Cell Microencapsulation for Tissue Engineering and Regenerative Medicine
Jian Du and Kevin J. Yarema, Department of Biomedical Engineering and the Translational Tissue Engineering Center, The Johns Hopkins University, Baltimore, MD, USA
Cell encapsulation was originally envisioned decades ago as a way to protect cells from the host’s immune system to facilitate transplantation therapies and now serves additional roles such as localization of cells to a desired site in the body. In recent years, the pace of progress has increased as novel techniques for fabricating microcapsules have dovetailed with a growing number of biocompatible natural and synthetic polymers suitable for supporting cell growth, proliferation, and secretion of growth factors. This chapter provides an overview of general design parameters important for encapsulation, which include the size of the capsule and permeability to allow or exclude the passage of nutrients, small molecules, and larger proteins, and then discusses various biocompatible materials now used for cell encapsulation. Finally, an overview of emerging therapies—several of which are already in clinical trials—is given including those designed for the treatment of diabetes, cancer, neurological disorders, and liver disease.
Keywords
Cell microencapsulation; microencapsulation techniques; biomaterials; hydrogels; cell-based transplantation therapies
10.1 Introduction
Cell-based therapies have been attempted sporadically for well over a hundred years and became firmly established in the medical mainstream in 1968 when the first successful bone marrow transplantations were performed in humans [1]. During the same era, early efforts to treat diabetes with transplanted cells also flourished while in recent years, advances in stem cell biology have led to a rapidly increasing profusion of cellular therapies [2,3]. Throughout the past five decades, a primary obstacle to the clinical implementation of these otherwise exciting therapies has been avoidance of immune rejection of the implanted cells while secondary challenges, such as retention of the implanted cells at the desired location in the body, also persist. As will be outlined in this chapter, cell encapsulation technology offers a solution to these problems, as well as providing additional advantages such as ease of handling, a highly hydrated tissue-like environment for cell and tissue growth, and the ability to form complex structures in vivo. Emerging opportunities to include additional materials (growth factors, cells, drugs, even adsorbents, and magnetic materials) or other design features (e.g., topographical cues [4]) that control cell fate [5,6] are now complementing cell-based therapies and helping to propel them into the forefront of emerging therapeutic advances.
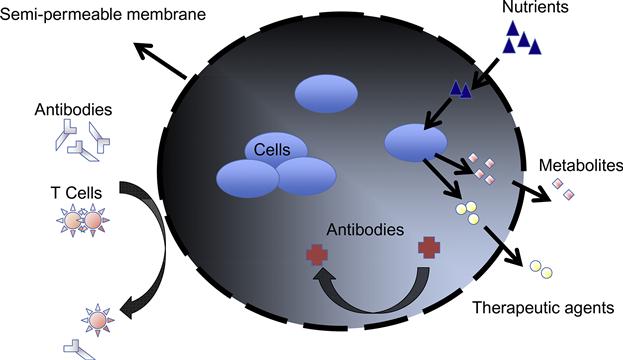
Cell encapsulation, a strategy that aims to physically isolate an implanted cell mass from the outside environment within the confines of a semipermeable membrane barrier [7–9], was originally inspired by the need to overcome a major obstacle associated with the transplantation of nonautologous cells or tissue, which is graft rejection [10,11]. To avoid graft rejection, transplant recipients currently are obliged to take immunosuppressive drugs for extended periods of time, which can result in severe side effects that include increased susceptibility to infections [12], negative effects on bone and cardiovascular function [13,14], and even cancer due to compromised immunosurveillance [14]. The immunosuppressive drugs also interact with other medicines and affect their metabolism and action causing wide-ranging medical difficulties [15]. The increasing number of options for achieving cell immunoisolation in microcapsules (as shown in Figure 10.1) negates the requirement for long-term administration of immunosuppressive agents and is opening the door for a growing number of cell-based therapies.
Before describing state of the art cell encapsulation applications, a brief history of this field begins with an early description of basic requirements for the technology by Chang in the early 1950s [16]. A primary requirement of microcapsule-surrounding membranes was to ensure that they were amenable to the diffusion of nutrients (e.g., glucose) and other molecules such as oxygen and growth factors essential for cell survival [17]. Twenty years later, Lim and Sun [18] presented the first implantable alginate–poly(L-lysine) microcapsules harboring rat islet cells, that naturally secreted insulin, for the treatment of diabetes. In the following three decades, the permselective capsule environment has been optimized for supporting cellular metabolism, proliferation, differentiation, and cellular morphogenesis.
Today’s ability to combine cells and polymer scaffolds by using microencapsulation methods creates “living cell medicines” that provide long-term drug delivery has opened new doors in the use of allografts. For example, encapsulated cells can deliver therapeutic proteins continuously for prolonged time periods of days, weeks, or in some cases even months. These features made this technology particularly attractive for islet transplantation for treatment of diabetes [19–22]. Moreover, cell encapsulation allows the use of cells from a variety of sources such as primary or stem cells, or genetically engineered cells which can be modified to express any desired protein in vivo without the modification of the host’s genome [9].
Recent advances in biotechnology, molecular biology, nanotechnology, and polymer chemistry are now opening up further exciting possibilities in this field, making increasingly sophisticated applications of cell encapsulation technologies feasible such as forming functional new tissues. For example, the Elisseeff group has developed cell compatible, chemically functionalized hydrogels that can function as “glues” to encapsulate cells for cartilage reconstruction [23–25]. The robustness of this technique is evidenced by its ability to paste living cells onto a beating heart, as has been done in nascent efforts to achieve cell-based cardiovascular regeneration [26]. These encapsulation strategies not only protect the implanted cells from the host immune system, but also ensure cell localization and retention at a defined site in the body to an extent not yet possible even by using emerging glycoengineering techniques that guide cell migration [27,28].
This chapter describes the historical development and principles behind polymeric artificial cells, and discusses the potential of cell capsulation systems for efficiently utilizing the functions of transplanted cells or tissues. In our view, an understanding of the current status and limitations of cell implantation technology will enable the development of new strategies and increasingly successful cell-based transplantation therapies. Accordingly, this chapter provides an overview of encapsulation strategies and followed by a description of various biocompatible polymers used in this field. Finally, a survey of long-standing disease targets—primarily diabetes—is given followed by a synopsis of emerging efforts to use encapsulated cells to treat an expanded repertoire of conditions that include cancer, CNS disorders, and liver disease.
