2. Bacteria in history
Other than infectious disease, humanity’s early dealings with bacteria involved mainly the production of foods. Wheaton College biologist Betsey Dexter Dyer once noted that a meal can be assembled completely from bacteria-produced foods, such as the following items.
• Cheeses—Swiss from Propionibacterium and limburger from Brevibacterium
• Olives—Leuconostoc, Lactobacillus, and Pediococcus
• Dry sausages—Pediococcus
• Sourdough bread—Various lactic acid-producing bacteria
• Butter—Lactobacillus
• Cottage cheese—Streptococcus
A steak or a glass of milk results from the digestion of grasses by anaerobic bacteria in the rumen of cattle. The rumen fermentations are oxygen-free conversions of sugar into microbial energy with acid or alcohol as a by-product.
Olives may be the oldest food fermented specifically to make a new food. The Phoenicians brought olives throughout the Greek isles by 1600 BCE. The production of acids in the fermentation process helped preserve the product during long sea voyages. No one knows who made this discovery, but food historians assume that fermented foods were discovered by accident or perhaps by necessity by explorers who had already eaten all other food supplies.
Bacterial food spoilage takes the form of acid production, protein curdling, gas or toxin production, or decomposition. The latter two cause foodborne illness or a loss of food’s nutritional value, respectively, and render the food unusable. Properly controlled acid production, however, preserves fresh vegetables, fruits, and juices and retains most nutrients, while protein curdling does the same in dairy products.
Evidence of winemaking from alcohol-producing bacteria dates to 6000 BCE Mesopotamia and no doubt started earlier. Over the next two millennia, Hebrew, Chinese, and Inca cultures perfected yeast fermentations for wines and beers, but retained bacteria for fermenting crops to make sauerkraut, pickles, wine, soy sauce, silage, and other foods that lasted longer with an acid preservative than in the fresh form. The names of brave souls who tasted spoiled foods have been lost to history, but either by necessity or a sense of adventure, they invented food preservation.
Bacteria-made dairy products date to before 3000 BCE using milk from cows, yaks, goats, sheep, horses, camels, and even reindeer. Fermented milk products, the “mere white curd of ass’s milk” as described by 18th century poet Alexander Pope likely originated in more than one place. Traders used pouches made of cleaned animal entrails for carrying milk between villages and would not have realized that the stomach enzyme rennin (also called chymosin) remained active in the pouch lining. This enzyme helps nursing infants digest milk by curdling the milk proteins and thus slowing their passage through the digestive tract. In a pouch slung over a horse’s rump, the rennin made cheese.
Lactic acid-producing Lactobacillus, Lactococcus, Streptococcus, and Leuconostoc make up the main bacteria used in cheeses, yogurt, butter, buttermilk, and sour cream today as they did centuries ago. Manufacturers of salad dressings, coleslaw mixes, and mayonnaise now encourage the growth of lactic acid bacteria to produce an acidic tangy flavor and preserve the food.
When bacterial contaminants did not produce a tasty, edible product, the ancients froze, smoked, or dried the food or added salt, sugar, or honey. These preservation methods inhibit bacteria’s growth by making water molecules unavailable for cellular reactions. Food producers still use these ancient methods, but they now also use chemicals to inhibit the growth of microbes in food.
Bacteria have ploys for escaping physical injury from lack of water or harm from chemicals. Many bacteria enter a state of dormancy when water becomes scarce and grow again when water returns to their environment. The normal soil inhabitants Clostridium and Bacillus have evolved an adaptation that protects better than dormancy: the formation of endospores. More than any other type of cell in biology, endospores resist freezing, heating, boiling, chemicals, and irradiation. A microbiologist need only dilute a small amount of soil in nutrient broth and then incubate it to make the endospores germinate into actively growing cells. (Sometimes stubborn endospores need to be heat-shocked at 130°F for five minutes before they will germinate.)
In 1993, American microbiologists Raúl Cano and Monica Borucki found endospores resembling Bacillus sphaericus in an extinct bee that had been preserved in amber estimated at 25 to 40 million years old. As is customary in science when radically new discoveries are made, skeptics came forward suggesting the bacteria were contaminants from a later period. The critics charged that no living organism can survive that long. But in 2000, biologist Russell Vreeland found Bacillus endospores buried in 250-million-year-old salt deposits and showed they remained viable by growing the cells in his laboratory. Vreeland and his team then completed 16S rRNA analysis on the microbe and identified it as an ancestor of modern Bacillus. Perhaps expecting the same skepticism Cano had met with, Vreeland also calculated the chances of a contaminant invading the sterilized equipment or breeching his aseptic techniques at one in one billion. Assuming these bacteria are not contaminants, research like this demonstrates the astonishing durability of bacterial endospores and also hints at the challenges of protecting food from spore-forming pathogens.
The ancients
Paleopathology is the investigation of ancient artifacts for clues on history’s diseases (see Figure 2.1). Paleopathologists use fiber optics, X-ray imaging, and computerized tomography to see inside caskets without disturbing the contents. Only when they find evidence of damaged tissue do they open the casket and salvage DNA from a bit of tissue, bone, or tooth pulp. By comparing the ancient DNA with that of present-day pathogens, scientists have identified the main bacterial diseases that have haunted society for millennia: anthrax, bubonic plague (Yersinia pestis), cholera (Vibrio cholera), diphtheria (Corynebacterium diphtheria), leprosy (Mycobacterium leprae), syphilis (Treponema pallidum), tuberculosis (TB) (M. tuberculosis), and typhoid fever (Salmonella typhi). Facts gleaned from ancient writings have supplemented the technology of paleopathology. Pliny the Younger wrote of Roman society from 79 to 109 CE and in one essay described an illness affecting a close friend:
She has continued fever, her cough gets worse day by day, she is very thin and weak. Still she is mentally alert, and her spirit does not flag, a spirit worthy of her husband Helvidius....In everything else she is failing to such an extent that I not only fear but grieve.
