3. “Humans defeat germs!” (but not for long)
In bacteria, one mutated cell appears for every 100 million normal cells. Because some bacteria reproduce as quickly as every 20 minutes, new populations of mutants emerge literally overnight. Most mutations give no discernible advantages or disadvantages to the cell. Unfavorable mutations make bacteria vulnerable to other microbes or the environment, and these cells and their genes disappear forever. On rare occasions a mutation gives a bacterial cell a favorable characteristic called a trait that enables the bacterium to outperform others.
Most people remember from Biology class that a favorable mutation appears only because of a random event. “The survival of the fittest” comes not by plan but by luck. Chance mutations in bacterial DNA produce slight, random changes in a single gene, and this altered gene gives the cell the ability to grow faster, swim farther, absorb more nutrients, or withstand heat better than its brethren. When this special cell divides, two identical cells appear that also outcompete others until the new gene has become part of a new, evolved population.
In 1988, John Cairns found in E. coli a ploy that turned the concept of randomness on its head. Cairns’s E. coli used adaptive mutations, which occurred when a specialized mutator gene detected a stimulus in the environment. Mutator genes prompt the cell’s mutation rate to speed up, thus increasing the chance that one of E. coli’s 4,377 genes will mutate in a favorable direction. More than 30 mutator genes have now been located in E. coli and similar genes in Pseudomonas aeruginosa, a water-associated microbe and common invader of burns and invasive devices (intravenous tubes, catheters, and so on). Are bacteria choosing how and when they mutate? If so, an idea that once belonged only in science fiction may be a reality.
What is an antibiotic?
Antibiotic means “against life” and belongs to two groups: true antibiotics and bacteriocins. A true antibiotic is made by a microbe to kill other unrelated microbes. Penicillium mold produces the antibiotic penicillin to kill bacteria that venture too close to its territory. Bacteriocins come from bacteria to kill other bacteria. For example, E. coli produces the bacteriocin colicin that kills bacteria in E. coli’s family of enteric microbes. Some bacteriocins kill different strains of the very same species, all for the purpose of reducing competition for space, food, light, and water.
An antibiotic that kills bacteria outright is a “cidal” agent, or bactericidal. Weaker antibiotics that merely slow down bacterial growth are called bacteriostatic. Penicillin is bactericidal because it prevents susceptible bacteria from building a rigid cell wall, forcing the bacteria to succumb to toxins in their environment. Tetracyclines, by contrast, interfere with protein synthesis, which may not necessarily kill the cell. The cells might switch to an alternate synthesis pathway, but this slows their reproductive rate, so tetracycline has done its job. Figure 3.1 illustrates a simple laboratory test that determines the susceptibility of bacteria to various antibiotics.
Figure 3.1. Kirby-Bauer antibiotic testing. Small paper discs soaked in different antibiotics cause varying levels of inhibition against bacteria. This test is a refinement of Alexander Fleming’s discovery that mold spores can kill bacteria due to the secretion of antibiotic.
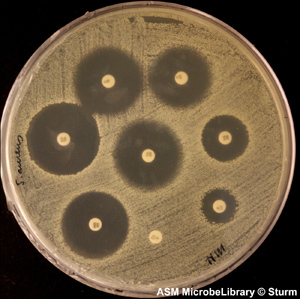
(Reproduced with permission of the American Society for Microbiology MicrobeLibrary, www.microbelibrary.org)
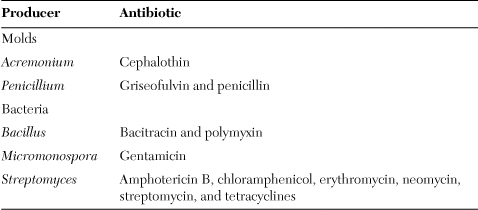
The structure of an antibiotic includes several carbons and hydrogens plus carbon rings and branches that make the molecule look complex. Nature developed the intricate structures to make it harder for bacterial enzymes to recognize and degrade an antibiotic. But humans interfered with nature’s plan by using increasing amounts of antibiotics and thus exposing bacteria to the compounds more frequently. Twenty years after the first commercial use of penicillin, antibiotic-resistant bacteria emerged. Resistant bacteria now exist for all of the natural antibiotics in Table 3.1. Today chemists try to stay ahead of bacteria by synthesizing new antibiotic molecules with more complexities in the hope of outwitting pathogenic bacteria.
