9
Microneedles in Improving Skin Appearance and Enhanced Delivery of Cosmeceuticals
Emma McAlister Maelíosa T.C. McCrudden and Ryan F. Donnelly
School of Pharmacy, Queen's University Belfast, Belfast, BT9 7BL, UK
9.1 Introduction
Since first postulated as a drug delivery mechanism in 1976, microneedle (MN) technology has garnered tremendous interest internationally. In the recent past, MNs have been exploited in the cosmeceutical industry. Cosmetic MN devices have been designed and developed with the distinct function of disrupting the barrier properties of the stratum corneum (SC), thus enabling skin rejuvenation and improved skin appearance via induced collagen synthesis and deposition. In addition, they are being used in combination with topical agents or light sources, and also as vehicles to deliver cosmeceutical molecules across the skin.
This chapter focuses on describing the evolution of skin microneedling technologies leading to the emergence and use of MN devices in this field. Commercially available MN devices used in cosmetic applications are described and areas of debate surrounding the safety profiles of MNs, including patient and healthcare providers acceptability of the same, are addressed. Finally, ongoing developments and new advancements in the MN‐mediated delivery of cosmeceuticals are highlighted.
9.2 The Skin
Skin health is influenced by numerous factors such as lifestyle, environment (chronic sun exposure and ultraviolet (UV) radiation), genetics, hormones and nutrition [1]. Aging of the skin is a multifactorial biological phenomenon. For example, extrinsic aging, so called “photoaging,” results from the cumulative effects of chronic exposure to the elements, primarily UV radiation, and intrinsic aging involves the degradation of elastin fibres and marked collagen reduction causing the development of wrinkles [2]. Facial scarring, arising for any number of reasons such as depigmentation, acne or burn related scars and the formation of large pores, is also a distressing phenomenon encountered by many people [3, 4]. In addition to all of these factors, innovative means of combating stretchmark development continues to undergo review [5]. One of the most common skin diseases that results in scaring is acne vulgaris, a potentially psychologically distressing condition, the pathogenesis of which is underpinned by active inflammation leading to damage to the elastic support structures beneath the skin surface [4, 6]. A recently published paper concisely details variability in acne scarring and documents different treatment approaches that can be used, with choice of treatment dependent on the type of acne scar presented [7]. Ablative methods such as laser resurfacing or dermabrasion are currently available for the treatment of scarred and aging skin but are invariably associated with significant post‐operative changes in the skin and require lengthy healing times. When considering this, it was highlighted by Aust et al. [8] that there was a need for less invasive treatment options to combat these conditions. A thorough review in 2014 charts the wide range of skin resurfacing therapies currently available to clinicians, in addition to innovative therapies under development [9]. Microneedling and subsequently MN devices are examples of such innovative therapies.
9.3 Microneedling Technologies: An Evolutionary Step Towards MN Usage
The concept of skin microneedling technology in dermatology was pioneered in 1995 when Orentreich and Orentreich successfully reported the use of needles in the treatment of acne scars by a methodology termed subcutaneous incisionless surgery or subcision [10]. This approach involves pricking or puncturing the skin and then scarifying the dermis with the needle to build up connective tissue beneath the scars. This technique could not be used over large surface areas however, due to bleeding and unacceptable bruising. Building upon his work, Camirand and Doucet in 1997 used a tattoo pistol (without ink) to “…needle abrade…” scars [11]. Although this work could have been used on larger areas, this technique was slow and laborious [12]. Based on these principles, an innovator, Fernandes, designed a drum‐shaped device with multiple fine protruding needles, initiating the new technological approach of microneedling [13]. Also referred to as MN therapy, percutaneous collagen induction (PCI) [12, 13], collagen induction therapy, dry tattooing (no pigment) and intra‐dermabrasion, this innovative microneedling technology has shown promising results as a simple means of minimising skin imperfections such as acne scars, surgical scars, fine lines, wrinkles, stretch marks and cellulite, in addition to improving skin texture, firmness and hydration [14, 15].
The principle of this technique is to break collagen strands, which tether the scar to the base of the dermis, via minute controlled injuries, caused by multiple microscopic needles piercing the skin. This technique induces angiogenesis and collagenesis via induction of the natural post‐traumatic inflammatory cascade. The basis of microneedling is the controlled mechanical stimulation of the wound healing response (Figure 9.1), which is divided into three phases: (I) initiation/inflammatory, (II) proliferation and (III) remodelling. Briefly, in phase I, after MN penetration, platelets, neutrophils and macrophages recruited to the injury site stimulate the release of growth factors, which in turn initiate the release of cytokines [16]. Growth factors including epidermal growth factor (EGF), transforming growth factor‐alpha (TGF‐α), TGF‐beta (‐β) and platelet derived growth factor (PDGF), in turn, activate fibroblasts and fibroblast proliferation, which all facilitate the production and propagation of intercellular matrix proteins [12, 16]. At this stage, neutrophils are the dominant leucocytes but are gradually replaced by monocytes, the dominant leucocytes in phase II. In phase II, fibroblast proliferation dominates and monocytes, keratinocytes and fibroblasts continue to release growth factors [12]. As a result, angiogenesis occurs whereby a fibronectin matrix is formed and fibroblasts ultimately deposit collagen, along with other matrix proteins including elastin, proteoglycans and glycosaminoglycans (GAGs) at the wound site [16]. In phase III, the conversion of collagen III, through tissue remodelling and vascular maturation, into collagen I ensues, resulting in skin tightening [12]. The final remodelling phase of healing after microneedling can take up to several months.

Figure 9.1 Schematic representation of the mode of action of MN devices. Phase I is characterised by growth factor release, following the initial microneedling injury. Phase II is dominated by fibroblast proliferation. Keratinocytes stimulate growth of the epidermis and release growth factors to promote collagen production by the fibroblasts. New blood vessels are created and there is a surge of collagen/matrix deposition. In the final remodelling phase, phase III, collagen III is converted into collagen I and the skin becomes tighter. Blood supply is normalised and the skin also becomes smoother.
9.4 Benefits of Microneedling
One of the most important advantages of microneedling is in overcoming the use of traditional ablative methodologies. Ablative methods including subcision, chemical peels, collagen injections, cortisone‐like injections, cryosurgery, dermabrasion and laser resurfacing are, by their nature, extremely physically disruptive to the targeted epidermis and superficial dermis [17]. In ablative methodologies such as these, undesired postoperative changes in the skin can result in significantly lengthened healing times. The destruction, rather than disruption, of the epidermis initiates an inflammatory response that stimulates fibroblasts to produce thick branches of scar collagen [18]. As a result, the skin has been shown to become more sensitive to photodamage and one study has stated that a side‐effect of such treatment may be the development of dyschromias, the common skin disorder whereby there is a marked alteration in normal skin pigmentation, resulting in discolouration of the skin, hair and nails [19]. In contrast, microneedling (a non‐ablative method), with needles of appropriate heights, would negate the risks and negative side‐effects often seen with invasive ablative approaches. For example, in one study, microneedling was compared with an ablative methodology, carbon dioxide (CO2) fractional laser, for the treatment of striae distensae (stretchmarks, a form of atrophic dermal scarring with overlying epidermal atrophy) in the abdomen and lower limbs [20]. By definition, a CO2 fractional laser involves the emission of light energy, in the form of a beam of photons from CO2 gas. This fractional approach results in the lasering of columns of skin in a grid‐like manner, leaving the skin surrounding each column intact, thus aiding healing [21] (https://www.advdermatology.com/c02re‐‐co2‐fractional‐laser‐‐pages‐235.php (accessed 7 June 2017). In this study, the results supported the use of microneedling over a CO2 fractional laser, as 90% of microneedling treated patients showed improvement of striae, whereas only 50% of patients showed improvement of striae after treatment with a CO2 fractional laser [20]. The outcome of this study, therefore, demonstrates that ablative methods are not necessarily the best option for such skin treatments, both in terms of significant recovery time and end results.
The suitability of non‐ablative approaches for use on skin areas where laser treatments and chemical peels cannot be performed, for example, areas very close to the eyes, is another benefit of microneedling compared with the traditional ablative methods. Furthermore, in one comparative study, greater collagen deposition was compared between two non‐ablative methods, intense pulsed light and microneedling to combat scarring [14]. A study was conducted using 54 imprinting control region mice and four weeks after the last treatment, skin thickness measurements using calipers, microscopic examination, western blot analysis for type I collagen and enzyme‐linked immunosorbent assay for total collagen content were performed. Microneedling was determined in this study to be more efficacious than intense pulsed light treatment, based on the fact that increased collagen deposition was observed from the biopsied specimens, as well as increased expression level of type I collagen and total collagen content, thus leading to improved smoothing of scars [14]. Therefore, this study highlights the successful outcomes of microneedling in comparison with an alternative non‐ablative method.
