Membranes, adsorbent materials and solvent-based materials for syngas and hydrogen separation
Abstract:
Advanced H2/syngas separation technologies have the potential to improve the cost and efficiency of future power plants for H2 production and/or CO2 capture. In this chapter, we will first present potential membrane materials used for syngas or H2/CO2 separation. Membranes with H2-permselective property will be discussed first, followed by CO2-permselective membrane materials. We will then review the recent developments in adsorbent materials for |H2 separation. Solvent-based materials will also be briefly covered. Finally, future trends and further source information will be commented on at the end of the chapter.
7.1 Introduction
Research on hydrogen and syngas production and separation technologies has received great attention in recent years, mainly due to increasing demand for hydrogen and syngas in refinery, petrochemical and energy industries. Refineries use hydrogen in their hydrotreating or hydroprocessing units to process increasingly heavier and sourer crudes. Petrochemical industries produce ammonia, methanol, synthetic liquid fuels via Fischer- Tropsch reactions with hydrogen and syngas as key building blocks. In energy industries, the increasing demand for clean energy and the pressing issues of resources depletion of oil have led to the emerging use of syngas and hydrogen. The fuel cell and the IGCC (integrated gasification combined cycle) are the two leading applications that are expected to require a significant amount of hydrogen and syngas. To sustain the future use of fossil fuels in power plants, CO2 capture and sequestration is considered one of the most promising strategies to reduce carbon emission. Converting fossil fuels to H2 or syngas and separating CO2 prior to combustion is one elegant way to achieve this objective.
Hydrogen in its molecular form is not available on Earth and is primarily separated from syngas, which consists of hydrogen and carbon monoxide with other components such as CO2, CH4, H2O and H2S depending on its source. Syngas can be generated from any carbonaceous material such as natural gas, coal, biomass, LPG, refinery off gas or naphtha. The thermal chemical reactions of syngas production can be classified into three main routes:
Several side reactions, such as water-gas shift reaction and carbon formation, accompany the above three reactions. Water-gas shift is a particularly important reaction in adjusting H2CO ratio for chemicals production or eliminating CO by converting it to CO2 before the H2/CO2 separation step: Water-gas shift reaction
In general, syngas needs to be conditioned, cleaned or purified before it can be used as a fuel or feedstock. For H2 production in refinery or chemicals application, separation of CO2 and hydrocarbons from H2 is a critical step to ensure high purity of H2 product. Removal of CO2 for methanol or Fisher-Tropsch liquid production is essential to prevent CO2 build-up in the recycle loop of the reactor (Probstein and Hicks, 1982). For energy applications, syngas can be used directly as a fuel in an oxygen combustion atmosphere or in a fuel cell device. More conveniently, the typically high-pressure syngas can first be separated to produce a pure CO2 stream for sequestration and a pure hydrogen stream for fuel source. In a polygenera-tion power plant, the separated hydrogen can also be delivered to power parks or filling stations for distributed power generation or transportation application. Separation of H2/CO2 is one enabling technology for hydrogen/ syngas applications. However, incorporating a CO2 capture unit in a power plant significantly reduces the energy efficiency and increases the cost of electricity (EPRI and NETL, 2000; Parsons, 2002; Gielen, 2003). This has also spurred the development of advanced H2/CO2 separation technologies recently.
For refinery and chemical industries, the pressure swing adsorption (PSA) process is the current state-of-the-art technology. Over 85% of current global hydrogen production units use PSA technology for hydrogen purification (Sircar and Golden, 2010). Research and development activities in this field have been extensive in recent decades. For syngas derived from coal, on the other hand, liquid absorption processes have been the choice for the acid gas removal, primarily due to the need to remove hydrogen sulfide. Selexol™ and Rectisol® are the two major physical solvent processes that are currently practiced commercially. In addition to their high costs, both adsorption and absorption separation techniques operate at low temperatures (< 50°C), and are difficult to integrate with modern power plants or upstream syngas generating units. In modern power plants such as IGCC, where hydrogen-containing syngas is generated at very high temperatures, high-temperature hydrogen separation technologies can eliminate the need of cooling the fuel gas and reheating it again for gas turbines. Further, high-temperature separation technologies can also combine both chemical reactions and separation in a single step to simplify the process, reduce the cost and improve the efficiency. There is a strong incentive to develop advanced hydrogen separation technologies which can operate at higher temperatures (> 200°C) and are cost effective.
In this chapter, we will first present potential membrane materials used for syngas or H2/CO2 separation. Membranes with H2-permselective property will be discussed first, followed by CO2-permselective membrane materials. We will then review the recent development in adsorbent materials for H2 separation. Solvent-based materials will also be briefly covered. The chapter will end with comments on future trends and further sources of information.
7.2 H2-selective membrane materials
Based on materials used, hydrogen membranes can be classified into organic and inorganic membranes. Organic membranes are mainly polymeric. Inorganic membranes can be made of metal, ceramic or carbon. Based on the transport mechanisms, membranes can be classified into dense and porous membranes.
In a dense membrane, hydrogen permeates through the bulk of the membrane material via a solution-diffusion mechanism. Essentially, gas molecules first need to dissolve or adsorb on the surface of the membrane. The dissolved or adsorbed gas molecules then diffuse or migrate through the bulk of the membrane, and finally desorb at the permeate side of the membrane. The membrane permeability P can be described as a product of the solubility coefficient S and the diffusivity coefficient D:
The membrane flux F is then related to the permeability by the following equation:
where ∆p is the partial pressure difference across the membrane for the permeating component and L is the membrane thickness.
In porous membranes, hydrogen diffuses through small pores of the membrane structure. As separation is determined by the molecular size or weight of the gas species, hydrogen can diffuse much faster than other larger gas components. Inside of the pores of the membrane, the diffusion process can be of Knudsen type, where the mean free path of the gas molecules is greater than the pore diameter. If the pore diameter is made even smaller, the diffusion process becomes an activated one, where the gas molecules interact strongly with the walls of the pore. If gas is adsorbed on the wall of the pores, surface diffusion can also occur, which enhances the gas diffusion process. The membrane pore diameters can also be made such that larger gas molecules are too big to enter the membrane pores, a phenomenon called molecular sieving effect. Almost all porous membranes use molecular sieving or activated diffusion to effect separation as the Knudsen diffusion does not give an acceptable separation factor.
In general, porous membranes tend to give higher flux than the dense ones; however, dense membranes can have better selectivity. As the membrane flux increases with the reduction of its thickness, it is desirable to have a very thin membrane. Composite membranes have been developed to increase the flux by placing a very thin dense or active layer on top of a porous support layer to improve the overall membrane strength. Asymmetric membranes can be considered as one type of composite membranes, where the active layer and the porous support layer are made of the same material. Membrane materials can also be combined or mixed to improve their performances. For example, mixed-matrix membranes contain inorganic particles inside the framework of the polymer membrane (Zimmerman et al., 1997; Mahajan and Koros, 2000; Jha and Way, 2008). We will first discuss three types of dense membrane for H2 separation: metal, polymer and ceramic. This will be followed by three types of porous membranes: ceramic, zeolite and carbon. Mixed-matrix membranes, typically CO2-selective, will also be discussed.
7.2.1 Metallic membranes
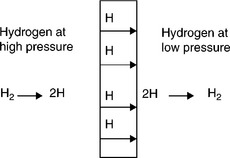
Metal membranes are all dense type, following the solution-diffusion mechanism with the hydrogen molecules first dissolving in the metal frame-work at the high-pressure feed side, dissociating into two atoms, atomic hydrogen transporting through the membrane and recombining back to the molecular hydrogen at the low-pressure permeate side, as shown in Fig 7.1. The hydrogen flux, F, across the membrane with a thickness L can be described by
where P is the permeability, and pfeed and pperm are the hydrogen partial pressure at the feed and permeate side, respectively. If n is equal to 0.5, Equation [7.7] becomes the well-known Siveret law. A deviation of n from 0.5 indicates presence of mass transfer resistances other than the bulk metal diffusion itself. Except for defects, metal membranes can produce very high purity hydrogen in the permeate side due to their ideal high selectivity. This contrasts greatly with the conventional thinking that membrane processes are considered primarily for bulk separation for enriching product rather than purification for high-purity product.
Palladium and its alloys are the most common metal membranes for hydrogen purification applications. Operating temperatures of the Pd membranes are in the range of 300–600°C. At low temperatures, Pd segregates into two phases and becomes brittle during hydrogen permeation (Shu et al., 1991). Binary alloys such as Pd-Ag (McCool et al, 1999; Tosti et al., 2003; Bhandari and Ma, 2009; Peters et al., 2009; Okazaki et al, 2011), Pd-Cu (Roa et al., 2002; Howard et al., 2004; Pomerantz et al., 2010) and Pd-Au (Gade et al, 2009; Chen and Ma, 2010) have been used to overcome this problem without sacrifice of hydrogen flux. Ternary alloy composite membranes have also been considered for the same purpose (Wang et al, 2007; Basile et al., 2008; Ryi et al., 2008; Coulter et al., 2010). Alloys have the advantage of reducing the amount of expensive Pd in the membrane. Further, by changing the surface morphology and the electronic structure of metal Pd, alloys exhibit better chemical stability than pure Pd, in terms of the resistance to poisoning by gaseous impurities such as H2S, CO, H2O (Gao et al, 2004). It is well documented that Pd-Cu alloy shows improved resistance to sulfur (Morreale et al, 2004; Kamakoti et al, 2005; Yang et al, 2008).
In addition to Pd, refractory metals in group IV and V elements such as V, Nb, Ta, Zr and Ti are all good candidate materials for hydrogen separation membranes. In fact their hydrogen permeabilities are higher than Pd (Buxbaum and Kinney, 1996). The major issue with these metals is that they can easily form an oxide layer on the surface in contact with air, rendering them incapable of dissolving or dissociating hydrogen molecules. Current practice uses a thin layer of Pd or Pd alloy coated on the surface of the refractory metal to catalyze the dissociation and reassociation of H2 (Buxbaum and Marker, 1993; Moss et al., 1998; Mundschau et al., 2006). These self-supported membranes are strong enough to sustain high-pressure differentials and are able to overcome the hydrogen-induced embrittlement problem commonly associated with the Group IV and V metals (Mundschau et al., 2005a, 2005b; Xie et al., 2006). Development of non-palladium-based metal alloy materials will continue to play a significant role in reducing the cost, increasing the permeability, and improving the durability of H2 separation membranes (Ozaki et al., 2003; Hashi et al, 2005; Phair and Donelson, 2006; Ryi et al, 2006; Dolan, 2010; Song et al, 2010).
In actual practice of metallic membranes, intermetallic diffusion at high temperatures is one concern that needs to be addressed. Due to the high cost of Pd metal, it is very desirable to fabricate an ultrathin membrane, which is then supported by a porous substrate. If a metal such as stainless steel is chosen as the support material, Pd and Fe or Cr intermetallic diffusion becomes difficult to avoid. One alternative approach is to use ceramic materials for support. However, ceramics have the drawbacks of difficult fabrication, lower pressure rating and poor sealing resulting in delamination between the metal and the support. A good compromise is to insert a thin layer of ceramic barrier between the metal membrane and the stainless steel support (Nam and Lee, 2001; Ma et al, 2004; Bosko et al, 2009; Zhang et al, 2009). Membranes produced by this approach have been shown to be stable after over 6000 h of continuous testing in the temperature range of 350–450°C (Ma, 2008). For palladium-coated vanadium membranes, hydrogen flux decay was also observed due to intermetallic diffusion, and inserting a layer of porous aluminum oxide has improved the membrane stability (Edlund and McCarthy, 1995).
Among all high temperature inorganic membranes for hydrogen separation, Pd membranes are in the most advanced stage of development. In fact, the Pd membrane system was successfully scaled up to 25 tonnes per day H. capacity in the early 1960s (McBride and McKinley, 1965). Smallscale palladium membranes are commercially available for hydrogen purification in the semiconductor industry. Membrane reactors incorporating Pd have also been considered for H2 production integrated with CO2 capture in power generation cycles, although this has only been tested experimentally on a laboratory scale (Tong et al, 2006; Barbieri et al, 2008; Tosti et al, 2008). Cost and stability are still the major barriers for commercialization of metallic membranes on a large scale.
