2 Low-Power Energy Harvesting Solutions for Biomedical Devices
CONTENTS
2.2.1 Types of Energy Harvesting Sources and Power Ranges
2.2.2 Fields of Application: From Bridge Monitoring to Medical Implants
2.2.3 Powering Solutions for Human Wearable and Implantable Devices
2.1 Introduction
Interest in the recovery of available energy from the environment is constantly increasing. The greenhouse effect in relation to climate change is a present and future concern. The possibility of converting energy available in the human environment and the capacity to transform it into useful energy has resulted in the creation of infrastructures capable of recovering tens of megawatts in the form of electrical energy—thanks, for example, to wind turbines or wave energy systems. In this way we can speak of energy harvesting on the macroscale, as is perhaps best known to the general public, but the importance of energy recovery on the microscale is even greater. What does one understand by recovery on the microscale? We should think of energy recovery that can vary in the range of nanowatts to milliwatts [1]. Evidently, approximation to these levels of recovery and interest in it depend largely on the field of application. The clearest example is in the field of portable consumer products. The possibility of keeping a mobile telephone charged without the need for a battery, with all the attendant environmental advantages, gives us a clear perspective. If electronic equipment generally could avoid the need for a battery, being charged instead by the user’s own movements or by the difference in temperature between it and the environment, the existing ambient light, or the electromagnetic waves it produces in the environment, and if we extend this to millions of mobile telephones, we can extrapolate the enormous advantages of being able to develop efficient electronic systems without batteries.
In existing applications, we can speak of small-scale recovery when we refer to intelligent sensors capable of self-charging and able to transmit from the field to a remote center of communication. The concept and development of these smart wireless sensors is referred to in the present chapter. The fields of application are broad, as we will see, from the monitoring of the structural condition of bridges and highways [2] to the level of so-called intelligent buildings [3]. In general, the conception and development of intelligent and autonomous sensors that do not require the use of batteries bring many advantages from the point of view of cost, maintenance [4], etc. But the investigation and conception of these self-powered units also brings us to the field of medical healthcare in various aspects, such as the remote monitoring of chronic illnesses; avoiding continuous hospital admissions is a notable aspect [5], known as pervasive healthcare [6]. This chapter will review the principal sources of energy present in the environment that are capable of recovery and conversion to electrical energy, with particular attention to those orientated to the microscale or the nanoscale.
2.2 Energy Harvesting
2.2.1 Types of Energy Harvesting Sources and Power Ranges
There exist various sources for the recovery of energy present in the environment, and those sources as well as the type of conducting element determine the admissible levels of recoverable energy, as do the fields of application. Among the typical sources and energy transducers available in the environment there are, for example, vibrations [7], heat [8], light [9], and radio waves [10]. The available energy per unit area, or volume, for each one of these sources depends heavily on the size, operating conditions, and technologies available [1]. Table 2.1 introduces some values for vibrational sources, ambient light, radio frequency (RF) and thermoelectrical sources. Vibrational energy is a typical approach to harvesting energy. Three main solutions are developed based on the type of transducer or microgenerator: piezoelectric (∼200 μW/ cm3), electrostatic (∼50–100 μW/ cm3), and electromagnetic (<1 μW/ cm3). There are wide ranging scenarios where this type of harvesting is or could be applied basically to monitor structural integrities. We can think of different fields of application in terms of the following mapping:
Stiff structures that themselves produce movement (ships, containers, mobile devices, housings of fans, escalators and elevators in public places, appliances, refrigerators, bridges, automobiles, building structures, trains)
Elastic structures that show an elastic deformation of their walls (rotor blades, wind mill blades, aircraft wings, pumps, motors, HVAC ducts, rotorcraft)
Soft structures with very low elastic modulus and high deformation ratios (different textiles, leather, rubber membranes, piping with internal fluid flow)
TABLE 2.1 Mapping of available energy sources
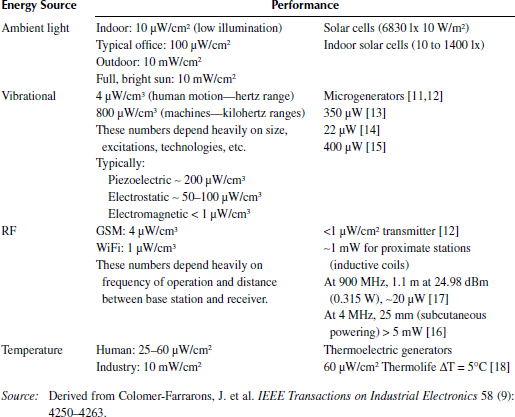
For industrial applications, it is possible to take advantage of “fixed frequency” vibrations because AC-driven motors and pumps produce vibration harmonics from their drive frequency (for example, 60 Hz in the United States and 50 Hz in Europe). For vehicles, the result is more random vibrations than in the other categories. Although nearly all vehicular applications provide significant vibration amplitudes and sufficient levels for energy harvesting, this energy is available more through random occurrences such as bumps, rough surfaces, and dynamic frequencies than through one particular frequency of interest.
Different industrial solutions exist in the market and some examples are introduced. Advanced Linear Devices® has developed different energy harvesting modules. EnOcean GmbH defines self-powered sensor networks and monitors energy harvesting from natural motion; MicroStrain®, the Volture system from Mide Technology®, and the Perpetuum PMG® series harvest the vibrations in their installed environment to generate electricity for wireless sensor and other applications.
Thermal generators are based on the Seebeck effect and convert the temperature difference or heat into an electrical energy. An interesting example is the thermoelectric generator (LPTG) by Thermo Life (Thermo Life Energy Corp, California) [18], measuring 0.5 cm2 and 1.6 mm thick, that can supply energy to a biosensor when in contact with the skin.
Interesting research has been presented by IMEC [19], which has demonstrated the integration of a wireless autonomous sensor system in clothes. The system is fully autonomous for its entire life and requires no service—such as replacing or recharging the battery—from the user. The shirt with integrated electronics can be washed in a regular washing machine. The device is powered from a rechargeable battery. The battery is constantly recharged, mainly through thermoelectric conversion of the wearer’s body heat. The thermoelectric generator is divided into 14 modules to guarantee user comfort. It occupies less than 1.5% of the shirt area and typically generates a power of 0.8–1 mW at about 1 V at regular sedentary office activity. However, if the user walks indoors, the power increases up to 2.7 mW at 22°C due to forced convection. The thermoelectric generator is neither cold nor intrusive for the user. In colder environments where other clothes need to be worn on top of the shirt, the power generation is typically not affected. In summary, this is one of the major fields with a huge rate of growth expected in the following years.
Ambient light is another typical example. In Nasiri, Zabalawi, and Mandic [20], the utilization of indoor cells for extremely low conditions of illumination and low voltages of operation is shown for a cell of 55 mm × 20 mm × 1.1 mm with a power of 5 μW at 10 lx to 200 μW at 1450 lx for a voltage drop of 2 V. RF harvesting is also applied, but depends heavily on frequency of operation and distance between the base station and the receiver, where the energy conversion takes place. Typical values for standard bands are 4 μW/ cm2 (GSM [global system for mobile communications]) and 1 μW/ cm2 (WiFi). In the case of inductive coils, an interesting case is reported in Carrara et al. [16], where a complementary metal–oxide semiconductor (CMOS) (solution is implemented in a 0.18 μm technology), operating with an input carrier of 900 MHz and an input voltage of 250 mV in the integrated antenna in the tag, will theoretically generate an average power of 25 μW, with an output voltage of 1.8 V to supply the tag electronics.
Attention is especially focused on body harvesting. The technological evolution is defining a new scenario in which it will be possible to monitor patients anywhere at any time (see Figure 2.1), which is of increasing interest. The traditional approach, where patients are monitored during hospital or surgery visits, would be replaced by continuous and remote monitoring, which could have a great impact on patients’ quality of life. This is the concept of pervasive monitoring [21,22]. Different scenarios can be envisaged in the continuous search to meet technological challenges through miniaturization, intelligence, and autonomy of the biomedical devices [23], looking for new implantable devices or capsules, like ultrasound pill cameras. Here resides the importance of the autonomy of the devices and the importance of body harvesting, which is addressed in more detail in Section 2.2.2, along with new approaches. Some examples are introduced.
The conversion, for instance, of the natural motion of the body based on mechanical (vibration) energy to electrical energy is one of the main research topics. In this context, the power that can be harvested when a human being walks or runs has been studied at different locations, with an average of 0.5 mW/ cm3 for hip, chest, elbow, upper arm, and head, and a maximum of 10 mW/ cm3 for the ankle and knee. The mechanical energy can be converted to electrical energy based, for instance, on electromagnetic, electrostatic, or piezoelectric principles. But following these approaches just a few microwatts are available [23]. The other classical source of harvesting is body heat. Taking into account that the total amount of power that is waste in the form of heat is around 100 W, the energy that can be recovered, typically using the Carnot engine, is in the range of 2.4 to 4.8 W, but just a few milliwatts are really available [22].

