8 Droplet-Based Microfluidics for Biological Sample Preparation and Analysis
CONTENTS
8.2.2 In-Droplet Reagent Combination and Mixing
8.2.4 Droplet Readout Strategies
8.3 Perspectives for Droplet-Based Microfluidics
8.3.1 Enhanced LC/MS-Based Proteomic Analysis
8.1 Introduction
Modern biological research often requires massively parallel experiments to analyze a large number of samples in order to find biomarkers, screen drugs, or elucidate complex cellular pathways. These processes frequently involve time-consuming sample preparation and expensive biochemical measurements. Another constraint frequently encountered in bioanalysis is limited amounts of available sample. Microfluidics or lab-on-a-chip platforms offer promise for addressing the challenges encountered in biological research because a large number of small samples can be handled and processed with different functional elements in an automated fashion.
Droplet-based microfluidics, in which reagents of interest are compartmentalized within femtoliter-to-nanoliter-sized aqueous droplets or plugs that are encapsulated and dispersed in an immiscible oil phase, has emerged as an attractive platform for small-volume bioanalysis [1,2,3,4,5,6,7,8 and 9]. This new platform elegantly addresses challenges encountered with conventional continuous flow systems by, for example, limiting reagent dilution caused by diffusion and Taylor dispersion and minimizing cross-contamination and surface-related adsorptive losses [10]. The microdroplets isolated by the immiscible liquid can serve as microreactors, allowing for high-throughput chemical reaction screening and extensive biological research [1]. Droplet-based microfluidics also offers great promise for reliable quantitative analysis because monodisperse microdroplets can be generated with controlled sizes and preserve temporal information that is easily lost to dispersion in continuous flow systems [11,12].
Biological analysis begins with sample selection and preparation. The initial sampling can comprise cell sorting, tissue dissection, or extraction of protein or other analytes of interest from cells or tissues [13]. The biological samples are then prepared by, for example, combining reagents, mixing, incubating, purifying, and enriching. Depending on the complexity of the sample, the subsequent analytical measurements can be very simple, employing, for example, laser-induced fluorescence (LIF) to detect a single labeled analyte. With more complex samples having multiple analytes of interest, chemical separation techniques including capillary electrophoresis (CE) and liquid chromatography (LC), and information-rich detection methods such as mass spectrometry (MS) become necessary. To date, many operational components for microdroplets have been well-developed to perform most of these basic operations. For example, stable aqueous droplets dispersed in an oil phase can be generated using various droplet generator designs for sampling in a confined small volume, the most common of which are the T-junction [14,15] and flow focusing [16,17] geometries. Addition of reagents to existing droplets can be realized by fusion with other droplets, enabling the initiation and termination of the compartmentalized reactions confined in the microdroplets [18,19]. Rapid mixing of fluids within droplets enables a homogeneous reactive environment to be achieved and can be enhanced by means of chaotic advection [20]. In addition, droplets can be incubated in delay lines [21] or stored in reservoirs [22,23] or traps [24,25] for extended periods of time to complete reactions or facilitate biological processes.
Droplet-based microfluidic platforms have been successfully applied in a variety of chemical and biological research areas. For example, a droplet-based platform for polymerase chain reaction (PCR) amplification has proven able to significantly improve amplification efficiency over conventional microfluidic formats [26], which is mainly due to the elimination of both reagent dilution and adsorption on the channel surfaces. Droplets have also been employed to encapsulate, sort, and assay single cells [12,27] or microorganisms [24], study enzyme kinetics [11] and protein crystallization [28], and synthesize small molecules and polymeric micro- and nanoparticles.
Although droplet-based microfluidic technology has developed to a degree where droplets can be generated and manipulated with speed, precision, and control, some real challenges still exist that limit the widespread use of these systems. One challenge is how to extract and acquire the enormous amount of chemical information that can be contained in the picoliter-sized droplets. Detection of droplet contents has historically been limited to optical methods such as LIF, while coupling with chemical separations and nonoptical detection has proven difficult. Combining the advantages of droplet-based platforms with more information-rich analytical techniques including LC, CE, and MS can greatly extend their reach. This requires extraction of the droplets from the oil phase for downstream analysis and detection.
This chapter focuses primarily on the integrated droplet-based microsystems having the ability to couple with chemical separations and nonoptical detection, allowing for ex situ analysis and identification of the biochemical components contained in the microdroplets. Some unit operations for microdroplets will be briefly introduced, including droplet generation, fusion, and incubation. All approaches and techniques developed for droplet detection, droplet extraction, coupling CE separation, and electrospray ionization (ESI)-MS detection will be reviewed. An example of integrated droplet-based microfluidics, including on-demand droplet generation and fusion, robust and efficient droplet extraction, and a monolithically integrated nano-ESI emitter, will be given to demonstrate its potential for chemical and biological research.
8.2 Droplet-Based Operations
8.2.1 Droplet Generation
Currently, most planar microfluidic droplet generators are designed using T-junction [14,15] and flow focusing [16,17] geometries, in which small droplets are spontaneously formed at an intersection taking advantage of the interface instability between oil and aqueous streams. Using these approaches, droplets can be generated over a broad range of frequencies ranging from ~0.1 Hz to 10 kHz and using flow rates on the order of 0.1–100 μL/min [29]. Droplet volume and generation frequency depend on several factors, including the physical properties of the immiscible phases, flow rates, intersection geometry, and so on. For a given geometry and solvent composition, flow focusing and T-junction interfaces exhibit interdependence between flow rate and droplet generation frequency and cannot be easily modulated over short timescales.
