Ceramic biomaterials for tissue engineering
J. Huang, University College London, UK
S. Best, University of Cambridge, UK
Abstract:
This chapter reviews the range of ceramics currently used in skeletal repair and tissue regeneration and covers the bioinert, bioactive and resorbable ceramics, glasses and glass ceramics. The scope of the chapter includes the relationships between microstructure (crystalline and non-crystalline) and properties (mechanical properties, surface properties, biocompatibility and bioactivity). The processing (porous tissue engineering scaffolds and surface modification) of bioceramics is also considered. Based on the stringent requirements for clinical application, prospects for the development of advanced ceramic materials for tissue engineering are highlighted for the future.
Key words
bioceramics; hydroxyapatite; bioactive glasses; mechanical properties; biocompatibility; bioactivity
1.1 Introduction
Ceramic materials, such as porcelain, cement and glass, have been part of everyday life for thousands of years; advanced ceramics have been used in recent times in telecommunications, the environment, energy, transportation and health. Generally speaking, ceramic materials are solid materials composed of inorganic, non-metallic substances, exist as both crystalline and non-crystalline (amorphous) compounds, and glasses and glass-ceramics (partially crystallised glasses) are subclasses of ceramics.
A biomaterial is a non-viable material used in a medical device; intend to interact with biological systems (Williams, 1987). Various engineering materials, including ceramics, metal (alloys), polymer and composites, have been developed to replace the function of the biological materials. The focus of this chapter is to consider ceramics used in biological applications, now generally referred to as bioceramics, and their applications in implants and in the repair and reconstruction of diseased or damaged body parts. Most clinical applications of bioceramics relate to the repair of the skeletal system, comprising bone, joints and teeth, and to augment both hard and soft tissue. According to the types of bioceramics and host tissue interactions, they can be categorised as either bioinert or bioactive, the bioactive ceramics may be resorbable or non-resorbable, and all these may be manufactured either in porous or dense in bulk form, or granules or coatings.
The chapter begins by introducing various ceramics used in medical applications, including bioinert ceramics (i.e. alumina and zirconia), and bioactive ceramics (i.e. calcium phosphates, bioactive glasses and glass-ceramics). To understand the nature and formation of ceramic structures, it is essential to have an understanding of the atomic arrangements, the forces between atoms and the location of atoms in a crystalline lattice. The difference between crystalline and non-crystalline materials with the examples of hydroxyapatite ceramics and bioactive glasses, the most widely applied bioceramics, is discussed in Section 1.2. The properties of a ceramic are determined by its microstructure (e.g. grain size and porosity). A brief summary of the common techniques for characterisation of the microstructure of ceramics is included in Section 1.3. This is followed by a review of the properties of ceramics, particularly mechanical properties, surface properties, biocompatibility and bioactivity, which are crucial for the biological application of the ceramics. Alumina and zirconia have excellent mechanical properties for the load-bearing applications, while the bioactivity of glass and ceramics leads to the potential for osteoconduction. A brief review of the processing of ceramics with an example of hydroxyapatite (HA) is presented in Section 1.5. The processing of porous ceramics scaffolds and surface modification of surface using coating and thin film deposition is also discussed. The chapter finishes with a summary highlighting the importance of understanding of the clinical requirement and relationships between processing, microstructure and properties, which will help to develop better ceramic materials for tissue engineering.
1.1.1 Bioinert ceramics
Alumina and zirconia have been used as an important alternative to surgical metal alloys in total hip prostheses and as tooth implants. The main advantages of using ceramics over the traditional metal and polymer devices are lower wear rates at the articulating surfaces and the release of very low concentrations of ‘inert’ wear particles. For example, using femoral heads of alumina ceramic bearing against alumina cup sockets significantly reduces wear debris when against ultrahigh molecular weight polyethylene cups. Excessive wear rates can contribute to loosening and eventual implantation failure. Alumina ceramics have been used successfully for many years. Zirconia ceramics have advantages over alumina ceramics in terms of higher fracture toughness and higher flexural strength, combined with a relatively lower Young’s modulus (Table 1.1). Therefore, zirconia ceramics were developed for bearing surfaces in total hip prostheses. However, concerns about in-service failures (particularly the premature fracture of a batch of ceramic femoral heads) resulted in a Food and Drug Administration (FDA) recall. For this reason, the use of zirconia for strengthening and toughening of alumina matrix composites has been developed. One example is Biolox® delta (CeramTec), which has FDA approval for use in femoral head components.
Table 1.1
A summary of mechanical properties of various biomaterials (Kokubo, 1991; Hench and Andersson, 1993; Hulbert, 1993; Hench and Best, 2004)
| Materials | Density (g cm− 3) | Hardness (Vickers, HV) | Young’s modulus (GPa) | Bending strength (MPa) | Compressive strength (MPa) | Fracture toughness KIC (MPa m1/2) |
| Bioglass® 45S5 | 2.66 | 458 | 35 | 40–60 | 0.4–0.6 | |
| A-W glass-ceramic | 3.07 | 680 | 118 | 215 | 1080 | 2.0 |
| Sintered HA | 3.156 | 500–800 | 70–120 | 20–80 | 100–900 | 0.9–1.3 |
| Alumina | 3.98 | 2400 | 380–420 | 595 | 4000–4500 | 4–6 |
| Zirconia (TZP) | 6.05 | 1200 | 150 | 1000 | 2000 | 7 |
| Zirconia (Mg-PSZ) | 5.72 | 1120 | 208 | 800 | 1850 | 8 |
| Ti6Al4V | 4.43 | 340 | 110 | 900 | 970 | ~ 80 |
| 316 stainless steel | 8 | 200 | 540–1000* | ~ 100 |
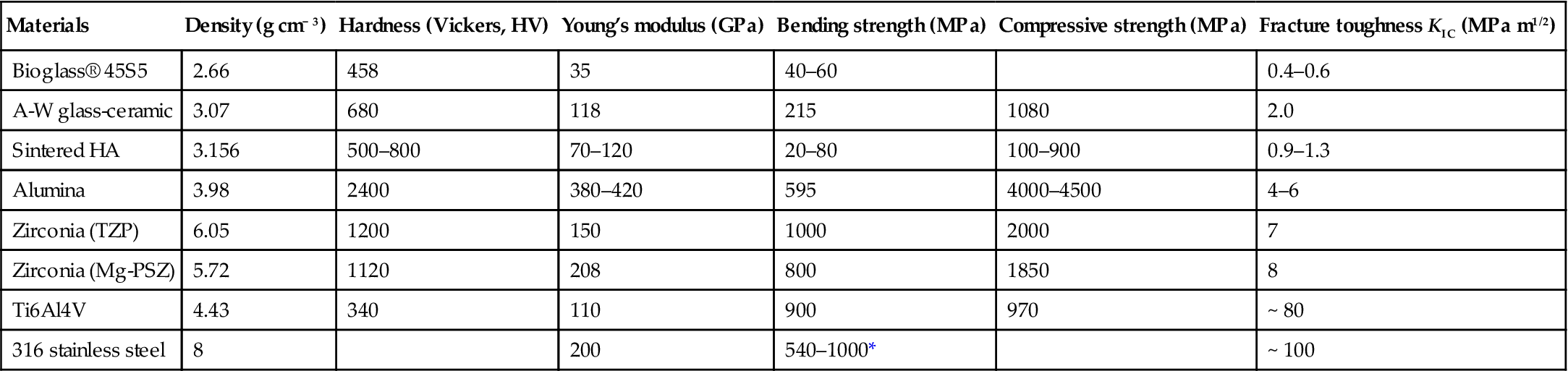
*Tensile strength.