10.2 Encapsulation Requirements and Strategies
10.2.1 Purpose and Requirements of Cell Encapsulation
To achieve the promise inherent in cell-based therapies, a capsule must satisfy a stringent set of dichotomous requirements. The capsule membrane, to serve as an immunoisolation device, must be able to keep various components of the immune system away from the living cells while at the same time allowing nutrients such as glucose and oxygen, as well as proteins such as growth factors, to pass through without much impedance. The membrane must also be biocompatible to the host and to the cells it encloses and when capsulation occurs, it must be cell friendly. At the same time, however, the membrane must be sufficiently robust to survive handling, transplantation, and the hostile environment inside the human body. Furthermore, in many cases, a biodegradable membrane is useful, but care must be taken to ensure that its degradation products do not adversely affect the encapsulated cells [29]. In addition, the diameter and surface charge of the capsule also needs to be taken into consideration because they also influence immune response and nutrient diffusion [30].
10.2.2 Categories of Cell Capsules
Numerous strategies have been pursued over the years to physically isolate cells from the immune system to avoid immunorejection. Immunoisolational capsules can be categorized very broadly as macrocapsules, which are usually millimeter-sized flat-sheet and hollow-core fibers or spheres or as microcapsules comprised of small spherical vehicles and conformally coated tissues. Regarding microcapsules [31], their smaller size (ranging from 100 to 500 μm) is particularly advantageous from a mass transport perspective, offering optimal surface-to-volume ratio for macromolecule (e.g., protein) and nutrient (e.g., glucose or oxygen) diffusion thereby enhancing cell viability. The small size of the capsules also allows their implantation in close contact to the bloodstream where oxygen transfer into their interiors is optimized further, which is critical for applications that require the long-term functionality of the enclosed cells [32]. Moreover, microcapsules are typically more durable than macrocapsules and are difficult to disrupt mechanically [17].
10.2.3 Classification of Cell Microencapsulation
10.2.3.1 Matrix-Core/Shell Microcapsules
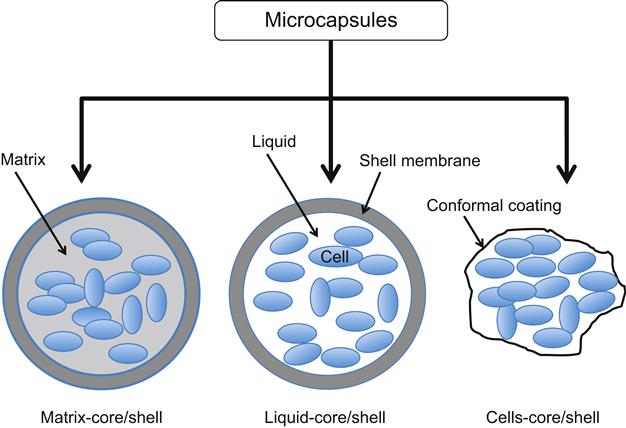
Microcapsules can be classified into three categories (Figure 10.2): matrix-core/shell microcapsules, liquid-core/shell microcapsules, and cells-core/shell microcapsules (or conformal coating) [29]. Matrix-core/shell microcapsules are produced by placing polymer/cell droplets in a gelling solution. Basically, there are two methods to prepare the matrix-core/shell microcapsules. One method produces covalently crosslinked capsules, which attain an equilibrium swelling state that depends on the polymer–water interaction parameter and the crosslink density [33]. This type of capsule has significant advantages in terms of stability; however, the toxicity of the reactants and the time required to complete chemical crosslinking can be incompatible with cell survival. The other method involves the production of “reversible” or “physical” gels where the constituent polymer networks are held together by various degrees of molecular entanglements and secondary forces including ionic charges, hydrogen bonding, or hydrophobic interactions. All of these interactions are reversible and can be disrupted by changes in physical conditions or application of stress [33]. This class of microcapsules has been well studied due to its several advantages that include the maintenance of the cells in an aqueous environment where they are in contact with a soft and biocompatible material and for the long-term release of therapeutic molecules. Despite ability of the hydrogel matrix to support facile cell growth, the in vivo stability of electrostatic and hydrogen bonds maintaining the structure of this type of microcapsule is limited and the mechanical resistance of capsules also remains a concern [34].
10.2.3.2 Liquid-Core/Shell Microcapsules
Many technical improvements in microcapsule design and production have been introduced over the years. For example, the hydrogel network found within a capsule can be tuned to promote cell growth and protein production by including a liquid or semiliquid environment that supports higher diffusion of gases and nutrients than possible in a gel [35,36]. As a result, cells show increased survival and protein production. Liquid-core/shell microcapsules were first manufactured by dropping a cell/gelling solution into a polymer bath and have been pursued with different types of hollow polymeric microcapsules such as polymersomes, multilayered capsules, and hollow microspheres. Methods to fabricate this type of microcapsule have grown increasingly sophisticated beyond the self-assembly of microgels in droplets [37] to now include layer-by-layer polyelectrolyte deposition [38], interfacial polymerization [39], precipitation by phase separation [40], surface polymerization [41], copolymer vesicles [42], multiphase microfluidics [43], and a combination of molecular self-assembly and precipitation [44].
10.2.3.3 Cells-Core/Shell Microcapsules
Both of the techniques mentioned above (matrix-core and liquid-core shells) typically start with a scheme to generate a controlled-size droplet, followed by an interfacial process to stabilize the droplet and to obtain a solid microcapsule membrane around the droplet. Aside from these long-established approaches, new techniques are emerging that overcome shortcomings of the existing methods. For example, core/shell microcapsules that can be generated by conformal coating of cells been proposed [45,46]. Conformal coating constitutes a special case of microencapsulation where the term describes a method of forming a barrier directly around a small cell mass or a small piece of tissue to eliminate unutilized space in a microcapsule core. By closely surrounding the cell mass with the encapsulation membrane, this approach provides improved mass transport between the capsule exterior and the cell mass and also minimizes implant size by increasing the effectiveness of cell packing. The thickness of the poly(ethylene glycol) (PEG) layer formed on the cell surface in this strategy can be as thin as several nanometers thereby drastically reducing total graft volume compared with conventional microcapsules.
