Chapter 6
Toxicity of Metals
Mad as a Hatter.
Introduction
The process of felting, employed in making hats many years ago, required the use of mercurial compounds and many hatters suffered from the CNS disturbances, including behavioral disorders, associated with mercury toxicity. Metal intoxication as an occupational disease may be 4000 years old. Lead was produced as a by-product of silver mining as long ago as 2000 BC. Hippocrates described abdominal colic in a man who worked as a metal smelter in 370 BC and arsenic and mercury were known to the ancients even if their toxicity was not. In 1810 a remarkable case of mass poisoning with mercury occurred. The 74-gun man-o’-war HMS Triumph salvaged 130 tons of mercury from a Spanish vessel wrecked while returning from South America, where the mercury had been mined. The mercury was contained in leather pouches, which became damp and rotten, allowing it to escape and vaporize. Within 3 weeks 200 men were affected with signs of mercury poisoning including profuse salivation, weakness, tremor, partial paralysis, ulcerations of the mouth, and diarrhea. Almost all animals onboard died, including mice, cats, a dog, and a canary. Five men died. When the vessel put in at Gibraltar for cleaning, all those working in the hold salivated profusely.
The common nineteenth-century practice of adulterating foods and beverages (wine, beer, etc.) to increase profit led Accum to publish a treatise on the subject in 1820. Lead, copper, and mercury were frequently detected. Methods were not yet in place to detect arsenic, which was found to be a widespread adulterant later in the century. In 1875, the British parliament passed the first Food and Drugs Act as a result of these investigations.
In the past it was common to refer to heavy metal toxicity, as it was those metals that first emerged as industrial hazards. Heavy metals are arbitrarily defined as those having double-digit specific gravities and they include platinum (21.45), plutonium (19.84), tungsten (19.3), gold (18.88), mercury (13.55), lead (11.35), and molybdenum (10.22). These are in contrast to iron (7.87), manganese (7.21), chromium (7.18), zinc (7.13), selenium (4.78), and aluminum (2.70). Intermediate are copper (8.96) and cadmium (8.65).
In general, it can be seen that metals with sp.gr. less than eight are mostly essential trace nutritional elements (copper also is one and therefore the exception, as is aluminum, which is not a nutritional element), whereas those having sp.gr. greater than eight are the more toxic ones. It must be stressed once again that dose is all-important. Aluminum, with a sp.gr. of 2.70, has toxic properties. Arsenic exists in two solid forms: yellow arsenic (1.97) and grey or metallic arsenic (5.73). Both are highly toxic.
Lead
The Latin word for lead is plumbum, hence the chemical designation Pb. This word also gave origin to such English ones as plumbob (a mason’s line with a metal ball attached for establishing vertical trueness), plummet (to fall as if leaden), and aplomb (to be as calm and undeviating as a plumb line). Lead was obviously well known to the ancients. In fact, they spent a lot of time trying to turn it into gold (alchemy). Lead toxicity was also familiar to them. Diascorides described its CNS toxicity as delirium.
Despite early knowledge of lead’s toxic effects, the low melting point of the metal, coupled with its density, made it popular and useful. Well into the 1940s and early 1950s it was possible to buy lead toys, and kits were available to cast lead soldiers and lead fishing weights. An 1885 description of chronic lead poisoning is as good as any to be found in a modern text:
The chief signs of chronic poisoning are those of general ill health; the digestion is disturbed, the appetite lessened, the bowels obstinately confined, the skin assumes a peculiar yellowish hue, and sometimes the sufferer is jaundiced. The gums show a black line from two to three lines in breadth, which microscopical examination and chemical tests alike show to be composed of sulphide of lead; occasionally the teeth turn black. The pulse is slow and all secretions are diminished. Pregnant women have a tendency to abort. There are also special symptoms one of the most prominent of which is lead colic. This colic is paroxysmal and excruciating.
Modern-day sources of lead are numerous. In the eighteenth century the industrial West discovered what the Chinese had known for centuries, namely that lead glazes produce crockery with a richer, smoother look. From this source and from lead solder in cans and kettles and water pipes leached by soft (but not hard) water, we consume about 150 μg/day. In some areas the figure may reach 1–2 mg. Occupational exposures were common in the past from lead mining, smelting, and the manufacture of products employing lead such as lead-based paints, toys, fuels, etc. Such exposures still occur in developing countries. Children are more vulnerable as all dirt and dust contain lead, especially in cities where lead from auto exhaust (tetraethyl lead) settles out on the ground. This will persist long after the conversion to lead-free auto fuel. Children may also consume old lead-based paint, common in older buildings and which may also be on cheap wooden toys. In children, CNS toxicity is the dominant feature. This starts with vertigo and irritability, progressing to delirium, vomiting, and convulsions. The mortality rate is about 25% if treated and about 65% if untreated. In infants, exposure produces progressive mental deterioration after 18 months, with loss of motor skills, retarded speech development, and hyperkinesis in some cases. In the United States, the Lead Paint Poison Prevention Program was introduced in 1970. Since that time, the mean blood lead level of U.S. children has fallen from over 1 μmol/L (20.7 μg/dL) to less than 0.25 μmol/L (5.2 μg/dL). Only two deaths in children from acute lead encephalopathy have been reported in the past 30 years.
Children are not the only victims of lead poisoning from lead paint. Sandblasting of old, lead-painted buildings may, over time, cause chronic lead poisoning in workers who inhale the dust. Proper respirators and protective clothing are required for sandblasters. Heating of lead pint to a sufficiently high temperature can release lead fumes that can be inhaled. Cutting torches can produce sufficient heat to do this.
In all exposed individuals, subchronic toxicity can involve interference with mitochondrial heme synthesis at several levels, with resultant hypochromic (pale) microcytic (small) anemia. The pathway involved in this is illustrated in Figure 6.1.