7.2.2 Polymeric membranes
The only hydrogen membrane technology that has been widely used on a commercial scale is based on polymeric materials. In the early 1980s, Monsanto commercialized the first hydrogen membrane process – Prism®(MacLean et al., 1986). Since then, polymeric membranes have been used in hydrogen recovery from purge streams in ammonia plants and refineries. In syngas plants, they have also been used for adjustment of the hydrogen/carbon monoxide ratio. The major suppliers are Air Products, Air Liquide, Praxair, UOP, UBE (Japan).
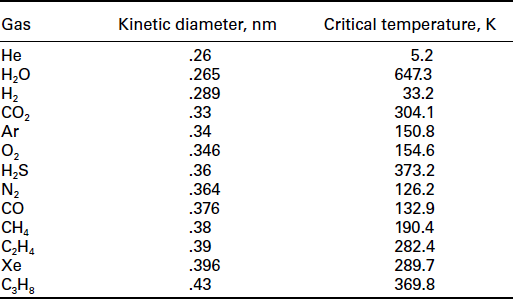
Dense polymer membranes are typically made of polysulfone, polystyrene, cellulose acetate, polyimide or polyaramade among others (Koros and Fleming, 1993). Separation of hydrogen is mainly due to the fast-diffusion coefficients of smaller hydrogen molecules being transported through the bulk of the membrane. Other gas molecules can still permeate through the membrane, depending on the relative solubility and diffusivity in the polymer material. Therefore, polymeric membranes are rarely used for producing high-purity hydrogen. In general, improving the performance of H2-selective membrane relies on increasing diffusivity selectivity of H2 and decreasing the solubility selectivity of CO2. In addition to designing new molecules for H2-selective polymers, other techniques include blending, doping and crosslinking (Shao et al, 2009a). However, there is always a trade-off between the selectivity and the permeability and an 'upper bound', as first introduced by Robeson (1991), is difficult to cross, as illustrated in Fig. 7.2. Figure 7.3 shows the gas permeabilities for several different gas pairs at two different selectivities, 5 and 50. The figure is calculated based on the correlation of the Robeson's upper bound curves (Robeson, 2008). H2/CO2 is difficult to separate because of the similar kinetic diameters of the two molecules and the preferential sorption of CO2. Table 7.1 lists the molecular diameters and critical temperatures for several gases. The higher the critical temperature is, the more easily the gas can be condensed and hence the higher the solubility of the gas in the polymers. Note that the difference of the molecular sizes between H2 and CO2 is relatively small. Further, CO2 has a much higher critical temperature than H2 and therefore higher solubility.
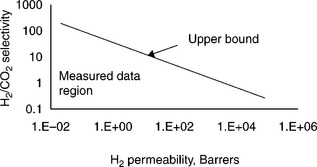
7.2 Representative Robeson upper bound for H2/CO2 separation for polymeric membranes (Robeson, 2008). 1 Barrer = 10− 10 (cm3 STP cm)/ (cm2 s cm Hg).
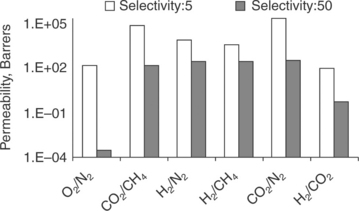
7.3 Comparison of permeabilities for various gas pairs at selectivity of 5 and 50, based on Robeson's upper bound curves (Robeson, 2008). 1 Barrer = 10− 10 (cm3 STP cm)/(cm2 s cm Hg).
Polymeric membranes are very susceptible to swelling effect and are easily plasticized by highly soluble gases such as heavy hydrocarbons or CO2. Pretreatment of the feed gas before the membrane can be complicated and costly. Pretreatment is also used to prevent condensation of hydrocarbon vapor on the membrane surfaces. Because hydrocarbons and other larger molecules are enriched on the feed side of the membrane after hydrogen permeation, the dew point of the residue stream can increase.
The possibility of condensation is further increased as the membrane temperature drops due to Joule-Thomson effect when hydrogen permeates from the high-pressure feed side to the low-pressure permeate side. Other problems include poor resistance to certain chemicals such as hydrochloric acid (HCl) and sulfur oxides (SOx) (Adhikari and Fernando, 2006). The pretreatment issue and the low temperature capability make the polymeric membrane less attractive for applications in the power sector. The maximum operating temperature can reach about 110°C. The maximum pressure differential across the membrane, depending on the temperature, can be as high as 100 bars at 40°C (Air Products, 2011). The low H2/CO2 selectivity (< 10) is another disadvantage for power plant application, as the feed gas tends to contain a large amount of CO2, especially after the water-gas shift reaction. Nevertheless, polymeric membranes have the advantages of low cost, easy fabrication and extensive field experience. Research on hightemperature (to 300°C) polymeric membranes has been reported (Costello et al, 1994; Rezac et al, 1995; Pesiri et al, 2003), but they are only limited to laboratory testing.
7.2.3 Mixed ionic-electronic membranes
Dense ceramic membranes are mainly of perovskite type, which has the following formula:
where A´is selected from the group consisting of Ba, Sr, Ca, Mg, and
A' is selected from the lanthinide series such as Ce, Pr, Nd, Gd, Yb;
B and B' are selected from any transition metal such as Ce, Y, Co, Ti, V, Cr;
x and y are numbers between 0 and 1;
z is a number sufficient to neutralize the charge in the mixed metal oxide.
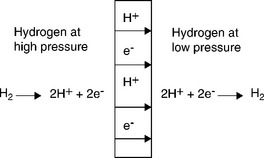
This class of material exhibits a mixed protonic–electronic conductivity at high temperatures, 600–1000°C (Iwahara et al., 1981; Norby and Larring, 2000; Qi and Lin, 2000; White et al, 2001; Hamakawa et al, 2002; Norby, 2007). When a H− - containing gas mixture is introduced on one side of the membrane, H2 dissociates into proton (H+) and electron (e +) on the surface. The dissociated species are transported through the membrane to its opposite side where the species recombine to a H2 molecule, as illustrated in Fig. 7.4. This type of membrane has a very high selectivity as only hydrogen can permeate through the membrane. However, the hydrogen flux currently demonstrated in the lab is very low (Doong et al., 2005). Its unique high-temperature operating range makes it possible to closely couple with the coal or biomass gasifier without further cooling down the syngas. On the other hand, the high-temperature operation makes the robust design of membrane support, module and housing more challenging. The tolerance to contaminants in the coal-derived syngas is another concern, especially the stability issue in the presence of CO2. Certain perovskites can be converted to more stable carbonate compounds in contact with CO2. Dense perovskite membranes are still in an early stage of development.
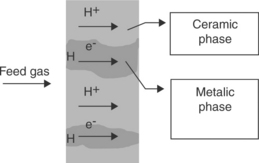
To increase the hydrogen flux of dense ceramic membranes, a hydrogenpermeable metallic material can be incorporated into the dense mixed conducting ceramic phase, as a cermet (Dorris et al., 2003; Mundschau (2005). The transport mechanism is illustrated in Fig. 7.5. The hydrogen transport is predominantly carried by the metallic phase, which offers a high flux. Therefore, typical ceramic materials without ion-conducting property such as Al2O3 and stabilized ZrO2, can also be used for the ceramic phase (Balachandran et al., 2005). With metals dispersed in the ceramic framework, the shape integrity of the metal phase is no longer an issue. The ceramic/metal composite structure offers better mechanical, chemical and thermal stability than metal membranes and can afford a high temperature operation (~ 900°C). At such a high temperature, it has better tolerance to sulfur compounds in the feed gas (Balachandran et al., 2006)
7.2.4 Porous ceramic membranes
Porous ceramic membranes are usually amorphous, made of alumina, silica, zirconia or titania in a form of metal oxide. These materials possess good thermal and chemical stability in harsh operating conditions. Other advantages include easy fabrication and low cost of production. In order to efficiently separate hydrogen, pore diameters of the membrane must be less than 1 nm (Diniz da Costa et al, 2002; Lee and Oyama, 2002; Verweij et al, 2006). As in dense membranes, the active layer with small pore size needs to be supported by a thick layer with a pore size between 0.5 and 50 μm. An intermediate layer with a pore size between 0.005 and 0.5 μm is also used to bridge the support and the active layer (Judkins and Bischoff, 2005). Figure 7.6 shows a SEM micrograph of a mesoporous silica membrane (pore size 20 Å) deposited on a coarse pore alpha-alumina (200 nm) support. Hydrogen product purity in porous ceramic membrane is almost always less than 100% as microporous membranes with uniform pore size and defect-free properties are difficult to fabricate. The improvement of selectivity comes with a reduction of flux for microporous ceramic membranes. One possible remedial solution to the H2 purity deficiency problem is to use a multi-stage approach, where the microporous membrane is used for bulk removal of CO2 and other larger contaminants, followed by a highly selective but more expensive Pd or metallic membrane.

7.6 SEM micrograph of a 1-pm-thick mesoporous silica membrane (pore size 20 A) deposited on a coarse-pore (200 nm) alpha-Al2O3 support. (Source: Courtesy of G. Xomeritakis, the University of New Mexico.)
Two main problems of the amorphous microporous membranes are their tendency to densify at high temperatures and the hydrothermal stability of silica membranes in the presence of steam (Yoshino et al., 2005). Supported silica membranes can suffer delamination and structural instability of the intermediate layer. Improvement in stability has been reported by incorporating methyl groups in the silica microstructure (de Vos et al., 1999), using metal doping such as Ni (Tsuru, 2008), introducing Si-C bonds to form silica organic hybrid structure (Kanezashi et al., 2009), or addition of TiO2 to SiO2 structure (Gu and Oyama, 2009).
7.2.5 Zeolite membranes
Zeolites are porous crystalline aluminosilicates with their framework consisting of an assemblage of SiO4 and AlO4 tetrahedra. The unique feature of uniform pore size distribution distinguishes the zeolites from other amorphous microporous materials. Zeolite membranes typically are prepared by growing polycrystalline zeolite into a continuous film on the surface of a porous support. More than 14 zeolite structures have been investigated for H.-selective membranes (Ockwig and Nenoff, 2007). Gas permeation is based on the diffusion of the gas molecules through the interconnected channels or intracrystalline pores (Lin, 2001). Gas separation could be affected both by the molecular sizes of the gases and by their adsorption properties (Hong et al, 2005; Kanezashi et al, 2008). Therefore, for hydrogen separation, high-temperature operation is used to suppress the adsorption of CO2 and improve the H2/CO2 separation factor. Current research on zeolite membranes focuses on tailoring the micropore size and fabricating defect-free membranes (Caro and Noack, 2008).
7.2.6 Carbon molecular sieve membranes
Carbon molecular sieve is another group of material, similar to zeolite, which possesses a pore size in the range of gas molecular dimensions and a very sharp pore size distribution. The slit-shaped pores are formed from packing imperfections among planar aromatic structures (Kiyono et al, 2010). It is produced by pyrolysis of organic precursors such as polymers, coke, coal or biomass. Polyimides are the most used precursors with good H2/CO2 separation performance surpassing the Robeson's upper bound, as recently reported by Hosseini and Chung (2009). Flat, tubular or hollow fiber carbon molecular sieve membranes of asymmetrical structure can be fabricated from the precursors of the same geometry (Jones and Koros, 1994; Ismail and David, 2001; Saufi and Ismail, 2004). However, these may have the disadvantage of brittleness and difficulty to handle for scale-up. Alternatively, the membranes can be produced by coating on a porous ceramic or stainless steel support. Carbon molecular sieve membranes supported on ceramic tubes have been tested in a refinery pilot plant using hydrocracker off-gas for about 100 h (Liu, 2005). Operating at 220°C, the membranes were stable in the presence of significant H2S, NH3 and higher hydrocarbons, presumably due to low adsorption of these contaminants on carbon at high temperatures. The hydrogen flux was quite comparable to the dense Pd membranes. Like any microporous membrane, the carbon molecular sieve membrane has the difficulty of producing ultrahigh purity hydrogen from a feed gas containing a significant amount of CO2.
7.3 CO2-selective membrane materials
There are three major applications for developing CO2-selective membranes: (1) CO2 removal from natural gas, CO2/CH4 separation, (2) carbon capture from flue gas, CO2/N2 separation and (3) CO2 removal from syngas, CO2/H2 separation. Only the first application has been commercialized on a large scale. The requirements and characteristics of membrane materials for each application are quite different. We focus on the syngas application in this section.
