1. Why the world needs bacteria
What is a bacterium? Bacteria belong to a universe of single-celled creatures too small, with rare exceptions, to be seen by the unaided eye, but exist everywhere on Earth. Being small, simple, and many confers on bacteria advantages that allow them to not only survive but also to affect every mechanism by which the planet works. Bacteria influence chemical reactions from miles above the Earth’s surface to activities deep within the Earth’s mantle.
Bacteria range in size from Thiomargarita namibiensis, which reaches 750 micrometers (μm) end to end and is visible to the naked eye, to Francisella tularensis measuring only 0.2 μm in diameter. Since 1988, microbiologists have explored a new area involving “nanobacteria.” These microbes measure 0.05 μm in diameter or one-thousandth the volume of a typical bacterial cell. Excluding these unusual giants and dwarfs, most bacteria are between 0.5 and 1.5 μm in diameter and 1 to 2 μm long, or less than one-twentieth the size of the period at the end of this sentence. The volume of bacterial cells ranges from 0.02 to 400 μm3. One of many advantages in being small involves the ability to sense environmental changes with an immediacy that large multicellular organisms lack.
Bacterial simplicity can deceive. The uncomplicated structure actually carries out every important biochemical reaction in Earth’s ecosystems. Bacteria have an outer cell wall that gives them their distinctive shapes (see Figure 1.1) and overlays a membrane, which holds in the watery cytoplasm interior and selectively takes in nutrients, restricts the entry of harmful substances, and excretes wastes. This membrane resembles the membranes of all other living things. That is, it is consists of a bi-layer of proteins and fats that communicates with the aqueous environment and confines the cell contents to the cell interior. Inside the membrane bi-layer proteins and fats line up in a way that hydrophilic or water-attracting portions of the compounds face out or into the cytoplasm, and hydrophobic compounds point into the membrane. The character of membrane fats enables them to assemble spontaneously if put into a beaker of water. The ease with which membranes assemble likely helped the first cells to develop on Earth.
Figure 1.1. Bacteria shapes. Cell shape is hardwired into bacteria genetics. No animal life adheres as strictly to a standard shape as bacteria and algae called diatoms.
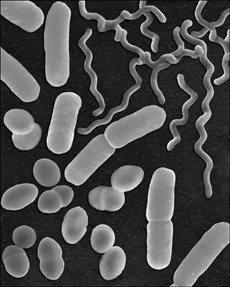
(Courtesy of Dennis Kunkel Microscopy, Inc.)
The bacterial cytoplasm and membrane hold various enzymes that keep the cell alive. Bacterial deoxyribonucleic acid (DNA), the depository of information formed over the millennia, appears in the cytoplasm as a disorganized mass (seen only with an electron microscope), but it actually contains precise folds and loops that decrease the chances of damage and facilitate repair. Tiny protein-manufacturing particles called ribosomes dot much of the remainder of the cytoplasm.
Bacteria require few other structures. Motile bacteria have whiplike tails called flagella for swimming, photosynthetic cyanobacteria contain light-absorbing pigments, and magnetotactic species, such as Aquaspirillum magnetotacticum, contain a chain of iron magnetite particles that enable the cells to orient toward Earth’s poles. These micro-compasses help Aquaspirillum migrate downward in aqueous habitats toward nutrient-rich sediments.
Though tiny, bacteria occupy the Earth in enormous numbers. Microbiologists estimate total numbers by sampling soil, air, and water and determining the bacterial numbers in each sample, and then extrapolating to the size of the planet with the aid of algorithms. Guesswork plays a part in these estimates. Bacteria exist 40 miles above the Earth and 7 miles deep in the ocean, and most of these places have so far been inaccessible. The total numbers of bacteria reach 1030. Scientists struggle to find a meaningful comparison; the stars visible from Earth have been estimated at “only” 7 × 1022. The mass of these cells approaches 2 × 1015 pounds, or more than 2,000 times the mass of all 6.5 billion people on Earth. Of these, the overwhelming majority lives in the soil.
Bacteria can stretch the limits of our imagination with small size and massive numbers. Both of these attributes help bacteria, and by the biological processes they carry out, bacteria also ensure that humans survive.
Tricks in bacterial survival
Bacteria and bacterialike archaea survive challenging conditions through the benefit of adaptations accrued in evolution. Survival techniques might be physical or biochemical. For example, motility in bacteria serves as an excellent way to escape danger. In addition to flagella that help bacteria swim through aqueous environments, some bacteria can glide over surfaces, and others start twitching frantically to propel themselves. Certain bacterial species develop impregnable shells called endospores. Others use biochemical aids to survival to counter the effects of acids, bases, salt, high or low temperature, and pressure.
A large number of bacteria use a modified version of a capsule for protection. The cells build long, stringy lipopolysaccharides, which are polysaccharides (sugar chains) with a fatty compound attached and which extend into the cell’s surroundings. The bacteria that make these appendages, called O antigens, construct them out of sugars rarely found in nature. As a consequence, protozoa that prey on bacteria do not recognize the potential meal and swim past in search of “real” bacteria.
Archaea seem to be Earth’s ultimate survivors because of the extreme environments they inhabit. Archaea and bacteria both belong to the prokaryotes, one of two major types of cells in biology, the other being more complex eukaryotic cells of algae, protozoa, plants, and animals. Because archaea inhabit extreme environments that would kill most terrestrial animal and plant life, the archaea are sometimes thought of as synonymous with “extremophile.” The outer membrane of archaea living in boiling hot springs contain lipid (fatlike) molecules of 30 carbons or more, larger than most natural fatty compounds. These lipids and the ether bonds that connect them stabilize the membrane at extremely high temperatures. News stories often tell of new bacteria found at intense pressures 12,000 feet deep on the ocean floor at vents called black smokers. These hydrothermal vents spew gases at 480°F, release acids, and reside at extreme pressures, so any organisms living there would truly be a news item. The organisms living near black smokers are usually archaea, not bacteria. Archaea also dominate habitats of high salt concentration, such as salt lakes, or places completely devoid of oxygen, such as subsurface sediments. Because of the difficulty of getting at many archaea and their aversion to growing in laboratory conditions, studies on archaea trail those completed on bacteria.
