Kidney tissue engineering
A. Saito, Tokai University School of Medicine, Japan
Abstract:
Bioartificial tubule devices consist of tubular epithelial cells and porous membranes. Although bioartificial tubule device treatment is yet to be approved by any government in the world, investigation of its use is now considered an essential step in the future of kidney regeneration. This chapter outlines the basic form and function of tubular epithelial cells, interactions between matrices, porous membranes and tubular epithelial cells, the development of tubular epithelial cells attached to the surface of porous membranes, and in vitro evaluation of the transport and metabolic function of bioartificial tubule devices. Bioartificial tubule device treatments for acute kidney injuries with multiple organ dysfunction syndrome are also considered before, finally, the potential future development of a bioartificial glomerulus is discussed.
Key words
kidney tissue engineering; bioartificial tubule device; porous membrane; bioartificial glomerulus
13.1 Introduction
Over the past two decades, increasing focus has been placed on the potential role of regenerative medicine as the next generation treatment for diseased tissues or failed organs. Human embryonic stem cells and induced pluripotent stem (iPS) cells1 have, to date, never been used clinically because of ethical hurdles or risk of carcinogenesis, although there have been several clinical trials in which bone marrow stem cells or progenitor cells in adult tissues were utilized to repair diseased tissues. In general, the application of regenerative medicine is expected to become the first step in the treatment of organs and tissues in which one cell type plays the main role in the development and functioning of the organs or tissues.
Generating an organ such as a kidney by using regenerative medicine is complicated by the complexity of the basic functional unit of the kidney – the nephron. This consists of a glomerulus, the filtration unit containing endothelial, mesangial and epithelial cells; and a tubule, the metabolic and endocrinologic unit that comprises tubular epithelial cells (proximal and distal tubules, Henle loop and collecting duct). Therefore, development of an artificial kidney using kidney cells and artificial membranes as the scaffold should be considered an essential step in kidney regeneration.
This chapter describes the limitations of haemodialysis as renal replacement therapy in Section 13.2, and the concept and configuration of bioartificial kidneys in Section 13.3. Past and current developments of a bioartificial renal tubule device (BTD) are described in Sections 13.4 and 13.5, which outline the history of BTD development as well as research into the construction of functional BTDs and their metabolic activity. In vivo evaluation of BTD treatments for severe acute kidney injury (AKI) patients and bilateral nephrectomized goats with endotoxinemia are described in Section 13.6. A new technology involving blending methacryloyloxyethyl phospholylcholine (MPC) polymer with polysulfone (PSf) to produce a BTD membrane with a cytocompatible surface at tubule cell site and haemocompatible surface at blood cell site is then described in Section 13.7. Development of a bioartificial glomerulus, consisting of CD133+ cells obtained from cord blood and a semipermeable polymer membrane, is introduced in Section 13.8. Finally, future trends in the development of bioartificial kidneys are discussed in Section 13.9.
13.2 Limitations of hemodialysis (HD) as renal replacement therapy
The kidney functions to maintain the homeostasis of body fluids by controlling (1) excretion of metabolites, (2) blood volume, (3) electrolyte concentrations, (4) acid–base balance, and (5) metabolic and endocrinologic roles in renal tubules. Patients with chronic kidney diseases2 in whom the renal function has deteriorated to less than 15% of the normal functionality have to be treated by dialysis or undergo surgical intervention for renal transplantation to reduce the risk of mortality. It was reported that there were 1.371 million patients who were treated on dialysis and 412 000 patients who received renal transplantation at the end of 2004.3
In haemodialysis (HD), accumulated metabolites in a patient’s blood are removed by diffusion across a semipermeable artificial membrane, and excessive or low densities of electrolytes are normalized by moving them to/or from dialysate according to the Donann’s membrane equation. Excessive body fluids are removed under negative pressure added to the dialysate, and the acidic pH of the blood is neutralized by the presence of bicarbonate or sodium acetate in the dialysate. In Japan, patients with renal failure undergo HD three times per week, and each session lasts for 4 h. Currently, treatment by HD cannot substitute normal kidney function as the effects last for only 12 (7.2%) of the 168 h in a week, whilst some components used in HD are, additionally, harmful to patients. Severe complications, such as bone mineral disorder, renal anaemia, atherosclerosis/cardiovascular diseases, dialysis-related amyloidosis and malnutrition, are observed in patients undergoing long-term HD due to the lack of proper kidney function. Whilst the current HD treatment can imperfectly replace the glomerular filtration, it cannot replace tubular function at all. In order to overcome this problem and prevent dialysis-related complications, intermittent HD has to be replaced by continuous haemofiltration (HDF) and the tubular function has to be included in the new treatment. Development of a bioartificial kidney with bioartificial tubules capable of continuous haemofiltration (CHF) is therefore essential, unless the development of functional kidneys from stem cells or the healing of diseased kidney tissues by such treatments as bone marrow stem cells, become usable techniques.
