Thin-film solid oxide fuel cell (SOFC) materials
Abstract:
The focus of this chapter is on the materials used in micro solid oxide fuel cells based on thin films. Micro fuel cells have attracted interest because they have the potential to provide high storage capacities per unit volume and weight for applications in portable electronic devices. Thin-film syntheses and properties of the individual materials components (electrolytes, anodes, cathodes) are discussed, followed by examples of specific device structures.
15.1 Introduction
The last few years have seen a large increase in the use of small portable electronic devices including laptop computers, wireless telephones, CD players, noise reduction headphones and Ebook readers. At present these devices are powered by rechargeable batteries but ‘micro’ fuel cells have attracted interest because they have the potential to provide higher storage capacities per unit volume and weight and consequently longer operating times between recharges, and can be recharged simply by replacing the fuel container. Micro-fuel cells based on polymer membranes (PEMs), direct methanol fuel cells (DMFCs) and solid oxide fuel cells (SOFCs) have all been considered and prototype systems constructed. As with larger systems, each fuel cell type has advantages and disadvantages that mostly have to do with the fuel and fuel processing. While for portable electronics applications, the lower operating temperature is an advantage, PEMs require pure hydrogen which is difficult to store and DMFCs operate with methanol which has some toxicity issues. SOFCs, in contrast, can operate on a variety of hydrocarbon fuels but at elevated temperature (≥ 500°C) which presents problems with start-up and heat management. The driving force for the introduction of micro fuel cells is the potential to replace primary and secondary batteries with systems that can store more energy (Evans et al., 2009b).
The focus of this review is on materials and design of micro SOFCs based on thin films. The topic has been of recent interest and a number of excellent review articles have appeared in the last few years (Litzelman et al., 2008; Evans et al., 2009a, 2009b; Hertz and Tuller, 2009; Tuller et al., 2009). Because of space limitations micro-tubular and single chamber fuel cell systems are not considered; further information can be found in recent reviews (Buergler et al., 2007; Suzuki et al., 2010a, 2010b).
15.1.1 Materials for thin-film solid oxide fuel cells (SOFCs)
Techniques for the preparation of crystalline oxide thin films using physical and chemical vapor deposition techniques have been developed independently of SOFCs because of their importance in many device applications including, dielectric and ferroelectric materials for memory applications (Martin et al., 2010), passive electrodes (Qin et al., 2009), resistive memory (Lin et al., 2008), magnetoresistance (Liu et al., 2010) and superconducting electronics (Claeson et al., 1999). Most of the properties of these films have been studied at ambient temperature or below. In the last decade, however, more effort has been dedicated to the investigation of the high-temperature properties of oxide thin films where phenomena such as changes in stoichiometry, order–disorder, and ionic conductivity become important. The high-temperature behavior is relevant to the performance of devices which interact chemically with their environment via chemical reaction and ion diffusion – for example, chemical sensors, ion transport membranes and SOFCs. The use of thin films in such devices makes possible the effective elimination of bulk ionic diffusion as a rate-limiting process leading to faster overall kinetics and lower operating temperatures. Lowering the operating temperature to ≤ 500°C is of key importance to the successful development of micro SOFCs. For very thin films or films containing very small grains (nano-dimensioned), grain boundaries become important and the overall kinetics become limited by transport across solid–solid interfaces and by reaction at gas–solid interfaces. The presence of such interfaces can either enhance or degrade the overall performance. A general discussion of the importance of interfaces in the context of nano-ionics can be found in the review articles by Maier (2005, 2009).
In addition to potential applications as components in micro-SOFCs, dense oxide thin-film electrodes are of interest for more fundamental studies of reaction mechanisms. Thin films simplify the electrode architecture and eliminate uncertainties associated with porous microstructures found in bulk electrodes (Adler, 2004). Crystalline thin films also make possible the separation of bulk and grain boundaries and direct determination of surface reaction rates; epitaxial films permit the study of the effects of strain and, for anisotropic materials, of property anisotropy. In the following, some of the recent work on the materials aspects of thin-film electrolytes and electrodes is reviewed.
15.2 Electrolytes
The oxide ion electrolytes used in micro-SOFCs are the same materials as those used in their larger-scale counterparts, namely yttria-stabilized zirconia (YSZ), gadolinium-, samarium- or yttrium-doped ceria (GDC, SDC, YDC), and lanthanum strontium magnesium gallate (LSGM). Most of the micro-SOFC devices studied to date have used YSZ. YSZ is preferred for free-standing membranes or larger area-supported membranes because of its better mechanical properties (Baertsch et al., 2004; Tarancon et al., 2010).
15.2.1 Yttria-stabilized zirconia
A variety of different deposition techniques have been used to make YSZ thin films including dc and rf reactive sputtering from metal alloy targets (Thiele et al., 1991; Wang et al., 1992; Jankowski et al., 2002; La O’ et al., 2004; Kang et al., 2006; Huang et al., 2007; Rey-Mermet and Muralt, 2008; Johnson et al., 2010; Kerman et al., 2011), pulsed laser deposition (PLD) (Muecke et al., 2008b; Noh et al., 2009b; Tarancon et al., 2009; Johnson et al., 2010) atomic layer deposition (ALD) (Shim et al., 2007; Prinz, 2008; Jee et al., 2010), spray pyrolysis (Wilhelm et al., 2005), chemical vapor deposition (Chun and Mizutani, 2001), electron beam evaporation (Baertsch et al., 2004) and solgel techniques (Kosacki et al., 2000; Peters et al., 2009). A review of thin-film deposition methods is given by Beckel (Beckel et al., 2007).
The method of deposition has a significant effect on the microstructure of the films, as does the nature of the substrate (amorphous, polycrystalline or single crystal) and the substrate temperature used during deposition. For example, PLD on amorphous Si3N4 gave a columnar microstructure with 10 nm grains at 400°C (Tarancon et al., 2009), whereas PLD on c-cut sapphire single crystals at 600°C gave polycrystalline films with a columnar micro-structure and some degree of (111) orientation (Heiroth et al., 2008, 2010). Deposition on single-crystal MgO at 500°C gave (100)-oriented epitaxial films with a narrow rocking curves (< 1°) (Kosacki et al., 2005). Physical deposition techniques using heated substrates usually give crystalline material whereas those prepared by chemical deposition are initially amorphous and require further heat treatment to crystallize and control the grain size.
In addition to the synthesis of free-standing membranes for application in devices, many investigations have been made of electrolyte films on dense substrates in order to make more fundamental studies of the effects of micro- and nano-structure, epitaxial strain and impurities on the electronic and ionic conductivity. Specific studies of nanoscale effects on the conductivity of YSZ have been made by a number of authors. Kosacki and co-workers studied highly textured (cube-on-cube) YSZ films deposited on MgO by PLD (Kosacki et al., 2004, 2005). For films < 60 nm thick, an increase in the conductivity was observed and attributed to the increased importance of the interface. Dense YSZ films were also prepared by a polymeric precursor spin coating method with grain sizes of 1–400 nm on single-crystal sapphire and polycrystalline Al2 O3 substrates. Nanocrystalline samples were reported to show enhanced electrical conductivity compared with micro-crystalline material (Kosacki et al., 2000). Chao et al. (2009) prepared 8–55 nm thin films of YSZ by atomic layer deposition (ALD) and measured inplane oxide ion conductivities as a function of temperature, film thickness and yttria concentration in the film. Higher conductivities were observed for thinner films which were thought to be due to increased oxide conduction along the surface. Increasing the yttrium concentration at the surface also led to higher conductivity.
Recently, Peters et al. (2009) made a detailed study of grain size effects in YSZ thin films. Films of 8.3 mol% YSZ, 400 nm thick, were prepared on sapphire by a sol–gel method and grain sizes were systematically adjusted to 5 nm ≤ d ≤ 782 nm by subsequent processing. High-resolution electron microscopy was used to show that the grains and grain boundaries were chemical homogeneous without contamination. Oxygen transport was found to be impeded by the grain boundaries. No evidence for an enhanced conductivity in 400 nm thin films with grain sizes in the range 5 nm ≤ d ≤ 36 nm was observed in contrast to the behavior of very thin films where surface conduction plays a large role. A survey of the previous experiments in more detail than given here can be found in Peters et al. (2009). It is worth noting that the calculated depletion width in YSZ due to space charge effects was estimated by Hertz and Tuller (2009) to be only 0.4 nm which is too small to produce significant electronic conductivity, consistent with the experimental data. In general, the variations observed in the properties of thin films with different thickness and grain size illustrate the critical importance of interfaces in nanoscale materials and is an area that needs further study. Some further aspects of interfaces are discussed in Section 15.2.4.
15.2.2 Doped ceria
The electrolytes studied in most detail after YSZ are based on doped ceria (GDC, SDC and YDC). Ceria electrolytes are an attractive alternative to YSZ because of their higher conductivity; at temperatures of ≤ 500°C, the electronic contribution to the conductivity is expected to be small. GDC has been used in some micro-fuel cell devices (Section 15.5) but most thin-film studies have concerned fabrication and properties. Measurements have been made on both highly epitaxial films and on nanocrystalline and polycrystalline films. The latter have been synthesized by physical deposition techniques with amorphous or polycrystalline substrates held at ambient or low temperatures or by chemical methods at ambient temperature followed by annealing at higher temperature to induce crystallization and grain growth.
Highly oriented GDC (Ce0.8Gd0.2O2 – x) thin films were grown on single-crystal (001) MgO substrates by PLD (Chen et al., 2003). The films were highly c-axis-oriented with cube-on-cube epitaxy despite the extremely large lattice misfit of > 28%. The electrical conductivity is ionic down to pO2 = 1 × 10−19 atm at 500–800°C. The activation energy changes from 0.86 eV for as-deposited films to 0.74 eV after annealing and the conductivity increases to a value very close to that of bulk grains in polycrystalline material, indicating the absence of any significant contribution from grain boundaries. Single-crystal epitaxial thin films with similar properties were deposited on NdGaO3 (NGO) and LaAlO3 (LAO) substrates by PLD. Jacobson and co-workers studied the microstructure of GDC films grown on different substrates including MgO, YSZ, LAO, NGO and SrTiO3 (STO) with different film-substrate lattice mismatch ratios (Huang et al., 2004a, 2004b, 2005). The highest film crystallinity was obtained on LAO with a small compressive strain. This single-crystal GDC film shows no columnar grain growth but contains directionally aligned Gd-rich nanoparticles which act to relax strain fields induced during the film growth.
Highly crystalline epitaxial films of Sm0.2Ce0.8O1.9 (SDC) films were grown by PLD on MgO single-crystal substrates but with a buffer layer of STO to provide a proper lattice match. At 700°C, SDC/STO films have an ionic conductivity 7 × 10−2 S cm−1 with an activation energy of 0.74 eV; an electronic contribution becomes apparent at pO2 = ~ 10−15atm. These values are comparable to those reported for GDC on NdGaO3. Similar results were recently obtained for polycrystalline GDC prepared by rf sputtering which remains a pure ionic conductor at 700°C down to 10−17 atm (Podpirka and Ramanathan, 2009).
Yttria-doped ceria is the third system that has received attention (Tian and Chan, 1996, 1997, 1998, 1999, 2002). YDC films containing 0.58 and 4 mole% Y2O3 were prepared by e-beam evaporation and by PLD and were deposited on different substrates. Both polycrystalline and textured films were obtained with grain sizes ranging from 50 to 500 nm. The ionic conductivities of the films were dominated by grain boundaries.
Nanocrystalline films on sapphire substrates were prepared by spin coating a substrate with polymeric precursor (Suzuki et al., 2002). The grain size of these films depends upon the annealing temperature and the dopant content; higher dopant levels give smaller grains. The ionic conductivity was found to increase as the grain size decreases, and the activation energy for the ion mobility decreases from 1.3 eV for 36 nm grains to 1.0 eV for 9 nm grains. Chiodelli et al. (2005) prepared thin films of GDC, 80–400 nm thick, by off-axis reactive sputtering on polished polycrystalline alumina and quartz substrates which were held at ambient temperature or heated to 300 or 600°C. Samples were annealed at 800°C before conductivity measurements. On quartz the conductivity is comparable to polycrystalline material but is much lower on alumina, apparently because of the substrate roughness.
Rupp and co-workers prepared nanocrystalline films by both spray pyrolysis and PLD (Rupp and Gauckler, 2006). PLD typically gives columnar microstructures whereas spray pyrolysis give more isotropic grains. Spray pyrolysis onto a sapphire single-crystal substrate held at 310°C, followed by annealing at 800–1100°C, was used to obtain different grain sizes in a stable microstructure. The conductivities decreased with increasing grain size as observed previously by Suzuki et al. (2002). Similar results were obtained for samples with comparable grain sizes on sapphire substrates. A comparison of these results with the data of Suzuki et al., is given by Beckel (Beckel et al., 2007). Very high conductivities were observed in ultrathin films of nanocrystalline GDC with thicknesses comparable to the grain size (20–50 nm) prepared by dc reactive sputtering (Huang et al., 2006a). Conductivities were determined to be 3–4 orders of magnitude higher than those of thicker films (> 500 nm). The unusual properties were attributed to the reduction of cross grain boundary resistance and the segregation of the Gd dopant in the vicinity of grain boundaries.