Figure 2.1. Leprosy. Mycobacterium leprae preferentially attacks the cooler extremities of the body, mainly skin and peripheral nerves. The disease erodes the skeleton, such as these feet dated to a leprosy sufferer from c. 1350.
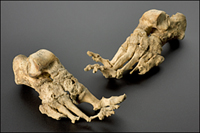
(Courtesy of Science and Society Picture Library, Science Museum, London)
The mention of coughing and weakness without reference to fever or delirium suggested to medical historians that Pliny wrote of tuberculosis. Studies on the emergence of diseases have been aided by the knowledge that cancer and heart disease were rare in antiquity; most deaths from disease can be attributed to infectious diseases.
Some people sensed that hygiene affected quality of life 1,000 years before microbiologists connected bacteria with disease. Mesopotamia’s Sargon I decreed the construction of privies for the ruling class in the 3rd century BCE, and the Greeks and Egyptians devised similar toiletlike receptacles to protect drinking water and food from human waste. The Roman Empire’s largest cities established a model for sanitation infrastructure with freshwater aqueducts, public baths, and sewers for the wealthy. (Rome’s poor endured squalid conditions that led to chronic infections and short lives.) Romans sprinkled spices and herb oils into bath waters for fragrance. These substances are now known to kill bacteria when used in low concentrations.
Hygiene practices changed when the Roman Empire declined. The Roman Catholic Church took a bigger role in influencing public opinion as well as science, teaching that disease came from God as punishment for evil; some present-day clergy continue to embrace this belief. Human behavior certainly influences disease transmission, but evil has nothing to do with it.
The legacy of bacterial pathogens
During World War II, scientists in Germany and Great Britain raced to find a “magic bullet.” They sought not a weapon but a drug to stop needless fatalities from infections of battlefield injuries. Before the magic bullet arrived, herbs served as the main way to fight infectious disease, with mixed results.
Evidence of TB predates written historical records. Mycobacterium bovis may have entered the human population between 8000 and 4000 BCE with the domestication of cattle. Samples from the spinal column of Egyptian mummies from 3700 BCE have shown signs of damage from the disease, but no one has determined if the infection had come from M. bovis or the cause of modern TB, M. tuberculosis. The distinction is small because these two species share more than 99.5 percent of their genes.
In 400 BCE Hippocrates identified the most widespread disease in Greece as phthisis and in so doing described classic signs of consumption or TB. In Aphorisms, he warned, “In persons affected with phthisis, if the sputa which they cough up have a heavy smell when poured upon coals, and if the hairs of the head fall off, the case will prove fatal.” The infected transmit the TB pathogen when they cough, sneeze, or merely breathe, and dense population centers have always acted as breeding grounds. Today TB infects one-third of the world’s population, and humans, not cattle, serve as the reservoir.
Bubonic plague, syphilis, and anthrax began appearing with regularity about 2000 BCE. Historians have debated the message intended by historian Ipuwer, who sometime between 1640 and 1550 BCE wrote of the Egyptian plagues. In referring to the Fifth Egyptian Plague he wrote, “All animals, their hearts weep. Cattle moan.” This passage seems to describe anthrax, a disease as deadly in animals as in humans.
Mobile societies enabled infectious diseases to reach wider distribution. Infectious agents traveled with Egyptian and Phoenician traders crisscrossing the Mediterranean between 2800 and 300 BCE. Both cultures sent ships into the Red Sea and to Persia, but the Phoenicians also sailed north along Europe’s coast. If local residents managed to evade syphilis-infected sailors, they might have contracted disease from some of the tradable goods infested with parasites and pathogens. Anthrax endospores, for instance, hid in hides, pelts, and wool that held bits of soil. As each ship docked, rodents undoubtedly clambered down gangways and brought bubonic plague to shore.
Anthrax became a disease of laborers. Anyone who worked their hands into the soil had a much greater chance of inhaling B. anthracis endospores or infecting a cut. Shearers, tanners, and butchers also had higher incidences of the disease than the rest of society. Since livestock also picked up the microbe from the ground, anthrax caused occasional epidemics in agriculture. The Black Bane of the 1600s killed nearly 100,000 cattle in Europe. People do not transmit anthrax to each other; infection comes mainly by inhaling endospores from the environment. When B. anthracis germinates in the lungs, mortality rates reach 75 percent of infected individuals. Today, the United States has less than one case of anthrax a year. Slightly higher rates occur in the developing world.
Syphilis-causing Treponema might also have entered the human population from animals, perhaps in tropical Africa. Bacteria similar to the one causing human syphilis—the Great Pox—were isolated in 1962 from a baboon in Guinea, but few other clues about the origin of syphilis exist. Ancient explorers brought syphilis throughout the Mediterranean and Europe. Its migration mirrored the spread of the major bubonic plagues that followed trade routes west from Asia to Europe, and later followed the slave trade from Africa to the western hemisphere. Syphilis additionally accompanied each of history’s armed invasions.
Disease historians diagnose syphilis in skeletons by looking for the presence of caries sicca (bone destruction) of the skull, which gives the bone a moth-eaten appearance plus characteristic thickening of the long bones. Corkscrew-shaped T. palladium wriggles into the testicles where it reproduces and then infects a sexual partner by similarly burrowing into the skin. The bacteria then enter the lymph system and bloodstream. As syphilis progresses, the skin, aorta, bones, and central nervous system are affected, but the disease’s early-stage signs are so nebulous that misdiagnosis persisted for centuries. Physicians could not distinguish syphilis from leprosy until Europe’s first major syphilis outbreak from 1493 to 1495 in Naples, which remains one of history’s worst syphilis epidemics. The siege of Naples has also been implicated as the origin of syphilis in the New World, a debate that continues to this day.
