Functional materials for hydrogen storage
Abstract:
Hydrogen is the lightest and most abundant element in the universe. The overall amount of hydrogen on Earth is around 0.74 wt%; it is mostly bound in water and carbon-based materials. The future challenges of a hydrogen-based energy economy include the production, distribution and safe storage of hydrogen. Of these, one of the most important is the safe storage of hydrogen for use by consumers in electronic devices, automotive applications or heating and burning processes. A simple technical- or chemical-based solution, which fulfills all the requirements in terms of size, weight and safety for these different applications, is not yet in sight. Therefore, a number of possible solutions for the storage of hydrogen in a solid state are currently being researched.
8.1 Introduction
Hydrogen is the lightest and most abundant element in the universe. The overall amount of hydrogen on earth is around 0.74 wt%; it is mostly bound in water and carbon-based materials, like plants, natural gas or crude oil. Due to its high enthalpy of combustion (− 286 kJ mol−1) and the absence of any environmental hazardous gases released from combustion, hydrogen is an attractive alternative as a secondary energy carrier. Future challenges of a hydrogen–based energy economy include the production, distribution and safe storage of hydrogen (Züttel et al., 2010). Hydrogen can be produced from a number of different sources and by a variety of methods. These include electrolysis of water, steam processing using natural gas, water splitting with thermo-chemical processes utilizing concentrated solar power or the production of hydrogen using certain species of algal. Large–scale distribution of hydrogen can be achieved by putting hydrogen gas under pressure in pipelines and high–pressure gas tanks, or alternatively using hydrogen in liquid form in super-insulated tank systems. One of the most important aspects is the safe storage of hydrogen for use by consumers in electronic devices, automotive applications or for heating and burning processes. A simple technical or chemical−based solution, which fulfills all the requirements in terms of size, weight and safety for these different applications, is not yet in sight. Therefore, a number of different solutions for the storage of hydrogen in a solid state are currently undergoing investigation. In this chapter, different promising examples of solid state hydrogen storage are summarized and some technical applications are presented.
8.2 Hydrogen storage with metal hydrides: an introduction
During the exposition of a pure metal or metal alloy, a metal hydride is formed according the following reaction scheme [8.1]:
∆H expresses the heat of the reaction, which is released during formation of a hydride. From a thermodynamic point of view the same amount of heat must be applied to the metal hydride during its decomposition. The thermodynamic properties determined by the hydrogen equilibrium pressure at a given temperature are important for the description and comparison of different metal hydride materials. These thermodynamic properties are usually described in terms of pressure-composition isotherms (PCI curves). An idealized description of this behavior is shown in Fig. 8.1. During the hydrogen uptake three different regions can be distinguished on the PCT curves:
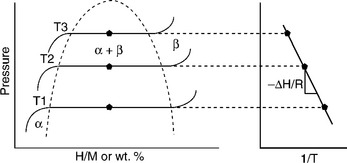
8.1 Schematic of a pressure–concentration isotherm. From the Van’t Hoff plot on the right the enthalpy of the hydride formation can be found.
• At low hydrogen concentrations the hydrogen is exothermically dissolved into the metal lattice, producing a solid solution of hydrogen in the lattice (α–phase).
• With increasing hydrogen pressure the α–phase becomes saturated and transforms into the β–phase, until the whole α–phase has disappeared. This region is described as a plateau, with the corresponding hydrogen pressure defined as the equilibrium pressure at the temperature T.
• At higher concentrations the hydrogen gas is dissolved into the β–phase (solid solution). No α–phase is present and the β–phase is enriched with hydrogen due to increasing pressure.
By measuring the isotherms at different temperatures the thermodynamic values of a metal hydride can be calculated with the Van’t Hoff equation, where T is the absolute temperature, ∆H and ∆S are the enthalpy and entropy of the system, and R is the gas constant, assuming ∆H and ∆S are constant over a small range of temperature:
Plotting ln peq versus the 1/T leads directly to the thermodynamic values of ∆H and ∆S (Fig. 8.1).
The reversibility of the metal hydride is important if this method of hydrogen storage is to function in practice. Reversibility means that a common material can be de- and re-hydrogenated under variable temperature and hydrogen pressure conditions. A reversible material always displays an endothermic decomposition, meaning that energy is necessary for the release of hydrogen. On the other hand, the re-hydrogenation, or refilling of the material with hydrogen gas, is an exothermic reaction, meaning that heat is produced. By varying the temperature and hydrogen pressure, a reversible metal hydride system can be pushed into hydrogen uptake or hydrogen release, whilst non–reversible materials cannot be manipulated in this way. Therefore these materials must be regenerated outside the tank system through high energy consumption coupled with chemical reactions. It may be possible for non-reversible materials to produce hydrogen in niche applications, but widespread distribution in mobile or stationary applications is not possible at this time.
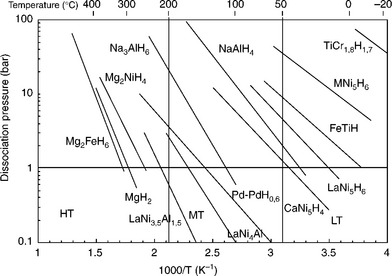
The temperature at which an equilibrium pressure of 0.1 MPa is reached is important for the classification of metal hydride compounds. This temperature is correlated with the energy uptake during the decomposition of a metal hydride and can be deduced from the Van’t Hoff equation. Metal hydride systems are divided into three different classes of materials. Low-temperature (LT) metal hydride systems can reach an equilibrium pressure of 0.1 MPa at temperatures lower than 50°C. These compounds have energy values lower or close to 40 kJ mol−1 H2 for the decomposition process. Medium-temperature metal hydrides are more stable compared to the LT metal hydrides. The equilibrium pressure of 0.1 MPa can be reached at a temperature range between 50°C and 200°C. This process is accompanied by an energy uptake amount of 40–70 kJ mol−1 H2. High-temperature (HT) metal hydrides can reach their equilibrium pressure at temperatures higher than 200°C and have reaction enthalpies greater than 70 kJ mol−1 H2 (Fig. 8.2).
8.3 Hydrogen storage with interstitial hydrides, AlH3 and MgH2
Three different types of interstitial hydrides are important for hydrogen storage (Sandrock, 1999). They are classified on the basis of their crystal structure and belong to AB2 (Laves phase) compounds, AB5 compounds and Ti-based body centered cubic (BCC) materials.
8.3.1 AB 5–compounds
A wide range of compounds with a composition of AB5 are known for their applications in hydrogen storage. Element A is often a lanthanide metal (or the much cheaper mischmetal, an alloy composed of variable amounts of rare earth metals), calcium or yttrium. In most cases the B element is nickel, but can be partially or completely substituted with ele– ments like Ti, Sn or Al. The properties of the materials can vary widely, depending on the substitutions on both the A side or the B side. The most significant material with regard to hydrogen storage is LaNi5, which can absorb up to 1.4 wt% of hydrogen, producing a material with the composition LaNi5H6. The enthalpy of the formation is − 31 kJ mol−1 H2, resulting in a plateau pressure of 0.16 MPa at 25°C. The AB5 hydrides have been widely used in commercial applications in the form of nickel-metal hydride Ni/MH-batteries. The AB5 hydrides represent the negative electrode of the battery.
8.3.2 Laves phases
Laves phases have the ideal composition AB2. A Laves phase can be described as a tetrahedrally closed packed structure from atoms A and B with the ideal ratio of the radii ra/rb = (3/2)1/2. These phases form the largest class of intermetallic compounds with more than 1400 different examples. Examples of ternary and multinary phases are also possible, with A or B in excess. Important factors affecting the stability of these materials are the geometry and packing of the atoms, the density of the material and the differences in electronegativity of both atoms. The hydrogen storage amount which can be achieved is in the range of 1–2 wt%; this is too low for broad usage in fuel cell systems. The Laves phase hydrides show good kinetics for hydrogenation and dehydrogenation and have high cycle stability (Shaltiel et al, 1977). Depending on the composition of the Laves phase, the hydrogen storage capacity and the heat of formation can vary a great deal. However, most are too stable for hydrogen storage at, or close to, room temperature (Bououdina et al, 2006).