13.3 Concept and configuration of bioartificial kidneys
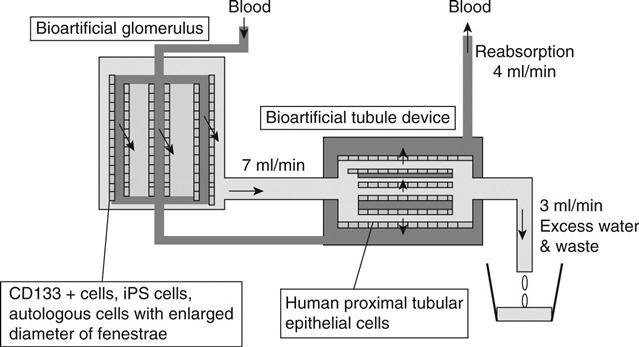
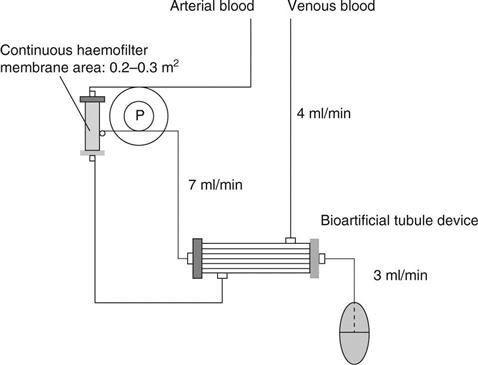
A bioartificial kidney should mimic the structure and function of the nephron. To achieve this, a continuous haemofilter has been used as a replacement for glomerulus, and a tubular cell-attached hollow fibre module was used as a BTD. CHF (10 l/day) was reported by the author to facilitate the maintenance of the patients’ plasma urea, creatinine, uric acid, inorganic phosphate and β2-microglobulin at levels lower than those of the same eight patients on conventional haemodialysis.4–6 At the first step of treatment using bioartificial kidneys, the authors intended to develop the system in conjunction with CHF, using 10 l/day of filtrate (7 ml/min) and a BTD that uses proximal tubular epithelial cells and porous membrane hollow fibre modules to reabsorb 6 l/day (4 ml/min) and to discard 4 l/day (3 ml/min) (Fig. 13.1).5 The ultrafiltrate obtained from the patient is eluted inside the hollow fibre capillaries and the blood that goes through the haemofilter is eluted outside the tubular epithelial cell-attached capillaries in the BTD.6 These two parts are connected with blood tubing and filtrate tubing, whilst roller pumps control the blood and HF amounts/min. Humes7 and the author and coworkers8 have confirmed that this system functions as a nephron, capable of transporting water, sodium and glucose, and operating like renal tubules in vitro. The author’s team6 aimed to develop a wearable type of bioartificial kidney for chronic renal failure patients that could function for prolonged periods whilst also being compact. We had to overcome two hurdles while developing an antithrombogenic haemofilter; firstly, the system needed to be capable of functioning for a week without using systemic anticoagulation and, secondly, it needed to be small and light enough to be transportable.
While developing a bioartificial kidney, the construction of both a bioartificial glomerulus device and a BTD should be considered. A bioartificial glomerulus in which confluent endothelial cells are attached on polymer membranes has never been successfully developed, because a sufficient amount of filtrate could not be obtained with the endothelial cell-attached porous membranes. Therefore, researchers working on bioartificial kidneys initially focused on the development of BTDs. The BTD is considered to play an important role in contributing toward metabolic function and preventing the progress of inflammation in AKI and chronic renal failure. It does this by improving oxidative stress and removing inflammatory cytokines, as well as employing other unexpected roles of proximal tubular epithelial cells. However, the BTD is only a partial replacement for tubule function. Because it only includes proximal tubular epithelial cells, among the many kinds of cells that constitute a kidney, the tubular metabolic function can be replaced but the endocrine function cannot. The essential substances of human metabolism are reabsorbed from a filtrate in a BTD, while some end metabolites are wasted. The proximal tubular epithelial cells endocytically absorb proteins and peptides, including glutathione,9 albumin-linked pentosidine,10 β2-microglobulin, and cytokines.11 These are degraded into amino acids and eventually reabsorbed into blood. Cytokines,12 however, are synthesized in the proximal tubule cells under certain environments. The current BTD cannot perform endocrine functions such as the production of rennin or erythropoietin, because these hormones are not secreted from tubule cells.
Several researchers have investigated CHF and BTDs first in order to improve the prognosis in AKI patients with multiple organ dysfunction syndrome (MODS). It might take much time to develop the BTD for preventing deterioration of dialysis-related complications in chronic haemodialysis patients.
