2.9. FERROELECTRICITY 47
Orientational polarization is the key phenomenon contributing to ferroelectric properties,
including tunability. Recall that in many molecules, their constituent ions have different affini-
ties of electrons, and this asymmetry in charge gives rise to permanent dipoles. Well-known
examples are water and some oils used in transformers, where one end of the molecules is ef-
fectively positive and the other end is effectively negative [41]. ermal agitation tends to ran-
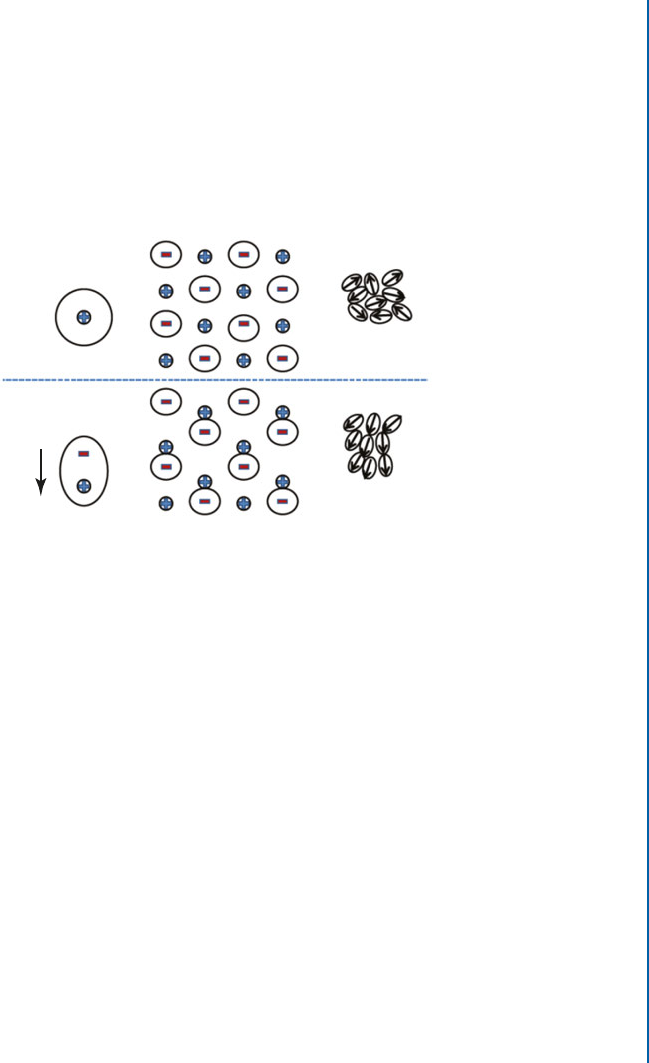
domize these dipole orientations. e application of an electric field tends to align them along
the field’s direction as shown in Figure 2.16c [41]. However, orientational polarization in ferro-
electrics is due to asymmetrically located ions in the crystal structure.
E = 0
E
(a) (b) (c)
Figure 2.16: Schematic representation of the physical mechanisms producing electric dipoles
for: (a) electronic, (b) ionic or atomic, and (c) orientational polarizabilities.
e relative contribution of the three polarization mechanisms to the dielectric constant is
presented in Figure 2.17. It can be observed that orientational polarizability is effective below the
far infrared. Ionic polarizability contributes up to the infrared region, and electronic polarizabil-
ity is observed in almost the whole frequency spectrum—specifically up to the ultraviolet region.
is dependence of the dielectric constant is also known as the material frequency dispersion.
e loss tangent is also presented at some indicative frequencies in Figure 2.17. It is obvious
that each polarization mechanism is accompanied by corresponding losses. ese losses sum up
at the lower part of the spectrum, where all three mechanisms are effective. Alternatively, it can
be said that a higher dielectric constant is accompanied by higher losses.
2.9 FERROELECTRICITY
Electronic and ionic polarizations revert to an unpolarized state when the electric field is re-
moved. Materials supporting only electronic and ionic polarizations are called “nonpolar mate-
rials.” is is because they do not exhibit individual electric dipoles in the absence of an applied
electric field. In contrast, orientational polarization can be divided into two classes. One class
..................Content has been hidden....................
You can't read the all page of ebook, please click here login for view all page.