Some bacteria also survive in the same extreme conditions favored by archaea. The aptly named Polaromonas inhabits Antarctic Sea ice where temperatures range from 10°F to –40°F by slowing its metabolism until it reproduces only once every seven days. By comparison, E. coli grown in a laboratory divides every 20 minutes. Polaromonas is a psychrophile or cold-loving microbe. Thermus aquaticus is the opposite, a thermophile that thrives in hot springs reaching 170°F by synthesizing heat-stabile enzymes to run its metabolism. Enzymes of mesophiles, which live in a comfortable temperature range of 40°F to 130°F, unfold when heated and thus lose all activity. Mesophiles include the bacteria that live on or in animals, plants, most soils, shallow waters, and foods. The bacteria that live in harsh conditions that mesophiles cannot endure are the Earth’s extremophiles.
The genus Halococcus, a halophile, possesses a membrane-bound pump that constantly expels salt so the cells can survive in places like the Great Salt Lake or in salt mines. Barophilic bacteria that hold up under intense hydrostatic pressures from the water above are inexorably corroding the RMS Titanic 12,467 feet beneath the Atlantic. These barophiles contain unsaturated fats inside their membranes that make the membrane interior more fluid than the fats in other bacterial membranes. Unsaturated fats contain double bonds between some of the carbon atoms in the chainlike fat rather than single bonds that predominate saturated fats. At pressures of the deep ocean, normal membrane liquids change into the consistency of refrigerated butter, but the special membrane composition of barophiles prevents such an outcome that would render the membrane useless. A later chapter discusses why red-meat animals store mainly saturated fats and pork and chicken store more unsaturated fats.
The acidophile Helicobacter pylori that lives in the stomach withstands conditions equivalent to battery acid of pH 1 or lower by secreting compounds that neutralize the acid in their immediate surroundings. Even though an acidophile lives in strong acids that would burn human skin, it remains protected inside a microscopic cocoon of about pH 7. Additional extremophiles include alkaliphiles that live in highly basic habitats such as ammonia and soda lakes; xerophiles occupy habitats without water; and radiation-resistant bacteria survive gamma-rays at doses that would kill a human within minutes. Deinococcus, for instance, uses an efficient repair system that fixes the damage caused to the DNA molecule by radiation at doses that would kill a human. This system must be quick enough to complete the repair before Deinococcus’s next cell division.
All bacteria owe their ruggedness to the rigid cell wall and its main component, peptidoglycan. This large polymer made of repeating sugars and peptides (chains of amino acids shorter than proteins and lacking the functions of proteins) occurs nowhere else in nature. Peptidoglycan forms a lattice that gives species their characteristic shape and protects against physical damage. A suspension of bacteria can be put in a blender, whipped, and come out unharmed.
Archaea construct a cell wall out of polymers other than peptidoglycan, but their cell wall plays the same protective role. Furthermore, because archaea have a different cell wall composition than bacteria, they resist all the antibiotics and enzymes that attack bacterial cell walls. This quirk would seem to make archaea especially dangerous pathogens to humans, but on the contrary, no human disease has ever been attributed to an archaean.
In a microscope, bacteria present an uninspiring collection of gray shapes: spheres, rods, ovals, bowling pins, corkscrews, and boomerangs. Microbiologists stain bacteria with dyes to make them more pronounced in a light microscope or use advanced types of microscopy such as dark field or phase contrast. Both of these latter methods create a stunning view of bacteria illuminated against a dark background.
When bacteria grow, the cell wall prevents any increase in size so that bacterial growth differs from growth in multicellular organisms. Bacteria grow by splitting into two new cells by binary fission. As cell numbers increase, certain species align like a strand of pearls or form clusters resembling grapes. Some bacteria form thin, flat sheets and swarm over moist surfaces. The swarming phenomenon suggests bacteria do not always live as free-floating, or planktonic, beings but can form communities. In fact, bacterial communities represent more than a pile of cells. Communities contain a messaging system in which identical cells or unrelated cells respond to each other and change their behavior. This adaptation is called quorum sensing.
Quorum sensing begins when cells excrete a steady stream of signal molecules resembling amino acids. The excreted signal travels about 1 μm so that neighboring cells can detect it with specific proteins on their surface. When the receptors clog with signal molecules, a cell gets the message that other cells have nudged too close; the population has grown too dense. The proteins then turn on a set of genes that induce the bacteria to change their behavior. Different types of bacterial communities alter behavior in their own way, yet throughout bacteriology communities offer bacteria a superb survival mechanism. Some communities swarm, others cling to surfaces, and yet others can cover a pond’s surface and control the entire pond ecosystem.
Bacterial communities
Swarm cells start growing like any other bacterium on laboratory-prepared nutrient medium. (Media are liquids or solids containing gel-like agar that supply bacteria with all the nutrients needed for growth.) They metabolize for a while, split in two, and repeat this until nutrients run low. Rather than halting the colony’s growth, swarm cells signal each other to change the way they reproduce. The swarmer Proteus develops a regular colony when incubated, each cell about three μm in length. After several hours, cells on the colony’s outer edge elongate to 40 to 80 μm and sprout numerous flagella. Ten to 12 flagellated cells team up and then squiggle away from the main colony. By forming teams of cells lined up in parallel, 50 times more flagella power the cells forward than if one Proteus headed out on its own. Several millimeters from the main colony, the swarmers stop and again begin to reproduce normally. As generations of progeny grow, they build a ring of Proteus around the original colony, shown in Figure 1.2. At a certain cell density in the ring, Proteus repeats the swarming process until a super-colony of concentric rings covers the entire surface. When two swarming Proteus colonies meet, they do not overrun each other. The two advancing fronts stop within a few μm of each other, repelled by their respective defenses. Proteus produces an antibacterial chemical called bacteriocin. The specific bacteriocin of each swarmer colony protects its turf against invasion.