Table 3.1. The main natural antibacterial antibiotics
The United States produces 25,000 tons of antibiotics annually. Most of the drugs go to human medicine and agriculture. Cattle, hogs, sheep, goats, and poultry raised for meat receive 70 percent of the supply to promote growth rate and repel infections that travel fast through factory farms. The remainder of the antibiotic supply goes to dogs, cats, horses, and other domesticated animals, pelt animals, fish, and plants and trees.
Meat producers have suffered strident criticism for giving the animals they raise a constant intake of antibiotics. When I began my college career as an animal science major, we took for granted the benefits of antibiotic use in meat animals. Beef, pork, and poultry received subtherapeutic levels of more than one drug for no specific reason other than the possibility of increased weight gain. The mounting questions regarding this practice spurred researchers to study bacteria in the digestive tract of healthy ruminant and nonruminant animals receiving antibiotics. Antibiotic-resistant bacteria have been recovered from these animals, but it can be difficult to prove that the antibiotics led to resistance.
Food producers insisted for years that antibiotics are needed for efficiency in meat production. Meat producers give these drugs to animals to prevent the spread of infection in a population of animals living in very close quarters from birth to slaughterhouse—this is the reason behind the term “factory farming.” Factory farming increases the nebulous condition we call stress when animals spend their entire lives squeezed together, and stress weakens immunity. The high density of individuals creates a higher risk for infection. Perhaps the logic behind factory farming and administering antibiotics to livestock makes no sense, and halting both would be a better choice.
The agriculture industry argues that efficient mass-production style farming keeps food costs low. Researchers have discovered shifts in the proportions of intestinal bacteria in antibiotic-fed animals. It has been more difficult, however, to determine the connection between altered bacterial populations and faster growth in animals.
Large-scale agriculture has been reluctant to share its antibiotic methods, so the public will have a hard time learning which antibiotics, if any, are in the meat they buy.
The environmental effects of subtherapeutic antibiotics in meat animals remain largely unknown. Two outcomes seem likely, however. First, antibiotic-resistant bacteria shed in manure enter the environment and cause harmful consequences in ecosystems, and second, eating rare meats or runny eggs increases a person’s chance to ingest resistant bacteria. Food is not sterile and cooking does not guarantee the removal of all potential pathogens; cooking reduces bacterial numbers to safer levels. We get away with ingesting a pathogen here or there throughout the week because the dose of the microbe is lower than required to cause infection. At the same time, our native bacteria and immune system protect the body from exposure to low numbers of pathogens.
The European Union and Canada ban antibiotic use in meat animals, and the World Health Organization has taken a stance that conveys its concern over antibiotics in agriculture. The United States still uses antibiotics, and meat-producing states continue to contend that no indisputable evidence exists to prove that meat antibiotics lead to drug resistance in people. Indisputable evident is exceedingly difficult to find in any field of science, so consumers have been left with making their own decisions on the safety of meat products.
Antibiotics that escape farms in runoff from manure piles enter surface waters. In a perfect world, the wastewater would be directed to a wastewater treatment plant without contaminating the environment. This is impractical considering the magnitude of daily manure output in the United States alone. Wastewater treatment and drinking water disinfection provide poor protection against antibiotics. In 2005, researchers from the University of Wisconsin detected six antibiotics in treated wastewater:
• Tetracycline—For skin, urinary tract, and some sexually transmitted diseases
• Trimethoprim—Childhood ear infections, urinary tract infections
• Sulfamethoxazole—Used in combination with trimethoprim for treating ear, bronchial, or urinary tract infections
• Erythromycin—Treats respiratory tract infections
• Ciprofloxacin—Lower respiratory, urinary, and other infections
• Sulfamethazin——For respiratory and other infections in animals
I am not suggesting that treated water is a source of danger in every community or that antibiotics in water definitely cause harm. The drugs in the study described here had, furthermore, been detected in parts per billion levels, equivalent to one corn kernel in a nine-foot silo of corn.
Drugs entering the environment year after year are affecting ecosystems, but scientists do not yet know all the details. Therefore, the public has no way of knowing. But imagine an antibiotic injected into a sick horse ending up miles away in a glass of tap water or a plate of oysters Rockefeller.
