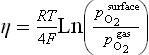Novel cathodes for solid oxide fuel cells
Abstract:
In this chapter, after a brief overview of the oxygen reduction reaction at the cathode side of a solid oxide fuel cell (SOFC), the main requirements for cathode materials are discussed as well as the conventional compounds that have been intensively studied during the last two decades. In the second part of the chapter, innovative materials (mixed ionic and electronic conducting oxides) are considered from the viewpoint of structural aspects and transport properties. Special attention is devoted to the layered compounds, Ln2NiO4 + δ, which exhibit promising properties (oxygen diffusivity, electrocatalytic properties). Their performances as SOFC cathode materials are compared to those of other materials. Finally, the flexibility of these materials as oxygen electrodes in protonic ceramic fuel cells and electrolyzers is emphasized. This is discussed in terms of the materials’ structural features.
13.1 Introduction
It is now an acknowledged fact that there is a worldwide interest in developing a hydrogen-based economy in order to reduce environmental pollution; fuel cell technology commands attention in particular as fuel cells convert electrochemically and efficiently the chemical energy of a fuel directly into electrical energy (Singhal, 2000). Similarly, high-temperature steam electrolysis is the subject of more and more interest (Erdle et al., 1992).
Depending on the nature of the electrolyte and on the diffusing species, which determines the operating temperature and the application fields, various kinds of fuel cells have been developed. However, current research is mainly devoted to mature technologies:
• the low-temperature systems, namely PEMFCs (proton exchange membrane fuel cells) used mainly for transportation applications,
• the high-temperature systems, namely solid oxide fuel cells (SOFCs), used for stationary applications and combined heat production.
Figure 13.1 summarizes the various types of fuel cells and their corresponding ranges of operating temperatures.
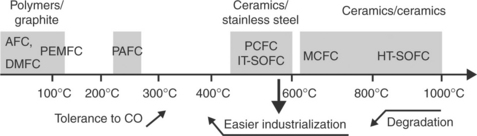
13.1 Panorama of the various fuel cell technologies and their corresponding ranges of operating temperatures (AFC: alkaline fuel cell; DMFC: direct methanol fuel cell; PEMFC: proton exchange membrane fuel cell; PAFC: phosphoric acid fuel cell; PCFC: protonic ceramic fuel cell; IT-SOFC: intermediate-temperature SOFC; MCFC: molten carbonate fuel cell; HT-SOFC: high-temperature SOFC).
All systems still exhibit drawbacks that often have delayed large-scale commercialization. For instance for the PEMFCs, reducing the Pt catalyst content and the cost of the Nafion membrane are still crucial issues, as is increasing the operating temperature significantly higher than 100°C. Concerning SOFCs, the high operating temperatures (T > 800°C) accelerate the reactions between cell materials and cause extensive degradation of the single cells. This imposes drastic restrictions on materials, which increases the cost of the systems (for instance the use of specific ceramics or alloys for interconnects and stacks).
Lowering the operating temperature of SOFCs down to intermediate temperature range (750–500°C) and targeting high performance, power density and efficiency have become the main challenges for this technology during the last 15 years (Steele and Heinzel, 2001; Tietz et al., 2002). By decreasing the operating temperature the following changes are expected:
• lifetime of the cells to be increased due to lowered reactivity of materials,
• thermal cycling to be facilitated,
• the use of inexpensive stainless steel or metal alloys as interconnects to be possible (Fergus, 2005),
• manufacturing costs to be substantially lowered by simplifying the production processes (Menzler et al., 2010).
However, at lower operating temperatures, one has to face other problems: the ionic conductivity of the electrolyte decreases whereas the overpotentials at the electrodes greatly increase due to slower reaction kinetics, especially at the air electrode (Ivers-Tiffée et al., 2001).
In order to overcome these major problems, two main strategies have been deployed:
• First, new cell architectures and morphologies of electrodes have been developed: for instance thin electrolyte membranes for lessening the ohmic losses, composite electrodes with gradients of composition and/or porosity, improved electrode/electrolyte interfaces (Menzler et al., 2010; Tietz, 2008).
• Second, the search for innovative materials has been intensified concerned with electrolytes as well as with electrodes, especially cathode materials (Sun; Orera, 2010; et al., 2010; Tarancon et al., 2010).
It is this last point which is emphasized in this chapter.
First, the general context of the oxygen reduction reaction (ORR) will be recalled.
Conventional cathode materials based on the TPB (triple phase boundary) concept are then described. Decreasing the operating temperature requires to use MIEC (mixed ionic and electronic conducting) oxides. Perovskite compounds were the first components of SOFC cathodes; however, innovative materials for operating at intermediate temperatures (typically 600–700°C) have been intensively studied during the last decade. A detailed description is given. From a general viewpoint, the operating temperature of a SOFC is mainly determined by the temperature required to achieve sufficient ionic conductivity in the electrolyte membrane: as a consequence, the choice of the cathode material is very dependent on it.
New cathode materials for protonic conducting fuel cell PCFC (or H+-SOFC), as well as anode for HTSE (high-temperature steam electrolyzers) require specific features; this is discussed in the last part of the chapter.
13.2 The oxygen reduction reaction in solid oxide fuel cells (SOFCs) and implications for cathode materials
13.2.1 The oxygen reduction reaction mechanism
The electrochemical ORR taking place at the cathode of a SOFC can be summarized as follows:
Using the Kröger–Vink notation, it can be also written as
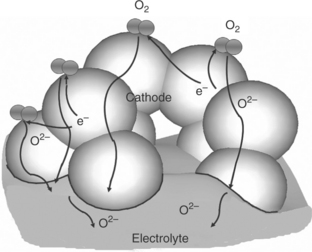
This reaction requires the simultaneous presence of three species, that is, gas/electrons from the cathode/electrolyte, which is only met at the so-called TPB. As illustrated in Fig. 13.2, the agreed macroscopic reaction pathways for oxygen reduction processes on porous cathode/solid electrolyte are (Pizzini, 1973):
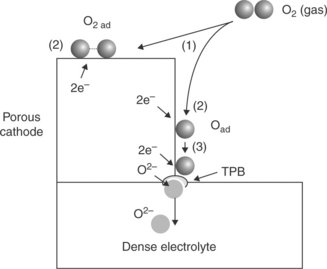
13.2 Schematic representation of the various reaction pathways of the electrochemical reduction of oxygen at the cathode/electrolyte interface.
1. reaction of molecular oxygen with electrode surface,
2. dissociative-adsorption of oxygen molecules and surface diffusion towards the TPB,
3. surface reaction followed by dissolution and diffusion of oxygen ions towards cathode/electrolyte interface – then oxide ions are transported through the electrolyte towards the anode (Steele, 1996).
When the cathode is an electronic conducting material, this reaction can be achieved only in the vicinity of the TPB as summarized by Fleig (2003). As a consequence, the oxygen reduction is restricted to a nearly one-dimensional (1D) region and the performance of such electrodes is thereby poor (Srinivasan et al., 1967; Siebert et al., 1995). This is the case of the most classical and early cathode material, the strontium lanthanum manganite perovskite-type oxide (La1 − x SrxMnO3, so-called LSM); its properties are described in Section 13.3 (Jorgensen and Mogensen, 2001).
It is obvious that the performance of such electrodes is highly dependent on their morphology and microstructure, which clearly affect the size and distribution of the TPBs.
To reduce the cathodic overpotential, it is necessary to operate at high temperature, typically T > 800°C. Another option is to employ designed composite electrodes with appropriate composition and porosity gradients that can provide both ionic and electronic conductivities, and thus a significant extension of the three-phase area, as long as open porosity and continuous paths of the electron-conducting and ion-conducting phases are guaranteed (Kenjo and Nishiya, 1992; Holtappels and Bagger, 2002; Menzler et al., 2010).
A more sophisticated approach is to choose an electrode material that has both intrinsic electronic and ionic conductivity (mixed ionic and electronic conducting, MIEC). Instead of multiplying the TPB ‘reaction lines’, the catalytically active area is extended to the entire surface of the cathode (the so-called DIB, double interface boundary or IDE, internal diffusion electrode) as schematized in Fig. 13.3. In such a way, one can expect electrode polarization losses to be significantly lowered.
From a general viewpoint, MIEC oxides can be formulated as (A1 − x A’x)m(Mn+M(n + 1)+)p Oz ± δ; such oxides exhibit simultaneously:
• a mixed valence of the metallic cations M giving rise to electronic conductivity σe,
• an oxygen non-stoichiometry, either oxygen vacancies (VO⋅ ⋅) or additional oxygen atoms Oi, which induces ionic conductivity σion,
• the large counter-cations (usually alkaline-earths or lanthanides) as well as m/n ratio determine the structural type and play on the oxygen stoichiometry amount.
Many different materials have been studied as SOFC cathode, most of them belonging to the perovskite or perovskite-related oxide families (Chroneos et al., 2010b; Sun; Orera, 2010; et al., 2010; Tarancon et al., 2010).
13.2.2 Basic requirements for SOFC cathode materials
Whatever the cathode active material – single phase (MIEC oxide) or composite (electronic conducting oxide mixed with ionic conducting oxide) – various requirements must be met:
• a high electronic conductivity, preferably more than 100 S cm− 1 under air,
• a high anionic conductivity, preferably similar to that of the electrolyte,
• a chemical and mechanical compatibility with the electrolyte and interconnect materials, that is, its thermal expansion coefficient should be as close as possible to those of usual electrolytes (for instance TEC ≈ 11.10− 6 K −1),
• a thermal stability during the sintering process and limited chemical reactivity with the electrolyte,
• a high catalytic activity for the ORR,
• the ability to shape a microstructure with an adequate porosity in order to allow oxygen molecules to easily diffuse through the cathode,
In order to select materials able to fulfil the SOFC cathode functionality, one has first to determine some basic intrinsic properties of the compounds, usually bulk properties.
Determination of the transport properties (electronic and ionic conductivities)
It is well known that in MIEC oxides, especially those selected for SOFC cathodes, the electronic conductivity σe is at least 103 times higher than the ionic conductivity σion, which imposes to be determined using indirect ways. A number of different experimental techniques have been used based on oxygen diffusion measurements, such as isotopic exchange depth profiling (IEDP) using SIMS (based on a gradient of isotopic oxygen) (Ezin et al., 1998; Ruiz-Trejo et al., 1998; Lane and Kilner, 2000), oxygen permeation measurements (Chen et al., 1997; Kharton et al., 2001), electrical conductivity relaxation and gravimetric relaxation (Flynn and Dickens, 1976; Yasuda and Hishinuma, 1996; Lane et al., 1999; Mauvy et al., 2004) – the two last relaxation methods being based on the fact that these oxides have a variable oxygen non-stoichiometry with temperature and oxygen partial pressure.
It follows that a sharp change in oxygen pressure or temperature results in gradual transition into a new equilibrium state that differs in oxygen content. The oxygen concentration in the samples changes with time and the observed relaxation time depends on two processes:
• The oxygen exchange at the interface gas/solid quantified by the surface exchange coefficient k, involving complex chemical processes as described earlier. This parameter represents the proportionality coefficient between the oxygen flux and the difference in the oxygen concentration at the interface. It is usually determined from IEDP (18O/16O exchange) by fitting the 18O concentration profile on the basis of the Crank model (Kilner and Shaw, 2002; Boehm et al., 2003).
• The oxygen bulk diffusion described by the oxygen diffusion coefficient, namely DO.
Using the Nernst relation, the ionic conductivity can be calculated from the DO values according to the following relation:
where zOF represents the electrical charge of a mole of oxygen ions. DO and CO are the diffusion coefficient and the concentration of oxygen ions, respectively.
The DO coefficient can be measured using various techniques. From relaxation measurements, one can determine the chemical diffusion coefficient, Dchem or ![]() . One can also use IEDP (18O/16O exchange) technique by fitting the 18O concentration profile and calculate the tracer self-diffusion coefficient DO⁎. These experimental independent methods give values of diffusion coefficients that can be linked using the Haven ratio
. One can also use IEDP (18O/16O exchange) technique by fitting the 18O concentration profile and calculate the tracer self-diffusion coefficient DO⁎. These experimental independent methods give values of diffusion coefficients that can be linked using the Haven ratio ![]() (Steele, 1973) and the following relation:
(Steele, 1973) and the following relation:
where γ isa thermodynamic factor that can be obtained by thermogravimetric analysis (TGA) (Mauvy et al., 2004).