9.5 Commercially Available MN Devices
9.5.1 Dermaroller®
The original concept design, developed by Fernandes, that is used for microneedling is known commercially as a Dermaroller®. Manufactured by Dermaroller® Deutschland GmbH and registered with the Food and Drug Administration (FDA) as a class I medical device, a Dermaroller® (also known as a skin roller or MN roller) is described as a “…simple, hand‐held device equipped with medical grade solid steel needles, projecting from a cylindrical roller…” (http://www.dermarollersystem.com/how‐the‐derma‐roller‐system‐works.html (accessed 26 April 2017) [22]. Typically, a Dermaroller® consists of 24 circular arrays of 8 needles each located on the roller (total 192 needles) [23] (Figure 9.2A). The roller device is applied directly across the skin, vertically, horizontally and diagonally (Figure 9.2B). Dermarollers® can be used as homecare treatment MN devices or as a treatment conducted by a trained professional. Needle lengths are dependent on the nature of the treatment being employed [24]. For example, for treating acne and other scars, needle lengths of 1500–2000 µm are routinely employed. When microneedling is used as a procedure to treat aging skin and wrinkles, needle lengths of 500–1000 µm are usually recommended [25].
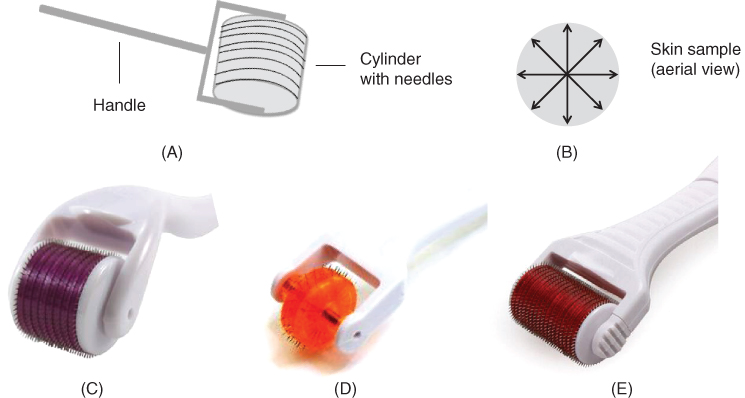
Figure 9.2 (A) Schematic representation of the Dermaroller® based upon the principles of Fernandes initial PCI innovation. It consists of 24 circular arrays of 8 needles each located on the roller (total 192 needles). (B) Schematic representation of an exemplar skin sample. The application of a roller MN device is applied across the skin, vertically, horizontally and diagonally, as depicted. (C, D and E) Commercially available Dermarollers®. (C) Royal Derma Roller with 540 needles. Reproduced with permission from [26] Royal Derma Roller (2015). Derma Rollers. https://www.royaldermaroller.com/collections/derma‐rollers/products/derma‐roller‐1‐0‐acne‐scars (accessed 25 May 2017). (D) Dermaroller® with narrow drum width, namely the DNS® Classic 3 Line Roller with 75 needles. Reproduced with permission from [27] DNS® (2006). DNS Classic 3 Line Roller. http://www.dnsroller.com/dns‐classic‐roller‐dermaroller‐micro‐needling‐derma‐system‐titanium‐biogenesis‐dns‐london/dns‐classic‐3‐line‐roller‐p10002#.WTgFf4WcHIU (accessed 25 May 2017). (E) Dermaroller® with large width of drum, namely Body Roller with 1080 needles.
Reproduced with permission from [28] MTS Roller.com (2012). Derma Rollers, MTS Derma Roller, Body Roller. https://www.mtsroller.com/1080‐titanium‐needles‐body‐roller (accessed 7 June 2017).
There are currently five types of Dermarollers® registered with the FDA, categorised according to their needle lengths. Home Dermarollers® consist of the C‐8 and the C‐8HE models. The cosmetic, C‐8 and C‐8HE models have needle lengths ranging between 130 and 200 µm, with a penetration diameter of 70 µm (http://www.dermarollersystem.com/how‐the‐derma‐roller‐system‐works.html) (accessed 26 April 2017). The C‐8HE was specifically designed for hair‐bearing surfaces, such as the scalp whereas the C‐8 model is described as the “…basic Dermaroller®…” [25]. The medical models, CIT‐8, MF‐8 and MS‐4, are intended for use by trained professionals only. The CIT‐8 has needle lengths of 500 µm and the MF‐8 has needle lengths up to 1500 µm [23]. The MS‐4 is the only Dermaroller® that has a small cylinder, 4 circular arrays of 24 needles (total 96 needles) that can have needle lengths up to 1500 µm. It is used on areas where better precision and deeper penetration is required, such as on facial acne scars [25].
As the therapeutic use of microneedling is now being extended beyond scar treatment, various modifications and advancements have evolved since the initial Dermaroller® was introduced. The current Dermaroller® market is ever‐increasing with an assortment of Dermaroller® devices based on various needle lengths and drum sizes being introduced onto the market from a multitude of different manufacturing companies. Some such companies include Hansderma, White Lotus, Royal Derma Roller, bioGenesis London and MTS Roller. Hansderma have developed Genosys® rollers that feature needles 25% thinner than other brands along with more needles per device (450 needles per unit) [29]. They also provide Dermarollers® with detachable heads to facilitate re‐use of the device handles. White Lotus have developed a hypoallergenic Dermaroller® (or Lotus Roller), which is metal‐free and made from a biocompatabile polymer and therefore suitable for use by end‐users who are allergic to metals [30]. Royal Derma Rollers manufacture Dermaroller® products that are made using titanium alloy needles [29] (Figure 9.2C) and bioGenesis London produce the DNS® Classic 3 (Figure 9.2D) and 8 Line Roller [31]. Lastly, MTS Roller manufacture a host of different Dermaroller® devices, namely the Dr. Roller, MT Roller, MRS Roller, Body Roller, Elimiscar Roller and the ZGTS Titanium Derma Roller [32]. These Dermarollers® differ according to handle and roller design. For example, the Body Roller, see Figure 9.2E, has a larger roller head (total 1080 titanium needles) to accommodate larger skin areas such as the chest, back, buttocks, thighs and arms [27].
Therefore, the microneedling field is growing constantly. The conventional Dermaroller® developed by Fernandes has evolved dramatically over the past 15 years through a variety of advancements. To this end, other MN devices will now be explored and described in more detail.
9.5.2 Beauty Mouse®
Listed as a medical device on the Australian Register of Therapeutic Goods (ARTG), the Beauty Mouse® is an approved MN device intended for home use, and again its usage is based on the same principles of the Dermaroller® technology (http://www.genuinedermarollerclinic.co.uk/beauty‐mouse.html (accessed 5 June 2017)). It contains a total of 480 needles of length approximately 200 µm on three separate Dermaroller® heads strategically placed inside a computer mouse‐shaped device (Figure 9.3A). Like the Body Roller, it has been developed to ensure coverage of larger skin surface areas, such as the arms, legs and buttocks, for the treatment of stomach or thigh stretch marks and cellulite [25, 33].

Figure 9.3 Examples of various MN devices now available for use. (A) Image of Beauty Mouse®. Reproduced with permission from [34] Dermaroller®. Beauty Mouse® Micro‐Needling Home Body. https://dermarollerus.com/color‐box‐product/891?width=600&height=600 (accessed 6 June 2017). (B) Image of White Lotus Dermastamp™. Reproduced with permission from [35] White Lotus Holistic Microneedling. Scar Dermastamp 1.0 mm. https://www.whitelotusantiaging.co.uk/scar‐dermastamp‐1‐0mm (accessed 6 June 2017). (C) eDermastamp®.
Reproduced with permission from [36] Consulting Room. eDermastamp™. http://www.consultingroom.com/Treatment/eDermastamp‐eDS‐Skin‐Rejuvenation (accessed 6 June 2017).
