Liver tissue engineering
J. Bierwolf and J.-M. Pollok, University Hospital Bonn, Germany
Abstract:
Due to the lack of human organs required for liver transplantation, alternative methods including tissue engineering have been established over the last decades. This chapter describes all the relevant elements concerning liver tissue engineering using polymeric scaffolds. The chapter begins with a brief review on liver diseases and current treatments. The special demands, which hepatocytes in three-dimensional culture require, are then reviewed. Furthermore, an overview on tests and assays to analyse hepatocyte-specific function in vitro is given and potential clinical applications using engineered liver tissue are mentioned. Limitations of liver tissue engineering and the challenges that may occur in prospective applications are thoroughly discussed at the end of the chapter.
Key words
liver transplantation; liver tissue engineering; scaffold; three-dimensional (3D) culture; hepatocyte culture
19.1 Introduction
The liver participates in synthesis of nearly 20 000 individual proteins, some of which are exclusively produced in the liver such as albumin and clotting factors (Larsen and Bjerring, 2011). Given this fact it is not surprising that fulminant liver failure often results in multiple organ dysfunction syndrome and rapid death of the afflicted patient. Due to the lack of high quality human organs required for liver transplantation, alternative methods including tissue engineering have been established worldwide to stabilise patients with liver failure or to bridge them until regeneration occurs or a donor organ is available (Diekmann et al., 2006). In this chapter we describe all elements relevant to liver tissue engineering using polymeric scaffolds. The chapter begins with a brief review of liver diseases to give an overview on hepatic failure and currently available treatments. Hepatocytes in three-dimensional (3D) culture have special demands. They are reviewed here in a separate section, focusing on polymer constitution and cell seeding as well as culture medium and culture conditions. Furthermore, an overview on tests and assays to analyse hepatocyte-specific function in vitro is given and potential clinical applications using engineered liver tissue are described. Of those, extracorporeal bioartificial liver devices as well as toxicology and drug screening are most important in transplantation. Limitations of liver tissue engineering and the challenges that may occur in prospective applications are extensively discussed at the end of the chapter, clearly supporting the assumption that the current technical problems can be solved.
19.2 Liver diseases and current treatments
19.2.1 Acute and chronic liver failure
Acute or chronic liver failure and its complications cause morbidity and mortality all over the world. Treatment of liver failure is associated with huge costs in the health care system. Acute liver failure is a clinical syndrome with high mortality (60–80%) leading to death by brain edema, systemic inflammatory response syndrome and multiple organ failure (Chamuleau, 2009). Acute liver failure is mainly caused by viral infections (i.e. hepatitis A and B), drug overdose or intoxication. Further reasons include autoimmune hepatitis, Budd-Chiari syndrome, Wilson disease, ischaemic hepatitis, cancer or liver diseases associated with pregnancy such as HELLP syndrome. Chronic liver failure with the histological feature of irreversible fibrosis or cirrhosis is most often caused by excessive alcohol consumption, biliary obstructions, biliary cirrhosis, chronic hepatitis (infections or autoimmune diseases) or haemochromatosis. Furthermore, several inherited metabolic disorders such as α1-antitrypsin deficiency or urea cycle disorders result in chronic liver failure and therefore require an adequate treatment in reasonable time.
19.2.2 Current treatments
Liver transplantation
Liver transplantation is the most commonly used curative treatment for patients with end-stage liver disease or liver-based inherited metabolic disorders. Unfortunately, this preferred therapy is limited by organ shortage. Hence, for both full and split liver transplantation from a deceased donor, transplant centres around the world have established living liver donation programmes to enable partial liver donation from a living-related donor to a liver-diseased patient. However, it is not simple for physicians to make a decision that achieves a balance between risks and benefits for the donor and the patient (Surman, 2002). To further reduce the discrepancy between the number of available organs and patients waiting for liver transplantation, extended donor criteria liver allografts are transplanted (Nickkholgh et al., 2007). Although many studies implicate no negative impact on patient outcome after liver transplantation, a potential higher risk of poor function and graft failure is accepted using these marginal organs. Further strategies to enhance the pool of available donor organs including donation after cardiac death, old for old programmes, paired organ donation or domino liver transplantation have achieved some success but have not solved the huge problem of organ shortage.
