Natural fiber-reinforced polymer-based composites
Alan Kin-tak Lau1 and Karen Hoi Yan Cheung2, 1Swinburne University of Technology, Hawthorn, Melbourne, VIC, Australia, 2Hong Kong Green Building Council, Kowloon, Hong Kong, SAR China
Abstract
Recently, the mankind has realized that unless environment is protected, he himself will be threatened by the over consumption of natural resource as well as substantial reduction of fresh air produced in the world. Conservation of forests and optimal utilization of agricultural and other renewable resources like solar and wind energies, and recently, tidal energy have become important topics worldwide. In such concern, the use of renewable resources such as plant and animal based fiber-reinforced polymeric composites, has been becoming an important design criterion for designing and manufacturing components for all industrial products. Research on biodegradable polymeric composites can contribute to green and safe environment to some extent. In the biomedical and bioengineered field, the use of natural fiber mixed with biodegradable and bioresorbable polymers can produce joints and bone fixtures to alleviate pain for patients. In this chapter, a comprehensive review on different kinds of natural fiber composites will be given. Their potential in future development of different kinds of engineering and domestic products will also be discussed in detail.
Keywords
Biocomposites; natural fiber; animal fiber; environmentally friendly
1.1 Introduction
Over the past few decades, research and engineering interest has been shifting from traditional monolithic materials to fiber-reinforced polymer-based composites due to their unique advantages of high strength to weight ratio, noncorrosive property, and high fracture toughness. These composite materials consisting of high strength fibers, such as carbon, glass, and aramid, and low strength polymeric matrix, have now dominated the aerospace, leisure, automotive, construction and sporting industries. Unfortunately, these fibers have serious drawbacks such as (1) nonrenewable; (2) nonrecyclable; (3) high energy consumption in the manufacturing process; (4) health risk when inhaled; and (5) nonbiodegradable. Biodegradation is the chemical breakdown of materials by the action of living organisms which leads to a change in physical, mechanical, and chemical properties. It is a concept of vast scope, ranging from the decomposition of environmental wastes involving microorganisms to host-induced biomaterials.
Although glass fiber-reinforced polymer composites have been widely used due to their advantages of low cost and moderate strength for many years to provide solutions to many structural problems, the use of these materials, in turn would induce a serious environmental problem that is now of concern in most Western countries. Recently, due to a strong emphasis on environmental awareness worldwide, it has brought much attention in the development of recyclable and environmentally sustainable composite materials. Environmental legislation as well as consumer demand in many countries are increasing the pressure on manufacturers of materials and end-products to consider the environmental impact at all stages of their life cycle, including recycling and ultimate disposal. In the United State, it encourages manufacturers to produce materials and products by practicing the 4Rs, which are (1) Reduce the amount and toxicity of trash to be discard (sourced reduction); (2) Reuse containers and products; (3) Repair what is broken; and (4) Recycle as much as possible, which includes buying products with recycled content. After these processes are gone, the materials finally are entitled to be disposed into landfill.
The most common types of conventional composites are usually composed of epoxy, unsaturated polyester resin, polyurethanes, or phenolic reinforced by glass, carbon, or aramid fibers. These composite structures lead to the problem of conventional removal after the end of their life time, as the components are closely interconnected, relatively stable, and thus difficult to be separated and recycled. The recent development of aircraft, such as Boeing 787 and AIRBUS 350, use over 50% of composites as their structural components. A serious problem that brings a strong debate is on the recyclability of the composites after the end of their service life. During the production process, the use of energy to make fibers and resins is another arguable item. However, the advantage of using these materials is due to their light-in-weight, noncorrosive properties, and the ease of manufacturing in different forms and shapes without involving the use of heavy equipment. Therefore, if the natural fiber and biodegradable matrix are used for a new type of biocomposite and can achieve similar functions and strength as glass fiber-reinforced polymer (GFRP) composites, it would help solve many environmental problems addressed above and help improve the living environment in our planet.
Within the past few years, there has been a dramatic increase in the use of natural fibers, such as leaves from flax, jute, hemp, pineapple, and sisal, for making a new type of environmentally-friendly composite. Recent advances in natural fiber development, genetic engineering, and composite science offer significant opportunities for improved materials from renewable resources with enhanced support for global sustainability. In general, two types of natural fibers are identified for making fiber-reinforced polymer; they are (1) plant-based fibers and (2) animal-based Fibers. For the former, due to their abundant supply in the natural environment, the raw material cost is relatively low and can compete with synthetic fibers, such as glass to make the composites. Animal fibers, however, are difficult to collect from wildlife and, normally, have to be obtained from home-fed animals, such as spiders and cocoons.
By using the plant-based natural fiber as reinforcement of polymer-based materials the reduction of the use of synthetic fibers and undegradable polymer for composite structures can be targeted. Excessive use of petroleum-based plastics induces huge amounts of nondecomposable solid waste which causes a serious depletion of landfill capacities. The awareness of the soaring waste problems on the environment has awakened a new interest in the area of materials science and engineering. Because of the increasing environmental consciousness in the society, it is a critical topic for researchers to study different alternatives to replace nonrenewable materials, especially for petroleum-based plastics. Therefore, different types of fully biodegradable materials have been developed recently, as substitutions for nonbiodegradable petroleum-based plastics, and even metallic components [1–4].
