Cell Surface Engineering by Chemical Reaction and Remodeling
Wade M. Fox1 and Debanjan Sarkar1, 2, 1Department of Chemical and Biological Engineering, University at Buffalo, The State University of New York, Buffalo, NY, USA, 2Department of Biomedical Engineering, University at Buffalo, The State University of New York, Buffalo, NY, USA
Cell surface modification with covalent approaches provides novel bioengineering tools to conjugate functional molecules and structures on the cell surface. Immobilization of specific molecules and structures can precisely control the interactions of the cell with its extracellular microenvironment. Since cell membrane contains functional molecules with specific chemical signatures, covalent transformation of cell surface molecules can form stable adducts to immobilize molecules with controlled density, defined organization, and spatiotemporal control. Particularly, covalent approaches for cell surface engineering are advantageous for long-term application where enhanced stability of the immobilized molecules is required. Depending on the nature of the target molecule and on the availability of cell surface functionalities, conjugation strategies should be selected, developed, and optimized. This strategy allows new molecular engineering tools to interrogate fundamental biological properties of cells and to develop cell-based therapeutics and diagnostic tools.
Keywords
Cell membrane; covalent reaction; cell surface functional groups; amine; thiols; carbonyls; polymers; nanoparticles
2.1 Introduction
Molecular engineering of the cell membrane is a powerful tool to manipulate surface composition and for controlling the interactions between the cell and its surrounding environment. Since cells interact with the extracellular environment through the molecular receptors and ligands present on the membrane, it is important to control the molecular composition to regulate these interactions. Enzymatic strategies and genetic manipulation of cellular machineries are widely used to achieve this goal, particularly in molecular biology [1–3]. But recent advancements in material science and engineering have provided novel technologies to alter cell membranes through chemical methods [4,5]. Since cell membranes are molecularly composed of wide-ranging chemical functionalities, it provides a versatile ground for modification with characteristic molecules through defined pathways [5,6]. This enhances our ability to augment functioning of cells and manipulate their fate during different biological processes with precise control and defined mechanisms. As a result, chemical engineering of the cell membrane has been a powerful tool to interrogate fundamental cellular process and has emerged as a technology to develop cellular therapeutics and diagnostics. Particularly, chemical engineering of cell membranes has been used to investigate cell–cell and cell–matrix interactions [7–10] and to design targeted delivery [10], biosensing, and imaging techniques [11].
Cell membranes are chemically heterogeneous and dynamic in nature. In generic terms, cell membranes are characterized by the presence of different proteins, lipids, and carbohydrates. However, molecular structure and three-dimensional organization of these molecules on the cell surface are highly specific and overly complex and are dependent on the cell type and external environment. Importantly, cell membranes are continuously remodeled and reorganized in response to external conditions and stimuli to mediate cell–cell and cell–niche communication and intracellular signaling [12]. This indicates the dynamic nature of cell membranes where chemical signatures are continuously changing. Opposed to this process, which is recognized as a natural cellular mechanism derived from internal cellular machineries, exogenous modification of cell membrane is considered as a synthetic mechanism to alter cell surface composition.
Chemical modification of cell membranes by synthetic methodologies can be categorized either as a biological or physicochemical process. Genetic engineering and enzymatic transformations of cells are common biological mechanisms to alter the cell surface molecules. While the genetic approach involves introduction of exogenous genetic materials into the cell genome to express or regress specific cell surface molecules, the enzymatic approach transforms specific molecules into distinct types with defined functionality. Both of these approaches are widely used to study the fundamental biology of cell functioning, but these strategies have also found acceptance in developing cellular therapeutics and diagnostics. However, there are several limitations which potentially restrict the use of these approaches as generic methods. Particularly, genetic engineering of cells alters genomics of the host cell which can permanently alter genotype and can potentially induce malignant character in the long term with safety concerns. Alternately, enzymatic transformations are highly complex and require the presence of specific molecules on the cell surface for modifications. In contrast, cellular engineering using physicochemical principles can be broader and can provide platform technologies for developing molecular tools to modify the cell surface [4,10]. Manipulating living cells by physical and chemical techniques offers exciting avenues to decorate the cell membranes with molecules and specialized structures, for example, particles and patches. Physical processes utilize noncovalent molecular interactions, for example, hydrogen bonding, hydrophobic interactions, and electrostatic interactions to attach molecules and structures to cell membranes. Since these interactions are physical in nature, this technique does not involve any permanent chemical change on cell membranes to engineer cell surface. As a result, cell surface engineering by physical process is often transient in nature and probably not useful where permanent and long-term cell modification is required. Furthermore, physically immobilized ligands cannot sustain in a mechanical environment where a large force is exerted on the ligands. These limitations can be addressed by performing chemical reactions on cell membranes to attach molecules through covalent bond formation.
