Electromigration-induced microstructural evolution in lead-free and lead–tin solders
Abstract:
The electromigration-induced microstructural variation at the joint interface and within the bulk of Pb-free and Pb–Sn solders is examined. The accumulation of solder alloy elements accelerates the interfacial reaction and thus the formation of interfacial intermetallic compound. The formation of intermetallic compound results in stress accumulation and enhances whisker growth within the solder. The torque induced by electron wind results in grain rotation of the solder bulk. The current stress causes the dissolution and thus supersaturation of second phase in the matrix, for example Zn in Sn of a Sn-Zn solder. Dissolution and thus recrystallization of the second phase occurs. The recrystallization behavior of the second phase has been observed for Sn–9Zn.
9.1 Introduction
A number of factors affect the microstructure of material. The variation in composition of a material gives rise to diffusion upon thermal aging owing to chemical potential. Diffusion results in the dissolution of alloying elements in the matrix and thus diminishing second phases. Diffusion also enhances interactions among constituent elements and thus the formation of compound such as intermetallics. The momentum transferred from electrons to atoms during current stressing causes migration of the atoms and thus the occurrence of electromigration, a directional forced atomic movement (Huntington, 1975). The elemental diffusion owing to chemical potential in conjunction with the electron wind force owing to electric current raises the energy level of the atoms in a metallic material. Consequently, there are a variety of interactive behaviors occurring induced by electromigration. In view of the excess energy impinged by the electric current, it is reasonable to anticipate further behaviors other than those caused by the conventional diffusion process. In addition, the heterogeneity in lattice and properties of the solder alloy, compared with the homogeneous property of pure metal like Al and Cu, further causes complexity to the microstructure variation during electromigration. The well recognized Black’s equation also needs appropriate amendment to accommodate the complexity of solder alloy (Choi et al., 2003). The accelerated failure, faster than the mean time to failure (MTTF) predicted by Black’s equation, of a solder joint resulted from a combination of current crowding, dissolution of IMC, and Joule heating (Choi et al., 2003). The major occurrences reflected in microstructure variation include phase separation, compound formation, grain extrusion and rotation, whisker and hillock formation, void nucleation and growth, recrystallization of second phase, and lattice reorientation. These occurrences together result in the formation of defects and accelerated failure of the electronic components.
9.2 Intermetallic compound formation
The atomic flux in a metallic material under the influence of electric current stressing is given by the Nernst–Einstein equation. This is the flux of the atoms traveling through the bulk under the influence of electric field. However, the electric field and the current flow induce a polarity effect on the electrode which causes different intermetallic compound (IMC) formation behaviors to cathode and anode, Fig. 9.1 (Wu and Chan, 2005). The thickness of IMC layers formed in diffusion couples such as Cu/Sn and Ni/Sn (Chen et al., 1998) is governed by the electric current. For Sn–Pb solder, the Pb atoms move in the same direction as the electron flow, whereas the Sn atoms move in the opposite direction. A 97Pb3Sn solder bump thus becomes 83Pb17Sn in the cathode region (Nah et al., 2004) at a current density of 2.55 × 104 A cm−2 and causes the formation of Cu6Sn5 IMC. The electric current does not result in significant change to the initial anodic compound formed after reflow.
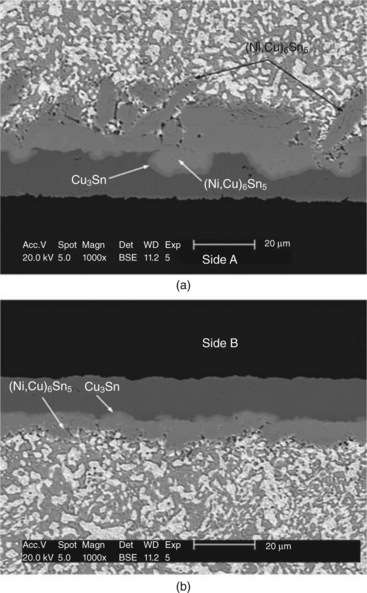
9.1 The polarity effect during current stressing causes different reaction behavior at (a) the anode and (b) the cathode. The 63Sn37Pb BGA solder joint was stressed with 1.5 A for 11 h (Wu and Chan, 2005).
