Novel anode materials for solid oxide fuel cells
Abstract:
This chapter aims to chart the development of novel anode materials for solid oxide fuel cells (SOFCs), mainly perovskite oxides. The requirements of efficient anode materials are also discussed alongside the trade-off between certain requirements. We conclude with a short discussion of the current state in the development of SOFC anode materials and the outlook for future development.
14.1 Introduction
The key parts of a solid oxide fuel cell (SOFC) are cathode, electrolyte, anode and interconnector. The typical electrolyte materials for SOFCs are oxygen-ion-conducting materials such as yttria-stabilised zirconia (YSZ) (Minh, 1993), Ce0.9Gd0.1O2-δ21078(CGO) (Steele, 2000) and La0.85Sr0.15 Ga0.9Mg0.1 O3 −δ « (LSGM) (Ishihara et al., 1994; Kuroda et al., 2000). On the other hand, proton-conducting materials such as Y-doped BaCeO3, BaZrO3 can also be used as electrolyte for SOFCs (Iwahara et al, 1981; Ishihara et al, 1994; Kreuer, 2003; Tao et al, 2005; Tao and Irvine, 2006).
As for the SOFC anode, a number of substantial reviews have been published in this area in the previous decade, notably those by Atkinson et al. (2004), Tao and Irvine (2004a) and Sun and Stimming (2007). In this chapter, we focus on novel anode materials for SOFCs but will briefly cover the traditional cermet composite anode as well.
The anode reaction is different when different types of ionic-conducting electrolytes are applied in a SOFC. When hydrogen is used as fuel in a fuel cell based on an O2_ ion conducting electrolyte, the anode reaction is
when an O2_ ion-conducting electrolyte is used. In an SOFC based on H + ion-conducting electrolyte, the anode reaction is
Not only hydrogen but also hydrocarbons such as methane can be directly used as the fuel in a SOFC. When a hydrocarbon is used as the fuel in an SOFC based on an O2_ ion-conducting electrolyte, the anode reaction is
When an H+ conducting electrolyte is used, the reaction is
Therefore, water is required at the anode in order to generate protons at the anode (Coors, 2003).
14.2 Requirements for solid oxide fuel cell anode materials
Anode materials for SOFCs are usually formed from single-phase metal oxides or cermets, defined as a mixture of metals and metal oxide ceramics. Owing to the operating conditions and requirements of SOFC anode materials, there has been great difficulty in synthesising efficient anode materials. An ideal anode material must conform to several requirements, some of which must be balanced to create effective materials.
14.2.1 Electronic/ionic conductivity
Electronic conductivity in anode materials is considered as the primary selec- tion criterion, with an absolute minimum of 1 S cm− 1 and an ideal conductivity superior to 10 S cm− 1(Atkinson et al., 2004). High conductivity in anode materials is necessary for transfer of electrons. Most efficient anode materials are, therefore, highly conductive semiconductors or metallic conductors.
Ionic conductivity in anode materials is achieved through the use of an ionically conductive material in a cermet or through the use of a mixed ionic–electronic conductor (MIEC). Instead of cermets, an ideal solution is to identify a redox stable material which simultaneously exhibits high ionic and electronic conductivities.
The inherent efficiency of a fuel cell is partially derived from the size of the electrode triple phase boundary (TPB) (OØHayre et al, 2005). The TPB is the specific spatial position at which the hydrogen oxidation reaction (HOR) and the oxygen reduction reaction (ORR) occur, where the electrolyte, gas, and catalytically active regions are electrically connected. Figure 14.1 illustrates the TPB regions of different SOFC anode materials, those with a pure electronic conductor (a) and with an O2_/e' mixed conductor (b). High ionic conductivity in the anode will extend the TPB thus increasing the number of active sites for the HOR and reducing the electrode polarisation resistance thus improving fuel cell power density.
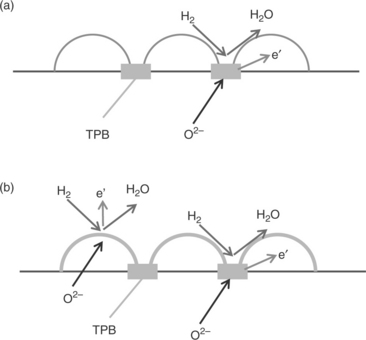
14.1 I llustration of the three-phase boundary regions of different SOFC anode materials. Schematic diagram of the anode surface (a) with a pure electronic conductor and (b) with an O2−/e' mixed conductor. Triple phase boundary (TPB) is extended in mixed conducting anode materials.
When Cu-CeO2-YSZ cermet was used as anode, it was observed that gas-phase pyrolysis reactions on anode can lead to tar formation on copper/ceria cermets; however, the compounds that form on copper tend to be polyaromatics, such as naphthalene and anthracene, rather than graphite. It has been suggested that the polyaromatic compounds enhance anode performance by providing additional electronic conductivity in the anode (McIntosh et al., 2003; Atkinson et al., 2004).
14.2.2 Catalytic activity
In theory, pure hydrogen is the ideal fuel for fuel cells; however, current infrastructure, supply and production of high-purity hydrogen gas is limited (Backhaus-Ricoult, 2008). Research into direct use of alternative fuels has been ongoing and much of the current investigation into anode materials utilises materials with catalytic activity for hydrocarbon oxidation. Previously, precious metal catalysts (Pt, Pd, Au) were used for both fuel cell electrodes but the cost of implementation was an obstacle to commercialisation.
Thus, many of the SOFC anode materials currently under development are designed in such a way to incorporate active catalytic sites within their structures, as catalytically active anode materials or as intercalated metals and metal oxides. For example, the introduction of manganese or iron at the B-site of La1 _ xSrxCrO3 can dramatically increase the catalytic property of the perovskite oxides and make them viable anode materials for SOFCs (Tao and Irvine, 2003, 2004b; Tao et al, 2004). Good catalytic properties are an essential requirement in order to facilitate the anode reaction and reduce the anode polarisation resistance.
14.2.3 Stability and compatibility
As SOFCs operate at very high temperatures, both the electrolyte and the electrodes have to be stable at operating temperatures and also withstand thermal and redox cycling upon start-up and shut-down. This is vital for use of SOFCs as auxiliary power units (APU) for transportation applications (H. Yokokawa, 2003).
An additional consideration is the thermal expansion coefficient (TEC), which is the specific change in volume per change in temperature at a constant pressure. As some materials may expand/contract more than others upon heating/cooling cycles, it is essential to match the TECs of components which contact to avoid stresses building at the interface, possibly causing delamination and cracking.
Chemical reactivity and stability is also an important aspect of SOFC materials as it governs durability and constitutes the most critical challenge for stationary power units (H. Yokokawa, 2003). Ideally, the material will be chemically compatible with contacting components and fuel impurities. However, materials currently employed are subject to inter-diffusion (Yokokawa et al, 2006) and poisoning (Matsuzaki and Yasuda, 2000).
14.2.4 Microstructure
Requirements for SOFC anode materials are not only on the materials themselves, good microstructure is also very important to achieve excellent performance. For a Ni-YSZ anode, a porosity of about 40–50% is ideal to reduce the anode polarisation and achieve the best performance (Sakamoto et al., 2008). Pore formers such as acrylic and starch are normally mixed with anode materials to form the right microstructure (Haslam et al, 2005). Suzuki et al. (2009) reported that in three cells with anode porosities 57% (Cell A), 47% (Cell B) and 37% (Cell C), Cell A exhibits the best performance. The microstructures of anode with different porosities are shown in Fig. 14.2. Not only porosity, but also cell performance is related to the particle size of NiO in the cermet. NiO particle size in Cell A was < 100 nm while >500 nm in Cells B and C due to the different firing temperature. The produced Ni is also smaller from reduction of smaller NiO leading to higher catalytic activities, smaller anode polarisation and high fuel cell performance (Fig. 14.2).
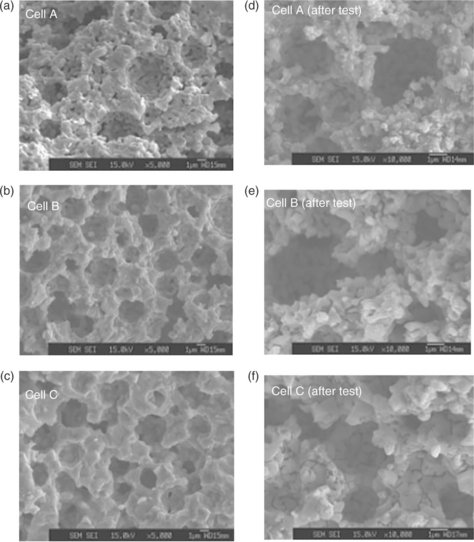
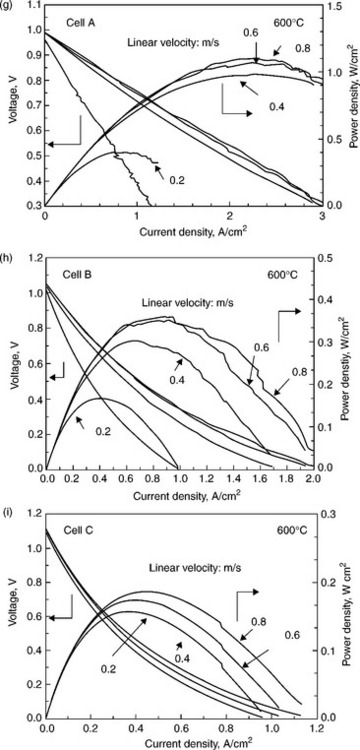
14.2 SEM images of the anode microstructure before the cell test (not reduced) (a−c) and after the cell test (reduced) (d–f) for cell A, cell B, and cell C. Because of the reduction of NiO to Ni, which expands the lattice parameter, the porosity preferably increased for SOFC operation. The corresponding fuel cell performances are listed together (g–i). (Source: Re-drawing from Fig. 2 in Reference Suzuki et al., 2009.)
qAtkinson et al. (Busawon et al, 2008) found that infiltration of nickel in a porous YSZ can effectively enhance the dimensional stability of anode and thus the redox stability.
14.3 Cermet solid oxide fuel cell anode materials
Single-phase materials investigated in the earliest SOFC developments included graphite, iron oxide, platinum group and transition metals (Baur and Preis, 1937; MoÈbius, 1997). Graphite is corroded electrochemically and platinum spalls off in service, presumably due to water vapour evo- lution at the metal oxide interface. As for the transition metals, iron is oxidised when the oxygen partial pressure at the anode exceeds a critical value, and cobalt is somewhat more stable, but also more costly. Nickel has a significant thermal expansion mismatch to stabilised zirconia, and at high temperatures the metal aggregates by grain growth, finally obstruct- ing the porosity of the anode and eliminating the three-phase boundaries required for cell operation. As a consequence, all-metal anodes have not found acceptance (Atkinson et al, 2004). This was the context for the introduction of the nickel–zirconia cermet anode (Tragert, 1967; Spacil, 1970). A cermet consisting of Ni and YSZ has many of the properties required for an efficient anode material. It has high electronic conductivity, reasonable ionic conductivity and high catalytic activity for hydrogen oxidation. The cermet, however, has multiple problems to effective implementation. Ni/YSZ is prone to carbon deposition, or coking, when using hydrocarbon fuels (Nikooyeh et al., 2008), sulphur poisoning (Rasmussen and Hagen, 2009), nickel agglomeration upon prolonged usage (Iwata, 1996) and is not redox stable (Cassidy et al, 1996). Reduction of coking and sulphur poisoning was achieved by transition metal substitution for nickel. However, this was coupled with a significant reduction in the anodic efficiency (Benyoucef et al, 2008; Grgicak et al, 2008).
