Namesa |
Oral LD50 mg/kg |
Adult Lethal Dose (mL) |
|
Insecticides |
||
|
Aldicarb (a carbamate) Temik |
5 |
0.3 |
|
Carbaryl (a carbamate) Sevin |
500 |
30 |
|
Chlorpyrifos (organophosphate) Lorsban |
92–276 |
3–30 |
|
Diazinon (organophosphate) |
300–400 (tech)t |
To 25 |
|
Methoxychlor (chlorinated hydrocarbon) |
6000 |
300–900 |
|
Lindane (chlorinated hydrocarbon) |
88–125 |
3–30 |
|
Permethrin (a pyrethrin) Ambush, Pounce |
techt (>4000) |
30–300 |
|
Pyrethrum |
1500 |
30–300 |
|
Herbicides |
||
|
Alachlor, Degree |
techt 930–1550 |
30–300 |
|
Diquat |
600 |
30 |
|
Glyphosphate, Roundup, Touchdown |
>5000 |
>300 |
|
Oxyfluorfen, Goal |
>5000 |
>300 |
|
Paraquat, Gramoxone Max |
150 |
3–30 |
|
2,4-D (acid) |
375 |
3–30 |
|
2,4-DB (Butyrac) |
>2000 |
150 |
|
Fungicide |
||
|
Captan |
9000 |
500 |
|
Fluazinam, Omega |
>5000 |
>300 |
Sources: This table was compiled from data on websites provided by the British Columbia Ministry of Agriculture, http://www.agf.gov.bc.ca/b_1.htm ; Pennsylvania State University, http://pubs.cas.psu.edu/FreePubs/pdfs/uo222.pdf
a Chemical names are followed by trade names.
t Technical grade.
Problems Associated with Pesticides
Development of Resistance
Insects, like microorganisms, possess the most important characteristics for the evolution of resistant strains; an extremely short reproductive cycle and the production of vast numbers of progeny. Most species of insects can go through many generations in one season, producing millions of offspring. There is, thus, the capacity for multiple, sequential mutations to occur, and a good chance that some of these will be resistant to one or more insecticide. The development of resistance requires the presence of the appropriate insecticide to select out the resistant strain (by killing off the susceptible ones) with the means of detoxifying the chemical or excluding it from absorption. Only 3 years after the introduction of DDT, houseflies and mosquitoes were showing signs of resistance and, by 1951, DDT, methoxychlor, chlordane heptachlor, and benzene hexachloride (of which lindane is the active isomer) no longer had any effect on houseflies, which proliferated abundantly. By the end of 1980, 428 species of insects and acarines (mites, ticks) were classified as resistant.
In the pre-DDT era, relatively few species developed resistance. This has been attributed to the multisite mechanisms of action of earlier pesticides (making single mutation resistance unlikely) and to their ionic nature, making detoxification by metabolism nearly impossible.
Closely related insecticides may be detoxified by the same mechanism and generally act at the same target site, so that if resistance evolves to one, either by the evolution of a detoxification process or by modification of the target molecules, cross-resistance to the others will occur. This type of resistance tends to be under the control of a single gene allele or closely linked genes. Cross-resistance to DDT and methoxychlor, aldrin, and heptachlor has developed in this manner. One study reported that 216 weeds in 45 countries were resistant to a variety of herbicides including the old 2,4-D and the newer glyphosate. This problem has led to the use of combinations of herbicides to make it less likely that adaptation to them all can develop. This is much like the use of combinations of antibiotics in an effort to thwart the emergence of resistant strains of bacteria.
Multiple Pesticide Resistance
In common with bacteria, protozoa, and cancer cells, insects can develop resistance to several insecticides. This is referred to as multiple resistance. It is the result of the existence of several, independent gene alleles producing resistance to unrelated agents (e.g., organochlorines and synthetic pyrethrins, called pyrethroids) with different modes of action and different detoxification pathways. It can be a very serious problem of insect control. The mechanism of multiple resistance is obviously quite different from that of bacteria, which is discussed in Chapter 8.
Multiple resistance to herbicides in weeds is also becoming a problem. Like the development of resistance in other organisms, from bacteria to rats, the greater the degree of exposure of the target organism to the pesticide, the greater the likelihood that a resistant mutation will be selected out. Although motivated more by concerns about adverse health effects, the ban or regulation of the use of pesticides for cosmetic purposes should help slow the emergence of resistant strains.
