Cell Engineering with Nanoparticles for Cell Imaging
David C. Yeo, Riddhima Minocha and Chenjie Xu, Division of Bioengineering, School of Chemical and Biomedical Engineering, Nanyang Technological University, Singapore
Genetic engineering of cells is a popular approach to create permanent labels for cells (e.g., the green fluorescent protein). However, it is limited by labeling efficiency, technical complexity, and crucially, the risk of incorporating random mutations which makes them unsuitable in the clinic. Nanoparticle (NP) engineering, an alternative to genetic engineering, is similarly able to incorporate imaging labels on cells at a high efficiency while being less detrimental to the cell. This chapter gives an overview of work accomplished in the burgeoning field of NP engineering for cell imaging purposes. The most popular imaging modalities are described, before efforts in engineering cells for imaging using NPs are described. Crucial to NP engineering are cell internalization, surface membrane functionalization, and extracellular matrix modification approaches with their distinct advantages and limitations. For NP engineered cells to have a greater chance at acceptance in the clinic, NP engineering methods cannot interfere with cell implant function, must maintain sufficiently strong contrast for longitudinal tracking, and report on implanted cell function. This will enable a greater amount of information to be accumulated regarding in vivo behavior of implanted cells, thereby reducing eventual trial costs.
Keywords
Nanoparticles; imaging; cell internalization; cell surface functionalization; extracellular matrix functionalization; noninvasive monitoring
11.1 Introduction
The cell is generally considered as the basic building block of life. Understanding its structure, spatial location, and function is critical in revealing biological and physiological phenomena such as growth, proliferation, regeneration, cancer, and death. Since the first observations made by Van Leeuwenhoek and Hooke in the seventeenth century, advances in cellular biology have become closely intertwined with that of imaging technology [1]. Whereas compound microscopes were state of the art 300 years ago, the field of imaging has vastly expanded to encompass a wide range of imaging modalities such as fluorescence, luminescence, Raman, magnetic resonance, radionuclide, (photo)-acoustic, and X-ray based imaging [2]. This multitude of imaging techniques, with their specific advantages and limitations, have since been utilized in biology and medicine to study both biological and physiological phenomena from singular cellular units to whole organisms.
To be visualized and distinguished from the background by an imaging modality, cells of interest have to generate a contrast signal. Although some cells may generate endogenous contrast agents (e.g., the metabolic cofactor, nicotinamide adenine dinucleotide or NADH) [3–5], such biophysical phenomena is comparatively uncommon. Instead, a more reliable method is to engineer cells to produce detectable signals. Currently, the most widely used and reproducible approach is centered upon genetic engineering of cells with fluorescent proteins [6,7]. Proximity-based imaging probes (e.g., Förster resonance energy transfer, FRET) which rely on donor and acceptor fluorescent proteins are yet another technique commonly adopted to decipher cell signaling dynamics [8–10]. The major limitation of these methods is the usage of genomic modification to express fluorescent proteins within cells. Not only are gene transfection techniques considerably inefficient (e.g., 5–10% transfection rate for human embryonic stem cells [11]), they are also unsuitable for clinical usage since cells with random mutation sites might be detrimental to patient health [12].
An alternative way to engineering cells for imaging is to utilize nanoparticles (NPs) that exhibit unique optical, magnetic, or electronic properties due to their basic structural units, grains, particle fibers or other constituent components <100 nm in one or more dimensions. Unlike genetic engineering, NP-based cell engineering does not require the alteration of cellular genotype. Following 20 years of investigation, cells of interest can be engineered with NPs through one of the following strategies: intracellular internalization [13,14], functionalization of the plasma membrane [15,16], and the modification of extracellular matrix (ECM) [14,17–19]. The most common modification strategy, intracellular particle internalization has already been adopted in the clinic (e.g., the labeling and tracking of dendritic cells) [20,21], this preference may be attributed to their ease in usage and high labeling efficiency (>97% cells are labeled using NPs with cationic surface modification [22,23]).
