6. The invisible universe
The field of microbial ecology focuses on the role microorganisms play in all of nature. Microbial ecologists study bacteria in small habitats of a few dozen species as well as global systems that circulate elements through continents and oceans. These systems called biogeochemical or nutrient cycles make carbon, nitrogen, sulfur, phosphorus, and metals available for humans and all other life. Microbial ecology now includes technologies aimed at reversing global warming, pollution, and biodiversity loss.
Microbial ecologists continually uncover new Earth-human-bacteria relationships. Despite the importance of good bacteria in the environment, microbial ecology is a new science compared with food and medical microbiology.
During the Golden Age of Microbiology, battles against disease inspired microbiologists more than finding out what grew in a clump of dirt. Joseph Lister introduced aseptic techniques for surgical procedures, Edward Jenner developed the smallpox vaccine, and Florence Nightingale promoted hygiene practices for preventing infection. It may have seemed as if the only good bacterium was a dead bacterium.
Late in the Golden Age, botanists Martinus Beijerinck and Sergei Winogradsky took roads less traveled by studying the beneficial bacteria of soil and water. In the Netherlands Beijerinck studied the symbiotic relationships between plants and bacteria. Winogradsky, from Russia, explored bacterial metabolism in soil and water.
Martinus Beijerinck was born in 1851 and grew up in modest surroundings as the son of a tobacco farmer. After pursuing an education in botany and agriculture, he became head of the Netherlands’ first laboratory devoted to industrial microbiology. At this position Beijerinck investigated contagious viruses that infected tobacco plants and nitrogen-metabolizing bacteria that live in association with legume plants.
In 1888, Beijerinck discovered bacteria living inside small lumps or nodules on the roots of Vicia and Lathyrus (yellow pea) plants. Beijerinck performed the difficult tasks of isolating these bacteria from the nodules and growing them in his laboratory. He took on the painstaking process of formulating a nutrient mixture to favor the root nodule bacteria while inhibiting thousands of other bacteria in soil. This method, called enrichment medium, remains a key part of environmental microbiology. Beijerinck spent several years piecing together the metabolism of these bacteria (later to be named to the genus Rhizobium) and their role in nature.
Martinus Beijerinck revealed what is now known to be a critical step in the Earth’s nitrogen cycle: Rhizobium pulls nitrogen from the air, a process called nitrogen fixation, and converts the element into a form that legume plants (peas, beans, peanuts, and alfalfa) can use. The plant incorporates the nitrogen into proteins, nucleic acids, and vitamins, which a diversity of animal life then takes in for nutrition. The Rhizobium-legume union represents symbiosis in which two unrelated organisms live in close association. In this case, the type of symbiosis is termed mutualism because both organisms cooperate in giving each a benefit. The root gives the bacteria a safe haven, and Rhizobium supplies the plant with an essential nutrient. Not all types of symbiosis are as beneficial as mutualism:
• Commensalism—One organism benefits and the other receives neither a benefit nor harm.
• Amensalism—One organism benefits by exerting a harmful effect on another.
• Parasitism—One organism living on or in a host organism benefits at the expense of the second organism’s health.
Beijerinck also studied the sulfur cycle in soil bacteria. The step called sulfate reduction occurs in anaerobic places in soil. Beijerinck devised methods for growing fastidious sulfate-reducing bacteria, a feat that other microbiologists had believed to be too difficult or even impossible.
Winogradsky, born 5 years after Beijerinck, enjoyed a more privileged upbringing. Young Sergei found classes in Greek and Latin “not only uninteresting and unpleasant, but depressing, both physically and mentally.” As he grew older he tried law, then music, but inspired by neither he turned to the natural sciences. In 1885, Winogradsky took a position in botany at the University of Strasbourg and began at once to study the sulfur-using bacterium Beggiatoa, the bacterium that shuttles between sunlit and dark layers in microbial mats.
Louis Pasteur offered Winogradsky a position at his famed research institute in Paris, but the Russian declined, preferring to return to his homeland to build the microbiology profession there. The Great War disrupted the progress of most professions; in 1917, wealthy families such as Winogradsky’s barely escaped death at the hands of the Bolsheviks.
Winogradsky took a position at the University of Belgrade where no science laboratories or even a library existed, but at least it provided some stability for his family. He perused the only scientific journal he could find, Centralblatt dür Bakteriolge and thus kept abreast with bacteriology research in Europe. Few microbiologists were examining in depth the bacteria of natural environments. He plunged into studies on the organism he knew best, Beggiatoa, examining the microbe’s use of iron compounds for energy. The Institut Pasteur called again, and this time Winogradsky accepted, perhaps tempted by the well-funded and stocked laboratories in Paris.
During his career, Winogradsky would discover at least eight new bacterial species in addition to Beggiatoa: endospore-forming Clostridium pasterianum; the gliding, cellulose-digesting Cytophaga of freshwater, estuarine, and marine habitats; and nitrogen-metabolizing Nitrosococcus, Nitrosocystis, Nitrosomonas, Nitrosospira, and Nitrobacter. The five nitrogen-utilizing bacteria differed from those studied by Beijerinck: these bacteria live free in soil and run separate steps in the nitrogen cycle from those carried out by Rhizobium.