Nonspecificity
Broad-spectrum pesticides, as most are, make no distinction between true pests and species that are harmless or even beneficial. They, therefore, may disrupt the natural competition among species to permit the proliferation of one previously held in check or, as has been observed, they may kill off predator species and permit the expansion of a prey species, which then becomes a pest. This has happened with spider mites.
The problem of emerging resistance has actually been exploited in recent years to address the nonspecificity of herbicides. By developing genetically modified crops with resistance to a specific herbicide it allows the use of that herbicide for weed control in that crop. This is most commonly done with glyphosate (Roundup). Canola, in 1995, was the first crop plant to be genetically modified in this way but others have followed including corn. The approach is not without its own problems, however. The proliferation of naturally resistant strains and the emergence of resistance in previously susceptible ones have limited this usefulness of this approach. Recently, the U.S. Department of Agriculture decided not to allow the growing of glyphosate-resistant sugar beet and wheat because of these factors.
Environmental Contamination
A greater danger than direct toxicity to humankind is probably the contamination of the environment and the subsequent bioaccumulation and biomagnification which occur with persistent pesticides. These chemicals may end up in soil, water, air or all three, depending on their characteristics (see Chapters 3 and 5). The Great Lakes are accumulating hazardous chemicals as a result of agricultural runoff and industrial discharges, frequently accidental ones. It must be stressed, however, that the greatest source of chemical contamination is residential sewage. Even after treatment, phosphates and other household chemicals may enter the water system. The water table in many areas has been contaminated with the herbicide atrazine, commonly used in corn fields.
Callous disregard for the environment can be the result of greedy individuals attempting to increase their profit margin. There have been numerous cases in Toronto of trucks dumping toxic wastes in Lake Ontario after dark, in violation of provincial and municipal laws. In the United States, careless dumping of chlordecone in the James River by a chemical company resulted in a ban on fishing. This cyclodiene is used in ant and roach baits.
There is no doubt that persistent poisons can have a catastrophic effect on the environment and this is probably the most compelling argument for limiting their use. In 1996, over 4000 Swainson’s hawks were found dead in a 50 km2 area in Argentina. The deaths were attributed to the spraying of crops to kill grasshoppers. The hawks follow tractors that stir up the hoppers and the hawks then feast on them. Ecologists feared that losses may reach 20,000 birds out of a world population of about 400,000.
Balancing the Risks and Benefits
The widespread use of pesticides means that there are trace quantities present in or on almost all foodstuffs. Major advances in analytical techniques over the last 30 years mean that chemicals can now be detected at levels never before possible. Headlines proclaiming that dioxins (or PCBs, etc.) have been detected in Lake Erie fish seldom go on to say that the quantities were at the parts-per-billion level. The detection of dioxins in milk, leached from the carton paper, is an example of this. Actual levels were comparable to a drop in an olympic-sized swimming pool.
Nonetheless, there is a growing feeling in the public that the use of pesticides should be greatly curtailed because the risks are unacceptable or, what is almost as bad, unconfirmed. In recognition of this, the Ontario Ministry of Agriculture and Food established in 1983 the Food Systems 2002 program that attempted to reduce the use of pesticides in agriculture in Ontario by 50% by the year 2002. In 2008, the Ontario Ministry of Agriculture, Food, and Rural Affairs looked at the impact of the program on pesticide use in agriculture from 1983 to 2008. Pesticide use, excluding greenhouse of pesticides and growth regulators, was, overall, 40% lower in 2008 than in 1983. It was, however, higher than in 2003 when a 54% reduction in use was observed. The greatest reductions occurred in the use on corn and tobacco but it should be noted that tobacco farming had greatly declined in the intervening years. Pesticide use on fruits and vegetables fell by only 5% but the health risk fell much more, 23%, due to the use of lower risk pesticides.
The impact of pests on food production is so great that temporary approval is sometimes granted to new agents before all of the required tests have been completed. In 1977 it was discovered that an American testing company, Industrial Bio-Test Laboratories, had misrepresented toxicological data on numerous agents. Of the 405 pesticides registered in Canada, 106 had been approved partly on the basis of Bio-Test data. In 1983 Health and Welfare Canada announced that five of these were to be withdrawn, one being the fungicide Captan, which was found to be teratogenic and carcinogenic.