9.5.3 Dermastamp™
The evolution of microneedling technologies is highlighted by the emergence onto the UK market of miniature versions of the Dermaroller®, termed Dermastamps™. These are sterile medical MN devices with a range of different needle heights (200–3000 µm) (Figure 9.3B) [25]. They are specifically designed to accommodate small, localised, confined areas where it is difficult for the conventional Dermaroller® to achieve optimal stimulation, for example, the upper lip. The device uses “…vertical penetration to create infusion channels…” in the skin and is considered ideal for use on isolated scars and wrinkles [37]. Another area where this device is being considered is in the stimulation of hair growth but no research studies have yet been published on this topic and MN device. Very recently, a more advanced device called the eDermastamp® has been developed, which is manufactured by the same makers of Dermaroller®, Dermaroller® Deutschland GmbH (Figure 9.3C) [38] (http://www.original‐dermaroller.de/en/edermastamp.html (accessed 6 June 2017)). This device is an electronically powered stamping device consisting of six fine precision needles of maximum length 1500 µm, arranged in a circular design, the needles of which are made from medical grade stainless steel. Owing to the nature of this device, the manufacturers have recommended that it must only be used by trained practitioners and authorised clinics (http://www.original‐dermaroller.de/en/edermastamp.html) (accessed 6 June 2017).
9.5.4 Dermapen®
Designed to overcome the issues of varying pressure application by physicians/users and the subsequent needle depth penetration achieved, an MN device has been launched termed the Dermapen®. This ergonomic device is a spring loaded, oscillating MN device, consisting of two parts: an electric hand piece and a sterile, individual, disposable needle cartridge, which carries out the function of “…fractional mechanical resurfacing…” (http://dermapen.com/safe‐effective‐dermapen‐leads/ (accessed 26 April 2017), http://www.dermapenworld.com/dp‐family/dermapen‐3?tab=1 (accessed 26 April 2017)). It uses an electrically powered pen to deliver a vibrating, vertical, stamp‐like motion to the skin, creating a series of micro‐channels in it. Consisting of 12 stainless‐steel needles, the penetration depth of the needles can be adjusted from 250 to 2500 µm by simply turning the adjustment ring on the MN device (http://www.dermapenworld.com/dp‐family/dermapen‐3?tab=1) (accessed 26 April 2017). Treatment in acne scarring, burn scars and photoaging is being investigated by the manufacturers, although no available research studies focusing on this MN device have yet been published.
Owing to the substantial interest in these MN devices, more commercial products based on the same microneedling technology are currently being developed, of which four will be discussed. Within the Dermapen® family, AOVN™ technologies launched Dermapen 3™, the latest and most advanced model (Figure 9.4A) (http://www.dermapenworld.com/dp‐family/dermapen‐3?tab=1) (accessed 26 April 2017). Compared with the conventional Dermapen®, its key new feature is that it is able to produce over 1300 micro‐holes in the skin per second, therefore allowing shorter treatment times. The manufacturers deem it to be more robust, more user‐friendly and better suited for the rigors of clinical applications than previous models of the Dermapen®, with clinical reviews stating that it “…glides better because there is less rattling and vibration…” (http://www.dermapenworld.com/dp‐family/dermapen‐3?tab=1) (accessed 26 April 2017). There are currently two models of the Dermapen 3™ device available on the market, Dermapen 3MD™ and Dermapen 3PRO™. Not intended for home use, the Dermapen 3MD™ and Dermapen 3PRO™ are for clinical and professional use, respectively. Penetrating to depths up to 2500 µm, the Dermapen 3MD™ is suited for more aggressive skin remodelling treatments such as scars whereas the Dermapen 3PRO™ (penetration depth up to 1000 µm) is suitable for use in improving the appearance of fine lines, pigmentation and enlarged pores.
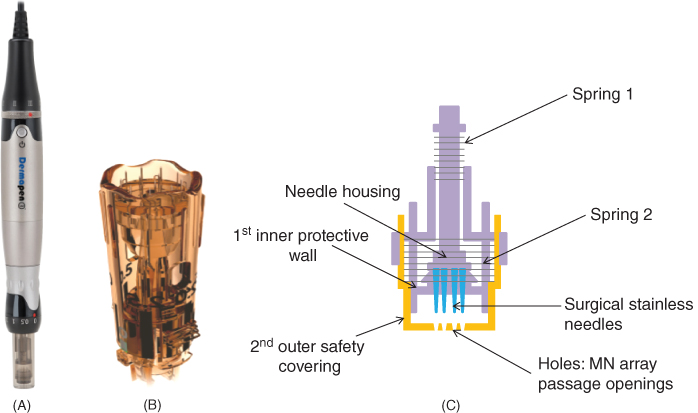
Figure 9.4 Commercially available Dermapens®. (A) Image of a Dermapen 3™.
Reproduced with permission from [39] Advanced Skin Innovations (2017). Dermapen™. http://www.equipmed.com/LiteratureRetrieve.aspx?ID=150517 (accessed 7 June 2017). (B) Image of a MDerma™ FDS disposable needle cartridge. Reproduced with permission from MDerma™ marketing PDF – The most advanced, effective and safest micro‐needling tip. https://www.mdermaaustralia.com.au/wp‐content/uploads/2015/12/MDermaFeatures.pdf (accessed 7 June 2017). (C) Schematic diagram of the patented disposable needle cartridge INNOTip™, highlighting the double‐protective, sealed tip and dual‐spring system.
A new and improved version of the conventional Dermapen® that uses the patented microneedling tip technology, SurSpace™, is the MDerma™ FDS (http://dermapen.com/mderma‐pen/ (accessed 27 April 2017)). Its patented 12 needle tip arrangement creates maximum pressure efficacy. Its disposable needle cartridge includes a scalloped edge and two opposing vents to eliminate suction and prevent device contamination (Figure 9.4B). The oscillating function of the treatment needle tip is also improved, using a new elastomeric spring with an updated motor for maximum power and performance. From the patented INNO™ Technology, another Dermapen®‐like MN device that has been combined with the patented disposable needle cartridge, termed the INNOTip™ (engineered by Clinical Resolution Lab, Inc.), is the INNOPen™ (https://www.uniqueskin.co.uk/products/innopen‐microneedling‐professional‐use‐ only (accessed 27 April 2017)). In contrast to competitor MN devices that use single‐walled open tip cartridges, the INNOTip™ provides a unique, double‐protective, sealed tip system, see Figure 9.4C, which consists of an inner protective wall and an outer safety covering made of sterile, medical grade polycarbonate resin (https://www.uniqueskin.co.uk/products/innopen‐microneedling‐professional‐use‐only and http://www.clinicalresolution.com/main/pdf/INNOTip.pdf (accessed 6 June 2017)). Consisting of 13 surgical stainless‐steel needles, the first inner protective wall stabilises the INNOTip™ at all speeds, which serves to prevent the needle housing from shaking and becoming off‐centred due to vibrations caused by the device's motor. Furthermore, it ensures safety via uniformity in the vertical lining of the needles therefore preventing unnecessary needle trauma and injury from slanted needle insertion. The second outer safety covering provides complete enclosure of the system with only the 13 passage openings. As a result, all 13 needles are required to pass through these tiny holes before perforating the skin. This outer safety covering detects any deformity and/or misalignment of the needles therefore ensuring accurate needle penetration and patient safety (https://www.uniqueskin.co.uk/products/innopen‐microneedling‐professional‐use‐only and http://www.clinicalresolution.com/main/pdf/INNOTip.pdf) (accessed 6 June 2017). The dual‐spring system is another unique feature of the INNOTip™ that allows the user to have full control over the needle motion. The INNOPen™ is available in two models, INNOPen MD™ and INNOPen PRO™. The INNOPen MD is for medical professionals whereas the INNOPen PRO™ is for clinical use only. Like the Dermapen 3™ models, the INNOPen MD™ penetrates up to 2500 µm and therefore is used for deep wrinkles and atrophic scars. Similarly, the INNOPen PRO™ has a maximum needle length of 1000 µm, so is suitable for treating skin conditions such as anti‐aging and hyperpigmentation.
As the microneedling market continues to grow and develop, new innovations will emerge which will expand upon the unique engineering features of the aforementioned MN devices. In line with this, the combinatorial use of MN devices with other novel cosmeceutical treatments will undoubtedly be explored. On this theme, MN devices have undergone exploration in the field of photodynamic therapy (PDT) for enhanced topical treatments.