Nanotechnology has also been applied to improve the properties of implant materials with the aim of extending the longevity of implant devices in the body, with no revision surgery necessary at the later time. To improve the fracture toughness of alumina ceramics, nanophase alumina with grain size of 23 nm were synthesised. The modulus of elasticity of nanophase alumina decreased by 70% (Webster et al., 1999). The fracture toughness of alumina can then be controlled through the use of nanophase formulations; furthermore, enhanced biological responses of osteoblast cells to the nanophase materials were found, indicating the improved osseointegration potential for nanophase alumina (Webster et al., 2000).
Alumina and zirconia have good biocompatibility, and adequate mechanical strength, but are relatively biologically inactive (nearly inert) and lack direct bonding with host tissue. Bioactive materials are conceptually different from bioinert materials in that chemical reactivity is essential. A series of bioactive ceramics, glasses and glass-ceramics are capable of promoting the formation of bone at their surface and of creating an interface, which contributes to the functional longevity of tissue.
1.1.2 Bioactive ceramics
Bioactive ceramics include several major groups, such as calcium phosphate ceramics, bioactive glasses and glass-ceramics.
Calcium phosphate ceramics
Calcium phosphates are the major constituent of bone mineral. Table 1.2 lists several calcium phosphates with their chemical formula and Ca/P ratio (from 0.5 to 2). These calcium phosphates can be synthesised by mixing calcium and phosphate solution under acid or alkaline conditions. Only certain compounds are useful for implantation in the body: compounds with a Ca/P ratio less than 1 are not suitable for biological implantation due to their high solubility.
Table 1.2
Ca/P ratio of various calcium phosphates (Aoki, 1991)
| Name | Abbreviation | Formula | Ca/P ratio |
| Tetracalcium phosphate | TTCP | Ca4O(PO4)2 | 2.0 |
| Hydroxyapatite | HA | Ca10(PO4)6(OH)2 | 1.67 |
| Tricalcium phosphate (α,α′,β,γ) | TCP | Ca3(PO4)2 | 1.50 |
| Octacalcium phosphate | OCP | Ca8H2(PO4)6•5H2O | 1.33 |
| Dicalcium phosphate dihydrate (brushite) | DCPD | CaHPO4•2H2O | 1.0 |
| Dicalcium phosphate (montite) | DCP | CaHPO4 | 1.0 |
| Calcium pyrophosphate (α,β,γ) | CPP | Ca2P2O7 | 1.0 |
| Calcium pyrophosphate dihydrate | CPPD | Ca2P2O7•2H2O | 1.0 |
| Heptacalcium phosphate | HCP | Ca7(P5O16)2 | 0.7 |
| Tetracalcium dihydrogen phosphate | TDHP | Ca4H2P6O20 | 0.67 |
| Calcium phosphate monohydrate | CPM | Ca(H2PO4)2•H2O | 0.5 |

The most extensively used synthetic calcium phosphate ceramic for bone replacement is HA because of its chemical similarities to the inorganic component of bone and teeth. HA with a chemical formula of Ca10(PO4)6(OH)2 has a theoretical composition of 39.68 wt% Ca, 18.45 wt% P; Ca/P wt ratio of 2.151 and Ca/P molar ratio of 1.667. It is much more stable than other calcium phosphate ceramics within a pH range of 4.2–8.0.
The stoichiometry of HA is highly significant where thermal processing of the material is required. Slight imbalances in the ratio of Ca/P can lead to the appearance of extraneous phases. If the Ca/P is lower than 1.67, β-tricalcium phosphate (TCP) and other phases, such as tetracalcium phosphate (TTCP), will be present with HA. If the Ca/P is higher than 1.67, calcium oxide (CaO) will be present with the HA phase. The extraneous phases may adversely affect of the biological responses of the implants.
TCP is a biodegradable bioceramic with the chemical formula of Ca3(PO4)2. TCP dissolves in wet media, can be replaced by bone during implantation, and has been commonly used as degradable bone graft.
In an ideal situation, a biodegrable implant material is slowly resorbed and replaced by natural tissue. However, to match the rate of resorption with that of the expected bone tissue regeneration for a biodegradable material is a great challenge. When the solubility of calcium phosphate is higher than the rate of tissue regeneration, the material will not be of use in cavity filling. TCP with Ca/P ratio of 1.5 is more rapidly resorbed than HA. A mixture of HA and β-TCP, known as biphasic calcium phosphate (BCP), has been used as bone substitute (Daculsi et al., 2003). Its chemical properties can be tailored, such as varying the ratio of HA/β-TCP. The higher the TCP content in BCP, the higher the dissolution rate. The resorption rate of BCP can then be monitored and controlled.
Calcium phosphate cement is another important type of bioceramic (Fernandez et al., 1998; Bohner, 2000; Chow and Takagi, 2001; Tamimi et al., 2012). By mixing with various calcium phosphates, an injectable paste can be formed, which will be cured over time. The final product is a carbonate apatite. The cements cure in situ, and are gradually resorbed and replaced by the newly formed bone.
Bioactive glasses and glass-ceramics
The concept of a bioactive glass was initiated by Hench and colleagues (Hench et al., 1971). The composition of Bioglass® is a series of special designed glasses, consisting of a Na2O–CaO–SiO2 glass with the addition of P2O5, B2O3 and CaF2 (Hench and Andersson, 1993). A biologically active hydroxy-carbonate apatite (HCA) layer was formed on the surface of bioactive glasses in vitro and in vivo. This HCA phase is chemically and structurally equivalent to the mineral phase in bone, so it provides a direct bonding by bridging host tissue with implants. It is possible to control a range of chemical properties in bioactive glasses and the rate of bonding to tissue. Some specialised compositions of Bioglass® (e.g. 45S5) can bond to soft tissue as well as bone, in either bulk or particulate form (Hench, 1991).
An attractive feature of glass is that the properties of a glass system can be varied by the adjustment of the composition: for example, the degradation rate or the solubility of phosphate glasses can be controlled by altering the glass composition. The biodegradability as well as the chemical composition similar to inorganic component of bone has led to the applications of phosphate-based glasses in both hard and soft tissue repair and regeneration, such as phosphate glass fibres for muscle and ligament replacements (Abou Neel, 2009).