This chapter starts with a brief overview of major imaging modalities for cell imaging and their most relevant NP-based contrast agents. Next, the focus switches to describe relevant strategies to engineer cells with NPs, namely: intracellular internalization of NPs, NP functionalization of the plasma membrane, and ECM NP immobilization. A schematic of cell engineering methods as well as detection methods discussed in the book chapter is provided in Figure 11.1. Their usage, especially for cell tracking in vivo is highlighted. Finally, the challenges during the translation from bench to bedside using NP engineering will be discussed.
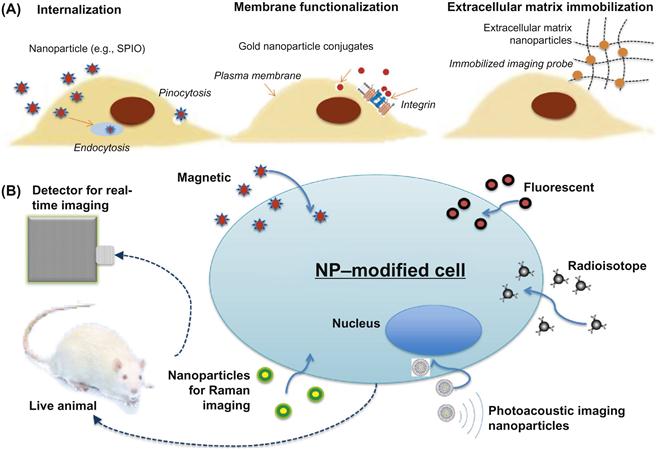
11.2 Imaging Modalities for NP Engineered Cells
The most common imaging modalities for cell imaging in laboratory are fluorescence imaging, Raman imaging, and photoacoustic imaging. While these give high imaging resolution, they are less amenable for imaging in deeper tissue locations. Instead, modalities like magnetic resonance and radionuclide contrast can be applied for whole body clinical applications.
Fluorescence imaging modalities covered in this chapter include epifluorescence, confocal, and multiphoton microscopy. Commonly used NPs for fluorescence imaging encompass quantum dots (QDs), upconversion NPs, nanodiamonds, silica NPs, graphene QDs, and fluorescent-labeled NPs [24]. QDs are semiconductor NPs typically comprised of materials such as cadmium selenide (CdSe) and germanium. Upconversion is a process where sequential absorption of two or more photons leads to light emission at shorter wavelengths—anti-Stokes nonlinear emission behavior, aiding higher sensitivity and penetration depths. Nanodiamonds are biologically inert with low exocytosis activity and possess higher fluorescence lifetimes compared to organic fluorophores [14]. Silica, an extremely versatile material for NP fabrication, has been used as biocompatible shells for reducing cytotoxicity of many substances, such as QDs [24].
Raman imaging is based on red-shifted (Stokes) and blue-shifted (anti-Stokes) light scattering. Its advantages lie in its specificity (narrow peaks), allowing Raman signals to be easily differentiated from autofluorescence. On the other hand, Raman signals are considerably weaker, 12–14 orders of magnitude weaker than fluorescent signals. The introduction of single-walled carbon nanotubes (SWNTs), graphene, gold (Au) NPs, and silver NPs significantly enhances Raman signals [24].
Photoacoustic detection is based on optical excitation and ultrasonic detection, typically using pulsed nanosecond laser excitation and acoustic impulse response detection. Unlike other optical imaging methods, photoacoustic can achieve up to 7 cm imaging depth due to the low acoustic scattering in tissue (approximately 103 fold lower than optical scattering) [25]. Of NPs that enable photoacoustic detection: dielectric core Au-coated nano shells, Au nanorods, and Au nanocages have been commonly applied. Silicate matrices also afford a greater range of flexibility in particle fabrication by incorporating sensor dyes, imaging contrast agents, drugs, and cell targeting ligands [26].
