Probe and Control of Cell–Cell Interactions Using Bioengineered Tools
Feng Li1, 2, Rangoli Aeran1, Egest J. Pone1, Dong-Ku Kang1, Linan Liu1, X. Chris Le2 and Weian Zhao1, 1Department of Pharmaceutical Sciences, Chao and Family Comprehensive Cancer Center, Sue and Bill Gross Stem Cell Research Center, Edwards Lifesciences Center for Advanced Cardiovascular Technology, Department of Biomedical Engineering, University of California, Irvine, CA, USA, 2Department of Laboratory Medicine and Pathology, University of Alberta, Edmonton, AB, Canada
Understanding how cells interact and communicate with each other in multicellular organisms is essential to understanding diverse life and biological processes. However, the complicated nature of cells, their various in vivo microenvironments, and the multitude of changing environmental and biological signals that confront cells make it challenging to probe and control cell–cell interaction and communication using conventional macro-scale and population-averaged tools. With the rapid advances in bioengineering, we now have ever finer tools to peek increasingly closely at cellular processes, including the interaction and communication of cells with each other. In this chapter, we discuss established as well as emerging micro-, nano-, and molecular-scale genetic, chemical, and engineering tools designed to address remaining challenges in cell–cell interactions and communication. Microengineering tools, such as microfluidic chips, microwells, and microdroplets, make possible the manipulation of the spatial and temporal distribution of individual cells or defined homotypic and heterotypic cell clusters, thus simplifying the complicated in vivo microenvironments into well-controlled in vitro platforms. Molecular tools based on genetic or chemical approaches, frequently utilizing fluorescent or luminescent reporter signals, are being used at increased resolution to interrogate target cells. Moreover, with advances in complementary imaging systems, such as two-photon imaging, it is now possible to probe cell–cell interactions in their in vivo microenvironments. The knowledge derived from these studies is expected to not only increase the information content on various biological processes but also reveal novel paradigms of cellular and physiological processes, thereby also helping in the design of novel strategies to diagnose and treat diseases.
Keywords
Cell–cell interactions; cell–cell communications; single cell assays; microfluidics; cell engineering; cell surface sensors
15.1 Introduction
Cell–cell interactions broadly comprise the exchange of signals between various cells of multicellular organisms that underpin the diverse biological processes and responses [1,2]. The benefits of having groups of specialized cells working together for a supreme purpose—the survival, growth and reproduction of the organism—required continuous evolution of, on the one hand ever more specialized cells, and on the other, a more complex and robust means [3] of ensuring their cooperation with each other by means of communication through cellular and molecular signals [4]. The fundamental mechanism that ensures appropriate cooperation of individual cells and cell types is cellular communication: just like individuals within various groups each perform their specialized functions for common, overarching goals, so do cells of metazoa. Appropriate real-time communication ensures that each cell performs the function that most benefits the survival of the entire organism. This usually includes homeostasis and reproduction of individual cells (to replace aging and dying ones). Conversely, aberrant cell–cell communication is thought to give rise to devastating diseases such as cancer [5] (runaway cell proliferation) or autoimmunity [6] (immune attack on own healthy cells). Thus, understanding cell–cell communication is essential for understanding life and developing therapeutics.
Cellular interactions can be guided by physical contact between cells [7], by various secreted extracellular vesicles known as microvesicles and exosomes carrying protein or nucleic acid messengers [8,9], by soluble factors secreted by cells to act locally or distantly [1,10], electrical charges and pulses [11], etc. Physical cell–cell interactions comprise receptor–ligand pairs that tether cells together and to their underlying matrix. Besides giving rise to characteristic tissue tensile strength [12], such close cell–cell interactions ensure that some cell types can interact with each other by exchanging molecular messages through gaps in their membranes, referred to as gap junctions. These are permanent physical connections that provide a free passageway for ions and small molecules between adjacent cells but prevent the flow of larger proteins and nucleic acids [5,13]. As a result, they couple cellular metabolic status, signal transduction state, or electrical responses. Gap junctions are thought to be numerous and important particularly to cells of the same type within a tissue. In effect, a response initiated in one cell can spread to neighboring cells to synchronize their responses, as happens with calcium waves in cardiomyocytes during heart beating. Moreover, unlike most cells of a tissue that never migrate for their entire lifetime, cells of the immune system are motile, and therefore communicate primarily not through gap junctions but through receptor–ligand pairs [7]. The receptor and ligand could be expressed on the membranes (particularly on the plasma membrane), could be soluble intracellular, or could be soluble in the extracellular environment (interstitial fluids or blood) [14]. A case in point is the characteristic migration of leukocytes from bone marrow, lymph nodes, and spleen as they undergo various phases of maturation, and their subsequent recirculation and patrolling between lymphoid organs and periphery. Upon encountering signals for the presence of pathogen or cancer (released by the pathogen or cancer itself), various immunocytes of the innate and adaptive arms of the immune system migrate to the site of infection or cancer to eradicate the threat [15,16]. Clearly, these are complex scenarios that involve the action of a multitude of conserved microbial molecules, cytokines, chemokines, lipid mediators, other signaling molecules, and immune cells must constantly communicate with each other as well as with the surrounding tissue [17]. A decrease in cell–cell communication, or worse, its breakdown as in the case of ambiguous or conflicting signals, would have catastrophic consequences for the organism, as in the case of cancer, autoimmunity, or immunodeficiency. Likewise, integration and coordination of various responses through the endocrine system is essential for proper homeostasis and adaptive responses, whereas its breakdown results in various diseases, most notably diabetes. Finally, cross-system cellular communication is also critical for homeostasis and adaptation, and its breakdown has severe consequences: a misdirected immune attack on pancreatic insulin-producing β cells results in type 1 diabetes mellitus (T1DM) whereas a misdirected immune attack on the nervous system results in diseases such as multiple sclerosis (MS) [6].