Hepatocyte transplantation
Transplantation of single hepatocytes might be an option, especially for patients with metabolic defects, where a full organ is not needed to replace missing function (Fisher and Strom, 2006; Fitzpatrick et al., 2009). In addition, hepatocyte transplantation offers the chance to bridge patients with acute hepatic failure until endogenous liver regeneration occurs (Walker and Bumgardner, 2005). A lack of benefit is anticipated in patients with chronic hepatic failure due to the altered liver micro-architecture followed by fibrosis or cirrhosis. In those cases incorporation of donor cells into the patients′ native liver appears less likely. During hepatocyte transplantation freshly isolated or cryopreserved hepatocytes are transplanted into the liver via portal infusion. Unfortunately, the success of this procedure is limited by low cell engraftment rates and marginal effects in liver-diseased patients. One of the biggest barriers seems to be the low number of hepatocytes, which can be delivered to the liver without causing cell embolisation to the lung or portal hypertension (Fox et al., 2006; Navarro-Alvarez et al., 2010). Techniques of serial hepatocyte transplantation into the portal system did not lead to significant improvements in cell engraftment rates (Rozga et al., 1995). Despite all the problems associated with this procedure, hepatocyte transplantation found its way into clinical application and more than 80 case reports from different transplantation centres worldwide have been published, as summarised by Fitzpatrick et al. (2009). Nevertheless, there is a need for controlled clinical trials to make the results more comparable and to establish a safe and effective transplantation protocol. Experiences from animal models indicate that successful transplantation of approximately 5–10% of liver cell mass is required to correct metabolic defects (Pietrosi et al., 2009) and as less as 1–5% is needed for hepatic regeneration in patients suffering from acute liver failure (Turner et al., 2010).These findings have encouraged scientists to establish new techniques using tissue engineering for the purpose of hepatocyte transplantation.
Extracorporeal liver support devices
In fulminant liver failure, extracorporeal liver support devices can achieve short term support of patients. Two types of liver assist devices exist. Artificial liver (AL) devices use non-living components to remove toxins from the plasma by physical or chemical gradients and adsorption (Carpentier et al., 2009). However, in bioartificial liver (BAL) systems the patient’s blood or plasma is perfused through a bioreactor containing primary human or animal hepatocytes or hepatocytes from cell lines (Allen et al., 2001). It is commonly concluded that most of the currently available devices are not sufficient for a life-saving treatment in patients with acute liver failure, because they are only supporting the failing detoxification function of the diseased liver. Only if the extracorporeal liver support system replaces both the failing detoxification and the lack of liver protein synthesis and regulatory function of the diseased liver, will there be a beneficial long-term effect in patients with liver failure (Chamuleau, 2009). This prerequisite is better fulfilled by BAL systems and therefore they may have a future. Large numbers of bioreactors with specific designs have been described for application in BAL systems and several in vivo experiments were established, as summarised in Hui et al. (2001) and Park and Lee (2005). Altogether, there are four types of bioreactor design, each with its advantages or disadvantages as detailed in a review by Allen et al. (2001): hollow fibre, flat plate and monolayer, perfused beds or scaffolds, and beds with encapsulated or suspended cells. Bioreactors having undergone clinical trials are thoroughly described in a review by Yu et al. (2009). It is difficult to compare the clinically used liver support systems due to their technical variability and differences in patient setup and outcome parameters (Diekmann et al., 2006).
Xenotransplantation
Xenotransplantation, defined as the transplantation of functioning organs, tissue or cells between different species in general and more specifically from animal to human (German Reference Centre for Ethics in the Life Sciences), is not only flawed by ethical issues. The use of xenogeneic livers in the clinical setting is mainly limited by hyperacute rejection caused by the immune response of the recipient against the graft, the possibility of transferring zoonotic infectious agents (Kanazawa and Platt, 2000), and by major differences in physiological normal ranges of metabolic pathways. Recently, there has been only one clinical attempt of xenotransplantation, published by Makowa et al. (1995). The authors transplanted a pig liver xenograft into a 26-year-old woman with fulminant hepatic failure and a history of immune hepatitis for temporary metabolic support prior to attempted transplantation with a human donor organ. The patient died 34 hours after pig liver xenotransplantation by reason of irreversible brain damage. However, recent developments of less immunogenic transgenic pigs or cellular encapsulation systems represent encouraging advances in the field of xenotransplantation and renewed interest in clinical application (Bonavita et al., 2010).