13.4 Early developments in bioartificial kidney design
In 1987, Aebischer13,14 first explored the feasibility of tubular epithelial cell-attachment on the outer surface of semipermeable hollow fibre capillary membranes, in order to function as a bioartificial tubule. Aebischer also evaluated the transport of water, phenol red and several biological substances across the Madin-Darby canine kidney (MDCK) and Lewis-lung cancer porcine kidney (LLC-PK1) cell-attached membranes.15 A number of researchers, including the authors, conducted functional evaluations of several types of renal tubular cells and investigated their interaction with extracellular matrices and polymer membranes,16–18 whilst simultaneously exploring the concept and configuration of bioartificial kidneys. Humes7 developed renal assist devices (RAD) in which proximal tubular epithelial cells formed confluent monolayers on the inner surfaces of polysulfone hollow fibre membranes. In addition, Humes evaluated glucose transport, bicarbonate production, ammonia excretion, 1,25, hydroxyvitamin D production, etc. by the proximal tubular epithelial cells (LLC-PK1 cells and human primary tubular epithelial cells present on the polymer membranes). Humes succeeded in scaling up the human proximal tubular epithelial cell-attached membrane area of the RAD to 0.8 m2, and treated renal failure dogs by using the RAD for 24 h under an anaesthesia.19 Survival of the dogs was shown to be prolonged using the RAD treatment.
In 2004, Humes treated 10 patients with AKI admitted to the ICU. Using CHF and RAD for 24 h led to six patients surviving for 28 days after the RAD treatments.20 Although the reason why good prognosis was observed in these patients treated with tubular epithelial cell-based RAD has never been completely clarified, plasma levels of G-CSF, interleukin (IL)-6, IL-10, and the ratio of IL-6/IL-10 tended to decrease after the treatment.20
13.5 Present developments in bioartificial tubule devices
13.5.1 Maintenance of confluent monolayer tubular epithelial cells on polymer membrane
In general, the BTD requires a production time of approximately 2 weeks; hence, a BTD must be prepared before a patient with severe AKI is brought to the intensive care unit (ICU). A BTD may therefore need to be kept for a new AKI patient for several weeks. During this period, the viability and condition of the proximal tubular epithelial cell layers are presumably changing inside the devices, resulting in the functional deterioration of the device. Detachment of the cells or multilayer formation on the surface of hollow-fibre membranes in the device might occur after the formation of the cell monolayer. Detachment of the tubular cells, however, can be prevented by maintaining an appropriate environment with adequate nutrition, optimal O2 and CO2 supplementation, and an optimal pH level. Specific attention must be paid to overgrowth, presumably 1 or 2 weeks after the confluent monolayer formation, during the waiting time for AKI patients. We have confirmed that immortalized tubular epithelial cell lines and human proximal tubular epithelial cells (HPTEC) proliferate on the tubular cell layer after forming a confluent monolayer, resulting in the formation of a multilayer.21
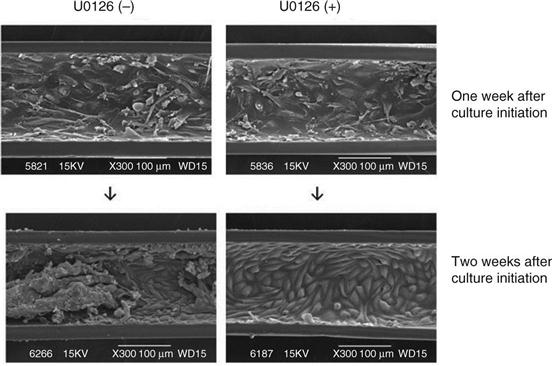
Maintenance of a confluent monolayer of LLC-PK1 cells after the formation of a confluent monolayer in the BTD for a long duration was succeeded by the addition of an mitogen-activated kinase kinase (MEK) 1/2 inhibitor, U0126, to the cell culture medium opposite the site of the cell layer, across porous membrane.22 Figure 13.2 shows that the number of LLC-PK1 cells that initially formed a confluent monolayer varied according to incubation with different constituents of Dulbecco’s Modified Eagle Medium (DMEM). The cell number was maintained at a confluent monolayer level in DMEM with 50 mmol/l U0126, but not in normal DMEM, DMEM with 1% dimethyl sulfoxide, or DMEM with 20 or 30 mmol/l U0126 (Fig. 13.2). Western blot analysis demonstrated that the addition of 50 mmol/l U0126 inhibited extracellular signal-regulated kinase (ERK) 1/2 phosphorylation, but the total ERK1/2 level was not affected.21 A confluent monolayer of HPTECs was maintained by the addition of 50 mmol/l U0126 to DMEM medium outside of EVAL membrane hollow fibres (Fig. 13.3).22 For clinical use, the HPTEC layers on an artificial membrane will be cultured with a U0126-containing medium in the hollow fibre modules to maintain the cell layers at confluent state before patient use, and the medium will be washed and replaced by a U0126-free medium when an AKI patient is brought to the ICU.