Just as erroneous cellular interaction and communication leads to disease, many treatments rely on restoring cell–cell communication. Thus, in order to understand at a fundamental level the various types of cell–cell interaction and communication, we utilize appropriate molecular and cellular tools at our disposal. Traditionally, our need to investigate cell–cell interactions and communication has been limited by approaches of low resolution, the use of bulk cell population methods that obscures individual cellular state heterogeneity, and lack of ability to manipulate individual cells and their signaling molecules [18]. However, with the advent of molecular biology techniques on the one hand and the emergence of micro- and nano-scale engineering on the other, we now possess ever finer tools to probe cell–cell interaction and communication [19,20]. In particular, these new tools allow us to elucidate and manipulate cell–cell interactions at a single cell level at an unprecedented temporal and spatial resolution both in vitro and in vivo [21,22]. In this chapter, we summarize recent and emerging micro-, nano-, and molecular tools for the investigation of different types of cell–cell interactions. We will limit our discussion to selected examples that best illustrate recent advances in the cell–cell interaction methodology, and which have applications to diseases such as cancer, those of immune dysfunction, and endocrine disorders. We will first describe the engineered micro-scale tools to pattern cells in well-defined fashion and study cell–cell interactions at cellular and subcellular levels, particularly in a 3D context. We will then describe recent advances in engineered tools at the “molecular” level, including genetic and chemical tools, used to probe and control cellular interactions with other cells as well as their surrounding microenvironments. Finally, we will conclude with future directions in which our ever-growing list of high-resolution tools both informs, and is inspired, by cellular and subcellular behavior.
15.2 In Vitro Study of Cell–Cell Interactions Using Engineered Microdevices
Although in vivo studies of cell–cell interactions are ideal in maintaining the native microenvironment during the targeted biological process, the complexity of biological environment and limitations to the current probing techniques usually hinder the accurate and quantitative understanding of cell–cell interactions. As important complementary tools for the investigation of cell–cell interactions, in vitro techniques simplify the complicated in vivo microenvironment into well-controlled experimental conditions, leading to better understanding of many cell–cell interactions. Here, we emphasize the applications of microfabricated devices, due to their advantages in terms of control over the cell spatial and temporal arrangement, as well as their relatively high sensitivity and resolution in analyzing cell–cell interactions at single cell levels, while mimicking to a great extent the in vivo microenvironments where cell interactions occur.
15.2.1 Control Over Cell Spatial and Temporal Arrangements Using Engineered Microdevices
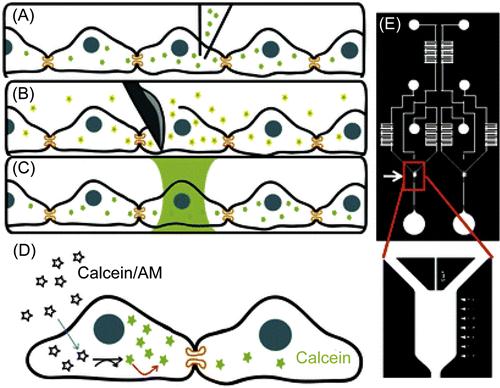
Cell–cell interactions depend on both physical contacts and soluble and particulate factors. Therefore, it is imperative to utilize devices that provide both defined spatial arrangements of each cell type and the spatial/temporal control of cellular secretions. Engineered microdevices are particularly advantageous due to their customizable size, shape, material composition, and design to introduce stimuli as well as monitor cellular responses [23,24]. Numerous microdevices have been engineered to study the roles of gap junctions in permanent cell–cell interactions [25–28]. Gap junctions are nano-scale protein channels that connect the cytosolic compartments of adjoining cells, allowing for the transfer of ions, metabolites, and other small molecules between cells below typical cutoff sizes [5,13,29]. Understanding the physiological roles of gap junctions is essential to uncover how cells synchronize and communicate signals among large cell populations; however, it remains challenging to achieve quantitative characterization of gap junctions in conventional macro-scale assays due to the lack over spatial control. To address this, Lee and coworkers [25] have developed a quantitative microfluidic assay to measure dye diffusion through gap junctions in connected cells (Figure 15.1). In this study, the microfluidic device was designed to allow cells to adhere to the surface as well as express functional gap junctions. Calcein dye was used as the diffusion tracer because its acetomethoxy (AM) form is nonfluorescent and membrane permeable, but when cleaved by intracellular esterases it becomes fluorescent and can freely move through cellular gap junctions (Figure 15.1D). To achieve spatially targeted delivery of calcein dye in the device, a noninvasive hydrodynamic focusing approach was developed to focus fluids at low Reynolds number into a column of C6 cells within a confluent cell culture chamber while leaving their neighboring cells unperturbed (Figure 15.1C). Subsequent time-lapse fluorescent microscopy was used to quantitatively monitor the dynamics of dye transfer via gap junction to neighboring cells, yielding a calculated effective diffusivity of 3.4 × 10−13 m2/s. In a similar study, Hua et al. [27] developed a tri-stream microfluidic system for multiplexed measurements of connexin-43 (Cx43) gap junctions with several dyes, including calcein AM, 5-(6)-carboxyfluorescein diacetate (CFDA), Oregon green 488, in the absence or presence of Cx43 inhibitors 1-heptanol, 2-APB, and MFA. The use of multistream laminar flow to selectively expose cultured cells with these dyes and inhibitors enabled measurement of the diffusion coefficient of the four dyes. This device could also be used to screen multiple inhibitors in parallel, demonstrating the potential of micro-engineered tools for high-throughput cell analysis.