Like Beijerinck, Winogradsky studied the sulfur bacteria and became the first microbiologist to isolate pure cultures of sulfur-oxidizing bacteria from soil. These bacteria turn the element sulfur into a usable inorganic form that Beijerinck’s bacteria then convert to a molecule useful to higher organisms. A bit of a Renaissance man of microbiology, Winogradsky also became the first bacteriologist to study biofilms in aqueous habitats, and he sparked interest among microbiologists in iron-metabolizing bacteria living in deep aqueous sediments.
Winogradsky continued writing on microbial ecology into his nineties. His daughter Helen would join him at the institute and carry on his work on nitrogen-using bacteria after his death at age 97.
Versatility begets diversity
Communities such as biofilms and microbial mats make life easier for their members than living alone as a single cell. But all species of bacteria spend some part of their existence free from a microbial community. Cells break away from communities when the density grows too high. Motile cells escape toxins by themselves or migrate toward nutrients by using flagella, cilia, or twitching movements. During the periods of growth in which cells fend for themselves separate from a microbial community, they often meet their toughest challenges for survival.
Bacteria grown in laboratories encounter few of the discomforts found in nature. Rich nutrient broths, incubators set at perfect temperature, and culture vessels bathed in the bacteria’s preferred gas make laboratory life plush compared with life in soil or water. In the lab, bacteria grow faster and bigger than in nature.
Out in the real world bacteria confront scant nutrients, inadequate adherence sites, toxic chemicals, and predators. But with diversity comes versatility, and bacteria have developed a multitude of tactics to ensure their survival in the environment.
In nature, bacteria wage constant competition with protozoa, algae, plants, insects, and worms for nutrients in the soil or natural waters. Unlike these eukaryotes, bacteria become dormant, construct an endospore, or select an alternative metabolism to ride out tough conditions. When nutrients are few, bacteria hold cell size to a minimum; cells that in the lab grow to three or four μm in diameter might reach only one to two μm in nature. This downsizing reduces the amount of nutrients a cell needs, increases the number of safe hiding places on surfaces, and might help bacteria to go airborne and thus move to better environments. Small size also leads to faster reproduction so that a species survives in part by producing enormous numbers of progeny.
Single bacterial cells weather the harsh conditions in their environment and rejoin a community as soon as they can. Part of community-building involves the ability to stick to surfaces. Pathogens and nonpathogenic bacteria both rely on adherence as a key part of their survival mechanism. Like pathogens, environmental bacteria use tiny appendages called fimbrae to attach to things such as rock, soil particles, leaves, or decomposing matter. On surfaces lacking a topography good for attachment, bacteria use electrical charges to help them stick.
Bacteria have a small negative charge on their outside due to the chemistry of the carbon and phosphorus in their proteins and acidic portions of the cell wall. In aqueous environments where most bacteria live, the negative cell attracts positively charged molecules. A negatively charged cell therefore travels through the environment wrapped in a positively charged suit. The minerals in rock and soil also have a positive charge. Organic matter in nature carries a negative charge like bacteria and also attracts its own suit of positive particles. Bacteria would seem to have no chance of adhering to a surface because of all the positive-positive repulsion. But matter behaves differently at the nanoscale level than it does at visible or microscopic sizes measured in micrometers.
At certain nanometer (nm) distances, positive-positive repulsion prevents bacteria from sticking to positively charged objects. At about 10 nm from a surface, a pebble for instance, bacteria detect a small electrical attraction to the surface, but repulsion increases as a cell comes nearer to the pebble. The amount of repulsion wavers due to additional chemical forces existent between 10 nm and 2 nm from the surface. If the cell manages to reach within 1 nm of the pebble, the attractive forces win out and the cell can adhere.
Not only must bacteria overcome the competing chemical forces that occur between 10 nm and 2 nm, they also must find a site not already occupied by other cells, settle in a spot far from microbes secreting antibiotics, and locate a place that affords nutrients, light, and air.
Evading the action of natural antibiotics takes additional guile. Most of the natural antibiotics in use today came from soil microbes. Soil bacteria must resist not only the naturally produced antibiotics in their environment but also synthetic antibiotics that contaminate water coming from human-populated places. Chlorine-containing pollutants, toxic metals (such as mercury, cadmium, silver, and copper), and radioactive chemicals also harm bacteria except for the relatively small number of species that have adapted to these substances. As the amount of pollutants increases in soil, the number and diversity of bacterial species decrease. Adaptations, in fact, provide bacteria with their most powerful survival mechanism. Because of their fast reproductive rate, bacteria can make vital adaptations such as antibiotic resistance part of their genetic makeup more efficiently than any other organism.
After overcoming the travails of starvation, lack of sites to live, and toxic substances, bacteria still must deal with predation. Protozoa roam aqueous environments in nature as they do inside the rumen, engulfing and digesting bacteria. A protozoal cell gobbles 1,000 to 10,000 bacteria for each cell division. Bacteria’s greatest defense against extinction is a reproductive rate faster than that of protozoa. The size diversity of bacteria helps, too. Larger protozoa (100 to 1,000 μm in length) capture larger bacteria, leaving most of the small bacteria for small protozoa (5 to 100 μm in length). In many other areas of nature, organisms of similar type diversify the prey they target. Wolves target elk and leave smaller prey such as jackrabbits to the coyotes. This hierarchy of prey and predators ensures the survival of biodiversity. In the microbial world, the protozoa size-to-prey size ratio is about ten to one. On rare occasions, however, protozoa try to take in food larger than their size with deadly consequences.