Among all the natural fibers, sisal [5], hemp [5], basalt [6], kenaf [7], flax [8], and bamboo fibers [9] are the most common types to be used due to their abundant supply in the natural environment. However, the skill of how to extract the fibers with consistent physical, material, and mechanical properties is key. Besides the surface treatment and processing temperature of the fibers, also their high moisture absorbability is a factor that makes them difficult to be used in high-end engineering products and structures. In some scenarios, the processing temperature of thermoset or thermoplastics during the injection modeling process may cause the thermal degradation of fibers, which substantially reduces the mechanical strength of the composites.
Vaisanen et al. [10] have addressed the advantages of using organic waste and residues from agricultural and industrial processes to develop a new class of composites, in which some of them are good at a relatively high servicing temperature condition (~300°C). Fig. 1.1 shows the thermal decomposition ranges for natural fiber polymer-based composites at different temperatures. In general, natural fibers are degraded at temperatures starting from 200°C up to 500°C (from hemicelluloses to lignin). In the civil engineering industry, the use of natural fibers to reinforce cementitious materials have become more popular due to the advantages of low cost, low density, moderate strength, and local availability in different countries. However, the moisture absorption problem of plant-based fibers is still a critical issue that affects the resultant strength of the composites [11].
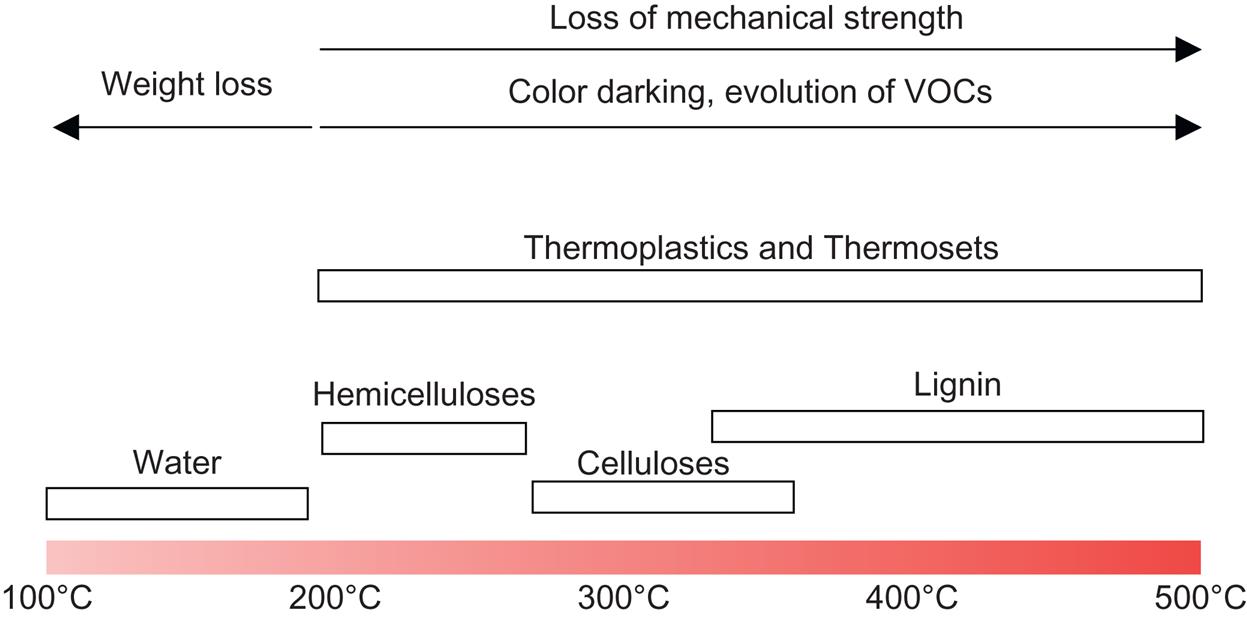
To popularize the usage of plant-based natural fiber in civil infrastructure applications, durability, ultraviolet (UV) degradation, and corrosion resistant and inflammable properties are important [12]. Fire-retardant filler compounds are normally used for plant-based natural fiber-reinforced polymer composites. The criteria of selecting the fillers are low cost, relatively easy addition into the polymer, and high fire resistance. Aluminum trihydroxide (ATH), which is also known as alumina triydrate, is an active fire-retardant filler compound most often used in polymers and polymer composites. This compound is decomposed during the dehydration process, to form carbon-inorganic residues and finally becomes a foam-like structure to isolate heat release. It was proved that using a small amount of fire-retardant compound (ammonium polyphosphate [APP]) with kenaf and hydrophobic plastic (PP) can improve the fire-retardant ability by 200%, with wool it is 250%.