Antibiotics made immediate and profound effects on human health when they first became available, and few people foresaw trouble. Trouble would come, and the first warning came from a surprising source. Unfortunately, the world missed the message concerning antibiotic resistance until it was too late.
Inventing drugs is like making sausage
In 1897, a 23-year-old doctoral student submitted his graduation thesis to the Institut Pasteur, alluding to a new drug that might be helpful in fighting bacterial infections. The document described a Penicillium mold that killed E. coli in Petri dishes and cured laboratory animals injected with live typhoid bacteria. The reviewing faculty found Ernest Duchesne’s work uninspired, but they granted Duchesne a diploma along with little encouragement for a career in science. He enlisted in the French army. Before leaving, Duchesne discarded his laboratory notes; his thesis disappeared into a corner of the institute.
World War I mimicked all prior wars by costing millions of lives from infections, many of them minor, received on the battlefield. On the front, nurses stretched their bleach supply by diluting it until it had no effect against any germ. About half of the war’s 10 million fatalities came from infections. Duchesne did not get the chance to alert the world of his anti-infection drug. He caught tuberculosis soon after joining the French army and died at age 37 in 1912.
Another medical student in Germany had already begun his own hunt for a “magic bullet,” a drug to kill pathogens without harming the patient. Paul Ehrlich tested 605 different substances in an effort to find a drug that killed many different types of pathogens but did not cause harmful side effects in patients. When he tested the arsenic-containing compound, salvarsan, he found it inhibited the Treponema bacteria that cause syphilis. The promising new drug became known as Compound 606. Prior to the discovery of salvarsan as an antibiotic, Western medicine depended on an antibacterial substance that Spanish conquistadors had learned of in South America. Peru’s Quechua Indians had been using an extract from the cinchona tree to treat “ague.” In the mid-17th century, Jesuit priests brought the Peruvian powder to Europe. The substance to become known as quinine caused little stir in the medical community until it cured England’s Charles II of ague, now known as malaria. The new drugs energized physicians, biologists, and chemists toward finding other disease-curing compounds hidden in nature.
Chemists soon emulated Ehrlich, whom they had nicknamed Doctor 606, by testing hundreds of synthetic compounds against bacteria. In the early 1900s, however, chemical companies had little practice in drug research. Their chemical stockpiles were limited to fabric dyes for protecting threads against decomposition by bacteria. The compounds did not work well in laboratory tests against bacterial cultures, and in later years most of these substances were shown to cause cancers. Ehrlich would not realize his dream of finding a single magic bullet to kill all infectious disease.
Sixteen years after Duchesne’s death, Scottish microbiologist Alexander “Alec” Fleming prepared for a short September vacation from his lab at London’s St. Mary’s Hospital. Historians have shaped the ensuing tale. Fleming had a reputation as a dedicated scientist but terrible housekeeper. His lab overflowed with Petri plates, tubes, beakers—certainly the makings of a contaminated experiment. While Fleming was away, rogue mold spores contaminated Petri dishes filled with Staphylococcus bacteria. When Fleming returned, he noticed clear zones in the film of Staph cells where a spore had landed, and he concluded that the mold had disintegrated the bacteria. No one is sure where the mold originated. Spores likely drifted from a floor below where mycologist C. J. La Touche’s laboratory was chock-full of molds. Fleming’s habit of messiness gave the spores plenty of places to land and grow.
More than one stroke of luck converged to propel Alexander Fleming into history. The early-fall temperatures were warm enough for bacterial growth but cool enough for mold contaminants such as Penicillium; Staph cells prefer body temperature while molds prefer room temperature. Fleming had been studying Staph cultures, which are particularly susceptible to the action of Penicillium. Perhaps the most fortuitous break occurred when lab assistant D. Merlin Pryce came by for a casual hello and spotted the Penicillium-inhibited Staph cells among the cultures.
To Alec Fleming’s credit he investigated odd occurrences that others might dismiss as aberration. He continued studying Penicillium. Fleming had assumed that the mold had lysed the bacteria when spores landed on the bacterial film. Only later did microbiologists learn that Penicillium targets young, growing bacteria. The mold spores had probably contaminated Fleming’s Staph cultures before he began his vacation.