10.3 Materials Used for Cell Encapsulation
10.3.1 Hydrogels
One of the key issues in the design of a microencapsulation strategy is the choice of a suitable material for delivering cells to the host or immobilizing them for long-term delivery of molecules that they produce to the surrounding tissue. In the last few decades, the field of cell microencapsulation has greatly benefited from advances and optimization of biomaterials used to elaborate the capsule [47]. Microcapsules are almost exclusively produced from hydrogel-forming polymers because these materials hold a number of appealing features. For example, hydrogels are comprised of 3D networks of hydrophilic polymers, which provide a highly hydrated microenvironment for embedded cells that can also be designed to present biochemical, cellular, and physical stimuli that guide cellular processes including cell attachment, growth, and differentiation [48]. The unique properties of hydrogels that make them a material of choice for biomedical applications include minimal interfacial tension with surrounding biological fluids, permeation, and diffusion of low molecular weight compounds. Hydrogels also match the flexibility of human tissue, due to their water content, which reduces mechanical and frictional irritation to tissues and cells [49].
An important advantage of hydrogel-based microcapsules is a variety of administration options; in particular, they can be administered as implantable forms (preformed scaffolds) or injectable forms (in situ formed scaffolds). Unlike tissue engineering approaches that utilize prefabricated scaffolds, injectable systems have garnered great interest as a unique therapeutic method for administration to difficult-to-reach areas of the body using minimally invasive procedures. Another advantage is the ability of implanted microcapsules to conform to any shape irrespective of the defect geometry [50]. Overall, incorporating microencapsulated cells within hydrogel-based scaffolds improves administration options and local retention at the desired site within the body while reducing posttransplantation inflammation [51].
10.3.2 Natural Polymers
A variety of natural and synthetic polymers listed in Table 10.1 have been used for cell microencapsulation. Natural polymers that form semipermeable membranes suitable for cell encapsulation include polysaccharides (e.g., alginate, chitosan, hyaluronic acid (HA), and agarose) and extracellular matrix (ECM) proteins (e.g., collagen, gelatin, and fibrin); a sampling of these materials will be discussed in more detail in the following sections.
Table 10.1
Biomaterials for Cell Encapsulation
| Material | Encapsulated Cells | Application | References |
| NATURE POLYMERS | |||
| Alginate | Mesenchymal stem cells | Bone tissue engineering | [52] |
| Pancreatic islet | Diabetes | [18,53] | |
| Fibroblast | Cardiac disease | [54] | |
| Embryonic stem cells | Diabetes | [55] | |
| Neonatal porcine choroid plexus cells | Neurodegenerative diseases | [56] | |
| Alginate–agarose | BHK fibroblast and C2C12 myoblast | [57] | |
| Feline kidney cells | Subsieve-size capsules | [58] | |
| Alginate–chitosan | Mesenchymal stem cells | Cartilage regeneration | [59] |
| Chondrocytes | Bone tissue engineering | [60] | |
| Alginate–collagen | Mesenchymal stem cells | Bone tissue engineering | [61] |
| Alginate–gelatin | Hepatocytes | Liver tissue engineering | [62] |
| Alginate–poly(L-lysine) | HepG2/C3A cells | Liver tissue engineering | [63] |
| Chromaffin cells | Neuropathic pain | [64] | |
| CHO cells | Cardiovascular disease | [65] | |
| Porcine aortic endothelial cells | Cancer | [66] | |
| Genetically modified hamster kidney cells | Cancer | [67] | |
| Mouse genetically engineered myoblasts | Cancer | [68] | |
| Alginate–poly(L-ornithine) | Pancreatic islet | Diabetes | [69] |
| Choroid plexus | Huntington’s disease | [70] | |
| Chitosan | PC12 cells and R208F cells | [71] | |
| Chondrocytes | Orthopedics | [72] | |
| C2C12 myoblasts | Myocardial infarction | [73] | |
| Mesenchymal stem cells | Cartilage regeneration | [74] | |
| Collagen | Mesenchymal stem cells | Cartilage regeneration | [75] |
| Mesenchymal stem cells | Bone tissue engineering | [76] | |
| Hyaluronic acid (HA) | 3T3 fibroblasts | [77] | |
| Chondrocytes | Cartilage regeneration | [78] | |
| Adipose-derived stem cells | Adipogenic differentiation | [79] | |
| Chick dorsal root ganglia | Neuronal injury | [80] | |
| Embryonic stem cells or fibroblasts | [81] | ||
| SYNTHETIC POLYMERS | |||
| Polyethersulfone | C2C12 myoblasts | Focal epilepsies | [82] |
| Polyethylene glycol (PEG) | Chondrocytes | Cartilage regeneration | [83,84] |
| Osteoblasts | Bone tissue engineering | [85] | |
| Mesenchymal stem cells | Bone tissue engineering | [86] | |
| Aortic valvular interstitial cells | Cartilage regeneration | [87] | |
| Neural cells | Neuronal regeneration | [88] | |
| Poly(2-hydroxyethyl methacrylate) | 3T3 fibroblasts CHO-K1 | CNS diseases | [89] |
| Poly(lactic-co-glycolic acid) | P19 embryonic carcinoma cells | Nerve tissue engineering | [90] |
| Poly(vinyl alcohol) | Islets | Diabetes | [91]. |
10.3.2.1 Alginate
Alginates are a frequently employed biomaterial for cell immobilization due to their abundance, easy gelling properties, and apparent biocompatibility; the significant breadth of literature available (reviewed in detail elsewhere [17,92]) drives home how important this compound has now become for cell and tissue encapsulation. Despite continuing relevance to the present day, alginate was first discovered in the late nineteenth century by Edward Stanford [93] and commercial production began in the 1920s through the harvest and processing of cell wall components of various species of marine brown algae [94]. This material was used in the pioneering study by Chang in 1964 [16] that laid a foundation for encapsulation as a strategy for immunoprotection of transplanted cells and the subsequent groundbreaking work of Lim and Sun in 1980 [18]. In the three decades since then, alginate has become a ubiquitous matrix material for cell, tissue, and macromolecule encapsulation with applications in many aspects of tissue engineering and regenerative medicine.