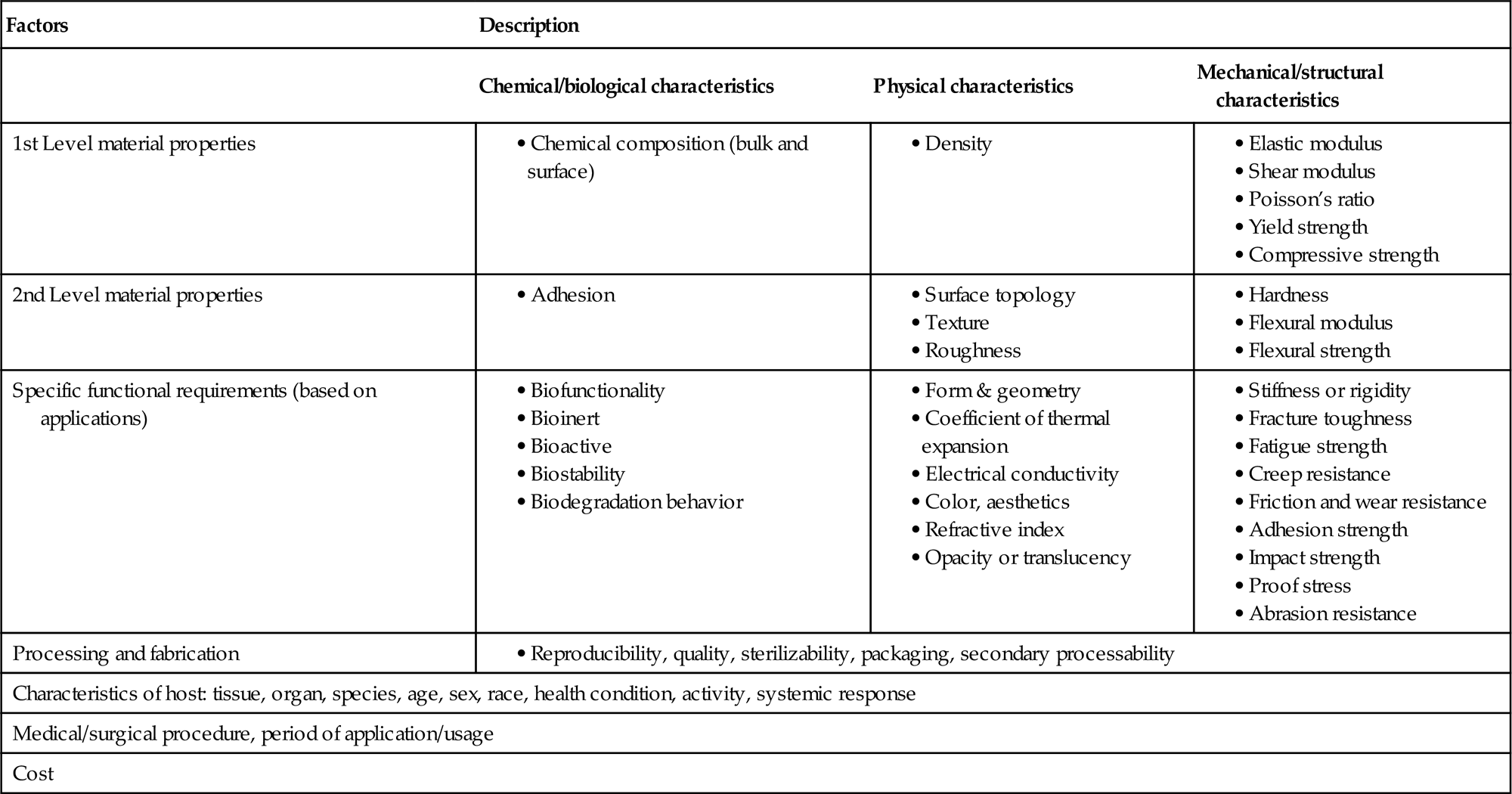
Animal fibers, due to their high protein-based content, are suitable for biomedical engineering applications, particular for the design of implant structures, like bond fixators. A material that can be used for medical applications must possess a lot of specific characteristics. The most fundamental requirements are related to biocompatibility, i.e., not to have any adverse effect on the host tissues; therefore, those traditional composite structures with nonbiocompatible matrix or reinforcement are substituted by bioengineered composites. Table 1.1 summarizes several important factors that need to be considered in selecting a material for the biomedical applications [13]. Spider and silkworm silks are identified as good reinforcements for making composites for tissue scaffolds. The mechanical properties of these fibers as compared with Nylon is shown in Fig. 1.2. It is obviously seen that the spider silks (Nephila inaurata and Argiope trifasciata) possess high tensile modulus and strength as compared with Nylon 6.6 and cocoon silks [14,15]. However, the main concern for the use of these silks is the consistency of where the silks have originated.
Table 1.1
Key factors for the selection of materials for biomedical applications [2]

Bioengineering refers to the application of concepts and methods of the physical sciences and mathematics in an engineering approach towards solving problems in the repair and reconstructions of lost, damaged, or deceased tissues. Any material that is used for this purpose can be regarded as a biomaterial. According to Williams [16], a biomaterial is a material used in implants or medical devices, intended to interact with biological systems. Those common types of medical devices include substitute heart valves and artificial hearts, artificial hip and knee joints, dental implants, internal and external fracture fixators, and skin repair templates etc. One of the major features of composite materials is that they can be tailor-made to meet different applications’ requirements.
1.2 Silkworm silk fiber
Natural fibers are subdivided based on their origins, coming from plants, animals, or minerals. Generally, plant-based natural fibers are lignocelluloses in nature and are composed of cellulose, hemicellulose, and lignin, such as flax, jute, sisal, and kenaf; whereas animal-based natural fibers are of proteins, like wool, spider and silkworm silks, and chicken feathers. The enhanced environmental stability of silk fibers in comparison to globular proteins is due to the extensive hydrogen bonding, the hydrophobic nature of much of the protein, and the significant crystallinity. Many researchers have studied the use of Bombyx mori cocoon silk fabrics as a reinforcement to make a composite. Sericin protein on the surface of silk fiber can serve as a binder to bond the fabric together by the action of pressure and heat. However, for making an advanced composite, this layer of sericin has to be removed by boiling water to ensure a good bonding between the fibers and a polymer matrix results.
Silk proteins—known as silk fibroins—are stored in the glands of insects and spiders as an aqueous solution. During the spinning process, a silkworm accelerates and decelerates its head in arcs for each change of direction, and the concentration of silk in the solution is gradually increased and finally an elongation stress is applied to produce a partly crystalline, insoluble fibrous thread in which the bulk of the polymer chains in the crystalline regions are oriented parallel to the fiber axis. Faster spinning speed leads to stronger but more brittle fibers, whereas a slower speed leads to weaker and more extensible fibers. At even greater speed, silk toughness decreases, mainly due to the loss of extensibility [17].