MRI is the most popular modality for cellular imaging in large animals and humans due to its superior temporal and spatial contrast, absence of harmful ionizing radiation, and unlimited penetration depth. Magnetic contrast can be generated through the exchange of water protons with gadolinium (Gd3+) resulting in T1—nuclear spin–lattice relaxation time, also known as “positive contrast.” On the other hand, paramagnetic agents generate T2—spin–spin relaxation time or “negative contrast.” NPs used in MRI as contrast agents include magnetic NPs (such as iron oxide NPs (IONPs)), NPs containing Gd3+, and micro-sized particles containing magnetic NPs or Gd3+ [12]. It should be noted that seven IONP-based products have been approved for clinical usage. Despite this success, some clinically approved cell labeling products (e.g., Feridex IV) have ceased operations, impeding the reliable supply of clinical grade cell imaging agents [20,27].
11.3 Strategies for Cell Engineering with NPs
11.3.1 Intracellular Internalization of NPs
Cellular internalization of NPs is an endocytosis process, which is closely related with several parameters such as (i) size, (ii) surface coating, and (iii) labeling condition of particles. The usage of different particle sizes for cell labeling can affect the manner in which cells are internalized. Cellular mechanisms responsible for internationalization of micro/nanoparticles include: (i) phagocytosis—“cell eating” (particles in the range of 50 nm–4 μm [28]); (ii) macropinocytosis (particles are bigger than 200 nm [29]); and (iii) clathrin/caveolae pit endocytosis (particles are smaller than 200 nm [29])—“cell drinking” [30]. To ensure high intracellular loading capacity, NPs typically are 200 nm in size for better cellular retention [31]. Furthermore, shape is another consideration in particle design with macrophages favoring ellipsoidal shapes over spherical ones during attachment and uptake. The endocytosis capacity of cells can be further enhanced by incorporating ligand targeting/binding modifications on cell membrane receptors, as well as cationic polymers such as poly-L-lysine [22,23,31]. Other parameters to optimize cell internalization include varying parameters such as NP concentration, serum concentration, and labeling time [32].
Upon NP internalization, cells can be visualized through relevant imaging modalities. For example, using fluorescence imaging (the most prevalent imaging modality due to high resolution (single cell imaging resolution), low cost, and availability), lung stem cells were labeled with 100 nm fluorescent nanodiamonds (FNDs) to monitor their engraftment in both injured and uninjured lung alveoli following transplantation [14]. Promisingly, labeled cells were still detected 15 days after labeling. Nanoflares (Au NPs functionalized with a dense layer of fluorescent emitting aptamers) have also been utilized in live cell applications for studying cell signaling pathways [33,34]. These nanostructures consist of a gold NP functionalized with a high concentration of oligonucleotides (~8.4 pmol/cm2) hybridized to fluorophores. These aptamer nanoflares were developed to detect physiological changes in ATP levels and readily entered cells. The detected fluorescent signal levels correlated closely with changes of the intracellular ATP levels [33].
Similar to fluorescence imaging, the burgeoning popularity of photoacoustic and Raman imaging modalities can also be improved through NP internalization [24]. For example, SWNTs have been conjugated with protamine (SWNT–PEG–PRO) to enable efficient cellular uptake and detection using Raman imaging [35]. Crucially, the addition of arginine-enriched protamine enhanced the uptake of SWNTs in stem cells. Subsequent incorporation of metallic catalysts facilitated enhanced magnetic and photoacoustic contrast [35]. This versatility of SWNTs as labeling agents in stem cells was further exploited by Gambhir et al., fabricating optical dye-doped SWNTs that enhanced photoacoustic contrast 100-fold. This enhancement improved the sensitivity during the detection of αVβ3 positive tumors in mouse models [36].