9.5.5 Light Emitting MN Devices
Microneedling has previously been used in combination with PDT to enhance topical delivery of aminolevulinic acid (ALA), in the treatment of actinic keratosis, the dry scaly patches of skin caused by long term sun exposure [40]. In this study, skin was pre‐treated with an MN device, Roll‐CIT™, (MN width = 108 µm, MN length = 300 µm) and ALA was then applied to the skin for a defined period. Following this, the use of red light and broadband pulsed light allowed for deeper activation of ALA, resulting in statistically significant improvements in photoaging scores [40]. For a more detailed review of the use of MNs in conjunction with PDT, please refer to Kearney et al. [41] and Chapter 8 of the present book. Other research teams have also developed microscale optical diffusers, or fibre optic MNs, for the enhancement of clinical laser procedures and homogeneous light emission, while minimising photothermal damage in non‐target tissues [42]. In one such study, results indicated that MNs with needles of smaller diameter (base width = 33–48 µm) were favourable, in terms of their light delivery abilities [42]. The authors agreed, however, that further research is required to mechanically strengthen these MNs due to the risk of damage/breakage whilst being inserted into the skin. Evolving from these innovations, new CE approved MN devices, termed light emitting diode (LED) MicroNeedling Rollers (MN length of 1000 µm) have been launched. These incorporate titanium needles and LED light to combat wrinkles and scarring and are used in a fashion akin to that described for the Dermaroller® device (http://www.dhgate.com/product/new‐led‐540‐needle‐roller‐acne‐scars‐cellulite/133208643.html#sb1‐8‐1b;hot|4293359023 (accessed 30 April 2017)). Worth noting, however, is that despite the fact that these MN devices are readily available for purchase online, there is very limited technical information available regarding their development and use.
9.6 Patient Factors Relating to MN Devices
Despite the promising therapeutic benefits of MN devices and their increasing availability and usage, issues surrounding patient acceptability of such devices, in addition to queries surrounding potential erythema, irritation and patient safety in usage and sterilisation considerations have been raised [43–45].
9.6.1 Acceptability of MN Devices by Patients and Healthcare Providers
The future commercial and clinical success of MN devices will undoubtedly depend not only upon their ability to fulfil a designated function, but also on their acceptability by both patient and healthcare professionals [46]. To this end, initial user perspectives of MNs were documented in an informative study carried out by the Birchall group [47] and this data has been built upon by recent pilot studies conducted by the Donnelly research team [48–50]. With reference to one of those studies, in all instances, study participants acknowledged the potential benefits of MN delivery systems with 80% of the participants having a “strongly positive” perception of the MNs [48]. Potentially detrimental to the impact of such studies however are reports, such as that published in the Daily Mail newspaper in 2009, which stated that the Dermaroller®, specifically, resembled a “…miniature medieval instrument of torture…” [43]. Despite this poor description of the MN device, the article concluded that the use of the Dermaroller® resulted in signs of improved skin appearance [43]. Media reports, such as this, reinforce the importance of clear message dissemination to the general public about these MN devices and their uses so that their reputation may not be harmed unnecessarily.
9.6.2 Potential Irritation and Erythema
With reference to the potential for local irritation or erythema of the skin following the use of an MN device (a fundamental concern based on the potent immune‐stimulatory nature of the skin) [23], research has proven that prompt recovery of skin barrier function is achieved within a matter of hours of MN device usage [3, 51]. These studies also address the issue that the time frame for skin recovery is dependent on age, skin elasticity, skin application site and application pressure (Dermaroller®) [3, 51]. In relation to the reddening of the skin, one study proved that erythema normalised within 24–48 h in all groups under investigation with no significant difference observed in the erythema index ratios between the different needle lengths employed in the study [3].
9.6.3 Patient Safety
The potential long‐term effects of MN application, or indeed repeated MN application, on skin have not yet been fully evaluated with only one study having addressed this question. This study described the local and systemic effects of repeat applications of polymeric MNs on hairless Crl: SKH1‐Hrhr mice in vivo [52]. Two control groups of mice had no MNs inserted into their skin, while the remaining groups had one of two polymeric MN formulations (dissolving MNs or hydrogel‐forming (swelling) MNs) inserted into their skin repeatedly. Dissolving MNs were inserted once per week for five consecutive weeks whereas hydrogel‐forming MNs were inserted twice weekly for three successive weeks. Skin appearance and skin barrier function, as illustrated by measurement of transepidermal water loss (TEWL), were not measurably altered during the entire study period. Biomarkers of infection (C‐reactive protein (CRP)), immunity (immunoglobulin G (IgG)) and inflammation/irritation (tumour necrosis factor‐alpha (TNF‐α) and interleukin 1‐β (IL‐1β)) were also statistically unchanged, regardless of the MN formulation, needle density or number of applications of the MN [52]. Therefore, this preliminary study suggests that repeated use of the specific polymeric MN investigated does not cause undesirable local or systemic side‐effects in the animal model employed. It is clear that similar studies must be carried out for all MN delivery platforms under development to ensure safety profiles in advance before proceeding to MN usage with patients.
Moving beyond this, it is without question that inappropriate use of any of these novel technologies could cause problems. With specific reference to cosmetic MN devices, three cases of allergic granulomatous and systemic hypersensitivity in female patients, following application of a non‐sterile topical product and subsequent MN treatment, have been reported [44]. Worth noting however is that, in each case, medical supervision was absent. In all three cases, the deleterious side‐effects witnessed were determined to have been due to the inappropriate intradermal tattooing of the skin with antigenic topical products, rather than usage of the MN device alone [44]. The combined use of these topical products with MN application is unlicenced and so the artificially enhanced delivery of these products to the dermis in this report resulted in the hypersensitivity reactions observed. To explain this further, as the name suggests, these topical products were licenced for use topically rather than designed specifically to penetrate across the SC. In all instances, full or partial recovery of the skin was achieved following corticosteroid or tetracycline treatments [44]. These cases highlight however the need for caution with regards to inappropriate use of topical medicines in combination with enhanced transdermal delivery methods. As a result, MN application should only be used in conjunction with fully licenced and tested therapeutics or cosmetics, designed for this intended purpose. Furthermore, despite the fact that MN devices are often categorised into home and medical use in the product literature, they are widely available for purchase by any individual online from a multitude of commercial websites (https://www.amazon.co.uk/Derma‐Roller‐Anti‐Ageing‐Stretch‐Cellulite/dp/B00L63C468/ref=sr_1_7_a_it?ie=UTF8&qid=1496847802&sr=8‐7&keywords=derma+rollers (accessed 7 June 2017)). With no restriction on purchasing of these products, it is clearly evident that there is potential for abuse and misuse of these MN devices.
9.6.4 Sterilisation Considerations
The question of whether MN devices, for use in cosmetic applications, should be terminally sterilised has also been brought to the fore. This question must be addressed in line with the recommendations of regulatory bodies, as inappropriate use of MN devices could potentially lead to the early, unwarranted rejection of these devices. An example of one such damaging report, which involved the inappropriate use of non‐adequately sterilised MN devices between patients, was published in the Daily Mail in August 2011 [45]. Regardless of how MN devices are classified by the pharmaceutical and cosmeceutical industries, for example as drug delivery systems, consumer products or medical devices, they are not equivalent to conventional transdermal patches, in that they do not simply adhere to the skin surface [46]. Considered to be more akin to a conventional hypodermic injection, this may mean that they will be required to be terminally sterilised. The Dermaroller®, Beauty Mouse®, Dermastamp™ and Dermapen® are supplied as sterile products and as they are all fabricated from metal, they can be easily re‐sterilised. In particular, the Dermapen® explicitly highlights this issue by describing it as a “…patent pending MN tip…” (http://dermapen.com/safe‐effective‐dermapen‐leads/ (accessed 26 April 2017)) that is supplied sterilised in individual packages which are easily replaced and loaded into the spring automated device. It has been demonstrated in published work that the holes created in the skin by the individual needles on the MN, elicit minimal microbial penetration [2, 53, 54]. In order to ensure this is the case, particularly in a home environment, we, the authors, feel that appropriate sterilisation of such MN devices prior to and following use is essential. With this in mind, the UK Health Centre does provide guidance for at‐home sterilisation of Dermaroller® devices, whereby it is suggested that they be sterilised using those agents routinely used to sterilise babies' bottles (http://www.healthcentre.org.uk/cosmetic‐treatments/derma‐roller‐sterilising.html (accessed 9 May 2017)).
