Chapter 5
Halogenated Hydrocarbons and Halogenated Aromatic Hydrocarbons
Introduction
Halogens are the related elements chlorine (Cl), bromine (Br), fluorine (F), and iodine (I). They may exist as gases (Cl2, F2), a liquid (Br2), or a solid (I2). Halogenated hydrocarbons, also known as organohalogens, are a group of organic compounds of diverse structure to which one of these halogens has been attached. The core structure may be either simple, consisting of one or two carbons, or it may be a more complex aromatic one (halogenated aromatic hydrocarbons or HAHs). Because they have been implicated almost universally in toxic reactions in mammals and lower species (including carcinogenesis in some), it is appropriate to consider them as a group despite their chemical diversity. Polycyclic aromatic hydrocarbons (PAHs) are multiringed, planar chemicals that share many of the same toxicological properties as HAHs.
Early Examples of Toxicity from Halogenated Hydrocarbons
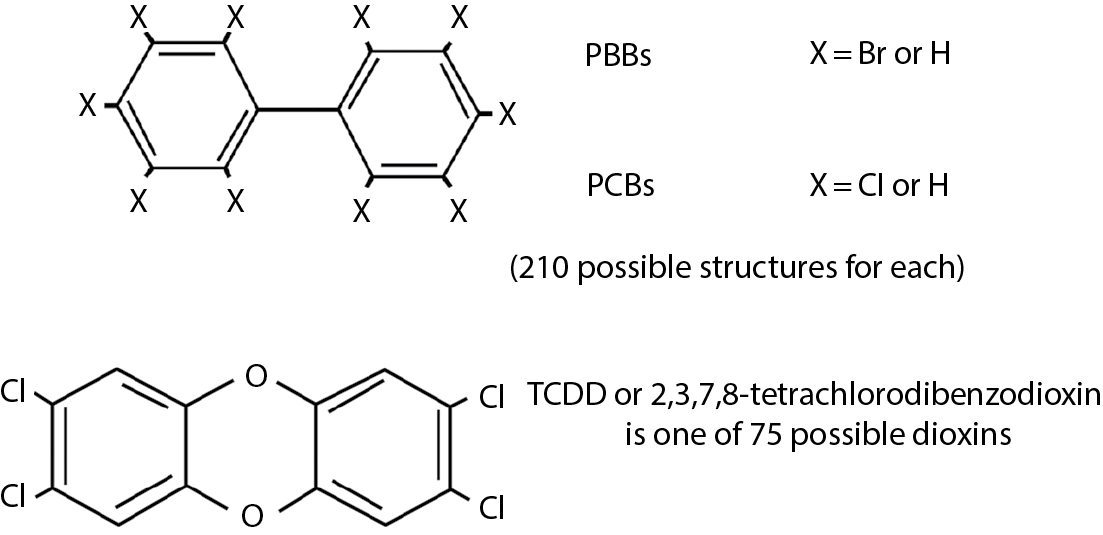
One of the oldest and simplest of these compounds is carbon tetrachloride (CCl4), which was used extensively as a solvent and as a dry-cleaning agent until its hepatotoxic nature was discovered. In fact, it was used originally as a treatment for hookworm in humans and domestic animals and as a component in fire extinguishers. These uses may still exist in some parts of the world. CCl4 owes its toxicity to the fact that it is converted in the liver to carbon trichloride (CCl3), which is a free radical capable of inducing peroxidation of lipid double bonds and of poisoning protein-synthesizing enzymes. Older, halogenated hydrocarbons include trichloroethylene, and the anesthetics halothane and chloroform (now abandoned because of its toxicity). Figure 5.1 shows the variety of structural formulae included in this class of compounds. The earliest form of poisoning associated with a halogen was probably “bromism,” a condition resulting from the use or abuse of sodium or potassium bromide as a sedative and sleeping potion early in this century. Symptoms included severe headache, stupor, delirium, cardiac problems, very bad breath (from the bromine), and an acneform skin rash of the type now called “chloracne.” The expression “bromide” is used now to indicate a soothing but meaningless statement of the sort frequently uttered by certain politicians.
Physicochemical Characteristics and Classes of Halogenated Hydrocarbons
The characteristics of halogenated hydrocarbons that make them useful for a variety of applications are generally the same ones that make them hazardous to the environment and to humans. These include
- High lipid solubility
- Ability to survive heat >800°C
- High resistance to chemical breakdown
- Toxicity to microorganisms
These agents are used for a variety of purposes (see in the following).
Antibacterial Disinfectants
Hexachlorophene has been used for many years as a surgical scrub and, as a 3% solution, as a hospital disinfectant. It is also used as the active ingredient in deodorant soaps. In the late 1960s, a change was proposed in US-FDA regulations to permit the use of hexachlorophene as an antifungal wash for fruit and vegetables.
In light of the then-recent thalidomide tragedy, extensive testing was required for approval to be granted. Rats fed high levels of hexachlorophene developed weakness, ataxia, paralysis, and evidence of a type of brain pathology known as status spongiosus, indicative of axonal degeneration. In 1971, a study was done in which infant monkeys were washed daily for 90 days in 3% hexachlorophene. Neurological symptoms were observed and status spongiosus was seen in all specimens at post mortem. Significant blood levels have been detected in infants washed with 3% hexachlorophene, and those with severe diaper rash, burns, or congenital skin disorders are especially prone to absorb it, as the natural permeability barrier has been disrupted (see Chapter 1). Autopsies of infants dying from a variety of causes and who received high exposures showed evidence of status spongiosus. In 1972, hexachlorophene was accidentally added in high concentration to baby powder during its manufacture in France. Forty-one deaths of infants and young children were attributed to this error. A few years later, Dr. Hildegard Halling, a Swedish physician, published a report indicating that nurses who washed frequently in hexachlorophene (10–60 times daily) had a higher incidence of birth defects in their offspring (25/460 births) than those who did not (nil/233 births). Although this clinical study was criticized for design flaws, others with rats have revealed teratogenic effects. This product is no longer used in nurseries in North America and pregnant women are advised to avoid it.