FIGURE 2.1 Typical full wireless body sensor networks (WBSNs). (From Yang, G.-Z. 2006. Body Sensor Networks. London: Springer. With permission.)
Furthermore, in the function of the field and the application, if the quantity of energy available for recovery is small, it is useful to be able to combine different sources at the same time. In this way they are combined and at the same time the system does not depend entirely on one source. A platform that combines different sources [24] present in the environment to charge a microsystem is presented, in a form that could, at the conceptual level, be autonomous. The combination of four sources is envisaged [1]: the vibrational, based on the use of a piezoelectric generator as the conducting element; light, based on the use of interior solar cells; RF signals, through electromagnetic coupling between inductors; and, finally, a thermoelectric element. This system is implemented and described in more detail in Section 2.3 of this chapter. The ability to recover energy on the micro-or nanoscale requires the development of efficient conduction elements and this is a topic of great interest [25] in which the great importance of energy modeling in the design of these new self-powered nanodevices and the conception of nanonetworks is envisaged [26]. Recent references exist in the field of MEMS (microelectromechanical sensors) as much on the aspect of the sensor as of the energy conductor. In Khoshnoud and de Silva [27], a full review of new approaches is revised by the authors. Special interest is focused on the field of energy harvesting for self-powered sensors and their expected impact against the greenhouse effect [28].
Recent developments have been published regarding new approaches for micro-and nanoharvesting purposes in the field of transducers based on the piezoelectric effect [29]. In Trolier-McKinstry et al. [30], the authors present a report on piezoelectric thin films, looking for ultra low-voltage applications and low frequencies of operation (from 50 to 500 Hz), with power densities as high as 10–4 W/ cm2 at 1 g acceleration. Other energy harvesting sources and transducers are investigated. Some solutions based on the electrostatic effect have been reported recently. In Liu, Lye, and Miao [31], a sandwich parallel-plate combination of two capacitors is implemented, with a final size of 1 cm2, capable of operating at low accelerations and low frequencies, up to 50 Hz, with a maximum recovered power of 20 nW for a maximum acceleration of 10 ms–2. This is a technological approach, but in Peterson and Rincon-Mora [32] a circuitry approach to improve the recovered energy is presented that lowers conduction losses and increases the voltage across the plates.
In the field of light, there are new technological approaches [33] and circuitry solutions to improve the maximum power point tracking (MPPT) technique, which maximizes the power that is harvested. This technique represents an excessive overhead of power when it is applied to systems recovering energy at the microscale. Then, it is necessary to develop MPPT techniques with lower power consumption, as in Lu et al. [34], where from an available power of 140 μW, it is possible to recover 46.65 μW: an efficiency of 33.15%—and with power consumption, in average lighting conditions, from 784 to 2152 lx, of 180 μW, and efficiencies up to 39.51%.
New technological advances in nanomaterials present new opportunities in the future development of the storage element, with special interest in grapheme because it presents high electrical conductivity and mechanical stability, which are ideal for new storage elements, such as supercapacitors [35]. At the nanoscale, there is interesting work regarding nano-antennas, which are dipole antennas of 60 nm of width, operating at the terahertz range, where small MOM (metal–oxide–metal) tunneling diodes of 50 nm of range are envisaged for the AC rectification to recover energy from the incoming signal [36].Special interest, at the micro-and nanoscale, is focused on thermoelectric generators (TEGs), for energy harvesting purposes. In Paul et al. [37], Si/ SiGe heterostructure nanoscale materials, grown on Si substrates, are derived to improve the thermoelectric performances at room temperature, improving the Seebeck coefficient compared with a bulk p-type Ge generator, with comparable doping density.
In general, at the level of the implementation of the electronics associated with these systems, they must present a trade-off between their level of intelligence and the level of energy they can recover, and their own consumption. For instance, in the case of TEG, a power management unit (PMU), which generates a DC voltage level suitable for the rest of the electronics [38], is necessary and loads present in a self-powered sensor node. A PMU [28] capable of setting up a low-input voltage from the TEG—as low as 150 mW to an output voltage for ultra low-voltage circuitries of 0.85–0.90 V is presented. In the case of vibrations coming from piezoelectric or electrostatic transducers, the PMU unit presents a sensible circuitry, which is defined by the integrated rectifier, as in the case of RF harvesting [36]. There is an interesting possibility of combining full-wave active rectifiers [39] capable of working with low-voltage amplitudes and with high efficiencies up to 90% with a charge-pump module to work with scavenged low voltages and generate regulated DC levels [40]. The charge pump, as a DC–DC converter, is designed to track the maximum power point transfer, in the range of 10 to 200 μW, for specific load conditions, operating from 0.5 to 2.5 V with efficiency close to 50%.
A more recent work by the same authors presents a different PMU with higher efficiencies, up to 93% with an MPPT also implemented, and a greater input voltage range of operation [41], from 0.44 to 4.15 V. In this case, the active rectifier also acts as voltage doubler. In the field of RF harvesting, a key point is the design also of the ACDC stage [42]. A very interesting setup is presented in Frizzell-Makowski et al. [43], where a full battery-free dynamic on/ off time (DOOT) control, with a buck converter, is implemented, with efficiencies up to 95% and a low-power static consumption, as low as 217 nW.
2.2.2 Fields of Application: From Bridge Monitoring to Medical Implants
We have broadly introduced the types of sources that can recovery energy from the environment, and among them we have introduced an application of interest for monitoring the mechanical condition of infrastructures (e.g., bridges [2]) in the application of sensors in the environment of intelligent buildings [3], surveillance systems [43], and medical implants [22,23]. The solutions implemented extend:
From the use of indoor solar cells [44], from the light present in the environment, for application in control of the environment in buildings in which the low consumption of the electronics would permit the system to operate with only 100 lx to solutions in which the possibility of using electromagnetic energy present is the environment is analyzed [45]
From the electrical network, such as radiation from radio stations for mobile telephones, to more revolutionary solutions such as those found in Zhu et al. [46], in that of the automatic sensor based on a miniature airflow energy harvester generator that consists of a wing attached to a cantilever capable of generating up to 90 μW in conditions of airflow speed as low as 2 ms–1
An interesting work on very small self-powered sensors is reported in Lee et al. [47]. This is a cubic millimeter approach rather than bulky solutions, greater than 1 cm3.
But particular interest is focused in self-powered devices destined for medical applications. In the last 5 years, interest in the development of so-called body sensor networks (BSNs) has increased [5]. The miniaturization of the main electronic systems involved in such systems (as depicted in Figure 2.2), such as the instrumentation, the signal processing module, communications devices, and sensors), has opened up the increasing evolution of this field, where not only single wearable sensors are conceived, but also implantable solutions in the conception of e-health, where new trends are introduced into the medical industry. The traditional approach, where patients are monitored during hospital or surgery visits, would be replaced by continuous and remote monitoring, which could have a great impact on patients’ quality of life. This is the concept of pervasive monitoring [21]. Such an approach should represent a lower cost for health systems, thanks to the reduced number of visits and hospital stays patients will require. Different scenarios can be envisaged in the continuous search to meet technological challenges through miniaturization, from micro-to nanotechnologies, intelligence, and autonomy [22]. As has been pointed out, special interest has been taken in developments that address chronic illness [48], where traditional approaches are not capable of ruling out sudden death events— particularly in the case of cardiovascular illness [49]. The opportunity of detecting symptoms early in patients who are at risk, of monitoring patients who are following a course of treatment, or of tracking how a disease is progressing offers the possibility of preventing the worst case scenarios. In particular, it is of special interest to track the medical parameters of patients who are not following the traditional clinical observation in hospital. The trick is to monitor patients in their routine daily activities.

FIGURE 2.2 Miniaturization of the main involved electronics.
Another scenario focuses on elderly patients, who constitute a population at risk; as people live longer, the need for medical resources increases. The possibility of monitoring this substantial proportion of the population, remotely, from their homes, will reduce medical cost [50]. However, BSN monitoring is also opening up new options of interest to hospitals. People who undergo surgery may need to be tracked via intensive monitoring immediately before and after the operation—not just while they are confined to bed. Furthermore, the possibility of monitoring the patient’s specific intervention zone after surgery is increasingly becoming a topic of interest [51]. However, the development of such solutions is quite complex.
Different papers have been published recently that focus on applications in different fields. These include, for instance, approaches that present multisensing platforms for patients who could be monitored in their daily lives at home [52]. A variety of sensors are placed in a monitored home laboratory, where different activities are tracked to provide information regarding the motion of the patient; however, this is not limited to an option based on wearable sensors. The authors combine such bodily devices with ambient sensors programmed for specific algorithms that have the capability to identify just one activity at a time. Other approaches have focused on single units that consist of different multisensors [53]. In such approaches a single unit placed on the chest of the patient is able to monitor body motion, activity intensity, and other parameters, such as heart rate, or provide electrocardiography (ECG) data, etc.