9.7 Delivery of Cosmeceutical Compounds
9.7.1 A Role for Hyaluronic Acid in MN Delivery Systems
Hyaluronic acid (HA), also termed hyaluronan, is a ubiquitous component of the extracellular matrix, similar to collagen and elastin, which maintains the suppleness of the skin. HA is a naturally occurring linear, polyanionic, polysaccharide with repeating disaccharide units composed of β‐glucuronic acid and N‐acetyl glucosamine [55], see Figure 9.5. HA is lost from the skin upon aging but has been deemed to play a pivotal role in tissue rejuvenation [56]. It has previously been shown, when applied topically, to be absorbed from the surface of the skin through the epidermis, thus restoring moisture and elasticity [57]. It was, at that time, suggested by the authors that HA could be a suitable candidate to act as a unique vehicle for the transport of other drugs into the deeper layers of the dermis. Prior to this, a HA‐based gel was implanted intradermally in augmentation therapy studies of facial soft tissues, once again highlighting the potential of this compound in skin rejuvenation [58]. Based on these previous studies and the unique, versatile properties of HA, it has been studied as a suitable candidate for MN formulation and subsequent delivery of incorporated active pharmaceutical ingredients (APIs) [56, 59, 60]. One such HA‐based MN product is MicroHyala®. Based on the dissolving MN design, as previously addressed in earlier studies [61], the salt form of HA, namely, sodium hyaluronate, acts as the base material for these MNs [56]. Despite abstract reference to the use of this product in cosmetic applications [56], there are, to date, no publications on the use of this, or similar HA‐based patches, in cosmetic applications specifically. The mechanical properties of MicroHyala®, incorporating model drugs, has been evaluated however and the authors have conceded that the product, although promising, does require further development [56]. With the emergence of HA as a novel cosmetic and MN scaffold material, it was agreed recently that a fuller understanding of its inherent biochemical functions and interactions is warranted, in order to ensure HA homeostasis [62]. Although it is evident that further research must be performed on HA‐based MN products, the viscoelastic properties of this compound, together with the excellent biocompatibility and non‐immunogenicity of HA, suggest it to be an ideal candidate for investigation in MN‐based cosmetic, medical and pharmaceutical products.

Figure 9.5 Schematic representation of the structure of hyaluronic acid (HA), highlighting the β‐glucuronic acid and N‐acetyl glucosamine repeating subunit pattern.
9.7.2 MN‐mediated Peptide Delivery
Protein‐based compounds do not readily permeate across the skin, due to their large molecular weights and hydrophilic nature [77]. The delivery of protein‐ and peptide‐based therapeutics across the skin continues to undergo extensive investigation, with current research focusing on the delivery of insulin and vaccines across the skin [24, 64, 65]. In relation to the cosmetic industry, the intradermal delivery of peptide cosmeceuticals via MNs is now undergoing investigation. Three such peptides are melanostatin, rigin and pal‐KTTKS. Melanostatin is a novel melanin synthesis inhibitor and rigin can reduce inflammation. Pal‐KTTKS is a peptide that, when delivered into the dermis, has been proposed to stimulate collagen production [66]. A recent study used confocal scanning laser microscopy and fluorescently tagged melanostatin, rigin and pal‐KTTKS to assess the influence of MN usage on the penetration and distribution of these peptides in the skin, compared with passive diffusion of the same peptides [66]. Pre‐treatment of the skin with solid, stainless‐steel MNs, followed by peptide application, resulted in increased penetration and distribution of the lowest molecular mass peptide, namely melanostatin. This trend was not maintained with the peptides of higher molecular masses however. The authors, in interpreting these findings, perhaps could have commented on the MN modality enlisted in this work. It would have been interesting to compare and contrast delivery of the same peptides using various MN approaches, such as dissolving and hydrogel‐forming MNs. These alternative approaches may, in time, prove more suitable for the delivery of protein‐based therapeutics and cosmeceuticals when compared with solid MNs.
9.7.3 The Delivery of Other Cosmeceutical Agents
The exploitation of MN technologies in facilitating efficient delivery of cosmeceuticals across the skin is constantly advancing with many interesting studies having been published in the recent past. A study carried out in mice detailed the in vivo efficacy of eflornithine cream, a topical product used to reduce facial hirsutism, following pre‐treatment of mouse skin with MNs [67]. The positive results achieved in this preliminary study promoted the continuation of work in this area. In this respect, MN devices have also been used in combination with topical products, such as minoxidil, to stimulate hair regrowth. Following on from pilot studies carried out in mice [68, 69], a 2013 study evaluated the influence of microneedling and minoxidil topical treatment, on human subjects suffering from androgenetic alopecia (AGA) [70]. A Dermaroller®, with needle heights of 1500 µm, was rolled over the shaven scalp in longitudinal, vertical and diagonal directions and minoxidil was applied 24 h post‐procedure. Using the three primary efficacy measures of hair count and patient/investigator assessment, the Dermaroller® and minoxidil treated group were determined to exhibit statistically superior hair growth when compared with those treated with minoxidil only [70].
With a view to improving skin appearance, an interesting study was conducted in vitro on the delivery of epigallocatechin‐3‐gallate (EGCG), a high molecular weight and lipid binding flavanol that has UV radiation protection, photoaging and collagen degradation prevention properties, using solid maltose MNs [71]. Permeation of EGCG from an aqueous solution, as well as a rheologically optimised hydrogel, through dermatomed porcine ear skin (untreated and MN treated) was evaluated. To quantitatively determine the amount of EGCG retained in the skin layers, after 24 h, the SC was separated from the underlying epidermis and dermis. MN treated skin showed significant enhancement in the delivery of EGCG to the viable epidermis and dermis from the aqueous solution (38.67±2.96 µg/cm2) as well as the hydrogel (24.60±2.62 µg/cm2) in comparison with the untreated skin (24.16±2.11 and 15.62±0.24 µg/cm2 for hydrogel and aqueous solution, respectively) [71]. To explain this, due to EGCG aqueous solubility, EGCG was able to diffuse through the hydrophilic micro‐channels, but at the same time, due to the lipophilicity rendered by the gallate group (in the molecule) and very high binding affinity to the skin tissues, particularly the collagen network, EGCG was found to have concentrated in the dermis. This is the ideal site of action for its antioxidant and photoprotective activities [71]. Therefore, this study provided evidence to indicate that solid maltose MNs facilitated the penetration of EGCG across the SC into the deeper skin layers.
In another study, skin depigmentation was investigated on the facial area of 45 women who had facial hyperpigmentation [72]. Subjects in this study were divided into two groups and dissolving HA‐MNs containing the depigmentation agent, 4‐n‐butylresorcinol, were applied to the faces of the participants every four days for eight weeks (Group 1) and every three days for eight weeks (Group 2). In both groups, a control MN patch (containing no 4‐n‐butylresorcinol) was applied to the other side of the face. For effective skin depigmentation, the skin depigmentation agent must be delivered to melanocytes, where melanin is synthesised. This study showed that this novel HA‐MN patch was twice as effective in delivering 4‐n‐butylresorcinol than the control patch in both the four‐ (Group 1) and three‐day (Group 2) interval tests, with no allergic reactions and only mild skin irritation observed throughout the study [72]. Comparing the four‐ and three‐day interval tests, the three‐day interval was more effective, therefore indicating that more frequent patch application would lead to a better skin depigmentation effect [72]. Thus, the authors concluded that this novel dissolving HA‐MN has the potential to be fabricated using other depigmentation and anti‐aging agents because of its usability, safety and efficacy.
Two complementary examples of successful MN‐mediated cosmeceutical delivery were published recently, evaluating the anti‐wrinkle effect of an ascorbic acid‐loaded dissolving HA‐MN patch [73, 74]. In one of the studies, two anti‐wrinkle compounds of different hydrophilicities, namely ascorbic acid and retinyl retinoate (Figure 9.6A and B, respectively) were incorporated into dissolving HA‐MNs, separately, for evaluation in combating wrinkles, on 24 women for 12 weeks [73]. Patients were randomly allocated to either the ascorbic acid or the retinyl retinoate group. All subjects applied dissolving HA‐MN patches to the “crow's feet” area of the face twice daily and these were left in place for 6 h. These HA‐MN patches were deemed to have efficiently delivered their payload as both MN formulations displayed improved skin appearance in terms of reduction of roughness and diminished wrinkle appearance. In addition, the MN demonstrated sufficient mechanical robustness for insertion into the skin at drug loadings of 60% for ascorbic acid and 35% for retinyl retinoate [73]. However, in this study, skin problems including irritation and sensitisation, which are critical issues in development of the MN patch for cosmetic purposes, were not fully assessed. Therefore, leading on from this study, a second study, from the same research group, was conducted, evaluating the anti‐wrinkle effect of the ascorbic acid‐loaded dissolving MN patch via a double‐blind, placebo‐controlled clinical study [74]. The anti‐wrinkle effect was similarly determined (as in the previous described study) using a Visiometer® [73] but additionally, skin irritation and sensitisation assessments were also performed using the modified Shelanski and Shelanski procedure [74]. There were no skin reactions, allergic contact dermatitis, irritant contact dermatitis or other side‐effects observed as a result of the treatment in any of the 51 test subjects [74]. Both studies serve as promising indications of proof‐of‐concept for the incorporation of other anti‐aging compounds of varying hydrophilicities into dissolving HA‐MNs. In addition, this work supports the possibility of using ascorbic acid‐loaded dissolving MN devices in future cosmetic treatments.