13.5.2 Evaluation of the metabolic and transport properties of a proximal tubular epithelial cell layer on porous polymer membrane
The transport and metabolic abilities of a renal tubular epithelial cell layer on a polymer membrane have been evaluated by Humes7 and Saito and coworkers.8,23–30 Messenger RNA (mRNA) expression and production of physiologically active proteins21,25 and metabolites, activation of vitamin D in proximal tubular epithelial cells7 and incorporation of pentosidine, a moiety of advanced glycation end products (AGEs), into the proximal tubular epithelial cells,26 etc. on polymer membranes were examined by in vitro studies.
Existence of Na+/K+ ATPase protein in MDCK23 and JTC-12 cell layers on polymer membrane were first evaluated by Fujita et al., using anti-Na+/ATPase antibody by confocal laser scanning microscopy. Na+/K+ ATPase protein was observed to be located at the lateral and basal sides of the plasma membrane in those cells one or two weeks after the confluent monolayer on polymer membrane.
Expression of sodium-glucose cotransporter-1 (sGLT-1) mRNA at the apical side and facilitated glucose transporter-1 (GLUT-1) mRNA at the basal side of membranes of tubular epithelial cell layers on porous polymer membranes were investigated by Sato,25 in order to clarify whether or not tubular epithelial cell layers maintain transport ability on porous polymer membranes for a long time. Expression of sGLT-1 and GLUT-1 mRNA in LLC-PK1 cell layers on cellulose acetate and polysulfone membranes were examined via reverse transcription-polymerase chain reaction (RT-PCR) 1, 2 and 3 weeks after formation of the confluent monolayers. The sGLT-1 and GLUT-1 mRNA expression were detected on both cellulose acetate and polysulfone membranes, and were maintained for at least 3 weeks.
Long-term culture for increasing the number of the tubular epithelial cells has been generally thought to reduce cell viability. Evaluation of the expression ability of mRNA from channels and transporters of a HPTEC layer on non-porous dishes, porous membranes, and porous hollow-fibre membranes in the BTD modules were investigated. As shown in Fig. 13.4,21 the expressions of γ-glutamyl-transpherase-1, sGLT-1, and aquaporin-1 mRNA were reduced in HPTEC cultured on conventional nonporous culture dishes, but these mRNAs were elevated again when cells were grown on porous membranes and semipermeable hollow-fibre membranes in the BTD modules. Furthermore, the expression of these mRNAs was still maintained at a higher level in cells in the BTD after usage in a 24 h treatment of bilaterally nephrectomized goats. These results demonstrated that mRNA expression of these functional genes decreased when cells were cultured on a nonporous membrane, but the expression increased again when the cells were transferred to culture on a porous membrane. These expression levels were also maintained after a 24 h treatment with a BTD was performed for acute renal failure goats, which were intravenously injected with lipopolysaccharide 48 h after bilateral nephrectomy. Our data suggest that HPTEC layers can maintain cell viability on a porous membrane for a long time under appropriate culture conditions.

Uremia plasma containing considerable amounts of pentosidine was added to the apical portion of the medium in which JTC-12, MDCK and BALB3T3 cells (mouse embryonic fibroblasts) were cultured. Subsequently, pentosidine plasma levels were detected by high performance liquid chromatography (HPLC) using a fluorescence detector at excitation/emission wavelengths of 335/385 nm over time for the culture, and the incorporation of pentosidine into those cells was examined by histochemical staining with antipentosidine rabbit IgG.26 Pentosidine was incorporated only into the proximal tubular epithelial cells, JTC-12, 8 h after the addition of uremic plasma to the apical side of the medium. Pentosidine was not incorporated into any of the cells when it was added in the basal side.
Humes7 demonstrated that 1,25 hydroxy vitamin D production of LLC-Pk1 cell layers was significantly stimulated in the medium containing the parathyroid hormone, in comparison to that in the medium without the parathyroid hormone. Further, 1,25 hydroxy vitamin D production of LLC-Pk1 cell layer was stimulated significantly more in the medium without inorganic phosphate than in the medium with 3.0 mg/dl of inorganic phosphate. Glucose, bicarbonate and ammonia production of LLC-PK1 cell layers on the polymer membrane were also confirmed.