Herbicides
This group includes 2,4-dichlorophenoxyacetic acid (2,4-D), 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), and dioxins such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD = dioxin). Agent Orange, used as a defoliant in Vietnam, was equal parts of 2,4,-D and 2,4,5,-T and contained TCDD as a contaminant. One of the best-documented human exposures to dioxin was the explosion at Seveso, Italy (see Chapter 2). Hundreds of individuals suffered from chloracne, which is the hallmark of toxicity of halogenated hydrocarbons.
The herbicides 2,4,-D and 2,4,5-T are used to control broad-leafed plants along highways, railways, and utility rights-of-way. They are hormonal growth promoters and force plants to consume energy at a greater rate than it can be replaced. The humans at greatest risk of toxic exposure are the workers who apply the sprays, and poisoning from dermal and respiratory absorption has occurred as well as from accidental ingestion. Signs and symptoms include peripheral neuritis, muscular weakness, and chloracne. Although 2,4,5-T is a weak teratogen in some animals, it is the presence of TCDD, or dioxin, that is the greatest source of public concern.
Dioxin (TCDD) Toxicity
Dioxins are a family of compounds of which TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) has received the most public attention. There is significant species variation in TCDD toxicity with the guinea pig being most sensitive (LD50 1 μg/kg) and the rat quite insensitive (LD50 22 μg/kg). There are other manifestations of dioxin toxicity.
Hepatotoxicity
All species show enlarged livers and microsomal monooxygenase enzyme induction occurs in most. Rats develop fatty livers with triglyceride deposition. Hepatic fibrosis has been reported in humans. People exposed at Seveso had elevated serum enzyme levels (serum glutamic-oxaloacetic transaminase, SGOT, and serum glutamic-pyruvic transaminase, SGPT), indicating liver damage, for several weeks after the accident.
Porphyria
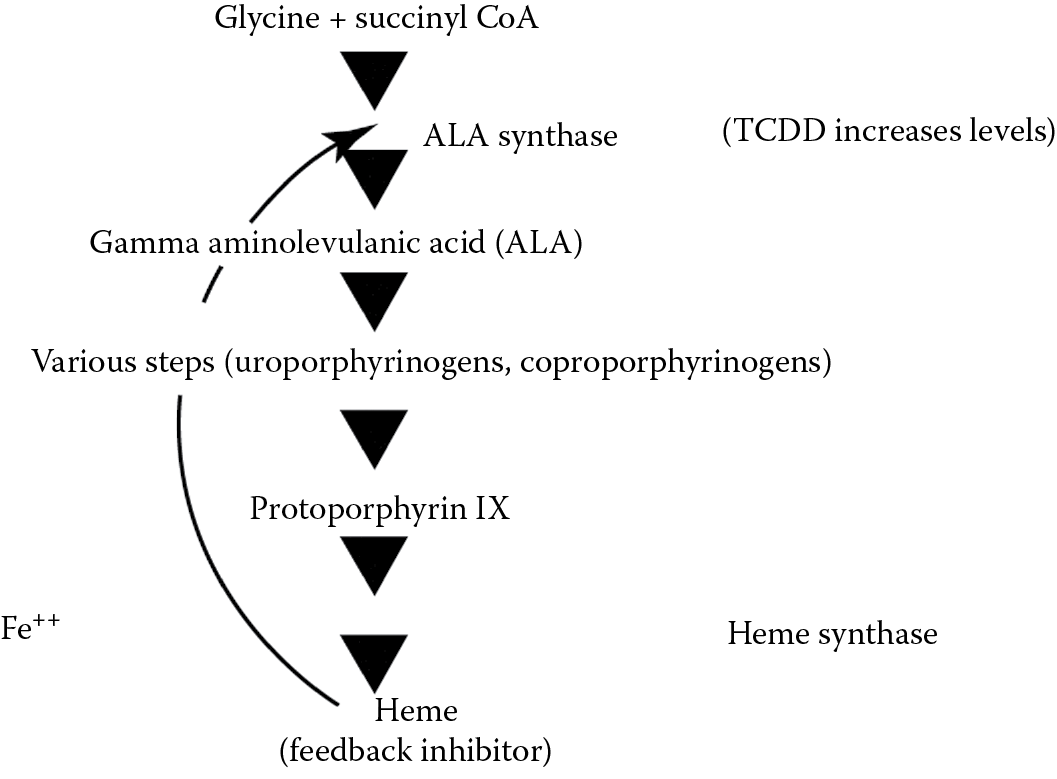
Porphyrins are pigments widely distributed in nature and they are present in the body as by-products of heme synthesis that is required for the formation of hemoglobin, myoglobin, and cytochromes. Heme is ferrous protoporphyrin IX. Hematin, the iron-containing molecule in catalase and peroxidases, is ferric protoporphyrin IX. The rate-limiting step in the synthesis of heme is ALA synthetase (ALA = gamma-aminolevulinic acid). Dioxin significantly increases the levels of ALA synthetase and hence ALA levels and the synthesis of porphyrins. This is not enzyme induction, but probably is due to interference with a feedback control system. The excess porphyrins are excreted in the urine, giving it a port wine color, and they are deposited in the skin, producing pigmentation. Because porphyrins are photoreactive, a condition known as porphyria cutanea tarda develops, which is characterized by photosensitivity, blistering, fragility of the skin, pigmentation, and hirsutism (hairiness). Congenital defects in porphyrin metabolism cause the same syndrome and this has been suggested as the explanation for the vampire and werewolf myths of Europe (characterized by hirsutism and the avoidance of sunlight). There is even an explanation of why drinking blood might have a therapeutic effect. Heme is the feedback substance that turns off ALA synthetase. Absorption of sufficient heme might thus inhibit porphyrin synthesis. In the late 1950s an extensive outbreak of porphyria cutanea tarda occurred in Turkey during a famine as the result of consuming seed grain treated with hexachlorobenzene as an antifungal agent. A simplified scheme of the steps involved in porphyrin synthesis is shown in Figure 5.2.