For lower frequencies and applications for which the ability to rapidly change droplet size and generation frequency is desirable, on-demand droplet generation strategies become more favorable, as they ensure precise control and fine manipulation of individual droplets. Various approaches have been developed to generate droplets on demand, for example, by carefully balancing the pressure and flow in the system [27], as well as electrical [30] or laser pulsing [31] and piezoelectric actuation [32]. Pneumatic valving has also been explored and has been found to provide easy, independent control over both droplet size and generation frequency [33,34,35 and 36]. Galas et al. utilized a single pneumatic valve that was embedded in an active connector and assembled close to a T-junction to regulate the flow of the dispersed phase [33]. Constant pressures were applied on the inlets of two immiscible liquids to drive the flow in the microchannel. Individual droplets were created by briefly opening the valve. The aqueous droplet size depended on the valve actuation time and frequency, as well as the pressure applied at the oil inlet. Therefore, the droplet volume, spacing, and speed could be controlled accurately and independently. This device not only generated periodic sequences of identical droplets, but also enabled the production of nonperiodic droplet trains with different droplet sizes or spacing. Lin and coworkers also reported a similar platform for pneumatic valve-assisted on-demand droplet generation [34]. Negative pressure was applied at the outlet of the device to drive the flow of the two immiscible liquids through the microchannel. The dependence of droplet size on the valve actuation time and applied pressure was investigated. In addition, they utilized several aqueous flow channels, each with independently controlled microvalves, to generate arrays of droplets containing different compositions by alternately actuating the valves.
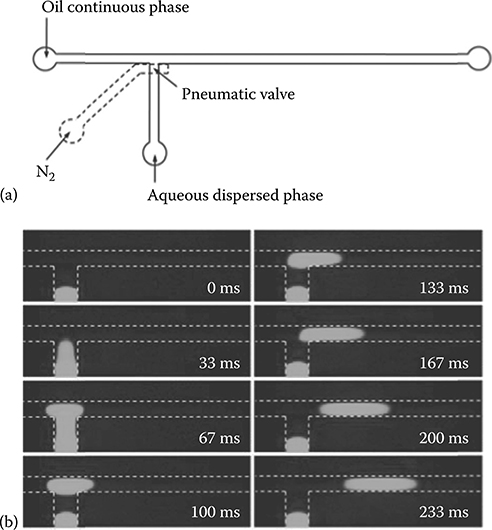
FIGURE 8.1 (a) Schematic depiction of a T-junction droplet generator controlled by a pneumatic valve. (b) Micrograph sequences depicting pneumatic valve-controlled generation of an individual fluorescein droplet. The width of the oil flow channel was 100 μm, the oil flow rate was 0.5 μL/min, and the sample injection pressure was 8 psi. Valve actuation time and pressure were 33 ms and 25 psi, respectively.
We have also investigated valve-controlled on-demand droplet generation. To minimize the dead volume and control droplet volume precisely, the pneumatic valve was placed over the side channel exactly at the T-junction (Figure 8.1a). Carrier oil flow was driven by a syringe pump, and the dispersed aqueous phase was injected by finely controlled air pressure. Figure 8.1a shows the generation process of an individual droplet. The valve is initially closed and the aqueous fluorescein solution is confined in the side channel. When the valve is opened briefly, a small volume of aqueous solution is dispensed into the oil channel to form a droplet, which is then flushed downstream by the carrier oil flow. Compared with conventional microfluidic droplet generation techniques based on a T-junction or flow focusing, the valve-integrated system can generate droplets with precise control over droplet volume, generation frequency, and velocity. Droplet velocity is determined by the syringe pump driving the oil stream, while the droplet generation rate is controlled by the valve actuation frequency as defined in the software. The droplet spacing is determined by the interval between valve openings and the oil flow velocity. Droplet volume depends on several parameters including the valve actuation time, the pressure of the aqueous solution, the oil phase flow rate, and the valve control pressure (Figure 8.2).
8.2.2 In-Droplet Reagent Combination and Mixing
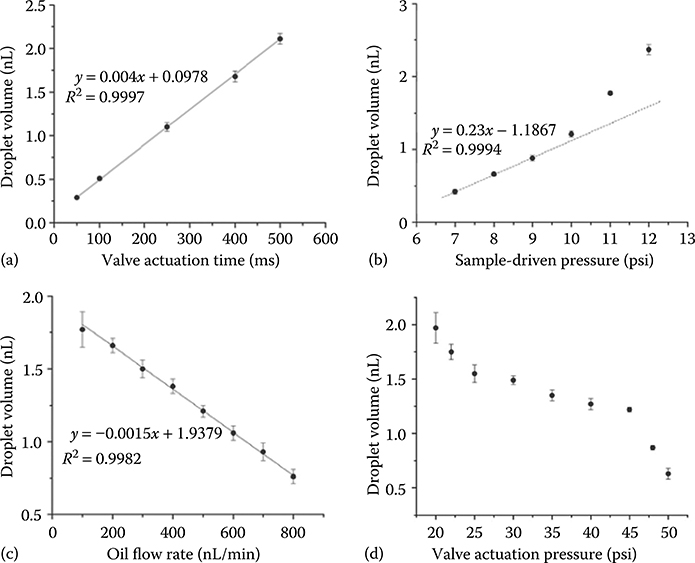
FIGURE 8.2 Plots of droplet volume dependence on (a) valve actuation time, (b) sample driven pressure, (c) oil flow rate, and (d) valve actuation pressure. Channel dimensions were the same as for Figure 8.1.
Besides controlled droplet generation, droplet fusion is of crucial importance in relation to the development of microreactors because it allows precise and reproducible mixing of reagents at well-defined points to initiate, modify, and terminate reactions [6]. Ismagilov and coworkers carried out the pioneering work to combine different reagents into individual droplets by causing two reagent solutions to flow in a micro-channel as two laminar streams [37]. To prevent the prior contact of two reagents before droplet generation, an inert center stream was used to separate them. Thus, three streams were continuously injected into an immiscible carrier oil phase to form droplets. The gradient droplets in the reagent concentrations were achieved by varying the relative flow rates of the three streams [11,38]. A subsequent winding channel was designed to accelerate mixing by chaotic advection [20,37]. This approach has been widely employed to control networks of chemical reactions [37], study reaction kinetics [11], screen protein crystallization conditions [38], and investigate single-cell-based enzyme assays [39] and protein expression [12].
Recently, Weitz and colleagues presented a robust picoinjector to add reagents to droplets in microfluidic systems [40]. The picoinjector was controlled by an electric field to trigger the injection of a controlled volume of reagents into each droplet. The injection volume was precisely controlled by adjusting the droplet velocity and injection pressure. Selective injection was realized by switching the electric field on and off at kilohertz frequencies.