In 1493, France’s Charles VIII claimed Naples as his by birthright and sent his army to wrest it from Spain. During the clash, syphilis spread from Naples to the rest of Europe. The timing of the siege and Christopher Columbus’s voyages from Spain have convinced some historians that Columbus’s men brought syphilis to the Americas. Columbus left the port of Palos, Spain, with three ships and 150 men in August, 1492, and returned in March, 1493, leaving dozens of his crew on the island of Hispaniola. The next two excursions from Cadiz to Hispaniola totaled 30 ships and at least 2,000 men with return crossings in 1494 and 1495. After each voyage, most of Columbus’s men wanted no more part of the open ocean and earned money by joining ranks with the Neapolitan troops to fight the French. When Naples finally rebuffed the invasion in 1496, Charles’s troops returned home and syphilis went with them.
Writings by European physicians from 1497 to 1500 indicate that they had never before seen the disease that had first victimized Naples. The French called syphilis “the disease of Naples,” but the Italians felt equally sure of the source. In 1500, Spanish physician Gaspar Torella wrote, “On this account it was christened the morbus Gallicus by the Italians, who thought it was a disease peculiar to the French nation.” The argument would never be resolved and the finger pointing probably continued for years.
Did Christopher Columbus’s ships bring syphilis to the Americas as many historians believe? Martín Alonso Pinzón captained the Pinta in Columbus’s first voyage. A bitter rival of Columbus throughout their sailing careers, Pinzón died of syphilis in 1493 soon after returning home to Spain. Although symptoms begin earlier, the fatal stages of the disease can arise 10 to 20 years after infection, which suggests that Pinzón may have contracted syphilis well before 1492. Before sailing with Columbus, Pinzón had voyaged along the African coast and to the Azores, widening the possibilities of where he caught syphilis. Although speculation on whether Columbus brought venereal disease to the Americas persists, the establishment of settlers’ colonies would have increased the chance for disease transmission. If not Columbus, then certainly others who followed brought with them contagious diseases.
The plague
Justinian I, 6th-century ruler of the Byzantine Empire, devoted himself to spreading Byzantine architecture from his throne in Constantinople, along the Mediterranean rim, up the Nile, and deep into Europe. To prepare for his fleet’s voyages, Justinian ordered continuous stocking of the massive granaries on the city’s outskirts. The grain sustained the ships, but also fed an exploding rat population.
By 540 CE Justinian had succeeded in expanding Constantinople’s influence. But at each new port, residents fell nauseous and developed chills, fever, and headache, some within only two days of a ship’s arrival. Their abdomen would swell with pain and bloody diarrhea followed. Their lymph nodes (or buboes) clogged with necrotic tissue and by six days of the first discomfort, many had died, the skin covered with dark purple lesions. The same occurred in Constantinople where deaths grew to 10,000 daily. Many who felt the first symptoms of illness panicked and fled to the countryside. Within days the fatalities rose in those rural places, too.
Justinian blinded himself to the misery and more than one assassination attempt. He drained the coffers to expedite his dream and perhaps also to entice new sailors from a dwindling labor pool. The Plague of Justinian would kill 60 percent of the empire or 100 million people by the time it had run its course in 590 CE. Justinian himself avoided the plague and died of natural causes at age 38.
Did a mitigating factor suddenly appear to cause the first bubonic plague in recorded history to arise? Rodents then as now carry the intestinal bacterium Y. pestis that can contaminate the animal’s fur or skin. Fleas ingest Y. pestis each time they bite and so engorge themselves that their digestive tract fills with bacteria. The insect must regurgitate some bacteria just to stay alive. When a flea upchucks on an uninfected rodent, it transmits Y. pestis and creates an ever-expanding reservoir of disease. Poor sanitation leading to large rat populations in metropolises like Constantinople increased the probability of receiving flea bites. Justinian gave the epidemic a boost by building granaries that all but guaranteed a massive rat colony to serve as the disease’s reservoir.
Following Justinian’s rule, bubonic plagues mysteriously disappeared for the next 700 years. As the last remnants of Roman influence faded, disease control also declined, and many contradictions took root. People believed that retaining wastes and even an animal carcass in the home repelled evil and thus disease, yet many also assumed that bad odors brought illness—a cadaver in the living room surely smells. Even with the building of the first centralized hospitals at the dawn of the Middle Ages, medicine remained the domain of healers who used leeches for extracting the body’s pains. Faulty birthing methods caused a high incidence of mental illness that further threatened good personal hygiene.
Beginning in the 14th century, four plague epidemics would decimate Europe, none more brutal than the Black Death, named for the black-purplish lesions formed by hemorrhaged vessels under the skin. Between 1346 and 1352 the Black Death killed more than 25 million people in Europe or about 30 percent of the total population. Combined with the loss of life as the plague followed trade routes from Asia in the 13th century and to northern Africa and the Crimea before reaching Europe, the global Black Death killed a total of 200 million. As in Justinian’s day, survivors could not bury the dead fast enough. Survivors carried the corpses on long poles—“I wouldn’t touch that with a 10-foot pole”—to mass graves outside the towns. The epidemic slowed only when it reached the Alps where colder weather repelled rats, and the pathogen had likely mutated to a less virulent form.
An epidemic that destroys 100 million lives in less than a decade and reduces Europe’s population by one-third, as the Black Death did, certainly impacts society in ways that are felt for generations. Even art and music reflected the looming presence of Death, which usually triumphed over mortals (see Figure 2.2). Some cities lost 75 percent of their children, and entire family trees had been reduced to one individual—the plague had created a parentless generation. Craftsmen, artists, farmers, and clergy disappeared. A plunge in economic vitality caused birth rates to drop.
Figure 2.2. Dance of Death. Death became an everyday occurrence, by the hundreds in some towns, in the Middle Ages in Europe. Artists, writers, and composers depicted bleak futures where Death overwhelms the living.

