7. Climate, bacteria, and a barrel of oil
A band of rock called the Isua formation runs along the edge of an inland ice cap from western Labrador to southwestern Greenland. The formation holds the oldest known rock found so far on Earth, dated at 3.8 billion years. Traces of fossilized life lace the Isua formation and analyses of its carbon content point to photosynthetic ancestors of cyanobacteria.
During the period in which the Isua formation developed, the Earth’s atmosphere held no oxygen. Primitive photosynthetic microbes used the sun, carbon dioxide, and the Earth’s elements (nitrogen, sulfur, phosphorus, salts, and metals) to sustain life. Their rudimentary photosynthetic reactions released little oxygen. Chemically unstable compounds in the atmosphere quickly captured what little oxygen the microbes liberated, and the oceans absorbed the rest. By 2.2 billion years ago, however, the oceans had accumulated enough dissolved oxygen to allow the gas to begin building up in the atmosphere. The oxygen levels in the atmosphere began to stabilize about 2 billion years ago.
Evolution is a change in an entire population due to small and discrete adaptations that favor species survival. The accumulation of oxygen on Earth signaled the development of a stable population of photosynthetic microbes that we now identify as primitive cyanobacteria. The cyanobacteria split into two evolutionary paths at least two billion years ago. One branch gave rise to plants. (Gene analysis suggests that the archaea branched off this path.) The second branch led to modern cyanobacteria and other bacteria.
By analyzing bacterial DNA, scientists have found that almost all bacteria contain DNA base sequences that are remnants of earlier evolutionary paths. In other words, bacteria have exchanged genes for so long that their evolution may resemble more of a network than a straight path. A few microbiologists have half-jokingly proposed that instead of estimating the thousands of bacterial species in the world, we should think of all bacteria as belonging to one giant species with a huge family tree of relatives.
The evolution of photosynthesis by whichever multiple paths it used accelerated the development of other biota. Microbial ecologist Patrick Jjemba has justifiably concluded, “The evolution of photosynthesis is the most important metabolic invention in the history of life on Earth.” Bacterial diversity increased as oxygen levels rose from 0.1 percent (about 2.8 billion years ago) to 1 percent (2 billion years) to 10 percent (1.75 billion years). Not until the Cambrian Period, 543 to 490 million years ago, did oxygen reach its present concentration. The sudden increase in the diversity of life has prompted scientists to call it the Cambrian Explosion. The evolution of today’s higher plants and animals took less time than the evolution of the Earth’s first bacterial cell.
Although life developed into hundreds of millions of different forms, the variety of aerobic or anaerobic energy-production schemes inside cells remained disproportionately small. The pathway called glycolysis is life’s universal pathway because it exists in every living thing. Bacteria use glycolysis as humans and other animals do by getting a small amount of energy from the breakdown of glucose to pyruvate. After glycolysis, various bacteria use a varied but limited choice of metabolisms. In addition to photosynthesis and glycolysis, bacteria use anaerobic fermentations, anaerobic or aerobic respiration, plus a small number of specialized metabolisms that branch off from these main metabolic pathways.
The story of oil began when oxygen accumulated in the atmosphere and fed the respiration of aerobic organisms. Food chains made of a widening diversity of life developed on continents and in the oceans. Bacteria, protozoa, algae, worms, and crustaceans built a hierarchy of prey and predators. The oceans took in dead bacteria, invertebrates, plankton, and the remains of prehistoric multicellular creatures. The majority of expired macro- and microscopic life never reached the ocean floor; other animals ate the organic matter as it sunk. But over millennia the populations of marine organisms increased, and more organic matter drifted down and built up in the sediments under the ocean.
The diversity of species that ended up in the organic sediments contributed to sediments’ various forms of carbon. The Earth has been estimated to hold at present about 1.4 million known species and at least 10 times that number of undiscovered, uncharacterized species. Many times more species have already gone extinct than the number that survive today, yet today’s biodiversity resulted directly from the Cambrian Explosion, the period in Earth’s history when oxygen systems expanded faster than anaerobic systems.
The story of oil
Plant and animal matter decomposed due to the action of bacteria millions of years ago as it does today. As each layer of organic matter under the ocean crushed the layers below, the pressure expelled water molecules. The sediments accumulated a dense mixture of carbon compounds, the majority of which were hydrocarbons, long carbon chains in which each carbon is saturated with hydrogen. Over millions of years, the pressure pushed the hydrocarbons deeper into the Earth and caused them to harden into a brownish-black solid. A chunk of this material viewed through a microscope would reveal fossilized bacteria—hence the name fossil fuel—with other bits of plant life, marine invertebrates, and shells.
