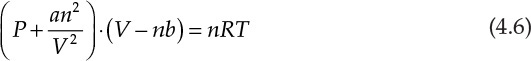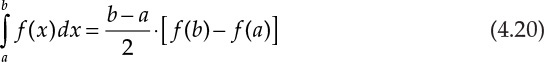Chapter 4. Introduction to Computations in Chemical Engineering
It is unworthy of excellent men to lose hours like slaves in the labor of calculation which could be safely relegated to anyone else if machines were used.
—Gottfried Wilhelm von Leibniz1
1. Leibniz’s fame as the co-inventor of calculus overshadows his contributions in many other fields, including those in the field of computations. He was one of the earliest pioneers in the development of mechanical calculators. Quotation source: http://www-history.mcs.st-and.ac.uk/Quotations/Leibniz.html
Chemical engineering, like all engineering disciplines, is a quantitative field; that is, it requires accurate solutions of problems having high mathematical complexity. A chemical engineer must be able to model—develop quantitative mathematical expressions that describe the processes and phenomena—any system of interest, and simulate—solve the equations—the model. The solutions so obtained allow the engineer to design, operate, and control the processes. The courses described in Chapter 3, “Making of a Chemical Engineer,” provide the students with the theoretical basis for modeling the processes. The nature of the resulting equations and tools used for solving the equations are presented in this chapter.
4.1 Nature of Chemical Engineering Computational Problems
Chemical engineers deal with a multitude of equations ranging in complexity from simple linear equations to highly involved partial differential equations. The solution techniques accordingly range from simple calculations to very large computer programs. The classification of the problems based on the mathematical nature is presented in the following sections.
4.1.1 Algebraic Equations
Algebraic equations comprise the most common group of problems in chemical engineering. Linear algebraic equations are algebraic equations in which all the terms are either a constant or a first-order variable [1]. The straight line is represented by a linear algebraic equation. Linear algebraic equations are often encountered in phase equilibrium problems associated with separation processes. Figure 4.1 is a representation of one such separation operation, wherein a high-pressure liquid stream is fed to a flash drum where the system pressure is reduced, resulting in the formation of a vapor and a liquid stream that exit the drum. The compositions of the liquid and the vapor stream depend on the process conditions, and a chemical engineer has to calculate these compositions.
The governing equations for the system follow:
Equations 4.1 and 4.2 state that the mole fractions of all components, numbering n, in each phase add up to 1. xi and yi represent the mole fractions of component i in the outlet liquid and gas phases, respectively. The mole fractions in the feed stream are denoted by zi. (Typically, x is used to represent the mole fraction when the phase is liquid, and y is used when the phase is gaseous.) These two equations should be intuitively clear, as the mathematical statements of the concept that all fractions of any quantity must add up to the whole. Equation 4.3 is actually a system of n equations relating the mole fraction of a component in the gas phase to the mole fraction of the same component in the liquid phase. Ki is a characteristic constant for component i and is dependent on pressure, temperature, and the nature of the component mixture. Solution of this system of equations allows us to calculate the compositions of the two different phases, which is necessary for designing the separation scheme for the mixture. Each term in the system of equations is linear (variables having power of 1) in x or y.
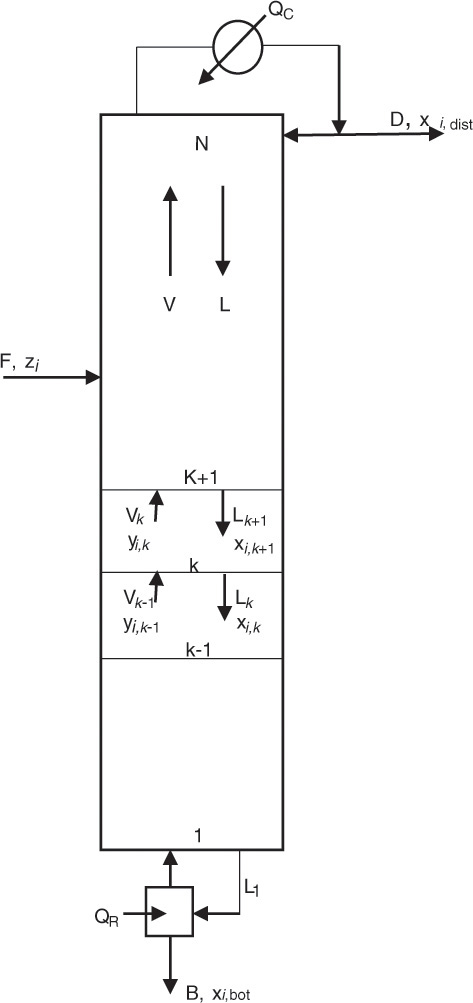
A similar system of equations is used to model a stagewise gas-liquid contactor, such as a distillation column, described in Chapter 3. Figure 4.2 represents a distillation column containing N equilibrium stages [2]; the vapor and liquid inlet and outlet flows can be seen in the figure for stage k.
