Polymeric biomaterials for tissue engineering
G. Wei, Medtronic, Inc./Osteotech, USA
P.X. Ma, University of Michigan, USA
Abstract:
Polymeric scaffolds play a pivotal role in tissue engineering. This chapter will focus on the state-of-the-art polymeric scaffolds and exciting recent advancements. First, the general concepts and requirements of tissue engineering biomaterials are introduced. Second, various types of polymeric biomaterials used in tissue engineering are reviewed. Third, important fabrication technologies of 3D scaffolds and their structural characteristics are discussed. Fourth, surface modification and composite scaffold fabrication techniques are presented as ways to improve scaffold properties. Then advanced nanostructured polymer scaffolds with biomolecule-delivering capacity are highlighted. Finally, the future perspectives of polymeric scaffolds for tissue engineering are discussed.
Key words
polymer; biodegradable; scaffold; porous; nanofiber; controlled release; tissue engineering; regeneration
2.1 Introduction
Biomaterials are substances other than food or drugs contained in therapeutic or diagnostic systems that are in contact with tissue or biological fluids (Peppas and Langer, 1994). The categories of biomaterials include metals, ceramics, carbons, glasses, modified natural biomolecules, synthetic polymers, and composites consisting of various combinations of these material types (Dumitriu, 1996). Biomaterials are utilized to manufacture various medical devices, diagnostic products, and pharmaceutical preparations, offering solutions to medical and healthcare problems. This chapter will primarily review polymeric biomaterials for tissue engineering.
In the past few decades, tremendous advances have been made in the biomaterials field, and biomaterials development has evolved through several different focus areas. Early biomaterials research was aimed at achieving the passive and permanent replacement of damaged tissues with matching physical properties and minimal toxicity to the host. Conventional metals, ceramics, and non-degradable polymers were the most representative. Their common features are biologic inertness and inability to adapt to growth. The second focus area was the development of bioactive materials that have the ability to interact with the biological environment to enhance biological responses and promote the material–host tissue integration. Bioglass (BGs) and calcium phosphates (CPs) reached clinical application in the 1980s and remain very active research areas. Surface-modified metallic and polymer implants are also widely used for tissue repair.
Unlike a living tissue that undergoes dynamic changes or remodeling, the majority of these bioactive materials have little capability of responding to physiological loads and biochemical stimuli. Advances in medical technologies have changed the concept from the deletion of damaged tissues for the preservation of remaining healthy tissues or the filling of tissue defects with permanent foreign materials to regenerative medicine; that is, the repair and replacement of lost tissues by initiating the natural regeneration process. Most approaches currently pursued or contemplated within the framework of regenerative medicine, including cell-based therapies and engineered living tissues, are dependent on the ability to synthesize or otherwise generate novel materials, to fabricate or assemble materials into appropriate 2D and 3D forms, and to precisely tailor the physical, chemical, structural, and biological properties so as to achieve desired clinical responses. This conceptual change has inspired the current development of various advanced biomaterials, among which biodegradable polymeric biomaterials are becoming more and more important in drug delivery, tissue engineering, and regenerative medicine (Langer, 1990; Langer and Vacanti, 1993; Ma, 2004a; Saito et al., 2005; Zhang et al., 2012). The greatest advantage that the biodegradable polymeric biomaterials offer is the ability to be eliminated from the body after fulfilling their intended delivery, templating, or scaffolding functions. The degradability results in no foreign materials left in the body after the repair and regeneration processes.
In the previous edition, this ‘Polymeric Biomaterials’ chapter reviewed advances in biodegradable polymer scaffolds for tissue engineering and drug delivery applications (Wei and Ma, 2007). It discussed the scaffold fabrication from biodegradable polymers, the 30 porous structures, the surface modification, and highlighted drug delivering polymer scaffold and its applications in tissue engineering. While still focusing on biodegradable synthetic biomaterials for tissue engineering applications, the chapter updates the most recent new developments in scaffolding technologies and provides a more thorough review of scaffold characteristics on the successful engineering of tissues and organs, especially at the nanometer scale. Also added are the applications of polymeric scaffold for cell delivery (including stem cells) and their applications in musculoskeletal tissue regeneration.
2.2 Polymeric scaffolds for tissue engineering
In a tissue engineering strategy, an ideal synthetic scaffold should be capable of presenting a physiochemically biomimetic environment that actively promotes desirable or suppresses undesirable physiological responses, while biodegrading as native tissue integrates. Scaffolds promote new tissue formation by providing an appropriate surface and adequate space (volume) to foster and direct cellular attachment, migration, proliferation, and desired differentiation towards the specific cell phenotypes throughout the scaffold where new tissue formation is needed. The design and manufacturing of a scaffold are critical because they affect not only the ultimate function of regenerated tissues but also its clinical applications. There are many design criteria for an ideal scaffold for tissue engineering applications, which vary among different tissue types (Ma, 2004a, 2004b). Critical variables in scaffold design and function include the bulk material composition, the mechanical properties, the 30 architecture, the surface morphology and chemistry, and the scaffold environments during and after fulfilling its function, which is determined by the degradation characteristics (Muschler et al., 2004). Generally, a tissue engineering scaffold should be: (1) biocompatible, that is, non-immunogenic and non-toxic to living cells and tissues; (2) biodegradable or capable of being remodeled in tune with regeneration or repair process; (3) porous to provide a suitable 3D environment for cell penetration as well as mass (nutrients and wastes) transportation; (4) surface conductive to facilitate cellular functions; (5) mechanically stable for surgical handling; and (6) easy to manufacture at a large scale and sterilize without loss of stability and function.
