Processing of lignocellulosic fiber-reinforced biodegradable composites
Saurabh Chaitanya1, Amrinder P. Singh2 and Inderdeep Singh1, 1Indian Institute of Technology Roorkee, Roorkee, Uttarakhand, India, 2University Institute of Engineering and Technology, Punjab University, Chandigarh, India
Abstract
Lignocellulosic fiber-reinforced biodegradable composites (LFBC) offer several distinct advantages of having high specific strength, biodegradability, recyclability, etc. compared to conventionally used glass fiber-reinforced polymer composites. However, despite several advantages, commercial applicability of LFBC is still a challenge to overcome. For commercial applicability of LFBC, manufacturing processes capable of rapid processing, precision, and repeatability are required. Hence, LFBC can be processed using techniques like injection and compression molding. The manufacturing process selected for development of LFBC should be able to effectively disperse and orient the lignocellulosic fibers within the product. This chapter provides an insight into the processing of LFBC, for the industrial and research fraternity working in the area of LFBC. Also, several preprocessing techniques like fiber surface modification and precompounding (using extrusion, pultrusion, melt blender, and roll mill) prior to processing of LFBC have been discussed.
Keywords
Lignocellulosic fibers; biocomposites; injection molding; compression molding; extrusion; pultrusion; melt blending; fiber treatment
9.1 Introduction
During the last few decades, plastics have replaced several traditional materials used in various engineering applications, owing to advantages such as high strength to weight ratio, low cost, ease of processing, and better productivity. Conventionally used polymers majorly comprise of petroleum-derived epoxy or synthetic polymers like polypropylene (PP), high density poly-ethylene (HDPE), high impact polystyrene (HIPS), nylon, etc. For use in structural or engineering applications, the properties of these polymers are modified using some reinforcing filler material to enhance their performance. Conventionally, high strength synthetic fibers, such as glass, carbon, and aramid, were used to develop reinforced polymer composites. These offer high specific properties for use in sports, building-construction, aerospace, automobiles etc. However, petroleum-derived polymers as well as synthetic fibers, being nondegradable and nonrenewable resources, act as a serious environmental hazard (Verma et al., 2013). Increased environmental awareness and strict environmental regulations worldwide have forced the nations to reduce their carbon footprint. In order to achieve this, the replacement of synthetic polymers and synthetic fibers with biodegradable polymers (biopolymers) and biodegradable fibers, respectively, is being explored as one of the possible alternative (Mohanty et al., 2000; Petinakis et al., 2013).
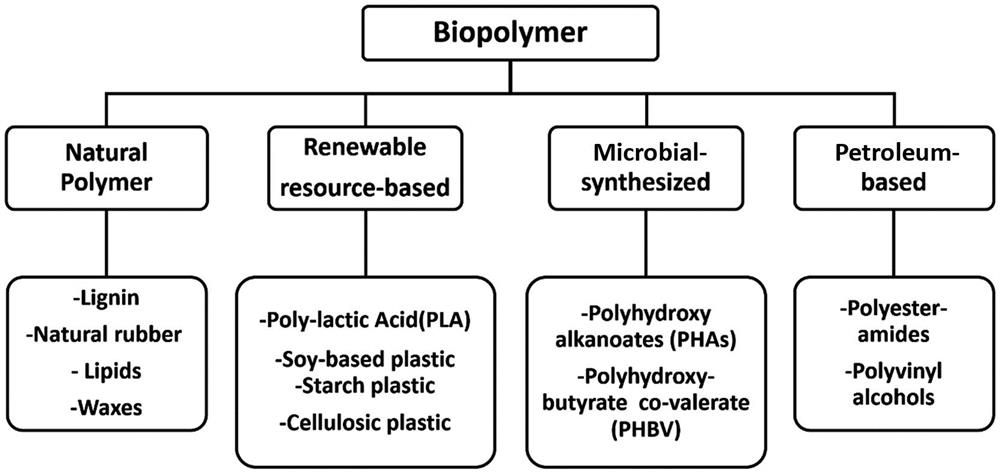
Biopolymers are naturally occurring as well as being derived from renewable and petroleum-based resources as classified in Fig. 9.1. Important characteristics of biopolymers is their ability to degrade naturally in landfills or under forced ambient conditions. Naturally occurring as well as biopolymers developed from renewable resources generally have low strength and/or high production cost. For use in commercial applications, biopolymers have to compete with traditionally used commodity and engineering plastics in terms of strength and commercial availability. Poly(lactic acid) (PLA) has been identified as a potential alternative meeting these requirements. PLA, an aliphatic (thermoplastic) polyester, is derived from starch-rich natural resources (Bajpai and Singh, 2013). PLA is now being commercially developed and supplied worldwide by several industries globally. NatureWorks LLC, USA, is one of the primary producers of PLA in the world with a production capacity of 150,000 metric tons per year. NatureWorks LLC develops a wide variety of PLA grades to suite different applications. However, its higher cost and brittle behavior is restricting its widespread application. To overcome these drawbacks to some extent, several feasibility studies on the use of biodegradable fibers as a reinforcement into PLA have been reported in the literature (Jaszkiewicz et al., 2013; Huda et al., 2006a,b).