19.3 In vitro conditions for hepatocytes
19.3.1 Fundamentals
Although most of the biochemical functions are accomplished by parenchymal hepatocytes, there is a strong functional interaction between hepatocytes and non-parenchymal cells such as sinusoidal endothelial cells, Kupffer cells, stellate cells, intrahepatic lymphocytes and bile duct cells (Kmiec, 2001) demonstrating that hepatic tissue is organised in a complex manner. As commonly known, hepatocytes are very demanding cells with special requirements on culture technologies and conditions. One of the most relevant problems during cell culture is that hepatocytes lose their proliferative ability in vitro, although they have an enormous replicative capacity in vivo after liver damage or injury (Fausto, 2000). Although Walldorf et al. (2004) proposed that in vitro proliferative capacity of primary human hepatocytes is critically related to the fraction of diploid cells and reflected by the expression of regulatory cell cycle proteins, the real cause for this limitation has not been found. An additional problem related to primary hepatocyte culture is the loss of hepatocyte-specific function. During hepatocyte monolayer culture a strong perturbation of cell morphology with fibroblast-like protrusions, increasing nuclear volume and granulated cytoplasm have been found after 72 hours (Hewitt et al., 2007). Application of scaffolds and 3D culture conditions can preserve hepatocyte differentiation and enables long-term culture with maintenance of hepatospecific functions for up to 2 months (Riccalton-Banks et al., 2003). However, a technique of culturing primary hepatocytes without loss of differentiation for a prolonged period has not yet been developed.
The hepatocyte is a highly polarised cell with apical and basolateral membranes that are the structural fundament for its exceedingly specific function. Facing the sinusoid it has a cuboidal shape with two or three basal surfaces in contrast to the apical domain, where a bile canaliculi network between adjacent hepatocytes can be observed (Abu-Absi et al., 2002). The loss of polarisation during the cell isolation procedure from liver tissue seems to have large effects on viability, differentiation and reorganisation and may therefore be the first target in improving hepatocyte culture. During cell culture, liver tissue specific reorganisation of single hepatocytes to 3D clusters (spheroids) requires replacement of extracellular matrix serving as a scaffold. It is well accepted that formation of 3D spheroids during cell culture stimulates hepatospecific metabolic function (Dvir-Ginzberg et al., 2003; Elkayam et al., 2006; Lee et al., 2009), avoids the loss of differentiation (Tong et al., 1994; Bierwolf et al., 2011) and, furthermore, shows higher resistance to stress compared to cells in monolayer culture (Olive and Durand, 1994). Due to the requirement of high cell density and close cell-to-cell contacts, formation of highly differentiated spheroids with optimised function is observed in 3D culture using polymeric scaffolds as matrix.
The above facts confirm that hepatic tissue engineering is very laborious and requires an excellent replacement of extracellular matrix and optimal culture conditions.
19.3.2 Special demands on the matrix
Utilisation of polymer matrices as culture surface and scaffold for hepatocytes requires specific matrix composition and architecture. First, the matrix has to facilitate cell attachment to allow a high degree of hepatocyte incorporation after cell seeding. This seems to be one of the most difficult prerequisites, because several of the synthetic matrix components such as poly(lactic acid) (PLA) have a hydrophobic surface, requiring special treatments before use in hepatocyte culture. In contrast to other cell types no interaction between hepatocyte and matrix seems to be necessary due to self-assembly between hepatocytes with spheroid formation. Second, pore size as well as pore connectivity have large effects on spheroid formation and hepatocyte-specific function, especially under static culture conditions using culture dishes. This fact may be based on the special in vivo situation in the liver, where the hepatocytes are surrounded by the portal venous blood, which is rich in oxygen and nutrients.
To meet these criteria, interconnections between matrix micro-pores or high matrix permeability are essential for excellent cell nutrition and waste removal in high density 3D culture using static culture conditions. Furthermore, both nutrition of incorporated cells as well as pore size control the size of the emerging spheroids. However, as large as possible is not always the most favourable option. In flow culture, 3D engineered spheroids with a diameter of more than 200 μm showed central necrosis, due to lack of oxygen and nutrition (Torok et al., 2001b). In general, a spheroid diameter not exceeding 100 μm seems to be most advantageous for viability and function during in vitro hepatocyte spheroid culture (Glicklis et al., 2004). A high surface-to-volume ratio of scaffolds is a further necessity in liver tissue engineering and may enhance hepatocyte adhesion and attachment especially immediately following cell seeding using high density hepatocyte suspensions.
To further increase the quality of the matrix, there is the idea of preparing scaffolds with controlled time-appropriated release of nutritional hepatotrophic factors as offered in vivo by portal blood circulation. Smith et al. (2004) developed an implantable system with sustained delivery of vascular endothelial growth factor (VEGF), an inducer of angiogenesis, from a porous polymer matrix. Subcutaneously implanted hepatocytes using VEGF-containing matrices demonstrated significantly better survival as compared with cells implanted on matrices without growth factors. The group further developed a polylactide-co-glycolide scaffold system with epidermal growth factor (EGF) and hepatocyte growth factor (HGF) release to promote long-term survival of hepatocytes after transplantation. Surprisingly, long-term engraftment of the subcutaneously transplanted hepatocytes was not improved under these conditions (Smith et al., 2006). Sequential delivery of angiogenic factors VEGF, platelet-derived growth factor-BB (PDGF-BB) and transforming growth factor-β1 (TGF-β1) from affinity-binding alginate scaffolds resulted in a three-fold higher blood vessel density after subcutaneous implantation in rats (Freeman and Cohen, 2009). Further details on growth factor delivery for hepatic tissue engineering are given in an excellent review by Babensee et al. (2000).