Alginates are linear block polymers consisting of α-L-guluronic acid (G block) and β-D-mannuronic acid (M block). The gelation of alginate from a solution containing divalent cations results in the formation of the calcium junctions of GG-GG, MG-GG, and MG-MG between alginate molecules. Calcium and barium are the most commonly used as the complexation ions to produce the gelled structure because of their selectivity and cooperative binding to the G blocks. The G and M content varies with the alginate source and can affect gel properties such as mechanical strength, biocompatibility, and permeability [95]. The main limitation of using alginate as an encapsulation matrix is poor mechanical stability in the presence of monovalent and certain divalent cations commonly found in cell culture media or a physiological milieu, which can have an adverse effect on hydrogel structure by destabilizing the polyelectrolyte interactions involved in gel integrity [36]. To increase the stability and to reduce the wall permeability (for immunoisolation) of the microcapsules, alginate microcapsules are often coated first with a polycation and then with another layer of alginate. Various polycations have been used for this purpose including poly-L-lysine (PLL), poly-L-ornithine (PLO), chitosan, lactose modified chitosan, and photopolymerized materials [96].
Cell adhesion cues have been incorporated into the alginate network either through the addition of Arg–Gly–Asp (RGD), a cell adhesion oligopeptide [97,98], or by copolymerizing alginate with gelatin as culture time increased and complete degradation of the gel occurred in 5 weeks [62]. In recent work, Liu et al. [99] used crosslinked gelatin and alginate derivatives to prepare cell sheets and spherical tissues wrapped in living cell sheaths. The microcapsules not only supported growth of the enclosed cells into spherical tissues but also provided a cell adhesive outer surface. Light-activated gelation of alginate was recently employed to encapsulate rat bone marrow cells through the release of calcium chloride from light-sensitive liposomes suspended in the alginate–cell solution [100].
10.3.2.2 Chitosan
Chitosan, a derivative of the extremely abundant chitin polysaccharide, has structural characteristics similar to mammalian glycosaminoglycans (GAGs) and has attracted much attention as an alternative polycation in preparing microcapsules. Chitosan is a biocompatible, biodegradable, and nontoxic cationic linear polysaccharide composed of β-(1-4)-linked D-glucosamine and N-acetyl-D-glucosamine units and is obtained by the extensive deacetylation of chitin by hydrolyzing the amino-acetyl groups using NaOH [101]. Chitosan can form hydrogels by ionic interactions [102] or through chemical cross-linking [103] with the bifunctional agent glutaraldehyde and it degrades via enzymatic hydrolysis [104].
Due to its weak mechanical properties and lack of bioactivity, chitosan is often combined with other materials, such as poly(lactic-co-glycolic acid) (PLGA) [105] to achieve more desirable mechanical properties for encapsulation. Chitosan has also been used to coat capsules to enhance stability and modify permeability as an alternative to PLL because it is regarded as being more biocompatible [106]. Under dilute acid conditions (pH<6), chitosan is positively charged and water soluble, while at physiological pH, chitosan is neutral and hydrophobic, leading to the formation of a solidified, physically crosslinked hydrogel. The addition of polyol salts enables encapsulation of cells at neutral pH, where gelation becomes temperature dependent [102]. The addition of the adhesion-promoting RGD tripeptide has been necessary to promote cell attachment to chitosan, allowing myoblast and cardiomyocytes to be successfully encapsulated in crosslinked gel made from this polysaccharide [73]. In a separate study, polylysine was grafted to chitosan to enhance the microenvironment for neural cell growth. When fetal mouse cortical cells were encapsulated in chitosan-g-polylysine gels, neurite extensions were observed in the 3D gel cultures [107]. Examples that illustrate the versatility of chitosan include the use of this material for microencapsulation of human red blood cells [108], HepG2 cells [109], BHK-21 cells [110], chondrocytes [111], bone marrow stem cells [112], and PC12 and R208F cells [71].
10.3.2.3 Hyaluronic Acid
HA, or hyaluronan, is a natural GAG that is nonimmunogenic in its high molecular weight form and also nonadhesive [113]. HA is a linear polysaccharide composed of repeating disaccharide units of N-acetyl-D-glucosamine and D-glucuronic acid and is found in many tissues, such as skin, cartilage, and the vitreous humor. HA is a component of the ECM in mammalian connective tissues and participates in many important biological processes during wound repair [81]. Colloids of HA can be gelled by prior chemical modification of hyaluronan with thiols [114], methacrylates [115], or tyramines [116], or can be crosslinked in situ using formaldehyde or divinyl sulphone. One of the key advantages of using HA gels for tissue engineering is that their degradation can be mediated by hyaluronidase, an enzyme secreted by a multitude of mammalian cell types [117]. These properties make HA an interesting biomaterial for cell-based scaffolds and several cell types (chondrocytes, fibroblasts, and murine embryonic stem cells (ESCs)) encapsulated into either HA/alginate beads [118] or HA-microfabricated devices have been observed to proliferate and behave correctly [81].
10.3.2.4 Collagen
Collagen gels, as well as other mammalian-derived protein-based polymers covered in the next section, are effective matrices for cellular growth because these materials contain many cell-signaling domains present in the in vivo ECM [119]. Collagen type I is one of the most studied materials for tissue engineering in part because of its low immunogenicity [120]. An advantage of collagen gels is that they can be created through natural means without chemical modifications; the resulting collagen matrices, however, do have drawbacks including mechanical weakness and low resistance to the enzymatic degradation. Various strategies, therefore, have been pursued to improve the stability and mechanical properties of collagen, including chemical (glutaraldehyde and water-soluble carbodiimide) [121], physical (UV irradiation and dehydrothermal) [122], and enzymatic [123] crosslinking approaches. As an example of the promise held by collagen microcapsule technology, self-assembled collagen microspheres provide superior hyaline cartilage reproduction for the microencapsulation of human mesenchymal stem cells (hMSCs) based on the stability of these devices, their ability to be injected in vivo, and their provision of a protective, growth- and migration-supporting environment for hMSC both in vitro and in vivo [75].