Oil formation from the hard, black material required a precise combination of organic substances, pressure, time, and characteristics of the surrounding rock. Pressure from above pushed clumps of organic matter toward the Earth’s center where it rose to about 180°F. Given enough time, the heating and the pressure turned the black rock into a liquid in which the hydrocarbon chains broke into a heterogeneous mixture of smaller chains. The pressure then pushed the liquid into pores in the surrounding rock. As the liquid squeezed through the network of pores, membrane constituents from bacteria mixed with it and increased its water-repelling property. The entire process produced crude oil.
At about 18,000 feet deep, oil remains in liquid form. Deeper, the intense pressure and heat further decompose the hydrocarbons into methane or natural gas. At shallower depths, the hydrocarbons remain solid and make up coal.
Microbiologists know that bacteria have played an integral part in formation of fossil fuels, but they still do not know all the ways in which bacterial metabolism altered oil’s hydrocarbon composition from site to site. Oil shale, the rock that contains crude oil in a network of pores, contains chlorophyll pigments resembling those of modern photosynthetic bacteria. The late microbial ecologist Claude ZoBell has added that without bacteria oil would never have been formed. ZoBell theorized that subterranean bacteria acted on the longest hydrocarbons to make shorter (but still long) hydrocarbons. Crude oil contains hydrocarbon lengths from 8 to 80 carbons, and the relative composition varies between reserves and within the same reserve. For centuries, hydrocarbon-digesting anaerobic bacteria saturated the carbon atoms with hydrogen. These anaerobes also helped form natural gas, the very same methane produced inside ruminants and termites.
Fossil fuels can be viewed as renewable resources because the sedimentation process is continual. Organic matter continues to sink underfoot, and bacteria will eventually make more oil. But the process unfolds on a timescale that humanity does not comprehend.
Most people should by now understand that the rate of oil consumption outpaces the Earth’s available oil. Saudi Arabian oil expert Sadad I. Al Husseini calculated in 2000 that the world’s oil reserves would level off about the year 2004, and the plateau might last for no more than 15 years. After this plateau, the remaining oil becomes too difficult and/or too expensive to extract. The United States already passed this Rubicon in the early 1970s. A clue that signaled an oil deficit arose during that decade: increasing numbers of oil tankers bringing crude to the United States from halfway around the globe, often accompanied by spills.
Bacteria can help build second and third generations of alternative energies. Could bacteria be engineered to manufacture hydrocarbon fuels on a scale needed by the human population?
Bacteria power
Unrefined crude oil poisons marine and terrestrial animals that ingest it. Oil’s aromatic hydrocarbons, compounds with a carbon ring structure (benzene, toluene, xylene, and so on) damage tissue, enzymes, and nervous systems. Bacteria view crude oil as a carbon-rich and digestible food. Bioengineers have begun to turn to this process on its head.
Entrepreneurial companies such as LS9 in California have bioengineered E. coli and other bacteria to produce hydrocarbons that refineries can then turn into fuel without emitting sulfur gases made by conventional refineries. Microbiologists know how to make a minor alteration to bacterial fatty acid synthesis so that the cells produce gasoline instead of fats. Bioengineered species might soon churn out hydrocarbons of specific chain lengths as a way to adjust octane level.
The proportion of heavy, hard-to-extract oil has risen in oil reserves as Big Oil draws off the lighter, cleaner crude. Geomicrobiologists now search for bacteria that convert heavy oil to higher quality fuel for combustion engines. Increasing today’s oil recovery rate by as little as 5 percent would make a substantive impact on world oil supply.
The bacteria that fix nitrogen, that is, capture nitrogen gas directly from the air, release hydrogen gas, which has been touted as an alternative to fossil fuel. Heterotrophs, some photosynthetic bacteria, and anaerobes make hydrogen as part of their normal metabolism. Bacterial hydrogen production for future fuels would require large fermenters designed to allow in sunlight for photosynthesis and possibly more than one species working in concert. For example an anaerobe that produces hydrogen might pair with anaerobic photosynthetizers that energize the system by absorbing sunlight.
Current chemical methods for making hydrogen involve breaking apart water molecules in a costly and technologically challenging process. Bacteria use the enzyme hydrogenase to split water into hydrogen and oxygen with less energy demand than the same reaction in a manufacturing plant. Some bacterial hydrogenases need only a small supply of selenium, iron, and nickel to stabilize the reaction. Biochemists are already working on a thermophile Clostridium that performs the reaction at about 140°F and dispenses with the need for added metals.