As cell membranes present a rich repertoire of wide-ranging functionalities, different chemical reaction methodologies can be used to react with cell membranes for covalent cell surface modifications. Contrary to biological transformation, chemical reaction engineering of cell membranes is not dependent on a specific surface molecule or receptor. Rather chemical reaction depends on having specific functional groups, independent of the constituent molecule, which can be modified through covalent reactions. This provides enhanced versatility of chemical reaction based cell engineering where different molecules can be attached to cell membranes through a diverse range of chemical reactions. Thus, chemical reaction based cell surface engineering is technologically a more adaptable method and is currently being explored as a molecular engineering tool to manipulate cell function and phenotype. In this chapter, we will primarily focus on the methods used to covalently engineer cell membranes and the resultant modifications. Use and effectiveness of these cell surface modifications will be briefly mentioned but not in detail.
2.2 Methods and Technology of Covalent Cell Surface Reaction
Chemical reactions on cell surfaces are dependent on the availability of specific functional groups on the surface molecules which can chemically react under acceptable conditions without impairing native cell functions. Since cell membranes display a diverse range of molecules, researchers have covalently modified the functional groups present in these molecules to attach specific molecules and structures. Furthermore, chemical reactions on the cell surface should be selective without any nonspecific modifications under guided cell-compatible conditions which are feasible in an aqueous environment.
2.2.1 Direct Chemical Modification of Cell Membrane
Selection of chemical reactions to covalently modify a cell membrane is dependent on the availability of a specific functional group which can react under defined conditions. The presence of proteins, carbohydrates, and lipids on cell membranes offers multiple sites to external reagents which can react to form a covalent linkage. However, based on feasibility and practical methods of modification, only a few selectable functional groups on cell membranes have been used for chemical reactions and conjugation. Most popular chemical targets include amines, sulfhydryl, carboxyl, and carbonyl groups present in proteins and other cell surface molecules. Proteins, with an unique composition and sequence of amino acids, on cell surfaces provide a rich environment for these functional groups. Although many amino acids do not have functional groups available for external chemical modifications, several amino acids possess these functional moieties which have been chemically modified on cell surfaces.
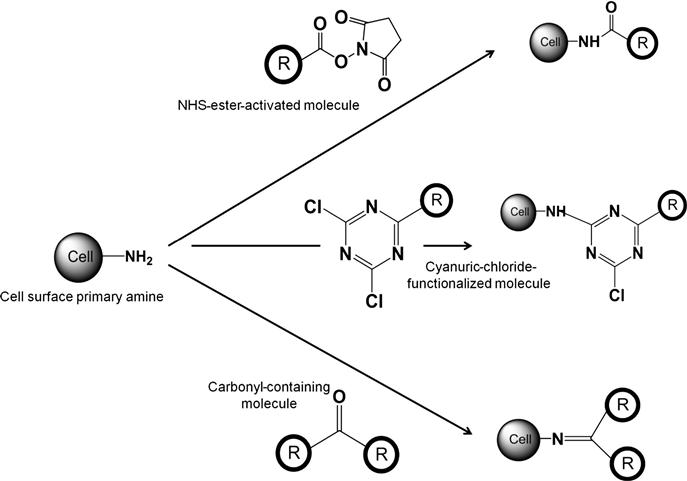
Amine groups, predominantly primary amines, are widely used for chemical modification of cell membranes due to well-established protocols, easily available conjugation linkers (which can conjugate cell membrane groups to the external molecules), and cell-compatible reaction conditions. Figure 2.1 schematically shows the chemical reactions which are primarily used for covalent transformation of primary amine groups on the cell surface. The most common reaction to modify primary amines involves reaction with N-hydroxysuccinimide (NHS) ester. NHS-ester-activated compounds react with primary amines in slightly alkaline conditions (pH 7.2–8.5) to form amide bonds with release of NHS which can be removed easily by dialysis or desalting. Since NHS-ester cross-linking reactions are usually performed in phosphate buffer at pH 7.2–8.0 for 0.5–4 h at room temperature or 4°C, this reaction has been used extensively to covalently modify primary amines on the cell surface. To improve the solubility of NHS-ester molecules in aqueous medium, sulfo-NHS-esters are used which are identical to NHS-esters except that they contain a sulfonate (![]() SO3) group on the NHS ring. This charged group has no effect on the reaction chemistry, but it increases the water solubility of the reaction reagent. In particular, the charged group prevents sulfo-NHS compounds from permeating cell membranes, enabling them for cell surface chemical modifications.
SO3) group on the NHS ring. This charged group has no effect on the reaction chemistry, but it increases the water solubility of the reaction reagent. In particular, the charged group prevents sulfo-NHS compounds from permeating cell membranes, enabling them for cell surface chemical modifications.