During the plagues, clergymen insisted as they had for centuries that sickness came as penance from God. Their ineffective efforts to administer to the dying by combining faith and sorcery caused the church to lose its customary privileged status in society. The banking profession gained stature, however, for two reasons. The plague’s survivors understood the need for protecting assets for the next generation, especially when death could strike so suddenly. At the same time, serfs abandoned fields controlled by feudal landowners and took advantage of monetary pay to fill labor shortages in the cities. This in turn helped create a mobile workforce that covered Europe in search of the highest wages in labor-starved towns. Young adults left as the sole managers of family property opted against traveling to traditional centers of learning in Paris, Vienna, or Bologna. New centers of education thus developed in Oxford, Cambridge, Edinburgh, Amsterdam, Copenhagen, and Stockholm by the 14th century. The depopulation of the European continent also opened up new land for cultivation or development and laid the foundation for the industrial centers of today’s Europe.
Surgeons had been as useless as clergymen during the plagues. The status of the surgeon would decline and not rebound until the mid-19th century when Joseph Lister invoked the need for sterile conditions in hospitals. Barbers came to the fore as more trustworthy medical practitioners despite their penchant for bloodletting as an all-purpose cure. But what is now known as western medicine also advanced. Medical schools grew and students for the first time learned anatomy and physiology. As a result, the medical community began learning about the effects of infectious disease on internal organs.
With each of these historical plagues, survivors learned better precautions for escaping infection. Bubonic plague is not contagious, but streets filled with the dead and dying certainly showed that anyone could fall victim. Plague survivors gingerly removed the bodies and took them to the countryside where funeral pyres awaited. This had been the commonest method of disposal throughout the Middle Ages, but on occasion people used more imaginative ways to dispose of corpses.
From 1344 through 1347, Tartars laid siege repeatedly to the port city of Caffa (now Feodosija, Ukraine), home to diverse nationalities and political persuasions. The plague had already laid waste to the Tartars’ homeland of eastern Asia, and deaths among them mounted even as they surrounded the city. With a body count mounting, the Tartars disposed of their deceased by the simple expediency of catapulting the cadavers over Caffa’s walls. Caffa’s healthy residents would be infected when they collected the bodies for burial. Thus bacteria and humans forged a complex relationship involving disease, sustenance, evil, and God.
Microbiologists save the day
In 1822 Louis Pasteur was born into a family that had made its living tanning hides for generations. A lackluster student, only chemistry held Pasteur’s interest. By the time he reached college, Pasteur would spend hours studying structures of organic compounds and this pursuit likely awakened a curiosity about biology. Still, Pasteur thought of himself as foremost a chemist.
After winning election as France’s president in 1848, Napoleon Bonaparte III made transportation, architecture, and agriculture the country’s priorities. New edicts pressured university scientists to follow commercial pursuits. As a professor at the University of Lille, Pasteur grudgingly tucked away his chemistry equipment and brought a microscope into his lab without a clear plan for using it. He decided to teach students about biology’s relationship to agriculture until the time came when he could return to his chemistry experiments.
Pasteur’s “temporary” foray into biology initiated the most accomplished career in microbiology’s history. His publication list lengthened, and his reputation grew inside and outside of science. By the 1850s, Pasteur had been recruited by France’s alcohol manufacturers to improve their fermentation methods. He began by investigating yeast fermentations, perhaps because brewers had not studied it in detail. Pasteur noticed that a drop of liquid from the fermentation flasks gave a curious result in the microscope. When Pasteur put a glass cover slip on top of the drop, some of the microbes avoided the edges of the slip where the liquid was exposed to the air. Pasteur introduced biology to anaerobic bacteria.
By describing processes taking place in fermentation, Pasteur gave the wine and brewing industries greater control over their manufacturing steps. His reputation soared when he diagnosed a disease that had been decimating France’s silk industry. By the 1860s, Pasteur reached national hero status. (Pasteur had incorrectly identified bacteria as the cause of the silkworm disease. Electron microscopes were not yet available to enable him to find the real cause: a virus. Pasteur nevertheless made the crucial and previously overlooked connection between microbes and infection.)
The public adored Louis Pasteur. Napoleon III invited the microbiologist to his table to hear the latest theories on microbes, and Pasteur happily obliged. He, in fact, had developed the habit of dismissing anyone who questioned his work. Pasteur also cultivated the ability (or flaw according to some) of drawing scientific conclusions even while producing little data to back them. Louis Pasteur possessed such a rare and keen insight into biology that his conclusions almost always proved to be correct. One famous misstep occurred in 1865 during a cholera outbreak in Paris. Pasteur believed the pathogen Vibrio cholerae transmitted through the air though it is a waterborne pathogen. The French nonetheless felt relieved to know that Pasteur was hard at work trying to save them from cholera. The Paris epidemic ran its course and disappeared on its own.
During a rabies scare in 1885, Pasteur concocted a treatment and gave the untested drug to a nine-year-old boy, Joseph Meister, who had been bitten by a grocer’s dog. Three weeks later, Meister had almost fully recovered. Pasteur’s legend received considerable help by the fact that Meister hailed from Alsace, a region controlled by Germany but claimed by France. The tricolor declared a victory for French science and for Pasteur who had beaten the German, Robert Koch, who had like Pasteur been working on vaccines. As a grown man, Joseph Meister took a job as a guard at the Institut Pasteur after Pasteur’s death. When German troops entered Paris in 1940, they swarmed the institute’s grounds and ordered that Pasteur’s crypt be opened. Meister likely had been one of several men who defended the crypt against the Wehrmacht and prevented its defilement. Shortly after, Meister inexplicably shot himself through the head. Even this act became part of Pasteur’s celebrity. Historians would write that Meister committed suicide in front of the Germans rather than disturb Louis Pasteur, France’s hero.