Fleming published his results in 1929 and gave lectures on the new substance he called penicillin. But because of acute shyness, Fleming reduced the most riveting topics to a monotonous drone, and he failed to inspire his peers. His colleague at St. Mary’s, pathologist Almroth Wright, openly disparaged Fleming’s work. Alec Fleming retreated to his lab and his main interest, a new compound called lysozyme that he had discovered in human tears. (Fleming developed most of the knowledge biologists have today on lysozyme. This enzyme serves as a first-line defense against pathogens on the skin or near the eyes. Fleming’s important discovery would be overshadowed by his research on penicillin.)
When the British entered World War II, German bacteriologist Gerhard Domagk had already discovered sulfa drugs. Britain’s doctors saw the advantage these drugs gave the German infantry for treating wounds, but their own laboratories offered nothing similar. In 1938, Oxford University pathologist Howard Florey had teamed with a recent refugee from Germany, Ernst Chain, to find an anti-infection drug for Britain. Chain unearthed Fleming’s 1929 article on the effect of mold on Staph, and the two suspected they had a diamond in the rough. Florey and Chain extracted penicillin from the mold, and then began the lengthy, tedious task of purifying and scaling it up to useful amounts. Back in London, Fleming alternated penicillin experiments with lysozyme studies. During the London Blitz he expanded the list of bacteria susceptible to penicillin and designed clever tests to differentiate mildly susceptible bacteria from the highly susceptible.
In late 1940, Florey and Chain published a brief article in a medical journal on a Penicillium extract hundreds of times stronger than Domagk’s sulfa drugs in killing gas gangrene Clostridium. Not until August of 1942 did the London Times pick up the story, but it mentioned no scientists by name. Almroth Wright who had so harshly criticized Fleming 13 years earlier pounced on an opportunity. He wrote the Times to inform them of penicillin’s discoverer Alexander Fleming, with special credit to St. Mary’s Hospital. The headlines “Professor’s Great Cure Discovery,” “Miracle from Mouldy Cheese,” and “Scottish Professor’s Discovery” began appearing. St. Mary’s hospital basked in the recognition (and the increased donations) that other London hospitals coveted.
The public had never heard of Florey or Chain, but Fleming and the scientific community kept abreast of their attempts to scale-up penicillin production. In August, Fleming, who had never developed the knack for making large quantities of purified penicillin, asked Florey for some of his drug to treat his friend Harry Lambert, suffering with a severe streptococcus infection. Florey rushed to London with his entire stockpile of pure penicillin and showed Fleming how to inject it. Although Fleming bungled Florey’s instructions, he nonetheless saved Lambert from certain death.
Everyone now wanted to know about the new drug, and Fleming may have felt obligated to offer uplifting news. He hinted at penicillin’s promise for saving the lives of Britain’s troops. Florey knew better. Britain had reached the limits of its manufacturing capacity. In his view, Fleming and St. Mary’s Hospital reaped publicity and donations based on false hopes. Between air raids, Florey and colleague Norman Heatley had been scrounging jars, bottles, even bedpans to keep up with the demand for new batches of penicillin. In 1941 both men obtained coveted tickets for Pan Am’s Dixie Clipper flight across the Atlantic. On the trip Florey carried a briefcase stuffed with mold cultures and a handful of vials of pure penicillin with the hope to get help from a large American drug company—they visited Merck, Pfizer, E. R. Squibb, and Lederle Laboratories—for mass-producing penicillin. As late as 1942, Britain’s version of mass production involved collecting Penicillium extract in bathtubs, and then rigging milking equipment for the purification steps.
Florey’s campaign for penicillin took a lucky turn when he visited Yale medical researcher John Fulton during his second year in the United States. Fulton told Florey of a local woman, Anne Miller, who had been dying with a seemingly incurable Streptococcus infection. Fulton had cajoled a few grams of penicillin from Merck in New Jersey, which Florey had visited the previous year. At 3:30 in the afternoon on a cold March day in 1942, Miller had been consigned to death with a fever over 100 degrees when she received her first dose of penicillin. By 4:00 the next morning her temperature had returned to normal. Miller’s recovery shocked even Fulton. He preserved her hospital charts, which now belong to the Smithsonian Institution. By the close of the war, American drug companies were producing 30 pounds of penicillin a year, enough to treat a quarter-million patients for a month.