For large tissue defect regeneration or the fabrication of a large piece of tissue ex vivo for implantation, a scaffold may be required to be able to carry biological signals including cytokines, growth factors, and nucleic acids, and to deliver them in a temporally and spatially controlled manner. The combination of a scaffold, biological signals, and stem cells forms a practical tissue engineering platform, where a predesigned scaffold with the patient-specific anatomy is preferred (Chen et al., 2006; Wang et al., 2009; Smith and Ma, 2012). In cases of irregular shaped defects and wounds that need to be filled and repaired, an injectable scaffold can be advantageous because it allows for easy manipulation and minimally invasive procedures by surgeons to reduce complications and to improve patient comfort and satisfaction (Elisseeff et al., 1999).
2.2.1 Polymeric scaffold fabrication
The search for an ideal scaffolding material and appropriate scaffolding structure that fulfill the above design criteria continues to be important and challenging in tissue engineering (Burg et al., 2000; Liu and Ma, 2004; Ma, 2004a; Guarino et al., 2007; Moroni et al., 2008; Liu et al., 2012; Sun et al., 2012; Zhang et al., 2013; Mallick and Cox, 2013). Polymeric biomaterials, particularly biodegradable polymers such as the family of poly(α-hydroxy esters) including poly(lactic acid) (PLA), poly(glycolic acid) (PGA) and their copolymers (PLGA), have been used extensively in medical and surgical applications. These biodegradable polymers have been of interest primarily because of their biocompatibility and biodegradability, their established safety as suture materials (approved by the US Food and Drug Administration, FDA), and the versatility and flexibility that they offer for producing well-defined highly porous scaffolds with different geometries and structures to meet the needs of specific tissue engineering applications (Mikos et al., 1994; Whang et al., 1995; Harris et al., 1998; Giannobile et al., 2001; Borden et al., 2002; Smith and Ma, 2012; Bhamidipati et al., 2013). Because degradation of these polymers occurs mainly by hydrolysis, the degradation rate can be modulated over a wide range by tailoring the composition, molecular weight, end groups in copolymer, and geometry of the device (Hollinger and Leong, 1996; Shive and Anderson, 1997; Liu et al., 2012; Pan and Ding, 2012; Loh and Choong, 2013).
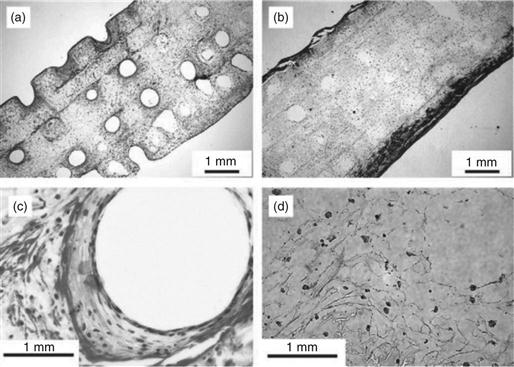
A number of scaffolding techniques have been explored to fabricate biodegradable polymers into 3D porous scaffolds with different porosities, porous architectures and orientations, pore sizes, pore interconnection, and pore wall surface morphologies (Mikos et al., 1994; Zhang and Ma, 2000; Ma and Choi, 2001; Chen and Ma, 2004; Wei and Ma, 2006; Wei and Ma, 2009). Solvent-evaporation/particulate leaching is a conventional technique that has been widely used to fabricate porous scaffolds for tissue engineering applications (Mikos et al., 1994). It is technically simple and easy to carry out. The pore size can be controlled by the size of salt particles and the porosity by the polymer/particulate ratio. However, this technique has limited controls over the pore shape and the pore interconnectivity. To prepare scaffolds with well-controlled interconnected porous structures, paraffin spheres, or sugar spheres are used as alternative porogen materials to salt particles. Because paraffin or sugar spheres can be heat treated to form a bound template, interconnected spherical pore structures can be obtained after the removal of the bound porogen template (Fig. 2.1a, b). The heat treatment time and temperature of porogen control the pore interconnectivity (or interpore opening area) of the scaffold (Chen and Ma, 2004; Wei and Ma, 2006).

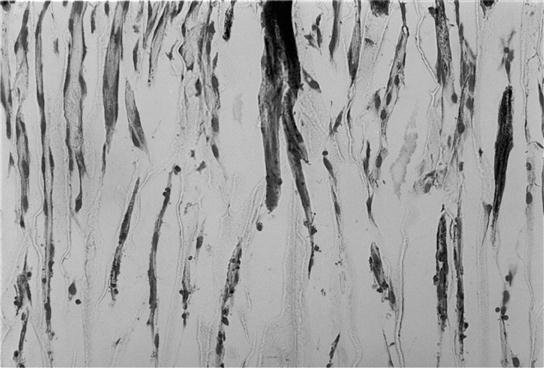
Porous structures can be introduced into a material using a phase separation technique without the use of a porogen material. A thermally induced solid–liquid phase separation is achieved by lowering the temperature of a homogeneous polymer solution to induce solvent crystallization. Subsequent removal of solvent crystals results in porous polymer scaffolds (Fig. 2.1c). The characteristics of pores vary with the polymer material, the concentration of the polymer solution, the solvent, the phase separation temperature, and the heat transfer direction (Ma and Zhang, 1999, 2001; Wei and Ma, 2009). For example, manipulation of the heat transfer direction can control the direction of solvent crystal growth during the phase separation, resulting in a scaffold with anisotropic microtubular structures (Fig. 2.1d). Such a parallel array of microtubules facilitates the organization and regeneration of certain tissues (such as nerve, muscle, tendon, ligament, dentin, and so on) that naturally have oriented tubular or fibrous bundle architectures (Fig. 2.2).