Pasteur’s influence on microbiology cannot be captured in a few pages. Early in his career, Pasteur had disproved the long-held theory of spontaneous generation, the belief that microbes and all other life arose from inanimate things: rocks, water, or soil. Biologists had already begun taking sides on this issue prior to Pasteur. As their science matured, many microbiologists doubted the logic behind spontaneous generation—science was increasingly distancing itself from spiritual dogma. Pasteur developed an experiment that unequivocally showed that a flask of sterilized broth could not produce life on its own. Pasteur modified this flask with an S-shaped tube to serve as the opening. This configuration let in air but prevented any particles from the air to enter. A second sterile flask left open to the air was soon teeming with bacteria but the S-flask remained sterile. Elegant in its simplicity, Pasteur’s experiment earned him respect from his contemporaries.
During his career, Pasteur also distinguished between anaerobic and aerobic metabolism, invented the preservative method to be known as pasteurization, and developed the first rabies and anthrax vaccines (see Figure 2.3). As a postscript, the original S-flask is on display at the Institut Pasteur today and remains sterile.
Figure 2.3. Bacillus anthracis, the anthrax pathogen. B. anthracis and all other Bacillus species form a tough, protective endospore. In this picture, endospores in phase-contrast microscopy look like bright ovoid balls inside an elongated cell.
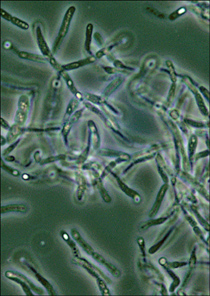
(Courtesy of Larry Stauffer, Oregon State Public Health Laboratory)
When bubonic plague erupted in Asia in the late 1800s, Pasteur dispatched Alexandre Yersin of the French Colonial Health Service to investigate. Microbiologists had by that time a century of using ever-improved microscopes, and they had become skilled at diagnosing disease by examining patient specimens to detect pathogens. In 1894, Yersin and a bacteriologist sent by Japan’s government, Shibasaburo Kitasato, rushed with other public health officials to Hong Kong where a localized plague outbreak was emerging. Within a week Yersin isolated a rod-shaped bacterium from a plague victim. Kitasato found a similar microbe, but because the two men conversed only in broken German, they shared little of their findings. Yersin sent his report to the Institut Pasteur in Paris. Kitasato forwarded his results to Robert Koch in Berlin. In most circumstances, two scientists having attained the prominence in their profession as Pasteur and Koch had would have shared their data and drawn mutually agreed-upon conclusions. But Yersin and Kitasato’s place in history would hinge on a rivalry between Pasteur and Koch that began 12 years earlier.
Pasteur and Koch held different perspectives on bacteria. Pasteur focused on the interplay between the body’s immune system and bacterial pathogens and felt that virulence in pathogens changed over time, creating more or less virulent strains depending on environmental influences. Koch believed pathogens to be less variable and always capable of releasing virulence factors if opportunities for infection arose. Their differences would have made for lively and good-natured discussions had not Pasteur accidentally insulted Koch’s “German arrogance” at a meeting in Geneva in 1882. Pasteur had actually praised Koch’s body of work on anthrax and tuberculosis bacteria to the audience, but a scientist sitting next to Koch struggled to keep up with Pasteur’s speech and translate it into German for his colleague. Unbeknown to Pasteur or Koch, the translator had made a mistake in going from French to German. In an age lacking telecommunications gadgets, the misunderstanding persisted. Koch returned to Berlin with contempt toward Pasteur that he made no effort to conceal.
When Pasteur published details of his successful rabies vaccine in 1885, Koch dismissed the work, insisting that a vaccine made of attenuated viruses needlessly endangered patients. But an underlying animosity likely arose out of each man’s patriotism and the border conflicts between France and Germany over the Alsace and Lorraine regions. Koch undoubtedly remembered that Pasteur had received an honorary degree from the University of Bonn in 1868, but returned it later during the height of French-German tension. “Today this parchment is hateful to me,” Pasteur wrote to the university dean, “and it offends me to see my name, which you have decorated with the qualification virum clarissimum, placed under the auspices of a name that will henceforth be loathed by my country, that of Guillermus Rex.” The Germans responded with equal vitriol with both letters ending up printed in local newspapers.
With this history as a backdrop, Yersin and Kitasato hardly stood a chance of reaching an agreement on who had discovered the plague pathogen. Kitasato would unsuccessfully argue for the rest of his career that his discovery was the same as Yersin’s, but Yersin received the accolades. He named the microbe Pasteurella pestis in honor of his boss. (Microbiologists still treasure the compliment of having a lethal pathogen named after them. The species would be renamed Yersinia pestis in 1944.) Historians have had trouble finding evidence in Kitasato’s notes to confirm his discovery of the plague bacterium. A new generation of microbiologists would try to smooth the prickles by conceding that Kitasato had probably seen the same bacteria in his microscope that Yersin had spotted.
Pasteur and Koch never resolved their differences and Pasteur remained a French patriot to the end. In 1895, the Berlin Academy of Sciences extended a peace offering to Pasteur by inviting him to accept the medal of the Prussian Order of Extreme Merit. The Frenchman refused any invitation to Germany as long as it still held Alsace and Lorraine.
Unheralded heroes of bacteriology
The names Pasteur, Lister, and Fleming represent significant advances in bacteriology but, as in today’s technical fields, the rise to prominence results as much from personality as it owes to scientific merit. Generations of scientists since van Leeuwenhoek’s day pursued the secrets of bacteria with the same devotion as more famous microbiologists. Many of their stories have been all but lost due to oversight, misunderstanding of their discoveries, and sometimes jealousy.
Robert Hooke
In the 17th century, Robert Hooke corresponded with Antoni van Leeuwenhoek on the assembly of lenses for viewing the natural world on a microscopic scale. Both men developed similar instruments, but van Leeuwenhoek would become known as the Father of Microbiology while Robert Hooke’s name has faded into near obscurity. A brilliant biologist and engineer, Hooke also mastered physics, the arts, architecture, geology, and paleontology over his long career.