In-channel droplet fusion is another attractive approach to combine different reagents in individual droplets to initiate or terminate the confined reactions. The process of droplet merging introduces convective flows into the system, resulting in far more rapid mixing than relying on diffusion alone [41]. In-channel droplet fusion is readily achieved by bringing two or more surfactant-free droplets into contact. Both passive and active methods have been developed to control droplet fusion. For passive fusion devices, droplet coalescence is usually initiated by utilizing specially designed fusion elements in the channel. For example, Bremond et al. incorporated an expanded coalescence chamber in the channel network in which two droplets were brought into close proximity and merged together before they entered a narrow channel [42]. Fidalgo et al. reported a method for droplet fusion based on a surface energy pattern inside a microfluidic channel where the segmented flow was disrupted and the droplets were trapped and fused together [43]. In this case, full control of droplet fusion could be achieved by varying channel and pattern dimensions, as well as the fluid flow. This surface-induced droplet fusion method enabled the merging of multiple droplets containing different reagents to form a large droplet. However, this approach could potentially cause cross-contamination between droplets from the patterned surface. Niu et al. developed a pillar-induced droplet merging device, in which rows of pillars were constructed in the channel network serving as passive fusion elements or chambers [44]. The pillar array trapped droplets and drained the carrier oil phase through the apertures between pillars. The first trapped droplet was suspended and merged with succeeding droplets until the surface tension was overwhelmed by the hydraulic pressure. The merging process depended on the droplet size, and the number of droplets that could be merged relied on the mass flow rate and volume ratio between the droplets and merging chamber.
Active fusion methods that can be controlled externally and selectively have also been developed using, for example, electric fields [45,46,47 and 48] and laser pulses [49] to trigger coalescence. To perform active droplet fusion effectively, synchronization of droplets is a key factor because fusion efficiency relies on the droplets being in very close proximity [50]. Currently, special designs are often employed to synchronize droplets in two parallel channels, which then merge into a single channel downstream to realize droplet coalescence [34,42,51]. However, this system can potentially be disturbed by a few factors such as flow rate and back pressure in the channel, which may reduce the fusion efficiency. Recently, Jambovane et al. used valve-based droplet generation for multiple reagents to perform controlled reactions and establish chemical gradients among arrays of droplets [52]. Droplets were generated at a valve-controlled side channel and then different reagents were added to the droplets as they passed by similar side channels downstream.
An efficient method for reagent combination that we have recently developed employed two pneumatic valves integrated at a double-T intersection (Figure 8.3a). Reagents were introduced through the different side channels, each controlled by a separate valve, and simultaneous opening of the valves resulted in the creation of an aqueous plug containing both reagents. Upon actuation, the oil between the two side channels is quickly displaced, and the two aqueous streams collide and combine. No sample cross-contamination was observed because of the applied pressures, the rapid valve actuation, and the offset between the two side channels. The two liquids mixed together by a combination of diffusion and convection caused by an equalization of internal pressures following the combining of the aqueous streams. The linear dependence of droplet volume on valve actuation time and the independent valving for the two aqueous streams provide a high degree of control over droplet composition. Figure 8.3b shows arrays of six droplets containing different ratios of two dyes that were created by controlling the operation of two valves. Such control should be useful for optimizing or screening reactions and for studying reaction kinetics.

FIGURE 8.3 (a) Schematic depiction of the droplet generation, fusion, and mixing portion of the device. (b) Relative intensity of an array of six droplets containing different volume ratios of colored dyes.
8.2.3 Droplet Incubation
Many biological assays involving, for example, enzymatic reactions, have relatively slow kinetics, requiring microdroplets to be incubated for minutes to hours for efficient reaction. Similarly, studies involving cell incubation or protein expression require extended incubations. A straightforward method for microdroplet incubation is to simply increase the channel length following droplet generation [53,54], but increased back pressure and disruption of droplet formation can quickly become an issue. Frenz et al. incorporated deeper and wider delay lines following the droplet generation section, which enabled the length of times for reactions in the droplets to increase from 1 min to >1 h [21]. Similarly, Kennedy and coworkers have interfaced capillaries or Teflon tubing with the droplet generation devices to collect and store sample plugs for 1–3 h [55]. For longer-term online incubation, droplets can be stored in reservoirs, traps, or dropspot arrays. For example, Courtois et al. fabricated a large reservoir for storing droplets for periods of up to 20 h to study the retention of small molecules in droplets [23]. Huebner et al. designed a droplet trapping array to store and incubate picoliter-sized droplets for extended periods of time to investigate the encapsulated cells and enzymatic reactions [25]. Weitz and colleagues introduced a “Dropspots” device to immobilize and store thousands of individual droplets in a round chamber array over a 15 h incubation period [56]. Droplets can also be incubated off-chip for periods ranging from minutes to several days when appropriate surfactants are used to stabilize the droplets [57,58]. The incubated droplets can then be reinjected into microfluidic devices for further processing and detection.
8.2.4 Droplet Readout Strategies
To date, in-droplet fluorescence detection remains the most widely used method for analyzing the contents of droplets due to its ability to measure in real time and with high sensitivity. Fluorescence detection has been implemented to study enzyme kinetics within droplets [11,59,60], characterize the behavior of encapsulated single cells [12,27], detect PCR products [61,62], and investigate the interactions between biological samples [63]. Fluorescence detection is ideally suited to rapid, sensitive detection of a small number of distinct species. For cases in which a large number of analytes need to be detected and identified (e.g., proteomics and metabolomics) and where fluorescent labeling is not desirable, alternative measurement strategies are needed.