8.3.3 The BCC compounds
Alloys with BCC structures can absorb hydrogen up to more than 3 wt% H2. These alloys are often prepared from the following compositions: Ti–V–Mn; Ti–V–Cr; Ti–V or Cr–Mn. The BCC systems usually exhibit two different PCI, but in most cases the lower plateau cannot be used for hydrogen storage purposes (Akiba and Okada, 2002). Multi-phase alloys consisting of a BCC and a Laves phase are known as Laves phase-related solid solutions (Akiba, 1999). These materials form two types of hydrides. The monohydride phase which is usually very stable and not useable for hydrogen storage under practical conditions and the dihydride phase which is useable under practical conditions. To increase the storable amount of hydrogen it is necessary to find new solutions to destabilize the monohydride system. This was shown for instance with the Ti–Cr–V system, where a small varia– tion in the amount of Cr had a large influence on the PCI curves (Tamura et al., 2003).
8.3.4 AlH3
With a hydrogen content of about 10 wt% AlH3 is an interesting candidate for solid hydrogen storage. The volumetric hydrogen capacity is 148 g L−1, which is twice the volumetric capacity of liquid hydrogen. It decomposes into Al metal and releases 1.5 moles of H2 [8.3].
From a thermodynamic point of view AlH3 is metastable at room temperature. The reaction enthalpy is around 7 kJ mol−1 H2 depending on the molecular structure of AlH3. This means that AlH3 is a kinetically stabilized compound, but the decomposition is hindered because of the kinetic restrictions (Graetz and Reilly, 2007). In ambient conditions it does not exists in a monomeric form and produces polymeric chains. It exists in six different polymorphic structures with different molecular connections depending on the synthesis procedure and conditions.
The direct synthesis of AlH3 from Al metal and hydrogen pressure is only possible under conditions which are very far from possible during technical applications (more than 700 MPa H2) (Sandrock et al, 2005). AlH3 requires off-board refueling which makes it an irreversible hydrogen storage material for mobile applications. This is one of the most significant restrictions of AlH3 as a hydrogen storage material in consumer friendly technical systems.
AlH3 decomposes rapidly at temperatures around 100°C. The rate of kinetics is high enough to feed a polymer electrolyte membrane (PEM) fuel cell for mobile applications, but demonstration projects which have shown that AlH3 could be a viable hydrogen source for fuel cells have lacked specific application.
The addition of alkali metal hydrides or catalysts based on LiH can accelerate the decomposition of AlH (Fig. 8.3). The decomposition temperatures of these mixtures are close to the working temperatures of a PEM fuel cell.
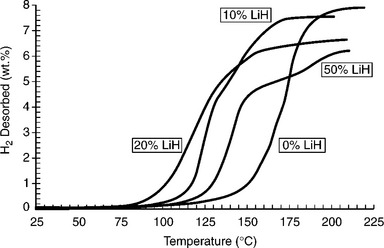
8.3 Effect of LiH doping on the TPD scans of ball-milled AlH3–LiH mixtures. LiH levels expressed in mol percent. Scan rate: 2°C min−1 (Source: With permission from Springer.)
One of the most important factors with regard to the usage of AlH3 as a hydrogen storage material is that the method for recovering Al metal in an off-board process back to AlH3 is inexpensive.
A simple laboratory preparation method for AlH3 is the reaction of one mole of AlCl3 with three moles of LiAlH4 according to the reaction:
This kind of reaction is much too expensive for large-scale preparation of AlH3. Electrochemical methods for the regeneration of AlH3 from Al metal are currently under investigation (Martinez-Rodriguez et al, 2012).
8.3.5 MgH2
Magnesium metal is an interesting material for the storage of hydrogen because of its low cost and high hydrogen storage capacity. One mol H2 is stored per mol Mg metal resulting in an overall amount of 7.7 wt% H2:
The pure MgH2 has an energy density of 9 MJ kg−1 Mg. This is the highest value of all metal hydride systems applicable for reversible hydrogen storage. The decomposition enthalpy of MgH2 is around 75 kJ mol−1 H2, meaning that this material belongs to the HT metal hydrides. It reaches an equilibrium pressure of 0.1 MPa at a temperature of 300°C. Therefore, it cannot be used for the storage of hydrogen in mobile systems or in combination with fuel cells. However, it could be used in stationary systems for the storage of hydrogen or heat. One possible example for this application is presented at the end of the chapter. The pure MgH2 shows slow kinetics for the de- and re-hydrogenation, but this can be significantly enhanced through a mechanochemical treatment (ball milling) or through the addition of different types of catalysts. The diffusion process of hydrogen into the bulk is critical for hydrogen absorption in magnesium and other metals. During the process of hydride formation the diffusion of hydrogen is not possible and it limits the rate of hydrogenation. Ball milling can change the microstructure of the metal/metal hydride, increasing the surface area and producing a large number of cracks and defects inside the bulk and on the surface layer. In addition to the mechanical treatment, the kinetics of the hydrogenation and desorption can be significantly increased by the addition of metals (for instance Ti, Ni, Co) (Ivanov et al, 1987), transition metal oxides (TiO2, Nb2O5) (Oelerich et al, 2001) or mixtures of metals and non–metal compounds. Hydrogenation times of only 500 s at a temperature of 200°C and 2 MPa H2 pressure have been obtained by utilizing ball milled MgH2 + 5 at % V. Under these conditions it is possible to achieve a hydrogen amount of 5.5 wt% (Liang et al, 1999).
MgH2 has been commercialized for stationary applications in different industrial processes by the company McPhy.
8.4 Hydrogen storage with complex metal hydrides
Complex hydrides are salt-like substances and exhibit ionic bonding between a positive metal ion (often alkaline or alkaline earth ions) with a molecular anion containing the hydride. The bonding of the hydrogen to the second metal (often Al or B) is significantly covalent, producing the anion. They have the general formula MxM’yHn. The most important of these materials are the light-weight compounds based on aluminum and boron, because of their high hydrogen weight amount.
Complex aluminum hydrides have been known for more than 50 years, but found only limited interest as materials for hydrogen storage. They show a high kinetic barrier for decomposition therefore requiring high temperatures and showing low rates for the hydrogenation reaction. The situation changed after the important observation that addition of Ti catalysts lowers the temperature for both reaction steps which become reversible under acceptable technical conditions (Bogdanovi![]() and Schwickardi, 1997).
and Schwickardi, 1997).
8.4.1 Introduction to aluminum hydrides
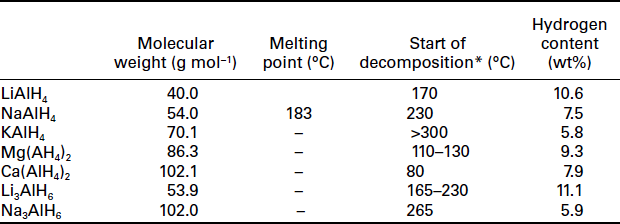
Complex aluminum hydrides are materials with high hydrogen content. Several physical properties of these compounds are shown in Table 8.1.
8.4.2 LiAlH
The overall hydrogen amount in LiAlH4 is 10.6 wt%. LiAlH4 undergoes a multistep process involving structural phase transformations and several decomposition steps with increasing temperature. The decomposition follows the expected mechanism of complex aluminum hydrides, with an octahedral intermediate in the first step:
Non–catalyzed LiAlH4 shows endothermic melting between 165 and 175°C, followed by an exothermic decomposition (∆H = − 10 kJ mol−1 H2) of LiAlH4 to Li3AlH4 and Al metal between 175°C and 220°C. Theoretically 7.9 wt% can be released during this process. The second decomposition step is endothermic (∆H = 25 kJ mol−1 H2) with the formation of LiH and Al metal. The last dehydrogenation step is the decomposition of LiH between 370°C and 483°C. Due to the exothermic decomposition of LiAlH4 to Li3AlH6, a re–hydrogenation is impossible and LiAlH4 cannot be used as a reversible hydrogen storage material. LiAlH4 is a thermodynamically unstable material at room temperature with restricted kinetics for the solid-state transformation from the tetra-to the hexahydride.