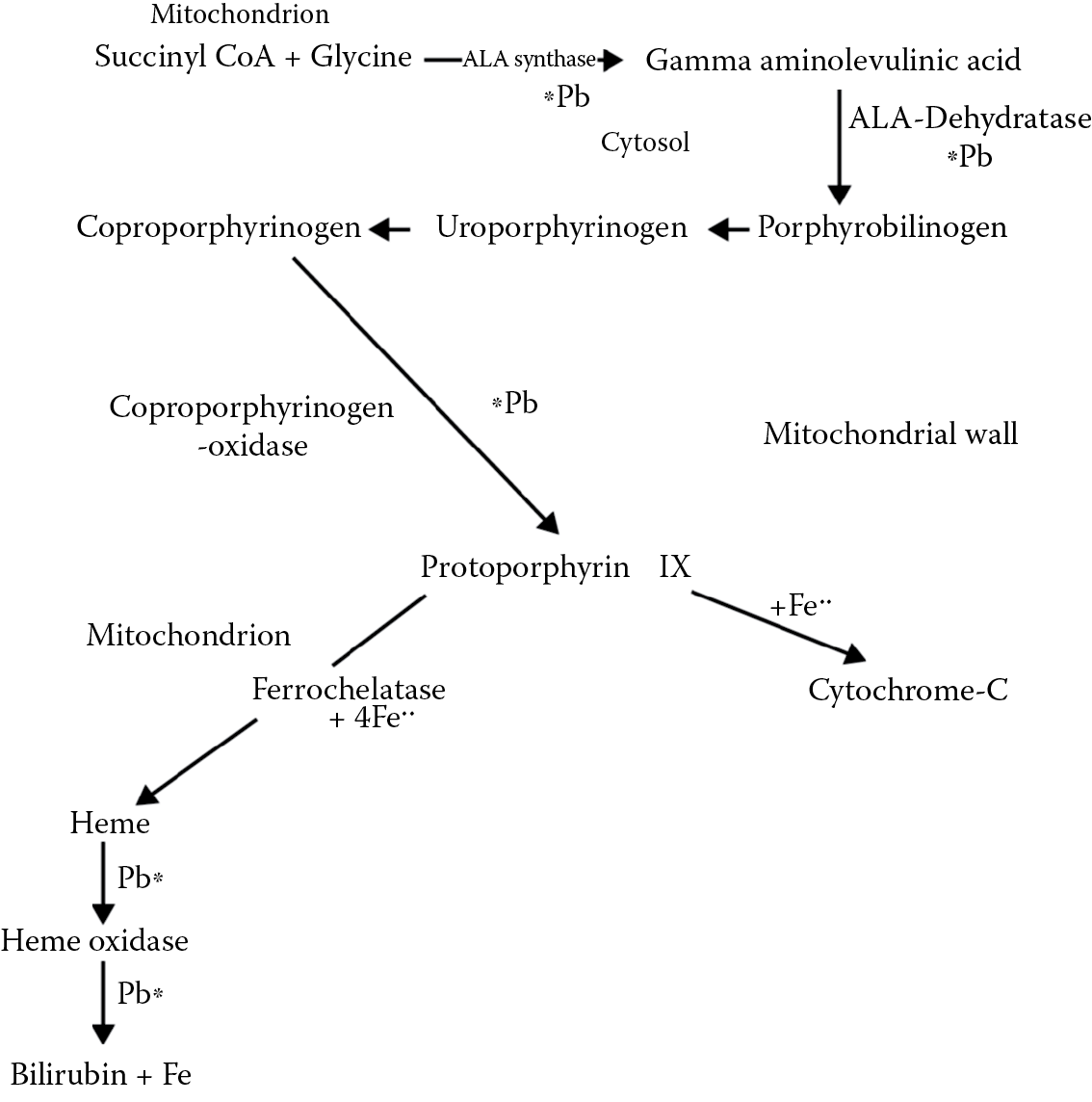
A simplified scheme showing points of interference of lead in heme synthesis (see also Figure 5.2 ) for ALA synthase and heme inhibition.
Toxicokinetics of Lead
Elemental lead is not absorbed by the skin or through the alveoli of the lungs. Inhaled particulate lead is returned to the pharynx by the bronchial cilia and swallowed. Tetraethyl lead, however, may be absorbed across the skin and alveoli and readily penetrates CNS. Most of it is destroyed in exhaust emissions but sniffing leaded gasoline can result in severe CNS damage.
Gastrointestinal absorption of lead probably occurs via calcium channels as lead is a divalent cation (Pb2+). It first appears in red blood cells, then hepatocytes, and then the epithelial cells of the renal tubules. It is gradually redistributed to hair, teeth, and bones where 95% of it is stored harmlessly. The T1/2 in blood is about 30 days, in bone, 25 years. Little reaches the adult brain but much more enters the infant brain. Renal excretion is the main route of elimination.
Cellular Toxicity of Lead
Lead affects oxidative phosphorylation and ATP synthesis in the mitochondrion. It also increases red cell fragility and inhibits sodium/potassium ATPase. Kidney tubular cells become necrotic, and chronic exposure may lead to interstitial nephritis. Nuclear inclusion bodies, consisting of lead bound to a protein, may be formed in renal cells. This may be considered as a protective mechanism. Carcinogenesis has been demonstrated in experimental animals and chromosomal abnormalities have been observed, but evidence of tumor production in humans is scarce. Most of the toxic effects of lead and other heavy metals can be explained by their affinity for thiol groups. This is also the basis of chelation therapy.
Fetal Toxicity
A characteristic of all metals is their ability to penetrate the placental barrier, so that fetal toxicity can occur as a result of maternal exposure. Lead is considered to be a human carcinogen and pregnant women are generally removed from jobs where exposure may occur.
Treatment
Lead chelators are the treatment of choice. These bind lead (and other divalent cations) so that it can be excreted. Calcium/sodium ethylene diamine tetra-acetate (CaNa2EDTA) and dimercaprol (British antilewisite, BAL) are given intramuscularly followed by oral penicillamine for several weeks. BAL was developed during World War II as a treatment for lewisite, a vesicant arsenical poison gas. A newer chelator is meso-2,3-dimercaptosuccinic acid (DMSA). The chemical structures of these chelators are shown in Figure 6.2. In the case of EDTA, Pb is exchanged for Ca2+ whereas with the others, the Pb is bound to sulfhydryl (SH) groups. The complexes are excreted, mostly in urine. A disadvantage of chelation therapy is that it does not remove lead from the brain very efficiently. Cuprimine, another chelator, is d-penicillamine, 3-mercapto-D-valine.
Despite 50 years of use, objective evidence for benefit of chelation therapy for lead poisoning is scanty. It is widely agreed that it has drastically reduced the mortality from lead encephalopathy if diagnosis and treatment are started early. It also relieves lead colic, malaise, basophilic stippling, and it rapidly restores red-cell ALA dehydratase. It does not influence the residual manifestations of chronic lead poisoning such as peripheral neuropathy. A recent study showed that chelation with Cuprimine in lead-intoxicated patients significantly lowered blood Pb levels (initially 5.58 ± 2.02 μg/dL) and total Pb body burden as well as reducing blood zinc protoporphyrin. Cuprimine was given intramuscularly at 25–35 mg/kg of body weight per day in divided doses.