Determination of electrocatalytic properties
Using electrochemical impedance spectroscopy (EIS), the electrocatalytic properties of MIEC oxide porous electrodes can be determined. For instance, the polarization resistance has been modelled by Adler (1998); the so-called ALS model proposed a strategy for optimizing the composition and structure of electrodes in order to lower the electrode polarization losses (Rchem) and to enhance the performances. It is stated that the main and limiting parameters are both the oxygen surface exchange (k) and diffusion in the bulk (DO). Providing that the values of DO (cm2 s− 1) and k (cm s− 1) as well as appropriate microstructural parameters are available, the heterogeneous chemical reaction contribution (Rchem) can be calculated by the following relation:
where τ, ε, and α are tortuosity, fractional porosity and internal surface area/unit volume, respectively, cV is the vacancy concentration and DV is the vacancy diffusion coefficient. On the basis of numerous data, it is now well established that low electrode polarization values seem to occur when D*k* > 10− 14 cm3 s− 2 (Adler et al., 1996; Manning et al., 1996; Adler, 1998).
EIS, especially the area-specific resistance of an electrode of a geometric surface area S (ASR = Rchem · S in Ω cm2), as well as i− V polarization curves provide additional useful criteria for definitively choosing the cathode material for further development in complete single cells (Huang et al., 2007).
13.3 Conventional cathode materials: perovskitetype oxides
The perovskites are the most important class of SOFC cathode materials; they have been extensively studied and industrially developed and commercialized. Among various compounds, the strontium-substituted lanthanum manganite, La1 − xSrxMnO3 − δ (LSM), appears as the earliest conventional cathode material (Jiang, 2008).
13.3.1 The structure of lanthanum manganite-related oxides
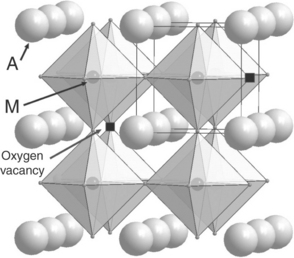
The lanthanum manganite LaMnO3 belongs to the perovskite family of compounds formulated AMO3 (Mitchell, 2002); they crystallize with a structure (usually of cubic symmetry) made of MO6 octahedra sharing corners along the three directions of the space; M is usually a 3d metal cation. The largest cation, A (rare- or alkaline-earth), is located in the central cage of the 3D network of octahedral in a cuboctahedron of 12 oxygen atoms (Fig. 13.4). This site can be partially vacant (as in the tungsten bronzes NaxWO3 or the titanate La2/3TiO3), whereas the M one is very seldom deficient. Conversely, the structure can quite easily show oxygen deficiency in compounds formulated as AMO3 − δ (Grenier et al., 1981; Hagenmuller et al., 1991; Mitchell, 2002). The amount of vacancies can reach values as high as δ = 1 and are ordered in such a way that new structural types are formed – as for instance in LaNiO2 or YBa2Cu3O6 (Hewat et al., 1987). For small values of δ (typically lower than 0.15), the vacancies are considered as statistically disordered, almost without significant interactions and with no long-range ordering. The presence of these oxygen vacancies induces an ionic conductivity thanks to the mobility of the O2 − oxide ions in the incomplete network.
13.3.2 Early cathode materials: manganites and composite electrodes
In LaMnO3, substituting the lanthanum with a lower-valence cation such as Sr2 + (up to 20–30%) leads to the formation of Mn4 +. The mixed valence of the manganese considerably enhances the p-type electrical conductivity of the La1 − xSrxMnO3 manganites as well as its electrocatalytic properties (Anderson, 1992). These compounds show a noteworthy stability in air and their apparent oxygen overstoichiometry, improperly formulated (La, Sr)MnO3 + δ, has given rise to many controversies (Mizusaki et al., 2000; Miyoshi et al., 2002). Actually, there is almost no occurrence of oxygen interstitials at high temperature and they are preferably expressed as cation-deficient compounds (La, Sr)1 − yMn1 − yO3, which makes these compounds almost pure electronic conducting oxides. Indeed, the oxygen diffusivity measured for LSM is very low, which involves very small ionic conductivity values, even at high temperatures (Yasuda et al., 1996). However, the good performance of this compound at high temperature was assigned to the fact that the ORR activity is enhanced under high polarization (‘cathodic biasing’) which leads to the creation of oxygen vacancies able to promote ionic transfer at the interface between cathode and electrolyte (Wang and Jiang, 2006; La O’ et al., 2009).
As LSM had acceptable performance only at temperatures higher than 900°C, the early air electrodes that have been industrialized for high-temperature SOFCs (HT-SOFCs) were formed as composite electrodes of LSM (a highly electronic conducting oxide) mixed together with a highly ionic conducting oxide – usually the electrolyte, yttrium-doped zirconia (YSZ) or gadolinium-doped ceria (GDC) – in order to increase the active surface area. Typically, composites LSM/YSZ with 50–70 wt% of YSZ seem to be the most efficient in terms of electronic conductivity and ionic conduction (Kim et al., 2001; Yang et al., 2003). They are used for operating at temperatures lower than 800°C (Sun et al., 2010).
13.3.3 From high-temperature to low-temperature SOFC cathode compounds: oxygen-deficient perovskites
As mentioned previously, to be able to operate at lower temperatures, an alternative approach is to use MIEC perovskite oxides. These compounds are obtained either by replacing La3 + (or another lanthanide) by large amounts of Sr2 + or Ba2 +, or by substituting Mn by other metallic cations such as Fe, Co, Ni or Cu with the aim of creating oxygen vacancies. As a consequence, basic properties such as electronic conductivity, TEC, and chemical and thermal stability are significantly affected. Useful references can be found in Sun et al. (2010, p. 6806). The most relevant results are summarized in the following.
Replacing strontium for lanthanum in the perovskite LaCoO3 leads to a mixed-valence Co3 + 4 + (and high electronic conductivity), as well as to the occurrence of oxygen vacancies, resulting in enhanced mixed electronic and ionic conducting as well as electrochemical properties with respect to LSM (Gödickemeier et al., 1996; Kawada et al., 1999). Although these materials have been much studied and even compositions such as La0.6Sr0.4CoO3 − δ commercialized, they show some drawbacks:
• Their thermal expansion coefficient (TEC ≈ 23 × 10− 6 K− 1) is much higher than those of usual SOFC electrolytes (≈ 12 × 10− 6K− 1) (Sun et al., 2010).
• They react with YSZ forming highly resistive La2Zr2O7 or SrZrO3 compounds. These detrimental reactions can be avoided by using a ceria-based diffusion layer of a few μms (Chen et al., 1995). Other compositions, for example, Gd1 − xSrxCoO3 − δ or Sm1 − xSrxCoO3 − δ, are also known for having attractive electrochemical properties (Takeda et al., 1996; Xia et al., 2002).
In order to reduce the thermal expansion mismatch while maintaining good electrochemical performance, the best compromise is to substitute iron for cobalt, an optimized composition being La0.6Sr0.4Fe0.8Co0.2O3 − δ (Sahibzada et al., 1997). With regard to electrocatalytic activity, a rough classification of cathode materials has been stated to follow the order: cobaltites > manganites > ferrites > chromites (Takeda et al., 1987; Menzler et al., 2010), which makes possible the design of many different compositions. For instance, ferro-nickelates appear quite attractive as some compositions, for example La(Ni,Fe)O3, do not contain strontium, which is interesting with respect to chromium poisoning caused by interconnects (Basu et al., 2003; Millar et al., 2008). Replacing strontium by barium also results in excellent electrochemical properties, but with a large TEC and a high sensitivity to CO2 from oxidant gas leading to rapid performance loss (Yan et al., 2006; Bucher et al., 2008).
In order to overcome these drawbacks, new cathode materials were developed during the past decade. Instead of oxygen-deficient oxides, compounds with oxygen interstitials were sought.
13.4 Innovative cathode materials: structural aspects of 2D non-stoichiometric perovskite-related oxides
Numerous oxides are currently considered for the next generation of IT-SOFC (intermediate temperature) cathodes with the aim of designing electrodes with improved performances at lower temperatures and significant resistance to degradation during operation. Most of these compounds are perovskite-related oxides whose main feature is a reduced dimensionality character with respect to the classical perovskites that show a 3D character in terms of vacancy distribution.
In the following we describe some of these compounds: the double perovskites, the brownmillerite-type phases and the Ruddlesden-Popper (RP) series of layered oxides (formulated An + 1MnO3n + 1).
13.4.1 Double perovskites
As previously mentioned, the perovskite compounds AMO3 − y may exhibit large amounts of oxygen vacancies. For y values equal to 0.50, due to large electrostatic interactions, the vacancies order. According to the involved cations (their size, valence state, electronic configuration, etc.) various structural types can be formed. For instance, A2M2O5 compounds may crystallize with structures as different as the brownmillerite, the so-called 112 double perovskite or even other structural types (Mitchell, 2002). Some of these materials exhibit good performance as SOFC cathode materials.
The structure of the double perovskites
The general formulation of these materials is AA′M2O5 + δ, A being a lanthanide cation, A′ an alkaline-earth and M = 3d element (preferably Mn or Co). In most perovskite compounds, the A and A′ cations are statistically disordered and are conventionally labelled A0.50A′0.50MO3 − x. However, when A and A′ order, peculiar structural and electronic properties may be observed, such as giant magnetoresistance and superconductivity. Ordered structures are observed only in the presence of a large difference between ionic radii of A and A′, typically for barium and a small lanthanide cation.
A detailed study of the structure of these oxygen-deficient perovskites was reported for the first time by Maignan et al. (1999) for the cobaltites formulated LnBaCo2O5 + δ, Ln being rare-earths from Pr to Ho.Their structure derives from the ‘112’ type structure of YBaFeCuO5 (Er-Rakho et al., 1988). Using HRTEM (high-resolution transmission electron microscopy), these authors established that these compounds present two main structural features: the first is that lanthanide and Ba ions order in alternating (001) layers; the second is that the oxygen vacancies locate at the lanthanide layers with a pronounced tendency to form ordered patterns. They also showed that the δ value can be easily varied by means of heat treatments under appropriate atmospheres (variable pO2) and is directly related to the Ln3 + ionic radius, δ decreasing with the decreasing size of Ln3+. As stressed by Frontera et al. (2005), temperature and annealing time are key parameters for vacancy ordering; various types of ordering were evidenced.
For δ = 0, the structure results in double-pyramidal cobalt layers containing the barium cations, interleaved with lanthanide layers in which are located all the oxygen vacancies (Fig. 13.5). When δ increases, Co ions are coordinated in pyramids and some octahedra in a random way.
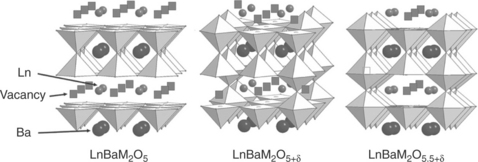
13.5 Representation of the structures of the double perovskites LnBaM2O5, LnBaM2O5 + δ, LnBaM2O5.5 + δ, with Ln = Gd, Pr, Nd, Sm; M Co, Fe, Mn, Cu.
For δ = 0.5, additional oxygen atoms lead to the formation of octahedra, the vacancies remaining more or less ordered in the lanthanide layer. An ordered situation corresponds to vacancy lines along the (1 0 0) direction (LnBaM2O5.5 in Fig. 13.5). For higher δ values, the oxygen vacancies remain in the Ln layer but are disordered (LnBaM2O5.5 + δ in Fig. 13.5).
Mixed ionic and electronic properties
The transport properties are highly dependent on the oxygen content, directly related to the valence state of the metal cation: for δ = 0, 50% of M3 + and 50% of M2 +, for δ = 1, 50% of M3 + and 50% of M+, passing through 100% of M3 + for δ = 0.5.
An interesting relationship between the oxygen content (5 + δ) in ALnCo2O5 + δ double perovskites and the difference in ionic radii between A = (Ba1 − xSrx)2 + and Ln3 + (rA2 + − rLn3 +) (Ln = La, Nd, Sm, Gd and Y) samples synthesized in air was given by Kim et al. (2008) (Fig. 13.6). It was observed that the larger the size difference between (Ba1 – xSrx)2+ and Ln3 +, the greater the tendency of (Ba1 – xSrx)2 + and Ln3 + cations to order in alternate planes along the c-axis and the lower the oxygen content, as Ln3 + tends to have less than 12 coordination. Such a trending may be useful to predict the oxygen content on the basis of the size difference between the A2 + and Ln3 + ions.

13.6 Variation of the oxygen content (5 + δ) value as a function of the difference in ionic radii rA2 + − rLn3 + (A = alkaline-earth, Ln = rare-earth) in various double perovskites. (Source: Data from Kim et al., 2008.)