BSN solutions are not just conceived as discrete external resources. Specific and highly complex solutions are envisaged in the search for devices that are truly implantable in the body and not just positioned on the body. The power consumption is therefore one of the main issues to consider [54] in a BSN mode. Such systems have been powered—but in external devices, where the approach is different than it is for implantable devices [55], as is presented later. Ideally, the change toward removing the use of batteries would be a priority. Concerns about the role of batteries are also presented in Zheng et al. [56], where computing platforms based on electronic textiles are considered as one of the fields of future development. Batteries have a limited lifetime that may be as long as 10 years in some applications, but the ideal approach would be to remove this element and ensure the reliability and operability of the systems without the need to use an element that must be replaced. This is particularly salient when considering implantable devices. The possibility of ensuring a long-term working life of such devices, without the use of the batteries, can improve the quality of life of patients without the need for any surgery.
Nowadays, the concept of new e-health systems already imposes very low-power consumption restrictions on the electronic instrumentation, processing devices, and communication systems in order to extend the operating life of batteries. If the batteries are removed, then the system must be powered by different kinds of energy sources present in the immediate environment—that is, the body itself or specific solutions that will depend on the placement of the sensors. This situation produces new challenges for engineers. Systems that rely on just one power source could be a problem. If that energy source is not always available, the power could fail. So, different approaches should be considered. In one case, if a battery is still used, that power source could be combined with other possible scavenging sources in such a way that, in terms of the operating protocol of the system, the battery lifetime is extended. This could be achieved by recharging the battery when enough energy is recovered, or the battery could be in an open-load configuration in which the required operating energy is supplied by the scavenger module. If the battery is removed altogether, the system must rely on a combination of different scavenger energy sources.
The types of sensors, their location, and the amount of data that must be processed and transmitted define the power budget that must be considered in order to define the power module.
The approach depicted in Figure 2.1 shows not only the monitoring objective of such a system, but also the concept of a closed loop. In such a system, monitoring the patient is not the only objective; the system also has to be able to generate electrical stimulation. This is a new approach. In some sense the monitoring sensor nodes must take measurements periodically while sensors that also have a treatment functionality can operate either periodically or be event driven, which thereby defines different power consumption scenarios. The typical paradigm example of a sensor node with functionality is the artificial pancreas [57]. Another key example is related to micro nerve stimulation and recording, as in previous work [58], but for which bioamplifier circuits and stimulator output stage circuits are being developed nowadays [59] for regenerative microchannel interfaces.
Many examples are currently being developed in the field of implantable devices. Some of them are presented in what follows, paying special attention to the power involved, power supply, and operating ranges. In the field of pacemakers, Lee et al. [60] present a programmable, implantable microstimulator with wireless telemetry for endocardial stimulation in order to detect and correct cardiac arrhythmias. That option lists the global power consumption as 48 μW, but it relies on a rechargeable battery based on RF coupling. Later, an implantable device that is placed deep in the body is presented. Another interesting case is presented in Langel et al. [61]. There, a transcutaneous implantable device is developed based on an infrared radiation (IR) link just a few centimeters from the emitter. The device is completely autonomous, battery free, and powered through an AC signal operating at the industrial, scientific, and medical (ISM) radio band. It incorporates low-frequency operation at 13.56 MHz, with a global power consumption of 270 μW and an application-specific integrated circuit (ASIC) size of 1.4 × 1.4 mm2.
An interesting approach is presented in Liao et al. [62]. There, for the particular case of glucose monitoring, a noninvasive solution is presented based on an active contact lens, whose size is limited to 0.5 × 0.5 mm2. In that case, the implant is powered by a rectified 2.4 GHz RF power signal source at a distance of 15 cm with a global power consumption of 3 μW. Some specific examples in the case of neuro-science applications are also introduced. In Chiu et al. [63], a CMOS implementation is presented that operates as a stimulator of the dorsal root ganglion, which has been experimentally probed with rats. A battery-free solution is envisaged with an induced 1 MHz RF signal at a distance of 18 mm, at the standard medical implanted communication system frequency (MICS) of 402 MHz. Another example of an inductively powered neural SoC (system on chip) system is presented in Lee et al. [64]. In a 0.5 μm technology, a 4.9 × 3.3 mm2 SoC is designed for a 32-channel wireless integrated neural recording system, with a power dissipation of 5.85 mW.
There are other implantable devices that are more oriented toward biomechanical applications: in particular, the resources needed for monitoring prosthetic implants that are used in human beings to replace joints and bones (Figure 2.3). Such implants are designed to have a duration of 20 years, but degradation can lead to the necessity to replace them, with all the associated inconveniences and risks to the patients. The possibility of integrating electronics to monitor fatigue in the implants has clear advantages in protecting against premature mechanical failures. The amount of wear that implants suffer depends of many variables, but, in particular, on the level of activity and weight of the patient. The typical locations of such implants are knee joints, carpal and tarsal, scapula, and hip-socket joints. Arthroplasty applications with monitoring electronics must ensure genuine autonomous long-term operation, in terms of capturing measurement data that are to be transmitted and powering the system.

FIGURE 2.3 Implantables for biomechanical applications.
Interesting applications have been reported where self-powered systems are based on piezoelectric transducers that use mechanical deformation to sense the strain, but are also a harvesting power source for the system [65]. In such cases, human motion is used to power the electronics and the battery is removed, as is presented later. In Almouahed et al. [66], a knee implant is presented based on the use of piezoceramics able to deliver a maximum average electrical power of 12 mW at the tibial base plate. In Lahuec et al. [67], based on another approach, the estimated power is 1.8 mW. The power levels are low, so the power consumption of the integrated electronics must be very low [68].
The design of an implantable device must contemplate different modules for implementation. The energy levels that are involved in the development of an integrated solution will vary in terms of the final application and the level of intelligence of the device. A system that must work continuously is not the same as a system that works in bursts. Four modules are usually present in an implantable device:
The sensor or sensors involved, which fix the type of measurement and the complexity of the front-end instrumentation, which is the signal conditioning
The data processing
The wireless module
The power management unit
If the system has to close the loop, the stimulation electronics—based on DC–DC converters and drivers—are also involved in the power budget. The design of the front-end electronics [69] depends on the type of medical signals that must be considered. It can be stated that these signals may vary from a few microvolts to several millivolts, with a frequency band that varies from a few hertz to kilohertz, as depicted in Figure 2.4. Typically, electroencephalogram (EEG) signals ranges from 1 to 50 μV and have low frequencies from 1 to 100 Hz. Electrocardiogram (ECG) signals present the highest voltage level: in the range of millivolts. Electromyogram (EMG) signals and action potentials present the highest frequencies: up to 10 kHz.

FIGURE 2.4 Some types of medical signals.
The power consumption levels (in terms of energy per operation) depend on the electronics involved. If a microprocessor is needed, then a typical power consumption of 300 μW for commercial solutions, such as the Texas Instruments® MSP430 microprocessor is far from the desired power budget. Favorable examples of specific microprocessors have been presented in Hanson et al. [70] and Zhai et al. [71]. Hanson et al. [70] present the Phoenix Processor, in a 180 nm technology, with a power consumption of 226 nW, but with emphasis on the standby consumption, which is as low as 35.4 pW. It has an area of 915 × 915 μm2 and operates at 0.5 V. It evolved from Zhai et al. [71], where the subthreshold operating region is explored.
Analog–digital converter (ADC) conversion in terms of the needs of sample conversion rates and the submicrowatt transmitters (as a function of the distance between the implanted device and the exterior on the one hand and the data transmission rate on the other) are two of the key elements of these designs. Some pertinent examples show how research aims to find ADCs with lower power consumption. In Song et al. [72], ultra low-power front-end signal conditioning is implemented for an implantable 16-channel neurosensor array, with a maximum power consumption of 50 μW per channel. The ADC is based on a commercial solution derived by Analog Devices®; a 12-bit AD7495 micro-SO8 standard packaged chip. The complete system has a total consumption of 12 mW.
Specific examples of ADCs for implantable applications have been also derived as in Cheong et al. [73]. There, a 400 nW SAR ADC converter, with 8 bits of ENOB (effective number of bits) and 80 kS/ s is presented in 180 nm technology. The implantable blood pressure sensing microsystem developed in Cong et al. [74] achieves 10-bit resolution with an integrated cyclic-ADC converter with 11-bit resolution and a power consumption of 12 μW at 2 V. The pressure sensor has a full-power dissipation of 300 μW. In Trung and Häfliger [75], an ADC converter, based on an integrated ADC, for a blood glucose monitoring implant presents even better performance at just 10.2 nJ per sample, with 10 ENOB, operating at very low frequencies. Another example, in this case in the field of neural signal acquisition, is presented in Muller, Gambini, and Rabaey [76], where the ADC has a power consumption of 240 nW based on a boxcar sampling ADC [77] operating at 20 kS/ s.
Regarding the wireless module, different approaches depending on the type of signal captured and the amount of data related to the types of complex signals define a wide range of data transmission constraints from a few samples by second, as in the case of heart rating (typically 25 Sps) or scenarios with data transmission rates of several megabits per second, such as medical imaging [78] or recent developments in endoscopic capsules [79,80].