If the syngas contains more H2 than CO2, it is also more efficient to permeate CO2 than H2 to reduce the membrane area. The other advantage of CO2- permselective vs H2-permselective membranes is that the hydrogen product is at a high pressure, reducing the need for recompression. This advantage may no longer be valid if CO2 capture and sequestration is required as CO2 needs to be compressed to a very high pressure as well.
As both H2 and CO2 have very close kinetic diameters, CO2 -selective membranes are almost exclusively dense materials with higher solubility of CO2 than H2. Membrane materials can be organic (polymeric), inorganic or hybrid. The hybrid group can be divided into mixed-matrix membrane and facilitated transport membrane.
7.3.1 Polymeric membranes
To increase the solubility of CO2 vs H2, one approach is to incorporate polar groups such as ethylene oxide (EO), propylene oxide (PO) or ethylene glycol (EG) units to increase their interactions with quadrupolar CO2 molecules. The affinity of the membrane material for CO2 must not be so high as to inhibit its mobility. For example, the high molecular weight polyethylene oxide (PEO) has low CO2 permeability due to its tendency to crystallize. The other issue is the negative effect of plasticization by CO2, resulting in an increase of H2 permeability and hence reduced CO2 selectivity. To over-come this shortcoming, uses of cross-links, copolymers, or polymer blends have been suggested. Freeman's research group has developed a series of cross-linked and highly branched PEOs, containing different terminal functional groups and network copolymers (Lin et al, 2006; Kusuma et al, 2009; Reijerkerka et al., 2011). They showed the effect of free volume and glass transition temperature or polymer chain mobility on CO2 permeability. Plasticization actually increases both CO2 permeability and CO2/H2 selectivity. Poly (amide-b-ether) or Pebax is a well-known copolymer consisting of flexible polyether segments (PEO) and the rigid amide blocks. Reijerkerka et al. (2010) studied the tuning of mass transport properties of multi-block copolymers using PEO and/or PPO as a soft segment and a monodisperse di-amide as a hard segment. They demonstrated the influence of the length of the soft segment and the type of soft segment on CO2 permeability. Other copolymer examples include poly (ether-urethane) and poly (ether-urea) block that contains glycol units (Simmons, 2005). Car et al. (2008) blended Pebax with low molecular weight PEG resulting in a two-fold increase of CO2 permeability and 20% increase in CO2/H2 selectivity.
Very high free-volume polymers such as poly(1-trimethylsilyl-1-propyne) (PTMSP), poly(4-methyl-2-pentyne) (PMP) and polydimethylsiloxane (PDMS) are more permeable to the more condensable gases such as CO2 and H2S than H2, at room temperature (Merkel et al, 2001; Shekhawat et al, 2003; Bernardo et al, 2009). Their high CO2 permeability allows these polymers to be further modified to increase the selectivity by blending or crosslinking. Another option is to introduce inorganic particles to form hybrid membranes, which will be discussed in Section 7.3.3.
The design of CO2-selective polymeric membrane faces two counter-effects of higher CO2 solubility and higher H2 diffusivity. Rubbery polymers exhibit high solubility selectivity for CO2 over H2, but lack the diffusivity difference. Glassy polymers offer good diffusivity selectivity for H2 over CO2, but fall short of the solubility difference. One alternative approach is to resort to chemical reaction as in a facilitated transport membrane.
7.3.2 Facilitated transport membranes
Facilitated transport membranes (FTMs) offer high selectivity and high flux by incorporating a carrier agent into a polymer matrix to react with CO2 reversibly. There are generally two types of FTMs: mobile carrier and fixed carrier. The mobile carrier can move freely across the membrane. This type of membrane is also called supported liquid membrane (SLM) or 'immobi-lized' liquid membrane (ILM). The carrier first reacts with CO2 on the feed side and the reaction product moves across the membrane. On the permeate side, CO2 is released while H2 or other gas species is not affected by this facilitated transport. The fixed carrier is covalently bonded to the polymer backbone and its mobility is limited. The CO2 molecule reacts with one carrier site and then hops to the next site until it reaches the permeate side. Figure 7.7 illustrates the two types of CO2 transport mechanism in FTMs.
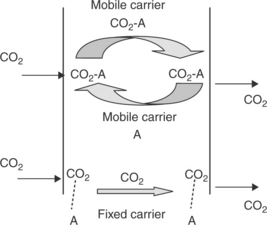
7.7 CO2 transport in facilitated transport membranes showing two types of mechanism, mobile carrier (top) and fixed carrier (bottom).
The most commonly used mobile carriers are amine and carbonate-bicar-bonate solutions. The primary drawback for SLMs is the loss of solvent due to evaporation or wash out under a high trans-membrane pressure. Ionic liquids, which are liquid salts at room temperature with negligible vapor pressure and are chemically and thermally stable, have recently been explored as carriers for SLMs (Hanioka et al., 2008; Myers et al., 2008; Scovazzo, 2009). They can be synthesized with specific functional groups to enhance CO2 solubility. Furthermore, certain ionic liquids are polymerizable and can be blended with another polymeric membrane to form fixed carrier FTMs (Tang et al.. 2005; Bara et al.. 2007; Carlisle et al., 2010).
In general, fixed carrier membranes are considered more stable. Poly vinyl alcohol, poly(allylamine) or polyethyleneimine have been investigated for FTMs for CO2 separation due to the presence of reactive functional groups of amines (Matsuyama et al, 1999; Zou and Ho, 2006; Huang et al, 2010). Hydroxy groups in poly (vinyl alcohol) or chitosan is another example (El-Azzami and Grulke, 2008, 2009). Ion-exchange membranes are intermediate between the mobile and the fixed carrier types (LeBlanc et al, 1980). They typically can be made by neutralizing an ionomer membrane with organic amine counter ions such as monoprotonated ethylenediamine (EDA) (Hagg and Quinn, 2006). Most data were reported for CO2/N2 separation, but the barrier to N2 in ionic membrane can be extended to H2 as well.
7.3.3 Inorganic membranes
A few zeolite membranes, instead of H2 -selective as discussed in Section 7.2.5, actually show CO2 selectivity over H2. This is mainly due to stronger CO2 adsorption. Figure 7.8 shows that zeolite membranes can be CO2-selective due to competitive adsorption or H2-selective due to molecular sieving effect. Lindmark and Hedlund (2010) reported a CO2/H2 separation factor of 6.2 for a BaZSM-5 membrane at room temperature. CO2/H2 selectivity greater than 100 was reported for a SAPO-34 membrane at 253 K and 1.7 MPa by Hong et al. (2008). As the temperature is increased, the CO2/H2 separation factor is always reduced because the adsorption of CO2 is decreased. Other microporous membranes such as silica, alumina or carbon have also been considered for CO2./N2 separation (Shekhawat et al, 2003) due to their molecular sieving capabilities. For CO2/H2 separation, the materials need to be modified to increase CO2 adsorption, as mentioned earlier.
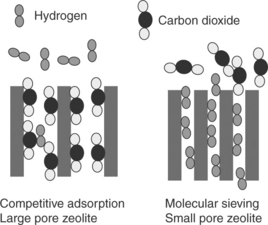
7.8 Zeolite membranes can be CO2-selective due to competitive adsorption or H2-selective due to molecular sieving effect.
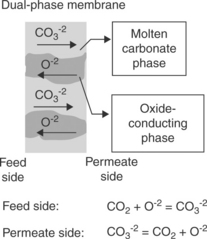
A new class of carbonate–ceramic dual-phase membrane has been discovered recently by Yamaguchi et al. (2007), Anderson and Lin (2010) and Wade et al. (2011). These membranes are composed of porous solid oxide electrolyte filled with molten carbonate, operating at a temperature exceeding 600°C. Carbonate and oxide ions are transported through the liquid phase electrolyte and the solid phase substrate, respectively, in opposing currents, as shown in Fig. 7.9. The CO2 permeability was quite high at about 1 × 10− 10 mol m/(m2 s Pa). If leak-tight, this type of membrane should have infinite CO2/H2 selectivity. Similar to the mobile carrier FTMs, this material has the issue of retaining the liquid electrolyte during prolonged operation.
7.3.4 Mixed-matrix membranes
Mixed-matrix membranes (MMMs) enhance the properties of polymeric membranes by combining the easy processability of polymers and the superior selectivity of inorganic materials. The difficult and costly fabrication of inorganic membranes can be avoided by using MMMs, which use polymers as the continuous phase and the inorganic particles as the dispersed phase. The presence of the solid particles modifies the polymer membranes through the following different effects: (a) increasing the sorption of CO.. (b) creating a barrier to reduce the permeability, especially for H2, (c) disrupting the polymer structure, and hence its free volume and chain mobility and (d) improving the overall thermal and mechanical strength. Figure 7.10 is a simplified diagram showing how MMMs can improve the selectivity of CO2/H2.
The successful development of the MMMs depends on the judicious selection of polymer matrix and inorganic filler. The major challenge is to improve the poor contact or eliminate the interfacial defect between the two phases. This is still a very wide open area, as numerous pairs of polymers/ inorganic fillers have been investigated. MMMs can be H2-selective (Zhang et al., 2008a, 2008b; Khan et al., 2010; Ordõnez et al., 2010; Zornoza et al, 2011) or CO.-selective (Jha and Way, 2008; Shao et al, 2009b; Ahn et al, 2010). Xing and Ho (2011) blended fumed silica with cross-linked polyviny-lalcohol-polysiloxane containing both fixed and mobile amine carriers. They reported a CO2 permeability of 1296 barrers (1 Barrer = 3.35 × 10− 16 mol m/ (m2 s Pa)) and a very high CO2/H2 selectivity of 87. Fumed silica with PMP or PTMSP can potentially increase CO2/H2 selectivity and/or permeability as the presence of the particles can increase the diffusivity of CO2 or the larger molecule more than H2, thus reducing the diffusivity selectivity for H2, based on the results from Merkel et al. (2003a, 2003b, 2003c). A comprehensive review on MMMs was recently published by Chung et al. (2007), in which more details can be found.
7.4 Adsorbent materials for H2/CO2 separation
Activated carbon and zeolite are arguably the two most commonly used adsorbents for H2 separation. Improvement in these two types of materials for H2 PSA application is quite significant, but mainly through incremental progress by industries over the years (Sircar and Golden, 2010). Research has focused on increasing the surface area and the porosity of the materials to increase the adsorption rate and capacity (Drage et al., 2009; Liu et al, 2009; Lopes et al., 2009; Ducrot-Boisgontier et al., 2010). Improvement in CO2/H2 selectivity can sometimes be achieved if the increase of CO2 capacity outweighs that of H2. The real significant increase in selectivity will need to come from chemical modification of the sorbents or the development of new classes of materials.
Immobilized amine sorbent is one possible pathway to achieve high selectivity by impregnation or physisorption of liquid amines within highly porous substrates, such as silica, alumina, zeolite, carbon or polymers. The amines can be those typically used in commercial solvent absorption systems, MEA or DEA. The problems with those lower amines are their high volatility and consequently these materials are not stable after heat regeneration due to the release of considerable amounts of amines. Polymeric amines such as polyethylenimie (PEI) supported on poly(methylmethacrylate) (PMMA), molecular sieve or silica substrates have been reported and were found to have reduced or no amine leaching problem (Xu et al, 2005; Goeppert et al, 2010). The selection of the substrate, especially with respect to the meso and macro porosity also plays a significant role in affecting the CO2 capacity.
Instead of amines impregnated or physisorbed onto porous substrates, amine-functionalized materials have been synthesized by covalently tethering the amine compound to the surface of silicas. These include amine-grafted MCM-48 and silica xerogel (Huang et al, 2003), hexagonal mesoporous silicas (HMS) (Knowles et al., 2005, 2006), pore expanded MCM-41 (Harlick and Sayari, 2007; Serna-Guerrero et al., 2008) and hyperbranched aminosilica (HAS) (Hicks et al, 2008). Those materials are capable of binding CO2 reversibly with no changes in capacity after multiple regeneration cycles. Furthermore, the amine groups on the surface are stable in the temperature range between 25°C and 130°C due to the covalent attachment between the support and the organic groups. The adsorption capacity of CO2 has exceeded that of common 13X zeolite at a low CO2 partial pressure < 0.05 bar (Belmabkhout et al., 2010).