In nonindustrial cases of lead poisoning, it is essential to identify the source of the lead and remove it or remove the possibility of people contacting it. This is most important where children are involved.
Mercury
Mercury (Hg) exists in three forms: elemental mercury, inorganic compounds, and organic compounds. Elemental mercury causes toxicity when the mercury vapor is inhaled, as exemplified by the episode described at the beginning of this chapter. The major source of elemental mercury in the environment is the natural degassing of the earth’s crust. Estimates of the level of mercury reaching the atmosphere range from 25,000 to 150,000 tons/year, and the atmosphere represents a major mechanism for global transport of metallic mercury. Conversely, anthropogenic sources account for only 10,000 tons/year, but because industrial effluent tends to be concentrated, these are the sources usually associated with toxicity. Metallic mercury and its vapor can be an industrial hazard. Mercury is used in the manufacture of chlorine and sodium hydroxide by the mercury cell process, in paint preservatives, and in the electronics industry. It is a by-product of smelting processes (most mineral ores contain mercury) and it is released during fossil fuel combustion.
Elemental Mercury Toxicity
In vapor form, elemental mercury is well absorbed across both the alveoli of the lungs and the blood–brain barrier. Acute poisoning usually occurs within several hours. Weakness, chills, metallic taste, salivation, nausea, vomiting, diarrhea, labored breathing, cough, and tightness in the chest may ensue. If the exposure is more prolonged, interstitial pneumonitis may develop. Recovery is usually complete except that residual loss of pulmonary function may persist. Chronic exposure to mercury vapor results in CNS disturbances including tremor and a variety of behavioral changes that can include depression, irritability, shyness, instability, confusion, and forgetfulness. Mercury vapor from mercury nitrate formerly used in the felting process accounted for the “mad hatter” syndrome. The behavioral abnormalities of the “Mad Hatter” in Lewis Carroll’s The Adventures of Alice in Wonderland were really quite mild, compared to the other characters, which is in keeping with the topsy-turvy world that Carroll created. Thyroid disturbances may be present.
Inorganic Mercurial Salts
Inorganic salts such as mercuric chloride can cause severe, acute toxicity. The proteins of mucous membranes are precipitated, giving them an ash-gray color in the mouth and pharynx. Intense abdominal pain and vomiting are common. Loss of blood and fluid from the gastrointestinal tract results from sloughing of the mucosa in the stool and may lead to hypovolemia and shock. Renal tubular necrosis occurs after acute exposure and glomerular damage is more common after chronic exposure. A phenomenon called “pink disease” or acrodynia commonly follows chronic exposure to mercury ions. It is a flushing of the skin that is believed to have an allergic basis.
Organic Mercurials
Methylmercury is the commonest cause of organic mercurial poisoning and the most important one environmentally. It is extremely well absorbed from the gastrointestinal tract (90%) and deposited in the brain. Because of its high affinity for SH groups, methylmercury binds to cysteine and this may then substitute for methionine and be incorporated into proteins. This can result in the formation of abnormal microtubules required for cell division and neuronal migration. Many enzymes, such as membrane ATPase, are SH dependent and mercury may thus interfere with their function. Renal toxicity is a common manifestation of poisoning. Renal uptake of mercury is rapid and it accumulates in the kidneys. Mitochondrial dysfunction appears to be involved in cell damage in the proximal tubule.
The main signs and symptoms are neurological and consist of visual disturbances, weakness, incoordination, loss of sensation, loss of hearing, joint pain, mental deterioration, tremor, and in severe cases, paralysis, and death. Infants exposed in utero may be deformed and intellectually challenged. Experimentally, methylmercury has been shown in cell cultures to mobilize Ca2+ from intracellular stores that are sensitive to inositol 1,4,5-triphosphate.
Mercury is a waste product of many industrial processes. Hg is methylated in sediment by bacteria and cyanocobalamin. Several outbreaks of methylmercury poisoning have occurred. The most widely known began in Minimata, Japan, in 1953 near a plant that manufactured acetaldehyde and which discharged methylmercuric chloride (MeHg+Cl−) into Minimata bay. People who ate mollusks and large fish from the bay developed the symptoms that came to be known as Minamata disease. Nine hundred cases developed and there were 90 fatalities. Because of the high fetal toxicity of mercury many deformed infants were born. Another source of mercury toxicity is the consumption of seed grains treated with methylmercuric chloride as a fungicide. Several mass poisonings have occurred around the world. In Iraq in 1972, one such episode resulted in over 6500 cases of poisoning and 500 deaths.
Mechanism of Mercury Toxicity
Mercury toxicity can be explained entirely by its ability to bind with the hydrogen of SH groups to form mercaptides (i.e., X-Hg-SR and HgSR2 where X = an electronegative radical and R = a protein). Organic mercurials such as methylmercury form mercaptides, R-Hg-SR′. The term mercapto means “to capture mercury” and refers to sulfur-containing groups. Since SH groups are important components of many enzymes, mercury acts as an enzyme poison and interferes with cell function at many levels. Mercury can also combine with other physiologically important ligands such as phosphoryl, carboxyl, amide and amine groups. Metallic Hg vapor may be oxidized by catalase enzyme in red blood cells to the less toxic divalent form. Alcohol competitively inhibits this process. Mercury was an important pharmaceutical agent for centuries, and its pharmacological properties also depend on its affinity for SH groups. It was used as an antibacterial agent (Ehrlich’s 606 or Salvarsan) for syphilis, as a laxative, in skin creams, and in diuretics. Mercurial diuretics were still in use in the 1960s. They were replaced eventually by safer agents. Aminomercuric chloride may still appear in freckle-removing creams, and daily application for years may result in increases in 24 h urine mercury excretions from 10 μg to 1 mg and the development of symptoms such as excessive salivation and insomnia.
Treatment of Mercury Poisoning
Mercury poisoning continues to be a significant industrial problem. A 2012 report from Iran compared 46 workers exposed to low levels of Hg vapors in a chlor-alkali plant to 65 healthy, unexposed employees. Atmospheric Hg levels were 3.98 ± 6.28 μg/m3. Urinary Hg levels were threefold higher in the exposed workers. Symptoms reported included fatigue, anorexia, memory loss, erethism (a state of agitation), blurred vision, and dental problems.
Chelation therapy is recommended for elemental, inorganic mercury poisoning. Dimercaprol and penicillamine are SH-containing chelators. Dimercaprol is given intramuscularly and penicillamine, orally. Hemodialysis may also be used, and vomiting may be induced if there has been recent ingestion of mercury. These treatments are of little use in methylmercury poisoning, however. Dimercaprol actually increases brain levels of methylmercury and penicillamine and hemodialysis do not relieve symptoms. Some success has been achieved with binding resins taken orally. Since there is a significant enterohepatic recirculation of methylmercury (i.e., it is excreted in the bile and reabsorbed from the intestinal tract), binding it to a polythiol resin prevents its reabsorption because it is excreted in the feces.
