Engineering the Surface of Cells Using Biotin–Avidin Chemistry
Kawther K. Ahmed, Sean M. Geary and Aliasger K. Salem, College of Pharmacy, University of Iowa, Iowa City, IA, USA
This chapter is dedicated to the use of biotin-avidin chemistry to functionalize cell surfaces for a range of biomedical and biotechnological applications. The importance of different avidin analogs and methods of cell surface biotinylation are discussed. The valuable role of biotin-avidin chemistry in augmenting tissue engineering is highlighted as well as its role in generating cell-particle hybrids for downstream medical purposes such as cancer vaccines and targeted drug delivery.
Keywords
Avidin; Biotin; streptavidin; Tissue engineering; Biotinylation; Noncovalent binding; Cell surface modification
7.1 Introduction: Rationale for Engineering the Cell Surface
The interplay between cells and their surroundings plays a major role in directing cell fate in terms of differentiation, adherence, spreading, and at times, the outcome of their interactions with cells of the immune system [1]. Thus, precise patterning of the cellular environment can be used to control and manipulate cell behavior. The ability to manipulate the cell surface to impart nonnative properties to cells has been used as a tool for enhancing cell therapy [2]. Apart from the benefit cell surface engineering can have in the field of cell therapy, cells can be modified for research purposes to expand our understanding of cellular processes in both healthy and diseased states [3,4]. Examples of applications for which cell function and behavior have been modified include: promotion of cell adhesion and migration for tissue engineering [1], modulating cell trafficking and tissue homing [5,6], providing autocrine growth factors for cells used in cell therapy [2,7], and prolonged persistence of modified cells to promote immune responses for cancer vaccine applications [8–11]. While genetic engineering of cells enables the introduction of proteins (such as surface receptors or cytokines), there is the issue of safety when implementing viral transduction, the generally most efficient form of transfection. In addition, transfection methods cannot be used to functionalize cells with nonproteins such as drugs or even particles [2]. Thus, there is a need for alternative methods to surface engineer cells with the desired functionality. One of these approaches being implemented and showing promise in a range of applications is cell surface engineering using biotin–avidin crosslinking. Advantages to the biotin–avidin system include that it is inherently adjustable, relatively inexpensive, simple, and nontoxic.
7.2 Biotin and Avidin: An Overview
The interaction between avidin and biotin was recognized as early as 1940 by Esmond Snell with the observation that chicks fed on a diet of raw egg white were deficient in biotin, regardless of its availability in their diet [12]. The discovered interaction exhibited unique strength and stability that made it become a widely used interaction in biomedical applications. The avidin–biotin complex is one of the strongest known noncovalent interactions in biological systems. The high association constant (Ka≈1015 M−1 [13]) and the exceptionally high stability of the avidin–biotin crosslink [14] have made it an indispensable tool in biomedical and biotechnological applications. Avidin–biotin binding is rapid and very stable, being resistant to: high temperature, extreme pH, organic solvents, and proteolytic enzymes [14,15]. The list of applications is long and still growing due to the advances made in cell and tissue engineering. A highly utilized methodology involving the avidin–biotin interaction is cell surface protein enrichment prior to their identification with other detection techniques like western blotting and liquid chromatography–mass spectroscopy (LC–MS) [16]. Signal amplification in enzyme-linked immunosorbent assays (ELISA), immunocytochemistry, and bioimaging have also made great use of the strong affinity and stability of the avidin–biotin interaction [17–20]. More novel uses of avidin–biotin chemistry include cell surface engineering where the cell membrane is coated with biotin for applications such as tissue engineering [21] and cell–particle hybrid synthesis [22].
7.2.1 Avidin–Biotin Complex at the Structural Level
Avidin (Mr 67,000) is a tetrameric glycoprotein with each of the four identical 128 amino acid polypeptide subunits having a carbohydrate group [23]. Primary natural sources of avidin, which possess some antibacterial activity, are the egg whites of birds, amphibians, and reptiles [24]. Each subunit has been characterized by X-ray crystallography and was found to have a barrel-like shape and contain one biotin-binding site, thus one avidin molecule has the capacity to bind four biotin molecules [13,25–27]. Research involved in determining the three-dimensional structure of the biotin-binding site and binding kinetics has identified specific amino acids essential for biotin binding and stability of the tetrameric structure [13,23,27–30]. This knowledge has enabled manipulation of the structural features of avidin to tune its binding properties in terms of biotin-binding capacity and specificity (see Section 7.2.2).