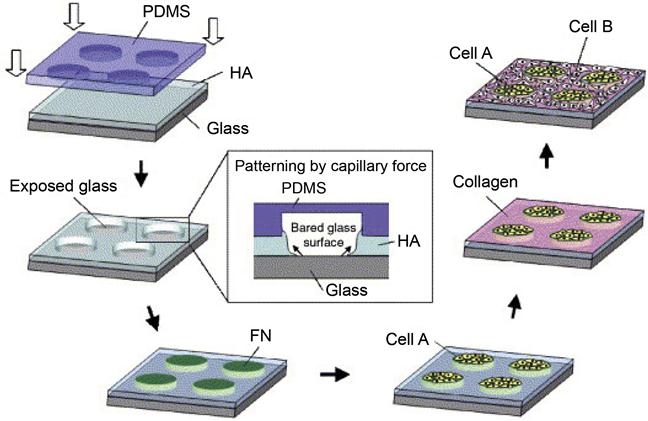
Besides studying interactions between homogeneous cell types, engineered microdevices are also suitable for studying heterotypic cell interactions by arranging different cell types in spatially defined cocultures. The precise arrangements are achieved typically by controlling the micropatterned surfaces on engineered devices [30–33]. A number of fabrication strategies, including photolithography, microcontact printing, micromodeling, inkjet printing, and dip-pen spotting, have been used to create micropatterned surfaces to localize cells to defined regions. To construct patterned cocultures, selective adhesion of one cell type is commonly utilized. For example, hepatocyte–fibroblast cocultures can be fabricated on collagen-patterned substrates by allowing hepatocytes to adhere to the patterned collagen-coated regions with fibroblasts only adhering to the non-collagen-coated regions [34]. Layer-by-layer deposition of biopolymers, such as polysaccharides and proteins, is another commonly used strategy to create such cell patterning. For example, Langer et al. [35] developed a cell coculture system by using layer-by-layer deposition of three major extracellular matrix (ECM) components: hyaluronic acid (HA), fibronectin (FN), and collagen (Figure 15.2). HA was micropatterned on a glass substrate by capillary force lithography, and the regions of exposed glass were coated with FN to generate cell adhesive islands. To achieve the coculture of multiple cells on this micropatterned substrate, murine embryonic stem (ES) cells were first cultured onto FN-coated islands, forming cell islets with 100 µm diameters. Once ES cells were immobilized on FN islands, the HA-patterned surface and the seeded ES cells were treated with collagen. This treatment switched the non-adherent HA surface to adherent, thus allowing the seeding of the second NIH-3T3 fibroblasts. Such coculture systems of ES cells with other differentiated cell types could potentially be used to study the effect of heterotypic cell–cell interactions on ES cell fate decisions. In a separate study, Jiang et al. [36] developed a method for patterning multiple types of cells on the same substrate using electrochemical desorption of self-assembled monolayers (SAMs) in a microfluidic system. Importantly, this technique allows the patterning of different types of cells without the presence of physical constraints, therefore enabling control over cell motility. This method first employed alkanethiol (EG6) to form an inert SAM on gold surface to resist protein adsorption and cell adhesion. By using a PDMS stamp with embedded microfluidic channels to selectively electrochemically desorb EG6 from the gold substrate, each cell type could be patterned on the same substrate and confined to the activated regions. Because there is no physical barrier between these cells, the free exchange of substances between these different types of cells facilitates studies of cell–cell interactions. Subsequently, an electrochemical desorption step can be applied to convert the previously “inert” surface to an “active” that allows cells to move under the influence of each other. Having established this method, the same group patterned mammalian cells on a fabricated microfluidic device for modeling three types of naturally occurring cell–cell interactions: (i) those between two types of immobilized cells, (ii) those between immobilized and freely moving cells, and (iii) those between two types of freely moving cells [37].
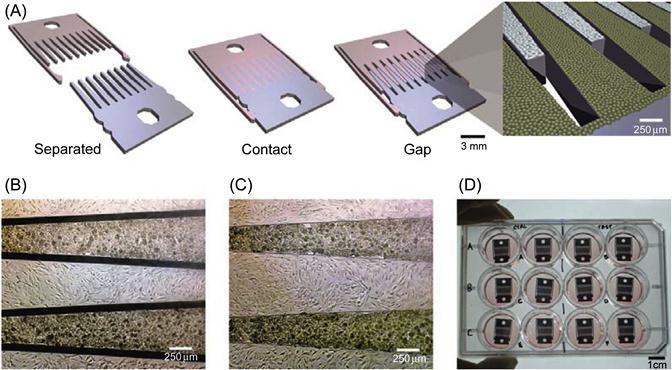
In addition to using chemical deposition to control cell patterning, microenvironments consisting of defined physical substrates have also been used to control cell–cell interactions. Typically, different cells are grown on different substrates, which can be rearranged to change the spatial organization of the culture. Therefore, such mechanically controlled microdevices allow the decoupling of cell–cell interaction processes into manipulations mimicking biological events such as cell differentiation based on physical topology and other cues. Hui and Bhatia [38] have developed such a device to study the intracellular communication between hepatocytes and supportive stromal cells in coculture. In their approach, cells were grown on an array of microfabricated plates that were physically separated or locked to change the spatial organization of the culture (Figure 15.3). By imposing a small micrometer-scale separation between the plates, cell–cell contact could be abrogated while soluble signaling is unperturbed. By increasing separation distances, the extent of soluble signaling could be further modulated. In addition, cells could be exchanged by removing one set of fingers and inserting in a replacement seeded with another cell type. Using this device, the maintenance of the hepatocellular phenotype by stroma required direct contact for a limited time followed by a sustained soluble signal that had an effective range of <400 µm. This work demonstrated the power of using a mechanical devices to investigate dynamic cell–cell interactions in a multitude of applications, spanning embryogenesis, homeostasis, and when dysregulated, pathogenic processes. Likewise, Nishizawa et al. [39] developed a mechanically controlled device to study interactions between HeLa cells and human umbilical vein endothelial cells (HUVECs). HeLa cells and HUVECs were cultured separately on two detachable substrates, and coculturing was initiated by mechanically assembling the two complementary substrates. Using this coculture system as a tumor/endothelium model, the authors found that HeLa cells migrated toward HUVECs, whereas HUVECs retreated. Additionally, when direct contact between the two cell types was prevented, HUVECs initially migrated toward the HeLa cells and then retreated. In a separate study, the same group further integrated this mechanically controlled cell culture device with a microfluidic component to control the direction of medium flows [40]. By using this integrated device, they further observed that the retreat of HUVECs only happened when medium flow was directed from the HeLa side toward the HUVEC side, suggesting that the migration of HeLa cells and HUVECs was likely mediated by soluble factors produced by HeLa cells. Indeed, increased levels of VEGF produced by HeLa cells were observed in the presence of HUVECs. The increasing levels of VEGF could attract the HUVECs, leading to the initial HUVEC migration toward HeLa cells. However, since the tumor cells also produced repulsive signals, such as reactive oxygen species (ROS), the initial HUVECs approach halted and actually turned into a retreat. The characteristics of the cell movements including the direction and speed observed from the mechanically controlled cell culture devices revealed the significant role of cell–cell communication through secreted soluble factors, frequently with contrasting activities, in the processes of tumor cell intra- and extravasations.