10.3.2.5 Protein-Based Gels
Proteins are polymers of amino acids that form structures attractive for cell encapsulation including β-sheet-rich fibrils that can be nanometers in width and micrometers in length that also further self-organize and entangle to form 3D hydrogels under appropriate conditions [124,125]. As an alternative to natural proteins, synthetic peptides hold great promise in designing hydrogel carriers with a wealth of bioactive signals programmed directly into the hydrogel matrix. For example, one could envision incorporation of cell adhesion moieties, biochemical cues to promote tissue deposition, and specific enzyme-sensitive sequences for cell-mediated degradation [126]. Several groups have explored synthetic peptides that are relatively short ~15- to 20-mer amino acid sequences designed to self-assemble into a hydrogel for cell encapsulations. One example was an amphiphilic peptide containing positively charged lysine residues that was demonstrated to successfully encapsulate mesenchymal stem cells (MSCs) [127]. Another example is provided by elastin-like peptides (ELPs) used to encapsulate human adipose-derived adult stem cells [128] and as a preosteoblastic cell carrier for bone regeneration [129].
10.3.3 Synthetic Polymers
10.3.3.1 General Considerations
In general, natural polymers, including those discussed above, have attractive features that provide them with therapeutic relevance. For example, natural polymers, as well as their degradation products, are usually not toxic or carcinogenic, nor do they elicit adverse reproductive and developmental effects and in most cases entrapment of living cells in matrices made from natural polymers can be accomplished under physiological conditions. In addition to natural biomaterials, however, many synthetic polymers as well as inorganic compounds have also been explored for cell encapsulation [130]. The mechanical and chemical properties of synthetic polymers can be tailored for different applications without having the immunogenicity-related concerns of some naturally occurring polymers (e.g., low molecular weight HA), which constitutes an important advantage of these materials over their natural counterparts [130].
Although synthetic materials provide researchers with large flexibility in material design, one drawback is that they do not have an intrinsic mechanism for interacting with cells and cell attachment typically relies on nonspecific adhesion, which tends to be weak [131]. This limitation, which restricts the use of synthetic materials in applications that require defined control over cell–matrix interactions, can be overcome in some cases by functionalizing these materials with bioactive molecules such as the RGD tripeptide adhesion motif. A number of synthetic hydrogels, e.g., poly(ethylene glycol), poly(acrylic acid), poly(vinyl alcohol), poly(N-isopropyl acrylamide), and their derivatives have been developed and successfully employed for use in tissue engineering and encapsulation of cells [49,132,133]. PEG will be discussed in more detail below as a well-studied representative of synthetic polymers used for cell encapsulation.
10.3.3.2 Poly(Ethylene Glycol)
PEG has been the most widely used synthetic material to create macromeric polymers for cell encapsulation. PEG hydrogels can be formed via the photoinitiated chain polymerization of functionalized PEG macromers, specifically dimethacrylated PEG. Photoencapsulation within these gels has been shown to be cytocompatible with multiple cell types, including fibroblasts, chondrocytes [83], osteoblasts [85], mesenchymal stem cells [86], aortic valvular interstitial cells [87], and neural cells [88]. Even though PEG gels are inherently cell repellent [117], certain cell types such as chondrocytes and osteoblasts encapsulated in PEG hydrogels survive without the addition of any adhesion or biological-signaling molecules [134,135]. However, many cell types are attachment dependent and require cell adhesion sites to survive necessitating chemical modification strategies to incorporate various peptides or other signaling molecules into the gels that reduce their cell-repulsion behavior. For example, hMSCs encapsulated in PEG hydrogels require the addition of a cell adhesion component, such as the oligopeptide sequence RGD, for cell survival [136]. Alternatively, strategies have developed natural hydrogel environments by mimicking the native ECM, which not only promote cell–matrix interactions with the hydrogel but also sequester important growth factors that can augment tissue growth [137].
PEG hydrogels are very stable in vitro and are refractory to degradation in biological systems. This drawback—when biodegradation of an implanted material is advantageous—has been addressed by exploiting the versatile nature of PEG polymer chemistry. For example, the incorporation of copolymers enables the network structure and stability of PEG-based materials to be modified conveniently [138]. In particular, polylactic acid (PLA) and poly(caprolactone) (PCL) have been widely used in poly(α-hydroxy esters) for creating biodegradable PEG macromers for cell encapsulation because degradation profiles can be controlled by tuning chemistry and size of the degradable block [139]; in one example, Martens et al. [140] demonstrated that biodegradation time was enhanced when PEG hydrogels contained labile PLA linkages. In another strategy, the degradation of PCL-b–PEG-b–PCL hydrogels was temporally controlled by exogenously delivering lipase to match tissue growth for cartilage tissue engineering [141].
10.4 Therapeutic Applications of Encapsulated Cells
Although challenges remain, considerable progress has been made during the past decade toward understanding the biological and technological requirements for successful transplantation of encapsulated cells in experimental animal models. These studies have provided a foundation for clinical trials with many of the long-standing translational efforts directed toward treating diabetes. Additional promising therapeutic options are currently on the horizon for cancer, neurodegenerative diseases, and liver failure. Examples of therapy directed to each of these endpoints are provided in the following sections and are listed in Table 10.2.