19.3.3 Seeding methods
Frequently used static seeding methods, where a defined number of cells is seeded on a scaffold by pipetting, are often associated with low seeding efficiency. To resemble the complex in vivo liver network, close cell-to-cell contact of the hepatocytes has to be achieved. Thus, large initial cell numbers are required in hepatic tissue engineering. To obtain high cell density and to facilitate cell-to-cell interaction centrifugal force is used in several studies (Yang et al., 2001; Dvir-Ginzberg et al., 2003; Bierwolf et al., 2011). Applying this method, hepatocytes are suspended in culture medium to obtain a cell suspension. The scaffolds are then placed within the suspension and centrifuged at 40–100 g to entrap the cells inside the pores of the scaffold. Repeated centrifugation and resuspension is recommended in some studies. Utilisation of moderate centrifugal force during cell seeding permits high density cell culture and may furthermore result in a decrease of hepatocyte leakage for the period of 3D cell culture. Another method to immobilise hepatocytes within a scaffold is perfusion cell seeding as described in Shvartsman et al. (2009). The authors used a novel perfusion bioreactor system equipped with a flow-distributing mesh for online cell seeding. Uniform hepatocyte distribution within the scaffolds led to the regeneration of homogeneous functional liver neo tissue. An additional technique includes hepatocyte injection via a 22-gauge needle into the scaffold centre (Dvir-Ginzberg et al., 2003).
19.3.4 Special demands on culture medium
There are several culture media specific for hepatocyte culture available yet most labs prepare their own medium, consisting of a basic medium supplemented with glucocorticoids, hormones, amino acids and growth factors. A typical composition of a medium applied in our lab during 3D culture of primary hepatocytes is the following: Williams’ Medium E supplemented with 200 mM low endotoxin l-alanyl-lglutamine, 1 M HEPES buffer, 100 mM sodium pyruvate, 4 mg/mL of insulin, 5 nM dexamethasone, 10 ng/mL EGF, 10 ng/mL recombinant human thrombopoietin, 10 ng/mL recombinant human HGF and 1% penicillin/streptomycin. Addition of 10% heat-inactivated fetal bovine serum is optional and depends on the intended use. It is commonly known, that medium composition severely influences hepatocyte morphology and gene expression in cell culture. Tuschl and Mueller (2006) detected that serum addition resulted in a strong decrease in gene expression of the drug-metabolising cytochrome P450 isoenzyme 1A1 (CYP1A1). This point might become of particular importance if the engineered liver tissue is engineered for utilisation in toxicology and biotransformation. 3D culture at standard conditions under 37 °C and 5% CO2 is recommended for hepatic tissue engineering. A daily medium change is suggested and may influence hepatocyte function.
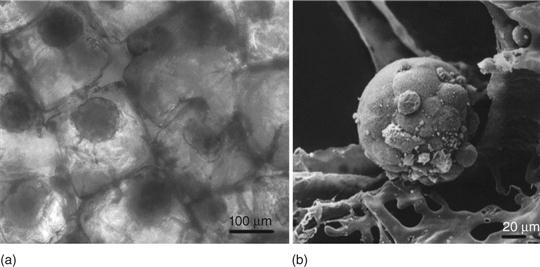
Another opportunity to meet the nutritional and oxygen need in high density 3D culture is the use of special bioreactors enabling flow culture conditions. The flow system contains a computer-assisted peristaltic pump, a medium reservoir and a culture chamber, where the matrices seeded with hepatocytes are exposed to pulsatile flow mimicking blood flow in vivo (Fig. 19.1). Silicone tubing connecting the medium reservoir with culture chamber allows for gas exchange (Torok et al., 2001a, 2001b). Moreover, flow culture conditions seem to enhance hepatocyte aggregation (Fig. 19.2). Spheroid formation in flow culture is already observed after 1 to 2 days (Torok et al., 2006, 2011) whereas under static culture conditions spheroid formation occurs after 7 days (Elkayam et al., 2006). It is important to know that in flow culture some cytochrome P450 (CYP) genes are induced through fluid-flow mediated shear stress. Flow culture conditions may therefore up-regulate the detoxification function in primary human hepatocytes (Vinci et al., 2011).