Apatite–wollastonite (A-W) glass-ceramic, with an assembly of small apatite particles effectively reinforced by β-wollastonite, exhibits not only bioactivity, but also a fairly high mechanical strength (Kokubo et al., 1986). The bending strength, fracture toughness and Young’s modulus of A-W glass-ceramic are the highest among bioactive glasses and glass-ceramics (Table 1.1), enabling it to be used in some compression load-bearing applications (Kokubo, 1991). A comprehensive review of bioactive glasses and glass-ceramics is covered in Chapter 3, by M. N. Rahaman.
In general, the advantages of bioactive glasses are the speed of their surface reactivity and the ability to alter the chemical composition, thus enabling bonding with a variety of tissues. Their mechanical properties are disadvantageous, as these materials have relatively low bending strength compared with other ceramic materials.
Bonfield et al. (1981) proposed the concept of matching the mechanical behaviour of an implant with the tissue to be replaced in order to eliminate the problem of stress shielding of conventional biomaterials. The composite approach can potentially meet the challenge of a longer lifetime, as required for the new generation implant materials (Bonfield, 1988; Wang 2003). For tissue engineering applications, biodegradable composite scaffolds with high mechanical strength as well as enhanced bioactivity and resorbability have been developed (El-Ghannam 2005; Rezwan et al., 2006). Dorozhkin (2009) reviewed the developments of calcium phosphate-based biocomposites and hybrids for medical applications. The bioactive coating of metallic implant (de Groot et al., 1998) is a successful way to overcome the limitation of the relatively poor mechanical properties while utilising the high bioactivity of bioactive glasses and ceramics.
1.2 Characteristics of ceramics
The major characteristics of ceramics are their brittleness, high hardness, thermal and electrical insulation, and corrosion resistance. Chemical inertness is an initial criterion for the selection of suitable materials in biological applications, as the human body is a hostile environment for any material.
Fundamentally, the properties of a material are controlled by the type of bonding between atoms. There are three primary interatomic bonds – metallic, ionic and covalent – and secondary bonds such as van der Waals and hydrogen. Metallic bonding is the predominant bond mechanism for metals. Atomic bonding in ceramics is mainly ionic or covalent or a combination of the two (Kingery 1976).
The crystal structure of a material is the periodic arrangement of atoms in the crystal. A basic concept in crystal structures is the unit cell. It is the smallest unit of volume that permits identical cells to be stacked together to fill all the space. By repeating the pattern of the unit cell, the entire crystal lattice can be constructed. The spatial arrangement of individual atoms in a ceramic depends on the type of bonding, the relative sizes of the atoms and the need to balance the electrostatic charges. The brittle nature of ceramic material stems from its crystal structure.
1.2.1 HA and substituted HA
HA possesses a hexagonal lattice and a P63/m space group. This space group is characterised by a six-fold c-axis perpendicular to three equivalent a-axes at angles 120° to each other with cell dimension of a = b = 0.9418 nm and c = 0.6884 nm (Posner, 1969).
The mineral phase of bone, biological apatite, is not stoichiometric HA. The apatite is hospitable to a variety of cationic and anionic substitutions, and the type and amount of these ionic substitutions in the apatite phase varies from the wt% level (e.g. 3–8 wt% CO3). to the ppm–ppb level(e.g.Mg2 + or Sr2 +). Substitution in the apatite structure for (Ca), (PO4) or (OH) groups results in changes in properties, such as lattice parameters, morphology and solubility, without significantly changing the hexagonal symmetry.
The substitution of fluoride (F− for OH−) has the consequence of increasing the crystallinity, crystal size and the stability of the apatite, which in turn reduces solubility. Fluoride substitution has been implicated in caries prevention, where its presence in enamel crystals increases stability. This helps to resist dissolution in the acidic oral environment (LeGeros and LeGeros, 1993).
Carbonate, CO3, can substitute for either the hydroxyl (OH) groups, or the phosphate groups, and the resulting apatite is designated as Type A or Type B respectively. An important effect of carbonate substitution in HA is on crystal size and morphology. An increase in carbonate content leads to changes in the size and shape of apatite crystal (LeGeros et al., 1967) and the carbonate substituted apatites are more soluble than carbonate-free synthetic apatites.
Although silicon has only been found in trace quantities in bone mineral (up to a level of ~ 0.5 wt%), it has been shown to have a crucial role in bone mineralisation, and believed to be essential in skeletal development (Carlisle, 1970, 1972). In vitro and in vivo bioactivity was enhanced with the incorporation of silicate groups into the HA lattice (Gibson et al., 1999a; Patel et al., 2002, 2005). The silicate substitution in HA inhibited densification and grain growth at higher sintering temperatures (Gibson et al., 1999b, 2002), thus increasing the total surface area/volume ratio of grain boundaries (Porter et al., 2003, 2004), this may also have an effect on the in vivo responses. The surface charge of silicate substituted HA (SiHA) was significantly more negative than that of pure HA (Botelho et al., 2002), and may contribute to the faster bone-like apatite formation in vitro induced by SiHA. A higher concentration of protein absorption onto SiHA than HA was related to the formation of a silicate network structure, which interacts with integrins, thus triggering a signalling cascade and leading to consequent cell attachment, proliferation and differentiation. The addition of silicate in SiHA provides an extra chemical cue to stimulate and enhance bone formation, which promotes the attachment and proliferation of human osteoblast (HOB) cells (Fig. 1.1). The enhanced bioactivity of SiHA is the result of the increase in availability of Si as well as the favourable topography from increased grain boundaries with decrease in the grain size. Therefore, SiHA is a highly attractive alternative to conventional HA in bone replacement. SiHA as bone graft, commercially known as Actifuse™, has been used successfully for spinal fusion.

Porous strontium (Sr) and magnesium (Mg) co-substituted HA has been exploited to prolong the beneficial Mg release during the bone regeneration process as well as to utilise the anti-osteoporotic and cariostatic properties of Sr ions (Landi et al., 2013). Enhanced adhesion of osteoblasts was found on HA doped with yttrium (Y) compared with those doped with cadmium (Cd), zinc (Zn) or magnesium (Mg) (Webster et al., 2002).
Ti-substituted HA nanoparticles were found to inhibit the growth of four bacterial strains, including multi-antibiotic resistant epidemic MRSA (EMRSA) 15 and EMRSA 16 ‘superbugs’, which is the first step in the development of multifunctional dental and orthopaedic prostheses (Huang et al., 2011). Recent studies have shown that zinc-substituted HA (ZnHA) resulted in increased proliferation and differentiation of human adipose-derived mesenchymal stem cells, while there is a significant decrease in the number of viable Staphylococcus aureus bacteria after being in contact with ZnHA (Thian et al., 2013).
Besides the substitutions mentioned above, there are other substitutions, both cationic (substituting for calcium with barium, lead) and anionic (substituting for the phosphate with vanadates, borates, manganates). Shepherd et al. summarised some of the key effects of substitutions with magnesium, zinc, strontium, silicon and carbonate on physical and biological characteristics of HA, such as thermal stability, solubility, osteoclastic and osteoblastic response in vitro and degradation and bone regeneration in vivo (Shepherd et al., 2012).