Being the most popular imaging modality for large animals and humans [37], MRI has received plenty of interest for the design and application of contrast agents. To facilitate the potential translation from bench to bedside, most of the current cell labeling uses iron oxide-based magnetic NPs ranging from ultra-small (35 nm) to micron dimensions. The surface of those NPs is often functionalized with targeting ligands (e.g., antibody [38,39], polycationic polymer [40,41], viral capsid [42–44]) for improved uptake. One early study used superparamagnetic iron oxide (SPIO) NPs to label dendritic cells ex vivo. This strategy relied upon the ability of dendritic cells to endocytose SPIO NPs. This modality was as sensitive as radionuclide 111In agents with a greater tracking accuracy in a melanoma patient [45]. In another recent report, three FDA approved substances (heparin, protamine, and ferumoxytol—carbohydrate-coated SPIO) were combined to form self-assembled NPs (~200 nm) that act as clinically relevant cell tracers. The individual particle constituents were incubated within serum-free media and allowed to self-assemble and label the respective cell types. These contrast agents were considerably inert and did not affect functional behavior (viability, proliferation, freeze thaw, migration, and differentiation) to any significant degree. Crucially, as few as 103 cells could be detected by T2 weighted MRI, when implanted into a rat brain [46].
Although IONPs are more commonly studied, it should be brought to the reader’s attention that non-IONPs like gadolinium oxide NPs are generating great interest as labeling agents for MRI imaging. Although SPIOs primarily generate T2 contrast, they induce hypo-T1 intensity that can be misinterpreted as blood clots or hemorrhages. On the other hand, Gd3+ paramagnetic contrast agents typically generate increased T1 signal intensity through the exchange of water protons with the Gd3+ containing complex which reduces this confusion. Recent advances in the implementation of T1 contrast agents include the generation of a gadolinium hexanedione NPs with greater than three times accumulation and greater than or equal to twofold contrast in human mesenchymal stem cells (MSCs) compared to commercial gadolinium labeling agents. In addition, by conjugating folate receptor targeting ligands, the in vitro and in vivo detection of tumor cells can be achieved using T1 contrast imaging [47]. NP internalization methods to enable cell imaging are widely used and will likely be mainstays for cell imaging in the coming years.
11.3.2 Cell Membrane Functionalization with NPs
The second strategy for engineering cells with NPs is to label the cell plasma membrane with NPs. The cell surface contains a multitude of biological moieties that can be utilized as recognition sites for modification. In one recent case, mouse embryonic stem cells (mESCs) were engineered by labeling the plasma membrane with poly-lactic-glycolic acid (PLGA) NPs conjugated with an antibody for the oligosaccharide antigen stage-specific embryonic antigen 1 (SSEA-1). PLGA NPs eluted leukemia inhibitory factor (LIF) cytokine to support long-term embryonic stem cell renewal [16]. Karp et al. also utilized the covalent attachment of fluorescent aptamer (fApt) sensors on the MSC surface for the recognition of platelet-derived growth factor (PDGF) in vivo. Unlike other imaging agents that emit a passive signal, this novel “cell surface” sensor facilitated the monitoring of intercellular signaling and increased expression when homing to PDGF rich sites [15]. Based on similar principles, fApts immobilized on glia cell surfaces were able to monitor extracellular adenine (Ade)—a crucial gliotransmitter. Remarkably, the choice of fApt was able to achieve rapid (10 s) response time in sensing proximal Ade transmission dynamics, a significant improvement over existing techniques, albeit not at the order of milliseconds demonstrated in physiological neurotransmission [48].
A surface energy transfer (SET) “nanoruler” principle was used to measure the distance of cell membrane receptor protein tyrosine kinase (PTK7) receptor and their ligand binding sites. This system comprised of an antibody-coupled fluorophore and an aptamer–Au NP conjugate. By employing varying sizes of Au NPs, different signal emissions were recorded, providing information of the distance between the antibody binding site and the aptamer binding sites [49]. Using similar NP engineering principles, cell surface features can be measured using Au NP-labeled surface receptors. To detect direct interactions of surface receptors on live cells, 40 nm Au NPs were conjugated using fibronectin to integrins expressed on the plasma membrane of HeLa cells. The authors suggested that plasmon coupling between individual Au NPs could be used to examine dynamic or transient interactions below the diffraction limit [50].