The Grassy Narrows Story
In 1969 Norvald Fimreite, a PhD candidate in the Department of Zoology at the University of Western Ontario, first made public his findings on the mercury contamination of fish in Canadian and border lakes. The highest levels were recorded from a small lake, Pinchi, in British Columbia (10 ppm) and from Lake St. Clair (7.03 ppm) in the Great Lakes waterway. The (Canadian) federal standard for export and consumption was 0.5 ppm. His report was a bombshell coming on the heels of reports of Minamata disease from Japan. Fimreite estimated that Canadian industry was releasing 200,000 lb of mercury annually into the environment. Most of it came from chlor-alkali plants and from pulp and paper mills that used mercurials as antisliming (antialgal) agents, and the chlorine and alkali as bleaching agents. The question of mercury discharge from the Dow (Canada) Chemical plant had been raised 6 years earlier in the Ontario Legislature but nothing had been done. In 1970, the Ontario Water Resources Commission took steps to reduce Dow’s output, but in Dryden, near the Manitoba border, the Dryden Pulp and Paper Co. (owned by the British Reed Group) had been emitting mercury vapor since 1962, and some workers developed bleeding gums and muscle twitches. By 1970 it had pumped an estimated 20,000 lb of mercury into the surrounding environment, including discharges described as a brown froth into the Wabigoon River. Raw sewage also was discharged into the Wabigoon, providing a rich source of anaerobic bacteria to methylate elemental mercury. The Wabigoon is part of the English River system, and about 50 km downstream lie the Grassy Narrows and Wabaseemong Anishanaabe First Nations reserves. The residents gleaned a slim but adequate living as fishing guides and lived largely off the land, eating fish, deer, and moose supplemented with garden vegetables. In March of 1970, contamination of fish in Lake Erie was detected and the Lake St. Clair and Lake Erie fisheries were closed. Chlor-alkali plants and pulp mills were ordered to stop using mercury by the end of May after a concerted attack in the Ontario Legislature by opposition parties. Mercury, however, is not biodegradable, and it is only when it is buried by uncontaminated sediment that it ceases to be a threat. In June of 1970, the Lamms, owners of Ball Lake Fishing Lodge, hired Fimreite to conduct a survey of mercury levels in the fish of the English–Wabigoon system. The findings were appalling. Levels ranged from 13 to 30 ppm, as high as those from Minamata Bay. The government lifted Fimreite’s license to collect specimens for scientific purposes and ignored his appeals to test the residents of the reserves until his data were made public, when it conceded that it had similar findings. A ban was placed on eating fish from the contaminated area but otherwise the government continued to downplay the problem. Tourist fishing dried up and the Indians went on welfare. Blood levels of mercury were not seriously studied until 1973, and ranged from 45 to 289 ppb (normal is about 20 ppb for a city dweller). Some residents were showing signs of mercury poisoning and the incidence of stillbirths was going up.
The social costs of this tragedy were perhaps even greater than the direct effects of mercury. In the years surrounding the discovery of mercury in the Grassy Narrows area, the death rate rose to 1 in 50, three times the national average. Most were alcohol related. Many of the deaths were newborn or very young infants. Violence became rampant. Dr. Peter Newbury, also a graduate of U.W.O., conducted a study for the Society of Friends (Quaker) and the National Indian Brotherhood and felt that the CNS effects of mercury were a contributing factor in the violence. Gasoline sniffing became common amongst young people. (It remains a problem on many reserves.) The Grand Council of Treaty Three District, which includes Grassy Narrows and Kenora, completed a study in 1973. They found that in the preceding 42-month period, there had been 189 violent deaths of native people. They reported 38 from gunshot, stabbing, or hanging; 30 in fires; 42 drownings; 25 from exposure; and 16 from car accidents. In the same year, members of the Ojibwa Warrior Society occupied Anicinabe Park on the outskirts of Kenora. Barricades were erected and manned by armed warriors. The park was claimed as First Nations land. The standoff lasted for several weeks but achieved little.
No follow-up of mercury contamination of fish in the river system was done since the 1970s until a report was published in 2007. This report was done at the request of the Grand Council treaty #3. Several species of fish were assayed for mercury including Walleye, Northern Pike, Large-mouth Bass, and Whitefish. A total of 851 fish samples were collected along the length of the river system. They found that mercury levels had declined over the preceding 25 years but a gradient was still observed with the highest levels detected in specimens collected closest to the original source of contamination. There also was a correlation between the length of the fish (an indicator of age) and the level of mercury. Moreover, the predatory species (the piscivores large-mouth bass, walleye, and northern pike) had much higher levels of mercury than the whitefish that survive on zooplankton. From Clay Lake the levels in the predators were all in excess of 2 mg/kg (ppm), whereas Whitefish levels were 0.2 mg/kg or less. It was recommended that fish consumption from Clay Lake should be discouraged. Whitefish from all other areas could be consumed regularly but consumption of the other species should be limited to smaller fish on a restricted basis. This study clearly illustrates how mercury contamination can persist for decades.
Cadmium
Cadmium (Cd) is present naturally in the environment in very low levels, being solubilized during the weathering of rock (levels are about 0.03 μg/g of soil, 0.07 μg/mL of freshwater, and 1 ng/m3 of air). Dissolved cadmium may form a number of soluble and insoluble organic and inorganic compounds. Cadmium is chemically similar to zinc and it is present in zinc ore in a ratio of about 1/250. Most cadmium is produced as a by-product of electrolytic zinc plants. It is used in metal plating, in the manufacture of nickel-cadmium batteries, in the manufacture of pigments, in plastic stabilizers and small amounts are used in photographic chemicals, in catalysts, and in fungicides used on golf courses. Environmentally significant emissions come mainly from smelting operations for copper, lead, and zinc, from auto exhaust, and manufacture of pigments and alloys (most nickel-cadmium batteries are imported into Canada). Cadmium is readily taken up by plants and stored in the leaves and seeds. It is present in sewage sludge fertilizers (recommended maximum 20 ppm). Water pollution with cadmium may result in high levels in fish and especially in mollusks. The main sources in the human diet are organ meats (cadmium accumulates in liver and kidney), cereal grains, shellfish, and crustaceans.