Biotin, also known as vitamin H or vitamin B7, is small relative to avidin with a molecular weight of 244 [31]. It contains a cyclic urea group and a valeric acid side chain and plays an essential role as a coenzyme for carboxylases involved in a range of metabolic processes including anabolism of fatty acids [31,32].
The strong avidin–biotin interaction is provided mainly by van der Waals forces driven by the exquisite orientation of the amino acid residues in the biotin-binding sites of the avidin molecule [15,29,33], the small size of the biotin molecule [29], and the structural relation of avidin to the cyclic ureido group of biotin [30].
7.2.2 Avidin and Avidin Analogs
One limitation to the use of natural avidin is its nonspecific adsorption to some biological molecules. Avidin has a basic isoelectric point (pI~10) [25] rendering the protein positively charged at physiological pH [34] which can result in nonspecific binding to cells and negatively charged molecules. In addition, the carbohydrate content of avidin can contribute to nonspecific adsorption to lectins [35,36]. Although nonspecific binding may be stringently controlled in certain in vitro applications, it becomes more problematic for tissue engineering purposes and other biomedical applications. Therefore, alternatives to avidin have been investigated, with two of the most prevalently implemented being streptavidin and neutravidin [37] (other alternatives are genetically engineered avidin and streptavidin and they will be discussed later). Streptavidin and neutravidin are nonglycosylated analogs of avidin, each having a molecular weight of 60,000 [38,39]. Both have biotin-binding affinities equivalent to that of avidin; in a variety of applications they have greater specificities and have been successfully used as an alternative to avidin [25]. Streptavidin, extracted from Streptomyces avidinii [27], has an isoelectric point close to 6 and is therefore anionic at physiological pH. This, combined with the lack of glycosylation, contributes to the enhanced specificity of streptavidin compared to avidin [28,30,40].
Neutravidin has a near to neutral isoelectric point (pI 6.3) [25,39] and exhibits even lower nonspecific binding than streptavidin due to the lack of an RGD-mimicking sequence, RYD, found in streptavidin [41].
While the four to one binding capacity of biotin to avidin plays an important role in using avidin–biotin chemistry to synthesize cell aggregates [42], it becomes troublesome in some applications where the aggregation of biotinylated cells with the avidin-modified ligand is undesirable, such as in tissue engineering. The solution to this problem is provided by the use of monomeric or monovalent avidin or avidin analogs. These analogs are produced by inducing mutants in the genes encoding for the protein. A good portion of the genetic engineering efforts goes to limiting the protein-binding capacity to one biotin molecule per avidin molecule, however, studies generating avidin and streptavidin mutants with enhanced binding specificity [43] and reversible biotin-binding properties for affinity chromatography have also been reported [44]. These modified biotin-binding proteins have the potential to be used in controlling cell growth and spreading in tissue engineering allowing more precise control of cell–cell and cell–matrix interaction.
An avidin monomer was synthesized by Kulomaa and coworkers by inducing a mutation to change one of the amino acid residues at the subunits interface. However, these monomers reassociated upon the addition of biotin [45]. This problem of reassociation was solved by inducing a second mutation such that two amino acids at the interface are changed in the new monomer [46]. Another group had also reported the development of stable monomeric avidin by inducing amino acid changes, although different amino acids were changed [47]. However, these stable monomers showed less affinity to binding biotin relative to the tetrameric protein and had lower stability to proteolytic enzymes. Another study has reported the synthesis of a stable streptavidin monomer that has a biotin-binding affinity higher than the previously reported monomers [48]. The synthesized monomer produced was an analog to rhizavidin, a biotin-binding protein from the bacterium Rhizobium etli and exists naturally as a dimer [48].
Another approach to overcome the aggregation associated with the use of tetrameric biotin-binding proteins is the use of monovalent protein developed by Alice Ting’s group [18]. In monovalent avidin and streptavidin, a tetramer of three inactive subunits and only one biotin-binding subunit is the final product [49]. The process involves forming inclusion bodies in Escherichia coli that express active or inactive units. These units are then purified and folded with a ratio of inactive:active units of 3:1. Tetramers having three inactive units and one biotin-binding unit are then isolated using a specific tag on the active subunit to identify its multiplicity (number of active sites folded in the product) [49]. The conservation of binding affinity in the monovalent protein compared to the monomeric product was attributed to the preservation of the subunits interface at which the biotin-binding site resides [49–51].