8.4.3 NaAlH4
There has been considerable interest in sodium aluminum hydride over the last 15 years, due to its high hydrogen content and its reversibility under acceptable technical conditions. The two important reversible steps of the decomposition/re–hydrogenation of NaAlH4 are
The first step releases 3.7 wt% H4, producing Na3AlH6 as an intermediate compound, Al metal and three moles of hydrogen. The enthalpy of the reaction was measured to be ∆H = 37 kJ mol−1 H2, meaning that in this reaction, sodium aluminum hydride is a typical LT-metal hydride. At higher temperatures the intermediate Na3AlH6 releases 1.9 wt% of hydrogen and produces NaH and again Al metal. This reaction step is typical for MT-metal hydrides with a decomposition enthalpy of 47 kJ mol−1 H2. The decomposi– tion temperature for reaction [8.11] is too high and therefore not used for the delivering of hydrogen. Under the influence of a catalyst the system is reversible at higher hydrogen pressure.
NaAlH4 shows structural changes like most of the complex aluminum hydrides. During the decomposition a tetrahedral coordinated Al atom in sodium alanate is converted into an octahedral coordinated Al atom in Na3AlH6. As a separated phase pure Al-metal is produced during the decomposition. The mechanism of this segregation process is still a point of discussion (Frankcombe, 2012; Felderhoff and Zibrowius, 2011).
NaAlH4 can be produced from the constituent elements in a hydrocarbon solvent according to
Elevated temperatures of 100–200°C and a hydrogen pressure of 10–20 MPa are necessary. For an accelerated reaction rate a catalyst-like triethyl aluminum is added.
A simple ball milling method uses NaH, Al metal and TiCl3 as catalyst (Fig. 8.4). Under hydrogen pressure of 8 MPa a Ti-doped NaAlH4 suitable for hydrogen storage can be formed (Bellosta von Colbe et al, 2005).
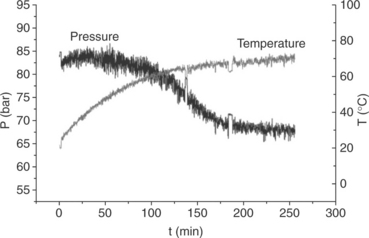
8.4 Evolution of hydrogen pressure and temperature during the ball milling synthesis of Ti-doped NaAlH4. (Source: Reproduced by permission of The Royal Society of Chemistry.)
Doping agents and methods
Titanium trichloride (TiCl3) is one of the most important doping agents for NaAlH4 with ball milling the method of choice for a fast and efficient doping procedure. During the doping process of NaAlH4 with TiCl3, a reduction to the zero-valent state of the titanium compound and irreversible evolution of hydrogen gas can be observed [8.13]. The theoretical capacity of 5.6 wt% cannot be reached with doped samples due to the presence of dead weight from NaCl and loss of Al metal. A higher amount of dopant, which is necessary for increased kinetic rates, results in lower hydrogen storage capacities. A doping level of 2–4 mol% is considered reasonable. Similar to TiCl3, other transition metal chlorides, for example CeCl3 and ScCl3, can be used as doping agent (Bogdanovi![]() et al, 2006; Wang et al, 2008). Re-hydrogenation times of several minutes can be achieved with these materials. Similar to the TiCl3 reduction during the ball milling process, a reduction to the metallic state can be expected for these chlorides.
et al, 2006; Wang et al, 2008). Re-hydrogenation times of several minutes can be achieved with these materials. Similar to the TiCl3 reduction during the ball milling process, a reduction to the metallic state can be expected for these chlorides.
The nature of titanium precursors is important for performance. Titanium trifluoride shows the highest storage capacities of the titanium halides, since NaF, produced instead of NaCl, is much less in weight (Majzoub and Gross, 2003).The high activity of the metallic state for the de- and rehydrogenation process was also shown with Ti13 nanoparticles (Bogdanovi![]() et al, 2003; Fichtner et al, 2003). With these dopants, re-hydrogenation times of only 10 min at 10 MPa H2 pressure and 100°C can be achieved. Unfortunately, the reaction rates decrease over time and the synthesis of these colloids is expensive. Titanium colloids are no longer used as preferred doping materials. Using the cerium aluminum alloy CeAl2 as dopant for NaAlH4 a dramatic enhancement in the hydrogen release and uptake can be observed. Re-hydrogenation amounts of 4.9 wt % can be achieved under moderate conditions in 20 min, which is very close to the theoretically expected values (Fan et al, 2011).
et al, 2003; Fichtner et al, 2003). With these dopants, re-hydrogenation times of only 10 min at 10 MPa H2 pressure and 100°C can be achieved. Unfortunately, the reaction rates decrease over time and the synthesis of these colloids is expensive. Titanium colloids are no longer used as preferred doping materials. Using the cerium aluminum alloy CeAl2 as dopant for NaAlH4 a dramatic enhancement in the hydrogen release and uptake can be observed. Re-hydrogenation amounts of 4.9 wt % can be achieved under moderate conditions in 20 min, which is very close to the theoretically expected values (Fan et al, 2011).
State of the dopant
Up to now the exact nature of the titanium catalyst and how the catalyst acts in the Ti–NaAlH4 system are still unclear.
Hydrogen is evolved during the doping process of NaAlH4 with titanium compounds in different oxidation states [8.13–8.14]. The amount of hydrogen depends on the oxidation state of the titanium compound and is proportional to a reduction of titanium and aluminum to a zero-valent metallic state (Bellosta von Colbe et al, 2004):
The above finding is supported by extensive X–ray absorption studies of Ti-doped NaAlH4. It has been concluded that the formal titanium valence is zero and does not change during the cycling process (Felderhoff et al., 2004; Graetz et al., 2004; Léon et al., 2004).
It was shown that the Ti-atoms are dispersed in the metallic Al phase in a state which resembles a TiAl3 alloy. During the whole de-and re-hydrogenation process the Ti metal remains dispersed in the Al alloys. Dehydrogenation produces Al metal and dilutes the TiAl alloy. During the re-hydrogenation Al metal is used for the generation of NaAlH4 and the concentration of Ti inside the alloy increases. It could be also shown that the amount of dopant changes the thermodynamics of the system (Streukens et al., 2006). This was determined by the equilibrium pressure of Ti-doped NaAlH4 depending on the doping concentration. It is this dilution process which gives an additional contribution to the free energy of the system and destabilizes it at higher dopant concentrations.
Doped NaAlH4 has been one of the most researched hydrogen storage materials over the last 15 years. It shows good re-hydrogenation performance and is stable over hundreds of cycles. One major disadvantage is the two step decomposition, which excludes this material from use as hydrogen storage in mobile systems with fuel cells. Nevertheless, the first decomposition step alone has twice the storage capacity of interstitial metal hydride systems. The possible use of this material as a hydrogen storage material in tank systems was shown in several research projects with amounts of more than 50 kg of doped NaAlH4 (Johnson et al, 2011; Bellosta von Colbe et al, 2012).
8.4.4 Complex borohydrides
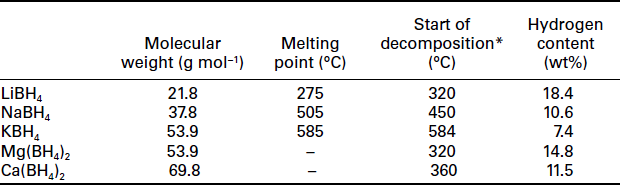
Complex borohydrides belong to a class of hydrogen materials with very high gravimetric hydrogen density, because of the low atomic weight of boron and the high amount of bound hydrogen atoms. Chemical and physical data from a number of important examples of complex borohydrides are summarized in Table 8.2.
The hydrogen content in LiBH4 is 18.4 wt%. From this value it seems that LiBH4 is attractive for hydrogen generation or hydrogen storage, but possible applications are limited because of the thermal stability of LiBH4 and small amounts of B2H6 liberated in addition to the released H2. Most of the hydrogen is released from the liquid phase (melting point of LiBH4 is 280°C) in a multistep decomposition process at temperatures higher than 320°C. Up to 600°C an overall amount of 9 wt% can be observed (Züttel et al., 2003). From Raman spectroscopy, X–ray diffraction studies and NMR investigation it has been shown that the extremely stable B12H122− cluster is formed as an intermediate during the decomposition. The formation of stable B.2H122− anion makes the re−hydrogenation difficult (Züttel et al., 2007). It is in principle possible, but a re−hydrogenation starting from LiH + B metal needs rigorous conditions (690°C and 20 MPa H2) and is still incomplete after 12 h.