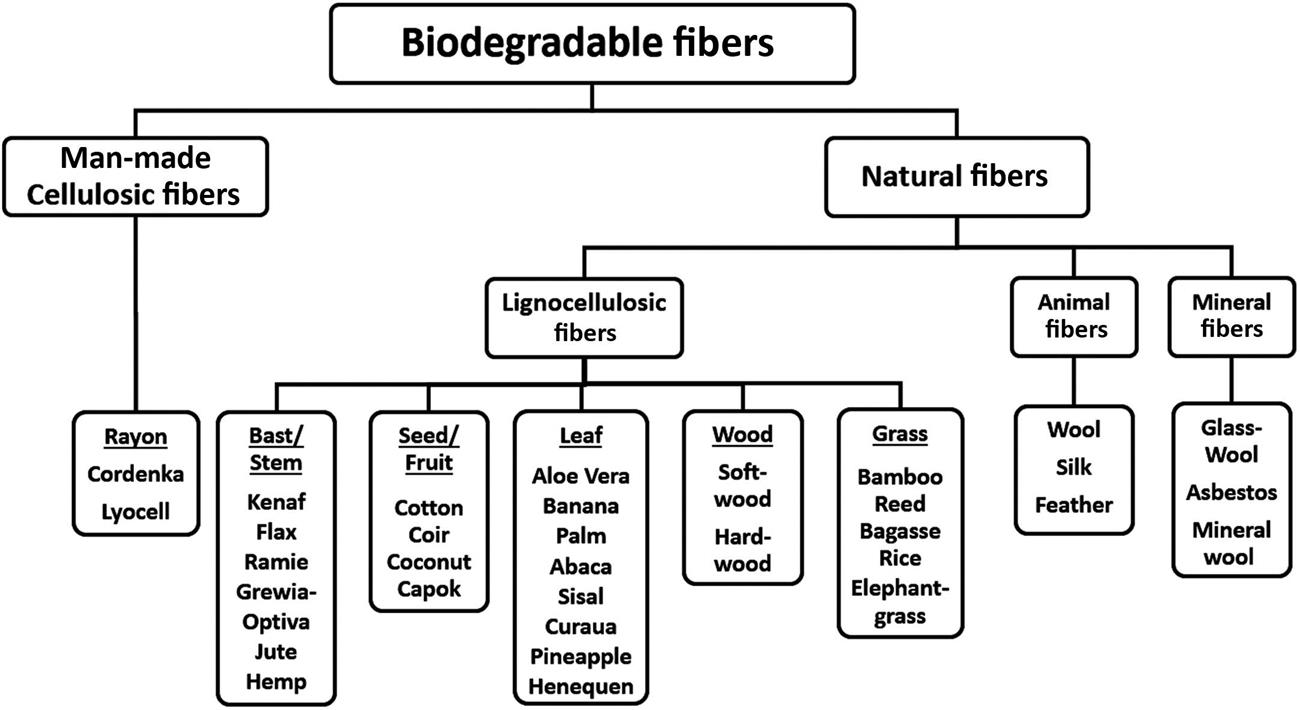
Biodegradable fibers have gained attention as a potential reinforcement material in polymer composites, due to their distinct advantages over the conventional synthetic fibers. Lignocellulosic fibers possess distinct advantages of being readily available at low cost, lower density, fairly good mechanical properties, higher specific strength, renewability, recyclability, biodegradability, and nonabrasiveness compared to glass fibers (Akil et al., 2011; Joshi et al., 2004). Lignocellulosic fibers reduce the weight as well as impart higher specific properties, such as high specific modulus, to the biocomposites. Biodegradable fibers can be broadly classified into natural fibers and man-made cellulosic fibers. Natural fibers can be further classified into three types as plant-based lignocellulosic fibers, animal-derived fibers, and mineral-derived fibers. Lignocellulosic fibers can be further classified based on the part of plant they are derived from, as depicted in Fig. 9.2. Lignocellulosic fibers consists of cellulose, hemicellulose, lignin, pectin, waxes, and other water soluble substances (Ramamoorthy et al., 2015). The microstructure of lignocellulosic fibers consists of several cellulose fibrils running along the length of the fiber, bonded together by an amorphous matrix of lignin and hemicelluloses (John and Thomas, 2008). The hemicellulose present in the fibers is bound to cellulose fibrils by strong hydrogen bonding between them (Mohanty