Certain bacteria in nature prey on other bacteria each in their unique way. Bdellovibrio lives in a broad range of habitats from soil to fresh and salt waters and in sewage. This gram-negative genus preys on other gram-negatives by attaching to a cell and secreting enzymes that bore a hole in the cell wall. The predator then squeezes into the space between the prey’s cell wall and membrane. The prey cell dies but the Bdellovibrio stays and wears it like a coat that somehow resists any new predators.
The aptly named Vampirococcus attaches to its prey but does not penetrate the bacterium. It excretes enzymes to partially degrade the prey cell, preferably photosynthetic species. After sucking out all of the prey’s cytoplasm, Vampirococcus leaves behind an empty cell wall.
Myxobacteria have their own distinctive type of predation. Motile myxobacteria form “wolf packs” of a few dozen to hundreds of cells that glide through the soil in search of prey. The long, thin rods line up in parallel with a few leader cells extending a bit in front of the pack. Myxobacteria packs gracefully patrol the waters for food. After devouring all bacteria in an area, the myxobacteria cells aggregate into a huge funguslike structure called a fruiting body that grows up to 75 millimeters in height. This body, like nothing else in the bacterial world, contains pigments that color the colonies red, orange, yellow, or brown. The fruiting body’s stalk raises a sac of cells above the soil’s surface. Wind or rain liberates the myxobacteria and carries them to a new location. If conditions at the new site look good, the myxobacteria begin a new life cycle. Fruiting bodies are easy to spot on decaying organic matter, particularly beech and elder trees.
Microbial ecologists have not determined the role of predation in the microbial world. Predation certainly benefits predators in places with low nutrient supply. The predator lets the prey do the work of absorbing and concentrating nutrients, and then gulps the entire meal. Some predators take in bacteria but do not digest them. In the termite gut for instance, bacteria inside protozoa inside the insect digest the woody fibers that termites ingest.
Three hallmarks of bacteria contribute to their versatility. First, the huge size of bacterial populations increases the chance of developing mutants with one or more new, favorable traits. Second, short generation times help a species make the new trait part of its genetic makeup. Third, because bacteria are compact, they have developed enzymes that can do more than one function. For example, enzymes that degrade common organic compounds in nature might also decompose pollutants. The principle behind bioremediation is to use microbes that prefer decomposing a pollutant even when other foods are available.
Large numbers of organisms with different nutrient needs, energy generation, and adaptations would be expected to create a diverse population such as found among microbes. Microbial diversity dwarfs that of other life forms, and microbial ecologists suspect that the diversity is highest in the belt that circles the globe near and at the equator. Biodiversity of higher organisms, plant and animal, in the equatorial tropics exceeds that in other regions on Earth. In this region, an abundance of sunlight might lead to higher numbers of photosynthetic bacteria, which give rise to food chains on land and in water. Because of the tropics’ higher overall biodiversity, the region is environmentally stable. This allows countless small and specialized populations to exist. Diverse populations in turn offer bacteria more options for creating symbiotic relationships. Finally, a stable tropical climate compared with the seasonal changes of temperate regions gives bacteria better opportunities to evolve and develop useful adaptations.
Microbial ecology challenges microbiologists because the bacteria studied in laboratories are not necessarily the most abundant. This is due to VBNC, meaning viable but not culturable. Craig Venter’s genetic analysis of marine microbes supported the idea long suspected by biologists, that microbial diversity is far greater than even the highest estimates. VBNC bacteria either do not grow in lab conditions or microbiologists have yet to discover the things these species need. As a result, microbiology must base most of its theories on how a small minority of the world’s bacteria behave in a laboratory. Genetic testing, such as Venter’s, will help solve this problem because whole bacteria need no longer be the focus of experimental study. By analyzing gene diversity, microbiologists will learn more about microbial diversity.
Cyanobacteria
No single bacterium can be thought of as more important than any other, but if pressed to select one above others, I would pick cyanobacteria. These microbes that biologists originally misidentified as blue-green algae almost single-handedly symbolize bacterial diversity.
The bacteria that began providing the Earth with oxygen three and a half billion years ago show wonderful versatility that spans terrestrial and aquatic environments, freshwater and marine. Cyanobacteria (see Figure 6.1) have few constraints on where they live other than needing sunlight for photosynthesis. On land, cyanobacteria often pair with fungi to form lichens that live on inorganic surfaces. The cyanobacterium Anabaena forms a similar relationship in water with Azolla, a small floating fern. In this association, the plant supplies about 90 percent of the photosynthesis, and Anabaena takes responsibility for pulling nitrogen from the air to supply itself and Azolla.
Figure 6.1. Cyanobacteria. Cyanobacteria contain a diversity of species and activities, all having photosynthesis in common. This string of Anabaena cells contains larger cystlike cells that fix nitrogen.
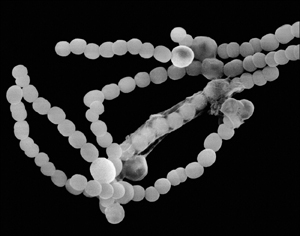
(Courtesy of Dennis Kunkel Microscopy, Inc.)