Cadmium Toxicokinetics
Cadmium intake in Canada averages 50–100 μg/day from inhaled and ingested sources. Inhaled, unpolluted air may contribute up to 0.15 μg/day, whereas breathing air near a smelter can raise the level to 10 μg/day. Cigarettes contain cadmium and smoking increases exposure still further. About 50% of inhaled cadmium is absorbed. Only about 6% of ingested cadmium is absorbed, but it contributes most of the daily load. The FAO/WHO recommends a maximum weekly intake of 500 μg. Absorbed cadmium is bound to plasma albumin and cleared rapidly from the plasma. It is found in red cells only after high exposures. It is rapidly distributed to the liver, pancreas, prostate, and kidney, with slow redistribution to the kidney until, over time, it contains most of the cadmium. Renal levels increase up to age 50 and depend on the cumulative exposure. The T1/2 in humans is about 20 years. Cadmium is trapped in the kidney and liver by a cysteine (i.e., SH-rich) protein called metallothionein with a high affinity for cadmium and zinc. Cadmium normally binds to metallothionein, the synthesis of which is induced by the presence of the cadmium. High doses, however, exceed the binding capacity of the protein and the cadmium is free to bind to other essential cell components such as the basement membrane of the renal glomerulus.
Cadmium Toxicity
The kidney is the major organ of toxicity. About 200 μg/g wet weight of kidney appears to be the critical concentration in the renal cortex for damage to occur in the form of proximal tubule dysfunction. Once renal disease develops, cadmium is lost from the kidney. Nutritional deficiencies of zinc, iron, and calcium may predispose cadmium toxicity by increasing absorption from the gastrointestinal tract. Calcium deficiency increases the synthesis of calcium-binding proteins and cadmium absorption. Cadmium causes cellular damage probably by binding to SH groups of essential cell proteins. The production of reactive oxygen species can cause oxidative stress. The complexing of cadmium with metallothionein can increase renal toxicity because the complex is more readily taken up by the kidney than the free metal. It protects, however, against testicular damage, which can occur after a single exposure that can cause necrosis, degeneration, and loss of spermatozoa. This appears to be related to cadmium’s effect on testicular vascular supply.
Workers in metal refineries may be exposed to high levels of cadmium fumes and develop respiratory difficulties. Chronic exposure may lead to obstructive pulmonary disease and emphysema. A major exposure occurred in Japan in the late 1940s. Effluent from a lead processing plant washed into adjacent rice paddies over decades and the rice accumulated high levels of cadmium. Because the people were calcium deficient due to a poor diet, they developed acute cadmium toxicity with severe muscle pain, malabsorption, anemia, and renal failure. The outbreak was named “Itai-Itai” (ouch-ouch) disease. The fetus appears to be protected from cadmium toxicity by placental synthesis of metallothionein, but heavy exposures can overwhelm this defense.
Animal studies have shown cadmium to be carcinogenic and there is a suggestion that it may increase the incidence of prostate cancer in elderly men. Other metals, notably arsenic, chromium, and lead, also have been implicated as carcinogens. A recent study provides convincing evidence that cadmium dietary intake is a significant risk factor for breast cancer in postmenopausal women. Cadmium is a common contaminant of food stuffs especially cereal grains. This Swedish study examined the incidence of estrogen receptor-positive (ER+) and estrogen receptor-negative (ER−) breast cancer in about 56,000 postmenopausal women. Dietary cadmium intake, adjusted for confounders such as whole grains and vegetables, which, although they are a source of cadmium, also contain anticancer phytochemicals, was associated with an increased incidence of total breast cancers that was statistically significant as was the association with ER+ ones.
Treatment
Chelation therapy is not effective. Treatment consists of removing the patient from the source of exposure and supportive measures.
Arsenic
Arsenic (As) pollution of water courses and groundwater is a major health issue in some parts of the world and in some countries it is the most important chemical pollutant in groundwater and drinking water. Up to 200 million people in 70 countries are exposed to arsenic from contaminated drinking water. They are at risk from debilitating disease and fatal cancers. In the Bengal delta region, it is estimated that about 35 million people have been drinking arsenic-contaminated water for decades and 15% had arsenic-related skin lesions. Arsenic, at a concentration of 50 μg/L in drinking water, can cause health problems after drinking it for 10–15 years. The Mekong River delta is another area of concern affecting Cambodia and Vietnam. Groundwater arsenic concentrations ranged from 1 to 3050 μg/L (average 159 μg/L) in Cambodia, and 1–845 μg/L in southern Vietnam. The Red River delta area also was affected (2–33 μg/L). Deep (12–40 m) core sediment samples contained arsenic 2–33 μg/g.
Arsenic is an age-old pharmaceutical preparation. It was believed to be a tonic because it causes facial flushing (rosy cheeks) and fullness (edema). It is still used in the treatment of trypanosomiasis. Ehrlich studied organic arsenicals and developed the first effective treatment for syphilis (Ehrlich’s 606). The chemistry of arsenic is exceedingly complex since it can exist as a metallic form and as trivalent and pentavalent compounds. Trivalent forms include arsenic trioxide, arsenic trichloride, and sodium arsenite. Pentavalent forms include arsenic pentoxide, arsenic acid, lead arsenate, and calcium arsenate. Organic arsenicals also may be trivalent or pentavalent. Arsenic in the environment arises from weathering of rock and from emissions from smelting of gold, silver, copper, zinc, and lead ores, combustion of fossil fuels, and the use of arsenicals in agriculture as herbicides and pesticides. Airborne particles may travel considerable distances and penetrate deeply into the lungs. Arsenic is taken up by plants and the degree of uptake varies with the soil type. Fine soils high in clay and organic material inhibit uptake. Arsenic also enters the water system through runoff and fallout. Wells drilled through rock containing arsenic will yield water high in arsenic. Chronic poisoning may result, and this is a problem in some parts of Nova Scotia (see above regarding water contamination in other countries). Tobacco contains arsenic. The average daily intake of arsenic in North America is about 25 μg.
Toxicokinetics of Arsenicals
Arsenic may be absorbed from the gastrointestinal tract, the lungs, and across the skin and mucous membranes. It penetrates intracellularly by an uptake mechanism used in phosphate transport. Like mercury, arsenic binds to SH and disulfide groups to poison numerous cell enzymes and respiration. Chromosomal breakage has been observed experimentally. In general, the order of toxicity is organic arsenicals > inorganic arsenicals > metallic arsenic. The trivalent arsenite has a high affinity for SH groups and interferes with the enzyme pyruvate dehydrogenase. Plasma pyruvate levels will increase. Some biotransformation between trivalent and pentavalent forms may occur. The T1/2 of arsenic is about 10 h and excretion is mainly by the kidneys. Arsenic is an effective uncoupler of oxidative phosphorylation. Numerous arsenite-oxidizing bacteria exist in the environment, forming arsenic trioxide. Arsenic trioxide has been reintroduced recently as a chemotherapeutic agent.