19.4 In vitro analysis of hepatocyte function
Maintaining hepatocyte-specific function is the first aim during 3D hepatocyte culture (Gebhardt et al., 2003). There are several options beyond simple microscopic analyses to investigate hepatocyte-specific in vitro function of engineered liver neo tissue.
19.4.1 Metabolic function
For examination of metabolic functions a broad range of immunoassays is available with specific antibodies against apolipoproteins, albumin, α1-antitrypsin or urea, for example. To detect not only a trend of secretion during cell culture, and simultaneously be able to compare different culture systems, results have to be calculated on cell number or DNA content per scaffold. In addition, hepatocyte-specific function can also be determined by immunohistochemical or immunoblotting methods. A multitude of specific antibodies against proteins synthesised by hepatocytes are available for this purpose. Glycogen storage capacity is another hepatocyte-specific function and can simply be analysed by periodic acid Schiff (PAS) reaction. In addition, expression of genes involved in hepatocyte-specific metabolism can be detected via real-time polymerase chain reaction (PCR).
One of the most important functions of hepatocytes is their ability to metabolise drugs and toxic substances. Measurement and display of CYP enzyme activity is well established for this purpose. The CYP gene family contains a number of families and subfamilies. However, families 1, 2 and 3 are largely involved in the biotransformation of xenobiotics and metabolise most pharmaceuticals, of which CYP3A4 is the most commonly occurring isoform representing approximately 30–40% of the CYP proteins in the human liver (Donato and Castell, 2003). The strong effect of culture conditions on CYP expression has to be considered, particularly in 3D culture. Furthermore, a lot of publications reported down-regulation of CYP isoenzyme expression in monolayers from the third culture day as consequence of dedifferentiation (Donato and Castell, 2003; Tuschl and Mueller, 2006; Hewitt et al., 2007). In addition, Tuschl and Mueller (2006) found strongly increased CYP1A1 levels in serum-free and considerably decreased CYP levels in serum-containing cultures. Last, Shvartsman et al. (2009) revealed a 50-fold increase in CYP3A4 expression in perfused hepatocellular constructs as compared to the level in statically cultivated cells, indicating the strong effect of environment on CYP3A4 expression in 3D cell culture.
To display CYP action, enzyme activity has to be chemically induced by drugs or xenobiotics. The articles published by Pichard-Garcia et al. (2002) or LeCluyse et al. (2000) are particularly pertinent to give insight into drug-mediated CYP induction in long-term primary hepatocyte culture. The capabilitity of hepatocytes to respond to the inducer with increasing CYP expression seems to depend once more on the presence of the proper matrix. Primary human hepatocytes respond well to enzyme inducers throughout the first 2–3 days in monolayer culture but lose this ability thereafter (Donato and Castell, 2003). However, although the metabolic capacity of cells from hepatoma cell lines is usually limited due to very low CYP expression levels (Donato and Castell, 2003), Elkayam et al. (2006) identified stable expression during 14 days of 3D culture using C3A/HepG2 spheroids on alginate scaffolds compared to monolayer culture with the same cells. In contrast, LeCluyse et al. (2000) found no effect of matrix conditions on CYP expression, suggesting a possible interaction of still unknown factors.
CYP enzyme expression in primary human hepatocytes depends mainly on cell-to-cell than cell-matrix contact during 3D culture (Hamilton et al., 2001). This was also confirmed for 3D culture of C3A/HepG2 cells on polymeric scaffolds by Elkayam et al. (2006). Additionally, a study of Hamilton et al. (2001) furthermore indicates that the disappearance of cell-to-cell contact as assessed by connexin-mediated gap junction or membrane-associated cadherin expression decreases the response to CYP3A4 induction in 3D culture requiring high initial cell density on scaffolds. Nevertheless, there are gender-related differences in CYP3A4 expression of primary human hepatocytes demonstrating the large effect of donor gender on CYP activity.
19.4.2 Morphology
Other interesting features regarding engineered liver neo tissue include fine structure and morphology, which should at its best in closely resemble the in vivo morphology of normal liver tissue. Hepatocytes organised to spheroids show high levels of hepatocyte nuclear factor 4 (HNF-4), one of the major liver-enriched nuclear hepatocyte transcription factors in normal liver tissue (Lindros et al., 1997; Schrem et al., 2002; Dean et al., 2010), as well as a liver-like micro-architecture as demonstrated by staining with Cytokeratin (CK) 18 or 8, both cytoskeleton markers (Fig. 19.3a). To investigate a potential bile canaliculi network in between hepatocytes within a spheroid, colour-labelled phalloidin can be used detecting actin filaments in bile canaliculi (Fig. 19.3b). Additional markers for bile canaliculi are CK 9 and 19. Bipolar configuration by re-establishment of apical and basolateral membranes can be observed by depicting the tight junction protein zonula occludens (ZO) (Abu-Absi et al., 2002; Fanning and Anderson, 2009) (Fig. 19.3c).