Redox instability occurred upon anodic re-oxidation and was a result of the expansion of Ni to NiO which produced stresses at the anode–electrolyte interface (Faes et al, 2009; Laurencin et al, 2009). This has been found to be partially alleviated by modification of the microstructure and distri- bution of the Ni and YSZ phases in the cermet (Waldbillig et al, 2005; Kim et al, 2006).
Many materials based on CeO2 demonstrate mixed ionic and electronic conductivity in reducing atmospheres due to the mixed valence of cerium, Ce3 + and Ce4 +, under these conditions. Doping with lanthanides was found to significantly increase the ionic conductivity, especially for Gd (forming GDC) and Sm (forming SDC) (Van Herle et al., 1996). For SOFCs based on doped ceria as the electrolyte, the ionic conducting oxide YSZ in the cermet is normally replaced by the ceria-based oxide such as Sm- or Gd-doped ceria. Cermets of GDC or SDC with nickel were found to have increased performance compared to Ni/YSZ when methane was used as a fuel (Wang et al., 2003). Cerates have also been found to promote catalytic activity for hydrocarbon oxidation reactions without coking (Xu and Wang, 2005). Formation of a cermet consisting of a cerate coupled with both a transition metal and a noble metal was found to be especially effective for anodic hydrocarbon reforming (Hibino et al., 2003).
In the study of Qiao et al. (2007) using Ni/YSZ and Ni-CeO2/YSZ as anodes, it was demonstrated that at least 25 wt% of Ni was needed to ensure low ohmic resistance. If doped with titanium, YSZ exhibits an increased electronic conductivity with a smaller ionic conductivity in reduced atmosphere and compounds with high yttrium content and low titanium content are better conductors than YSZ both in air and hydrogen (Tao and Irvine, 2002a, 2004b; Mantzouris et al., 2007, 2008). Concerning the use of other metals, Venancio et al. (2008) and Cimenti et al. (Cimenti and Hill, 2010) reported new advances on carbon deposition issues when dealing with alcohol fuels. Using a copper cermet, the former applied a cell to ethanol and found no degradation after 200 h with no carbon deposition at all; while using a copper/cobalt catalyst on zirconium-doped ceria. If copper is used with ceria as the anode, operability up to 250 h without degradation was observed (Gross et al, 2009). This latter behaviour was also observed even with a Ni–scandia-stabilised zirconia (ScSZ) system given that a layer of copper–ceria was present (Ye et al, 2009).
Finally, cermet anodes are also used in combination with the perovskitetype electrolyte LSGM. If Ni–Pd alloys or interlayers are used in conjunction with a mixed lanthanum strontium cobalt (LSC)–SDC anode (Nabae and Yamanaka, 2009) or if Cu–Pd is added to lanthanum-doped ceria (LDC) anode (Bi and Zhu, 2010), results obtained show respectively that the meth- ane oxidation over Pd–Ni involves non-electrochemical formation of hydro- gen and carbon monoxide from methane and water and that if impregnated with Cu-Pd, a better performance for LDC is obtained than when using pure copper as well as less carbon deposition than when using pure palladium.
A study with silver (Wang et al., 2008) was also conducted and results included a high electrical conductivity in pure CO, no carbon deposition during fuel cell testing, a high porosity and generally a better performance than the copper–ceria/SDC anode. However, the nature of the electrode itself is a major obstacle to its commercialisation due to the cost of silver. The typical fuel cell performances of SOFCs based on cermet anode are listed in Table 14.1.
In brief, the introduction of copper or silver to partially or completely replace nickel or form an alloy can effectively suppress coking when a carbon-containing fuel is used. Nickel alloys are not redox stable therefore may cause problems on redox cycling of an SOFC.
One of the methods to overcome the volume change is to prepare a porous anode matrix on dense electrolyte through a tape-casting or pressing method. The NiO catalyst can be formed later through impregnation (Gorte et al, 2000; Park et al, 2000). Another approach is to develop a redox-stable anode for SOFCs.
14.4 Perovskite-structured solid oxide fuel cell anode materials
14.4.1 (La0.75Sr0.25)Cr0.5Mn0.5O3-based anode materials
Much of the research into novel anode materials has focused on the production of perovskite-type compounds (Marina et al., 2002; Tao and Irvine, 2003; Huang et al, 2006). Perovskite is a naturally occurring structure which has the general formula ABO3, in which the larger A-site cation occupies a 12-coordinate site and the B-site cation occupies a 6-coordinate site (Tao and Irvine, 2004a).
The first example of an efficient redox-stable anode material, (La0.75Sr0.25) Cr0.5Mn0.5O3 (LSCM), was developed in 2003 (Tao and Irvine, 2003), building on previous research into interconnect, LaCrO3, and cathode, La1 _ xSrxMnO3. materials (Minh, 1993). LSCM is a p-type conductor with a conductivity of approximately 38 S cm− 1 in air and 1.5 S cm− 1 at 900°C in 5% H2/Ar (Tao and Irvine, 2004b). Similar performance was found when methane was used, with 3% H2O, to that found for 5% H2/Ar. The material also shows a high resistance to coking when using hydrocarbon fuels. Due to similarities between cathode and anode requirements, LSCM has been employed to form symmetrical/reversible fuel cells (Bastidas et al, 2006), which utilise the same compound for both anode and cathode.
Previous reports (Tao and Irvine, 2003) noted that coking was not observed when operating pure LSCM in methane, thus investigation into alternative hydrocarbon fuels was undertaken. When operating in ethanol a minor reduction in fuel cell performance was found when switching between humidified H2 and a gasified 2:1 ethanol and water mixture (Huang et al., 2007). Stability testing in the gasified 2:1 ethanol and water mixture showed no significant performance degradation over 60 h, suggesting that carbon deposition during operation was minimal.
Kobsiriphat et al. (2010) found that substitution of nickel or ruthenium into LSCM introduced nanoscale metal particles onto the oxide surface upon anode reduction. According to previous reports (Madsen et al, 2007; Kobsiriphat et al, 2009), both nickel and ruthenium nanocluster formation was found to reduce the anodic polarisation resistance, albeit with much greater efficacy for ruthenium. Nickel substitution for manganese was also found to increase the catalytic activity of LSCM for total oxidation of methane, while substitution for either chromium or manganese increased the electronic conductivity in both air and 5% H2/Ar (Jardiel et al, 2010). A maximum conductivity of 2.4 S cm− 1 was found for La0.75Sr0.25Cr0.5Mn0.44Ni0.06O3 in 5% H2/Ar at 800°C, with a partial protonic contribution to the total conductivity observed under reducing atmospheres.
Fuel cell testing with a composite LSCRu-GDC anode yielded maximum power densities between 400 and 535 mW cm− 2 at 800°C in humidified H2 (Jardiel et al., 2010). Impregnation of LSCM with metallic Ru was also effective at improving the anode properties, with significant increases in the catalytic activity for propane reforming/oxidation (Barison et al, 2010). Additionally, the addition of Ru improved the H2 and syn-gas (the mixture of H2 and CO) yield, giving a maximum syn-gas yield of ~ 90% at 800°C, whilst maintaining good chemical stability.
Composite anodes, comprising of GDC and LSCM, exhibited reasonable catalytic activity for the oxidation of humidified H2 and CH4 without observed carbon deposition or microstructural alteration (Chen et al, 2007a). Further work by Chen et al. (2007b) demonstrated that impregnation of LSCM with GDC forms an effective anode material for operation in humidified H2 and CH4. Reasonable fuel cell performance, of 419 and 158 mW cm− 2 at 800°C in humidified H2 and CH4, respectively, was observed.
Zhu et al. (2010a) discovered reasonable fuel cell performance for a LSCM-impregnated YSZ composite anode, a maximum power density of 567 mW cm− 2 at 850°C in dry H2, and little change in fuel cell performance when using dry CH4, as a fuel. The fuel cell performance was significantly improved without any observed carbon deposition or degradation through impregnation of the composite with 2 wt% Ag and 6 wt% Ni due to the improved catalytic activities. Co-impregnation of a YSZ scaffold with LSCM and Ni improved the fuel cell performance compared with sequen- tial impregnation of YSZ (Zhu et al, 2010c). The enhancement was attributed to the differing microstructures observed and the obvious disparate distribution caused by sequential impregnation. The fuel cell performance of the co-impregnated Ni–LSCM–YSZ anode reached 1151 mW cm− 2 in dry H2 at 800°C (Zhu et al, 2010b). The fuel cell performances with LSCM–YSZ anode (Cell I) and Ni–LSCM–YSZ anode (Cell II) are shown in Fig. 14.3. Reasonable fuel cell performance was also observed when operating with dry methane, with no drop in open-circuit voltage (OCV), carbon deposition or compound deterioration noted after 6 h of operation. It should be noted that longer term testing is required for real applications.
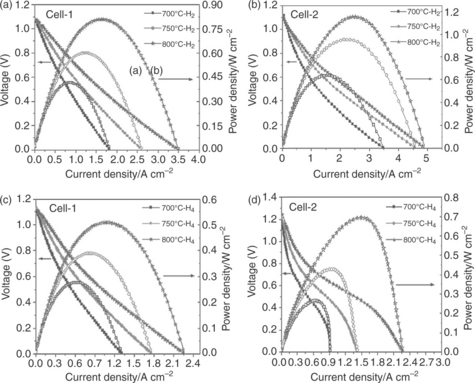
14.3 Voltage–current density and performance curves of the Cell-1 (La0.75Sr0.25Cr0.5Mn0.5O3 − δ anode) and Cell−2 (La0.75Sr0.25Cr0.5Mn0.5O3 − δ and Ni anode) at different testing temperatures in (a and b) dry H2 and (c and d) dry CH4. La0.8Sr0.2MnO3 − δ was used as cathode (Zhu et al., 2010b).
Impregnation of Pd nanoparticles in a LSCM/YSZ anode was found to improve fuel cell performance slightly in humidified H2 and significantly in humidified CH4 and ethanol-saturated nitrogen (Jiang et al, 2008). The lack of carbon deposition on the anodes and the improved fuel cell performance leads to the conclusion that Pd promotes the partial oxidation reaction of both methane and ethanol. Further increases in fuel cell performance were noted with the addition of a metal catalyst, cerium oxide and the use of a LSCF–YSZ cathode (Zhu et al., 2010d). The addition of 0.5 wt% palladium and 5 wt% cerium oxide produced the highest maximum power density of 520 mW cm− 2 at 700°C in humidified H2.