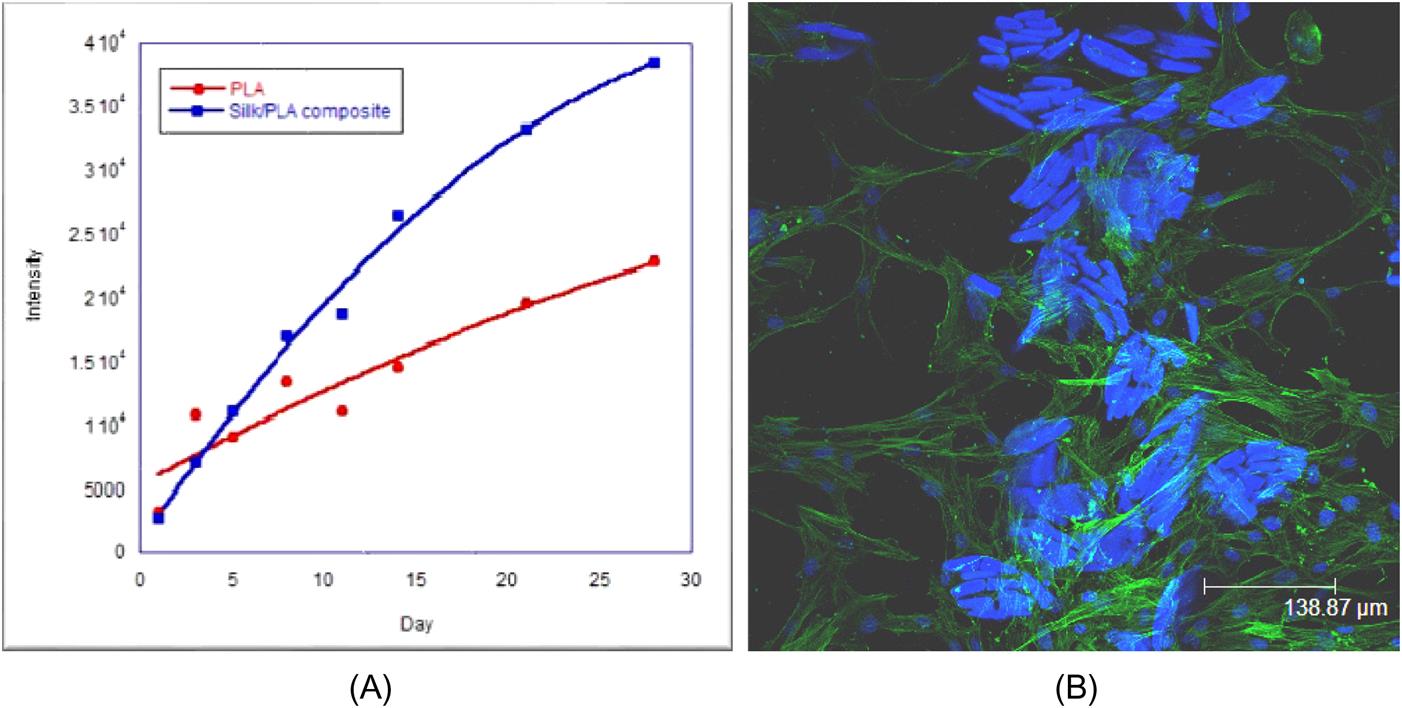
Cocoons are natural polymeric composite shells made of a single continuous silk strand with length in the range of 1000–1500 m and conglutinated by sericin [18]. This protein layer resists oxidation, is antibacterial and UV resistant, and it also absorbs and releases moisture easily. This protein layer can be cross-linked, copolymerized, and blended with other macromolecular materials, especially artificial polymers, to produce materials with improved properties. In average, the cocoon production is about 1 million tonnes worldwide, and this is equivalent to 400,000 tonnes of dry cocoon (see Fig. 1.3). In the tissue engineering area, silks have been identified as a kind of biomaterial, used in the healing process for bone, tendons, or ligament repairs. Slowly degrading biomaterials which can maintain tissue integrity following implantation, while continually transferring the load-bearing burden to the developing biological functional tissue are desired. In such phenomena, the gradual transfer of the load-bearing burden to the developing and/or remodeling tissue should support the restoration and maintenance of tissue function over the life of the patient. A series of tests were conducted to investigate the cell growth properties by considering fetal human osteoblasts (fHobs), which are cells that are responsible for bone formation. It was found that silk fiber/PLA composites could enhance cell growth on their surface. Fig. 1.4 shows an AlamarBlue assay on fHobs seeded on neat PLA and silk/PLA composite, and it is obvious seen that the many cells are grown with time.
Silk fibers spun out from silkworm cocoons consist of fibroin in an inner layer and sericin in an outer layer; all are protein-based. From the outside to the inside layers of the cocoon, the volume fractions of sericin decreases while the relative content of fibroin increases. Also, it is known that silk fibroin consists of both hydrophilic and hydrophobic regions, which is a block-like polymeric system. These fibers have a highly nonuniform cross-sectional geometry with respect to both shape and absolute dimensions. By changing the reeling conditions, silkworm silks can be stronger, stiffer, and more extensible, approaching to the properties of spider dragline silks [19]. Each raw silk thread has a lengthwise striation, consisting of two separate but irregularly entwined fibroin filaments (brin) embedded in sericin. Silk sericin is a minor protein that envelops silk fibroin fibers and glues them together to form cocoon shape. Fibroin and sericin in silk account for about 75 and 25 wt% respectively. Silk fibers are biodegradable and highly crystalline with a well-aligned structure.