et al., 2000). It is also responsible for thermal and biological degradation of lignocellulosic fibers. Table 9.1 shows chemical composition of commonly used lignocellulosic fibers. The amount of the presence of each of these constituents significantly affects the behavior of lignocellulosic fibers (Faruk et al., 2012; John and Thomas, 2008).
Table 9.1
Chemical composition of commonly used lignocellulosic fibers
| Lignocellulosic fiber | Lignin (%) | Cellulose (%) | Hemicellulose (%) | Pectin (%) |
| Flax | 2.2 | 71 | 18.6–20.6 | 2.3 |
| Ramie | 0.6–.07 | 68.6–91 | 5–16.7 | 1.9 |
| Coir | 41–45 | 36–43 | 0.15–0.25 | 3–4 |
| Jute | 12.1–26 | 44–71 | 13.7–22 | 0.2 |
| Bagasse | 25.3 | 40–55.2 | 16.8 | – |
| Bamboo | 21–31 | 26–43 | 30 | – |
| Henequen | 13.1 | 77.6 | 4–8 | – |
| Wheat Straw | 12–20 | 38–45 | 15–31 | – |
| Seed flax | 21–23 | 43–47 | 24–26 | – |
| Cotton | – | 82.7 | 5.7 | – |
| Sisal | 7–11 | 47–78 | 10–24 | 10 |
| Hemp | 3.7–13 | 57–77 | 14–22.4 | 0.9 |
| Abaca | 7–9 | 56–63 | 15–17 | – |
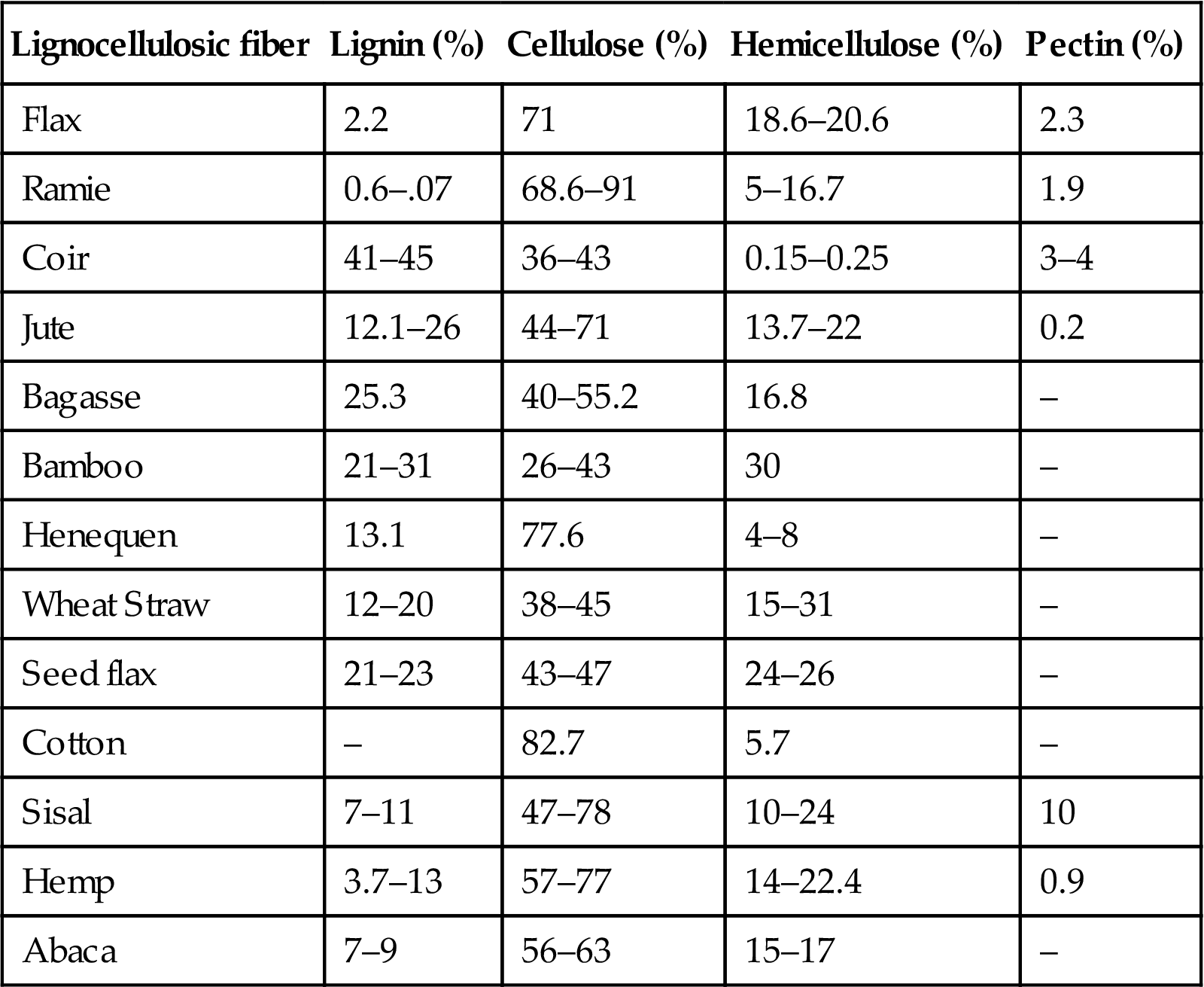
Source: Faruk et al., 2012; Mohanty et al., 2000; Ramamoorthy et al., 2015; Yao et al., 2008.
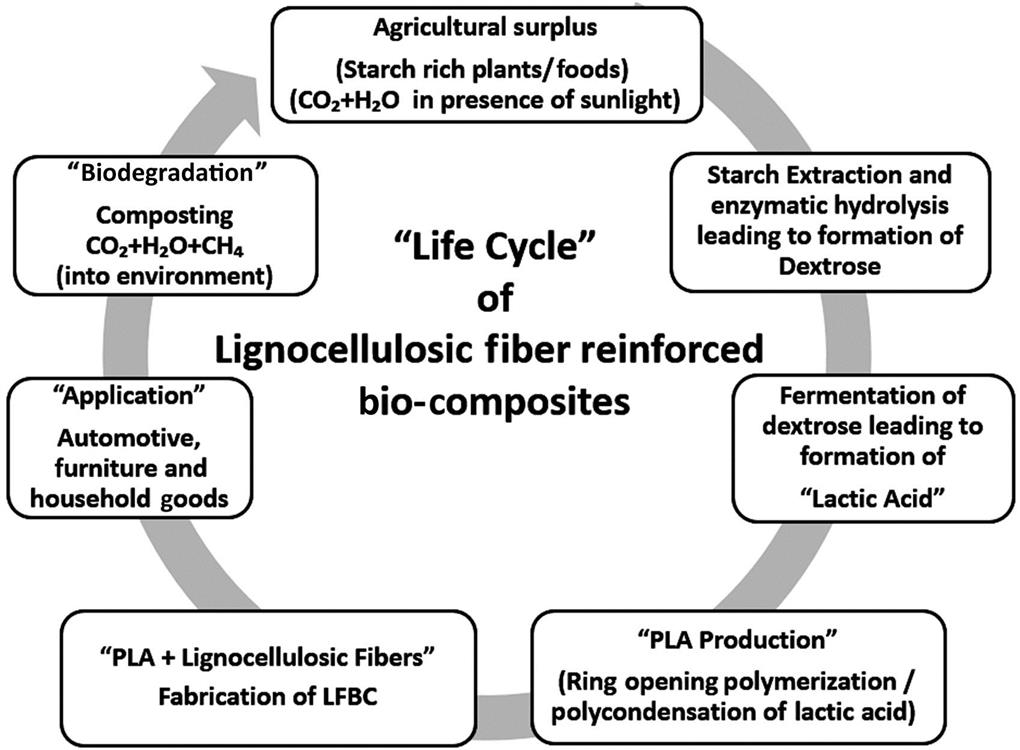
A typical life cycle of PLA-based LFBC is depicted in Fig. 9.3. These biocomposites, developed using biopolymer and lignocellulosic fibers, result in a significant reduction in carbon footprint. However, despite several advantages, widespread commercial application of LFBC is still a challenge to overcome.
