Multifunctional scaffolds for bone tissue engineering and in situ drug delivery
V. Mouriño and J.P. Cattalini, University of Buenos Aires, Argentina
W. Li and A.R. Boccaccini, University of Erlangen-Nuremberg, Germany
S. Lucangioli, University of Buenos Aires, Argentina
Abstract:
This chapter provides an overview about the development of bone tissue engineering scaffolds with the ability to provide the controlled delivery of therapeutic drugs. Typical drugs considered include gentamicin and other antibiotics generally used to combat osteomyelitis as well as anti-inflammatory drugs and bisphosphonates. Special attention has been given to the technology used for controlling the release of the loaded drugs. A detailed summary of drugs included in bone tissue scaffolds is presented and the many approaches developed to combine organic and inorganic biomaterials in composites for drug-delivery systems are discussed. The remaining challenges in the field are summarized, suggesting also future research directions.
Key words
multifunctional scaffolds; drug delivery; bone tissue engineering; therapeutic drugs
22.1 Introduction
Although bone tissue has the unique ability to heal, repair and remodel, there is a growing need to tackle several debilitating and deadly conditions, due to trauma or disease, which require the replacement of the affected bone (Buckwalter et al. 1996a, 1996b). In addition the increasing aging population is leading to more requirements for the effective treatment of bone defects and the need to accelerate the healing of large bone fractures. Tissue engineering (TE) offers an alternative to donor graft tissue (autografts, allografts, or xenografts) for the treatment of bone diseases (Goessler et al. 2007; Lee and Shin 2007; Bran et al. 2008; Kanczler and Oreffo 2008; Guarino et al. 2012; Nguyen et al., 2012). In this sense, bone TE can potentially improve the lives of those patients who are suffering from bone diseases associated with tissue loss by providing a controlled environment that promotes and directs cell attachment, proliferation and differentiation, and for supporting new tissue growth (Mouriño et al. 2013). A common approach to bone tissue engineering involves the application of biodegradable and biocompatible scaffolds to create in the first instance sufficient space for new tissue formation, e.g. a three-dimensional (3D) engineered porous biomaterials which must: (a) promote the infiltration and proliferation of host cells for tissue regeneration (Duarte et al. 2007), (b) exhibit high porosity, high pore interconnectivity and uniform pore distribution to enable vascularization (Lee and Shin 2007; Cipitria et al. 2012; Guarino et al. 2012; Nguyen et al. 2012) and (c) have the ability to be resorbed at a rate similar to the rate of new tissue formation. Therefore, the selection of the most appropriate biomaterials for the preparation of scaffolds should be done considering the mechanical and physicochemical requirements as well as their degree of biocompatibility and absence of adverse immune responses (Hutmacher 2000; Mouriño et al. 2013). The desired characteristics of traditional scaffolds for bone TE (BTE) and their fabrication technologies have been described in several review articles (for example in Garg et al., 2012; Blackwood et al., 2012; Hutmacher, 2000; Guarino et al., 2007; Rezwan et al., 2006). In addition, scaffolds have to be suitable for sterilization without losing their properties.
A convenient alternative being increasingly investigated to improve the scaffold biological functionality is to load therapeutic drugs in scaffolds to support the treatment of bone disorders and/or to combat possible infections. Such drugs incorporated into tissue scaffolds must be released with an adequate therapeutic concentration level and for a desired time frame (Gomes and Reis 2004; Duarte et al. 2007; Baroli 2009; Mouriño and Boccaccini 2010; Hafeman et al. 2010; Wende and Guelcher 2011). The increasing research activities associated with this approach, which promotes the development of matrices with a dual function: scaffolds for the growth of new tissue and carriers for controlled drug delivery in situ, is leading to the novel research field called TE therapeutic (Baroli 2009; Mouriño and Boccaccini 2010). In this chapter, which follows from our previous review paper (Mouriño and Boccaccini 2010), special attention has been paid to the latest developments related to control the release rate of relevant drugs from bone TE scaffolds based on different organic and inorganic biomaterials. However, the development of bone TE scaffolds with the specific capability to deliver growth factors or other bioactive molecules, being a very important subtopic in bone TE strategies (Wende and Guelcher 2011; Vo et al. 2012; Ekenseair et al. 2013), will not be covered in this chapter. The document is organized as follows: Section 22.2 is dedicated to summarizing the biomaterials utilized and scaffold designs proposed as drug delivery vehicles. Section 22.3 details several approaches adopted to develop bone TE scaffolds with drug delivery capability considering antimicrobial agents, anti-inflammatory and antiresorptive drugs. Finally, the remaining challenges in the field are summarized in Section 22.4, where also directions for future research efforts are highlighted.