NaBH4 contains 10.4 wt% H2, but also shows unfavorable thermodynamics for reversible hydrogen storage. Unlike LiBH4, liberation of hydrogen from NaBH4 is observed from the solid state. It starts at about 240°C and proceeds in several steps. Up to 450°C only 1 wt% of the total amount is released. Temperatures of more than 450°C are necessary for the decomposition of most of the material. NaBH4 was discovered as a non-reversible hydrogen releasing material in alkaline solutions.
One other borohydride with a high hydrogen content is Mg(BH4)2, with an overall amount of 14.8 wt% H2. It can be simply prepared from a metathesis reaction starting from MgCl2 and 2 LiBH4 in the solid state or in ether solutions. Mg(BH4)2 shows high thermal stability, starting decomposition around 300°C. Hydrogen is liberated in a multistep process according to Equations [8.15]–[8.17], with an overall amount of 13 wt% H2 up to 500°C:
Similar to LiBH4, the formation of very stable B12H122− clusters as intermediates is detected during the decomposition of Mg(BH4)2. Re-hydrogenation is difficult and leads to an amount of only 3 wt% at temperatures of 350°C and 10 MPa, because of the high kinetic barrier to hydrogenate Mg(B12H12) (Soloveichik et al., 2009). Under rigorous conditions (95 MPa, 400°C, 108 h) it is possible to store around 11 wt% H. in Mg(BH.)2 starting from the decomposition product MgB2. (Severa et al., 2010). Decomposition of Mg(BH4)2 at 200°C in vacuum results in the formation of magnesium triborane Mg(B3H8)2, which can be nearly completely re-hydrogenated at 250°C under a pressure of 12 MPa H2 in 48 h (Chong et al, 2011).
8.5 Hydrogen storage using other chemical systems
8.5.1 Amide–imide systems
The hydrogen storage process in the Li–N–H system involves a two–step process according to
The overall gravimetric capacity is 10.4 wt% starting with lithium nitride Li3N. In the first step one mole of hydrogen can be absorbed producing a mixture of the lithium imide and lithium hydride. The additional absorption of one mol of hydrogen produces lithium amide and one additional mole of lithium hydride. A first description of reversible hydrogen release and uptake was more than 100 years ago (Dafert and Miklauz, 1909), but the systematic discovery of the hydrogen storage process started with the research activities of Chen and co-workers (2002). Under practical conditions only the second step in Equation [8.18] can be used for reversible hydrogen storage. Therefore one mole of LiH can be eliminated and the reversible hydrogen storage process can be described with Equation [8.19]. Under these conditions the theoretical storage capacity is 6.5 wt.%:
The decomposition enthalpy ∆H for this process was determined from PCI measurements to be − 66 kJ mol−1 H2 for the hydrogenation process (Chen et al, 2002; Kojima and Kawai, 2005). The temperature necessary for the decomposition is higher than 200°C. The Li–N–H system requires unsuitable thermal conditions for practical applications, therefore several other metal–N–H systems have been developed over the last few years (Chen et al, 2007). One of the most promising appears to be a Li–Mg–N–H system, which can achieve a reversible hydrogen storage capacity of 5.5 wt% (Leng et al., 2004).
A drawback of all amide–imide systems is the possible emission of small amounts (up to several hundreds ppm) of NH3 during the decomposition process, which reduces the hydrogen storage capacity over time and can poison a fuel cell system. Strategies for the absorption or complete depression of NH3 production are necessary for the potential application of these materials as hydrogen storage systems.
8.5.2 Hydrolysis reactions
In principle, any metal hydride can be used in a hydrolysis reaction with water in order to release hydrogen, but only a limited number of materials have been found to be applicable. The overall amount of evolved hydrogen is double that of the number of hydrides in the metal compound; this is because half of the hydrogen released comes from water. The big disadvantage of this process is that it is irreversible, meaning that almost all of the hydride cannot be recovered from the solution. Simple metal hydrides like CaH2 are used in a hydrolysis process for the filling of weather balloons, where heavy gas pressure bottles are difficult to transport.
NaBH4
Complex sodium borohydride can deliver hydrogen at higher temperatures, but it is also used for the production of hydrogen in a hydrolysis reaction with water (Amendola et al, 2000). According to Equation [8.20] two moles of water are necessary for a full decomposition of NaBH4, producing sodium metaborate NaBO2 and four moles of hydrogen. Half of the evolved hydrogen comes from the water increasing the amount of theoretically delivered hydrogen to 10.8 wt%. A ruthenium catalyst is often used to decompose the mixture. The decomposed solution is then stored in a second tank system. In practice, the solubility of NaBH4 and NaBO2 in water are the limiting factors of this type of storage system. Precipitation of solid particles from the solution must be prevented, as this can inhibit the active sides of the catalyst. Typically a solution of 20 wt% in NaBH4 is used (1 wt% NaOH for stabilization of the solution). This reduces the overall storage capacity to roughly 4 wt% H2 for the whole system:
The decomposed solution can in principle be recovered, but an energy extensive chemical operation is required. First the decomposed materials NaBO2 must be separated from the water solution, carefully dried and then recovered in a chemical reaction with other metal hydride compounds. This makes the whole process expensive and therefore not attractive for wide distribution as a hydrogen releasing compound.
Zinc
Gas cells can be used to provide small amounts of hydrogen necessary for micro fuel cells (Hahn, 2008). The hydrogen is produced by the simple reaction of zinc with water [8.21]. The gas cell is very similar to a zinc–air cell, composed of a zinc electrode with KOH as the electrolyte. Hydrogen production begins through an electric short-cut of the external poles. The hydrogen produced leaves the cell through a small outlet and can then be piped to the fuel cell. The gas production is in direct correlation with the power of the electric current flowing through the cell. Gas production stops instantly when the electric circuit is interrupted. These systems are commercially available, producing up to 1000 mL H2, depending on the size of the cells:
This amount is high enough to feed a micro fuel cell with hydrogen for several months. The empty gas cell can be cast off like a similar zinc air cell.
8.5.3 Ammonia (NH3) and ammonia compounds
Ammonia is a well-known chemical substance with a gravimetric hydrogen capacity of 17.8 wt% H2. From this perspective it is a very attractive opportunity for the storage and transportation of hydrogen (Faleschini et al, 2000). It has a boiling point of − 33°C, but can be liquefied under slightly higher pressure. Ammonia is one of the most important base chemicals in the world and more than 130 million tonnes are produced every year. The industrial process for the synthesis of ammonia is known as the Haber–Bosch process. This process consists of the reaction of nitrogen and hydrogen gas under the influence of iron or ruthenium catalysts at pressures in the range of 15–25 MPa, at temperatures between 300 and 550°C. Given that pure hydrogen is necessary for the production of ammonia, the usage of ammonia as a hydrogen storage material is realistic for limited applications. One disadvantage of this is the toxic properties of ammonia through inhalation, which makes broad distribution as a hydrogen storage material difficult.
There have been several designs of fuel cells which can operate without cracking the ammonia. For example, solid oxide fuel cells can use ammonia directly as a feed (Wojcik et al, 2003). The recently developed DAFCs (direct ammonia fuel cells), based on proton-conducting ceramic electrolytes and molten salts, have shown highly efficient conversion of ammonia to electric power (Rees and Compton, 2011).
Solid storage of ammonia can be done using metal ammines. One important example of this is the reversible storage of ammonia into MgCl2 (Christensen et al, 2005). This proceeds in three steps, resulting in the absorption of six moles of ammonia in one mole of MgCl2:
Ammonia can be released within a temperature range from 100°C up to 300°C. The absorption and desorption of ammonia are reversible processes which can be repeated several times. The ammonia released can then be used in a cracking process for the production of hydrogen or as a feed for a DAFC.