In his acceptance speech for the 1945 Nobel Prize in medicine Fleming shared with Florey and Chain, he commented on the future of antibiotic drugs. Perhaps, Fleming mused, a time would come when anyone with real or perceived illness could get penicillin. “The ignorant man,” he warned, “may underdose himself and by exposing his microbes to non-lethal quantities of the drug, make them resistant.” Fleming described a hypothetical scenario of resistant bacteria infiltrating families, and then entire communities. On that December day the story of penicillin’s discovery captured the public’s imagination more than the remote oddity of resistant bacteria.
Mutant wars
Alec Fleming’s fear of antibiotic overuse and misuse soon became reality. Doctors began prescribing antibiotics for minor injuries, headaches, colds, flu, and other ailments. Even perceptive physicians who worried over indiscriminate use of the drugs could be badgered into prescribing them by patients who felt lousy. The patients did not know or perhaps did not care that antibiotics had no effect on colds, the flu, and other viral infections.
In the 1960s, rather than slowing down to do more research on antibiotics, agriculture stepped up the use to fight imaginary infections and put more weight on livestock or plump poultry before sending them to market. Resistant bacteria began showing up in places in addition to hospitals. A microbiologist taking a sample of bacteria from a person’s digestive tract, skin, or mouth, or from natural waters and soil had a very good chance of finding more than one resistant species. Antibiotic-resistant bacteria now settle on kitchen counters, gym equipment, and in locker rooms. Franz Reinthaler showed in 2003 that antibiotic-resistant E. coli exists at every step in wastewater treatment, and most of the strains tested have resistance to more than one antibiotic. The microbial world has become almost saturated in antibiotics and thus in antibiotic-resistant microbes.
Bacteria excel at adaptability. Bacteria carry genes that confer antibiotic resistance in their large DNA molecule, the chromosome, and also on small circular strands of DNA called plasmids that stay separate from the chromosome in the cytoplasm. Resistance genes give bacteria the ability to fight antibiotics in five ways: (1) by cleaving antibiotics into pieces, (2) blocking an antibiotic’s penetration into the cell by altering the drug’s normal entry site, (3) pumping the antibiotic out of the cell as soon as it penetrates, (4) repairing any damage the drug does inside the cell, or (5) altering metabolism to lessen the antibiotic’s damaging effects. Put another way, bacteria have at least as many tactics for resisting antibiotics as antibiotics have modes of action.
Penicillin, sulfa drugs, and the other new antibiotics introduced in the 1940s and 1950s delivered some remarkable cures. Doctors treating very sick patients were probably tempted to try antibiotics on nonbacterial diseases in the hope that the drug helped kill secondary infections even while they knew the primary infection had been caused by a virus. If a doctor noticed that an antibiotic began losing strength, he would simply prescribe a new antibiotic. Sometimes a patient received both drugs at the same time. Two antibiotics together stymied bacteria for a while, but this strategy created problems. Any two random antibiotics cannot be paired and expected to work better than either drug alone. Certain antibiotics lower the activity of the second: streptomycin inhibits chloramphenicol’s activity; erythromycin blocks penicillin’s activity. When tetracycline acts on Staphylococcus, for instance, it inhibits protein synthesis in mature cells. But penicillin requires new, growing cells to exert its activity against the cell wall. By slowing bacteria’s growth, tetracycline neutralizes penicillin’s mode of action.
Multiple antibiotics, even when paired correctly, also led to multidrug resistance. Bacteria now evade many antibiotics at the same time. This is not an extraordinary talent as nature already exposes bacteria to more than one antibiotic or bacteriocin at a time, and multidrug resistance probably already existed in a minority of bacteria. Soil bacteria face a dense community of antibiotic-producing fungi and bacteria that make antibiotic resistance essential for survival. The proliferation of antibiotic use from the 1950s to the1980s merely accelerated the evolution of antibiotic defenses.
Some bacteria began carrying additional resistance genes for more than one antibiotic. Methicillin-resistant Staphylococcus aureus (MRSA) has separate genes that control resistance for the penicillin family of antibiotics as well as genes for resisting tetracycline, clindamycin, aminoglycoside, and erythromycin.