19.5 Potential applications of engineered liver tissue
19.5.1 In vivo transplantation
Transplantation of engineered liver tissue into a recipient for replacement of liver function is the most important potential application. There are mainly two opportunities to accomplish this. First, precultured hepatocytes on biodegradable polymer scaffolds can be transplanted heterotopically into extrahepatic well-perfused areas such as the muscle or the mesenterial cavity. This option is preferred in patients with chronic liver disease such as cirrhosis. Since the first studies of Vacanti et al. (1988), Mooney et al. (1994), Kaufmann et al. (1994), Johnson et al. (1994), Uyama et al. (1993) and Fontaine et al. (1993), several in vivo reports have become available on heterotopic transplantation of engineered liver tissue in animal models. Navarro-Alvarez et al. (2010) reported intramuscular transplantation of hepatic tissue constructs sufficiently reversing acute and chronic liver failure in mice. Gwak et al. (2004) identified conglomerates of transplanted hepatocytes and fibrin matrix on the intestinal mesentery 1 week after injection of hepatocytes seeded on fibrin matrix into the peritoneal cavity of athymic mice. Those hepatocytes maintained liver cell specific functions like albumin synthesis and glycogen storage. Other authors used the subdermal layer for incorporation of precultured hepatocytes on biomaterials. However, 3 weeks after implantation of hepatocyte-polymer constructs into subcutaneous tissue of nude mice, a strong decrease in the number of transplanted hepatocytes was observed by Zavan et al. (2005). In contrast, transplantation of collagen hydrogel-based hepatic units into the subcutaneous space of Sprague-Dawley rats resulted in significant cell engraftment with formation of large revascularised hepatic systems (Zhao et al., 2010). Lastly, ectopic implantation of engineered human artificial liver tissue into the intraperitoneal site of athymic nude mice was shown to maintain their detoxification ability in vivo (Chen et al., 2011).
Taken together, the current results suggest that the mesentery provides superior qualities compared to a subcutaneous site for hepatocyte transplantation. It has been suggested that the vasculature in this area carries nutrient-rich blood that supplies a better approximation of the soluble signals present in the liver circulation (Smith et al., 2006). To our knowledge, there is only one publication on human hepatocyte transplantation on polymer scaffolds in clinical application. Schwarz et al. (2011) reported the utilisation of autologous hepatocyte and islet cell transplantation on poly(L-lactic acid) (PLLA) scaffolds for the therapy of human liver cirrhosis. The authors isolated hepatocytes and pancreatic cells from human tissue samples, seeded them onto a PLLA matrix and re-implanted them into the mesentery of the same patient. The average survival rate of 57 patients was 75% at a year after transplantation. Model for End-stage Liver Disease (MELD) dependent survival rate was even as high as 91% for patients with a MELD equal or less than 10. Laboratory values like transaminases, liver synthesis parameters and blood coagulation improved in the majority of the reported patients within 12 months post-treatment. The patients also reported an improved quality of life 1 year post-treatment. Nevertheless, because of the surgical risk of the procedure this kind of treatment is not recommended for patients with a MELD score above 10.
Intrahepatic transplantation of in vitro created hepatocyte spheroids without their polymer matrix is the second option in hepatocyte transplantation and may become feasible in the near future. The use of alginate scaffolds during 3D culture of primary human hepatocytes allows scaffold dissolving and therefore harvesting of the precultured spheroids (Bierwolf et al., 2012). Intrahepatic transplantation of the spheroids via portal infusion as an alternative for single cell infusion may enhance integration of hepatocytes within the recipient liver tissue, improve cell engraftment rates in the recipient liver and for that reason solve the problem of low repopulation rates observed in clinical single cell transplantation. Nevertheless, it is only recommended for fulminant liver failure or inherited metabolic disorders, where the fine structure of the native liver is not compromised. In patients with cirrhosis or chronic liver disease the altered micro-architecture of the recipient liver is not an appropriated site for hepatocyte or spheroid transplantation (Navarro-Alvarez et al., 2010).