This chemical reaction has been used to covalently conjugate biotin on the cell membranes by using NHS-ester-activated biotin. Commercial availability of NHS-biotin and sulfo-NHS-biotin has enabled extensive use of this approach to covalently biotinylate the cell surface which can subsequently be functionalized through (strept)avidin. Biotin–streptavidin bridges on the cell surface allow immobilization of different functional molecules and enables this approach as a platform technology for covalent modification of cell membranes [13,14]. Since the biotin–streptavidin linkage is exceptionally strong and stable, this is an effective way to attach a multitude of molecules for adding new functionalities to cells. For example, this approach has been used to functionalize mesenchymal stem cells (MSCs) where cells were covalently biotinylated with NHS-activated biotin followed by addition of streptavidin and subsequent addition of biotinylated functional molecules. Using this approach, Sarkar et al. [15] immobilized Sialyl Lewis X (SLeX), a functional moiety expressed by leukocytes for their rolling response on activated endothelium, on MSCs and demonstrated that SLeX modified MSCs exhibited the rolling response on activated endothelium both in vitro and in vivo. Most importantly, this covalent modification was stable and did not induce any adverse effect on MSC functioning including their ability to adhere, differentiate, or secrete paracrine factors. The generic nature of this approach was further substantiated when similar covalent modifications of MSCs were used to immobilize platelet-derived growth factor (PDGF) sensing aptamer (single-stranded oligonucleotides) and modified MSCs were able to sense PDGF in a spatiotemporal manner [16]. Use of the NHS-biotin approach has also been applied to conjugate antibodies, quantum dots, and nanoparticles to the cell surfaces [17,18]. Cell surface functionalization, particularly covalent labeling of primary amines in cell surface proteins, by NHS-biotin has also been used as an analytical tool for enrichment and protein profiling studies [19,20]. This shows the wide-ranging utility for covalent functionalization of cell membranes with NHS-biotin.
Although use of NHS-biotin has gained acceptance due to well-established biotin–(strept)avidin conjugation technology, several other NHS-ester-activated molecules have been used for covalent modification of cells. NHS-activated small molecule, for example, 4-pentynoic acid, has been used to functionalize cells with alkyne groups which enable performing click chemistry on the cell membrane [21]. Similarly, NHS-activated macromolecules are developed for covalent functionalization of cells. In particular, NHS-activated polyethylene glycol (PEG) has been used to link PEG for several applications [22]. This approach has been used to functionalize islet cells, and no change in cell morphology and function was observed after PEG was covalently immobilized. Furthermore, NHS-PEG-modified islets were transplanted into the liver which showed transient normalization of blood glucose [23]. However, use of PEG is limited in its ability to link to therapeutically relevant molecules due to lack of functionalities which reduces the effectiveness in a wide range of applications [24]. This limitation can be addressed by using bifunctional PEG where one group specifically interacts with functional groups on the cell membrane while the other group is available for functionalization with an external reagent. To achieve this, amine groups on the cell surface were reacted with the linker molecule NHS-PEG-maleimide where NHS reacts with amines on the cell surface, leaves maleimide to react with a thiol group, the target molecule. This process allowed conjugating target peptides on the cell surface which induced interaction with E-selectin on endothelial cells for enhanced cell rolling [25]. However, a potential concern in this approach remains because cell surface thiols can also react to maleimide groups which can be within the same cell or in between multiple cells (leading to cross-linking). If this is the case, availability of second functional groups of bifunctional PEG is limited for further conjugation. This potential problem can be addressed by using multifunctional PEG where hyperbranched PEG with multiple functionalities can be conjugated to the cell membrane. Although it was not hyperbranched PEG, Chapanian et al. [24] have proposed a method of chemical conjugation to the cell surface using hyperbranched polyglycerol (HPG) molecules. The rationale for this approach was that while HPG and PEG have similar biocompatibility, HPG eliminates some of the shortcomings of PEG due to its functionality. In this study, red blood cells (RBCs) were decorated with HPG. The carboxyl groups were activated by addition of NHS, and the molecule was subsequently attached to the cell surface through reaction with the exposed amines of proteins present on the surface [24]. In addition to conjugation of small molecules and synthetic macromolecules, NHS-ester-based reactions have been used to conjugate biomolecules. For example, single-stranded DNA (ssDNA) was conjugated to cells by preparing NHS-DNA conjugates through reaction of NHS-PEG-maleimide and thiolated DNA [26]. Reaction of cells with NHS-DNA enabled conjugation of ssDNA to the cell surface through a reaction between NHS and cell surface amine groups. Using this approach, cellular assembly and cell–cell interaction were examined. This covalent immobilization is generic as it can be performed on any cells under cell-compatible conditions without eliciting any adverse reactions.