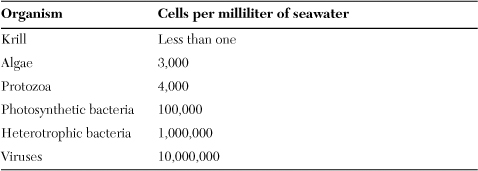
Cyanobacteria dominate microbial mats and attach to terrestrial surfaces (the periphyton form). They live in the greatest abundance, however, as free cells in aquatic habitats and make up a large portion of marine plankton as shown on Table 6.1. At normal ocean concentrations of about 100,000 cells per milliliter, the microbes are invisible, but in larger, denser populations called blooms, cyanobacteria can turn the waters red, perhaps the inspiration for naming the Red Sea. The oceans receive ample sunlight at the surface, but the light penetrates no deeper than about 320 feet. For this reason, cyanobacteria and all of the world’s marine photosynthesis occurs in this layer called the photic zone.
Table 6.1. Main constituents of marine plankton
Green plants, algae, and cyanobacteria act as the Earth’s main conduits for converting the sun’s energy into usable energy for animals. The oceans contribute the major share of this process. Just as cyanobacteria provide the energy that powers the metabolism of microbial mats, they play a similar vital role as the foundation of marine food chains. From the marine or freshwater cyanobacteria, energy transfers to small organisms and to progressively larger animals until the food chain reaches the top predator, referred to as the “top of the food chain.”
As Earth’s oldest bacteria still in existence, cyanobacteria played a part in the rise of algae, primitive plants, and today’s higher plants. The oldest known fossils are of cyanobacteria from the Archaean period before oxygen began accumulating in the atmosphere. These fossils dated to 3.5 billion years are almost as old as the oldest rocks, dated to 3.8 billion years.
During the Archaean and Proterozoic Eras, cyanobacterial photosynthesis changed the atmosphere’s composition from oxygenless to oxygenated. Sometime during the transition from the Proterozoic to the Cambrian Era, some of the large cells dependent on oxygen engulfed a few cyanobacterial cells. A portion of the engulfed cyanobacteria managed to resist being digested inside the predator, and they evolved with succeeding predator generations to become an organelle of the host cell. Plant life descending from these primitive cells evolved into more complex structures, and the vestiges of ancient cyanobacteria would become chloroplasts, the sites in plant cells that convert sunlight energy to chemical energy in the form of sugars.
Cyanobacteria grow slower than most other microbes, doubling about once per day, but they nevertheless compete well against other bacteria because of their durability and the capacity to exist on almost no nutrients; cyanobacteria need only sunlight for energy, carbon dioxide for carbon, and miniscule amounts of salts. These bacteria have larger than normal cell size that reaches several μm in diameter, and they possess a more complex internal structure than other bacteria. The cytoplasm contains a network of membranes that support the enzymes and pigments that run photosynthesis. Cyanobacteria cell shapes also present a unique collection of blocks, chains, and long filaments that microscopically resemble algae more than bacteria.
In the 16th century, Swiss physician Paracelsus—his birth name was Aureolus Phillipus Theostratus Bombastus von Hohenheim!—made one of the earliest observations on cyanobacteria. He noted the mucuslike colonies growing on plants and named the growth Nostoc, a term generally meaning nasal discharge. If Nostoc was the first cyanobacterium studied, the marine species Prochlorococcus marinus is one of the newest. Discovered in 1986, P. marinus is possibly the most abundant organism of any type on the planet. P. marinus is also the smallest cyanobacterium and one of the smallest known bacteria at 0.6 μm in diameter. The species acts as a photosynthesis machine with few other ecologically important activities. It contains only 1,716 genes. Because the ocean conditions change slowly, P. marinus can survive with only a few genes to help it respond to its environment.
The best places to see cyanobacteria in nature are rocky shorelines and on seashells. Microbial ecologist Betsey Dexter Dyer described cyanobacteria on shorelines as a slippery, brown-black, velvety coating on rocks. In aquatic environments, the microbe is evident by its blue-green, green, yellow-red, orange, or violet pigments.
Cyanobacteria in all forms serve the Earth as a tremendous storage site for carbon and nitrogen. Photosynthesis converts carbon dioxide to sugar, which the plant uses to make structural fibers and starches. All bacteria that decompose organic matter on land or in the sea add to the large stores of the Earth’s carbon.
Bacterial protein factories
Ruminant animals and to a lesser extent humans and other single-stomach animals (monogastric animals) get a large portion of their amino acid requirements from bacteria. Enzymes in the intestines digest bacteria, and then specific enzymes called proteases break down the bacterial proteins to liberate individual amino acids, which the animal absorbs. These amino acids or the nitrogen they contain serve as the basis for the animal’s own protein synthesis.
This process, complicated though it may seem, is but one step in a global nitrogen recycling system. The nitrogen cycle is perhaps the most-studied nutrient cycle due to its importance to agriculture and the health of humans and other animals. Most people do not suffer from a lack of carbon in their diet. Nitrogen in the form of protein is another story. Nitrogen often occurs in limited supply in diverse environments, making the nitrogen cycle all the more crucial for Earth’s organisms.
The proteins in a steak dinner result from a global cycle of nitrogen use and reuse. Without exaggeration, the steak’s nitrogen may have come from cyanobacteria in a distant ocean or soils on another continent. Nitrogen gas makes up 78 percent of the atmosphere, almost four times as much as the next most abundant constituent, oxygen. Despite this apparent abundance of nitrogen, living things expend much more energy to get nitrogen into their bodies than they do to absorb oxygen. Except for bacteria, no life takes nitrogen gas directly into the body like oxygen enters. Bacteria, including cyanobacteria, make nitrogen available for all other life by taking in the gas in a process called nitrogen fixation and converting the nitrogen into a form usable by plants. Grazers such as rabbits convert the plant nitrogen (mainly in vitamins and nucleic acids such as DNA) to animal nitrogen (mainly as protein in muscle).