Nevertheless, the consumption of cathodic electroless nickel under bump metallurgy (UBM) was accelerated to form Ni3Sn4. Current stressing at an average current density of 0.4 × 104 A cm−2 at 25 °C through a Sn0.7Cu ball grid array (BGA) package causes complete dissolution of the Au/Ni (5 μm thick) UBM and the 17 μm Cu pad (Zhang et al., 2008). The current density increases up to 5.83 × 104 A cm−2 in the current entry corner area. Ni forms the Ni–Sn IMC Ni3Sn4 in the nearby area. The formation of the IMC was partially attributed to the inevitable temperature rise as a result of Joule heating owing to current crowding at the entry corner. Meanwhile, the unprotected Cu pad dissolved rapidly in the solder and flowed to form (Cu,Ni)6Sn5 at the anode area.
In a μBGA (300 μm diameter solder ball) 63Sn37Pb solder joint with Cu UBM on the component side, the IMC Cu6Sn5 and Cu3Sn were formed. The thickness of the IMC depends on the current direction as a result of polarity effect (Alam et al., 2006). The IMC is thicker when the component acts as the anode, with a current density of 1.3 × 104 A cm−2, whereas it is thinner when it is the cathode. Similar occurrence was observed for the (Cu,Ni)6Sn5 IMC formed on the boardside where electroless Ni–P was applied as the barrier layer. Temperature enhances the IMC growth. Electric current stressing at higher temperature, 150 °C versus 20 °C, significantly accelerated the IMC growth even operated at lower current density, 9 × 103 A cm−2 versus 1.3 × 104 A cm−2. The polarity effect on IMC growth may be affected by the testing structure and the Cu concentration in the solder joint. The polarity effect was prominent in a stripe structure with Cu/Sn–3.8Ag–0.7Cu/Cu system (Gan and Tu, 2002), but not in a Ni/Sn–Ag/Cu system (Ebersberger et al., 2005).
In a Cu/Sn–3Ag–0.5Cu/Cu solder bump system with Cu pad, replacing the Ni/Au finish in both the anode and the cathode areas accelerated IMC growth upon current stressing at 104 A cm−2 at 180 °C (Yamanaka et al., 2007). The current stressing enhances the thickness growth of Cu6Sn5 more than Cu3Sn. Yet the enhancement in IMC growth is retarded when there are voids formed which interrupt the Cu transport. The polarity effect also stops when the Cu concentration in the solder bump reaches a certain level after current stressing. In addition to the polarity effect, current circulation through the bump enhances IMC growth at the bottom of the joint even when the current was not passing through it owing to the structural design of the component (Yamanaka et al., 2007). The polarity effect in a Ni(P)/SnPb/Ni(P) BGA package shows fast depletion of Ni and Cu at the cathode but it enhances the growth of Ni3Sn4 at the cathode and (Cu,Ni)6Sn5 at the current crowding triple point (Lu et al., 2009a). The effect of current stressing on IMC growth was probably suppressed by alloying element. The addition of 0.6%Zn in Sn–Ag solder attracts Cu elements and surpasses the current influence on Cu transport. The addition of Zn stabilized the Ag3Sn and Cu6Sn5 IMC in the solder joint under current stressing and gave rise to better reliability than Sn–Ag solder having up to 1.8% Ag (Lu et al., 2009a). The dissolution of cathode metallization produces an atom flux toward the anode as pushed by the current stressing. The atom flux may not completely reach the anode as the atoms may be trapped during the flow towards anode. The Au metallization of the cathode side in the Cu/Au/SnAgCu/Cu combination dissolves rapidly during the current stressing at 103 A cm−2 at ambient temperature. The solder matrix contains longitudinal Cu6Sn5 after reflow. It is of interest that the Au forms AuSn4 which precipitates at the tip end of the longitudinal Cu6Sn5 IMC after 72 h of current stressing, Fig. 9.2 (Chiu and Lin, 2008). It is apparent that the formation reaction of the Au–Sn IMC takes place in the path of Au flux. The tip of the Cu–Sn IMC, owing to its high surface energy, serves as the nucleation site for the AuSn4 IMC.
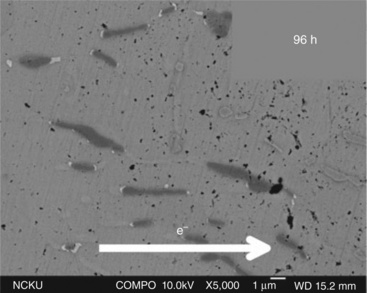
9.2 The Au flux from the dissolution of the metallizaton layer reacts with Sn of the Sn4Ag0.5Cu to form AuSn4 IMC (white precipitate at the tips of the Cu6Sn5 IMC) and precipitate at the tips of the Cu6Sn5 IMC (dark precipitate) (Chiu and Lin, 2008).