15.2.2 Cell Interactions Studies at Single Cell Levels Using Engineered Microdevices
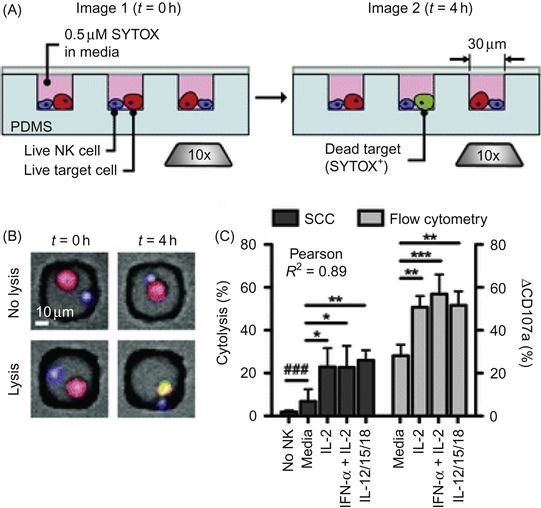
An additional advantage of using engineered microdevices to study cell–cell interactions is their adaptation for use in a high-throughput manner, i.e., to interrogate many cell interactions, one at a time, and then draw conclusions based on individual and rare events, as well as use statistical analysis to understand cellular responses at a population level. High-throughput single cell studies enabled by engineered microdevices give insights on the heterogeneity of intercellular interactions. One simple but effective approach is to segregate target cells into arrays of micro/nanoliter scaled wells [41]. One immune cell type that is amenable to high-throughput microfluidic analysis is the natural killer (NK) cell. The latter are granular lymphocytes lacking antigen receptors yet having the ability to recognize and kill tumor or virally infected cells and other non-self cells (e.g., transplanted or donor grafted cells) [42]. The efficacy of the NK cell-mediated immune response is heterogeneous with cell-to-cell variability in their cytolytic and secretory activities. To study NK cell responses at single and population levels, Guldevall et al. [43] measured immune surveillance by individual NK confined in microwell arrays. The authors designed dense arrays of microwells (100×100 square wells with 80 μm sides and a depth of 45 μm) to spatially confine NK cells, in effect allowing the analysis of many single NK cell events. Time-lapse imaging of NK cell cytotoxicity in these microwells revealed heterogeneity in the rate of 293T cell killing upon NK cell encounter, indicating heterogeneity in individual NK killing efficiency. In a separate study using a similar array of subnanoliter microwells, Love et al. [44] investigated the individual NK cell–target cell interactions and quantified the resulting cytolytic and secretory responses (Figure 15.4). As shown in Figure 15.4, NK cells and K562 target cells were co-deposited onto an array of 30 µm cubic nanowells to obtain small, isolated groups of cells (0–3 cells of each type). In these single cell cytolysis assays, measurements were performed in 1000–5000 wells per array containing exactly one NK cell and one target cell. NK cells were shown to behave with individual variations when lysing single target cells and lysis was most probable during an NK cell’s first encounter with target. Furthermore, the secretion of interferon-γ (IFN-γ), a key inflammatory immunomodulator that coordinates immune responses particularly during viral infection or cancer, occurred most often among NK cells that became the least motile upon contacting a target cell. However, the secretion of IFN-γ was unexpectedly largely independent of cytolysis. Furthermore, the frequencies of cytolytic events measured from thousands of individual NK cell–target cell pairs in the nanowells were strongly correlated with the expression levels of target cell-induced degranulation marker CD107a, as measured by flow cytometry analyses of bulk cultures (Figure 15.4C). Both cytolytic and degranulation frequencies are increased upon stimulation by exogenously added cytokines including IL-2, IFN-α plus IL-2, or a combination of IL-12, IL-15, and IL-18. This study represents a good example of how integrated analysis of cell–cell interaction parameters, cytolytic activity, and secretory activity enabled by micro-engineered tools can reveal new insights into the correlations of these complex functions in individual cells.
Microfluidic devices can also be engineered to trap single cells and study single cell interactions using hydrodynamic trappings. For example, Lee et al. developed a microfluidic device for selective trapping of cell pairs. Specifically, their microfluidic channel contains a single pair of trapping sites that are separated by 20 µm on opposite sides. Since the mouse fibroblast cell line used in their study averaged 12 µm in diameter and were rather homogenous in size distribution, the channel was wide enough to allow cells to flow through but narrow enough to ensure cell membrane contact when the cell pairs were trapped across from each other. This device enabled monitoring of cell–cell communications through gap junctions using the calcein transfer system as described previously [45]. Moreover, the same group developed a coculture device that can maintain and track single cell pairwise interactions [28]. As shown in Figure 15.5(A), by combining hydrodynamic trapping with a semi-isolated chamber that is accessible to continuous medium supplementation, this device can efficiently isolate cell pairs using minimal physical restraints to study cell migration, proliferation, and cell–cell interactions through multiple generations. Specifically, the first cell is trapped at the trapping junction, and thereafter fluidic resistance is increased such that subsequent cells will pass by the filled chamber due to self-variable fluidic resistance. Subsequently, the trapped cell migrates and opens the trapping junction, enabling the loading of second cells. Using this system, the intercellular communication between mouse embryonic stem cells (mESCs) and mouse embryonic fibroblast cells (MEFs) was investigated. Two distinctive cellular behaviors in migration and proliferation emerged: (i) the paired cells’ migration patterns depended on their initial cell distance, and (ii) heterotypic pairing led to distinct proliferation patterns compared to homotypic, single cell cultures. Microfluidic-based hydrodynamic trapping also provides a powerful approach for cell–cell fusion that is important, for example, for the generation of hybridomas and somatic cell reprogramming. For example, Voldman et al. [46] developed a device to achieve cell paring and fusion using an array of weir-based passive hydrodynamic cell traps on a microfluidic platform. Specifically, thousands of PDMS cell traps were densely arrayed within a flow-through channel (Figure 15.5B). Each cell trap consisted of a weir structure that extended vertically into the channel and contained front-side and back-side capture cups. Using a unique three-step loading protocol, cells were trapped individually once they entered the capturing cup. In this manner, different cell types, including fibroblasts, mESCs, and myeloma cells, were paired with efficiencies up to 70%. Furthermore, this device is compatible with both chemical and electrical fusion protocols, providing flexible cell fusions with high efficiency of 89%, or a fivefold increase compared to available commercial electrofusion chambers.