22.2 Scaffolds as drug carriers
Scaffolds for bone TE are made from a variety of biodegradable polymers, bioactive and resorbable inorganic materials including calcium phosphates, mesoporous silica and bioactive glasses, and their composites, as discussed in several chapters of this book. There are increasing investigations focusing on loading engineered scaffolds with therapeutic drugs, generating a dual function for the matrices: (i) scaffolds in the ‘classical’ TE approach, e.g. for the delivery of cells and to support the growth of new tissue (Langer and Vacanti 1993; Freed et al. 1994; Bonassar and Vacanti 1998; Shea et al. 1999; Hutmacher 2000; Hoffman 2002; Guarino et al. 2012) and (ii) carriers for controlled in situ drug delivery (Drury and Mooney 2003; Sokolsky-Papkov et al. 2007; Kretlow and Mikos 2008; Lyons et al. 2008; Makarov et al. 2010; Kankilic et al. 2011; Lee et al. 2011; Wende and Guelcher 2011; Hum and Boccaccini 2012). Therapeutic drugs used in the treatment of bone diseases administered locally have several advantages over systemic administration such as the reduction of adverse effects and the risk of overdose, while enhancing the bioavailability of the drug with the appropriate therapeutic concentration effectively reaching the target site (Baroli 2010; Mouriño and Boccaccini 2010; Mouriño et al. 2013).
In order to design and develop multifunctional scaffolds, several variables must be taken into account. Generally, multifunctional scaffolds must enable the delivery of therapeutic drugs to the nearby tissues, and must provide adequate control of the rate of release of the loaded drugs in order to sustain the expected concentration level in their target sites for the required period of time. The selection of processing methods to develop highly porous scaffolds with suitable mechanical and structural properties is one of the challenges in the field of bone TE (Hutmacher 2000; Rezwan et al. 2006; Guarino et al. 2012; Liu et al. 2013). The effect of the drug incorporation on the physicochemical and mechanical properties of scaffolds as well as the controlled release of drugs from the matrices must be taken into account when multifunctional scaffolds are designed. From a pharmaceutical standpoint, the type of interaction between drug and scaffold, the mechanism of degradation of the scaffold and the selection of the fabrication process will be determined by the type of drug, its stability and the required release kinetics. Further, the level and duration of the therapeutic drug may need to be modulated to avoid excessive drug activity at the target site and it may also be useful a drug delivery with time varying concentrations.