Table 10.2
Clinical Studies of Encapsulated Cells
| Biomaterials | Therapeutic Agents | Application | Results/Status | References |
| Alginate | Human islets | Diabetes | Insulin independence with tight glycemic control was demonstrated after 9 months | [142] |
| Parathyroid tissue | Hypocalcaemia | 2 patient, parathyroid allotransplantation without immunosuppression |
[143] | |
| Diabecell®; neonatal porcine islets; insulin | Diabetes | 1 patient; 9.5 years functionality tested A new opened investigation of the safety and effectiveness of Diabecell® started from 2009 and will complete on 2014 | [144] Clinical trial number NCT00940173 www.clinicaltrials.gov | |
| Cellulose | Pancreatic cancer cells, ifosfamide | Cancer | 2 independent clinical trials, entering phase III trials | http://www.stockmarketmediagroup.com/nuvilex-inc-will-enter-phase-iii-trials-with-advantages-using-cell-encapsulation/ |
| Gelatin | Human retinal pigment epithelium, spheramine | Parkinson’s disease | The 1-year, open-label, single center study evaluated the safety and efficacy of gelatin microcarrier | [65,145,146] |
| Polyethersulfone | Engineered kidney cells | Huntington’s disease | 6 patients, phase I study evaluated the safety and tolerability of the procedure | [147] |
| Engineered baby hamster kidney cells | Amyotrophic lateral sclerosis | 6 patients, neurotrophic factors can be continuously delivered within the CSF of humans by an ex vivo gene therapy approach | [148] | |
| Polyethylene glycol (PEG) | Novocell®; allogeneic human islets; insulin | Diabetes | A single center phase I/II study, encapsulated islet allografts can be implanted safely in the subcutaneous tissues without the use of long-term immunosuppression | Clinical trial number NCT00260234 www.clinicaltrials.gov |
10.4.1 Diabetes
10.4.1.1 Cell Encapsulation Technologies were Pioneered by Efforts to Treat Diabetes
It has long been recognized that diabetic patients, who suffer from a plethora of secondary complications such as blindness, neuropathy, and end-stage renal failure, benefit from insulin treatment. Relevant to this chapter, difficulties in obtaining and administering insulin provided impetus to many of the historical developments in the field of cell encapsulation dating back to 1964 when Chang [16] proposed the idea of using ultrathin polymer membrane microcapsules for the immunoprotection of transplanted cells and introduced the term “artificial cells” to define the concept of bioencapsulation. Based on efforts started in the early 1970s that employed alginates for the immobilization of insulin-secreting pancreatic islet cells (PICs), which remained morphologically and functionally intact for as long as 4 months in vitro [18], this approach subsequently was implemented in vivo when it was used to xenograft islet cells in a rat model of diabetes [149]. Importantly, the microencapsulated PICs had a superior ability to maintain normoglycemia in animal models compared to nonencapsulated cells; for example, in a study conducted in streptozotocin-induced diabetic Wistar Lewis rats, encapsulated PICs maintained normoglycemia for 3 weeks compared to only 6–8 days with nonencapsulated cells [149]. More recently, Luca et al. [150] immobilized Sertoli cells in alginate-based microcapsules and transplanted the newly formed functional islets β-cells into nonobese diabetic mice thereby reversing the disease.
10.4.1.2 Expanding the Types of Cells Available for Treating Diabetes
Intensified insulin treatment, in particular, the delivery of insulin-secreting PICs as discussed above has proven promising for the treatment of type 1 diabetes [151]. Unfortunately, transplantation of whole human pancreases, isolated islets, or PICs into patients has been severely hampered by the scarcity of cadaveric human donor organs, which has mandated a search for insulin producing cells/tissue source alternatives. Taking one direction toward overcoming this hurdle, Abalovich et al. [152] performed a preclinical study investigating the potential of encapsulated pig islet cells for xenotransplantation. Type 1 diabetic dogs transplanted with encapsulated PICs experienced a significant reduction (20–80%) in insulin necessity after transplantation. Elliott et al. [144] showed that porcine islet cells survived and insulin production was maintained in a human patient 10 years after transplant of pig islet cells. In a second direction, recent progress in stem cell biology has provided promising leads for the conversion of progenitor cells into functionally competent, insulin-secreting cells. Currently, several studies have reported that MSCs [153], ESCs [55], and recently induced pluripotent stem cells (iPSCs) [154] can differentiate into insulin producing cells potentially useful for the treatment of diabetes. Tuch et al. [155] have proposed replacing pancreatic β-cells of diabetic recipients with pancreatic progenitors derived from human ESCs (hESCs).
10.4.1.3 Diabetes Leads the Way in Clinical Translation of Cell Encapsulation Therapies
Translation of microencapsulated cell systems, as evidenced by preclinical trials, now is under way (listed in full in Table 10.2) and has often focused on diabetes. Encapsulated islet transplantation was first performed in 1999 in a 38-year-old male patient who had severe diabetes [20]. In 2000, Elliott et al. [19] transplanted encapsulated porcine islets into diabetic patients and confirmed no PERV infection in patients. Calafiore et al. [21] subsequently performed human islets microencapsulation using alginate and transplanted them into 10 T1DM patients in Italy in an approach approved by the Italian Ministry of Health [21] and an Australia group transplanted barium-alginate encapsulated human islets into four T1DM patients without immunosuppression and followed them for over 2 years in a phase I study. Similarly, Tuch et al. [22] investigated the transplantation of barium-alginate microcapsules containing human islet cells in four type 1 diabetic patients and demonstrated the safety of this method, with little C-peptide—which reflects the level of blood sugars—detected, normal renal function, negligible cytokine release, and no major infection detected during the trial. Although insulin independence was not achieved, glycemic control was improved, with a reduction in insulin daily requirement. To quickly summarize clinical testing, a high level of safety has been demonstrated with an absence of adverse events in four phase I trials showing that intraperitoneally infused microencapsulated human islets can be considered safe for up to 3 years [22,156]; conversely, efficacy remains marginal.
10.4.1.4 Status of Clinic Translation
Important lessons have been learned and precedents set from efforts to clinically translate cell encapsulation therapies to treat diabetes; in particular, this approach provides protection from immunorejection and facilitates the long-term health and function of the transplanted cells. Although the (yet to be achieved) Holy Grail of specific induction of transplant tolerance would be ideal, in vivo testing has demonstrated cell encapsulation is a promising technology for achieving sufficient immunoprotection to allow β-cell replacement [157,158]. The early studies to date have confirmed that an encapsulation approach limits undesirable immunological responses to the nonhost islet cells and increases the access of cell replacement therapy to a broader patient base by minimizing the need for chronic administration of immunosuppressive drugs. Furthermore, the use of a macroencapsulation device will enable retrievability of the entire graft, which may be a desirable feature for products using hESC-derived cells as a potential safety benefit, allowing removal of the cells if complications do arise [159]. Overall, as the examples of clinical testing of cell encapsulation therapies outlined in the previous paragraph illustrates, hurdles remain to make this therapeutic approach reproducible and effective. On the other hand, a high degree of safety has been achieved, making this approach attractive to treat an expanded set of diseases; examples include cancer, CNS disorders, and liver disease as discussed next.