In general, the conductivities for nanocrystalline ceria increase with decreasing grain size and are lower than for epitaxial films which approach the value found for bulk crystalline grains. The electronic contribution to the total conductivity is not known in all cases. Overall, the conductivities reported vary by more than two orders of magnitude (10−4−10−2 S cm−1 at 600°C) indicating that further work is needed to understand the effect of the deposition method on microstructure, segregation at grain boundaries and impurities.
15.2.3 Lanthanum strontium magnesium gallate
A limited amount of work has been reported on the synthesis of LSGM films. Mathews et al. (2000) used PLD to deposit films of LSGM on quartz and silicon substrates held at room temperature. The as-deposited films were amorphous but on annealing at 700°C gave a single-phase cubic structure. Subsequently, ~ 500 nm thick LSGM films were deposited by PLD on quartz, STO, sapphire, and calcia-stabilized zirconia (CSZ) followed by post-annealing in air at 730°C (Joseph et al., 2002). Stoichiometric films were obtained under high-vacuum conditions but not in an oxygen environment. Highly oriented films of LSGM were obtained on STO but the other films were polycrystalline. The oriented film on STO was less conductive than bulk LSGM by a factor of 10 at 730°C. Ishihara and co-workers also used PLD to make thin films with the composition La0.8Sr0.2Ga0.8Mg0.15Co0.0.5O2.8 on silica and LAO substrates (Mitsugi et al., 2004). Films were amorphous when the substrate temperature was less than 750°C. The films were poly-crystalline on silica and oriented on LAO. The conductivities measured for the films were comparable on both substrates and were very high (1 S cm−1 at 600°C). In more recent studies, LSGM thin films were deposited by PLD on a NiO–Fe2O3 substrate which was reduced in situ to a porous Ni–Fe alloy. High power densities were measured for single cells with porous thick-film Sm0.5Sr0.5CoO3 cathodes (Ishihara et al., 2008; Ju et al., 2009, 2010).
15.2.4 Interface effects
One approach to study the influence of surface and interface contributions on the conductivity of very thin films is to generate controlled interfaces by making superlattices of two different materials. The presence of internal interfaces can modify properties through space charge effects, by the presence of disordered regions due to mismatched lattice dimensions of the two phases, and strain when the interfaces are coherent. Space charge effects that lead to a variation in the density of majority charge carriers in the space charge region were first experimentally demonstrated and modeled for nanocrystalline CeO2 (Chiang et al., 1997; Tschope, 2001; Kim and Maier, 2002). Subsequently the concept was extended to epitaxial hetero-structures prepared by sequential deposition of CaF2/BaF2 layers. When the thickness of the layers was reduced to ~ 20 nm while keeping the total thickness constant, the F− conductivity parallel to the interfaces was increased by several orders of magnitude (Sata et al., 2000; Guo and Maier, 2009a, 2009b). In situations where the mobile defect concentration is high, the Debye length is correspondingly short and structural effects (a combination of lattice strain and extended defects due to lattice mismatch) are responsible for any variations in conductivity. The effects of coherent, semi-coherent and disordered interfaces have been studied for a number of different fluorite oxygen ion conductors in superlattices with ZrO. (Azad et al., 2005; Wang et al., 2009) or with RE2 O3 oxides (RE = Y, Lu, Sc) (Peters et al., 2007; Korte et al., 2008, 2009; Schichtel et al., 2009, 2010). For example, the multilayer system CSZ (ZrO2 + 8.7 mol% CaO)/Al2O3, represents a system with incoherent interfaces. The total conductivity of the CSZ increases by two orders of magnitude when the thickness of the individual CSZ layers is decreased from 780 to 40 nm. Changes in the activation energy for the interfacial transport suggest that the ionic mobility is much higher in the disordered regions of the incoherent interfaces than in the bulk. In contrast, in multilayer samples of YSZ/Sc2O3 with layer thicknesses between 8 and 250 nm prepared on (0001) Al2O3 substrates, the interfaces between YSZ, Sc2O3, and the substrate are sharp and the layers are highly textured. As the individual layers in the multilayer get thinner, the conductivity of the multilayers decreases due to the presence of compressive stress in YSZ at the YSZ/Sc2O3 interfaces. This observation agrees well with the results for YSZ/Y2O3 superlattices where tensile misfit strain leads to a conductivity increase. An overview of the effect of strain and disorder is shown in Fig. 15.1, adapted from Janek and co-workers (Korte et al., 2009). The MgO/YSZ system investigated by Kosacki et al. (2005) is also included.
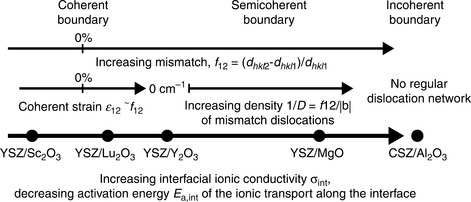
15.1 Classification of the multilayer systems Sc2O3/YSZ, Lu2O3/YSZ, Y2O3/YSZ and Al2O3/CSZ.) Source: From Korte et al. 2009.
In recent work, Garcia-Barriocanal et al. (2008) reported a very large enhancement (eight orders of magnitude) in the conductivity of superlattices in which 10 nm thick STO films alternated with YSZ layers of thickness between 62 and 1 nm. The epitaxial growth of the YSZ on STO gives a large, tensile strain (7%) in the thin YSZ layers in the plane of the layer. The dc conductivity of the 1 nm YSZ layer showed a record value of 0.014 S/cm at 357 K, with an activation energy of 0.64 eV. These results have been the subject of much debate concerning the ionic or electronic nature of the observed conductivity (Garcia-Barriocanal et al., 2009; Guo, 2009). Janek and co-workers (Schichtel et al., 2009) concluded that the maximum enhancement in ionic conductivity that could be expected from the 7% strain was about 2.5 orders of magnitude and that this could not explain the observed behavior. Cavallaro et al. recently prepared YSZ/STO multilayers by PLD with comparable layer thicknesses to the earlier work but with a different microstructure (YSZ islands in a matrix of STO) (Cavallaro et al., 2010). The absolute value of the conductivity found for these films, along with the apparent activation energies are, however, very similar. The dependence of the electrical conductivity on pO21/4 and the absence of 18O diffusion along the interfaces led this group to conclude that the enhancement is related to electronic rather than ionic conductivity.
15.3 Anode materials
Electrodes are critical components in all SOFCs in that they provide the interface between the chemical energy associated with fuel oxidation and electrical power. Several materials requirements are general to both anodes and cathodes. Both electrodes must have high electrocatalytic activity and electronic conductivity to minimize the effective resistance. The anode acts as an electrocatalyst for the oxidation of fuel components by oxide ions transported through the electrolyte and the cathode catalyzes the oxygen reduction reaction. Electrons produced or consumed by the chemical reactions at the electrode surfaces must be transported to or from the external circuit requiring high electronic conductivity. The electrodes must be chemically stable at the operating temperature with respect to the electrolyte and the current collector and must have stable microstructures under operating conditions with respect to both porosity and surface area. Most micro-SOFC devices have used platinum electrodes because of its activity, stability and ease of fabrication though there are issues with microstructural stability. In the future, other complex oxide electrode materials may be preferred and in particular the use of cermets to provide stable microstructures. Electrode performance is of critical importance in micro-SOFCs which must operate at reduced temperatures (≤ 500°C).
15.3.1 Platinum
In order to obtain suitably structured electrodes with a large triple phase boundary length for use with thin-film electrolytes, porous Pt fabricated by sputtering has been most frequently used. Porous platinum has good catalytic activity but unfortunately the microstructure at operating temperature is not stable, grain growth occurs depending on time, temperature and film thickness which reduces the length of the triple phase boundary, and eventually the performance degrades. As an example, Muecke et al. (2008b) observed accelerated grain growth of platinum under hydrogen with associated performance loss. The average grain and pore size of the film on the anode side was several times larger than on the cathode side. Similar studies by Kerman et al. (2011) found that overall performance showed extreme sensitivity to Pt anode porosity and microstructure. The poor thermal stability of platinum films probably will require the use of anode materials with more stable microstructures. Some improvement in stability has been observed for Pt-Ni alloys (Wang et al., 2008).
ALD has been used as an alternative to sputtering to deposit Pt films on electrolytes (Jiang and Bent, 2007; Jiang et al., 2008a). Using (methylcyclo-pentadienyl) trimethylplatinum (MeCpPtMe3) and oxygen as precursors, Pt films were deposited on YSZ with thicknesses of ≤ 30 nm. Peak power densities were achieved for ALD Pt anodes with only one-fifth of the platinum loading relative to dc-sputtered Pt anodes. The sputtered and ALD Pt films had very different microstructures, which accounted for the difference in their performance.
15.3.2 Cermets
In conventional fuel cells, electrodes can be thick, porous composites, with large triple phase boundary lengths. In micro-SOFCs, the equivalent thin-film composite electrode of a metal with an ionically conducting ceramic phase will be needed. The ceramic network in the composite can limit metal grain growth as observed in conventional SOFC Ni/YSZ anodes. A number of groups have investigated the formation of both precious metal (Ag, Pt) cermets with YSZ and Ni cermets with both YSZ and GDC. Barnett and co-workers employed reactive magnetron sputtering to deposit Ag/YSZ cermet films from Ag and Zr/Y targets in Ar–O2 mixtures (Wang and Barnett, 1992, 1995; Wang et al., 1992). Evaporation of Ag was observed during long-term annealing at 750°C, but at lower temperatures, they are potential cathodes with low resistivity. Thin-film composite electrodes with nanosized grains of Pt and YSZ were prepared by reactive sputtering onto single-crystal YSZ by Hertz and Tuller (2007). Area-specific electrode polarization resistances of < 500 Ω cm2 were achieved at 400°C in a dense thin-film electrode. By turning on and off the platinum deposition, a thin-film stack of Pt–YSZ/YSZ/Pt–YSZ with a total thickness of ~ 1 μm was constructed.
Nickel cermet anodes have been prepared on YSZ and on GDC. Tsai and Barnett used dc reactive magnetron sputtering of Ni–Zr–Y targets in Ar–O2 mixtures to form porous Ni–YSZ films with grain sizes of ~ 35 nm which gave low specific area resistances (0.15–0.35 Ω cm.) at 750°C in 97% H. + 3% H2O (Tsai and Barnett, 1995). La O’ et al. prepared Ni–YSZ cermets by rf sputtering (La O’ et al., 2004). The films were porous with microstructures consisting of nanosized columnar grains and showed no cracks after treatment at 600°C under a reducing atmosphere. Also by using reactive rf sputtering, Jou and Wu prepared porous Ni/YSZ films on YSZ and evaluated their performance as the anode with a La0.7Sr0.3MnO3 thick-film cathode (Jou and Wu, 2008). The maximum power density at 700°C was lower than that obtained using a screen-printed Ni–YSZ thick anode.
Ni/YSZ cermet films have also be made by PLD from NiO/YSZ targets followed by reduction of the NiO in hydrogen. The oxide films deposited at 700°C contain very small grains (~ several nm) which results in nickel agglomeration on reduction. Post-annealing the films at temperatures in the range of 800–1200°C to induce some grain growth was found to be necessary to obtain a stable microstructure (Noh et al., 2009a, 2010). PLD was also used to deposit NiO/GDC films on YSZ, SiO2/Si and sapphire substrates held at temperatures from 25°C to 800°C. Upon reduction of the NiO phase in H2 at 600°C, a stable three-phase, three-dimensional interconnecting microstructure was obtained. Higher reduction temperatures led to coarsening and segregation of the Ni to the surface of the film. Ni grain growth can be minimized by annealing in air at 1000°C after deposition (Infortuna et al., 2009). Muecke et al., measured the electrochemical performance of nanocrystalline Ni–GDC anodes with a thickness of 500–800 nm prepared by spray pyrolysis (Muecke et al., 2008a, 2008c). The polarization resistance at 600°C decreased from 1.73 to 0.34 cm2 when the grain size was decreased from 53 to 16 nm, respectively. The polarization resistances of the Ni–GDC sprayed thin films were comparable to those prepared by PLD and were similar to state-of-the-art thick films.
In summary, platinum anodes, while convenient, are unlikely have sufficiently stable microstructures for long-term performance stability. As discussed earlier, several recent studies have shown that cermets may provide a good alternative for use with both YSZ and GDC electrolytes as indeed they do in full-scale SOFCs. Recently, oxides with both electronic and ionic conductivity in the reducing atmosphere at the anode and which are catalytically active have been discovered and evaluated as alternatives to metalcermets (Tao and Irvine, 2003; Huang et al., 2006b). Whether materials of this type can be developed with sufficient catalytic activity to operate at the lower temperatures (350–500°C) needed for thin-film devices is an open question.
