High-Entropy Alloy Solid Solutions
High-entropy alloys (HEAs), being multicomponent equiatomic or near equiatomic in nature, have a high mixing entropy. They have a strong tendency to form disordered and partially ordered solid solutions instead of stoichiometric intermetallic compounds or strong elemental segregation, when they are prepared through various processing routes. Most of the HEAs have FCC or BCC structures, while a few HEAs have shown the formation of HCP structures. Under certain conditions, when the interactions between some constituent elements differ a lot with others in nature, the formation of intermetallic compounds or elemental segregation has been observed because mixing entropy effect is not sufficient to mix them with larger mutual solubility. This chapter gives a comprehensive account of solid solution formation in HEAs.
Keywords
Solid solution; BCC; FCC; HCP; microstructure; thermal stability
6.1 Introduction
HEAs tend to form disordered and partially ordered solid solution phases having FCC, BCC, or HCP structures, instead of a mixture of many intermetallics. It is attributed to the high configurational entropy possessed by these alloys. For equiatomic alloys, the configurational entropy is given as ΔSconf=R ln(n) per mole, where n is the number of elements in equiatomic alloy and R is the universal gas constant. The high configurational entropy reduces the free energy of solid solutions in accordance with the relation ΔGmix=ΔHmix−TΔSconf and thus stabilizes the solid solutions, especially at high temperatures. This was first reported by Yeh and his coworkers, who showed that as-cast CoCrCuFeNi alloy has a single-phase FCC structure and AlCoCrCuFeNi alloy has a two-phase mixture of FCC and BCC structures. The BCC phase in the latter alloy spinodally decomposes into a modulated structure composed of ordered BCC (B2) and disordered BCC (A2) phases during cooling from the liquid state. They also emphasized that the phases at higher temperature are often solid solutions which might undergo phase transformation, such as spinodal decomposition (SD), ordering, or precipitation during cooling, because the importance of high mixing entropy in stabilizing solid solutions is reduced with decreasing temperature. In addition, sluggish diffusion will reduce the substitutional diffusion and lower the nucleation and growth rate and thus rate of phase transformation at lower temperatures (Yeh et al., 2004b). All these have been substantiated by several other works in later years. This chapter deals with solid solution phase formation, microstructural features, and thermal stability of these phases in equiatomic and nonequiatomic HEAs.
6.2 Solid Solution Formation in Equiatomic HEAs
Because materials with second-phase or multi-phase strengthening often have practical applications especially for those requiring high strength, high hardness, high-temperature softening resistance, and creep resistance, it is of considerable interest to identify the HEAs which can form a stable single phase and study their properties. A few conventional examples of single-phase alloys are α-brass, stainless steels, cupronickel, and nichrome. They have moderate strength and high ductility. A basic study of single phase HEAs leads to a better understanding of the mechanisms and properties of alloys containing more phases. The formation of a single-phase random solid solution is favored in the alloys where the majority of constituent binary pairs have a large mutual solid solubility and a small enthalpy of mixing. HEAs obtained through nonequilibrium processing routes like splat quenching, magnetron sputtering, and laser cladding usually exhibit single-phase structure because of lack of time available for the formation of second phases or even intermetallic phases. MA also promotes the formation of single-phase structure as it extends the mutual solid solubility of elements. Most of the HEAs contain either BCC, FCC, or a mixture of these two phases.
6.2.1 Solid Solutions with BCC Structure
BCC HEAs are important in high-strength applications as they usually show better mechanical properties like higher yield strength than FCC HEAs. Formation of BCC structure is favored when most of the binary pairs present in the alloy crystallize in BCC lattice. For example, AlCoCrFeNi alloy has been studied widely by a variety of processing routes such as arc melting and casting (Manzoni et al., 2013b; Wang et al., 2008), suction casting (Qiao et al., 2011), Bridgman solidification (Zhang et al., 2012b), and electro-spark deposition (Li et al., 2013c). Interestingly, this alloy shows a BCC/B2 structure irrespective of the processing route adopted, although only Cr and Fe have a BCC structure out of all the constituent elements. Zhang et al. (2012b) have shown that AlCoCrFeNi alloys processed by casting and Bridgman techniques, while having different solidification rates, show a single phase BCC structure. Interestingly, nanoparticles of B2 have been observed in the grains of BCC (A2) phase in this alloy made by Bridgman solidification.
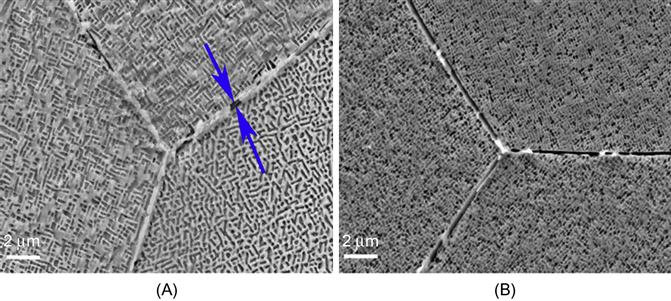
Phase formation in HEAs appears to be governed more by the binary constituent pairs which evolve first rather than the individual elements themselves. This can be explained by considering phase evolution in AlCoCrFeNi alloy in greater detail. Qiao et al. (2011) reported that the framework of the crystal structure is Cr because of the closeness of lattice parameter of Cr (0.28847 nm) and that calculated for AlCoCrFeNi alloy (0.289675 nm), and the fact that except Cr and Fe, none of the other constituent elements have BCC structure. However, it can be argued that the parent crystal structure is AlNi (B2 structure, which is an ordered structure based on BCC) rather than Cr. AlNi also has a lattice parameter of 0.28810 nm, which is also close to that of AlCoCrFeNi. The presence of superlattice peaks similar to that of AlNi in XRD patterns of AlCoCrFeNi also substantiates that the parent crystal structure is AlNi in which the other elements dissolve. The order parameter for the (Al,Ni)-rich phase will not be unity, as the crystal structure is disordered by substitution of other elements into the lattice. Further, considering the phase diagram of Cr (which is the first to solidify as it has the highest melting point) and Al (which has the highest diffusivity at solidification temperature as it has the lowest melting point) (ASM Handbook, 1992), Cr remains segregated from the liquid mixture up to 1350°C at the equiatomic composition. On the other hand, Al–Ni phase diagram indicates that at equiatomic composition solid AlNi begins to form at 1638°C itself. AlNi has the largest negative enthalpy of formation among all binary pairs in AlCoCrFeNi alloy, as shown in Table 2.6 (de Boer et al., 1988), remains stable over a wide composition field down to room temperature, and is able to dissolve other constituent elements. Thus, it is also an intermediate solid solution. In fact, Wang et al. (2014b) have investigated the structural evolution of AlxCoCrFeNi (x=0–1.8) alloys in detail and concluded that AlCoCrFeNi alloys have a spinodal microstructure below 873 K, in which (Cr,Fe)-rich disordered (A2) solid solution and (Al,Ni)-rich (B2) solid solution form a modulated structure from high-temperature (Al,Ni)-rich (B2) solid solution. Zhang et al. (2012b) have prepared this alloy by Bridgman solidification and found fine B2 precipitates in BCC (A2) grains formed by SD (Figure 6.1). Higher withdrawal velocity under the same temperature gradient ~70 K/mm could lead to a finer modulated structure.