Most compounds show rather poor electrical conductivity (a few S cm− 1) below their insulator-metal transition (≈ 200°C) but it significantly increases at high temperatures up to a few hundreds of S cm− 1. Taskin et al. studied the electronic properties and oxygen-exchange behaviour in both GdBaMn2O6 – x and GdBaCo2O5 + δ, pointing out a close relation between vacancy ordering and charge ordering (Taskin and Ando, 2005; Taskin et al., 2007). Using re-oxidation kinetics of these compounds, they found that the oxygen diffusivity is enhanced by orders of magnitude in the ordered compounds with respect to the disordered cubic phase in which the Gd and Ba cations as well as oxygen vacancies are disordered. They found high values of the oxygen diffusion coefficient (Taskin et al., 2005).
Later, the oxygen diffusion coefficient as well as the surface exchange coefficient were measured on GdBaCo2O5 + δ ceramics by Tarancon et al. (2007) using the 2 8O/16O IEDP method. Their measurements were carried out with accuracy, using a FIB-SIMS technique for measuring the 18O profiles in the samples. They did not confirm the very promising results previously reported by Taskin et al. (2005), which was explained by the fact that their approaches were different, resulting in discrepancies. More recently, Choi et al. (2010) determined the ionic conductivity from oxygen permeation measurements and found very small values in air (the material being highly oxidized, with correspondingly high values of δ), whereas σ reaches 0.01 S cm− 1 under N2 (pO2 ≈ 10− 4 atm). Finally, all these data and values of D* of about 10− 9 cm2/s at 700°C higher than those of disordered perovskites suggested that a two-dimensional (2D) structure could be interesting for the oxide ion conductivity in perovskite-type compounds, the oxygen bonding strength being reduced in the vacancy planes in which motion channels are created.
In the LnBaCo2O5 + δ series, the praseodymium compound PrBaCo2O5 + δ was also carefully examined, at first by Frontera et al. (2005) who studied the phase diagram as a function of oxygen content, δ varying from 0.2 to 0.9 for temperatures in between 250°C and 900°C. On the basis of in situ TGA measurements and neutron diffraction data, they confirmed that oxygen exclusively diffuses within the PrOx planes. Oxygen diffusivity measurements were performed by Kim et al. (2007), unfortunately, on poorly dense ceramics (≈ 90%); the unusual very high values of D and k coefficients found by these authors cannot be considered as reliable. For Ln = Sm, Zhou et al. (2008) reported high electrical conductivity (815–434 S cm− 1) in the temperature range 500–800°C and a good chemical compatibility with the SDC and LSGM electrolytes.
13.4.2 Brownmillerite-type phases
Another kind of ion vacancy ordering in oxygen-deficient perovskites may lead to the formation of superstructures whose typical example is the structure of the brownmillerite. It belongs to the family of compounds formulated as AnMnO3n – 1 (n = 2 to ∞). Their structures consist of (n – 1) alternating perovskite-type layers of corner-sharing MO6 octahedra and one layer of MO4 tetrahedra rows (Grenier et al., 1981; Berastegui et al., 1999). In the structure of the brownmillerite A2M2O5 (n = 2, Fig. 13.7), the oxygen vacancies are ordered along (010) planes, forming a one-dimensional (1D) diffusion pathway for oxygen ion migration in the tetrahedral layer. Its orthorhombic unit cell is related to the basic cubic perovskite cell by the following relations: ![]() . This kind of material has attracted much attention, due to its ability to accommodate additional oxygen atoms in the lattice, which in turn results in fast oxygen ionic conductivity (Kendall et al., 1995). Some brownmillerite structure compounds have been found to exhibit relatively high ionic-electronic mixed conductivity, promising oxygen permeability and compatible thermal expansion coefficients (TECs) with the usual solid electrolytes (≈ 11.3−13.6 × 10− 6 K− 1) (Kharton et al., 2003; Shaula et al., 2006; Vente et al., 2006). These properties might be of interest for applications as SOFC cathode. For the first time, the oxygen electrode properties of the brownmillerite-type Ca2Fe2 – xCoxO5 compounds have been recently reported; these materials show good electrochemical properties, the lowest polarization resistance being 0.23 Ω cm2 at 700°C in air. These first results evidence that brownmillerite-type oxides are likely to be new promising cathode materials for IT-SOFCs. They emphasize again that reduced dimensionality could be interesting for improving the oxygen diffusivity.
. This kind of material has attracted much attention, due to its ability to accommodate additional oxygen atoms in the lattice, which in turn results in fast oxygen ionic conductivity (Kendall et al., 1995). Some brownmillerite structure compounds have been found to exhibit relatively high ionic-electronic mixed conductivity, promising oxygen permeability and compatible thermal expansion coefficients (TECs) with the usual solid electrolytes (≈ 11.3−13.6 × 10− 6 K− 1) (Kharton et al., 2003; Shaula et al., 2006; Vente et al., 2006). These properties might be of interest for applications as SOFC cathode. For the first time, the oxygen electrode properties of the brownmillerite-type Ca2Fe2 – xCoxO5 compounds have been recently reported; these materials show good electrochemical properties, the lowest polarization resistance being 0.23 Ω cm2 at 700°C in air. These first results evidence that brownmillerite-type oxides are likely to be new promising cathode materials for IT-SOFCs. They emphasize again that reduced dimensionality could be interesting for improving the oxygen diffusivity.
13.4.3 Ruddlesden-Popper-type oxides: A2MO4 compounds
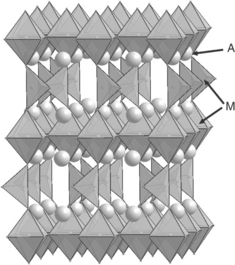
Oxides belonging to the so-called Ruddlesden–Popper (RP) series are formulated as An + 1MnO3n + 1 (n ≥ 2) (Ruddlesden and Popper, 1958). Their structures consist of n AMO3 perovskite layers separated by one AO rocksalt layer, the value of n determining the number of perovskite layers. Figure 13.8 represents the n = 1, 2, 3 and ∞ terms. With respect to the characteristics of their atomic arrangement, these compounds can show various kinds of oxygen non-stoichiometry (oxygen deficiency or additional oxygen atoms), which makes them very attractive with regard to their transport properties (electronic and ionic conductivities).
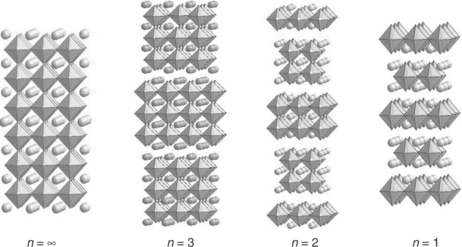
13.8 Representation of the structures of the n = 1, 2, 3 and ∞ terms of the Ruddlesden–Popper (RP) series formulated An + 1MnO3n + 1.
Commonly, A is a rare earth/alkaline earth cation and M is, a transition metal cation, which makes it possible to obtain a large variety of compounds with fitted properties.
The most widely studied systems are the n = 1 phases, which crystallize with the well-known K2NiF4 structure; they are considered as very promising cathode materials.
Some preliminary studies have been devoted to n > 1 terms, however, they are not considered in this review.
The structure of A2MO4 phases
The structure of the A2MO4 phases can be depicted in two different ways according to the features that are emphasized (Fig. 13.9).
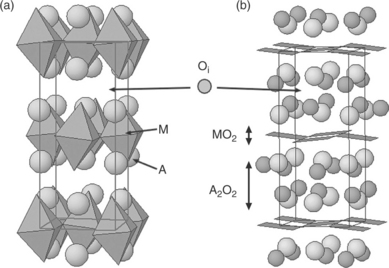
13.9 Structure of A2MO4 + δ-type phases (Cmca space group): (a) AMO3 octahedra shifted layers (b) succession of MO2 (square planar sites) and A2O2 rock salt type layers.
First, it can be described as a succession of AMO3 octahedra layers that are shifted (½,½,½) according to others in such a way that they appear to be linked by AO rock salt type plane.
As for the perovskite compounds, the main parameter governing the stability of the A2MO4-type compounds is the well-known Goldschmidt tolerance factor, t:
where rX is the ionic radius of the X ion (A, M or O) (Mitchell, 2002).
In the ideal condition (t = 1), the structure is not distorted and shows a tetragonal symmetry, T-type (Space Group, I4/mmm). However, the octahedra exhibit a slight elongation, the apical oxygen atoms being distant from the equatorial plane. Such a feature is emphasized when the metallic cation, M, is a ‘Jahn-Teller’ cation (d9, d7(LS), d4(HS)). It follows that, in such a way, the M cation can be considered in a square planar coordination (4 + 2).
The second way to describe the structure is to consider a succession of MO2 (square planar sites) and A2O2 rock salt type layers (Fig. 13.9b).
However, in most of the A2MO4 oxides, as in the perovskite compounds, the size of the A cation is too small, which markedly lessens the t value (0.87 < t < 1) and a strong orthorhombic distortion appears, as for instance in La2CuO4 or in Ln2NiO4 (Ln = La, Pr, Nd). The structure undergoes a rather important deformation due to a ‘mismatch’ resulting from the different sizes of the MO2 and A2O2 layers. The ‘size accommodation’ leads to a wrinkling of the too large MO2 layers and a cooperative tilting of the MO6 octahedra all around the c-axis (Fig. 13.9a). The symmetry becomes orthorhombic (T/O type), the space group being then Cmca.
When rA decreases (for instance for smaller rare-earth cations), t becomes lower than 0.87, and the T/O-type structure is no longer stable. A new structure, so-called ‘T’, is formed: the apical oxygen atoms shift towards tetrahedral sites formed by lanthanide ions, which results in the formation of A2O2 fluorine-type layers instead of rock salt-type ones. The M cations are then in square planar coordinations. Nd2CuO4 adopts this structure but does not accept additional oxygen.
Oxygen intercalation: structural features
The T/O-type A2MO4 stoichiometric phases insert oxygen atoms into the A2O2 layers; considering the previously described structural characteristics, this can be easily explained in terms of structural constraints existing in the compound, which are released thanks both to an increase of the volume of the A2O2 layers and to a decrease of the one of MO2 layers resulting from the oxidation of the Mn+ into M(n+ 1)+ cations. The symmetry remains orthorhombic but, as the octa-hedra are no longer tilted, the space group becomes Fmmm. The orthorhombic structures Fmmm (T/O) and tetragonal I4/mmm (T) are very similar, the cell parameters a and b having very close values. Ln2NiO4 compounds that are of interest as SOFC cathode materials are typical examples of such features.
Oxygen insertion can be also achieved using electrochemical oxidation in alkaline solution as was shown for the first time by Wattiaux and Grenier for La2CuO4, and later for La2NiO4 (Wattiaux et al., 1990; Grenier et al., 1992). Under anodic polarization, high local oxygen activity is generated thanks to the following reaction:
Significantly oxidized compounds were prepared in such a way: the superconducting cuprate La2CuO4.10, and the long-range ordered La2NiO4.25 (i.e., La8Ni4O17) as well as hyperoxidized Nd2NiO4 + δ, δ up to 0.30) (Demourgues et al., 1992, 1993).
These results show the capability of these oxides to insert high contents of oxygen.
Basic properties of A2MO4-type oxides
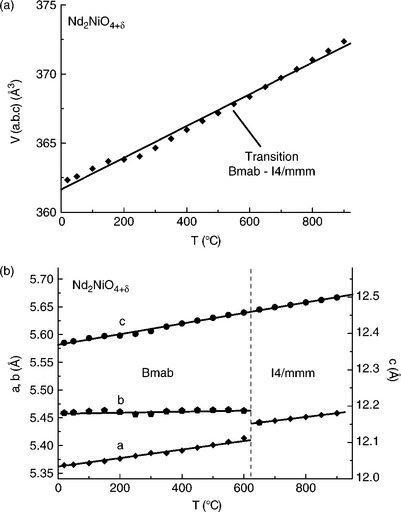
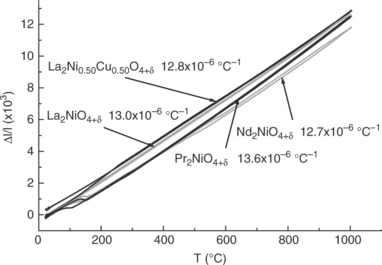
The A2MO4 phases showing the T/O-type symmetry (either Bmab or Fmmm), undergo at rising temperature a structural transition towards tetragonal symmetry (SG: I4/mmm). Detailed structural studies (high-temperature X-rays or neutron diffraction) have shown that this transition temperature can occur in a rather large temperature domain (150–650°C) and depends not only on the nature of the metal cation but also on the oxygen stoichiometry, that is, on the oxygen partial pressure. A remarkable feature of these compounds is that their volume changes very little at the transition despite the structural changes (internal atomic shifts) (Fig. 13.10a). Even though there is a thermal expansion of the volume on heating, it follows a linear variation with temperature, which is of great interest as no mechanical consequence is expected on manufactured SOFC electrodes (Skinner, 2003; Aguadero et al., 2006; Skinner and Amow, 2007). A typical example is given for Nd2NiO4 + δ in Fig. 13.10b, for which the structural transition Bmab/I4/mmm occurs at about 620°C.