In-droplet Raman spectroscopy has recently been used to detect and analyze droplet contents [64,65]. It is a nondestructive and label-free detection approach with high molecular selectivity which can track the droplets in real time to determine fundamental droplet properties and chemical contents, including droplet sizes, encapsulated species, structures, and concentrations. Surface-enhanced Raman spectroscopy (SERS) can offer higher sensitivity and reproducible quantitative analysis of the droplets due to the enhancement of the Raman signal intensity [66]. Electrochemical detection is an inexpensive and label-free approach to collect information on the physical and chemical properties of droplets and can monitor droplet production and measure droplet length, frequency, and velocity [67]. It can provide chemical information when the reaction within the droplets involves an electrochemically active reactant or product [68]. Another advantage of electrochemical measurement is its compatibility with alternative chip materials, including opaque substrates, which are difficult to implement for conventional optical detection strategies such as fluorescence. Nuclear magnetic resonance (NMR) has also been used for droplets or segmented flow analysis. Karger and coworkers developed a microcoil NMR probe for high-throughput analysis of sample plugs in dimethyl sulfoxide (DMSO) [69].
While the above detection strategies can be employed in situ, others require the contents to be removed from the droplet for subsequent analysis. Once extracted to an aqueous stream, the droplet contents can be analyzed using more information-rich techniques including LC, CE, and MS. MS is an especially attractive technique for in-depth, label-free biological analysis because of its ability to identify and provide structural information for hundreds or more unique species in a given analysis [70]. Below we detail methods used for droplet extraction and subsequent analysis.
Ismagilov and colleagues used a microfluidic system to screen and optimize organic reaction conditions in microdroplets detected using matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) [71]. The incubated reaction plugs were deposited onto a sample plate for MALDI-MS analysis. Kennedy and coworkers directly pumped nanoliter plugs of sample into a mass spectrometer for analysis through a metal-coated capillary nanospray emitter, separating the analyte from the carrier at the emitter itself [72,73]. Teflon tubing was placed close to the emitter tip to siphon the accumulated oil away from the tip, and could maintain stable electrospray at flow rates as high as 2000 nL/min. However, it is generally necessary to extract the aqueous droplet from the oil phase for further separation or on-line MS analysis to avoid contamination of the mass spectra with peaks from the oil and to maintain the electrospray Taylor cone in the most efficient cone-jet mode of operation.
Edgar et al. first reported the extraction of aqueous droplet contents into a channel for CE separation [74]. A femtoliter-volume aqueous droplet was directly delivered to fuse with the aqueous phase in the separation channel for CE separation. Niu et al. employed a similar method to inject the droplets in which the LC eluent was fractionated into a CE channel for comprehensive two-dimensional separations in both time and space [75]. A pillar array was constructed at the interface to evacuate the carrier oil phase prior to loading samples into the separation channel. In these two cases, it was very difficult to maintain a robust extraction because the segmented flow was perpendicular to the CE separation channel.
Kennedy and colleagues exploited a surface modification method to form a stable interface at the junction between two immiscible phases in the microchannel [76,77 and 78]. They selectively patterned glass surfaces in the segmented flow channel to be hydrophobic in order to stabilize the oil–water interface and facilitate droplet extraction. But in some cases only part of each droplet was extracted due to the presence of a “virtual wall,” and was not suitable for quantitative analysis because of irreproducibility and loss of information [76]. Fang and coworkers employed a similar surface modification technique to obtain a hydrophilic tongue-based droplet extraction interface that could control the droplet extraction by regulating the waste reservoir height [79]. The extracted droplet contents were then detected by MS through an integrated ESI emitter. More recently, Filla et al. used a corona treatment to hydrophilize a portion of a polydimethylsiloxane (PDMS) chip to establish an extraction interface [80]. Aqueous droplets were transferred into the hydrophilic channel when the segmented flow encountered the interface. The droplet contents were subsequently analyzed by electrochemistry or microchip-based electrophoresis with electrochemical detection.
Huck and coworkers employed electrocoalescence to control droplet extraction [81,82]. The segmented flow and continuous aqueous flow met at a rectangular-shaped chamber where an interface between two immiscible phases was built up. A pulsed electric field was applied over the chamber to force droplets to coalesce with the continuous aqueous stream, which then delivered the droplet contents to a capillary emitter for ESI-MS detection [82]. This droplet extraction approach required careful adjustments of the flow of the two immiscible phases to maintain a stable interface in the extraction chamber and avoid cross-contamination of the aqueous and oil streams. In addition, the severe dilution of the droplet contents resulted in high detection limits (~500 μM bradykinin). Lin and coworkers used an electricity-based method to control the droplet breaking and extraction at the stable oil–water interface [83]. One reported issue in this case was the difficulty of achieving complete extraction with high efficiency, which limited its compatibility with quantitative analysis.
Kelly et al. invented a droplet extraction interface that was constructed with an array of cylindrical posts to separate the segmented flow channel and the continuous aqueous phase channel [84]. When the aqueous stream and carrier oil phase flow rates were well controlled to balance the pressure at the junction, a stable oil–aqueous interface based on interfacial tension alone was formed to prevent bulk crossover of the two immiscible streams. The droplets could be transferred through the apertures to the continuous aqueous stream and finally detected by ESI-MS with virtually no dilution, enabling nanomolar detection limits.
Most of the reported methods and techniques for droplet extraction, as mentioned above, need to adjust two immiscible liquid flow rates to stabilize the interface and extract entire droplets. It is desirable to perform effective and complete droplet extraction independent of the flow rates, which would provide added flexibility for device operation. Recently, we have developed a robust interface for reliable and efficient droplet extraction, which was integrated in a droplet-based PDMS microfluidic assembly. The droplet extraction interface consisted of an array of cylindrical posts (Figure 8.4a), the same as was previously reported [84], but the aqueous stream microchannel surface was selectively treated by corona discharge to be hydrophilic. The combination of different surface energies and small flow-through apertures (~3 × 25 μm) enabled a very stable liquid interface between two immiscible steams to be established over a broad range of aqueous and oil flow rates. All aqueous droplets were entirely transferred to the aqueous stream (Figure 8.4b) and detected by MS following ionization at a monolithically integrated nanoelectro-spray emitter.