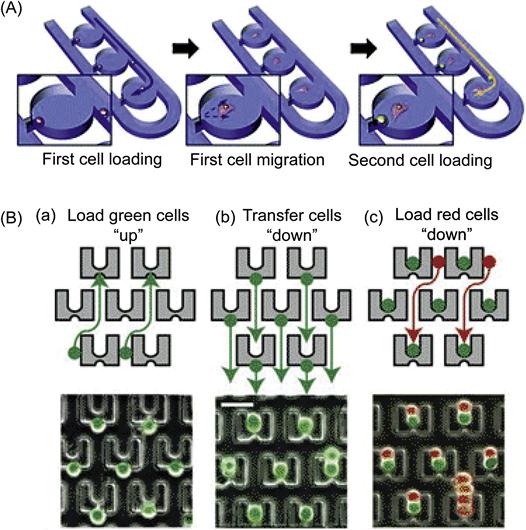
In addition to microwell devices, microdroplet devices have also been used to study cell–cell interactions at single cell levels. Yarmush et al. [47] recently developed a device for monitoring interactions between T cells and dendritic cells (DCs) at single cell levels. Specifically, monodisperse nanoliter droplets that encapsulated individual cell pairs were generated through a nozzle formed with three inlet microchannels containing an oil phase, an aqueous suspension of naïve T cells, and an aqueous suspension of LPS-stimulated DCs, respectively. Tubulin marker assays were performed in each droplet to monitor the polymerized tubulin in live cells. This system allowed monitoring the remodeling of the cytoskeleton and microtubule polymerization in DCs at the immunological synapse (IS) formed between co-encapsulated pairs of DCs and T cells. Importantly, it was revealed that not all DC and T cells were able to interact and create IS, demonstrating heterogeneity in cell–cell interaction at a single cell level that may be missed in traditional bulk population measurements.
15.2.3 Mimicking the In Vivo Microenvironment for Cellular Interactions Using Engineered Microdevices
Cells integrate and respond to cues in their in vivo microenvironments, including cell organizations, contacts with neighboring cells, soluble factors, as well as mechanics of the surrounding fluid and ECM. In cancer, in particular, the microenvironment, or the niche, has been shown to play critical roles in cancer development, metastasis, and drug resistance. Therefore, microdevices that offer 3D controls over multiple cues of cancer microenvironment have attracted great attention to exploit cell–cell interactions under a more physiologically relevant condition [48–53]. For example, to examine the role of tumor–endothelial cell interactions during cancer metastasis, Zervantonakis et al. [54] developed an in vitro 3D microfluidic model of the tumor–vascular interface that integrates live imaging, precise control of microenvironmental factors, and endothelial barrier measurement. In this system, the relationship between tumor cell intravasation and endothelial activation and permeability as a function of cytokines present was shown to also depend on paracrine signaling involving macrophages interacting with tumor cells. As shown in Figure 15.6, this microdevice consisted of two independently addressable microchannels where tumor and endothelial cells are seeded. The two channels were connected through a 3D ECM hydrogel. The tumor cells invaded in 3D hydrogel in response to externally applied growth factor gradients or paracrine signals produced by the endothelial cells or macrophages. Macrophages were shown to regulate endothelial barrier function and paradoxically increase tumor cell intravasation through secreted tumor necrosis factor alpha (TNF-α).
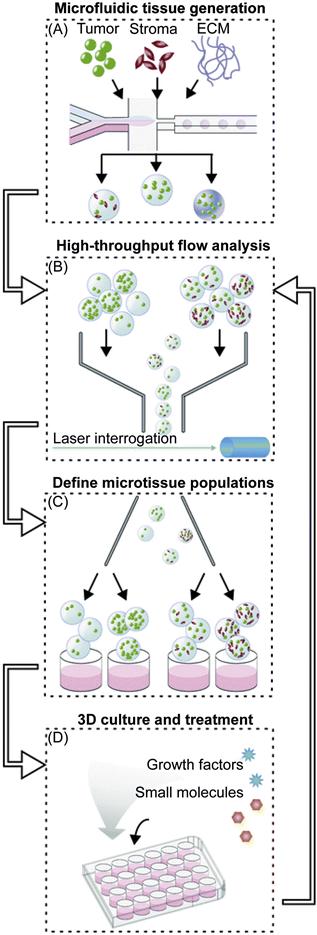
In a related approach, the Bhatia group developed a microfluidic platform for high-throughput analysis of microtissues that allows systematic probing of tumor microenvironmental cues, including ECM, soluble factors, and stromal cells, in a 3D context [55]. As shown in Figure 15.7, the workflow could be divided into three phases. First, fluorescently labeled tumor cells were encapsulated with the desired combination of stromal cells or ECM into synthetic 3D microtissue using a droplet microfluidic device (Figure 15.7A). In the second phase, a cytometry-like flow analyzer was used to sort out defined populations of freshly generated microtissues by various parameters, including cell proliferation and cell density (Figure 15.7C). This system therefore allowed selection of ECM molecules and soluble factors that lead to a range of cell proliferation profiles. In the third phase, sorted microtissues were cultured in the presence of drugs to examine tumor response in a 3D-defined niche. Importantly, TGFβ, a benchmark immunomodulatory factor used in their study, had an antiproliferative effect that is unique to the 3D tumor microtissue compared to the 2D monolayer culture. Such 3D microtissues that more closely resemble in vivo systems compared to the traditional 2D monolayer cultures are therefore advantageous in studying new and emerging anticancer drugs and approaches.
15.3 Probing and Manipulation of Cell–Cell Interactions Using Engineered Molecular Tools
In addition to above-mentioned microfabricated tools, new genetic approaches allow the study of cells at unprecedented temporal and spatial resolution. In cell therapy, in particular, engineering of cells with molecular sensors allows tracking and monitoring of transplanted cells to elucidate cell–cell and cell–niche interactions in vivo using imaging techniques such as intravital two-photon fluorescent microscopy (IVM). Furthermore, the ability to engineer novel and enhanced cell–cell interactions makes it possible to manipulate and control the fate and function (e.g., homing) of transplanted cells. In this section, we will summarize the recent advances in genetically and chemically engineered tools that have opened up possibilities to explore and control cell–cell interactions in vitro and in vivo.