Numerous compositions have been considered for SOFC application; from a general viewpoint, they can be formulated as
where Ln stands for the lanthanides, La, Pr, Nd, with the A for a lanthanide or/and an alkaline-earth (Ca, Sr or Ba), and M and M′ for transition metal cations (Fe, Co, Ni or Cu). The symbol ⃞y represents the content y of possible cationic vacancies on the Ln site.
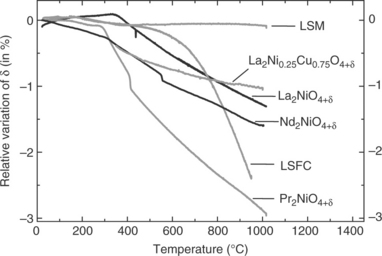
Their oxygen stoichiometry as a function of temperature and oxygen partial pressure has been studied in detail by TGA. Typical examples are given in Fig. 13.11. From a general viewpoint, the relative thermal variation of the oxygen stoichiometry in air is smaller for the Ln2MO4 + δ compounds than for the perovskite ones (except for LSM, which remains almost stoichiometric vs temperature), especially in the usual operating temperature range (600–800°C). In addition, the structural transition of the Ln2MO4 + δ compounds is characterized by a small abrupt change in oxygen content, but it does not seem to significantly influence the thermal expansion coefficient whose variation remains almost linear (Fig. 13.12). Their oxygen overstoichiometry can be modulated by substituting Sr for Ln, and even reduced down to zero. For low oxygen pressures, oxygen vacancies may appear (Nakamura et al.,2009, 2010).
13.5 Comparative transport properties and electrochemical performances of 2D non-stoichiometric oxides
13.5.1 Electrical conductivity
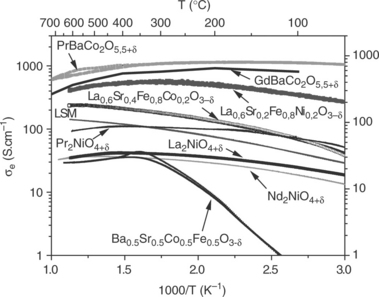
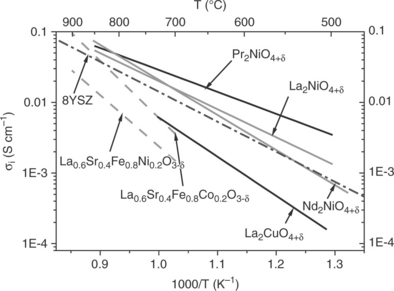
Figure 13.13 shows the thermal variation of the electrical conductivity of some perovskite-related oxides interesting as SOFC cathode materials and having the mixed valence of metallic cations (Dailly, 2008; Kim et al., 2008). Most of the compounds exhibit a semiconducting-type behaviour, that is, an Arrhenius-type thermally activated conductivity, at temperatures lower than 400°C. At higher temperatures, the materials lose some oxygen (as previously quoted), which leads to decrease of the M3 + or M4 + content and correlatively the carrier density (i.e., electron holes). These competing phenomena result in the observed maximum of σe and in its decrease at high temperature. In the operating temperature domain, the values of conductivity range between 50 and 1000 S cm− 1, which meets the requirement for a SOFC cathode.
13.5.2 Oxygen diffusivity: effect of dimensionality
Oxygen diffusivity in oxides may arise from various processes depending on the oxygen exchange type:
• in oxygen-deficient perovskite, the oxygen exchange occurs through vacancies in the oxide network according to the reaction
• in A2M2O5 + δ (brownmillerite), AA′M2O5.5 + δ (double perovskite) or A2MO4 + δ compounds, the oxidation of the lattice leads to the creation of additional oxygen atoms according to
Compared to the perovskite compounds, the oxygen transport in these materials is supposed to be quite different with respect to the oxygen non-stoichiometry type which involves oxygen interstitials instead of oxygen vacancies. Indeed, the fact that electrochemical oxygen intercalation is possible at room temperature in these compounds suggests that inserted oxygen atoms are likely to be highly mobile in such networks. Early calculation of the migration energy of oxide ions in interstitial sites of La2CuO4 by Allan and Mackrodt (1991) gave a rather small value of the activation energy, Ea ≈ 0.55 eV. Using the model of Cottrell, the value of the diffusion coefficient (DO) was determined to be about 10− 9− 10− 11 cm2 s− 1, at room temperature, which is somewhat higher than the value usually found for O2 − ion diffusion in oxides (DO ≈ 10− 15 cm2 s− 1) (Arrouy et al., 1991; Grenier et al., 1996).
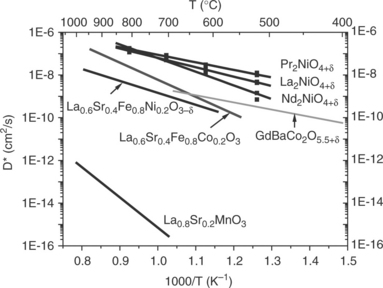
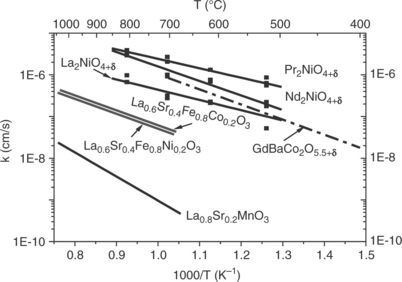
Numerous data concerned with the measure of the oxygen diffusion coefficient DO as well as that of the exchange coefficient kO, at high temperature, are available. Authors have used various techniques and the most reliable results seem to be obtained by the isotopic 18O/16O exchange technique; results are reported in Figs 13.14 and 13.15 for polycrystalline samples
(Yasuda et al., 1996; De Souza et al., 2000; Skinner and Kilner, 2000; Boehm et al., 2003, 2005; Tarancon et al., 2010). These values agree with those determined by other techniques. It can be noticed that:
• The MIEC oxides show the highest values of DO* and kO*, several orders of magnitude higher than those of the electronic conducting LSM compound.
• One should also point out that oxides showing a 2D structural character have DO values larger than the 3D-type perovskites.
• More particularly, Ln2NiO4 + δ compounds evidence coefficients about one order of magnitude higher than the best perovskite oxides, which suggests that these materials may be potential excellent candidates as SOFC cathodes.
• In addition, their activation energy (≈ 0.8 eV) is lower than that of the perovskite MIEC oxides.
On the basis of the Nernst-Einstein relation, the oxygen ionic conductivity of some perovskites and Ln2NiO4 + δ compounds has been calculated and plotted in Fig. 13.16 (Boehm et al., 2005). It is of the same order of magnitude as that of 8YSZ, the stabilized zirconia (σ ≈ 10− 2Ω− 1cm− 1 at 700°C).
From a general viewpoint, the activation energies are lower for the Ln2NiO4 + δ compounds, which means that the lower the temperature, the better the expected performances of these 214 materials.
Experiments have also been performed on single crystals of both cuprates (La2CuO4 + δ) and nickelates (La2NiO4 + δ) as well as on thin-films using the IEDP-SIMS method (Claus et al., 1996; Bassat et al., 2004; Burriel et al., 2008). All data clearly show anisotropy of the oxygen diffusivity (Fig. 13.17). The values of DO* as well as of the activation energy measured in the a-b planes of crystals are close to those of ceramic samples, the DO* along the c-direction being about one to two orders of magnitude smaller. However, the values of the activation energy i the c-direction appear questionable and the experiments need to be repeated. This structural anisotropy seems also to induce anisotropy in the catalytic activity of these SOFC cathode materials, which depends on the crystalline surface as shown by Yamada et al. (2010).
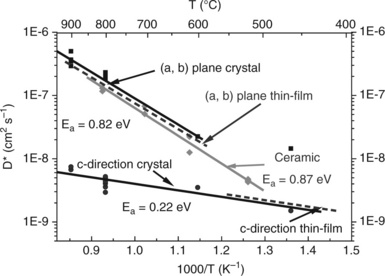
13.17 Thermal dependences of the diffusion coefficient D* along different directions for a crystal (Bassat et al., 2004) and a thin-film (Burriel et al., 2008) of La2NiO4 + δ.
Several simulations have been performed in order to explain these results. Early simulations were carried out on cuprates in relation to their superconducting properties. For the oxygen-deficient cuprates (strontium substituted), it was shown that the oxygen defects are located at the equatorial positions (i.e., in the MO2 plane, Fig. 13.9). On the other hand, for oxygen-excess materials, it was established, especially using molecular dynamics, that interstitial oxygen atoms are mobile within the (a–b) La2O2 layer (Allan and Mackrodt, 1991; Mazo and Savvin, 2004; Savvin et al., 2008). Simulations (using atomistic computer simulation techniques, DFT, molecular dynamics, etc.) were also performed on Ln2NiO4 + δ compounds (Read et al., 1999; Minervini et al., 2000; Cleave et al., 2008); they definitively showed:
• the anisotropy of the oxygen diffusion, that is, the easier diffusion of the oxide ions in the (a-b) plane rather than along the c-axis,
• the migration of oxygen interstitials as O2 − species (instead of O− that was previously suspected (Grenier et al., 1996) via a mechanism involving the neighbouring apical oxygen atoms, which appears more likely than a direct jump from one interstitial site to the next one, requiring more energy (Frayret et al., 2005).
Recent molecular dynamics simulations with La-NiO4 + δ and Pr-NiO4 + δ as well as neutron scattering studies and synchrotron diffraction data in doped Pr2NiO4 + δ by Yashima et al. have nicely confirmed the large anisotropy of both the electronic conduction taking place in the NiO2 plane and the ionic diffusion in the Ln2O2 layer (Yashima et al., 2008, 2010; Chroneos et al., 2010a; Parfitt et al., 2010).
It is now clearly established that the oxygen diffusion mechanism involves both the interstitial oxygen atoms as well as the apical oxygen Oap atoms (Figs 13.9 and 13.23) with an activation energy of about 0.85–0.90 eV.
Finally, with regard to the values of D* and k*, it appears that a 2D character of the oxygen diffusion (instead of a 3D character as in usual perovskite-type compounds) seems to be a preponderant factor for improving the basic properties required for a SOFC cathode material. Most of these materials have been integrated in single cells for tests.
13.5.3 Comparative electrochemical performance of 2D non-stoichiometric oxides and fuel cell tests
The electrochemical performance of these compounds has been evaluated, first, through electrochemical measurements by EIS of the ASR of porous electrodes deposited on usual electrolytes (ZrxY1 − xO2 − δ (YSZ), Ce1 – xGdxO2 − δ (CGO)), then, for the best materials, through single-cell tests. Numerous electrochemical studies and single-cell tests have been reported in the literature but their comparison is difficult as the results strongly depend on the method used to fabricate the cell (Menzler et al., 2010).
The most widely utilized cathode material LaxSr1 – xMnO3 (LSM) shows very good performance around 800°C, but due to its poor ionic conductivity, overpotential is dramatically increased at lower temperatures (Brandon et al., 2003). The replacement of Mn by Co or Fe leading to MIEC perovskites is worthwhile in terms of activation of the oxygen reduction. One can mention for instance LaxSr1 – xCo1 – yFeyO3 – δ (LSCF) (Takeda et al., 1987; Riza et al., 2001; Tietz et al., 2006) and Sm0.5Sr0.5CoO3 – δ (SSC) (Xia et al., 2002) which have been commercialized.
Recent studies devoted to the new double perovskites LnBaCo.O5 + δ (Ln = Nd, Pr, Gd, Sm) report cathodic ASRs that meet the targets, that is, lower than 0.25 Q cm2 at operating temperatures such as 625°C for GdBaCo2O5 + δ or even as low as 550°C for PrBaCo.O5 + δ deposited on CGO (Tarancon et al., 2007, 2008; Kim et al., 2007). The samarium compound, SBCO, deposited on Sm0.2Ce0.8O1.9 (SDC) or La0.9Sr0.1Ga0.8Mg0.2O3 – δ (LSGM) exhibits ASR values of 0.098 and 0.054 Ω cm2, respectively, at 750°C and maximum power densities of single cells, at 800°C, of 641 and 777 mWcm− 2, respectively (Zhou et al., 2008).