Some species, such as Azotobacter and Beijerinckia, live independently in the soil and perform nitrogen fixation there. These bacteria convert the gas to ammonia by adding hydrogen atoms to each nitrogen atom. The bacteria Rhizobium (discovered by Beijerinck) and Bradyrhizobium also absorb nitrogen from the air, but they do so from inside bumps on the roots of plant cells, called root nodules. Martinus Beijerinck discovered this bacteria-plant process in 1888. The bacteria-plant relationship in nitrogen fixation represents symbiosis, which is the cooperative association of two organisms living in close proximity. The intestinal bacteria in humans, other animals, and insects also illustrate a symbiotic relationship.
After nitrogen gas has been converted into ammonia, the nitrogen passes through a sequence of reactions each carried out by the bacteria that Sergei Winogradsky discovered more than a century ago. Nitrosomonas converts the ammonia to compounds called nitrites (two oxygens attached to a nitrogen), Nitrobacter turns nitrites into nitrates (three oxygens attached to nitrogen), and plant roots then absorb the nitrates for their own nitrogen needs. An entirely different group of bacteria takes excess nitrates from the soil, turns it into the gas nitrous oxide, and releases this gas back into the atmosphere.
The nitrogen that ends up in plants is used by the plant to build vitamins, nucleic acids, and proteins. Cattle grazing on grasses and clovers take in the plant nitrogen, and then rumen bacteria begin their task of building microbial proteins. Humans benefit from the entire process when they ingest proteins in beef. The environment also receives a share of nitrogen when plants die and decay (by the action of soil bacteria such as Bacillus), releasing nitrogen into the soil, and manure from cattle farms leaches into the soil and surface waters.
The nitrogen cycle, so essential for all life, takes up a lot of space. Meat-producing cattle and sheep take up thousands of square miles of land across the world. Countries with a large land area like the United States or Canada can manage this problem, but water-stressed and tropical regions find that meat production makes impossible demands on their environment. Meat animals compete with humans for water in an increasingly large portion of the world. In the tropics, meanwhile, farmers are cutting down or burning jungle to clear land for cattle. As the tropics shrink so does biodiversity.
Many environmentalists feel that large animal meat production threatens the environment, prompting scientists to investigate bacteria as a direct protein source. The nitrogen cycle will continue running, of course, but humans might put less demand on it by using alternate protein sources. The cyanobacterium Spirulina (see Figure 6.2) has drawn interest as a potential microbial protein source. Dried Spirulina powder serves as a vitamin and protein supplement. Spirulina cells are up to 70 percent protein. Most other bacteria consist of about 50 percent protein. Furthermore, Spirulina protein is high-quality protein, meaning it contains all of the essential amino acids for humans. Spirulina resembles all other photosynthetic organisms by supplying a variety of vitamins and minerals. The enzymes that run photosynthesis require a constant input of vitamins and minerals that act as co-factors, supplementary molecules that participate in chemical reactions. Consider the following attributes of Spirulina as a food:
• More beta-carotene, which the body converts to vitamin A, than carrots
• 28 times more iron than beef liver
• Higher concentration of vitamin B12 than any other food.
Figure 6.2. Spirulina pacifica. This filamentous cyanobacterium has been used for centuries as food. Masses of growth are collected from water, sun-dried, and patted into flat cakes to cook or eat directly.
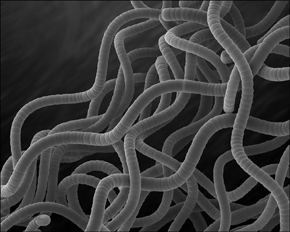
(Courtesy of Dennis Kunkel Microscopy, Inc.)
Does Spirulina have a future as a new protein source for undernourished regions of the world? On a per-acre basis, the cyanobacterium supplies 200 times the protein yield of beef and consumes 315 times less water. NASA has experimented with the use of Spirulina, misidentified as algae, as a food for space flights. Spirulina farms have grown in number worldwide from Thailand, India, and to the United States. These farms contain large ponds for growing the bacteria in a continuous flow system of incoming nutrients and outgoing final product. Microbiologists maintain a narrow range of growth conditions to enhance cyanobacteria growth and inhibit the growth of contaminants.
The environment’s current precarious condition requires people to make hard choices regarding the materials they consume. Spirulina may become an important aspect of sustainability, but it has not yet arrived there.
How to build an ecosystem
A pond, meadow, or tidal pool is an example of an ecosystem. Each ecosystem contains a network of interactions between multicellular plants and animals, tiny invertebrates, microbes, and inanimate objects such as soil, water, rocks, and air. Bacteria operate in ecosystems by interacting with other microbes as well as with the immediate microscopic environment composed of liquid and solid surfaces.
In liquids, bacteria contend with the positively or negatively charged substances dissolved or suspended in the microenvironment. Some cells turn on their chemotaxis mechanism to swim toward favorable conditions or away from harmful conditions. Other bacteria float in the milieu and absorb any nutrients they meet, or these cells are swept toward communities of mixed species and settle down there.