Chloracne
This skin disorder is typified by rash, cysts, and hyperpigmentation and this is the hallmark of poisoning with all halogenated hydrocarbons. It was the predominant toxic manifestation at Seveso.
Cardiovascular effects—A 10 year mortality study of the population exposed to TCDD after the Seveso explosion of 1976 revealed a significantly increased mortality from cardiovascular events.
Carcinogenicity
Dioxin is a potent hepatocarcinogen in mice and to a lesser extent in rats. There is a latency period before the emergence of the liver tumors. Evidence of cancer in several studies of Vietnam veterans, for whom claims of increased incidence of cancer have been made, has been inconclusive. A retrospective cohort study was conducted by scientists at the (U.S.) National Institute for Occupational Safety and Health (NIOSH) on 5172 workers at 12 U.S. plants in which TCDD was a chemical contaminant of the manufacturing process. Exposure was well documented and serum TCDD levels were obtained from 253 workers. The mortalities from several cancers previously associated with TCDD (stomach, liver, and nasal cancers, Hodgkin’s disease and non-Hodgkin’s lymphoma) were not significantly different from the overall population but the incidence of all cancers taken together was slightly but significantly increased. In a subcohort of 1520 workers with more than 1 year of exposure and more than 20 years of latency, mortality from soft tissue sarcoma was significantly higher than for the general population. The authors concluded that the results were not suggestive of the high relative risks of cancer reported for TCDD in previous studies. The slight risk of increased soft tissue sarcoma is weakened by the small numbers involved (only three cases) and confounding factors such as smoking and exposure to other chemicals.
In another epidemiologic study of 754 Monsanto employees exposed to high levels of TCDD in a 1949 accident, 122 of whom developed chloracne, there was no increased incidence of cancer in those who developed chloracne, although they were presumably the group with the highest exposure. Conversely, workers who were also potentially exposed to 4-aminobiphenyl, a potent bladder carcinogen, had increased mortality from bladder cancer, lung cancer, and soft tissue sarcoma. This suggests that TCDD might act as a co-carcinogen or promoter. Again, the effects of confounders such as smoking and exposure to other chemicals could not be ruled out, but recent experimental evidence supports the suspicion that TCDD could act in this way.
Walsh et al. studied the cell toxicity of aflatoxins in cultured human epidermal cells. AFB1 was markedly toxic at 1 μg/mL. Neither AFB2 nor AFB1 dihydrodiol was toxic. TCDD alone was not toxic to the cells but at 5 nM, it dramatically stimulated AFB1 toxicity at levels as low as 0.1 μg/mL. It also increased the formation of AFB1 epoxides and a 20-fold increase in DNA adduct formation was observed. AFB1 is the most carcinogenic of the aflatoxins (see Chapter 10).
The most recent report from Seveso (see Chapter 2 regarding this industrial accident) is suggestive of a carcinogenic effect in humans. It re-examined the data in 1996 and confirmed an excess risk of lymphatic and hematopoietic tissue neoplasms in the most exposed zones (A and B). The exposed population was divided into three groups according to their likely level of exposure, zone A being nearest the explosion and zone C furthest away. Unlike earlier studies, the 1996 one also found an increased risk of breast cancer in zone A and noted a need for further monitoring of this effect.
Weaknesses of this study include the inability to control for confounding factors such as smoking, and lack of hard data regarding real exposure levels. Despite the conflicting results of several epidemiological studies, most authorities now agree that there is a high index of suspicion for TCDD carcinogenicity in humans, especially for non-Hodgkin’s lymphoma. A very recent (2011) critical review of epidemiologic cancer studies of TCDD determined that evidence for carcinogenicity was inconclusive and that the practice of comparing all-cancer incidences was not justified epidemiologically. The definitive word, however, remains to be heard, and the existing evidence comes from high industrial and occupational exposures that may not be relevant to environmental exposures encountered by the general population. If a threshold truly exists because of the TCDD/Ah receptor story, which follows, environmental exposures may never reach that threshold.
The mechanism of carcinogenesis in animals appears to be epigenetic. Recent evidence indicates that TCDD binds to a specific receptor, the Ah (for aryl or aromatic hydrocarbon) receptor and that a minimum number of Ah receptors must be occupied for TCDD to exert its effect (see the following). The implication of this is that there is, thus, a threshold dose and that the linear multistage carcinogenesis model is inappropriate for TCDD and for any other agent that works by this mechanism, such as PCDDs and PCDFs (see Chapter 3 and the following). The EPA is now reconsidering the use of the linear multistage model at least for TCDD and perhaps for a few other agents.
Neurotoxicity
Many toxic effects have been observed including impaired vision, hearing, and smell; depression; sleep disturbances; and others. These were observed in workers exposed to TCDD in the 1949 Monsanto accident.
Reproductive Toxicity
Testicular atrophy, necrosis, and decreased spermatogenesis have been seen in laboratory animals.
Metabolic Disturbances
Weight loss and depletion of adipose tissue occur in laboratory animals.