Although these cobalt-based cathodes exhibit good performance, they suffer from the problems previously mentioned (high reactivity with electrolytes, high thermal expansion coefficients (TECs), as well as the high cost of cobalt element (Sun et al., 2010). Developing cobalt-free cathodes with good catalytic activity at reduced temperatures for IT-SOFCs is clearly significant. Several cobalt-free oxides with the simple perovskite structure, such as LaxSr1 – xFeO3 − δ (LSF) (Simner et al., 2002) or LNF (Chiba et al., 1999; Komatsu et al., 2006) have been proposed as cathode materials for IT-SOFC cathodes. In the same way, double perovskites such as LnBaFe2O5 + δ exhibit interesting properties. By EIS, in a symmetrical cell with Sm0.2Ce0.8O1.9 (SDC) electrolyte, the observed ASR of GdBaFe2O5 + δ was 0.15 Ω cm2 at 700°C and 0.39 Ω cm2 at 650°C; a maximal power density of 861 mWcm− 2 was achieved at 700°C (Ding and Xue, 2010).
The 214 nickelates have been intensively studied in recent years with the same strategy to avoid the presence of cobalt in the hope that they may be a promising alternative to the traditional perovskite cathode materials. Electrochemical studies have been performed using all the classical electrolytes, ZrxY1 – xO2 – x/2 (YSZ), Ce1 − xGdxO2 – x/2 (CGO), La1 − xSrxGa1 − yMgO3 − δ (LSGM) and the apatite La9Sr(SiO4)O2, with Ln2NiO4 + δ (Ln = La, Nd or Pr), being the porous cathode material.
Although these nickelates showed promising basic properties in terms of D* and k* coefficients compared to those of perovskite compounds, the first measured ASR values were not as small as expected. Using YSZ as electrolyte, Zhao et al. (2008) and Escudero et al. (2007) found values of 4 Ω cm2 at 708°C and 2.5 Ω cm2 at 800°C, respectively. These ASRs were much improved when making graded electrodes (0.11 Ω cm2 at 800°C (Rieu et al., 2010) and thanks to the addition of a doped ceria interfacial layer (Sayers et al., 2011). Power densities up to 2.2 Wcm− 2 were measured at 800°C by Laberty et al. when using a composite electrode made of 1/1 nickelate/Sm0.2Ce0.8O1.9 (SDC), even though the kinetics of ORR was claimed to be rather slow (Laberty et al., 2007).
Earlier experiments on the neodymium nickelate Nd2NiO4 + δ reported an ASR value of about 1 Ω cm2 at 700°C (Mauvy et al., 2003). Later, Lalanne et al. (2008) obtained with the selected neodymium-deficient Nd1.95NiO4 + δ composition, screen-printed on anode-supported SOFC half-cell (HTceramix® commercial cell based on co-tape cast 8YSZ/Ni–8YSZ), current density up to 1.31 Acm− 2, at 0.70 V – that is, a power density of 1W cm− 2 at 800°C. As shown by Haanappel et al. (2009), again the use of an interlayer of CGO improved the performance and durability of the cell. The feasibility to produce cathode-supported SOFCs on porous neodymium nickelate substrates, that is, cathode tubes of 4.9 mm inner diameter, the electrolyte layers being applied by dip-coating, was demonstrated by Luebbe et al. (2009).
Up to now, only few data have been reported with the praseodymium nickelate which seems to show the best results, especially at low temperatures of about 600°C. A polarization resistance value as low as 0.08 Ω cm2 has been measured on a symmetrical cell configuration (with commercial YSZ as electrolyte) by Ferchaud et al. (2011); the electrodes made of powder, with average particle size of 0.4 μm, were sintered at 1130°C on a barrier layer made of gadolinium-doped ceria (CGO) that was shown to be better than the yttrium-doped one (YDC). The best cell performance – that is, a maximum power output density of approximately 400 mW cm− 2 at 600°C – was obtained for a cell combined with a thin screen-printed Co-doped GCO layer.
In general, substitution of an alien cation for nickel does not seem to significantly improve the electrochemical properties of these nickelates (Kharton et al., 2004a, 2004b); it usually increases the Ni3 + content and consequently the electrical conductivity but lessens the additional oxygen interstitials (S decreases, i.e. the ionic conductivity decreases) (Sun et al., 2008).
13.6 Ln2NiO4 + δ oxides: innovative and flexible materials for air electrodes of protonic ceramic fuel cells (PCFCs) and electrolyzers
Nickelates with the 214 structure have appeared to be innovative materials as cathodes for oxide conducting O2 −-SOFC with regard to their ASR values as well as to single-cell performance. In the following we focus on these materials used for different applications. Recent studies have been devoted to their behaviour as cathodes for PCFC (or H+-SOFC) and as anode for high-temperature steam electrolyzers (O2 −-HTSE based on oxide conducting electrolyte).
13.6.1 Nickelates as PCFC cathodes
A specific feature of protonic-conducting H+-SOFCs is that water vapour is produced at the cathode side, due to the fact that the electrolyte is a proton-conducting oxide. Such a feature involves some additional requirements for the cathode material with respect to the usual ones for oxide conducting O2 −-SOFC application, the first one being their chemical stability in moist air.
Additionally, according to Tao et al. (2000) suitable cathode compounds must exhibit both high electronic and oxygen-ion or/and proton conductivities as well as good catalytic activity for the following electrode reaction:
The reaction [13.1] can be decomposed into two steps:
When the cathode material is an oxygen-ion mixed conducting oxide (Fig. 13.18a), reaction [13.2] may occur all over its surface, but water is formed only at the TPB near the electrolyte surface and has to flow out through the electrode.
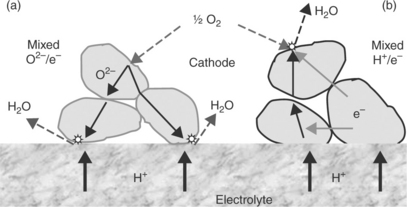
13.18 Schematic representation of the oxygen reduction mechanism and water production for: (a) a mixed anionic–electronic conducting oxide (b) a mixed protonic–electronic conducting oxide.
Using a protonic mixed conducting oxide (Fig. 13.18b) allows the water to be produced not only at the TPB sites, but over the whole cathode surface. From a practical viewpoint, the last system would obviously exhibit faster electrode processes.
Nickelates were recently studied as cathodes for protonic fuel cells. They were found to be stable in moist atmosphere: their electrical conductivity is unchanged and, according to TGA experiments and IR spectroscopy, a small quantity of water is inserted (e.g. less than 0.03 mole H2O/mole for La2NiO4 + δ) (Dailly et al., 2010). Their electrochemical characteristics were determined on symmetrical half-cells under zero dc conditions and under dc polarization (three-electrode cell): Pr2NiO4 + δ shows the lowest ASR at 600°C.
A single cell was fabricated starting from an anode-supported cell made of Ni cermet as anode, BaCe0.9Y0.1O2 95 as electrolyte and Pr2NiO4 + δ as cathode; a power density of 130 mW cm− 2 was obtained at 923 K (Taillades et al., 2010). Improving the microstructure of the cathode recently made it possible to reach values of up to 180 mW/cm2 (Grimaud et al., 2010).
Again these materials showing oxygen over stoichiometry appear to be very efficient as H+-SOFC cathode; however, their mixed protonic-electronic conductivity has not yet been evidenced.
13.6.2 Nickelates as high-temperature steam electrolyzer anodes
Hydrogen being considered as a promising energetic vector for the future (Winter, 2009), its massive production using clean processes has become crucial. HTSE is one of the most promising ways to achieve this target (Dönitz, 1984). It supposes the components constituting the solid oxide electrolysis cell (SOEC based on oxide-conducting electrolyte) where the HTSE reactions occur, to be optimized, especially the oxygen electrode (anode) where oxygen is produced according to the following reaction:
Most current studies concerned with HTSE consider reversible solid oxide cells (fuel cell/electrolysis cell), constituted of a hydrogen electrode (a nickel-YSZ cermet), YSZ electrolyte and perovskite-type oxygen electrode (usually LSM) (Hauch et al., 2008). Recently, it has been reported that alternative oxygen electrode materials with Ln.NiO4 + δ (Ln = La, Nd,) compositions show excellent performances (Pérez-Coll et al., 2009; Chauveau et al., 2010). For instance, Chauveau et al. tested, in both fuel cell (SOFC) and electrolysis (HTSE) modes, a TZ3Y-supported cell (TZ3Y as electrolyte) with a Ni–CGO as hydrogen electrode and Ln2NiO4 + δ (Ln = La, Nd) as oxygen electrode; they found ASR values to be slightly lower in the electrolysis mode. Compared to a commercial electrolyte-supported cell containing the same hydrogen electrode and electrolyte membrane but with a conventional LSM-based oxygen electrode, improved performances have been evidenced for both nickelates over the temperature range 750–850°C. For instance, at 750°C, the current densities at 1.3 V (the so-called thermoneutral potential), are 1.8 and 4.4 times larger than the one observed for LSM (Fig. 13.19). Such results show that these materials are very promising for this application, which can be explained by the beneficial effect of using mixed conducting electrode materials, allowing the oxygen formation reaction to be delocalized over the entire electrode surface.
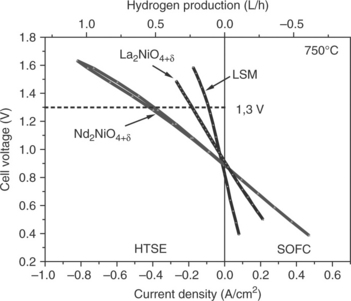
13.19 I–V curves measured at 750°C on cells containing La2NiO4 + δ, Nd2NiO4 + δ or LSM in both either SOFC (right) or HTSE mode (left). (Data from Kim et al., 2008.)
13.6.3 K2NiF4-type oxides: flexible oxides as oxygen electrodes
Previous examples pointed to an interest in using K2NiF4-type compounds, especially Ln2NiO4 nickelates as oxygen electrodes in various systems, such as cathode for either O2 −-SOFC or H+-SOFC and as anode for HTSE.
Therefore, it is interesting to analyse why these materials are so flexible and efficient.
First, one should recall some basic features of these compounds:
• They are stable in dry air and can be used as cathode for O2 −-SOFC as well as anode for HTSE even though the operating conditions are quite different in terms of oxygen pressure generated at the electrode surface ![]() . Indeed, assuming that the concentration polarization only occurs at the electrode surface, the local pO2 can be estimated using the Nernst relation
. Indeed, assuming that the concentration polarization only occurs at the electrode surface, the local pO2 can be estimated using the Nernst relation
which correlates the oxygen partial pressure to the overpotential at the electrode, with ![]() atm. Figure 13.20 represents the correspondence between the electrode overpotential η and the oxygen pressure calculated according to Equation [13.4].
atm. Figure 13.20 represents the correspondence between the electrode overpotential η and the oxygen pressure calculated according to Equation [13.4].
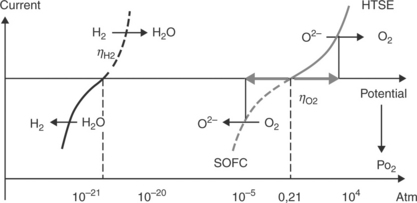
13.20 Correspondence between the electrode overpotential η and the oxygen pressure generated at an electrode and calculated according to the Nernst relation (see Section 13.6.34).
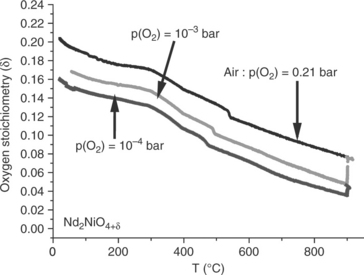
• With respect to the usual overpotentials undergone by oxygen electrodes, it follows that in the SOFC mode, the oxygen partial pressure at the surface of the material can reach values as low as 10− 4 atm and lead to the depletion of oxygen. According to TGA measurements as well as XRD data, it is stated that these oxides can lose reversibly some additional oxygen atoms and remain stable in such conditions; an example is given in Fig. 13.21 (Grenier et al., 2009). These results are also confirmed by coulometric experiments that show these materials to be stable at high temperatures down to oxygen pressures as low as 10− 10 atm (Fig. 13.22) (Nakamura et al., 2010).