A variety of chemisorbents for CO2. at higher temperatures (> 100°C) have been developed for (a) integration with the upstream syngas reaction or shift reaction and (b) fuel gas CO2 capture. Sorption enhanced reaction concept has been explored with these high-temperature sorbents for lowering the reforming reaction temperature and increasing the conversion in the area of hydrogen production. Based on their reaction mechanisms with CO2, the high-temperature chemisorbents can be grouped into (a) bulk reaction and (b) surface reaction. The first group of materials comprises mainly metal oxide or carbonate. The mechanism of chemisorption for this group of materials is diffusion of CO2 into the pores of the solid sorbent matrix followed by bulk chemical reaction. Table 7.2 summarizes the thermodynamic properties for various metal oxide and carbonate reactions. In the table, the temperatures at which the equilibrium constant equals one indicate the approximate temperature ranges that adsorption and desorption processes for each sorbent can occur. Despite very favorable forward reaction equilibrium, the three alkali metal hydroxides (Na, K, Li) may not be feasible sorbents for CO2. due to very high regeneration temperature required. If mixed or bound with other materials, NaOH has been suggested for CO2 removal from syngas (Siriwardane et al, 2007; Siriwardane, 2008).
Table 7.2
Thermodynamic properties for various metal oxide and carbonate reactions, as calculated from HSC Chemistry 5
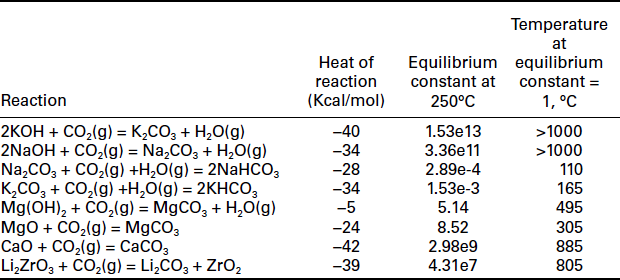
Calcium oxide and sodium carbonate are the two chemisorbents that have received most attention (Sun et al., 2008; Harrison, 2009; Symonds et al., 2009). Ca-based materials such as limestone or dolomite are abundant and inexpensive. They require very high temperature (> 800°C) for regeneration and consequently sintering of the sorbent is one major issue. On the other hand, the regenerable Na2 CO3 adsorbs CO2 to form bicarbonate at 50-80°C, while regeneration occurs in the range of 120-200°C (Liang et al, 2004). The reaction temperature may be too low to integrate with the syngas reactions. K2 CO3 is quite similar (Hayashi et al., 1998). The main application of both Na and K carbonate sorbents is for CO2 capture from flue gas. Other metal oxide sorbents that have been investigated include MgO or Mg(OH)2 (Lee et al., 2008a; Siriwardane and Stevens, 2009; Hassanzadeh and Abbasian, 2010) and Li2ZrO3 (Ida and Lin, 2003; Xiong et al, 2003; Nair et al., 2004).
The second group of chemisorbent materials includes hydrotalcite and alumina, which adsorb CO2 by reaction with active sites on the surface. Hydrotalcites belong to a class of anionic clays also known as layered double hydroxides (LDHs). The structure consists of magnesium-aluminum hydroxide-like layers with interlayer space containing charge compensating anions (typically CO3−) and water molecules (Yang et al, 2002; Hutson et al, 2004). The adsorption capacity of CO2 on LDHs at 300-400°C is not high, but can be greatly enhanced if promoted by potassium or sodium (Mayorga et al, 2001; Yong et al, 2002; Lee et al, 2007a; Singh et al., 2009). The thermal stability of promoted hydrotalcites in the presence of steam has been demonstrated from a few cycles of operation (Hufton et al, 1999; Lee et al, 2007b). They have fast kinetics and moderate heats of sorption and therefore are good candidate materials for sorption-enhanced reaction applications in a PSA or TSA process mode. Basic alumina and doped alumina with alkaline metals also offer reversible CO2 chemisorption at 200-450°C and have been evaluated for sorption-enhanced reaction for syngas and CO2 removal from flue gas (Gaffney et al, 1999; Yong et al, 2000; Lee et al., 2007c; Lee and Sircar, 2008). A good review article on reversible chemisorbents for CO2 can be found in Lee et al. (2008b).
Other new classes of sorbents that have been considered for CO2 adsorption include metal-organic frameworks (MOFs) and a subset of MOFs, zeolitic imidazolate frameworks (ZIFs) or zeolite-like MOFs (Rowsell and Yaghi, 2004; Millward and Yaghi, 2005; Liu et al., 2006; Wang et al, 2008). These are highly porous, chemically and thermally stable crystalline materials with a geometrically well-defined structure. One important attribute of these materials is the seemingly endless design flexibility offered by inorganic clusters linked together by functionalized organic components, similar to organic copolymers with their properties deriving from their building blocks. They have tremendous potential in areas such as gas separation, gas storage, catalysis or sensor. While it is still in early stage of development, good CO2. capacity and CO2./H2 selectivity have recently been reported (Babarao and Jiang, 2009; Banerjee et al, 2009; Jiang, 2009).
7.5 Solvent-based materials for H2/CO2 separation
The absorption process using solvents is the most widely used technology to remove CO2 from mixed gas streams on large commercial scales. For low partial pressures of CO2 as in flue gas applications, chemical solvents are favored, while for high concentrations of CO2 as in high-pressure fuel gas applications, physical solvents are preferred. The majority of chemical solvents are organic amine-based compounds, which can be primary amines (MEA, DGA), secondary amines (DEA, DIPA) or tertiary amines (TEA, MDEA). The amine solvents operate at low temperatures, < 40°C, and are regenerated at about 120°C. There are also inorganic chemical solvents that can operate at some-what higher temperatures such as potassium carbonate (80-120°C) or sodium carbonate (50-80°C). The former has a dominant market share as in UOP's Benfield™ process. To increase the efficiency of the potassium carbonate process, various promoters or activators and corrosion inhibitors are added to the solution (Field, 1975; Cullinane and Rochelle, 2006; Mani et al, 2008). Sodium carbonates are mainly targeted for the flue gas application.
Chemical solvents are seldom recommended for syngas applications unless the purpose is to remove only H2S. Physical solvents have been employed in industrial processes such as IGCC for acid gas removal. Typical physical solvents have high boiling points and low vapor pressures, and include glycols (Selexol™ process), cold methanol (Rectisol®) and n-formyl/acetyl morpholine (Morphysorb®). The hybrid solvent Sulfinol® uses a mixture of sulfolane and DIPA or MDEA (Korens et al., 2002). Recently, Heintz et al. (2008) investigated the use of perfluoriated compounds for selective CO2 capture from syngas at elevated pressures (to 30 bar) and temperatures (to 500 K). Their study proved the chemical and thermal stability of the solvent. The high temperature absorption at 500 K offers the advantage of thermal integration with the upstream syngas production; however, the relatively high solvent loss is one drawback due to the closeness of the solvent boiling point (533 K) and the operation temperature.
Ionic liquids, in addition to being explored for facilitated transport membranes mentioned earlier, can also be used in the absorption process (Baltus et al, 2005). The most commonly investigated ionic liquids are based on imidazolium salts. They possess attractive physical properties for the absorption process, low viscosity, non-volatility and good stability. The CO2 solubility for ionic liquids is primarily a physical phenomenon without any chemical reaction and is generally lower than in common organic solvents (Bara et al, 2009). However, the structure of ionic liquids can be tuned by matching different cations and/or anions and thus offers a great potential for future development. One approach to increase CO2 solubility is to tether an amine moiety to the cation, similar to the amine-functionalized adsorbent mentioned earlier (Bates et al, 2002). Although the CO2 capacity from this task-specific ionic liquid is comparable to the common MEA solvent, the increasing viscosity may limit its practical application. The other technique is simply to dissolve commercial amines in ionic liquids to have non-aqueous amine systems (Camper et al., 2008). Very rapid and reversible CO2 removal has been achieved, but the precipitates generated due to insolubility of carbomate in the ionic liquid may present some challenges for process/equipment design. Due to their high CO2/H2 selectivity and the low volatility at room temperature, ionic liquids can also be treated as solid adsorbents, for example in PSA processes (Brennecke and Maginn, 2003; Yokozeki and Shiflett, 2007).
7.6 Future trends
The H2/ syngas separation market will be mainly driven by the increasing demand for H2 in the refineries and petrochemical industries and the regulations on CO2 emissions in the energy industries. These two some- what different objectives may require different technology attributes, which affect the selection or development of the suitable separation technologies. For example, the hydrogen purity requirement may not be very high if the goal is only to reduce CO2 emissions, as opposed to H2 production. H2 PSA will remain as the benchmark process for developing advanced separation technologies for H2 production, while the solvent process will be the basis for comparison for developing CO2 capture technologies.
Among the three separation technologies that are covered in this chapter, solvent processes, in general, are perceived as very mature with relatively limited new development recently. On the other hand, membrane separation technologies have been very actively pursued by researchers globally. In reality, the boundaries between these three technologies have become indistinct as more advanced materials have been developed by mixing and matching different characteristics from membrane, adsorbent and solvent groups. Membrane materials have progressed beyond the conventional organic polymers by incorporating inorganic entities as in MMMs and FTMs. Organic functional groups can be introduced into solid adsorbent as in MOFs. Solvent-based compounds such as amines, if added to the membrane or adsorbent materials, can enhance their functionality or selectivity. New separation materials sometimes can find applications in all three separation processes as in the example of ionic liquids. This trend can be expected to continue and lead to discovery of new classes of materials.
Increasing the operation temperature will be at the forefront of H2/syngas separation material research. The high-temperature capability of separation materials can realize the benefit of process intensification by combining H2 separation and chemical reactions such as shift and reforming reactions in a single reactor. Membrane reactor technology not only offers operational simplicity, but it also raises the conversion of reactions and improves the energy efficiency (Drioli, 2004). The challenge is to package catalysts in an already complicated membrane module, which adds another dimension of design complexity. In this regard, the sorption-enhanced reactor may have the advantage by simply mixing the sorbents and the catalysts in a conventional fixed-bed reactor mode. The challenge here is the requirement of sorbent regeneration, which presents operational complexity. Reactive separation technology, in either material or process area, will receive increasing attention.
Improving the stability and durability of the materials should also be a priority issue for the new separation technologies to be successfully employed. The fossil fuel-derived syngas can contain contaminants, such as sulfur, nitrogen or carbon (coke) compounds, which are harmful to the separating agents. In some cases, the materials may not be stable in the presence of steam, CO, or even CO2. In addition to focusing on a robust separation system, developing gas clean-up technologies operating at temperatures comparable to the reactive separator is also important.
The literature survey conducted in this chapter reveals that there is no shortage of new concepts in the H2/syngas separation area, covering a wide range of membrane, adsorbent and solvent materials. What is lacking are pilot-scale or long-term demonstrations of these promising materials and technologies. At present, there are still no new technologies that have come close to replacing the current H2 PSA or solvent processes. The uncertain or unknown knowledge on cost is another hurdle for the success of advanced separation technologies. System analysis and economic evaluation for a H2 plant or an IGCC plant incorporating these advanced hydrogen/syngas separation technologies would be a valuable study.
7.7 Sources of further information and advice
There are numerous books and monographs on the subject of membranes. For a general overview of membrane fundamentals and applications, see Mulder (1997) and Baker (2004). For gas separation by polymeric membranes, the latest monograph edited by Freeman et al. (2007) provides a good state-of-the-art review. Recently, research on inorganic membrane materials and applications has expanded rapidly, especially in the energy and fuel areas. Bose (2008), Mallada and Menendez (2008), Kanellopoulos (2000), Burggraff and Cot (1996) and Hsieh (1996) are excellent references in the field of inorganic membranes. The book by Marcano and Tsotsis (2002) specifically deals with the area of the membrane reactor. In the area of adsorption, the books by Yang (1997, 2003), Ruthven (1984) and Ruthven et al. (1994) will be most useful. For hydrogen and syngas production and separation technologies, see the review article by Ritter and Ebner (2007) and the recent book by Liu et al. (2010). Discussion on general CO2 capture technologies is available in Maroto-Valer (2010).
The US Department of Energy (www.energy.gov) has been championing membrane research for many years. The development of novel membranes for separation and purification is included in several key publications: Hydrogen Posture Plan (US DOE and DOT, 2006), A National Vision of America's Transition to Hydrogen Economy - To 2030 and Beyond (US DOE, 2002), Hydrogen, Fuel Cells & Infrastructure Technologies Program: Multi-Year Research, Development and Demonstration Plan (US DOE, 2007), and Hydrogen from Coal Program (US DOE, 2010). The DOE has held annual meetings on merit review and peer evaluation for hydrogen projects. Report and proceedings are available on their website (www.annu- almeritreview.energy.gov).