9.3 Void formation
In a metallic material, voids may form through a variety of mechanisms and thus affect the mechanical properties and physical properties of a material. In addition, the formation of voids in the solder joint of an electronic component affects the reliability in use. The formation of voids in a metallic material, apart from those formed by poor manufacturing, is generally induced by an unbalanced atomic diffusion flux. The Kirdendall void forms after prolong unbalanced atomic counter diffusion at the interface between two contact phases. The counter diffusion occurs as a result of the chemical potential difference between the two phases.
Electromigration, however, receives contributions from chemical potential and electron wind force. The electron wind force, induced by electric current, is the factor that accelerates the atomic flux. The migration of atoms away from an interface causes depletion and thus void formation if there is insufficient supply. The electron entry in a solder joint is a typical location for void nucleation and growth (Yeh et al., 2002). There is no solder constituent supply from the chip or substrate side during electric current stressing.
In a flip chip eutectic SnPb solder bump structure, void nucleation occurs at the corner of current entry after an incubation period of 30–50 min with 4 × 104 A cm−2 at 30 °C (Lin et al., 2005). The void nucleation initiates at the corner as a result of current crowding and thus Joule heating. The void exists between the Cu6Sn5 IMC and solder. During current stressing, the IMC initially formed upon reflow dissolves and new IMC forms with the Cu supplied from the UBM. The electric current detours and moves forward to the front of the void, thus forcing the void to grow. The void grows and propagates rapidly along the UBM/solder interface after nucleation. Thermomigration does not show a significant contribution to the void growth. Nevertheless, the formation of void increases local resistance and thus a fast temperature rise that causes melting to the solder (Lin et al., 2005).
Voids also formed at both the IMC/solder and the UBM/IMC interfaces in a flip chip BGA using Sn3Ag1.5Cu solder with Ti/NiV/Cu UBM when stressed at current density 1 × 104 A cm−2. The extent of voids formed at the solder/IMC interface near the substrate side depends on the metal finish. Ni exhibits a slower dissolution rate in solder than Cu. There it tends to form more voids with the Cu/Ni/Au metal finish than with the Cu-OSP pad when the substrate serves as cathode (Jen et al., 2009).
The behavior of void formation was also reported to occur at the low current density side of a Cu/Sn/Cu solder bump joint. A high current density at the current entry corner of this structure consumed more Cu UBM which caused high Cu concentration and thus the extensive formation of IMC. On the other end of the same Cu UBM, however, the relatively low current density did not raise the temperature significantly. Consequently, the void was formed at the cathodic IMC/solder interface in contact with the Cu UBM (Tseng et al., 2010).
The void growth was simulated and studied with a flip chip solder joint Cu/Sn–3Ag–0.5Cu/Cu system, Fig. 9.3 (Yamanaka et al., 2007). In this system the Kirkendall voids were observed at the Cu/Cu3Sn interface. However, the void induced by current stressing nucleates at the solder/Cu6Sn5 interface. The finite elemental simulation of void propagation was conducted with a specific focus on its rate at the currently entry side but without consideration on Joule heating. According to the result of simulation, a high current density front edge exists between the void area and the residual layer. The current density can increase up to four times the initial value. The joint resistance increases in proportion to the void area ratio increase. The formation of void at the electrode causes an increase in local resistance and thus a temperature rise. A higher temperature also accelerates void formation and growth. The propagation of the void across the entire interface results in an abrupt jump in the voltage as observed for a Cu/42Sn–58Bi/Cu structure (Guo et al., 2009). The accumulation of voids after prolong current stressing gives rise to the interfacial crack. The void itself may be regarded as an accumulation of vacancy flux driven by current stressing. The void serves as the vacancy sink and helps to keep the vacancy at thermal equilibrium during current stressing across a Cu/Sn–3.0Ag–0.5Cu/Cu joint at a current density of 4.9 × 103 A cm−2 (Kinney et al., 2009). Void formation also correlates to the activation of electromigration. The activation energy, 0.84 eV, of electromigration in eutectic SnAg solder bump with Cu-Ni UBM is related to void formation, although it is related to IMC formation when Cu UBM was used for eutectic SnAg and SnPb solder bumps (Chen and Chen, 2010). In a Cu/Sn9Zn/Cu structure the void forms at the Cu5Zn8 IMC/solder interface when the current density is 103 A cm−2. Needle-like voids initiated at the cathode/solder interface as a result of the outward diffusion of Zn atoms of the Zn-rich phase (Kuo and Lin, 2008).