The review in Bashirullah [81] analyzes in detail the parameterization of the different implantable devices in terms of three key variables: the size of the implantable device, the power involved, and functionality and performance. Two main approaches to communications are considered depending on the type of placement of the implant in the body: inductive coupling [82], when there is short distance between the implant and the exterior of the body, and far field electromagnetic communication [83]. The approach for a subcutaneous implant based on inductive links, where coil misalignment and the effects of the geometry have a great impact on performance [84], is not the same as solutions operating at high frequencies following the MICS protocol [85,86]. For inductive link circuits, the operating frequencies are in the range of a few megahertz, typically in the 13.56 MHz band, following the ISM protocol, with characteristic data modulation methods for transmission, such as BPSK (binary phase-shift keying), ASK (amplitude shift keying), or OOK (on-off keying), and a data transfer rate that is not very fast: from a few kilobits per second (100) to several megabits per second (1.6) [87].
In Simard, Sawan, and Massicotte [88], a high-speed OQPSK is presented with a bit rate of 4.16 Mbps based on an inductive link, with high power levels and multiple coils, operating at 1 MHz for the power link and at the ISM 13.56 MHz frequency for the data. In Jung et al. [89], an implant OOK transmitter is presented that operates at 2.4 GHz in 180 nm technology and is able to transmit at 136 Mbps with a power consumption of 3 mW. As has been pointed out [68], for the particular case of an orthopedic implant, the power consumption for the full electronics must be low. The total power consumption of the full electronics is not stated, but the DC–DC that recovers energy from PZT (lead zirconate titanate) and the ADC converter that presents a quiescent power consumption of 150 nW and an operating consumption of 12.5 μW at 1.8 V for a sample frequency of 4 kHz and an ENOB > 7 bits merit special attention.
2.2.3 Powering Solutions for Human Wearable and Implantable Devices
Thus far, we have introduced the concept of energy harvesting and of the basic sources that permit the recovery of energy present in the environment (Figure 2.5). Several applications in the biomedical environment that have been presented have enabled us to visualize the energy needs of these solutions. Specific cases have been discussed, but at this point of development, more specifically, resources that could be used in the strictly biomedical environment. The type of energy source required to power a sensor node varies according to the final application and where the biomedical device is placed. This brings us to three approximations: (a) the possibility of utilizing discrete elements of energy storage, (b) being able to use energy resources present in the environment, and (c) being able to use energy from the human body as a resource. The use of batteries could be a limitation for the envisaged sensor nodes of the future, either on the outside of or implanted within the human body, as in Figure 2.1. The use of large batteries ensures the duration of the system, but the sensor nodes may be too large and heavy. So, smaller solutions with a high enough energy density are needed, combined with ultra low-power electronics solutions that ensure a trade-off between the autonomy of the system and the smart functionality of the sensor, in terms of the sensor, signal processing and communications modules.
FIGURE 2.5 Combined power sources in a sensor node.
Ultra low-power electronics for biomedical applications based on lithium ion batteries are common for nonimplantable solutions, such as BSNs, and also in implantable solutions [90,91]. Lithium ion batteries are divided into two types: (a) single-use batteries, which are placed, for instance, in drug pumps, cardiac defibrillators, and pacemakers; and (b) rechargeable batteries, which are used in artificial hearts. Both types of batteries present an adequate energy density (around 1440 to 3600 J/ cm3) and, unlike some other batteries, they do not present the memory effect; that is, they do not need to be discharged completely before a recharge phase. These batteries have a better life cycle than other types, typically with 20,000 discharges and recharge cycles, but with a finite lifetime limitation, which is typically several years for a battery of 1 cm3.
A key aspect for these batteries is the need for battery-management circuitry to ensure the range of operation, as lithium ion batteries are extremely sensitive to over-voltage (maximum 4.2 V) and deep discharge (minimum 2 V), as well as to ensure high energy efficiencies. An application conceived for biomedical applications has been reported [92] with an average power efficiency of 89.7% and a voltage accuracy of 99.9%. Other approaches are under development. Interest is especially focused on fuel cells, such as the methanol fuel cell [93], but these also have their drawbacks. One is the need to replace the external reactant and the oxidant, which is analogous to the problem of recharging batteries. Although higher levels of energy are expected, based on the use of fuels such as methanol with an energy density of 17,600 J/ cm3, the design issues are highly complex and proving to be very expensive.
Supercapacitors are another field that is being explored as an option for biomedical sensors instead of batteries, but they have a low energy density, which is a problem for systems that require a constant power source for long periods of time. In Pandey et al. [94], a sensor is powered via a supercapacitor that is charged wirelessly; in Shanchez, Sodini, and Dawson [95], an energy management ASIC is implemented and tested to manage supercapacitors for implants.
The next issue concerns the power sources that are available in and around the human body. With reference to sources of energy present in the vicinity of the human body that permit the charging of implantable devices, we are able to emphasize recent works, as in Ayazian and Hassibi [108], in which the possibility of using sub-cutaneous photovoltaic (PV) cells for superficial implants with a recovery capacity of a density to the power of 0.1 μW/ mm2 in conditions of strong light (sunlight) is discussed. Another interesting approximation is at the level of ultrasound [109], with advantages compared to solutions based on RF.
But we can consider other scenarios. For instance, is it possible to recover energy from human bodily motion? Is it possible to recover energy from the body itself? These are two questions addressed in this section. First, we introduce the concept of a harvester system based on an energy harvester, an energy storage element, and power management regulation (Figure 2.6). Four types of energy harvester based on ambient sources— that is, systems that acquire electrical energy from environmental energy sources—are introduced when this system is described: mechanical, solar, thermal, and RF (the details of which are beyond the scope of the present chapter). The power management unit has two main roles: (a) to generate a raw DC voltage from the harvester unit, essentially acting as a conversion module; and (b) to regulate the output voltage from the energy storage unit to the sensor node electronics. The energy storage unit receives energy from the conversion module and stores it.
Attention is especially focused on body harvesting. The energy can be generated passively or actively. The natural motion of the body, where the conversion is based on mechanical (vibration) energy to electrical energy, is one of the main research topics [24]. In this context, the power that can be harvested when a human being walks or runs has been studied at different locations, with an average of 0.5 mW/ cm3 for hip, chest, elbow, upper arm, and head, and a maximum of 10 mW/ cm3 for the ankle and knee. The mechanical energy can be converted to electrical energy based, for instance, on electromagnetic [24], electrostatic [13], or piezoelectric [15] principles.

FIGURE 2.6 Schematic for an energy harvesting solution.
TABLE 2.2 Examples of Human Power Actively Generated
| Activity | Power Generation |
Finger (pushing pen) |
0.3 W |
Legs (cycling at 25 km/ h) |
100 W |
Hand and arm (Freeplay) |
21 W |
Hand (AladdinPower) |
3.6 W |
There are several references to types of design that convert this mechanical energy to electrical energy based on the three methods of transduction just mentioned [96]. The piezoelectric approach is of special interest because high voltages are obtained for low strains with a maximum energy density of 17.7 mJ/ cm3. Several examples are presented in the literature. In particular, in Mhetre et al. [97], piezoelectric microgenerators [98] are envisaged for drug delivery devices or dental applications. Examples of power generated actively are human power through peddling activity [99], or examples such as the Freeplay® or AladdinPower® rechargeable products (see Table 2.2). Walking alone has been analyzed and a prototype implemented [100], but that prototype is excessively large for our purposes.
An extended analysis in terms of the placement of the harvester and the type of everyday activity is presented in Olivares et al. [101]. Based on a commercial cantilever beam harvester from Midé Technologies [102]—the PEH20W Volture— these different scenarios have been analyzed. The greatest level of power extracted (28.74 μW) was from a shank at the instep of the foot while the subject ran fast, and the lowest level of power (0.02 μW) was also from a shank but while performing knee rehabilitation exercises sitting down. As expected, the maximum levels are obtained for activities and placements where higher amplitudes and impacts are produced.
An example of a wearable wireless sensor based on a kinetic energy harvester has been presented [103]. Electromagnetic transduction is used for an average human motion at 0.5 Hz, with a simple architecture implemented via commercial components and a supercapacitor, but not used as a final storage element of the system. It is used to transfer the charge to a smaller supply capacitor, thus improving system start-up. In Zainal Abidin, Hamzah, and Yeop Majlis [104], a MEMS piezoelectric generator is used to harvest energy from vibrations; it also uses supercapacitors as storage elements. An example of a MEMS designed for implantable devices is given in Martínez-Quijada and Chowdhury [105], where it is stated that the microgenerator is able to generate more energy per unit volume than conventional batteries—that is, an RMS power of 390 μW for 1 mm2 of footprint area and a thickness of 500 μm, which is smaller than the volume of a typical battery in a pacemaker.