1.2.2 Bioactive glasses
Polycrystalline ceramics are solids in which the atoms or ions are arranged in regular array. In contrast, the regularity (order) is only short range in glass (amorphous materials), because a glass formed when a molten ceramic composition is rapidly cooled while the atoms do not have time to arrange themselves in a periodic structure. A number of bioactive glasses have been developed and investigated for tissue engineering and probably the best known of these is Bioglass® (Hench, 2006), while a three-dimensional (3D) SiO2 network is modified by incorporation of Na2O, CaO and P2O5. The composition of Bioglass® makes the surface highly reactive when exposed to an aqueous medium, leading to in vitro and in vivo bioactivity.
Furthermore, sol–gel-derived bioactive glasses have a porous texture in the nanometre range, giving them a surface area of 150–600 m2 g−1, which is two orders of magnitude higher than that of melt-derived glasses. Dissolution is therefore more rapid for sol–gel glasses at similar composition, and more silanol groups are on sol–gel glass surfaces to act as nucleation sites for formation of apatite layer, leading to high bioactivity. A comprehensive review of bioactive glass has recently been done by Jones (2013).
1.2.3 Bioactive glass-ceramics
A glass-ceramic is polycrystalline solid prepared by the controlled crystallisation or devitrification of a parent glass. It generally consists of fine grains (with crystal sizes ranging from 0.1 to 10 μm) and has a small volume of residual glass sited at the grain boundary. One advantage of glass-ceramics is that the crystallisation and formation of the crystal phases can be controlled to develop materials with a combination of special properties, such as bioactivity, machinability and improved mechanical properties. A-W glass-ceramic is the most extensively studied glass-ceramic for use as bone substitutes (Kokubo, 1991). It combines high bioactivity with desirable mechanical properties and has been successfully in the load-bearing spinal area of the body.
1.3 Microstructure of ceramics
The microstructure of ceramics determines their mechanical and biological properties. Ceramics are commonly polycrystalline; phases are physically or chemically distinguishable from each other, and may vary in the crystal structures. The arrangement of crystals (or grains) and phases constitutes the microstructure of the ceramics. Various grain sizes are observed, which depend on the manufacture method, raw materials and grain growth during sintering; a glassy phase, grain boundaries and gas-filled pores may also exist.
Medical grade alumina with an average grain size of less than 4 μm and 99.7% purity exhibits good flexural and compressive strength (Table 1.1). The strength, fatigue resistance and fracture toughness of alumina are a function of grain size and percentage of sintering aid (Hulbert, 1993): a high concentration of sintering aids for limiting grain growth must be avoided because sintering aids remain in the grain boundaries and reduce fatigue resistance, especially in a corrosive physiological environment. This is particularly important for orthopaedic prostheses to be used in younger patients.
The grain structure of ceramics can be observed by optical and electron microscopy after polishing and etching. Glass-ceramic microstructures are characterised by a dispersion of crystals in a continuous glassy matrix. In contrast, no microstructural features can be observed in glasses. In addition to microscopy, other analytical methods, such as X-ray diffraction (XRD), infra-red spectroscopy and spectrochemical analysis for detecting impurities, are equally important for understanding the microstructure of ceramics.
XRD is a common technique for structure determination, phase analysis, detection of preferred orientation and determination of crystal size. Rietveld refinement can be carried out after the collection of X-ray diffraction data. It involves comparing the experimental data with data derived from a theoretical model; the lattice parameters are allowed to vary and are refined to match the experimental data. In carbonated substitute HA, the substitution of larger planar CO3 group for smaller liner OH group causes an increase in the a-axis and a decrease in the c-axis in the Type A substitution; while for Type B, the substitution of smaller planar CO3 group for a larger tetrahedral PO4 group causes a decrease in the a-axis and an increase in the c-axis (LeGeros and LeGeros, 1993). The larger silicate ions (![]() ) substitute for the smaller phosphate (
) substitute for the smaller phosphate (![]() )group causes a decrease in the a-axis and an increase in the c-axis with increasing silicate content (Gibson et al., 1999b).
)group causes a decrease in the a-axis and an increase in the c-axis with increasing silicate content (Gibson et al., 1999b).
Solid-state nuclear magnetic resonance (NMR) spectroscopy is another technique for analysing the structure of materials. The dissolution of glass network and the formation of Si-O groups after soaking 20Na2O · 80SiO2 glass in SBF was confirmed using 23Na, 29Si and 31P NMR (Hayakawa et al., 1999). This study showed that the hydrated silica gel layer with Si(OSi)3 O− units provided the negative charged sites to promote the precipitation of calcium phosphates and lead to apatite nucleation and crystallisation. A 31P and 1H NMR study showed that the surface of nanocrystalline was different from the bulk composition as the nanocrystals consisted of a crystalline HA cores covered by a disordered surface layer (Jager et al., 2006). The surface region was dominated by hydrogen phosphate anions with no HA-like structural motif and structural water.
X-ray photoelectron spectroscopy (XPS) is a useful tool to detect the chemical composition and evaluate the chemical bonding states (or oxidation state) as well as the electronic structure of the surface (outermost 5 to 10 nm of ceramic materials). It has been used to study the formation of apatite on Ti–6Al–4 V alloy in SBF (Takadama et al., 2001). The bonding energy of an electron is characteristic of the atom and orbital from which the electron was emitted, and the elemental composition of the surface is then determined.
Infrared spectroscopy is a non-destructive technique enabling the presence of certain bonds in a material to be established. It has been used to study the reactions between aqueous solutions and the surfaces of bioactive glasses and glass-ceramics. The reaction stages occurring on the material side can be clearly delineated by changes in the vibrational modes of the chemical species in the surface. The presence of carbonate substitution in HA can be observed directly in infrared spectra in the form of weak peaks at between 870 cm−1 and a stronger doublet at between 1470 cm−1 (Rehman and Bonfield, 1997).
Raman spectroscopy is another analytical technique that can detect chemical bonds present in a material. It has been used to compare the bone-like apatite formed on Bioglass® surfaces in vitro with biological apatite (Rehman et al., 1994) and the crystal imperfection in silicate substituted HA (Zou et al., 2005).
Scanning electron microscopy (SEM) has the advantages of high resolution and depth focal length. Fractography of ceramics is important to understand the performance of materials. The size, shape and connectivity of pores of a HA ceramic scaffold can be revealed (Fig. 1.2). The selection or optimisation of suitable material for required application can be carried out accordingly. Combining SEM with X-ray microanalysis makes it possible to examine the changes of structure and composition on the material surface and on the implant–bone interface, i.e. the thickness of each layer in the reaction zone on the surface of implant in vivo (Kitsugi et al., 1987). Such quantitative analysis is useful in designing a new material.
Complementary to SEM, laser scanning confocal microscopy, with the advantage of non-destructive optical sectioning, has been used to generate the 3D surface structure of A-W glass ceramics in vitro (Akhshi et al., 2005); the detailed structure in the Z-direction will provide further understanding of the in vitro responses of the material.