11.3.3 ECM Modifications
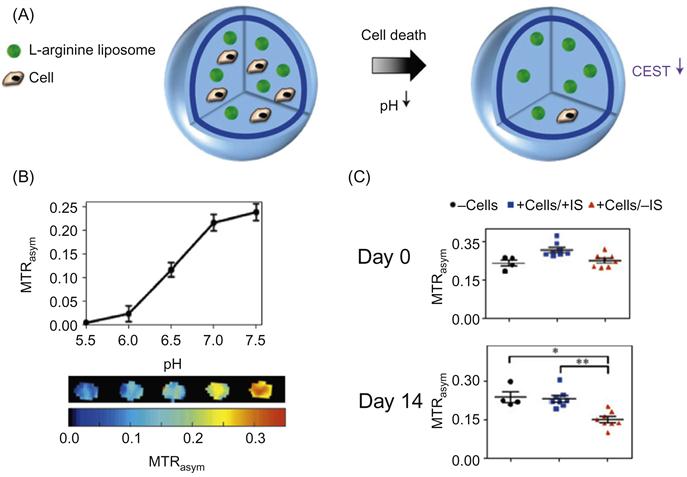
The ECM is another location with the potential for immobilizing imaging probes. An advantage of this strategy compared to internalization and plasma membrane methods is the retention of imaging probes, thus reducing the loss of probe density through cell division and exocytosis [17]. A highly successful approach utilizing the ECM to sequester probes is the co-encapsulation of L-arginine liposomes that exhibit chemical exchange saturation transfer (CEST) contrast with hepatocytes to function as a bioartificial liver (BAL) [18]. The numerous N–H bonds on the L-arginine structure facilitate exchange with secreted acidic species to detect pH changes noninvasively through MRI. This is a highly promising cell tracking method with clinical translational potential. A similar optical-based pH NP sensor strategy has been developed to be compatible for sequestration in agarose dishes, potentially useful as cheap, robust, and contactless bacteria sensors for the food industry [19]. Imaging through ECM immobilization has also been carried out to detect matrix metalloproteinase activity in two disease models—tumor xenografts and an inflammation model. This was achieved through the conjugation of the dabcyl luciferase quencher and luciferase to a GPLGVRGC peptide (a MMP-2/9 substrate) immobilized on collagen in vivo [17]. The addition of an inert 64Cu isotope normalizes sensor concentration, making relevant to image disease progression in real time.
11.4 Challenges and Outlook
Despite the promise demonstrated by the above techniques, existing limitations still remain. The authors envision the following as the most pressing of challenges: cytotoxicity of NPs; decreased signal strength caused by NP exocytosis and dilution during cell division; and the inability to report cellular function following transplantation [17].
Ideally, NPs for cell engineering should have either minimal or no influence over the original properties of the implanted cell. Evaluating cell function consists of evaluating viability, proliferation, “stemness,” cytokine secretion profile, gene profile, differentiation, and homing parameters. For example, although SPIOs with cationic coatings (e.g., poly-L-lysine) displayed minimal influence on MSC viability and proliferation, they impaired the osteogenic and chondrogenic differentiation of MSCs [51,52]. Unexpectedly, the internalization of silica NPs into MSCs led to the increased secretion of cytokines critical for cardiac regeneration [13]. The trend to develop NP imaging agents that have few detrimental effects on cellular function is the preference for incorporating FDA approved materials. In a recent example, the self-assembly of three FDA approved materials—ferumoxytol, heparin, and protamine—did not exhibit significant toxicity and damage on a variety of cells (neural stem cells, T cells, MSCs, and hematopoietic stem cells), and enabled the detection of ~1000 cells with MRI in a rat brain model [53].