As a youngster, a case of smallpox had disfigured Hooke, but he compensated with a gregarious nature. By the time Hooke graduated from Oxford, scientists in England sensed a luminary had arrived to raise public opinion of their profession. In 1662, the Royal Society of London elected Hooke at age 27 as Curator of Experiments, a role suited to his intellect and penchant for innovation. As Curator, Hooke performed an impressive array of demonstrations in biology, chemistry, and physics for the Royal Society but had an increasingly hard time staying focused on details from month to month. He often bolted to new projects before finishing the last, leaving other Society members to the drudgery of completing his studies.
Hooke tinkered with van Leeuwenhoek’s microscope design and began a detailed study of the world he found under its lens. Hooke drew sketches of insects, feathers, plants, and leaves as well as snowflakes and mineral crystals and published them in Micrographia in 1665. (The book’s full title is Micrographia: Or Some Physiological Descriptions of Minute Bodies Made by Magnifying Glasses with Observations and Inquiries Thereupon.) In it he coined the term “cell” to describe similar but separate units that composed a thin slice of cork. Overlooked at the time, this remark laid the foundation for all of biology: the cell is the simplest basic unit of every living thing on Earth, and without cells life does not exist.
The breadth of Hooke’s accomplishments in architecture and engineering are no less impressive, yet Royal Society records contain little mention of the man or his work. In 1672, mathematician Isaac Newton out of Cambridge joined the Royal Society. Hooke had already begun developing equations to describe the gravitational forces of Earth’s elliptical orbit around the Sun when Newton arrived at the Society with expertise in this same subject. The frail, introverted Newton developed a rapport with the outgoing Hooke as they pondered the mathematics of planetary movement. Their alliance permanently dissolved when in 1672 Hooke publically criticized a presentation to the Society by Newton on the properties of light and color. The animosity grew over the next decade. Hooke accused Newton of claiming credit for theories Hooke felt he had already developed. When in 1687, Newton published a thesis on planetary orbits with no mention of Hooke, the rift seemed irreparable.
Hooke’s personality disintegrated in later years for reasons unknown. Isaac Newton would be one on a long list of people to whom Hooke directed his animosity. Newton took the Curator position shortly after Hooke’s death in 1703 and almost at once struck Hooke’s name from Society documents. Hooke’s portrait disappeared under suspicious circumstances as well as many of his laboratory notes. Some historians believe those missing notes contain evidence that Hooke invented the compound microscope rather than van Leeuwenhoek, and questions persist on whether Hooke had developed the theory of gravity before Newton. Hooke would sadly become known as much for his rivalry with Isaac Newton as for his contributions to science.
John Snow
Epidemiology owes its beginning to a London doctor’s dogged attempt to stem one of several cholera outbreaks that had tormented London in the 1800s. Physician John Snow wrote in his journal in September 1854, “The most terrible outbreak of cholera which ever occurred in this kingdom is probably that which took place in Broad Street, Golden Square, and the adjoining streets, a few weeks ago.” Snow’s nonplussed colleagues knew of his tedious house-by-house assessment of family health and daily habits near the outbreak’s center in Soho. The details he collected from the interviews seemed to have nothing to do, however, with the debilitating diarrhea that claimed many of the afflicted.
Snow persevered and sifted through his stacks of notes. He found that 73 of the outbreak’s 83 deaths occurred within two blocks of a pump (see Figure 2.4) that dispensed water free to the public. The incidence of diarrhea related to the frequency in which families used the pump. By simply removing the pump’s handle to make it unusable, Snow stopped the 1854 Soho cholera outbreak. He would become known as the Father of Epidemiology. Today’s epidemiology follows the same path used by Snow. Epidemiologists track the locations where disease incidence are highest and search for commonalities among the sick. They gather other clues, such as an increased reporting by doctors and hospitals of common symptoms. Epidemiologists have even identified the presence of a waterborne outbreak by the uptick in sales of toilet paper in a community.
Figure 2.4. The Broad Street pump. London has preserved the Broad Street pump as a historic site where John Snow stopped a deadly cholera outbreak. Prior to Snow’s accomplishment, most doctors did not believe water carried disease.

(Courtesy of Peter Vinten-Johansen, et al., Cholera, Chloroform, and the Science of Medicine: A Life of John Snow, 2003, 289; and http://johnsnow.matrix.msu.edu/images/online_companion/chapter_images/fig11-2.jpg)
Snow conducted his study without any idea of the pathogen coming from the pump. His contemporaries had not connected water with many of the diseases of the day. Thirty years after the Soho outbreak, German microbiologist Robert Koch identified C. vibrio as the cause of the waterborne disease.
George Soper
In 1883 Irish immigrant Mary Mallon arrived in New York City and found work cooking for well-to-do families. In the summer of 1906, Mary escaped the city heat and took a job at the rented cottage of banker Charles Warren in Oyster Bay, Long Island. Soon afterward Warren’s family and staff suffered headaches, lethargy, loose bowels, and debilitating fever. The family doctor recognized the symptoms of typhoid fever but doubted an inner-city disease would afflict suburbia’s wealthy.
By summer’s end the Warrens had recuperated and returned to the city. The house’s owner, George Thompson, heard of the outbreak and made a brilliant assumption: He suspected that a dangerous germ had entered his home. The Thompsons called on a public health officer, the fastidious, systematic, and humorless George Soper. Soper went straight to hands and knees at the Thompsons’ in search of dirt, an undertaking depicted in the satirical cartoon in Figure 2.5. He sat for hours perusing household records and the comings and goings of staff and visitors. Soper scoured details that few epidemiologists had in the past. In a meal log, Soper noticed the Warrens’ fondness for ice cream and sliced fresh fruit, excellent carriers of germs. He also noticed Mary’s name in the records at the time the Warrens got sick. Soper hurried back to New York and unearthed health records showing that in seven of the eight families for whom Mary cooked, typhoid fever broke out; 28 cases in all and three deaths.