Transmission electron microscopy (TEM), with its high resolution and selected area electron diffraction, is a powerful tool for ultrastructural analysis (Fig. 1.3). The use of high-resolution TEM has enabled dislocations and grain boundaries to be characterised in HA and SiHA, with a significant increase in density of triple junctions per unit area in SiHA over HA (Porter et al., 2003, 2004). Dissolution was observed to follow the order 1.5 wt% SiHA > 0.8 wt% SiHA > HA, and was prevalent at grain boundaries and triple junctions, suggesting that an increased number of defects in SiHA leads to an increased rate of dissolution of SiHA. The findings will help to understand the mechanisms by which silicate ions increase the in vivo bioactivity, as the incorporation of silicate ions into HA has been shown to increase the rate of bone apposition to HA bioceramic implants.
Atomic force microscopy (AFM) is another high resolution imaging technique, which is based on the detection of surface forces (repulsive or attractive) between a probe (tip) and the sample surface. It is highly versatile, and can be operated in number of different imaging modes, non-contact, tapping and contact. A height image can be captured when a tip is raster-scanned across the surface. As a result of the movement of the z-piezo crystal in the z-direction to maintain the set deflection of the cantilever, the surface properties of materials, such as surface roughness, can be measured (Fig. 1.4).
Porosity and pore size, distribution and interconnectivity are important parameters for porous glasses and ceramics materials, which determined the mechanical properties and biocompatibility. For a porous material, the pore sizes and volume can be measured from mercury intrusion porosimeter. X-ray microtomography (XMT) is a technique to characterise material structure three-dimensionally and non-destructively. For a tissue engineering scaffold, it is important to obtain pore size and distribution and interconnectivity in 3D. Figure 1.5 shows the pore size and connectivity of a HA scaffold obtained from XMT. It can be seen that X-ray microtomography is a valuable tool for studying 3D structure of porous scaffolds non-destructively.

In general, none of these analysis techniques may adequately be used in isolation to characterise ceramics, but they complement each other and reveal the various aspects of characteristics of tissue engineering materials in vitro and in vivo.
1.4 Properties of ceramics
1.4.1 Mechanical properties
The mechanical properties of a material are important as they determine its structural applications. Table 1.1 summarises some of mechanical properties of bioactive and bioinert ceramics.
Strength can be measured in a number of different ways, such as uniaxial tension, three-point bending, four-point bending and uniaxial compression. Tensile strength testing is typically used for characterising ductile metals. Ceramics are not normally characterised by tensile testing due to the high cost of test specimen fabrication and the requirement for extremely good alignment of the load during testing. Compressive strength is the crushing strength of a material, and is commonly measured for ceramics, especially those that must support loads. The strength of ceramics materials is generally characterised by bending testing, also referred to as flexural testing. Apart from understanding these uniaxial stresses of a material, biaxial strength provides the data under a biaxial stress condition (e.g. under both tensile and shear stresses) since many applications for materials impose multi-axial stress fields. The strength value is dependent on the type of test conducted, flaw size distribution of the material and the stress distribution in the test specimen. Based on the concept of the failure of the weakest link, the strength distribution of ceramic materials can be described as a Weibull modulus, a dimensionless number used to characterise the variability in measured strength of brittle materials (ceramics) which arises from the presence of flaws with a distribution in size and orientation. The higher the Weibull modulus is, the more consistent the material, which means uniform defects are evenly distributed throughout the entire volume.
Fracture toughness is one of the key measures of the mechanical properties of ceramic materials. Due to the brittle nature of ceramics, it is essential to consider fracture in terms of crack surface displacement and the stresses at the tip of the crack using a fracture mechanics approach. The stress concentration at a crack tip is denoted in terms of the stress intensity factor K. Additional subscripts refer to the direction of load application with respect to the position of the crack. In the tensile and bending tests the load is perpendicular to the crack, and Mode I is most frequently encountered for ceramic materials. KIC is the stress intensity factor at which the crack will propagate and lead to fracture. It is also referred to as fracture toughness and is considered as a basic property of ceramic materials. The higher the fracture toughness, the more difficult it is to initiate and propagate a crack.
The hardness of a material is a measure of its resistance to localised deformation by indentation or scratching. A small indenter is pressed into the surface of the material and the size of the indent is measured to calculate a hardness value. The hardness and fracture toughness of a ceramic influence its wear behaviour. The wear properties of bioceramics are important for their application in joint replacement, as wear debris can trigger the inflammatory responses in the surrounding tissue and eventually lead to failure of the implant.
1.4.2 Surface properties
The physicochemical properties of implant materials, such as surface energy and charge, hydrophilicity or hydrophobicity, tend to affect cellular response by influencing protein absorption and cell attachment. Therefore, the surface properties of materials can have a great impact on cellular responses. The nature and development of a stable interface between an implanted bioceramic and bone, which is crucial for the clinical success of the implant, are affected by many factors. It is becoming recognised that a key aspect is the surface modification of the bioceramic, which occurs due to interaction with the local environment. For materials, such as bioactive glasses, a series of surface reactions occur when immersed in physiological solution. Exchanges of Na+ and K+ with H+ and H3O+ ions from solution at the glass surface lead to the loss of soluble silica and the formation of Si-OH (silanols) at the glass–solution interface. This stage is followed by the migration of calcium and phosphate ions through the silica-rich layer to the surface and the formation of an amorphous calcium phosphate layer, which can then crystallise by the incorporation of hydroxyl, carbonate and fluoride ions to create an apatite layer. In the presence of osteogenic precursors, bioactive glasses favour the formation of osteoblasts which govern the further steps of bone development (Hench, 2006).
Enhancing the interaction between tissue and biomaterials is another way to achieve a desirable tissue response to implant materials. Cells recognise surface features and react to them, resulting in contact guidance (Curtis and Wilkinson, 1997). A key design parameter for achieving maximal cell responses is material topography. There is increasing evidence that surface topography both on the micro and nano-scale are important in determining the cell response to biomaterials (Gray et al., 1996; Dalby et al., 2002, 2007). The creation of micro- and nano-scaled surface topography using HA has been attempted by electrohydrodynamic spraying and print-patterning with the aim of up-regulating cell activity (Huang et al., 2004; Ahmad et al., 2006; Munir et al., 2011).
In order to understand the mechanism of bonding of a bioactive material with host tissue, it is necessary to characterise the surface of a material in vitro, both acellular (in physiological solutions) and cell cultures, as the specific tissue compatibility of a material is highly dependent on the composition and structure of surface layers. Such in vitro analysis will help in understanding the potential in vivo host tissue responses and will provide the material characteristics information for developing new tissue engineering scaffold materials.
There are two approaches, solution analysis and surface analysis, for studying the mechanism and reaction at material surface. In solution analysis, the constituents released into the surrounding environment (potentially those will be released into the tissue) are examined. The leaching of alkali and alkaline earth ions from bioactive glass and ceramic surface, the dissolution of silica and the precipitation of calcium phosphates are revealed by this method. The dissolution products of bioactive glasses have a positive effect on the expression of genes regulating osteogenesis (Xynos et al., 2000, 2001). Therefore, the quantification of the release products from bioactive glasses and ceramics is important for their application as scaffolds for the formation of bioengineered bone tissue.