The International Energy Association has been issuing high-level research reports on a broad range of energy areas. Hydrogen separation research can be found in the Greenhouse Gas R&D program (www.ieagreen.org.uk) and Hydrogen Implementation Agreement (www.ieahia.org). The Energy Research Center of the Netherlands (www.ecn.nl/) also supports a strong membrane research program as they focus on pre-combustion CO2 capture technologies. The International Adsorption Society (ias.vub.ac.be), North American Membrane Society (www.membranes.org), European Membrane House (www.euromemhouse.com) and European Membrane Society (www.emsoc.eu) regularly organize international conferences on adsorption/membrane research. Interested readers can go to their websites for lists of conferences and programs.
7.8 References
Adhikari, S., Fernando, S. Hydrogen membrane separation techniques. Industrial & Engineering Chemistry Research. 2006; 45:875–881.
Ahn, J., Chung, W.J., Pinnau, I., Song, J., Du, N., Robertson, G.P., Guiver, M.D., Gas transport behavior of mixed-matrix membranes composed of silica nano-particles in a polymer of intrinsic microporosity (PIM-1). Journal of Membrane Science 2010; 346:280–287, doi: 10.1016/j.memsci.2009.09.047.
Air Products and Chemicals (2011), Available from www.airproducts.com/products/Gases/supply-options/prism-membrane-hydrogen-recovery-and-purification.aspx.
Anderson, M., Lin, Y.S., Carbonate-ceramic dual-phase membrane for carbon dioxide separation. Journal of Membrane Science 2010; 357:122–129, doi: 10.1016/j.memsci.2010.04.009.
Babarao, R., Jiang, J., Unprecedentedly high selective adsorption of gas mixtures in rho zeolite-like metal-organic framework: A molecular simulation study. Journal of the American Chemical Society 2009; 131:11417–11425, doi: 10.1021/ja901061j.
Baker, R.W. Membrane Technology and Applications. West Sussex: Wiley; 2004.
Balachandran, U., Lee, T.H., Chen, L., Song, S.J., Picciolo, J.J., Dorris, S.E., Hydrogen separation by dense cermet membranes. Fuel 2006; 85:150–155, doi: 10.1016/j,fuel.2005.05.027.
Balachandran, U., Lee, T.H., Chen, L., Song, S.J., Picciolo, J.J., Dorris, S.E. Current status of dense cermet membranes for hydrogen separation. 22nd Annual Pittsburgh Coal Conference, Pittsburgh, USA, 12–15, September 2005. 2005.
Baltus, R.E., Counce, R.M., Culbertson, B.H., Luo, H., DePaoli, D.W., Dai, S., Duckworth, D.C., Examination of the potential of ionic liquids for gas separations. Separation Science and Technology 2005; 40:525–541, doi: 10.1081/SS-200042513.
Banerjee, R., Furukawa, H., Britt, D., Knobler, C., O'Keeffe, M., Yaghi, O.M., Control of pore size and functionality in isoreticular zeolitic imidazolate frameworks and their carbon dioxide selective capture properties. Journal of the American Chemical Society 2009; 131:3875–3877, doi: 10.1021/ja809459e.
Bara, J.E., Lessmann, S., Gabriel, C.J., Hatakeyama, E.S., Noble, R.D., Gin, D.L., Synthesis and performance of polymerizable room-temperature ionic liquids as gas separation membranes. Industrial & Engineering Chemistry Research 2007; 46:5397–5404, doi: 10.1021/ie0704492.
Bara, J.E., Carlisle, T.K., Gabriel, C.J., Camper, D., Finotello, A., Gin, D.L., Noble, R.D., Guide to CO2 separations in imidazolium-based room-temperature ionic liquids. Industrial & Engineering Chemistry Research 2009; 48:2739–2751, doi: 10.1021/ie8016237.
Barbieri, G., Brunetti, A., Tricoli, G., Drioli, E., An innovative configuration of a Pd-based membrane reactor for the production of pure hydrogen: Experimental analysis of water gas shift. Journal of Power Sources 2008; 182:160167, doi: 10.1016/j.jpowsour.2008.03.086.
Basile, A., Gallucci, F., Iulianelli, A., Tereschenko, G.F., Ermilova, M.M., Orekhova, N.V., Ti-Ni-Pd dense membranes - The effect of the gas mixtures on the hydrogen permeation. Journal of Membrane Science 2008; 310:44–50, doi: 10.1016/j. memsci.2007.10.028.
Basile, A., Iulianelli, A., Advanced membrane separation processes and technology for carbon dioxide (CO2) capture in power plantsMercedes Maroto-Valer, M., eds. Developments and innovation in carbon dioxide (CO2) capture and storage technology: Carbon dioxide capture, transport and industrial applications; Volume 1. Woodhead Publishing, Cambridge, UK, 2010:203. [Chapter 7].
Bates, E.D., Mayton, R.D., Ntai, I., Davis, J.H., Jr., CO2 capture by a task- specific ionic liquid. Journal of the American Chemical Society, 2002;124(6):9, doi: 10.1021/ja017593d.
Belmabkhout, Y., Serna-Guerrero, R., Sayari, A., Adsorption of CO2- containing gas mixtures over amine-bearing pore-expanded MCM-41 silica: Application for gas purification. Industrial & Engineering Chemistry Research 2010; 49:359–365, doi: 10.1021/ie900837t.
Bernardo, P., Drioli, E., Golemme, G., Membrane gas separation: A review/ state of the art. Industrial & Engineering Chemistry Research 2009; 48:4638–4663, doi: 10.1021/ie8019032.
Bhandari, R., Ma, Y.H., Pd-Ag membrane synthesis: The electroless and electro-plating conditions and their effect on the deposits morphology. Journal of Membrane Science 2009; 334:50–63, doi: 10.1016/j.memsci.2009.02.014.
Bose, A.C. Inorganic Membranes for Energy and Fuel Applications. New York: Springer; 2008.
Bosko, M.L., Ojeda, F., Lombardo, E.A., Cornaglia, L.M., NaA zeolite as an effective diffusion barrier in composite Pd/PSS membranes. Journal of Membrane Science 2009; 331:57–65, doi: 10.1016/j.memsci.2009.01.005.
Brennecke J F and Maginn E J (2003), 'Purification of gas with ionic liquids', US Patent 6579343
Burggraff, A.J., Cot, L. Fundamentals of Inorganic Membrane Science and Technology. Amsterdam: Elsevier; 1996.
Buxbaum, R.E., Kinney, A.B. Hydrogen transport through tubular membranes of palladium-coated tantalum and niobium. Industrial & Engineering Chemistry Research. 1996; 35:530–537.
Buxbaum, R.E., Marker, T.L. Hydrogen transport through non-porous membranes of palladium-coated niobium, tantalum and vanadium. Journal of Membrane Science. 1993; 85:29–38.
Camper, D., Bara, J.E., Gin, D.L., Noble, R.D., Room-temperature ionic liquid-amine solutions: Tunable solvents for efficient and reversible capture of CO2. Industrial & Engineering Chemistry Research 2008; 47:8496–8498, doi: 10.1021/ ie801002m.
Car, A., Stropnik, C., Yave, W., Peinemann, K.V., PEG modified poly(amide- b-ethylene oxide) membranes for CO2 separation. Journal of Membrane Science 2008; 307:88–95, doi: 10.1016/j.memsci.2007.09.023.
Carlisle, T.K., Bara, J.E., Lafrate, A.L., Gin, D.L., Noble, R.D., Main-chain imidazolium polymer membranes for CO. separations: An initial study of a new ionic liquid-inspired platform. Journal of Membrane Science 2010; 359:37–43, doi: 10.1016/j.memsci.2009.10.022.
Caro, J., Noack, M. Zeolite membranes - Recent developments and progress. Microporous and Mesoporous Materials. 2008; 115:215–233.
Chen, C.H., Ma, Y.H., The effect of H.S on the performance of Pd and Pd/Au composite membrane. Journal of Membrane Science 2010; 362:535–544, doi: 10.1016/j.memsci.2010.07.002.
Chung, T.-S., Jiang, L.Y., Li, Y., Kulprathipanja, S., Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Progress in Polymer Science 2007; 32:483–507, doi: 10.1016/j. progpolymsci.2007.01.008.
Costello, L.M., Walker, D.R.B., Koros, W.J. Analysis of a thermally stable polypyrolone for high temperature membrane-based gas separations. Journal of Membrane Science. 1994; 90:117–130.
Coulter, K.E., Way, J.D., Gade, S.K., Chaudhari, S., Sholl, D.S., Semidey-Flecha, L., Predicting, fabricating, and permeability testing of free-standing ternary palladium-copper-gold membranes for hydrogen separation. Journal of Physical Chemistry C 2010; 114:17173–17180, doi: 10.1021/jp1039628.
Cullinane, J.T., Rochelle, G.T., Kinetics of carbon dioxide absorption into aqueous potassium carbonate and piperazine. Industrial & Engineering Chemistry Research 2006; 45:2531–2545, doi: 10.1021/ie050230s.
Diniz da Costa, J.C., Lu, G.Q., Rudolph, V., Lin, Y.S. Novel molecular sieve silica (MSS) membranes: Characterization and permeation of single-step and two-step sol–gel membranes. Journal of Membrane Science. 2002; 198:9–21.
Dolan, M.D., Non-Pd BCC alloy membranes for industrial hydrogen separation. Journal of Membrane Science 2010; 362:12–28, doi: 10.1016/j. memsci.2010.06.068.
Doong, S.J., Ong, E., Lau, F. Direct extraction of hydrogen from coal using a membrane reactor integrated with a gasifier. 22nd Annual Pittsburgh Coal Conference, Pittsburgh, USA, 12–15, September 2005. 2005.
Dorris S E, Lee T H and Balachandran U (2003), 'Metal/ceramic composites with high hydrogen permeability', US Patent 6,569,226 B1.
Drage, T.C., Blackman, J.M., Pevida, C., Snape, C.E., Evaluation of activated carbon adsorbents for CO2 capture in gasification. Energy & Fuels 2009; 23:2790–2796, doi: 10.1021/ef8010614.
Drioli, E., Membrane reactors. Chemical Engineering and Processing 2004; 43:1101–1102, doi: 10.1016/j.cep.2004.04.002.
Ducrot-Boisgontier, C., Parmentier, J., Faour, A., Patarin, J., Pirngruber, G.D., FAU-type zeolite nanocasted carbon replicas for CO. adsorption and hydrogen purification. Energy & Fuels 2010; 24:3595–3602, doi: 10.1021/ ef100011q.
Edlund, D., McCarthy, J. The relationship between intermetallic diffusion and flux decline in composite-metal membranes: implications for achieving long membrane lifetime. Journal of Membrane Science. 1995; 107:147–153.
El-Azzami, L.A., Grulke, E.A., Carbon dioxide separation from hydrogen and nitrogen by fixed facilitated transport in swollen chitosan membranes. Journal of Membrane Science 2008; 323:225–234, doi: 10.1016/j.memsci.2008.05.019.
El-Azzami, L.A., Grulke, E.A., Carbon dioxide separation from hydrogen and nitrogen facilitated transport in arginine salt-chitosan membranes. Journal of Membrane Science 2009; 328:15–22, doi: 10.1016/j.memsci.2008.08.038.
EPRI and NETL (2000), 'Evaluation of innovative fossil fuel power plants with CO2 removal', Technical Report, 1000316, EPRI, Palo Alto and NETL, Pittsburgh
Field J H (1975), 'Separation of CO2 from gas mixtures', US Patent 3,907,969
Freeman, B., Yampolskii, Y., Pinnau, I. Materials Science of Membranes for Gas and Vapor Separation. West Sussex: Wiley; 2007.
Gade, S.K., Payzant, E.A., Park, H.J., Thoen, P.M., Way, J.D., The effects of fabrication and annealing on the structure and hydrogen permeation of Pd-Au binary alloy membranes. Journal of Membrane Science 2009; 340:227–233, doi: 10.1016/j. memsci.2009.05.034.
Gaffney T R, Golden T C, Mayorga S G, Brzozowski J R and Talyer F W (1999), 'Carbon dioxide pressure swing adsorption process using modified alumina adsorbents', US Patent 5917136
Gao, H., Lin, Y.S., Li, Y., Zhang, B., Chemical stability and its improvement of palladium-based metallic membranes. Industrial & Engineering Chemistry Research 2004; 43:6920–6930, doi: 10.1021/ie049722f.