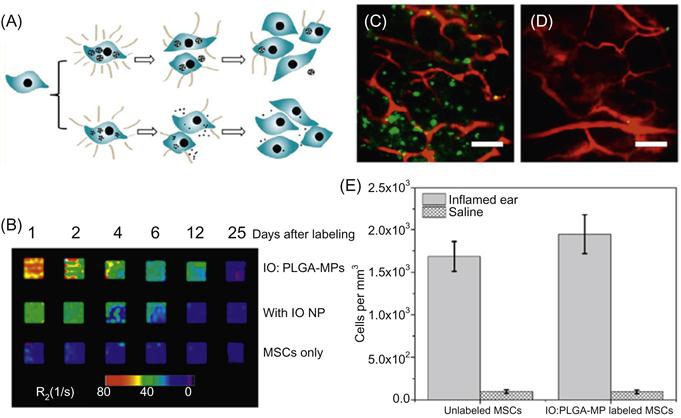
The second challenge is the loss of signal due to NP dilution impairing its longitudinal imaging ability. This is especially the case for stem cells that not only exocytose NPs but also undergo frequent rounds of cell division. Moreover, magnetic contrast is less reliable over longer periods of tracking, since iron oxide degrades in vivo, contributing to signal attenuation [37]. A promising method to minimize these effects is to label cells with particles of larger dimensions, which can reduce exocytosis rates [54]. As demonstrated in our previous achievements, the entrapment of IONPs (<20 nm) within PLGA microspheres (1–2 μm) resulted in an increase in R2 weighted MRI signal (fivefold) and prolonged signal duration beyond that of naked IONPs (threefold) [23]. These achievements are illustrated in Figure 11.2, which covers the experimental scheme, MRI signal enhancement using submicron particles, and in vivo achievements. A greater understanding of micro/nanoparticle interactions with cells may facilitate the design of imaging agents [28,30,32,55] with a sufficient period of intracellular residence to enable longitudinal tracking postimplantation.
Finally, developing NP imaging strategies that report on in vivo cellular function can increase the yield of data content from a single study (i.e., clinical trial). Currently, most strategies are not able to reveal postimplantation behavior such as viability and differentiation status. For example, during islet transplantation, magnetic NPs were used to label islets and track in real time an islet graft in diabetic mice [56,57]. This strategy is limited in its ability to report the survival of transplanted islets and the level of insulin secretion, since transplant efficacy was gauged by measuring physiological blood glucose levels. Such passive cell tracking technologies are unable to determine specific insulin secretion rates or the extent of cell death. Instead, the ability to monitor postimplantation behavior can assist the optimization of islet transplantation by determining if insulin secretion is mediated by a small subset of cells or the majority. A potential solution for this hurdle lies in CEST MRI imaging. This method utilizes CEST probes (FDA approved L-arginine) immobilized in the extracellular environment of an alginate capsule. Changes in proton content (pH 5.5–7.5) led to CEST contrast increase, enabling in vivo detection of cell death through indirect means [18]. The experimental schematic as well as key findings of this report are shown in Figure 11.3. On the other hand, this method may have limited usage for cells that secrete high levels of lactic acid (thereby low pH microenvironment) such as embryonic stem cells. Other promising solutions may be based on technologies that contain molecular recognition elements such as Au NP-based nanoflares [33,34] and molecular beacons [58]. Their precision in detecting specific molecular sequences as well as their versatility suggests a strong potential for reporting cell function.
11.5 Conclusion
This chapter gives a brief overview on NP-based cell engineering strategies including intracellular internalization, plasma membrane, and ECM modifications. These strategies are universal to many imaging modalities as well as cell types. Their biggest advantage is their simplicity, convenience, high efficiency, and “inertness”—minimal change to the cell’s original properties. Nevertheless, while several of these strategies are poised for clinical usage, they can be further improved by incorporating designs that: (i) provide stable signal intensity; (ii) report cell function; yet have (iii) minimal influence on cellular function. Returning to the themes discussed at the beginning of this chapter, advancements in NP engineering methods to enable clinically viable imaging tools will usher in significant advances for medicine, biology, and healthcare.
Acknowledgments
Project was supported by the Nanyang Technological University Start-Up Grant, Singapore and the Singapore MOE AcRF Tier 1 Research Fund.