9.2 Challenges in primary processing of LFBC
Three major factors influencing the behavior of LFBC are depicted in Fig. 9.4. Thermal, physical, and chemical behavior of biopolymer matrix and lignocellulosic fibers play a significant role in determining the characteristics of biocomposites. As observed from Table 9.1 and Table 9.2, the physical properties as well as chemical composition of lignocellulosic fibers vary significantly for the same type of fiber. Lignocellulosic fibers exhibit variation in properties depending upon geographical regions of plant cultivation, age of plant, extraction process, the part of the plant from which the fiber is extracted, etc.
Table 9.2
Properties of some lignocellulosic fibers and conventional fibers used in polymer composites
| Lignocellulosic fiber | Tensile strength (MPa) | Young’s modulus (Gpa) | Elongation (%) | Density (g/cm3) |
| Abaca | 400 | 12 | 3–10 | 1.5 |
| Flax | 345–1500 | 27.6–80 | 1.2–3.2 | 1.4–1.5 |
| Cotton | 287–597 | 5.5–12.6 | 3–10 | 1.5–1.6 |
| Jute | 393–800 | 10–30 | 1.5–1.8 | 1.3–1.46 |
| Kenaf | 930 | 53 | 1.6 | 1.45 |
| Coir | 175–220 | 4–6 | 15–30 | 1.2 |
| Bagasse | 290 | 17 | – | 1.25 |
| Sisal | 400–700 | 9–38 | 2.0–14 | 1.33–1.5 |
| Bamboo | 140–230 | 11–17 | – | 0.6–1.1 |
| Ramie | 220–938 | 44–128 | 2.0–3.8 | 1.5 |
| Pineapple | 400–627 | 1.44 | 14.5 | 0.8–1.6 |
| Hemp | 550–900 | 70 | 2–4 | 1.48 |
| Carbon | 4000 | 230–240 | 1.4–1.8 | 1.4 |
| E-glass | 2000–3500 | 70 | 2.5–3.0 | 2.5 |
| Aramid | 3000–3150 | 63–67 | 3.3–3.7 | 1.4 |

Source: Faruk et al., 2012; Mohanty et al., 2000; Ramamoorthy et al., 2015.
Fiber–fiber as well as fiber–matrix interactions within LFBC are crucial. Sometimes lignocellulosic fibers within the biocomposite do not act as effective reinforcement due to poor adhesion at the fiber–matrix interface. Lignocellulosic fibers, as discussed above, are comprised of hemicellulose, lignin, pectin, waxes, and cellulose. The presence of these noncellulosic substances on the fiber surface hinders an adequate fiber–matrix interaction and affects the wettability of fibers by the matrix (Petinakis et al., 2013). Lignocellulosic fibers are also prone to agglomeration due to strong interfiber hydrogen bonding. These fibers being hydrophilic have the tendency to absorb moisture, which in general can vary between 5 and 10% by weight. The presence of moisture during processing of LFBC can lead to dimensional variations, porosity defects, and biopolymer degradation. Lignocellulosic fibers are prone to biological degradation due to the presence of carbohydrates present within the fiber structure. Exposure to ultraviolet radiation also results in biodegradation of lignin present in the lignocellulosic fibers. Lignocellulosic fibers are also prone to thermal degradation during processing. Thermogravimetric analysis of dry lignocellulosic fibers reveal that their thermal degradation is a typical two-stage process. The first stage of thermal degradation occurs between 190 and 360°C, wherein hemicellulose (190–280°C) and cellulose (280–360°C) degrades (Chaitanya and Singh, 2016a). Thermal degradation of lignin (second stage) occurs beyond 360°C. As the chemical composition of each type of lignocellulosic fiber differs, knowledge of their thermal degradation behavior prior to processing becomes imperative.
Apart from fiber–fiber and fiber–matrix interactions, the processing technique used and the interaction of tooling with the fiber–matrix melt mixture during processing also play significant roles in the behavior of the developed biocomposites. During processing fibers might experience high shear rates resulting in severe fiber attrition. Control of fiber attrition rate is one of the major challenges associated with the processing of biocomposites. The processing route followed also affects the fiber orientation and distribution behavior within the developed biocomposite. Fiber orientation and distribution influences the mechanical behavior of the biocomposites significantly. However, there is no set of standard guidelines available for the selection of processing route, preprocessing technique, and processing parameters to achieve the desired properties in the biocomposite.
9.3 Processing of biocomposites
The selection of the processing route to develop commercial use LFBC products depends upon various factors, such as (1) size and shape of the desired product; (2) thermal processing range of the biopolymers and the lignocellulosic fibers being used; (3) desired properties of the product; (4) desired production output; and (5) development cost (Singh and Chaitanya, 2015). LFBC can be processed using the traditional processing routes used to fabricate neat polymer based products. However, the addition of lignocellulosic fibers adds to the complexity of the process and require some modifications in tooling and processing parameters to obtain the desired defect-free products. Injection and compression molding processes are commercially viable fabrication routes for development of LFBC (Ku et al., 2011). However, prior to processing using these routes, several preprocessing techniques have been employed to improve the fiber–matrix, fiber–fiber, and fiber–matrix–tooling interactions.
9.3.1 Preprocessing of lignocellulosic fibers and biopolymer matrix
Prior to the processing of biocomposites into desirable products, important preprocessing steps have been reported in the literature depending upon the type of lignocellulosic fiber, biopolymer matrix, and processing route selected. These preprocessing techniques are recommended to overcome the various challenges encountered during fabrication of LFBC as mentioned in Section 9.2.
9.3.1.1 Fiber surface modification
Fiber surface modification techniques help to improve the fiber–matrix and fiber–fiber interactions within the LFBC. Surface modification of lignocellulosic fibers can be generally achieved through a physical or chemical treatment route. However, fiber surface modification using a chemical route is highly preferred over physical treatment (John and Thomas, 2008; La Mantia and Morreale, 2011). Alkaline treatment (mercerization), silane treatment, acetylation, benzoylation, acrylation, permanganate treatment, peroxide treatment, isocyanate treatment, maleated coupling, etc. can be used for modifying the fiber surface (Faruk et al., 2012).