AlCoCrCuFeNi alloy has been produced by a number of processing routes. When the alloy is prepared by nonequilibrium techniques such as splat quenching (Singh et al., 2011b), DC magnetron sputtering (Dolique et al., 2009, 2010), and MA (Tariq et al., 2013; Zhang et al., 2009b, c), it shows only a single-phase BCC. When the alloy is prepared by more or less equilibrium routes such as arc melting and casting (Hsu et al., 2007; Tung et al., 2007; Yeh et al., 2007b; Wen et al., 2009), induction melting and casting (Kuznetsov et al., 2012, 2013; Singh et al., 2011a), and suction casting (Zhuang et al., 2012), Cu segregation is observed in ID region due to its positive enthalpy of mixing with other constituent elements as indicated in Table 2.6 (de Boer et al., 1988).
Therefore, the parent crystal structure observed in alloys containing significant amounts of aluminum and nickel is that of AlNi because it has the highest ordering energy among the binary pairs, which makes it form readily. In addition, other elements dissolve into the lattice subsequently due to their chemical compatibility and mixing entropy effect. The degree of ordering of the multicomponent alloy depends on the number and type of other elements present. This may be applicable to other HEAs as well, that is, a binary solid solution which forms most readily can evolve first and other elements dissolve into it subsequently.
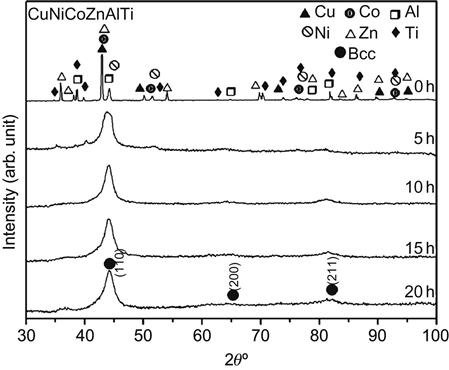
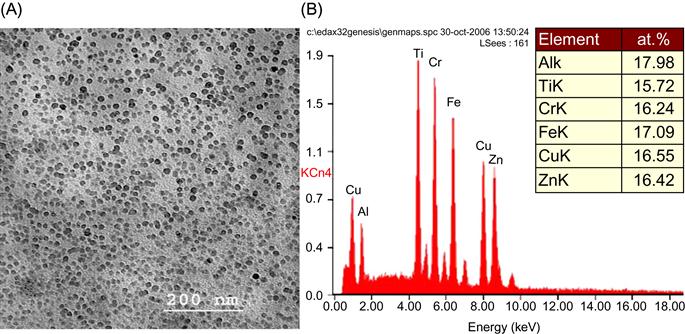
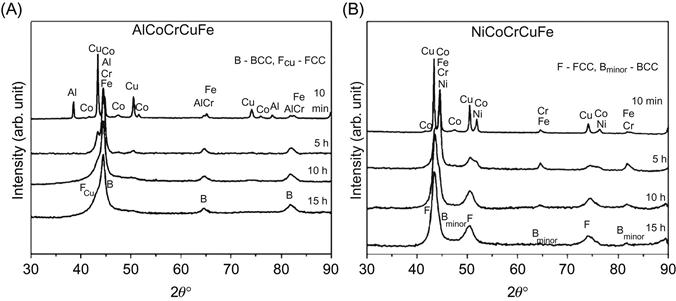
Another interesting case to note is of AlCoCuNiTiZn which has been reported to form a single-phase BCC structure, although none of the constituent elements has a BCC structure at room temperature. Varalakshmi et al. (2010b,c) reported that this alloy can be formed by MA. Figure 6.2 shows the XRD pattern of AlCoCuNiTiZn with milling time which shows a single-phase BCC structure. Transmission electron microscopy (TEM) studies confirmed the nanocrystalline nature of as-milled AlCoCuNiTiZn alloy powder. The crystallite size was reported to be less than 10 nm after 20 h of milling. It is also important to note that the nanocrystallites of HEA obtained by MA have excellent homogeneity in their composition, which is demonstrated by the EDS spectrum (Figure 6.3) from one of the nanoparticles obtained in another AlCrCuFeTiZn HEA with BCC solid solution prepared by MA (Varalakshmi et al., 2008).
HEAs of refractory elements have been synthesized and are shown to have single-phase BCC structure in the as-cast state. Senkov et al. (2010 and 2011b) have reported that MoNbTaW and MoNbTaVW alloys exhibit BCC structure without formation of any complex phases or intermetallics. The lattice parameters reported for these alloys are 0.32134 and 0.31238 nm, respectively. Addition of vanadium reduces the lattice parameter of the quinary alloy as its lattice constant is the least (0.3039 nm) of all elements and also less than that of the quaternary alloy. This effect is similar when compared to the conventional alloys where incorporation of larger radii element causes lattice expansion and substitution of small radii element decreases the lattice constants. The formation of single phase is attributed to the conformance of individual elements to Hume-Rothery rules. Mo, Nb, Ta, V, and W have similar atomic radii, valency, and BCC crystal structure. Feng et al. (2012) have processed thin films of NbTaTiW which exhibit single-phase BCC structure. Although Ti has a HCP structure at room temperature, its BCC structure above 880°C, the complete solid solubility of Nb–Ta, Nb–W, and Ta–W and large solubility of Ti in Nb, W, and Ta lead to the formation of BCC crystal structure in this alloy.
From the above, it can be summarized that individual elements promoting BCC phase formation are Al, Cr, Fe, Ti, Mo, Nb, Ta, V, and W. Al is unique because it has a FCC structure. It has been shown that Al stabilizes FCC structure when its concentration is less than 11 at.% and promotes BCC phase formation when present in a greater amount (Wang et al., 2014b). This is because, although Al has a FCC structure, many of its binary compounds (AlNi, AlFe, AlCo, AlTi) crystallize in BCC lattice due to the formation of d–p hybrid orbital. Cr, Fe, Mo, Nb, Ta, V, and W have BCC structure at room temperature and hence tend to stabilize BCC structure in HEAs. Ti is HCP at room temperature but is BCC at higher temperatures and its large solubility in many BCC stabilizing elements like Nb, Ta, W, Al promotes formation of BCC HEAs. Table A1.1 in Appendix 1 gives a listing of various HEAs in which a single-phase BCC has been observed.
It should be mentioned that phase type prediction is done in a qualitative manner from constituent-element’s features and/or unlike-atomic pair’s features, which is similar to Hume-Rothery rules. While it is comprehensive, it is not possible to make an accurate prediction. This is simply because HEAs involve multielements and many unlike atomic pairs. As a result, various quantitative ways using parameters such as mixing entropy, mixing enthalpy, atomic size difference, and valence electron concentration are proposed for the prediction. Besides, various computational material science techniques including molecular dynamics simulation, ab initio calculation, and phase diagram calculation are proposed for pursuing direct outcomes. All these have been presented and discussed in Chapter 4. They are in fact complementary to each other for better understanding and prediction.
6.2.2 Solid Solutions with FCC Structure
FCC HEAs, owing to their closed-packed structure, are expected to show slower diffusion kinetics at elevated temperatures than BCC HEAs and hence are a better suited for high-temperature applications.