15.4 Cathode materials
15.4.1 (La,Sr)MnO3
The perovskite lanthanum strontium manganite (LSM) is the most important electrode material in large-scale SOFCs and remains the practical choice for operation in the 700–900°C range because of its high electrical conductivity, high electrochemical activity for the O2 reduction reaction, compatibility with YSZ at operating conditions and long-term performance stability (Jiang, 2008).
In the context of micro fuel cells, operating at lower temperature, LSM has not been studied in much detail. Holme et al. (2008) used ALD to prepare a 95 nm thick, dense cathode, on 100 nm thick film of dense YSZ as electrolyte supported on a Si3 N4/Si as described in Section 15.5. Porous platinum was used as the anode. Only a limited power density was obtained for this cell and the authors concluded that while ALD is a good route to high-quality films, even thin LSM cathodes are limited by slow bulk diffusion at 450°C.
In contrast, a considerable effort has been made to use dense films to understand the mechanistic aspects of LSM electrode performance. Early experiments had problems with the preparation of crack-free films (Gharbage et al., 1994, 1995) but in 1996 Mizusaki and co-workers (Mizusaki et al., 1996) successfully deposited 1–2 μm thick films of La0.63Sr0.27MnO3 on polycrystalline partially stabilized YSZ by PLD. Electrochemical measurements at 700–900°C showed that the reaction kinetics were controlled by chemical diffusion of oxygen through the oxide layer. Ioroi et al. (1997) used electrochemical vapor deposition to prepare dense LSM electrodes on YSZ. Impedance measurements showed that the electrode resistance was proportional to the film thickness, and the inverse of the electrode resistance was proportional to the electrode surface area, showing again the importance of bulk diffusion. In an extensive series of experiments (Brichzin et al., 2000, 2001, 2002; Fleig, 2003; Fleig et al., 2008), Maier, Fleig and co-workers used thin-film microelectrodes of LSM on YSZ to showed that at 800°C, the electrode resistance scales with the area of the microelectrode indicating that diffusion through the electrode area rather that the length of the triple phase boundary is rate determining. The electrode polarization also increases linearly with thickness, confirming the bulk path. On application of an anodic bias potential, however, the resistance scales with the inverse of the microelectrode diameter, indicating a change in the mechanism from bulk to surface due to a decrease in the oxygen vacancy concentration. The role of triple phase boundaries was also studied by Horita and co workers who prepared a square LSM mesh with ~ 2 μm lines separated by ~ 9 μm on YSZ with a Pt anode (Horita et al., 1998, 2000, 2001). The application of a potential leads to oxygen incorporation. Switching the gas from 16O2 to 18O2 followed by quenching and SIMS analysis revealed the active areas for oxygen incorporation. Measurements were made either on the surface of the LSM pattern on YSZ or on the YSZ after removing the LSM. Under cathodic polarization, high 18O concentrations were found on the surface of the LSM and as peaks at the edges of the O2/LSM/YSZ three-phase boundary. After removal of the LSM, 18O was also observed in the region where the LSM had been attached but only at high cathodic polarization. For large cathodic bias, the increased vacancy concentration in LSM results a strong contribution of the bulk path to the oxygen reduction rate in accordance with the results obtained from the microelectrode measurements.
15.4.2 (La,Sr)CoO3 – x
Lanthanum cobaltate substituted with strontium to various degrees is probably the most extensively studied thin-film system. In early work, Kawada et al. (1999) carried out oxygen isotope exchange experiments with a dense 0.5 ^m thick film of La0.6Sr0.4CoO3 – x deposited on a Ce0.9Ca01O19 polycrystalline substrate by PLD. The isotope exchange profile was measured from the surface into the electrolyte by SIMS. No gradient in the oxygen isotope concentration inside the film was observed because the oxygen diffusion through the La0.6Sr0.4CoO3 – x film is so fast. A similar experiment was carried out by Mims et al., for La..5Sr0.5CoO3 – x deposited as oriented ~ 300 nm thick films on single-crystal YSZ by PLD (Mims et al., 2000). Isotope exchange and SIMS depth profiling showed a uniform 18O concentration in the film and a significant barrier to transport across the solid–solid interface perhaps due to reaction of YSZ with LSC. In a series of papers, Yang et al., reported measurements of the surface exchange rate and interfacial transport by AC impedance measurements for La0.5Sr0.5CoO3 – x on 100-oriented YSZ and electrical conductivity relaxation for La0.5Sr0.5CoO3 – x deposited on LAO, all films were prepared by PLD (Chen et al., 2000, 2002; Yang et al., 2000, 2001a, 2001b). The electrical conductivity relaxation data were used to obtain the surface exchange rates assuming that the kinetics were surface-limited as shown by the 18O2 exchange results. As synthesized, the film was epitaxial with a smooth surface (rms roughness ~ 5 Å). After prolonged heating at 900°C, the surface exchange rate increased substantially due to a change in the thin-film surface morphology (rms surface roughness of 70 A) and a decrease in grain size. The change is too large to be due to an increase in surface area and must reflect a change in surface activity due to the increase in number of grain boundaries. The surface reaction rates were determined also from the low-frequency feature observed in the impedance spectra (Fig. 15.2) and are in the range of those measured by ECR; again higher surface reaction rates were obtained on films with smaller grains and more interfaces. The results obtained from four different films are shown Fig. 15.2; substantial variations are observed for films with different degrees of perfection. For La0.6Sr0.4CoO3 – x films on GDC, Sase et al. found that the reaction rate on an electrode with 30–50 nm grains was larger by half an order of magnitude compared with 300–500 nm grains (Sase et al., 2006).

15.2 (a) Impedance spectra of La0.5Sr0.5CoO3 – x/YSZ at pO2 = 1 atm and 750°C (b) Surface exchange rate (kchem) vs temperature at pO2 = 1 atm. Open and solid circles, impedance. ECR measurements: solid triangles pointing down LSC/LaAlO3(100) before annealing, solid triangles pointing up LSC/LaAlO3(100) after annealing at 900°C. (Source: Data from Yang et al. (2000, 2001b).)(Source: Chen et al. 2002.)
The medium-frequency feature observed in the impedance spectra is associated with an electrode–electrolyte interface resistance due to reaction of LSC with YSZ at the deposition temperature. The interface resistance of La0.6Sr0.4CoO3 on YSZ is almost independent of pO2, and increases with time according to a parabolic rate law due to formation of a strontium-rich layer between the electrode and the electrolyte (Sase et al., 2005). In contrast, a La0.7Sr0.3CoO3 – x film deposited on YSZ by rf magnetron sputtering with the substrate at ambient temperature did not show this feature and no interfacial reactions were observed below 530°C. Two features were observed in the impedance spectra; a low-frequency semicircle due to the surface reaction and a Warburg-like feature due to diffusion (Ringuede and Fouletier, 2001; Ringuede and Guindet, 1997).
The oxygen vacancy concentration in a dense film varies with the pO2 or applied voltage. This accumulation or depletion of electrical charge is detected in an impedance measurement as a large capacitance sometimes referred to as a ‘chemical capacitance’ (Jamnik and Maier, 1999, 2001). The equilibrium oxygen vacancy concentration for a dense film of La0.6Sr0.4CoO3 – x on polycrystalline CGO was determined from this chemical capacitance (Kawada et al., 2002; Masuda et al., 1997) and found to be much smaller than the values determined for bulk samples by thermo-gravimetry (Mizusaki et al., 1989). More recently, further measurements were made on films with the same composition but a different microstructure (20–200 nm grains, YAG laser). The vacancy concentration determined from the capacitance measurements was again smaller than the bulk value but not so small as for the previous sample prepared using a Kr–F laser. Similar results were obtained for La0.5Sr0.5CoO3 – x PLD films on GDC, SiO2 and MgO substrates using a XeCl excimer laser (Hemmi et al., 2009). Clearly, microstructural effects significantly modify the thermodynamic properties. Nanostructured films show similar effects.
15.4.3 (La,Sr)CoO3 – x nanoparticles
Since thin films containing grain boundaries are inherently nanostructured, deliberately engineering nanostructures is of interest. Two groups have successfully done this by different methods and obtained very high performance electrodes. One approach is to use what have been referred to as vertically aligned nanoporous thin films prepared by off-axis PLD (Yoon et al., 2007, 2009; Wang et al., 2010). This technique produces thin films less than 1 μm thick that are nanoporous and contain very small particles (8 nm in the example shown in Fig. 15.3 for La0.5Sr0.5CoO3 – x). The high surface area (80 μm−1) results in an electrode with very low area-specific resistance (0.09 O cm2 at 600°C) measured with a symmetrical cell with a polycrystalline GDC electrolyte. The chemical capacitance deduced from the impedance data indicates a very low oxygen vacancy concentration and is comparable to the results of Kawada et al. (2002) discussed above. Table 15.1 shows a comparison of results.
Table 15.1
A comparison of bulk and thin-film thermodynamics for La1 – x SrxCoO3 – δ
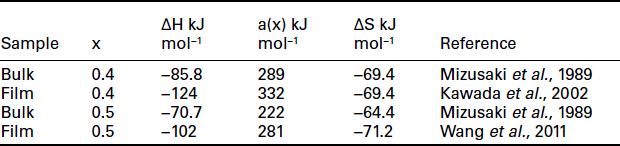
Thermodynamic model assumes that ΔH(ξ) = ΔH0(ξ) – α(ξ)δ (Mizusaki et al., 1989).
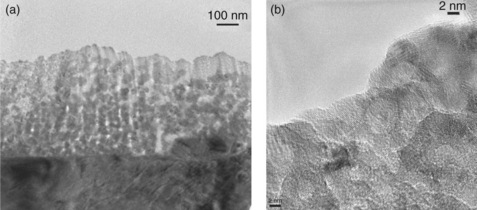
15.3 Nanostructured La0.5Sr0.5CoO3 – x. (a) Cross-sectional low magnification image for the entire film area and (b) HRTEM image around the film surface of a LSC/GDC film after impedance measurements. Source: Reproduced with permission from Wang et al. 2010.
The large difference in the enthalpy of vacancy formation for the film relative to the bulk material is striking. It is also interesting that these nano-structured films appear to be quite stable at least over a period of 240 h (Wang et al., 2011).
Peters et al. (2008) did similar experiments on nanoporous (La0.5Sr0.5) CoO3 – x thin-film cathodes (film thickness, 200–300 nm; grain and pore size ~ 50 nm). The films were made by metal–organic deposition and deposited on YSZ or GDC electrolytes. Low polarization resistances were observed on both electrolytes and the polarization resistance of the LSC/YSZ interface remained constant for 100 h at 500°C. In related work, La0.6Sr0.4CoO3 – x thin-film electrodes were deposited on YSZ substrates by PLD at different temperatures (Januschewsky et al., 2009). The decrease in the film crystallinity that occurs when the deposition temperature is lowered is accompanied by an increase in the oxygen exchange rate. For X-ray amorphous electrodes deposited between 340°C and 510°C, polarization resistances as low as 0.1 O cm2 at 600°C were obtained. The amorphous films also exhibited the best stability of the polarization resistance with time.
15.4.4 Epitaxial films: effect of strain
Unusual effects are observed in epitaxial films when the cell constants of the film are constrained by the lattice constants of the substrate and the films are as a result in either tensile or compressive strain. Typically this is only observed for quite thin films (typically < 100 nm but the thickness depends on the lattice mismatch) before the strain is relaxed by the introduction of defects such as edge dislocations. Both experimental and theoretical studies have been made for LSC cathode materials. Kushima et al., employed first-principles calculations (DFT + U) to obtain a microscopic description of the mechanisms by which strain influences oxygen vacancy formation as well as oxygen adsorption on LaCoO3 (Kushima et al., 2010). A significant lowering in the oxygen-vacancy formation energies in the bulk and surface were found as the tensile strain was increased. An experimental study of a 40 nm thick strained film of La0.5Sr0.5CoO3 – x on SrTiO3 using synchrotron X-ray diffraction showed the formation of an ordered phase at 650 K consisting of La and Sr cations in planes parallel to the surface associated with coherent expansion in the c-direction of about 5% (Donner et al., 2011). This chemical ordering is ascribed to the epitaxial strain imposed by the substrate. The origin of the chemical ordering in LSC films was assessed using density-function-theory-based ab initio methods (GGA + U) on LaCoO3 and La0.875Sr0.125CoO3 – x. A key factor that drives the A-site ordering is the presence of a higher concentration of vacancies in the strained film than are present in bulk samples under the same conditions. A high concentration of oxygen vacancies was also observed for thin strained films of La0.8Sr0.2CoO3 – x (20, 45, 130 nm) deposited on YSZ single crystals with intermediate 5 nm GDC layers to prevent interfacial reactions (La O’ et al., 2010). The films were evaluated by electrochemical impedance spectroscopy using patterned microelectrodes. The oxygen non-stoichiometries in the LSC films was estimated by using the chemical capacitance, and are approximately 100 times greater than those of bulk LSC at pO2 = 1 atm, whereas they are about five times higher at 10−4 atm. The surface exchange rates determined from the impedance data are much higher than observed in the bulk, presumably as a result of the high oxygen vacancy concentration in the films. Clearly, epitaxial strain has a profound effect on the thermo-kinetic behavior of these cathode materials.