Figure 2.5. Health inspectors react to newspaper headline, “Looseness of the Bowels is Beginning of Cholera.”

(Courtesy of Wellcome Library, London; The John Snow Archive and Research Companion, Center for the Humane Arts, Letters, and Social Sciences online at Michigan State University)
Soper tracked down Mary the next year working in a Park Avenue apartment. With little formality he accused her of spreading death and disease and ordered her to surrender a fecal, urine, and blood sample on the spot. Husky and with a lightning temper, the cook hustled Soper out the door and into the street. Undaunted, he showed the city’s Health Department his evidence and demanded action against the cook. Authorities felt Park Avenue was as unlikely a place for typhoid fever as Long Island, but Soper’s meticulous notes swayed them. Police wrestled Mary out of the apartment and took her to Willard Parker Hospital, the main center for treating contagious diseases. There, doctors found unusually high concentrations of Salmonella typhi in her stool and the legend of “Typhoid Mary” was born.
Soper had all but called Mary Mallon an evil genius and put equal blame on upper class women who brought people like Mary into their homes. He irrationally likened them all to murderers. In 1928, he told the New York World, “She knew that when she cooked she killed people, and yet she deliberately sought employment as a cook.” In fact, Mary Mallon never believed she had made anyone sick. Soper fought the prevailing beliefs of the day to stop the Typhoid Mary outbreaks. He also spearheaded the inspection of New York’s sewers, water supply, and garbage pickup and became an advocate for good personal hygiene and community sanitation as the best ways to break the transmission of pathogens.
I thought of Mary Mallon in 1998 at a San Francisco crafts festival. Waiting in the lunch line, I noticed a young woman behind the salad-mixing station. During a lull in the action, she fished around in her mouth with her fingers and cleaned her teeth. She then plunged her unwashed, gloveless hands into a trough of lettuce, mixing the leaves and scooping out salad. I stepped out of line and said to her, “Do you realize you just contaminated all that salad with your dirty hands?” She looked confused at first then glanced at the lettuce. “Good,” I thought, “I’ve taught someone about hygiene and averted a possible health disaster.”
In 1909, New York quarantined Mallon on an island in the East River. Miserable, angry, and convinced she had nothing to do with typhoid, she lamented her role as “a peep show for everybody.” After her release Mary changed her name and began cooking again, this time at Sloane Maternity Hospital. By 1915, she had caused 25 new cases of typhoid until a health inspector spotted her in the hospital kitchen. Police took Mary back to the island where she died in 1938.
Unlike other pathogens, S. typhi lacks multiple strains of varying virulence; the species is fairly uniform the world over. S. typhi’s survival rests on asymptomatic carriers who efficiently spread the pathogen through a population. Research has not yet uncovered all of the secrets of typhoid susceptibility in those who are asymptomatic. In carriers the bacteria multiply in the gallbladder, bile duct, and intestines, and then spread in drinking water and food contaminated with miniscule bits of fecal matter, a more common occurrence than most people think. I had no more success in convincing the salad vendor to change her hygiene habits than Soper had with Mallon because no one wants to believe they disseminate contamination.
Because of the prevalence of fecal bacteria everywhere, people would be wise to take a bacteriocentric view of the world, “seeing” bacteria on the places they exist even though they remain invisible. Suspicious foods, dirty floors, or murky water shout the presence of bacteria as do people who avoid washing their hands. In the 1970s, bacteriocentricity helped solve one of modern epidemiology’s most puzzling outbreaks.
Joseph McDade
Mid-July in Philadelphia is sticky, sweaty, and heavy with odors that seem to seep from the concrete. In 1976, the 70-year-old Bellevue Stratford on South Broad Street opened its doors to 4,000 World War II Legionnaires in town for their annual convention. An influenza outbreak that had killed a soldier in nearby Fort Dix, New Jersey, that summer put many of the visitors on edge, especially because the virus resembled one that took 40 million lives in 1918 to 1919, the worst single flu outbreak in history. The Legionnaires and hotel staff likely took extra care washing their hands and staying on guard for the sounds of sneezing or coughing. But trouble came from a different direction.
The Bellevue Stratford’s air conditioning system had developed a thick biofilm in the condensation-wetted distribution lines. There, amoeba, a type of protozoa, multiplied in the moist habitat they need for survival, feeding on the biofilm. Hidden inside the amoeba lived a bacterium that most microbiologists did not know existed. Because of the biofilm, the air conditioning vents began to emit moisture droplets filled with microbes.
No one knew they were inhaling contaminated air. Hotel guests and even people who had strolled past the building’s open doors became congested and weak, developed muscle pain and headaches, and suffered with diarrhea. The dreaded flu virus obviously had returned and with it, near panic—someone blamed the communists. Congress ordered into place an emergency vaccination program, but the year closed with few weapons against the mysterious disease.
During the holidays, Joseph McDade from the Centers for Disease Control and Prevention (CDC) stared into his microscope searching blood samples from the hotel’s guests for Rickettsia bacteria. Rickettsia bacilli would be easy to miss because it lives only inside other cells, such as human cells. Weary with eyestrain he went to a holiday celebration, but for McDade, bacteria were more compelling than office parties. He returned to his laboratory and re-examined the Legionnaires’ samples. In the early hours he spotted a cluster of bacilli inside white blood cells. The bacteria were not stubby, short rods of 1 μm like Rickettsia, however, but long thin rods stretching to 10 μm or more.
McDade had found a new species, Legionella pneumophila. The CDC unraveled the bacteria’s pathology. L. pneumophila enters the lungs and then infects the bloodstream. The immune system releases cells called macrophages for the specific purpose of destroying infectious agents such as bacteria, but like Rickettsia, Legionella is a “stealth pathogen.” L. pneumophila slips inside macrophages and multiplies in the phage’s cytoplasm. A new generation of cells bursts free and continues the infection cycle. Microbiologists had noted bacteria that fit L. pneumophila’s description years earlier, but the microbe’s finicky growth requirements made laboratory studies almost impossible.