Although NHS-ester-based modification of primary amines is widely used for covalent modification of cell membranes, there are other reaction mechanisms which have been explored to modify cell surface amine groups. In this category, reaction of cell surface amines with cyanuric chloride is most prominent. Cyanuric-chloride-activated molecules have been used to covalently modify cell membranes due to their malleable chemistry, the efficacy in membrane protein modification, the chemical stability of the modified proteins, and the feasible reaction conditions [27]. Using this strategy, blood cells, mainly RBCs, are covalently modified with PEG [28]. Conditions for this covalent modification have been optimized with respect to pH, temperature, and reagent concentration. Using covalently modified RBC with PEG provides a tool to create “universal blood” by camouflaging the immune system as PEG provides a protective shell around the RBC that potentially excludes large molecules, such as antibodies, but does not appear to inhibit the passage of small molecules, such as glucose and oxygen [29,30]. Another strategy to modify cell surface amine group is through reaction with carbonyl functionality resulting in formation of Schiff’s base. This approach has been primarily investigated by reacting a carbonyl-containing small molecule drug, tucaresol, with primary amine groups on T-cell surfaces to understand the immune response of T cells [31]. Reaction between cell surface amine and carbonyl functionality resulted in imine bond (Schiff’s base) which mimicked the formation of Schiff bases between constitutive carbonyl and amino groups expressed by macromolecules at the surface of the T cell. However, this approach has not been explored as a platform technique for cell surface modification.
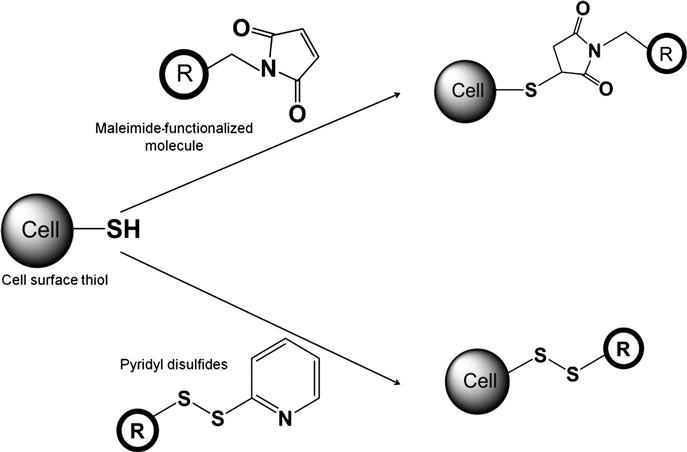
The second most important functionality on cell surfaces used for covalent modification is the sulfhydryl groups, that is, thiols. Cell surface thiols are present either in oxidized disulfide bridges or in reduced thiol groups. Common covalent reactions for cell surface thiol modification are schematically shown in Figure 2.2. Most widely used covalent reactions involve maleimide-activated molecules which specifically react with thiol groups (![]() SH) at near neutral conditions (pH 6.5–7.5) to form stable thioether linkages. These reactions are typically carried out at ambient temperature or at 37°C for 90–120 min and in serum-free media, typically phosphate buffer saline, to avoid any cross-reaction with thiol-containing compounds in the media. Maleimide-based covalent modification has been used for covalent immobilization of molecules and different structures, such as nanoparticles. For example, maleimide-functionalized dye, Alexa488, has been covalently linked to the surface of KG1a cells through covalent reaction which shows specific immobilization of the dye on the cell membrane [32]. In contrast, control dye with inactivated maleimide did not produce any signal indicating the specificity of this reaction. This approach has also been used to covalently conjugate macromolecules. For example, maleimide-functionalized PEG is used for covalent functionalization of RBCs to develop universal blood [33]. Maleimide-based covalent mechanisms have also been employed for covalent immobilization of nanoparticles on cell surfaces. Stephan et al. [34] have developed a method of nanoparticle conjugation in which the exposed free thiols of surface proteins on T cells were utilized. The nanoparticles are 100–300 nm in diameter and have a phospholipid surface layer which features maleimide headgroups available for reaction with the thiols on cell surface. This chemical conjugation is completed through a simple two-step process beginning with incubation of the nanoparticles with the cells to allow for maleimide–thiol bonding, and subsequent exposure of the nanoparticles to PEG molecules containing a thiol end to eliminate any remaining functional groups on the nanoparticle surface. Through this method, the nanoparticles can be attached to the cell at a count of approximately 140±30 per cell without affecting the functionality of the cell [34]. This approach enabled sustained delivery of therapeutic molecules to control the immune function. In addition to maleimide-based covalent linking, other functional groups reactive to surface thiols are bromoacetamide and dithiopyridyl functionalities which can covalently bind to cell surface thiols [35]. This study also indicated that the reactivity of these reagents and maleimide-based compounds, particularly in neutral pH, was in the order of maleimide→dithiopyridyl→bromoacetamide (based on their reaction with mercaptoethanol) which can probably be extrapolated to analyze the relative potency of these reagents to cell surface thiols. Furthermore, it is important to note a delicate balance exists between the oxidized and reduced form of thiols depending on the redox environment [36,37]. Based on this microenvironmental condition, exchange between these two forms can lead to the formation of covalent linkages.