Ozgen et al.8 investigated the long-term effectiveness of active transport in proximal tubular epithelial cell-attached polysulfone hollow fibre modules with an area of 0.4 m2. Prior to seeding LLC-PK1 cells, the inner surface of hollow fibres was coated with Pronectin-F. After 107/ml of LLC-PK1 cells was seeded in the hollow fibres four times while the module was rotated by 90° in 1 h interval, the modules were incubated for 14 days with O2 and CO2 supplementation detecting medium O2, CO2 content and pH (Fig. 13.5).27 Two types of medium were used; one was eluted inside the hollow fibres containing 50 mg/dl of urea and 5.0 mg/dl of creatinine, and the other was eluted outside the hollow fibres with and without 2.5 g/dl of albumin. The leak rates of urea and creatinine and transport rates of water, glucose and sodium for 90 min were calculated inside and outside the medium. Further, the concentrations of urea, creatinine, glucose and sodium in both media were calculated.
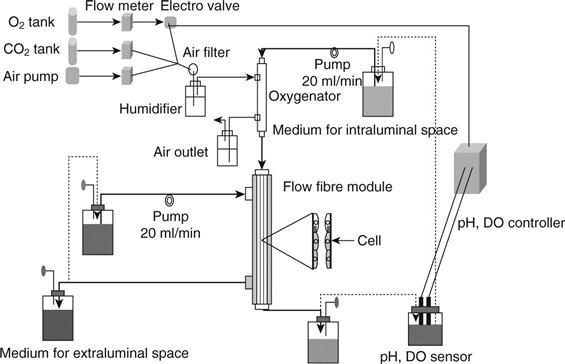
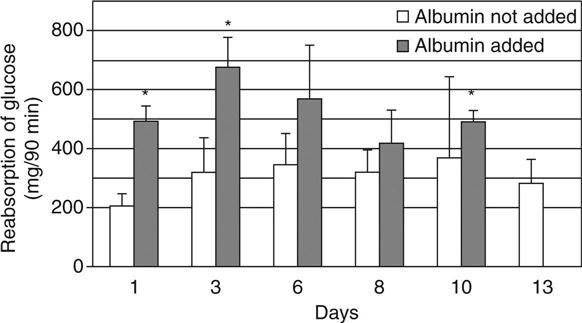
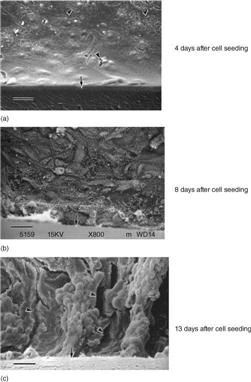
The transport rates of the water without added albumin gradually increased until 8 days after the cell seeding and gradually decreased thereafter. With the addition of albumin, the rate was significantly higher than without albumin 8 days after the cell seeding, and then the transport rate gradually decreased. The sodium and glucose transport patterns were similar to those of water transport under the conditions with and without albumin addition (Fig. 13.6).27 The glucose transport was significantly decreased when phlorizin, the inhibitor of sGLT, was added to the medium inside the hollow fibres. Glucose transport in the cell-attached hollow fibres was considered to be facilitated by the inhibitor of sodium/glucose cotransporter. Scanning electron microscopic findings of the cell-attached hollow fibre at day 4, day 8 and day 13 are shown in Fig. 13.7.27 Although LLC-PK1 cells grew confluently at day 4 and at a high density on a PSf hollow fibre membrane at day 8, the dense cell layer with uneven cell mass was observed at day 13 after the cell-seeding.
13.5.3 Preparation of human proximal tubular epithelial cells for bioartifical tubule devices
The bioartificial renal tubule device is a cell therapy system for acute and chronic renal failure patients. The major obstacle in the development of the bioartificial renal tubule device is the obtainment of a large number of viable renal tubule cells to seed on the inner surface of hollow fibres (membrane area: 0.8 m2). Although our previous studies had used a transformed cell line, these may not be safe for clinical use. Different approaches to amplification of HPTEC in culture without oncogenes, vectors and carcinogens have therefore been required. We developed BTD prepared with lifespan-extended HPTEC, enhancing the ability of normal HPTEC replication by silencing cell cycle-related genes, using ribonucleic acid interferance (RNAi) technology. The limited replicative lifespan of HPTEC, which is ~12 population doublings (PDs), was extended by invalidating the messenger RNA of cell cycle-related genes with antisense oligonucleotide (ASOs) or small interfering RNA (siRNA).
HPTEC were cultured to subconfluence, transfected with a control double-stranded RNA (dsRNA) or siRNA to p53 or p16INK4a at a final concentration of 50 nM, and subcultured on the following day. Transfection and subculture were repeated periodically at ~2 week intervals when cells were subconfluent. Control cells were cultured without transfection. PDs were determined at each subculture and presented as a function of days of culture. The siRNA for p53 and p16INK4A were individually transfected into HPTEC every 2 weeks. Periodic transfection with siRNAs to p53 or p16INK4a reduced the target mRNA levels to 10–20% of that in nontransfected cells.28 The control dsRNA had no effect. Nontransfected and control dsRNA-transfected cells ceased proliferation after ~12 PD. p53 or p16INK4a siRNA increased the replicative lifespan by 33 PD and 63 PD, respectively, in 3 months of culture prior to culture termination. Figure 13.8 shows the mean values of a representative five experiments performed in triplicate. This experiment was terminated after 249 days of culture when siRNA-transfected cells showed no signs of senescence.