Gielen D (2003), 'The future role of CO2 capture and storage: Results of the IEA- ETP model', International Energy Agency Working Paper, EET/2003/04, Paris.
Goeppert, A., Meth, S., Prakash, G.K.S., Olah, G.A., Nanostructured silica as a support for regenerable high-capacity organoamine-based CO2 sorbents. Energy & Environmental Science 2010; 3:1949–1960, doi: 10.1039/c0ee00136h0.
Gu, Y., Oyama, S.T., Permeation properties and hydrothermal stability of silica-titania membranes supported on porous alumina substrates. Journal of Membrane Science 2009; 345:267–275, doi: 10.1016/j.memsci.2009.09.009.
Hagg, M.-B., Quinn, R. Polymeric facilitated transport membranes for hydrogen purification. MRS Bulletin. 2006; 31:750.
Hamakawa, S., Lin, L., Li, A., Iglesia, E. Synthesis and hydrogen permeation properties of membranes based on SrCeYbO. thin films. Solid State Ionics. 2002; 48:71.
Hanioka, S., Maruyama, T., Sotani, T., Teramoto, M., Matsuyama, H., Nakashima, K., Hanaki, M., Kubota, F., Goto, M., CO2 separation facilitated by task- specificionic liquids using a supported liquid membrane. Journal of Membrane Science 2008; 314:1–4, doi: 10.1016/j.memsci.2010.06.047.
Harlick, P.J.E., Sayari, A., Applications of pore-expanded mesoporous silica. 5. Triamine grafted material with exceptional CO2 dynamic and equilibrium adsorption performance. Industrial & Engineering Chemistry Research 2007; 46:446–458, doi: 10.1021/ie060774+.
Harrison, D.P., 'Calcium enhanced hydrogen production with CO 2 capture. Energy Procedia 2009; 1:675–681, doi: 10.1016/j.egypro.2009.01.089.
Hashi, K., Ishikawa, K., Matsuda, T., Aoki, K., Hydrogen permeation characteristics of (V, Ta)-Ti-Ni alloys. Journal of Alloys and Compounds 2005; 404–406:273–278, doi: 10.1016/j.jallcom.2005.02.085.
Hassanzadeh, A., Abbasian, J. Regenerable MgO-based sorbents for high- temperature CO. removal from syngas: 1. Sorbent development, evaluation, and reaction modeling. Fuel. 2010; 89:1287–1297.
Hayashi, H., Taniuchi, J., Furuyashiki, N., Sugiyama, S., Hirano, S., Shigemoto, N., Nonaka, T. Efficient recovery of carbon dioxide from flue gases of coal-fired power plants by cyclic fixed-bed operations over K2CO3-on-carbon. Industrial & Engineering Chemistry Research. 1998; 37:185–191.
Heintz, Y.J., Sehabiague, L., Morsi, B.I., Jones, K.L., Pennline, H.W. Novel physical solvents for selective CO2 capture from fuel gas streams at elevated pressures and temperatures. Energy & Fuels. 2008; 22:3824–3837.
Hicks, J.C., Drese, J.H., Fauth, D.J., Gray, M.L., Qi, G., Jones, C.W., Designing adsorbents for CO2 capture from flue gas-hyperbranched aminosilicas capable of capturing CO. reversibly. Journal of the American Chemical Society 2008; 130:2902–2903, doi: 10.1021/ja077795v.
Hong, M., Li, S., Falconer, J.L., Noble, R.D., Hydrogen purification using a SAPO-34 membrane. Journal of Membrane Science 2008; 307:277–283, doi: 10.1016/j.memsci.2007.09.031.
Hong, M., Falconer, J.L., Noble, R.D. Modification of zeolite membrane for H2 separation by catalytic cracking of methyldiethoxysilane. Industrial & Engineering Chemistry Research. 2005; 44:4035–4041.
Hosseini, S.S., Chung, T.S., Carbon membranes from blends of PBI and polyimides for N2/CH4 and CO2/CH4 separation and hydrogen purification. Journal of membrane Science 2009; 328:174–185, doi: 10.1016/j.memsci.2008.12.005.
Howard, B.H., Killmeyer, R.P., Rothenberger, K.S., Cugini, A.V., Morreale, B.D., Enick, R.M., Bustamante, F., Hydrogen permeance of palladium-copper alloy membranes over a wide range of temperatures and pressures. Journal of Membrane Science 2004; 241:207–218, doi: 10.1016/j.memsci.2004.04.031.
Hsieh, H.P. Inorganic Membranes for Separation and Reaction. Amsterdam: Elsevier; 1996.
Huang, H.Y., Yang, R.T., Chinn, D., Munson, C.L., Amine-grafted MCM-48 and silica xerogel as superior sorbents for acidic gas removal from natural gas. Industrial & Engineering Chemistry Research 2003; 42:2427, doi: 10.1021/ie020440u.
Huang, J., Zou, J., Ho, W.S.W. CO2-selective membranes for hydrogen fuel processing'. In: Liu K., Song C., Subramani V., eds. Hydrogen and Syngas Production and Purification Technologies. Hoboken, New Jersey: Wiley and AIChE; 2010:385. [Chapter 9].
Hufton, J.R., Mayorga, S., Sircar, S. Sorption-enhanced reaction process for hydrogen production. AIChE Journal. 1999; 45(2):248.
Hutson, N.D., Speakman, S.A., Payzant, E.A., Hutson, N.D., Speakman, S.A., Payzant, E.A. Structural effects on the high temperature adsorption of CO. on a synthetic hydrotalcite. Chemistry and Materials Science. 2004; 16:4135–4143. [0.1021/cm040060u].
Ida, J.-I., Lin, Y.S., Mechanism of high-temperature CO2 sorption on lithium zirconate. Environmental Science & Technology 2003; 37:1999–2004, doi: 10.1021/ es0259032.
Ismail, A.F., David, L.I.B. A review on the latest development of carbon membranes for gas separation. Journal of Membrane Science. 2001; 193:1–18.
Iwahara, H., Esaka, T., Uchida, H., Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics. 1981; 3/4:359.
Jha, P., Way, J.D., Carbon dioxide selective mixed-matrix membranes formulation and characterization using rubbery substituted poly- phosphazene. Journal of Membrane Science 2008; 324:151–161, doi: 10.1016/j. memsci.2008.07.005.
Jiang, J., Charged soc metal-organic framework for high-efficacy H2 adsorption and syngas purification: Atomistic simulation study September. AIChE Journal, 2009;55(9):2422–2432, doi: 10.1002/aic.11865.
Jones, C.W., Koros, W.J. Carbon molecular sieve gas separation membranes-I. Preparation and characterization based on polyimide precursors. Carbon. 1994; 32:1419–1425.
Judkins, R., Bischoff, B., Scale-up of microporous inorganic hydrogen- separation membrane. 22nd Annual Pittsburgh Coal Conference, Pittsburgh, USA, 2005.
Kamakoti, P., Morreale, B., Ciocco, M., Howard, B., Killmeyer, P., Cugini, A., Sholl, D. Prediction of hydrogen flux through sulfur-tolerant binary alloy membranes. Science. 2005; 37:569–573.
Kanellopoulos, N.K. Recent Advances in Gas Separation by Microporous Ceramic Membranes. Amsterdam: Elsevier; 2000.
Kanezashi, M., O'Brien-Abraham, J., Lin, Y.S., Suzuki, K., Gas permeation through DDR-type zeolite membranes at high temperatures. AIChE Journal, 2008;54(6):1478–1486, doi: 10.1002/aic.11457.
Kanezashi, M., Yada, K., Yoshioka, T., Tsuru, T., Design of silica networks for development of highly permeable hydrogen separation membranes with hydrothermal stability. Journal of the American Chemical Society 2009; 131:414415, doi: 10.1021/ja806762q.
Khan, A.L., Cano-Odena, A., Gutiérrez, B., Minguillón, C., Vankelecom, I.F.J., Hydrogen separation and purification using polysulfone acrylate-zeolite mixed matrix membranes. Journal of Membrane Science 2010; 350:340–346, doi: 10.1016/j.memsci.2010.01.009.
Kiyono, M., Williams, P.J., Koros, W.J., Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes. Journal of Membrane Science 2010; 359:2–10, doi: 10.1016/j.memsci.2009.10.019.
Knowles, G.P., Graham, J.V., Delaney, S.W., Chaffee, A.L., Aminopropyl- functionalized mesoporous silicas as CO2 adsorbents. Fuel Processing Technology 2005; 86:1435–1448, doi: 10.1016/j.fuproc.2005.01.014.
Knowles, G.P., Delaney, S.W., Chaffee, A.L., Knowles, G.P., Delaney, S.W., Chaffee, A.L., Diethylenetriamine[propyl(silyl)]- functionalized (DT) mesoporous silicas as CO2 adsorbents. Industrial & Engineering Chemistry Research 2006; 45:2626–2633, doi: 10.1021/ie050589g.
Korens N, Simbeck D R, Wilhelm D J, Longanbach J R and Stiegel G J (2002), 'Process screening analysis of alternative gas treating and sulfur removal for gasification', Revised final report. SFA Pacific, Inc., Mountain View, CA; U.S. Department of Energy, National Energy Technology Laboratory, Pittsburgh, PA, Task Order 739656–00100.
Koros, W.J., Fleming, G.K. Membrane-based gas separation. Journal of Membrane Science. 1993; 83:1–80.
Kusuma, V.A., Matteuccia, S., Freeman, B.D., Danquahb, M.K., Kalika, D.S., Influence of phenoxy-terminated short-chain pendant groups on gas transport properties of cross-linked poly(ethylene oxide) copolymers. Journal of Membrane Science 2009; 341:84–95, doi: 10.1016/j.memsci.2009.05.043.
LeBlanc, O.H., Jr., Ward, W.J., Matson, S.L., Kimura, S.G. Facilitated transport in ion-exchange membranes. Journal of Membrane Science. 1980; 6:339–343.
Lee, C.S., Chae, H.J., Lee, S.J., Choi, B.Y., Yi, C.K., Lee, J.B., Ryu, C.K., Kim, J.C., Development of regenerable MgO-based sorbent promoted with K2CO3 for CO2 capture at low temperatures. Environmental Science and Technology 2008; 42:2736–2741, doi: 10.1021/es702693c.
Lee, D., Oyama, S.T. Gas permeation characteristics of a hydrogen selective supported silica membrane. Journal of Membrane Science. 2002; 210:291–306.
Lee, K.B., Verdooren, A., Caram, H.S., Sircar, S., Chemisorption of carbon dioxide on potassium-carbonate-promoted hydrotalcite. Journal of Colloid and Interface Science 2007; 308:30–39, doi: 10.1016/j.jcis.2006.11.011.
Lee, K.B., Beaver, M.G., Caram, H.S., Sircar, S., Novel thermal-swing sorption-enhanced reaction process concept for hydrogen production by low- temperature steam-methane reforming. Industrial & Engineering Chemistry Research 2007; 46:5003–5014, doi: 10.1021/ie0701064.
Lee, K.B., Beaver, M.G., Caram, H.S., Sircar, S., Chemisorption of carbon dioxide on sodium oxide promoted alumina. AIChE Journal 2007; 53:2824, doi: 10.1002/aic.11312.
Lee, K.B., Sircar, S., Removal and recovery of compressed CO2 from flue gas by a novel thermal swing chemisorption process. AIChE Journal 2008; 54:2293, doi: 10.1002/aic.11531.
Lee, K.B., Beaver, M.G., Caram, H.S., Sircar, S., Reversible chemisorbents for carbon dioxide and their potential applications. Industrial & Engineering Chemistry Research 2008; 47:8048–8062, doi: 10.1021/ie800795y.
Liang, Y., Harrison, D.P., Gupta, R.P., Green, D.A., McMichael, W.J., Carbon dioxide capture using dry sodium-based sorbents. Energy & Fuels 2004; 18:569–575, doi: 10.1021/ef030158f.
Lin, H., van Wagner, E., Freeman, B.D., Toy, L.G., Gupta, R.P., Plasticization- enhanced hydrogen purification using polymeric membranes. Science 2006; 311:639–642, doi: 10.1126/science.1118079.
Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Separation and Purification Technology. 2001; 25:39–55.
Lindmark, J., Hedlund, J., Carbon dioxide removal from synthesis gas using MFI membranes. Journal of Membrane Science 2010; 360:284–291, doi: 10.1016/j. memsci.2010.05.025.