Toxicity of Arsenicals
As noted, the most poisonous forms are arsenic trioxide (As2O3) and sodium arsenite (NaAsO2). Arsenic tends to accumulate in the liver, kidney, heart, and lung. It is also deposited in bone, teeth, hair, and nails and these become important tissues for diagnostic and forensic analysis. The average human intake is about 300 μg/day but it may be much higher if fish is a large part of the diet, as they accumulate the poison through biomagnification. In 2012 the U.S. Food and Drug Administration (FDA) released a report on arsenic contamination of fruit juices. Importation of several sources of pear juice and concentrate was blocked because the arsenic content was greater than 23 ppb, their level of concern. Contaminated apple juice and concentrate were also found.
Acute arsenic poisoning causes severe abdominal pain and it is rare. Chronic poisoning causes muscle weakness and pain, skin pigmentation, gross edema, gastrointestinal disturbances, kidney and liver damage, and peripheral neuritis with eventual paralysis. The fingernails develop white lines, called Mee’s lines, which can be used to determine when exposure occurred.
Arsine is the gaseous form of arsenic resulting from electrolytic processes and it is extremely toxic, producing rapid and often fatal hemolysis. It has a garlic-like odor.
When Napoleon Bonaparte died in exile on the island of St. Helena in 1821 suspicions immediately fell on royalists fearful of his return to France. This theory was reinforced when tests in 1961 revealed high levels of arsenic in a sample of his hair. Subsequent studies, however, found that virtually everyone in the early nineteenth century had elevated arsenic in hair samples. Arsenic was used in dyes, cosmetics, as a pharmaceutical, and in many manufacturing process. Even the wallpaper in Bonaparte’s damp, shabby home-in-exile contained it. In the hot climate the action of microorganisms could have caused off-gassing of arsine.
Treatment
Chelation therapy is used for arsenic poisoning. Both dimercaprol and penicillamine have been used.
Environmental Effects of Arsenic
Arsenic is toxic to a wide range of plants and animals including marine species. Of the plants, beans, peas, and rice are especially sensitive. Algae are sensitive, as well as some protozoa such as Daphnia magna. Finned fish are quite susceptible.
Chromium
Chromium (Cr) is used in the production of stainless steel, chrome plating, pigments, and in the chemical industry. Chromium has two oxidation states: Cr+3 and Cr+6. The latter is much more toxic, causing severe respiratory irritation when inhaled, and possibly lung cancer after long chronic exposure. Kidney damage also occurs. The trivalent form binds readily to electron-donating ligands such as macromolecules like RNA but it does not readily cross the cell membrane. Conversely, the hexavalent form readily crosses cell membranes and is reduced to the trivalent form intracellularly. Toxicity is thus normally related to the presence of the hexavalent form in the environment. As the oxyanion it is taken up by the cell, probably by a sulfate transport system as shown later. Air levels as low as 10 μg/m3 of Cr+6 can produce respiratory irritation. Chromium is distributed in the biosphere much like arsenic and can have similar effects.
Other Metals
Virtually any metal, if taken in excessive amounts or by an unusual route, can manifest toxicity. Thus, the inhalation of any metal dust can cause pulmonary fibrosis.
Aluminum
The kidney does not clear aluminum very efficiently and in renal insufficiency it may accumulate to toxic levels. Minute levels of aluminum are present in food and water, and if aluminum-free water is not used in dialysis machines, patients may accumulate high blood levels over time. This can cause microcytic anemia. This can be treated with the chelator desferoxamine, which removes the aluminum from the blood. Before the problem with aluminum and dialysis was identified, some patients developed very high blood levels and signs and symptoms of dementia similar to Alzheimer’s disease.
Manganese
Public concern has developed over a potential source of inhaled manganese, the fuel additive MMT. Methylcyclopentadienyl manganese tricarbonyl is an octane booster employed by the petroleum industry and manufactured by the Ethyl Corporation. Automobile manufacturers object to it because it defeats antipollution devices. Environmentalists object to it because they believe it constitutes a threat to human health. The petroleum industry claims that it would be too costly to eliminate its use. A WHO group conducted a study of workers in Quebec who had been exposed to manganese fumes and dust during the manufacture of metal alloys. The workers demonstrated problems with motor coordination and mentation. Manganism is a condition in which Parkinson-like signs and symptoms appear; tremor, memory loss, irritability, insomnia, difficulties with speech, and, in severe cases, insanity. The workers were believed to be suffering from “micro-manganism.” Attempts to ban MMT use in both Canada and the United States have foundered on the absence of definitive evidence that it constitutes a hazard and on concerted legal assaults by its defenders. Uncertainty regarding the health risk associated with the use of MMT as a fuel additive persists. However, a study released in 2012 indicates that new pharmacokinetic data for MMT will allow the prediction of tissue manganese levels for several portals of entry, exposure levels, and durations of exposure. This should permit a more accurate risk assessment.
Uranium
Uranium is nephrotoxic. It binds to albumin and to bicarbonate anion that is filtered by the kidney where it dissociates. The free uranyl cation binds to proteins in the proximal tubule and damages them. Toxic metals may also substitute for physiological ones, as when lead and strontium 90 are deposited in bones and teeth like calcium.
Antimony
Antimony is an industrial contaminant with distribution and toxicity essentially similar to that of arsenic’s.
Nutritional Elements
Even essential metals like iron can be very toxic, especially to young children who may ingest iron-containing vitamin preparations, mistaking them for candy. Vomiting occurs and vomitus and stool may contain blood. Acidosis and shock develop. Kidney and liver damage can occur. In adults, iron overload sometimes occurs and hemosiderin is deposited in tissues including muscle. Desferroxime is a specific iron chelator with a low affinity for calcium that is used to remove systemic iron in both types of poisoning.