13.6 Bioartificial tubule devices in the treatment of acute kidney injuries with endotoxinaemia
A phase II a randomized, open label study was carried out in 58 ICU patients with dialysis-dependent AKI by Tumlin et al. in 2008 in order to determine whether the RAD alters patient mortality. After 6 h of CHF, the patients were randomized (2:1) to receive CHF with or without the RAD for 72 h.31 The primary end point compared 28 day all-cause mortality in patients receiving conventional CHF with those receiving CHF and RAD. Forty patients were randomized to receive RAD therapy. The 28 day mortality rate of patients receiving any duration of RAD therapy was 34.3% compared with 55.6% for patients receiving only CHF. The significantly improved mortality rates in AKI patients treated with the HPTEC-based BTD compared with the patients treated with the HPTEC-less BTD observed in the phase IIa clinical trial were not, however, observed in the patients treated with the BTD with or without HPTECs in the phase IIb clinical trial. The tubular cell therapy, therefore, has never been approved by the Food and Drug Administration (FDA) in the United States. Multilayer formation was suggested to cause the deterioration of the result.
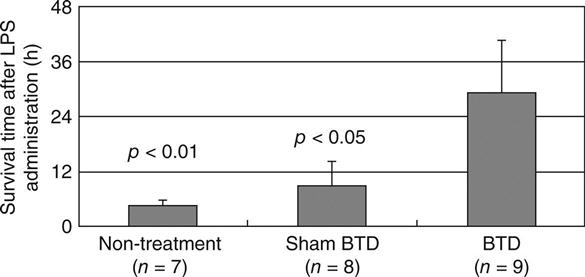
The authors established renal failure goats as an AKI animal model by subjecting goats to bilateral nephrectomy, followed by lipopolysaccharide (LPS) administration, in order to confirm the efficacy of the treatment with CHF and a BTD. After the nephrectomy, uraemia progressed gradually, starting the extracorporeal circulation with haemofilter and BTD later. The administration of LPS (5 × 105 IU/kg; Sigma, St. Louis, MO) induced endotoxin shock, with sepsis and MODS. To evaluate the function of BTD, we established three groups. These groups were as follows: AKI goats without circulation (non-treatment, n = 6). AKI goats treated with BTD with HPTEC (BTD, n = 8), and AKI goats treated without the cells (sham-BTD, n = 9) in the extracorporeal circulation. The goats were randomly designation to each group by the supplier. In the treatment extracorporeal circuit, a continuous haemofilter was set in series with BTD. Arterial blood from a goat was perfused inside the hollow fibres of a continuous haemofilter (blood flow rate: 150 ml/min) and then perfused outside hollow fibres of a BTD. Ultrafiltrate obtained from goat’s blood was perfused inside the hollow fibres of the BTD (filtrate flow rate: 14 ml/min) in which HPTEC was attached on inner surfaces and the waste discarded.
The results show that the survival times of AKI goats treated with LPS were lengthened by the extracorporeal circulation, especially by the BTD. The average survival time after the start of LPS administration was 5.5 ± 1.6 h without circulation. Circulation with sham-BTD tended to lengthen the survival time to 9.8 ± 5.0 h. BTD in the extracorporeal circulation for the AKI goat significantly extended the survival time to 29.1 ± 10.9 h as compared to the non-treatment or sham-BTD (p < 0.05). No AKI goat survived 24 h following LPS administration without the cell BTD, expiring by 8 h for non-treatment and 19 h for sham-BTD. In contrast, half of the AKI goats with BTD survived when the extracorporeal circulation terminated after 24 h from the initiation of LPS administration (Fig. 13.9). The BTD treatment significantly extended the lifetime of AKI goats (p < 0.05).