SH) at near neutral conditions (pH 6.5–7.5) to form stable thioether linkages. These reactions are typically carried out at ambient temperature or at 37°C for 90–120 min and in serum-free media, typically phosphate buffer saline, to avoid any cross-reaction with thiol-containing compounds in the media. Maleimide-based covalent modification has been used for covalent immobilization of molecules and different structures, such as nanoparticles. For example, maleimide-functionalized dye, Alexa488, has been covalently linked to the surface of KG1a cells through covalent reaction which shows specific immobilization of the dye on the cell membrane [32]. In contrast, control dye with inactivated maleimide did not produce any signal indicating the specificity of this reaction. This approach has also been used to covalently conjugate macromolecules. For example, maleimide-functionalized PEG is used for covalent functionalization of RBCs to develop universal blood [33]. Maleimide-based covalent mechanisms have also been employed for covalent immobilization of nanoparticles on cell surfaces. Stephan et al. [34] have developed a method of nanoparticle conjugation in which the exposed free thiols of surface proteins on T cells were utilized. The nanoparticles are 100–300 nm in diameter and have a phospholipid surface layer which features maleimide headgroups available for reaction with the thiols on cell surface. This chemical conjugation is completed through a simple two-step process beginning with incubation of the nanoparticles with the cells to allow for maleimide–thiol bonding, and subsequent exposure of the nanoparticles to PEG molecules containing a thiol end to eliminate any remaining functional groups on the nanoparticle surface. Through this method, the nanoparticles can be attached to the cell at a count of approximately 140±30 per cell without affecting the functionality of the cell [34]. This approach enabled sustained delivery of therapeutic molecules to control the immune function. In addition to maleimide-based covalent linking, other functional groups reactive to surface thiols are bromoacetamide and dithiopyridyl functionalities which can covalently bind to cell surface thiols [35]. This study also indicated that the reactivity of these reagents and maleimide-based compounds, particularly in neutral pH, was in the order of maleimide→dithiopyridyl→bromoacetamide (based on their reaction with mercaptoethanol) which can probably be extrapolated to analyze the relative potency of these reagents to cell surface thiols. Furthermore, it is important to note a delicate balance exists between the oxidized and reduced form of thiols depending on the redox environment [36,37]. Based on this microenvironmental condition, exchange between these two forms can lead to the formation of covalent linkages.
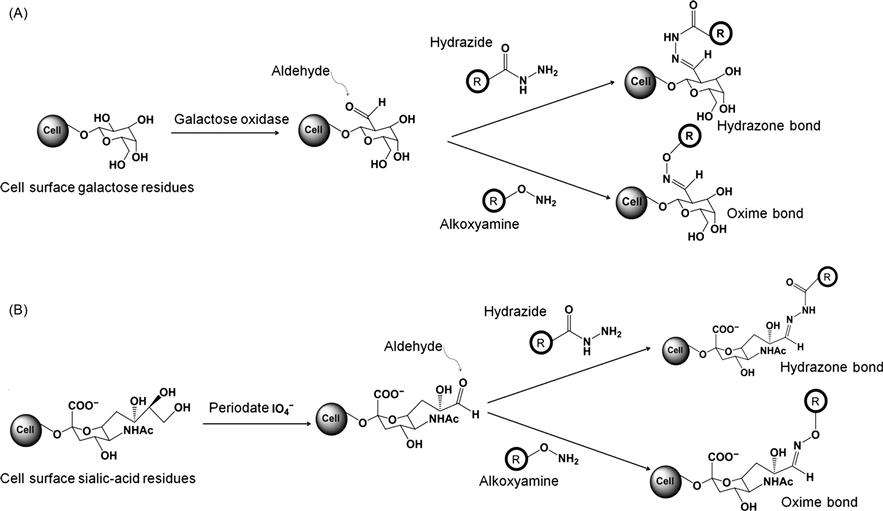
Although amine and thiol-based cell surface modification has been used extensively for cell surface modifications, cell surface carbonyls from aldehydes and ketones can also be covalently functionalized. Carbonyl groups can react with hydrazides at pH 5–7, typically carried out in phosphate buffer saline and at ambient temperature or 37°C, resulting in formation of hydrazone bonds. Similarly, under the same reaction conditions, carbonyl groups can react with alkoxy amines to form oxime bonds. However, the limited presence of carbonyl groups on cell surfaces requires an additional step through which carbonyls groups can be enhanced in enough surface concentration for covalent modification [38,39]. This process usually involves oxidation of diols in polysaccharides and glycoproteins which is either chemically mediated through periodate or enzymatically mediated through galactose oxidase. Carbonyl groups induced to the cell surface through the oxidation can react with hydrazide-activated molecules to form covalent adducts