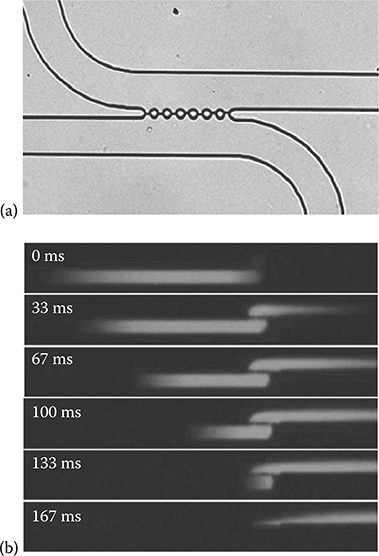
FIGURE 8.4 (a) Photograph of the droplet extraction region of the device. Water and oil fill the top and bottom channels, respectively, and the interface for the two liquids can be seen between the circular posts. (b) Micrograph sequences depicting the extraction of an individual fluorescein droplet. The flow rate in both channels was 400 nL/min.
8.3 Perspectives for Droplet-Based Microfluidics
As mentioned above, droplet-based microfluidics have been employed for a wide range of analyses and due to their unique advantages, their use will undoubtedly grow. The following subsections outline a few promising applications that will leverage the strengths of the platform.
8.3.1 Enhanced LC/MS-Based Proteomic Analysis
MS-based proteomics studies are vital for biomarker discovery, identification of drug targets, and fundamental biological research. In a typical “bottom-up” proteomics workflow [85], proteins are extracted from a sample, purified, and enzymatically digested into peptides. The peptides are then separated by LC, ionized by ESI, identified by MS, and those identified peptides are then matched to their corresponding proteins based on genomic information. Alternatively, for “top-down” proteomics [86], intact proteins are separated and identified directly by MS, providing potentially more complete sequence information and the ability to characterize posttranslational modifications. However, MS identification of intact proteins is far more challenging and has a lower throughput, limiting the widespread use of top-down approaches at present.
As top-down and bottom-up proteomics approaches each have unique and complementary advantages, it would be especially attractive to obtain both intact protein and peptide-level information from a single analysis. We propose that this could be achieved by encapsulating separated proteins into droplets as they elute from an LC column, thus preserving temporal information and separation resolution while enabling further processing. For example, using our droplet-on-demand and droplet merging technologies, we could encapsulate eluting proteins into droplets, and selectively add reagents for digestion to alternating droplets. The droplets could then be incubated in a delay line to allow sufficient reaction time prior to extracting and ionizing the droplets. The result would be that each droplet containing unreacted protein would be followed by a droplet containing digested peptide such that conventional bottom-up MS would be complemented with the intact molecular mass.
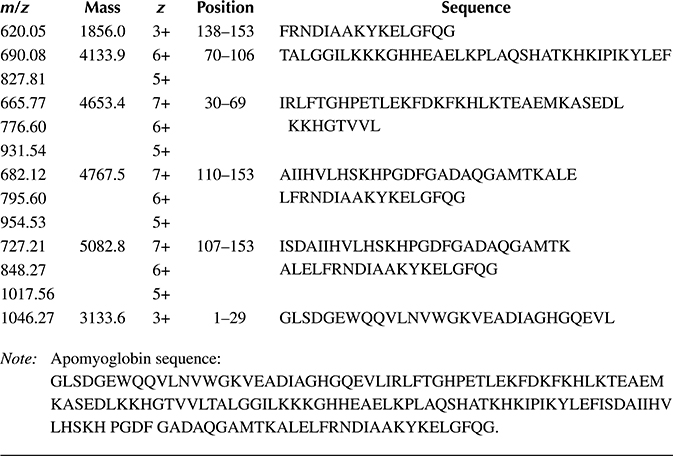
To this end, we have begun combining proteins with proteases in droplets to evaluate the conditions needed for digestion. The platform incorporated our integrated droplet-on-demand interface that enabled controlled in-droplet reactions, incubation in the oil stream, extraction from the aqueous stream, and ionization of the droplet contents at an integrated nanoelectrospray (nano-ESI) emitter [87] for MS analysis (Figures 8.5 and 8.6). This integrated microfluidic platform has been successfully utilized to combine myoglobin and pepsin from separate aqueous streams into droplets to perform rapid in-droplet digestions that were detected and identified on-line by nano-ESI-MS following droplet extraction (Figure 8.7). Given the short incubation time (18 s), the digestion did not go to completion such that peaks from the intact protein are still evident in the mass spectrum, but numerous peptides are confidently identified based on their m/z ratio as well (Table 8.1). We expect that simply extending the incubation time will dramatically improve digestion efficiency and enable the application of the platform to combined top-down/bottom-up proteomic analyses.
8.3.2 Single-Cell Chemical Analysis
Sensitivity limitations on biochemical measurements typically dictate that large samples are required comprising populations of cells. These ensemble measurements average over important cell-to-cell differences. Direct chemical analysis at the single-cell level will enable the heterogeneity that is currently obscured to be better understood. The sensitivity of MS instrumentation used for proteomic and metabolomic studies has increased to the point that such single-cell measurements are now feasible. For example, while inefficient ionization and transmission of ions generated at atmospheric pressure to the high vacuum region of the mass spectrometer previously were serious problems, recent improvements have produced combined efficiencies that can exceed 50% in some cases [88]. Indeed, around 50 proteins have been identified from samples containing just 50 pg of protein [89], which is as much protein as is contained in an average eukaryotic cell [90]. However, despite having adequate analytical sensitivity, existing methods for sample preparation, involving manual pipetting and multiple reaction vessels, are incompatible with single cells.
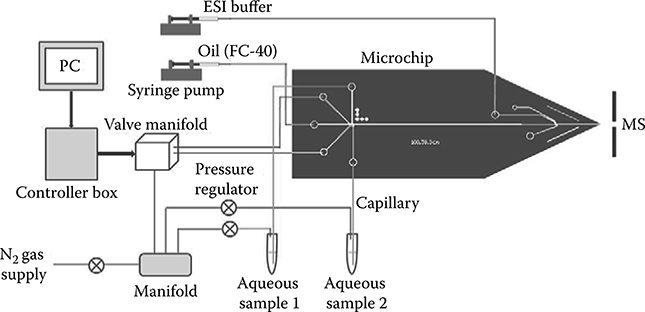
FIGURE 8.5 Schematic of the experimental set-up for droplet generation, fusion, mixing, extraction, and MS detection.