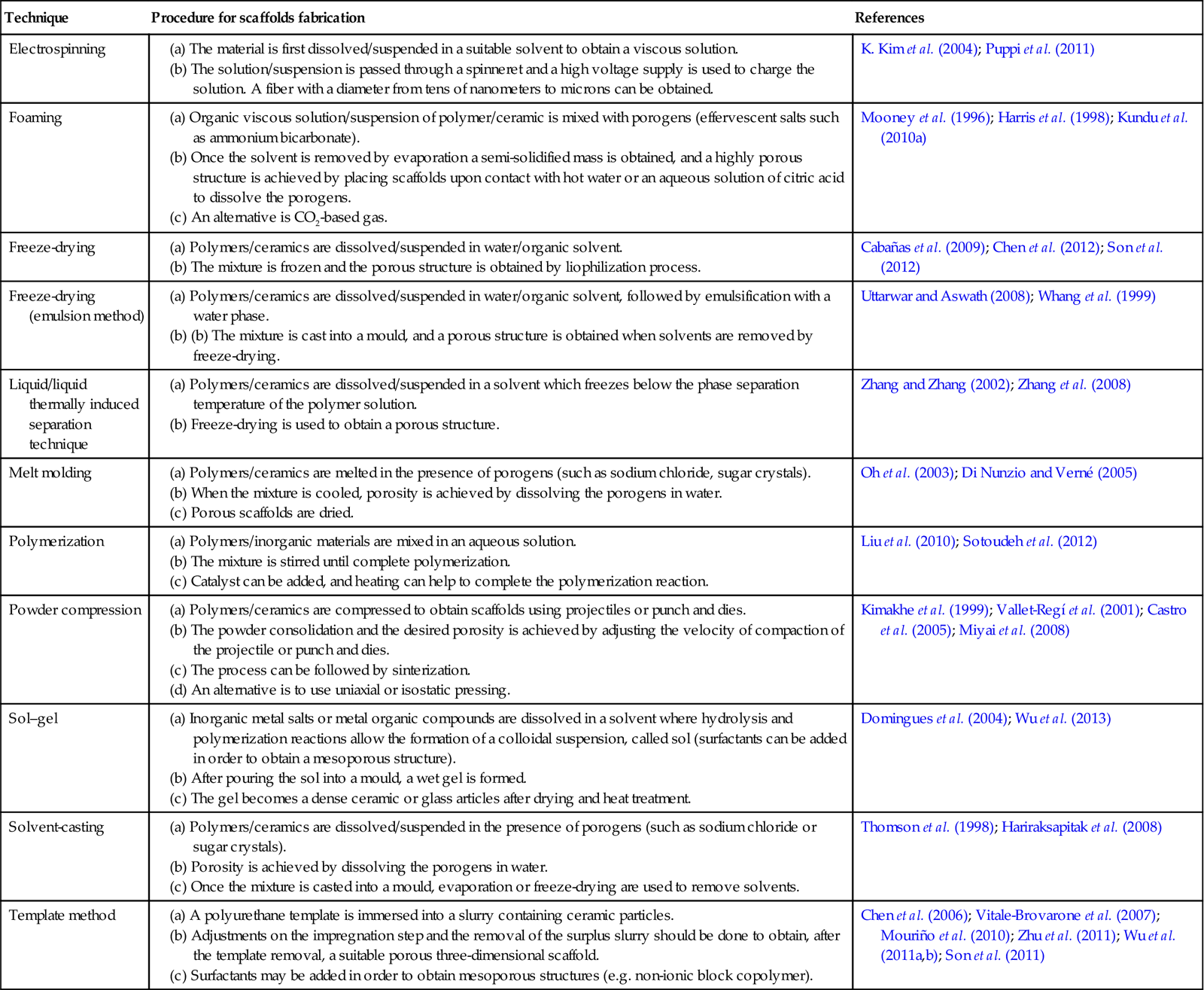
Processing methods for TE scaffolds usually involve processes that are incompatible with the incorporation and stability of organic drugs (Drury and Mooney 2003). Fabrication conditions such as high temperatures, use of certain organic solvents, application of pressure and free radicals may lead to drug decomposition and should be avoided (Mandal and Kundu 2008). The techniques more frequently used to fabricate bioactive bone scaffolds with potential drug release capability are listed in Table 22.1 and a summary of the evolution of experimental research carried out on the development of 3D scaffolds for bone tissue engineering with controlled release capability is schematized in Fig. 22.1. These scaffolds can be developed with the potential to provide not only the physicochemical environment and the structural integrity required for bone regeneration (the main scaffold function), but also with the added function of local regulator to control the dose and kinetics of drug release effectively acting as the drug carrier (Berger et al. 1997; Mouriño and Boccaccini 2010).