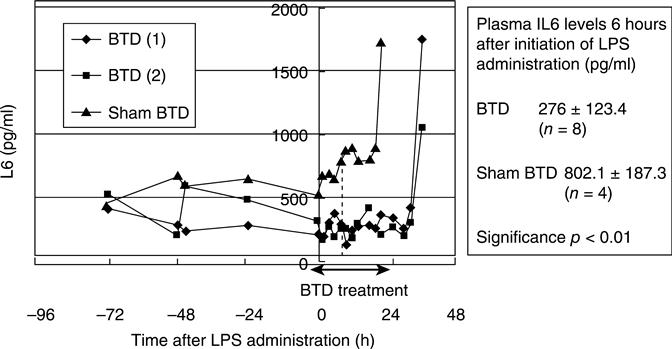
The expression levels of inflammatory cytokines such as IL-6, IL-1β, tumor necrosis factor (TNF) α, interferon (IFN) γ, and anti-inflammatory cytokine, IL-10 in peripheral blood mononuclear cells (PBMC) during the extracorporeal circulation were analysed using RT-PCR detection methods. The cytokines were produced at low levels before LPS treatment, but were then dramatically increased and produced maximally at 2 h after the start of LPS administration. Cytokine expression was maintained at nearly the same level, up to 8 h of LPS administration during sham-BTD treatment. In contrast, cytokine expression in PBMC was reduced at 4 h after the start of LPS administration in BTD-treated goats (Fig. 13.10). It therefore seemed that the cytokines were expressed during LPS injection, but were suppressed immediately by the BTD treatment. The serum IL-6 level was also reduced significantly by BTD. The IL-6 levels in sham-BTD-treated goats were 802.1 ± 187.3 pg/ml, while those in BTD-treated goats were 276.7 ± 123.4 pg/ml at 6 h after the LPS treatment, although number of alive goats in each group was 4 and 8 (Fig. 13.11).
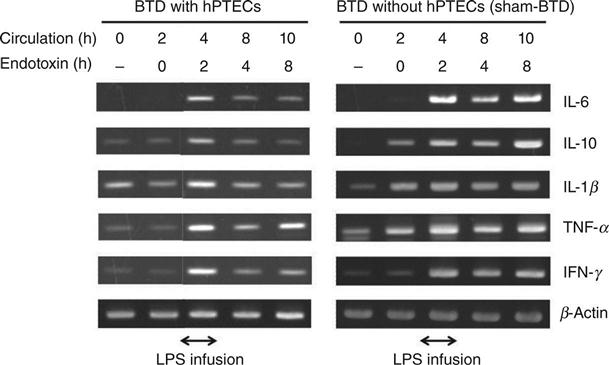
The amount of serum IL-6 in BTD-treated goats was almost half the level of that measured in sham-BTD goats. Similar results in the expression of mRNA and serum levels of cytokines were obtained in further study, in which lifespan extended HPTECs were used in the BTDs for the treatment of bilateral nephrectomized goats with MODS.32 These results suggest that the BTD treatment not only reduces the cytokine expression levels, but also the secretion level. The reduction of these cytokines may have alleviated severe inflammation and improved survivability from AKI. Although the mechanism for the suppression of inflammatory cytokine production is still not clear, if the mechanism is elucidated, a new treatment will be possible for AKI and other inflammatory diseases. Further experiments are required to examine the observed mechanism of inflammatory cytokine production suppression (Fig. 13.12).
13.7 Development of bioartificial renal tubule devices for long-term treatment
The methacryloyloxyethyl phospholylcholine (MPC) polymer, which mimics the phospholipid of cell membranes, was invented by Ishihara et al.33 The application of an MPC polymer coating on the surface of the hollow fibre membrane in a hemodialyzer was shown to produce antithrombogenic properties.34 However, it will be essential for the porous membrane in the BTD to offer both antithrombogenic properties on the blood-side surface and cytocompatible properties on the cell-side surface of the membrane if the BTD function is to be maintained for a long time. MPC blended with PSf has been developed as a cytocompatible and antithrombogenic membrane for the long-term functioning of the BTD.35 This polymer blend contains 1 weight% of the MPC polymer in PSf. The MPC unit composition is seven times larger at the sponge layer surface than at the skin layer surface and the amount of protein adsorbed on the surface has been shown to correspond to the MPC unit composition.