FIGURE 8.6 (a) MS detection of the extracted 1 μg/μL apomyoglobin droplets. Oil phase flow rate was 100 nL/min, ESI buffer flow rate was 400 nL/min, and droplet generation frequency was 0.1 Hz. (b) Detailed view of the MS-detected extracted apomyoglobin droplets. (c,d) Mass spectra obtained from the peak and baseline indicated as a and b in (b), respectively.

FIGURE 8.7 (a) MS detection of the fused droplets mixing 1 μg/μL apomyoglobin with 1 μg/μL pepsin in water containing 0.1% formic acid (pH ~3). The flow rates of oil and ESI buffer streams were 0.1 and 0.4 μL/min, respectively. (b) and (c) MS spectra of the fused droplet and 1 μg/μL apomyoglobin, respectively. The sequences of some digested peptide fragments labeled in (b) are listed in Table 8.1.
Table 8.1 Sequence of Apomyoglobin and Identification of Peptide Fragments from In-Droplet Digested Apomyoglobin Shown in Figure 8.7b.
This is another area where droplet-based microfluidics should be able to meet the need. Droplets have been previously used for single-cell encapsulation, and cells have also been lysed within droplets, with the surrounding oil preventing further dilution of the contents. Using such technologies for encapsulation and lysis in combination with our approaches for reagent mixing and droplet compatibility with ultrasensitive MS should enable us to dig deeper into the proteome and metabolome of single cells than has been accomplished previously.
8.4 Conclusions
Droplet-based microfluidics has developed substantially as a technology and will likely assume a higher-profile role in biological analyses in the future. Not only are much smaller amounts of reagents and samples consumed, but also thousands of reactions and screening experiments can be performed within droplets simultaneously. Perhaps more importantly, droplet-based microfluidics is a promising tool to help us understand some fundamental biological processes such as enzymatic reactions in a confined and crowding environment, protein–protein and protein–ligand interactions, interfacial functions in biological systems, and single-cell proteomics and metabolomics. A number of operational units have been well developed for droplet-based microfluidics, including droplet generation, fusion, and incubation. Others, such as droplet extraction for subsequent analysis of the contents, have been developed recently and promise to add versatility to the platform. Robust integration of multiple functions to create a true “lab-on-a-chip” continues to be a challenge, but the unique advantages of droplets for sample-limited biological analyses will undoubtedly spawn further development and we anticipate significant growth in the number of applications that rely on this technology in coming years.
References
1. Song, H., D.L. Chen, and R.F. Ismagilov, Reactions in droplets in microfluidic channels. Angew. Chem. Int. Ed., 45: 7336–7356, 2006.
2. Huebner, A., et al., Microdroplets: A sea of applications? Lab Chip, 8: 1244–1254, 2008.
3. Teh, S.-Y., et al., Droplet microfluidics. Lab Chip, 8: 198–220, 2008.
4. Chiu, D.T., R.M. Lorenz, and G.D.M. Jeffries, Droplets for ultrasmall-volume analysis. Anal. Chem., 81: 5111–5118, 2009.
5. Chiu, D.T. and R.M. Lorenz, Chemistry and biology in femtoliter and picoliter volume droplets. Accounts Chem. Res., 42(5): 649–658, 2009.
6. Theberge, A.B., et al., Microdroplets in microfluidics: An evolving platform for discoveries in chemistry and biology. Angew. Chem. Int. Ed., 49(34): 5846–5868, 2010.
7. Yang, C.-G., Z.-R. Xu, and J.-H. Wang, Manipulation of droplets in microfluidic systems. Trends Anal. Chem., 29: 141–157, 2010.
8. Kintses, B., et al., Microfluidic droplets: New integrated workflows for biological experiments. Curr. Opin. Chem. Biol., 14: 548–555, 2010.
9. Casadevall i Solvas, X. and A.J. deMello, Droplet microfluidics: Recent developments and future applications. Chem. Commun., 47: 1936–1942, 2011.
10. Roach, L.S., H. Song, and R.F. Ismagilov, Controlling nonspecific protein adsorption in a plug-based microfluidic system by controlling interfacial chemistry using fluorous-phase surfactants. Anal. Chem., 77: 785–796, 2005.
11. Song, H. and R.F. Ismagilov, Millisecond kinetics on a microfluidic chip using nanoliters of reagents. J. Am. Chem. Soc., 125: 14613–14619, 2003.
12. Huebner, A., et al., Quantitative detection of protein expression in single cells using droplet microfluidics. Chem. Commun., 28: 1218–1220, 2007.
13. Aebersold, R. and M. Mann, Mass spectrometry-based proteomics. Nature, 422(6928): 198–207, 2003.
14. Thorsen, T., et al., Dynamic pattern formation in a vesicle-generating microfluidic device. Phys. Rev. Lett., 86: 4162–4166, 2001.
15. Garstecki, P., et al., Formation of droplets and bubbles in a microfludic T-junction-scaling and mechanism of break-up. Lab Chip, 6: 437–446, 2006.
16. Anna, S.L., N. Bontoux, and H.A. Stone, Formation of dispersions using “flow focusing” in microchannels. Appl. Phys. Lett., 82: 364–366, 2003.
17. Ward, T., et al., Microfluidic flow focusing: Drop size and scaling in pressure versus flow rate driven pumping. Electrophoresis, 26: 3716–3724, 2005.
18. Baroud, C.N., F. Gallaire, and R. Dangla, Dynamics of microfluidic droplets. Lab Chip, 10(16): 2032–2045, 2010.
19. Gu, H., M.H.G. Duits, and F. Mugele, Droplets formation and merging in two-phase flow microfluidics. Int. J. Mol. Sci., 12(4): 2572–2597, 2011.
20. Song, H., et al., Experimental test of scaling of mixing by chaotic advection in droplets moving through microfluidic channels. Appl. Phys. Lett., 83: 4664–4666, 2003.