Oxford University chemists have also attached hydrogenase and a light-sensitive dye to microscopic titanium dioxide beads. In this system, photosynthetic microbes supply their own energy by capturing solar energy. No conventional chemical companies can make the same claim.
Similar nonsunlit systems include E. coli hydrogenase with the enzyme carbon monoxide dehydrogenase (CMD) from Carboxy-dothermus hydrogenoformans. CMD splits carbon monoxide. The overall reaction is
carbon monoxide (CO) + water (H2O) → carbon dioxide (CO2) + hydrogen (H2)
C. hydrogenoformans that catalyzes this reaction is an anaerobe isolated in 1991 from a freshwater hot spring on Kunashir Island in the Sea of Japan. The German Collection of Microorganisms in Braunschweig owns one of the world’s few cultures of this obscure microbe.
Astute readers will notice that the preceding reaction gets rid of the greenhouse gas carbon monoxide but produces another culprit in global warming, carbon dioxide. Scientists have dreamed up various ways to pull carbon dioxide from the air. Ideas include giant filters strewn across the landscape to suck in carbon dioxide and then pump it deep into the earth. Others have proposed seeding the oceans with nutrients so that algae and cyanobacteria increase and thus consume more carbon dioxide.
Carbon-dioxide consumers in the bacterial world occupy special niches. Chemolithotrophs (growing only on inorganic salts and carbon dioxide) and photolithotrophs (growing on sunlight and carbon dioxide) draw some of this gas from the atmosphere. The other important consumers of carbon dioxide live in dark places where they digest organic matter and prevent the Earth from becoming choked in waste.
How is a cow like a cockroach?
The methane gas emanating from sewage treatment plants, landfills, and the muck submerged in swamps comes mainly from methane-producing archaea. These so-called methanogens interact with bacteria in a way that allows both to thrive and keep ecosystems running.
Methanogens sustain cattle, goats, sheep, deer, elephants, and all other ruminants plus cockroaches, termites, beetles, and millipedes—thousands of arthropod species in all. Their digestive tracts contain a heterogeneous mixture of microbes from the three domains of living things: Archaea, Bacteria, and Eukarya. The bacteria and archaea cling to the rumen wall and to feedstuffs entering the rumen while protozoa tend to stay in the liquid.
A cow’s four-part digestive organ—rumen, reticulum, omasum, and abomasum—evolved for fermentation. Ruminant animals scarcely chew their food after yanking it from soil; they masticate just enough to mix the grasses with saliva, and then send the bolus into the esophagus leading to the rumen. The cow rumen holds up to 20 gallons, the interior resembling a perpetual washing machine lined with small protuberances called papillae. These structures increase the rumen’s inner surface area to make absorption more efficient and to increase attachment sites for microbes. Rumen fluid ranges from Kelly green from grass diets to olive-green when the cow gets mainly a hay diet. Every minute or so the esophagus launches a bolus into the mix like a torpedo. The fluid softens the bolus, and then the animal regurgitates and rechews it. After “chewing the cud,” the bolus goes back to the rumen where bacteria and protozoa continue the fiber digestion. As the rumen contents slosh about, the animal regurgitates larger pieces and sends smaller denser pieces to the intestines where a different population of bacteria continues the digestion.
A microscope slide holding a drop of rumen fluid or a speck from a cockroach’s innards reveals a mob of microbial life. Cocci and rods bob in the currents. Every second or so a spirillum twirls through the microscope’s field; blink and you have missed it. Protozoa come and go, looking massive next to the bacteria. These eukaryotes range from 20 to more than 100 times the volume of bacteria. Some flagellated protozoa poke through the liquid in fits and starts while other protozoa blanketed in cilia whiz past.
Most of the digestive anaerobes differ from E. coli because they cannot tolerate even miniscule amounts of oxygen. These über-anaerobes (obligate anaerobes to microbiologists) include Bacteroides, Butyrivibrio, Clostridium, Eubacterium, Lactobacillus, Peptostreptococcus, Ruminococcus, Selenomonas, Streptococcus, Succinimonas, Succinivibrio, and Veillonella. Cattle also harbor Lactobacillis, Clostridium, and E. coli—cattle is humans’ main source of the deadly E. coli O157 when farm waste contaminates food. At least 20 species of archaea and 50 species of protozoa also inhabit the digestive tract.