Lu et al. (Lu and Zhu, 2007) produced a series of Cu and Pd impregnated LSCM anodes with optimal Cu loading of 20 wt%. Additional loading of 1.5 wt% Pd showed a slight improvement of the maximum power density in dry H2. However, a significant increase was noted for dry CH4. No additional increase in performance was noted for further impregnation of Pd. The maximum power density achieved at 850°C was 890 and 600 mW cm− 2 in dry H2 and dry CH4, respectively. No carbon deposition was observed upon operation, although exposure of the anode to 50 ppm H2S initiated a significant drop in fuel cell performance, attributed to the low sulphur tolerance of LSCM.
Ye et al. (2008) investigated Cu-LSCM-ScSZ composite anodes, achieving poor maximum power densities albeit with good stability when operating with either H2 or a 2:1 mixture of C2H5OH–steam fuel. Grading of the anode
was used to increase the anodic performance, producing a complicated mul- tilayer anode: Cu–LSCM–YSZ/Ni–YSZ/Ni–ScSZ. An increase of ~ 5 × was noted in the maximum power density, reaching 534 and 384 mW cm− 2 at 800°C in H2 and 2:1 C2H5OH–H2O, respectively. Stable operation over 120 h was observed in the ethanol-steam mixture without any observed carbon deposition.
Ni–LSCM–YSZ composite anode has been successfully demonstrated in a single-chamber SOFC. A maximum power density of 285 mWcm− 2 is achieved at a gas composition of CH4:O2 approximate to 2:1 and a furnace temperature of 800°C. The redox cycling test demonstrates that the stability of the LSCM-based anode is better compared to the conventional Ni–YSZ anode (Zhu et al, 2010e).
14.4.2 SrTiO 3-based anode materials
Another material which has been under extensive investigation is stron- tium titanium oxide (STO). In 1997, Slater and Irvine first proposed to use La-doped SrTiO3 (LST) as an anode for SOFCs (Slater et al., 1997). Early research into B-site doping of LST found that niobium doping produced a redox-stable material with a metallic-type electronic conductivity of ~ 7 S cm− 1 at 930°C (Slater et al. 1997), though, as in LST, the catalytic activity for H2 oxidation is not good (Blennow et al, 2009). Research in the last few years has been dedicated to improving the electronic conductivity and the catalytic activity of anode materials based on SrTiO3.
These materials have shown a high sulphur tolerance, resistance to coking and have good chemical and redox stability (Mukundan et al, 2004). The main difficulty with the use of STO is low electronic conductivity in a reducing atmosphere (Balachandran and Eror, 1981).
A-site doping of yttrium in SrTiO3 (forming YST) has been shown to sig- nificantly improve the electronic conductivity, up to 73.7 S cm− 1 at 800°C in forming gas with good thermal stability (Li et al, 2007). Lanthanum substitution on the strontium site (forming LST) was also found to significantly increase the electronic conductivity in a reducing atmosphere, to 16 S cm− 1 at 1000°C. However, the catalytic activity for the oxidation of H2 was unsatisfactory (Marina et al, 2002).
Recent reports have found significantly higher electronic conductivity for Sr0.94Ti0.9Nb01O3 > 120 S cm− 1 at 1000°C in 9% H2/N2; however, these compounds were sintered at high temperature in a reducing atmosphere and had a high pellet density (Blennow et al., 2008). Gross et al. (2009) found similar conductivity values for dense pellets of SrTi0.98Nb0.02O3 sintered under similar conditions. However, the enhanced conductivity was found to be nullified after oxidation at 1200°C.
A composite niobium-doped strontium titanate (SrTi0.99Nb0.01O3) − yttrium-doped zirconate anode demonstrated stable conductivity of ~ 1 S cm− 1 in humidified H2 at 800°C over successive redox cycles. Infiltration of this material with 1 wt% Pd and 3 wt% CeO2 produced an anode material with promising fuel cell performance, 640 mW cm− 2 at 800°C in humidified H2 (Gross et al, 2009). Further A-site doping was investigated by Vincent et al. (2010) with the introduction of Ba to LST. Increases in the amount of Ba were correlated with increases in fuel cell performance in dry hydrogen. However, the opposite effect was observed in dry methane. Introduction of 5000 ppm of H2S was observed to significantly increase the fuel cell performance in both dry H2 and dry CH4. Additionally, exposure to hydrogen sulphide did not have any observable effect on the cell microstructure. Despite all of this, fuel cell performance was universally poor, with a maximum power density of 60 mW cm− 2 at 900°C in 0.5% H2S/H2.
Introduction of A-site deficiency, with maximum deficiency forming (Y0 .08Sr0.92)0.94TiO3, was found to increase the ionic conductivity at the expense of the electronic conductivity (Zhao et al., 2009). The same trend is also found with increasing B-site deficiency in Y0.08Sr0.92Ti1 − xO3, with the maximum deficiency occurring for the x = 0.05 compound (Gao et al, 2008). The introduction of A-site deficiency retained greater electronic conductivity than introduction of B-site deficiency, 73.7 S cm− 1 compared to ~ 35 S cm− 1 at 800°C in a reducing atmosphere, while achieving similar ionic conductivity. Stability testing of both the A-site and B-site deficient compound with YSZ found that no impurity peaks were observed after firing at temperature > 1200°C for 10 h in forming gas. Previous uncertainty over the conductivity of the A-site deficient YST was ascribed to different synthesis methods and the purity of the reagents (Lu et al, 2007).
Further work into A-site and B-site deficient YST was undertaken by Ma et al. (2010), confirming that changing the sintering temperature of the samples did not affect the conductivity of the A-site deficient samples but increased that of the B-site deficient samples. Redox cycling of the samples showed a significant reduction in the conductivity of the B-site deficient samples, with a lesser effect observed for A-site deficiency. As noted in previous research (Fu and Tietz, 2008), the conductivity of A-site deficient samples maintains acceptable electronic conductivity despite the reduction upon successive redox cycles.
Similar trends in ionic and electronic conductivity were found for introduction of A-site deficiency in LST (Li et al., 2010). The electronic conductivity was found to be superior to that found for A-site-deficient YST with the expected consequence of lower ionic conductivity. An additional effect of A-site deficiency was the promotion of pellet densification and an improvement in the thermal stability of the compound. Pre-reduction of the compound was found to enhance the conductivity, albeit only for the first redox cycle. Preparation of a composite anode with A-site-deficient LST, Cu and GDC gave a maximum power density of 550 mW cm− 2 at 750°C in humidified H2 (Savaniu and Irvine, 2010).
Formation of composite anode materials from La-doped STO (LST) and CeO2 exhibited increased fuel cell performance, with maximum power densities of 172.3 mW cm− 2 in H2 and 139.6 mW cm− 2 in C2H6 at 900°C, despite a reduction in the electronic conductivity of the anode (Sun et al, 2008). Incorporation of Ni into an LST–GDC anode was found to significantly increase the fuel cell performance in comparison to the LST–GDC cermet (Yoo and Choi, 2009). The highest performance was found for Ni–50% La0.2Sr0.8TiO3–50% Gd0.2Ce0.8O2 which had a maximum power density of 275 mW cm− 2 at 800°C in humidified H2. Unfortunately, the anodic polarisation resistance was found to significantly increase within 10 h of operation, which was attributed to nickel agglomeration due to the high level of nickel components.
Fu et al. (2007) prepared a composite Ni–YST–YSZ anode with a stable electronic conductivity of ~ 10 S cm− 1 at 800°C in 4% H2/Ar. Impregnation of the nickel into the porous YST–YSZ framework was found to give good dimensional stability upon redox cycling, as the volume change of the nickel was nullified due to homogenous NiO distribution throughout the pores of the framework.
Pd was also investigated for use in LST–YSZ cermets as a promoter of catalytic activity (Kim et al., 2008). Pd and CeO. impregnated LST–YSZ (75:25) composite anodes demonstrated reasonable fuel cell performance, 570 mW cm− 2 at 800°C in humidified H2, despite the low conductivity values (Kim et al, 2008). Further increasing the amount of CeO2 impregnated into the scaffold did not have a significant effect on fuel cell performance, suggesting catalytic performance was not the limiting factor in increasing fuel cell performance.
Further development of strontium titanates by Ruiz-Morales et al. (2006) in 2006 led to the formation of LaoSr8Ti11Mn0.5Ga0.5O38-δ (LSTMG). No changes in OCV or electrode performance were observed upon redox cycling at 850°C and 950°C for 48 h, with maximum power densities of ~500 and 350 mW cm− 2 in wet H2 and wet CH4, respectively (Ruiz-Morales et al, 2007a). Additionally, no carbon deposition was detected after operation in wet CH4. Substitution of Ga by Mn was found to reduce the degree of oxy- gen loss on reduction and, subsequently, reduce the electronic conductivity. The maximum electronic conductivity, 7.9 S cm.' at 900°C in 5% H./Ar, was noted for the pure gallium sample, although the TEC of the 1:1 Mn-Ga sample was closest to that of most SOFC electrolytes (Escudero et al., 2009). The best reported performance of SrTiO3-based anode was 1066 mW cm− 2 at 850°C when H. was used as fuel and 1.5 wt% Pd was impregnated at the anode, with La0.8Sr0.2Ga0.83Mg017O3 − δ and Sm0.5Sr0.5CoO3 − δ as electrolyte and cathode (Fig. 14.4) ' (Lu et al, 2009b).
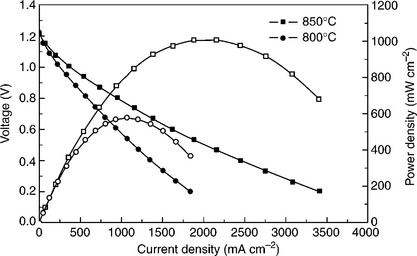
14.4 Voltage and power density of the cell at different temperatures with H2 as the fuel. The anode was 1.5 wt % Pd-Sr0.88Y0.08TiO3–Ce0.6La0.4O2 − δ (Lu et al., 2009b).
14.4.3 Double perovskite-based anode materials
The conductivity and redox stability of double perovksite oxides such as Sr2FeNbO6 (Tao et al., 2004) and Sr2MnNbO6 (Tao and Irvine, 2002b) as potential anode materials for SOFCs have been investigated by Tao and Irvine since 2002. Double perovskites such as Sr.MgMoü6 (SMM) were investigated for anode applications by Huang et al. (2006). Recent research investigated Sr2MgMoO6 and Sr2MnMoO6 and determined that both compounds have electronic conductivities of ~ 10 S cm− 1 in both H2 and CH4 at 800°C, in addition to redox stability and stability to sulphurous impurities (Huang et al., 2006). Fuel cell testing established that the maximum power densities in CH. were 438 and 118 mW cm− 2 for Sr.MgMoO6 and Sr2MnMoO6, respectively (Huang et al, 2006). Huang et al. investigated substitution of Mg in SMM yielding both Sr2NiMoO6 and Sr2CoMoO6 (Huang et al., 2006). The total conductivity for Sr.CoMoO6 was low, ~ 1 S cm− 1 at 800°C in H2. However, fuel cell performance in H2. reached a maximum power density of 735 mW cm− 2. Fuel cell performance in dry methane was significantly lower than that in wet methane, suggesting that the anode is active for internal reforming of methane, although the performance was still lower than in H2. Power densities of 1017 and 634 mW cm− 2 have been achieved at 800°C when H2 or CH4 was used as the fuel, respectively, based on a cell with Sr2CoMoO6 anode, La0.8Sr0.2Ga0.83Mg0.17O2.815/La0.4Ce06O2 − δ as electrolyte, and SrCo0.8Fe02O3 − δ as cathode (Fig. 14.5) (Zhang et al, 2011a). Despite the promising fuel cell results, further research into Sr.MgMoO6 found degradation of the compound at 900°C in a reducing atmosphere (Bernuy-Lopez et al, 2007). Doping with La was found to improve the fuel cell performance, giving a maximum power density of 550 mW cm− 2 at 800°C in wet CH4. However, subsequent research discovered compound degradation upon reoxidation (Ji et al, 2007, Marrero-Lopez et al, 2009).