21. Frenz, L., et al., Reliable microfluidic on-chip incubation of droplets in delay lines. Lab Chip, 9: 1344–1348, 2009.
22. Courtois, F., et al., An integrated device for monitoring time-dependent in vitro expression from single genes in picolitre droplets. ChemBioChem, 9: 439–446, 2008.
23. Courtois, F., et al., Controlling the retention of small molecules in emulsion microdrop-lets for use in cell-based assays. Anal. Chem., 81: 3008–3016, 2009.
24. Shi, W., et al., Droplet-based microfluidic system for individual Caenorhabditis elegans assay. Lab Chip, 8: 1432–1435, 2008.
25. Huebner, A., et al., Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip, 9: 692–698, 2009.
26. Schaerli, Y., et al., Continuous flow polymerase chain reaction of single copy DNA in microfluidic microdroplets. Anal. Chem., 81: 302–306, 2009.
27. He, M., et al., Selective encapsulation of single cells and subcellular organelles into picoliter- and femtoliter-volume droplets. Anal. Chem., 77: 1539–1544, 2005.
28. Lau, B.T.C., et al., A complete microfluidic screening platform for rational protein crystallization. J. Am. Chem. Soc., 129: 454–455, 2007.
29. Yobas, L., et al., High performance flow focusing geometry for spontaneous generation of monodispersed droplets. Lab Chip, 6: 1073–1079, 2006.
30. He, M., J.S. Kuo, and D.T. Chiu, Electro-generation of single femtoliter- and picoliter-volume aqueous droplets in microfluidic systems. Appl. Phys. Lett., 87: 031916, 2005.
31. Park, S.-Y., et al., High-speed droplet generation on demand driven by pulse laser-induced cavitation. Lab Chip, 11: 1010–1012, 2011.
32. Bransky, A., et al., A microfluidic droplet generator based on a piezoelectric actuator. Lab Chip, 9: 516–520, 2009.
33. Galas, J.C., D. Bartolo, and V. Studer, Active connectors for microfluidic drops on demand. New J. Phys., 11: 075027, 2009.
34. Zeng, S., et al., Microvalve-actuated precise control of individual droplets in microfluidic devices. Lab Chip, 9: 1340–1343, 2009.
35. Choi, J.-H., et al., Designed pneumatic valve actuators for controlled droplet breakup and generation. Lab Chip, 10: 456–461, 2010.
36. Abate, A.R., et al., Valve-based flow focusing for drop formation. Appl. Phys. Lett., 94: 023503, 2009.
37. Song, H., J.D. Tice, and R.F. Ismagilov, A microfluidic system for controlling reaction networks in time. Angew. Chem. Int. Ed., 42: 768–772, 2003.
38. Zheng, B., L.S. Roach, and R.F. Ismagilov, Screening of protein crystallization conditions on a microfluidic chip using nanoliter size droplets. J. Am. Chem. Soc., 125: 11170–11171, 2003.
39. Huebner, A., et al., Development of quantitative cell-based enzyme assays in microdroplets. Anal. Chem., 80: 3890–3896, 2008.
40. Abate, A.R., et al., High throughput injection with microfluidics using picoinjectors. Proc. Natl. Acad. Sci. USA, 107: 19163–19166, 2010.
41. Rhee, M. and M.A. Burns, Drop mixing in a microchannel for lab on a chip platforms. Langmuir, 24: 590–601, 2008.
42. Bremond, N., A.R. Thiam, and J. Bibette, Decompressing emulsion droplets favors coalescence. Phys. Rev. Lett., 100: 024501, 2008.
43. Fidalgo, L.M., C. Abell, and W.T.S. Huck, Surface-induced droplet fusion in microfluidic devices. Lab Chip, 7: 984–986, 2007.
44. Niu, X., et al., Pillar-induced droplet merging in microfluidic circuits. Lab Chip, 8: 1837–1841, 2008.
45. Priest, C., S. Herminghaus, and R. Seemann, Controlled electrocoalescence in microfluidics: Targeting a single lamella. Appl. Phys. Lett., 89: 134101, 2006.
46. Link, D.R., et al., Electric control of droplets in microfluidic devices. Angew. Chem. Int. Ed., 45: 2556–2560, 2006.
47. Zagnoni, M. and J.M. Cooper, On-chip electrocoalescence of microdroplets as a function of voltage, frequency and droplet size. Lab Chip, 9: 2652–2658, 2009.
48. Niu, X., et al., Electro-coalescence of digitally controlled droplets. Anal. Chem., 81: 7321–7325, 2009.
49. Baroud, C.N., M.R. de Saint Vincent, and J.P. Delville, An optical toolbox for total control of droplet microfluidics. Lab Chip, 7: 1029–1033, 2007.
50. Thiam, A.R., N. Bremond, and J. Bibette, Breaking of an emulsion under an ac electric field. Phys. Rev. Lett., 102: 188304, 2009.
51. Frenz, L., et al., Microfluidic production of droplet pairs. Langmuir, 24: 12073–12076, 2008.
52. Jambovane, S., et al., Creation of stepwise concentration gradient in picoliter droplets for parallel reactions of matrix metalloproteinase II and IX. Anal. Chem., 83: 3358–3364, 2011.
53. Agresti, J.J., et al., Ultrahigh throughtput screening in drop based microfluidics for directed evolution. Proc. Natl. Acad. Sci. USA, 107: 4004–4009, 2010.
54. Brouzes, E., et al., Droplet microfluidic technology for single-cell high throughput screening. Proc. Natl. Acad. Sci. USA, 106: 14195–14200, 2009.
55. Slaney, T.R., et al., Push-pull perfusion sampling with segmented flow for high temporal and spatial resolution in vivo chemical monitoring. Anal. Chem., 83: 5207–5213, 2011.
56. Schmitz, C.H.J., et al., Dropspots: A picoliter array in a microfluidic device. Lab Chip, 9: 44–49, 2009.
57. Mazutis, L., et al., Multi-step microfluidic droplet processing: Kinetic analysis of an in vitro translated enzyme. Lab Chip, 9: 2902–2908, 2009.