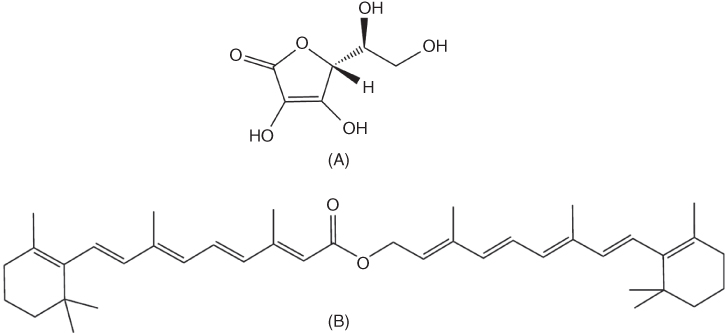
Figure 9.6 Schematic representation of the structures of (A) ascorbic acid, water‐soluble vitamin C and (B) retinyl retinoate, a practically water‐insoluble derivative of vitamin A.
9.8 Recent Developments
9.8.1 Human Stem Cells
The combinatorial effects of microneedling and human stem cell delivery as an innovative anti‐aging treatment, is a new and exciting area of research. This combinatorial approach gained momentum when it was demonstrated that endothelial precursor cells (EPCs), differentiated from human embryonic stem cells (hESCs), improved blood perfusion in damaged tissues as a result of the secretion of high levels of growth factors and cytokines [75]. Conditioned medium (CM) of hESC‐derived EPCs (which are comprised of several growth factors and cytokines), significantly enhanced the proliferation and migration of dermal fibroblasts and epidermal keratinocytes, as well as increasing collagen synthesis by fibroblasts [75]. In this respect, growth factors may promote the reduction of the signs of skin aging, with the beneficial role of growth factors in skin rejuvenation having been studied and discussed recently [76, 77]. With this in mind, a study conducted on 25 women investigated the effects of the secretory factors of hESC‐EPC CM (compared with saline, a control medium) in the treatment of wrinkles and depigmentation [78]. The left and right sides of the face of each participant were randomly assigned to treatment with microneedling plus saline (control) or microneedling plus hESC‐EPC CM. DTS Dermarollers® (MN length of 250 µm) were used to enhance the penetration of hESC‐EPC CM into the skin layers [78]. In terms of wrinkle and pigmentation improvement, treatment with microneedling plus hESC‐EPC CM, measured by a Visiometer® and a Mexameter®, respectively, resulted in a significantly greater decrease in maximum roughness, average roughness and melanin index (MI), two weeks after the final session. In terms of MI, a decrease in this value provides confirmation of skin lightening. Of note, no serious adverse events were encountered during this study protocol. This is the first, and only, in vivo experiment known to the authors that demonstrates the efficacy of microneedling plus hESC CM for improving the signs of skin aging with regards to reducing wrinkles and pigmentation. It signals an innovative move forwards in the use of stem cells and microneedling.
9.8.2 Fractional Radiofrequency
In 2009, non‐ablative fractional radiofrequency (RF) microneedling was introduced as a new approach in facilitating facial rejuvenation, and it is continuing to receive much recognition for its unique “…deep dermal heating with epidermal sparing…” feature [79, 80]. RF is non‐ionising electromagnetic radiation in the frequency range of 3 kHz to 300 GHz [81]. Fractional RF microneedling uses insulated MNs at a pre‐set depth to penetrate the skin and release radiofrequency currents only from the needle tips, producing thermal zones in the dermal structural components and accessory glands [25, 82]. The production of thermal energy at fixed spacing induces long‐term dermal remodelling, neoelastogenesis and neocollagenesis, allowing cells to promote healing, resulting in skin tightening [83]. Since RF microneedling allows the delivery of energy at a very specific point, tissue damage is minimised, thus sparing the epidermis and skin adnexal structures that contribute to rapid healing, such as growth factors and fibroblasts. Moreover, as fractional RF microneedling can control the depth and subsequently the induction of RF thermal zones, this allows for deeper penetration into dermal layers but lowers the risk of pain and adverse events such as blistering, burns and post‐inflammatory hyperpigmentation associated with epidermal injury [81]. With these advantages in mind, this technology has been very successfully used in the treatment of acne vulgaris and acne scars, with the treatment of hyperhidrosis also being considered [84, 85].
Expanding upon this, wrinkles as a result of photoaging are currently being explored using a similar approach. Specifically, a study carried out in 20 patients examined the in vivo efficacy of this system for the treatment of periorbital wrinkles (wrinkles around the eyes) [80]. The patients were treated three times at four‐week intervals with the fractional RF MN system and evaluated using a five‐point Wrinkle Assessment Scale. Digital imaging confirmed dramatic improvement of periorbital wrinkles, with only mild hyperpigmentation documented [80]. In an extension of this work, a more thorough study was then undertaken evaluating the in vivo efficacy of MN fractional RF, in comparison with the synergistic effects of the same technique used in combination with stem cell CM [81]. In a split‐face comparative study, one side of each of 15 subjects' faces were treated with fractional RF alone, and the other sides of the faces were treated with fractional RF, in addition to stem cell CM. Patients received three sessions of treatment at four‐week intervals. Skin roughness was measured using a Visiometer® and histologic evaluations were obtained via quantitative assessments of procollagen‐I using biopsy specimens. Among these measurements, the combined treatment using fractional RF microneedling and stem cell CM provided a significant improvement in skin roughness, dermal thickness and dermal collagen content [81]. It was also very promising to note that there were no serious adverse events documented in this study. Therefore, these two studies demonstrate the success of combining microneedling and RF energy.
Building upon these preliminary studies and highlighting a new dimension in microneedling technology, a 2‐in‐1 MN device, combining RF and microneedling, resembling the Dermapen® has been launched onto the market, named the INTRAcel™ (http://www.intraceluk.com/intracel‐fractional‐rf‐microneedling‐videos.htm (accessed 27 April 2017)). Each needle on this device is insulated, with only 300 µm of the apex emitting a high‐tensioned RF pulse onto the target area of the skin. Therefore, this innovative device provides a new treatment option in skin rejuvenation.
9.9 Conclusion
Research on the use of MN devices in the cosmeceutical industry has intensified in the recent past and continues to evolve and develop, with the emergence of superior materials, fabrication methods and designs. One area of MN research which is undergoing sustained development is the facilitated delivery of cosmeceutical agents. In this chapter, the integration and use of MNs in cosmetic procedures has been discussed. A wide range of MN devices have been described, including the Dermaroller®, Beauty Mouse®, Dermastamp™ and Dermapen®, with further commercial products currently in development. Additionally, the role of HA in MN delivery systems, the use of MN platforms in the delivery of cosmetic peptides and emerging fractional RF/MN combinatorial treatments were also discussed. As this field progresses towards further commercialisation of MN devices, however, it is clear from the safety and public perception studies outlined herein, that interaction and engagement with the relevant regulatory agencies is essential in order to address issues regarding MN sterile manufacture and to avoid misuse of these devices.
References
- 1 S.K. Schagen, V.A. Zampeli, E. Makrantonaki and C.C. Zouboulis (2012). Discovering the link between nutrition and skin aging. Dermato‐Endocrinology 4 (3): 298–307.
- 2 M.E.I. Domyati and W. Medhat (2013). Minimally invasive facial rejuvenation: current concepts and future expectations. Expert Review of Dermatology 8 (5): 565–580.
- 3 T.Y. Han, K.Y. Park, J.Y. Ahn, et al. (2012). Facial skin barrier function recovery after microneedle transdermal delivery treatment. Dermatologic Surgery 38 (11): 1816–1822.
- 4 I. Majid (2009). Microneedling therapy in atrophic facial scars: an objective assessment. Journal of Cutaneous and Aesthetic Surgery 2 (1): 26–30.
- 5 L. Liu, H. Ma and Y. Li (2014). Interventions for the treatment of stretch marks: a systematic review. Cutis 94 (2): 66–72.
- 6 A. Harvey and T.T. Huynh (2014). Inflammation and acne: putting the pieces together. Journal of Drugs in Dermatology 13 (4): 459–463.
- 7 M. Sanchez‐Viera (2015). Management of acne scars: fulfilling our duty of care for patients. British Journal of Dermatology 172 (suppl. 1): 47–51.
- 8 M.C. Aust, D. Fernandes, P. Kolokythas, et al. (2008). Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plastic and Reconstructive Surgery 121 (4): 1421–1429.