When fibers (cellulose, hemicelluloses) and polysaccharides enter the rumen, bacteria degrade these large compounds into smaller sugars for energy. Protozoa subsist on sugars but also graze on the variety of bacteria and archaea. The archaeal methanogens use carbon dioxide plus vitamins and minerals available in the rumen fluid.
The cow uses relatively little of the nutrients in grass and grain directly. Ruminants live mostly on the volatile compounds emitted by the bacteria. These so-called volatile fatty acids (VFAs) named acetic (two carbons), propionic (three), and butyric (four) acids pass through the animal’s gut lining and enter the bloodstream. The fat and flavor of fresh cow’s milk result from the mammary gland’s synthesis of long fats from the short VFAs. Goats produce a different array of fats from the same three VFAs, which results in distinctive flavors in products made from goat’s milk.
Cows receive most of their amino acids and vitamins from bacteria that the animal’s digestive enzymes degrade. Unlike humans, ruminants survive on very poor quality protein, meaning the protein contains a limited variety of amino acids, because the bacteria improve the variety of amino acids available for absorption.
Cattle spend one-third of their time eating, one-third ruminating or chewing the cud, and one-third resting. During rest, bacterial activity reaches its peak: Large molecules decompose in fermentation to VFAs, carbon dioxide, and a little hydrogen. These reactions would soon stop if carbon dioxide built up in the gut. Methanogenic archaea play the vital role of absorbing carbon dioxide as it appears and turning it into methane:
CO2 + H2 → CH4
A dairy cow with a 15-gallon rumen belches 65 to 130 gallons or 5,370 to 10,740 cubic feet of methane a day. The world’s domesticated and wild ruminants produce about 22 percent of the atmosphere’s methane, one million tons of methane put into the atmosphere a year. Since methane exerts more than 20 times the atmosphere-warming effect of carbon dioxide, ruminants contribute to global warming. When the media make coy references to ruminant flatulence as a major cause of global warming they really should blame belching.
Microbiologists study the goings on inside cow rumens by using fistulated animals. A fistula is an opening about the diameter of an orange leading from the outside of the animal to the inside of the rumen. The left wall of a cow’s rumen lies against the animal’s left side, making the distance from outside to inside less than 3 inches. After a veterinarian surgically fistulates the left side of the animal, the patient recovers quickly and begins eating again within the first few hours after surgery. (Humans can last days without food, but a ruminant cannot go 24 hours without food before becoming deathly ill.) The fistula, like a rubberized doughnut, can be closed with a tight-fitting plastic plug. When opening a fistula plug, a rush of methane bursts from within.
Cockroaches use processes similar to ruminants but with a more active role by protozoa. Bacteria and archaea living inside the protozoa that live inside the insect’s gut carry out the chemical reactions of digestion. Protozoa presumably take in nutrients that sustain the prokaryotes and protect them from predation by other protozoa. As a result, 80 percent of the (American) cockroach’s methane emissions comes from its protozoa.
The protozoa inside ruminants, cockroaches, and termites live in mutualistic symbiosis with the host. Termites contain symbionts within symbionts. The insect lacks fiber-degrading enzymes, so it depends on gut protozoa to digest the wood fibers. But the protozoa, such as Trichonympha sphaerica, also make little progress in digesting wood. T. sphaerica relies on spirochete (spiral-shaped) bacteria living inside it. The bacteria produce the enzyme cellulase that decomposes the cellulose so that the insect, the protozoa, and the bacteria all benefit.
A second group of bacteria live on the outside of termite protozoa. Some spirochetes and other rod-shaped cells line up in precise rows in grooves between the protozoan’s cilia. Electron microscopy has revealed that the curvy spirochetes line up end-to-end and undulate in unison. The protozoan moves by the combined action of its cilia and the coordinated beating of thousands of spirochete flagella, creating a smooth wave of propulsion. No one has yet figured out if the protozoa tell the bacteria where to swim or if the bacteria control where protozoa go. Regardless of the answer, protozoa need their bacteria; if the bacteria disappear, the protozoan stops dead in the water.
Microscopic power plants
In the 1990s, Al Gore’s tireless campaign to address global warming prompted scientists to identify the world’s main sources of methane. Twenty times more active in warming the atmosphere than carbon dioxide, methane became a strategic target in the global warming campaign. The scientists estimated that enteric fermentations of ruminants and insects account for almost 25 percent of the atmosphere’s methane. Cattle manure accounts for another 7.5 percent.