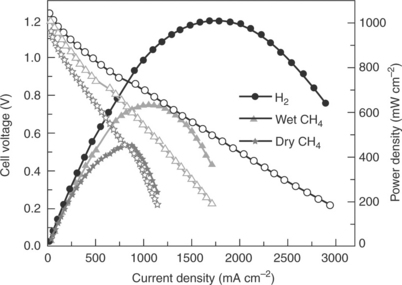
14.5 Power density and cell voltage as functions of current density at 800°C in H2, dry CH4 and wet CH4 for Sr2CoMoO6 − δ anode (Zhang et al., 2011a).
Research by Bernuy-Lopez et al. suggested that SMM may have limited redox stability at temperatures above 900°C (Bernuy-Lopez et al, 2007). This has been refuted by various authors who have suggested that the compound is stable and the RP-phase observed was due to the high-temperature synthesis method (Marrero-López et al, 2008). High chemical reactivity was also observed for other molybdenum-containing compounds, for example La2Mo2O9 electrolyte materials (Marrero-López et al., 2010). Sintering of SMM with YSZ at 1000°C was found to form SrMoO3, SrZrO3 and MgO while sintering with LSGM at 1000°C produced SrMoO4 and (Sr,La)Ga3O7 (Lu et al, 2009a). No significant interfacial reactivity was observed for SMM and GDC and fuel cells using a GDC interlayer showed improved performances.
Zhang et al. (2010) found that Fe substitution in SMMO increased the fuel cell performance. However, the improvement was less than was found for Ni or Co substitution. Substitution of strontium with calcium was found to increase the conductivity of the sample, although degradation of the compound was observed in an N. atmosphere at ~ 400°C. Sr.FeMoO6 had the highest fuel cell performance, 835 mW cm− 2 in dry H2 at 850°C, and had 20 h of stable operation in commercial city gas, which contains impurities of H2S, ammonia and naphthalene (Zhang et al, 2010).
Substitution of Mo in SMM by WVI and NbV was attempted by Vasala et al. (2010a). Substitution with either niobium or tungsten decreased the conductivity in 5% H2/Ar, with greater reductions noticed for tungsten than niobium. All compounds synthesised were found to be redox stable, although the range of oxygen content variation decreased with increased doping. The same authors also investigated the effect of substitution of Mg with various transition metals (Vasala et al., 2010b). Only the compound with pure magnesium content was stable in both oxidising and reducing conditions at temperatures greater than 900°C, with those with Mn or Fe stable in reducing conditions only and those with Co, Ni or Zn stable in oxidising conditions only. These data do not correlate with the previously determined stability of Sr2NiMoO6 (Wei et al, 2008). However, no plausible explanation for this discrepancy could be proffered by the authors.
14.4.4 Other perovskite-based anode materials
Lanthanum strontium cobalt ferrite (LSCF) is widely used as a SOFC cathode material. However, in recent years it has become of interest for anode applications due to its catalytic activity for the electrochemical oxidation of methane (Fisher et al, 2009). As LSCF does not have significantly high ionic conductivity, Haag et al. (2008) produced a composite anode of LSCF and GDC. A maximum power density of 365 mW cm− 2 was achieved at 800°C under humidified H2. LSCF is known to be at the limits of its stability and low conductivity under anodic conditions.
The activity of LSCF cermets with alternative fuels, such as propane and syn-gas, was investigated by Lo Faro et al. (2009a). The fuel cell performance using syn-gas increases with decreasing amounts of LSCF in LSCF–GDC cermets and increases with increasing proportions of carbon monoxide (Huang et al, 2009). When propane was used as a fuel, a maximum of 300 mW cm− 2 was observed for the 7 wt% Ni-impregnated LSCF–GDC cermet. Gradual increases in the fuel cell performance are observed over 100 h of operation, although the anode was found to partially degrade into La2NiO4 and SrFe1 − xCoxO3 (Lo Faro et al, 2009b).
Calcium-doped lanthanum chromite, a common interconnect material, was investigated as a potential symmetrical electrode material due to high electronic conductivity in both oxidising and reducing atmospheres and limited catalytic activity for both the anode and cathode reactions (Ruiz-Morales et al., 2007b). Symmetrical fuel cell performance with La0.7Ca0 3CrO3 as both anode and cathode, with a maximum power density of 110 mW cm− 2 in humidified H2 at 950°C, was underwhelming when compared to the performance of symmetrical LSCM electrodes.
Xu et al. utilised separate electronic conducting, ionic conducting and catalytically active components to form a cermet anode: LaCrO3, YSZ and VOx. respectively (Xu et al., 2007). Fuel cell performance was significantly improved with the addition of CO to the H2 feed gas, with a maximum power density of 190 mW cm− 2 at 850°C in syn-gas. Addition of H2S did not affect fuel cell performance and XRD showed no changes to the anode structure after firing in syn-gas for 24 h at 900°C. Extended fuel cell operation exhib- ited reduction of the maximum power density of the cell over time, with rapid degradation after 10 h at 900°C. Carbon deposition was observed through XPS and is expected to be the cause of the performance degradation.
La0.7Ca0.3CrO3 (LCC)–Ce0.8Gd0.2O1.9 (GDC) composites have also been investigated as symmetrical electrodes for SOFCs on LSGM electrolyte. The electrical conductivity of the LCC-GDC composites decreases with increasing GDC content. The best electrical conductivities of 18.64 S cm− 1 in air and 1.86 S cm− 1 in H2 at 850°C are achieved for an 80 wt% LCC-20 wt% GDC (LCC–GDC20) composite. The maximum power density is 573 mW cm− 2 in dry H2 and 333 mW cm− 2 in humidified commercial city gas containing H2S at 900°C, respectively. These results suggest that the LCC–GDC20 composite can potentially serve as an electrode for symmetrical SOFCs operated on H2 and commercial city gas containing H2S (Zhang et al, 2011b). The typical fuel cell performances of SOFCs based on perovskite anode are listed in Table 14.2.
14.5 Other oxide anode materials
Both pyrochlore and tungsten-bronze-structured compounds were the focus of investigations into suitability for utilisation as an anode material. A series of tungsten-bronze-structured niobates, (Ba, Sr, La, Ca)06Nb1 − xMxO3, M = Ti, Na, Mg (Slater and Irvine, 1999a, 1999b), were investigated in 1999 with conductivities of less than 5 S cm− 1. B-site doping increased the electronic conductivity, although these compounds suffered from either poor oxygen exchange kinetics or poor redox stability. The most promising compound, Sr0.2Ba0.4Ti0.2Nb0.8O3, was found to have a conductivity of 10 S cm− 1 at 930°C with pO2 of 10–20 bar, however an interfacial reaction was observed with YSZ (Kaiser et al., 2000).
Gadolinium titanate was firstly investigated as an electrolyte material for SOFCs due to its high ionic conductivity (Kramer and Tuller, 1995). Mo doping was used to increase the electronic conductivity although this also introduced redox instability (Porat et al., 1997). Replacement of Mo with Mn increased both the electronic conductivity and redox stability (Sprague and Tuller, 1999). Holtappels et al. (2000) found that Gd.TiMoO7 did not exhibit an interfacial reaction with YSZ.
No further development of these pyrochlore or tungsten-bronze-struc- tured compounds has been attempted due to either poor redox stability or low electronic conductivity.
In our recent research, it was found that Sr- and Ca-doped CeVO4 are redox stable at temperatures below 600°C although their electronic con- ductivity is not high enough to be used as SOFC anodes (Petit et al, 2010a, 2010b). However, at higher temperature, it was found that Ce0.85Sr0.15VO4 + δ can be reduced to Ce0.85Sr0.15VO3 − δ and this reaction is reversible under a successive redox process (Petit et al, 2011a). Similar behaviour was found in Ce0.8Ca0.2VO4 + δ (Petit et al, 2011b). To use a redox reversible oxide such as Ce85Sr0.15VO4 + δ could be a new approach to develop efficient SOFC anodes. Co2V2O7 (Cowin et al, 2011a) and FeVO4 (Cowin et al, 2011b) were also investigated as potential SOFC anode but it was found that they are unstable in a reducing atmosphere therefore are not suitable candidates.
14.6 Non-oxide anode materials
As much of the research in this area was focused on development of perovskite and fluorite materials, minimal research was conducted into prospective alternative anode structures.
Sulphurous impurities, mainly in the form of H2 S, have been found to degrade anode materials through the formation of poorly conducting metal sulphides, as some oxides can react with sulphur to form sulphides which causes performance degradation. One possible solution is to develop anodes based on conductive sulphides. Xu et al. (2009) developed an anode catalyst for removal of the H2S in syn-gas, formed of gold nanoparticles deposited on a MoS2 framework with 10% Ag. The presence of Au was necessary to prevent CO poisoning of the MoS2 catalyst. When utilised as an anode material for SOFCs with syn-gas fuel, the maximum power density observed was 75 mW cm− 2 at 900°C. The performance dropped to 58 mW cm− 2 when H2S- containing H2 was used as the fuel, suggesting that CO was also utilised at the anode. Operation without Au was found to slightly decrease fuel cell performance in H2S-containing H2 but caused a significant decrease when used with syn-gas, caused by CO poisoning of MoS2. Degradation of fuel cell performance was noted on extended operation with Au-containing anodes, which was attributed to sintering of the Au nanoparticles.
Previous work by Vorontsov et al. (2008a) utilised nickel and molybdenum sulphides for operation of SOFCs with H2S fuel. Oxidation of MoS2, occurring at 450°C, was found to cause problems for MoS2-rich anodes due to volatility above 600°C. Addition of Ni3 ± xS2 was found to suppress MoS2 volatility by preventing MoO3 formation. Initial anode performance was promising for an anode composition of 1:1 ratio of MoS2 to Ni3 ± xS2, reaching 300 mW cm− 2 at 850°C. Severe degradation of anode performance was noted for all composi- tions, albeit the decline in performance was less for those anodes with greater MoS2 content. Anode degradation was ascribed to nickel sulphide agglomeration, reducing the catalytically active surface area in the anode.