Surface analysis is used to examine the surface of the material using instrumental tools such as AFM, Auger electron spectroscopy, infrared reflection spectroscopy, thin-film X-ray diffraction and SEM with X-ray analysis. Solution analysis can provide information about the total depths of reaction on the surface of a material; however, if precipitation occurs in the system (on the material surface or elsewhere), it will yield misleading information about the surface reactions of a material. By using instrumental tools, the surface structure regarding either the top 1.5 μm or only the top 0.5–5 nm of the sampling depth can be obtained. Combining surface or solution analyses, the characteristics of a reactive material throughout top and deep zones of the surface can be revealed.
1.4.3 Bioactivity and biocompatibility
Bioactivity
To assess the bioactivity of a material, Hench proposed an in vivo bioactivity index IB, which is defined as IB = 100/t50bb, where t50bb is the time required for more than 50% of the interface to be bonded (Hench, 1991). The rate of bone bonding to implant and the strength and stability of the bond vary with the composition and microstructure of bioactive materials. Generally, the higher the value of bioactivity index of a material, the faster the rate of apatite formation on its surface and the better bonding with bone.
Deionised water is the simplest solution for studying the behaviour of a material in vitro, but it is unlikely to reflect the in vitro and in vivo situation, due to a lack of buffer capacity to the changes in the pH of the solution. A variety of buffers, such as phosphate-, carbonate-, tris- and HEPES, have been introduced to maintain the pH of the solution in the physiological range (7.2 to 7.4) and form physiological solutions or pseudo-extracellular fluids. Kokubo developed and named the solutions as simulated body fluid (SBF) K1 to K9 in ascending order of ion content and concentration (Kokubo et al., 1990). SBF K9 is considered as a suitable medium for initial in vitro study, as its ion concentrations is close to those of human blood plasma, and new recipes have been developed and tested (Kokubo and Takadama, 2006).
The interaction of Bioglass® with the solution was modified by adsorption protein from the medium, which influenced the development of the surface structure. The crystallisation of hydroxyl carbonate apatite (HCA) on the surface of Bioglass® became more complex or delayed in the solutions containing proteins (Radin and Ducheyne, 1996). In contrast, the lag time needed for the nucleation and formation of apatite on HA in normal physiological solution was significantly retarded, or even blocked, in the media with serum proteins and other organic molecules. The surface reactivity of bioactive materials influences the attachment, proliferation, differentiation and mineralisation of bone cells (Ducheyne and Qiu, 1999). The adsorption of proteins on materials surface is critical for the sequence of biological activities through this protein layer that cells sense and respond to implant materials (Dos Santos et al., 2008; Wang et al., 2012). The role of various proteins and enzymes on the bonding of implant materials with host tissue and bone mineralisation needs to be considered thoroughly.
Biocompatibility
The mechanism of tissue attachment is directly related to the type of tissue response at the interface. There are four types of materials according to the type of tissue response at the material–tissue interface (Table 1.3).
Table 1.3
Various types of materials and tissue response at implant–tissue interface
| Type of material | Tissue response | |
| Toxic | Death of the surrounding tissue | |
| Non-toxic | Biologically inactive (bioinert) | Formation of a fibrous tissue |
| Biologically active (bioactive) | Formation of an interfacial bond | |
| Resorbable or degrades (biodegradable) | Replacement of the surrounding tissue |
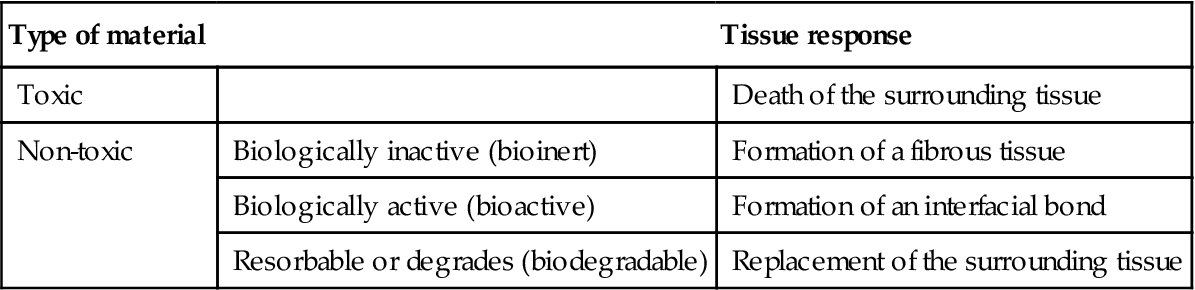
Biocompatibility has been defined as ‘the ability of a material to perform with an appropriate host response in a specific application’ (Williams, 1989), and is a critical property for a biomaterial. Biological assessment of a biomaterial usually involves two stages, in vitro and in vivo. Although a direct study of the host tissue response to a scaffold material in vivo would be ideal, the high complexity of the in vivo processes could cause difficulty in understanding a specific cellular response. Therefore, instead of studying the complex in vivo response, the in vitro assessment of the responses of isolated cell lines to a biomaterial is performed, which allows a controlled study of a specific cellular response to a test material. The knowledge obtained in vitro is useful for tailoring the material to the host site, which aids the screening of a new biomaterial. It cannot, however, replace the in vivo evaluation completely. Especially for a bioactive system, a conflict exists for a bioactive glass tested in vitro and in vivo.
A wide range of cell lines are available for use in the in vitro modelling of biological responses. They are derived either from animals (mouse, rat) or humans, and can be primary cells, transformed sarcoma cells or stem cells. For a bone replacement material, the osteoconductive potential is related to the biological responses from HOB cells, such as the attachment, adhesion and spreading at the initial stage of cell/material interactions, and the subsequent cell proliferation, differentiation and gene expression.
However, tissues and organs are not made up of a single cell type and the interactions of different cells play essential roles in the physiological functions of cells. Various co-culture models, closely resembling the potential interaction of scaffolds with the host tissues, have been developed, such as endothelial cell/osteoblast (Unger et al., 2011), osteoclast/osteoblast (Spence et al., 2009) and chondrocytes/osteoblast (Jiang et al., 2005).
The in vitro biological responses of a biomaterial can be assessed qualitatively, i.e. assessment of the organisation of cytoskeletal proteins, and quantitatively, i.e. cytotoxicity testing, measurement of the growth, proliferation and differentiation, phenotype and gene expression of cells.
The function of cells grown in contact with a material is affected by the physico-chemical characteristics of the material, such as crystallinity and surface roughness. It was also found that micro-molar concentration of inorganic ions, such as Si, could stimulate osteoblast proliferation, differentiation and gene expression (Xynos et al., 2001; Reffitt et al., 2003).