Table 22.1
Different techniques to elaborate porous three-dimensional scaffolds with drug-delivery capability for bone tissue engineering therapeutics
| Technique | Procedure for scaffolds fabrication | References |
| Electrospinning | K. Kim et al. (2004); Puppi et al. (2011) | |
| Foaming | (a) Organic viscous solution/suspension of polymer/ceramic is mixed with porogens (effervescent salts such as ammonium bicarbonate). (b) Once the solvent is removed by evaporation a semi-solidified mass is obtained, and a highly porous structure is achieved by placing scaffolds upon contact with hot water or an aqueous solution of citric acid to dissolve the porogens. |
Mooney et al. (1996); Harris et al. (1998); Kundu et al. (2010a) |
| Freeze-drying | Cabañas et al. (2009); Chen et al. (2012); Son et al. (2012) | |
| Freeze-drying (emulsion method) | Uttarwar and Aswath (2008); Whang et al. (1999) | |
| Liquid/liquid thermally induced separation technique | Zhang and Zhang (2002); Zhang et al. (2008) | |
| Melt molding | Oh et al. (2003); Di Nunzio and Verné (2005) | |
| Polymerization | Liu et al. (2010); Sotoudeh et al. (2012) | |
| Powder compression | (a) Polymers/ceramics are compressed to obtain scaffolds using projectiles or punch and dies. (b) The powder consolidation and the desired porosity is achieved by adjusting the velocity of compaction of the projectile or punch and dies. (c) The process can be followed by sinterization. (d) An alternative is to use uniaxial or isostatic pressing. |
Kimakhe et al. (1999); Vallet-Regí et al. (2001); Castro et al. (2005); Miyai et al. (2008) |
| Sol–gel | (a) Inorganic metal salts or metal organic compounds are dissolved in a solvent where hydrolysis and polymerization reactions allow the formation of a colloidal suspension, called sol (surfactants can be added in order to obtain a mesoporous structure). (b) After pouring the sol into a mould, a wet gel is formed. (c) The gel becomes a dense ceramic or glass articles after drying and heat treatment. |
Domingues et al. (2004); Wu et al. (2013) |
| Solvent-casting | Thomson et al. (1998); Hariraksapitak et al. (2008) | |
| Template method | (a) A polyurethane template is immersed into a slurry containing ceramic particles. (b) Adjustments on the impregnation step and the removal of the surplus slurry should be done to obtain, after the template removal, a suitable porous three-dimensional scaffold. (c) Surfactants may be added in order to obtain mesoporous structures (e.g. non-ionic block copolymer). |
Chen et al. (2006); Vitale-Brovarone et al. (2007); Mouriño et al. (2010); Zhu et al. (2011); Wu et al. (2011a,b); Son et al. (2011) |
22.3 Controlled release of therapeutic drugs for bone tissue engineering
Different strategies have been proposed to enable the release in a controlled manner of relevant therapeutic drugs for the treatment of diseases associated with bone repair process in 3D scaffolds used in bone TE. A detailed summary of the most common drugs loaded in such scaffolds and both in vitro and in vivo studies carried out is presented in Table 22.2. Typical drugs considered include gentamicin and other antibiotics generally used to combat osteomyelitis such as tetracycline, polymyxin B, gatifloxacine and ciprofloxacine; as well as silver, anti-inflammatory drugs and bisphosphonates. From Table 22.2 it appears that porous matrices based mainly on well-characterized biocompatible polymeric scaffolds or, in some cases, composites comprising polymeric matrices and added inorganic particles, represent convenient systems to incorporate therapeutic drug delivery in bone TE approaches. A schematic diagram summarizing the different strategies proposed is shown in Plate XIV (between pages 354 and 355). Although several novel techniques have been developed to introduce therapeutic