Solid freeform fabrication (SFF) or rapid prototyping which is adopted from industrial computer-assisted design and computer-assisted manufacture (CAD/CAM) appears promising for polymeric scaffold fabrication to provide precise control over 3D shapes at the macro-scale level. The overall shape of the scaffold is ultimately controlled by anatomical data files taken from the patient’s defective part using computed tomography (CT), magnetic resonance imaging (MRI) or other medical-imaging techniques, so that the resulting scaffold shape matches exactly the specific anatomic shape (Yang et al., 2002; Hutmacher et al., 2004). While this technology allows customized fabrication with high reproducibility, the SFF techniques also have their own drawbacks, such as inadequate resolution, limited material selections, and structural heterogeneity due to their inherent ‘pixel assembly’ nature (Ma, 2004a).
Many living tissues have a hierarchical structure that varies over length scales from nanometers to millimeters (Griffith, 2002). One particular fabrication technology sometimes may not be able to fabricate a scaffold that meets all requirements for a specific tissue engineering application. As a result, two or more scaffolding techniques can be combined to overcome the limitations of each technology. For example, the phase separation has been used in conjunction with porogen leaching, injection molding or SFF techniques (Chen and Ma, 2004; Chen et al., 2006; Wei and Ma, 2006; Sun et al., 2012). Gas foaming has been combined with the traditional salt-leaching method. The combined techniques can generally control porous architecture over a broader size scale which can meet more tissue engineering requirements (Chen and Ma, 2004; Chen et al., 2006; Wei and Ma, 2006; Wei and Ma, 2009; Smith and Ma, 2012).
2.2.2 3D porous architectures
In the body, nearly all cells are embedded in or exposed to a 3D microenvironment that is tightly regulated by interactions with the surrounding cells, soluble factors, and extracellular matrix (ECM) molecules. Chondrocytes in monolayer culture lose their phenotypic properties, while cultures in 3D agarose gels lead to re-expression of chondrocytes’ phenotype (Benya and Shaffer, 1982). The in vitro results demonstrated that cells expressed very different phenotypic characteristics when cultured on a 3D scaffold versus a 2D substrate. A 3D scaffold offers a local microenvironment that mimics natural ECM-cell context more closely and where the functional properties of cells can be manipulated and optimized (Zhang, 2004; Ma, 2008).
Consequently, the degree of success of various tissue engineering strategies depends significantly on the 3D architecture of the polymeric scaffold (3D environmental cues for cells). 3D scaffold architecture refers to the way in which a bulk material is distributed in space from the macro-, micro- to the nano-scales (corresponding to tissue, cellular and molecular scales in a specific tissue, respectively) (Muschler et al., 2004). Such hierarchical porous properties affect not only cell seeding, survival, migration, proliferation, and organization but also their gene expression and phenotypic characteristics (Chen et al., 2006). They also define the mechanical structure of the scaffold and the initial void space that is available for progenitor cells to form new tissues, including new blood vessels, as well as the pathways for mass transport via diffusion and/or convection.
The importance of macroporosity (>100 μm) of polymer scaffolds on the neo-tissue formation has been investigated (Tsuruga et al., 1997; Kuboki et al., 2001; Zeltinger et al., 2001; Roy et al., 2003). PLGA scaffolds with a higher porosity (>80%) promoted more tissue ingrowth and new tissue formation (Roy et al., 2003). However, porosity is not the only factor that needs to be considered when choosing suitable scaffolds for 3D cell culture and tissue regeneration (Kumar et al., 2011). The interconnection between macropores (interpore opening size, density, and pathway) is also critical for cellular activity and bone tissue formation. Scaffolds with open pore structures favored cell and tissue penetration, blood vessel invasion and new bone formation (Lu et al., 1999). Failures of cell/tissue ingrowth often resulted from insufficient inter-pore connection where cell colonization was limited to the very peripheral and superficial layers (e.g. about 240 μm from the surface of a 1.9 mm thick scaffold made from traditional solvent-evaporation/particulate leaching technique) (Ishaug-Riley et al., 1998). With comparable porosity and pore size to a scaffold made with traditional salt-leach technique, a gyroid-like scaffold made with stereolithography showed a more than 10-fold higher permeability (Melchels et al., 2010). Increase in interconnected macroporosity, however, may adversely affect important mechanical properties of a scaffold, which requires more advanced scaffold design and fabrication technique. For example, scaffolds prepared by thermally induced phase separation offer both higher porosity (as high as 98%) and improved mechanical properties over scaffolds produced by traditional salt-leaching technique (Zhang and Ma, 1999a). The oriented microtubular scaffolds have shown anisotropic mechanical properties similar to fibrillar and tubular tissues, and have been demonstrated to facilitate cell organization into oriented tissues (Ma and Zhang, 2001) (Fig. 2.2).