As discussed earlier, the formation of FCC phase is promoted when most of the binary constituents crystallize in FCC structure. For example, AlCoCrFeNi alloy shows an A2+B2 structure. But, when Al is replaced by Cu to form CoCrCuFeNi alloy, FCC phase is formed (Cui et al., 2011b; Li et al., 2009). The binary constituents in CoCrCuFeNi alloy, namely, CoNi, CoFe, CuNi, CuCo, and FeNi, all have FCC structure and hence stabilize this phase. Similar behavior can be seen when Al is replaced by Mn to form CoCrFeMnNi alloy which exhibits single-phase FCC structure (Ye et al., 2012).
Varalakshmi et al. (2010b) studied the effect of elemental addition in HEAs. They synthesized CuNi, CuNiCo, CuNiCoZn, CuNiCoZnAl, and CuNiCoZnAlTi alloys by MA. Up to quinary alloys, a single-phase FCC structure is observed. CuNi is a well-known isomorphous system which crystallizes in the FCC lattice. Co is HCP at room temperature and it transforms to FCC above 450°C and is easily accommodated in the parent CuNi structure (FCC). Zn has a HCP structure, yet CoCuNiZn has a FCC structure which can be attributed to the presence of other three FCC elements (Co, Cu, and Ni). A particular case to notice is that of AlCoCuNiZn which crystallizes in a FCC lattice, even though Al content is 20 at.%. This indicates that formation of a CuNi isomorphous structure takes place readily before AlNi formation, and thus parent crystal structure is FCC, instead of B2, which is further stabilized by the presence of Co. In another similar interesting situation, Dolique et al. (2009, 2010) have observed the formation of a FCC phase in the DC magnetron sputtered AlCoCrCuFeNi alloy, when the deposited film has an Al content less than 15 at.% Al.
Praveen et al. (2012) have shown that CoFeNi and CoCuFeNi, formed by MA, show a single-phase FCC structure. It has been discussed that the parent structure in these alloys is that of Ni in which other elements dissolve. Complete solubility of Co–Ni and Cu–Ni and sufficient solubility of Fe in Ni stabilize the FCC structure in these alloys. Hence, whenever these elements are present together, we are more likely to get a FCC HEA except for a few cases where Al is present in larger amount.
Equiatomic alloys such as CoCrFeNi (Praveen et al., 2012) and CoCrFeMnNi (Liu et al., 2013; Otto et al., 2013a; Zhu et al., 2013; Tsai et al., 2013b) are important FCC alloys which predominantly exhibit a single-phase FCC structure. Here all the constituent elements are similar in sizes, valencies, and electronegativity. However, as discussed previously, uniqueness of HEAs lies in the fact that even elements of different crystal structure might combine to form a single-phase solid solution. For example, in CoCrFeNi and CoCrFeMnNi alloys mentioned above, not all the elements have FCC structure at room temperature, yet the alloys exhibit a single-phase structure. This non-conformance to Hume-Rothery rules in multicomponent system is, as discussed above for AlCoCrFeNi, because the phase evolution in HEAs is largely dominated by the binary constituent with highest driving force of formation. High-entropy effect enhancing the mixing of elements also has an important contribution.
Bhattacharjee et al. (2014) studied the texture development in CoCrFeMnNi alloy on deformation and annealing. They reported that the alloy exhibited a single phase FCC structure and developed submicron cell structure with a strong brass type texture on heavy cold rolling to 90% reduction. Annealing the alloy at 650°C showed ultrafine recrystallized grain structure with an average grain size of about 1 μm. In the recrystallized grain structure, a large fraction of annealing twins were formed. Remarkable resistance to grain growth up to 800°C and very large fraction of boundaries at misorientation angle about 60°(corresponding to the 60° <111> twinning relationship) have also been observed as shown in Figure 6.4. This study suggests that FCC HEAs generally have low-stacking fault energy and tend to have profuse twins and fine stable grain structure after rolling and annealing.
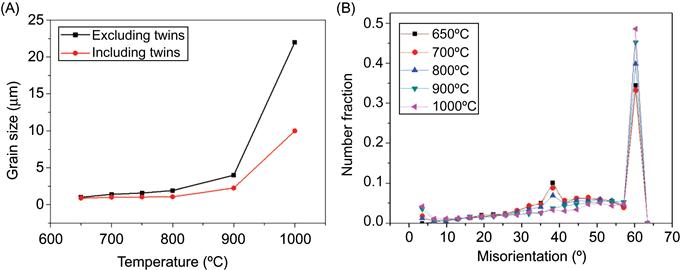
Table A1.2 in Appendix 1 lists the HEAs in which a single FCC phase has been observed.
6.2.3 Mixture of FCC and BCC Solid Solution Phases
As pointed out previously, nonequilibrium processing route is more likely to give rise to single phase. Most HEAs that show the presence of more than two phases are processed through equilibrium processing routes like arc melting and furnace melting. The rate of solidification in these processes is slow and allows sufficient time for different phases to grow or for elemental segregation. For example, AlCoCrCuFeNi shows a single-phase structure when processed by MA or sputtering techniques but exhibits a mixed-phase structure (BCC+FCC) when synthesized through the arc melting route.
Praveen et al. (2012) discuss the phase formation behavior in AlCoCrCuFe and CoCrCuFeNi alloys processed by MA. The XRD patterns for these alloys are shown in Figure 6.5. AlCoCrCuFe crystallizes into a more open BCC structure which can accommodate an increased number of elements without much expansion, and hence no peak shift is observed after MA. On the other hand, the elemental peaks merge into Ni and peak shift of Ni has been observed in CoCrCuFeNi alloy (Figure 6.5B), which is explained on the basis of lattice expansion with increasing number of elements entering the basic Ni lattice during milling. Lattice expansion occurs as a consequence of more elements being accommodated in close-packed FCC structure. In addition, a minor BCC phase exists in CoCrCuFeNi alloy while a minor FCC phase exists in AlCoCrCuFe alloy. This is due to the difference in affinities between Al–Cu and Ni–Cu, which results in partial solubility and complete solubility of Cu during MA in AlCoCrCuFe and CoCrCuFeNi alloys, respectively. The presence of the minor FCC phase is attributed to Cu segregation in AlCoCrCuFe while Cr leads to the formation of the minor BCC phase in CoCrCuFeNi alloy. However, longer milling time is expected to further promote the mixing and chemical homogeneity.