Source: Adapted from Wankat, P. C., Separation Process Principles, Third Edition, Prentice Hall, Upper Saddle River, New Jersey, 2012.
The material balances for each component yield the following system of n equations for stage k:
V and L represent the molar flow rates of the vapor and liquid stream, respectively. The subscripts for these flow rates represent the stage from which these flows exit. For example, Vk and Lk are the vapor and liquid flow rates exiting stage k, respectively. Lk+1 is the liquid flow rate exiting stage k + 1 and entering stage k, and Vk−1 is the vapor flow rate exiting stage k − 1 and entering stage k. The mole fractions are doubly subscripted variables, the first subscript representing the component, the second one the stage. Equation 4.4 is the mathematical representation of the steady-state nature of the system for each component: the amount of component i entering the stage through vapor and liquid flows is the same as the amount leaving through the exiting vapor and liquid flows. Each stage is assumed to be an equilibrium stage; that is, the exiting vapor and liquid flows are in equilibrium with each other. This allows us to utilize the equilibrium relationships of the form shown by equation 4.3 to complete the system description. The total number of equations for the entire column is N × n, which can be significantly large depending on the number of components present in the process stream and the number of stages needed to obtain the desired separation.
Algebraic equations encountered in chemical engineering can also be polynomial equations; that is, they can have variable orders greater than one. Equation 4.5 represents a typical polynomial equation of interest to chemical engineers:
This equation is an example of a cubic equation of state, V being the volume of the substance under the given conditions of temperature (T) and pressure (P). Constants a, b, c, and d are functions of the system pressure, temperature, number of moles, and fluid properties. An equation of state represents the relationship between the system temperature, pressure, and volume; the ideal gas law represented by the mathematical expression PV = nRT is the simplest of the equations of state. These equations of state are further used in thermodynamic calculations involving interconversion between energy and work, and phase equilibrium. It is readily apparent that an accurate equation of state is critical for superior process design and performance. Unfortunately, the volumetric behavior of most substances does not conform to the ideal gas law, and more complex equations are needed for accurately describing the P-V-T relationships for these substances. The cubic equations of state represent one of the developments addressing this need for improved accuracy. Equation 4.6 is an example of the cubic equation of state and is called the van der Waals equation [3].
In this equation, a and b are constants characteristic of the substance, and n is the number of moles present in the system.
Several other more complex equations have also been developed, many of them polynomial in nature. A chemical engineering student encounters polynomial equations in practically every subject described in Chapter 3.
4.1.2 Transcendental Equations
Many of the equations in chemical engineering involve functions of variables more complex than simple powers. An equation containing exponential, logarithmic, trigonometric, and other similar functions is not amenable to solution by algebraic means—that is, by simple addition, multiplication, or root extraction operations. Such equations “transcend” algebra and are called transcendental equations [4]. Equation 4.7, the Nikuradse equation, often used in fluid flow calculations, is an example of a transcendental equation [5].
Re in the equation represents Reynolds number, a dimensionless quantity of enormous significance in fluid mechanics and transport phenomena. The Nikuradse equation allows us to calculate f, the friction factor, a quantity that further leads to the estimation of pressure drop for a flowing fluid and, ultimately, the power requirements for material transfer.
Equation 4.8 is another example of a transcendental equation that is used in the design of chemical reactors [6].
XA represents the conversion (extent of reaction) of the reactant A, τ the residence time (the time spent by the fluid in the reactor), and A and E the characteristic parameters that describe the rate of reaction. The equation can be used to calculate one of the three quantities XA, τ, or T when the other two are specified.