Figure 1.2. The swarming bacterium Proteus mirabilis. Proteus swarms outward from a single ancestor cell and forms concentric growth rings with each generation.
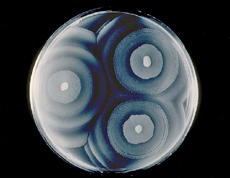
(Courtesy of John Farmer, CDC Public Health Image Library)
Other swarmer bacteria use hairlike threads called pili rather than flagella, and cast their pili ahead to act as tethers. By repeatedly contracting, the cells drag themselves forward to up to 1.5 inches per hour. Petri dishes measure only 4 inches across, but if dishes were the size of pizzas, swarm cells would cover the distance.
Communities such as biofilm grow on surfaces bathed in moisture. Biofilms cover drinking water pipes, rocks in flowing streams, plant leaves, teeth, parts of the digestive tract, food manufacturing lines, medical devices, drain pipes, toilet bowls, and ships’ hulls. Unlike swarming colonies, biofilm contains hundreds of different species, but they too interact via quorum sensing. (Bacteria that merely attach to surfaces such as skin are not true biofilms because they do not coalesce into a community that functions as a single entity.) Biofilm begins with a few cells that stick to a surface by laying down a coat of a sticky polysaccharide. Other bacteria hop aboard and build the diverse biofilm colony.
Biofilms facilitate survival by capturing and storing nutrients and excreting more polysaccharide, which protects all the members against chemicals such as chlorine. Eventually fungi, protozoa, algae, and inanimate specks lodge in the conglomeration of pinnacles and channels. When the biofilm thickens, signals accumulate. But because many different species live in the biofilm, the signals differ. Some bacteria stop making polysaccharide so that no more cells can join the community. The decrease in binding substance causes large chunks to break from the biofilm, move downstream, and begin new biofilm. (This constant biofilm buildup and breakdown causes great fluctuations in the number of bacteria in tap water. Within a few hours tap water can go from a few dozen to a thousand bacteria per milliliter.) Meanwhile, other bacteria ensure their own survival by increasing polysaccharide secretion, perhaps to suffocate nearby microbes and reduce competition.
Pathogens likely use similar strategies in infection by turning off polysaccharide secretion. With less polysaccharide surrounding the bacteria, the cells can reproduce rapidly. Then when pathogen numbers reach a critical level in the infected area, polysaccharide secretion returns to quash competitors.
A second type of multispecies community, the microbial mat, functions in complete harmony. Microbial mats lie on top of still waters and are evident by their mosaic of greens, reds, oranges, and purples from pigmented bacteria. Two types of photosynthetic bacteria dominate microbial mats: blue-greenish cyanobacteria and purple sulfur-using bacteria. During the day, cyanobacteria multiply and fill the mat’s upper regions with oxygen. As night falls and cyanobacteria slow their metabolism, other bacteria devour the oxygen. Purple bacteria prefer anoxic conditions, so they live deep in the mat until the oxygen has been depleted. In the night, the purple bacteria swim upward and feast on organic wastes from the cyanobacteria. The sunlight returns, and the purple bacteria descend to escape the photosynthesis about to replenish the upper mat with oxygen. As they digest their meal, these bacteria expel sulfide compounds that diffuse to the top layer. There, sulfur-requiring photosynthetic bacteria join the cyanobacteria (and some algae) in a new cycle. An undisturbed mat literally breathes: absorbing oxygen and emitting it, expelling carbon dioxide and inhaling it one breath every 24 hours. Microbial mats’ diurnal cycle makes them a distinctive microbial community.
Communities are mixtures of species within an ecosystem. Ecosystems contain living communities that interact with the nonliving things around them: air, water, soil, and so on. Bacteria participate in every phase of ecosystem life, but to learn about bacteria microbiologists must remove them from the environment and study one species at a time in a laboratory. A collection of bacterial cells all of the same species is called a population, or in lab talk a pure culture.
Microbiologists learn early in their training the tricky job of keeping all other life out of a pure culture by using aseptic technique. Aseptic—loosely translated as “without contamination”—technique requires that a microbiologist manipulate cultures without letting in any unwanted bacteria. They accomplish this by briefly heating the mouth of test tubes over a Bunsen burner flame, similarly flaming metal inoculating loops, and learning to keep sterilized equipment from touching unsterilized surfaces. Surgeons follow the same principles after they scrub up for surgery.
Under the microscope
For the two centuries following van Leeuwenhoek’s studies, microscopes improved, but microbiologists still needed a way to distinguish cells from inanimate matter in a specimen. They tested a variety of chemical dyes on bacteria with usually unsatisfactory results. In 1884, Danish physician Hans Christian Gram formulated through trial and error a stain for making bacteria visible in the tissue of patients with respiratory infection. On a glass slide, Gram’s recipe turned some of the bacteria dark purple and others pink. The new method served Gram’s purposes for diagnosing disease, but he had no notion of the impact the Gram stain would have on bacteriology.
The Gram stain divides all bacteria into two groups: gram-positive and gram-negative. This easy procedure serves as the basis for all identifications of bacteria from the sick, from food and water, and from the environment. Every student in beginning microbiology commences her education by learning the Gram stain.
Bacteria with thick cell walls of peptidoglycan retain a crystal violet-iodine complex inside the wall. These cells turn purple and are termed gram-positive. Other species cannot retain the stain-iodine complex when rinsed with alcohol. These gram-negative cells remained colorless, so Gram added a final step by soaking the bacteria in a second stain, safranin, that turned all the colorless cells pink. All bacteriologists now use the Gram stain as the first step in identification, monitoring food and water for contamination, and diagnosing infectious disease.