Various metal vanadium sulphides were tested for operation with H.S fuel, with MoV2S4 found to have the lowest polarisation resistance and fin- est microstructure (Vorontsov et al, 2008b). Under pure H2S fuel, a maximum power density of 50 mW cm− 2 was observed for a MoV2S4–YSZ/YSZ/Pt fuel cell at 800°C, an improvement on previous MoS2:Ni3 ± xS2 anodes at this temperature. Stable performance of the fuel cell was observed for three days with XRD and SEM of the material after operation demonstrating chemical and micro-structural stability. As most sulphides are unstable in air at high temperatures, normally the anode precursors are oxides and sulphides were in situ formed between the oxide and H2S in the fuel. To date, high performances have not been achieved based on sulphide anodes.
Further work is also continuing on the development of liquid tin anodes, first proposed in 1998, for direct use of carbon fuels (Tao et al, 2007a, 2007b), however these anodes still suffer from poor power density and fuel efficiency (Tao et al, 2008). Jayakumar et al. (2010) examined the use of either Sn or Bi as an anode at 700-800°C with YSZ/YSZ–LSCF cells. The cell was operated in battery mode for these tests, without fuel at the anode side, to complete oxidation of the anode. To some extent, this is more close to a battery instead of a SOFC as the Sn or Bi cannot be continuously fed to the anode for extended duration operation.
14.7 Poisoning of solid oxide fuel cell anode materials
Real hydrocarbon fuels such as natural gas, coal gas and biogases always contain impurities such as sulphur, phosphor, chloride and arsenic (Shiratori et al., 2008). To develop impurity-tolerant anodes is very important for operation of SOFCs with real fuels. There are some reports on the effects of impurities such as H2S (Kuhn et al, 2008; Rasmussen and Hagen, 2009), HCl (Trembly et al, 2007b), H3As (Trembly et al, 2007a) and H3P (Zhi et al, 2008; Martinez et al., 2010) on conventional Ni-based anodes when using coal gas as the fuel in SOFCs. Almost all impurities have severe negative effects on the Ni-based anode and fuel cell performance due to the reaction between nickel and these impurities. However, oxide anodes may be a better choice compared to Ni-based cermets. Kurokawa et al. (2007a) found that the impregnation of Ni–YSZ with nanoparticles of ceria rendered the former much more stable to sulphur-containing hydrogen as the initial cell voltage could almost be fully recovered by exposing the cell to sulphur-free hydrogen after 500 h of operation under poisonous conditions.
Redox stable oxides such as La0.75Sr0.25Cr0.5Mn0.5O3 (Tao and Irvine, 2003) are promising alternative anodes for SOFCs. In recent developments, performance results of 1100 mW cm− 2 have been achieved using humidi- fied (3% H2O) H2 at 800°C and 710 mW cm− 2 using methane, based on a redox-stable La0.75Sr0.25Cr0.5Mn0.5O3 anode (Kim et al., 2008). However, it was found that LSCM can react with sulphurous impurities (10% H2S) to form MnS, La2O2S and o-MnOS (Zha et al, 2005). The stability of LSCM at lower concentration of H2S is not clear yet. Gong et al. (2010) investigated the stability of LSCF–GDC composites to phosphine-containing fuels and observed a significant degradation in anode performance upon exposure. Phosphine was found to react with iron and lanthanum in the anode, form- ing FePx and LaPO4, and degradation was calculated to occur at 800°C at PH3 levels as low as 1 ppm. The reaction was irreversible, despite flushing of the anode with non-phosphine containing fuels.
Kurokawa et al. (2007b) produced Ru–CeO2–50% Sr0.88Y0.08TiO3–50% Y0.08Zr0.92O2 which gave maximum power densities of 510 mW cm− 2 in humidified H2 at 800°C. A slight drop in cell voltage, 0.01 V, was observed upon exposure of the cell to 10 ppm H2S in H2; however, this was entirely reversible. The maximum power density dropped to 470 mW cm− 2 upon exposure to 10 ppm H2S in H2 at 800°C, although this was again found to be reversible upon removal of the H2S.
Lu et al. (2009a) demonstrated even further improvement in fuel cell performance by incorporation of Pd into a YST–LDC cermet, exhibiting maximum power densities of 1006 and 577 mW cm− 2 in dry H. at 850°C and 800°C, respectively. Further testing showed no observable anode degrada- tion when operating with 50 ppm H2S/H2 fuel, with any reduction in performance attributed to cathodic degradation.
In terms of sulphur tolerance, La.1− xSrxTiO3-based anode materials are a better choice. It was observed that the presence of H.S in the fuel cell can enhance the fuel cell performance no matter whether CH4 or H2 were used as fuel when lanthanum strontium titanate (La0.4Sr0.6 − xBaxTiO3,0 < x < 0.2; LST, x = 0; LSBT, x > 0) was used as the anode (Vincent et al, 2010). Enhanced coking and sulphur tolerance was also observed when a mixed H+/e' conductor, BaZr0.1Ce0.7Y02 − xYbxO3 − δ was used as a part of a composite anode (Yang et al, 2009).
Redox reversible oxides such as Ce0.85Sr0.l5VO4 + δ could be a potential sulphur-tolerant anode for SOFCs. Peng et al. (2009) reported on the fuel cell performance and stability of strontium-doped lanthanum vanadate. The maximum power density of a La0.7Sr0.3VO3/YSZ/Pt cell was determined to be ~ 140 mW cm− 2 at 850°C in syn-gas. After fuel cell operation for 24 h in syn-gas at 900°C no carbon or sulphur deposition was observed, although performance was seen to degrade by 1.3% h− 1, which was attributed to anode coarsening. Taking account of the redox reversibility between orthovanadate to metavanadate, this is promising for the development of efficient sulphur and other impurity-tolerant anodes based on vanadates. Further research into sulphur-tolerant SOFC anode materials proceeded with the investigation of Ce0.9Sr0.1VO3 (Danilovic et al, 2009). Stability testing in 0.5% H2S/CH4 for 24 h at 950°C revealed no structural changes in the anode, how- ever carbon deposition through methane cracking was observed.
Substitution of Ce0.9Sr0.1VO3 with Cr3 +, forming Ce0.9Sr0.1Cr0.5V0.5O3 (CSCV), was found to increase the fuel cell performance (Danilovic et al, 2009). The maximum power density for CSCV at 850°C with 0.5% H2S/N2 was 75 mW cm− 2, while Ce0.9Sr0.1VO3 only reached ~ 55 mW cm− 2 at 950°C. Further increases in fuel cell performance with methane were achieved through the formation of Ni/CSCV/YSZ cermets. However, much of the fuel cell performance was still due to H2S conversion.
Addition of oxidation catalysts such as CeO2 in a composite anode can help to improve sulphur tolerance. Oxide containing Mn, Fe, Co, Ni and Cu normally exhibits good catalytic activities to various fuels due to the change of oxidation states which may form oxygen vacancies to be used as catalytically active sites for anode reactions. However, oxides contain- ing these elements are very active and tend to react with impurities to form sulphides or phosphides. This will lead to loss of catalytic activity and increase anode polarisation resistance. As sulphur is very reactive, to avoid the formation of sulphide, perovskite oxides with strong metal ions such as Ti, V, Cr at the B-sites are suitable anode for sulphur-tolerant anode. The strategy to develop a good redox-stable anode is to combine stable elements such as Ti, Cr, V, Mo, Nb and less stable elements such as Mn, Fe, Co, Ni, Cu at the B-sites in an oxide. A successful example is Sr2FeMoO6 which exhibits stable performance in the presence of H.S, ammonia and naphthalene (Zhang et al, 2010) although the redox stability of Sr2 FeMoO6 is poor.
14.8 Conclusions and future trends
In recent years there has been good progress in the area of SOFC anode materials. Anode materials without carbon deposition and sulphur poisoning are being developed with great success in both cermet and oxide based composite materials. Recent improvements with the incorporation of ceria and metal catalysts, typically copper, have had encouraging results.
Many of the recent materials developed have the ideal combination of high electronic and ionic conductivity and good chemical stability. The development of composite perovskite and fluorite cermets is especially promising, with some performances reaching ~ 1 w cm− 2.
Research into the direct use of methane as the fuel has been widely reported. However, research into the direct use of other hydrocarbons, liquid metal anodes, and hydrogen sulphide fuels is at an early stage but appears to provide wide scope for alternative research routes.
The operating temperatures of most of the developed redox-stable anode materials are above 700°C. It is believed that we should focus on developing redox-stable anodes for intermediate temperature (below 700°C) SOFCs.
14.9 References
Atkinson, A., Barnett, S., Gorte, R., Irvine, J., Mcevoy, A., Mogensen, M., Singhal, S., Vohs, J. Advanced anodes for high-temperature fuel cells. Nature Materials. 2004; 3:17–27.
Backhaus-Ricoult, M. SOFC-A playground for solid state chemistry. Solid State Sciences. 2008; 10:670–688.
Balachandran, U., Eror, N. Electrical conductivity in strontium titanate. Journal of Solid State Chemistry. 1981; 39:351–359.
Barison, S., Fabrizio, M., Mortalo, C., Antonucci, P., Modafferi, V., Gerbasi, R. Novel Ru/La0.75Sr0.25Cr0.5Mn0.5O3.δ catalysts for propane reforming in IT-SOFCs. Solid State Ionics. 2010; 181:285–291.
Bastidas, D.M., Tao, S.W., Irvine, J.T.S. A symmetrical solid oxide fuel cell demonstrating redox stable perovskite electrodes. Journal of Materials Chemistry. 2006; 16:1603–1605.
Baur, E., Preis, H. Uber Brennstoff-ketten mit Festleitern. Zeitschrift für Elektrochemie und angewandte physikalische Chemie. 1937; 43:727–732.
Benyoucef, A., Klein, D., Coddet, C., Benyoucef, B. Development and characterisation of (Ni, Cu, Co)–YSZ and Cu–Co–YSZ cermets anode materials for SOFC application. Surface and Coatings Technology. 2008; 202:2202–2207.
Bernuy-Lopez, C., Allix, M., Bridges, C., Claridge, J., Rosseinsky, M. Sr2MgMoO6_δ: Structure, phase stability, and cation site order control of reduction. Chemistry of Materials. 2007; 19:1035–1043.
Bi, Z.H., Zhu, J.H. Cu1-xPdx/CeO2-impregnated cermet anodes for direct oxidation of methane in LaGaO3-electrolyte solid oxide fuel cells. Journal of Power Sources. 2010; 195:3097–3104.
Blennow, P., Hagen, A., Hansen, K.K., Wallenberg, L.R., Mogensen, M. Defect and electrical transport properties of Nb-doped SrTiO3. Solid State Ionics. 2008; 179:2047–2058.
Blennow, P., Hansen, K., Wallenberg, L., Mogensen, M. Electrochemical characterization and redox behavior of Nb-doped SrTiO3. Solid State Ionics. 2009; 180:63–70.
Busawon, A.N., Sarantaridis, D., Atkinson, A. Ni infiltration as a possible solution to the redox problem of SOFC anodes. Electrochemical and Solid State Letters. 2008; 11:B186–B189.
Cassidy, M., Lindsay, G., Kendall, K. The reduction of nickel–zirconia cermet anodes and the effects on supported thin electrolytes. Journal of Power Sources. 1996; 61:189–192.
Chen, X., Liu, Q., Khor, K., Chan, S. High-performance (La, Sr)(Cr, Mn) O3/(Gd, Ce)O2-δ composite anode for direct oxidation of methane. Journal of Power Sources. 2007; 165:34–40.