The other main source of power from the human body is heat, which is limited by the Carnot efficiency that states that the maximum power that can be recovered is in the range of 2.4 to 4.8 W when other possible sources are available, like arm motion (0.33 W), exhalation (0.40 W), or breathing band (0.42 W). In the case of body heat, thermoelectric generators are used [106]. Specific designs must be considered when working with thermoelectric generators in these conditions, as the voltages generated are very small and DC–DC converters with a specific step-up are conceived in order to boost the voltage [107]. An example of a thermogenerator is Thermolife®, which generates 60 μW/ cm2 for a temperature difference of 5°C [11]. It should also be stated that the recovery depends on the specific placement of the thermoelectric generator. Placing it in the neck is not the same as placing it in the head, both of which are parts of the body that are warmer than other zones. The recovery range is 200 to 320 mW for the neck and 600 to 960 mW for the head (three times the surface area of the neck).
There are other approximations in the area of the recovery of energy from the human body itself. From the concept of a fuel cell, the biogenerator for implantable devices has emerged. In some ways, the basic concept is the use of fluids in the body as a fuel source for the fuel cell; this would be an inexhaustible energy source. An interesting approach is the use of glucose as a fuel source and the oxygen dissolved in blood [110,111]. Advanced approaches also explore a shift to the use of white blood cell capacities in biofuel cells [112] or approaches such as that in Muller, Gambini, and Rabaey [76], where the fuel cell is based on the use of a micro organ ism to convert the chemical energy of glucose into electrical energy in a PDMS (polydimethylsiloxane) (plasma desorption ionization mass spectrometry) structure [113].
2.3 Multisource Self-Powered Device Conception
At this point, a sensor node could be powered by following different approaches; the choice depends on the placement of the sensor, which defines its accessibility, its size, and its weight. A battery, a supercapacitor, and a fuel cell are possible choices on the one hand. On the other, the use of some kind of energy harvesting source that recovers energy from the body, mostly based on vibration to electrical conversion or thermoelectric generation, is also envisaged. However, there are some other approaches that can also be considered, depending on the placement of the sensor; these include, the possibility of using environmental resources to harvest energy from for the sensor node, such as light [9,114] or radio waves [10].
The approach introduced previously is RF coupling, both as a way to supply energy to the sensor node and to use the RF link for communications purposes, typically for short distances between the master and slave modules and typically in the 13.56 MHz ISM band. For instance, at 4 MHz and a distance of 25 mm, for sub-cutaneous powering of a biomedical device, the energy recovered was 5 mW [115]. A particular example of this is introduced in Zhang et al. [116]. There, the rectifier module is designed to work at the 915 MHz ISM bandwidth (only in region 2). The performance of the RF source is quite small, from a typical 4 μW/ cm2 for GSM to 1 μW/ cm2 for the WiFi band. For coils, typical values are lower than 1 μW/ cm2, but with as much as 1 mW for close inductive coils (a few centimeters). In the 915 MHz ISM bandwidth, at 1.1 m, the energy recovered is around 20 μW [12]. New approaches are being developed. In particular, the use of ultrasonic powering instead of RF powering is of great interest. In Zhu, Moheimani, and Yuce [117] and Yong, Moheimani, and Yuce [109], 1 V is generated with a power capability of 21.4 nW.
At this point the issue that we need to consider is the possibility of placing or using more than just one energy harvester. This approach, combining indoor solar cells, RF coupling at the ISM 13.56 MHz band, and mechanical vibration based on piezoelectric transducer, was presented in Colomer-Farrarons et al. [1], together with experimental results. The system does not rely on only one harvesting source, and the objective is to use different energy harvesters. A specific power management unit has been designed for a multisource and multiload energy harvesting system with the aim of establishing a minimum number of conversion stages and magnetic components, and of developing a specific algorithm [118]. An approach to the envisaged platform architecture for a multiharvesting WSN has been presented [119]. Another interesting example, commented on in Lahuec et al. [67], has the particularity that it combines the possibility of recovering energy from the motion of a human being based on PZTs, with recovering energy from the RF signal, depending on the operating mode; however, no more details have been reported.
The combination of a vibration energy harvester and charging circuitry for a lithium ion battery has been presented [120]. The architecture proposed in references 1 and 121 has been used to demonstrate the feasibility of combining different harvester sources for a 1 cm3 multisensor node. This is a generic architecture envisaged for a generic self-powered smart sensor that could be applied in different fields of applications, such as the integrity of structures, surveillance, and medical devices and implants. Electromagnetic coupling, with an indoor solar-cell module and a piezoelectric generator, was used. The platform envisaged is defined as a multiharvesting power chip (MHPC) with a total power consumption of 160 μW. The integrated circuit is designed with 0.13 μm technology, which is a low-voltage technology up to 3.3 V. The capacity of a small system to recover a few microwatts from the energy present in the environment of the multisensor node, combining vibration, light, and RF, is a proof of concept and recovers a total power of more than 1 mW.
The system is initially analyzed with each different source working alone. The system recovers 360 μW from the piezoelectric generator, which is a commercial PZT (QP40W) [122], operating at 7 m/ s2 at 80 Hz. Then, two indoor solar cells [123] under indoor conditions of 1500 lx are used, with a total harvesting power of 2.76 mW. Finally, an RF link generator (TRF7960) emitting at full power (200 mW) with a distance between the base station and the antenna receiver of 25 mm is used and recovers 4.5 mW. The total power expected to be recovered from the combination of the three harvested sources would be 7.8 mW, but the experimentally recovered power was 6.2 mW [1,121]. The difference of 1.6 mW between the theoretical total and the actual amount of power that was recovered is due to variations in the effective load conditions for the light and RF modules.
This suggests that research is needed into the trade-off between the implementation of peak power tracking circuitry for the light and RF modules and the improvement that could be achieved, compared with the cost in silicon area and the power consumption of each module. The three main elements in the design of the MHCP ASIC are as follows:
A bandgap reference circuit is based on peak regulation.
A linear dropout (LDO) regulator i based on the bandgap reference circuit, which uses a PMOS switch and an error amplifier [1]. The PMOS ( p-type metal–oxide semiconductor) switch of the LDO regulator is placed at the output stage of the simple two-stage amplifier, where MN1 and MN2 define the ground current. These elements have a power requirement of 30 μW.
Two integrated rectifiers must be placed in the MHCP chip, one for AC/ DC rectification of the piezoelectric generator signal and the other one for the RF-coupled signal.
The MHCP has its own control unit that plays the role of the power management unit, based on the concept presented in Colomer-Farrarons et al. [4]. The system combines a control switch for each of the energy harvester channels in order to be able to operate as a single and independent harvesting channel, with independent storage elements for each channel—that is, multiple storage device configuration (MSD mode of operation) [1] or combining of all them into a single storage device (SSD mode of operation). In SSD mode, the PMU controls the energy stored in the single storage element (a supercapacitor) and transfers energy when an adequate voltage is reached in the capacitor. When the voltage reaches the low-value threshold (Vmin), the system opens the charge transfer to the load until the maximum voltage level (Vmax) is reached again. The PMU also incorporates power on reset (POR). This circuit is used to reduce the power consumption of the module during the start-up phase.
Based on this MHCP ASIC conception, a CMOS architecture for an implantable device [124] is envisaged. Nowadays, interest in nanobiosensors is increasing in the field of medical diagnosis. The development of such devices and the telemedicine environments that can be derived from them has great market potential, as has been pointed out before. Different approaches are required for discrete, small cubic centimeter devices and for implantable devices; the performance, communications capabilities, etc. are very different. The size of the implantable device is envisaged as that of a capsule, ideally less than 4.5 cm long and 2.5 cm in diameter, following the same philosophy as some subcutaneous implantable contraceptive devices such as Norplant®, Jadelle®, and Implanon®.
One proposal is an event-detector implantable system, or an event detector that works as an alarm. When the concentration analyzed moves out of the range of accepted values and reaches a threshold value, the alarm is activated. The proposed generic implantable architecture is presented in Colomer-Farrarons and Miribel-Catala [121]. It is composed of three BioSensor electrodes, an antenna, and the electronic modules. Such a system combines different modules. The antenna and the AC/ DC module are used to supply energy to the device (inductive powering) and the communication setup (backscattering) based on AM modulation. Then, a low-voltage and low-power potentiostat is integrated. The BioSensor is the only part of the implantable device interacting with the biological environment. It detects the desired target generating an electrical signal. The BioSensor’s design must be carefully selected as a function of the target sample to be detected and the total size of the device.
Several BioSensor configurations formed by simply two, three, or four electrodes can be used for single target detection, and more complex array structures of micro-sensors can be introduced for multianalyte detection. The antenna and its associated electronics are used for two main aspects. Firstly, it is used to supply energy to the implantable device (inductive powering) working together with an integrated AC/ DC module. Secondly, the same antenna transmits the information to the external reader through the communications electronic setup (backscattering). In this scenario, one antenna is used for both power generation and communication. This reduces the antenna operation frequency to tens of millihertz due to the inductive power drop caused by the human skin. Moreover, the amount of transmitted information is limited and the size of the antenna is considerable.
Another scenario considers the use of two antennas in the same implantable device: one for the communication and the other for powering. In this case, the communication link can be established around hundreds of megahertz (usually in the 400 MHz ISM band), allowing higher communication rates and reducing the size of the antenna. On the other side, the second antenna is focused to power the electronics through a dedicated inductive link operating at lower frequencies than the communication antenna. In that way, each antenna can be optimized for its functionality. Then, the integrated electronics is introduced to drive the BioSensor and to generate the data to be transmitted. Usually, a low-voltage, low-power potentiostat circuit or similar instrumentations are used to control the implantable sensors.