Many processes involve consecutive chemical reactions that can be represented by the equation A → R → S. A is the starting reactant, which upon undergoing the reaction yields the specie R, which is often the desired product. However, R may undergo further reaction forming S. The typical concentration profiles for the three species in a reactor as a function of time are shown in Figure 4.3. As can be seen, the concentration of A decreases continuously, while that of S increases continuously. The concentration of the desired product R increases first, reaches a maximum, and then starts decreasing. The concentration-time relationship for R when both the reactions are first order2 with respect to the reactants is shown in equation 4.9 [7].
2. A first-order reaction is one in which the rate of reaction is proportional to the concentration of the reactant. These concepts are presented in more detail in Chapter 9, “Computations in Chemical Engineering Kinetics.”
Here, CR is the concentration of R, CA0 is the initial concentration of A, and k1 and k2 are the rate constants for the two reactions. Calculating the concentration of R at any specified time, when the rate constants and initial concentration of A are known, is straightforward. However, calculation of time needed to achieve a certain specified concentration of R is more challenging and requires use of techniques needed for the solution of transcendental equations.
4.1.3 Ordinary Differential Equations
Modeling—developing a set of governing equations—of systems of interest to chemical engineers often starts with defining a differential element of the system. This differential element is a subset of the larger system, but with infinitesimally small dimensions. All the processes and phenomena occurring in the larger system are represented in the differential element. The modeling approach involves writing conservation of mass and/or conservation of energy equations for the differential element. These equations yield ordinary differential equations when all the quantities are functions of a single independent variable. For example, equation 4.10 is a first-order differential equation relating the rate of change of concentration to time in a chemical reaction [6]. The equation indicates that the rate at which the concentration of species A, CA, changes with time t is linearly dependent on the concentration of A itself—an example of a first-order reaction. The parameter k is called the rate constant.
Solution of this equation yields the concentration-time profile for the reactant A in the reaction, which provides the basis for the design of the reactor.
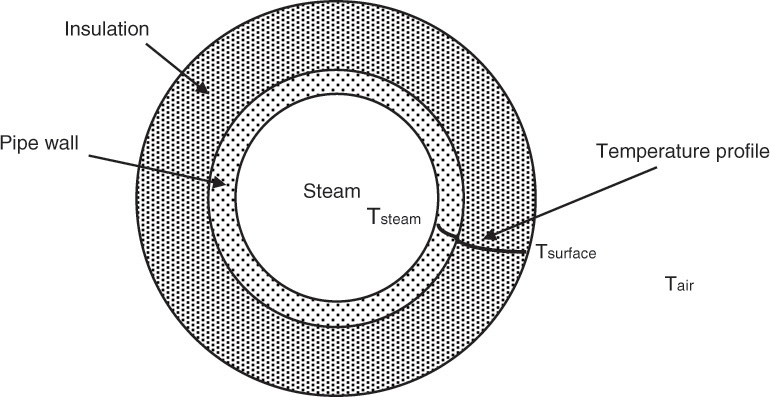
Higher-order differential equations are very common in chemical engineering systems. Figure 4.4 shows the cross-sectional view of a pipe conducting steam, the ubiquitous heat transfer medium in chemical plants. The pipe will inevitably be covered with insulation to minimize heat loss to the surroundings. Note that the heat loss can be reduced but not completely eliminated. Obviously, choosing proper insulation and determining the resultant heat loss is extremely important for estimating the energy costs. Heat loss can be calculated from the temperature-distance profiles existing in the system [5].
The governing equation describing the heat transfer for a cylindrical pipe follows:
Equation 4.11 is a second-order ordinary differential equation that governs the relationship between temperature T and radial distance r from the center of the pipe. kT is the thermal conductivity of the material, which depends on the temperature. As the temperature varies with respect to the radial position, the thermal conductivity is also a function of the radial position. Solution of this equation yields the temperature profile within that object, which in turn allows us to determine the heat lost to the surroundings.
The solution of differential equations requires specifying values of dependent variable(s) at certain values of the independent variable. These specifications are termed boundary conditions (at a specific location, with respect to dimensional coordinate) or initial conditions (with respect to time). Complete solution requires as many boundary/initial conditions as the order of the differential equation [4].
Frequently, modeling of a system leads to a set of ordinary differential equations, consisting of two or more dependent variables that are functions of the same independent variable. These equations need to be solved simultaneously to obtain the quantitative description of the system.