58. Clausell-Tormos, J., et al., Droplet based microfluidic platforms for the encapsulation and screening of mammalian cells and multicellular organisms. Chem. Bio., 15: 427–437, 2008.
59. Damean, N., et al., Simultaneous measurements of reactions in microdroplets filled by concentration gradients. Lab Chip, 9: 1707–1713, 2009.
60. Bui, M.P.N., et al., Enzyme kinetic measurements using a droplet based microfluidic system with a concentration gradient. Anal. Chem., 83: 1603–1608, 2011.
61. Beer, N.R., et al., On chip, real time, single copy polymerase chain reaction in picoliter droplets. Anal. Chem., 79: 8471–8475, 2007.
62. Beer, N.R., et al., On chip single copy real time reverse transcription PCR in isolated picoliter droplets. Anal. Chem., 80: 1854–1858, 2008.
63. Srisa-Art, M., et al., Monitoring of real time streptavidin biotin binding kinetics using droplet microfluidics. Anal. Chem., 80: 7063–7067, 2008.
64. Marz, A., et al., Droplet formation via flow through microdevices in Raman and surface enhanced Raman spectroscopy: Concepts and applications. Lab Chip, 11: 3584–3592, 2011.
65. Cristobal, G., et al., On line laser Raman spectroscopic probing of droplets engineered in microfluidic devices. Lab Chip, 6: 1140–1146, 2006.
66. Strehle, K.R., et al., A reproducible surface enhanced Raman spectroscopy approach. Online SERS measurements in a segmented microfluidic system. Anal. Chem., 79: 1542–1547, 2007.
67. Liu, S., et al., The electrochemical detection of droplets in microfluidic devices. Lab Chip, 8: 1937–1942, 2008.
68. Han, Z., et al., Measuring rapid enzymatic kinetics by electrochemical method in droplet based microfluidic devices with pneumatic valves. Anal. Chem., 81: 5840–5845, 2009.
69. Kautz, R.A., W.K. Goetzinger, and B.L. Karger, High throughput microcoil NMR of compound libraries using zero-dispersion segmented flow analysis. J. Comb. Chem., 7: 14–20, 2005.
70. Liu, T., et al., Accurate mass measurements in proteomics. Chem. Rev., 107(8): 3621–3653, 2007.
71. Hatakeyama, T., D.L. Chen, and R.F. Ismagilov, Microgram-scale testing of reaction conditions in solution using nanoliter plugs in microfluidics with detection by MALDI-MS. J. Am. Chem. Soc., 128: 2518–2519, 2006.
72. Pei, J., et al., Analysis of samples stored as individual plugs in a capillary by electro-spray ionization mass spectrometry. Anal. Chem., 81: 6558–6561, 2009.
73. Li, Q., et al., Fraction collection from capillary liquid chromatography and off-line electrospray ionization mass spectrometry using oil segmented flow. Anal. Chem., 82: 5260–5267, 2010.
74. Edgar, J.S., et al., Capillary electrophoresis separation in the presence of an immiscible boundary for droplet analysis. Anal. Chem., 78(19): 6948–6954, 2006.
75. Niu, X.Z., et al., Droplet based compartmentalization of chemically separated components in two dimensional separations. Chem. Commun., (41): 6159–6161, 2009.
76. Roman, G.T., et al., Sampling and electrophoretic analysis of segmented flow streams using virtual walls in a microfluidic device. Anal. Chem., 80: 8231–8238, 2008.
77. Wang, M., et al., Microfluidic chip for high efficiency electrophoretic analysis of segmented flow from a microdialysis probe and in vivo chemical monitoring. Anal. Chem., 81: 9072–9078, 2009.
78. Pei, J., J. Nie, and R.T. Kennedy, Parallel electrophoretic analysis of segmented samples on chip for high-throughput determination of enzyme activities. Anal. Chem., 82: 9261–9267, 2010.
79. Zhu, Y. and Q. Fang, Integrated droplet analysis system with electrospray ionization-mass spectrometry using a hydrophilic tongue-based droplet extraction interface. Anal. Chem., 82: 8361–8366, 2010.
80. Filla, L.A., D.C. Kirkpatrick, and R.S. Martin, Use of a corona discharge to selectively pattern a hydrophilic/hydrophobic interface for integrating segmented flow with microchip electrophoresis and electrochemical detection. Anal. Chem., 83: 5996–6003, 2011.
81. Fidalgo, L.M., et al., From microdroplets to microfluidics: Selective emulsion separation in microfluidic devices. Angew. Chem. Int. Ed., 47: 2042–2045, 2008.
82. Fidalgo, L.M., et al., Coupling microdroplet microreactors with mass spectrometry: Reading the contents of single droplets online. Angew. Chem. Int. Ed., 48(20): 3665–3668, 2009.
83. Zeng, S., et al., Electric control of individual droplet breaking and droplet contents extraction. Anal. Chem., 83: 2083–2089, 2011.
84. Kelly, R.T., et al., Dilution-free analysis from picoliter droplets by nano-electrospray ionization mass spectrometry. Angew. Chem. Int. Ed., 48(37): 6832–6835, 2009.
85. Swanson, S.K. and M.P. Washburn, The continuing evolution of shotgun proteomics. Drug Discov. Today, 10(10): 719–725, 2005.
86. Zhou, H., et al., Advancements in top-down proteomics. Anal. Chem., 84(2): 720–734, 2012.
87. Sun, X., et al., Ultrasensitive nanoelectrospray ionization-mass spectrometry using poly(dimethylsiloxane) microchips with monolithically integrated emitters. Analyst, 135: 2296–2302, 2010.
88. Marginean, I., et al., Achieving 50% ionization efficiency in subambient pressure ionization with nanoelectrospray. Anal. Chem., 82(22): 9344–9349, 2010.
89. Shen, Y., et al., Ultrasensitive proteomics using high-efficiency on-line micro-SPE-nanoLC-nanoESI MS and MS/MS. Anal. Chem., 76(1): 144–154, 2004.
90. Zhang, Z.R., et al., One-dimensional protein analysis of an HT29 human colon adeno-carcinoma cell. Anal. Chem., 72(2): 318–322, 2000.