2.4 Summary and Conclusions
This chapter introduces a review in the state of the art of energy harvesting and focuses interest on the idea of using different types of energy harvesting sources to drive ultra low-power electronics. This approach is followed in order to develop new portable biodevices for human wearable and implantable devices. In that way, it would be possible to envisage new autonomous smart healthcare portable instruments able to be adapted easily to daily human activity. The importance of harvest energy from normal human activities introduces the enormous advantage of reducing bulky batteries from small handheld devices, allowing miniaturization and weigh reduction of the portable instruments.
References
1. Colomer-Farrarons, J., Miribel-Catala, P., Saiz-Vela, A., and Samitier, J. 2011. A multiharvested self-powered system in a low-voltage low-power technology. IEEE Transactions on Industrial Electronics 58 (9): 4250–4263.
2. Sazonov, E. 2009. Self-powered sensors for monitoring on highway bridges. IEEE Sensors Journal 9 (11): 1422–1429.
3. Wang, W. et al. 2010. Autonomous wireless sensor network based building energy and environment monitoring system design (paper presented at the International Conference on Environmental Science and Information Application Technology, Wuhan, China, July 17–18).
4. Colomer-Farrarons, J. et al. 2008. Power-conditioning circuitry for a self powered system based on micro PZT generators in a 0.13 μm low-voltage low-power technology. IEEE Transactions on Industrial Electronics 55 (9): 3249–3257.
5. Yang, G.-Z. 2006. Body sensor networks. London: Springer.
6. Dey, A. K., and D. Estrin. 2011. Perspectives on pervasive health from some of the field’s leading researchers. IEEE Pervasive Computing 10 (2): 4–7.
7. Mehraeen, S., S. Jagannathan, and K. A. Corzine. 2010. Energy harvesting from vibration with alternate scavenging circuitry and tapered cantilever beam. IEEE Transactions on Industrial Electronics 57 (3): 820–830.
8. E. Carlson, K. Strunz, and B. Otis. 2009. 20 mV Input boost converter for thermoelectric energy harvesting (paper presented in the Symposium on VLSI Circuits, Seattle, WA, June 16–18).
9. Nasiri, A., S. A. Zabalawi, and G. Mandic. 2009. Indoor power harvesting using photo-voltaic cells for low-power applications. IEEE Transactions on Industrial Electronics 56 (11): 4502–4509.
10. Sogorb, T., J. V. Llario, J. Pelegri, R. Lajara, and J. Alberola. 2008. Studying the feasibility of energy harvesting from broadcast RF station for WSN (paper presented in the IEEE Instrumentation and Measurement Technology Conference, Victoria, BC, Canada, May 12–15).
11. Roundy, S., D. Steingart, L. Frechette, P. Wright, and J. Rabaey. 2004. Power sources for wireless sensors networks (paper presented in the First European Workshop on Wireless Sensors Networks, Berlin, Germany, January 19–21).
12. Yeatman, E. M. 2007. Energy scavenging for wireless sensor nodes (paper presented in the 2nd International Workshop on Advances in Sensors and Interface, Bari, Italy, June 17–26).
13. Kiziroglou, M. E., C. He, and E. M. Yeatman. 2009. Rolling rod electrostatic micro-generator. IEEE Transactions on Industrial Electronics 56 (4): 1101–1108.
14. Le, T. T. et al. Piezoelectric micro-power generation interface circuits. IEEE Journal of Solid-State Circuits 41 (6): 1411–1420.
15. Garbuio, L. et al. 2009. Mechanical energy harvester with ultralow threshold rectification based on SSHI nonlinear technique. IEEE Transactions on Industrial Electronics 56 (4): 1048–1056.
16. Carrara, S. et al. 2012. Developing highly integrated subcutaneous biochips for remote monitoring of human metabolism (paper presented in the IEEE Sensors, Taipei, Taiwan, October 28–31).
17. Curty, J. P., N. Noehl, C. Dehollain, and M. Declercp. 2005. Remotely powered addressable UHF RFID integrated system. IEEE Journal of Solid-State Circuits 40 (11): 2193–2202.
18. Thermo Life LPTG. Thermo Life Energy Corp. http://www.poweredbythermolife.com
19. Van Hoof, C., V. Leonov, and R. J. M. Vullers. 2009. Thermoelectric and hybrid generators in wearable devices and clothes (paper presented in the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, June 3–5).
20. Nasiri, A., S. A. Zabalawi, and G. Mandic. 2009. Indoor power harvesting using photo-voltaic cells for low-power applications. IEEE Transactions on Industrial Electronics 56 (11): 4502–4509.
21. Garcia-Morchon, O., T. Falck, T. Heer, and K. Wehrle. 2009. Security for pervasive medical sensor networks (paper presented in the International Mobile and Ubiquitous Systems Conference: Networking & Services, MobiQuitous, Toronto, Canada, July 13–16).
22. Penders, J. et al. 2008. Human++: From technology to emerging health monitoring concepts (paper presented in the International Summer School and Symposium on Medical Devices and Biosensors, Hong Kong, China, June 1–3).
23. Mhetre, M. R., N. S. Nagdeo, and H. K. Abhyankar. 2011. Micro energy harvesting for biomedical applications: A review (paper presented in the IEEE International Conference on Electronics Computer Technology, Kanyakumari, India, April 8–10).
24. Christmann, J. F., E. Beigné, C. Condemine, and J. Willemin. 2010. An innovative and efficient energy harvesting platform architecture for autonomous microsystems (paper presented in the IEEE International NEWCAS Conference, Montreal, Canada, June 20–23).
25. Lu, C., V. Raghunathan, and K. Roy. 2011. Efficient design of micro-scale energy harvesting systems. IEEE Journal on Emerging and Selected Topics in Circuits and Systems 1 (3): 254–266.
26. Jornet, J. M. 2012. A joint energy harvesting and consumption model for self-powered nano-devices in nanonetworks (paper presented in the International Conference on Communications, Ottawa, Canada, June 10–15).
27. Khoshnoud, F., and C. W. de Silva. 2012. Recent advances in MEMC sensor technology-mechanical applications. IEEE Instrumentation & Measurement Magazine 15 (2): 14–24.
28. Benecke, S., J. Ruckschloss, N. F. Nissen, and K.-D. Lang. 2012. Energy harvesting on the way to a reliable and green micro energy source (paper presented in the Electronics Goes Green Conference, Berlin, Germany, September 9–12).
29. Lu, J., Y. Zhang, T. Itoh, and R. Maeda. 2011. Design, fabrication and integration of piezoelectric MEMS devices for applications in wireless sensor network (paper presented in the International Symposium on Design, Test, Integration and packaging of MEMS/MOEMS, Aix-en-Provence, France, May 1–13).
30. Trolier-McKinstry, S. et al. 2011. Designing piezoelectric films for micro electromechanical systems. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control 58 (9): 1782–1792.
31. Liu, S. W., S. W. Lye, and J. M. Miao. 2012. Sandwich structured electrostatic/electrets parallel-plate power generator for low acceleration and low frequency vibration energy harvesting (paper presented in the International Conference on Electro Mechanical Systems, Cancun, Mexico, June 23–27).
32. Peterson, K., and G. A. Rincon-Mora. 2012. High-damping energy-harvesting electro-static CMOS charger (paper presented in the IEEE International Symposium on Circuits and Systems, COEX, Seoul, Korea, May 20–23).
33. Martorell, J. 2012. A photonic nano-structuring approach to increase energy harvesting for organic photovoltaic cells (paper presented in the International Conference on Transparent Optical Networks, Coventry, England, July 2–5).
34. Lu, C., S. P. Park, V. Raghunathan, and K. Roy. 2012. Low-overhead maximum power point tracking for micro-scale solar energy harvesting systems (paper presented in the International Conference on VLSi Design, Hyderabad, India, January 7–11).
35. Chen, B. 2012. Nanomaterials for green energy: Next generation energy conversion and storage. IEEE Nanotechnology Magazine 9: 4–7.
36. Bareiss, M. et al. 2011. Energy harvesting using nano antenna array (paper presented in the IEEE International Conference on Nanotechnology, Portland, OR, August 15–19).
37. Paul, D. J. et al. 2012. Si/SiGe nanoscale engineered thermoelectric materials for energy harvesting (paper presented in the IEEE International Conference on Nanotechnology, Birmingham, U.K., August 20–23).
38. AbdeElFattah, M., A. Mohieldin, A. Emira, and E. Sanchez-Sinencio. 2011. A low-voltage charge pump for micro scale thermal energy harvesting (paper presented in the IEEE International Symposium on Industrial Electronics, Gdansk, Poland, June 27–30).
39. Zargham, M., and P. G. Gulak. 2012. High-efficiency CMOS rectifier for fully integrated mW wireless power transfer (paper presented in the IEEE International Symposium on Circuits and Systems, COEX, Seoul, Korea, May 20–23).