2.2.3 Nanofibrous scaffolds
While the interconnected macroporosity of a scaffold is important to provide sufficient space for cellular activity, interactions between cells and biomaterials occur at the interface, i.e. the internal/external pore walls of the 3D scaffold. Manipulation of surface morphology or topography on the macropore walls can directly and significantly affect cell–scaffold interaction and eventually tissue formation and function.
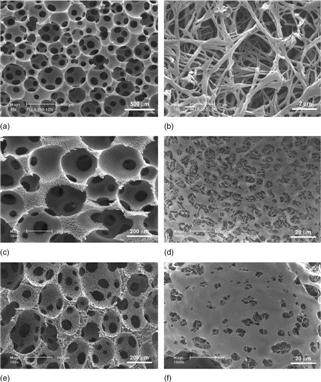
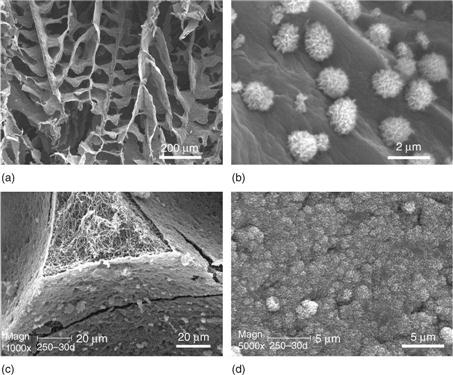
Collagen is the major ECM component of many tissues and has been actively investigated as a substrate or scaffold for cell attachment, proliferation and differentiation (Elsdale and Bard, 1972; Strom and Michalopoulos, 1982). Importantly, the nanoscaled collagen fibrillar structure has been recognized for the enhancement of cell/matrix interaction (Grinnell and Bennett, 1982; Kuntz and Saltzman, 1997). To mimic collagen fiber bundles on the nanometer scale (50–500 nm) while eliminating the possible immunogenicity brought by collagen, nanofibrous features have been introduced into a synthetic biodegradable polymer scaffold (Zhang and Ma, 2000; Woo et al., 2003; Chen and Ma, 2004; Chen et al., 2006; Wei and Ma, 2006). A combined technique of sugar sphere template leaching and phase separation has been developed to prepare spherical macroporous and nanofibrous scaffolds (Wei and Ma, 2006) (Fig. 2.3a, b). The resulting polymer nanofibers had a diameter between 50 and 500 nm, which is similar to that of collagen nanofibers. The nanofibrous scaffold had high surface area of about 100 m2/g, which is more than 100 times higher than of the non-fibrous solid scaffold with the same macroporosity and pore structure. By using PLLA/PDLLA polymer blends or solvent mixtures, macroporous polymer scaffolds with varying macropore wall architectures from smooth solid, microporous, partially nanofibrous/nanostructured, to pure nanofibrous can be prepared (Fig. 2.3c–f) (Wei and Ma, 2009). In particular, phase separation of PLLA/PDLLA or PLLA/PLGA blends led to partially nanofibrous scaffolds, in which PLLA and PDLLA (or PLGA) formed nanofibers and smooth (solid) surfaces on the macropore walls, respectively. Although all scaffolds had similar macroporosity and pore size, the surface area of the partially nanofibrous scaffold increased linearly with the PLLA content in the polymer blend. Macroporous scaffolds with adjustable pore wall surface architecture may provide a platform to study the specific cellular response to different surface properties and serve as excellent substrates for various tissue engineering applications (Wei and Ma, 2009).
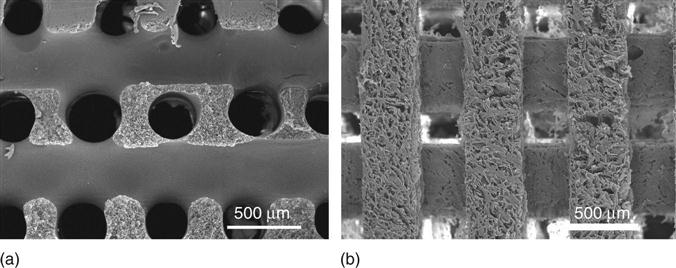
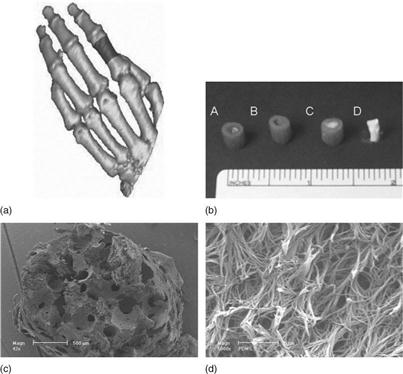
Alternatively, phase separation can be induced in polymer solutions in a specifically designed template or mold. For, example, nanofibrous scaffolds with interconnected macro-tubular or channeled structures can be fabricated using a sugar fiber bound mold or a wax mold generated from 3D printing (Zhang and Ma, 2000; Chen et al., 2006) (Fig. 2.4a, b). More excitingly, patient-specific and anatomically shaped nanofibrous scaffolds have been prepared by integrating a reverse SFF technology with phase separation (Fig. 2.5). The resulting scaffold had hierarchical pore structures and mimicked the natural ECM more closely on the nanometer scale, which had been shown to promote tissue regeneration (Chen et al., 2006; Wang et al., 2009).