15.4.5 Heterostructured interfaces
Sase et al., investigated large-grained polycrystalline (La0.6Sr0.4)CoO3 – x (113) with (La,Sr)2CoO4 (214) grains as a secondary phase by 18O exchange and SIMS analysis and by impedance spectroscopy. The oxygen surface exchange rate was found to dramatically increase at the interface between the two phases (Sase et al., 2008b; Yashiro et al., 2009). The observation was confirmed for a thin-film structure in which PLD was used to deposit La1.5Sr0.5CoO4 on La0.6Sr0.4CoO3 on a polycrystalline GDC substrate (Sase et al., 2008a). After 18O isotope exchange, the SIMS images (Fig. 15.4) show clearly that fast oxygen incorporation occurs along the hetero-phase interface as found in the ceramic samples. Similar results were obtained recently for ~ 85 nm thick La0.8Sr0.2CoO3 – x films prepared by PLD on YSZ single crystals with GDC as the buffer layer (Crumlin et al., 2010). The films were subsequently decorated by depositing layers of (La0.5Sr0.5)2CoO4+x of ~ 0.1, ~ 0.8, ~ 5, ~ 15 nm thickness on top. Partial coverage (< 15 nm) on the perovskite film leads to the formation of nano-islands of 214 on 113 and a large number of interfacial 113/214 boundaries on the film surface. Impedance measurements showed that the oxygen reduction rate was dramatically enhanced (~ 3–4 orders of magnitude above bulk 113) for partial coverage. Increasing LSC214 the thickness to ~ 15 nm led to a fully dense surface layer with a 214 reaction rate. While the mechanism for the enhancement in reaction rate is not yet understood, clearly the interfaces are important.
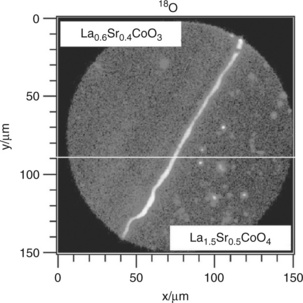
15.4 SIMS image of 18O intensity at the surface of La0.5Sr0.5CoO4/La0.6Sr0.4CoO3/GDC, after diffusion annealing at 773 K for 180 s, in 0.2 bar 18O2 gas. Source: Reproduced with permission from Sase et al. 2008a.
Thin films of several other perovskite oxide compositions have been investigated, including La1 – xSrxCo1 – yFeyO3 – x (Baumann et al., 2006a, 2007), Ba0.5Sr0.5Co0.8Fe0.2O3 – x (Baumann et al., 2006b; Burriel et al., 2010b), Sm0.5Sr0.5CoO3 – x (Baumann et al., 2008) and SrTi1 – xFexO3 – x (Jung and Tuller, 2008, 2009), but are not discussed here.
15.4.6 Anisotropic materials
In addition to perovskite oxides, other oxide structure types that have anisotropic bulk properties have been investigated for application as thin-film cathodes. The main ones are the K2NiF4 structure type exemplified by La2NiO4 and the A-site-ordered double perovskites AA″B2O5+x.
K2NiF4 structures
Ln2NiO4 + x (Ln = La, Pr, Nd) oxides are of interest for SOFC cathodes because of the high diffusivity of the interstitial oxygen ions. The structure of Ln2NiO4 + x can be described as a succession of LaNiO3 perovskite layers alternating with LaO rock salt layers which contain interstitial oxygen anions. In La2NiO4 + x at ambient temperature, x can be as high as 0.18 and in Pr2NiO4 + x, the maximum value of x is 0.22. As expected from the structure, the diffusion in the plane of the interstitial oxygen is much higher than along the perpendicular direction. Thin films of La2NiO4 + x. 300 nm thick, were deposited on LAO single crystals by PLD and studied by electrical conductivity relaxation (Kim et al., 2006a). Unlike similar measurements on isotropic perovskites, the experimental data were not well described by a single time constant model for oxygen surface exchange, but a good fit was obtained using two independent time constants. X-ray diffraction, after the measurements, indicated that some reconstruction had occurred with formation of the energetically more favored (100) surface suggesting that the two different rates correspond to reactions on different crystal faces. The rate constants were significantly lower than values measured for bulk samples. The same group also made impedance measurements on a symmetrical cell of La2NiO4 deposited on single crystal YSZ by PLD (Kim et al., 2007). Because of the lattice mismatch, the film ends up being polycrystalline though with some orientation. The oxygen reduction reaction rate at the film surface in this case agrees well with values for bulk samples. Interestingly and somewhat unexpectedly, a (110)-oriented flat epitaxial film of Nd2NiO4 + x was successfully grown on the (100) surface of YSZ (Yamada et al., 2008). The most detailed study of the properties of La2NiO4 + x films was made by isotope exchange and depth profiling (Burriel et al., 2008). Measurements were made on c-axis-oriented La2NiO4 + x films grown on STO and NdGaO3 (110) by pulsed injection metal organic chemical vapor deposition, with different thicknesses ranging from 33 to 370 nm. Tracer diffusion and surface exchange coefficients were measured along c and in the ab plane; the measurements in the ab plane were made by masking the top surface of the film with gold and then cutting a line though the film to expose the basal surface (see Fig. 15.5). The diffusion coefficients were observed to increase with film thickness as the strain in the film relaxed but the surface exchange coefficient did not change. As anticipated, the tracer diffusion and surface exchange coefficients are approximately two orders of magnitude higher along the ab plane than along the c-axis. The surface exchange coefficients have quite different activation energies, as observed previously for the values measured by ECR.
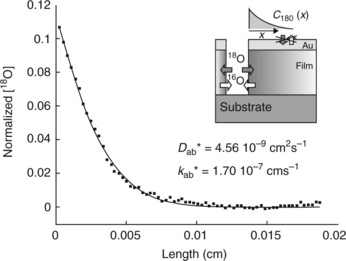
15.5 Normalized isotope fraction profile along the ab plane of a 170 nm La2NiO4 film deposited on STO, exchanged at 420°C. Inset: configuration scheme for oxygen transport measurements in the ab plane. Source: Reproduced with permission from Burriel et al. 2008.
Ordered double perovskites
The structures of A-site-ordered double perovskites of general formula AA′Co2O5+x (A = RE, Y and A′ = Ba, Sr) consist of double layers of square pyramidally coordinated cobalt cations separated by layers of lanthanide cations. The A-site ordering was shown by Taskin et al., to be associated with rapid oxygen ion transport in bulk samples of GdBaB2O5 + x (B = Mn, Co) (Taskin et al., 2005). Subsequently, dense epitaxial PrBaCo2O5 + x (PBCO) and LaBaCo. O5 + x (LBCO) thin films were deposited on STO and LAO single-crystal substrates by PLD (Kim et al., 2006b; Jiang et al., 2008b). These films were shown to have high electronic conductivity and rapid but more complex surface exchange kinetics than the perovskites due to the structural anisotropy. As yet the influences on the kinetics of the degree of A-site order and the film orientation with respect the substrate are not well understood and further work is needed. In PBCO on STO the film is oriented such that the ab plane containing the oxygen vacancies is perpendicular to the substrate. The structural complexities that can be present in the microstructures of double perovskite films were recently shown in studies of GdBaCo2O5 5 + x epitaxial films (Burriel et al., 2010a). The films mainly consist of single- and double-perovskite regions that are oriented in different directions depending on the deposition temperature. Depletion of cobalt ions induces the formation of a high density of stacking defects in the films in the form of additional GdO planes along the c-axis. The films closer to the stoichiometric composition show p-type electronic conductivity with values as high as 800 S/cm at 330°C at pO2 = 1 atm, comparable to PBCO.
15.5 Device structures
Several different approaches have been used for the fabrication of micro-SOFCs that differ in how the anode/electrolyte/cathode membrane structure is supported and the nature of the support material (Evans et al., 2009a). In general, membranes are synthesized by thin-film deposition techniques such as sputtering or PLD and are either free-standing or are supported on a porous substrate or grid structure. The support material must be inert and thermally and mechanically stable during the deposition and any subsequent processing. Free standing membranes and some grid-supported structures require lithography to expose the membrane surface after film deposition. Most studies have used silicon/silicon nitride substrates (Si/Si3N4) which are attractive because lithography and processing can use standard methods of the semiconductor industry. In addition, Gauckler and co-workers at ETH Zurich have made extensive studies of membranes deposited on Foturan®, a photo-structurable glass ceramic (Muecke et al., 2008b; Tolke et al., 2009), and nickel metal has been used both as the substrate and the anode for fuel oxidation. Some of the recent work on each substrate type is briefly reviewed.
15.5.1 Silicon
General processing schemes for SOFCs on Si/Si3N4 have been described by several authors and there are a number of variations (Jankowski et al., 2002). The method used by Huang et al. (2007) is briefly described and illustrated in Fig. 15.6. A 500-nm thick silicon nitride film was deposited on both sides of a Si wafer by low-pressure chemical vapor deposition to provide an insulating layer and to prevent reaction of Si with YSZ. One side of the Si wafer was coated with photoresist using a mask, then exposed and developed. The exposed silicon nitride was removed by reactive ion etching and the residual photoresist stripped off. Then 50 nm YSZ was deposited on the other side by rf sputtering at 200°C. Windows in the Si were etched with 30% KOH at 85–90°C and silicon nitride on both sides removed by reactive ion etching. Finally, by using physical masks, 80 nm porous Pt layers were patterned on both sides of the electrolyte as cathode and anode. Other variations of this scheme have been used to produce more complex structures such as with a corrugated thin-film electrolyte (Su et al., 2008) and with a nickel grid support (Rey-Mermet and Muralt, 2008; Johnson et al., 2010).
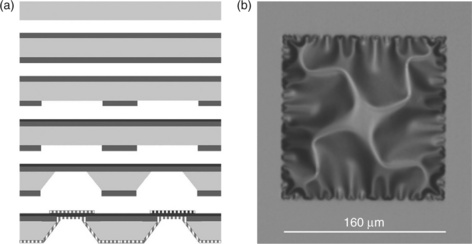
15.6 (a) Processing scheme for SOFCs on Si/Si3N4 (for a discussion see text). (b) An optical micrograph of a YSZ free-standing membrane showing a characteristic buckling pattern. (Source: Reproduced with permission from reference Huang et al., 2007.)(Source: Reproduced with permission from Kerman et al., 2011.)
A variety of film deposition methods have been used to deposit the electrolyte layers, the most common being reactive sputtering from an Y–Zr metal target but pulsed laser deposition (Tarancon et al., 2009), atomic layer deposition (Shim et al., 2007) and pulsed injection metal–organic chemical vapor deposition have also been used (Garbayo et al., 2010).
The performances of silicon-based devices have been discussed in a recent excellent review and are only briefly summarized here with some updates (Evans et al., 2009b). As noted by Evans et al., it is very difficult to make comparisons of device performance since different temperatures and fuel conditions have been used. The electrolyte layers differ in thickness ranging from 50 to 150 nm and the membrane areas range from 20 × 20 μm to 240 × 240 μm; corrugated nickel grid supported membranes have been made in mm sizes. In general higher performance is obtained for thinner electrolyte layers provided that they are pinhole free to avoid reduction in the open-circuit voltage and the addition of a GDC layer helps charge transfer on the cathode side leading to better performance. High power densities have been observed for corrugated membranes (677 mWcm−2 on a 600 × 600 μm membrane at 400°C) and most recently a value of 1037 mWcm−2 was reported for a 160 × 160 μm membrane with 100 nm of YSZ and Pt electrodes at 500°C (Kerman et al., 2011). In that study, the overall stability during 12 h of continuous use was investigated. The power density was reduced by ~ 50% at 400°C after 12 continuous hours of testing due to coarsening of the Pt electrodes particularly the anode. In the majority of cells that have been evaluated platinum electrodes have been used. More recently, efforts have been made to introduce complex oxides such as La0.32Sr0.68CoO3 (Rey-Mermet and Muralt, 2007) and La0.6Sr0.4Co0.8Fe0.2O3 (Xiong et al., 2009; Lai et al., 2011) into silicon-based cells to address the problem with Pt stability.