Liu, K., Song, C., Subramani, V. Hydrogen and Syngas Production and Purification Technologies. Hoboken, New Jersey: Wiley and AIChE; 2010.
Liu, P. Hydrogen production via a commercially ready inorganic membrane reactor. US DOE Annual Hydrogen Program Review Meeting, Washington, DC, 23–26. (May 2005):2005.
Liu, Y., Kravtsov, V.C., Larsen, R., Eddaoudi, M., Molecular building blocks approach to the assembly of zeolite-like metal-organic frameworks (ZMOFs) with extra-large cavities. Chemical Communications 2006; 14:1488–1490, doi: 10.1039/b600188m.
Liu, Y., Xu, J., Jin, L., Fang, Y., Hu, H., Synthesis and modification of zeolite NaA adsorbents for separation of hydrogen and methane. Asia-Pacific Journal of Chemical Engineering 2009; 4:666–671, doi: 10.1002/apj.315.
Lopes, F.V.S., Grande, C.A., Ribeiro, A.M., Oliveira, E.L.G., Loureiro, J.M., Rodrigues, A.E., Enhancing capacity of activated carbons for hydrogen purification. Industrial & Engineering Chemistry Research 2009; 48:3978–3990, doi: 10.1021/ ie801132t.
Ma, Y.H., Composite Pd and Pd/alloy membranesBose A., ed. Inorganic Membranes for Energy and Fuel Applications. Springer: New York, 2008:241–254, doi: 10.1007/978-0-387-34526-0_13. [chapter 13].
Ma, Y.H., Akis, B.C., Ayturk, M.E., Guazzone, F., Engwall, E.E., Mardilovich, I.P. Characterization of intermetallic diffusion barrier and alloy formation for Pd/Cu and Pd/Ag porous stainless steel composite membranes. Industrial & Engineering Chemistry Research. 2004; 43:2936–2945.
MacLean, D.L., Bollinger, W.A., King, D.E., Narayan, R.S., 'Gas separation design with membranesLi, N.N., Calo, J.M., eds. Recent Development in Separation Science; Vol. IX. CRC Press, Boca Raton, FL, USA, 1986:227–244. [Chapter 12].
Mahajan, R., Koros, W.J., Factors controlling successful formation of mixed- matrix gas separation materials. Industrial & Engineering Chemistry Research 2000; 39:2692–2696, doi: 10.1021/ie990799r.
Mallada, R., Menendez, M. Inorganic Membranes: Synthesis, Characterization and Applications. Amsterdam: Elsevier; 2008.
Mani, F., Peruzzini, M., Stoppioni, P., Combined process of CO2 capture by potassium carbonate and production of basic zinc(II) carbonates: CO2 release from bicarbonate solutions at room temperature and pressure. Energy & Fuels 2008; 22:1714–1719, doi: 10.1021/ef7006936.
Maroto-Valer, M.M. Developments and Innovation in Carbon Dioxide (CO2) Capture and Storage Technology: Carbon Dioxide Capture, Transport and Industrial Application; Volume 1. Woodhead Publishing, Cambridge, UK, 2010.
Marcano, J.G.S., Tsotsis, T.T. Catalytic Membrane and Membrane Reactors. Weinheim: Wiley-VCH; 2002.
Matsuyama, H., Terada, A., Nakagawara, T., Kitamura, Y., Teramoto, M. Facilitated transport of CO2 through polyethylenimine/poly(vinyl alcohol) blend membrane. Journal of Membrane Science. 1999; 163:221–227.
Mayorga S G, Weigel S J, Gaffney T R and Brzozowski J R (2001), 'Carbon dioxide adsorbents containing magnesium oxide suitable for use at high temperatures', US Patent 6,280, 503
McBride, R.B., McKinley, D.L. A new hydrogen recovery route. Chemical Engineering Progress. 1965; 61:81–85.
McCool, B., Xomeritakis, G., Lin, Y.S. Composition control and hydrogen permeation characteristics of sputter deposited palladium-silver membranes. Journal of Membrane Science. 1999; 161:67–76.
Merkel, T.C., Gupta, R.P., Turk, B.S., Freeman, B.D. Mixed-gas permeation of syngas components in poly(dimethylsiloxane) and poly(1-trimethylsi- lyl-1-propyne) at elevated temperatures. Journal of Membrane Science. 2001; 191:85–94.
Merkel, T.C., He, Z., Pinnau, I., Freeman, B.D., Meakin, P., Hill, A.J., Effect of nanoparticles on gas sorption and transport in poly(1-trimethylsilyl-1-pro- pyne). Macromolecules 2003; 36:6844–6855, doi: 10.1021/ma0341566.
Merkel, T.C., He, Z., Pinnau, I., Freeman, B.D., Meakin, P., Hill, A.J., Sorption and transport in poly (2,2-bis(trifluoromethyl)-4,5-difluoro-1,3-dioxole-co- tetrafluoro-ethylene) containing nanoscale fumed silica. Macromolecules 2003; 36:8406–8414, doi: 10.1021/cm020672j.
Merkel, T.C., Freeman, B.D., Spontak, R.J., He, Z., Pinnau, I., Meakin, P., Hill, A.J., Sorption, transport, and structural evidence for enhanced free volume in poly(4-methyl-2-pentyne)/fumed silica nanocomposite membranes. Chemistry of Materials 2003; 15:109–123, doi: 10.1021/cm020672j.
Millward, A.R., Yaghi, O.M., Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. Journal of the American Chemical Society 2005; 127:17998–17999, doi: 10.1021/ja0570032.
Morreale, B.D., Ciocco, M.V., Howard, B.H., Killmeyer, R.P., Cugini, A.V., Enick, R.M., Effect of hydrogen-sulfide on the hydrogen permeance of palladium- copper alloys at elevated temperatures. Journal of Membrane Science 2004; 241:219–224, doi: 10.1016/j.memsci.2004.04.033.
Moss, T.S., Peachey, N.M., Snow, R.C., Dye, R.C. Multilayer metal membranes for hydrogen separation. International Journal of Hydrogen Energy. 1998; 23:99–106.
Mulder, M. Basic Principles of Membrane Technology. Dordrecht: Kluwer; 1997.
Mundschau M V (2005), 'Hydrogen transport membranes', US Patent 6,899,744 B2.
Mundschau, M.V., Xie, X., Evenson, C.R., IV., Sammells, A.F., Dense inorganic membranes for production of hydrogen from methane and coal with carbon dioxide sequestration. Catalysis Today 2006; 118:12–23, doi: 10.1016/j. cattod.2006.01.042.
Mundschau, M.V., Xie, X., Evenson, C.R., IV., Sammells, A.F. Membranes for the purification of hydrogen produced from coal-derived water-gas shift mixtures. 22nd Annual Pittsburgh Coal Conference. Pittsburgh, USA, 12–15, September 2005. 2005.
Mundschau, M.V., Xie, X., Sammells, A.F., Hydrogen transport membrane technologies for simultaneous carbon dioxide capture and hydrogen separation in a membrane shift reactorThomas, D.C., Benson, S.M., eds. Carbon Dioxide Capture for Storage in Deep Geologic Formation; Vol 1. Elsevier, Amsterdam, 2005:291–306. [Chapter 16].
Myers, C., Pennline, H., Luebke, D., Ilconich, J., Dixon, J.K., Maginn, E.J., Brennecke, J.F., High temperature separation of carbon dioxide/hydrogen mixtures using facilitated supported ionic liquid membranes. Journal of Membrane Science 2008; 322:28–31, doi: 10.1016/j.memsci.2008.04.062.
Nair, B.N., Yamaguchi, T., Kawamura, H., Nakao, S.-I. Processing of lithium zirconate for applications in carbon dioxide separation: Structure and properties of the powders. Journal of the American Ceramic Society. 2004; 87(1):68–74.
Nam, S.-E., Lee, K.-H. Hydrogen separation by Pd alloy composite membranes: Introduction of diffusion barrier. Journal of Membrane Science. 2001; 192:177–185.
Norby, T. Ceramic proton and mixed proton-electron conductors in membranes for energy conversion applications. Journal of Chemical Engineering of Japan. 2007; 40:1166–1171.
Norby, T., Larring, Y. Mixed hydrogen ion-electronic conductors for hydrogen permeable membranes. Solid State Ionics. 2000; 136–137:139–148.
Ockwig, N.W., Nenoff, T.M., Membranes for hydrogen separation. Chemical Reviews 2007; 107:4078–4110, doi: 10.1021/cr0501792.
Okazaki, J., Ikeda, T., Tanaka, D.A.P., Sato, K., Suzuki, T.M., Mizukami, F., An investigation of thermal stability of thin palladium-silver alloy membranes for high temperature hydrogen separation. Journal of Membrane Science 2011; 366:212–219, doi: 10.1016/j.memsci.2010.10.011.
Ordõnez, M.J.C., Balkus, K.J., Jr., Ferraris, J.P., Musselman, I.H., Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes. Journal of Membrane Science 2010; 361:28–37, doi: 10.1016/j.memsci.2010.06.017.
Ozaki, T., Zhang, Y., Komaki, M., Nishimura, C. Hydrogen permeation characteristics of V-Ni-Al alloys. International Journal of Hydrogen Energy. 2003; 28:1229–1235.
Parsons. Evaluation of fossil fuel power plants with CO2 recovery, 2002. [Final Report, DOE Contract No. DE-AM26-99FT40465].
Pesiri, D.R., Jorgensen, B., Dye, R.C., Thermal optimization of polyben- zimidazole meniscus membranes for the separation of hydrogen, methane, and carbon dioxide. Journal of Membrane Science 2003; 218:11–18, doi: 10.1016/ S0376-7388(03)00129-7.
Peters, T.A., Tucho, W.M., Ramachandran, A., Stange, M., Walmsley, J.C., Holmestad, R., Borg, A., Bredesen, R., Thin Pd-23%Ag/stainless steel composite membranes: Long-term stability, life-time estimation and post-process characterization. Journal of Membrane Science 2009; 326:572–581, doi: 10.1016/j. memsci.2008.10.053.
Perry, J.D., Nagai, K., Koros, W.J. Polymer membranes for hydrogen separations. MRS Bulletin. 2006; 31:745.
Phair, J.W., Donelson, R., Development and design of novel (non-palladium-based) metal membranes for hydrogen separation. Industrial & Engineering Chemistry Research 2006; 45:5657–5674, doi: 10.1021/ie051333d.
Pomerantz, N., Ma, E.Y.H., Payzant, A., Isothermal solid-state transformation kinetics applied to Pd/Cu alloy membrane fabrication. AIChE Journal, 2010;56(12):3062, doi: 10.1002/aic.12217.
Probstein, R.F., Hicks, R.E. Synthetic Fuels. New York: McGraw-Hill; 1982.
Qi, X., Lin, Y.S. Electrical conduction and hydrogen permeation through mixed proton-electron conducting strontium cerate membranes. Solid State Ionics. 2000; 130:149.
Reijerkerka, S.R., Arun, A., Gaymans, R.J., Nijmeijera, K., Wessling, M., Tuning of mass transport properties of multi-block copolymers for CO2 capture applications. Journal of Membrane Science 2010; 359:54–63, doi: 10.1016/j. memsci.2009.09.045.
Reijerkerka, S.R., Nijmeijera, K., Ribeiro, C.B., Jr., Freeman, B.D., Wessling, M., On the effects of plasticization in CO2/light gas separation using polymeric solubility selective membranes. Journal of Membrane Science 2011; 367:33–44, doi: 10.1016/j.memsci.2010.10.035.
Rezac, M.E., Koros, W.J., Miller, S.J., Chemical stability of polyimide membranes at temperature near Tg. Journal of Applied Polymer Science 1995; 58:165170, doi: 10.1002/app.1995.070580118.
Ritter, J.A., Ebner, A.D., State-of-the-art adsorption and membrane separation processes for hydrogen production in the chemical and petrochemical industries. Separation Science and Technology 2007; 42:1123–1193, doi: 10.1080/01496390701242194.
Robeson, L.M., The upper bound revisited. Journal of Membrane Science 2008; 320:390–400, doi: 10.1016/j.memsci.2008.04.030.
Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. Journal of Membrane Science. 1991; 62:165.
Roa, F., Block, M.J., Way, J.D. The influence of alloy composition on the H2 flux of composite Pd-Cu membranes. Desalination. 2002; 147:411–416.