7.2.3 Safety of the Avidin–Biotin Complex for Clinical Use
Being xenoproteins, avidin and its analogs are, not surprisingly, immunogenic [52,53]. The level of avidin-specific antibodies that are induced increases with increasing exposure to avidin and streptavidin [54]. However, the immunogenicity of avidin and streptavidin does not necessarily constitute a health hazard since 15 years of use of avidin in clinical settings has had no major side effects [55]. In addition, melanoma patients treated with two or three doses of a vaccine formulation incorporating streptavidin–biotin complexes induced, at worst, low-grade allergic reactions [53]. Furthermore, it was shown that the presence of these antibodies did not abrogate or weaken the function of the avidin–biotin complex [55]. Nevertheless, it has been suggested that the immunogenicity of avidin can be reduced by pegylation [56].
7.3 Methods for Engineering Cell Surfaces with Avidin–Biotin Complexes
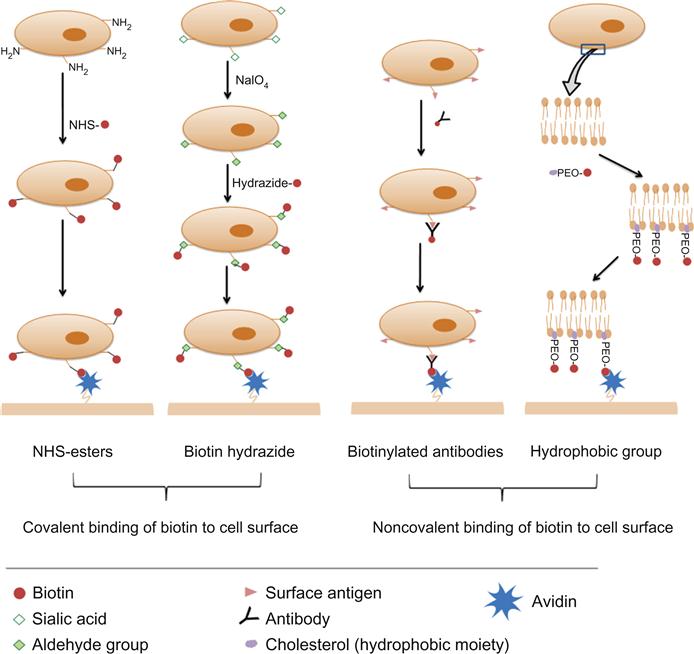
The literature is rich with different approaches and variable protocols for decorating cell surfaces with biotin. However, they can be categorized generally into covalent and noncovalent binding of biotin to cell surfaces (Figure 7.1).
7.3.1 Covalent Binding of Biotin to Cell Surfaces
Biotin can be covalently bound to cell surfaces through its valeric acid side chain. To elaborate, the carboxylate functional group of the valeric acid side chain can be activated to bind different native or induced residues on the cell surface. Different activated groups are available for substrate biotinylation such as N-hydroxysuccinimide (NHS) ester, hydrazide, BMCC (1-biotinamido-4-[4′-(maleimidomethyl)cyclohexane-carboxamido]butane), and HPDP (N-[6-(biotinamido)hexyl]-3′-(2′-pyridyldithio)propionamide) [57]. However, NHS and hydrazide biotin conjugates are the reagents most commonly used for cell surface biotinylation and thus only these two reagents will be discussed.
7.3.1.1 NHS Esters
The valeric side chain of the biotin molecule is not required for avidin binding; therefore, the carboxylic group can be activated with carbodiimides so as to bind to amine groups present on the cell surface. Alternatively, an acylating active group like NHS can be conjugated to the valeric acid side chain to bind cell surface amines [57,58]. These biotinylating reagents (activated biotin) are commercially available as NHS–biotin, which is water insoluble, and the water-soluble form sulfo-NHS biotin [57], which is cell membrane impermeable [59]. One limitation to the use of biotin alone or NHS–biotin is the possible unavailability of the conjugated biotin for avidin binding due to steric hindrance by adjacent cell membrane proteins. In such circumstances, in order for biotin to effectively bind to avidin, a spacer is required between the NHS ester group and the valeric acid side chain [57]. NHS-LC-biotin and sulfo-LC-NHS biotin have 6-aminocaproic acid spacers that provide greater length between the modified cell surface and the biotin [57]. The method for cell surface biotinylation with NHS esters is easy and involves incubating cells (up to 2.5×107/ml) with a solution of the biotinylating reagent (0.5 mg/ml) for approximately 30 min at room temperature in a protein-free isotonic solution [53,60–62]. A range of cell types (adherent and nonadherent) have been biotinylated by this method and no significant effect on cell viability or cell adhesion properties (for adherent cells) was observed [53,59,61,62].