through hydrazine bond formation or with alkoxy-amine-activated molecules to form covalent adducts through oxime bond formation. This process was utilized to fluorescently label cells where hydrazide-modified dyes were allowed to react with the cell after oxidation which allowed covalent immobilization of dyes on the cell membrane [40]. Similar approaches have also been used to biotinylate cell surfaces by using hydrazide-activated biotin [41]. Apart from small molecule conjugation, researchers have used this strategy to covalently link nanoparticles to cell surfaces. Particularly, sialic acid residues of macrophage surfaces were oxidized with periodate to introduce aldehydes which were covalently conjugated to amine-functionalized polyamidoamine (PAMAM) and quantum dots [42]. With this approach, a targeted cell-based drug delivery system was developed. In addition to hydrazides, alkoxyamine-activated molecules can also react to the carbonyls to covalently label the cell membrane through formation of oxime bonds, for example, biotin was immobilized on the cell surface via oxime bond following oxidation of glycoprotein [43]. Most importantly, this covalent conjugation method represents a feasible chemical approach to detect cell surface glycoproteins for biomolecular tools in glycoproteomics [44]. Thus, covalent modification of the cell surface through aldehyde and ketone-based reactions is a practical approach for conjugation of molecules to the cell surface. Figure 2.3 schematically summarizes these covalent reactions on the cell surface. Since carbonyl groups are not readily available on cell surfaces and it requires an additional oxidation step to introduce the carbonyl groups, this approach is not extensively used for cell surface modification. In particular, oxidation of the cell even under mild condition can induce an adverse effect on cells [45].
Direct chemical reaction of cell surface molecules is primarily centered on these functional groups: amine, thiol, and oxidation-induced carbonyls. Relatively simple reaction techniques performed under cell-compatible conditions, high selectivity, and minimal reaction steps are the primary reasons for the use of these approaches for direct covalent modification of cell membranes.
2.2.2 Indirect Chemical Modification of Cell Membrane
Although cell membranes represent a rich repertoire of chemical groups, only a few functionalities are available for direct covalent reactions under feasible conditions. This has limited the use of the covalent modification technique as a platform technology to immobilize wide ranges of molecules and functionalities. Direct covalent modification is dependent on the development of functionalized targets which can specifically react to cell surface functionalities. Thus to enhance the capability of covalent modification on cell surfaces, approaches are adopted where functionalities are introduced on cell surfaces through a noncovalent mechanism followed by a covalent reaction. Although, in a strict sense, this approach is not to be considered as a covalent modification of the cell membrane, the postmodification step is a covalent reaction and often enables performing a wide range of covalent chemistries on cell surfaces.
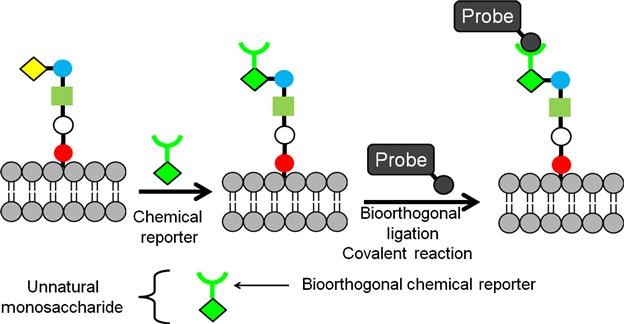
This indirect chemical modification strategy has been studied and explored in detail by the Bertozzi group which is schematically presented in Figure 2.4 [46]. In this approach, chemical functionality is introduced on the cell surface through a biosynthetic or a metabolic pathway. First, a chemically reactive moiety is incorporated into target glycans by metabolic labeling with an unnatural monosaccharide substrate. This chemically reactive group is known as the “chemical reporter.” In the second step, the reporter group is covalently reacted with a target molecule through bioorthogonal ligation. Functionalities