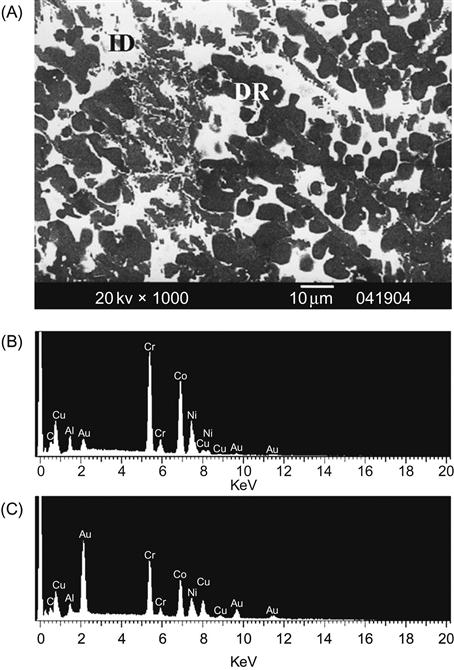
Strong elemental segregations might occur when some elements have large positive mixing enthalpy in the interactions with other elements. Hsu et al. (2007) studied the effect of noble elements gold (Au) and silver (Ag) on phase formation in AlCoCrCuNi alloy. Ag, owing to its large positive enthalpy of mixing with other elements, leads to the formation of two layers and enhances Cu and Ag segregation in the liquid. The microstructure of these two layers along with EDX results shows that Ag segregates to Au layer and depleted in Ag layer. The Au layer enriched in Ag and Cu has a hypoeutectic structure of Ag-rich FCC phase and Cu-rich FCC phase whereas the silver layer enriched in Al, Co, Cr, and Ni has A2+B2 structure. On the other hand, Au, due to its less positive enthalpy of mixing with other principal elements, combined well with the other elements and also reduced Cu segregation. Figure 6.6 shows DR microstructure of AlAuCoCrCuNi with EDX results showing good mixing of Au with other elements. In addition, XRD analysis shows two constituent phases of FCC+AuCu. This study also highlighted the importance of the mixing enthalpy between unlike atom pairs on the structural evolution and related properties of HEAs. Table A1.3 in Appendix 1 summarizes the compositions of HEAs in which a mixture of BCC and FCC phases has been observed.
6.2.4 Solid Solutions with HCP Structure
Very few HEAs are shown to crystallize in an HCP structure. Tsau and Chang (2013) reported the formation of HCP phase in quaternary TiCrZrNb alloy with a c/a ratio of 1.61; however, this phase is not present alone but is accompanied by a BCC phase with a lattice parameter of 0.298 nm. The HCP phase was present along the ID region while the BCC phase formed the matrix.
Huang et al. (2007) observed HCP phase formation in the oxide film of AlCoCrCu0.5FeNi HEA when the oxygen content is in the range of 10–50%. Tsau (2009) observed that the HCP phase gets stabilized when Ti is added to CoFeNi alloy. The as-cast CoFeNiTi alloy showed hard ordered HCP DR with a eutectic mixture in the ID region consisting of soft ordered FCC phase as matrix and hard ordered HCP phase as particles. The HCP phase is found to be stable on annealing up to 1000°C, while the FCC phase gets disordered and softens on annealing for 2 h at 1000°C. Annealing of the alloy for 24 h at 1000°C has resulted in an ultimate compressive strength of 2.60 GPa and a plastic strain of 20%.
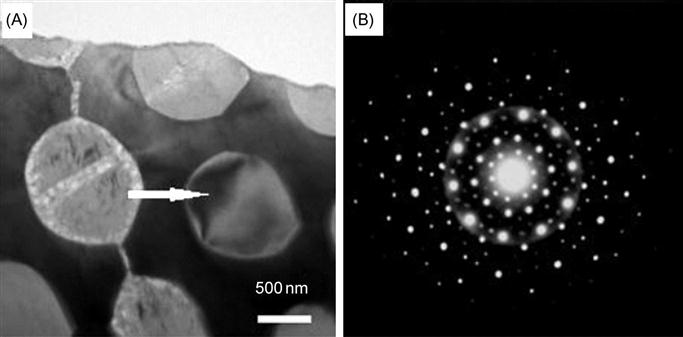
In another interesting study, Li et al. (2010b) showed the formation of an HCP phase along with Al–Mn type quasicrystalline phase in as-cast Mgx(AlCuMnZn)100−x (x=20, 33, 43, 45.6, and 50) alloys. It is important to note that the quasicrystalline phase in this alloy has been observed without the need for rapid solidification. In another report, Li et al. (2011) have provided an electron diffraction evidence for the quasicrystalline phase formation (Figure 6.7) along with an HCP phase in AlCuMgMnZn HEA.
Shun et al. (2010b) observed an ID HCP phase and DR FCC phase in Al0.3CoCrFeNiTi0.1 alloy. Shun et al. (2010a) have also seen similar (Ni,Ti)-rich HCP phase in the ID regions of CoCrFeNiTi0.3 alloy. Tsai et al. (2010c) have reported an HCP phase in the CrTiVZrY HEA coatings obtained by magnetron sputtering. The nitride coatings obtained from this HEA have shown NaCl-type FCC structure. The HCP phase in the HEA coating was attributed to an HCP structure of three of the five elements (Ti, Zr, and Y) in the alloy. In an attempt to search for a single-phase HCP, Gao and Alman (2013) used CALPHAD approach to predict the HCP phase formation in CoOsReRu equiatomic alloy. However, they did not provide any experimental evidence for this prediction.
6.3 Solid Solution Formation in Nonequiatomic HEAs
The original concept of designing HEAs used the principle that configurational entropy, given by ΔSconf=−RΣXi ln Xi per mole, is maximum for Xi=1/n, where Xi is the mole fraction of the ith element, R is the universal gas constant, and n is the number of elements in the alloy. Nonequiatomic alloys have a lower configurational entropy as their composition deviates from equiatomic one. This implies that tendency for solid solution formation would be the greatest for an equiatomic HEA. Yet, we see a large number of researchers are working on nonequiatomic HEAs. The reasons for this are threefold. Firstly, the difference between configurational entropy of equiatomic and nonequiatomic HEA is not very large. For example, configurational entropy (ΔSconf) of six-component AlCoCrCuFeNi alloy comes out to be 1.93R, while ΔSconf for Al0.2CoCrCuFeNi alloy would be 1.78R. Secondly, many nonequiatomic HEAs have been shown to have superior mechanical properties than the corresponding equiatomic alloys. Thirdly, it has been of interest to determine the effects of various elements on structural evolution and properties of HEAs. Yeh et al. (2004b) studied the effect of aluminum in as-cast AlxCoCrCuFeNi alloys and showed that aluminum when added in smaller quantities (<0.5), the alloy has a FCC structure but promotes BCC phase formation when its content is higher. This helps in designing HEAs with required microstructure and properties.
The main constituent phases in nonequiatomic alloys are not significantly different from that of the equiatomic alloys. It is also very probable to find single-phase HEA from nonequitaomic composition. For example, equiatomic AlCoCrCuFeNi HEA has a mixture of FCC and BCC besides Cu-rich ID phase whereas nonequiatomic Al0.3CoCrCu0.4FeNi HEA has a single FCC phase and no Cu-rich ID phase. Similarly, CoCrCu0.5FeNi forms a single-phase FCC structure (Lin et al., 2010). AlxCoCrFeNi forms an ordered BCC structure even when x is higher than 1 (Li et al., 2009). According to Gibbs phase rule, for a sexinary alloy, there are six degrees of freedom in the composition range for the single-phase field. As a result, a large number of nonequiatomic compositions can give raise to a single phase. However, in some cases, these alloys might show typical DR microstructure with ID regions having different composition in comparison to the DR. This DR structure is related to the freezing range as seen in conventional as-cast alloys.
Obviously, nonequiatomic HEAs might also have two or more phases. AlCoCuxNiZnTi exhibits a simple solid solution structure (FCC+BCC) for different copper contents (Varalakshmi et al., 2010b). In all these cases, the phases observed are similar to those observed for corresponding equiatomic HEAs. Ren et al. (2012) studied a series of nonequiatomic HEAs containing Cr, Cu, Fe, Mn, and Ni by varying two elements at a time. In all the alloys, they observed either FCC or FCC+BCC structures without the formation of any complex phases or compounds.
