Preface
The worldwide transdermal patch market approached $32 billion in 2015, despite limited innovation in this area over the previous five years. Indeed, the entire market is still based on only 20 drugs. This rather limited number of drug substances is attributed to the excellent barrier function of the skin, which is accomplished almost entirely by the outermost 10–15 µm (in the dry state) of tissue, the stratum corneum (SC). Before being taken up by blood vessels in the upper dermis and prior to entering the systemic circulation, substances permeating the skin must cross the SC and the viable epidermis. There are three possible pathways leading to the capillary network: through hair follicles with associated sebaceous glands, via sweat ducts, or across continuous SC between these appendages. As the fractional appendageal area available for transport is only about 0.1%, this route usually contributes negligibly to apparent steady state drug flux. The intact SC thus provides the main barrier to exogenous substances, including drugs. The corneocytes of hydrated keratin are analogous to “bricks,” embedded in a “mortar” composed of highly organised, multiple lipid bilayers of ceramides, fatty acids, cholesterol and its esters. These bilayers form regions of semicrystalline gel and liquid crystal domains. Most molecules penetrate through skin via this intercellular micro‐route. Facilitation of drug penetration through the SC may involve by‐pass or reversible disruption of its elegant molecular architecture. The ideal properties of a molecule that can penetrate the intact SC well are:
- Molecular mass less than 600 Da.
- Adequate solubility in both oil and water so that the membrane concentration gradient, which is the driving force for passive drug diffusion along a concentration gradient, may be high.
- Partition coefficient, such that the drug can diffuse out of the vehicle, partition into, and move across, the SC, without becoming sequestered within it.
- Low melting point, correlating with good solubility, as predicted by ideal solubility theory.
Clearly, many drug molecules do not meet these criteria. This is especially true for biopharmaceutical drugs, which are becoming increasingly important in therapeutics and diagnostics of a wide range of illnesses. Drugs that suffer poor oral bioavailability or susceptibility to first‐pass metabolism, and are thus often ideal candidates for transdermal delivery, may fail to realise their clinical application because they do not meet one or more of these conditions. Examples include peptides, proteins and vaccines, which, due to their large molecular size and susceptibility to acid destruction in the stomach, cannot be given orally and, hence, must be dosed parenterally. Such agents are currently precluded from successful transdermal administration, not only by their large sizes, but also by their extreme hydrophilicities. Several approaches have been used to enhance the transport of drugs through the SC. However, in many cases, only moderate success has been achieved and each approach is associated with significant problems. Chemical penetration enhancers allow only a modest improvement in penetration. Chemical modification to increase lipophilicity is not always possible and, in any case, necessitates additional studies for regulatory approval, due to generation of new chemical entities. Significant enhancement in delivery of a large number of drugs has been reported using iontophoresis. However, specialised devices are required and the agents delivered tend to accumulate in the skin appendages. The method is presently best‐suited to acute applications, with several commercialised products intended for regular at‐home use by patients being withdrawn relatively quickly after market approval for a range of reasons. Electroporation and sonophoresis are known to increase transdermal delivery. However, they both cause pain and local skin reactions and sonophoresis can cause breakdown of the therapeutic entity. Techniques aimed at removing the SC barrier, such as tape‐stripping and suction/laser/thermal ablation are impractical, while needle‐free injections have so far failed to replace conventional needle‐based insulin delivery. Clearly, a robust alternative strategy is required to enhance drug transport across the SC and thus widen the range of drug substances amenable to transdermal delivery.
Microneedle arrays are minimally invasive devices that can be used to by‐pass the SC barrier and thus achieve transdermal drug delivery. Microneedles (MN) (50–900 µm in height, up to 2000 MN/cm2) in various geometries and materials (silicon, metal, polymer) have been produced using recently developed microfabrication techniques. Silicon MN arrays are prepared by modification of the dry‐ or wet‐etching processes employed in microchip manufacture. Metal MN are produced by electrodeposition in defined polymeric moulds or photochemical etching of needle shapes into a flat metal sheet and then bending these down at right angles to the sheet. Polymeric MN have been manufactured by micromoulding of molten/dissolved polymers. MN are applied to the skin surface and pierce the epidermis (devoid of nociceptors), creating microscopic holes through which drugs diffuse to the dermal microcirculation. MN are long enough to penetrate to the dermis but are short and narrow enough to avoid stimulation of dermal nerves. Solid MN puncture the skin prior to application of a drug‐loaded patch or are pre‐coated with drug prior to insertion. Hollow bore microneedles allow diffusion or pressure‐driven flow of drugs through a central lumen, while polymeric drug‐containing microneedles release their payload as they biodegrade in the viable skin layers. In vivo studies using solid MN have demonstrated delivery of oligonucleotides, desmopressin and human growth hormone, reduction of blood glucose levels from insulin delivery, increase of skin transfection with DNA and enhanced elicitation of immune response from delivery of DNA and protein antigens. Hollow MN have also been shown to deliver insulin and reduce blood glucose levels. MN arrays do not cause pain on application and no reports of development of skin infection currently exist.
MN have been considered for a range of other applications, in addition to transdermal and intradermal drug/vaccine delivery. These include minimally invasive therapeutic drug monitoring, as a stimulus for collagen remodelling in anti‐ageing strategies and for delivery of active cosmaceutical ingredients. MN technology is likely to find ever‐increasing utility in the healthcare field as further advancements are made. However, some significant barriers will need to be overcome before we see the first MN‐based drug delivery or monitoring device on the market. Regulators, for example, will need to be convinced that MN puncture of skin does not lead to skin infections or any long‐term skin problems. MN will also need to be capable of economic mass production.