Other techniques that have the capability of nanofiber production are electrospinning (Huang et al., 2003; Xu et al., 2004; Barnes et al., 2007) and self-assembly (Whitesides and Grzybowski, 2002). However, well-designed 3D macroporosities are difficult to incorporate into electrospun scaffolds and the thickness of the scaffold is limited to 1 mm (Nair et al., 2004). Recently, self-assembly has drawn much interest in developing nanofibrous materials with potential as tissue engineering scaffolds. However, it is mainly limited to the use of biological molecules such as peptides in the form of hydrogels (Whitesides et al., 1991; Whitesides and Grzybowski, 2002).
Due to the dramatic increase in surface area, the macroporous and nanofibrous scaffold adsorbed significantly more serum proteins in vitro as compared to solid scaffold without nanofibrous structures (Woo et al., 2003; Chen et al., 2006). In the series of partially nanofibrous scaffolds, the amounts of adsorbed proteins from serum showed linear increase with the surface area of the scaffolds (Wei and Ma, 2009). Interestingly, nanofibrous and solid scaffolds showed different serum protein adsorption profiles. Significant amounts of fibronectin and vitronectin were adsorbed on nanofibrous scaffold while much lower amounts of these cell-adhesion proteins were found on the solid-walled scaffolds (Woo et al., 2003).
In a study comparing cellular activities on nanofibrous scaffolds with those on solid scaffolds, osteoblastic progenitor cells (MC3T3-E1) attached on nanofibrous scaffolds at a level of 70% higher than that on solid scaffolds (Woo et al., 2003). The nanofibrous scaffolds also displayed more uniform matrix and mineral production throughout (Fig. 2.6), and cells on nanofibrous scaffolds showed significantly higher expression of osteocalcin and bone sialoprotein mRNAs. The nanofibrous matrix architecture promoted alkaline phosphatase activity and calcium deposition of human embryonic stem cells cultured under osteogenic conditions (Hu et al., 2010; Smith et al., 2010). In a critical-sized rat calvarial defect repair model, nanofibrous scaffolds supported substantially more new bone tissue formation than did solid-walled scaffolds. Histological analysis also revealed abundant collagen deposition on nanofibrous scaffolds but not on the control solid-walled scaffolds (Woo et al., 2009). The high surface area, the microporosity between nanofibers (several μms), and the selective adsorption of ECM proteins on nanofibrous scaffolds all contribute significantly to the enhanced cell response in vitro and the bone formation in vivo. In a number of other studies, PLLA nanofibrous scaffolds were also found to be a versatile 3D substrate for many other cell types to promote proliferation, to induce differentiation, and to facilitate ECM deposition (Wang et al., 2011; Feng et al., 2012).
Due to the significant benefits of nanofibers on a 3D porous scaffold to the cellular response and tissue regeneration, the unique nanofibrous features have been incorporated into an injectable scaffold other than a pre-fabricated tissue scaffold. In a recent study, a nanofibrous microsphere scaffold has been developed as an injectable chondrocyte carrier for cartilage regeneration (Liu et al., 2011). The injectable nanofibrous microspheres were self-assembled from a star-shaped biodegradable poly(L-lactic acid) (SS-PLLA) polymer. Compared to conventional PLLA microspheres, the new microsphere showed hollow structures with high porosity (96.7%) and were composed entirely of nanofibers at the same scale as collagen fibers (160±67 nm) (Fig. 2.7). The nanofibrous hollow microspheres were shown to efficiently accommodate cells, enhance cartilage regeneration over control microspheres, and support a significantly larger amount and higher quality cartilage regeneration over the chondrocytes alone control in an ectopic implantation model. In a critical-size rabbit osteochondral defect repair model, the nanofibrous hollow microspheres/chondrocytes mixture achieved substantially better cartilage repair and integration than the chondrocytes alone group that simulates the clinically available autologous chondrocyte implantation (ACI) procedure (Plate I, between pages 354 and 355).

2.2.4 Surface modification of polymeric scaffolds
Besides the manipulation of surface physical structures such as the morphology and topography, the scaffold surface can also be chemically modified to improve its physiological functions. Plasma exposure is a widely used approach to modify the hydrophilicity of a surface or introduce certain functional groups onto the surfaces of a substrate for further modification using other chemical methods (Chim et al., 2003; Yamaguchi et al., 2004; Yildirim et al., 2011). Because of its limited penetration depth, plasma treatment is more effective on 2D substrates but has limited capability to modify a 3D porous scaffold.
In a complex 3D porous scaffold, the cell– or tissue–material interface is not just the outside surface of the scaffold, but also the entire internal pore wall surfaces. Surface modification of a scaffold needs to modify the entire surfaces throughout while maintaining the designed 3D porous architecture at the same time. One strategy for surface modification is to introduce functional groups in polymer chains before the scaffold fabrication which can later couple with ECM molecules, cell adhesion peptides, or sugar moieties (Yoon et al., 2004). A PLGA porous scaffold was functionalized with a primary amine group to which Gly-Arg-Gly-Asp-Tyr (GRGDY) was immobilized. Bone marrow cell adhesion was substantially enhanced by the introduction of GRG peptide on the scaffold pore surfaces. The GRG-immobilized PLGA scaffold also showed enhanced differentiation as determined through alkaline phosphatase activity (Yoon et al., 2004). PLGA scaffolds modified with hyaluronic acid showed great potential for cartilage regeneration since they were able to support the growth of chondrocytes and to maintain their phenotype (Yoo et al., 2005). Surface coating is a technically simple approach to adsorb the required surface-modifying species from a solution onto pre-fabricated 3D scaffold surface. In one study, a nano-layer of collagen was coated onto a PLGA scaffold when the excessive collagen solution was removed by centrifuge (G. Chen et al., 2009). Generally, the modified surface from the surface coating method is unstable because of the weak interaction between bulk material and surface-modifying species (Wu et al., 2006).