The most popular fiber surface modification technique practiced is mercerization using alkaline solution (Petinakis et al., 2013). Mercerization process removes the noncellulosic content off the fiber surface decreasing its water absorption capability. The removal of noncellulosic content from the fiber surface also results in improved interaction between the fibers and the matrix due to increased availability of hydroxyl bonds. It also imparts a rough fiber surface resulting in additional sites for mechanical interlocking between fibers and the matrix (Chaitanya and Singh, 2016a). In addition to surface treatment of the lignocellulosic fibers, the addition of a coupling agent or compatibilizer can further enhance the transfer of stress at the fiber–matrix interface during loading. Coupling agents or compatibilizers acts as a bridge between fibers and the matrix by reacting with the hydroxyl group present in the fibers and functional group of biopolymer matrix (Gonzalez et al., 2011; Shih and Huang, 2011).
Thermal stability of lignocellulosic fibers is also improved post the removal of noncellulosic content from the fiber surface. The removal of hemicellulose from the fiber surface enhances the onset temperature for thermal degradation and as a result enables the fibers to be processed at higher temperatures (Chaitanya and Singh, 2016a).
9.3.1.2 Precompounding of lignocellulosic fibers and biopolymer matrix
To improve the fiber–matrix interaction and ensure uniform distribution of lignocellulosic fibers within the biocomposites, the use of various compounding techniques prior to the processing of LFBC have been reported in literature. These precompounding techniques are usually employed in the case of short fiber-reinforced biocomposites. Commonly used compounding techniques used prior to compression or injection molding process are extrusion, melt blending, pultrusion, two-roll mill, etc.
9.3.1.3 Extrusion
The use of extrusion for precompounding of the fiber–matrix mixture prior to injection or compression molding has been reported by several researchers. During the extrusion process, as depicted in Fig. 9.6A, the biopolymer and lignocellulosic fibers are mixed together and introduced into an extruder. The extrusion machine consists of a heated barrel with a rotating screw in it. Extruders can be classified as single or twin-screw extruders based on the number of screws. Twin-screw extruders can be further classified as corotating and counterrotating extruders based on the rotational direction of individual screws. The fiber–matrix mixture fed through the hopper is sucked into the heated barrel by the rotating screw(s) and is carried towards the die end. During this travel, the matrix melts and the fiber–matrix compounding takes place. The temperature of various zones of the barrel can be adjusted as per the optimum temperature range for a particular fiber–matrix combination to avoid thermal degradation of the melt. The biocomposite extruded strands are then pelletized and injection molded into desired products. The major processing parameters which affect the quality of the extruded biocomposite strands are barrel temperature, screw speed, and fiber reinforcement concentration. The extrudate quality also depends upon the screw profile and strand die design.
Bledzki et al. (2009) employed twin-screw and single screw extruders for coating and compounding of PLA-based biocomposites, respectively, prior to injection molding. During the compounding process, the melt temperature of PLA was kept at 200°C at a screw speed of 100 rpm, while compounding was carried out at a temperature of 180°C and screw speed of 20 rpm. Bledzki and Jaszkiewicz (2010) in a different study used single and twin-screw extruders for compounding PHBV- and PLA-based biocomposites, respectively. Kim et al. (2010) compounded bamboo flour and wood flour-reinforced PLA and PBS biocomposites in the presence of compatibilizer, using a laboratory size, corotating twin-screw extruder. Fiber weight fraction of 30% was compounded at a screw speed of 250 rpm and temperature of 190 and 145°C for PLA- and PBS-based biocomposites, respectively. The extrudate was subsequently pelletized and dried in an oven at 80°C for 24 h. Moigne et al. (2014) employed a corotating twin-screw extruder for compounding of flax fibers (20 wt%) and PLA pellets prior to the development of biocomposites. The fiber–matrix mixture was compounded at a barrel temperature profile and screw speed of 60–175°C and 300 rpm, respectively. Anstey et al. (2014) compounded PBS-based biocomposites using a 15 cc micro twin-screw extruder. The PBS–filler mixture in the ratio of 3:1 was compounded at 160°C at a screw speed of 100 rpm. Huda et al. (2006a,b) also employed a mini twin-screw extruder having screw L/D and length of 18 and 150 mm, respectively, for compounding wood flour-reinforced PLA biocomposites. Tokoro et al. (2008) compounded PLA–bamboo fiber biocomposites using a twin-screw extruder (screw L/D=36 and screw diameter=30 mm) at 180°C. Borchani et al. (2015) compounded alfa fiber-reinforced poly(butylene terephthalate-co-butylene adipate) and starch-based Mater-Bi® biocomposites using an intermeshing corotating twin-screw extruder (screw L/D ratio of 36 and screw diameter=25 mm), at a melt temperature of 150°C. The extrudate was extruded at a rate of 10 mm/s in the form of 1 mm strands. These strands were cooled and subsequently pelletized using a pelletizer. Asaithambi et al. (2014) compounded untreated and treated hybrid banana–sisal fiber-reinforced PLA biocomposites using a twin-screw extruder having a screw L/D ratio and screw diameter of 40 and 28 mm, respectively. The barrel temperature profile, screw speed, and residence time was fixed at 140–150–155–160–165–170–175–170–165–160°C, 100 rpm, and 15 min, respectively. Sykacek et al. (2009) compounded wood flour (up to 65 wt%) and five different biopolymers using a conical counterrotating twin-screw extruder having a screw diameter of 45–90 mm and two shear generating elements. The temperature profile and screw speed during extrusion was selected as 150–170–180–180 (feed to die) and 75 rpm, respectively.