4.1.4 Partial Differential Equations
Properties of systems are frequently dependent on, or are functions of, more than one independent variable. Modeling of such systems leads to a partial differential equation [4]. Temperature within a rod, for example, may vary radially as well as axially. Similarly, concentration of a species within a system may depend on the location as well as vary with time. Figure 4.5 shows batch drying of a polymer film cast on a surface. The solvent present in the polymer diffuses through the film to the surface, where it is carried away by an air sweep.
The concentration of the solvent within the film is a function of time as well as distance from the surface. Equation 4.12 is the fundamental equation3 for governing the solvent mass transport within the film, a partial differential equation that is first order with respect to time t and second order with respect to location x.
3. This is called the Fick’s second law equation.
DA is the diffusivity of solvent A in the polymer film, which depends on the properties of the system.
The solution of this (and other partial differential equations) requires an appropriate number of specifications (boundary and initial conditions) depending on orders with respect to the independent variables.
4.1.5 Integral Equations
The differential equations representing the behavior of the system are obtained by the application of conservation principles to a differential element. Integration of these differential equations leads to expressions that describe the overall behavior of the entire system. Many of the differential equations can be integrated analytically, yielding algebraic or transcendental equations. However, such analytical integration is not always possible, and numerical computation is necessary for obtaining the integrals [4]. The determination of reactor volume often involves equations of the following form [6]:
Here, FA0 is the molar flow rate of species A, and −rA is the rate of reaction, which is a function of conversion XA. Equation 4.13 is represented by Figure 4.6, where the shaded region represents the integral and is equal to the quantity V/FA0.
When the reaction rate cannot be easily integrated analytically, the shaded region—the area under the curve—is evaluated numerically.
4.1.6 Regression Analysis and Interpolation
Chemical engineers routinely collect discrete data through various experiments, which they further use for design, control, and optimization. This often requires obtaining the value of the function (or dependent variable) at some value of the independent variable within the domain of experimental data where direct measurement is not available. Regression analysis involves fitting a smooth curve that approximates the data, yielding a continuous function [4]. It is then possible to interpolate—obtain the function value at any intermediate value of the independent variable. It is also possible to extrapolate—obtain the function value at a value of the independent variable that is outside the data range used for regression analysis. Linear regression involves approximating the data using a straight line, whereas nonlinear regression involves using polynomial or transcendental functions for the same purpose. Multiple regression involves performing regression analysis involving two or more independent variables that determine the value of the function. For example, equation 4.10 can be integrated to obtain the following mathematical relationship between concentration and time:
To determine the rate constant k, experiments are conducted obtaining the concentration-time data and a linear regression carried out between ln(CA) and t, as shown in Figure 4.7.
It can readily be seen that a chemical engineer must have skills to deal with and solve problems ranging from simple arithmetic calculations to those requiring highly sophisticated and involved algorithms. Further, the solution must be obtained fairly rapidly for the individuals and organizations to maintain their competitive edge and respond to changing conditions. Section 4.2 presents a brief overview of solution algorithms developed for numerical solutions of different types of problems. Section 4.3 describes the different tools including the machines and software available to chemical engineers to perform these computations.
4.2 Solution Algorithms
The theoretical basis and approach to developing the solutions of various types of computational problems is briefly described in this section. This discussion is not meant to be exhaustive or comprehensive, but rather introductory, in nature. Several alternative techniques are available for solving the various types of problems; the following discussion is in most cases confined to presenting an outline of one of the techniques.
4.2.1 Linear Algebraic Equations
It should be clear that systems of linear algebraic equations can range in size from very small (fewer than five equations) to very large (several hundreds), depending on the number of components and complexity of operations. For example, a system consisting of four components being separated in a distillation column containing five stages yields a system of 20 material balance equations. Typically, the system of equations is rearranged into the following matrix form:
In this equation, [X] is the column matrix of n variables; [A], the n × n matrix of coefficients; and [B], a column matrix of n function values.