In liquid environments with high content of organic matter, bacteria aggregate into a thin film that covers the surface. In this position, cells take in oxygen from the air and absorb nutrients from below. Bacteria in surface films must regulate the surface tension of the air-water interface to stabilize the film’s structure and reduce breakup.
Some bacteria, such as Pseudomonas, secrete surfactants to help regulate surface tension. These detergentlike substances allow hydrophobic compounds that land on the film to become miscible with the water, and this provides the film with a potential new nutrient source. Surfactants also help nutrients enter microbial cells, thus helping the cells stay in the fragile surface film. Surfactants play a similar role for bacteria in soil on root surfaces.
Soil bacteria live in a microenvironment made mainly of silicon, the Earth’s most abundant element. Soil also has substantial amounts of aluminum, iron, calcium, sodium, potassium, and magnesium. Most of these elements occur in a positively charged form that influences the cells’ attachment to soil particles. Sometimes bacteria live in a community attached to the inanimate particles, but in other instances they inhabit moist micropores varying from micrometers to millimeters in size. These tiny microenvironments are often the places where nutrients begin cycling through the Earth, atmosphere, oceans, and to every living thing.
The sulfur cycle consists of more chemical conversions than any other known nutrient cycle. Sulfur as sulfur dioxide gas enters the atmosphere when released from volcanic activity, including hot sulfur springs. Fossil fuel combustion also adds large amounts to the atmosphere. Most of the Earth’s sulfur is held in the planet’s core with lesser amounts in biological matter. The Earth’s crust contains almost 2 × 1016 tons of sulfur; the terrestrial and marine biological matter holds about 1 × 1010 tons.
Two groups of bacteria nicknamed the green sulfurs and purple sulfurs for the type of pigments they contain convert elemental sulfur to sulfate compounds. Elemental sulfur—this is pure sulfur unattached to any other element—is a solid that sticks to soil particles as well as the surface of bacterial cells. Yellowish sulfur granules cover these bacteria, which secrete enzymes to convert the granules to more soluble sulfate compounds. A wide variety of soil microbes then use the sulfates.
Still ponds or swamps that give off a rotten egg smell, characteristic of hydrogen sulfide, provide evidence of active sulfur cycling taking place underground. Because this cycle depends on anaerobic bacteria, it occurs in sediments and the deepest waters lacking oxygen. If a light were to be lowered into a swamp and clicked on, the sulfur bacteria would be green and pinkish-purple.
Aside from his studies on nitrogen and sulfur bacteria, Winogradsky examined the bacteria that generate energy by oxidizing or reducing iron. The iron cycle takes place in waters that drain from slow-moving bodies such as swamps and ponds and involves continual conversions of the element’s chemical form by releasing or accepting electrons from other atoms. In soils suspected of having high iron levels, orange to reddish soil indicates that more oxidation (releasing electrons) is occurring than reduction (accepting electrons). Three prevalent bacteria that carry out this step are Thiobacillus ferrooxidans, which works in acidic conditions, Gallionella that prefers neutral conditions, and Sulfolobus, which grows best in acidic and high-temperature conditions. Microbiologists find T. ferrooxidans in areas that contain drainage from mining operations and Sulfolobus in sulfur hot springs. High-iron soils that are dark green or black contain more reduction than oxidation. The anaerobic Geobacter, Desulfuromonas, and Ferribacterium perform this reaction.
Winogradsky’s legacy has been captured in a simple experiment that pulls together all of these metabolisms and mimics bacterial activities in nature. The “Winogradsky column” is a tall cylinder or jar filled with wet mud from a pond, lake, or ocean shore and topped with water. The amateur scientist adds shredded and chopped newspaper (as carbon source) and egg yolk (sulfur), and then puts the glass in a well-lighted place. After six weeks, bacteria from the mud settle into layers defined by oxygen levels with anaerobic mud below and aerated water above. The bacterial numbers start out low, but the appearance of colored striations in the column indicate the populations have grown to high densities. The colors give clues to the organization of bacteria in the column:
• Blue-green cyanobacteria receiving sunlight at the top
• Sulfide-using bacteria Beggiatoa and Thiobacillus in a light brown layer
• Photosynthetic Rhodospirillum in a large, nutrient-rich, rust-colored layer
• Red Chromatium in a low-oxygen layer using filtered light for photosynthesis
• Green Chlorobium absorbing hydrogen sulfide gas rising from the mud
• Brown anaerobic mud filled with hydrogen sulfide-producing Desulfovibrio and cellulose (newspaper)-degrading Clostridium
Iron-reducing bacteria, if present, live in the anaerobic sediment at the bottom of the column, and iron-oxidizing bacteria develop a rusty-red zone above the sediment.
In a single glass container, a person can watch the real activities of bacteria in nature. The Winogradsky column also creates a simplified microcosm of evolution; anaerobic actions beginning life in an oxygenless environment, and then progressing to photosynthesis and oxygen-respiring organisms.
Winogradsky columns can metabolize for months to years to decades. Microbiologists now build modified columns to emphasize a certain type of metabolism. For example, columns containing sediments from iron bogs or iron springs have more iron metabolism than a standard Winogradsky column.
Feedback and ecosystem maintenance
Beijerinck and Winogradsky made a pivotal decision to study mixed populations of bacteria as they are in nature. By doing so, they helped define the concept of an ecosystem. The Winogradsky column contains numerous interdependencies between bacteria, yet it is a simple example of larger natural ecosystems.