In the more than 100 years since Gram invented the technique, microbiologists have yet to figure out all the details that make some cells gram-positive and others gram-negative. The thick peptidoglycan layer in gram-positive cell walls has an intricate mesh of cross-links. This structure acts as a net to retain the large crystal violet-iodine aggregate and might keep the alcohol from reaching the stain and washing it out. By contrast, the gram-negative cell wall is more complex. The thin peptidoglycan in gram-negatives lies in between membranes on both the outer and inner surfaces of the cell. The thinness of the layer has been proposed as one reason why gram-negative cells cannot hold onto the stain.
Few hard and fast rules can be attributed to gram-positive and gram-negative populations. Gram-negative bacteria were once thought to be more numerous than gram-positives and have a higher proportion of pathogens, but these generalizations probably hold little merit. The Gram reaction nevertheless helps gives clues to microbiologists about potential trouble. Food, water, consumer products such as shampoo, and skin with high concentrations of gram-negative bacteria signal possible fecal contamination. That is because E. coli and all other bacteria in its family come from animal intestines. But gram-positive bacteria are not totally benign. Gram-positive bacteria recovered from a person’s upper respiratory tract might indicate strep throat (from Streptococcus) or tuberculosis. Skin wounds infected with gram-positives range in seriousness from Staph infections (from Staphylococcus) to anthrax. In the environment, the known gram-negative and gram-positive species distribute almost evenly in soils and waters.
During the time Gram worked out his new procedure, German physician Walther Hesse left his job of ten years tending to uranium miners in Saxony who were dying of lung cancer (although the disease had not yet been identified). After two years in Munich working in public hygiene, he became an assistant to Robert Koch who was second only to Louis Pasteur as the world’s eminent authority on microbes. Originally a country doctor in a small German village, Koch had already immersed himself in the behavior of anthrax and tuberculosis bacteria in test animals. From these studies he began developing a procedure for proving that a given bacterial species caused a specific disease. In 1876, Koch established a set of criteria that a bacterium must meet in test animals to be identified as the cause of disease. The criteria to become known as Koch’s postulates laid the foundation for diagnosis of infectious disease that continues today.
Medical historians have debated whether the criteria attributed to Robert Koch should be called the Henle-Koch postulates. Koch received his early training under German physician Jacob Henle who in 1840 published a list of criteria for confirming the cause of infectious disease. The criteria proposed by Koch were similar to Henle’s, but the origin of Koch’s postulates probably came by a gradual evolution of ideas with each new experiment on pathogens. I explain Koch’s postulates here:
- The same pathogen must be present in every case of a disease.
- The pathogen must be isolated from the diseased host and grown in a laboratory to show it is alive.
- The pathogen should be checked to confirm its purity and then injected into a healthy host (a laboratory animal).
- The injected pathogen must cause the same disease in the new host.
- The pathogen must be recovered from the new host and again grown in the laboratory.
Some bacteria do not conform to Koch’s postulates. For example Mycobacterium tuberculosis, the cause of tuberculosis, also infects the skin and bones in addition to the lungs. Streptococcus pyogenes causes sore throat, scarlet fever, skin diseases, and bone infections. Pathogens that cause several different disease conditions can be difficult to fit into the criteria for diagnosing a single disease.
In developing these criteria, Koch made another contribution to the fundamentals of microbiology by introducing a way to obtain pure cultures. For Koch’s postulates to work, a microbiologist needed a pure culture of the potential pathogen. Without bacteria in pure form, no one would be able to prove bacterium A caused disease A, bacterium B caused disease B, and so forth. Koch used potato slices for growing bacterial colonies and for his studies used only colonies that were isolated from all other colonies. This concept seems elementary today, but it helped microbiologists of Koch’s time rid their experiments of contaminants. To this day, prominent researchers have reported results only to make an embarrassing retraction months later because all of the data were collected on a contaminant.
When Hesse joined Koch’s laboratory, Koch had stopped using potato slices and substituted gelatin as a handier surface for growing pure colonies. Soon both men were grousing about gelatin’s flaws. In hot summers, the gelatin turned to liquid. Most other times, protein-degrading bacteria turned it into a useless blob. Hesse’s wife, Angelina, often came to the lab to help—this was a period in Germany when women were taking their first steps into professions. Lina, as Hesse called her, was an amateur artist and helped Koch and Hesse by drawing the bacterial colonies they had grown in the laboratory. She soon understood why the two microbiologists needed something better than gelatin. Lina suggested that they try agar-agar, a common ingredient at the time for solidifying puddings and jellies. Wolfgang, the Hesses’ grandson recalled in 1992, “Lina had learned about this material as a youngster in New York from a Dutch neighbor who had immigrated from Java.” People living in the warm East Indian climate noticed that birds gathered a substance from seaweed and used it as a binding material in nests. The material did not melt and did not appear to spoil—bacteria cannot degrade it.
Hesse passed on to Koch the idea of replacing gelatin with agar-agar. Koch immediately formulated the agar with nutrients into a medium that melted when heat-sterilized and solidified when cooled (see Figure 1.3). Koch published a short technical note on the invention but mentioned neither of the Hesses. Lina lived for 23 years after her husband’s death in 1911 and saved as many of his lab notes as she could find. A few of those notes showed that Hesse and Lina had originated the idea of agar in microbial growth media, and they have since been recognized for their part in microbiology.
Figure 1.3. Pouring molten agar. Agar melts when sterilized, and then solidifies when it cools to below 110°F. The microbiologist here pours the agar aseptically from a sterile bottle to a sterile Petri dish.
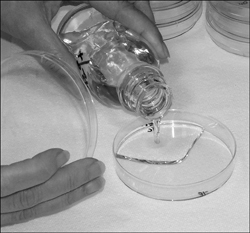
(Courtesy of BioVir Laboratories, Inc.)
Three years after Koch and Hesse switched to agar-based media, another assistant in the laboratory, Richard J. Petri, designed a shallow glass dish to ease the dispensing of the sterilized molten media. The dishes measured a little less than a half-inch deep and 4 inches in diameter. This Petri dish design has never been improved upon and is a staple of every microbiology lab today.