The Gauss elimination technique for solving this system of equations involves progressive elimination of variables from the equations such that at the end only a single linear equation is obtained in one variable. The value of that variable is then obtained and back-substituted progressively into the equations in reverse order of elimination to obtain the values of the rest of the variables that satisfy equation 4.15. For example, if the system consists of n equations in variables x1, x2,…, xn, then the first step is elimination of variable x1 from equations 2 to n using equation 1 to express x1 in terms of the rest of variables. The result is a system of n − 1 equations in n − 1 variables x2, x3,…, xn. Repeating this procedure then allows us to eliminate variables x2, x3, and so on, until only an equation in xn is left. The value of xn is calculated, and reversing the calculations, values of xn−1, xn−2,…, x1 are obtained [4].
Iterative procedures offer an alternative to elimination techniques. The Gauss-Seidel method involves assuming an initial solution by guessing the values for the variables. It is often convenient to assume that all the variables are 0. Based on this initial guess, the values of the variables are recalculated using the system of equations: x1 is calculated from the first equation, and its value is updated in the solution matrix; x2 is calculated from the second equation; and so on. The steps are repeated until the values converge for each variable [8]. The Gauss-Seidel method is likely to be more efficient than the elimination method for systems containing a very large number of equations or systems of equations with a sparse coefficient matrix, that is, where the majority of coefficients are zero [9].
Many sophisticated variations of the elimination and iteration techniques are available for the solution. One other solution technique involves matrix inversion and multiplication. The effectiveness of these and solution techniques is dependent on the nature of the system of equations. Certain techniques may work better in some situations, whereas it might be appropriate to use alternative techniques in other instances.
4.2.2 Polynomial and Transcendental Equations
The complexity of solutions for polynomial and transcendental equations increases with increasing nonlinearity. Quadratic equations can be readily solved using the quadratic formula, provided such equations can be readily rearranged in the appropriate form. Formulas exist for obtaining roots of a cubic equation, but these are rarely used. No such easy formulas are available for solution of higher-order polynomials and transcendental equations.
These equations are typically solved by guessing a solution (root) and refining the value of the root on the basis of the behavior of the function. The principle of the Newton-Raphson technique, one of the most common techniques used for determining the roots of an equation, is represented by equation 4.16 [4]:
Here, xn and xn+1 are the old and new values of the root; f(xn) and f’(xn) are values of the function and its derivative, respectively, evaluated at the old root.
The calculations are repeated iteratively; that is, so long as the values of the roots do not converge, the new root is reset as the old root and a newer value of the root evaluated. It is obvious the new root will equal the old root when the function value is zero. In practice, the two values do not coincide exactly, but a tolerance value is defined for convergence. For example, the calculations may be stopped when the two values differ by less than 0.1% (or some other acceptable criteria).
The computations for this technique depend on not only the function value but also its behavior (derivative) at the root value. The initial guess is extremely important, as the search for the root proceeds on the basis of the function and derivative values at this point. Proper choice of the root will yield a quick solution, whereas an improper choice of the initial guess may lead to the failure of the technique.
The iterative successive substitution method can also be used to solve such equations [9]. The method involves rearranging the equation f(x) = 0 in the form x = g(x). The iterative solution algorithm can then be represented by the following equation:
Here, xi+1 is the new value of the root, which is calculated from the old value of the root xi. Each successive value of x would be close to the actual solution of the equation. The key to the success of the method is in the proper rearrangement of the equations, as it is possible for the values to diverge away from the solution rather than toward a solution.
Finding the roots of polynomial equations presents a particular challenge. An nth-order polynomial will have n roots, which may or may not be distinct and may be real or complex. The solution technique described previously may be able to find only a single root, irrespective of the initial guess. The polynomial needs to be deflated—its order reduced by factoring out the root discovered—progressively to find all the n roots. It should be noted that in engineering applications, only one root may be of interest, the others needed only for mathematically complete solution. For example, the cubic equation of state may have only one real positive root for volume, and that is the only root of interest to the engineer. A complex or negative root, while mathematically correct as an answer, is not needed by the engineer.
4.2.3 Derivatives and Differential Equations
Some computational problems may involve calculating or obtaining derivatives of functions. Depending on the complexity of the function, it may not be possible to obtain an explicit analytical expression for the derivative. Similarly, some of the problems may involve obtaining the derivative from observed data. For example, an experiment conducted for determination of the kinetics of a reaction will yield concentration-time data. An alternative method of determining the rate constant for the reaction involves regressing the rate of the reaction as a function of concentration. The rate of the reaction is defined as ![]() ; thus, the problem involves estimating the derivative from the concentration-time data. One of the numerical techniques for obtaining the derivative is represented by equation 4.17.