- 9 M.M. Loesch, A.K. Somani, M.M. Kingsley, et al. (2014). Skin resurfacing procedures: new and emerging options. Clinical, Cosmetic and Investigational Dermatology 7: 231–241.
- 10 D.S. Orentreich and N. Orentreich (1995). Subcutaneous incisionless (subcision) surgery for the correction of depressed scars and wrinkles. Dermatologic Surgery 21 (6): 543–549.
- 11 A. Camirand and J. Doucet (1997). Needle dermabrasion. Aesthetic Plastic Surgery 21 (1): 48–51.
- 12 D. Fernandes and M. Signorini (2008). Combating photoaging with percutaneous collagen induction. Clinics in Dermatology 26: 192–199.
- 13 D. Fernandes (2002). Percutaneous collagen induction: An alternative to laser resurfacing. Anaesthetic Surgery Journal 22 (3): 307–309.
- 14 S.E. Kim, J.H. Lee, H.B. Kwon, et al. (2011). Greater collagen deposition with the microneedle therapy system than with intense pulsed light. Dermatologic Surgery 37 (3): 336–341.
- 15 Dermaroller Planet (2009). What a dermaroller does. http//:www.dermaroller‐planet.com/new‐to‐dermarollers/what‐a‐dermaroller‐does (accessed 3 May 2017).
- 16 M.C. Aust, K. Reimers, H.M. Kaplan, et al. (2011). Percutaneous collagen induction ‐ regeneration in place of cicatrisation. Journal of Plastic, Reconstructive and Aesthetic Surgery 64 (1): 97–107.
- 17 K.M. Hassan and A.V. Benedetto (2013). Facial skin rejuvenation: ablative laser resurfacing, chemical peels, or photodynamic therapy? Facts and controversies. Clinics in Dermatology 31 (6): 737–740.
- 18 J.M. Rawlins (2006). Quantifying collagen type in mature burn scars: a novel approach using histology and digital image analysis. Journal of Burn Care and Research 27 (1): 60–65.
- 19 M.H. Gold and J.A. Biron (2012). Treatment of acne scars by fractional bipolar radiofrequency energy. Journal of Cosmetic and Laser Therapy 14 (4): 172–178.
- 20 M.H. Khater, F.M. Khattab and M.R. Abdelhaleem (2016). Treatment of striae distensae with needling therapy versus CO2 fractional laser. Journal of Cosmetic and Laser Therapy 18 (2): 75–79.
- 21 T. Omi and K. Numano (2014). The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Therapy 23 (1): 49–60.
- 22 Dermaroller® (2013). Dermaroller® Germany. https://dermarollerus.com/about‐dermaroller‐germany (accessed 28 May 2017).
- 23 M.M. Badran, J. Kuntsche and A. Fahr (2009). Skin penetration enhancement by a microneedle device (Dermaroller®) in vitro: Dependency on needle size and applied formulation. European Journal of Pharmaceutical Sciences 36 (4‐5): 511–523.
- 24 Y.C. Kim, J.H. Park and M.R. Prausnitz (2012). Microneedles for drug and vaccine delivery. Advanced Drug Delivery Reviews 64 (14): 1547–1568.
- 25 A. Singh and S. Yadav (2016). Microneedling: Advances and widening horizons. Indian Dermatology Online Journal 7 (4): 244–254.
- 26 Royal Derma Roller (2015). Derma Rollers. https://www.royaldermaroller.com/collections/derma‐rollers/products/derma‐roller‐1‐0‐acne‐scars (accessed 25 May 2017).
- 27 DNS® (2006). DNS Classic 3 Line Roller. http://www.dnsroller.com/dns‐classic‐roller‐dermaroller‐micro‐needling‐derma‐system‐titanium‐biogenesis‐dns‐london/dns‐classic‐3‐line‐roller‐p10002#.WTgFf4WcHIU (accessed 25 May 2017).
- 28 MTS Roller.com (2012). Derma Rollers, MTS Derma Roller, Body Roller. https://www.mtsroller.com/1080‐titanium‐needles‐body‐roller (accessed 7 June 2017).
- 29 Hansderma (2013). Genosys devices. http://www.hansderma.biz (accessed 27 April 2017).
- 30 White Lotus Holistic Microneedling (2016). Dermaroller. https://www.whitelotusantiaging.co.uk/dermaroller/ (accessed 27 April 2017).
- 31 DNS® (2006). DNS Classic Series. http://www.dnsroller.com/dns‐classic‐roller‐dermaroller‐micro‐needling‐derma‐system‐titanium‐biogenesis‐dns‐london#.WThDfoWcHIU (accessed 7 June 2017).
- 32 MTS Roller (2012). Derma Rollers, MTS Derma Roller. https://www.mtsroller.com/ (accessed 25 May 2017).
- 33 Dermastamp® (2010). Beauty Mouse. http://dermastamp.com.au/beauty‐mouse/ (accessed 26 April 2017).
- 34 Dermaroller® (2013). Beauty Mouse® Micro‐Needling Home Body. https://dermarollerus.com/color‐box‐product/891?width=600&height=600 (accessed 6 June 2017).
- 35 White Lotus Holistic Microneedling (2016). Scar Dermastamp 1.0 mm. https://www.whitelotusantiaging.co.uk/scar‐dermastamp‐1‐0mm (accessed 6 June 2017).
- 36 Consulting Room (2013). eDermastamp™. http://www.consultingroom.com/Treatment/eDermastamp‐eDS‐Skin‐Rejuvenation (accessed 6 June 2017).
- 37 Dermique (2014). Micro Dermastamp Treatment. http://www.dermique.com.my/micdermtreatment.php (accessed 5 June 2017).
- 38 Aestheticare® (2013). EDS®. http://www.aestheticare.co.uk/devices‐treatments/eds/ (accessed 5 June 2017).
- 39 Advanced Skin Innovations (2017). Dermapen™. http://www.equipmed.com/LiteratureRetrieve.aspx?ID=150517 (accessed 7 June 2017).
- 40 M.T. Clemmentoni, M.B. Roscher and G.S. Munavalli (2010). Photodynamic photorejuvenation of the face with a combination of microneedling, red light and broadband pulsed light. Lasers in Surgery and Medicine 42: 150–159.
- 41 M.C. Kearney, S. Brown, M.T.C. McCrudden, et al. (2014). Potential of microneedles in enhancing delivery of photosensitising agents for photodynamic therapy. Photodiagnosis and Photodynamic Therapy 11(4): 459–466.
- 42 M.A. Kosoglu, R.L. Hood, J.H. Rossmeisl, et al. (2011). Fiberoptic microneedles: novel optical diffusers for interstitial delivery of therapeutic light. Lasers in Surgery and Medicine 43 (9): 914–920.
- 43 Mail Online (2009). It costs £250 and looks like an instrument of medieval torture ‐ but can the Dermaroller make you look younger? http://www.dailymail.co.uk/femail/beauty/article‐1226193/Can‐Dermaroller‐make‐look‐younger.html (accessed 21 October 2014).
- 44 R. Soltani‐Arabshahi, J.W. Wong, K.L. Duffy and D.L. Powell (2014). Facial allergic granulomatous reaction and systemic hypersensitivity associated with microneedle therapy for skin rejuvenation. JAMA Dermatology 150 (1): 68–72.
- 45 Mail Online (2011). Chinese women warned over ‘potentially lethal’ microneedle roller after beauty treatment takes Hong Kong by storm. http://www.dailymail.co.uk/femail/article‐2026700/Microneedle‐Therapy‐System‐potentially‐lethal‐Chinese‐women‐warned.html (accessed 21 October 2014).
- 46 R.F. Donnelly and A.D. Woolfson (2014). Patient safety and beyond: What should we expect from microneedle arrays in the transdermal delivery arena? Therapeutic Delivery 5 (6): 653–662.
- 47 J.C. Birchall, R. Clemo, A. Anstey and D.N. John (2011). Microneedles in clinical practice‐‐an exploratory study into the opinions of healthcare professionals and the public. Pharmaceutical Research 28 (1): 95–106.
- 48 R.F. Donnelly, K. Moffatt, A.Z. Alkilani, et al. (2014). Hydrogel‐forming microneedle arrays can be effectively inserted in skin by self application: A pilot study centred on pharmacist intervention and a patient information leaflet. Pharmaceutical Research 31 (8): 1989–1999.
- 49 E.M. Vicente‐Pérez, H.L. Quinn, E. McAlister, et al. (2016). The use of a pressure‐indicating sensor film to provide feedback upon hydrogel‐forming microneedle array self‐application in vivo. Pharmaceutical Research 33 (12): 3072–3080.