The size of life
Bacteria need only be big enough to hold their vital enzymes, proteins, and genetic machinery. Evolution has eliminated all extraneous structures. Also, a small, simple architecture allows for rapid reproduction, which aids adaptation. Bacterial metabolism is a model of efficiency because of a large surface-to-volume ratio that smallness creates. No part of a bacterial cell is very far from the surface where nutrients enter and toxic wastes exit. Eukaryotic cells that make up humans, algae, redwoods, and protozoa contain varied organelles each surrounded by a membrane. The surface-to-volume ratio in these cells is one-tenth that of bacteria, so shuttling substances across all those organelle membranes, the cytoplasm, and the outer membrane burns energy. Bacterial structure is less demanding and more efficient. Finally, small size contributes to massive bacterial populations that dwarf the populations of any other biota.
Large multicellular beings that produce small litters with long life spans—think whales, elephants, and humans—take a long time to make new, favorable traits part of their genome. Insects evolve faster and can develop a new trait within a few years. In bacteria, evolution occurs overnight. Often, the progeny contain a new trait that makes them better equipped for survival.
No one knows the number of bacterial species. About 5,000 species have been characterized and another 10,000 have been partially identified. Biodiversity authority Edward O. Wilson has estimated that biology has identified no more than 10 percent of all species and possibly as little as 1 percent. Wilson’s reasoning would put the total number of bacterial species at 100,000, probably a tenfold underestimate. Most environmental microbiologists believe that less than one-tenth of 1 percent of all bacteria can currently be grown in laboratories so that they can be identified.
Microbial geneticist J. Craig Venter’s studies on microbial diversity have correctly pointed out that the number of species may be less important than their diversity and roles in the Earth’s biosphere. Venter concluded from a two-year study of marine microbes that for every 200 miles of ocean, 85 percent of the species, judged by unique genetic sequences, changed. The ocean appears to contain millions of subenvironments rather than one massive marine environment, and each milliliter holds millions of bacteria. The actual number of bacteria in the oceans alone may exceed any previous estimates for the entire planet. In future studies of Earth’s microbial ecology, the absolute number of species will probably never be determined.
Microbiologists begin defining the microbial world by taking samples from the environment and determining the types of bacteria found there. One of the first questions to answer is: Are any of these bacteria new, previously undiscovered species? To answer this, microbiologists must understand the species that have already been characterized, named, and accepted in biology, such as E. coli.
Taxonomists assign all living things to genus and species according to outward characteristics and the genetics of an organism. Until the late 1970s, microbiologists identified bacteria through enzyme activities, end products, nutrient needs, and appearance in a microscope. In 1977 Carl Woese at the University of Illinois proposed using fragments of a component of cell protein synthesis, ribosomal ribonucleic acid (rRNA). Cellular rRNA takes information contained in genes and helps convert this information into proteins of specific structure and function. Because the genetic information in rRNA is unique to each species, it can act as a type of bacterial fingerprint. Woese’s method specifically used a component called 16S rRNA, which relates to a portion of the ribosome, the 16S subunit. This analysis led to a new hierarchy of living things (causing considerable consternation among traditional taxonomists) with bacteria, archaea, and eukaryotes comprising the three domains shown in Figure 1.4. Prior to the new rRNA classifications, biology students had been taught five-, six-, and even eight-kingdom classifications for organizing all plants, animals, and microbes. When I took my first biology classes, the five-kingdom system being taught looked like this:
• Monera, containing the bacteria
• Protista, containing protozoa and algae
• Plantae, containing green plants descended from algae
• Fungi descended from specific members of the Protista
• Animalia descended from specific members of the Protista
Figure 1.4. The three domains. Classification of the world’s organisms does not remain static; new technologies constantly force taxonomists to reevaluate and reclassify species.
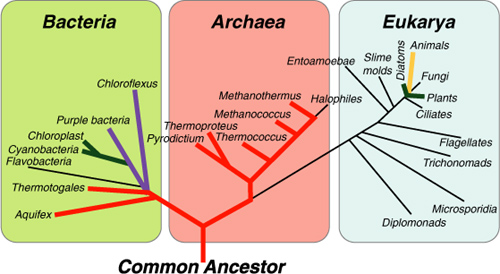
New technologies for classifying organisms have yet to end confusion that ensues when attempting to organize the world’s biota, and for good reason. Taxonomists and philosophers have been trying to figure out organisms’ relationships to each other since Aristotle’s first attempts. Additionally, since the emergence of DNA analysis in the 1970s, geneticists have discovered more diversity in biota but also a dizzying amount of shared genes, especially among bacteria. The rRNA analysis introduced by Woese showed the degree to which different species shared genes. The studies revealed a significant amount of horizontal gene transfer, which is the appearance of common genes across many unrelated species.
The evolutionary tree we all learned in which families, genera, and species branched from a major trunk does not depict horizontal gene transfer. The evolutionary tree may look more like a bird’s nest than an oak. Nowhere may that be truer than in the bacteria. Gene sharing or gene transfer is now known to take place in bacteria, and possibly archaea, more than ever before imagined. In 2002, the 16S rRNA system became further refined by focusing on certain protein-associated genes. But as biologists dig deeper into the genetic makeup of bacteria, they find more shared genes. Some microbiologists have begun to think that the term “species” makes no sense when speaking about bacteria. Currently, if two different strains of bacteria have less than 97 percent identical genes determined by 16S rRNA analysis, then they can be considered two different species. Some microbiologists suggest that only a 1 percent difference in genes differentiates species, not 3 percent.
When microbiologists first developed the bacterial groups known today as species, they let common characteristics of bacteria guide them. Gram reaction, nutrient requirements, unique enzymes, or motility served as features for putting bacteria into various species. Modern nucleic acid analysis has shown whether the traditional classification system still makes sense. With a high percentage of shared genes among bacteria and the ease with which diverse cells transfer genes around, some microbiologists have suggested that classifying bacteria by species is futile. It seems as if all bacteria belong to one mega-species, and different strains within this species differ by the genes they express and the genes they repress. By classifying bacteria into a single species, all bacteria would obey the definition for a species first proposed by Ernst Mayr in 1942: Members of the same species interbreed and members of different species do not.