Role of the Aryl Hydrocarbon Receptor (AhR) and Enzyme Induction
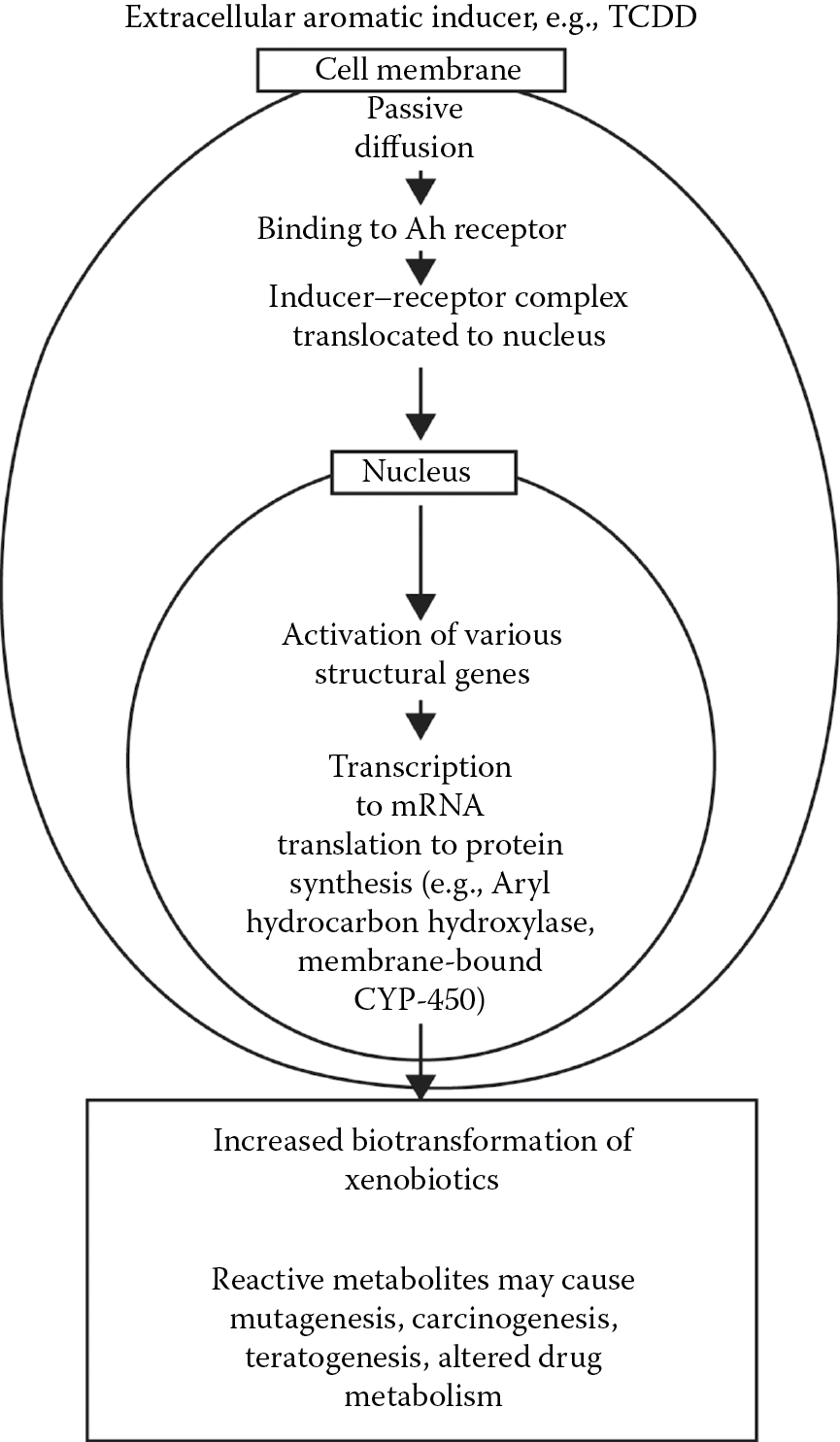
TCDD is one of the most potent inducers of aryl hydrocarbon hydroxylase (AHH) so far discovered. In some species it is effective at 1 μg/kg. It induces synthesis of hepatic microsomal cytochrome P450 (CYP1A1 and possibly 1B1). TCDD uptake into the cell is passive (i.e., concentration gradient-dependent). Intracellularly, it binds to the aromatic hydrocarbon cytosolic receptor (AhR), which is the product of a regulatory gene. The unliganded, aryl hydrocarbon receptor complex (AhRC) contains the aryl hydrocarbon receptor (AhR). Binding of an Ah ligand causes release of the AhR that is translocated to the nucleus by a translocator protein. The unliganded AhRC is heteromeric, but after binding to a HAH or a PAH the AhR is released and associates with the AhR nuclear translocator protein to form heterodimeric, transformed AhRC. Ligands for the AhR are typically hydrophobic aromatic compounds including the HAHs dibenzo-p-dioxins, dibenzofurans, biphenyls, and PAHs such as benzo[a]pyrene and many other benzo derivatives (products of combustion), naphthalene, naphthacene, and many others (see http://chrom.tutms.tut.ac.jp//JINNO/DATABASE/00alphabet.html).
In the nucleus, AhRC activates numerous, xenobiotic-responsive, structural genes. The information is transcribed to m-RNA and translated to protein synthesis and the production of cytochrome P-450 (1A1, 1A2, 1B1). This is known as a pleiotropic response, i.e., it results in more than one phenotypic effect. TCDD is the most potent known inducer of AHH. The consequence of this induction is that several drug (xenobiotic)-metabolizing enzymes are induced, and reactive metabolites may be formed, which react with proteins and nucleic acids to cause mutations, teratogenesis, and carcinogenesis, as well as altered drug metabolism. Conversely, the reactive metabolites may be excreted or detoxified, as by conjugation with glucuronide. A schematic representation of this pathway is shown in Figure 5.3. This system has been most studied in the mouse, where it is inherited as an autosomal dominant pattern. Similar systems have been identified in the rat, rabbit, and some fish. It is most heavily concentrated in the liver. In humans, there is considerable variation in the Ah locus. To date, no endogenous substrate has been identified for the Ah receptor. AhR “knockout” mice, however, have small, fibrosed livers. In the absence of an exogenous ligand, AhR has been found in the nucleus of cultured Hela cells, behaving as if they were ligand-bound. It seems apparent that the AhR has an important physiological role. It is known that primitive species (bacteria, yeasts) utilize polycyclic hydrocarbons as an energy source and possess P450 metabolizing enzymes for them (camphor in Pseudomonas, benzo-[a]-pyrene in yeast), so these may have evolved as a detoxication system. The mechanism of TCDD toxicity is not known, but if there is a natural substrate for Ah receptors, its displacement by TCDD could be involved in the latter’s toxicity. It is not clear whether TCDD itself or its metabolite(s) are responsible.