like ketones and azide groups are introduced through this strategy by biosynthesis of unnatural sialic acid (Figure 2.5) [47,48]. The ketone group was introduced by using a mannosamine analog derivatized with a levulinoyl side chain (ManLev) which transformed into the corresponding cell surface sialoside in human cells. This ketone group can undergo selective reaction with aminooxy, hydrazide, or related functional groups to form stable covalent adducts (Figure 2.5). As a result, cell surfaces can be covalently modified with an epitope of interest generated by chemical synthesis. At least 107 ketones on a single cell surface were achieved, offering significant potential for remodeling cells for specific cell–surface interactions [49]. This approach was further extended by introducing azide groups to cell surfaces using N-azidoacetylmannosamine. The presence of azide groups was verified through reaction of the azide group with a phosphine group and alkynes (Figure 2.5). Azides were ligated with triaryl phosphine using Staudinger reaction through formation of a stable amide bond. This approach was tested through attaching biotin to the cell surface by using biotinylated phosphine. Additionally, azides were covalently modified with linear alkynes via Cu-catalyzed azide–alkyne cycloaddition and with a variety of cyclooctynes via the strain-promoted azide–alkyne cycloaddition (also termed “Cu-free click chemistry”) [50–52]. Since this cycloaddition avoids the usage of toxic metal catalysts, Cu-free click chemistry is more acceptable as a cell-compatible reaction. This biosynthetic metabolic labeling of cells is a versatile route to present chemical functionalities which are otherwise not present on the cell surface in appreciable surface concentrations for covalent modification. Additionally, this approach is highly chemoselective and can be performed in cell-compatible conditions without inducing any changes to the surrounding environment. Similar to this strategy, another approach involves enzymatic transformation of the cell surface proteins to introduce functional molecules for covalent transformation of the cell surface, for example, posttranslational modification of acyl carrier protein (ACP) on the cell surface by phosphopantetheine transferase (PPTase) transfer 4′-phosphopantetheine from co-enzyme A (CoA) to a serine residue of ACP [53,54]. By using several CoA derivatives, it was possible to functionalize cells with chemically diverse compounds such as fluorophores, affinity ligands, or quantum dots [54]. Immobilizing functional groups on cell surfaces through these strategies can provide tools for sensing and imaging, understanding cellular interactions, and enabling new molecular engineering tools for synthetic biology.
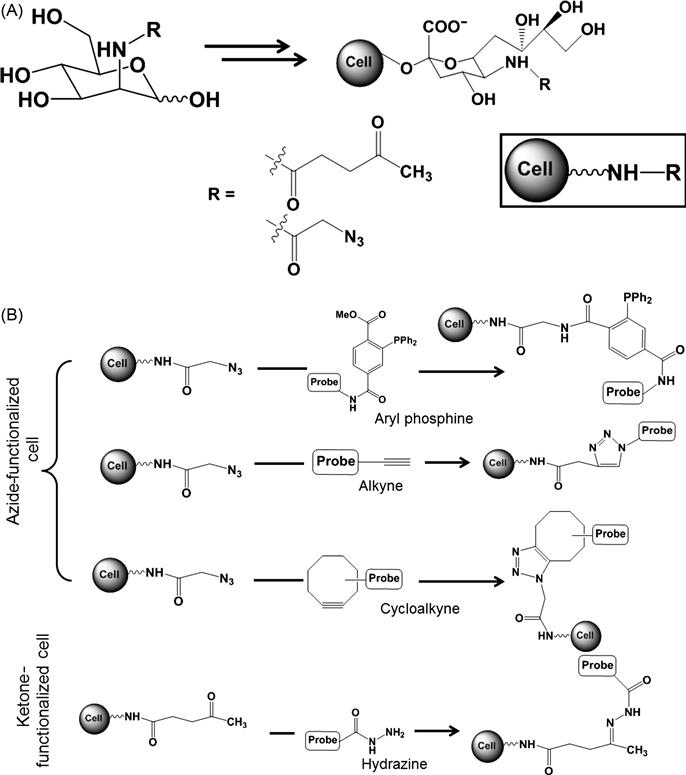
In addition to the introduction of functional groups by biosynthetic mechanism, functional groups can be introduced to cell membranes through physical methods for subsequent covalent cross-linking. By using this approach, ketone functionalities were physically introduced to the cell surface through vesicle-based delivery by spontaneous fusion of lipid to the cell membrane. Physically immobilized ketone was subsequently reacted to oxyamine for covalent modification, for example, covalent reaction of oxyamine-functionalized dye with ketone-functionalized cells resulted in dye conjugated cells [55]. This strategy is also useful for promoting cellular assembly where covalent reactions between ketone-functionalized cells and oxyamine-functionalized cells promoted three-dimensional cellular aggregations [8].
Thus, indirect covalent modification to the cell membrane via noncovalent approaches has significant importance. This approach is particularly relevant when appropriate functional groups, which can participate in direct covalent modification, are not available on the cell surface.