A properly working ecosystem does not remain static but rather evolves in a process called succession. On a large scale, deforested land offers the best visible illustration of succession. New life takes hold on denuded land when cyanobacteria begin to grow in numbers. Some of the cyanobacteria team with new fungi entering the environment to form lichens that begin to cover the nutrient-scarce land. Mosses follow, and in turn small plants follow them. Over a period of months, higher plants such as bushes become established. Small trees and progressively larger, longer-lived trees establish over the next years. As this succession progresses, some species disappear as new more complex species emerge. Microscopic ecosystems follow a similar type of succession.
When bacteria enter a pristine habitat, nutrients may be plentiful and competition low. (Nature has no completely pristine habitats, but some natural events like floods and fires can create habitats that have lost a lot of their life and are ripe for recolonization.) The first bacteria to colonize the habitat are usually microbes that start out in the highest numbers or grow faster than the other microbes. Equally important, these bacteria have already adapted to the environmental conditions. They begin to change the habitat according to their specific type of metabolism. Some bacteria alter the pH, others remove all the oxygen, and some excrete simple organic compounds.
The altered conditions might favor another group of bacteria over the original species. For example, an acid-producing bacterium eventually chokes under the buildup of acids. But another species that uses organic acids as a carbon source sees the habitat as a nutrient-rich place to colonize. In rare instances, the original colonizer alters the environment so much that no other organism can live there. For example, areas exposed to mine drainage become increasingly acidic when T. ferrooxidans grows there and produces sulfuric acid as a by-product of the iron-sulfur compound pyrite. An ecosystem of diverse life cannot develop in situations like this, and the area turns into an extreme environment where only acid-loving extremophiles can live.
In the development of a healthy ecosystem, bacteria provide the foundation for food chains. Increasingly complex organisms become established. In healthy ecosystems, the new food chains develop associations that link them horizontally as well as vertically. In other words, a food web develops.
The more complex an ecosystem, the better it withstands changes in the environment. In simple ecosystems with few food chains all of the members depend on a relatively few species. If one or two species disappear, the entire ecosystem collapses. By contrast, complex ecosystems with many alternate paths for energy- and nutrient-sharing are versatile and can adjust to change. Rich biodiversity benefits all life, and this biodiversity extends all the way to microscopic life.
Ecosystems are hardwired to control the number and variety of species they contain. Two types of control processes operate: bottom-up and top-down. Bottom-up control uses microbes as the primary determinant of ecosystem health. If bacteria begin to disappear, the foundation of the ecosystem’s food chains also disappears. Top-down ecosystem control theorizes that predators control the health of an ecosystem. By regulating the size of its prey, each member of an ecosystem prevents an exploding population of another member. Nature rarely follows hard and fast rules, so ecosystems tend to utilize a mixture of both control mechanisms.
In any ecosystem, organisms depend on feedback to help them regulate their activities. The simplest feedback mechanism to understand is food supply; when a person is full, he stops eating (hopefully). Being highly attuned to their environment, bacteria constantly interpret the immediate surroundings and respond by using feedback systems. For example, under starvation conditions, Bacillus turns into an endospore and myxobacteria produce fruiting bodies. When an ecosystem undergoes dramatic changes, even feedback may not be sufficient to save all of the system’s members.
Microbial blooms are an example of an ecosystem gone out of balance. A bloom is a rapid overgrowth of microbes that drastically change an environment to the harm of other species. Blooms are caused by a sudden increase in numbers of aquatic algae, protozoa, or bacteria. Cyanobacteria and purple sulfur-metabolizing bacteria create most bacterial blooms, but bacteria play a part in algal blooms, too. Cyanobacteria and algae bloom in fresh and marine water when large, sudden influxes of nitrogen and/or phosphorus enter the environment. Runoff carrying fertilizer or manure from farmland acts as the main cause of blooms. Nitrogen and phosphorus wash into waters that usually contain low levels of these nutrients. The sudden bounty of nutrients causes an equally sudden explosion of microbes. As the microbial population grows increasingly dense, the cells release oxygen into the water as well as nutrients in the form of dead cells. Heterotrophic bacteria (bacteria that use sugars, fibers, amino acids, and fats) begin feasting, and they create a second bloom.
Bacteria in the second bloom are not photosynthetic so do not produce oxygen. Instead the fast-growing heterotrophs suck up all the oxygen from water around them. The oxygenless conditions soon cause other life to disappear; fish, crustaceans, and small invertebrates suffocate. Nutrient influx followed by ecosystem imbalance is called eutrophication. Cyanobacteria Anabaena and Nostoc are two common causes of blooms.
Cyanobacterial blooms now develop yearly in coastal areas and specific rivers worldwide and cause a health threat beyond the harm caused to aquatic life: cyanotoxins. Cyanotoxins are poisons released by cyanobacteria and that remain in the water after the bacteria subside. A serious occurrence of cyanotoxin contamination took place in Brazil in 1993. Fifty hospitalized dialysis patients died because their therapy used a water source contaminated with microcystin from the cyanobacterium Microcystis. (Water treatment technology has improved for removing pathogenic bacteria, but it remains poor at removing antibiotics, hormones, chemicals, and toxins.)