Bacteria with pump mechanisms could eject an antibiotic as soon as the drug passed through the cell wall and membrane. These bacteria developed more sophisticated systems to resist multidrug treatment with an adaptation called the ABC transporter, for “ATP-binding cassette transporter.” (A cassette is a set of genes that work as a team.) Present in bacteria, archaea, and eukaryotes, ABC transporters are proteins that help pump certain harmful molecules out of the cell. (Cancers that do not respond to chemotherapy resist the treatment in part by employing ABC transporters to eject the drug from tumor cells.)
ABC transporters consist of two proteins that span the bacterial membrane from the inner surface surrounding the cytoplasm to the membrane’s outer surface. The two proteins form a pore through the membrane. By expending energy, the cell uses this pore to expel a variety of chemicals, including more than one type of antibiotic. About 30 different types of ABC transporters exist among bacteria to eject from cells the diverse chemicals that can harm them in their environment. In addition to antibiotics and bacteriocins, transporters carry bile salts, immune system factors, hormones, and carried chemicals called ions, and they have recently been shown to adapt to, and eject, human-made antibiotics.
Multidrug resistance among bacteria has now become more prevalent than resistance to a single antibiotic. Some bacteria carry so many defenses it seems as if they were designed specifically to defeat drug companies’ best efforts. The tuberculosis bacterium Mycobacterium tuberculosis contains 30 different ABC transporters that provide the species with a defense that acts as a backup to other defensive schemes. First, the microbe’s unusual cell wall composition prevents the penetration of many antibiotics that work on other bacteria. The ABC transporter system acts on any antibiotic that manages to get past the cell wall. Second, M. tuberculosis’s capability to hide inside cells of the immune system enables it to elude antibiotics circulating the bloodstream. Third, these bacteria grow like the tortoise compared with E. coli’s hares. Slow growth may not in itself be a defensive tactic, but this characteristic of the species forces doctors to lengthen the antibiotic treatment for TB. Because most antibiotics work best on actively dividing bacteria, M. tuberculosis’s growth rate lessens an antibiotic’s killing efficiency. Typical TB treatment lasts six months or longer, and this alone favors the pathogen because even diligent patients have a hard time staying on a drug regimen for that long.
The multiple defenses of M. tuberculosis necessitated more than one antibiotic when doctors began treating the disease with antibiotics in the 1940s. Two antibiotics worked well for many years, but now this species requires four different drugs to kill it, and many strains already resist all four, leaving doctors with a dwindling choice of antibiotics that still work against TB. Like other bacteria, when M. tuberculosis has developed a favorable trait, it keeps the gene for that trait in its DNA. Multidrug resistance has also become common in skin infections, sexually transmitted diseases, and pneumonia.
Following Germany’s 1936 introduction of sulfa drugs to cure gonorrhea, resistant strains of Neisseria gonorrhoeae had spread throughout the country by 1942. Doctors turned to penicillin as soon as U.S. drug companies made large quantities available. Before the 1960s had arrived, resistant N. gonorrhoeae capable of cleaving penicillin into pieces had spread around the globe. Almost all Staphylococcus species had already become resistant to penicillin 15 years earlier. Bacteria have become so efficient in building and sharing resistance that they no longer need months or years to adapt. Four days after streptomycin therapy begins, for a kidney infection for instance, streptomycin-resistant bacteria outnumber the susceptible bacteria in patient urine samples.
Bacteria possess an effective defense against antibiotics: the plasmid. Bacteria of the same species or sometimes dissimilar species pass plasmids back and forth and thereby give each other useful traits they would not normally possess. Sometimes bacteria insert a resistance gene from their chromosome into a plasmid before passing the plasmid to other cells. Cells also share entire DNA segments from the chromosome by absorbing pieces when another cell dies and breaks apart or by connecting cell-to-cell in a version of bacterial sex.
Microbiologists have tried various approaches to outmaneuver bacterial defenses against antibiotics. One ploy called the Trojan Horse takes advantage of the competition for iron among living things in nature. Because iron can be scarce in many habitats, bacteria produce compounds called siderophores to seize hold of precious iron molecules and bring the metal into the cell through a specific pore. Microbiologists have designed siderophores that instead of grabbing iron will bind to an antibiotic. When bacteria recognize the siderophore, they open the pore to let it in and thus allow the antibiotic to enter.