Simulating the complexities of the host–scaffold interface poses considerable challenges. The local in vivo conditions of cellular activity, pH and ionic concentration and the transportation of soluble products away from the scaffold site are difficult to replicate in vitro. To assess the biocompatibility/bioactivity of scaffold materials, suitable in vivo studies are required. There are difficulties in selecting appropriate animal models for evaluation, as tissue responses to scaffold materials may differ with species and anatomical location. Large animals show bone growth and remodelling similar to that observed in human, smaller animals (mice or rats) rarely exhibit lamellar cortical bone remodelling. Rats have accelerated bone metabolism and are able to spontaneously regenerate proportionally greater bone defects than humans. It should be considered carefully in choosing an appropriate animal model for in vivo study. Smaller animals have been used for studying initial bone formation (Patel et al., 2002; Hing et al., 2006), while larger animals are preferable for studies of bone growth and remodelling. Sheep are often ideal candidates for the study of load-bearing effects on bone healing, and larger defect sizes can be created. The sheep model has been used successfully to evaluate the bioactivity of calcium phosphate implants (Patel et al., 2005).
In addition to histological examination of implant–tissue interface, quantitative histomorphometry is also used to estimate the percentage of bone ingrowth and bone coverage within the implant, which is performed on a toluidine blue-stained section using point counting and linear intercept. Mineral apposition rates can be calculated by dividing the distance between two time-spaced fluorochrome labels with the time between the administration of the labels. Commonly used fluorochromes for bone mineral are tetracycline, alizarin red and calcein green. Statistical analysis or ranking of test materials can then be performed based on these quantitative measurements.
1.5 Processing of ceramics
The objective of ceramic processing is to make a specific form of the material that will perform a specific function, such as space-filling, tissue bonding or replacement. This requires the production of a solid object, a coating or particulates. There are various ways of making a specific shape, including casting from the liquid state or pre-forming the shape from fine-grained particulates followed by consolidation. When a shape is made from powders it is called forming. The powders are usually mixed with water and an organic binder to achieve a plastic mass that can be cast, injected, extruded or pressed into a mould of the desired shape. The green body formed is subsequently subjected to a rising temperature to be densified. After cooling and finishing steps (e.g. grinding and polishing), a product with the required properties is obtained.
An example of preparation of HA ceramics is given in the following. It consists of HA powder preparation, consolidation to a compact and densification (sintering).
1.5.1 Preparation of HA ceramics
There are numerous methods for the preparation of synthetic apatites, which can be grouped as aqueous reactions, solid-state reactions and hydrothermal reactions. The aqueous reactions may be divided into chemical precipitation and hydrolysis methods. Chemical precipitation is the most commonly used method, because of its simplicity and ability to produce a wide variety of particle sizes and morphologies.
Methods based on those described by Akao et al. (1981) (equation 1.1) and Hayek and Newesely (1963) (equation 1.2) are the most frequently used. They consist of the dropwise addition of phosphate solution into a stirred solution of calcium solution. The addition of ammonium hydroxide is needed to keep the pH of the reaction alkaline to ensure the formation of HA after sintering the precipitate.
[1.1]
[1.2]
 [1.2]
[1.2]
The concentrations of reagents must be such that the Ca/P molar ratio is maintained at 1.67 for stoichiometric HA. The concentration of calcium can be adjusted if substitution for calcium (strontium, magnesium, etc.) is required. Similarly, the phosphate concentration can be adjusted and replaced with required amount of carbonate or silicate when carbonate or silicate substitution is desired. Fluoride or chloride substitute apatite can be prepared by addition of fluoride or chloride ions in the reactions.
The next step of ceramic processing is to break down the materials received from chemical synthesis, which is a solid aggregation of particles in a dried, filtered precipitate. The agglomerates have a deleterious effect on the properties of the ceramic, and therefore need to be broken down by crushing and grinding. Milling is then used to further reduce the particle size. Particle size reduction is vital for good sinterability, but the ability to handle the powder is equally important, as it ensures the powder flows properly and can be compacted practicably. Spray drying is a widely used method to transform powder to soft agglomerates for easy handling. Calcination, a heat treatment, is another means of improving powder handling.
The HA powder obtained can then be made into dense or macroporous products using compaction (die pressing, isostatic pressing, slip casting, etc.) followed by solid-state sintering. The properties of powder, such as morphology, surface area, mean particle size and particle size distribution, need to be adequately characterised, as this will greatly influence the handling and processing.
Consolidation or compacting is the final stage in the powder preparation. The compacted or compressed body is usually sintered at temperatures of 950 to 1300 °C. The processing of densifying a powder compact without the presence of a liquid phase is called solid-state sintering. During solid-state sintering the material moves to eliminate the pores and open channels that exist between the grains of the compact, the crystals become tightly bonded together at their grain boundaries and the density, strength, toughness and corrosion resistance of sintered material increase greatly.
1.5.2 Porous ceramics
Porous ceramics have attracted great interest as scaffolds for tissue engineering, particularly bioactive ceramics and glasses, as they are able to bond to the host tissues. To be able to regenerate a tissue, a scaffold should act as a template for tissue to grow in three dimensions. The template must be a network of large pores (macropores, at least 100 μm in size) and the pores must be connected to each other, thus allowing essential nutrients to reach the whole network and stimulating blood vessels to grow inside the pore network.
Macroporosity can be introduced by mixing the powder with a volatile component, e.g. hydrogen peroxide or naphthalene, or adding polymethyl methacrylate (PMMA) beads to the powder slurry, using porous polymer (polyurethane foam) as templates for impregnating with ceramic suspensions. The volatile components or polymer phases with low decomposition temperature are removed during the evaporating and sintering process. The ceramics component with porous structure remain.
Ceramic slurries can also be foamed to obtain a porous structure. The incorporation of bubbles is achieved by injection of gases though the fluid medium, mechanical agitation, blowing agents and evaporation of compounds. A surfactant is generally used to stabilise bubbles formed in the liquid phase by reducing the surface tension of the gas–liquid interface. The gel-casting method has been used to produce macroporous HA with interconnected pores. The compressive strengths of HA foams were above 10 MPa, which is similar to that of trabecular bone (Sepulveda et al., 2000).
Porous HA has also been produced by hydrothermal transformation from reef-building corals. These methods employ the use of elevated temperatures, pressures and controlled atmospheres to convert calcium carbonate skeleton into HA. The route has the benefit of preserving the original architecture, the corals serving as a template to make a porous structure (Roy and Linnehan, 1974).
The design and control of the internal architectural of the porous HA structure influence the tissue regeneration (Chu et al., 2002). Rapid prototyping (RP) has emerged as a new processing technique for making scaffold, which allows highly complex structures to be built as a series of thin 2D slices using computer-aided design (CAD) and computer-aided manufacturing (CAM) programs. This technique allows properties such as porosity, interconnectivity and pore size to be predefined. Rapid prototyping, especially 3D printing, has been developed for making custom-made 3D porous HA scaffolds for bone replacement, and the repair of osseous defects from trauma or disease. A 3D HA structure with controlled patterns or porosity was built by direct-write assembly (Michna et al., 2005). A complex-shaped porous HA ceramic with fully interconnected channel was generated from HA powder (Seitz et al., 2005), which was printed with a binder solution layer by layer. Unglued powder was removed and the obtained ceramic green body was consolidated by sintering at high temperature. It is possible to design and manufacture parts according to individual patient’s anatomy. Patient-derived cells can then be seeded onto the scaffolds for tailor-making tissue engineering implants.