The influence of processing routes on nonequiatomic HEAs is similar to that of equiatomic HEAs. Nonequilibrium processing routes like MA and rapid solidification techniques are more likely to give raise to single-phase structures whereas equilibrium synthesis techniques like casting, in some cases, can lead to the formation of intermetallic phases. Al0.5CoCrCuFeNi exhibits a single-phase FCC structure when prepared by magnetron sputtering (Chen et al., 2005a) whereas the formation of ordered L12 phase is observed when the same alloy is processed through arc melting route (Hemphill et al., 2012). Huang et al. (2004) showed that AlCrFeMo0.5NiSiTi exhibits a mixed-phase structure containing one B2 phase and two FCC phases when processed through arc melting and casting, while it shows almost a single-phase BCC structure when prepared by plasma spraying.
6.3.1 Effect of Aluminum
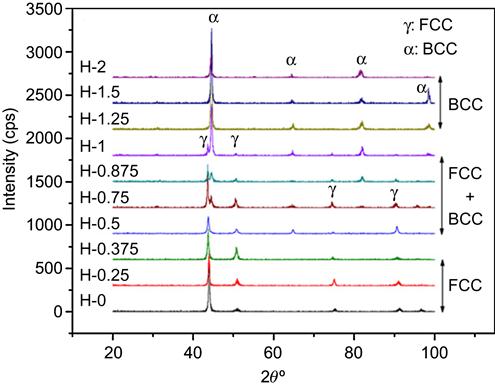
Aluminum has been a major alloying element in most of the HEAs studied till date. It is believed to impart strength and good oxidation resistance. At lower Al concentrations, it stabilizes FCC phase since it has the same structure. When added in higher amounts, it tends to stabilize ordered BCC (B2) structure which can be attributed to the fact that it forms stable binary compounds with many of the common elements like AlNi, AlFe, AlCo, etc. The XRD studies of AlxCoCrCuFeNi alloys prepared by arc melting and casting by Yeh et al. (2004b) showed this trend. The alloy with Al content between 0 and 0.5 has an FCC structure, but a BCC phase appears after x=0.5. The amount of BCC phase increases after this, but the FCC phase starts decreasing after x=2.8. At higher concentration, the FCC phase disappeared and a single-phase B2 structure was observed. Similar results have been reported by Chou et al. (2009) for AlxCoCrFeNi alloys (Figure 6.8). The alloy shows FCC structure with x up to 0.375. For alloys with x=0.5–1.0, the alloy shows a FCC+BCC structure. When the Al content is higher than x=1.25, the alloy has a BCC structure. Hsu et al. (2013b) have shown that addition of Al not only promotes the formation of B2 structure but also hampers the evolution of σ phase in AlxCoCrFeMo0.5Ni alloy. This can be attributed to the fact that most of the elements have negative interaction energy with Al and binary constituents like AlNi can readily evolve which further can dissolve other σ phase forming elements like Co, Cr, and Fe.
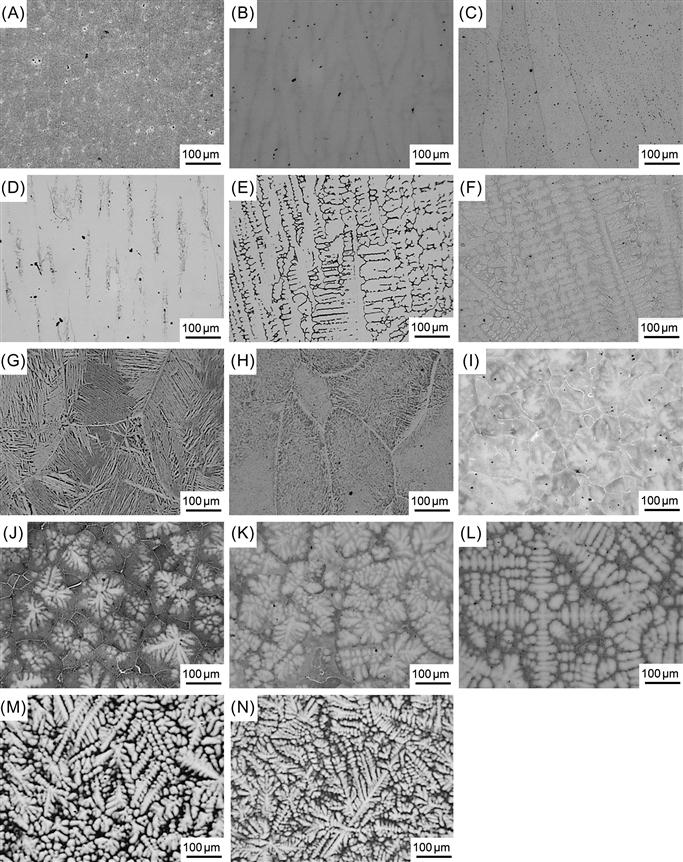
The morphology of cast structure of HEAs also changes with the addition of aluminum. Figure 6.9 shows the different microstructures observed when Al content is varied from 0 to 2 for AlxCoCrFeNi alloys (Wang et al., 2012b). The cast structure is cellular for x=0–0.3, columnar DR for x=0.4–0.6, equiaxed non-DR for x=0.7–0.8, equiaxed DR for x=0.9–1.5, and nonequiaxed DR for x=1.8–2.
In a recent report, Tang et al. (2013) discuss the mechanism of interaction between Al and transition metals in HEAs. Aluminum has three electrons in the outer shell, a small work function, and a low ionization energy. Consequently, it prefers to transfer electron to vacant d orbitals of transition metals like Fe, Co, Ni, Cr, and Mn to form strong covalent bonds and intermetallic compounds. In addition, Al, being a larger atom, causes a lattice distortion in crystal, particularly in HEA consisting of transition metals. The lattice distortion can be better accommodated if the lattice has a more open structure than a close-packed one. Therefore, it is seen that BCC phase formation is favored at higher Al contents.
6.3.2 Effect of Transition Elements (Co, Cr, Cu, Mo, Ni, Ti, and V)
Transition elements form important constituents of HEAs. They stabilize the HEA phase similar to their own crystal structure. Co (above 450°C), Cu, and Ni are FCC elements and stabilize FCC phase in HEAs while Cr, Mo, V, and Ti (high-temperature BCC) favor the formation of BCC structure. Wang and Zhang (2008) showed that increasing the cobalt content in AlCoxCrFeNiTi0.5 alloy beyond x=1 leads to the appearance of FCC phase which increases in its intensity with further increases in Co content.
Copper tends to segregate in ID region due to its positive enthalpy of mixing with many common elements. This is sometimes reflected in the XRD patterns of HEAs with the presence of small FCC peak. The amount of copper decides the mechanism of segregation and hence influences the microstructural features of the alloys. Mishra et al. (2012) studied the phase evolution in Co–Cu–Fe–Ni–Ti alloys with different Ti/Cu atomic ratios. At lower ratios (Ti/Cu=9/11, 11/9, and 3/2), Cu-rich liquid segregates at ID region subsequently causing formation of eutectic mixture of Cu-rich phase and Laves phase of Ti2Co type. At higher atomic ratios (Ti/Cu=1/3, 3/7, and 3/5), two solid solution phases, one Co-rich and other Cu-rich phase, are observed. This occurs due to phase separation between Cu-rich and Co-rich liquid owing to their positive enthalpy of mixing. Formation of two solid solutions has also been observed in CuxZnyTi20Fe20Cr20 (Mridha et al., 2013) alloys synthesized by MA for different x/y ratios. One solid solution which is Cr-rich has BCC structure while the other solution is Cu-rich and has FCC structure.