; thus, the problem involves estimating the derivative from the concentration-time data. One of the numerical techniques for obtaining the derivative is represented by equation 4.17.
The subscripts refer to the time period. Thus, CAi is the concentration at time ti, and so on. The derivative is approximated by the ratio of differences in the quantities. This formula is termed the forward difference formula, as the derivative at ti is calculated using values at ti and ti+1. Similarly, there are backward and central difference formulas that are also applied for the calculation of the derivative [4, 10]. The comparative advantages and disadvantages of the different formulas are beyond the scope of this book and are not discussed further.
Similarly, the numerical techniques for integration of ordinary and partial differential equations are beyond the scope of this book. Interested readers may find a convenient starting point in reference [4] for further knowledge of such techniques.
4.2.4 Regression Analysis
The common basis for linear as well as multiple regression is the minimization of the sum of squared errors (SSE) between the experimentally observed values and the values predicted by the model, as shown in equation 4.19:
In this equation, yi is the observed value, and f(xi) is the predicted value based on the presumed function f. The function can be linear in a single variable (generally, what is implied by the term linear regression), linear in multiple variables (multiple regression), or polynomial (polynomial regression). Minimization of SSE yields values of model parameters (slope and intercept for a linear function, for example) in terms of the observed data points (xi, yi). The least squares regression formulas are built into many software programs.
4.2.5 Integration
As mentioned previously, numerical computation of an integral is needed when it is not possible to integrate the expression analytically. In other cases, discrete values of the function may be available at various points. Numerical integration of such functions involves summing up the weighted values of the function evaluated or observed at specified points. The fundamental approach is to construct a trapezoid between any two points, with the two parallel sides being the function values and the interval between the independent variable values constituting the height [4, 10]. If the function is evaluated at two points, a and b, then the following applies:
Decreasing the interval increases the accuracy of the estimate. Several other refinements are also possible but are not discussed here.
Section 4.3 describes various software programs that are available for the computations and solutions of the different types of problems just discussed. These software programs feature built-in tools developed on the basis of these algorithms, obviating any need for an engineer to write a detailed program customized for the problem at hand. The engineer has to know merely how to give the command in the language that is understood by the program. The previous discussion should, however, provide the theoretical basis for the solution as well as illustrate the limitations of the solution technique and possible causes of failure. A course in numerical techniques is often a required core course in graduate chemical engineering programs and sometimes an advanced undergraduate elective course.
4.3 Computational Tools—Machines and Software
The two components enabling the computations are the hardware—machines that perform the calculations—and the software—the instructions to run solution algorithms by the machines. Both these components are described in the sections that follow.
4.3.1 Computational Machines
A counting frame, or an abacus, is one of the earliest devices and has been used for more than three millennia for rapid calculations. This remarkable device continues to be used in various parts of the world to improve on manual hand calculations. Abaci are also popular as teaching tools in elementary schools. Calculations using an abacus involve physically moving beads on a wire, which clearly constrains the speed and type of calculations that can be performed.
A couple of tools developed in response to the ever-increasing demand for higher speeds worth mentioning are the log table and the slide rule. Figure 4.8 shows a page from the log table.
A log table enables all calculations, including highly complex ones, to be reduced to additions/subtractions, which can then be easily done by hand. The use of log tables is illustrated for the simple case of calculating the circumference C of a circle of diameter D. The concept and steps in calculations are as follows:
Equations 4.21, 4.22, and 4.23 demonstrate how a multiplication operation can be performed as an addition operation using the log tables. Logarithms of π and diameter are looked up in the log tables and added to obtain logarithm of the circumference. The answer is obtained by looking up the antilog of the sum in another set of tables.
The calculation will work with logarithms to any base; however, generally the tables are those of common logarithms (to the base 10) rather than natural logarithms (to the base e). An impressive compilation of common logarithms up to 24 decimal places of numbers leading to 200,000 was available by the end of the 18th century through the efforts of a large number of individuals.
Such compilations and early calculating machines were hardly portable, a disadvantage that was overcome by the slide rule. This elegant device was the size of a ruler and small enough to fit in a shirt pocket; a couple of examples are shown in Figure 4.9. As seen from the figure, a slide rule has a central sliding part, and both the fixed and sliding parts are marked with several scales. Multiplication, division, and logarithmic as well as trigonometric calculations can be rapidly performed using the slide rule, which became an indispensable tool for engineers until it was supplanted by inexpensive scientific calculators in the mid-1970s.