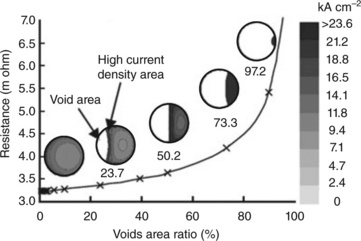
9.3 A simulated resistance change for a joint with respect to void area ratio. A high current density area exists in the front of the void progress (Yamanakas 2007).
9.4 Formation of whisker and hillock
The formation of Sn whisker in electronics is well recognized in the electronic packaging industry and occurs on the contingent of having adequate compressive stress (Tu, 1994). There are various sources of compressive stress, such as the interaction between Cu and Sn in a thin film. The reaction forms IMC that causes compressive stress because of volume change. The formation of whisker relieves the stress; Sn atoms sprout out from the crack of the surface oxide layer and thus grow as whisker (Chen et al., 2010, Tu, 1994).
As being a result of the compressive stress, both hillock and whisker are formed owing to the extrusion of Sn atoms and may be found in a same specimen. It is possible to distinguish between whisker and hillock by the difference in aspect ratio: whisker is defined as having an aspect ratio of greater than five, whereas that for hillock is less than two (Guo, 2009). Electromigration accelerates the diffusion of atoms within the matrix. The accumulation of atoms may give rise to compressive stress within the solder, either around electrodes, or at the solder/IMC interface. Electromigration thus serves as the direct driving force for building up compressive stress.
Although mass accumulation is of importance in the build up of stress, an in situ study with synchrotron X-ray microdiffraction showed that stress may also build up within a Sn0.7Cu flip chip solder joint owing to anisotropic diffusion of Sn (Chen et al., 2009). Sn crystal is body-centered tetragonal with quite different diffusivity in different lattice directions. The self-diffusivity of Sn in the a-direction is twice that in the c-direction (Dyson, 1967). Current stressing across the solder bump at 1.25 × 104 A cm−2 at 70 °C gradually builds up a compressive stress within the grain at the current crowding end (Chen et al., 2009). Electromigration of atoms in the solder matrix may be retarded by the existing stiff phase. The stiff structure does not migrate during current stressing. The migrating atoms accumulate at the interface between the solder matrix and the stiff structure. Portions of the Sn migration in a Sn9Zn solder under a current density of 105 A cm−2 at 80–140 °C was stopped in front of the Zn rich phase. The Sn accumulated at the interface between solder and Zn precipitate builds up compressive stress which extrudes Sn to form Sn whisker or hillock. The growth of Sn whisker was also enhanced by the current stressing temperature (Chen et al., 2009).
In a Cu/Sn3.5Ag/Au solder structure, AuSn4 IMC formed in the matrix after reflow. The IMC was seen to rotate after current stressing with a current density of 2.56 × 103 A cm−2 at 100 °C. Mechanical stress thus induced by IMC rotation may also be the major driving force for whisker and hillock formation (Chiu and Lin, 2009). On the other hand, the growth of whisker or hillock may be retarded by the IMC formed in the solder. The formation of larger amounts of IMC at higher temperatures in the Sn0.7Cu solder bump blocks the migration of Sn. Consequently, the growth of whisker or hillock at the anode is hindered owing to the diffusion barrier effect of the IMC in the solder matrix (Liang et al., 2010). The formation of Sn whisker is usually regarded as a deficit for reliability concern. However, it was reported that a solder bump with under-fill protection was not able to form whisker to relieve the stress inside the bump. Such circumstances result in the buildup of stress at anode and thus cracking (Lee et al., 2001).