Anaerobic blooms occur when purple sulfur-metabolizing bacteria Chromatium, Thiocapsa, or Thiospirillum grow out of control. These blooms usually develop in oxygen-depleted waters in bogs and lagoons, leaving a telltale pink-purple sheen over the water.
Many blooms disappear on their own when seasons change and the hours of sunlight decrease, but several sites worldwide develop annual cyanobacterial blooms. Problem blooms return every year to the Great Lakes, the western United States, many Pacific islands, and lakes and rivers in Europe.
Lake blooms can also come from the anaerobic purple bacteria that live in darkness. Nutrient-rich lakes with a deep layer of bottom sediments give rise to large anaerobic populations that support communities of Chromatium and Chlorobium just above the sediment layer. These two species possess the unusual ability to catch filtered sunlight that penetrates past the photic zone. As anaerobes proliferate, they can turn the lake conditions unsuitable for other life.
In the 1970s Lake Císo in Spain became a topic for study because it had developed an anaerobic bloom of sulfur-metabolizing bacteria. The sediments emitted so much hydrogen sulfide that the gas filled the lake’s entire water column, creating a rare type of anaerobic lake. Sulfate-rich water now runs into the bottom of the Lake Císo and water dense with bacteria flow from the upper layer. Most other anaerobic lakes have an upper layer of cyanobacteria, on top of a Chromatium layer, that sits atop much darker, sulfur-saturated water. Such an ecosystem is uninhabitable for fish and other animal life.
Macrobiology
In optimal conditions, ecosystems do not go out of balance. Whether in lakes, soils, rumens, or insects, ecosystem members tend to self-regulate their populations. These ecosystems receive extensive research and usually become study models for microbiology students. Other ecosystems have offered few hints on how they work.
The luminescent bacterium Vibrio phosphoreum was discovered in the 1970s in specialized glands of certain deep sea organisms (lantern fish, angler fish, and some jellyfish and eel), and they have since been found in Alaska salmon. Their role in water ecology has puzzled scientists. The bacteria emit bluish-green light generated by the pigment luciferin, the same compound that lights fireflies and creates the nighttime phosphorescence sailors see in their ship’s wake. Does V. phosphoreum benefit the fish or does the host benefit the bacterium? Perhaps neither organism cares about the other even though they live as a pair, a relationship called neutralism.
Microbial ecologists have barely scratched the surface of the relationships between bacteria and global ecology. Their challenges increase when considering almost inaccessible bacterial habitats deep in the Earth’s mantle or miles under the ocean surface.
Only within the past decade or so have microbiologists extended the reach of their studies to depths of about two miles into the earth or approaching a mile into the polar ice sheets. The information ecologists draw upon to describe the functions of bacteria on Earth has come entirely from species close to or at the surface. Subsurface microbiology seeks to answer the questions of how bacteria of the deep contribute to life at the Earth’s surface. What do these bacteria eat in the darkness? How do they relate to the evolution of life at the surface? Do they have any connection at all to life on other planets?
The U.S. Department of Energy launched a program on subsurface microbiology in 1986. Wells drilled to aquifers about 700 feet deep reached a population of diverse and novel bacteria. With the help of geologists and hydrologists, researchers either drilled to the depths or gained access to the deep subsurface via existing mines. As microbiologists probed deeper they found that bacteria became increasingly dependent on inorganic materials for survival and less on organic compounds.
Astrophysicist and NASA consultant Thomas Gold (who died in 2004) speculated in his book The Deep Hot Biosphere (1999) that the oceans’ food chains begin not with microscopic marine life in the water, but deep in the Earth’s lithosphere. These subsurface thermophiles, Gold proposed, exist on methane and hydrocarbons in massive untapped oil reserves and represent the closest relatives to life’s ultimate ancestors. A controversy rumbles, distant from the daily concerns of most microbiologists, as to whether life emerged on Earth’s surface or deep underground and grew outward to the surface. The bacteria that live in the deep-sea hydrothermal vents form the core of this argument because no one has as yet determined their origin.
A plan exists for building a subsurface physics laboratory in South Dakota’s Homestake gold mine one and a half miles down. Geomicrobiologists, who study the interactions of microbes with geological formations, anxiously await. First, the construction must overcome issues of water purification, equipment installation, and the possibility of higher than normal radioactive bombardment. Then microbiologists will face the hurdle of growing these specialized bacteria under lab conditions.
Microbiologists continue to learn about the connection between the Earth’s oil and subsurface bacteria. Some bacteria live in a world flooded with hydrocarbons, intense pressure at two and a half miles deep, and temperatures of 185°F: the world’s oil reserves. The earliest studies on species recovered from the reserves revealed that many were related to surface species, surprising since the oil bacteria have been sealed off from other life for 200 to 500 million years. To defuse the inevitable charges of contamination that skeptics made toward this discovery, scientists have constructed small sampling capsules that open only when they reach oil and enclose their sample before returning to the surface.
A new science in oil microbiology has begun. Bacteria will play a pivotal role in oil refining, invention of fossil fuel alternatives, and oil spill cleanup. Microbiology has plans for the bacteria that live on oil. By analyzing the genes of bacteria recovered from oil and comparing them to genes in soil species at the surface, biologists may be able to locate new oil reserves. A similar array of genes between both groups could indicate that the surface microbes are living on oil seepage from oil reserves below them.
The relationship between oil and global ecology, or macrobiology, is complex. But at the core of oil’s origin and its future sit the bacteria.