Clinical microbiologists deal with a short list of stealth pathogens in addition to Rickettsia and Legionella, including the foodborne pathogens Listeria monocytogenes, Shigella flexeri, and Salmonella enterica, and mycoplasmas. L. monocytogenes invades the epithelial cells lining the digestive tract, and when in the bloodstream is one of the few bacteria that cross the blood-brain barrier. Severe cases of listeriosis therefore damage the central nervous system. Salmonella and Shigella usually stay in the digestive tract.
On the front
Microbes have played a part in war before the Tartars deployed their unique bioweapon. Prior to the introduction of antibiotics, minor battlefield injuries led to about half of all wartime deaths. Marginal food, lack of sleep, and emotional stress reduced soldiers’ ability to fight infection. Without treatment of infected wounds, pathogens could enter the bloodstream and multiply—a condition called sepsis—and then infect major organs. Some pathogens stay at the wound site and cause severe infection there. Badly injured skin contains oxygen-free pockets in the tissue, which promotes the growth of anaerobes such as Clostridium perfringens, the cause of gas gangrene. Before World War II small scratches caked with soil and left untreated presented the risk of amputation or death.
Virulence factors aid the infection process. Some bacteria rely on only one approach, such as Mycoplasma that produces hydrogen peroxide and ammonia, both toxic to the body’s cells. After the two compounds damage cells lining the respiratory tract, Mycoplasma enters lung tissue. Staphylococcus aureus, by contrast, uses a battery of weapons:
• Coagulase enzyme clots the blood surrounding a wound and protects the bacteria from the body’s immune defenses.
• Nuclease enzyme breaks up exudates in the wound and thus helps the bacteria’s mobility.
• Hemolysins lyse red blood cells, causing anemia and weakened body defenses.
• Hyaluronidase enzyme degrades the binding material between human cells to aid passage of the pathogen throughout the body.
• Protein A binds the body’s antibodies and renders them inactive.
• Streptokinase enzyme activates a series of steps in blood clot destruction, allowing the bacteria to escape a clotted area.
Two champions of proper medical care died a few years before the First World War. British nurse Florence Nightingale called for reforms in treating combat injuries. During her service in the Crimean War, she reported on the diseases, poor food, and unsanitary conditions in medical hospitals. Her 1,000-page report compiled in 1858 convinced her superiors that the British Army was needlessly losing soldiers to treatable injuries. During the same period in Britain, surgeon Joseph Lister insisted that surgeries required sterile conditions and wounds must be kept clean with antiseptics. Lister used carbolic acid as an antiseptic; several years would pass before less irritating chemicals came into use.
Sterility and antiseptics were new ideas when war began in 1914. Not all surgeons wanted to put chemicals on patients’ skin, and they initially resisted using antiseptics. A second, more revolutionary, defense against infection soon surfaced. Microbiologist Felix d’Herelle had tried to fight a locust outbreak by infecting the insects with bacteria. He presumed that if a similar agent attacked pathogenic bacteria, it would fight infectious disease. d’Herelle knew that some microbiologists had discovered a substance in their bacterial cultures that infected and killed other bacteria. With little idea of the material’s identity, d’Herelle began collecting liquid medium from affected cultures. By 1917, he was using it to cure hundreds of cases of dysentery by injecting patients with his “antagonistic microbe.” Not until 1939 with the advent of electron microscopes did microbiology learn of bacteriophages, viruses that infect only bacteria. The treatment called phage therapy would be superseded by antibiotics in the next decade, but for a short period in history phages played the part of the magic bullet.
The consequences of war, upheaval of home life, and the creation of mass refugee migrations hampered sanitation and personal hygiene. The common body lice Pediculus humanus infested almost everyone in World War I. The lice carried the typhus bacterium Rickettsia prowazekii. This microbe behaves like a virus by living as a parasite inside other cells. The lice ingested R. prowazekii when they bit an infected person and after an incubation of six days they became infective to others. Unlike the plague, which spread via flea bites, typhus spread when lice defecated on the skin and the bacteria entered the body through a wound.
Typhus would blanket Europe and become an epidemic second only to the Black Death in fatalities. In Serbia, 20 percent of the population contracted typhus and 60 to 70 percent of those people died. Disease became so devastating to Austria, the Balkans, Russia, and Greece that the Central Powers delayed some maneuvers for fear of wiping out their own armies. At the close of the war, a four-year epidemic struck Russia and would kill half of the 20 million people who had been infected with typhus.
Many in the German army that invaded Poland to start World War II carried memories of the typhus outbreaks. Entering the third year of occupation, Polish physicians Eugene Lazowski and Stanislaw Matulewicz devised a way to stop some of the carnage and deportations to work camps. They knew that the Proteus strain OX19 looked similar to R. prowazekii to the body’s immune system. They thus began injecting healthy residents of the town of Rozvadow with killed OX19 cells. This ersatz vaccine induced the production of antibodies against the typhus bacterium. Lazowski and Matulewicz had created a fake typhus epidemic.
The Germans may have had suspicions of the isolated outbreak. A German medical team arrived in Rozvadow in 1942 to assess the situation, but their doctors so feared infection that they skipped giving physical exams; they collected blood samples and hurried back to Berlin. The antibodies in the samples convinced the German army to avoid typhus-ridden Rozvadow. The contrived typhus epidemic saved almost 8,000 lives, many of them Jews.
People and their pathogens have continuously traded victories and defeats. Sometimes the bacteria win, such as in plague and syphilis epidemics. Sometimes the guile of humans triumphs as in d’Herelle’s phage therapy. But do people ever truly defeat bacteria? The search for the magic bullet ended when a shy microbiologist discovered the “miracle drug” penicillin, or so it seemed at the time.