15.3.1 Genetically Encoded Proteins for Probing Cell–Cell Interactions
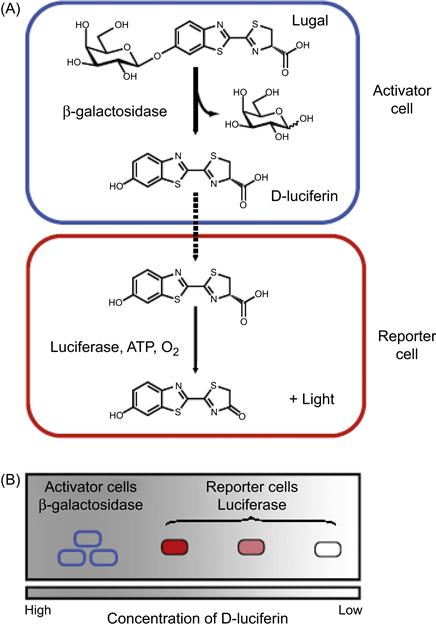
Genetic engineering, the direct manipulation of the genetic composition of cells and organisms, is a powerful technique to generate reporters for probing cell functions and interactions in vivo. By genetically engineering cells with fluorogenic or catalytic proteins, cells can be modified to produce optical signals inside a living system in controlled, frequently inducible, manner. Along with powerful in vivo optical imaging systems such as IVM, cell migration, trafficking, biodistribution, and cell–cell interactions can now be monitored in real time in vivo. Through these approaches, previously unknown intercellular interactions have been revealed with prominent examples including stem cell homing, stem cell–tumor cell interactions [56], immune cell–tumor cell interactions, immune cell interactions with each other or with stromal cells, endothelial cells or pathogens [57]. In particular, cell pairs can be engineered with fluorescent proteins or luciferase to report signals only upon cell–cell communication or if interactions occur, therefore minimizing nonspecific signals from complex biological systems. For example, Prescher et al. [57] recently developed a proximity reporter strategy that produced luminescent signals only when two different types of cells are physically in proximity to each other in vivo. As shown in Figure 15.8, the proximity reporter strategy was achieved by genetically engineering target cell pairs with β-galactosidase (β-gal) and luciferase to generate “activator cells” and “reporter cells,” respectively. “Caged” luciferins (lugal), i.e., a luciferin molecule attached to a steric appendage that precludes the binding of the molecule to luciferase, were used as substrates that are released only upon β-gal activity. Next, intracellular enzymes catalyze the cleavage of Lugal to release luciferin, which diffuses near neighboring reporter cells and is processed by the luciferase to produce bioluminescence. Thus, reporter cells closest to activator cells would consume the most substrate, resulting in a correlation between the signal intensity and reporter cell and activator cell proximity. This proximity reporter system was used to illuminate metastatic lesions and tumor–immune cell interactions in vivo. Specifically, mouse bone marrow from β-gal expressing transgenic mice was transplanted into irradiated immunodeficient mice. Three weeks following bone marrow transplantation, mouse breast carcinoma 4T1-luc2 reporter tumor cells were orthotopically allografted into the mammary fat pads. Metastatic sites were visualized after injection of Lugal and the resulting bioluminescence as described earlier. Potential sites of metastatic–immune cell interactions, especially at submandibular lymph nodes, could be observed in a living animal. This represents a good example in the use of genetically engineered tools to visualize cellular proximity and interactions in a variety of biological settings in the minimally perturbed in vivo microenvironments, and thus confirming their potential to better understand physiological processes such as immune responses or stem cell niche development.
15.3.2 Cell Surface Aptamer Sensors to Probe Cell–Cell Communications
Dynamic cell–cell interactions within an in vivo microenvironment largely rely on communications through ligand–receptor interactions at cell surface. The ability of chemical modifications to alter cell surfaces with synthetic ligands and sensors opens up a new venue to probe cell–cell interactions and communications. In this section, we describe representative approaches that make use of chemical engineering strategies to facilitate the study of cell interactions for emerging biological processes such as stem cell trafficking and chemical transmitter dynamics.
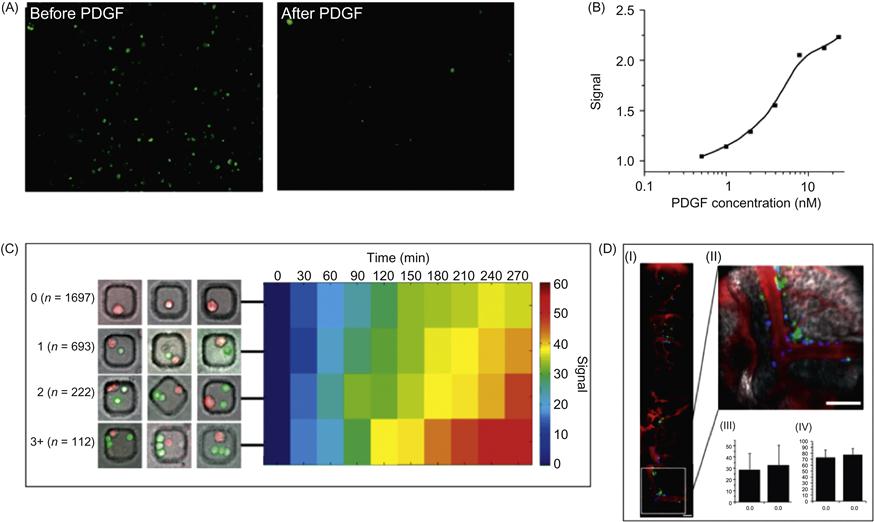
Although the ability to explore cellular interactions and communication is essential for understanding stem cell trafficking in vivo, it remains challenging to monitor the interaction of cells in their natural environments and niches in real time. To overcome these technical challenges, Zhao et al. [22] developed fluorescent cell surface sensors that quantitatively detect surrounding soluble factors with high spatial and temporal resolution (Figures 15.9 and 15.10). The sensor consists of a single strand DNA oligonucleotide aptamer modified with a pair of quenchable fluorescent dyes, frequently referred to as molecular beacons (Figure 15.9B and C). In the presence of the target analyte platelet-derived growth factor (PDGF), the aptamer binds to PDGF, triggering conformational changes that bring the two attached dyes in close proximity, thereby yielding a real-time fluorescent signal. This PDGF sensor was first attached on the mesenchymal stem cell (MSC) cell membrane by treating cell surface amines with sulfonated biotinyl-N-hydroxy-succinimide (NHS-Biotin), followed by streptavidin–biotin conjugation (Figure 15.9D). The engineered sensor MSCs are highly specific and able to quantitatively detect low local concentrations of PDGF that is either added into the medium or, more importantly, secreted by neighboring cells (Figure 15.10A–C). These cell surface sensors have unmatched spatiotemporal resolution at cellular and subcellular dimensions, opening new avenues to study cell–cell interactions by locally acting, i.e., autocrine, paracrine, or juxtacrine signaling factors. Furthermore, the engineered sensor MSCs retain their ability to home to the bone marrow and can be monitored in vivo at the single cell level using IVM (Figure 15.10D). The cell surface sensor approach makes use of functional aptamer sensors that dynamically monitor intercellular signaling in real time and therefore represents a new tool to elucidate unaddressed cell biology questions in the native cellular microenvironment.