To mimic both the nanofibrous structure and chemical composition of collagen fibers, a nanofibrous scaffold was surface modified with gelatin, a biomacromolecule derived from collagen (Liu et al., 2005a, 2005b, 2006). In a recent study, a new entrapment method was developed to effectively incorporate gelatin onto nanofibrous pore wall surfaces of both interior and exterior (Liu et al., 2005b). The entrapment modification method can be used for various geometries, morphologies, and thicknesses of 3D polymer scaffolds without interfering bulk properties and architectures of a scaffold. In a further study, gelatin spheres acted as both the porogen for scaffold fabrication and the surface-modification agent, where scaffold fabrication and surface modification were completed in a simple one-step process (Liu et al., 2006). The gelatin-modified porous nanofibrous scaffold significantly improved the initial osteoblast cell adhesion and the proliferation throughout the scaffold (Liu et al., 2005b, 2006). Since most biomacromolecules are charged cationic or anionic polyelectrolytes, a layer-by-layer self-assembly process has also been introduced for surface modification of 3D scaffold (Zhu et al., 2003, 2004; Liu et al., 2005a). One advantage of the self-assembly approach is that biomolecules can be immobilized onto 3D scaffold surfaces under mild conditions.
Broadly speaking, 3D polymer scaffolds can also be modified with a layer of bone-like apatite via a biomimetic process in a simulated body fluid (SBF) or growth factors through immobilization. The former modification is specifically designed to increase osteoconductivity and bone bonding properties in bone tissue regeneration. The latter (growth factor immobilization) can also be categorized into controlled drug delivery, which has been an exciting area in inductive tissue regeneration. We will discuss these two surface modification approaches separately in sections of composite scaffolds (Section 2.2.5) and polymer scaffolds with controlled release capacity (Section 2.3).
2.2.5 Polymer/apatite composite scaffolds for bone regeneration
Polymer/apatite composite materials have attracted tremendous interest in bone tissue regeneration. Being similar to the major inorganic component of natural bone, the inorganic component such as hydroxyapatite (HAP) or calcium phosphate (CaP) in a composite scaffold provides good osteoconductivity and bone bonding ability (Sun et al., 1998; LeGeros, 2002; Li et al., 2002) while polymer components offer strength and design flexibility to achieve the high porosity and high total surface area that are necessary for anchorage-dependent cells such as bone cells to survive and differentiate.
By blending and phase separation techniques, polymer/HAP composite scaffolds have been developed with improved mechanical properties and osteoconductivity (Fig. 2.8a, b) (Zhang and Ma, 1999a). The HAP-containing scaffolds improved osteoblastic cell seeding uniformity and showed significantly enhanced expression of osteocalcin and bone sialoprotein over the plain polymer scaffolds. Bone tissue formation throughout the scaffold has been demonstrated (Ma et al., 2001). A further study revealed that HAP addition significantly suppressed apoptotic cell death and provided a more favorable microenvironment for bone tissue regeneration, possibly by improving the serum protein adsorption (Woo et al., 2007). The HAP particle size in the composite scaffolds has significant effects on the scaffold properties. The nano-HAP/polymer composite scaffolds not only improved the mechanical properties, but also significantly enhanced protein absorption over micro-sized HAP/polymer scaffolds (Wei and Ma, 2004) (Fig. 2.8c, d). Enhanced protein adsorption improves cell adhesion and function (Webster et al., 2000). HAP particles have been combined with a number of other polymers and fabricated into 3D scaffolds using various fabrication techniques. Overall, the composite scaffold showed improved osteoconductivity as compared to the pure polymer scaffold (Thomson et al., 1998; Kim et al., 2007; Liu et al., 2009).

To efficiently modify the internal pore wall surface with bone-like apatite without altering the bulk structures and properties of the scaffolds, a biomimetic approach has been developed to grow bone-like apatite particles on pre-fabricated porous polymer scaffolds in an SBF (Zhang and Ma, 1999b, 2004; Wei and Ma, 2006). It has been observed that the growth of apatite crystals was affected greatly by the polymer materials, the porous structure, the ionic concentration of SBF as well as the pH value (Zhang and Ma, 2004). When macroporous nanofibrous scaffolds were used for bone-like apatite deposition, a uniform layer of nano-apatite crystals was found to cover the internal pore wall surface throughout the scaffold (Fig. 2.9). With the increase of the incubation time, the density and the crystal size of nano-HAP deposited on the porous surface also increased. One great feature of this process was that the interconnected macroporous architectures were maintained during the incubation and the mechanical properties were greatly improved. The nano-HAP deposition process can be further modulated by incorporating a small amount of nano-HAP particles into the polymer scaffold before the incubation process. Significantly greater amount of apatite particles were grown on the 90/10 PLLA/nHAP nanocomposite scaffold than on the plain PLLA polymer scaffold during the same period of SFB incubation (Wei and Ma, 2006).