Other pesticides that have been banned or which are under investigation include the following:
Chlordane. This cyclodiene was withdrawn in 1986 because it was considered to be an epigenetic tumor promoter. It is still registered for use against termites.
Alachlor. This herbicide was withdrawn in 1985 because of evidence of carcinogenicity in animals. It was introduced in 1969 and widely used on corn and soybean crops.
Cyhexatin. Dow chemical voluntarily withdrew this insecticide because of evidence of teratogenicity (hydrocephaly) in rabbits.
All of these agents may still be being used in other parts of the world, legally or otherwise.
Toxicity of Pesticides for Humans
Pesticide applicators are at risk primarily from inhalation and dermal contact with pesticides. Protective clothing and equipment are important means of reducing risk. Nonoccupational poisonings occur largely from oral ingestion of contaminated food although dermal exposure has resulted in poisonings in infants (the pentachlorophenol treatment of hospital linens) and inhalation exposure from spray drift can occur. Household pesticides can cause poisoning by all three routes.
There have been a few isolated fatalities in North America from acute pesticide poisoning, but elsewhere in the world, many cases of mass poisonings have occurred. Consumption of seed grains treated with hexachlorobenzene and organic mercury has resulted in mass outbreaks of poisoning in Turkey, West Pakistan, Iraq, and Guatemala. Accidental contamination of foodstuffs like flour, sugar, and grain with parathion and other agents has occurred in several places around the world.
Effects of long-term exposure to very low levels of pesticides on human health remain conjectural but evidence is accumulating that they exist. In one study in Great Britain, a battery of neuropsychological performance tests was administered to two groups of males, 16–65 years of age. One was a group of 146 sheep farmers exposed to organophosphates in sheep dip over several years. The other was a group of matched quarry workers not exposed to organophosphates. The farmers performed significantly worse on tests of short-term memory and cognitive function. The tests included simple reaction time, symbol digit substitution, digit span, serial word learning, and others. There is also the possibility that contaminants may emerge as a greater risk than the pesticide itself, as was the case with TCDD.
One epidemiological study reviewed several reports of human exposures and found an association with cancers of the blood, neurotoxicity (e.g., Parkinson-like symptoms), and behavioral and reproductive problems. There have been indications that agricultural workers have an increased incidence of brain tumors. The U. S. National Institute of Occupational Safety and Health (NIOSH) is conducting a long-term study on brain cancer incidence and its association with a selected group of 134 pesticides of the 600 or so currently in use. One recent study found that farmers and farm workers who spent 55 years or more on the farm had an increased incidence of the brain tumor glioma, especially in association with the use of paraquat, bufencarb, and chlorpyrifos. A modest association between pesticide use and breast cancer incidence has also been shown.
Epidemiological studies linking cancers to pesticide exposures, and reviews of such studies, continue to appear regularly in the scientific literature. Cancers so linked include testicular cancer, breast cancer, leukemia, non-Hodgkin’s lymphoma, prostate cancer, and most likely others. Most of these studies are vulnerable to criticism on several grounds including small group sizes, exposure to more than one pesticide, and the presence of confounding factors such as exposure to known carcinogens such as PCBs, PBBs, and dioxins. Indeed it seems probable that we live in a milieu of carcinogens and trying to identify a single one or even a group of them as a causative agent for a cancer type is nearly impossible. Nevertheless, there is a sufficiently high index of suspicion to warrant reducing pesticide use where possible and continuing to study the situation.
There is some hope that nature may be developing her own protective processes against pesticides. There is some new evidence that fields sprayed with the same chemicals year after year may develop a population of bacteria that break down the pesticides and may even adapt to the point that they utilize them as a food source. Tests on prairie soils indicated that the organism Rhodococcus breaks down thiocarbamate insecticides in the test tube within 2 h. It may be possible through gene splicing to develop plants that protect themselves against pesticide residues.
Case Study 18
A 43 year old male crop duster was admitted to the emergency department of a rural hospital following an accident in which the aircraft he was attempting to land on a grass strip hit hard, collapsed the undercarriage, and nosed over. The pilot suffered numerous lacerations and bruises but no serious injuries. He was restless and incoherent and he had to be physically restrained. A rapid breath alcohol test was performed and it was negative. His pupils were constricted, his heart rate was slowed and he was sweating profusely. His ground assistant volunteered the information that the pilot had complained of visual disturbances on several occasions during the previous few days. His clothing smelled strongly of a chemical.