1.2.1 Mechanical properties
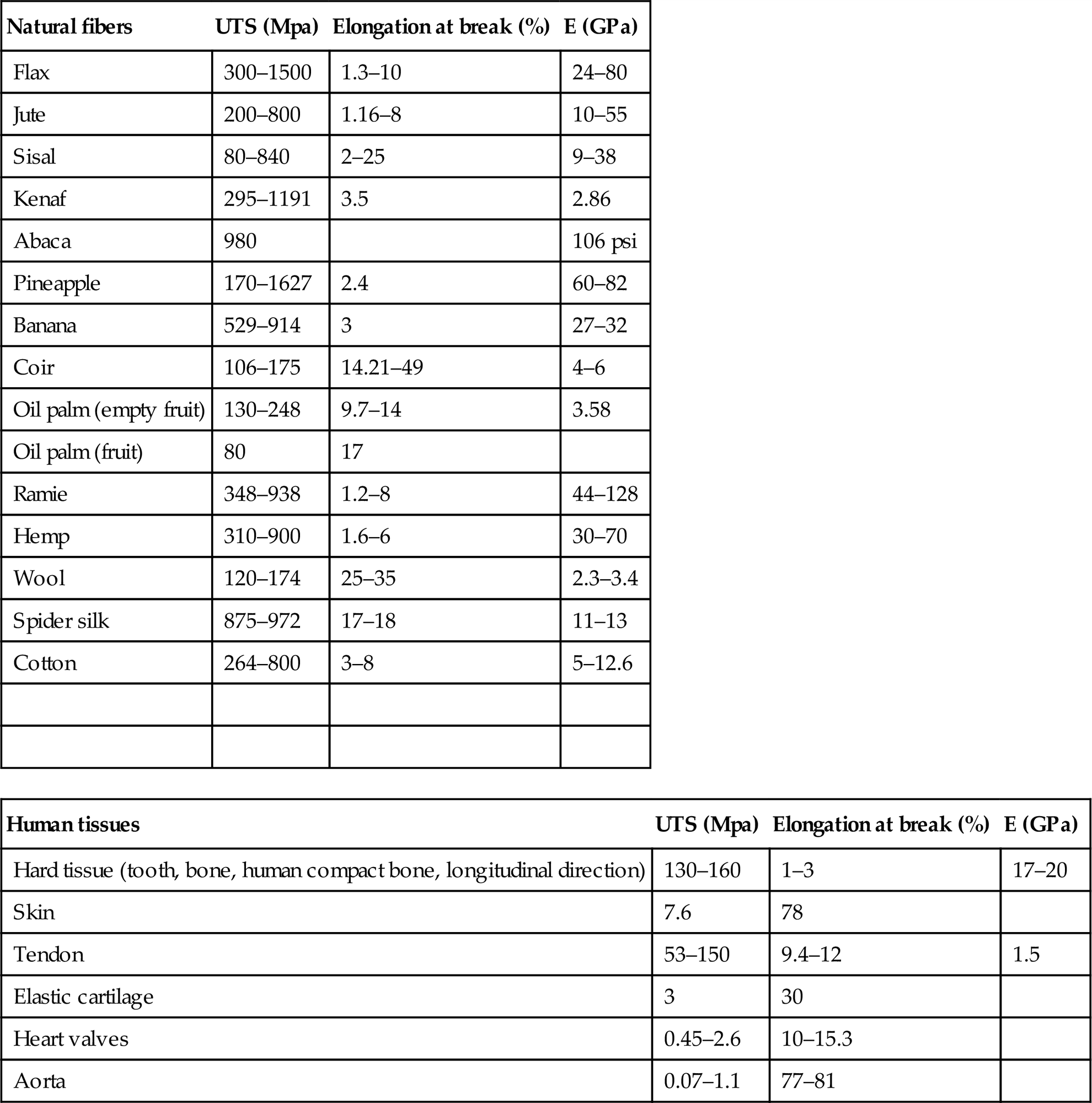
Composition, structure, and material properties of silk fibers produced by spiders, silkworms, scorpions, mites, and flies may differ widely depending on the specific source and the uncontrollable reeling conditions of those insects. Spinning under controlled conditions will have more uniform cross-sectional area of silk fibers, reproducible molecular alignment, and fewer microstructural flaws. The size and weight of cocoons decrease with an increase in temperature and cocoons can bear efficiently both external static forces and dynamic impact loadings [20]. A normal compact cocoon exhibits a high ability of elastic deformation with an elastic strain limit higher than 20% in both longitudinal and transverse directions. The anisotropic properties are mainly due to the nonuniform distribution and orientations of silk segments and the inner layer of cocoon has low porosity (higher silk density) and a smaller average diameter of silk, therefore, there is an increase in elastic modulus and strength from the outside to the inside layers. That is, the thinner the silk, the higher the elastic modulus and tensile strength and the maximum values at the innermost layer. On the other hand, at temperatures above the glass transition temperature, the cocoon and its layers become softer and softer and behave similar to a rubber-like material. Silk fibers have higher tensile strength than glass fiber or synthetic organic fibers, good elasticity, and excellent resilience [21]. They resist failure in compression, are stable at physiological temperatures, and the sericin coating is a water-soluble proteinaceous glue. Table 1.2 shows the comparison of the mechanical properties of common silk types (silkworm and spider dragline) to several types of biomaterial fibers and tissues commonly used today.