2.3 Polymeric scaffolds with controlled release capacity
Natural tissue formation and repair is an orchestrated molecular and cellular process, which is tightly regulated by the actions of a number of signaling molecules including growth factors and cytokines (Karsenty and Wagner, 2002; Peng et al., 2002). For example, the natural healing of an injured bone occurs in a series of phases, where a number of specific cytokines or growth factors are expressed in each phase (Gerstenfeld et al., 2003; Ito and Koefoed, 2005). In the wound healing process of skin, various signals are involved in triggering relatively sedentary cell lineages at the wound margin to proliferate, to become invasive, and then to lay down new matrix in the wound gap (Martin, 1997). In a tissue engineering strategy of mimicking the natural tissue formation and repair cascade, the supply of exogenous signaling molecules is necessary when endogenous signaling molecules are not sufficient in types and quantity. Owing to the rapid advances in recombinant technologies, a large variety of purified recombinant polypeptides, proteins, and growth factors are available for tissue engineering applications (Reddi, 1998; Babensee et al., 2000; Li and Wozney, 2001; Lee et al., 2002). However, the effective clinical applications of these bioactive factors has been limited by their short plasma half-lives, instability in the gastrointestinal tract, and low bioavailability due to their relatively large molecular weight and high aqueous solubility (Lee, 1988). The successful application of the growth factors depends critically on the controlled delivery technology to achieve their optimal therapeutic efficacy (Langer, 1990; Ferrara and Alitalo, 1999; Morley et al., 2001).
2.3.1 Micro-/nano-encapsulation
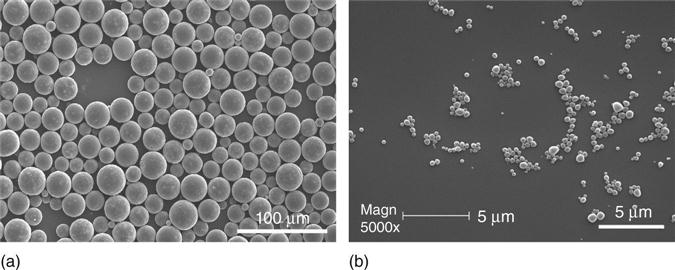
Polymeric particulate carriers (micro- and nanospheres) have been demonstrated to be effective to protect and release unstable biologically active molecules (Langer, 1990). Among the natural or synthetic polymers used for particulate carrier fabrication, PLLA and PLGA were found to be remarkable for applications in drug delivery due to their excellent biocompatibility and biodegradability through natural pathways (Uludag et al., 2000; Wei et al., 2004). Most importantly, the released proteins were able to maintain a high level of biological activity with the desired prolonged durations (Oldham et al., 2000; Wei et al., 2004). Spherical microspheres (20–50 μm) and nanospheres (200–500 nm) can be obtained depending on the concentration of surfactant used and the emulsion strength employed in the double emulsion process (Fig. 2.10). The release of a protein from microspheres was controlled in the first stage by the diffusion and in the second stage by the degradation of the polymer micro- or nanospheres. By varying the molecular weights and the ratio of LA/GA in PLGA copolymers, protein releases over days to months following sustained release kinetics can be achieved (Wei et al., 2004). Both in vitro and in vivo assays demonstrated that the bioactivity of parathyroid hormone (PTH) was retained during the fabrication of PLGA microspheres and upon release. Similarly, the released platelet-derived growth factor BB (PDGF-BB) from PLGA nanospheres was biological active and was able to stimulate the proliferation of human gingival fibroblasts (HGF) (Wei et al., 2006). These studies illustrate the feasibility of achieving local delivery of bioactive factors to induce cellular responses using a microsphere/nanosphere encapsulation and delivery technique.
2.3.2 Nano-/microsphere immobilization on three-dimensional scaffolds
As discussed above, biomimetic scaffolding using biodegradable polymers is able to mimic natural ECM for optimal 3D structures, surface morphologies and chemistry to achieve improved tissue regeneration from the material prospective. Growth factors, employed properly, are able to directly stimulate cellular activities. The delivery of growth factors from a 3D scaffold, therefore, is an attractive strategy for tissue regeneration. In this way, a 3D scaffold serves both as a temporary substrate for cell functions and as a delivery carrier for the controlled release of growth factors. The design of growth factor delivering scaffold aims to synergize the functions of the scaffold and the growth factors.
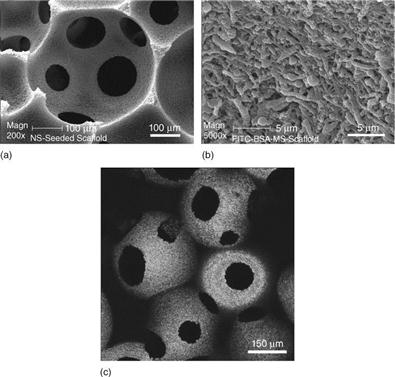
A novel immobilization technology has been developed to incorporate growth factor-encapsulated nanospheres into prefabricated porous polymer scaffolds (Wei et al., 2006, 2007). The nanospheres were uniformly distributed throughout the macroporous and nanofibrous scaffold without interfering with the macro-, micro-, and nanostructures of the scaffold (Fig. 2.11). Immobilization onto a scaffold significantly reduced the initial burst release of the growth factor. Various release profiles were achieved through the use of nanospheres with different degradation rates (Fig. 2.12) (Wei et al., 2006). The angiogenesis in a PDGF-releasing scaffold was investigated in a soft tissue wound repair model in the dorsa of rats. Angiogenesis and corresponding pericyte formation occurred in a PDGF dose-dependent manner. In the scaffolds with empty nanospheres, there was negligible new blood vessel formation. In the scaffolds containing PDGF-encapsulated nanospheres, there was substantial new blood vessel formation, increasing with PDGF dose (Plate II, between pages 354 and 355). In addition, blood vessel number and area in the scaffolds with slow-releasing PDGF nanospheres (PLGA with 64 K molecular weight) were significantly greater than those with fast-releasing PDGF nanospheres (PLGA with 6.5 K molecular weight), both with low and high doses (Fig. 2.13) (Jin et al., 2008).