1.5.3 Glass
Melting and sol–gel processing are two well-known methods for producing glasses. Traditional glass synthesis consists of melting the precursor mixture and quenching. New components can be added to the system to tailor the glass composition for clinical applications, i.e. to increase or decrease bioactivity, decrease the glass-forming temperatures, etc. An alternative approach is the use of sol–gel techniques to prepare glasses. This route can produce high purity glasses, which are more homogeneous than those obtained by melting, and require relatively low processing temperatures. It involves the transformation of a sol (suspension of colloidal particles) to a gel (3D, interconnected network). Colloids are solid particles with diameter less than 100 nm. Thermal treatment increases the density, strength and hardness of the gels and converts the network to a glass with properties similar to melt-derived glass. An advantage of the sol–gel process is the ability to control the surface chemistry of the material by the thermal treatment and make it possible to expand the bioactive compositional ranges studied in the phase diagram of melted glasses. The glasses obtained exhibit higher surface area and porosity, the critical factors in their bioactivity.
Glass in the fibre form can be produced by the melt-spinning approach. The fibre diameter is limited to micrometre-scale (e.g. tens to hundreds of micrometres). Electrospinning is the technique able to produce various polymer fibres in the range of 10–1000 nm, which has been applied to produce bioactive glass fibres in the micro-/nanoscale using sol–gel glass. Nanoglass fibres in the forms of bundled filaments, fibrous membranes and 3D scaffold have been produced (Kim et al., 2006).
Porous bioactive glasses have been produced by foaming of melt-derived and sol–gel-derived bioactive glasses (Sepulveda et al., 2002; Jones and Hench, 2003; Chen et al., 2006). During the foaming process of sol–gel glass, air was entrapped in the sol under vigorous agitation as viscosity increased and the silica network formed. As the porous foam became a gel, the bubbles were stabilised. The gel was then subjected to a controlled thermal processing of ageing (60 °C), drying (130 °C) and sintering to remove organic species (500–800 °C). The resulting bioactive glass foam scaffolds had macropores up to 600 μm in diameter and compressive strength up to 2.5 MPa (Jones, 2005). An overview of structural characteristics and mechanical properties of highly porous bioactive ceramic or glass for bone tissue engineering was summarised by Chen et al. (2006).
1.5.4 Glass-ceramics
Glass-ceramics are produced by the transformation of the glass into a ceramic. The glass is firstly heated at the temperature of 450–700 °C to produce a large number of nuclei, then the temperature is increased to 600–900 °C to promote crystal growth. The resulting microstructure is finegrained with a uniform size distribution.
1.5.5 Coating processing
Although the relatively poor mechanical properties of calcium phosphate ceramics limit their clinical applications to non-major load-bearing parts of the skeleton, calcium phosphate-coated metallic implants are used in the load-bearing parts. The most popular commercial routes of calcium phosphate coatings are based on plasma spraying (de Groot et al., 1987). The ceramic powder is suspended in the carrier gas and fed into the plasma, where it can be fired at a substrate. However, the high temperature involved in the processing can lead to the changes of phase purity and crystallinity of calcium phosphate ceramics. A variety of thin film and low temperature techniques have been developed to deposit a bioactive layer on the surface of bioinert materials, such as electrostatic atomisation spray deposition (Huang et al., 2005; Leeuwenburgh et al., 2005), sol–gel deposition (Gross et al., 1998), biomimetic deposition (Abe et al., 1990; Habibovic et al., 2002) and magnetron sputtering (Jansen et al., 1993; Thian et al., 2007). Condensation from a vapour by sputtering has been used to produce nanosized coatings, which are uniform, nonporous and very fine grains. Nanocrystalline SiHA coating by radiofrequency (RF) magnetron sputtering has been found to promote better biological responses (Thian et al., 2006).
1.6 Conclusions and future trends
Ceramic biomaterials have been widely used in biological applications as orthopaedic and dental implants and porous scaffolds for tissue engineering. Bioinert ceramics, such as alumina and zirconia, have excellent mechanical properties for load-bearing applications, while bioactive glasses and ceramics have the potential for osteoconduction. Therefore, it is of great importance to understand the clinical requirements and materials requirements to allow the production of tailor-made scaffolds. This chapter has described the microstructure and properties of ceramics with the intention of developing an understanding of the relationships between processing, microstructure and properties of ceramics. The most widely applied orthopaedic bioceramics, such as hydroxyapatite ceramics and Bioglass®, are discussed from the aspects of microstructure, processing, mechanical properties, surface properties, biocompatibility and bioactivity. An understanding of their properties and behaviour will help in developing better ceramic materials for tissue engineering.
Considerable efforts have been made towards developing and engineering structures and surfaces that could elicit rapid and desired reactions with cells and proteins for specific applications in addition to the activities of materials synthesis, optimisation, characterisation and the biological testing of host–material interactions. However, our knowledge of the physical and chemical functions of biomaterials, the response of these materials on people, and the interaction mechanism between the materials and the biological systems still need to be further understood (Anderson, 2006). The search for suitable materials with the desired degradation rates, products and mechanical properties for the desired tissue engineering scaffold is still on-going, so as to achieve the architectures with the desired pore size, morphology, surface topography and bioactivity. The advances in materials science, engineering, cell and molecular biology, and medicine will be able to offer new solutions. The incorporation of advanced fabrication technology and the synthesis of new materials will lead to the enhancement in the complexity and bioactivity of tissue engineering constructs. The emergence of biotechnology and nanotechnology fields will also offer great potential for calcium phosphate ceramics, bioactive glasses and glass ceramics to be further developed in regenerative medicine and tissue engineering (Hench and Polak, 2002; Hollister, 2005).
In addition to a wide range of tissue engineering applications of bioactive glasses, recently, specific therapeutic ions in the forms of metal oxide have been incorporated in the glass composition, such as boron, copper, cobalt, silver, zinc and strontium (Hoppe et al., 2013). The specific ions can then be delivered and the dose can be controlled for bone tissue engineering, particularly related to osteogenesis and angiogenesis, and drug delivery for cancer treatment.
Although bioactive glasses and ceramics bond to bone, the new bone growth is often limited due to their lacking osteoinductivity. In comparison with the usage of autogenous bone repair, most of the current biomaterials lack the abilities to self-repair, to maintain a blood supply and to modify their structures and properties in response to the physiological and mechanical environment.
More recent focuses have been on the development of new generation tissue engineering composite scaffolds with drug and cell delivery capacity and osteoinductive potentials. The paradigm in the development of biomaterial engineering is shifting from replacement to regeneration, and further towards the expansion of new generation materials to stimulate specific cellular and gene responses (Best et al., 2008; Hench, 2009; Dorozhkin, 2010). There are further drives for cell therapies, organ printing, advanced imaging and diagnostic systems and microelectronic devices for personalised treatment in the future (Williams, 2009).