It is accepted that the metabolic situation in patients with liver diseases and hepatic failure provides a hepatotrophic stimulus for hepatocytes transplanted in heterotopic locations (Fiegel et al., 2008). Portocaval shunt operation is a standard procedure to allow the delivery of hepatotrophic factors to the transplanted hepatocytes, resulting in higher survival rates for a restricted period of time (Kaufmann et al., 1994; Sano et al., 1996; Ohashi et al., 2001). The requirement for stimulation can also be met by co-transplantation of a small amount of pancreatic islets (Schwarz et al., 2011; Kaufmann et al., 1999). In summary, although some problems relating to in vivo transplantation of tissue engineered liver constructs have been solved, the technique is not available as a standard procedure in clinical application. The availability of functional primary human hepatocytes seems to be the limiting factor in human liver tissue engineering for transplantation purpose.
19.5.2 BAL devices
As mentioned above, in vitro created liver neo tissue on polymer scaffolds can be used in BAL systems for extracorporeal liver replacement, especially in patients with advanced liver disease until they receive a donated liver (Janorkar Amol, 2010). Nevertheless, there are still some problems with the use of perfused scaffolds in BAL, for example nonuniform perfusion, clogging and the cellular shear force (Allen and Bhatia, 2002). As calculated on human liver resections it is assumed that at least 20% of the liver mass is required for adequate liver support (Morsiani et al., 2002). Based on this assumption at least 10–20 × 109 hepatocytes are needed in BAL systems to treat patients with liver failure (Morsiani et al., 2002; van de Kerkhove et al., 2005). Primary human hepatocytes would be ideal for use in BAL systems, but the high demand for donor organs makes it unlikely that sufficient human liver tissue would be available for this application (Park and Lee, 2005). Thus, primary porcine hepatocytes are mainly used in BAL devices due to their unlimited source. Unfortunately, even in this case BAL systems serve only occasionally as temporary support for patients waiting for a donor liver or as a bridge to liver regeneration in the clinical setting of acute hepatic failure.
Scaffolds created for BAL systems need to fulfil different requirements from those required in 3D systems prior to transplantation. Importantly, scaffold biodegradability is not of major interest in BAL systems: mechanical resistance may be more favoured. Furthermore, the recipient’s immunological response is expected to be reduced due to the opportunity of lymphocyte removal filtration during plasmapheresis (Mayer et al., 2000). Scaffolds with smaller pore size might be more suitable, because cells might be flushed out by shear forces occurring during perfusion. To meet these criteria, several scaffolds have been created especially for BAL devices. Those scaffolds are often made of modified polyetherketone (De Bartolo et al., 2004), either chitosan-based (Pan et al., 2005) or galactose-carrying (Cho et al., 2006). The beneficial 3D environment of the scaffolds may help to establish BAL devices as future standard of care for patients with acute hepatic failure.
19.5.3 Toxicology and drug metabolism studies
Hepatocytes are also widely used in pharmaceutical research and toxicological studies based on their high expression of drug metabolising enzymes. Large differences in CYP expression are observed in cultured human hepatocytes from different donors and appear to reflect the heterogeneity of CYP expression in human liver among donors (Hewitt et al., 2007). In addition, large interindividual variability in phase II enzyme UDP glucuronosyltransferase (UGT) activity is observed caused by polymorphisms (Chung et al., 2005). The variability of expressed enzymes involved in drug metabolism is noticeably higher in human than in other species (Donato et al., 1999). These facts should influence the decision whether to use cells of animal or human origin for 3D culture for drug metabolism studies. In addition, the knowledge about species differences in drug metabolism and drug-induced hepatotoxicity has grown significantly (LeCluyse et al., 2005). Therefore, the appropriated advantages and disadvantages have to be taken into consideration in order to make a balanced decision with regard to the cell source.
To restore physiological cell communication and function during cell culture large cell numbers are initially required. This clinical demand conflicts with the requests of the pharmaceutical industry calling for effective high throughput assays preferably in 96- or 384-well format. During monolayer culture, morphological changes and the loss of hepatocyte-specific function occur (LeCluyse et al., 2005). To achieve a strong correlation between the in vivo and in vitro correlation, application of 3D hepatocyte culture on polymer scaffolds is therefore superior to monolayer culture also in the field of toxicology and pharmaceutical research. Nutrition of hepatocytes in high density 3D culture and under static culture conditions is one of the ultimate limiting factor based on the low amount of medium that fits into one well. This technical challenge can be overcome by the utilisation of a special culture dish with lengthwise oval-shaped wells increasing the well volume and initially suggested as a receiver tray (Bierwolf et al., 2011) (Fig. 19.4). This culture dish furthermore allows easy removal of the scaffolds from the wells for further analysis. Rat hepatocytes on nanofibrous scaffolds used in this study demonstrated highly differentiated hepatocyte-specific function with the capability to metabolise toxic substances.