More than half of the methane from human-made structures such as landfills and wastewater treatment already goes into systems that use it as an energy source. The methane from swamps, stagnant ponds, manure piles, and domesticated and wild ruminants goes lost to the atmosphere. An adult cow produces about 27 pounds of solid waste daily and the 100 million cattle in the United States add close to 14,000 tons of manure to waste piles every day. Central Vermont Public Service offers manure-derived methane, or “cow power,” to more than 3,000 homes and businesses. The state’s dairy farms supply the manure that produces the biogas, and the utility converts the gaseous energy to electrical energy and distributes the electricity.
Bacteria in nature or in test tubes always take the most efficient path for finding, absorbing, and metabolizing nutrients. Heterotrophs prefer sugars, fibers, amino acids, and fats for energy and building new cells. Other bacteria called autotrophs thrive on a less heterogeneous variety of nutrients, namely water and carbon dioxide for cell-building and sunlight or metal for energy. Autotrophs (also called lithotrophs) grow on a chunk of rock devoid of organic matter or in the nutrient-empty ultrapure water used in semiconductor manufacturing. The bacteria being discovered in subsurface microbiology are all autotrophs. They get small bits of energy from chemical reactions between water and basalt, and scavenge nitrogen and sulfur from tiny pockets of air.
Heterotroph and autotroph energy production happens in the cell membrane, a multilayered covering that lies just inside the cell wall. Energy generation in bacteria resembles that of humans in that it uses a stepwise transfer of electrons from compound to compound. Each transfer produces small spurts of energy. Humans use membrane-bound proteins called cytochromes to perform most of the electron transfers. Bacteria depend on pigments. The blue-green hues of ocean and freshwater cyanobacteria, the striking colors in hot springs from sulfur and iron metabolizers, and the green and purple intertidal flats populated by photosynthetic bacteria all give evidence that bacteria are hard at work.
Bacteria can be harnessed to produce energy directly rather than energy in the form of fuel. University of Massachusetts microbiologists Derek Lovley and Gemma Reguera have showed that biofilms grow tiny filaments between cells. These filaments act as “nano-wires” to transmit electrical current, which the cell consortium amplifies about tenfold when electrons travel through the film. Perhaps energy companies will one day feed sugar and oxygen to massive biofilm fields and thus produce electricity as well as clean water, a by-product of photosynthesis. Algae and cyanobacteria both possess the capabilities to do this. Biotechnology could also engineer the microbes to produce hydrogen or ethanol.
The waste problem
Bacteria degrade pollutants in soil, surface waters, and groundwaters. These pollutants include pesticides, vehicle and jet fuels, paints, organic solvents, and buried ammunition. Bioremediation scientists take genes from bacteria that metabolize these pollutants and insert the genes into bacteria that grow faster in nature. Bioremediation laboratories now have collections of bacteria that degrade chemical pollutants, detoxify metals such as mercury, or decompose radioactive compounds. Specialized bioreactors can grow biofilms on their interior surfaces (see Figure 7.1) and remove pollutants from water as it flows through the vessel. Bioremediation also seeks the bacteria that live in the acid drainage from ore and coal mines and which trickles 24/7 into more than 10,000 miles of U.S. rivers and streams. The traits enabling the bacteria to thrive in these places make them perfect gene donors for making bioengineered bacteria for mining pollution cleanup.
Figure 7.1. Community formation. In this series of photos, bacteria colonize the metal surface of a cooling system condenser, a process called fouling. (a) Scattered bacteria adhere to a copper-nickel surface; (b) cells and extracellular materials accumulate;
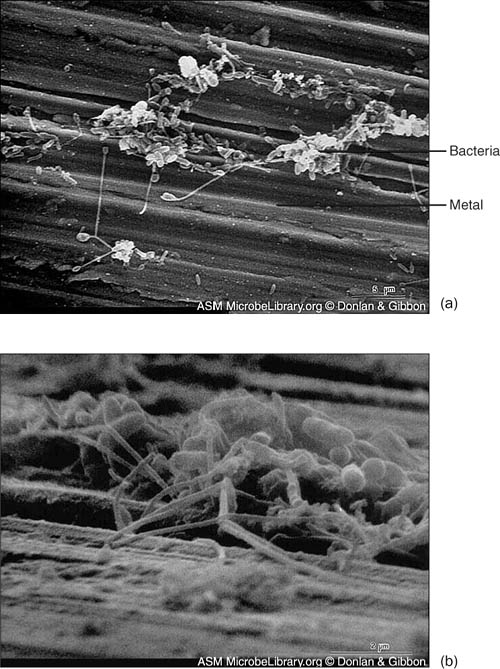
Figure 7.1. (c) filaments extend and trap more cells; and (d) bacteria, freshwater diatoms (type of algae), corrosion products, and clay particles imbed in intertwining filaments.