TCDD also has nonreceptor-mediated effects, including interference with calcium homeostasis and a variety of membrane-related changes. It is interesting that the chloracne associated with TCDD also occurs with bromides, which could not act as Ah ligands. For more on TCDD toxicity see Chapter 12.
Paraquat
This PAH herbicide (1,1′-dimethyl-4,4′-bipyridinium ion) is highly water soluble, therefore poorly absorbed across the skin or gastrointestinal mucosa. It is extremely toxic to humans when inhaled, however, and 5 g may be fatal. Pulmonary congestion, edema, and hemorrhage may result in almost complete functional destruction of the lung. In severe cases, lung transplantation has been tried as a last resort, with disappointing results. Although paraquat is poorly absorbed from the oral route, its highly toxic nature can result in lung toxicity days or weeks later. It is, thus, one of the “hit-and-run” class of toxicants. Liver and kidney damage and neurological damage also occur (see Chapter 9 on pesticides).
Insecticides
Chemical insecticides are used to increase food crop production, to protect livestock and household pets against insect pests (warble fly, botfly, screwworm, fleas), to control disease-carrying insects (anopheles mosquitoes that carry malaria), and to control destructive insects like termites. It was a chlorinated hydrocarbon insecticide DDT (dichlorodiphenyltrichloroethane) that first raised concerns over the impact of pesticides on the environment. In 1961, the author Rachel Carson brought out her book Silent Spring in which she documented the devastating effect that this chemical had on bird life because it weakened eggshells so that the eggs collapsed in the nest or the chicks were abnormal at hatching. The persistence of the chemical (it and its metabolite DDE have a biological T1/2 of 50 years) and its high lipid solubility result in its biomagnification up the food chain.
Predatory and fish-eating birds are especially vulnerable to DDT. The product was banned in the United States and Canada in 1972, but it is still used elsewhere in the world, including Mexico, and trace levels may be present in imported products. Levels of from 1 part/billion (ppb) to 1 part/million (ppm) may be present in fish, oysters, and other seafoods and can contribute to human tissue levels of up to 10 ppm.
Halogenated hydrocarbon insecticides (chlorinated hydrocarbons) are principally neurotoxic, interfering with axonal transmission by altering sodium and potassium transport across the axonal membrane to prevent normal depolarization. Evidence of carcinogenicity also has been obtained in animal experiments (see Chapter 9 on pesticides for more details).
Industrial and Commercial Chemicals
Biphenyls
Polybrominated biphenyls (PBBs) are used as fire retardants in thermoplastics for TV and office machine casings. The more familiar polychlorinated biphenyls (PCBs) are highly stable and resistant to degradation in acids, bases, by oxidation and by heat (to 800°C).
These characteristics make them ideal insulators in transformers in the electric power industry and as hydraulic fluid and in brake linings. They are also used as plasticizers in polymer films. The same characteristics, however, make these agents very persistent in the environment. Exposure to these compounds is largely an occupational hazard, but exposure in the environment can occur as a result of contamination of groundwater from spills, improper storage of waste PCBs from old transformers and capacitors, or from fires in storage sites. Although the manufacture of PCBs was banned in the United States in 1977, they remain a problem because of their persistence and resistance to destruction. Forty percent of North Americans have body fat levels of 1 ppm or higher (these agents have high lipid solubility). Prior to 1970, 500,000 ton of PCBs were produced in North America.
Toxicity
- Animal. The LD50 in rats may be 1–10 g/kg. Chronic toxicity involves skin lesions, hepatotoxicity, immunosuppression, and reproductive dysfunctions. Carcinogenicity has also been reported. Recently, genetic damage in cetaceans (whales, dolphins) and seals in the Baltic Sea has been ascribed to high levels of PCBs.
- Human. Characteristic chloracne, impaired immune response, liver damage, gastrointestinal disturbances (nausea, vomiting, loss of appetite), CNS disturbances (weakness, ataxia), as well as reproductive problems and cancer have been associated with exposures or induced in experimental animals.
Pharmacokinetics and Metabolism
Because of the high lipid solubility, these compounds are well absorbed from the gastrointestinal tract (>90%) and stored in body fat. They are secreted in milk (toxicity has been shown in nursing mice and rats) and they constitute a hazard for the nursing infant (see study of Michigan mothers in Chapter 3). They have been shown to induce cytochrome P448 through the Ah receptor pathway and to form reactive intermediates and to deplete glutathione. Conjugation with glucuronide and renal excretion are the final detoxification mechanisms.
Biodegradation
Recent studies indicate that solar photolysis (near-UV light) can reduce the T1/2 in surface water to 1–2 years by dechlorination of PCBs but this has no effect on bottom sediments that may contain high levels. Fortunately, chemical dechlorination can occur here, and, recently, subsequent oxidation by anaerobic bacteria has been discovered in sediments of the Hudson River. These discoveries offer some hope that biodegradation of PCBs may occur at a faster rate than anticipated.