9.3.1.4 Pultrusion
Pultrusion process is used to develop continuous fiber-reinforced composites based on thermosets as well as thermoplastic polymers (Akil et al., 2011; Velde and Kiekens, 2001). Long components having a fixed cross-sectional profile can be made using this process. However, some researchers have tried using it as a compounding process prior to injection molding. A schematic of the pultrusion process depicting various parts of the setup is depicted in Fig. 9.5. The lignocellulosic fibers to be reinforced are pulled through a preheater and a heated strand die, during which fibers are impregnated with the desired thermoplastic biopolymer (Kabir et al., 2012). The pultruded biocomposite strands thus obtained are either pelletized and injection molded or hot pressed into desired products. This process ensures minimum fiber length damage and helps in fabrication of long fiber-reinforced pellets. Ganster and Fink (2006) employed pultrusion process for compounding of spun cellulose fibers (viscose, lyocell, and carbamate) with PLA and other synthetic polymers (PP, PE, HIPS). Yang et al. (2012) also employed this process to obtain jute-reinforced PLA (long fiber) pellets prior to injection molding. The jute fibers were impregnated at different temperatures (235, 250, and 280°C) and it was reported that 250°C is the optimum impregnation temperature.
9.3.1.5 Miscellaneous compounding processes
Melt blenders having counter- or corotating rotors enclosed in a heated chamber are employed to melt blend the fiber–matrix mixture prior to injection or compression molding. A handful of studies have also reported the use of high speed mixers for the same purpose. These melt blenders or high speed mixers are ideal for small batch production and their output is in the form of biocomposite pellets. Two- or three-roll mill is another blending technique used for compounding of fibers and matrix prior to injection or compression molding. The fiber–matrix mixture is compounded by passing the mixture through heated counter-rotating rollers to form sheets (Joseph et al., 2005). The compounded biocomposite sheets thus obtained are either hot pressed using a compression molder or shredded into small fragments and injection molded. Yu et al. (2009, 2010) developed ramie and jute fiber-reinforced PLA biocomposites using a two-roll mill prior to hot pressing. The fiber–matrix mixture having varying fiber concentration (10–50%) was blended at 140°C for 5 min using a two-roll mill. Bledzki et al. (2005) conducted a comparative study of different compounding processes prior to compression molding. The fiber–matrix mixture, at a ratio of 1:1, was compounded using a twin-screw extruder (170–195°C for 4 min), a two-roll mill (180°C for 10 min), and high speed mixture (170–175°C and 10–15 mins). Ye et al. (2015) employed a two-roll mill for compounding of sisal fiber-reinforced PLA biocomposites having a fiber mass fraction of 20%. The biocomposites were processed at a temperature of 180°C for 5 min. Okubo et al. (2009) employed a laboratory scale three-roll mill with rollers rotating at varying angular velocities to efficiently disperse microfibrillated cellulose (MFC) (1 and 2 wt%) into a PLA matrix. During compounding the speed of the last roller was fixed at 100 rpm and the PLA/MFC mixture was passed through the rollers 10 times at progressively decreasing gap settings from 70 to 5 µm.
The interaction between tooling and fiber–matrix mixture is profound during compounding process and strongly influences the behavior of biocomposites. This interaction is observed to be more prominent in case of extrusion and high speed melt blending processes as it results in higher fiber attrition rates. Pultrusion process on the other hand exhibits maximum fiber length retention and is ideal for precompounding long fiber-reinforced pellets.
9.3.2 Injection molding of LFBC
Injection molding is the most extensively used industrial process for fabrication of plastic products. Injection molding process offers rapid processability and repeatability in fabricating products having convoluted geometries (Chaitanya and Singh, 2016a, 2016b). It is an ideal process for mass manufacturing of small to medium sized products. With the growing demand of LFBC, processes like injection molding, which have rapid processability, are desired for commercial applications. Fig. 9.6 depicts the extrusion–injection molding process. The biocomposite pellets are fed through the hopper into a heated barrel equipped with a compression screw which can rotate as well as reciprocate. The biocomposite pellets are melt blended in a heated barrel at a high shearing rate. As the fiber–matrix melt mixture progresses towards the nozzle, the reciprocating screw is pushed back (against back pressure) by the mixture accumulating in front of the screw tip. When the screw is pushed back up to the desired shot volume, the fiber–matrix melt mixture is then injected into the mold of the desired product at predefined processing parameters, i.e., injection pressure, injection speed, injection time, holding pressure, holding time, cooling time, and mold temperature. During the cooling phase, the charging of the next shot of the fiber–matrix mixture starts at predefined processing parameters, i.e., screw speed and pressure, back pressure, suck back distance, etc. The injection molding process produces near net-shaped products and has the ability to uniformly disperse lignocellulosic fibers within the biocomposite.