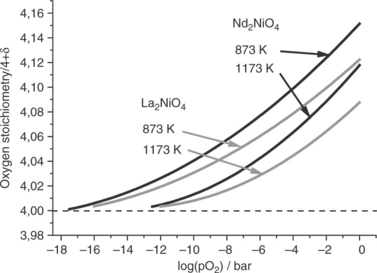
13.22 Oxygen stoichiometry of Ln2NiO4 + δ compounds (Ln = La, Nd) measured by coulometric titration as a function oxygen partial pressure, at different temperatures. (Source: Data from Nakamura et al., 2009–2010.)
• On the other hand, in HTSE operation, high oxygen activity is generated at the electrode, equivalent to a high oxygen pressure on its surface, estimated at about 104− 105 atm for usual overpotentials (Fig. 13.20). As quoted earlier, in previous works dealing with the electrochemical oxygen intercalation at room temperature, this feature was used for over-oxidizing these materials; values of inserted oxygen atoms as high as 0.25 could be obtained for La2NiO4 (Grenier et al., 1996). Using oxygen high pressures (200 bar, 350°C), Aguadero et al. even reached values of 0.30, which evidences the ability of these materials to easily incorporate high contents of additional oxygen (Aguadero et al., 2006).
It follows that, whatever the operating conditions, that is, the local oxygen pressure, additional oxygen atoms Oi will be more or less present in the Ln2O2 layers (Fig. 13.23).
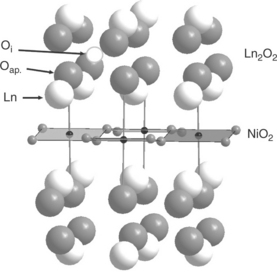
13.23 Representation of the Ln2NiO4 + δ structure showing the NiO2 square planar sites and Ln2O2 rock salt type layer with the apical oxygen atoms (Oap.) and the location of the interstitial oxygen.
• Furthermore, the presence of vacant sites in the structure allows the insertion of water molecules leading to the following formulation Ln2NiO4 − δ(OH)y (Grenier et al., 2009). Even though the value of y is small, it seems to be sufficient for inducing a protonic conductivity and to explain the good performances of these nickelates as H+-SOFC cathodes.
• The last feature of these compounds is concerned with their peculiar structural aspects. Indeed, as described previously, their structure can be considered as a succession of layers of square planar sites (NiO2) and rock salt type layers (Ln2O2) (Fig. 13.23). For stoichiometric Ln2Ni2 +O4 compounds containing only divalent nickel cations, there exists a structural misfit between both layers, that is, the size of the Ln2O2 layer is smaller than that of (NiO2). Stresses are induced in the structure giving rise to a buckling of the octahedra (i.e., to the orthorhombic Cmca space group) and to a tensile stress in the Ln2O2 layer; the Goldschmidt tolerance factor, t is << 1 (see Section 13.4.3). From a mechanical view-point, these stresses can be relaxed provided that the metallic cation is oxidized (for instance Ni2 + into Ni3 +), which lessens the size of the NiO2 plane, and/or by inserting oxygen into the Ln2O2 layer, which increases the size of this layer. Both phenomena occurring when the material is oxidized explains why these compounds easily insert oxygen atoms. This results in an increase of the Goldschmidt tolerance factor t.
As a consequence of the application as oxygen electrode, when the material operates as a SOFC cathode (i.e., in oxygen depletion conditions), the material is able to lose some oxygen atoms. But as δ decreases, stresses are generated and act to prevent a further reduction of the material, keeping the mixed valence of the nickel and additional mobile oxygen atoms. On the other hand, under anodic polarization in HTSE (i.e., under highly oxidizing conditions), oxygen can enter into the network, and the nickel is oxidized (the electrical conductivity is enhanced) until a mechanical limit in terms of size fitting of the layers is reached (t ≈ 1). The basic properties of the materials remain mobility of oxygen and mixed valence of the cations.
Thanks to these peculiar structural features, it appears that these compounds are genuinely flexible to be used in different systems, which is illustrated by the good performances reported in recent years. In particular, these materials seem to be especially interesting for electrolysis anode applications as, contrary to oxygen-deficient perovskites that completely oxidize in such conditions, they are never saturated in oxygen, which preserves their oxygen mobility and, therefore, their mixed ionic and electronic conduction properties.
13.7 Prospective conclusions
Given the fact that hydrogen is an important energy vector for the future, SOFCs as well as high-temperature steam electrolyzers have become active research topics. The aim of the research is to make efficient and robust systems. Most of the commercial systems use rather classical materials: fluorite type for the electrolyte, nickel cermet for the anode and a perovskite compound as cathode material, most of which were developed in the 1990s.
In this review, we have focused on cathode materials, especially on MIEC oxides that are currently considered the best compounds. We have also described the alternative materials that have been proposed during the last 10 years for the next generation of oxygen electrodes. They are characterized by the fact that:
• their electronic conductivity results from the presence of a mixed valence of the metallic cations;
• their ionic conductivity results from the presence of additional oxide ions instead of oxygen vacancies as in the usual cathode materials;
• their structures show a reduced dimensionality, two-dimensional for the A2MO4-type compounds and almost two-dimensional for the double perovskites.
The last two features could be an avenue worth exploring for the design of innovative cathode materials. However, electrochemical properties are not the only features to be considered; some materials have reached the targets, that is, ASR values lower than 0.10 Ω cm2 at the operating temperature and improving these values will be probably difficult. The long-term stability and chromium resistance of these cathode materials are also important issues to be taken into account for practical applications.
13.8 References
Adler, S. Mechanism and kinetics of oxygen reduction on porous La1.xSrxCoO3-δ electrodes. Solid State Ionics. 1998; 111(1–2):125–134.
Adler, S., Lane, J., Steele, B. Electrode kinetics of porous mixed-conduct-ing oxygen electrodes. J Electrochem Soc. 1996; 143(11):3554–3564.
Aguadero, A., Alonso, J., Martinez-Lope, M., Fernandez-Diaz, M., Escudero, M., Daza, L. In situ high temperature neutron powder diffraction study of oxygen-rich La2NiO4+δ in air: Correlation with the electrical behaviour. J Mater Chem. 2006; 16(33):3402–3408.
Allan, N., Mackrodt, W. Oxygen ion migration in La2CuO4. Philos Mag A. 1991; 64(5):1129–1132.
Anderson, H.U. Review of p-type doped perovskite materials for SOFC and other applications. Solid State Ionics. 1992; 52(1–3):33–41.
Arrouy, F., Wattiaux, A., Cros, C., Demazeau, G., Grenier, J.-C., Pouchard, M., Etourneau, J. Superconducting behaviour of the T/O structural type phase La2-xNdxCuO4 + δ (0<x<0.60) under oxidizing conditions. Physica C. 1991; 175(3–4):342–346.
Bassat, J., Odier, P., Villesuzanne, A., Marin, C., Pouchard, M. Anisotropic ionic transport properties in La2NiO4 + δ single crystals. Solid State Ionics. 2004; 167(3–4):341–347.
Basu, R., Tietz, F., Teller, O., Wessel, E., Buchkremer, H., Stöver, D. LaNi0.6Fe0.4O3 as a cathode contact material for solid oxide fuel cells. J Solid State Electrochem. 2003; 7(7):416–420.
Berastegui, P., Eriksson, S.G., Hull, S. A neutron diffraction study of the temperature dependence of Ca2Fe2O5. Mater Res Bull. 1999; 34(2):303–314.
Boehm, E., Bassat, J.-M., Dordor, P., Mauvy, F., Grenier, J.-C., Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2-xNiO4 + δ oxides. Solid State Ionics. 2005; 176(37–38):2717–2725.
Boehm, E., Bassat, J.-M., Steil, M., Dordor, P., Mauvy, F., Grenier, J.-C. Oxygen transport properties of La2Ni1-xCuxO4 + δ mixed conducting oxides. Solid State Sci. 2003; 5(7):973–981.
Brandon, N., Skinner, S., Steele, B. Recent advances in materials for fuel cells. Annu Rev Mater Res. 2003; 33:183–213.
Bucher, E., Egger, A., Caraman, G., Sitte, W. Stability of the SOFC cathode material (Ba, Sr)(Co, Fe)O3-δ in CO. containing atmospheres. J Electrochem Soc. 2008; 155(11):B1218–B1224.
Burriel, M., Garcia, G., Santiso, J., Kilner, J., Chater, R., Skinner, S. Anisotropic oxygen diffusion properties in epitaxial thin films of La.NiO4 + δ. J Mater Chem. 2008; 18(4):416–422.
Chauveau, F., Mougin, J., Bassat, J., Mauvy, F., Grenier, J. ‘A new anode material for solid oxide electrolyser: The neodymium nickelate Nd2NiO4 + δ. J Power Sources. 2010; 195(3):744–749.
Chen, C.H., Bouwmeester, H.J.M., van Doorn, R.H.E., Kruidhof, H., Burggraaf, A.J. Oxygen permeation of La20.3Sr0.7CoO3-δ. Solid State Ionics. 1997; 98(1–2):7–13.
Chen, C., Nasrallah, M., Anderson, H., Alim, M. Immittance response of La0.6Sr0.4Co0.2Fe0.8O3 based electrochemical cells. J Electrochem Soc. 1995; 142:491–496.
Chiba, R., Yoshimura, F., Sakurai, Y. An investigation of LaNi1-xFexO3 as a cathode material for solid oxide fuel cells. Solid State Ionics. 1999; 124(3–4):281–288.
Choi, M.-B., Jeon, S.-Y., Lee, J.-S., Hwang, H.-J., Song, S.-J. Chemical diffusivity and ionic conductivity of GdBaCo2 O5 + δ. J Power Sources. 2010; 195(4):1059–1064.
Chroneos, A., Parfitt, D., Kilner, J., Grimes, R. Anisotropic oxygen diffusion in tetragonal LaiNiO4 + δ: Molecular dynamics calculations. J Mater Chem. 2010; 20(2):266–270.
Chroneos, A., Vovk, R., Goulatis, I., Goulatis, L. Oxygen transport in perovskite and related oxides: A brief review. J Alloy Comp. 2010; 494(1–2):190–195.
Claus, J., Borchardt, G., Weber, S., Hiver, J.-M., Scherrer, S. Combination of EBSP measurements and SIMS to study crystallographic orientation dependence of diffusivities in a polycrystalline material: Oxygen tracer diffusion in La2–xSrxCuO4 + δ. Mater Sci Eng B. 1996; 38(3):251–257.
Cleave, A., Kilner, J., Skinner, S., Murphy, S., Grimes, R. Atomistic computer simulation of oxygen ion conduction mechanisms in La2NiO4. Solid State Ionics. 2008; 179(21–26):823–826.
Dailly, J. 2008, ‘Synthèse et caractérisation de nouveaux matériaux de cathode pour piles a combustible a conduction protonique PCFC (Protonic Ceramic Fuel Cell)’. Ph.D. thesis, University of Bordeaux.
Dailly, J., Fourcade, S., Largeteau, A., Mauvy, F., Grenier, J., Marrony, M. Perovskite and A2MO4-type oxides as new cathode materials for protonic solid oxide fuel cells. Electrochim Acta. 2010; 55(20):5847–5853.
De Souza, R.A., Kilner, J.A., Walker, J.F. A SIMS study of oxygen tracer diffusion and surface exchange in La0.8Sr0.2MnO4 + δ. Mater Lett. 2000; 43(1–2):43–52.
Demourgues, A., Weill, F., Darriet, B., Wattiaux, A., Grenier, J., Gravereau, P., Pouchard, M. Additional oxygen ordering in “La2NiO4.25 “ (La8NiO17,). I. Electron and neutron diffraction study; 2 Structural features. J Solid State Chem. 1993; 106(2):317–338.
Demourgues, A., Weill, F., Grenier, J., Wattiaux, A., Pouchard, M. Electron microscopy study of electrochemically prepared La2NiO4 + δ (0.17 ≤ S ≤ 0.26). Phys C Supercond Appl. 1992; 192(3–4):425–434.
Ding, H., Xue, X. Cobalt-free layered perovskite GdBaFe2O5+x as a novel cathode for intermediate temperature solid oxide fuel cells. J Power Sources. 2010; 195(15):4718–4721.
Dönitz, W. Economics and potential application of electrolytic hydrogen in the next decades. Int J Hydrog Energy. 1984; 9(10):817–821.