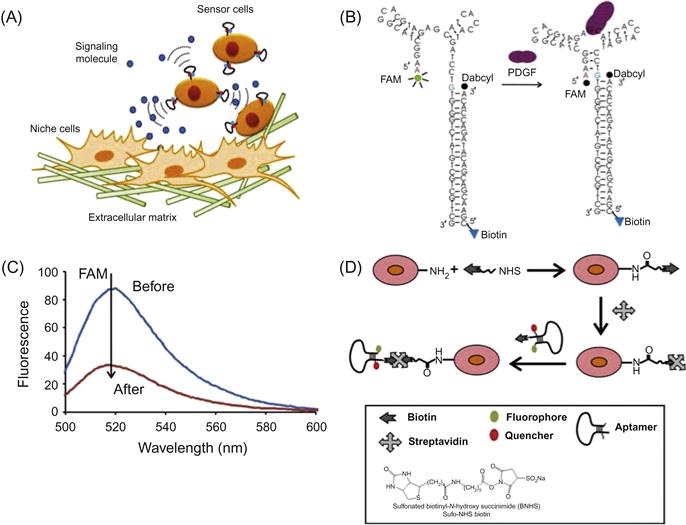
In a related approach, Tokunaga et al. [58] used a fluorescent aptamer immobilized on the surface of brain astrocytes to monitor in real time the release of adenosine triphosphate (ATP) as a gliotransmitter. The structure-switching fluorescent aptamer was conjugated to astrocyte cell surface through a tocopherol anchor which is a nontoxic lipophilic molecule and incorporates directly to cellular membranes. Upon mechanical stimulation of astrocytes, fluorescence could be monitored in real time, giving novel insights on the action of ATP as a neurotransmitter. Importantly, ATP release was synchronized with calcium waves in neighboring cells, confirming the unique utility of chemically engineered sensor cells in monitoring intercellular communications.
15.3.3 Chemical Modification of the Cell Surfaces with Adhesion Molecules to Control Cell–Cell Interactions
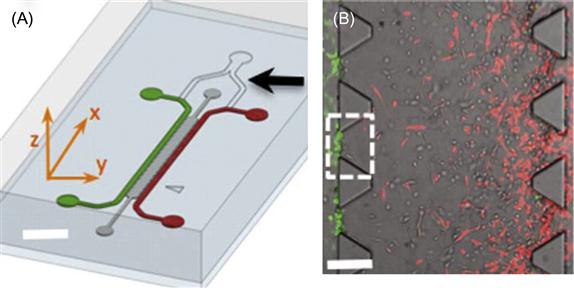
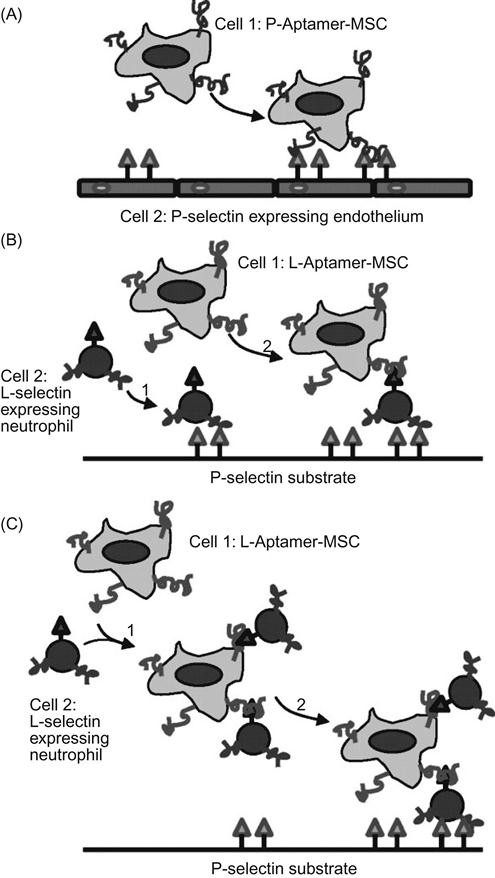
In addition to probes for cellular microenvironments and communications, cell surfaces engineered with exogenous ligands can alter cellular interactions with homotypic or heterotypic cells and the surrounding extracellular scaffolds. In cell-based therapies, in particular, one of the greatest challenges is to deliver modified cells to the injured sites in way that is minimally invasive and does not perturb normal host functions, especially immune functions [59,60]. For example, the inefficient homing of injected MSC is thought to be a major limitation to existing MSC-based therapy. In contrast, leukocytes can home efficiently to site of inflammation using a variety of homing and adhesion molecules. By mimicking this leukocyte homing cascade, Karp and coworkers chemically engineered MSCs with Sialyl LewisX (SLeX), a cell rolling ligand that is involved in leukocyte rolling and homing. They demonstrated that SLeX modification promotes a robust cell rolling response both in vitro on a P-selectin-coated dish and on inflamed endothelium in vivo [61]. In addition to naturally occurring ligands, synthetic adhesion molecules, including aptamers, have also been exploited to control the interactions between MSCs and endothelial cells. Our laboratory has developed a simple approach to study the interactions of MSCs with endothelial cells under dynamic flow conditions using cell surface-attached DNA aptamers [62]. MSCs were chemically engineered with P- or L-selectin aptamers using the NHS-Biotin protocol. As shown in Figure 15.11, aptamer-displaying MSC can replicate the three major types of cell–cell interactions under dynamic flow condition found in the leukocyte homing cascade including: (i) flowing P-selectin aptamer MSC tethers to adherent P-selectin expressing endothelial cells, (ii) flowing L-selectin aptamer MSC tethers to L-selectin expressing leukocyte that is first tethered on a P-selectin substrate, and (iii) flowing L-selectin aptamer MSC and L-selectin expressing leukocyte first complex in the flowing stream and then tether to a P-selectin-coated substrate. The concept of engineering specific cell–cell interactions by chemically engineering cell surface ligands has potential applications in cell delivery, tissue engineering, and construction of in vitro microtissues to study cellular interactions. Likewise, Tan et al. [63] showed that chemical modification of cell surfaces with artificial aptamer-lipid receptors could be used to alter cellular functions, including protein binding, enzymatic activity, and intercellular interactions.