NH3BH3
From a weight consideration, the simple ammonia borane NH3BH3 is very attractive for the storage of hydrogen, because the hydrogen content is 19.4 wt% (Stephens et al., 2007). The volumetric hydrogen density of NH3BH3 is more than 100 g L−1; much more compared to liquefied hydrogen, with a volumetric density of no more than 70 g L−1. The decomposition and hydrogen release show an exothermic behavior, making it different to the metal hydride systems. As a consequence, this hydrogen storage material must be regenerated outside of the storage tank by a chemical processing pathway. This makes the regeneration process more complicated and a completely new infrastructure is required for the distribution and the collecting of decomposed material.
The release of hydrogen from ammonia borane is dependent on the temperature during a number of decomposition steps (Baitalow et al, 2002). The complete thermal decomposition pathway is complicated and not fully understood. The formed reaction products and the reaction rates depend on the reaction conditions. A simple description is given in Equation [8.23], with the release of one mol H2 per formula unit. Depending on experimental conditions and temperature levels different volatile by–products are formed (Baumann, 2003):
The replacement of one of the hydrogen atoms in ammonia borane with alkaline or alkaline earth metals results in the formation of amidoborane compounds. These materials show high hydrogen content (up to 11 wt%) and moderate dehydrogenation conditions (Chua et al.,2011). Amidobranes are kinetically stabilized compounds with exothermic decomposition behavior. Because of this unfavorable thermodynamics these materials must be regenerated outside of the tank system using chemical synthesis methods.
Lithium amidoborane contains 11 wt% H2 and releases two moles of hydrogen according to
Lithium amidoborane starts to release hydrogen around 85°C, with the maximum release of hydrogen decomposition around 90°C. The thermodynamics of the decomposition are less exothermic with a value of − 3 kJ mol−1 H2 (Xiong et al., 2007). This value is close to a thermal neutral decomposition reaction. In order to stimulate reversibility in the materials they must be modified so that the decomposition steps become endothermic rather than exothermic.
8.6 Hydrogen storage with porous materials and nanoconfined materials
The adsorption (physisorption) of a gas molecule onto the surface of a material is the consequence of dispersive interactions between the gas molecule (adsorbate) with the surface (adsorbent) resulting from the fluctuation of the charge distribution between the surface and the molecule. Because these interactions are weak, significant adsorption is only observed at liquid nitrogen temperatures (77 K).
The hydrogen uptake of porous materials depends strongly on the specific surface area (SSA), the pore structure and pore size of the material. Adsorption enthalpies for hydrogen on the surfaces of porous materials like carbon or metal-organic frameworks are in the range of 2−5 kJ mol−1 H2. This means that significant adsorptive capacities can only be reached at liquid nitrogen temperatures and at pressures of several MPa of hydrogen. The hydrogen uptake seems to be limited by the SSA and the pore size of the material. Materials with a large surface area and pore sizes lower than 2 nm are ideal for high amounts of hydrogen adsorption. Small pore diameters mean that the adsorption potential in front of opposite pore walls can overlap resulting in increased adsorption energies.
The storage capacities of different types of porous materials depend on the surface area of these materials. A roughly linear correlation of the storage capacity with the specific surface area can be observed with a proportional constant of 1.9 × 10−3 wt% g m−2 at 77 K.
Different classes of materials with large surface areas have been tested for hydrogen storage at low temperatures (Panella et al., 2005; Thomas, 2007). Carbon-based materials and metal organic framework (MOF) materials are the most promising systems.
8.6.1 Carbon–based materials
A wide variety of different carbon structures can be synthesized, depending on the synthetic procedure used. These carbon nanostructures differ in the way the benzene hexagons are arranged in relation to each other. Carbon nanofibers, multi-walled carbon nanotubes, singlewall carbon nanotubes or carbon nanohorns are typical examples. It has been demonstrated that the hydrogen storage capacity of the material is more or less a function of the SSA of the carbon structure and that it is independent from the type of the carbon material (Fig. 8.5). Activated carbon can be prepared from the treatment of coke with KOH which has a specific surface of 2564 m2 g−1 and a pore diameter of 1.8 nm. The storage capacity is 0.5 wt% at room temperature and can be increased to 4.4 wt% at 77 K, which is one of the highest storage capacities of all carbon materials (Panella et al, 2005).
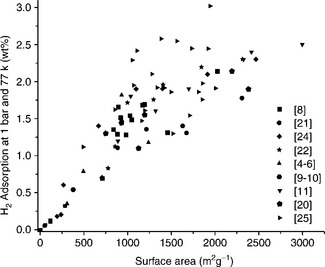
8.5 The variation of H2 adsorbed at 0.1 MPa and 77 K with the surface area for different carbon materials. (Source: With permission from Elsevier.)
Interest in carbon nanotubes and nanofibers increased a great deal several years ago in relation to hydrogen storage because of the exceptional hydrogen storage capacities which were reported by Chambers et al. (1998) and Dillon et al. (1997). But these reported values could never be reproduced by other researchers. Later on, it was shown that these values were attributed to experimental errors during measurements. Purified carbon nanotubes and nanofibers showed hydrogen storage uptake of no more than 0.6 wt% at room temperature and up to 4 wt% at 77 K.
8.6.2 Metal–organic frameworks (MOFs)
MOFs are porous materials with remarkably large surface areas. They consist of a metal ion connected by a bivalent organic linker. Typical linkers are polyvalent organic acids or compounds with nitrogen donors (Yaghi et al, 2003). One of the most widely researched materials is the so–called MOF-5. A ZnO4 cluster sits on every corner of a cube; these clusters are connected with a divalent 1,4−dicarboxylate ligand, producing a three−dimensional rigid network (Plate V – see color section between pages 238 and 239). Because of the high amount of free space inside the network the density of these materials is extremely low, down to 0.2 g cm−3.
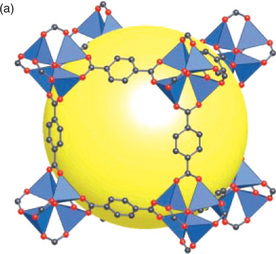
Plate V The MOF–5 structure is shown as ZnO4 tetrahedra (blue polyhedra) joined by benzene dicarboxylate linkers (O, red and C, black). The yellow sphere represents the pore diameter of 12 Å. (With permission from Nature Publishing Group.)
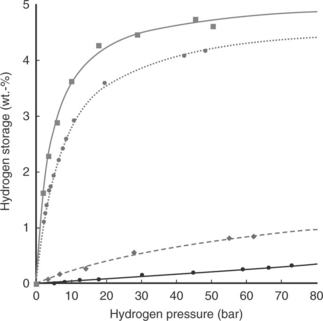
The maximum hydrogen which can be adsorbed is around 5 wt% at 77 K and 8 MPa H2, but at room temperature the capacity drops down to less than 0.2 wt% (Panella et al, 2006). The hydrogen uptake depends heavily on temperature and pressure. 8.6 shows the dependency of the adsorption isotherms of MOF-5 at various temperatures.
8.6.3 Nanoconfined materials
Nanoconfinement allows for the preparation and stabilization of small hydrogen storage particles with crystallite sizes of < 10 nm. A porous matrix or scaffold is necessary for this stabilization wherein the materials can be embedded. The impact of nanoconfinement on hydrogen sorption kinetics, thermodynamics and reversibility has been demonstrated over the last few years (Jongh de and Adelhelm, 2010).
Nanoconfined NH3BH3 in a nanoporous scaffold shows significant changes in decomposition behavior. The temperature required for hydrogen release is about 15°C less compared to that required for the neat material, and the activa tion energy of the decomposition is lowered from 184 to 67 kJ mol−1. The amount of borazine released is also significantly suppressed (Gutowska et al., 2005).
Nanoconfined LiBH4 in silica gel displayed a big decrease in the dehydrogenation temperature compared to the pure material. Even below the melting point of LiBH4, hydrogen is released (Kostka et al, 2008). The decomposition temperature can also be lowered using carbon aerogels as a porous matrix for LiBH4. The activation energy for the decomposition of LiBH4 in an aerogel with 13 nm pores has been shown to be 103 kJ mol−1. As a comparison, the activation energy was also measured for non-porous carbon at 146 kJ mol−1 (Gross et al, 2008).