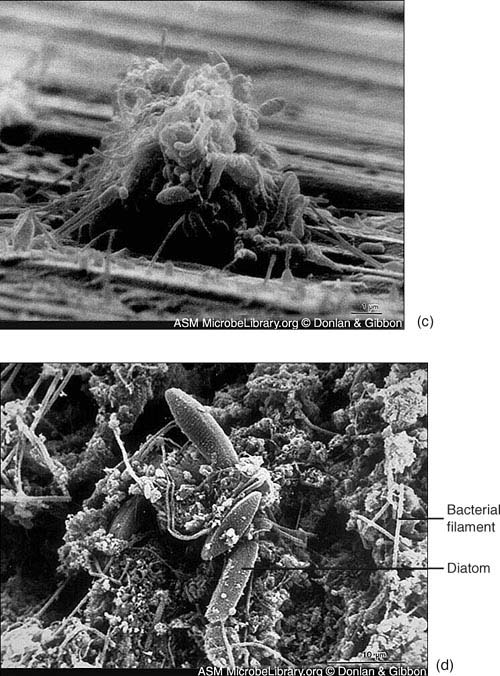
(Reproduced with permission of the American Society for Microbiology MicrobeLibrary (http://www.microbelibrary.org))
Wastewater treatment plants rely on mixtures of aerobic bacteria to degrade the substances in the incoming water. This step happens in the plant’s large outdoor pools filled with dark liquid. Wastewater plants mix the suspension with big paddles and constantly bubble air through the water to keep the bacteria growing. Treated wastewater gets a dose of chlorine to kill both pathogens and good bacteria before returning to the environment. The heavy sludge that settles to the bottom of wastewater pools receives extra digestion in closed tanks filled with anaerobic bacteria.
Wastewater treatment anaerobes break down tough substances like plant fibers and paper, but like rumen bacteria they emit methane gas. For many years, wastewater treatment plants burned off the methane stream from sludge digester tanks. Most now capture the gas and burn it for energy.
Methane production is a double-edged sword, a free energy source and a greenhouse gas. When too much methane enters the atmosphere, it can be viewed as a type of waste like carbon monoxide and hydrogen sulfides. A group of bacteria called methanotrophs use methane for both carbon and energy and thus remove some of the world’s excess methane. Methylobacteria, Methylococcus, and Xanthobacter live in many of the same places as methanogens and absorb the gas as it is produced. For example, still, swampy water contains small bubbles of methane from the methanogens living in the anaerobic sediments at the bottom. Methanotrophs live just above this region, capturing some of the methane as it rises. Xanthobacter possesses an additional ability to combine oxygen with hydrogen gas, also made by methanogens, in an explosive reaction called the knallgas reaction (O2 + 2 H2 → H2O). Knallgas bacteria have systems that control this reaction to make energy for the cell without blowing up!
Methanotrophs use the enzyme methane monooxygenase in its metabolism. This enzyme also breaks down a toxic chlorinated solvent called trichloroethylene (TCE). TCE is a pollutant in soil and groundwaters and harms almost every system in the body. Electroplating, metal degreasing, semiconductor manufacture, steel and rubber manufacturing, pulp and paper operations, and dry cleaning use TCE. Methanotrophs may soon become a tool for cleaning TCE out of contaminated aquifers and land. If microbiologists do put methanotrophs to work in labs, they will avoid Xanthobacter; knallgas bacteria require the explosive mixture of hydrogen and oxygen.
Thiobacillus ferrooxidans presents an equally important but more complex involvement with pollution. T. ferrooxidans thrives in very acidic conditions and gets energy from inorganic iron- and sulfur-containing compounds. All of these features occur in mine tailings, the fluids that flow from ore and coal mines. Mine tailings cause considerable damage to stream and river ecosystems. T. ferrooxidans reacts with iron pyrite to make tailings even more acidic and caustic to the environment.