drugs within scaffolds, in most cases the strategy followed has been the direct incorporation of the drug into the scaffold by immersion of the scaffold in a drug containing buffer aqueous solution. Nevertheless, thermo-labile drugs can also be loaded within 3D scaffolds in a one step process using room temperature compaction of powder mixtures, this being a solvent-free process which avoids the use of toxic solvents (Kimakhe et al. 1999; Vallet-Regí et al. 2001; Castro et al. 2005). Alternatively drug carriers in the form of biodegradable polymer microspheres can be loaded into the 3D scaffold structure (Francis et al. 2010). Such a structure is shown in Fig 22.2 where drug loaded P3HB microspheres have been deposited on the surface of bioactive glass scaffolds following an approach similar to that developed by Francis et al. (2010). Generally, it is observed that the release kinetics of drugs loaded in multifunctional scaffolds is not necessarily directly linked to the degradation kinetic of the biodegradable scaffold. Even though most of the 3D scaffolds reviewed in this chapter have been shown to have rates of degradation much lower than the required rate of drug release, it is important to highlight that drug release from most of the developed scaffolds is mainly driven by the process of diffusion through them. Moreover, few studies are focused on delivering the drugs within specific therapeutic levels over a predetermined period of time and taken into account the particular in vivo microenvironment including the effect of vascularization.
Table 22.2
Examples of drug delivery from three-dimensional scaffolds for bone tissue engineering
| Therapeutic effect | Therapeutic drug | Matrix composition | Matrix shape | Process technique | Type of Experimental trial | References |
| Antibiotic/antibacterial | Amoxicillin | Nano zeolite/PEG/poly acrylic acid/polyacrylamid | Composite pieces | Nano zeolite was added to a mixture containing polymers and drug + stirring until complete polymerization | in vitro | Sotoudeh et al. (2012) |
| Ceftazidime | EC microspheres/HA/PU | Porous matrix | A mixture of HA/PEG/catalyst was stirred under dry nitrogen atmosphere + a chain extender was added and the temperature was maintained at 65 °C + an aqueous suspension of drug-loaded microspheres was added with a catalyst + the mixture was poured into a mold at 80 °C to complete the polymerization + washing + drying | in vitro | Liu et al. (2010) | |
| Ceftriaxone/sulbactam | HA/β-TCP/chitosan | Porous matrix | Foaming method | in vitro | Kundu et al. (2010b) | |
| Ciproflozacin | HA/β-TCP/PLA | Porous matrix | Compression | in vitro/in vivo | Castro et al. (2005) | |
| Colistin | PLGA microspheres/PMMA/CMC | Porous construct | The mixture of loaded-PLGA microspheres + PMMA + CMC was cast in molds and allowed to harden | in vitro | Shi et al. (2010) | |
| Copper | Bioactive glass/alginate | Porous matrix | Polyurethane sponge technique + immersion in alginate solution + crosslinking | in vitro | Erol et al. (2012) | |
| Mesoporous bioactive glass | Porous matrix | Sol–gel | in vitro | Wu et al. (2013) | ||
| Gallium | Alginate/bioactive glass | Porous matrix | Bioactive glass scaffold made by foam replica technique + scaffold coating with gallium crosslinked alginate | in vitro | Mouriño et al. (2010) | |
| Alginate/bioactive glass nanoparticles | Films | The mixture containing alginate and bioactive glass nanoparticles was casted into a mould and left to dry + crosslinking with gallium | in vitro | Mouriño et al. (2011) | ||
| Gatifloxacin | β-TCP/PCL | Porous matrix | Compaction + sintering + immersion in drug-loaded PCL slurry | in vitro/in vivo | Miyai et al. (2008) | |
| Gentamicin | β-TCP/CP/chitosan | Porous matrix | Thermally induced phase-separation technique + immersion in drug-containing PBS solution | in vitro | Zhang and Zhang (2002) | |