Er-Rakho, L., Michel, C., Lacorre, P., Raveau, B. YBaCuFeO5 + δ: A novel oxygen-deficient perovskite with a layer structure. J Solid State Chem. 1988; 73(2):531–535.
Erdle, E., Dönitz, W., Schamm, R., Koch, A. Reversibility and polarization behaviour of high temperature solid oxide electrochemical cells. Int J Hydrog Energy. 1992; 17(10):817–819.
Escudero, M., Aguadero, A., Alonso, J., Daza, L. A kinetic study of oxygen reduction reaction on La2NiO4 cathodes by means of impedance spectroscopy. J Electroanal Chem. 2007; 611(1–2):107–116.
Ezin, A.N., Kurumchin, E.K., Murygin, I.V., Tsidilkovski, V.I., Vdovin, G.K. The types of surface exchange and diffusion of oxygen in La20.7Sr0.3CoO3 − δ. Solid State Ionics. 1998; 112(1–2):117–122.
Ferchaud, C., Grenier, J.-C., Zhang-Steenwinkel, Y., Van Tuel, M., Van Berkel, F., Bassat, J.-M. High performance praseodymium nickelate oxide cathode for low temperature solid oxide fuel cell. J Power Sources. 2011; 196(4):1872–1879.
Fergus, J. Metallic interconnects for solid oxide fuel cells. Mater Sci Eng A. 2005; 397(1–2):271–283.
Fleig, J. Solid oxide fuel cell cathodes: Polarization mechanisms and modeling of the electrochemical performance. Annu Rev Mater Res. 2003; 33(1):361–382.
Flynn, J.H., Dickens, B. Steady-state parameter-jump methods and relaxation methods in thermogravimetry. Thermochim Acta. 1976; 15(1):1–16.
Frayret, C., Villesuzanne, A., Pouchard, M. Application of density functional theory to the modeling of the mixed ionic and electronic conductor La2NiO4 + δ: Lattice relaxation, oxygen mobility, and energetics of Frenkel defects. Chem Mater. 2005; 17(26):6538–6544.
Frontera, C., Caneiro, A., Carrillo, A., Oro-Solé, J., Garcia-Munoz, J.L. Tailoring oxygen content on PrBaCo2O5 + δ layered cobaltites. Chem Mater. 2005; 17(22):5439–5445.
Gödickemeier, M., Sasaki, K., Gauckler, L.J., Riess, I. Perovskite cathodes for solid oxide fuel cells based on ceria electrolytes. Solid State Ionics. 1996; 86–88(Part 2):691–701.
Grenier, J., Mauvy, F., Lalanne, C., Bassat, J.-M., Chauveau, F., Mougin, J., Dailly, J., Marrony, M., A2MO4 + δ oxides: Flexible materials for solid oxide cells. 11th International Symposium on Solid Oxide Fuel Cells (SOFC-XI)-216th ECS Meeting, Vienna; Vol. 25, 2009:2537–2546.
Grenier, J.-C., Pouchard, M., Hagenmuller, P. Vacancy ordering in oxygen-deficient perovskite-related ferrites. Struct Bond. 1981; 47:1–25.
Grenier, J.-C., Pouchard, M., Wattiaux, A. Electrochemical synthesis: Oxygen intercalation. Curr Opin Solid State Mater Sci. 1996; 1(2):233–240.
Grenier, J.-C., Wattiaux, A., Doumerc, J.-P., Dordor, P., Fournes, L., Chaminade, J.-P., Pouchard, M. Electrochemical oxygen intercalation into oxide networks. J Solid State Chem. 1992; 96(1):20–30.
Grimaud, A., Mauvy, F., Bassat, J.-M., Grenier, J.-C., Dailly, J., Marrony, M., Electrochemical study of rare earth oxides used as cathode materials for SOFC-H+ applications. Proceedings of E-MRS 2010 Spring Meeting, 9–11 June, Strasbourg, France, 2010.
Haanappel, V., Lalanne, C., Mai, A., Tietz, F. Characterization of anode-supported solid oxide fuel cells with Nd2NiO4 cathodes. J Fuel Cell Sci Technol. 2009; 6(4):0410161–0410166.
Hagenmuller, P., Pouchard, M., Grenier, J.C. Nonstoichiometry in perovskite-type oxides. Influence of the electronic configuration on formation of extended defects. C-MRS Int. Symp. Proc. (Ed. by Kong, Meiying;Huang, Liji). 1991; 1:25–37.
Hauch, A., Ebbesen, S.D., Jensen, S.H., Mogensen, M. Highly efficient high temperature electrolysis. J Mater Chem. 2008; 18:2331–2340.
Hewat, A.W., Capponi, J.J., Chaillout, C., Marezio, M., Hewat, E.A. Structures of superconducting Ba2YCu3O7-δ and semiconducting Ba2YCu3O6 between 25°C and 750°C. Solid State Commun. 1987; 64(3):301–307.
Holtappels, P., Bagger, C. Fabrication and performance of advanced multilayer SOFC cathodes. J Eur Ceram Soc. 2002; 22(1):41–48.
Huang, Q.-A., Hui, R., Wang, B., Zhang, J. A review of AC impedance modeling and validation in SOFC diagnosis. Electrochim Acta. 2007; 52(28):8144–8164.
Ivers-Tiffée, E., Weber, A., Herbstritt, D. Materials and technologies for SOFC-components. J Eur Ceram Soc. 2001; 21(10–11):1805–1811.
Jiang, S. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J Mater Sci. 2008; 43(21):6799–6833.
Jorgensen, M., Mogensen, M. Impedance of solid oxide fuel cell LSM/YSZ composite cathodes. J Electrochem Soc. 2001; 148(5):A433–A442.
Kawada, T., Masuda, K., Suzuki, J., Kaimai, A., Kawamura, K., Nigara, Y., Mizusaki, J., Yugami, H., Arashi, H., Sakai, N., Yokokawa, H. Oxygen isotope exchange with a dense La0.6Sr0.4CoO3-δ electrode on a Ce0.9Ca0.1O1.9 electrolyte. Solid State Ionics. 1999; 121(1–4):271–279.
Kendall, K.R., Navas, C., Thomas, J.K., Loye, H.-C.Z. Recent developments in perovskite-based oxide ion conductors. Solid State Ionics. 1995; 82(3–4):215–223.
Kenjo, T., Nishiya, M. LaMnO3 air cathodes containing ZrO2 electrolyte for high temperature solid oxide fuel cells. Solid State Ionics. 1992; 57(3–4):295–302.
Kharton, V.V., Marozau, I.P., Vyshatko, N.P., Shaula, A.L., Viskup, A.P., Naumovich, E.N., Marques, F.M.B. Oxygen ionic conduction in brownmillerite CaAl0.5Fe0.5O2.5 + δ. Mater Res Bull. 2003; 38(5):773–782.
Kharton, V., Tsipis, E., Yaremchenko, A., Frade, J. Surface-limited oxygen transport and electrode properties of La2Ni08Cu02O4 + δ. Solid State Ionics. 2004; 166(3–4):327–337.
Kharton, V.V., Viskup, A.P., Kovalevsky, A.V., Naumovich, E.N., Marques, F.M.B. Ionic transport in oxygen-hyperstoichiometric phases with K2NiF4-type structure. Solid State Ionics. 2001; 143(3–4):337–353.
Kharton, V., Yaremchenko, A., Shaula, A., Patrakeev, M., Naumovich, E., Logvinovich, D., Frade, J., Marques, F. Transport properties and stability of Ni-containing mixed conductors with perovskite and K2NiF4-type structure. J Solid State Chem. 2004; 177(1):26–37.
Kilner, J.A., Shaw, C.K.M. Mass transport in La2Ni1, xCoxO4+8 oxides with the K2NiF4 structure. Solid State Ionics. 2002; 154–155:523–527.
Kim, J.-D., Kim, G.-D., Moon, J.-W., Park, Y.-I., Lee, W.-H., Kobayashi, K., Nagai, M., Kim, C.-E. Characterization of LSM-YSZ composite electrode by ac impedance spectroscopy. Solid State Ionics. 2001; 143(3–4):379–389.
Kim, J.-H., Prado, F., Manthiram, A. Characterization of GdBa1-xSrxCo2O5 + δ (0 < x < 1.0) double perovskites as cathodes for solid oxide fuel cells. J Electrochem Soc. 2008; 155(10):B1023–B1028.
Kim, G., Wang, S., Jacobson, A., Reimus, L., Brodersen, P., Mims, C. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered a cations. J Mater Chem. 2007; 17(24):2500–2505.
Komatsu, T., Arai, H., Chiba, R., Nozawa, K., Arakawa, M., Sato, K. Cr poisoning suppression in solid oxide fuel cells using LaNi(Fe)O3 electrodes. Electrochem Solid State Lett. 2006; 9(1):A9–A12.
La O’, G., Savinell, R., Shao-Horn, Y. Activity enhancement of dense strontium-doped lanthanum manganite thin films under cathodic polarization: A combined AES and XPS study. J Electrochem Soc. 2009; 156(6):B771–B781.
Laberty, C., Zhao, F., Swider-Lyons, K., Virkar, A. High-performance solid oxide fuel cell cathodes with lanthanum-nickelate-based composites. Electrochem Solid State Lett. 2007; 10(10):B170–B174.
Lalanne, C., Prosperi, G., Bassat, J.-M., Mauvy, F., Fourcade, S., Stevens, P., Zahid, M., Diethelm, S., Van herle, J., Grenier, J.-C. Neodymium-deficient nickelate oxide Nd1.95NiO4 + δ as cathode material for anode-supported intermediate temperature solid oxide fuel cells. J Power Sources. 2008; 185(2):1218–1224.
Lane, J., Benson, S., Waller, D., Kilner, J. Oxygen transport in La0.6Sr0.4Co0.2Fe0.8O3-δ. Solid State Ionics. 1999; 121(1):201–208.
Lane, J., Kilner, J. Measuring oxygen diffusion and oxygen surface exchange by conductivity relaxation. Solid State Ionics. 2000; 136–137:997–1001.
Luebbe, H., Van herle, J., Hofmann, H., Bowen, P., Aschauer, U., Schuler, A., Snijkers, F., Schindler, H.-J., Vogt, U., Lalanne, C. Cathode-supported micro-tubular SOFCs based on Nd 1.95NiO4 + δ. Solid State Ionics. 2009; 180(11–13):805–811.
Maignan, A., Martin, C., Pelloquin, D., Nguyen, N., Raveau, B. Structural and magnetic studies of ordered oxygen-deficient perovskites LnBaCo2O5 + δ, closely related to the “112” structure. J Solid State Chem. 1999; 142(2):247–260.
Manning, P., Sirman, J., Kilner, J. Oxygen self-diffusion and surface exchange studies of oxide electrolytes having the fluorite structure. Solid State Ionics. 1996; 93(1–2):125–132.
Mauvy, F., Bassat, J.M., Boehm, E., Manaud, J.P., Dordor, P., Grenier, J.C. Oxygen electrode reaction on Nd2NiO4 + δ cathode materials: Impedance spectroscopy study. Solid State Ionics. 2003; 158(1–2):17–28.
Mauvy, F., Bassat, J., Boehm, E., Dordor, P., Grenier, J., Loup, J. Chemical oxygen diffusion coefficient measurement by conductivity relaxation-correlation between tracer diffusion coefficient and chemical diffusion coefficient. J Eur Ceram Soc. 2004; 24(6):1265–1269.
Mazo, G., Savvin, S. The molecular dynamics study of oxygen mobility in La2.xSrxCuO4-δ. Solid State Ionics. 2004; 175(1–4):371–374.
Menzler, N., Tietz, F., Uhlenbruck, S., Buchkremer, H., Stöver, D. Materials and manufacturing technologies for solid oxide fuel cells. J Mater Sci. 2010; 45(12):3109–3135.
Millar, L., Taherparvar, H., Filkin, N., Slater, P., Yeomans, J. Interaction of (La1-xSrx)1-yMnO3-Zr1-zYzO2-δ cathodes and LaNi0.6Fe0.4O3 current collecting layers for solid oxide fuel cell application. Solid State Ionics. 2008; 179(19–20):732–739.
Minervini, L., Grimes, R., Kilner, J., Sickafus, K. Oxygen migration in La2NiO4 + δ. J Mater Chem. 2000; 10(10):2349–2354.
Mitchell, R. Perovskites, Modern and Ancient. Canada: Almaz Press Inc.; 2002.