- 50 A. Ripolin, J. Quinn, E. Larrañeta, et al. (2017). Successful application of large microneedle patches by human volunteers. International Journal of Pharmaceutics 521: 92–101.
- 51 H. Kalluri, C.S. Kolli and A.K. Banga (2011). Characterization of microchannels created by metal microneedles: formation and closure. AAPS Journal 13 (3): 473–481.
- 52 E.M. Vicente‐Pérez, E. Larrañeta, M.T.C. McCrudden, et al. (2017). Repeat application of microneedles does not alter skin appearance or barrier function and causes no measurable disturbance of serum biomarkers of infection, inflammation or immunity in mice in vivo. European Journal of Pharmaceutics and Biopharmaceutics 117: 400–407.
- 53 R.F. Donnelly, T.R.R. Singh, A.Z. Alkilani, et al. (2013). Hydrogel‐forming microneedle arrays exhibit antimicrobial properties: Potential for enhanced patient safety. International Journal of Pharmaceutics 451 (1‐2): 76–91.
- 54 R.F. Donnelly, T.R.R. Singh, M.M. Tunney, et al. (2009). Microneedle arrays allow lower microbial penetration than hypodermic needle in vitro. Pharmaceutical Research 26 (11): 2513–2522.
- 55 M. Farwick, P. Lersch and G. Strutz (2008). Low molecular weight hyaluronic acid: its effect on epidermal gene expression and skin ageing. SOFW Journal 134: 2–6.
- 56 Y. Hiraishi, T. Nakagawa, Y.S. Quan, et al. (2013). Performance and characteristics evaluation of a sodium hyaluronate‐based microneedle patch for a transcutaneous drug delivery system. International Journal of Pharmaceutics 441 (1‐2): 570–579.
- 57 T.J. Brown, D. Alcorn and J.R. Fraser (1999). Absorption of hyaluronan applied to the surface of intact skin. Journal of Investigative Dermatology 113 (5): 740–746.
- 58 F. Duranti, G. Salti, B. Bovani, et al. (1998). Injectable hyaluronic acid gel for soft tissue augmentation‐a clinical and histological study. Dermatologic Surgery 24 (12): 1317–1325.
- 59 S.G. Lee, J.H. Jeong, K.M. Lee, et al. (2014). Nanostructured lipid carrier‐loaded hyaluronic acid microneedles for controlled dermal delivery of a lipophilic molecule. International Journal of Nanomedicine 9: 289–299.
- 60 S. Liu, M.N. Jin, Y.S. Quan, et al. (2014). Transdermal delivery of relatively high molecular weight drugs using novel self‐dissolving microneedle arrays fabricated from hyaluronic acid and their characteristics and safety after application to the skin. European Journal of Pharmaceutics and Biopharmaceutics 86 (2): 267–276.
- 61 M.T.C. McCrudden, A.Z. Alkilani, C.M. McCrudden, et al. (2014). Design and physicochemical characterisation of novel dissolving polymeric microneedle arrays for transdermal delivery of high dose, low molecular weight drugs. Journal of Controlled Release 180: 71–80.
- 62 U. Anderegg, J.C. Simon and M. Averbeck (2014). More than just a filler ‐ the role of hyaluronan for skin homeostasis. Experimental Dermatology 23 (5): 295–303.
- 63 M.T.C. McCrudden, T.R.R. Singh, K. Migalska and R.F. Donnelly (2013). Strategies for enhanced peptide and protein delivery. Therapeutic Delivery 4 (5): 593–614.
- 64 M. Hultström, N. Roxhed and L. Nordquist (2014). Intradermal insulin delivery. Journal of Diabetes Science and Technology 8 (3): 453–457.
- 65 H. Suh, J. Shin and Y.C. Kim (2014). Microneedle patches for vaccine delivery. Clinical and Experimental Vaccine Research 3 (1): 42–49.
- 66 Y.H. Mohammed, M. Yammadda, L.L. Lin, et al. (2014). Microneedle enhanced delivery of cosmeceutically relevant peptides in human skin. PLoS One 9 (7): e101956.
- 67 A. Kumar, Y.W. Naguib, Y. Shi and Z. Cui (2016). A method to improve the efficacy of topical eflornithine hydrochloride cream. Drug Delivery 23 (5): 1495–1501.
- 68 K. Jeong, Y.J. Lee, J.E. Kim, et al. (2012). Repeated microneedle stimulation induce the enhanced expression of hair‐growth‐related genes. International Journal of Trichology 4: 117.
- 69 B.J. Kim, Y.Y. Lim, H.M. Kim, et al. (2012). Hair follicle regeneration in mice after wounding by microneedle roller. International Journal of Trichology 4: 117.
- 70 R. Dhurat, M.S. Sukesh, G. Avhad, et al. (2013). A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: A pilot study. International Journal of Trichology 5 (1): 6–11.
- 71 A. Puri, H.X. Nguyen and A.K. Banga (2016). Microneedle‐mediated intradermal delivery of epigallocatechin‐3‐gallate. International Journal of Cosmetic Science 38 (5): 512–523.
- 72 S. Kim, H. Yang, M. Kim, et al. (2016). 4‐N‐Butylresorcinol dissolving microneedle patch for skin depigmentation: a randomized, double‐blind, placebo‐controlled trial. Journal of Cosmetic Dermatology 15 (1): 16–23.
- 73 M. Kim, H. Yang, H. Kim, et al. (2014). Novel cosmetic patches for wrinkle improvement: retinyl retinoate‐ and ascorbic acid‐loaded dissolving microneedles. International Journal of Cosmetic Science 36 (3): 207–212.
- 74 C. Lee, H. Yang, S. Kim, et al. (2016). Evaluation of the anti‐wrinkle effect of an ascorbic acid‐loaded dissolving microneedle patch via a double‐blind, placebo‐controlled clinical study. International Journal of Cosmetic Science 38 (4): 375–381.
- 75 M.J. Lee, J. Kim, K.I. Lee, et al. (2011). Enhancement of wound healing by secretory factors of endothelial precursor cells derived from human embryonic stem cells. Cytotherapy 13 (2): 165–178.
- 76 W.S. Kim, B.S. Park, S.H. Park, et al. (2009). Antiwrinkle effect of adipose‐derived stem cell: activation of dermal fibroblast by secretory factors. Journal of Dermatologic Science 53 (2): 96–102.
- 77 B.S. Park, K.A. Jang, J.H. Sung, et al. (2008). Adipose‐derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatologic Surgery 34 (10): 1323–1326.
- 78 H.J. Lee, E.G. Lee, S. Kang, et al. (2014). Efficacy of microneedling plus human stem cell conditioned medium for skin rejuvenation: a randomized, controlled, blinded split‐face study. Annals of Dermatology 26 (5): 584–591.
- 79 B.M. Hantash, B. Renton, R.L. Berkowitz, et al. (2009). Pilot clinical study of a novel minimally invasive bipolar microneedle radiofrequency device. Lasers in Surgery and Medicine 41 (2): 87–95.
- 80 S.J. Lee, J. Kim, Y.J. Yang, et al. (2015). Treatment of periorbital wrinkles with a novel fractional radiofrequency microneedle system in dark‐skinned patients. Dermatologic Surgery 41 (5): 615–622.
- 81 K.Y. Seo, D.H. Kim, S.E. Lee, et al. (2013). Skin rejuvenation by microneedle fractional radiofrequency and a human stem cell conditioned medium in Asian skin: a randomized controlled investigator blinded split‐face study. Journal of Cosmetic and Laser Therapy 15 (1): 25–33.
- 82 B.J. Simmons, R.D. Griffith, L.A. Falto‐Aizpurua and K. Nouri (2014). Use of radiofrequency in cosmetic dermatology: focus on non‐ablative treatment of acne scars. Clinical, Cosmetic and Investigational Dermatology 7: 335–339.
- 83 G. Hruza, A.F. Taub, S.L. Collier and S.R. Mulholland (2009). Skin rejuvenation and wrinkle reduction using a fractional radiofrequency system. Journal of Drugs in Dermatology 8 (3): 259–265.
- 84 B.A. Chandrashekar, R. Sriram, R. Mysore, et al. (2014). Evaluation of microneedling fractional radiofrequency device for treatment for acne scars. Journal of Cutaneous and Aesthetic Surgery 7 (2): 93–97.
- 85 S.T. Kim, K.H. Lee, H.J. Sim, et al. (2014). Treatment of acne vulgaris with fractional radiofrequency microneedling. Journal of Dermatology 41 (7): 586–591.