The advent of computers enabled engineers to perform highly complex, involved, repetitive calculations and improve the accuracy of solutions. Mainframe computers were ubiquitous by the 1950s, with engineers writing programs and submitting them as jobs to be run on the mainframes.
Rapid technological advances in memory and data storage, materials, and processors resulted in lowering the cost of computers, making them affordable to most people. By the mid-1990s, personal computers (PCs) had become ubiquitous and an essential accessory for an engineering student. Continual reduction in the cost and size of devices has resulted in the availability of portable devices such as laptops and tablets that allow us to perform practically all types of computations except for the most complicated ones that require the use of supercomputers.
4.3.2 Software
As previously mentioned, computing machines need to be given instructions for performing calculations. These instructions are codified in a well-defined structure—the programming language. All the computational steps are written according to the rules of the programming language, resulting in a program, which can then be compiled and executed by the computer [4].
Developed in the 1950s, Fortran (from formula translation) became the dominant programming language for scientific computing, and even today remains the preferred language for highly intensive computations of large systems. Each line of the Fortran program used to be transferred onto a card using a keypunch machine. (An IBM punched card with 80 columns is shown in Figure 4.10. Each number, letter, and symbol was represented by a hole or holes punched in the column [11]). A deck of such punched cards would constitute a program, which along with the necessary data would be submitted to be run as a job on the mainframe computer. Subsequent developments eliminated the need for punch cards, and a Fortran program can now be run on a personal computer, similar to programs in other languages.

Figure 4.10 An IBM punched card illustrating representation of numbers and letters by punched holes.
Source: Ceruzzi, P. E., A History of Modern Computing, Second Edition, MIT Press, Cambridge, Massachusetts, 2003.
A large number of program modules developed over the past 60 years have resulted in a valuable library of programs in Fortran, and these modules are used every day throughout scientific and engineering computations.
Although Fortran remains indispensable for advanced computing, a large number of advanced software programs are available on the PC to perform most of the chemical engineering computations. The ubiquitous availability of these software programs has essentially eliminated the need for individuals to write their own software codes for all but only very specific problems [12]. Following are descriptions of a few of these software packages.
4.3.2.1 Spreadsheets
A spreadsheet program is an integral component of a software package for PCs, with Microsoft Excel being the dominant one. Spreadsheets store data in a grid of up to 1 million rows and 16,000 columns. Spreadsheets have built-in functions to perform practically all calculations, and also provide a programming capability to execute any other type of computations. The features of spreadsheet programs include, among others,
• Tools for solving algebraic and transcendental equations
• Graphing and regression tools
• Tools for solving iterative calculations
• Data and statistical analysis tools
Spreadsheets can be used to solve all types of computation problems, including differential and integral equations, described in section 4.1.
4.3.2.2 Computing Packages
A number of software packages that provide a computing environment have become available over the past 30 years. These include MATLAB (Mathworks, Inc., Massachusetts, USA), Mathematica (Wolfram Research, Illinois, USA), Maple (Waterloo Maple, Ontario, Canada), Mathcad (MathSoft, Massachusetts, USA), and others. These packages typically offer, to varying degrees, an ability to perform numerical and symbolic computations, graphing, as well as programming. Each package has its characteristic syntax—rules for constructing instructions—and degree of user-friendliness. As with spreadsheets, practically all types of computational problems can be handled by these software packages that also provide an additional benefit of having built-in scientific/engineering constants and units. Appendix A presents a comparative discussion of some of these programs.
4.3.2.3 COMSOL
COMSOL (Comsol Group, Sweden and USA) is a powerful software package specifically geared to solve coupled physics and engineering problems. Marketed as a multiphysics software, COMSOL offers a modeling and simulation environment for all engineering disciplines. The various modules available in COMSOL allow users to solve problems related to fluid flow, heat transfer, reaction engineering, electrochemistry, and many others.