Mining site remediation currently uses chemicals to absorb or neutralize the acid, but sulfate-reducing bacteria offer an option because they alter the acid-producing reactions of T. ferrooxidans. Sulfate-reducing bacteria begin with the prefix “Desulfo-”—such as, Desulfococcus, Desulfovibrio, and Desulfobacter.
Despite T. ferrooxidans’s penchant for making a bad environmental situation worse, the species has been put to good use too. T. ferrooxidans recovers metal from ore deposits and also reduces sulfur in coal, a step in making low-sulfur or “clean coal.” Conventional coal burning releases the greenhouse gas sulfur dioxide from pyrite and contributes to sulfuric acid formation in the atmosphere, the cause of acid rain.
Depletion of high-grade metal ores in the United States has made the recovery of low-grade ores critical to the metals industry. But high cost prevents the extraction of metals from low-grade ores by the usual smelting process. T. ferrooxidans and the similar species T. thiooxidans extract metals such as copper and uranium from ores filled with iron and sulfur compounds. For example, either of these species can recover copper from the copper-iron-sulfur compound chalcopyrite. The bacteria do the recovery exclusively with enzymes, an example of white biotechnology. Bacterial bioleaching also recovers some of the iron along with copper, and both are recycled by the metals industry into new products.
Similar mechanisms have been used to extract uranium from gold ore. Bioleaching can recover 90 percent of the desired metal from low-grade ores and saves on the high energy cost of smelting.
Bacteria on Mars
The ability of bacteria such as T. ferrooxidans to live surrounded by caustic chemicals or subsurface bacteria to eat rock keeps alive the notion that bacteria might live on other planets. Life on Mars represents more than an academic pursuit. Enzymes produced by potential Martian bacteria might have superior faculties to recover metals or make energy from Earth’s greenhouse gases. Mars’ atmosphere contains more than 95 percent carbon dioxide, about 3 percent nitrogen, and lesser amounts of oxygen, argon, and carbon monoxide. Other than argon, bacteria on Earth use all of these gases. Earth’s autotrophs live on energy sources much like the elements on Mars, that is, silicon, iron, magnesium, calcium, sulfur, aluminum, potassium, sodium, and chlorine.
Recently microbiologists have developed a theory that the Earth’s earliest bacteria may have degraded rock and thus carved out miniature caves that may have served as protective habitats. Earth’s atmosphere 2.75 billion years ago, the estimated age of the caves, held no ozone layer to protect the breakdown of macromolecules by ultraviolet radiation. The caves would have shielded bacteria, protected their DNA from destruction, and provided a probable site for water condensation. The ancient anaerobes possibly used the minerals in the rock to which they attached and absorbed methane percolating up from sediments. This scenario does not seem implausible knowing what we do about extremophiles.
Birger Rasmussen of John Curtin University in Australia has begun a global discussion on whether cave-dwelling bacteria indicate similar bacteria might live on Mars. By analyzing the chemistry of ancient microbial deposits attached to the cave ceilings, he has presumed that the early bacteria used sulfur and methane, probably had access to water, and likely lived in a biofilm community.
Many scientists have been unwilling to make the giant leap from bacteria on Earth to bacteria on other planets. Assuming extraterrestrial life follows the same biochemical principles as on Earth, bacteria on Mars would probably exist in the subsurface for the same reasons cave dwellers developed.
Theories abound on the possibilities of life on other planets in distant solar systems or on Mars. The three main themes of interplanetary under study in astrobiology are water, methane, and minerals.
In 1996, NASA added fuel to the “bacteria on Mars” fire when it announced that a meteorite that had crashed near Antarctica 13,000 years ago held traces of bacterial growth. Scientists named the meteorite ALH 84001 and recovered it in 1984. By the early 1990s, NASA scientists believed it had blasted off from Mars and traveled interplanetary space for 16 billion years. Astrobiologists, meanwhile, focused on tiny wormlike structures embedded in the rock that resembled fossilized microbes. Analysis of the structures’ elements suggested that the structures were more of biological origin than geological. With prior discovery of ancient rivers and seas on Mars’ surface, science seemed to hold circumstantial evidence of water and life on the Red Planet.
The analysis of Mars’ atmosphere has also provided evidence of methane. Considering that Earth’s methane is almost 95 percent of biological origin, the presence of this gas on Mars has been viewed by some astrobiologists as another point in favor of life on Mars. Earth’s atmospheric methane on a volume basis is 1,750 parts per billion, but that of Mars is only 10 parts per billion. No one knows why the disparity exists between the two planets or if any of Mars’ methane came from living things.