Metallothioneins
Metallothioneins (MTs) are low-molecular-weight (6000–7000 Da) proteins rich in SH groups and they are found in most mammalian cells. There are four classes of MTs based on their amino acid sequences. MT-I and MT-II are the most widely distributed, while MT-III and MT-IV are restricted to neurons, and squamous epithelial cells. MTs have a high affinity for many metals including Ag+, Cu+, Cd2+, Hg2+, and Zn2+. MTs are found primarily in the cytoplasm and their function is to serve as storage depots and buffers for copper and zinc. The MT-I gene can be induced by cadmium, copper, mercury, and zinc. Such induction makes them important defenses against heavy metal poisoning, and experiments with knockout mice have shown that the absence of MT-I and MT-II genes makes them more vulnerable to cadmium toxicity. The Agency for Toxic Substances and Disease Registry maintains an excellent website at www.atsdr.cdc.gov/tfacts22.html
Carcinogenicity of Metals
Arsenic has long been recognized as being associated with an increased risk of skin and respiratory cancer. In 1930, workers in a factory making an arsenical sheep dip were identified as having an excessive incidence of skin cancer. Arsenic levels in air >54.6 μg/m3 were associated with an increase in lung cancer incidence. High water content of arsenic also has been associated with increased cancer risk.
In 1976, a NIOSH study of 300 workers in a cadmium smelter revealed a significantly higher incidence of cancer. The incidence of lung cancer was twice normal and prostate cancer also was high. Some of these workers, however, were also exposed to arsenic. Inhalation of chromium dust by workers has been associated with an increased incidence of lung cancer. Nickel is also a respiratory carcinogen but it lacks other chronic toxic effects. There is some evidence suggesting that lead may be carcinogenic, or perhaps a co-carcinogen owing to its persistence in tissues. Case reports and epidemiological studies are difficult to sort out because exposures frequently involve more than one metal. This is true in the steel industry where increased cancer frequencies have been observed.
Many cationic metals will form complexes with thiol groups of cell components and the complex will mimic natural substrates to interfere with cell processes, mostly transport systems. Thus, methylmercury–cysteine complex mimics methionine and the complex is taken up into the brain by a transport system for neutral amino acids. Inorganic and organic mercury complexes with glutathione and is transported from liver cells into bile. Arsenic and copper do the same thing. Lead can substitute for Ca2+ in a number of transport and receptor-mediated processes. Voltage-activated calcium channels will admit a number of metallic cations, a fact that is exploited in research. Cadmium (Cd) and lanthanum (La) act in this way.
The case for mercury as a carcinogen is less clear-cut. Studies in experimental animals have confirmed carcinogenicity. Methylmercury chloride caused kidney tumors in male mice and mercury chloride was carcinogenic in male rats. Epidemiological evidence from dentists and dental nurses, chlor-alkali workers, and nuclear weapons workers is equivocal but suggests an increased risk of lung, kidney, and central nervous system tumors.
Unusual Sources of Heavy Metal Exposure
In 1988, the Texas Department of Health investigated illegal sales of drugs manufactured in Hong Kong. The tablets, sold as “chuifong tokuwan,” contained a veritable pharmacy of drugs, including diazepam, indomethacin, hydrochlorothiazide, mefenamic acid, dexamethasone, lead, and cadmium! This potpourri of tranquilizer, diuretic, anti-inflammatory agents, corticosteroids, and heavy metals was repackaged and marketed as “The Miracle Herb-Mother Nature’s Finest.” Twenty-four percent of 93 persons who took this preparation had elevated urine cadmium levels; 1.8 μg/mL compared to 0.5 μg/mL for random controls. The upper limit of normal is considered to be 2.5 μg/mL. No elevated (>25 μg/dL) blood lead levels were detected, but 42% of these individuals had elevated urine levels of retinol-binding protein, indicative of renal tubular damage. Ayurvedic medicines also have been implicated in metal contamination.
Some of the “health” supplements such as bone meal (for calcium) contain high amounts of lead, and some zinc supplements are contaminated with cadmium. See also Chapter 8 for more on herbal remedies.
In Ohio, several members of a household were hospitalized with a diagnosis of acrodynia, a form of metallic mercury poisoning in which neurological and psychological disorders occur as well as hypertension, rash, sweating, cold intolerance, tremor, irritability, insomnia, anorexia, and diminished performance at school. Twenty-four-hour urine collections revealed mercury levels of 850–1500 μg/mL (normal <20 mg/mL). Careful inquiry of neighbors indicated that a previous tenant had spilled a large jar of elemental mercury in the apartment. Treatment was instituted with the oral chelating agent 2,3-dimercaptosuccinic acid (DMSA). Some neurological disorders persisted.
Case Study 11
In early spring, two of five workers employed in demolishing an old iron bridge visited the company’s consulting physician complaining of muscle pain (myalgia), joint pain (arthralgia), headache, and nausea. These workers had been cutting up sections of the bridge using oxyacetylene torches. Large sections were lowered to a barge moored in the river below the bridge to be cut into smaller sections for hauling away. When the remaining three workers were questioned, it was discovered that they too had been suffering from similar symptoms as well as memory loss and irritability. A supervisor and a secretary who worked in the construction shack on shore were not affected, nor were four men involved in loading trucks or operating a small boom crane.
Q. What organ systems are involved in the affected workers?
Q. Could this be a toxicant causing the illness and, if so, what are its possible sources?
Q. What is the likely portal of entry?
Q. What diagnostic tests might be useful?
Q. What specific treatment might be appropriate?
Case Study 12
A 67-year-old man consulted his physician because of severe abdominal pain, weight loss, and fatigue. The doctor initially suspected gastric carcinoma but the patient was severely anemic, his red cells had basophilic stippling, and he had a blood lead level of 70 μg/dL. Six other household members also were affected, including an 8-year-old child. All had elevated blood lead levels. The home was located in a suburban residential area not near any industrial site.
Q. What is the probable portal of entry of the lead in these people?
Q. A search of the home revealed a ceramic jug as the offending agent. Why was it suspect?
Case Study 13
During a routine preemployment medical examination, a 46-year-old male was found to have a blood lead level of 50 μg/dL. He was subsequently investigated by a university hospital toxicology clinic that confirmed the same blood lead level 1 month later. Symptoms included numbness of the fingers and palms, tinnitus (ringing in the ears), and an apparent decrease in ability to do mental arithmetic and mild memory deficits. He had been taking ranitidine for indigestion. This is a histamine H2 receptor blocker that suppresses gastric acid secretion.
A detailed personal and employment history was obtained. He had spent 20 years as an electronics technician in the army and in civilian life but had had little exposure to lead from soldering or welding. He had no hobbies that could serve as a source of lead, no history of bullet or birdshot wounds and he denied drinking bootleg alcohol or using lead additives in his car.
Q. Is the source of lead likely to be work related or from the home environment? How could this be determined?
Q. What is the probable significance of the gastric distress?
Q. What therapeutic approach would be appropriate?
Review Questions
- For Questions 1–6 answer true or false:
- The term heavy metal usually refers to those with double-digit specific gravities.
- Aluminum, which has a specific gravity of only 2.7, has no toxic properties.