40. Maurath, D., P. F. Becker, D. Spreemann, and V. Manoli. 2012. Efficient energy harvesting with electromagnetic energy transducers using active low-voltage rectification and maximum power point tracking. IEEE Journal of Solid-State Circuits 47 (6): 1369–1380.
41. Leicht, J., D. Maurath, and V. Manoli. 2012. Autonomous and self-starting efficient micro energy harvesting interface with adaptive MPPT, buffer monitoring, and voltage stabilization (paper presented in the ESSCIRC Conference, Bordeaux, France, September 17–21).
42. Le, H., N. Fong, and H. C. Luong. 2011. An energy harvesting circuit for GHz on-chip antenna measurement (paper presented in the IEEE International Symposium on Radio-Frequency Integration Technology, Beijing, China, November 30–December 2).
43. Frizzell-Makowski, L. J., R. A. Shelsby, J. Mann, and D. Scheidt. 2011. An autonomous energy harvesting station-keeping vehicle for persistent ocean surveillance (paper presented in the OCEANS Conference, Hawaii, September 19–22).
44. Raisigel, H. et al. 2010. Autonomous wireless sensor node for building climate conditioning application (paper presented in the International Conference on Sensor Technologies and Applications, Venice/Mestre, Italy, July 18–25).
45. vom Boegel, G., F. Meyer, and M. Kemmerling. 2012. Batteryless sensors in building automation by use of wireless energy harvesting (paper presented in the IEEE International Symposium on Wireless Systems, Offenburg, Germany, September 20–21).
46. Zhu, D. et al. 2013. Novel miniature airflow energy harvester for wireless sensing applications in buildings. IEEE Sensors Journal 13 (2): 691–700.
47. Lee, Y. et al. 2013. A modular 1 mm3 die-stacked sensing platform with low power I2C inter-die communication and multi-modal energy harvesting. IEEE Journal of Solid-State Circuits 48 (1): 229–243.
48. Koutkias, V. G. et al. 2010. A personalized framework for medication treatment management in chronic care. IEEE Transactions on Information Technology in Biomedicine 14 (2): 464–472.
49. Zhou, H.-Y., and K.-M Hou. 2010. Pervasive cardiac monitoring system for remote continuous heart care (paper presented in the International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, June 18–20).
50. Zwifel, P., S. Felder, and M. Meiers. 1999. Ageing of population and health care expenditure: A red herring? Health Economics 8: 485–496.
51. Allen, M. G. 2005. Micromachined endovascularly implantable wireless aneurysm pressure sensors: From concept to clinic (paper presented in the 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Transducers, Seoul, Korea, June 5–9).
52. El Helw, M. et al. 2009. An integrated multi-sensing framework for pervasive healthcare monitoring (paper presented in the International Conference on Pervasive Computing Technologies for Healthcare, London, UK, April 1–3).
53. Chuo, Y. et al. 2010. Mechanically flexible wireless multisensor platform for human physical activity and vitals monitoring. IEEE Transactions on Biomedical Circuits and Systems 4 (5): 281–294.
54. Mandal, S., L. Turicchia, and R. A. Sarpeshkar. 2010. A low-power, battery-free tag for body sensor networks. IEEE Pervasive Computing 9 (1): 71–77.
55. Fang, Q. et al. 2011. Developing a wireless implantable body sensor network in MICS band. IEEE Transactions on Information Technology in Biomedicine 15 (4): 567–576.
56. Zheng, N. et al. 2010. Enhancing battery efficiency for pervasive health-monitoring systems based on electronic textiles. IEEE Transactions on Information Technology in Biomedicine 14 (2): 350–359.
57. Ricotti, L. et al. 2011. A novel strategy for long-term implantable pancreas (presented at the International IEEE Conference Engineering in Medicine and Biology Society).
58. Kovacs, G. T. A., C. W. Storment, and J. M. Rosen. 1992. Regeneration microelectrode array for peripheral nerve recording and stimulation. IEEE Transactions on Biomedical Engineering 39 (9): 893–902.
59. Gerald, F. et al. 2012. A regenerative microchannel neural interface for recording from and stimulating peripheral axons in vivo. Journal of Neural Engineering 9 (1): 016010.
60. Lee, S.-Y. et al. 2011. A programmable implantable micro-stimulator SoC with wireless telemetry: Application in closed-loop endocardial stimulation for cardiac pacemaker (paper presented in the International Solid-State Circuits Conference ISSCC, San Francisco, CA, February).
61. Langel, S. et al. 2011. An AC-powered optical receiver consuming 270 μw for transcutaneous 2 Mb/s data transfer (paper presented in the International Solid-State Circuits Conference ISSCC, San Francisco, CA, February).
62. Liao, Y-T., H. Yao, B. Parviz, and B. Otis. 2010. A 3 μW wirelessly powered CMOS glucose sensor for an active contact lens (paper presented in the International Solid-State Circuits Conference ISSCC, San Francisco, CA, February).
63. Chiu, H.-W. et al. 2010. Pain control on demand based on pulsed radio-frequency stimulation of the dorsal root ganglion using a batteryless implantable CMOS SoC. IEEE Transactions on Biomedical Circuits and Systems 4 (6): 350–359.
64. Lee, S. B. et al. 2010. An inductively powered scalable 32-channel wireless neural recording system-on-a-chip for neuroscience applications. IEEE Transactions on Biomedical Circuits and Systems 4 (6): 360–371.
65. Chao, P. C. 2011. Energy harvesting electronics for vibratory devices in self-powered sensors. IEEE Sensors Journal 11 (12): 3106–3121.
66. Almouahed, S. et al. 2011. The use of piezoceramics as electrical energy harvesters within instrumented knee implant during walking. IEEE/ASME Transactions on Mechatronics 16 (5): 799–807.
67. Lahuec, C. et al. 2011. A self-powered telemetry system to estimate the postoperative instability of a knee implant. IEEE Transactions on Biomedical Engineering 58 (3): 822–825.
68. Chen, H. et al. 2009. Low-power circuits for the bidirectional wireless monitoring system of the orthopedic implants. IEEE Transactions on Biomedical Circuits and Systems 3 (6): 437–443.
69. Yazicioglu, R. F., C. Van-Hoof, and R. Puers. 2009. Biopotential readout circuits for portable acquisition systems, Analog Circuits and Signal Processing Series. New York: Springer.
70. Hanson, S. et al. 2009. A low-voltage processor for sensing applications with picowatt standby mode. IEEE Journal of Solid-State Circuits 4 (4): 1145–1155.
71. Zhai, B. et al. 2009. Energy-efficient subthreshold processor design. IEEE Transactions on Very Large Scale Integration (VLSI) Systems 17 (8): 1127–1137.
72. Song, Y.-K. et al. 2009. Active microelectronic neurosensor arrays for implantable brain communication interfaces. IEEE Transactions on Neural Systems and Rehabilitation Engineering 17 (4): 339–345.
73. Cheong, J.-H. et al. A 400 nW 19.5 fJ/Conversion-step 8-ENOB 80 kS/s SAR ADC in 0.18 μm CMOS. IEEE Transactions on Circuits and Systems II: Express Briefs 58 (7): 407–411.
74. Cong, P., N. Chaimanonart, W. H. Ko, and D. J. Young. 2009. A wireless and batteryless 10-bit implantable blood pressure sensing microsystem with adaptive RF powering for real-time laboratory mice monitoring. IEEE Transactions of Solid-State Circuits 44 (12): 3631–3644.
75. Trung, N. T., and P. Häfliger. 2011. Time domain ADC for blood glucose implant. Electronics Letters 47 (26): S18–S20.
76. Muller, R., S. Gambini, and J. M. Rabaey. 2012. A 0.013 mm2, 5 μW, DC-coupled neural signal acquisition IC with 0.5 V supply. IEEE Journal of Solid-State Circuits 47 (1): 232–243.
77. Ezekwe, C. D., and E. B. Boser. 2008. A mode-matching ∑Δ closed-loop vibratory gyroscope readout interface with a 0.004 deg/s/rtHz noise floor over a 50 Hz band. IEEE Journal of Solid-State Circuits 43 (12): 3039–3048.
78. Liu, Y.-H. 2008. A 3.5 mW 15 Mbps O-QPSK transmitter for real-time wireless medical imaging applications (paper presented in the IEEE Custom Integrated Circuits Conference, San Jose, CA, September 21–24).
79. Diao, S. et al. 2011. A low-power, high data-rate CMOS ASK transmitter for wireless capsule endoscopy (paper presented in the Defense Science Research Conference and Expo, DSR, pp.1–4, Singapore, August).
80. Wang, Q., K. Wolf, and D. Plettermeier. 2010. An UWB capsule endoscope antenna design for biomedical communications (paper presented in the International Symposium on Applied Sciences in Biomedical and Communication Technologies, ISABEL, Rome, Italy, November 2010).
81. Bashirullah, R. 2010. Wireless implants. IEEE Microwave Magazine 2010:S14–S23.
82. Lenaerts, B., and R. Puers. 2009. Omnidirectional inductive powering for biomedical implants, Analog Circuits and Signal Processing Series. New York: Springer.