Compared to Co, Ni is a stronger FCC stabilizer. In fact, Ni is the strongest FCC stabilizer among all FCC stabilizers. Addition of Ni causes appearance or stabilization of FCC phase in HEAs. XRD studies of AlCoCrFeMo0.5Nix alloys for different x values by Juan et al. (2013) revealed that FCC phase appears at higher nickel content, confirming that Ni stabilizes FCC structure.
Addition of molybdenum tends to stabilize the formation of BCC structure and/or appearance of σ phase. When added to AlCrFeNiMoxalloys, Mo dissolves preferentially in Fe–Cr BCC phase. Cr is an important constituent in HEAs, and as already discussed in the context of equiatomic alloys, Cr stabilizes BCC structure and promotes formation of σ phase particularly in presence of Fe, Co, and Ni. Chen et al. (2006a) discussed the effect of vanadium on FCC Al0.5CoCrCuFeNi alloy. V when present in higher concentration (>0.2) causes phases separation and leads to a BCC phase with modulated structure by SD, which envelops the FCC DR.
Ti crystallizes in an HCP structure at room temperature which transforms to a BCC structure at higher temperatures. Ti is often added to improve corrosion resistance of alloy and increase strength by solid solution strengthening or precipitation hardening. Ti is usually found to favor the formation of BCC structure.
6.4 Microstructure of HEAs
HEAs processed through a casting route show typical cast microstructure consisting of DR and ID. DR region is often found to contain microstructural features like precipitates, nanostructured phases, and modulated structure arising from SD. Elements like Cu and Ag have been found to segregate in ID region of cast microstructure. ID regions are also shown to have two-phase eutectic structure. Tong et al. (2005b) have studied AlxCoCrCuFeNi HEAs (x=0–3.0) and pointed out that the occurrence of precipitation is because of the decreased solubility of the FCC and BCC matrix phases with lowering temperature due to the diminishing effect of high mixing entropy. Figure 6.10 (Tong et al., 2005b) depicts phase formation sequence during cooling of AlxCoCrCuFeNi alloy system with different aluminum contents.

Tung et al. (2007) discussed microstructural features of various HEAs having different elements in nonequiatomic proportion. Alloys having lesser content of copper have narrow ID regions which is evident from the segregation tendency of the element. SD leading to modulated structures is observed in the alloys containing BCC phase (DR regions) while ID regions have mixed FCC and BCC structures. It can be readily inferred from these results that although configurational entropy for different alloys is same, their microstructures vary in terms of phase fractions and compositions which further reinforces the fact that other thermodynamic factors also play a role in phase evolution in nonequiatomic HEAs.
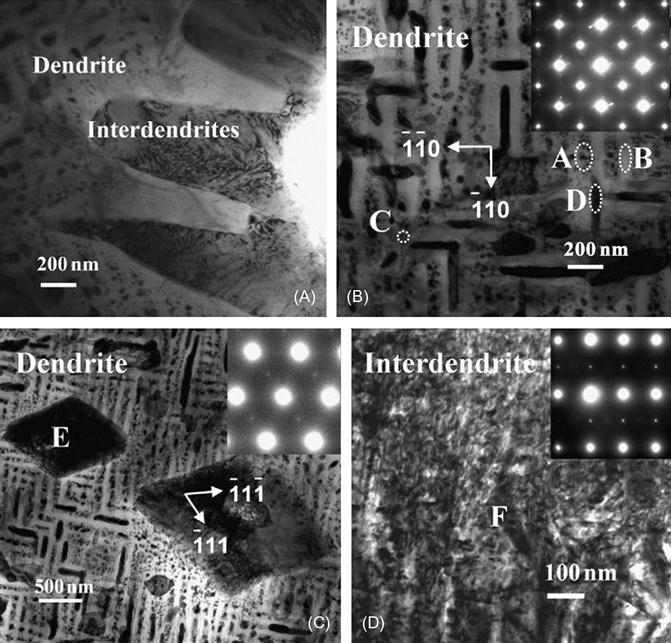
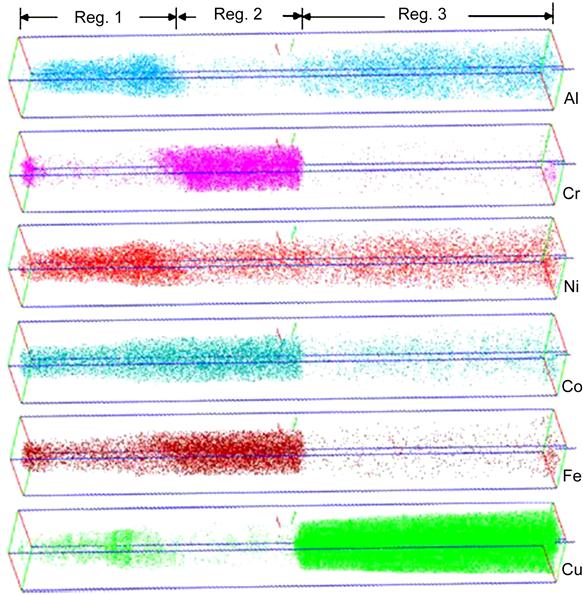
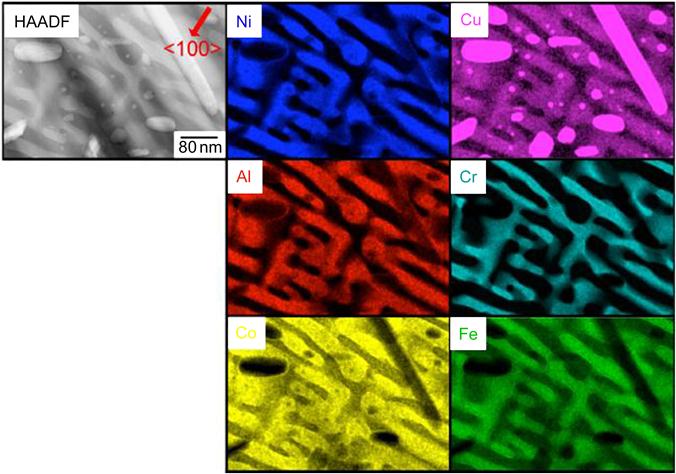
Singh et al. (2011b) have studied microstructure of AlCoCrCuFeNi alloy in detail (Figure 6.11). They have observed the DR microstructure in as-cast alloy (Figure 6.11A). It has been shown that DR region actually comprises of many minor phases like plate-like precipitates (Figure 6.11B), rhombohedral and spherical precipitates (Figure 6.11C), and weak superlattice reflections of L12 phase. The DR region was also shown to have Ni–Al, Cr–Fe, and Cu-rich plates as studied by 3D atom probe (Figure 6.12). On the other hand, splat-quenched AlCoCrCuFeNi alloy exhibited a polycrystalline microstructure with clear grains and grain boundaries. Figure 6.13 shows a high-angle annular dark-field (HAADF) image along with elemental EDS maps, obtained using image corrected TITAN, of Al1.5CoCrCuFeNi alloy prepared from elemental powders with a LENS device (Welk et al., 2013). Ni, Al, and Co appear to be more concentrated in one of the plate-like structures, while Cr and Fe are concentrated in the other plate-like phase. The cylindrical- and elliptical-shaped phases appear to be very rich in Cu. Furthermore, the nanoscale precipitates are also rich in Cu. This fine structure again confirmed the SD. The first of these plate-like phases corresponds to the B2 phase whereas the other plate-like phase corresponds to the disordered BCC (A2) phase.