Chen, X.J., Liu, Q.L., Chan, S.H., Brandon, N.P., Khor, K.A. Sulfur tol- erance and hydrocarbon stability of La0.75Sr0.25Cr0.5Mn0.5O3/Gd0.2Ce0.8O1.9 composite anode under anodic polarization. Journal of the Electrochemical Society. 2007; 154:B1206–B1210.
Cimenti, M., Hill, J. Direct utilization of methanol and ethanol in solid oxide fuel cells using Cu-Co (Ru)/Zr0.35Ce0.65O2.δ anodes. Journal of Power Sources. 2010; 195:3996–4001.
Coors, W. Protonic ceramic fuel cells for high-efficiency operation with methane. Journal of Power Sources. 2003; 118:150–156.
Cowin, P.I., Lan, R., Petit, C.T.G., Zhang, L., Tao, S.W. Conductivity and stability of cobalt pyrovanadate. Journal of Alloys and Compounds. 2011; 509:4117–4121.
Cowin, P.I., Lan, R., Zhang, L., Petit, C.T.G., Kraft, A., Tao, S.W. Study on conductivity and redox stability of iron orthovanadate. Materials Chemistry and Physics. 2011; 126:614–618.
Danilovic, N., Luo, J., Chuang, K., Sanger, A. Ce0.9Sr0.1VOx (x = 3, 4) as anode materials for H2S-containing CH4 fueled solid oxide fuel cells. Journal of Power Sources. 2009; 192:247–257.
Ding, J., Liu, J., Guo, W. Fabrication and study on Ni1-xFexO-YSZ anodes for intermediate temperature anode-supported solid oxide fuel cells. Journal of Alloys and Compounds. 2009; 480:286–290.
Escudero, M., Irvine, J., Daza, L. Development of anode material based on La-substituted SrTiO3 perovskites doped with manganese and/or gallium for SOFC. Journal of Power Sources. 2009; 192:43–50.
Faes, A., Nakajo, A., Hessler-Wyser, A., Dubois, D., Brisse, A., Modena, S. RedOx study of anode-supported solid oxide fuel cell. Journal of Power Sources. 2009; 193:55–64.
Fisher, I., James, C., Chuang, S. Investigating the CH4 reaction pathway on a novel LSCF anode catalyst in the SOFC. Catalysis Communications. 2009; 10:772–776.
Fu, Q.X., Tietz, F. Ceramic-based anode materials for improved redox cycling of solid oxide fuel cells. Fuel Cells. 2008; 8:283–293.
Fu, Q.X., Tietz, F., Sebold, D., Tao, S.W., Irvine, J.T.S. An efficient ceramic- based anode for solid oxide fuel cells. Journal of Power Sources. 2007; 171:663–669.
Gao, F., Zhao, H., Li, X., Cheng, Y., Zhou, X., Cui, F. Preparation and elec- trical properties of yttrium-doped strontium titanate with B-site deficiency. Journal of Power Sources. 2008; 185:26–31.
Gong, M., Bierschenk, D., Haag, J., Poeppelmeier, K., Barnett, S., Xu, C., Zondlo, J., Liu, X. Degradation of LaSr2Fe2CrO9-δsolid oxide fuel cell anodes in phosphine-containing fuels. Journal of Power Sources. 2010; 195:4013–4021.
Gorte, R.J., Park, S., Vohs, J.M., Wang, C.H. Anodes for direct oxidation of dry hydrocarbons in a solid-oxide fuel cell. Advanced Materials. 2000; 12:1465–1469.
Grgicak, C., Pakulska, M., O'brien, J., Giorgi, J. Synergistic effects of Ni1-xCox- YSZ and Ni1-xCux-YSZ alloyed cermet SOFC anodes for oxidation of hydrogen and methane fuels containing H2S. Journal of Power Sources. 2008; 183:26–33.
Gross, M.D., Carver, K.M., Deighan, M.A., Schenkel, A., Smith, B.M., Yee, A.Z. Redox stability of SrNbxTi1-xO3–YSZ for use in SOFC anodes. Journal of The Electrochemical Society. 2009; 156:B540–B545.
Haag, J.M., Madsen, B.D., Barnett, S.A., Poeppelmeier, K.R. Application of LaSr2Fe2CrO9-δ in solid oxide fuel cell anodes. Electrochemical and Solid- State Letters. 2008; 11:B51–B53.
Haslam, J., Pham, A., Chung, B., Dicarlo, J., Glass, R. Effects of the use of pore formers on performance of an anode supported solid oxide fuel cell. Journal of the American Ceramic Society. 2005; 88:513–518.
Hibino, T., Hashimoto, A., Yano, M., Suzuki, M., Sano, M. Ru-catalyzed anode materials for direct hydrocarbon SOFCs. Electrochimica Acta. 2003; 48:2531–2537.
Holtappels, P., Poulsen, F., Mogensen, M. Electrical conductivities and chemical stabilities of mixed conducting pyrochlores for SOFC applications. Solid State Ionics. 2000; 135:675–679.
Huang, B., Wang, S., Liu, R., Ye, X., Nie, H., Sun, X., Wen, T. Performance of La0.75Sr0.25Cr0.5Mn0.5O3-δ perovskite-structure anode material at lanthanum gallate electrolyte for IT-SOFC running on ethanol fuel. Journal of Power Sources. 2007; 167:39–46.
Huang, T., Chou, C., Chen, W., Huang, M. Coal syngas reactivity over Ni-added LSCF-GDC anode of solid oxide fuel cells. Electrochemistry Communications. 2009; 11:294–297.
Huang, Y., Dass, R., Xing, Z., Goodenough, J. Double perovskites as anode materials for solid-oxide fuel cells. Science. 2006; 312:254–257.
Ishihara, T., Matsuda, H., Takita, Y. Doped LaGaO3 perovskite-type oxide as a new oxide ionic conductor. Journal of the American Chemical Society. 1994; 116:3801–3803.
Iwahara, H., Esaka, T., Uchida, H., Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ionics. 1981; 3–4:359–363.
Iwata, T. Characterization of N-YSZ anode degradation for substrate- type solid oxide fuel cells. Journal of the Electrochemical Society. 1996; 143:1521–1525.
Jardiel, T., Caldes, M., Moser, F., Hamon, J., Gauthier, G., Joubert, O. New SOFC electrode materials: The Ni-substituted LSCM-based compounds (La0.75Sr0.25)(Cr0.5Mn0.5-xNix)O3-δ and (La0.75Sr0.25)(Cr0. 5-xNixMn0.5)O3-δ. Solid State Ionics. 2010; 181:894–901.
Jayakumar, A., Lee, S., Hornes, A., Vohs, J.M., Gorte, R.J. A comparison of molten Sn and Bi for solid oxide fuel cell anodes. Journal of the Electrochemical Society. 2010; 157:B365–B369.
Ji, Y., Huang, Y.H., Ying, J.R., Goodenough, J.B. Electrochemical performance of La-doped Sr2MgMoO6-δ in natural gas. Electrochemistry Communications. 2007; 9:1881–1885.
Jiang, S.P., Ye, Y., He, T., Ho, S.B., Jiang, S.P., Ye, Y., He, T., Ho, S.B. Nanostructured palladium- La0.75Sr0.25Cr0.5Mn0.5O3/Y2O3–ZrO2 composite anodes for direct methane and ethanol solid oxide fuel cells. Journal of Power Sources. 2008; 185:179–182.
Kaiser, A., Bradley, J.L., Slater, P.R., Irvine, J.T.S. Tetragonal tungsten bronze type phases (Sr1-xBax)0.6Ti0.2Nb0.8O3-δ: Material characterisation and performance as SOFC anodes. Solid State Ionics. 2000; 135:519–524.
Kim, G., Corre, G., Irvine, J., Vohs, J., Gorte, R. Engineering composite oxide SOFC anodes for efficient oxidation of methane. Electrochemical and Solid-State Letters. 2008; 11:B16–B19.
Kim, S., Moon, H., Hyun, S., Moon, J., Kim, J., Lee, H. Performance and durability of Ni-coated YSZ anodes for intermediate temperature solid oxide fuel cells. Solid State Ionics. 2006; 177:931–938.
Kobsiriphat, W., Madsen, B., Wang, Y., Marks, L., Barnett, S. La0.8Sr0.2Cr1, xRuxO3_5-Gda1Cea9OL95 solid oxide fuel cell anodes: Ru precipitation and electrochemical performance. Solid State Ionics. 2009; 180:257–264.
Kobsiriphat, W., Madsen, B., Wang, Y., Shah, M., Marks, L., Barnett, S. Nickel- and ruthenium-doped lanthanum chromite anodes: Effects of nanoscale metal precipitation on solid oxide fuel cell performance. Journal of the Electrochemical Society. 2010; 157:B279.
Kramer, S.A., Tuller, H.L. A novel titanate-based oxygen ion conductor: Gd2Ti2O7. Solid State Ionics. 1995; 82:15–23.
Kreuer, K. Proton-conducting oxides. Annual Review of Materials Research. 2003; 33:333–359.
Kuhn, J., Lakshminarayanan, N., Ozkan, U. Effect of hydrogen sulfide on the catalytic activity of Ni-YSZ cermets. Journal of Molecular Catalysis A: Chemical. 2008; 282:9–21.
Kuroda, K., Hashimoto, I., Adachi, K., Akikusa, J., Tamou, Y., Komada, N., Ishihara, T., Takita, Y. Characterization of solid oxide fuel cell using doped lanthanum gallate. Solid State Ionics. 2000; 132:199–208.
Kurokawa, H., Sholklapper, T., Jacobson, C., DE Jonghe, L., Visco, S. Ceria nanocoating for sulfur tolerant Ni-based anodes of solid oxide fuel cells. Electrochemical and Solid-State Letters. 2007; 10:B135–B138.
Kurokawa, H., Yang, L., Jacobson, C., DE Jonghe, L., Visco, S. Y-doped SrTiO3 based sulfur tolerant anode for solid oxide fuel cells. Journal of Power Sources. 2007; 164:510–518.
Laurencin, J., Delette, G., Morel, B., Lefebvre-Joud, F., Dupeux, M. Solid oxide fuel cells damage mechanisms due to Ni-YSZ re-oxidation: Case of the anode supported cell. Journal of Power Sources. 2009; 192:344–352.
Li, X., Zhao, H., Shen, W., Gao, F., Huang, X., Li, Y., Zhu, Z. Synthesis and properties of Y-doped SrTiO3 as an anode material for SOFCs. Journal of Power Sources. 2007; 166:47–52.
Li, X., Zhao, H., Zhou, X., Xu, N., Xie, Z., Chen, N. Electrical conductiv- ity and structural stability of La-doped SrTiO3 with A-site deficiency as anode materials for solid oxide fuel cells. International Journal of Hydrogen Energy. 2010; 35:7913–7918.
Lo Faro, M., La Rosa, D., Nicotera, I., Antonucci, V., ARIC, A. Electrochemical behaviour of propane-fed solid oxide fuel cells based on low Ni content anode catalysts. Electrochimica Acta. 2009; 54:5280–5285.