Okubo et al. (2009) used a microscale injection molder to fabricate tensile test specimens of PLA/MFC biocomposites. The PLA/MFC melt mixture was injected at an injection and holding pressure of 0.7 and 1.5 MPa, respectively, into a preheated mold (40°C). Wang et al. (2013) fabricated jute fiber-reinforced PLA biocomposites using an injection molding machine having a barrel temperature range of 140–190°C and mold temperature and cooling time of 30°C and 15 s, respectively. The biocomposites were injected by varying holding pressure. Bledzki and Jaszkiewicz (2010) developed abaca, jute, and man-made cellulose fiber-reinforced PLA and PHBV biocomposites using an extrusion–injection molding process. The biocomposite pellets obtained from extrusion process were injection molded using an injection molding machine having a nominal clamping force of 850 kN, screw L/D ratio of 21, screw diameter of 40 mm, and a screw speed of 120 rpm. The test specimens were injection molded at a barrel temperature of 180°C, injection pressure of 50 MPa, and injection speed of 0.2 m/s (Bledzki et al. 2009). Kim et al. (2010) injection molded PLA- and PBS-based precompounded biocomposite pellets at a melt temperature of 190 and 145°C, respectively. The injection pressure of 8.2 MPa was kept the same for all the biocomposites developed. Moigne et al. (2014) prepared flax fiber-reinforced PLA biocomposite test specimens using barrel and nozzle temperatures of 180 and 210°C, respectively. Extruded biocomposite pellets were injected into a mold (25°C) at an injection pressure of 50 MPa for 20 s and holding pressure of 80 MPa for 15 s. Tokoro et al. (2008) developed bamboo fiber-reinforced PLA biocomposite test specimens by injection molding at a pressure range of 50–60 MPa and melt temperature of 180°C. The mold temperature and cooling time was kept at 20°C and 30 s, respectively. Huda et al. (2006a,b) reported the use of a mini injection molder to prepare wood flour-reinforced PLA biocomposites at a melt and mold temperature of 183 and 40°C, respectively. Borchani et al. (2015) also employed a micro injection molder to fabricate MaterBi®-based biocomposite test specimens. The precompounded pellets of alfa fiber-reinforced MaterBi® biocomposites were injection molded at a temperature and pressure of 150°C and 10 MPa, respectively. Mofokeng et al. (2011) developed sisal fiber (1–3 wt%)-reinforced PLA biocomposites using injection molding process. The biocomposite pellets of PLA–sisal fiber were injected at an injection pressure of 60, 70, and 74 MPa for 1, 2, and 3 wt% of sisal fiber, at 190°C. The holding pressure, cooling time, back pressure, and mold temperature were fixed at 60 MPa, 30 s, 3 MPa, and 20°C, respectively. Asaithambi et al. (2014) developed treated and untreated banana–sisal fiber reinforced hybrid biocomposites. The precompounded extruded pellets were injection molded into test specimens using an injection molder having a maximum clamping force of 60 ton, screw L/D ratio of 20, and screw diameter of 30 mm. The processing parameters like barrel temperature profile, injection pressure, injection time and mold temperature were selected as 80–160–165–170–175°C (feed to nozzle), 19 MPa, 0.95 s, and 30°C, respectively. Sykacek et al. (2009) fabricated wood flour reinforced biocomposites based on five commercially available biopolymer matrices of Ecoflex®, PLA 7000D, Ecovio®, Bioflex 467-F, and Tenite Propionate 371 A 4000012. The extruded pellets with varying fiber content (up to 65%) were injection molded into test specimens at a temperature of 185–190°C and a back pressure of 10 MPa. The injection molding machine had a screw L/D ratio of 22 and screw diameter of 30 mm.
9.3.2.1 Issues and challenges in injection molding of LFBC
The behavior of the developed biocomposites is significantly influenced by the orientation, dispersion, and size of the lignocellulosic fibers within the biocomposite. Issues and challenges have thus been divided into the three following subsections.
9.3.2.2 Distribution and orientation of natural fibers
In order to develop biocomposites exhibiting exceptional mechanical behavior, the lignocellulosic fibers should ideally be oriented in the direction of applied load. The orientation of lignocellulosic fibers during injection molding is influenced by the fiber–matrix melt flow within the mold. However, in the case of injection molded LFBC, complex fluid flow is observed. During mold filling, the shear flow experienced by the fiber–matrix mixture close to the mold walls, tends to align the fibers in the direction of the flow. This phenomenon is observed for a certain thickness below the surface. However, near the core, bulk deformation of the fiber–matrix flow takes place, which orients the fibers randomly in the direction perpendicular to the applied load (Singh and Chaitanya, 2015). Fig. 9.7 depicts fiber orientation behavior of injection molded sisal fiber-reinforced PLA biocomposites. Fig. 9.7A represents a schematic of fiber orientation at a mid-cross-sectional plane parallel to the direction of flow. Fig. 9.7B represents the sisal fiber orientation in the cross-sectional plane perpendicular to the direction of flow. It can be observed from both the figures that the fibers near the mold walls tend to align in the direction of the applied load, while the fibers near the core align randomly. It was also observed that short lignocellulosic fibers are more likely to orient and disperse themselves in the direction of the applied load compared to long fibers (Chaitanya and Singh, 2016b). Hence, during loading, the fibers aligned in the direction of applied load bear more load as compared to the randomly aligned fibers, affecting the overall load-bearing capability of the injection molded biocomposite.
9.3.2.3 Fiber breakage/attrition
Another major factor effecting the performance of the biocomposites is the fiber attrition caused during processing. High shear rates experienced by lignocellulosic fibers during preprocessing as well as injection molding results in fiber twisting, bending, and breaking. All three modes of fiber damage reduces the effective fiber length of the lignocellulosic fibers available for load-bearing. Chaitanya and Singh (2016b) observed that long lignocellulosic fibers compared to short fibers are more prone to fiber attrition during processing. Also, the direct-injection molding process exhibits less fiber damage compared to the extrusion–injection molding process. To overcome these issues related to fiber–matrix–tooling interactions, some modifications in tool design are required. The nozzle diameter, sprue dimensions, gate dimensions, etc. should be increased to accommodate high lignocellulosic fiber reinforcement content. Modifying tool dimensions helps in reducing the shearing of fibers during processing.
9.3.2.4 Residual stresses
Residual stresses are generated within the developed biocomposites due to rapid solidification of the fiber–matrix mixture under high pressure. Residual stresses may result in warpage and stress cracking, resulting in permanent deformation of the product. However, these residual stresses can be kept under check by ensuring gradual and uniform cooling of the product in the mold. Proper mold design and careful selection of processing parameters like melt temperature and injection pressure can further help to reduce the residual stresses (Singh and Chaitanya, 2015).
9.3.3 Compression molding
Compression molding or hot pressing is the extensively employed processing route for the fabrication of lignocellulosic fiber-reinforced polymer composites by the research fraternity. The ease of processing and ability to make tailor-made biocomposites have attracted researchers towards this process. This process is usually employed to develop medium to large size products having flat or simple profiles. The schematics of the compression molding process depicting two variants of processing method are depicted in Fig. 9.8.