Table 1.2
Mechanical properties of different types of potential natural fibers for composite applications
| Natural fibers | UTS (Mpa) | Elongation at break (%) | E (GPa) |
| Flax | 300–1500 | 1.3–10 | 24–80 |
| Jute | 200–800 | 1.16–8 | 10–55 |
| Sisal | 80–840 | 2–25 | 9–38 |
| Kenaf | 295–1191 | 3.5 | 2.86 |
| Abaca | 980 | 106 psi | |
| Pineapple | 170–1627 | 2.4 | 60–82 |
| Banana | 529–914 | 3 | 27–32 |
| Coir | 106–175 | 14.21–49 | 4–6 |
| Oil palm (empty fruit) | 130–248 | 9.7–14 | 3.58 |
| Oil palm (fruit) | 80 | 17 | |
| Ramie | 348–938 | 1.2–8 | 44–128 |
| Hemp | 310–900 | 1.6–6 | 30–70 |
| Wool | 120–174 | 25–35 | 2.3–3.4 |
| Spider silk | 875–972 | 17–18 | 11–13 |
| Cotton | 264–800 | 3–8 | 5–12.6 |
| Human tissues | UTS (Mpa) | Elongation at break (%) | E (GPa) |
| Hard tissue (tooth, bone, human compact bone, longitudinal direction) | 130–160 | 1–3 | 17–20 |
| Skin | 7.6 | 78 | |
| Tendon | 53–150 | 9.4–12 | 1.5 |
| Elastic cartilage | 3 | 30 | |
| Heart valves | 0.45–2.6 | 10–15.3 | |
| Aorta | 0.07–1.1 | 77–81 |
Fibroin is a semicrystalline polymer of natural fibrous protein mainly consisting of two phases [22]: namely β–sheet crystals and noncrystalline, including microvoids and an amorphous structure, by which the structure of the sericin coating is amorphous and acts as an adhesive binder to maintain the fibroin core and the overall structural integrity of the cocoon. Degumming is a key process during which sericin is removed by thermochemical treatment of the cocoon. Although this surface modification can affect the tensile behavior and the mechanical properties of silk significantly, it is normally done to enhance interfacial adhesion between the fiber and the matrix.
In addition, according to Altman [23], silks are insoluble in most solvents, including water, dilute acid, and alkali. The reactivity of silk fibers with chemical agents is positively correlated to the size of the internal and external surface areas [24]. When fabricating silk-based composites, the amount of resin gained by fibers is strongly related to the degree of swelling of the noncrystalline regions, i.e., the amorphous regions and the microvoids inside the fibers.
1.2.2 Applications
1.2.2.1 Wound sutures
Silk fibers have been used in biomedical applications particularly as sutures by which the silk fibroin fibers are usually coated with waxes or silicone to enhance material properties and reduce fraying. But in fact, there are lots of confusing questions about the usage of these fibers as there is the absence of a detailed characterization of the fibers used, including the extent of extraction of the sericin coating, the chemical nature of wax-like coatings sometimes used, and many related processing factors. For example, the sericin glue-like proteins are the major cause of adverse problems with biocompatibility and hypersensitivity to silk. The variability of source materials has raised potential concerns with this class of fibrous protein. Yet, silk’s knot strength, handling characteristics, and ability to lay low to the tissue surface make it a popular suture in cardiovascular applications where bland tissue reactions are desirable for the coherence of the sutured structures [25].
1.2.2.2 Scaffolds tissue engineering
A three-dimensional scaffold permits the in vitro cultivation of cell–polymer constructs that can be readily manipulated, shaped, and fixed to the defect site [26]. The matrix acts as the translator between the local environment (either in vitro or in vivo) and the developing tissue, aiding in the development of biologically viable functional tissue. However, during the 1960s to the early 1980s, the use of virgin silk negatively impacted the general acceptance of this biomaterial from the surgical practitioner perspective, e.g., the reaction of silk to the host tissue and the inflammatory potential of silk. Recently, silk matrices are being rediscovered and reconsidered as potentially useful biomaterials for a range of applications in clinical repairs and in vitro as scaffolds for tissue engineering.
Silk, as a protein, is susceptible to proteolytic degradation in vivo and over a longer period of time in vivo will slowly be absorbed. Degradation rates mainly depend on the health and physiological status of the patient, the mechanical environment of the implantation site, and the types and dimensions of the silk fibers. The slow rate of degradation of silk in vitro and in vivo makes it useful in biodegradable scaffolds for slow tissue ingrowths since the biodegradable scaffolds must be able to be retained at the implantation site, including maintaining their mechanical properties and supporting the growth of cells, until the regenerated tissue is capable of fulfilling its desired functions. The degradation rate should be matched with the rate of neotissue formation so as not to compromise the load-bearing capabilities of the tissue.
Additionally, scaffold structures, including the size and connective of pores, determine the transport of nutrients, metabolites, and regulatory molecules to and from cells. The matrix must support cell attachment, spreading, growth, and differentiation. Meinel et al. [26] concentrated on cartilage tissue engineering with the use of silk protein scaffolds and the authors identified and reported that silk scaffolds are particularly suitable for tissue engineering of cartilage starting from human mesenchymal stem cells (hMSC), which are derived from bone marrow, mainly due to their high porosity, slow degradation, and structural integrity.