7.3.1.2 Biotin Hydrazides
Hydrazides bind spontaneously to aldehyde and ketone groups to form covalent hydrazone linkages [57]. Although aldehydes and ketones are not expressed on the surfaces of cells, they can be introduced by the oxidation of a diol group in a sugar moiety on the cell surface using sodium periodate [63,64]. The sugar moiety targeted in such cases is sialic acid. Sialic acids are a group of monosaccharides widely expressed on the cells of vertebrates [65]. This method of cell surface biotinylation (oxidizing sialic acid residues on the cells to aldehydes followed by incubating treated cells with biotin hydrazide) was employed to independently biotinylate HEK293 cells [22], live embryonic stem cells [66], and murine L6 myoblasts [67]. As with NHS–biotin, biotin-LC-hydrazide with a 6-aminocaproic acid spacer is also available [57]. The reported method for cell surface biotinylation involves initially incubating cells with a solution of sodium periodate (NaIO4) at a concentration of 1–2 mM for 5–20 min at 4°C. Then treated cells are incubated in a solution of 5 mM biotin hydrazide, pH 6.5, for 90 min at room temperature [22,67,68]. Incubation with biotin hydrazide for 30 min at 37°C has also been reported [66]. This method was also cited as not resulting in major cell toxicities, however, a study comparing NHS–biotin and biotin hydrazide demonstrated that, at least for red blood cells, biotinylation with the former provided higher viable cell recovery and better cell surface biotinylation [69].
Generating reactive ketone groups as substrates for biotinylation has been suggested as a potential methodology for in vivo biotinylation. To explain, it was suggested that metabolic generation of ketones can provide a method for in vivo engineering of cell surfaces [70–72]. The method involves the use of cellular sialic acid synthesis machinery to metabolize an external precursor, N-azidoacetylmannosamine, to provide a substrate for cell surface ligation with biotin hydrazide [63]. In vivo engineering of cell surfaces was suggested to provide a tool for targeted drug delivery with ligand-labeled drug delivery systems or drugs [70,71]. It should be noted, however, that in the study that involved metabolic engineering of cell surfaces, the substrate was injected i.p. and cell surface ketones were evident in the spleen, heart, liver, and kidney of the mice [73].
7.3.2 Noncovalent Binding of Biotin to Cell Surfaces
Covalent binding ensures strong association of biotin to cell surfaces; however, noncovalent biotinylation has also been reported and shown to be effective. However, to the best of our knowledge, no direct comparison between the two approaches has been reported in terms of biotinylation efficiency and effect on cell viability.
7.3.2.1 Cell Surface Biotinylation via Biotinylated Antibodies
This methodology has been mainly used to biotinylate cells for engineering cell surfaces with particles and involves incubating cells with biotinylated antibodies at 4°C for 25–60 min in cell culture media [74]. Concentrations of antibodies used ranged between 4 and 5 µg/2–5×106 cell/ml. Cells are then washed and incubated with an avidin-modified ligand. The choice of the antibody depends on the cell type being engineered. Some examples of biotinylated antibodies used cell surface engineering via avidin–biotin linkage are anti-beta 1 (CD29) for murine melanoma (B16-F10) and prostatic cancer (RM11) cells (unpublished work from our laboratory), and anti-CD19 for B-cell lymphoma cell biotinylation [74]. The cell-bound biotin that does not complex with avidin is unlikely to have any biological consequences.
7.3.2.2 Use of Biotin-Tagged Hydrophobic Molecules to Coat Cell Surfaces
Another approach to anchor biotin to the cell surface, though not widely adopted, is through the insertion of a biotin-labeled hydrophobic moiety into the cell lipid membrane [21,75]. This approach involves the synthesis of a polyethylene oxide (PEO) polymer that is functionalized with biotin at one end and cholesterol at the other. Upon incubating this polymer with living cells, biotin is anchored via the insertion of the hydrophobic cholesterol into the lipid cell membrane. The efficiency of the cell surface biotin to subsequently bind streptavidin was found to increase with increasing polymer chain length. Similar to cholesterol, other biocompatible anchoring molecules (e.g., BAM90) have been utilized to anchor biotin on hepatic cell surfaces [21].