One of the main drawbacks of nanoconfinement is the reduced storage capacity through the addition of ‘death’ materials. However, these materials show enhanced kinetics and sometimes different reaction pathways compared to the bulk systems. A complete understanding of these system is presently too far away in scientific research terms. The effect of size needs to be researched, and a better understanding of the interaction between the scaffold and the embedded materials needs to be investigated.
8.7 Applications of hydrogen storage
8.7.1 Hydrogen storage for fuel cell car applications
One crucial problem with the implementation of a hydrogen-based fuel cell for applications in cars is the on–board storage of hydrogen. In principle, hydrogen storage in solid-state materials (adsorption or absorption materials) offers an interesting and safe method for the storage of large quantities of hydrogen. However, after more than ten years of intensive research a ubiquitous solution for the on-board storage of hydrogen in the solid state has not been found.
A crucial point for automotive applications is the fast on-board regeneration of the storage material. Materials with very high amounts of hydrogen (for instance AlH3, NH3BH3) are non−reversible and must be regenerated outside of the tank system using energy consuming chemical methods. During the refilling of reversible hydrogen storage materials (for instance Ti−doped NaAlH4), large amounts of heat must be compensated for in a short filling time of less than 5 min. For a material with an enthalpy of formation ∆H of about 20 MJ kg−1 H2 (typical for many hydrides) the overall amount of thermal load of 100–120 MJ (5–6 g H2) is produced. This has to be compensated for using high power heat exchanger. Because of cost, volume and weight reasons, this does not seem to be a realistic solution for mobile applications. The drawbacks of using porous materials are their low volumetric density and the requirement of low temperatures (77 K) necessary to produce sufficient storage amounts. With all these drawbacks in mind, the car companies have chosen the 70 MPa high-pressure gas tank as a compromise solution for the first generation of fuel cell cars instead of a chemical solution for the storage of hydrogen. The implementation of a high-pressure infrastructure has a significant impact on the prospective chemical storage systems. Newly developed materials for hydrogen store should be manageable under these restrictive conditions (Felderhoff et al, 2007).
8.7.2 Complex aluminum hydrides as hydrogen storage materials for applications involving high-temperature polymer electrolyte membrane (HT-PEM) fuel cells
Contrary to the working temperature of a PEM fuel cell (around 80°C), a HT-PEM fuel cell has a working temperature up to 180°C. This HT-PEM fuel cell uses membranes based on polybenzimidazole, treated with phosphoric acid for sufficient proton conductivity. Therefore, the HTPEM fuel cell can be operated at much higher temperatures than the PEM fuel cell with humidified membranes.
The operating temperature and the amount of heat produced from the HT-PEM fuel cell is high enough for the decomposition of doped NaAlH4 and to feed the hydrogen released back into the fuel cell. This is not possible at the lower working temperature of a normal PEM fuel cell. In a project it was recently demonstrated that a thermally coupled system of a NaAlH4 hydrogen storage tank and a HT-PEM fuel cell can independently operate producing electricity for several hours (Urbanczyk et al, 2011).
8.7.3 Fuel cell–driven submarines
One of the most impressive applications of hydrogen storage materials is the usage in fuel cell-driven submarines (Pommer et al, 2006). For the diving cruise the PEM fuel cell modules produce an overall electric power of 300 kW. The oxygen for the fuel cell is stored as a liquid, and the hydrogen is stored as a solid in a metal hydride compound. The water which is produced from the fuel can be used as raw water on-board the submarine. Advantages of this method are the noiseless energy conversion and prolonged cruising range during diving when compared to conventional submarines with diesel aggregates and battery systems (Fig. 8.7).
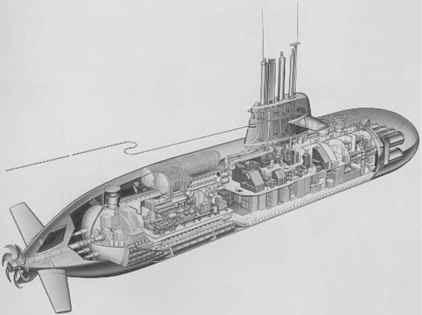
8.7 Schematic presentation of a fuel cell-driven submarine with a solid- state hydrogen stage system using metal hydrides and liquid oxygen storage. The fuel cell system is used during the diving cruise of the submarine. (Photo: HDW.)
The compound used for the storage of hydrogen is a typical low–temperature metal hydride and sold under the trademark Hydralloy. This alloys consists of titanium/zirconium (approximately 25–35 wt%), manganese (approximately 45–55 wt%) and vanadium/iron (approximately 15–20 wt%) and further possible alloying elements like chromium, nickel, depending on the desired properties of the hydrogen storage materials. The maximum hydrogen storage capacity is roughly 1.9 wt% H4. The equilibrium pressure at 20°C is adjust able within a range of approximately 0.5–20 bar, which is high enough for the required hydrogen pressure of the fuel cell. To achieve the full hydrogen amount from the desorption process, 30% of the waste heat from the fuel cell is used to compensate for the endothermic decomposition process.
8.7.4 High–temperature metal hydrides for heat storage
In addition to the application of metal hydrides as hydrogen storage materials, these compounds can be used for the storage of high amounts of solar or waste heat from industrial processes (Bogdanovi![]() et al, 1990; Felderhoff and Bogdanovic, 2009). Metal hydrides based on Mg compounds offer high heat storage capacities at a temperature of around 400°C. The amount of heat which is storable using reversible metal hydrides is higher compared to the storable heat capacity of sensible or latent heat storage materials. The storage of heat at this temperature level is essential for the continuous production of electricity with solar thermal power plants.
et al, 1990; Felderhoff and Bogdanovic, 2009). Metal hydrides based on Mg compounds offer high heat storage capacities at a temperature of around 400°C. The amount of heat which is storable using reversible metal hydrides is higher compared to the storable heat capacity of sensible or latent heat storage materials. The storage of heat at this temperature level is essential for the continuous production of electricity with solar thermal power plants.
According to Equation [8.25], a heat amount of 75 kJ mol−1 (0.9 kWh kg−1) is necessary for the decomposition of MgH2 (heat storing process). The same amount of heat can be recovered from the reversible process from the reaction of Mg metal with hydrogen (heat release):
Magnesium hydride as heat storage material can be used up to temperatures of roughly 440°C. At higher temperatures irreversible sintering processes can occur, resulting in heat capacity losses over the cycling process. In comparison to MgH2, the Mg2FeH6 compound can be used at higher temperatures, up to 550°C, without any capacity loss. The hydride decomposes into Mg and iron metal, releasing three moles of hydrogen (heat storage) [8.26]. The reaction of hydrogen with Mg and Fe metal produces the hydride with the release of the same amount of heat (77 kJ mol−1):
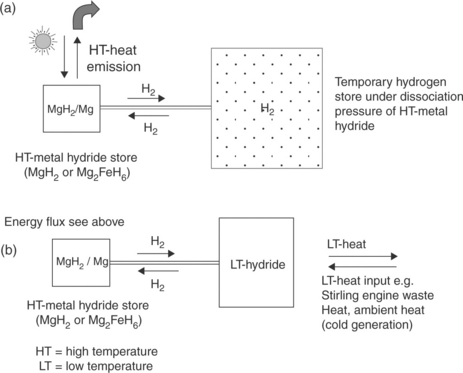
A schematic picture of a heat storage system based on MgH2 (or Mg2FeH6) is presented in Fig. 8.8. Such a heat storage system has to be constructed as a closed system. It consists of a MgH2 container, where the heat from the sun is stored and a high pressure gas container, in which the hydrogen gas released is temporarily stored under the dissociation pressure of MgH2 (Fig. 8.8a). At 400°C the hydrogen dissociation pressure is around 2 MPa. In Fig. 8.8b a second low-temperature metal hydride is used for up-take of the hydrogen released during the heat storage process. Desorption of hydrogen from the low-temperature hydride takes place with withdrawal of heat from the surrounding environment. This combination gives a much more compact construction compared to the system in Fig. 8.8a.