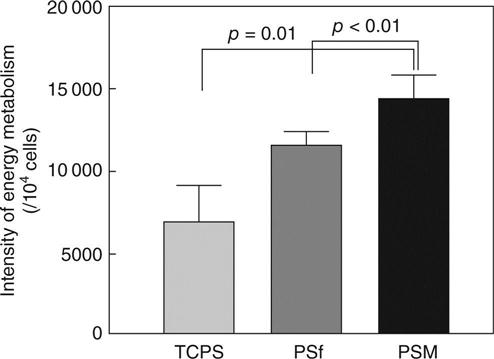
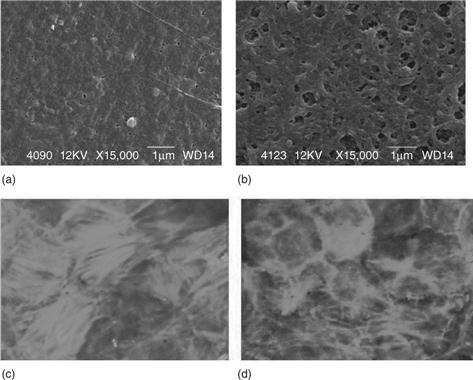
A significant reduction in the amount of platelets that adhere to the sponge layer surface of the MPC blend membrane (PSM) in comparison with the PSf membrane has also been confirmed, as shown in scanning electron micrographs of the adhered platelets on the sponge and skin layer surfaces (Fig. 13.13). The intensity of the energy metabolic activities of the mitochondrion of LLC-PK1 cells cultured on the skin layer surface of the PSM membrane, PSf membrane and tissue culture polystyrene (TCPS) plate as the control was compared. This revealed higher energy metabolic activities in the mitochondrion of the LLC-PK1 cell cultures on the PSM membrane than on the PSf membrane and TCPS plate, by 24% and 108% respectively (Fig. 13.14). These results suggest that the novel phospholipid polymer blend membrane with an asymmetrical functional surface, a haemocompatible sponge layer and a cytocompatible skin layer, is useful as a membrane in BTDs for long-term use.

13.8 Development of a bioartificial glomerulus
A bioartificial glomerulus, consisting of a porous polymer membrane and endothelial cell layer, should maintain key functional characteristics, including antithrombogenecity and enhanced permeability, for a long duration. The authors aimed to develop a bioartificial haemofilter as an artificial glomerulus using CD133 + cells, a progenitor of endothelial cells obtained from human cord blood, which formed a confluent monolayer on the surface of the haemofilter membrane. Pretreatment of CD133 + cells for 2 h with 50 mg/mL cytochalasin B (Cy B), an actin-microfilament polymerization inhibitor,36,37 resulted in enhancement of filtration across the cell-attached membrane (Fig. 13.15). Filtration is achieved by enlarging the diameter of the fenestrae of the attached cells (Fig. 13.16). This system enables a haemofilter to maintain its antithrombogenic property and considerable filtration volume for at least 1 week. In addition, the immature immune system of the cord blood cells ensures that the non-autologous cells will not be rejected.
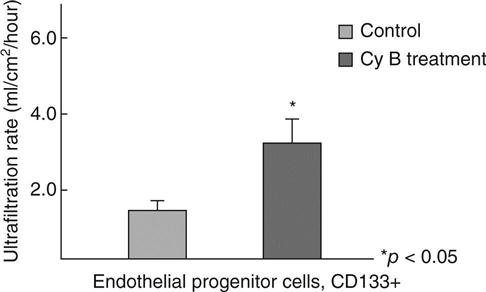
By the administration of an optimal dose of Cy B to the medium, the diameter of the fenestrae of CD133 + cells was appropriately enlarged. However, an excessive dose of Cy B not only changed the fenestrae diameter but additionally led to dislocation of functional channels and transporters, whilst also inducing disruption of the cytoskeleton of the cells, resulting in cell death and detachment of the cells from the polymer membrane surface. Cy B caused no significant effect up to 50 mg/ml on the viability of the cells. However, at the concentration of 70 mg/ml, the viability was significantly lower in CD133 + cells.37
13.9 Future trends
Treatment with a CHF and a BTD will produce significantly higher survival rates and lower plasma inflammatory cytokine levels in severe AKI patients with MODS in the controlled clinical trials. Such results will ensure that it is approved by the government as a treatment modality for AKI patients in any country in a few years. The treatment will then become a popular option among treatments for AKI patients worldwide within 10 years. However, a BTD used to prevent the progress of dialysis-related complications in chronic renal failure patients has to function for more than 7 days, despite a BTD for treatment of AKI patients being only required to work for 24 to 72 hours. A haemofilter and a BTD for long-term treatment are required to be more antithrombogenic and compact for easy wear. The antithrombogenic property of the membrane surface of a haemofilter should be further improved to ensure a clinically applicable level is reached. Novel factors by which membrane surfaces can maintain antithrombogenic properties for a longer duration than conventionally achievable have to be invented, and the function of the BTD should also be improved not just in terms of the cytocompatible and haemocompatible characteristics of both membrane surfaces, but also the metabolic and endocrinological activity. In addition, the BTD needs to include renal interstitial cells, such as erythropoietin-producing cells, as well as renal tubular epithelial cells.
As an alternative, a bioartificial glomerulus consisting of CD133 + cells and a semipermeable membrane linked to the bioartificial tubule device, in which HPTECs are attached on the MPC blend membrane, is a possible candidate as a wearable bioartificial kidney (Fig. 13.17). Miniaturized pumps for blood and filtrate flows, along with a powerful battery supplying electrical energy for the pumps and an alarm system, have recently been used clinically 38 and a wearable system capable of working for 7 to 14 days can potentially be realized within 10 years.