15.5.2 Foturan®
Foturan® is a photostructurable glass ceramic (Mikroglas Chemtech, n.d.) that has been used at ETH as a substrate for micro-SOFCs. Micropatterning is achieved by exposing through a shadow mask with holes 200 ^m in diameter to UV light (319 nm), while the rest of the sample is covered by a mask. During the exposure, the reactions Ce3 + + hv → Ce4 + + e′ and Ag+ + e′ → Ag occur. The UV exposure is followed by annealing at 500 and 600°C, when the reduced silver ions agglomerate and grow to larger nuclei at 500°C. At 600°C, the glass crystallizes around the silver nuclei forming lithium metasilicates (Livingston et al., 2007). The crystalline regions can then be removed by etching with aqueous hydrofluoric acid; the crystalline regions etch 20 times faster than the glassy matrix. Muecke et al., fabricated a micro-SOFC consisting of a 550 nm thick YSZ electrolyte (prepared by PLD) with a 50 nm thick sputtered Pt anodes deposited on a Foturan® substrate. Platinum paste, sputtered platinum and La0.6Sr0.4Co0.2Fe0.8O3 deposited by spray pyrolysis were used as cathodes. The arrangement is shown in Fig. 15.7 for a cell with a LSCF cathode. The Pt anode and the LSCF cathode form nanoporous electrodes that adhere well to the electrolyte. The YSZ electrolyte has a columnar structure with elongated grains perpendicular to the substrate. The power output from the cells was found to be limited by pinholes in the electrolyte and the electrode polarization resistance, but not by the resistance of the electrolyte. The best results were obtained with a LSCF cathode. Sputtering an additional 200 nm layer of YSZ on top of the PLD layer and using platinum electrodes gave a higher open-circuit voltage (1.06 V) and a maximum power density of 152 mWcm−2 at 550°C.
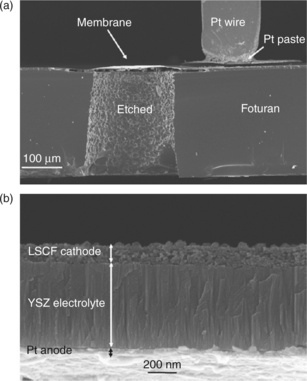
15.7 (a) SEM cross-section view of a μ-SOFC on a Foturan® substrate, (b) view of the membrane cross-section. Source: Reproduced with permission from Bieberle-Huetter et al., 2008.
15.5.3 Nickel
Several micro-SOFC devices have been constructed based on nickel anode supports. In one approach (Kang et al., 2006), a nanostructured Ni substrate was constructed from an anodic aluminum oxide template following a previously reported two-step replication process (Masuda and Fukuda, 1995). The template has pores 20 nm in diameter on one surface which increase to 200 nm at about 70 nm below this surface. The pores were filled with polymethylmethacrylate (PMMA) to obtain the negative replica and the aluminum oxide removed by dissolution in aqueous sodium hydroxide. The final structure was obtained by electroplating Ni into the negative structure followed by removal of PMMA with acetone. A thin-film SOFC was fabricated using on this substrate by reactively sputtering 200 nm of YSZ followed by sputtering a porous Pt layer. The final structure was 12 mm in diameter and 20 μm thick. The cell was operated on 3% H2 at 370–550°C and had a maximum power output of 7 mW/cm2 at 400°C. The same group also reactively sputtered a thin film of Y–Zr alloy on the surface of an AAO substrate. The volume expansion on oxidation of the alloy resulted in a dense gas tight YSZ thin film. The fuel cell performance was, however, modest, mostly due to the development of cracks due to the poor mechanical properties of AAO at high temperature (Park et al., 2006).
Ignatiev and co-workers used a lithographic approach to build a thin-film cell on a ~ 10 μm nickel foil substrate (Chen et al., 2004; Ignatiev et al., 2008). A 1–2 μm thick film of YSZ was deposited on the nickel foil by PLD. The Ni foil substrate was then converted into a porous anode by photolithographic patterning and etching with FeCl3. The electrolyte is polycrystalline when deposited on a polycrystalline substrate, and was (100)-textured when deposited on a textured nickel foil substrate. A nickel cermet layer was added to the foil to increase the length of the triple phase boundary and a porous La0.5Sr0.5CoO3 cathode was deposited by PLD. The resultant fuel cell achieved a maximum power density of 40 mW/cm2 at 515°C.
Most recently, another and simpler approach was developed that uses a nickel substrate but without lithography or etching (Joo and Choi, 2008). A porous nickel film was prepared by screen printing a layer of nickel oxide onto a ceramic substrate. After reduction in hydrogen at 700–750°C a porous nickel film was obtained (22 μm thick) which was easily removed from the substrate. The free-standing film was mounted on a nickel ring and 2 μm of GDC deposited on top by PLD (substrate temperature 500°C) followed by PLD deposition of porous La0.7Sr0.3CoO3 – x at ambient temperature (see Fig. 15.8). Contact to the LSC film was made with Pt. The cell was tested on 97%H2, 3% H2O at 450°C of 26 mW/cm2. The open-circuit voltage was lower than expected, possibly due to pin holes in the electrolyte layer.
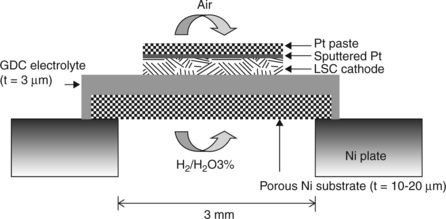
15.8 Schematic of the thin-film fuel cell supported on porous nickel. Source: Reproduced with permission from Joo and Choi, 2008.
15.6 Conclusions
The performance of single micro-SOFCs indicated in the earlier examples show that they can meet the power requirements for portable electronic devices but much work remains to overcome the technical challenges. At this stage of development little is known about long-term durability, performance degradation due to interface reactions and mechanical stability on thermal cycling. Further work will be needed to develop processes for fabricating fuel cell stacks and arrays to get sufficient power output for applications (1–20 W). The other system components including the fuel processing unit, efficient removal of non-combusted fuel, and thermal management need to be integrated with the fuel cell in order to produce a complete ‘battery replacement’ (Bieberle-Huetter et al., 2008).
Micro-SOFCs based on thin films are inherently nanostructured and dominated by the presence of interfaces both between and within individual components. Often such interfaces result in thermodynamic and kinetic properties that are very different from the bulk due to strain, disorder, composition variations and impurity segregation. Control of interface chemistry and structure can lead to materials with significantly enhanced properties but many aspects of the underlying science are not yet well understood. The wide variation in the reported conductivities for GDC films with different microstructures is an illustration of the difficulty of control whereas the enhancement of oxygen surface exchange kinetics in 113/214 cathode heterostructures is an example of a potential benefit. One of the future challenges in the characterization of materials for micro fuel cells, and of high-temperature electrocatalytic processes in general, is to develop better understanding of the surface and interface chemistry under operating conditions. Recent progress has been made in the application of a variety of in situ techniques including synchrotron X-ray diffraction (Donner et al., 2011), X-ray fluorescence (Fister et al., 2008), Raman spectroscopy (Blinn et al., 2010) and photoelectron spectroscopy (Vovk et al., 2005; Backhaus-Ricoult et al., 2008). Such techniques provide important information about how surface and interface structure and composition respond to changes in temperature, environment and electrode polarization, information that is needed to design and control thin-film material properties.
15.7 Acknowledgments
The author acknowledges support from the Robert A Welch Foundation (Grant No. E-0024) (writing) and the U.S. Department of Energy (U.S. DOE), Office of Basic Energy Sciences, Division of Materials Sciences and Engineering (under Award No. DE-SC0001284) (literature survey).
15.8 References
Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. (Washington, DC). 2004; 104:4791–4843.
Azad, S., Marina, O.A., Wang, C.M., Saraf, L., Shutthanandan, V., Mccready, D.E., El-Azab, A., Jaffe, J.E., Engelhard, M.H., Peden, C.H.F., Thevuthasan, S. Nanoscale effects on ion conductance of layer-by-layer structures of gadolinia-doped ceria and zirconia. Appl. Phys. Lett.. 2005; 86:131906/1–131906/3.
Backhaus-Ricoult, M., Adib, K., St. Clair, T., Luerssen, B., Gregoratti, L., Barinov, A. In-situ study of operating SOFC LSM/YSZ cathodes under polarization by photoelectron microscopy. Solid State Ionics. 2008; 179:891–895.
Baertsch, C.D., Jensen, K.F., Hertz, J.L., Tuller, H.L., Vengallatore, S.T., Spearing, S.M., Schmidt, M.A. Fabrication and structural characterization of selfsupporting electrolyte membranes for a micro solid-oxide fuel cell. J. Mater. Res.. 2004; 19:2604–2615.
Baumann, F.S., Fleig, J., Cristiani, G., Stuhlhofer, B., Habermeier, H.U., Maier, J. Quantitative comparison of mixed conducting SOFC cathode materials by means of thin film model electrodes. J. Electrochem. Soc.. 2007; 154:B931–B941.
Baumann, F.S., Fleig, J., Habermeier, H.-U., Maier, J. Impedance spectroscopic study on well-defined (La, Sr)(Co, Fe)O3-x model electrodes. Solid State Ionics. 2006; 177:1071–1081.
Baumann, F.S., Fleig, J., Habermeier, H.U., Maier, J. Ba0.5Sr0.5Co0.8Fe0.2O3–x thin film microelectrodes investigated by impedance spectroscopy. Solid State Ionics. 2006; 177:3187–3191.
Baumann, F.S., Maier, J., Fleig, J. The polarization resistance of mixed conducting SOFC cathodes: A comparative study using thin film model electrodes. Solid State Ionics. 2008; 179:1198–1204.
Beckel, D., Bieberle-Huetter, A., Harvey, A., Infortuna, A., Muecke, U.P., Prestat, M., Rupp, J.L.M., Gauckler, L.J. Thin films for micro solid oxide fuel cells. J. Power Sources. 2007; 173:325–345.
Bieberle-Huetter, A., Beckel, D., Infortuna, A., Muecke, U.P., Rupp, J.L.M., Gauckler, L.J., Rey-Mermet, S., Muralt, P., Bieri, N.R., Hotz, N., Stutz, M.J., Poulikakos, D., Heeb, P., Mueller, P., Bernard, A., Gmuer, R., Hocker, T. A micro-solid oxide fuel cell system as battery replacement. J. Power Sources. 2008; 177:123–130.
Blinn, K.S., Abernathy, H.W., Liu, M. Surface enhanced Raman spectroscopy for investigation of SOFC cathodes. Ceram. Eng. Sci. Proc.. 2010; 30:65–73.
Brichzin, V., Fleig, J., Habermeier, H.U., Cristiani, G., Maier, J. The geometry dependence of the polarization resistance of Sr-doped LaMnO3 microelectrodes on yttria-stabilized zirconia. Solid State Ionics. 2002; 152–153:499–507.
Brichzin, V., Fleig, J., Habermeier, H.U., Maier, J. Geometry dependence of cathode polarization in solid oxide fuel cells investigated by defined Sr-doped LaMnO3 microelectrodes. Electrochem. Solid-State Lett.. 2000; 3:403–406.
Brichzin, V., Fleig, J., Habermeier, H.U., Maier, J. Investigation of the cathodic polarization mechanism in SOFCs by means of LSM-microelectrodes. Proc. Electrochem. Soc.. 2001; 2001–16:555–563.
Buergler, B.E., Ochsner, M., Vuillemin, S., Gauckler, L.J. From macro- to micro-single chamber solid oxide fuel cells. J. Power Sources. 2007; 171:310–320.
Burriel, M., Casas-Cabanas, M., Zapata, J., Tan, H., Verbeeck, J., Solis, C., Roqueta, J., Skinner, S.J., Kilner, J.A., Van Tendeloo, G., Santiso, J. Influence of the microstructure on the high-temperature transport properties of GdBaCo2O5.5+x epitaxial films. Chem. Mater.. 2010; 22:5512–5520.
Burriel, M., Garcia, G., Santiso, J., Kilner, J.A., Chater, R.J., Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+x. J. Mater. Chem.. 2008; 18:416–422.
Burriel, M., Niedrig, C., Menesklou, W., Wagner, S.F., Santiso, J., Ivers-Tiffee, E. BSCF epitaxial thin films: Electrical transport and oxygen surface exchange. Solid State Ionics. 2010; 181:602–608.
Cavallaro, A., Burriel, M., Roqueta, J., Apostolidis, A., Bernardi, A., Tarancon, A., Srinivasan, R., Cook, S.N., Fraser, H.L., Kilner, J.A., Mccomb, D.W., Santiso, J. Electronic nature of the enhanced conductivity in YSZ-STO multilayers deposited by PLD. Solid State Ionics. 2010; 181:592–601.
Chao, C.-C., Park, J.S., Prinz, F.B. In-plane conductivities of atomic layer deposited yttria-stabilized zirconia electrolytes for solid oxide fuel cells. ECS Trans.. 2009; 16:157–164.