In this book, we review the work that has been carried out on MN to date in both the academic and industrial sectors. We have looked in detail at both in vitro and in vivo studies and covered the important area of MN‐based vaccines. We also consider safety and public perception aspects of MN and discuss newer applications of this exciting technology, such as delivery of gene therapies, photodynamic therapy, ocular delivery and enhanced administration of nanomedicines.
The MN field continues to expand, with ever‐increasing numbers of publications that are increasingly being cited (Figure 1). However, the number of commercialised products on the market remains disappointing. Indeed, no true MN array drug delivery system is currently available to patients. Research work is largely confined to universities and specialised drug delivery companies, many of which have been spun‐out from universities. It is hoped that the exciting data generated and published will encourage large pharmaceutical and medical device forms to make the considerable financial investment necessary for scaled‐up manufacture and comprehensive clinical trials over the coming years so that MN technology will finally deliver the impact envisioned by research scientists.
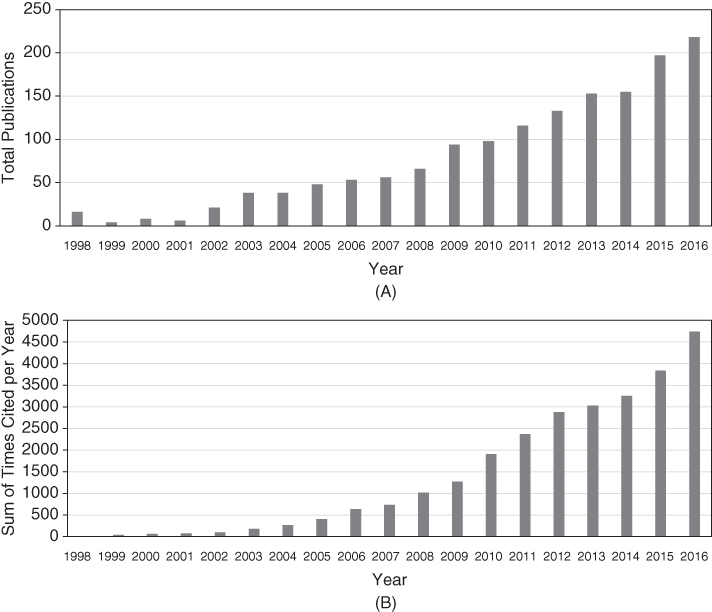
Figure 1 (A) Total number of journal articles published on microneedles, by year, since the first publication in 1998 and (B) total number of citations of microneedles articles, by year, since 1998.
We took a very different approach to the production of this book, as compared with how we wrote the first text on microneedles published by Wiley in 2012. The first book was written by four authors, with Desmond Morrow and David Woolfson coming on board with Raj and myself. Given the expansion of the field, Desy's move to New Zealand and David's well‐deserved retirement, I asked Eneko Larrañeta and Maelíosa McCrudden to join Raj and I as Editors of this new book. Instead of writing the entire book ourselves, we asked members of my research Group and close collaborators to each co‐author a chapter in an area of their specialisation. This approach worked extremely well, with our hectic schedules of research over the past year only slightly delaying delivery of the final text.
Editing this text took quite a considerable amount of time and I would like to thank my wife Johanne for her patience and support throughout the project. I am also grateful to Raj, Eneko and Maelíosa for agreeing to assist me in the editing of the book and for their work in co‐authoring several chapters. This is now the fourth book I have worked on with Raj and it has been every bit as enjoyable collaborating with him again this time. This has been a new experience for Eneko and Maelíosa and I hope that this is the first of many books for them.
I am highly appreciative of the past and present members of the Microneedles Group at Queen's for their hard work and imagination in the lab. Special mention must go to the current Group members: Maelíosa, Aaron Courtenay, Ismaiel Tekko, Lalit Vora, Patricia Gonzalez‐Vazquez, Helen Quinn, Mary‐Carmel Kearney, Aoife Rodgers, Bridie Dutton, Sara Cordeiro, Emma McAlister, Eman Migdadi, Rehan Al‐Kasasbeh, Iman Hamdan, Kurtis Moffatt, Dian Permana, Fabiana Volpe‐Zanutto, Boonnada Pamornpathomkul, Michelle Barreto‐Requena, Yadira Pastor‐Garcia, Ke Peng, Delly Ramadon, Sarah Stewart, Inken Ramöller, Heba Abdelazim and Alvaro Carcamo‐Martinez. I would also like to acknowledge BBSRC, EPSRC, MRC, The Wellcome Trust, PATH, USAID, Invest Northern Ireland, Action Medical Research, Prostate Cancer UK, Arthritis Research UK and The Royal Society for funding my work in this area. I have been fortunate to have many excellent industrial collaborators who continue to support the translation of my research towards commercialisation and patient benefit, but confidentiality prevents direct acknowledgement. You know who you are – thank you! Emma Strickland and Elsie Merlin from Wiley provided considerable help and encouragement as we completed this project and their support and guidance are greatly appreciated. Judith Egan‐Shuttler is also thanked for her work as the copyeditor. It is my hope that this book will serve as a comprehensive overview of the field and, hence, that it will be of use to those new to microneedles, as well as people already engaged in work in this area in both industry and academia.
Belfast, October 2017
Ryan F. Donnelly