Genetic analysis has blurred the lines between bacterial species so that the criteria used to classify other living things cannot apply to bacteria. To preserve their sanity, microbiologists need some sort of taxonomic organization so that they can speak the same language when discussing microbes. The traditional methods of grouping bacteria according to similar characteristics have turned out to be the handiest method regardless of DNA results. Microbiologists use the same classification and naming system for bacteria as used for all other life. The system has changed little since botanists in the mid-1800s, Carl Linnaeus being the most famous, developed it. Species classification and naming uses binomial nomenclature to identify every species by a unique two-part Latin name.
Bacteria of the same genus share certain genes, quite a few as mentioned, but different species have a few unique genes. For example, Bacillus is the genus name of a common soil bacterium. The genus contains several different species: Bacillus subtilis (shortened to B. subtilis), B. anthracis, B. megaterium, and so on. If I were a bacterium, my name would be Maczulak anne or M. anne.
To name a new bacterium, microbiologists have several conventions at their disposal. All that matters is that the new name be different from all other names in biology. Table 1.1 shows common naming conventions.
Table 1.1. Origins of bacteria names
Bacterial names will likely never be replaced regardless of scientific advances in classifying and reclassifying the species. Medicine, environmental science, food quality, manufacturing, and biotechnology depend on knowing the identity of a species that causes disease or contamination or makes a useful product. As microbiology fine-tunes its focus from the biosphere to the human body, species identity becomes more important.
The bacteria of the human body
Ten trillion cells make up the human body, but more than ten times that many bacteria inhabit the skin, respiratory tract, mouth, and intestines. Microbiologists are fond of pointing out that if all of a person’s DNA were mixed with the body’s entire bacterial DNA, that person would be genetically more bacterial than human.
About 1,000 different species belonging to 200 genera live on the body rather than in it. An animal’s body is a tube. The skin comprises the tube’s outer surface, and the gastrointestinal tract from mouth to anus makes up the inner surface. The body’s interior of blood, lymph, and organs normally contain no bacteria; these places are sterile. Urine and sweat exit the body as sterile fluids. In plants by contrast, bacteria live on but also inside the plant body.
The skin holds habitats that vary in moisture, oils, salts, and aeration. The scalp, face, chest and back, limbs, underarms, genitals, and feet make up the skin’s main habitats, and each of these contains smaller, distinct living spaces. The entire skin surface has about one million bacteria on each square centimeter (cm2) distributed unevenly among the habitats; the dry forearms contain about 1,000 bacteria per cm2, and the underarms have many millions per cm2.
Microbiologists sample skin bacteria by pressing a cylinder about the size of a shot glass open at both ends against the skin to form a cup, and then pouring in a small volume of water. By agitating the liquid and gently scraping the skin with a sterile plastic stick the microbiologist dislodges many of the bacteria. But no method or the strongest antiseptics remove all bacteria from the skin: The skin is not sterile. Staphylococcus, Propionibacterium, Bacillus, Streptococcus, Corynebacterium, Neisseria, and Pseudomonas dominate the skin flora.
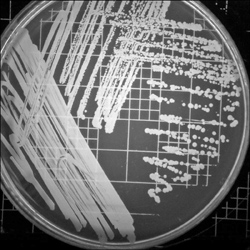
Figure 1.5. Staphylococcus aureus. A common and usually harmless inhabitant of skin, S. aureus can turn dangerous given the opportunity. This species can infect injuries to the skin, and the MRSA strain has become a significant antibiotic-resistant health risk.
(Courtesy of BioVir Laboratories, Inc.)
Some of these names are familiar because they also cause illness, and yet a person’s normal bacteria pose no problem on healthy, unbroken skin. The native flora in fact keep in check a variety of transient bacteria collected over the course of a day. Some of these transients might be pathogenic, but they do not settle permanently on the skin because the natives set up squatters’ rights by dominating space and nutrients, and producing compounds—antibiotics and similar compounds called bacteriocins—that ward off intruders. Such silent battles occur continually and without a person’s knowledge. Only when the protective barrier breaks due to a cut, scrape, or burn does infection gain an upper hand. Even harmless native flora can turn into opportunists and cause infection because conditions change in the body. Immune systems weakened by chemotherapy, organ transplant, or chronic disease increase the risk of these opportunistic infections:
• Staphylococcus—Wound infection
• Propionibacterium—Acne
• Bacillus—Foodborne illness
• Streptococcus—Sore throat
• Corynebacterium—Endocarditis
• Pseudomonas—Burn infection
Anaerobic bacteria do not survive in the presence of oxygen, but they make up a large proportion of skin flora. Though the skin receives a constant bathing of air, anaerobes thrive in miniscule places called microhabitats where oxygen is scarce. Chapped and flakey skin and minor cuts create anaerobic microhabitats. Necrotic tissue associated with major wounds also attracts anaerobes, explaining why gangrene (caused by the anaerobe Clostridium perfringens) and tetanus (C. tetani) can develop in improperly tended injuries. Of normal anaerobes inhabiting the skin, Propionibacterium acnes (the cause of skin acne), Corynebacterium, Peptostreptococcus, Bacteroides, and additional Clostridium dominate.
The mouth’s supply of nutrients, water, and microhabitats creates a rich bacterial community. Brushing and flossing remove most but not all food from between teeth, the periodontal pockets between the tooth and the gum, and plaque biofilm on the tooth surface, which holds a mixture of proteins, human cells, and bacterial cells. Anaerobes and aerobes find these places and their relative numbers vary from daytime to night as the level of aeration, flushing with drinks, and saliva production changes. During the day, more air bathes oral surfaces and aerobes flourish. At night or during long periods of fasting, the aerobes consume oxygen and anaerobes begin to prosper. By the nature of their fermentations, anaerobes make malodorous end products when they digest food. These bad-smelling, sulfur-containing molecules vaporize into the air and become bad breath.