Q. Some of these symptoms could be due to a head injury or to chemical intoxication. What facts point to the latter?
Q. What information would you want to seek from his assistant?
The ground crew revealed that the pilot had been spraying crops with parathion during the preceding 2 weeks.
Q. What class of pesticide is this?
Q. What drugs would be indicated for treatment?
Q. How would treatment differ if a carbamate insecticide such as Sevin had been used?
Q. What blood test might assist in confirming the diagnosis?
Case Study 19
During the months of June and August of 1993, 26 men, 19–72 years of age, were admitted to three different local hospitals with an array of symptoms that included nausea, vomiting, dizziness, visual disturbances, muscle weakness, abdominal pain, headache, sweating, and excessive salivation. The men all worked in apple orchards, 19 different ones in all.
Q. What do these symptoms suggest?
Q. What inquiries would you want to make of these men?
Q. What inquiries would you want to make of the orchard operators?
Review Questions
- For Questions 1–8, use the following code:
Answer A if statements a, b, and c are correct.
Answer B if statements a and c are correct.
Answer C if statements b and d are correct.
Answer D if only statement d is correct.
Answer C if all statements (a, b, c, d) are correct.
- Which of the following statements is/are true?
- Lindane is commonly used for the control of head lice.
- Toxicological testing is usually performed only on the active ingredient of an insecticide.
- Lindane is an organochlorine.
- A cause-and-effect relationship for progressive chronic disease resulting from prolonged pesticide use is well established.
- Which of the following is/are true?
- The herbicide “atrazine” has contaminated the water table in many areas.
- Eggshell strength is adversely affected by contact with pyrethroids by the female bird.
- The fungicide “Captan” is a teratogen and carcinogen in experimental animals.
- Natural pesticides are always safer than synthetic ones.
- The main reason for the carcinogenicity in animals of the herbicides 2,4,-D and 2,4,5,-T is
- Their action as growth hormones
- Their mutagenicity
- Their photosensitizing properties
- The presence of dioxin (TCDD) as a contaminant
- The mechanism of TCDD carcinogenicity in humans may involve
- Its pleiotropic response to the Ah locus
- Its conjugation with glutathione
- Its lack of gene restriction
- Its ability to cause chloracne
- The insecticide parathion
- Is an organochlorine
- Is biotransformed by a mixed function oxidase to its toxic form
- Biomagnifies in the environment
- Causes symptoms that can be treated with atropine
- Which of the following statements concerning DDT is/are incorrect?
- It has a low acute LD50.
- It is very persistent in the environment.
- It is very water soluble.
- It is still used for mosquito control in many places.
- Which of the following statements is/are true?
- Organophosphates are very persistent in the environment.
- Organophosphates are inhibitors of acetylcholinesterase.
- Carbamate insecticide poisoning can be treated with pralidoxime.
- Carbamate insecticides act as reversible inhibitors of acetylcholinesterase.
- Which of the following statements is/are true about insect resistance to insecticides?
- Over 400 species of insects, mites, etc., showed resistance to pesticides by 1980.
- Resistance to preorganic insecticides is especially prevalent.
- Cross-resistance to closely related chemicals may occur.
- Resistance is more likely to occur if an insecticide has several sites of action.
Answers
- A
- B
- D
- A
- C
- C
- C
- B
Further Reading
Alavanja, M.C. and Bonner, M.R., Occupational exposures and cancer risk: A review, J. Toxicol. Environ. Health B Crit. Rev., 15, 238–263, 2012.
Arbuckle, T.E. and Sever, L.E., Pesticide exposures and fetal death: A review of the scientific literature, Crit. Rev. Toxicol., 28, 229–270, 1998.
Baldi, M., Mohammed-Brahim, B., Brochard, P., Dartigue, J.F., and Salamon, R., Delayed health effects of pesticides: Review of current epidemiological knowledge, Rev. Epidemiol. Sante Publique, 46, 134–142, 1998.
Boada, L.D., Zumbado, M., Henriquez-Hernandez, L.A., Almeida-Gonzalez M., Alverez-Leon, E.E., Serra-Majem, L., and Luzardo, O.P., Complex organochlorine mixtures as determinant factor for breast cancer risk: A population-based case-control study in the Canary Islands (Spain), Environ. Health, 11, 28 (Epub ahead of print), 2012.