83. Alomainy, A., and Y. Hao. 2009. Modeling and characterization of biotelemetric radio channel from ingested implants considering organ contents. IEEE Transactions on Antennas and Propagation 57 (4): 999–1005.
84. Fotopoulou, K., and B. W. Flynn. 2011. Wireless power transfer in loosely coupled links: Coil misalignment model. IEEE Transactions on Magnetics 47 (2): 416–430.
85. Walk, J. et al. 2011. Remote powering systems of medical implants for maintenance free healthcare applications (paper presented in the 41st European Microwave Conference, Manchester, UK, October 10–13).
86. Abouei, J. et al. 2011. Energy efficiency and reliability in wireless biomedical implant systems. IEEE Transactions on Information Technology in Biomedicine 15 (3): 456–466.
87. Sawan, M., and B. Gosselin. 2008. CMOS circuits for biomedical implantable devices. In VLSI circuits for biomedical applications, 45–74. Norwood, MA: Artecht House, Inc.
88. Simard, G., M. Sawan, and D. Massicotte. 2010. High-speed OQPSK and efficient power transfer through inductive link for biomedical implants. IEEE Transactions on Biomedical Circuits and Systems 4 (3): 192–200.
89. Jung, J. et al. 2010. 22 pJ/bit Energy-efficient 2.4 GHz implantable OOK transmitter for wireless biotelemetry systems: In vitro experiments using rat skin-mimic. IEEE Transactions on Microwave Theory and Techniques 58 (12): 4102–4111.
90. Spillman, D. M., and E. S. Takeuhi. 1999. Lithium ion batteries for medical devices (paper presented in the Fourteenth Annual Battery Conference on Applications and Advances, Long Beach, CA, January 12–15).
91. Rubino, R. S., H. Gan, and E. S. Takeuchi. 2002. Implantable medical applications of lithium-ion technology (paper presented in the Seventeenth Annual Battery Conference on Applications and Advances, Long Beach, CA, January 18).
92. Valle, D. Do., C. T. Wentz, and R. Sarpeshkar. 2011. An area and power-efficient analog li-ion battery charger circuit. IEEE Transactions on Biomedical Circuits and Systems 5 (2): 131–137.
93. Yuming, Y. et al. 2011. Low-power fuel delivery with concentration regulation for micro direct methanol fuel cell. IEEE Transactions on Industry Applications 47 (3): 1470–1479.
94. Pandey, A., F. Allos, A. Patrick Hu, and D. Budgett. 2011. Integration of supercapacitors into wirelessly charged biomedical sensors (paper presented in the 6th IEEE Conference on Industrial Electronics and Applications, lloc, data).
95. Shanchez, W., C. Sodini, and J. L. Dawson. 2010. An energy management IC for bio-implants using ultracapacitors for energy storage (paper presented in the IEEE Symposium on VLSI Circuits, Honolulu, HI, June 16–18).
96. Roundy, S., P. K. Wright, and K. S. J. Pister. 2002. Micro-electrostatic vibration-to-electricity converters (paper presented in the International Mechanical Engineering Congress & Exposition, New Orleans, LA, November 17–22).
97. Mhetre, M. R. et al. 2011. Micro energy harvesting for biomedical applications: A review (paper presented in the International Conference on Electronics Computer Technology, lloc, data).
98. Zhang, J. Y. et al. 2012. Microstructure and piezoelectric properties of AIN thin films grown on stainless steel for the application of vibration energy harvesting. IET Micro & Nano Letters 7 (12): 1170–1172.
99. Kazazian, T., and A. J. Jansen. 2004. Eco-design and human-powered products (paper presented in the Electronics Goes Green Conference, Berlin, Germany, September 6–10).
100. Donelan, J. M., V. Naing, and Q. Li. 2009. Biomechanical energy harvesting (paper presented in the IEEE Radio and Wireless Symposium, San Diego, CA, January 18–22).
101. Olivares, A. et al. 2010. A study of vibration-based energy harvesting in activities of daily living (paper presented in the Pervasive Computing Technologies for Healthcare, Munich, Germany, March).
102. http://www.mide.comlproducts/volture/peh20w.php
103. Olivo, J., D. Brunelli, and L. Benini. 2010. A kinetic energy harvester with fast startup for wearable body-monitoring sensors (paper presented in the 4th International Conference on Pervasive Computing Technologies for Healthcare, Lausanne, Switzerland, March 22–25).
104. Zainal Abidin, H. E., A. A. Hamzah, and B. Yeop Majlis. 2011. Design of interdigitaed structures supercapacitor for powering biomedical devices (paper presented in the IEEE Regional Symposium on Micro and Nanoelectronics, Kota Kinabalu, Malaysia, September 28–30).
105. Martínez-Quijada, J., and S. Chowdhury. 2007. Body-motion driven MEMS generator for implantable biomedical devices (paper presented in the Canadian Conference on Electrical and Computer Engineering, lloc, data).
106. Lay-Ekuakille, A. et al. 2009. Thermoelectric generator design based on power from body heat for biomedical autonomous devices (paper presented in the IEEE International workshop on Medical Measurements and Applications, lloc, data).
107. Ramadass, Y. K., and A. P. Chandrakasan. 2011. A battery-less thermoelectric energy harvesting interface circuit with 35 mV startup voltage. IEEE Journal of Solid-State Circuits 46 (1): 333–341.
108. Ayazian, S., and A. Hassibi. 2011. Delivering optical power to subcutaneous implanted devices (paper presented in the IEEE International Conference Engineering in Medicine and Biology Society, lloc, data).
109. Yong Shu, R., S. O. Moheimani, and M. Rasit Yuce. 2011. A 2-DOF MEMS ultrasonic energy harvester. IEEE Sensors Journal 11 (1): 155–161.
110. Ravariu, C., C. Ionescu-Tirgoviste, and F. Ravariu. 2009. Glucose biofuels properties in the bloodstream in conjunction with the beta cell electro-physiology (paper presented in the International Conference on Clean Electrical Power, Boston, MA, August 20–September 3).
111. Stetten, F. V. et al. 2006. A one-compartment, direct glucose fuel cell for powering long-term medical implants (paper presented in the 19th International Conference on Micro Electro Mechanical Systems, MEMS, Istambul, Turkey, January 22–26).
112. Justin, G. A., Y. Zhang, M. Sun, and R. Sclabassi. 2005. An investigation of the ability of white blood cells to generate electricity in biofuel cells (paper presented in the IEEE 31st Annual Northeast Bioengineering Conference, Hoboken, NJ, April 2–3).
113. Siu, C. P. B., and M. Chiao. 2008. A microfabricated PDMS microbial fuel cell. Journal of Microelectromechanical Systems 17 (6): 1329–1341.
114. Bertacchini, A., D. Dondi, L. Larcher, and P. Pavan. 2008. Performance analysis of solar energy harvesting circuits for autonomous sensors (paper presented in the 34th Annual Conference of IEEE Industrial Electronics, Orlando, FL, November 10–13).
115. Sauer, C., M. Stanacevic, G. Cauwenberghs, and N. Thakor. 2005. Power harvesting and telemetry in CMOS for implant devices. IEEE Transactions on Circuits and Systems 52 (12): 2605–2613.
116. Zhang, X. et al. 2010. An energy-efficient ASIC for wireless body sensor networks in medical applications. IEEE Transactions on Biomedical Circuits and Systems 4 (1): 11–18.
117. Zhu, Y., S. O. R. Moheimani, and M. R. Yuce. 2010. Ultrasonic energy transmission and conversion using a 2-D MEMS resonator. IEEE Device Letters 31 (4): 374–376.
118. Saggini, S., and P. Mattavelli. 2009. Power management in multi-source multi-load energy harvesting systems (paper presented in the European Conference on Power Electronics and Applications, Barcelona, Spain, September 8–10).
119. Christmann, J. F., E. Beigné, C. Condemine, and J. Willemin. 2010. An innovative and efficient energy harvesting platform architecture for autonomous microsystems (paper presented in the International NEWCAS Conference, Montréal, Canada, June 20–23).
120. Torres, E. O, and G. A. Rincon-Mora. 2009. Electrostatic energy-harvesting and battery-charging CMOS system prototype. IEEE Transactions on Circuits and Systems I: Regular Papers 56 (9): 1938–1948.
121. Colomer-Farrarons, J., and P. Miribel-Catala. 2011. A CMOS self-powered front-end architectures for subcutaneous event-detector devices: Three-electrodes amperometric biosensor approach. Dordrecht: Springer.
122. Midé Engineering Smart Technologies. Mide Tehnology Corp., Medford, MA. http://www.mide.com/
123. IXYS Efficiency through Technology. http://www.ixys.com
124. Colomer-Farrarons, J., P. Miribel-Català, I. Rodríguez, and J. Samitier. 2009. CMOS front-end architecture for in-vivo biomedical implantable devices (paper presented in the 35th International Conference of Industrial Electronics, IECON, Porto, Portugal, November 3–5).