8.8 Conclusions
In the future, hydrogen storage will play an important role in the development of a hydrogen-energy-based society. The requirements for storage materials in different applications can vary widely, but the variety of solidstate materials for hydrogen storage is enormous. Metal hydride systems in particular have the potential to overcome the physical limitations of high pressured or liquefied hydrogen storage systems. A hydrogen storage material for mobile automotive applications has yet to be found and at present it is not certain if future research will find an acceptable solution to this. However, mobile car applications are only one part of the possibilities that would become available through the implementation of solid–state hydro– gen storage. Future applications will require different solutions. Significant progress has been made in the last few years concerning gravimetric and volumetric density, in operating temperatures and pressures, as well as in reversibility of solid state hydrogen storage materials. Further progress is needed to reach the technical requirements. For mobile applications, com– pletely new approaches are necessary in order to suit a new high pressure infrastructure. For several niche applications, different solutions utilizing solid–state hydrogen storage materials do exist. It will be an important chal– lenge in the near future to show the functionality of these materials in sev– eral technical applications.
8.9 References
Akiba, E. Hydrogen-absorbing alloys. Curr Opin Solid St Mater. 1999; 4:267–272.
Akiba, E., Okada, M. Metallic hydrides III: Body-centered-cubic solid solution alloy. MRS Bulletin. 2002; 27:699–703.
Amendola, S.C., Sharp-Goldman, S.L., Janjua, M.S., Spencer, N.C., Kelly, M.T., Petillo, P.J., Binder, M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int JHydrogen Energy. 2000; 25:969–975.
Baitalow, F., Baumann, J., Wolf, G., Jaenicke-Rößler, K., Leitner, G. Thermal decomposition of B-N-H compounds investigated by using combined thermoanalytical methods. Thermochim Acta. 2002; 391:159–168.
Baumann J (2003), PhD thesis, ‘Physikalisch-chemische Untersuchungen zur Wasserstoffabgabe von BNH-Verbindungen’, University Freiberg, Germany.
Bellosta von Colbe, J.M., Bogdanovi![]() , B., Felderhoff, M., Pommerin, A., Schüth, F. Recording of hydrogen evolution – a way for controlling the doping process of sodium alanate by ball milling. J Alloys Compd. 2004; 370:104–109.
, B., Felderhoff, M., Pommerin, A., Schüth, F. Recording of hydrogen evolution – a way for controlling the doping process of sodium alanate by ball milling. J Alloys Compd. 2004; 370:104–109.
Bellosta von Colbe, J.M., Felderhoff, M., Bogdanovic, B., Schüth, F., Weidenthaler, C. One-step direct synthesis of a Ti-doped sodium alanate hydrogen storage material. Chem Commun. 2005; 37:4732–4734.
Bellosta von Colbe, J.M., Metz, O., Lozano, G.A., Pranzas, P.K., Schmitz, H.W., Beckmann, F., Schreyer, A., Klassen, T., Dornheim, M. Behavior of scaled–up sodium alanate hydrogen storage tanks during sorption. Int J Hydrogen Energy. 2012; 37:2807–2811.
Bogdanovi![]() , B., Ritter, A., Spliethoff, B. Active MgH2-Mg systems for reversible chemical energy storage. Angew Chem Int Ed Engl. 1990; 29:223–234.
, B., Ritter, A., Spliethoff, B. Active MgH2-Mg systems for reversible chemical energy storage. Angew Chem Int Ed Engl. 1990; 29:223–234.
Bogdanovi![]() , B., Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. JAlloys Compd. 1997; 253254:1–9.
, B., Schwickardi, M. Ti-doped alkali metal aluminium hydrides as potential novel reversible hydrogen storage materials. JAlloys Compd. 1997; 253254:1–9.
Bogdanovi![]() , B., Brand, R.A., Marjanovic, A., Schwickardi, M., Tölle, J. Metaldoped aluminium hydrides as potential new hydrogen storage materials. J Alloys Compd. 2000; 302:36–58.
, B., Brand, R.A., Marjanovic, A., Schwickardi, M., Tölle, J. Metaldoped aluminium hydrides as potential new hydrogen storage materials. J Alloys Compd. 2000; 302:36–58.
Bogdanovi![]() , B., Felderhoff, M., Kaskel, S., Pommerin, A., Schlichte, K., Schüth, F. Improved hydrogen storage properties of Ti–doped sodium alanate using titanium nanoparticles as doping agents. Adv Mater. 2003; 15:1012–1015.
, B., Felderhoff, M., Kaskel, S., Pommerin, A., Schlichte, K., Schüth, F. Improved hydrogen storage properties of Ti–doped sodium alanate using titanium nanoparticles as doping agents. Adv Mater. 2003; 15:1012–1015.
Bogdanovi![]() , B., Felderhoff, M., Pommerin, A., Schüth, F., Spielkamp, N. Advanced hydrogen-storage materials based on Sc-, Ce-, and Pr-doped NaAlH4. Adv Mater. 2006; 18:1198–1201.
, B., Felderhoff, M., Pommerin, A., Schüth, F., Spielkamp, N. Advanced hydrogen-storage materials based on Sc-, Ce-, and Pr-doped NaAlH4. Adv Mater. 2006; 18:1198–1201.
Bououdina, M., Grant, D., Walker, G. Review on hydrogen absorbing materials – structure, microstructure and thermodynamic properties. Int J Hydrogen Energy. 2006; 31:177–182.
Chambers, A., Park, C., Baker, R.T.K., Rodriguez, N.M. Hydrogen storage in graphite nanofibers. J Phys Chem B. 1998; 102:4253–4256.
Chen, P., Xiong, Z., Luo, J., Lin, J., Tan, K.L. Interaction of hydrogen with metal nitrides and imides. Nature. 2002; 420:302–304.
Chen, P., Xiong, Z., Wu, G., Liu, Y., Hu, J., Luo, W. Metal-N-H system for hydrogen storage. Scripta Mater. 2007; 56:817–822.
Chua, Y.S., Chen, P., Wu, G., Xiong, Z. Development of amidoboranes for hydrogen storage. Chem Commun. 2011; 47:5116–5129.
Chong, M., Karkamkar, A., Autrey, T., Orimo, S., Jalisatgi, S., Jensen, C.M. Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions. Chem Commun. 2011; 47:1330–1332.
Christensen, C.H., Sørensen, R.Z., Johannessen, T., Quaade, U.J., Honkala, K., Elmøe, D., Køhler, R., Nørskov, J.K. Metal ammine complexes for hydrogen storage. J Mater Chem. 2005; 15:4106–4108.
Dafert, F.W., Miklauz, R. Über eine neue Verbindung von Stickstoff und Wasserstoff mit Lithium. Monatsh Chem. 1909; 30:981–996.
Dillon, A.C., Jones, K.M., Bekkedahl, T.A., Kiang, C.H., Bethune, D.S., Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature. 1997; 386:377–379.
Faleschini G, Hacker V, Muhr M, Kordesch K and Aronsson R R (2000), ‘Ammonia for high density hydrogen storage’, Fuel Cell Seminar 2000, Portland, OR, 30 October–2 November.
Fan, X.L., Xiao, X.Z., Chen, L.X., Li, S.Q., Ge, H., Wang, Q.D. Enhanced hydriding–dehydriding performance of CeAl2-doped NaAlH4 and the evolvement of Ce-containing species in the cycling. J Phys Chem. 2011; 115:2537–2543.
Felderhoff, M., Bogdanovic, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int J Mol Sci. 2009; 10:325–344.
Felderhoff, M., Zibrowius, B. Formation of Al2H7– anions – Indirect evidence of volatile AlH3 on sodium alanate using solid-state NMR spectroscopy. Phys Chem Chem Phys. 2011; 13:17234–17241.
Felderhoff, M., Klementiev, K., Grünert, W., Spliethoff, B., Tesche, B., Bellosta von Colbe, J.M., Bogdanovic, B., Hartel, M., Pommerin, A., Schüth, F., Weidenthaler, C. Combined TEM-EDX and XAFS studies of Ti-doped sodium alanate. Phys Chem Chem Phys. 2004; 6:4369–4374.
Felderhoff, M., Weidenthaler, C., Helmholt von, R., Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys Chem Chem Phys. 2007; 9:2643–2653.