Chen, L., Chen, C.L., Chen, X., Donner, W., Liu, S.W., Lin, Y., Huang, D.X., Jacobson, A.J. Electrical properties of a highly oriented, textured thin film of the ionic conductor Gd:CeO2-x on (001) MgO. Appl. Phys. Lett.. 2003; 83:4737–4739.
Chen, X., Wang, S., Yang, Y.L., Smith, L., Wu, N.J., Jacobson, A.J., Ignatiev, A. A study of the oxygen surface exchange coefficient on La0.5Sr0.5CoO3–x thin films. Mater. Res. Soc. Symp. Proc.. 2000; 606:275–280.
Chen, X., Wang, S., Yang, Y.L., Smith, L., Wu, N.J., Kim, B.I., Perry, S.S., Jacobson, A.J., Ignatiev, A. Electrical conductivity relaxation studies of an epitaxial La0.5Sr0.5CoO3–x thin film. Solid State Ionics. 2002; 146:405–413.
Chen, X., Wu, N.J., Smith, L., Ignatiev, A. Thin-film heterostructure solid oxide fuel cells. Appl. Phys. Lett.. 2004; 84:2700–2702.
Chiang, Y.M., Lavik, E.B., Kosacki, I., Tuller, H.L., Ying, J.Y. Nonstoichiometry and electrical conductivity of nanocrystalline CeO2-x. J. Electroceram.. 1997; 1:7–14.
Chiodelli, G., Malavasi, L., Massarotti, V., Mustarelli, P., Quartarone, E. Synthesis and characterization of Ce0.8Gd0.2O2-y polycrystalline and thin film materials. Solid State Ionics. 2005; 176:1505–1512.
Chun, S.Y., Mizutani, N. The transport mechanism of yttria-stabilized zirconia thin films prepared by MOCVD. Appl. Surf. Sci.. 2001; 171:82–88.
Claeson, T., Ivanov, Z., Winkler, D. Superconducting films and devices. Curr. Opin. Solid State Mater. Sci.. 1999; 4:45–52.
Crumlin, E.J., Mutoro, E., Ahn, S.-J., La O, G.J., Leonard, D.N., Borisevich, A., Biegalski, M.D., Christen, H,.M., Shao-Horn, Y. Oxygen reduction kinetics enhancement on a heterostructured oxide surface for solid oxide fuel cells. J. Phys. Chem. Lett.. 2010; 1:3149–3155.
Donner, W., Chen, C., Liu, M., Jacobson, A.J., Lee, Y.-L., Gadre, M., Morgan, D. Epitaxial strain-induced chemical ordering in La0.5Sr0.5CoO3–x films on SrTiO3. Chem. Mater.. 2011; 23(4):984–988.
Evans, A., Bieberle-Huetter, A., Galinski, H., Rupp, J.L.M., Ryll, T., Scherrer, B., Toelke, R., Gauckler, L.J. Micro-solid oxide fuel cells: status, challenges, and chances. Monatsh. Chem.. 2009; 140:975–983.
Evans, A., Bieberle-Huetter, A., Rupp, J.L.M., Gauckler, L.J. Review on microfabricated micro-solid oxide fuel cell membranes. J. Power Sources. 2009; 194:119–129.
Fister, T.T., Fong, D.D., Eastman, J.A., Baldo, P.M., Highland, M.J., Fuoss, P.H., Balasubramaniam, K.R., Meador, J.C., Salvador, P.A. In situ characterization of strontium surface segregation in epitaxial La0.7Sr0.3MnO3 thin films as a function of oxygen partial pressure. Appl. Phys. Lett.. 2008; 93:151904/1–151904/3.
Fleig, J. Solid oxide fuel cell cathodes: Polarization mechanisms and modeling of the electrochemical performance. Annu. Rev. Mater. Res.. 2003; 33:361–382.
Fleig, J., Kim, H.R., Jamnik, J., Maier, J. Oxygen reduction kinetics of lanthanum manganite (LSM) model cathodes: partial pressure dependence and rate-limiting steps. Fuel Cells (Weinheim, Ger.). 2008; 8:330–337.
Garbayo, I., Tarancon, A., Santiso, J., Cavallaro, A., Gracia, I., Cane, C and Sabate, N (2010). Silicon-based microplatforms for characterization of nanostructured layers with application in intermediate temperature micro solid oxide fuel cells. Mater. Res. Soc. Symp. Proc., 1256E, Paper #: 1256-N16–18.
Garcia-Barriocanal, J., Rivera-Calzada, A., Varela, M., Sefrioui, Z., Iborra, E., Leon, C., Pennycook, S.J., Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science (Washington, DC, U. S.). 2008; 321:676–680.
Garcia-Barriocanal, J., Rivera-Calzada, A., Varela, M., Sefrioui, Z., Iborra, E., Leon, C., Pennycook, S.J., Santamaria, J. Response to comment on “colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science (Washington, DC, U. S.). 2009; 324:465.
Gharbage, B., Mandier, F., Lauret, H., Roux, C., Pagnier, T. Electrical properties of La0.5Sr0.5MnO3 thin films. Solid State Ionics. 1995; 82:85–94.
Gharbage, B., Pagnier, T., Hammou, A. Oxygen reduction at La0.5Sr0.5MnO3 thin film/yttria-stabilized zirconia interface studied by impedance spectroscopy. J. Electrochem. Soc.. 1994; 141:2118–2121.
Guo, X. Comment on ‘colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures’. Science (Washington, DC, U. S.). 2009; 324:465.
Guo, X., Maier, J. Comprehensive modeling of ion conduction of nano-sized CaF2/BaF2 multilayer heterostructures. Adv. Funct. Mater.. 2009; 19:96–101.
Guo, X., Maier, J. Ionically conducting two-dimensional heterostructures. Adv. Mater. (Weinheim, Ger.). 2009; 21:2619–2631.
Heiroth, S., Lippert, T., Wokaun, A., Doebeli, M. Microstructure and electrical conductivity of YSZ thin films prepared by pulsed laser deposition. Appl. Phys. A Mater. Sci. Process.. 2008; 93:639–643.
Heiroth, S., Lippert, T., Wokaun, A., Doebeli, M., Rupp, J.L.M., Scherrer, B., Gauckler, L.J. Yttria-stabilized zirconia thin films by pulsed laser deposition: Microstructural and compositional control. J. Eur. Ceram. Soc.. 2010; 30:489–495.
Hemmi, D., Iwamoto, K., Hirai, N., Sase, M., Unemoto, A., Nakamura, T., Horikiri, F., Mori, Y., Sato, K., Yashiro, K., Kawada, T., Mizusaki, J. Electronic properties and oxygen nonstoichiometry of mixed-conducting oxide La1-xSrxCoO3–y thin films at high temperature. ECS Trans.. 2009; 16:311–316.
Hertz, J.L., Tuller, H.L. Nanocomposite platinum-yttria stabilized zirconia electrode and implications for micro-SOFC operation. J. Electrochem. Soc.. 2007; 154:B413–B418.
Hertz, J.L and Tuller, H.L (2009). Micro fuel cells. Edited by A. Mitsos and P. I. Barton, Microfabr. Power Gener. Devices, 51–80.
Holme, T.P., Lee, C., Prinz, F.B. Atomic layer deposition of LSM cathodes for solid oxide fuel cells. Solid State Ionics. 2008; 179:1540–1544.
Horita, T., Yamaji, K., Ishikawa, M., Sakai, N., Yokokawa, H., Kawada, T., Kato, T. Active sites imaging for oxygen reduction at the Laa9Sr01MnO3–x/yttria-stabilized zirconia interface by secondary-ion mass spectrometry. J. Electrochem. Soc.. 1998; 145:3196–3202.
Horita, T., Yamaji, K., Sakai, N., Yokokawa, H., Kato, T. Oxygen transport at the LaMnO3 film/yttria-stabilized zirconia interface under different cathodic overpotentials by secondary ion mass spectrometry. J. Electrochem. Soc.. 2001; 148:J25–J30.
Horita, T., Yamaji, K., Sakai, N., Yokokawa, H., Kawada, T., Kato, T. Oxygen reduction sites and diffusion paths at La0.9Sr0.1MnO3-x yttria-stabilized zirconia interface for different cathodic overvoltages by secondary-ion mass spectrometry. Solid State Ionics. 2000; 127:55–65.
Huang, D.X., Chen, C.L., Chen, L., Jacobson, A.J. Strain relaxation by directionally aligned precipitate nanoparticles in the growth of single-crystalline Gd-doped ceria thin films. Appl. Phys. Lett.. 2004; 84:708–710.
Huang, D.X., Chen, C.L., Jacobson, A.J. Single-crystal and nanocolumnar growth of gadolinium-doped ceria thin films on oxide substrates studied using electron microscopy. Mater. Res. Soc. Symp. Proc.. 2004; 795:223–228.
Huang, D.X., Chen, C.L., Jacobson, A.J. Interface structures and periodic film distortions induced by substrate-surface steps in Gd-doped ceria thin-film growth. J. Appl. Phys.. 2005; 97:043506/1–043506/5.
Huang, H., Gur, T.M., Saito, Y., Prinz, F. High ionic conductivity in ultrathin nanocrystalline gadolinia-doped ceria films. Appl. Phys. Lett.. 2006; 89:143107/1–143107/3.
Huang, H., Nakamura, M., Su, P., Fasching, R., Saito, Y., Prinz, F.B. Highperformance ultrathin solid oxide fuel cells for low-temperature operation. J. Electrochem. Soc.. 2007; 154:B20–B24.
Huang, Y.-H., Dass, R.I., Xing, Z.-L., Goodenough, J.B. Double perovskites as anode materials for solid-oxide fuel cells. Science (Washington, DC, U. S.). 2006; 312:254–257.
Ignatiev, A., Chen, X., Wu, N., Lu, Z., Smith, L. Nanostructured thin solid oxide fuel cells with high power density. Dalton Trans. 2008; 5501–5506.
Infortuna, A., Harvey, A.S., Muecke, U.P., Gauckler, L.J. Nanoporous Ni-Ce0.8Gd0.2O1.9-x thin film cermet SOFC anodes prepared by pulsed laser deposition. Phys. Chem. Chem. Phys.. 2009; 11:3663–3670.
Ioroi, T., Hara, T., Uchimoto, Y., Ogumi, Z., Takehara, Z.-I. Preparation of perovskite-type La1-xSrxMnO3 films by vapor-phase processes and their electrochemical properties. J. Electrochem. Soc.. 1997; 144:1362–1370.
Ishihara, T., Yan, J., Enoki, M., Okada, S., Matsumoto, H. Ni-Fe alloy-supported intermediate temperature SOFC using LaGaO3 electrolyte film for quick startup. J. Fuel Cell Sci. Technol.. 2008; 5:031205/1–031205/3.
Jamnik, J., Maier, J. Treatment of the impedance of mixed conductors. Equivalent circuit model and explicit approximate solutions. J. Electrochem. Soc.. 1999; 146:4183–4188.
Jamnik, J., Maier, J. Generalized equivalent circuits for mass and charge transport: Chemical capacitance and its implications. Phys. Chem. Chem. Phys.. 2001; 3:1668–1678.
Jankowski, A.F., Hayes, J.P., Graff, R.T., Morse, J.D. Micro-fabricated thin-film fuel cells for portable power requirements. Mater. Res. Soc. Symp. Proc.. 2002; 730:93–98.
Januschewsky, J., Ahrens, M., Opitz, A., Kubel, F., Fleig, J. Optimized La0.6Sr0.4CoO3-x thin-film electrodes with extremely fast oxygen-reduction kinetics. Adv. Funct. Mater.. 2009; 19:3151–3156.
Jee, Y., Chang, I., Son, J.-W., Lee, J.-H., Kang, S., Cha, S.W. Fabrication of thin solid oxide film fuel cells. J. Korean Ceram. Soc.. 2010; 47:82–85.
Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci.. 2008; 43:6799–6833.
Jiang, X., Bent, S.F. Area-selective atomic layer deposition of platinum on ysz substrates using microcontact printed SAMs. J. Electrochem. Soc.. 2007; 154:D648–D656.
Jiang, X., Huang, H., Prinz, F.B., Bent, S.F. Application of atomic layer deposition of platinum to solid oxide fuel cells. Chem. Mater.. 2008; 20:3897–3905.
Jiang, X. N., Wang, S., Kim, G., Liu, J., Liu, M., Gong, W. Q., Chen, C. L. and Jacobson, A. J. 2008b. Oxygen exchange kinetics of epitaxial PrBaCo2O5+x and LaBaCo2O5+x thin films. Mater. Res. Soc. Symp. Proc., 1126, Paper #: 1126-S01–05.
Johnson, A.C., Baclig, A., Harburg, D.V., Lai, B.-K., Ramanathan, S. Fabrication and electrochemical performance of thin-film solid oxide fuel cells with large area nanostructured membranes. J. Power Sources. 2010; 195:1149–1155.