If certain bacteria do not fall for the trick of smuggling an antibiotic into their cells, microbiologists try substituting the metal gallium for iron in siderophores—the two metals look similar to bacteria—to derive the bacteria of essential iron.
Microbiologists have another weapon at their disposal in bacteriophages or phages, which are viruses that attack only bacteria. In the microbial world, bacteria look like the mother ship to a phage’s fighter jets. A phage measures 225 nanometers (nm) at most at its longest end-to-end distance; a typical bacterial cell volume is 1,300 times the volume of a phage.
Microbiologists have revived Felix d’Herelle’s idea of a century ago by designing phages to enter bacteria and inactivate bacterial repair kits or shut down antibiotic pumps. This method has already been tried in humans to correct genetic diseases in the new science of gene therapy. In gene therapy, molecular biologists engineer viruses that infect humans to contain a specific gene that will repair a defect in human DNA. They inactivate the virus so that it cannot cause disease but can still infect the human host. When the engineered virus takes over the cell’s DNA replication, it inserts the new gene into the defective DNA.
Phages built to deliver antibiotics or foil the defenses of resistant bacteria have not been tried outside laboratory trials. But because of the constant evolution of bacteria to avoid harm from drugs, biology must stay abreast with new weapons of its own.
Bacteria share their DNA
Gene transfer confers on bacteria the capability to accept helpful genes from other microbes. In eukaryotes from algae all the way up to humans, gene transfer occurs by one mechanism, the fusion of gametes. One gamete from a female and one from a male creates a zygote that carries the DNA from both parents. Bacteria and archaea have three major routes whereby they exchange genes: transformation, transduction, and conjugation. All of these methods are called horizontal gene transfer because they occur between two or more adult cells rather than the standard sharing of genes by producing daughter cells.
Transformation occurs when bacteria take in DNA directly from the environment. The DNA may be either the molecule from the nucleoid or a plasmid. In either case, the DNA dissolves in an aqueous environment when a cell dies and lyses. A live bacterial cell encountering the DNA in its habitat may attach to the molecule and use an enzyme to unravel the large polymer. DNA is a double-stranded structure resembling a ladder. The enzyme cuts the ladder’s rungs to separate the DNA in half. One half degrades, but the cell pulls the other half inside where it will incorporate it into its own DNA.
Transduction occurs when a bacteriophage infects a bacterial cell and brings DNA from another microbe with it. If the phage commandeers the cell’s DNA replication steps but does not kill the bacterium, the bacterial cell makes new progeny containing some of the foreign DNA. New bacteria never before seen in nature begin growing.
When plasmids transfer between cells, they do so by conjugation. Conjugation has been called the bacterial version of sexual reproduction because two cells physically connect with one another by a tube called a sex pilus. After DNA has moved through the pilus from the first cell to the second, the pilus breaks. As a result of conjugation, the receptor cell incorporates new genes into its existing DNA. When the cell divides, the daughter cells and each successive generation can carry these genes.
Gene transfer in bacteria has its most profound effects in allowing antibiotic-resistance genes to move through a population of bacteria. The bacteria need not be closely related as long as they can use one of the three methods described above for passing DNA back and forth. Since plasmids have been shown to carry multiple genes for antibiotic resistance, plasmid transfer may be a major route for the expansion of antibiotic resistance in the past few decades. Biologists have not answered all their questions on the evolution of gene transfer in bacteria, but there can be no question of the advantages these systems give to bacteria.
The opportunists
Hospitals act as hot spots for antibiotic-resistant bacterial infections because hospital settings have high antibiotic use and a patient community weakened by disease, trauma, or surgery. These circumstances open the opportunities for bacterial infection. Nosocomial infections are infections picked up in hospitals. Many of these infections could well come from doctors, nurses, technicians, and other hospital staff who do not wash their hands properly between patient visits. Secret observations of hospital staff have revealed that healthcare professionals wash their hands properly only slightly more often than the general public of which less than 50 percent wash properly. Most of these poor habits (not enough time washing hands, not enough soap, no soap, or no hand wash at all) occurred in the public restroom! Most hospitals now have resident bacteria in proportions found nowhere else in society, and these nosocomial populations have a high incidence of multidrug resistance. No wonder that people believe that any bacterium is a dangerous bacterium. This thinking spawned not only antibiotic misuse, but a similar overuse of disinfectants and other antimicrobial products.