| Bioactive glass | Bioactive glass pieces | Uniaxial and isostatic compression at room temperature | in vivo | Vallet-Regí et al. (2001) | ||
| Antibiotic/antibacterial | Bioactive glass | Mesoporous bioactive glass/bioactive glass | Polyurethane sponge technique + immersion in drug-containing PBS solution | in vitro | Zhu and Kaskel (2009) | |
| Bioactive glass/P(3HB) microspheres | Porous matrix | Polyurethane sponge technique + emulsion solvent evaporation method to obtain gentamicin-loaded microspheres + immersion in microsphere slurry | in vitro | Francis et al. (2010) | ||
| HMS-HA/PLGA microspheres | Porous matrix | Tensioactive template to obtain HMS-HA + double-emulsion evaporation technique to obtain GS-loaded PLGA microspheres + sinterization at 70 °C | in vitro | Shi et al. (2009) | ||
| Zirconium/bioactive glass | Mesoporous bioactive glass | Polyurethane sponge technique + evaporation-induced self-assembly process + immersion in drug-containing solution | in vitro | Zhu et al. (2011) | ||
| Polymyxin B | Calcium phosphate | Ceramic pieces | Compaction | in vitro | Kimakhe et al. (1999) | |
| Silver | Bioactive glass | Bioactive glass pieces | Melting and sintering at high temperature + ion-exchange process to introduce the drug | in vitro | Di Nunzio and Verné (2005) | |
| Tetracycline | Bioactive glass/β-cyclodextrin | Bioactive glass pieces | Sol–gel | in vivo | Domingues et al. (2004) | |
| Chitosan/HA | Porous matrix | Freeze-drying | in vitro | Teng et al. (2009) | ||
| HA/PCL | Porous matrix | Polyurethane sponge technique + immersion in drug/HA/PCL slurry | in vitro | H. Kim et al. (2004c) | ||
| Vancomycin | β-TCP/agarose | Porous matrix | Freeze-drying and heat desiccation at 37 °C. | in vitro | Cabañas et al. 2009) | |
| (Gelatin/β-TCP | Porous matrix | A mixture of gelatin/β-TCP/drug/genipin was stirred + gelification + crosslinking + freeze-drying | in vivo | Zhou et al. (2012) | ||
| HA/PCL | Porous matrix | Polyurethane sponge technique + immersion in drug/HA/PCL slurry | in vitro | Kim et al. (2005) | ||
| PDLLA/BCP/alginate | Porous matrix | Particle: leaching/thermally induced phase separation method + immersion in alginate/vancomycin solution + crosslinking | in vitro | Zhang et al. (2008) | ||
| Antibiotic/antitumoral | Doxorubicin | PCL/chitosan/nanoclay/β-TCP | Porous matrix | PCL scaffolds were made by fused deposition modeling. The clay/DOX carrier was added to a chitosan/β-TCP mixture. Finally, PCL scaffolds were submerged into the mentioned mixture and freeze dried. | in vitro | Chen et al. (2012) |
| Antiinflammatory | Ibuprofen | Mesoporous bioactive glass | Porous matrix | Sol–gel + immersion in ibuprofen-hexane solution | in vitro | Wu et al. (2013) |
| HA | Porous matrix | Cellulose sponge technique + immersion in drug solution | in vitro | Palazzo et al. 2005) | ||
| Inductive effect in osteogenic culture | Dexamethasone | Starch/PLA | Porous matrix | Supercritical phase-inversion technique | in vitro | Duarte et al. (2009a) |
| Chitosan | Porous matrix | Freeze-drying + drug impregnation by supercritical fluid technology | in vitro | Duarte et al. 2009b) | ||
| Boron + bioactive glass | Mesoporous bioactive glass | Co-templates of nonionic block polymer + polyurethane sponge technique + immersion in drug-containing PBS solution | in vitro | Wu et al. (2011a) | ||
| SiO2 | Mesoporous structure | Co-templates of nonionic block polymer + polyurethane sponge technique + immersion in drug-containing PBS solution | in vitro | Wu et al. (2011b) | ||
| Drug-loaded PLGA nanoparticles/HA | Porous matrix | Polymeric template coating technique | in vitro/in vivo | Son et al. (2011) | ||
| Drug-loaded PLGA nanoparticles/HA/TCP | Granules | Freeze-drying | in vitro | Son et al. (2012) | ||