7.4 Applications of Cell Surface Engineering Using Avidin–Biotin Chemistry
As mentioned earlier, the avidin–biotin complex is widely used in different areas of biomedical and biotechnological applications. Cell surface engineering in particular is a process where the avidin–biotin complex has been exploited to tune cell behavior.
7.4.1 Spatial Control of Cell Growth on Solid Surfaces and Tissue Engineering
Spatial control of cell growth and spreading pattern is important in modulating cell properties and preserving cell phenotype [76]. In normal tissue, cell–cell and cell–ECM contact is mediated by transmembrane proteins (e.g., cadherins and integrins, respectively) [76,77]. However, these intrinsic adhesion molecules can only mediate weak cell attachment to synthetic ECM such as tissue engineering templates and solid surfaces [78]. Therefore, biomimetic surfaces coated with adhesion molecules have been developed to enhance cell adhesion and proliferation [79]. One example of applications where cell attachment needs to be augmented is the attachment of endothelial cell layers onto synthetic vascular grafts [78]. The Reichert group has utilized the avidin–biotin complex to improve the potency of synthetic vascular grafts by lining the graft lumen with a layer of endothelial cells [80–82]. Their studies have shown that the interaction between avidin and biotin is important in enhancing integrin-mediated cellular interaction with the ECM [80]. The studies involved biotinylating bovine aortic endothelial cells with sulfo-NHS-LC-biotin and using avidin to bridge biotinylated cells with a biotinylated growth surface [80,82]. The study emphasized the importance of the high affinity avidin–biotin interaction in supporting fibronectin–integrin-mediated adhesion.
The other application where spatial control of cell growth and enhancement of integrin-mediated cell adhesion becomes important is tissue engineering. In tissue engineering involving cell culturing on a polymeric templates, cell growth and spreading pattern need to be controlled and augmented to preserve cell phenotype and resemble normal tissue structures [83]. Hence a ligand–receptor patterning strategy is required to enhance cell attachment to the polymeric template. In a study carried out earlier by our group, a versatile procedure for cell growth patterning was developed [84]. The versatility stems from engineering the cell surface with biotin by oxidizing native sialic acid residues to generate reactive groups for biotin hydrazide coupling. The method was evaluated for patterning human dermal fibroblast growth on avidin-treated PLA–PEG–biotin (poly(lactic acid)–poly(ethylene glycol)–biotin) polymer surfaces. In a separate study in our group, a novel biotinylated nanotemplated degradable hydrogel was developed [85]. The fabricated hydrogel was suggested to have applications in targeted drug delivery and for cell patterning as the surface can be engineered with cell-specific receptors using the avidin–biotin interaction.
Besides cell growth on solid supports, tissue engineering involves the formation of cell aggregates between homo- and heterotypic cells as the first step toward the production of viable tissue [86]. The method of using the avidin–biotin interaction for such purposes was first described by the Kellam and Shakesheff groups where cells were aggregated into clusters for skeletal muscle engineering [42,67]. The method involved biotinylating cell surfaces with biotin hydrazide and crosslinking biotinylated cells with tetravalent avidin [68]. Cell aggregation through avidin–biotin interaction was also used to agglomerate hepatic cells and fibroblasts to form sheet-like liver tissue where the use of avidin to crosslink heterotypic biotinylated cells was shown to be effective in preserving cell viability and phenotype for nonproliferative cells such as primary cultures of hepatocytes [21].