Another research team examined the meteorite’s mineral content and found magnetite crystals similar to the magnetosomes in Earth’s Aquaspirllium magnetotacticum. Dennis Bazylinsky of the University of Nevada-Las Vegas has been studying magnetotactic bacteria for more than 20 years. When reviewing the meteorite data, he found the magnetic crystals in the meteorite to be identical to the crystals made by Earth’s magnetic bacteria. Again, scientists held circumstantial evidence in their hands, but the task of comparing Earth’s magnetic bacteria to extraterrestrial crystals would not be easy. Very few cultures of magnetic bacteria exist in laboratories worldwide. New strains are known to exist in nature but they live in difficult-to-reach marine sediments.
Naysayers to life on Mars have pointed out that methane and inorganic structures may resemble conditions on Earth but can also be explained in nonbiological terms, which is true. Microbiologists have questioned the meteorite’s “worm holes” because they are much smaller than the smallest Earth bacteria and thus unlikely to contain all the molecules needed for life. Of course, those scientists are speaking about Earth life, which may be a bit egocentric considering the size of the universe. By 2000, however, most astrobiologists concluded that the worm holes were probably fossilized debris, organic debris perhaps, but not microbial.
Conclusive studies on nanobacteria on Earth may recharge the life-on-Mars debate. Finnish researcher Olavi Kajander discovered nanobacteria in 1988, but the majority of microbiologists rejected the idea of their existence. (Notice how every new discovery mentioned in this book endured a period of denial?) More than a decade of study on nanobacteria suggested that these microbes played a pathogenic role in arterial and kidney calcification.
By 2005, literature had accumulated on Nanobacterium sanguineum, a gram-negative motile species with a calcium-coated outer shell. The bacterium measures only 20 to 200 nm across, but it is big enough to contain 16S rRNA. Studies on N. sanguineum have followed a similar path as studies in the Golden Age of Microbiology: The medical importance of the microbe has superseded environmental studies. But nanobiology will very soon be part of the growing science in interplanetary biology.
Shaping the planet
The Earth’s biosphere consists of millions of ecosystems. When ecosystems interrelate, they form large ecosystem communities. The Earth thus has grassland, rainforest, polar communities, and so on. Although members interact at boundaries called edges, such as the interface between marine and shore life, many of Earth’s communities remain separated by distance. Migrating herds and birds connect some communities but not all of them at once. Only bacteria connect all of Earth’s communities by the constant recycling of nutrients through soil, the oceans, and the atmosphere.
No one needs a degree in microbiology to find these microbes all around and performing their life-giving activities. In the country, notice the lichen growing on rocks, dead leaves decomposing underfoot, and the glimpses of color when a lake ripples. If you live in the city, bacteria live all around. Biofilms coat storm drains and metal-metabolizing bacteria weather bridges and buildings. Soot in the air carries bacteria from block to block. It is easier to detect the invisible universe than it is to find places having no bacteria.
You may never look at your surroundings the same way again, and that is a good thing. Appreciating bacteria is the best way to acknowledge the larger community of Earth. When I was in college in the 1970s, I realized microbiology is a hard subject. It encompasses the basics of cell biology, covers chemical and biochemical reactions, touches on the Earth sciences, and is intimately connected to genetics. Microbiology recruits only scientists willing to study organisms they cannot see. But it is impossible to delve deeper into the microbial world without seeing that bacteria run this planet. Humans reap the benefits of bacterial actions when they discard garbage, avoid infection (remember the skin’s good bacteria), and simply breathe.
Bacteria should not be synonymous with disease. Making cheese out of milk also seems to sell these microbes short. Because of bacteria, our lives are richer, healthier, and more hopeful. Hopeful because no matter what predicament humanity puts itself in, there is a very good chance that a bacterium somewhere can solve the problem.
Stop worrying about germs and start appreciating bacteria. Few pathogenic bacteria exist that cannot be stopped by simply washing hands, preparing food properly, and steering clear of others who are obviously sick. As for the good bacteria that fill the environment, we need not nurture them because they grow just fine without any help from humans. In the process, bacteria supply us with the nutrients we need. Bacteria shape the planet and they also shape us.
For safety’s sake, thinking of bacteria as occasional enemies as well as constant allies helps maintain your health. In the bigger picture, however, bacteria are your best friends. They welcomed humanity into their home tens of thousands of years ago, and they will stay with you to the end. Bacteria work behind the scenes to protect us, feed us, and decompose our wastes. I cannot think of a better ally than bacteria.