Accidental Human Exposures
In 1968, nearly 1700 people in the Fukuoka region of Japan developed chloracne as a result of using rice cooking oil contaminated with PCBs. The PCBs in turn were contaminated with tetrachlorodibenzofuran that is structurally and toxicologically similar to TCDD. These “Yusho” patients (Yusho means “oil disease”) constitute the largest human population known to be exposed to toxic levels of PCBs and their health continues to be monitored closely to identify delayed effects. Five years later, 22 deaths were reported in 1200 of these patients, 9 from cancer, 2 involving the liver. Calculated body burdens were 5.9 μg/kg for chloracne and 4.4 μg/kg for nausea and anorexia. These are 200 times higher than average current levels found in North American populations. Similar mortality statistics occurred after an outbreak in Taiwan. In neither case were the cancer deaths considered excessive, and they were not age adjusted (see also Chapter 3). Several cases of contamination of animal feeds have occurred. In North Carolina, a leaky heat exchanger contaminated 16,000 ton of chicken feed, only 10% of which was recovered. No human health problems were directly attributed to the accident, but there was some evidence that children might have been affected in utero (see also Chapter 3).
A major human exposure to PBBs occurred in Michigan in 1973 (the year of Seveso). A PBB product intended for use as a fire retardant and marketed as “Firemaster” was accidentally bagged as “Nutrimaster,” a magnesium supplement for dairy cattle. Whole herds of dairy cattle were afflicted with loss of appetite, open sores, weight loss, lack of milk production, sterility, and stillbirths. It took several months of detective work to trace the source of the problem; meanwhile, human food supplies were contaminated by milk containing the PBBs. Meat and eggs were also contaminated. By 1976 nearly 30,000 of Michigan’s best dairy cattle had died or been destroyed, along with thousands of sheep and hogs and millions of chickens. Thousands of Michiganders had consumed unknown quantities of contaminated food, and hundreds began to complain of headache, fatigue, joint pain and numbness in fingers and toes. Farm families were the most severely afflicted. Little was then known about the human toxicity of PBBs. Long-term effects were as yet unidentified.
In recent years it has become clear that they exist. Studies published in 2011 found that among women who were exposed to PBB in utero because their mothers consumed foods contaminated in the 1973 accidental exposure had a significantly greater risk of spontaneous abortion than women not so exposed. In the midrange exposure group (1–3.16 ppb) and high exposure group (≥3.17) the odds ratio was 2.75. Exposure to PPB contaminated breast milk increased this risk.
There was also some evidence that those women who actually consumed contaminated foods at the time of the accident (or in the early 1970s) had a slightly increased incidence of a positive Pap smear.
Problem of Disposal
Recent evidence from a disposal site in Great Britain suggested that current incineration temperatures might not be adequate as levels of PCBs were detected in the soil around the site. A complicating factor was the presence of a municipal incinerator within 100 m, but some authorities feel that incineration temperatures should reach 2700°C. The PCB fire (deliberately set) at St. Basile le Grand in 1988 highlights the dangers of unsecured storage. Public concerns over PCB hazards have led to the NIMBY response (Not In My Back Yard) and resulted in resistance to the establishment of proper disposal sites and to refusal to allow ships containing toxic wastes to unload. Such ships, wandering the seas in search of a berth, create a potential for a marine environmental disaster, with contamination of food fish that could be far worse than the hazards associated with well-run disposal sites.
Solvents
CCl4, chloroform, and methylene chloride (dichloromethane) are still popular industrial solvents because they are not flammable. Exposure is mainly an industrial problem but these agents may still appear as cleaning fluids in the home. A source of some concern is the presence of trace amounts of chloroform in drinking water as a result of the chlorination process.
Toxicity
These chemicals are hepatotoxic, causing central lobular necrosis with fatty degeneration of adjacent areas. They also can cause renal damage, and chronic exposure has been linked to neoplasms of the lung and liver. Cardiac arrhythmias have been reported, as has nausea and vomiting. The cardiac arrhythmias result from sensitization of the heart to catecholamines such as adrenaline and noradrenaline. Other halogenated anesthetics like cyclopropane and halothane will also do this, and hepatotoxicity has been reported as a toxic effect of halothane in some patients as well as in anesthetists routinely exposed to the drug. Liver toxicity can be increased in mice exposed to inducers of microsomal enzymes, suggesting that a toxic metabolite might be involved.
Mechanism of Toxicity
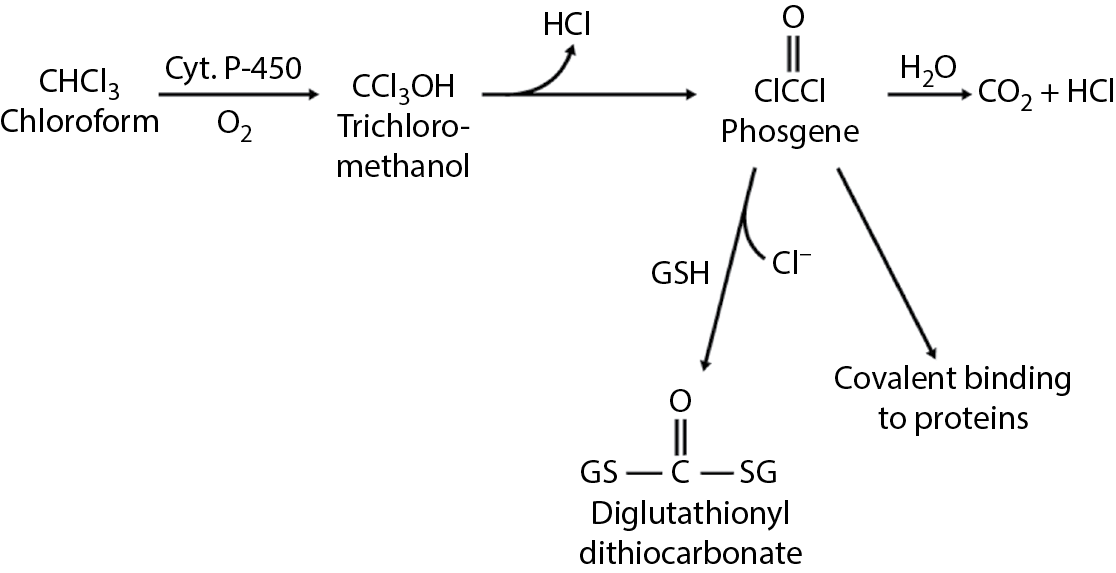
These substances are metabolized by cytochrome P-450 enzymes. Chloroform is converted to chloromethanol, phosgene, and CO2 + HCl. Phosgene is normally converted to glutathione for excretion, but if glutathione is depleted, covalent binding to proteins may lead to liver and kidney necrosis. The metabolic pathway for chloroform is shown in Figure 5.4. Methylene chloride is metabolized to CO2 + CO. Carbon monoxide poisoning through the formation of carboxyhemoglobin can occur. Chloroform is an example of a trihalomethane.