Recent research with silk has focused on the development of a wire rope matrix for the development of autologous tissue-engineered anterior cruciate ligaments (ACL) using a patient’s own adult stem cells [27]. Silk fibroin offers versatility in matrix scaffold design for a number of tissue engineering needs in which mechanical performance and biological interactions are major factors for success, including bone, ligaments, tendons, blood vessels, and cartilage. Silk fibroin can also be processed into foams, films, fibers, and meshes.
1.2.3 Silk-based biocomposites
Annamaria et al. [28] discovered that environment-friendly biodegradable polymers can be produced by blending silk sericin with other resins. Nomura et al. [29] identified that polyurethane foams incorporating sericin are said to have excellent moisture-absorbing and -desorbing properties. Hatakeyama [30] has also reported producing sericin-containing polyurethane with excellent mechanical and thermal properties. Sericin blends well with water-soluble polymers, especially with polyvinyl alcohol (PVA). Ishikawa et al. [31] investigated the fine structure and the physical properties of blended films made of sericin and PVA. Moreover, a recent patent reported on a PVA/sericin cross-linked hydrogel membrane produced by using dimethyl urea as the cross-linking agent had a high strength, high moisture content, and durability for usage as a functional film [32].
Silk fibroin film has good dissolved oxygen permeability in a wet state but it is too brittle to be used on its own when in a dry state; whereas for chitosan, it is a biocompatible and biodegradable material which can be easily shaped into films and fibers. Park et al. and Kweon et al. [33,34] have introduced an idea of silk fibroin/chitosan blends as potential biomedical composites as the crystallinity and mechanical properties of silk fibroin are greatly enhanced with increasing chitosan content.
Another type of biocomposite is the silk fibroin/alginate blend sponges. For biotechnological and biomedical fields, silk fibroin’s reproducibility, environmental and biological compatibility, and nontoxicity are of benefit in many different clinical applications. As the collective properties, especially mechanical properties, of silk fibroin sponges ina dry state are too weak to handle as wound dressing, they can be enhanced by blending silk fibroin films with other synthetic or natural polymers, e.g., the polysaccharide sodium alginate.
Furthermore, Katori and Kimura [35] and Lee et al. [36] examined the effect of silk/poly(butylenes succinate) (PBS) biocomposites. They found that the mechanical properties, including tensile strength, fracture toughness, and impact resistance, and thermal stability of biocomposites would be greatly affected by their manufacturing processes. Moreover, a good adhesion between the silk fibers and PBS matrix was found through the observation and analysis by scanning electron microscope (SEM) imaging.
The mechanical properties of Bombyx mori, twisted Bombyx mori, and Tussah silk fibers were also investigated through tensile property tests. It was found that Tussah silk fiber exhibited better tensile strength and extensibility compared with the others. However, the stiffness of all samples was almost the same. This may be due to the distinction of the silkworm raising process and the cocoon producing and spinning conditions. Based on the Weibull analysis, it was shown that the Bombyx mori silk fiber has a better reproducibility in terms of experimental measurement, than that of the Tussah silk fiber. This may be due to the degumming treatment which has an effect on the microstructure of the fiber.
By using silk fiber as reinforcement for biodegradable polymer, the mechanical properties change substantially. Cheung et al. [37] have demonstrated that the use of silk fiber to reinforce Poly(lactic acid) (PLA) can increase its elastic modulus and ductility by 40 and 53%, respectively, as compared with a pristine sample. It was also found that the biodegradability of silk/PLA biocomposites was altered with the content of the silk fiber in the composites. It reflects that the resorbability of the biocomposites used inside the human body can be controlled, in which this is the key parameter of using this new type of material for bone plate development.
1.3 Chicken feather fiber
Animal-based natural fibers, like chicken feather fiber (CFF), have attracted much attention to different product design and engineering industries recently, and the use of CFF as reinforcements for polymer-based biodegradable materials has emerged gradually. The advantages of using these natural fibers over traditional reinforcing fibers in biocomposite materials are their low cost, low density, acceptable specific strength, recycability, biodegradability etc. Natural fibers generally have high specific mechanical properties.
Due to an increasing public awareness of environmental protection, the application of natural fibers in biocomposite materials has been increased rapidly in the past few years. CFF, because of their renewable and recyclable characteristics, have been appreciated as a new class of reinforcements for polymer-based biocomposites. However, the full understanding of their mechanical properties, surface morphologies, environmental influences due to moisture and chemical attacks, bonding characteristics between silk fibroin and the surrounding matrix, and the manufacturing process is essential.
1.3.1 Chicken feather
According to the survey conducted recently, a chicken processing plant produces about 4000 pounds of chicken feathers every hour. In most western countries, these feathers are used as (1) feather fiber feed; (2) air filter elements that replace traditional wood pulps (retarding the tree cut-down rate); and (3) lightweight feather composites. Chicken feathers are approximately 91% protein (keratin), 1% lipids, and 8% water. The amino acid sequence of a chicken feather is very similar to that of other feathers and also has a great deal in common with reptilian keratin from claws [38]. The sequence is largely composed of cystine, glycine, proline, and serine, and contains almost no histidine, lysine, or methionine. In fact, a CFF is made up of two parts, the fibers and the quills (see Fig. 1.5). The fibers are thin filamentous materials that merge from the middle core material called quills. In simple terms, the quill is a hard, central axis off which soft, interlocking fibers branch. Smaller feathers have a greater proportion of fiber, which has a higher aspect ratio than the quill. The presence of quill among fibers results in a more granular, lightweight, and bulky material. A typical quill has dimensions on the order of centimeters (length) by millimeters (diameter). Fiber diameters were found to be in the range of 5–50 μm. The density of CFF is lighter than the other synthetic and natural reinforcements, thus, CFF inclusion in a composite could potentially lower composite density, whereas the density of a typical composite with synthetic reinforcing increases as fiber content increases. Hence, lightweight composite materials can be produced by the inclusion of CFF to plastics which even reduces the transportation cost. The barbs at the upper portion of the feather are firm, compact, and closely knit, while those at the lower portion are downy, i.e., soft, loose, and fluffy. The down feather provides insulation, and the flight feather provides an airfoil, protects the body from moisture, the skin from injury, and provides colors and shapes for displays. Fig. 1.6 shows the cross-sectional views of the flight and down feather fibers. It is obvious that flight feather fiber exists in a hollow form while down fiber is solid. In terms of fiber reinforcement, the use of down fiber appears much better than the use of flight fiber.