Chen et al. (2011) explored a new model in liver tissue engineering for toxicology. They established an in vivo model using humanised mice with ectopic artificial liver tissue for the use in various drug development and drug metabolism studies. More in detail, intraperitoneal implantation of tissue engineered human artificial livers prepared from functionalised polymer scaffolds that encapsulate primary human hepatocytes within a supportive microenvironment was achieved in mice. As a result animals exhibited humanised liver function persistent for weeks, including synthesis of human proteins, human drug metabolism, drug–drug interaction and drug-induced liver injury. Application of this model in toxicology and drug development could therefore promote the transferability of test results to the in vivo situation in humans.
Tissue engineering using hepatocytes on polymer scaffolds may further support the establishment of 3D culture systems in pharmaceutical research, toxicology and drug metabolism studies in future due to their morphological and functional stability, in contrast to the currently used monolayer systems.
19.6 Conclusions and future trends
During the last decades there has been a strong focus on in vitro culture conditions in liver tissue engineering to facilitate optimal precondition prior to transplantation. A large variety of biodegradable scaffolds was developed to mimic the special in vivo situation of hepatocytes within the native liver. Diverse culture technologies were established using flow culture in dynamic bioreactors to further enhance hepatocyte aggregation and hepatic tissue formation. Composition of culture medium was adapted to meet the special demand on hepatocyte nutrition. Different sources of human liver tissue were successfully used for hepatocyte preparation and 3D culture on polymer scaffolds, including discarded parts of donor livers after size reduction, healthy resection margins after partial hepatectomy or explanted livers after organ transplantation. Additional sources for 3D liver cell culture were used, including hepatoblastoma-derived cells, stem cells, progenitor cells and reprogrammed adult cells from skin. Nevertheless, the use of primary human hepatocytes is still the gold standard in hepatic tissue engineering. To avoid the in vitro loss of primary hepatocyte proliferative ability should therefore be the first aim in the future and requires further studies in basic sciences. To meet this criteria the matrix composition and configuration could play an important role.
Prior to clinical application safety and efficiency of engineered liver tissue transplantation has to be verified in large animal models for better estimation of risks and benefits for the potential recipient. Furthermore, in vivo behaviour of the scaffold, vascularisation, bile excretion and longtime survival of the tissue engineered liver have to be analysed. In addition, numerous questions concerning the recipient’s immunological response have to be clarified if allogenic hepatocytes are used.
Altogether there is still a long road until tissue engineered liver transplantation becomes a valid additional tool to orthotopic liver transplantation. However, the first important steps have been taken. An additional future aspect includes the establishment of ex vivo genetically modifications during 3D culture of human hepatocytes on scaffolds that can be dissolved. Our previously performed experiments using hepatocytes from metabolic disordered children for 3D culture without cell dedifferentiation could be an important step to application of gene therapy in the future (Weber et al., 2011; Bierwolf et al., 2012). Those systems may even act as an opportunity to bridge the availability of high quality hepatocytes from donor livers that are not suitable for transplantation due to mechanical lesions or long ischaemic time.
Clinical application of tissue engineered livers in BAL devices is currently limited by technical and logistical problems, as well as lacking availability of hepatocytes in large amounts, as required in BAL. In future BAL devices optimisation of logistical aspects such as storage, transportation and scale-up of the systems is necessary to offer flexible and independent devices for an effective clinical application (Diekmann et al., 2006). Several devices have undergone clinical trials, but have not confirmed the expected benefits. To date, currently available systems are not able to mimic the full metabolic, secretory, detoxification and regulatory function of native liver tissue. As a consequence, it has been asked whether BAL systems will ever be successful (Sussman et al., 2009). However, the greatest challenges for BAL devices in the future are to find the best cell type and to maintain viable and functional hepatocytes outside the body during an extended period of time (Strain and Neuberger, 2002). To develop biomaterials that meet the special demands on long-term hepatocyte culture in bioreactors seems to be an important step towards potential clinical application. Nevertheless, several technical difficulties will have to be resolved before BAL systems become a routine treatment for patients with fulminant hepatic failure.
Application of 3D engineered liver tissue in toxicology and drug development may become available in the near future. High throughput 3D systems with excellent hepatocyte-specific function and detoxification ability were developed especially for use in this field. Those systems are superior to monolayer culture and preserve hepatocyte-specific enzymatic function and morphology. Nevertheless, evidence is needed of strong correlation between 3D results and in vivo results.
Altogether, a lot of challenges are addressed in this chapter occurring in hepatic tissue engineering, but the chapter clearly supports the assumption that they are able to be solved.