Trihalomethanes
These are halogen-substituted, single-carbon compounds having the general formula CHX3 where X may be chlorine, fluorine, bromine, iodine, or a combination of these. They are formed during the process of water chlorination from naturally occurring organic compounds. The most common agents found in drinking water are chloroform (CHCl3), bromodichloromethane (CHBrCl2), chlorodibromomethane (CHClBr2), and bromoform (CHBr3).
These are liquid at room temperature, rather volatile, and only slightly soluble in water. Their octanol–water partition coefficients range from 1.97 for chloroform to 2.38 for CHBr3. All are sensitive to decomposition in air and sunlight. THMs are also released into the environment from industrial sources. Chloroform is the most common THM found in drinking water. Its major toxicity and metabolic transformation are noted earlier.
As a group, THMs are rapidly and well absorbed from the gastrointestinal tract, metabolized through di-halocarbonyl compounds via the cytochrome P450-dependent mixed function oxidases to CO2 and CO and eliminated through the lungs. Because of their high lipid solubility they accumulate in adipose tissue > brain > kidney > blood.
Several epidemiological studies have shown a correlation between chlorination of surface or groundwater and the incidence of many cancers, but correlations with actual measured levels of THMs have been harder to demonstrate. Exceptions are pancreatic cancer in white males, rectal cancer in males only, and stomach cancer in both sexes. When population migration patterns were considered, however, the correlation with stomach and rectal cancer could not be demonstrated, and other studies have suggested that other water quality parameters may be involved.
In animal studies, chloroform has been shown to be carcinogenic in rats and mice. CHClBr2 has been reported to be hepatotoxic in mice, but no evidence of carcinogenicity was obtained. These agents are probably mutagenic and teratogenic, as indicated in some studies.
In light of these facts, there are efforts directed to limiting the levels of THMs in drinking water. Standards vary widely throughout the world. In Canada, the current maximum standard is 350 μg/L, not to be exceeded. In the United States, the EPA has set a limit of 100 μg/L. This is an average based on quarterly samples, and is therefore more enforceable. The WHO sets a guideline of 30 μg/L, but with a warning that disinfecting efficiency should not be compromised in the pursuit of lower levels. The European Economic Community passed a directive that haloform levels in drinking water should be “as low as possible,” which is unenforceable.
In is thus evident that the problem of THMs in drinking water is another example of how cost–benefit analysis must be performed to weigh the potential risks of cancer from the chemicals against the known risks of epidemic infections if water supplies are not treated.
Case Study 9
Part 1. Three members of a family became dizzy and nauseated within 1 h of eating snacks (taquitos) consisting of tortillas wrapped around a meat filling. Two of them subsequently had grand mal epileptic seizures. The snacks were commercially prepared and sold in sealed bags of 48. They had been purchased a few days earlier. In an unrelated case a few weeks later, a 17-year-old male had four closely spaced seizures 30 min after consuming taquitos from the same manufacturer and purchased from the same store. The boy was on long-term antiepileptic therapy because he had been diagnosed as an epileptic the previous year. Following the initial episode, the manufacturer had voluntarily removed from shop shelves and destroyed all existing packages of the product.
Q. What organ system seems to be the primary site of toxicity?
Q. Was the most likely cause of the reaction bacterial or chemical?
Q. Was the likely site of contamination the factory or the retail store?
Part 2. Analysis of some remaining taquitos from the first case revealed traces of endrin. No source or trace of endrin was found at the factory.
Q. To what class of compound does endrin belong?
Review the toxicity of this class of chemicals.
A statewide press release turned up several other cases of seizures including five persons who had experienced seizures within 12 h of consuming taquitos purchased from the same store.
Q. What preventive or remedial measures might you recommend?
Case Study 10
A maintenance employee in a factory died after acute exposure to solvent fumes. He had been using a mixture of chlorinated solvents to remove grease from machinery. The principal component was trichloroethane (methyl chloroform).
Q. What was the immediate cause of death?
Q. What steps might you suggest to prevent this type accident?
Q. This substance is similar to CCl4. What would have been the nature of the toxic response if the exposure had been chronic rather than acute?
Review Questions
- For Questions 1–6, use the following code:
Answer A if statements a, b, and c are correct.
Answer B if statements a and c are correct.
Answer C if statements b and d are correct.
Answer D if statement c only is correct.
Answer E if all statements (a, b, c, d) are correct.