Few bacteria live in the esophagus and stomach with the exception of the spiral-shaped Helicobacter pylori, occurring in half of all people with peptic ulcers. The discovery of H. pylori in the stomach in 1975 dispelled the long-held belief that no microorganisms could withstand the digestive enzymes and hydrochloric acid in gastric juice. Most bacteria traverse the half gallon of stomach fluid at pH 2 by hiding in a protective coat of food particles on the way to the small intestine. H. pylori, however, thrives in the stomach by burrowing into the mucus that coats the stomach and protects the organ from its own acids. Inside the mucus, the bacteria secrete the enzyme urease that cleaves urea in saliva into carbonate and ammonia. Both compounds create an alkaline shield around H. pylori cells that neutralize the acids.
The pH rises in the intestines and bacterial numbers increase a millionfold from about 1,000 cells per gram of stomach contents, which to a microbiologist is a small number. Humans, cows, pigs, termites, cockroaches, and almost every other animal rely on intestinal bacteria to participate in the enzymatic digestion of food. The numbers reach 1012 cells per gram of digested material. Monogastric animals such as humans and swine absorb nutrients made available by the body’s enzymes as well as nutrients produced by bacteria. When the bacteria die and disintegrate in the intestines, the body absorbs the bacterial sugars, amino acids, and vitamins (B-complex and vitamin K) the same as dietary nutrients are absorbed. Cattle, goats, rabbits, horses, cockroaches, and termites, by contrast, eat a fibrous diet high in cellulose and lignin that their bacteria must break down into compounds called volatile fatty acids. Glucose serves as the main energy compound for humans, but volatile fatty acids power ruminant animals (cattle, sheep and goats, elephants, and giraffes) and animals with an active cecum (horses and rabbits).
Rumen bacteria carry out anaerobic fermentations. Almost every organic compound in the rumen becomes saturated there by fermentative bacteria before moving on to the intestines. As a result, ruminants such as beef cattle deposit saturated fats in their body tissue. Nonruminant animals, such as pigs and chicken, carry out fermentations to a lesser extent and their meat contains less saturated fat.
How important are all these bacteria in keeping animals alive? Germfree guinea pigs grow smaller than normal, develop poor hair coat, and show symptoms of vitamin deficiency compared with animals with a normal microbial population. Germfree animals also catch infections more than populated animals. On the upside, germfree animals never experience tooth decay!
Bacteroides, Eubacterium, Peptostreptococcus, Bifidobacterium, Fusobacterium, Streptococcus, Lactobacillus, and E. coli of the human intestines also produce heat in the same way wine fermentations produce heat. This heat loss is inefficient for the bacteria—any energy that dissipates before it can be used is lost forever—but the body uses it to maintain body temperature. The large numbers of normal intestinal bacteria also outcompete small doses of food illness bacteria such as Salmonella, Clostridium, Bacillus, Campylobacter, Shigella, Listeria, and E. coli.
E. coli is the most notorious of foodborne pathogens and also the most studied organism in biology. In fact, E. coli plays a minor role in the digestive tract; other bacteria outnumber it by almost 1,000 to one. E. coli has become the number one research tool in microbiology for two reasons. First, this microbe cooperates in the laboratory. E. coli is a facultative anaerobe, meaning it grows as well with oxygen as without it. It requires no exotic nutrients or incubation conditions, and it doubles in number so rapidly that a microbiologist can inoculate it into nutrient broth in the morning and have many millions of cells that afternoon. The second reason for using E. coli in biology relates to the ease of finding it in nature: The human bowel and that of most other mammals produce a constant supply.
The origins of our bacteria
Infants have no bacteria at birth but start establishing their skin flora within minutes and digestive tract populations soon after. E. coli, lactobacilli, and intestinal cocci latch on to a baby during birth and become the first colonizers of the infant’s digestive tract. Babies get additional bacteria for a reason that scares germophobes: fecal and nonfecal bacteria are everywhere, and people ingest large amounts each day. Fecal bacteria disseminate beyond the bathroom to countertops, desks, refrigerator handles, keyboards, remote controls, and copy machine buttons. Any object repeatedly touched by different people contains fecal bacteria. Newborns get these bacteria every time they handle toys or crawl on the floor, and then put their hands or other objects in their mouth. Adults similarly receive fecal bacteria, called self-inoculation, when touching their hands to the mouth, eyes, or nose. Adults touch their hands to their face hundreds of times a day, and children do it more frequently.
A baby’s digestive tract has some oxygen in it so aerobic bacteria and facultative anaerobes prosper there first. E. coli colonizes the gut early on and uses up the oxygen. A population of anaerobes then begins to dominate: Bacteroides, Bifidobacterium, Enterococcus, and Streptococcus make up the common genera. The adult digestive tract distal to the mouth will eventually contain 500 to 1,000 different species of bacteria and a lesser number of protozoa.
Pathogens make up a minority of all bacteria, but the word “germs” brings only bad connotations. A growing number of microbiologists have nonetheless begun to see the potential benefits of exposure to germs. In the 1980s German pediatrician Erika von Mutius investigated the apparent high incidences of asthma and allergies in industrialized nations compared with developing areas. She compared the health of children from households that received little housekeeping with counterparts in well-managed households with regular cleanings. Children who had been exposed to a dirty environment had fewer respiratory problems than children from cleaner surroundings. Von Mutius therefore proposed that a steady exposure to germs might help youngsters develop strong immune systems.
Von Mutius’s “hygiene hypothesis” drew criticism from microbiologists and, unsurprisingly, manufacturers of cleaning products. But pediatric allergist Marc McMorris supported the hypothesis, saying, “The natural immune system does not have as much to do as it did 50 years ago because we’ve increased our efforts to protect our children from dirt and germs.”