Lo Faro, M., La Rosa, D., Nicotera, I., Antonucci, V., Aric, A. Electrochemical investigation of a propane-fed solid oxide fuel cell based on a composite Ni-perovskite anode catalyst. Applied Catalysis B: Environmental. 2009; 89:49–57.
Lu, X., Pine, T., Mumm, D., Brouwer, J. Modified Pechini synthesis and characterization of Y-doped strontium titanate perovskite. Solid State Ionics. 2007; 178:1195–1199.
Lu, X., Zhu, J., Yang, Z., Xia, G., Stevenson, J. Pd-impregnated SYT/LDC composite as sulfur-tolerant anode for solid oxide fuel cells. Journal of Power Sources. 2009; 192:381–384.
Lu, X.C., Zhu, J.H. Cu (Pd)-impregnated La0.75Sr0.25Cr0.5Mn0.5O3-δ anodes for direct utilization of methane in SOFC. Solid State Ionics. 2007; 178:1467–1475.
Lu, X.C., Zhu, J.H., Yang, Z., Xia, G., Stevenson, J.W. Pd-impregnated SYT/LDC composite as sulfur-tolerant anode for solid oxide fuel cells. Journal of Power Sources. 2009; 192:381–384.
Lu, Z.G., Zhu, J.H., Bi, Z.H., Lu, X.C. A Co-Fe alloy as alternative anode for solid oxide fuel cell. Journal of Power Sources. 2008; 180:172–175.
Ma, Q., Tietz, F., ST Ver, D. Nonstoichiometric Y-substituted SrTiO3 materials as anodes for solid oxide fuel cells. Solid State Ionics. 2010; 192:535–539.
Madsen, W.K.B.D., Marks, L.D., Wang, Y., Barnett, S.A. 10th International Symposium on Solid Oxide Fuel Cells, ECS Proc. Series, Pennington, NJ, 2007. [Madsen, B., Kobsiriphat, W., Marks, L., Wang,Y. and Barnett, S. 10th International Symposium on Solid Oxide Fuel Cells. ECS Proc. Series, 2007, Pennington, NJ].
Mantzouris, X., Triantafyllou, G., Tietz, F., Nikolopoulos, P. Physical char- acterization of Y2O3-CeO2-TiO2 (YCT) mixed oxides and Ni/YCT cermets as anodes in solid oxide fuel cells. Journal of Materials Science. 2008; 43:7057–7065.
Mantzouris, X., Zouvelou, N., Haanappel, V., Tietz, F., Nikolopoulos, P. Mixed conducting oxides YxZr1-x-yTiyO2-x/2 (YZT) and corresponding Ni/YZT cermets as anode materials in an SOFC. Journal of Materials Science. 2007; 42:10152–10159.
Marina, O., Canfield, N., Stevenson, J. Thermal, electrical, and electrocata- lytical properties of lanthanum-doped strontium titanate. Solid State Ionics. 2002; 149:21–28.
Marrero-López, D., PE A-Martínez, J., Ruiz-Morales, J., Pérez-Coll, D., Aranda, M., Núñez, P. Synthesis, phase stability and electrical conductivity of Sr2MgMoO6-[delta] anode. Materials Research Bulletin. 2008; 43:2441–2450.
Marrero-López, D., Pena-Martínez, J., Ruiz-Morales, J., Gabas, M., Aranda, M., Ramos-Barrado, J. Redox behaviour, chemical compatibility and electro- chemical performance of Sr2MgMoO6- as SOFC anode. Solid State Ionics. 2010; 180:1672–1682.
Marrero-Lopez, D., Pena-Martinez, J., Ruiz-Morales, J., Martin-Sedeno, M., Nunez, P. High temperature phase transition in SOFC anodes based on Sr2MgMoO6-δ. Journal of Solid State Chemistry. 2009; 182:1027–1034.
Martinez, A., Gerdes, K., Gemmen, R., Poston, J. Thermodynamic analysis of interactions between Ni-based solid oxide fuel cells (SOFC) anodes and trace species in a survey of coal syngas. Journal of Power Sources. 2010; 195:5206–5212.
Matsuzaki, Y., Yasuda, I. The poisoning effect of sulfur-containing impurity gas on a SOFC anode: Part I. Dependence on temperature, time, and impurity concentration. Solid State Ionics. 2000; 132:261–269.
Mcintosh, S., Vohs, J.M., Gorte, R.J. Role of hydrocarbon deposits in the enhanced performance of direct-oxidation SOFCs. Journal of The Electrochemical Society. 2003; 150:A470–A476.
Meng, G.Y., Jiang, C., Ma, J., Ma, Q., Liu, X. Comparative study on the performance of a SDC-based SOFC fueled by ammonia and hydrogen. Journal of Power Sources. 2007; 173:189–193.
Minh, N. Ceramic fuel cells. Journal of the American Ceramic Society. 1993; 76:563–588.
Moèbius, H. On the history of solid electrolyte fuel cells. Journal of Solid State Electrochemistry. 1997; 1:2–16.
Mukundan, R., Brosha, E., Garzon, F. Sulfur tolerant anodes for SOFCs. Electrochemical and Solid-State Letters. 2004; 7:A5.
Murray, E.P., Tsai, T., Barnett, S.A. A direct-methane fuel cell with a ceriabased anode. Nature. 1999; 400:649–651.
Nabae, Y., Yamanaka, I. Alloying effects of Pd and Ni on the catalysis of the oxidation of dry CH4 in solid oxide fuel cells. Applied Catalysis A: General. 2009; 369:119–124.
Nikooyeh, K., Clemmer, R., Alzate-Restrepo, V., Hill, J. Effect of hydro- gen on carbon formation on Ni/YSZ composites exposed to methane. Applied Catalysis A: General. 2008; 347:106–111.
Oøhayre, R., Barnett, D.M., Prinz, F.B. The triple phase boundary. Journal of the Electrochemical Society. 2005; 152:A439–A444.
Park, S.D., Vohs, J.M., Gorte, R.J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature. 2000; 404:265–267.
Peng, C., Luo, J., Sanger, A., Chuang, K. Sulfur-tolerant anode catalyst for solid oxide fuel cells operating on H2S-containing syngas. Chemistry of Materials. 2009; 22:1032–1037.
Petit, C.T.G., Lan, R., Cowin, P.I., Irvine, J.T.S., Tao, S.W. Novel redox reversible oxide, Sr-doped cerium orthovanadate to metavanadate. Journal of Materials Chemistry. 2011; 21:525–531.
Petit, C.T.G., Lan, R., Cowin, P.I., Irvine, J.T.S., Tao, S.W. Structure, conductivity and redox reversibility of Ca-doped cerium metavanadate. Journal of Materials Chemistry. 2011; 21:8854–8861.
Petit, C.T.G., Lan, R., Cowin, P.I., Kraft, A., Tao, S.W. Structure, conductivity and redox stability of solid solution Ce1-xCaxVO4 (0 < x < 0.4125). Journal of Materials Science. 2010; 46:1–11.
Petit, C.T.G., Lan, R., Cowin, P.I., Tao, S.W., Petit, C.T.G., Lan, R., Cowin, P.I., Tao, S.W. Structure and conductivity of strontium-doped cerium orthovanadates Ce1-xSrxVO4 (0 < x < 0.175). Journal of Solid State Chemistry. 2010; 183:1231–1238.
Porat, O., Heremans, C., Tuller, H.L. Stability and mixed ionic electronic con- duction in Gd2(Ti1-xMox)2O7 under anodic conditions. Solid State Ionics. 1997; 94:75–83.
Qiao, J., Sun, K., Zhang, N., Sun, B., Kong, J., Zhou, D. Ni/YSZ and Ni-CeO2/YSZ anodes prepared by impregnation for solid oxide fuel cells. Journal of Power Sources. 2007; 169:253–258.
Rasmussen, J.F.B., Hagen, A. The effect of H2S on the performance of Ni-YSZ anodes in solid oxide fuel cells. Journal of Power Sources. 2009; 191:534–541.
Ruiz-Morales, J.C., Canales-Vazquez, J., Savaniu, C., Marrero-Lopez, D., Nunez, P., Zhou, W., Irvine, J.T.S. A new anode for solid oxide fuel cells with enhanced OCV under methane operation. Physical Chemistry Chemical Physics. 2007; 9:1821–1830.
Ruiz-Morales, J.C., Canales-Vazquez, J., Savaniu, C., Marrero-Lopez, D., Zhou, W., Irvine, J. Disruption of extended defects in solid oxide fuel cell anodes for methane oxidation. Nature. 2006; 439:568–571.
Ruiz-Morales, J. C., Lincke, H., Marrero-Lopez, D., Canales-Vazquez, J. and Nunez, P. 2007b. Lanthanum chromite materials as potential symmetrical electrodes for solid oxide fuel cells. Bolet®™n de la Sociedad Espa ola de Cer®(mica y Vidrio, 46, 218.
Sakamoto, Y., Shikazono, N., Kasagi, N. Effects of electrode microstructure on polarization characteristics of SOFC anodes. Proc. ASME Fuel Cell. 2008; 2008:1–6.
Savaniu, C., Irvine, J. La-doped SrTiO3 as anode material for IT-SOFC. Solid State Ionics. 2010; 192:491–493.
Shiratori, Y., Oshima, T., Sasaki, K. Feasibility of direct-biogas SOFC. International Journal of Hydrogen Energy. 2008; 33:6316–6321.
Slater, P., Fagg, D., Irvine, J. Synthesis and electrical characterisation of doped perovskite titanates as potential anode materials for solid oxide fuel cells. Journal of Materials Chemistry. 1997; 7:2495–2498.
Slater, P.R., Irvine, J.T.S. Niobium based tetragonal tungsten bronzes as potential anodes for solid oxide fuel cells: Synthesis and electrical characterisation. Solid State Ionics. 1999; 120:125–134.
Slater, P.R., Irvine, J.T.S. Synthesis and electrical characterisation of the tetragonal tungsten bronze type phases, (Ba/Sr/Ca/La)0.6MxNb1.xO3-δ (M = Mg, Ni, Mn, Cr, Fe, In, Sn): evaluation as potential anode materials for solid oxide fuel cells. Solid State Ionics. 1999; 124:61–72.
Spacil, H. S. 1970. Electrical device including nickel-containing stabilized zirconia electrode. US Patent 3558360
Sprague, J., Tuller, H. Mixed ionic and electronic conduction in Mn/Mo doped gadolinium titanate. Journal of the European Ceramic Society. 1999; 19:803–806.
Steele, B. Apraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500°C. Solid State Ionics. 2000; 129:95–110.
Sun, C., Stimming, U. Recent anode advances in solid oxide fuel cells. Journal of Power Sources. 2007; 171:247–260.
Sun, X., Wang, S., Wang, Z., Ye, X., Wen, T., Huang, F. Anode performance of LST-xCeO2 for solid oxide fuel cells. Journal of Power Sources. 2008; 183:114–117.
Suzuki, T., Hasan, Z., Funahashi, Y., Yamaguchi, T., Fujishiro, Y., Awano, M. Impact of anode microstructure on solid oxide fuel cells. Science. 2009; 325:852.