10.4.2 Cancer
Cell encapsulation technologies originally developed for the treatment of acquired and genetic diseases such as diabetes now are also proving to be attractive for the treatment of a variety of solid tumors. An advantage for using encapsulated cells for the treatment of cancers (and also other diseases) is that therapeutic molecules can be delivered in a sustained manner from implanted cells as the cells continuously produce the desired therapeutic products (e.g., endostatin as discussed below), which would otherwise have short residence times in the body.
Several microcapsule-based strategies are emerging to introduce and maintain immuno-protected cells in the body that secrete a variety of therapeutic products for the treatment of cancer. One approach is based on the idea that without angiogenesis, most solid tumors cannot grow past a critical size because of inadequate tissue oxygenation and nutrient supply; accordingly, strategies are being pursued based on encapsulation of cells that secrete anti-angiogenic proteins such as endostatin [67,160] and angiostatin [161]. In one study, genetically engineered HEK293 cells were encapsulated in alginate particles for the local production of endostatin for treatment of malignant brain tumors in a rat model [160]. In a follow-up study, tumor growth was reduced by 35% in treated animals that had received C6 glioma spheroids implanted either ectopically or orthotopically and, interestingly, tumor cell invasion into the surrounding tissue was also inhibited [162]. In a second set of studies, baby hamster kidney (BHK) cells were stably transfected with a human endostatin expression vector and were encapsulated in alginate–PLL microcapsules for long-term delivery of endostatin. A single local injection of encapsulated endostatin-secreting cells in nude mice subcutaneously xenografted with the human glioma cell line (U-87MG) resulted in a 72% reduction in tumor weight 21 days posttreatment [67]. Cells genetically modified to secrete angiostatin have also been encapsulated and implanted in tumor bearing mice resulting in a reduction of tumor volume by 70–80% in one study [161].
In a different approach, cell-based therapies have been designed to stimulate an immune response against tumors by encapsulating cells genetically modified to produce tumor necrosis factor α (TNF-α) [163] or interleukin-6 (IL-6) [164]. The encapsulated cells that were capable of releasing therapeutically significant amounts of functional TNF-α were intratumorally implanted into athymic nude mice tumors. The treated tumors showed extensive apoptosis and necrosis in response to TNF-α production resulting in significant tumor regression [163]. In another study, Chinese hamster ovary (CHO) cells producing IL-6 were encapsulated in alginate. This study demonstrated the viability of using cell encapsulation technology to generate short-term high levels of active circulating and intra-hepatic cytokines and also raised the possibility of modifying specific signal transduction cascades that contribute to tumor progression [164].
In a third approach, cells have been employed that over-express enzymes such as the cytochrome P450 enzyme to activate chemotherapeutic agents or prodrugs such as ifosfamide; such gene-directed enzyme prodrug therapy is one of the most encouraging approaches for cancer therapy [165]. The first demonstration of this method used genetically modified epithelial cells, which was later reproduced using HEK293 cells [166]. In addition, not only ifosfamide but also cyclophosphamide and related agents can be activated by the cytochrome P450 enzyme. Therefore, this strategy provides several options to treat tumors. Artificial cell encapsulated genetically modified cells expressing prodrug-activating enzymes, such as cytochrome P450, have been studied as a potential treatment for inoperable pancreatic carcinoma in patients [166,167]. These encapsulated cells are given intra-arterially so that they are located in the pancreatic carcinoma where they activate the drug ifosfamide. Therefore, the local concentration of activated ifosfamide is much higher than in the rest of the body, thus minimizing systemic toxicity and increasing local efficacy. Another approach involved polymeric artificial cells containing genetically modified myoblast cells that secrete IL-2 linked to the Fv region of a humanized antibody with affinity to tumors over-expressing the oncogene ERBB2; animal testing with these encapsulated cells delayed tumor progression and prolonged survival [168]. Finally, encapsulation of stem cells holds promise for cancer therapy. For example, Goren et al. [169] showed that encapsulated MSCs that were genetically engineered to secrete hemopexin-like protein (PEX), in alginate–PLL microcapsules. These microcapsules were implanted in a model of human glioblastoma reducing tumor growth, blood vessel formation, and tumor cell proliferation while increasing tumor cell apoptosis.
10.4.3 Central Nervous System
Targeted delivery of active biomolecules to the central nervous system (CNS) has therapeutic potential for a variety of disorders. Systemic drug delivery is inefficient because of poor passage of conventional therapeutic molecules through the blood–brain barrier while emerging cell and gene-based modalities also require alternative methods of administration. Cell microencapsulation technology provides an innovative strategy to deliver endogenous or engineered peptides/proteins directly to the diseased brain. Implantation of encapsulated producer cells ensures local de novo drug delivery, continuous and long-term release, and the possibility to combine several therapeutic agents [170].
10.4.3.1 Parkinson’s Disease
Initial proof of concept data for treating Parkinson’s disease (PD) with encapsulated cells came from studies in rodents and primates that demonstrated that encapsulated PC12 cells could be implanted into the striatum and deliver L-3,4-dihydroxyphenylalanine (L-DOPA) and dopamine in sufficient quantities to induce behavioral improvements [171,172]. Encapsulated PC12 cells survived in vivo for up to 6 months, while maintaining a typical morphology and production of catecholamines [172]. Other cell types, including encapsulated bovine adrenal chromaffin cells, survived for 1.5 years with continued production of catecholamines and met-enkephalin [173]. The most widely employed cell-based approach for the treatment of PD has entailed the administration of encapsulated choroid plexus (CP) cells in conjunction with glial-cell-derived neurotrophic factor (GDNF) or vascular endothelial growth factor (VEGF) secreting cells [174–176]. In one study using this strategy, encapsulated GDNF-secreting fibroblasts were implanted into the striatum of 6-hydroxydopamine (6-OHDA) injured rats. Long-term expression of GDNF, up to 6 months, was detected along with behavioral improvement and tissue biocompatibility of the capsules [175]. In another study, porcine CP cells were encapsulated in alginate–PLO–alginate capsules and implanted into the striatum of 6-OHDA lesioned rats. Results showed that the presumed neurorestorative proteins secreted by the encapsulated cells improved motor behavior and nigrostriatal dopaminergic activity [177].