In contrast to the above observation on cast alloys, atom probe studies on mechanically alloyed CoCrFeNi HEAs indicate very uniform distribution of alloying elements in the as-milled condition. However, segregation of certain elements in some alloys prepared by MA has been observed after hot consolidation. Elemental mapping using atom probe tomography in AlCoCrCuNiZn HEA prepared by MA followed by hot compaction at 600°C showed segregation of Cu to the grain boundaries. Retention of nanocrystalline grains of about 10 nm in size even after consolidation was also observed.
In addition to processing routes, microstructure of HEAs also depends on the alloying element because the phase equilibrium and kinetics in solidification stage are also changed. For example, Ti addition to AlCoCuFeNi changes morphology from DR to eutectic cell type (Wang et al., 2012c) whereas V addition exhibits DR region with ellipsoidal particles instead of modulated plate-like structure. They have observed morphology to change from DR to columnar on Ti addition.
Zhang et al. (2013a) reported the formation of lath-like martensite containing high density of dislocations in laser-solidified FeCoNiCrCuTiMoAlSiB0.5. Such martensite has been commonly observed in steels and shape memory alloys but not in HEA systems. The nucleation of martensite is mainly due to high cooling rates during laser-solidification process. In addition, boron atoms are believed to play a similar role as carbon does in steels. Boron atoms, being smaller in size, sit in octahedral voids and result in contraction and expansion along the shear modulus direction causing martensitic transformation. Interestingly, the martensite transforms to an ordered B2 structure for which the driving force is probably the reduction in strain energy and enhancement of crystal symmetry above a critical limit of solute concentration.
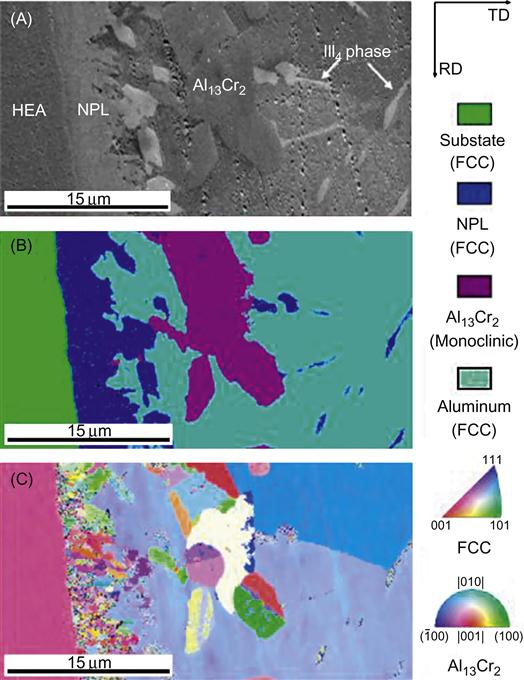
Advanced characterization techniques like high-resolution transmission electron microscopy, atom probe tomography, electron back-scattered diffraction (EBSD), etc. prove to be very helpful in observing the fine microstructural details of HEAs. Figure 6.14 (Yang et al., 2011) demonstrates the use of EBSD in the study of the interface between Al and AlCoCrFeMnNi HEA.
Mechanically alloyed powders of nonequiatomic HEAs show similar structures as that of equiatomic HEAs. Hard agglomerates containing nanocrystallites, smooth fine particles, and uniform morphology are some of the characteristic features of the microstructure. Chen et al. (2009c) prepared AlCoCrCu0.5FeMoNiTi alloy by MA. Consistent with the mechanisms of MA, the 2 h-milled powders show lamellar structures which transform to uniform featureless microstructure after 36 h of milling. In a similar study by Sriharitha et al. (2013) on Al5CoCrCuFeNi alloy prepared by MA, the microstructure showed an average particle size of around 0.5 microns with a crystallite size less than 50 nm.
6.5 Role of Sluggish Diffusion in Phase Evolution of HEAs
One of the distinct features of HEAs is the slow rate of diffusion due to the presence of a large variety of elements. This sluggish diffusion in HEAs not only improves the high-temperature properties but also plays a significant role in their phase evolution and thermal stability.
Varalakshmi et al. (2008) reported the synthesis of nanocrystalline BCC HEAs having two to six components. Among the AlFe, AlFeTi, AlFeTiCr, AlFeTiCrZn, and AlFeTiCrZnCu alloys made by MA of individual elements, it has been observed that phase formation is complete after longer milling time in quinary and sexinary alloys when compared to binary to quaternary alloys. It is attributed to sluggish diffusion kinetics with increasing number of elements. The slow rates of diffusion are also responsible for the formation of nanostructures in HEAs. Cantor et al. (2004) report that primary DR arm width (15 µm) seen for quinary CoCrFeMnNi alloy is larger than that (5 µm) observed for sexinary alloy CoCrCuFeMnNi alloy. In the same work, it is shown that when Nb and Ti are added to CoCrFeMnNi alloy, a single phase is still retained. Nb and Ti, both are not FCC elements and tend to stabilize BCC phase, yet they dissolve completely into single-phase FCC HEA. The slow diffusion rates in the alloy possibly prevents the formation of any second phase or complex precipitates as observed with Nb, Ti addition in conventional alloys.
The current understanding of mechanisms responsible for sluggish diffusion in HEAs is incomplete. The very first paper reporting diffusion measurements in HEAs appeared in 2013 (Tsai et al., 2013b). Pseudo binary approach using diffusion couple technique was adopted to measure interdiffusion coefficients in CoCrFeMnNi alloy. The diffusion coefficients obtained for different elements in HEAs are much lower when compared to that in conventional alloys. For example, Cr and Ni diffusion coefficients in CoCrFeMnNi alloy are calculated as 1.69×10−13 and 0.95×10−13 m2/s, respectively, whereas the values of these coefficients in FCC-Fe are 4.19×10−13 and 2.66×10−13 m2/s, respectively. The origin of slow diffusion rates is discussed in terms of varied interaction energies, also termed as Lattice Potential Energy (LPE) at different sites due to the presence of large number of elements in higher concentration. The larger LPE fluctuation in HEA leads to higher normalized activation energies and a lower diffusion rate, and thus the sluggish diffusion effect.
The sluggish diffusion can also be explained in terms of reduced activity of each element in the single-phase solid solution. Presence of different kinds of elements creates additional strain in the lattice, which further enhances the barrier for atom and vacancy migration. A detailed study of diffusion mechanisms by techniques like tracer diffusion, experimental determination of vacancy concentration by positron annihilation, etc. can provide better understanding of this field.