Tao, S.W., Canales-Vazquez, J., Irvine, J.T.S. Structural and electrical properties of the perovskite oxide Sr2FeNbO6. Chemistry of Materials. 2004; 16:2309–2316.
Tao, S.W., Irvine, J.T.S. Optimization of mixed conducting properties of Y2O3-ZrO2-TiO2 and Sc2O3-Y2O3-ZrO2-TiO2 solid solutions as potential SOFC anode materials. Journal of Solid State Chemistry. 2002; 165:12–18.
Tao, S.W., Irvine, J.T.S. Study on the structural and electrical properties of the double perovskite oxide SrMn0.5Nb0.5O3-δ. Journal of Materials Chemistry. 2002; 12:2356–2360.
Tao, S.W., Irvine, J.T.S. A redox-stable efficient anode for solid-oxide fuel cells. Nature Materials. 2003; 2:320–323.
Tao, S.W., Irvine, J.T.S. Discovery and characterization of novel oxide anodes for solid oxide fuel cells. The Chemical Record. 2004; 4:83–95.
Tao, S.W., Irvine, J.T.S. Investigation of the mixed conducting oxide Sc0.15Y0.05Zr0.62Ti0.18O1.9 as a potential SOFC anode material. Journal of the Electrochemical Society. 2004; 151:A497–A503.
Tao, S.W., Irvine, J.T.S. A stable, easily sintered proton-conducting oxide electrolyte for moderate temperature fuel cells and electrolyzers. Advanced Materials. 2006; 18:1581–1584.
Tao, S.W., Irvine, J.T.S., Kilner, J.A. An efficient solid oxide fuel cell based upon single-phase perovskites. Advanced Materials. 2005; 17:1734–1737.
Tao, T., Bateman, L., Bentley, J., Slaney, M. Liquid tin anode solid oxide fuel cell for direct carbonaceous fuel conversion. Ecs Transactions. 2007; 7:463–472.
Tao, T., Mcphee, W., Koslowske, M., Bateman, L., Slaney, M., Bentley, J. Advancement in liquid tin anode-solid oxide fuel cell technology. Ecs Transactions. 2008; 12:681–690.
Tao, T., Slaney, M., Bateman, L., Bentley, J. Anode polarization in liquid tin anode solid oxide fuel cell. Ecs Transactions. 2007; 7:1389–1397.
Tragert, W. E. 1967. Fuel cell with stabilized zirconia electrolyte and nicekl-silver alloy anode. US Patent 3296030.
Trembly, J.P., Gemmen, R.S., Bayless, D.J. The effect of coal syngas containing AsH3 on the performance of SOFCs: Investigations into the effect of operational temperature, current density and AsH3 concentration. Journal of Power Sources. 2007; 171:818–825.
Trembly, J.P., Gemmen, R.S., Bayless, D.J. The effect of coal syngas con- taining HCl on the performance of solid oxide fuel cells: Investigations into the effect of operational temperature and HCl concentration. Journal of Power Sources. 2007; 169:347–354.
VAN Herle, J., Horita, T., Kawada, T., Sakai, N., Yokokawa, H., Dokiya, M. Low temperature fabrication of (Y, Gd, Sm)-doped ceria electrolyte. Solid State Ionics. 1996; 86:1255–1258.
Vasala, S., Lehtim Ki, M., Haw, S., Chen, J., Liu, R., Yamauchi, H., Karppinen, M. Isovalent and aliovalent substitution effects on redox chemistry of Sr2MgMoO6-δ SOFC-anode material. Solid State Ionics. 2010; 181:754–759.
Vasala, S., Lehtim Ki, M., Huang, Y., Yamauchi, H., Goodenough, J., Karppinen, M. Degree of order and redox balance in B-site ordered double-per- ovskite oxides, Sr2MMoO6-δ (M = Mg, Mn, Fe, Co, Ni, Zn). Journal of Solid State Chemistry. 2010; 183:1007–1012.
Venancio, S., Gutierres, T., Sarruf, B., Miranda, P. Oxida o direta do etanol no anodo de PaCOS. Revista Materia. 2008; 13:560–568.
Vincent, A., Luo, J., Chuang, K., Sanger, A. Effect of Ba doping on performance of LST as anode in solid oxide fuel cells. Journal of Power Sources. 2010; 195:769–774.
Vorontsov, V., An, W., Luo, J., Sanger, A., Chuang, K. Performance and stability of composite nickel and molybdenum sulfide-based anodes for SOFC utilizing H2S. Journal of Power Sources. 2008; 179:9–16.
Vorontsov, V., Luo, J., Sanger, A., Chuang, K. Synthesis and characterization of new ternary transition metal sulfide anodes for H2S-powered solid oxide fuel cell. Journal of Power Sources. 2008; 183:76–83.
Waldbillig, D., Wood, A., Ivey, D. Thermal analysis of the cyclic reduction and oxidation behaviour of SOFC anodes. Solid State Ionics. 2005; 176:847–859.
Wang, F.Y., Jung, G.B., Su, A., Chan, S.H., Hao, X., Chiang, Y.C. Porous Ag-Ce0.8Sm0.2O1.9 cermets as anode materials for intermediate temperature solid oxide fuel cells using CO fuel. Journal of Power Sources. 2008; 185:862–866.
Wang, J.B., Jang, J.C., Huang, T.J. Study of Ni–samaria-doped ceria anode for direct oxidation of methane in solid oxide fuel cells. Journal of Power Sources. 2003; 122:122–131.
Wei, T., Ji, Y., Meng, X., Zhang, Y. Sr2NiMoO6-δ as anode material for LaGaO3- based solid oxide fuel cell. Electrochemistry Communications. 2008; 10:1369–1372.
Xu, S., Wang, X. Highly active and coking resistant Ni/CeO2-ZrO2 catalyst for partial oxidation of methane. Fuel. 2005; 84:563–567.
Xu, Z., Luo, J., Chuang, K. The study of Au/MoS2 anode catalyst for solid oxide fuel cell (SOFC) using H2S-containing syngas fuel. Journal of Power Sources. 2009; 188:458–462.
Xu, Z., Luo, J.L., Chuang, K.T., Sanger, A.R. LaCrO3–VOx–YSZ anode catalyst for solid oxide fuel cell using impure hydrogen. Journal of Physical Chemistry C. 2007; 111:16679–16685.
Yang, L., Wang, S., Blinn, K., Liu, M., Liu, Z., Cheng, Z. Enhanced sulfur and coking tolerance of a mixed ion conductor for SOFCs: BaZr0.1Ce0.7Y0.2- xYbxO3-δ. Science. 2009; 326:126.
Ye, X., Wang, S., Hu, Q., Chen, J., Wen, T., Wen, Z. Improvement of Cu-CeO2 anodes for SOFCs running on ethanol fuels. Solid State Ionics. 2009; 180:276–281.
Ye, X., Wang, S., Wang, Z., Hu, Q., Sun, X., Wen, T., Wen, Z., Ye, X., Wang, S., Wang, Z., Hu, Q., Sun, X., Wen, T., Wen, Z. Use of La0.75Sr0.25Cr0.5Mn0.5O3 materials in composite anodes for direct ethanol solid oxide fuel cells. Journal of Power Sources. 2008; 183:512–517.
Yokokawa, H. Handbook of Fuel Cells Fundamentals, Technology and Applications. Chichester: Wiley; 2003.
Yokokawa, H., Horita, T., Sakai, N., Yamaji, K., Brito, M., Xiong, Y., Kishimoto, H. Thermodynamic considerations on Cr poisoning in SOFC cathodes. Solid State Ionics. 2006; 177:3193–3198.
Yoo, K., Choi, G. Performance of La-doped strontium titanate (LST) anode on LaGaO3-based SOFC. Solid State Ionics. 2009; 180:867–871.
Zha, S., Tsang, P., Cheng, Z., Liu, M. Electrical properties and sulfur tolerance of La0.75Sr0.25Cr1-xMnxO3 under anodic conditions. Journal of Solid State Chemistry. 2005; 178:1844–1850.
Zhang, L., Zhou, Q., He, Q., He, T. Double-perovskites A2FeMoO6-δ (A = Ca, Sr, Ba) as anodes for solid oxide fuel cells. Journal of Power Sources. 2010; 195:6356–6366.
Zhang, P., Huang, Y.-H., Cheng, J.-G., Mao, Z.-Q., Goodenough, J.B. Sr2CoMoO6 anode for solid oxide fuel cell running on H2 and CH4 fuels. Journal of Power Sources. 2011; 196:1738–1743.
Zhang, Y., Huang, X., Lu, Z., Liu, Z., Ge, X., Xu, J., Xin, X., Sha, X., Su, W. Ni-Sm0.2Ce0.8O1.9 anode−supported YSZ electrolyte film and its application in solid oxide fuel cells. Journal of Alloys and Compounds. 2007; 428:302–306.
Zhang, Y., Zhou, Q., He, T. La0.7Ca0.3CrO3-Ce0.8Gd0.2O1.9 composites as symmetrical electrodes for solid-oxide fuel cells. Journal of Power Sources. 2011; 196:76–83.
Zhao, H., Gao, F., Li, X., Zhang, C., Zhao, Y. Electrical properties of yttrium doped strontium titanate with A-site deficiency as potential anode materials for solid oxide fuel cells. Solid State Ionics. 2009; 180:193–197.
Zhi, M., Chen, X., Finklea, H., Celik, I., Wu, N. Electrochemical and micro- structural analysis of nickel-yttria-stabilized zirconia electrode operated in phosphorus-containing syngas. Journal of Power Sources. 2008; 183:485–490.
Zhu, X., Lü, Z., Wei, B., Chen, K., Liu, M., Huang, X., Su, W. Fabrication and performance of membrane solid oxide fuel cells with La0.75Sr0.25Cr0.5Mn0.5O3-δ impregnated anodes. Journal of Power Sources. 2010; 195:1793–1798.
Zhu, X., Lü, Z., Wei, B., Liu, M., Huang, X., Su, W. A comparison of La0.75Sr0.25Cr0.5Mn0.5O3-[delta] and Ni impregnated porous YSZ anodes fab- ricated in two different ways for SOFCs. Electrochimica Acta. 2010; 55:3932–3938.
Zhu, X., Lü, Z., Wei, B., Liu, M., Huang, X., Su, W. A comparison of La0.75Sr0.25Cr0.5Mn0.5O3-δ and Ni impregnated porous YSZ anodes fabricated in two different ways for SOFCs. Electrochimica Acta. 2010; 55:3932–3938.
Zhu, X., Lü, Z., Wei, B., Zhang, Y., Huang, X., Su, W. Impregnated La0.75Sr0.25Cr0.5Mn0.5O3-δ -based anodes operating on H2, CH4, and C2H5OH fuels. Electrochemical and Solid-State Letters. 2010; 13:B91–B94.
Zhu, X.B., Lu, Z., Wei, B., Zhang, Y.H., Liu, M.L., Huang, X.Q., Su, W.H. Performance of the single-chamber solid oxide fuel cell with a La0.75Sr0.25Cr0.5Mn0.5O3-δ -based perovskite anode. Journal of the Electrochemical Society. 2010; 157:B691–B696.