10.4.3.2 Alzheimer’s Disease
Alzheimer’s disease (AD) is a progressive disorder with a successive decline in cognitive functions. One early symptom is episodic memory loss. The progressive memory loss in AD patients is associated with neurodegeneration and dysfunction of the central cholinergic [34]. Positive effects of nerve growth factor (NGF) on cell survival and neurological functionality have been demonstrated in several animal studies. Initial studies demonstrated that implants of encapsulated NGF-secreting BHK cells prevented cholinergic neuron loss following aspiration of the fimbria/fornix. Control-implanted animals had an extensive loss (88%) of ChAT-positive cholinergic neurons ipsilateral to the lesion that was prevented by NGF cell implants (14% loss) [178]. Nagahara’s [179] study indicated that sustained local delivery of NGF to the cholinergic basal forebrain can arrest and even reverse the degeneration of the basal forebrain cholinergic neurons involved in the cognitive decline in AD. The immobilization of genetically engineered cells to produce VEGF, ciliary neurotrophic factor (CNTF) or glucagon-like peptide-1 (GLP-1) have also been employed for the treatment of AD. Spuch et al. [170] implanted microencapsulated fibroblast cells releasing VEGF into a transgenic (APP/PS1) mouse model of AD with degenerative alterations in the microvasculature. The study reported increased angiogenesis, decreased presence of Aβ and tau protein, less apoptosis, and protection of the cognitive behavior in the APP/PS1 mice after the implantation of the microcapsules.
When encapsulated cells secreting CNTF were implanted intra-cerebroventricularly into mice expressing the mutant amyloid precursor protein, a significant improvement in cognitive function occurred [180]. In other studies, encapsulated MSCs expressing GLP-1 were evaluated in an AD double transgenic murine model for their ability to GLP-1 decrease amyloid deposition and A-β-induced toxicity [181]. Results suggested a reduction in A-β-induced toxicity in vitro along with anti-inflammatory and neuroprotective properties. Most recently, the authors describe the first clinical trial with encapsulated cell biodelivery implants that deliver NGF to the cholinergic basal forebrain with the intention of halting the degeneration of cholinergic neurons and the associated cognitive decline in patients with AD [182]. Follow-up MRI at 3 and 12 months postimplantation showed no evidence of inflammation or device displacement. At 12 months, implants were successfully retrieved and low but persistent NGF secretion was detected in half of the patients.
10.4.4 Liver Disease
Hepatic diseases, including acute liver failure, chronic liver disease, and congenital metabolic liver disease, require the restoration of liver function. In recent years, bone marrow stem cells have been used as feeder cells to enhance hepatocyte viability. Extending this concept from cell culture experiments, rat and immortalized human hepatocytes (IHHs) now can be encapsulated in 400 µm alginate–PLL–alginate membranes, stored using cryopreservation, and then later be transplanted into xenogeneic recipients with liver failure and sustain liver metabolic functions [183]. Unfractionated rat bone marrow stem cells coencapsulated with hepatocytes significantly enhances the viability of hepatocytes compared to encapsulation alone for time periods from 7 weeks to up to 4 months [184,185]. In a test of efficacy, rat bone marrow stem cells were encapsulated and then transplanted intraperitoneally into a 90% hepatectomy-induced acute liver failure rat model. After 2 weeks, survival of animals that received implants containing bone marrow stem cells was significantly prolonged comparable to the cohort that was transplanted with hepatocytes only [185]. Mechanistically, when coencapsulated with bone marrow stem cells, the hepatocytes attach to one another whereas most remained unattached when encapsulated in the absence of the bone marrow cells. Based on the need for cell–cell interactions for hepatocytes to function optimally, the bone marrow stem cells contribute to positive in vivo outcomes at least in part by promoting hepatocyte function.
10.5 Challenges and Future Perspectives
In the past 50 years since the idea of cell-based therapies was given impetus by early attempts to encapsulate cells before implantation into a patient to thwart immunorejection, considerable progress has been made as has been outlined in this chapter. However, there are remaining hurdles to surmount before this technology achieves its full potential that include overcoming technological and biological limitations, as well as conforming to ethical, political, and regulatory obstacles. A major focus of this chapter was on the “materials science” aspects of cell encapsulation-based therapies where considerable progress has been made over the past decades. However, there are still limitations in the production of microcapsules of defined size, certain size ranges, shape, and properties at a scale suitable for commercial application and under controlled environmental conditions. In addition, polymers used to encapsulate cells still hinder the development and application of cell-based therapies because these materials are often of natural origin and are highly variable in chemical composition and physical properties. It is therefore difficult to prepare reproducible encapsulation devices because of batch-to-batch variations in factors such as molecular weight distributions, a wide range of viscosities, purities, and the degree of chemical substitution. In many cases, synthetic polymers suffer from the same problems.
Another important consideration for clinical success of cell encapsulation therapies is an appropriate and reproducible source of functional cells. In this regard, the potential use of allogeneic versus xenogeneic cells has provoked important social and ethical debates [7]. Ultimately for a cell encapsulation strategy to be successful, a number of practical factors must be considered in addition to the fundamental design criteria described above. The process must be easily scaled up for manufacturing; marketable; accepted by surgeons, patients, and health-care providers; and receive FDA approval [92]. Nevertheless, the accelerating pace of technical and biological advances, together with the increasing experience in the field, offers hope that that full potential of bioactive cell encapsulation will reach fruition in the coming years.
Acknowledgments
Funding for this contribution was provided by the National Institutes of Health (R01CA112314 and R01AR054005).