Similarly, recombinant bone morphogenetic protein (rhBMP-7) delivered from nanosphere-immobilized scaffolds induced significant ectopic bone formation throughout the scaffold while simple adsorption of the same amount of rhBMP-7 onto the scaffold failed to induce bone formation due to either the loss of rhBMP-7 biological function or the insufficient duration of the factor present in the scaffold (Plate III, between pages 354 and 355) (Wei et al., 2007). Clearly, the new nanosphere immobilization technique protected growth factors from denaturation as compared with the simple adsorption of growth factors onto the scaffold, which has been reported to result in complete degradation of the growth factors (such as rhVEGF, BMP-4, and bFGF) during a very short release time of 3 days (Ziegler et al., 2002). In addition, the burst release is very high and temporal control over release kinetics is very limited when a simple adsorption method is used (Miyamoto et al., 1992; Tamura et al., 2001). Growth factors can also be incorporated into scaffold during emulsion freeze drying (Whang et al., 1998) or gas foaming (Sheridan et al., 2000) scaffold fabrications. The resulting rhBMP-2 or VEGF containing scaffolds were reported to release the growth factors to induce bone formation and angiogenesis, respectively. One disadvantage associated with these two incorporation techniques is the difficulty in achieving sufficient macroporosity and open pore structures in the scaffold. As an improvement, particulate leaching was combined with gas foaming to obtain an open porous structure of the scaffold (Murphy et al., 2000). However, significant loss of growth factor during the leaching process becomes a major concern.
The nanosphere-incorporated scaffolds were also used to deliver small molecules, such as an anti-bacterial drug, locally for reduced burst release and prolonged duration (Feng et al., 2010). In addition, the technology can potentially be utilized to deliver many other biologically active molecules such as DNAs, RNAs, siRNAs, and miRNAs.
Many current growth factor delivery approaches for tissue engineering have focused on delivering one potent growth factor to trigger a specific phenotypic repair process. The rationale for the use of single inductive molecules to engineer specific tissue is that a cascade of regeneration processes will follow the initial event. This may not be true or ideal for driving a highly and precisely regulated complex tissue regeneration (Rasubala et al., 2003; Franceschi, 2005; Wei, 2006). It has been suggested that specific growth factors and cytokines are required to regulate tissue regeneration during different stages. A few recent studies turn to the delivery of two or multiple factors that function synergistically by emulating the natural repair process (Richardson et al., 2001; Wei, 2006; Patel et al., 2008; F. M. Chen et al., 2009; Saif et al., 2010).
Multiple factor delivery, each with an individualized release profile, has been achieved by immobilizing multiple types of nanospheres onto the pores in an advanced macroporous and nanofibrous scaffold (Wei, 2006). Different biodegradable polymer nanospheres are utilized to individually control the release profiles of different biological molecules. Multiple factors with distinctly different release periods from days to weeks, months and even years are achievable from one single scaffold. The macro-, micro-, and nano-features of the scaffolds were retained after nanosphere incorporation. Such novel scaffolds, while supporting 3D neo-tissue genesis by cells, allow for the release of multiple biological factors in a spatially and temporally controlled fashion. The novel technology provides a platform to program biological signals into a 3D scaffold to regulate the cellular activities and to orchestrate predictable tissue regeneration.
2.4 Conclusions and future trends
Tissue engineering entails the interplay between cells, biomaterials, and bioactive factors to successfully regenerate tissues for therapies. In recent years, significant progress has been made in polymeric tissue-engineering biomaterials from synthesis, processing, and structural characterization to understanding their interactions with cells (especially stem cells) and their applications in tissue engineering. In addition to newly synthesized and chemically modified polymers, substantial amounts of research have been carried out to enhance the biological function of existing polymeric biomaterials. One such area is the integration of controlled release capacity into 3D scaffolds to achieve spatially and temporally controlled delivery of biomolecules. Another area is composite material development to utilize different material properties synergistically in facilitating tissue regeneration. In addition, a fast developing area is the generation of nano-sized features in biomaterials to mimic certain extracellular matrix structures to regulate cellular behavior and function. Furthermore, multi-sized design is rapidly growing, integrating macroscale shapes, microscale pores, and nanoscale architecture to systematically optimize scaffold function. These advances in 3D scaffold fabrication technologies have provided a platform for delivering both cells and biological signals to the site where tissue regeneration is needed. Combining the multiple types of information (material, physical, chemical, structural, and biological) into one single biomaterial system will more closely mimic the multifunctional features of the native extracellular matrix. Such technologies are converging to make it feasible to design microenvironments in 3D scaffolds to regulate the behavior and fate of stem cells for efficient and targeted tissue regeneration. We will likely see very exciting advances in these areas in the years to come.
2.5 Acknowledgements
The authors would like to acknowledge the financial support from the National Institutes of Health (NIDCR DE015384, DE017689 and DE022327: PXM), DOD (W81XWH-12-2-0008: PXM) and NSF (DMR-1206575: PXM).