9.3.3.1 Compression molding of precompounded fiber–matrix mixture
Fig. 9.8A represents a compression molding process in which a precompounded fiber–matrix mixture is hot pressed in the mold to form the desired product. Yu et al. (2009, 2010) reported the use of hot pressing to develop ramie and jute fiber-reinforced PLA biocomposites. The precompounded sheets obtained using a two-roll mill were hot pressed at 20 MPa and 170°C for 4 min and subsequent cooling at room temperature at 5 MPa. The biocomposites thus developed exhibited superior mechanical performance compared to neat PLA. Bledzki et al. (2005) fabricated 0.7 mm thick, PP–wood fiber sheets by compression molding of the fiber–matrix compound obtained from twin-screw extrusion, two-roll mill, and a high speed mixer. The fiber–matrix mixture was compressed at a mold temperature of 180°C under pressure of 5 MPa for 3 mins. The mold was subsequently cooled at room temperature at same pressure. Dong et al. (2014) developed compression molded PLA–coir biocomposites with varying fiber weight fraction (5, 10, 20, and 30%). PLA sheets were developed by placing heated PLA pellets (in an oven at 180°C for 15 min) between aluminum sheets and pressing them under a pressure of 1 MPa using a hydraulic press and subsequent cooling for 30 min. Evenly distributed predried coir fibers were then stacked between the PLA sheets and heated in an oven for 15–30 min and subsequently pressed at 1.5 MPa for 15 min. The biocomposite laminates were then cooled at room temperature for 30 min.
9.3.3.2 Film stacking method
The film stacking method is the simplest processing route for the fabrication of LFBC laminates (Fig. 9.8). In this process, biopolymer matrix films and lignocellulosic fiber mats (woven or plain or random) are alternatively stacked and compressed between the heated mold plates. Plackett et al. (2003) developed PLA–jute fiber biocomposites using the film stacking technique. PLA pellets were first converted into 0.2 mm thick PLA sheets using an extruder. Jute fiber mats (40 wt%) were then stacked between PLA films in a frame/mold and precompressed at 3.3 MPa for 15 s. The precompressed stack of PLA films and jute fiber mats was then placed between heated platens (180–220°C) under a pressure of 400 Pa for 3–10 min. After heating, the mold/frame was again compressed (3.3 MPa) at 60°C for 1 min to obtain 2 mm thick biocomposites. Sawpan et al. (2011, 2012) also used an extruder to convert PLA pellets into 5 mm thick sheets. A hand carding machine was used to align industrial hemp fibers. PLA films and industrial hemp fiber-based biocomposites were then prepared by the film stacking process at varying fiber weight fractions (30, 35, and 40%). The PLA films and industrial hemp fiber mats were then alternately stacked in a mold (220×150×3.5 mm3) and precompressed (2 MPa) at 185°C for 5 min using a compression molder. The mold pressure was then increased to 5 MPa and thereafter cooled at room temperature. Bajpai et al. (2012, 2013) developed 4 mm thick, PLA-based biocomposites incorporating sisal, Grewia optiva, and nettle fibers (fiber weight fraction of 20%), using the film stacking method. PLA pellets were compression molded into 1 mm thick PLA films. PLA films and fiber mats were then stacked alternatively within the mold. The PLA-fiber stack was then compressed at a pressure of 4 MPa for 8 min at 180°C. The compression pressure was further increased to 6 MPa and the mold was allowed to cool at room temperature. Huda et al. (2008) converted PLA pellets into 1 mm thick PLA sheets using a compression molder at 190°C. Three layers of kenaf fiber mats were stacked alternatively between four sheets of PLA films and compressed (4.8 MPa) at 190°C for 12 min. The mold was further compacted at 11.7 MPa for 5 min and cooled at room temperature. Three millimeter thick biocomposites were then removed from the mold at 90°C. Hu et al. (2010) developed randomly oriented short jute fiber (10–15 mm)-reinforced PLA biocomposites using the film stacking method. Jute fibers with varying fiber volume fractions (30, 40, and 50%) were stacked alternatively between PLA films and placed between a compression molder (1.3 MPa) at 170°C for 10 min to obtain 4–5 mm thick biocomposites. Islam et al. (2010) developed long hemp fiber (30 wt%)-reinforced PLA biocomposites, by stacking fiber mats in between PLA films and compressing the stack (1 MPa) at 170°C for 10 mins.
9.3.3.3 Fiber orientation and distribution
Compression molded specimens incorporating fiber mats can be tailor-made, using the film stacking method as it offers the best control to the designer over fiber orientation within the biocomposites. Continuous lignocellulosic fibers can be woven at different angles and types according to the load-bearing requirement. Research studies have reported the use of carding process to align nonwoven fibers before the film stacking method. No fiber–matrix–tooling interaction in the case of biocomposites developed using film stacking method is observed. The biocomposites developed using the film stacking method, having fibers aligned in the direction of applied load, are likely to bear more load as compared to the randomly aligned short fiber-reinforced (precompounded) biocomposites.
9.4 Conclusions
The incorporation of lignocellulosic fibers into a biopolymer matrix produces an economical and ecofriendly product. The use of LFBC in various applications is the need of the hour. To meet the increasing demand of LFBC, commercially viable manufacturing processes should be developed. The two most widely used processing routes in the industry which have the potential to develop LFBC commercially are injection and compression molding processes. However, prior to processing, preprocessing of lignocellulosic fibers and biopolymer matrix is required. The preprocessing techniques include fiber surface modification and various precompounding techniques. The behavior of the biocomposites is highly dependent on the preprocessing as well as processing route followed. Injection molding method is complex and is tedious to control compared to the compression molding method. However, for use in commercial applications, the rapid processability and repeatability of the injection molding process is an added advantage. Further, it can be concluded that the optimum selection of processing route, processing parameters, and tool design during the development of LFBC can result in the production of good quality products, leading to the enhancement of its application areas.