| Inhibition of the osteoclastic resorption | Alendronate | PLGA/HA | Microspheric scaffold | – | in vitro | Wang et al. (2010) |
| Poly(L-lactide-co-epsilon caprolactone)/bioactive glass | Microspheric scaffold | Emulsion-solvent + evaporation method | in vitro | Mondal et al. (2012) | ||
| Silica (SBA-15) | Mesoporous silica matrix | Triblock copolymers technique + functionalization + immersion in drug-containing buffer aqueous solution | in vitro | Nieto et al. (2008) | ||
| Inhibition of the osteoclastic resorption | Clodronate | PCL/HA | Porous matrix | Drug-loaded HA nanoparticles were added to a PCL solution + fibers obtained by electrospinning | in vitro | Puppi et al. (2011) |
| Pamidronate | PDLLA | Pellets | Solvent casting method + compression | in vivo | Yu et al. (2010) | |
| Zoledronate | CPA | Pellets | Suspension of CDA in drug containing water solution | in vitro | Faucheux et al. (2009) | |
| – | Ibuprofen | Bioactive glass/MCM-41 | Porous matrix | MCM-41 spheres + melting and sitering at high temperature to obtain bioactive glass scaffold + drug impregnation | in vitro | Mortera et al. (2010) |


b-TCP, b-tricalcium phosphate; CPA, calcium phosphate-deficient apatite; CMC, carboxymethylcellulose; CP, calcium phosphate invert glasses; ES, ethyl cellulose; GS, gentamicin; HA, hydroxyapatite; HMS, mesoporous silica; PCL, poly-1-caprolactone; PBS, phosphate-buffered saline; PCL, poly(ε-caprolactone); PDLLA, poly(D,L-lactic acid); PEG, poly(ethylene glycol); PLA, poly(L-lactic acid); PLGA, poly(lactide-co-glycolide); PMMA, polymethylmethacrylate; PU, polyurethane.

In addition, in most cases the effect of the formation of apatite surface layer and precipitation of hydroxyapatite, which is common in bioactive bone tissue scaffolds, on drug release kinetics has not been contemplated during in vitro release studies.
22.4 Conclusions and future trends
Research carried out, particularly in recent years, on bioactive bone TE scaffolds with additional drug-delivery capability has indicated the great potential of such multifunctional scaffolds for application in bone re-generation. Especially encouraging are the results obtained with combinations of materials, as well as improved 3D bone tissue scaffold designs based on novel processing techniques with the added value of drug-delivery capability. However, and despite the significant progress achieved, there is still the need to fully understand the correlation between in vitro and in vivo performance of the designed scaffolds, and probably it will take time to achieve in vivo results of relevance which can provide a rational for optimization of drug-delivery function of multifunctional scaffolds.
Further efforts should be made in developing strategies to establish the concentration and distribution of a therapeutic drug within a scaffold which is required for successful outcomes. At present, most of the investigated approaches present reduced ability to adjust drug dosages and most of the systems developed would not be easily scalable for commercial applications in terms of cost effectible manufacture process. In this sense, multifunctional scaffolds should be easy to produce, sterilize and handle. Finally, another important consideration, often overlooked, is the requirements needed to obtain approval from regulatory authorities.
It is clear that, in order to optimize these systems, various disciplines such as chemistry, biology, pharmacy, medicine and biomaterials science must come together in interdisciplinary approaches. The interdisciplinary character of TE – allowing the confluence of different scientific fields, backgrounds and knowledge – is mandatory for the great deal of further work required towards the development of more effective multifunctional bone TE.