15.4 Conclusions and Perspectives
Appropriate intercellular interactions and communication are essential to homeostasis and recovery, whereas erroneous signals would have catastrophic consequences for the organism, as in the case of cancer, diabetes, autoimmunity, or immunodeficiency. Traditional approaches that investigate parameters based on cell population averages are gradually being supplanted by emerging approaches with higher spatial and temporal resolution that take into account and can distinguish the heterogeneity of cellular and molecular systems. With recent advances in various fronts of the natural sciences, our tools for studying and manipulating life at the organismal, systems, cellular, subcellular, and molecular levels have sharpened considerably. This has enabled ever greater insight and control over cellular behavior, including cell–cell interactions and communication.
In particular, microengineering advances in the emerging field of biological microfluidics have greatly enhanced our ability to peer into the world of intercellular interactions in controlled and defined microenvironments that closely mimic their in vivo counterparts [19,20]. In addition, enhanced genetic, chemical, and micro- and nanotechnological tools have enabled the measurement of various membrane and soluble signals during homotypic and heterotypic intercellular interactions and communication. For example, the interactions of different types of immune cells with each other [64] or with pathogens [65] and cancer cells [66,67], the reciprocal communication of neural cells [68] with heterotypic cells, or the interaction of stem cells with immune cells and stressed cells during repair of tissue injury [69,70] are now being analyzed at cellular and subcellular resolution in defined in vitro, as well as in vivo, microenvironments. In particular, the micro-engineered tools and molecular-engineered tools are complementary and can be used in combination to explore cell–cell interactions. For example, cells that have been molecular engineered with fluorescent proteins have been applied to microdevices to generate signals in situ for real-time analysis of cell interactions [55]. Furthermore, micro-engineered microwell devices have been used to evaluate chemically modified sensor MSCs before injecting those cells into living systems [22]. Importantly, cells are not only being studied using advanced methods, but their own unique properties, including their sensing of external stimuli, communication with other cells and trafficking and homing to defined microenvironments—in short their adaptive behaviors—are in turn making possible their use as robust and customizable platforms for applications ranging from biological research to diagnostic biosensors and therapeutic delivery agents [21].
Challenges remain toward more clearly and comprehensively understanding cell–cell interactions. First, there are many types of signals that make up intercellular interactions and communications, including physical contact, soluble factors, electrical signal transmission, and mechanical cues. Therefore, better micro- and molecular-engineered tools that can precisely isolate or sense specific and a combination of signals will be required. As we have described in previous sections, several techniques have begun to show their potential in this endeavor. For example, the mechanically detachable microdevices are able to distinguish interactions through soluble factors from physical contacts [38]. Second, cell–cell interactions are highly dynamic in vivo, thus requiring better molecular-engineered tools to capture the dynamics of cell–cell interactions [71]. Genetic and chemical modifications are advantageous to light up target cells with fluorescence or luminescence; however, it will be beneficial to generate interaction-specific signals that can monitor dynamic biological and pathological processes in real time. The “caged” luciferin-based proximity reporter assay represents such a trend [57]. Third, for in vivo applications, novel nanomaterials have been synthesized and demonstrated to be useful in numerous in vivo imaging techniques, including MRI, ultrasound, PET, and optical imaging [72]. By chemically modifying target cells with novel nanomaterials, it is possible to further expand the current in vivo imaging techniques to probe cell–cell interactions in vivo.
In conclusion, in the coming years, it is anticipated that advances on several fronts—micro- and nano-scale engineering and fluidics, genetic and chemical approaches—will not only continue to enhance the resolution and information content of biological experiments but likely will reveal novel paradigms of cellular interactions and communication. For example, novel genetically encoded reporters, such as robust ratiometric genetically encoded calcium indicators (GECIs) [73], will likely give unique and novel time-resolved information on the in vivo interaction and communication of immune cells with each other and other cell types during immune responses. Similarly, novel indicators used to map neuronal interactions will greatly enhance our understanding of the nervous system and its interaction with other physiological systems and the environment [74]. Likewise, advances in stem cell research will likely merge genetic and materials engineering approaches to control the complex, multistep intercellular interactions and communication giving rise to tissue and organ formation for regenerative medicine. Synthetic biology is also expected to benefit from, as well as inform, the above-mentioned trends in experimental biology, bioengineering, and nanobiotechnology [75,76]. Advances in the related field of systems biology are likely to play an increasingly active and important role in uncovering previously unknown or obscure links between diverse and seemingly unrelated cellular and physiological processes [77]. Coupled to these advances in fundamental experimental biology and bioengineered tools, it is anticipated that our ability to diagnose, and therefore treat, diseases caused by aberrant intercellular interactions and communication, including cancer, immune, and endocrine disorders, will also continue to increase in efficacy and selectivity.