6.6 Thermal Stability of HEAs
HEAs have been explored for applications at high temperatures. Their performance as coating and structural materials at high temperatures depends on their creep behavior, stability of microstructure, and mechanical properties at elevated temperatures. Therefore, it is of critical importance to understand the thermal stability of phases and microstructure of HEAs.
Alloys processed through nonequilibrium processing routes like MA and sputtering show formation of metastable phases which may transform to new structures when subjected to thermal treatments. On the other hand, processing routes like casting result in the formation of equilibrium phases, consequently most of the cast alloys retain the solid solution phases on heat treatment. In some cases, however, a new second phase may evolve. Phase evolution upon heat treatment has been found to depend broadly on alloy composition, processing conditions, heat treatment process, and initial phases. Alloys having only a single solid solution phase in as-processed condition are more likely to be stable when subjected to heat treatment than alloys having mixed phases. If several phases are involved for strengthening and creep resistance purposes, the reinforced phases are also required to be thermally stable under operating condition as found in superalloys, for example, stable gamma prime in the gamma matrix is very important.
Varalakshmi et al. (2010c) have studied the effect of consolidation process for AlFeTiCrZnCu alloy. It has been shown that as-milled alloy contains a BCC and FCC phases, which transforms to two BCC phases depending upon consolidation process. Although the XRD patterns of HIPed (Hot Isostatic Pressed) and VHP (Vacuum Hot Pressed) are similar, the particles in HIPed samples are relatively smaller in size and spherical in shape due to application of uniform pressure from all sides.
The study of phase evolution in NiCoFe, NiCoCrFe, NiCoCuFe, NiCoCrCuFe, and AlCoCrCuFe after spark plasma sintering (SPS) at 900°C (Praveen et al., 2012) has demonstrated that Cu segregation (indicated by evolution of FCC peak) and formation of σ phase occur in AlCoCrCuFe and NiCoCrCuFe. In AlCoCrCuFe, the major BCC phase transforms to an ordered BCC phase. Formation of σ phase is observed in all Cr-containing alloys.
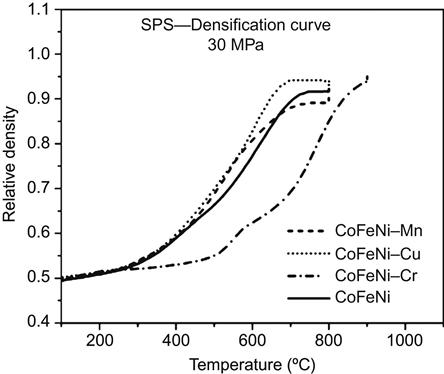
Praveen et al. (2013b) discuss the densification kinetics of CoCrFeNi alloy in detail. Figure 6.15 shows the densification curve obtained for different quaternary HEAs which indicates a delayed densification in CoCrFeNi alloy. The CoCrFeNi alloy shows a minor BCC phase in the XRD pattern. This phase transforms to a σ phase around 550°C, after which densification is also found to accelerate. BCC phase is a Cr-based phase and hence delays the densification of HEA because of slow diffusion rate of Cr owing to its high melting point. This alloy also demonstrated excellent stability for the nanocrystalline nature of the alloy and the crystallite size remained around 50 nm even after annealing for 25 days at 700°C, after SPS at 900°C.
One important difference that is observed in many HEAs when compared to conventional alloys is the nature of room temperature phase vis-a-vis the high-temperature phase. In conventional elements and alloys, a close-packed structure is more stable at room temperature and a more open structure is observed at higher temperatures. Also in conventional alloys an ordered phase is observed at lower temperature whereas disordered phase appears at higher temperature. However, for some HEAs a reverse trend is observed in both the cases (Praveen et al., 2012; Zhang et al., 2009b; Dolique et al., 2010). As discussed in previous section, crystal structure of HEAs is characterized by the presence of high strain due to severe lattice distortion. This strain is higher for a close-packed structure. Therefore, at room temperature, atoms tend to crystallize in a more open structure so that additional strain can be compensated. At higher temperature, the extra strain appears to be compensated, making the close-packed structure more stable.
MA usually leads to the formation of metastable phases which often disappear or transform to other phases at higher temperatures. Metastable phases are observed due to inherent nonequilibrium nature of the process. Sriharitha et al. (2013) studied the influence of heat treatment after MA of AlxCoCrCuFeNi alloys. They observed three phases, a major BCC and two minor FCC, in Al0.45CoCrCuFeNi in the as-milled samples. After differential scanning calorimetry (DSC) at 1480°C, the BCC phase disappears and two FCC phases form the structure. Formation of a new FCC phase is observed when Al2.5CoCrCuFeNi alloy is subjected to DSC. Al5CoCrCuFeNi retains its single-phase B2 structure even after DSC which suggests high thermal stability for the alloy. Fu et al. (2013a) reported that Al0.6CoCrFeNiTi0.4 shows BCC and FCC phases when processed through MA, and the phases transform to new BCC and FCC structures when subjected to SPS.
Alloys produced by casting route usually have equilibrium structures and hence are less likely to transform to new ones when subjected to higher temperatures. However, the presence of certain alloying elements can lead to precipitation and evolution of phases at elevated temperatures. For example, σ phase formation is often observed in Cr-containing HEAs. Age hardening of CuCr2Fe2NiMn alloy leads to the precipitation of ρ phase which contributes to high-temperature strength of the alloy (Ren et al., 2012). Also, the microstructure present often changes at high temperatures. For example, spherical nanoprecipitates are observed in as-cast Al0.3CoCrFeNi alloy, which change to platelets when the alloy is aged at 700°C and to micro-sized rod-shaped precipitates when aging is done at 900°C (Shun and Du, 2009).
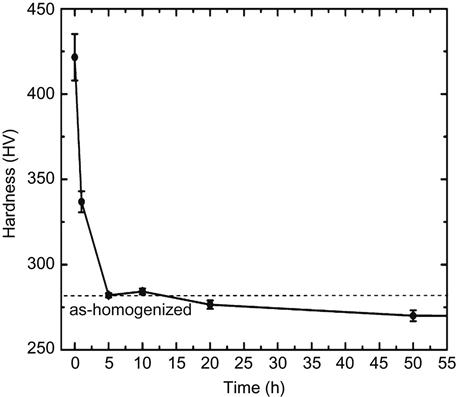
HEAs often have higher resistance to recovery, recrystallization, and grain growth. Tsai et al. (2009b) studied the behavior of deformation and annealing of Al0.5CoCrCuFeNi alloy. They found that significant work hardening occurred during forging even at 900°C, which indicates its low dynamic recovery. In addition, at least 5 h at 900°C were required to soften the sample to the hardness level of the as-homogenized state before cold rolling, as shown in Figure 6.16. Since the melting range of the present alloy is from 1279°C to 1362°C, its recrystallization temperature for 1 h annealing is estimated to be 545°C (~0.5Tm) according to the empirical rule for traditional alloys. Hence, the real recrystallization temperature of the alloy is much higher than the estimated one by at least 355°C. Bhattacharjee et al. (2014) studied the deformation and annealing behavior of CoCrFeMnNi alloy. They not only found that significantly higher recrystallization temperature than conventional alloy with similar melting point, but also found large resistance to grain growth up to 800°C as shown in Figure 6.4A. They attributed all the above phenomena to solute distorted matrices of HEAs, with high lattice distortion energy, sluggish diffusion effect, and low-stacking fault energy.