- All forms of arsenic are equally toxic.
- Lead poisoning may be manifested as both gastrointestinal and central nervous system toxicity.
- Acute, paroxysmal, colicky pain is common in severe lead poisoning in adults.
- Mental retardation does not occur in lead poisoning in children.
- For Questions 7–11 use the following code:
Answer A if statements a, b, and c are correct.
Answer B if statements a and c are correct.
Answer C if statements b and d are correct.
Answer D if statement d only is correct.
Answer E if all statements (a, b, c, d) are correct.
- a. Subchronic lead poisoning involves interference with mitochondrial heme synthesis at several levels.
- Anemia may accompany lead toxicity.
- Elemental lead is not absorbed through the skin or lungs.
- Elemental lead is not absorbed from the intestinal tract.
- a. All metals can cross the placenta and cause fetal toxicity.
- Examining a blood sample is the only way of detecting lead in the body.
- Chelation treatment with dimercaprol may be used in lead poisoning.
- Chelation therapy is of no use in elemental mercury poisoning.
- a. Elemental mercury poisoning never occurs.
- Methylmercury is the most important environmental source of mercury poisoning.
- Chelation therapy is useful in the treatment of methylmercury poisoning.
- Methylmercury poisoning may involve a variety of central nervous symptoms as well as signs of arthritis.
- 10. a. Cadmium accumulates in the liver and kidney.
- Cadmium induces the synthesis of the cadmium-binding protein metallothionein.
- The kidney is the major organ of toxicity for cadmium.
- Chronic inhalation of cadmium fumes may lead to pulmonary disease.
- 11. a. Like mercury, arsenic binds to sulfhydryl and disulfide groups.
- Trivalent forms of arsenic are the most toxic.
- Arsenic is readily absorbed from virtually all portals of entry.
- Arsenic does not accumulate up the food chain.
- For Questions 12–16, select, from the following list, the correct mechanism for the stated metal.
- (An answer may be used only once):
- Chelates with organic ligands containing SH groups
- Complexes with cysteine and competes with methionine in protein synthesis
- Mimics calcium and is deposited in bone
- Carried to the kidney by bicarbonate where it dissociates to cause renal damage
- The hexavalent form enters cells and is converted to the trivalent form that binds to, and poisons, macromolecules
- 12. Lead, arsenic.
- 13. Lead only.
- 14. Uranium (uranyl cation).
- 15. Methylmercury.
- 16. Chromium.
Answers
- True
- False
- False
- True
- True
- False
- A
- B
- C
- 10. E
- 11. A
- 12. a
- 13. c
- 14. d
- 15. b
- 16. e
Further Reading
Angle, C.R., Childhood lead poisoning and its treatment, Annu. Rev. Pharmacol. Toxicol., 32, 409–434, 1993.
Berg, M., Stengel, C., Trang, P.T.K., Viet, P.H., Sampson, M.L., Leng, M., Samreth, S., and Fredricks, D., Magnitude of arsenic pollution in the Mekong and red River deltas-Cambodia and Vietnam, Sci. Total Environ., 372, 413–425, 2007.
Blyth, A.W., Poisons; Their Effects and Detection, Wm. Wood & Co., New York, 1885.
Boffetta, P., Merler, E., and Vainio, H., Carcinogenicity of mercury and mercury compounds, Scand. J. Work Environ. Health, 19, 1–7, 1993.
Centers for Disease Control (CDC), Cadmium and lead exposure associated with pharmaceuticals imported from Asia, MMWR Morb. Mortal. Wkly Rep., 38, 612–614, 1989.
Centers for Disease Control and Prevention (CDC), Elemental mercury poisoning in a household, Morb. Mortal. Wkly Rep., 39, 424–425, 1990.
Clarkson, T.W., Molecular and ionic mimicry of toxic metals, Annu. Rev. Pharmacol. Toxicol., 32, 545–571, 1993.
D’souza, H.S., D’souza, S.A., Menezes, G., and Venkatesh, T., Diagnosis, evaluation and treatment of lead poisoning in general population, Indian J. Clin. Biochem., 26, 197–202, 2011.
Fimreite, N., Mercury contamination in Canada and its effects on wildlife, PhD thesis, Western University Library, London, Ontario, Canada, 1970.
Hutchison, G. and Wallace, D., Grassy Narrows, Van Nostrand Reinhold, Toronto, Ontario, Canada, 1977.
Jaworski, J. (ed.), Effects of chromium, alkali halides, arsenic, asbestos, mercury and cadmium in the Canadian environment. NRC Executive Reports, Publ # NRC 17585, 1980.
Julin, B., Wolk, A., Bergkvist, L., Bottai, M., and Akesson, A., Dietary cadmium exposure and risk of postmenopausal breast cancer: A population-based prospective cohort study, Cancer Res., 72, 1459–1466, 2012.
Kinghorn, A., Solomon, P., and Chan, H.M., Temporal and spatial trends in mercury in fish collected in the English-Wabagoon river system in Ontario, Canada, Sci. Total Environ., 372, 615–623, 2007.
Klassen, C.D. (ed.), Casarett and Doull’s Toxicology: The Basic Science of Poisons, 7th edn., McGraw-Hill Medical, New York, 2008.
Klassen, C.D. and Watkins, J.B. III. (eds.), Casarett and Doull’s Essentials of Toxicology, McGraw-Hill Medical, New York, 2010.
Marquardt, H., Schafer, S.G., McCellan, R., and Welsch, F., Toxicology, Academic Press, New York, 1999.
McPhee, S.J. and Papadakis, M.A. (eds.), Rabow, M.W. (assoc. ed.), Current Medical Diagnosis and Treatment, 51st edn., Lange Medical Books/McGraw-Hill, New York, 2010.
Neghab, M., Norouzi, M.A., Choobineh, A., Kardaniyan, M.R., and Zadeh, J.H., Health effects associated with long-term occupational exposure of employees of a chlor-alkali plant to mercury, Int. J. Occup. Saf. Ergon., 18, 97–106, 2012.
Santini, J.M. and Ward, S.A., The Metabolism of Arsenic, Taylor & Francis Group, Boca Raton, FL, 2012.
Timbrell, J.A., Biochemical Toxicology, 4th edn., Informa Healthcare, New York, 2009.
Walker, C.H., Hopkin, S.P., Sibly, R.M., and Peakall, D.B., Principles of Ecotoxicology, Taylor & Francis Group, London, U.K., 1996.
Zuber, S.L. and Newman M.C. (eds.), Mercury Pollution: A Transdisciplinary Treatment, Taylor & Francis Group, Boca Raton, FL, 2012.