Carson, R., Silent Spring, Fawcett Crest Books, New York, 1962.
Clapp, R.W., Jacobs, M.M., and Loechler, E.L., Environmental and occupational causes of cancer: New evidence 2005–2007, Rev. Environ. Health, 23, 1–37, 2008.
Cory-Slechta, D.A., Studying toxicants as single chemicals: Does this strategy adequately identify neurotoxic risks? Neurotoxicology, 26, 491–510, 2005.
Daniels, J.L., Olshan, A.F., and Savitz, D.A., Pesticides and childhood cancers, Environ. Health Perspect, 105, 1068–1077, 1997.
Duke, S.O., Taking stock of herbicide-resistant crops ten years after introduction, Pest Manage. Sci., 61, 211–218, 2005.
Flanders, R.V., Potential for biological control in urban environments, In Advances in Urban Pest Management, Bennett, G.W. and Owens, J.M. (eds.), Van Nostrand Reinhold, New York, 1986, pp. 95–129.
Georghiou, G.P. and Saito, T. (eds.), Pest Resistance to Pesticides, Plenum Press, New York, 1983.
Hall, S.H. and Dull, B.J., Comparison of the carcinogenic risks of naturally occurring and adventitious substances in food. In Food Toxicology: A Perspective on the Relative Risks, Taylor, S.L. and Scanlan, R.A. (eds.), Marcel Dekker, New York, 1989, pp. 205–224.
Klassen, C.D. (ed.), Casarett and Doull’s Toxicology: The Basic Science of Poisons, 7th edn., McGraw-Hill Medical, New York, 2008.
Klassen, C.D. and Watkins, J.B. III (eds.), Casarett and Doull’s Essentials of Toxicology, McGraw-Hill Medical, New York, 2010.
Lee, W.J., Colt, J.S., Heineman, E.F., McComb, R., Weisenburger, D.D., Lijinsky, W., and Ward, M.H., Agricultural use and risk of glioma in Nebraska, United States, Occup. Environ. Med., 62, 786–792, 2005.
McEwen, F.L. and Stephenson, G.R., The Use and Significance of Pesticides in the Environment, Wiley Interscience Publication, New York, 1979.
McGlynn, K.A. and Trabert, B., Adolescent and adult risk factors for testicular cancer, Nat. Rev. Urol., 9, 336–349, 2012.
Muir, K., Rattanamongkolgul, S., Smallman-Raynor, M., Thomas, M., Downer, S., and Jenkinson, C., Breast cancer incidence and its possible spatial association with pesticide application in two counties of England, Public Health, 118, 513–520, 2004.
Nelson, N.J., Studies examine whether persistant organic agents may be responsible for rise in lymphoma rates, J. Nat. Cancer Inst., 97, 1490–1491, 2005.
Ontario Ministry of Agriculture, Food and Rural Affairs, Evaluation of the changes in pesticide risk- executive summary, 2008, http://www.omafra.gov.on.ca/english/crops/facts/pesticide- use-exec.htm (accessed on May 16, 2012).
Owen, S.O. and Zelaya, I.A., Herbicide-resistant crops and weed resistance to herbicides, Pest Manage. Sci., 61, 301–311, 2005.
Palca, J., Libya gets unwelcome visitor from the west, Science, 249, 117–118, 1990.
Ribbens, P.H., Mortality study of industrial workers exposed to aldrin, dieldrin and endrin, Int. Arch. Occup. Environ. Health, 56, 75–79, 1985.
Sanderson, W.T., Talaska, G., Zaebst, D., Davis-King, K., and Colvert, G., Pesticide prioritization for a brain cancer case-control study, Environ. Res., 74, 133–144, 1997.
Sankpal, U.T., Pius, H., Khan, M., Shukoor, M.I., Maliakal, P., Lee, C.M., Abdelrahim, M., Connelly, S.F., and Basha, R., Environmental factors in causing human cancers: Emphasis on tumorigenesis, Tumour Biol., 33, 1265–1274, 2012.
Stephens, R., Spurgeon, A., Calvert, I.A., Beach, J., Levy, L.S., Berry, H., and Harrington, J.M., Neuropsychological effects of long-term exposure to organophosphates in sheep dip, Lancet, 345, 1135–39, 1995.