9.5 Grain reorientation and grain rotation
The resistance of a Sn stripe was found to decrease exponentially with time when stressed with a current of 6.25 × 104 A cm−2 at 150 °C (Lloyd, 2003). The decay in resistance was attributed to the reorientation of the grain structure. The anisotropic behavior of Sn crystal exhibits an electrical resistivity difference by more than 40% at 0 °C between the a-direction and c-direction of the body-centered tetragonal Sn crystal (Burckbuchler and Reynolds, 1968). The reorientation aligns the direction of low resistance of the Sn crystal in the direction of electromigration, thus reducing the electromigration-induced chemical potential (Lloyd, 2003). The results of synchrotron X-ray microdiffraction investigations grain by grain show the change in grain orientation. It indicates that the grain with low resistance grows whereas that of high resistance gradually shrinks. The grain growth therefore occurs at the expense of the high-resistance grains. This behavior was ascribed to electromigration rather than Joule heating effect (Wu et al., 2004).
The behavior of grain reorientation to reduce resistance, to a more macroscopic view, also appears to cause rotation of the grains. Grain rotation occurs in a Sn stripe after current stressing with a current density of 2 × 104 A cm−2 at 100 °C for 500 h. The rotation of a piece of grain needs a torque, which is thought to mainly come from the different direction of the vacancy flux driven by electromigraion (Wu et al., 2005). The diffusion of vacancy and atoms along the grain boundary is different for neighboring grains with different orientations. The vacancy becomes supersaturated at the anodic-side grain boundary and undersaturated at the cathodic-side grain boundary. The divergence in vacancy flux builds up a stress in the grain which serves as the origin of the torque (Wu et al., 2005). A theoretical expression indicates that the rotation speed, the angular velocity, of the grain is enhanced, thus affecting current and temperature. A higher current density produces a larger electron wind force, which drives larger atomic flux to the anode and a larger vacancy flux to the cathode. A larger vacancy flux divergence is thus produced to generate greater torque (Wu and Hsieh, 2008). An electrically isotropic material does not exhibit vacancy flux divergence and thus the grain rotation was not observed for Al and Cu. The grain rotation behavior was also observed for the AuSn4 IMC formed in a Cu/Sn3.5Ag/Au solder structure after current stressing with a current density of 2.56 × 103 A cm−2 at 100 °C. The AuSn4 IMC formed and grew rapidly. The large IMC within the joint essentially blocks the atom diffusion. Accumulation of Sn in front of the IMC induces the formation of both whisker and hillock (Chiu and Lin, 2009).
9.6 Dissolution and recrystallization
Current stressing through the solder joint encounters different metal structures including metallization and solder. The thin metallization at the electrode side, especially the cathode, may induce Joule heating because of its high resistance. The Joule heating induces dissolution of the metallization layer. In a μBGA solder joint the thin Cu trace on the component side encounters a higher current density and thus higher temperature rise than the solder joint. On the opposite side, the Cu trace on the board, larger in area and thicker, shows a lower temperature rise. A greater dissolution rate of the Cu trace on the component side is associated with the melting of the 63Sn37Pb solder joint when stressed at high current density (Alam et al., 2006, Wu and Chan, 2005). In such a case, dissolution of Cu trace on the anodic board side was observed owing to melting of the solder joint even though the current density on the board side is lower. When the solder joint remains in the solid state, the anodic copper trace dissolves and migrates to the anode (Tu et al., 2000, Hu et al., 2003). Cu6Sn5 IMC was formed on the anodic board side even though there is an electroless Ni–P metallization layer (Alam et al., 2006). The polarity behavior between the cathode and the anode side gives rise to thicker Cu6Sn5 and Cu3Sn IMC at the anode side than the cathode side, apparently owing to the dissolution of the Cu trace at the cathode side. Electromigration accelerates the dissolution of Ni and Cu of a BGA joint with Ni(P)/SnPb/Ni(P) structure when stressed with a current density of 3.0 × 103 A cm−2 at 120 °C. The fast dissolution enhances both the formation of (Cu,Ni)6Sn5 at the current crowding region and Ni3Sn4 at the anode side (Lu et al., 2009b). Cu6(Sn,In)5 compound formed in a Sn3Ag3Bi10In solder stripe after current stressing as a result of the dissolution of Cu into the solder to react with In and Sn. The Cu dissolution from the cathode resulted from the current stressing (Wu and Sun, 2009). Cu and Ni may dissolve rapidly in solder along the orientation of Sn grain (Lu et al., 2008). Both Cu and Ni exhibit several orders of faster diffusion along the c-axis than either the a- or the b-axis of Sn crystals (Dyson et al., 1967, Yeh and Huntington, 1984). It is reasonable to imagine that the synergism of electric current stressing and grain orientation will induce extreme fast dissolution of the metallization UBM into the solder. A dissolution flux of 9.98 × 1010 atoms cm−2 S−1 was calculated for Cu under electromigration through a Cu/Sn/Cu solder joint at a constant current density of 5.3 × 103A cm−2 (Tseng et al., 2010). The flux tends to be larger at the current crowing region.