4.3.2.4 Process Simulation Software
Chemical process plants are complex structures consisting of a large number of units. The software systems previously described support computations related to a single step or a single unit. Several comprehensive process simulation software packages are used by the chemical industry to design, control, and optimize operations of chemical process plants. The computational power offered by these software systems enables a chemical engineer to obtain integrated plant design in a fraction of the time needed before their advent. Following are some prominent software examples:
• Aspen Plus by AspenTech, Massachusetts, USA
• PRO/II by Invensys, Texas, USA (a division of Schneider Electric)
• ProSimPlus by ProSim, S.A., France
• CHEMCAD by Chemstations, Inc., Texas, USA
All of these software systems offer similar capabilities and user interfaces. A design engineer will invariably be using one of these environments for designing an operation, a unit, or the plant. Appendix B illustrates the capabilities of one of these software programs—PRO/II—for solving a complex separation problem to provide an idea of the amazing computational power at our disposal.
In addition to these general-purpose software programs, some specialized software for specific applications is also available, such as FLOTRAN/ANSYS (ANSYS Inc., Pennsylvania, United States) for pipe flow/fluid dynamics computations. Open source software such as Modelica (JModelica.org) is also available for modeling and simulation of complex systems.
4.4 Summary
Chemical engineers perform a wide variety of computations ranging from simple arithmetic calculations to solving partial differential equations and simulating entire process plants. Technological advances have enabled engineers to access high-performance computing machines and use advanced software for obtaining solutions rapidly. As envisioned by Leibniz, this has allowed them the freedom from time-consuming, laborious, repetitive computational tasks, and they can focus on the more important mission of developing technologies from concept to practical implementation.
References
1. Varma, A., and M. Morbidelli, Mathematical Methods in Chemical Engineering, Oxford University Press, Oxford, England, 1997.
2. Wankat, P. C., Separation Process Engineering, Third Edition, Prentice Hall, Upper Saddle River, New Jersey, 2012.
3. Kyle, B. G., Chemical and Process Thermodynamics, Third Edition, Prentice Hall, Upper Saddle River, New Jersey, 1999.
4. Chapra, S. C., and R. P. Canale, Numerical Methods for Engineers, Seventh Edition, McGraw-Hill, New York, 2014.
5. Welty, J. R., C. E. Wicks, R. E. Wilson, and G. L. Rorrer, Fundamentals of Momentum, Heat, and Mass Transfer, Fifth Edition, John Wiley and Sons, New York, 2008.
6. Fogler, H. S., Elements of Chemical Reaction Engineering, Fourth Edition, Prentice Hall, Upper Saddle River, New Jersey, 2006.
7. Doraiswamy, L. K., and D. Üner, Chemical Reaction Engineering: Beyond the Fundamentals, CRC Press, Boca Raton, Florida, 2014.
8. Rice, R. G., and D. D. Do, Applied Mathematics and Modeling for Chemical Engineers, Second Edition, John Wiley and Sons, New York, 2012.
9. Riggs, J. B., An Introduction to Numerical Methods for Chemical Engineers, Second Edition, Texas Tech University Press, Lubbock, Texas, 1994.
10. Pozrikidis, C., Numerical Computation in Science and Engineering, Second Edition, Oxford University Press, New York, 2008.
11. Ceruzzi, P. E., A History of Modern Computing, Second Edition, MIT Press, Cambridge, Massachusetts, 2003.
12. Finlayson, B. A., Introduction to Chemical Engineering Computing, Second Edition, John Wiley and Sons, New York, 2014.
Problems
4.1 Cramer’s rule and matrix inversion-multiplication offer alternative techniques to solve a system of linear algebraic equations. Conduct a literature search to collect information about these two techniques and the elimination and iteration techniques discussed in this chapter. Compare the various techniques regarding the complexity of algorithms, ease of implementation, and potential errors.
4.2 The Newton-Raphson technique may not converge to a solution. Inspecting equation 4.16, in what other possible way can the technique fail?
4.3 Roots of any equation can be found using what is known as the bracketing technique. Conduct a literature search and explain the principle behind such solution techniques.
4.4 The following data were obtained in an experiment where the concentration of a substance was monitored as a function of time. Calculate the first derivative of the concentration with respect to time for all possible times using the forward difference formula. Can the second derivative also be calculated numerically?

4.5 What is the area under the concentration-time curve obtained from the data shown for problem 4.4? Use the trapezoid method. An alternative technique is to use the rectangle method. What is the difference in the areas if the area is calculated using the rectangle method?