Medical microbiologist Stuart Levy has warned that overzealous cleaning with disinfectants merely increases the opportunity for bacteria to develop resistance. Might disinfectant- and antibiotic-resistant superbugs share their best defenses with each other by exchanging genes? Such sharing seems implausible because the chemicals in cleaning products (bleach, quaternary ammonium compounds) differ from large antibiotic molecules. Yet bacteria eject these chemicals much the same way they expel antibiotics: They use a pumplike mechanism. The term “pump” can be misleading. Bacterial antibiotic efflux pumps use transporters inside the cell. When an antibiotic enters the cell through a receptive pore in the bacterium’s outer membrane, the transporter moves toward the antibiotic and locks onto it. A bacterial protein (called a fusion protein) then recognizes the transporter now reconfigured by the antibiotic and swiftly carries the complex out through another pore. As long as bacteria have the nutrients needed to build transporters and fusion proteins, they can resist antibiotics by excreting them. Because the transporter must recognize all or part of the antibiotic for this system to work, chemists try to construct unique antibiotics, and biologists seek new natural substances that will throw a monkey wrench into the antibiotic efflux pump. If molecular biologists discover that the chemical pump and the antibiotic pump are one and the same, a new super-superbug may be around the corner, able to resist disinfectants as well as it resists antibiotics. No one yet knows which side will win the race to perfect resistance or a perfect drug.
Surely the rise in antibiotic resistance has made a difference to the bacteria that have always lived in harmony with their host. When the body’s good bacteria cause infection, they do so because circumstances change to invite them in. These circumstances usually have to do with a weakened or immature immune system, mainly in groups of people considered “high-risk” individuals:
• Chronic, debilitating disease
• Drug or alcohol abuse
• Poor nutrition
• Pregnancy
• Old age
• Young age (infants and children under 12 years)
• HIV/AIDS
• Organ transplantation
• Cancer chemotherapy or radiation.
Each of the stressors listed here increases the dangerous cycle of antibiotic-resistance causing infection that requires antibiotics, leading to more resistance. One of the prevalent bacteria on the body, Staphylococcus aureus, has already become one of the most multidrug-resistant microbes known. Because S. aureus is both a health risk and a prominent member of the body’s normal flora, good personal hygiene usually trumps antibiotics, disinfectants, and other weapons from the antimicrobial armory (see Figure 3.2).
Figure 3.2. Court at No. 24 Baxter Street, ca. 1890. Photographer Jacob Riis captured life in one of New York City’s tenement slums. Similar living conditions exist today worldwide. Poor nutrition and faulty hygiene have contributed to germ transmission throughout history.

(Courtesy of Museum of the City of New York, Jacob A. Riis Collection)
Drug companies have for the past decade introduced fewer and fewer new antibiotics. Because “all the easy antibiotics have been discovered,” research into new natural or synthesized compounds has grown more difficult and more expensive. Companies that once led in antibiotic production have now decreased the money they spend on new antibiotic research. The combination of skyrocketing research costs and patents that limit the profit-earning future of drugs has left doctors with a shrinking armamentarium against infectious disease.
Entrepreneurs have tried colloidal silver, copper, zinc, magnesium, medicinal herbs (cloves, echinacea, garlic, oregano, turmeric, and thyme), citrus oils, tea tree extracts, and grapefruit seed extract. I have tested most of these substances on laboratory cultures, and they do possess antibacterial activity. But inhibiting bacteria in a laboratory is much easier than stopping bacteria in nature or in the body. In a laboratory, bacteria are at their most vulnerable to damage because antibiotics work best on rapidly multiplying cells. In nature, bacteria turn on defensive mechanisms and slow their growth. Both actions take away some of the power of antimicrobials.
A new generation of antibiotics may yet emerge. If they do, they will probably come from the ocean. In the past decade scientists have recovered marine bacteria, algae, sponges, coral, and microscopic invertebrates that produce novel antibiotics. The new marine antibiotics might soon replace current antibiotics that are losing the battle against Staph infections, gonorrhea, strep, tuberculosis, and nosocomial infections.