Joo, J.H., Choi, G.M. Simple fabrication of micro-solid oxide fuel cell supported on metal substrate. J. Power Sources. 2008; 182:589–593.
Joseph, M., Manoravi, P., Tabata, H., Kawai, T. Preparation of La0.9Sr0.1Ga0.85Mg0.15O2.875 thin films by pulsed-laser deposition and conductivity studies. J. Appl. Phys.. 2002; 92:997–1001.
Jou, S., Wu, T.-H. Thin porous Ni–YSZ films as anodes for a solid oxide fuel cell. J. Phys. Chem. Solids. 2008; 69:2804–2812.
Ju, Y.-W., Eto, H., Inagaki, T., Ida, S., Ishihara, T. Improvement in thermal cycling durability of SOFCs using LaGaO3-based electrolyte by inserting convex Sm0.5Sr0.5CoO3 interlayer. Electrochem. Solid-State Lett.. 2010; 13:B139–B141.
Ju, Y.W., Eto, H., Inagaki, T., Ishihara, T. High power SOFC using LSGM film on NiFe porous bi-metal substrate. ECS Trans.. 2009; 25:719–726.
Jung, W., Tuller, H.L. Investigation of cathode behavior of model thin-film SrTi1-xFexO3.x (x=0.35 and 0.5) mixed ionic-electronic conducting electrodes. J. Electrochem. Soc.. 2008; 155:B1194–B1201.
Jung, W., Tuller, H.L. Investigation of cathode behavior and surface chemistry of model thin film SrTi1-xFexO3.x electrode. ECS Trans.. 2009; 25:2775–2782.
Kang, S., Su, P.C., Park, Y.I., Saito, Y., Prinz, F.B. Thin-film solid oxide fuel cells on porous nickel substrates with multistage nanohole array. J. Electrochem. Soc.. 2006; 153:A554–A559.
Kawada, T., Masuda, K., Suzuki, J., Kaimai, A., Kawamura, K., Nigara, Y., Mizusaki, J., Yugami, H., Arashi, H., Sakai, N., Yokokawa, H. Oxygen isotope exchange with a dense La0.6Sr0.4CoO3-x electrode on a Ce0.9Ca0.1O1.9 electrolyte. Solid State Ionics. 1999; 121:271–279.
Kawada, T., Suzuki, J., Sase, M., Kaimai, A., Yashiro, K., Nigara, Y., Mizusaki, J., Kawamura, K., Yugami, H. Determination of oxygen vacancy concentration in a thin film of La0.6Sr0.4CoO3–x by an electrochemical method. J. Electrochem. Soc.. 2002; 149:E252–E259.
Kerman, K., Lai, B.-K., Ramanathan, S. Pt/Y0.16Zr0.84O1.92/Pt thin film solid oxide fuel cells: Electrode microstructure and stability considerations. J. Power Sources. 2011; 196:2608–2614.
Kim, G., Wang, S., Jacobson, A.J., Chen, C.L. Measurement of oxygen transport kinetics in epitaxial La2NiO4+x thin films by electrical conductivity relaxation. Solid State Ionics. 2006; 177:1461–1467.
Kim, G., Wang, S., Jacobson, A.J., Yuan, Z., Donner, W., Chen, C.L., Reimus, L., Brodersen, P., Mims, C.A. Oxygen exchange kinetics of epitaxial PrBaCo2O5+x thin films. Appl. Phys. Lett.. 2006; 88:024103/1–024103/3.
Kim, G.T., Wang, S., Jacobson, A.J., Yuan, Z., Chen, C. Impedance studies of dense polycrystalline thin films of La2NiO4+x. J. Mater. Chem.. 2007; 17:1316–1320.
Kim, S., Maier, J. On the conductivity mechanism of nanocrystalline ceria. J. Electrochem. Soc.. 2002; 149:J73–J83.
Korte, C., Peters, A., Janek, J., Hesse, D., Zakharov, N. Ionic conductivity and activation energy for oxygen ion transport in superlattices-the semicoherent multilayer system YSZ (ZrO2 + 9.5 mol% Y2O3)/Y2O3. Phys. Chem. Chem. Phys.. 2008; 10:4623–4635.
Korte, C., Schichtel, N., Hesse, D., Janek, J. Influence of interface structure on mass transport in phase boundaries between different ionic materials. Monatsh. Chem.. 2009; 140:1069–1080.
Kosacki, I., Rouleau, C.M., Becher, P.F., Bentley, J., Lowndes, D.H. Surface/interface-related conductivity in nanometer thick YSZ films. Electrochem. Solid-State Lett.. 2004; 7:A459–A461.
Kosacki, I., Rouleau, C.M., Becher, P.F., Bentley, J., Lowndes, D.H. Nanoscale effects on the ionic conductivity in highly textured YSZ thin films. Solid State Ionics. 2005; 176:1319–1326.
Kosacki, I., Suzuki, T., Petrovsky, V., Anderson, H.U. Electrical conductivity of nanocrystalline ceria and zirconia thin films. Solid State Ionics. 2000; 136–137:1225–1233.
Kushima, A., Yip, S., Yildiz, B. Competing strain effects in reactivity of LaCoO3 with oxygen. Phys. Rev. B Condens. Matter Mater. Phys.. 2010; 82:115435/1–115435/6.
La O’, G.J., Ahn, S.-J., Crumlin, E., Orikasa, Y., Biegalski Michael, D., Christen Hans, M., Shao-Horn, Y. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid-oxide fuel cells. Angew Chem Int Ed Engl. 2010; 49:5344–5347.
La O’, G.J., Hertz, J., Tuller, H., Shao-Horn, Y. Microstructural features of RF-sputtered SOFC anode and electrolyte materials. J. Electroceram.. 2004; 13:691–695.
Lai, B.-K., Kerman, K., Ramanathan, S. Nanostructured La0.6Sr0.4Co0.8Fe0.2O3/Y0.0.8Zr0.92O1.96/La0.6Sr0.4Co0.8Fe0.2O3 (LSCF/YSZ/LSCF) symmetric thin film solid oxide fuel cells. J. Power Sources. 2011; 196:1826–1832.
Lin, C.-Y., Liu, C.-Y., Lin, C.-C., Tseng, T.Y. Current status of resistive non-volatile memories. J. Electroceram.. 2008; 21:61–66.
Litzelman, S.J., Hertz, J.L., Jung, W., Tuller, H.L. Opportunities and challenges in materials development for thin film solid oxide fuel cells. Fuel Cells (Weinheim, Ger.). 2008; 8:294–302.
Liu, M., Liu, J., Collins, G., Ma, C.R., Chen, C.L., He, J., Jiang, J.C., Meletis, E.I., Jacobson, A.J., Zhang, Q.Y. Magnetic and transport properties of epitaxial (LaBa)Co2O5.5+x thin films on (001) SrTiO3. Appl. Phys. Lett.. 2010; 96:132106/1–132106/3.
Livingston, F.E., Adams, P.M., Helvajian, H. Examination of the laserinduced variations in the chemical etch rate of a photosensitive glass ceramic. Appl. Phys. A Mater. Sci. Process.. 2007; 89:97–107.
Maier, J. Nanoionics: Ion transport and electrochemical storage in confined systems. Nat. Mater.. 2005; 4:805–815.
Maier, J. Nanoionics: Ionic charge carriers in small systems. Phys. Chem. Chem. Phys.. 2009; 11:3011–3022.
Martin, L.W., Chu, Y.H., Ramesh, R. Advances in the growth and characterization of magnetic, ferroelectric, and multiferroic oxide thin films. Mater. Sci. Eng., R. 2010; R68:89–133.
Masuda, H., Fukuda, K. Ordered metal nanohole arrays made by a two-step replication of honeycomb structures of anodic alumina. Science (Washington, D. C.). 1995; 268:1466–1468.
Masuda, K., Kaimai, A., Kawamura, K.-I., Nigara, Y., Kawada, T., Mizusaki, J., Yugamni, H., Arashi, H. Electrochemical reaction kinetics of mixed conducting electrodes on CeO2-based solid electrolytes. Proc. Electrochem. Soc.. 1997; 97–40:473–482.
Mathews, T., Manoravi, P., Antony, M.P., Sellar, J.R., Muddle, B.C. Fabrication of La1-xSrxGa1-yMgyO3-(x+y)/2 thin films by pulsed laser ablation. Solid State Ionics. 2000; 135:397–402.
Mikroglas Chemtech, G. http://www.mikroglas.de/.
Mims, C.A., Joos, N.I., VanderHeide, P.A.W., Jacobson, A.J., Chen, C., Chu, C.W., Kim, B.I., Perry, S.S. Oxygen transport in oxide thin film structures. Oriented La0.5Sr0.5CoO3-x on single-crystal yttria-stabilized zirconia. Electrochem. Solid-State Lett.. 2000; 3:59–61.
Mitsugi, F., Kanazawa, S., Ohkubo, T., Nomoto, Y., Takita, Y., Ishihara, T. Preparation of oxygen ion conducting doped lanthanum gallate thin films on amorphous and single crystal substrates by pulsed laser deposition. Mater. Res. Soc. Symp. Proc.. 2004; 796:105–110.
Mizusaki, J., Mima, Y., Yamauchi, S., Fueki, K., Tagawa, H. Nonstoichiometry of the perovskite-type lanthanum strontium cobalt oxides (La1-xSrxCoO3.x). J. Solid State Chem.. 1989; 80:102–111.
Mizusaki, J., Saito, T., Tagawa, H. A chemical diffusion-controlled electrode reaction at the compact La1-xSrxMnO3/stabilized zirconia interface in oxygen atmospheres. J. Electrochem. Soc.. 1996; 143:3065–3073.
Muecke, U.P., Akiba, K., Infortuna, A., Salkus, T., Stus, N.V., Gauckler, L.J. Electrochemical performance of nanocrystalline nickel/gadolinia-doped ceria thin film anodes for solid oxide fuel cells. Solid State Ionics. 2008; 178:1762–1768.
Muecke, U.P., Beckel, D., Bernard, A., Bieberle-Hutter, A., Graf, S., Infortuna, A., Muller, P., Rupp, J.L.M., Schneider, J., Gauckler, L.J. Micro solid oxide fuel cells on glass ceramic substrates. Adv. Funct. Mater.. 2008; 18:3158–3168.
Muecke, U.P., Graf, S., Rhyner, U., Gauckler, L.J. Microstructure and electrical conductivity of nanocrystalline nickel- and nickel oxide/gadolinia-doped ceria thin films. Acta Mater.. 2008; 56:677–687.
Noh, H.-S., Park, J.-S., Son, J.-W., Lee, H., Lee, J.-H., Lee, H.-W. Physical and microstructural properties of NiO- and Ni–YSZ composite thin films fabricated by pulsed-laser deposition at T? 700 DegC. J. Am. Ceram. Soc.. 2009; 92:3059–3064.
Noh, H.-S., Son, J.-W., Lee, H., Ji, H.-I., Lee, J.-H., Lee, H.-W. Suppression of Ni agglomeration in PLD fabricated Ni–YSZ composite for surface modification of SOFC anode. J. Eur. Ceram. Soc.. 2010; 30:3415–3423.
Noh, H.-S., Son, J.-W., Lee, H., Song, H.-S., Lee, H.-W., Lee, J.-H. Low temperature performance improvement of SOFC with thin film electrolyte and electrodes fabricated by pulsed laser deposition. J. Electrochem. Soc.. 2009; 156:B1484–B1490.
Park, Y.-I., Su, P.C., Cha, S.W., Saito, Y., Prinz, F.B. Thin-film SOFCs using gastight YSZ thin films on nanoporous substrates. J. Electrochem. Soc.. 2006; 153:A431–A436.
Peters, A., Korte, C., Hesse, D., Zakharov, N., Janek, J. Ionic conductivity and activation energy for oxygen ion transport in superlattices – The multilayer system CSZ (ZrO2 + CaO)/Al2O3. Solid State Ionics. 2007; 178:67–76.
Peters, C., Weber, A., Butz, B., Gerthsen, D., Ivers-Tiffee, E. Grain-size effects in YSZ thin-film electrolytes. J. Am. Ceram. Soc.. 2009; 92:2017–2024.
Peters, C., Weber, A., Ivers-Tiffee, E. Nanoscaled (La0.5Sr0.5)CoO3–x Thin film cathodes for SOFC application at 500 °C<T<700 °C. J. Electrochem. Soc.. 2008; 155:B730–B737.
Podpirka, A., Ramanathan, S. Transference numbers for in-plane carrier conduction in thin film nanostructured gadolinia-doped ceria under varying oxygen partial pressure. J. Am. Ceram. Soc.. 2009; 92:2400–2403.
Prinz, F.B. Opportunities of ALD for thin film solid oxide fuel cells. ECS Trans.. 2008; 16:15–18.