Fichtner, M., Fuhr, O., Kircher, O., Rothe, J. Small Ti clusters for catalysis of hydrogen exchange in NaAlH4. Nanotechnology. 2003; 14:778–785.
Frankcombe, T. Proposed mechanisms for the catalytic activity of Ti in NaAlH4. Chem Rev. 2012; 112:2164–2178.
Graetz, J., Reilly, J.J., Johnson, J., Ignatov, A.Y., Tyson, T.A. X-ray absorption study of Ti–activated sodium aluminum hydride. Appl Phys Lett. 2004; 85:500–502.
Graetz, J., Reilly, J.J. Kinetically stabilized hydrogen storage materials. Scripta Mater. 2007; 56:835–839.
Gross, A.F., Vajo, J.J., Van Atta, S.I., Olson, G.I. Enhanced hydrogen storage kinetics of LiBH4 in nanoporous carbon scaffols. J Phys Chem C. 2008; 112:5651–5657.
Gutowska, A., Li, L.Y., Shin, Y.s., Wang, C.M.M., Li, X.H.S., Lineham, J.C., Smith, R.S., Kay, B.D., Schmid, B., Shaw, W., Gutowski, M., Autrey, T. Nanoscaffolds mediates hydrogen release and the reactivity of ammonia borane. Angew Chem Int Ed. 2005; 44:3578–3582.
Hahn, F. Development of portable systems. In: Léon Aline, ed. Hydrogen Technology – Mobile and Portable Applications. Berlin Heidelberg: Springer-Verlag; 2008:409–438.
Ivanov, I., Konstanchuk, I., Stepanov, A., Boldyrev, V. Magnesium mechanical alloys for hydrogen storage. J Less-Common Met. 1987; 131:259–267.
Jongh de, P.E., Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. Chem Sus Chem. 2010; 3:1332–1348.
Johnson, T.A., Jorgensen, S.W., Dedrick, D.E. Performance of a full-scale hydrogen-storage tank based on complex hydride. Faraday Discuss. 2011; 151:327–352.
Kojima, Y., Kawai, Y. IR characterizations of lithium imide and amide. J Alloys Compd. 2005; 395:236–239.
Kostka, J., Lohstroh, W., Fichtner, M., Hahn, H. Diborane release from LiBH4/silica-gel mixtures and the effect of additives. J Phys Chem C. 2008; 111:14206–14209.
Leng, H.Y., Ichikawa, T., Hino, S., Hanada, N., Isobe, S., Fujii, H. New metal-N-H system composed of Mg(NH2)2 and LiH for hydrogen storage. J Phys Chem B. 2004; 108:8763–8765.
Léon, A., Kircher, O., Rothe, J., Fichtner, M. Chemical state and local structure around titanium atoms in NaAlH4 doped with TiCl3 using X-ray absorption spectroscopy. J Phys Chem B. 2004; 108:16372–16376.
Liang, G., Huot, J., Boily, S., Van Neste, A., Schuls, R. Hydrogen storage properties of the mechanically milled MgH2 + 5 at% V nanocomposite. J Alloys Compd. 1999; 315:237–242.
Majzoub, E.H., Gross, K. Titanium-halide catalyst-precursors in sodium aluminum hydrides. J Alloys Compd. 2003; 356:363–367.
Martinez-Rodriguez, M.J., Garcia-Diaz, B.L., Teprovich, J.A., Knight, D.A., Zidan, R. Advances in the electrochemical regeneration of aluminum hydride. Appl Phys A. 2012; 106:545–550.
McPhy. Available from: http://www.mcphy.com/en/index.php (Accessed 7 February 2012).
Oelerich, W., Klassen, T., Bormann, R. Metal oxides as catalysts for improved hydrogen sorption in nanocrystalline Mg–based materials. J Alloys Compd. 2001; 315:237–242.
Panella, B., Hirscher, M., Roth, S. Hydrogen adsorption in different carbon nanostructures. Carbon. 2005; 43:2209–2214.
Panella, B., Hirscher, M., Pütter, H., Müller, U. Hydrogen adsorption in met– al–organic frameworks: Cu-MOFs and Zn-MOFs compared. Adv Funct Mat. 2006; 16:520–524.
Pommer, H., Hauschildt, P., Teppner, R., Hartung, W. Außenluftunabhängiges Antriebsystem für Uboote. ThyssenKrupp techforum, Heft. 2006; 1:64–70.
Rees, N.V., Compton, R.G. Carbon-free energy: A review of ammonia- and hydrazine-based electrochemical fuel cells. Energy Environ Sci. 2011; 4:1255–1260.
Sandrock, G. A panoramic overview of hydrogen storage alloys from a gas reaction point of view. JAlloys Compd. 1999; 293:877–888.
Sandrock, G., Reilly, J., Graetz, J., Zhou, W.M., Johnson, M., Wegrzyn, J. Accelerated thermal decomposition of AlH3 for hydrogen-fueled vehicles. Appl Phys A. 2005; 80:687–690.
Severa, G., Rönnebro, E., Jensen, C.M. Direct hydrogenation of magnesium boride to magnesium borohydride: Demonstration of > 11 weight percent reversible hydrogen storage. Chem Commun. 2010; 46:421–423.
Shaltiel, D., Jacob, I., Davidov, D. Hydrogen absorption and desorption properties of AB2 Laves phase pseudobinary compounds. J Less-Common Met. 1977; 53:117–131.
Soloveichik, G.L., Gao, Y., Rijssenbeek, J., Andrus, M., Kniajanski, S., Bowman, R.C., Jr., Hwang, S.J., Zhao, J.C. Magnesium borohydride as a hydrogen storage material: Properties and dehydrogenation pathway of unsolvated Mg(BH4)2. Int J Hydrogen Energy. 2009; 34:916–928.
Stephens, F.H., Pons, V., Baker, R.T. Ammonia-borane: The hydrogen source par excellence? Dalton Trans. 2613-2626, 2007.
Streukens, G., Bogdanovi, B., Felderhoff, M., Schüth, F. Dependence of dissociation pressure upon doping level of Ti-doped sodium alanate – a possibility for ‘thermodynamic tailoring’ of the system. Phys Chem Chem Phys. 2006; 28:2889–2892.
Tamura, T., Kazumi, T., Kamegawa, A., Takamura, H., Okada, M. Protium absorption properties and protide formations of Ti-Cr-V alloys. J Alloys Compd. 2003; 356:505–509.
Thomas, K.M. Hydrogen adsorption and storage on porous materials. Catal Today. 2007; 120:389–398.
Urbanczyk, R., Peil, S., Bathen, D., Heßke, C., Burfeind, J., Hauschild, K., Felderhoff, M., Schüth, F. HT-PEM fuel cell system with integrated complex metal hydride storage tank. Fuel Cells. 2011; 11:911–920.
Wang, T., Wang, J., Ebner, A.D., Ritter, J.A. Reversible hydrogen storage properties of NaAlH4 catalyzed with scandium. JAlloys Compd. 2008; 450:293–300.
Wojcik, A., Middleton, H., Damopoulos, I., Van herle, J. Ammonia as a fuel in solid oxide fuel cells. J Power Sources. 2003; 118:342–348.
Xiong, Z., Yong, C.K., Wu, G., Chen, P., Shaw, W., Karkamkar, A., Autrey, T., Jones, M.O., Johnson, S.R., Edwards, P.P., David, W.I.F. High-capacity hydrogen storage in lithium and sodium amidoboranes. Nat Mater. 2007; 7:138–141.
Yaghi, O., O’Keeffe, M., Ockwig, N.W., Chae, H.K., Eddaoudi, M., Kim, J. Reticular synthesis and the design of new materials. Nature. 2003; 423:705–714.
Züttel, A., Wenger, P., Rentsch, S., Sudan, S., Mauron, P., Emmenegger, C. LiBH4 a new hydrogen storage material. J Power Sources. 2003; 118:1–7.
Züttel, A., Borgschulte, A., Orimo, S.I. Tetrahydroborates as new hydrogen storage materials. Scripta Mater. 2007; 56:823–828.
Züttel, A., Remhof, A., Borgschulte, A., Friedrichs, O. Hydrogen: The future energy carrier. Phil Trans R Soc A. 2010; 368:3329–3342.