Miyoshi, S., Hong, J.-O., Yashiro, K., Kaimai, A., Nigara, Y., Kawamura, K., Kawada, T., Mizusaki, J. Lattice creation and annihilation of LaMnO3 + δ caused by nonstoichiometry change. Solid State Ionics. 2002; 154–155:257–263.
Mizusaki, J., Mori, N., Takai, H., Yonemura, Y., Minamiue, H., Tagawa, H., Dokiya, M., Inaba, H., Naraya, K., Sasamoto, T., Hashimoto, T. Oxygen nonstoichiometry and defect equilibrium in the perovskite-type oxides La1.xSrxMnO3 + δ. Solid State Ionics. 2000; 129(1–4):163–177.
Nakamura, T., Yashiro, K., Sato, K., Mizusaki, J. Defect chemical and statistical thermodynamic studies on oxygen nonstoichiometric Nd2-xSrxNiO4 + δ. Solid State Ionics. 2009; 180:1406–1413.
Nakamura, T., Yashiro, K., Sato, K., Mizusaki, J. Structural analysis of La2-xSrxNiO4 + δ by high temperature X-ray diffraction. Solid State Ionics. 2010; 181(5–7):292–299.
Orera, A., Slater, P.R. New chemical systems for solid oxide fuel cells. Chem Mater. 2010; 22(3):675–690.
Parfitt, D., Chroneos, A., Kilner, J., Grimes, R. Molecular dynamics study of oxygen diffusion in Pr2NiO4 + δ. Phys Chem Chem Phys. 2010; 12(25):6834–6836.
Pizzini, S. Fast Ion Transport in Solids. New York: North-Holland Publishing Company; 1973.
Pérez-Coll, D., Aguadero, A., Escudero, M., Daza, L. Effect of DC current polarization on the electrochemical behaviour of La2NiO4 + δ and La2Ni2O7 + δ− based systems. J Power Sources. 2009; 192(1):2–13.
Read, M., Islam, M., King, F., Hancock, F. Defect chemistry of La2Ni1-xMxO4 (M = Mn, Fe, Co, Cu): Relevance to catalytic behavior. J Phys Chem B. 1999; 103(9):1558–1562.
Rieu, M., Sayers, R., Laguna-Bercero, M., Skinner, S., Lenormand, P., Ansart, F. Investigation of graded La2NiO4 + δ cathodes to improve SOFC electrochemical performance. J Electrochem Soc. 2010; 157(4):B477–B480.
Riza, F., Ftikos, C., Tietz, F., Fischer, W. Preparation and characterization of Ln0.8Sr0.2Fe0.8Co0.2O3-x (Ln=La, Pr, Nd, Sm, Eu, Gd). J Eur Ceram Soc. 2001; 21(10–11):1769–1773.
Ruddlesden, S., Popper, P. The compound Sr3Ti2O7 and its structure. Acta Crystallogr. 1958; 11:54–55.
Ruiz-Trejo, E., Sirman, J., Baikov, Y., Kilner, J. Oxygen ion diffusivity, surface exchange and ionic conductivity in single crystal Gadolinia doped Ceria. Solid State Ionics. 1998; 113–115:565–569.
Sahibzada, M., Steele, B., Zheng, K., Rudkin, R., Metcalfe, I. Development of solid oxide fuel cells based on a Ce(Gd)O2-x electrolyte film for intermediate temperature operation. Catal Today. 1997; 38(4):459–466.
Savvin, S., Mazo, G., Ivanov-Schitz, A. Simulation of ion transport in layered cuprates La2-xSxCuO4-δ. Crystallogr Rep. 2008; 53(2):291–301.
Sayers, R., Rieu, M., Lenormand, P., Ansart, F., Kilner, J., Skinner, S. Development of lanthanum nickelate as a cathode for use in intermediate temperature solid oxide fuel cells. Solid State Ionics. 2011; 192:531–534.
Shaula, A., Pivak, Y., Waerenborgh, J., Gaczynski, P., Yaremchenko, A., Kharton, V. Ionic conductivity of brownmillerite-type calcium ferrite under oxidizing conditions. Solid State Ionics. 2006; 177(33–34):2923–2930.
Siebert, E., Hammouche, A., Kleitz, M. Impedance spectroscopy analysis of La1-xSrxMnO3-yttria-stabilized zirconia electrode kinetics. Electrochim Acta. 1995; 40(11):1741–1753.
Simner, S., Bonnett, J., Canfield, N., Meinhardt, K., Sprenkle, V., Stevenson, J. Optimized lanthanum ferrite-based cathodes for anode-supported SOFCs. Electrochem Solid State Lett. 2002; 5(7):A173–A175.
Singhal, S. Advances in solid oxide fuel cell technology. Solid State Ionics. 2000; 135(1–4):305–313.
Skinner, S. Characterisation of La2NiO4 + δ using in situ high temperature neutron powder diffraction. Solid State Sci. 2003; 5(3):419–426.
Skinner, S., Amow, G. Structural observations on La2(Ni, Co)O4±δ phases determined from in situ neutron powder diffraction. J Solid State Chem. 2007; 180(7):1977–1983.
Skinner, S.J., Kilner, J.A. Oxygen diffusion and surface exchange in La2-xSrxNiO4 + δ. Solid State Ionics. 2000; 135(1–4):709–712.
Srinivasan, S., Hurwitz, H., Bockris, J. Fundamental equations of electrochemical kinetics at porous gas-diffusion electrodes. J Chem Phys. 1967; 46(8):3108–3122.
Steele, B. Fast Ion Transport in Solids. North-Holland: Elsevier; 1973.
Steele, B., Heinzel, A. Materials for fuel-cell technologies. Nature. 2001; 414(6861):345–352.
Steele, B.C.H. Survey of materials selection for ceramic fuel cells II. Cathodes and anodes. Solid State Ionics. 1996; 86–88(Part 2):1223–1234.
Sun, C., Hui, R., Roller, J. Cathode materials for solid oxide fuel cells: A review. J Solid State Electr. 2010; 14:1125–1144.
Sun, L.-P., Li, Q., Zhao, H., Huo, L.-H., Grenier, J.-C. Preparation and electrochemical properties of Sr-doped Nd2NiO4 + δ cathode materials for intermediate-temperature solid oxide fuel cells. J Power Sources. 2008; 183(1):43–48.
Taillades, G., Dailly, J., Taillades-Jacquin, M., Mauvy, F., Essouhmi, A., Marrony, M., Lalanne, C., Fourcade, S., Jones, D., Grenier, J.-C., Rozière, J. Intermediate temperature anode-supported fuel cell based on BaCe0.9Y0.1O3 electrolyte with novel Pr2NiO4 cathode. Fuel Cells. 2010; 10(1):166–173.
Takeda, Y., Kanno, R., Noda, M., Tomida, Y., Yamamoto, O. Cathodic polarization phenomena of perovskite oxide electrodes with stabilized zirconia. J Electrochem Soc. 1987; 134(11):2656–2661.
Takeda, Y., Ueno, H., Imanishi, N., Yamamoto, O., Sammes, N., Phillipps, M.B. Gd1- xSrxCoO3 for the electrode of solid oxide fuel cells. Solid State Ionics. 1996; 86–88(Part 2):1187–1190.
Tao, S., Wu, Q., Peng, D., Meng, G. Electrode materials for intermediate temperature proton-conducting fuel cells. J Appl Electrochem. 2000; 30(2):153–157.
Tarancon, A., Burriel, M., Santiso, J., Skinner, S., Kilner, J. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J Mater Chem. 2010; 20(19):3799–3813.
Tarancon, A., Pena-Martinez, J., Marrero-Lopez, D., Morata, A., Ruiz-Morales, J., Nunez, P. Stability, chemical compatibility and electrochemical performance of GdBaCo2O5+x layered perovskite as a cathode for intermediate temperature solid oxide fuel cells. Solid State Ionics. 2008; 179(40):2372–2378.
Tarancon, A., Skinner, S., Chater, R., Hernandez-Ramirez, F., Kilner, J. Layered perovskites as promising cathodes for intermediate temperature solid oxide fuel cells. J Mater Chem. 2007; 17(30):3175–3181.
Taskin, A., Ando, Y. Electron-hole asymmetry in GdBaCo2O5+x: Evidence for spin blockade of electron transport in a correlated electron system. Phys Rev Lett. 2005; 95(17):1–4.
Taskin, A., Lavrov, A., Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl Phys Lett. 2005; 86(9):1–3.
Taskin, A., Lavrov, A., Ando, Y. Fast oxygen diffusion in A-site ordered perovskites. Progr Solid State Chem. 2007; 35(2–4 SPEC. ISS.):481–490.
Tietz, F., Solid oxide fuel cells. Encyclopedia of Materials: Science and Technology. 2nd ed, 2008:1–8.
Tietz, F., Buchkremer, H.P., Stöver, D. Components manufacturing for solid oxide fuel cells. Solid State Ionics. 2002; 152–153:373–381.
Tietz, F., Haanappel, V., Mai, A., Mertens, J., Stöver, D. Performance of LSCF cathodes in cell tests. J Power Sources. 2006; 156(1 SPEC. ISS.):20–22.
Vente, J., McIntosh, S., Haije, W., Bouwmeester, H. Properties and performance of BaxSr1.xCo0.8Fe0.2O3-δ materials for oxygen transport membranes. J Solid State Electrochem. 2006; 10(8):581–588.
Wang, W., Jiang, S.P. A mechanistic study on the activation process of (La, Sr)MnO3 electrodes of solid oxide fuel cells. Solid State Ionics. 2006; 177(15–16):1361–1369.
Wattiaux, A., Park, J.-C., Grenier, J.-C., Pouchard, M. Une nouvelle voie d’accès aux oxydes supraconducteurs: l’oxydation électrochimique de La2CuO4. C R Acad Sci Paris. 1990; 310(II):1047–1052.
Winter, C.-J. Hydrogen energy – abundant, efficient, clean: A debate over the energy-system-of-change. Int J Hydrogen Energ. 2009; 34(14, Suppl. 1):S1–S52.
Xia, C., Rauch, W., Chen, F., Liu, M. Sm0.5Sr0.5CoO3 cathodes for low-temperature SOFCs. Solid State Ionics. 2002; 149(1–2):11–19.
Yamada, A., Saka, K., Uehara, M., Taminato, S., Kanno, R., Mauvy, F., Grenier, C. Anisotropic catalytic activity of the orientation controlled Nd2NiO4 + δ/ YSZ hetero-epitaxial system for SOFC cathode. Electrochem Commun. 2010; 12(12):1690–1693.
Yan, A., Cheng, M., Dong, Y., Yang, W., Maragou, V., Song, S., Tsiakaras, P. Investigation of a Ba0.5Sr0.5Co0.8Fe0.2O3-δ based cathode IT-SOFC. I.The effect of CO2 on the cell performance. Appl Catal B Environ. 2006; 66(1–2):64–71.
Yang, C.-C., Wei, W.-C., Roosen, A. Electrical conductivity and microstructures of La0.65Sr0.3MnO3-8mol yttria-stabilized zirconia. Mater Chem Phys. 2003; 81(1):134–142.
Yashima, M., Enoki, M., Wakita, T., Ali, R., Matsushita, Y., Izumi, F., Ishihara, T. Structural disorder and diffusional pathway of oxide ions in a doped Pr2NiO4 + δ−based mixed conductor. J Am Chem Soc. 2008; 130(9):2762–2763.
Yashima, M., Sirikanda, N., Ishihara, T. Crystal structure, diffusion path, and oxygen permeability of a Pr2NiO4-based mixed conductor (Pr0.9La0.1)2Ni0.74 Cu0.21Ga0.05O4 + δ. J Am Chem Soc. 2010; 132(7):2385–2392.
Yasuda, I., Hishinuma, M. Electrical conductivity and chemical diffusion coefficient of strontium-doped lanthanum manganites. J Solid State Chem. 1996; 123(2):382–390.
Yasuda, I., Ogasawara, K., Hishinuma, M., Kawada, T., Dokiya, M. Oxygen tracer diffusion coefficient of (La, Sr)MnO3 ± δ. Solid State Ionics. 1996; 86–88(Part 2):1197–1201.
Zhao, H., Mauvy, F., Lalanne, C., Bassat, J.-M., Fourcade, S., Grenier, J.-C. New cathode materials for ITSOFC: Phase stability, oxygen exchange and cathode properties of La2-xNiO4 + δ. Solid State Ionics. 2008; 179(35–36):2000–2005.
Zhou, Q., He, T., Ji, Y. SmBaCo2O5+x double-perovskite structure cathode material for intermediate-temperature solid-oxide fuel cells. J Power Sources. 2008; 185(2):754–758.