Qin, W., Xiong, J., Li, Y. Comparison on the effect of SrRuO3 and La0.5Sr0.5CoO3 bottom electrode on dielectric properties of Ba0.6Sr0.4TiO3 thin films prepared by pulsed laser deposition. Surf. Rev. Lett.. 2009; 16:493–497.
Rey-Mermet, S. and Muralt, P. 2007. Materials and design study for micromachined solid oxide fuel cells membranes. Mater. Res. Soc. Symp. Proc., 973E, Paper #: 0972-AA07–10-BB08–10.
Rey-Mermet, S., Muralt, P. Solid oxide fuel cell membranes supported by nickel grid anode. Solid State Ionics. 2008; 179:1497–1500.
Ringuede, A., Fouletier, J. Oxygen reaction on strontium-doped lanthanum cobaltite dense electrodes at intermediate temperatures. Solid State Ionics. 2001; 139:167–177.
Ringuede, A., Guindet, J. Ideal behavior of a thin layer of La0JSra3CoO3–x. Ionics. 1997; 3:256–260.
Rupp, J.L.M., Gauckler, L.J. Microstructures and electrical conductivity of nanocrystalline ceria-based thin films. Solid State Ionics. 2006; 177:2513–2518.
Sase, M., Hermes, F., Yashiro, K., Sato, K., Mizusaki, J., Kawada, T., Sakai, N., Yokokawa, H. Enhancement of oxygen surface exchange at the heterointerface of (La, Sr)CoO3/(La, Sr)2CoO4 with PLD-layered films. J. Electrochem. Soc.. 2008; 155:B793–B797.
Sase, M., Suzuki, J., Yashiro, K., Otake, T., Kaimai, A., Kawada, T., Mizusaki, J., Yugami, H. Electrode reaction and microstructure of La0.6Sr0.4CoO3-x thin films. Solid State Ionics. 2006; 177:1961–1964.
Sase, M., Ueno, D., Yashiro, K., Kaimai, A., Kawada, T., Mizusaki, J. Interfacial reaction and electrochemical properties of dense (La, Sr)CoO3-x cathode on YSZ (100). J. Phys. Chem. Solids. 2005; 66:343–348.
Sase, M., Yashiro, K., Sato, K., Mizusaki, J., Kawada, T., Sakai, N., Yamaji, K., Horita, T., Yokokawa, H. Enhancement of oxygen exchange at the hetero interface of (La, Sr)CoO3/(La, Sr)2CoO4 in composite ceramics. Solid State Ionics. 2008; 178:1843–1852.
Sata, N., Eberman, K., Eberl, K., Maier, J. Mesoscopic fast ion conduction in nanometre-scale planar heterostructures. Nature. 2000; 408:946–949.
Schichtel, N., Korte, C., Hesse, D., Janek, J. Elastic strain at interfaces and its influence on ionic conductivity in nanoscaled solid electrolyte thin films-theoretical considerations and experimental studies. Phys. Chem. Chem. Phys.. 2009; 11:3043–3048.
Schichtel, N., Korte, C., Hesse, D., Zakharov, N., Butz, B., Gerthsen, D., Janek, J. On the influence of strain on ion transport. Microstructure and ionic conductivity of nanoscale YSZ|Sc2O3 multilayers. Phys. Chem. Chem. Phys.. 2010; 12:14596–14608.
Shim, J.H., Chao, C.-C., Huang, H., Prinz, F.B. Atomic layer deposition of yttria-stabilized zirconia for solid oxide fuel cells. Chem. Mater.. 2007; 19:3850–3854.
Su, P.-C., Chao, C.-C., Shim, J.H., Fasching, R., Prinz, F.B. Solid oxide fuel cell with corrugated thin film electrolyte. Nano Lett.. 2008; 8:2289–2292.
Suzuki, T., Kosacki, I., Anderson, H.U. Microstructure-electrical conductivity relationships in nanocrystalline ceria thin films. Solid State Ionics. 2002; 151:111–121.
Suzuki, T., Yamaguchi, T., Fujishiro, Y., Awano, M., Funahashi, Y. Recent development of microceramic reactors for advanced ceramic reactor system. J. Fuel Cell Sci. Technol.. 2010; 7:031005/1–031005/5.
Suzuki, T., Zahir, M.H., Yamaguchi, T., Fujishiro, Y., Awano, M., Sammes, N. Fabrication of micro-tubular solid oxide fuel cells with a single-grainthick yttria stabilized zirconia electrolyte. J. Power Sources. 2010; 195:7825–7828.
Tao, S., Irvine, J.T.S. A redox-stable efficient anode for solid-oxide fuel cells. Nat. Mater.. 2003; 2:320–323.
Tarancon, A., Garbayo, I., Santiso, J., Cavallaro, A., Roqueta, J., Garcia, G., Gracia, I., Cane, C., Sabate, N. YSZ free-standing membranes for silicon-based micro SOFCs. ECS Trans.. 2009; 25:931–938.
Tarancon, A., Sabate, N., Cavallaro, A., Gracia, I., Roqueta, J., Garbayo, I., Esquivel, J.P., Garcia, G., Cane, C., Santiso, J. Residual stress of free-standing membranes of yttria-stabilized zirconia for micro solid oxide fuel cell applications. J. Nanosci. Nanotechnol.. 2010; 10:1327–1337.
Taskin, A.A., Lavrov, A.N., Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett.. 2005; 86:091910/1–091910/3.
Thiele, E.S., Wang, L.S., Masoh, T.O., Barnett, S.A. Deposition and properties of yttria-stabilized zirconia thin films using reactive direct current magnetron sputtering. J. Vac. Sci. Technol., A. 1991; 9:3054–3060.
Tian, C., Chan, S.-W. Ionic conductivities of doped CeO2 thin films as related to their microstructure. Mater. Res. Soc. Symp. Proc.. 1996; 411:277–282.
Tian, C., Chan, S.-W. Grain boundary conductivity and microstructure study of 4% Y2O3 doped CeO2 thin films. Mater. Res. Soc. Symp. Proc.. 1997; 453:555–560.
Tian, C., Chan, S.-W. Microstructural correlation with electrical properties for Y2O3 doped CeO2 thin films. Mater. Res. Soc. Symp. Proc.. 1998; 500:279–284.
Tian, C., Chan, S.-W. Grain-boundary conductivity of 0.58% Y2O3-doped CeO2 thin films. Mater. Res. Soc. Symp. Proc.. 1999; 548:629–634.
Tian, C., Chan, S.-W. Electrical conductivities of (CeO2)1-x(Y2O3)x thin films. J. Am. Ceram. Soc.. 2002; 85:2222–2229.
Tolke, R., Bieberle-Hutter, A., Rupp, J.L.M., Gauckler, L.J. Foturan glass ceramic – a substrate for power delivering free-standing micro-SOFC membranes. ECS Trans.. 2009; 25:983–988.
Tsai, T., Barnett, S.A. Sputter deposition of cermet fuel electrodes for solid oxide fuel cells. J. Vac. Sci. Technol., A. 1995; 13:1073–1077.
Tschope, A. Grain size-dependent electrical conductivity of polycrystalline cerium oxide II: Space charge model. Solid State Ionics. 2001; 139:267–280.
Tuller, H.L., Litzelman, S.J., Jung, W. Micro-ionics: Next generation power sources. Phys. Chem. Chem. Phys.. 2009; 11:3023–3034.
Vovk, G., Chen, X., Mims, C.A. In situ XPS studies of perovskite oxide surfaces under electrochemical polarization. J. Phys. Chem. B. 2005; 109:2445–2454.
Wang, L.S., Barnett, S.A. Deposition, structure, and properties of cermet thin films composed of silver and yttrium-stabilized zirconia. J. Electrochem. Soc.. 1992; 139:1134–1140.
Wang, L.S., Barnett, S.A. Ag-perovskite cermets for thin film solid oxide fuel cell air-electrode applications. Solid State Ionics. 1995; 76:103–113.
Wang, L.S., Thiele, E.S., Barnett, S.A. Sputter deposition of yttria-stabilized zirconia and silver cermet electrodes for SOFC applications. Solid State Ionics. 1992; 52:261–267.
Wang, S., Cho, S., Wang, H. and Jacobson, A. J. 2011. Oxygen non stoichiometry in nanocrystalline La0.5Sr0.55CoO3–x. thin films, ECS Transactions, 35(1, Solid Oxide Fuel Cells 12), 1891–1897.
Wang, S., Yoon, J., Kim, G., Huang, D., Wang, H., Jacobson, A.J. Electrochemical properties of nanocrystalline La0.5Sr0.5CoO3–x thin films. Chem. Mater.. 2010; 22:776–782.
Wang, X., Huang, H., Holme, T., Tian, X., Prinz, F.B. Thermal stabilities of nanoporous metallic electrodes at elevated temperatures. J. Power Sources. 2008; 175:75–81.
Wang, Y., An, L., Saraf, L.V., Wang, C.M., Shutthanandan, V., Mccready, D.E., Thevuthasan, S. Microstructure and ionic conductivity of alternating-multilayer structured Gd-doped ceria and zirconia thin films. J. Mater. Sci.. 2009; 44:2021–2026.
Wilhelm, O., Pratsinis, S.E., Perednis, D., Gauckler, L.J. Electrospray and pressurized spray deposition of yttria-stabilized zirconia films. Thin Solid Films. 2005; 479:121–129.
Xiong, H., Lai, B.-K., Johnson, A.C., Ramanathan, S. Low-temperature electrochemical characterization of dense ultra-thin lanthanum strontium cobalt ferrite (La0.6Sr0.4Co0.8Fe02O3) cathodes synthesized by RF-sputtering on nanoporous alumina-supported Y-doped zirconia membranes. J. Power Sources. 2009; 193:589–592.
Yamada, A., Suzuki, Y., Saka, K., Uehara, M., Mori, D., Kanno, R., Kiguchi, T., Mauvy, F., Grenier, J.-C. Ruddlesden-Popper-type epitaxial film as oxygen electrode for solid-oxide fuel cells. Adv. Mater. (Weinheim, Ger.). 2008; 20:4124–4128.
Yang, Y.L., Chen, C.L., Chen, S.Y., Chu, C.W., Jacobson, A.J. Impedance studies of oxygen exchange on dense thin film electrodes of La0.5Sr0.5CoO3-x. J. Electrochem. Soc.. 2000; 147:4001–4007.
Yang, Y.L., Chen, C.L., Luo, G.P., Ross, D.K., Chu, C.W., Jacobson, A.J. Impedance studies of oxygen exchange on a highly oriented thin film electrode of La0.5Sr0.5CoO3–x. Proc. Electrochem. Soc.. 2001; 2000–32:157–170.
Yang, Y.L., Jacobson, A.J., Chen, C.L., Luo, G.P., Ross, K.D., Chu, C.W. Oxygen exchange kinetics on a highly oriented La0.5Sr0.5CoO3–x thin film prepared by pulsed-laser deposition. Appl. Phys. Lett.. 2001; 79:776–778.
Yashiro, K., Nakamura, T., Sase, M., Hermes, F., Sato, K., Kawada, T., Mizusaki, J. Composite cathode of perovskite-related oxides, (La, Sr)CoO3-x/(La, Sr)2CoO4-x for solid oxide fuel cells. Electrochem. Solid-State Lett.. 2009; 12:B135–B137.
Yoon, J., Araujo, R., Grunbaum, N., Baque, L., Serquis, A., Caneiro, A., Zhang, X., Wang, H. Nanostructured cathode thin films with vertically-aligned nanopores for thin-film SOFCs and their characteristics. Appl. Surf. Sci.. 2007; 254:266–269.
Yoon, J., Cho, S., Kim, J.-H., Lee, J.-H., Bi, Z., Serquis, A., Zhang, X., Manthiram, A., Wang, H. Vertically aligned nanocomposite thin films as a cathode/electrolyte interface layer for thin-film solid oxide fuel cells. Adv. Funct. Mater.. 2009; 19:3868–3873.
15.9 Appendix: glossary
| ALD | atomic layer deposition |
| CSZ | calcia-stabilized zirconia |
| dc | direct current |
| DMFC | direct methanol fuel cell |
| GDC | gadolinium-doped ceria |
| LAO | lanthanum aluminate |
| LBCO | lanthanum barium cobalt oxide |
| LSC | lanthanum strontium cobalt oxide |
| LSCF | lanthanum strontium cobalt iron oxide |
| LSGM | lanthanum strontium magnesium gallate |
| LSM | lanthanum strontium manganite, La0.9Sr0.1MnO3 |
| NGO | neodymium gallate |
| PBCO | praseodymium barium cobalt oxide |
| PEM | polymer membrane fuel cell |
| PLD | pulsed laser deposition |
| PMMA | polymethylmethacrylate |
| rf | radio frequency |
| SDC | samarium doped ceria |
| SOFC | solid oxide fuel cell |
| STO | strontium titanate |
| YDC | yttria-doped ceria |
| YSZ | yttria-stabilized zirconia |