Rowsell, J.L.C., Yaghi, O.M., Metal-organic frameworks: A new class of porous materials. Microporous and Mesoporous Materials 2004; 73:3–14, doi: 10.1016/j.micromeso.2004.03.034.
Ruthven, D.M. Principles of Adsorption & Adsorption Processes. New York: Wiley; 1984.
Ruthven, D.M., Farooq, S., Knaebel, K.S. Pressure Swing Adsorption. New York: Wiley; 1994.
Ryi, S.-K., Park, J.-S., Kim, S.-H., Cho, S.-H., Kim, D.-W., The effect of support resistance on the hydrogen permeation behavior in Pd-Cu-Ni ternary alloy membrane deposited on a porous nickel support. Journal of Membrane Science 2006; 280:883–888, doi: 10.1016/j.memsci.2006.03.007.
Ryi, S.K., Park, J.S., Kim, S.H., Kim, D.W., Cho, K.I., Formation of a defect-free Pd-Cu-Ni ternary alloy membrane on a polished porous nickel support (PNS). Journal of Membrane Science 2008; 318:346–354, doi: 10.1016/j. memsci.2008.02.055.
Saufi, S.M., Ismail, A.F., Fabrication of carbon membranes for gas separation - A review. Carbon 2004; 42:241–259, doi: 10.1016/j.carbon.2003.10.022.
Scovazzo, P., Determination of the upper limits, benchmarks, and critical properties for gas separations using stabilized room temperature ionic liquid membranes (SILMs) for the purpose of guiding future research. Journal of Membrane Science 2009; 343:199–211, doi: 10.1016/j.memsci.2009.07.028.
Serna-Guerrero, R., Da'na, E., Sayari, A., New insights into the interactions of CO, with amine-functionalized silica. Industrial & Engineering Chemistry Research 2008; 47:9406–9412, doi: 10.1021/ie801186g.
Shao, L., Samseth, J., Hagg, M.B., Crosslinking and stabilization of nan-oparticle filled poly(1-trimethylsilyl-1-propyne) nanocomposite membranes for gas separations. Journal of Applied Polymer Science 2009; 113:3078–3088, doi: 10.1016/j.memsci.2008.09.053.
Shao, L., Low, B.T., Chung, T.S., Greenberg, A.R., Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. Journal of Membrane Science 2009; 327:18–31, doi: 10.1016/j.memsci.2008.11.019.
Shekhawat D, Luebke D R and Pennline H W (2003), 'A review of carbon dioxide selective membranes', A Topical Report, DOE/NETL-2003/1200.
Shu, J., Grandjean, B.P.A., van Neste, A., Kaliaguine, S. Catalytic palladium-based membrane reactors: A review. The Canadian Journal of Chemical Engineering. 1991; 69:1036–1060.
Simmons J W (2005), 'Block polyurethane-ether and polyurea-ether gas separation membranes', US Patent 6843829
Singh, R., Reddy, M.K.R., Wilson, S., Joshi, K., Diniz da Costa, J.C., Webley, P. High temperature materials for CO2 capture. Energy Procedia. 2009; 1:623–630.
Sircar, S., Golden, T.C. Pressure swing adsorption technology for hydrogen production'. In: Liu K., Song C., Subramani V., eds. Hydrogen and Syngas Production and Purification Technologies. Hoboken, New Jersey: Wiley and AIChE; 2010:414. [Chapter 10].
Siriwardane R V (2008), 'Regenerable sorbents for CO2 capture from moderate and high temperature gas streams', US Patent 7314847
Siriwardane, R.V., Stevens, R.W., Jr., Novel regenerable magnesium hydroxide sorbents for CO2 capture at warm gas temperatures. Industrial & Engineering Chemistry Research 2009; 48:2135–2141, doi: 10.1021/ie8011598.
Siriwardane, R.V., Robinson, C., Shen, M., Simonyi, T. Novel regenerable sodium-based sorbents for CO2 capture at warm gas temperatures. Energy Fuels. 2007; 21(4):2088–2097.
Song, G., Kellam, M.E., Liang, D., Dolan, M.D., Influence of processing conditions on the microstructure and permeability of BCC V-Ni membranes. Journal of Membrane Science 2010; 363:309–315, doi: 10.1016/j. memsci.2010.07.051.
Sun, P., Lim, C.J., Grace, J.R., Cyclic CO2 capture by limestone-derived sorbent during prolonged calcination/carbonation cycling. AIChE Journal, 2008;54(6):1668, doi: 10.1002/aic.11491.
Symonds, R.T., Lu, D.Y., Macchi, A., Hughes, R.W., Anthony, E.J., CO2 capture from syngas via cyclic carbonation/calcination for a naturally occurring limestone: Modelling and bench-scale testing. Chemical Engineering Science 2009; 64:3536–3543, doi: 10.1016/j.ces.2009.04.043.
Tang, J., Tang, H., Sun, W., Plancher, H., Radosz, M., Shen, Y., Poly(ionic liquid)s: A new material with enhanced and fast CO2 absorption. Chemical Communications 2005; 26:3325, doi: 10.1039/b501940k.
Tong, J., Su, L., Kashima, Y., Shirai, R., Suda, H., Matsumura, Y. Simultaneously depositing Pd-Ag thin membrane on asymmetric porous stainless steel tube and application to produce hydrogen from steam reforming of methane. Industrial & Engineering Chemistry Research. 2006; 45:648–669.
Tosti, S., Adrover, A., Basile, A., Camilli, V., Chiappetta, G., Violante, V. Characterization of thin wall Pd-Ag rolled membranes. International Journal of Hydrogen Energy. 2003; 28:105–112.
Tosti, S., Basile, A., Bettinali, L., Borgognoni, F., Gallucci, F., Rizzello, C., Design and process study of Pd membrane reactors. International Journal of Hydrogen Energy 2008; 33:5098–5105, doi: 10.1016/j.ijhydene.2008.05.031.
Tsuru, T., Nano/subnano-tuning of porous ceramic membranes for molecular separation. Journal of Sol–gel Science and Technology 2008; 46:349–361, doi: 10.1007/s10971-008-1712-5.
US DOE. A National Vision of America's Transition to Hydrogen Economy-To 2030 and Beyond, 2002. [Washington D.C.].
US DOE and DOT. Hydrogen Posture Plan: An Integrated Research, Development and Demonstration Plan, 2006. [Washington D.C.].
US DOE. Hydrogen, Fuel Cells & Infrastructure Technologies Program: Multi-Year Research, Development and Demonstration Plan, 2007. [Washington D.C.].
US DOE. Hydrogen from Coal Program: Research, Development, and Demonstration Plan, 2010. [Washington D.C.].
Verweij, H., Lin, Y.S., Dong, J. Microporous silica and zeolite membranes for hydrogen purification. MRS Bulletin. 2006; 31:756.
de Vos, R.M., Maier, W.F., Verweij, H. Hydrophobic silica membranes for gas separation. Journal of Membrane Science. 1999; 158:277–288.
Wade, J.L., Lee, C., West, A.C., Lackner, K.S., Composite electrolyte membranes for high temperature CO, separation. Journal of Membrane Science 2011; 369:20–29, doi: 10.1016/j.memsci.2010.10.053.
Wang, L., Yoshiie, R., Uemiya, S., Fabrication of novel Pd-Ag-Ru/Al, O3 ternary alloy composite membrane with remarkably enhanced H2 permeability. Journal of Membrane Science 2007; 306:1–7, doi: 10.1016/j.memsci.2007.08.057.
Wang, B., Cote, A.P., Furukawa, H., O'Keeffe, M., Yaghi, O.M., Colossal cages in zeolitic imidazolate frameworks as selective carbon dioxide reservoirs. Nature, 2008;453(8):207–211, doi: 10.1038/nature06900.
White J H, Schwartz M and Sammells F (2001), 'Solid state proton and electron mediating membrane and use in catalytic membrane reactors', US Patent 6,281,403
Xie, X., Evenson, C.R., IV., Mundschau, M.V., Wright, H.A., Grimmer, P.J., High-pressure operation of dense hydrogen transport membranes for pure hydrogen production and simultaneous CO2 capture. 23rd Annual Pittsburgh Coal Conference, Pittsburgh, USA, 2006.
Xing, R., Ho, W.S.W., Crosslinked polyvinylalcohol-polysiloxane/fumed silica mixed matrix membranes containing amines for CO2/H2 separation. Journal of Membrane Science 2011; 367:91–102, doi: 10.1016/j.memsci.2010.10.039.
Xiong, R., Ida, J., Lin, Y.S., Kinetics of carbon dioxide sorption on potassium-doped lithium zirconate. Chemical Engineering Science 2003; 58:4377–4385, doi: 10.1016/S0009-2509(03)00319-1.
Xu, X., Song, C., Miller, B.G., Scaroni, A.W., Adsorption separation of carbon dioxide from flue gas of natural gas fired boiler by a novel nanoporous "molecular basket" adsorbent. Fuel Processing Technology 2005; 86:1457–1472, doi: 10.1016/j.fuproc.2005.01.002.
Yamaguchi, T., Niitsuma, T., Nair, B.N., Nakagawa, K., Lithium silicate based membranes for high temperature CO2 separation. Journal of Membrane Science 2007; 294:16–21, doi: 10.1016/j.memsci.2007.01.028.
Yang, J.Y., Nishimura, C., Komaki, M., Hydrogen permeation of Pd,0Cu40 alloy covered V-15Ni composite membrane in mixed gases containing H/S. Journal of Membrane Science 2008; 309:246–250, doi: 10.1016/j.memsci.2007.10.036.
Yang, R.T. Gas Separation by Adsorption Processes. London: Imperial College Press; 1997.
Yang, R.T. Adsorbents: Fundamentals and Applications. New York: Wiley; 2003.
Yang, W., Kim, Y., Liu, P.K.T., Sahimi, M., Tsotsis, T.T. A study by in situ techniques of the thermal evolution of the structure of a Mg-Al-CO3 layered double hydroxide. Chemical Engineering Science. 2002; 57:2945–2953.
Yokozeki, A., Shiflett, M.B., Hydrogen purification using roomtemperature ionic liquids. Applied Energy 2007; 84:351–361, doi: 10.1016/j. apenergy.2006.06.002.
Yong, Z., Mata, V., Rodrigues, A.E. Adsorption of carbon dioxide at high temperature - A review. Separation and Purification Technology. 2002; 26:195–205.
Yong, Z., Mata, V., Rodrigues, A.E., Adsorption of carbon dioxide on basic alumina at high temperatures. Journal of Chemical & Engineering Data 2000; 45:1093–1095, doi: 10.1021/je000075i.
Yoshino, Y., Suzuki, T., Nair, B.N., Taguchi, H., Itoh, N., Development of tubular substrates, silica based membranes and membrane modules for hydrogen separation at high temperature. Journal of Membrane Science 2005; 267:8–17, doi: 10.1016/j.memsci.2005.05.020.
Zhang, K., Wei, X., Rui, Z., Li, Y., Lin, Y.S., Effect of metal-support interface on hydrogen permeation through palladium membranes. AIChE Journal, 2009;55(3):630, doi: 10.1002/aic.11760.
Zhang, Y., Musselman, I.H., Ferraris, J.P., Balkus, K.J., Jr., Gas permeability properties of Matrimid® membranes containing the metal-organic framework Cu-BPY-HFS. Journal of Membrane Science 2008; 313:170–181, doi: 10.1016/j. memsci.2008.01.005.
Zhang, Y., Balkus, K.J., Jr., Musselman, I.H., Ferraris, J.P., Mixed-matrix membranes composed of Matrimid® and mesoporous ZSM-5 nanoparticles. Journal of Membrane Science 2008; 325:28–39, doi: 10.1016/j.memsci.2008.04.063.
Zimmerman, C.M., Singh, A., Koros, W.J. Tailoring mixed matrix composite membranes for gas separations. Journal of Membrane Science. 1997; 137:145–154.
Zornoza, B., Téllez, C., Coronas, J., Mixed matrix membranes comprising glassy polymers and dispersed mesoporous silica spheres for gas separation. Journal of Membrane Science 2011; 368:100–109, doi: 10.1016/j.memsci.2010.11.027.
Zou, J., Ho, W.S.W., CO 2-selective polymeric membranes containing amines in crosslinked poly(vinyl alcohol). Journal of Membrane Science 2006; 286:310–321, doi: 10.1021/ie070449.