7.4.2 Surface Conjugation of Macromolecules
The use of cells for therapeutic purposes is an expanding area of research. For example, mesenchymal stem cells have been trialed as potential therapies for immune-based diseases such as multiple sclerosis [87], whilst dendritic cell preparations have been employed as cancer vaccines such as the recently FDA-approved sipuleucel-T for prostate cancer [88]. The employment of cells for therapeutic applications requires close control of cell trafficking, proliferation, adhesion, and other microenvironmental factors [2]. Such control is often achieved by specific molecules capable of adjusting cell behavior and/or microenvironments [7]. For example, hematopoietic stem cell (HSC)-based therapy can be augmented by co-administration of a glycogen synthase kinase-3 (GSK-3) inhibitor [89] and tumor cells can be stimulated (mostly by viral transfection) to secrete certain cytokines for cancer vaccine applications [90,91]. Another approach is the stable anchoring of these moieties to cell surfaces using crosslinking strategies [92] among which is the avidin–biotin complex. For example, surface engineering tumor cells with the cytokine granulocyte macrophage colony stimulating factor (GM-CSF) has been performed for cancer vaccine purposes [53]. GM-CSF is a pleiotropic cytokine that has gained interest as a vaccine adjuvant and proved effective in cancer immunotherapy and cancer vaccines [90,93]. GM-CSF is secreted by a range of cell types, which include macrophages, T cells, endothelial cells, and fibroblasts, and is known to play a role in the recruitment and maturation of dendritic cells (the main antigen-presenting cells) [94,95]. The study involved biotinylating melanoma cells using sulfo-NHS-LC-biotin and incubating biotinylated cells with streptavidin-tagged GM-CSF [53]. This method provides a safe alternative to viral transduction of cells to promote GM-CSF expression. However, it should be noted here that the conjugated GM-CSF is not meant to be released but rather to be constantly associated with the tumor cell surface. A separate study utilized biotin–avidin crosslinking to stably bind the sialyl Lewis X (SLeX) moiety on mesenchymal stem cells [59,96]. SLeX, a cell surface carbohydrate that has a role in tumor cell metastasis and mesenchymal cell trafficking [97,98], was used as a targeting moiety to promote mesenchymal stem cell tissue homing. Cell surface modification involved cells being biotinylated using sulfo-NHS biotin followed by treating biotinylated cells with streptavidin. Biotinylated SLeX was then introduced and stable binding was provided by the biotin–streptavidin–biotin crosslinkages. Modified cells demonstrated homing responses in vitro [59] as well as in vivo [99].
7.4.3 Cell–Particle Hybrid Synthesis
Engineering cells with particulated matter using avidin–biotin chemistry is an area that has only been remotely explored. Besides the use of avidin–biotin crosslinking in studying cell–particle binding kinetics [74], it has been used to stably anchor particles onto cell surfaces in order to synthesize cell–particle hybrids. Due to the presence of drug-containing particles, these hybrids can provide a sustained drug release profile [100]. In addition, the fabricated delivery system ensures co-delivery of the particles payload and the therapeutic cells to the same target. Cell–particle hybrids that have employed the avidin–biotin crosslinking were developed for cancer vaccine purposes and targeted drug delivery [22,60]. In terms of cancer vaccine applications, the assembled hybrid delivers cellular cancer antigens (provided by the cellular component) and the loaded vaccine adjuvant (provided by the particles) such as CpG to the same dendritic cell to maximize the prophylactic/therapeutic effect of the vaccine [92,101]. As a proof of principle, a cell–particle hybrid has been synthesized in our laboratory by attaching 1.4 µm PLA–PEG–biotin microparticles to biotinylated HEK 293 tumor cells using an avidin bridge [22]. Cells were biotinylated using biotin hydrazide following their treatment with sodium periodate. More recently, another cell–particle hybrid was synthesized in our laboratory by anchoring 500 nm polylactic-co-glycolic acid particles to murine B16-F10 melanoma cells (unpublished data). Particles were coated with streptavidin post-fabrication using carbodiimide chemistry. Cells were biotinylated using commercially available biotinylated anti-β1 integrin antibodies and the assembled hybrids were characterized using flow cytometry, laser scanning confocal microscopy, and scanning electron microscopy. The hybrids fabricated in this study are proposed to serve as potential cancer vaccines where the irradiated allogeneic cancer cells secreting GM-CSF act as an immunogen and the particles, loaded with an immune adjuvant, can act to promote a more robust antitumor immune response. Besides cancer vaccines, cell–particle hybrids can play a role in targeted drug delivery. A study has engineered human bone marrow-derived mesenchymal stem cells with 40 nm neutravidin-coated nanoparticles [60]. The fabricated cellular particle patches are suggested to be capable of targeting chemotherapeutic agents to tumor cells via the tumoritropic properties of stem cells. In this study, cells were biotinylated with sulfo-NHS-LC-biotin and the synthesized patches were characterized with laser scanning confocal microscopy and scanning electron microscopy.
7.5 Conclusion
Cell surface engineering is only one of many areas of research in which avidin–biotin crosslinking plays a vital role. As cell engineering technologies grow more complex, increasing numbers of researchers are relying on the strong and stable avidin–biotin interaction as their cementing material in modifying cell surfaces and building more complex hierarchal structures. The advances in genetic engineering have enabled more control over the binding properties of avidin and its analogs allowing more precise use of the avidin–biotin complex. Such advances will pave the way for more clinically relevant approaches using biotin–avidin crosslinking such as engineering cell surfaces for vaccine applications and targeted drug delivery.