- Halogenated hydrocarbons are characterized by
- High lipid solubility
- Susceptibility to chemical breakdown
- Toxicity for microorganisms
- Decomposition at temperatures greater than 200°C
- Dioxin (TCDD) toxicity is characterized by
- Chloracne
- Hepatotoxicity
- Porphyria
- None of the above (a, b, c, d)
- Dioxin (TCDD)
- Induces the enzyme gamma-aminolevulinic acid (ALA) synthetase
- Interferes with feedback inhibition of ALA synthetase
- Inhibits porphyrin synthesis
- Induces aryl hydrocarbon hydroxylase
- With regard to chloroform
- Phosgene is a major metabolite.
- Phosgene is detoxified by conjugation with glucuronide.
- Liver necrosis can occur if phosgene escapes the detoxification process.
- Phosgene is the only toxic metabolite of chloroform.
- With regard to the detoxification of PCBs while in the environment (biodegradation)
- Sunlight may break down PCBs in surface water.
- No breakdown of PCBs occurs in bottom sediments.
- Bacteria may detoxify them by oxidation.
- Chemical dechlorination does not reduce the toxicity of PCBs.
- With regard to the aromatic hydrocarbon (Ah) receptor
- TCDD attaches to it.
- Occupation of a certain minimum number of receptors is necessary for TCDD to be carcinogenic.
- The linear multistage assessment model for carcinogens may not be appropriate for TCDD.
- No other chemical is known to attach to the Ah receptor.
- For Questions 7–11, match the chemical listed in the following to the appropriate use.
- Hexachlorophene
- Polybrominated biphenyls (PBBs)
- Polychlorinated biphenyls (PCBs)
- 2,4-Dichlorophenoxyacetic acid (2,4-D)
- Dichlorodiphenyltrichloroethane (DDT)
- Insecticide
- Disinfectant
- Transformer insulator
- 10. Fire retardant
- 11. Herbicide
- For Questions 12–15 answer true or false.
- 12. Dioxin (TCDD) can cause behavioral abnormalities.
- 13. Porphyrins are by-products of heme synthesis.
- 14. Victims of TCDD poisoning at Seveso showed no evidence of hepatotoxicity.
- 15. Pentachlorophenol is used as a wood preservative. From its name, one would predict that it would cause chloracne if accidentally consumed.
Answers
- B
- A
- E
- A
- B
- A
- e
- a
- c
- 10. b
- 11. d
- 12. True
- 13. True
- 14. False
- 15. True
Further Reading
Axelson, O., Editorial: Seveso: Disentangling the dioxin enigma? Epidemiology, 4, 389–392, 1993.
Boffetta, P., Mundt, K.A., Adami, H.O., Cole, P., and Mandel, J.S., TCDD and cancer: A critical review of epidemiologic studies, Crit. Rev. Toxicol., 41, 622–626, 2011.
Collins, J.J., Acquavella, J.F., and Friedlander, B.R., Reconciling old and new findings on dioxin, Epidemiology, 3, 65–69, 1992.
Collins, J.J., Strauss, M.E., Levinskas, G.J., and Conner, P.R., The mortality experience of workers exposed to 2,3,7,8,-tetrachloro-p-dioxin in a trichlorophenol process accident, Epidemiology, 4, 7–13, 1993.
Denison, M.S. and Helferich, W.G. (eds.), Toxicant-Receptor Interactions, Taylor & Francis, Philadelphia, PA, 1998.
Fingerhut, M.A., Halperin, W.E., Marlow, B.S., Piacitelli, L.A., Honchar, P.A., Sweney, M.H., Griefe, A.L., Dill, P.A., Steenland, K., and Suruda, A.J., Cancer mortality in workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin, N. Engl. J. Med., 324, 212–218, 1991.
Fingerhut, M.A., Steenland, K., Sweeney, M.H., Halperin, W.E., and Piacitelli, L.A., Old and new reflections on dioxin, Epidemiology, 3, 69–72, 1992.
Hankinson, O., The aryl hydrocarbon receptor complex, Annu. Rev. Pharmacol. Toxicol., 35, 307–340, 1995.
Jamieson, D.J., Terrell, M.L., Aquocha, N.N., Small, C.M., Cameron, L.L., and Marcus, M., Dietary exposure to brominated flame retardants and abnormal Pap test results, J. Women’s Health, 9, 1269–1278, 2011.
Johnson, E.F., A partnership between the dioxin receptor and a basic helix-loop-helix-protein, Science, 252, 924–925, 1991.
Landers, J.P. and Bunce, N.J., The Ah receptor and the mechanism of dioxin toxicity, Biochem. J., 276, 273–278, 1991.
Pesatori, A.C., Consonni, D., Rubagotti, M., Grillo, P., and Bertazzi, P.A., Cancer incidence in the population exposed to dioxin after the “Seveso accident”: Twenty years of follow-up, Environ. Health, 8, 39–50, 2009.
Roberts, L., Dioxin risks revisited, Science, 251, 624–626, 1991a.
Roberts, L., EPA moves to reassess the risk of dioxin, Science, 252, 911, 1991b.
Ryan, J.J., Gasiewicz, T.A., and Brown, J.F., Human body burden of polychlorinated dibenzofurans associated with toxicity based on the Yusho and Yucheng incidents, Fundam Appl. Toxicol., 14, 722–731, 1990.
Small, C.M., Murray, D., Terrell, M.L., and Marcus, M., Reproductive outcomes among women exposed to a brominated flame retardant in utero, Arch. Environ. Occup. Health, 66, 201–208, 2011.
Walker, C.H., Hopkin, S.P., Silby, R.M., and Peakall, D.B., Principles of Ecotoxicology, Taylor & Francis Ltd., London, U.K., 1996.
Walsh, A.A., Hsieh, P.H., and Rice, R.H., Aflatoxin toxicity in cultured human epidermal cells: Stimulation by 2,3,7,8,-tetrachlorodibenzo-p-dioxin, Carcinogenesis, 13, 2029–2033, 1992.