Questions have not yet been answered on whether continued use of disinfectants and antimicrobial soaps change bacteria at the gene level. Medical microbiologist Stuart Levy has argued that antibiotic overuse combined with overzealous use of antimicrobials leads to bacteria resistant to the chemicals meant to kill them. These bacteria may develop subsequent resistance to antibiotics. Bacteria eject harmful chemicals and also antibiotics from inside the cell by using a pumplike mechanism. If bacteria use the very same pump for chemical disinfectants as for antibiotics, the vision of a new generation of super-resistant bacteria becomes probable. Imagine hospitals where no antibiotics can stop pathogens and few chemical disinfectants can kill them. Doctors and microbiologists have warned that medicine is inching closer to this very scenario.
The body helps native flora defend against pathogens that attach to the skin. The enzyme lysozyme in tears and saliva kills bacteria, and skin oils contain fatty acids that inhibit gram-positive bacteria. If those defenses fail, the immune system sets in motion a hierarchy of defenses meant to find and destroy any foreign matter in the bloodstream.
Dental caries can lead to more serious tooth decay and gum disease, or an infection of the blood if the oral lesions are severe. In plaque, Streptococcus mutans, S. sobrinus, and various lactobacilli (lactic acid-producing bacteria) initiate caries formation by producing acids. Lactic, acetic (also in vinegar), propionic, and formic acid diffuse into the tooth enamel and break it down by demineralization, meaning the removal of minerals such as calcium. Demineralization occurs several times a day in a cycle in which new dietary calcium and phosphate and fluoride from toothpaste replace the lost minerals. Dental caries offer an exception to the rule that native flora do not initiate infection.
On the skin, some bacteria create a nuisance. Skin bacteria consume amino acids, salts, and water excreted by eccrine sweat glands. These glands located all over the body produce copious amounts of watery sweat for cooling. The bacteria also feed on thicker sweat from apocrine glands in the underarms, ear canal, breasts, and external genitalia. These glands tend to activate in times of stress or sexual stimulation. Skin bacteria in these places degrade the sweat’s sebaceous oils to a mixture of small fatty acids and nitrogen- and sulfur-containing compounds, all of which vaporize into the air to cause body odor.
Some bacteria such as Staphylococcus live on everyone, but each person also has a unique population of native bacteria that produces a distinctive odor. Scientists have long sought elusive secretions called pheromones that foster communication between people through smell, but I suspect the secretions of native flora will prove to be the human version of quorum sensing. In 2009 anthropologist Stefano Vaglio analyzed the volatile compounds in the sweat of women shortly after childbirth and discovered unique patterns of odor compounds, perhaps to aid mother-infant recognition.
The deodorant and soap industries spend a fortune convincing people to block the natural products made by skin bacteria. Each week hundreds of deodorant-testing volunteers troop into deodorant companies’ odor rooms. The volunteers take positions like a police lineup and raise their arms. A team of trained sniffers works its way down the line to “score” the results. Women make up the majority of professional sniffers; the Monell Chemical Sciences Center confirmed in 2009 that women’s olfactory systems gather more information from body odors than men’s. (Sniffers have sworn that if blindfolded they could identify their mates.) The sniffers assess the best and the worst new deodorants based on underarm odor scores; 0 equals no odor and a score of 10 could clear a room.
One planet
During the Golden Age of Microbiology, bacteria were viewed as unrelated individualists. Pasteur studied the bacteria that made lactic acid by fermenting sugar. Joseph Lister focused on germs causing infections in hospital patients. Robert Koch discovered the anthrax pathogen, Bacillus anthracis, and delved into the processes of bacterial disease. He would develop a set of criteria (Koch’s postulates) that gave birth to today’s methods for diagnosing infectious disease. Not until microbial ecology developed did biologists recognize the interrelated world of bacteria as well as the relationship between environmental bacteria and humans.
Staphylococcus epidermidis contributes to body odor, a bacteria-human connection easily detected. But thousands of hidden bacterial activities shape the very ecology of the planet. In soil, Azotobacter pulls nitrogen from the air, chemically rearranges it, and hands it off to Nitrosomonas, which changes the nitrogen again and shuttles it to Nitrobacter. Nitrobacter then secretes the nitrogen in the form of nitrate, which disseminates throughout soils. Some of the nitrate reaches the roots of legumes such as clover or soybeans. Inside the plant roots anaerobic Rhizobium absorbs the nitrate and converts it to a form the plant can use. This process is vital in replenishing nitrogen that higher organisms need.
For carbon to make a similar cycle through the Earth’s organic and inorganic matter, the bacteria of decay must help decompose the planet’s fallen trees, plants, and animals. The common soil inhabitant Bacillus breaks down proteins, fats, and carbohydrates by excreting the enzymes protease, lipase, and amylase, respectively. Thousands of other species break down organic matter in similar ways. For example, Cellulomonas bacteria produce the enzyme cellulase—rare for bacteria—that digests plant cellulose fibers. Bacteria emit carbon dioxide as an end product, which enters the atmosphere. A massive population of photosynthetic bacteria in the Earth’s surface waters then captures this gas and inserts the carbon into a new food chain of bacterial cells, protozoa, invertebrates, and so on until the carbon ends up in tuna sashimi on a restaurant menu.
If clouds begin to form while a person lunches on sashimi, bacteria have a part in that, too. Photosynthetic marine bacteria and algae produce dimethyl sulfide gas as a waste product of their normal metabolism; they emit 50 million tons annually. When the gas rises and enters the atmosphere, it chemically rearranges into sulfate, which attracts water vapor. The vapor turns to droplets and forms clouds. On a global scale clouds inhibit the photosynthetic bacteria and less dimethyl sulfide forms. When the clouds thin, the cycle begins again.
Albert Kluyver of the Technical School of Delft—the town where van Leeuwenhoek discovered bacteria in 1677—praised the wonderful “unity and diversity” of microorganisms, a perfect description for dissimilar organisms that share more than 95 percent of their genes. The human body possesses its own unity and diversity of microbes that in most situations keep the body’s metabolism working at its best. Pathogens more than good bacteria gain the attention of researchers and doctors. For this reason, epidemics have expanded our knowledge of bacteria. Many of the discoveries in microbiology came about from a blend of genius and serendipity, a fair description of all science.