2.3 Relevance of Covalent Cell Surface Modification
Modification of cell membranes using covalent strategies is extensively used to decorate cell surfaces with target molecules, both synthetic and biological, nanoparticle probes, and different structures. These target molecules can be small molecules, for example, biotin, pharmaceutical drugs, fluorescent probes, or they can be macromolecules including synthetic polymers, such as PEG or polyvinyl alcohol. Biological macromolecules like proteins, antibodies, aptamers, are also attached to cell surfaces to impart unique biofunctionalities to cells [25,56]. Structural moieties such as nanoparticles and quantum dots are immobilized on the cell for delivery of therapeutic molecules to the cell and noninvasive tracking of cells, respectively [16,34,57]. Choice of reaction depends on the type of target and the feasibility of a particular reaction mechanism to achieve the conjugation under cell-compatible conditions with high selectivity. Furthermore, site density, distribution, mobility, and stability of the covalently attached molecules are dependent on the reaction mechanism. Most importantly, these modifications should be able to fulfill the functional requirements of the cell for a given application with precise control.
In the previous sections, the specific use of covalent cell modifications is mentioned in the context of particular approaches. For example, human MSCs covalently modified by a Sialyl Lewis X through biotin–streptavidin were able to target the selectins in activated endothelium [15]; conjugation of aptamer on human MSC membranes were able to sense the PDGF [16]; and nanoparticles conjugated to T-cell surfaces influenced cellular function through controlled delivery of drugs [34]. These examples demonstrate that the covalent remodeling of cell membranes has significant therapeutic applicability and is potentially useful for interrogating fundamental properties of cells both in vitro and in vivo. Specific applications of cell surface modifications and detailed analysis of these technologies are mentioned in other chapters. In general, covalent cell surface engineering has been used for a significant period of time for various applications and purposes. Molecular biology and immunology of cells are studied by selectively conjugating molecules to the cell surface. Pharmaceutical and toxicological effects of synthetic molecules and their mechanistic pathways of action are also elucidated by using covalent cell surface modification approaches. From an analytical perspective, cell surface conjugation is traditionally used to label cells for imaging and sensing purposes. Thus, covalent cell surface modification is an established technology with multifaceted goals. However, the use of these approaches has seen a significant surge in recent years for developing novel tools and technologies which can be used for therapeutic and diagnostic purposes.
In particular, covalent modification of cells allows controlling their interactions with the extracellular matrix and neighboring cells for regenerative applications in tissue engineering. Furthermore, this approach has enabled targeted delivery of cells to appropriate tissue microenvironments and also the ability to sense the cellular niche in a spatiotemporal manner. This approach has significant impact on developing cell-based therapies where cells are systemically delivered to a particular site. Most importantly, chemically functionalized cells, following their localization, can exert controlled cellular responses that are observed therapeutically and diagnostically. Controlling cell fate and functioning by redesigning the cell membrane with defined functional molecules and structures will continue to impact on fundamental science and will provide improved biomedical technologies for several applications.
2.4 Future Perspectives
With increased use of this covalent cell surface modification technique, it is important to understand the impact and usefulness of this strategy for a given purpose. Most studies indicate that covalent modification has minimal impact on cells, but it is important to examine its long-term effect in terms of toxicity and its potential to induce malignant features. Furthermore, localization and stability of covalently immobilized molecules and their fate should be critically investigated. These features vary significantly depending on the modification technique and the nature of the immobilized molecule. For example, cells modified with PEG-NHS are only stable for 48 h [22], whereas cells modified with streptavidin through covalent conjugation of biotin to cell surface showed enhanced stability up to 7 days [13]. Although long-term effects in terms of cell viability and functions were not detected, it is critical to monitor the cells for a longer period to maximize the efficiency of covalent cell surface modification. Thus, selection of proper modification techniques and optimization of strategies are crucial to ensure the availability and accessibility of the immobilized molecule for any given application. In the long term, it is important to characterize if the ligand is internalized or shredded by the cells and how cell division can change the density of functionalized ligand. Achieving proper spatial and temporal presentation of the ligands on the cell membrane is crucial for any application, particularly for therapeutic applications. Therefore, every covalent modification strategy needs careful examination to understand these features which are critical to ensure the effectiveness of this approach. Particularly, use of covalent cell engineering for in vivo and clinical applications requires proper study to ensure safety and higher efficacy. Finally, using cell surface modification in situ and in response to environmental stimulus can provide unique avenues to control cell functioning, both in physiological and disease conditions.
2.5 Conclusions
Cell surface modification is a powerful tool to modify the molecular composition of cell surfaces. Development of novel chemistries and material science tools has enabled the alteration of the cell surface through covalent modification techniques for a variety of applications. In particular, cell surfaces can be directly modified through covalent reactions if specific functional groups are present and can be accessed by external reagents under cell-compatible conditions. Alternately, covalent modifications can be performed following the noncovalent introduction of functional groups. Depending on the application, each reaction strategy needs optimization and complete investigation to ensure effective functionalization. In the future, development of novel molecules and conjugation strategies should be able to combine chemical mechanisms with biomolecular approaches to provide new avenues for controlling cell function and fate.