The moisture content of CFF is an important factor that can highly influence their weight and mechanical properties. The moisture content of processed CFFs can vary depending upon processing and environmental conditions. The glass transition temperature (Tg) of the feather fibers and inner quills is approximately 235°C while for the outer quills it is 225°C. High Tg represents that a tighter keratin structure is formed to which water is more strongly bonded. Fibers and inner quills do not begin to lose water below 100°C. The moisture evolution temperature of the CFF and quill occurs in the range of 100–110°C. This suggests that it may be possible to have fully-dry fibers and inner quills at 110°C.
The length and diameter (sometimes in the form of bundles) of CFF would highly affect their properties and the impregnatability of resin into a resultant composite. Therefore, the control of resin temperature (thus, its viscosity), while at the same time managing the sonication (ultrasonic vibration) time to facilitate the resin penetration rate into the fibers are essential. Short or longer fibers would highly affect the stress transferability as well as the shear strength of the composites. The fibers, themselves, also would be a barrier to the movement of polymer chains inside the composites and may result in increasing the strength and thermal properties, but reduce the fracture toughness. These properties will be studied in detail in this section.
1.3.2 CFF/PLA biocomposites
Mixing CFF with biopolymers, e.g., PLA, can form a biodegradable composite used for plastic products and implant applications. In preparation of the composite, chicken feather was immersed in alcohol for 24 h, then washed in a water-soluble organic solvent, and dried under 60°C for 24 h [38]. CFF with a diameter of about 5 μm and length of 10–30 mm were separated from the quill and then used. Fig. 1.4 shows an SEM photograph of a CFF. Fig. 1.7 shows the relations between CFF content and peak stress and modulus of elasticity, respectively. The modulus of elasticity of CFF/PLA composite increases with the CFF content and reaches the maximum modulus of 4.38 GPa (increment up to 35.6%) at the CFF content of 5 wt%. This reveals that the incorporation of CFF into the matrix is quite effective for reinforcement. The decrease of modulus for the composite with the CFF content above 5 wt% will be due to the insufficient filling of the matrix resin, designating 5 wt% CFF to be the critical content.
It also can be found from the peak stress that the tensile strength of PLA after the addition of CFF is lower than that of pure PLA. This phenomenon, also reported by other researchers [26,39], is an indication of poor adhesion between the CFF and the matrix. Although the CFF surface is rough, the hydrophobic properties of CFF and PLA would highly affect their bonding efficiency. Therefore, the adhesion property between them is an issue. And the stress could not be transferred from the matrix to the stronger fibers. Another possible explanation of this phenomenon could be that the CFFs were randomly oriented inside the composite; the failure of the composite might be initiated by the failure of the matrix and then followed by fiber breakage. Fig. 1.8 shows the stress–strain curves of the pure PLA and 5 wt% CFF/PLA composite. It is observed that a much longer plateau is located between a strain where the peak stress is reached and the strain at break. It can be concluded that the proper content addition of CFF shows a positive effect on elongation to break for PLA, which was expected because of CFFs acting as bridges to prolong the fracture process of the CFF/PLA composite, and that the failure of the composite was controlled by the bridging effect of CFF inside the composite. These conclusions could be proved by the fractured morphology of the microstructures observed by SEM. The thermal properties, such as glass transition temperature (Tg), crystallization temperature (Tc), melting temperature (Tm), crystallization enthalpy (ΔHc), and melting enthalpy (ΔHm), obtained from the DSC studies are summarized in Table 1.3.
1.4 Conclusion
The mechanical and thermal properties of silk fiber/PLA and CFF/PLA biocomposites have been investigated in depth in the past few years. The mechanical properties in terms of elastic modulus and ductility of these biocomposites increased substantially compared to the neat polymers. From the DMA results, incorporation of the fibers gave rise to a considerable increase in their storage modulus (stiffness) and to a decrease of their tan delta values. These results demonstrated the reinforcing effect of CFF on PLA matrix. The TGA thermograms reveal the thermal stability of the composites with respect to the pure PLA resin. In addition, the TMA results suggest that the biocomposite with a small amount of animal fiber provided also better thermal properties as compared with pristine polymer. The SEM investigations confirm that both fibers were well dispersed in the PLA matrix. However, concerns have been raised on their interfacial bonding properties, as normally it is difficult to have consistent physical properties between fibers collected from different suppliers. The surface of the fibers are rough, which enables a mechanical interlocking with surrounding matrix to be generated. Moisture absorption is another issue that restricts the wide usage of natural fibers for primarily structural components in industry.
Although plant- and animal-based fibers have attracted much attention in product design and engineering and bioengineering industries and have undergone comprehensive research in the past few years, many factors, such as their interfacial bonding and stress transfer properties, have not yet been solved to date. To broaden the application of these fibers in solving environmental problems, more studies have to be done in the future.