The electromigration-induced dissolution behavior not only occurs to the thin metallization layer of a solder joint but also to the IMC formed after reflow in the solder joint. A flip chip solder, 63Sn37Pb, joint with Cu/Ni(V)/Al UBM on the chip side and Au(30 nm)/Ni(10 μm) on the Cu trace of the FR4 substrate, was investigated with current stressing. The electromigration study was conducted at 100–140 °C with a current density of 1.90–2.75 × 104A cm−2. The Cu–Sn IMC at the cathode UBM side dissolved after current stressing with 2.25 × 104 A cm−2 at 125 °C. The dissolution of IMC reveals the underneath diffusion barrier Ni(V) layer followed by the exposure of Al layer (Choi et al., 2003). This dissolution behavior thus raises a concern about the reliability of the solder bump. The dissolution of IMC also occurs in the Sn3Ag3Bi10In solder system. Cu6(Sn,In)5 IMC and the ζ phase in the cathode region, formed after reflow of the Cu/solder/Cu joint in a Si U-groove, dissolve after current stressing at a current density of 1 × 103 A cm−2 at 150 °C for 120 h (Wu and Sun, 2009). Cu6(Sn,In)5 IMC and the ζ phase grew to less than half of the dimension with thermal aging at a higher temperature, 180 °C, for the same time duration. The occurrence of dissolution in the cathode area leads to atom flux toward the anode. The ζ phase in the solder matrix and the anode region grew during current stressing when encountering the atomic flux supplied by the dissolution. In view of the fact that the ζ phase is a thermodynamically stable phase, the disappearance of it at the cathode side was thought to be caused by the electron current from the cathode.
Electric current can refine the microstructure of a metal alloy. The effect was ascribed to the enhancement of the nucleation rate (Conrad, 2000). For the situation of deformed metallic specimen, the high current density at the deformed area causes nucleation by the pinch effect. Recrystallization occurs for a electric current pulse that provides an instantaneous high energy input that causes a high local temperature increase (Conrad, 2000, Song and Wang, 2008). Recrystallization also occurs in a current stressed Sn–9Zn solder joint. The stabilized equiaxial Zn-rich phase of the eutectic Sn-9Zn solder wire, 4.8 mm length with 1.2 mm diameter, gradually disappears when stressed with 2.6 × 103 A cm2 at 100 °C. The equiaxial crystals transform into a two-dimensional sheet structure of 200 nm thick of which the layer lattice stacks in the [0001] direction, compared with the [2000] and Zn[10ī0] textures of the stabilized equiaxial grains, Fig. 9.4 (Kuo and Lin, 2009). The driving force for the recrystallization behavior was not discussed in detail therein. The same group recently conducted a series studies on another solder. The in situ SEM results indicate an excess dissolution of the second-phase precipitates during electromigration. It is believed that the recrystallization results from the supersaturation which drives recrystallization. Nevertheless, the refinement as a result of the recrystallization seems to induce a high-energy state in the system. The mechanism of this recrystallization behavior still remains to be explored.
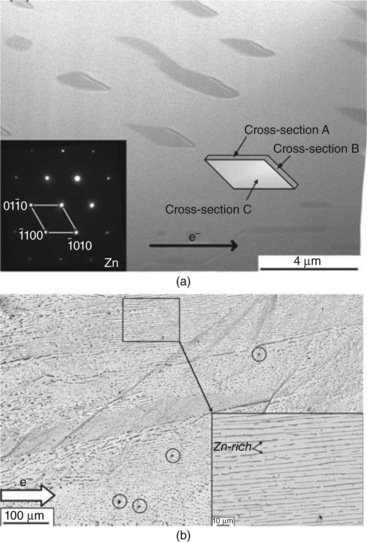
9.4 (a) The recrystallized Zn nanosheet grains in a Sn9Zn solder after current stressing, (b) morphology of the edge of the nanosheet grains at the lower magnification shows alignment of Zn-rich grains along the direction of the election flow after electromigration for 230 hours (Kuo and Lin, 2009).
9.7 References
Alam, M.O., Wu, B.Y., Chan, Y.C., Tu, K.N. High electric current density- induced interfacial reactions in micro ball grid array (μBGA) solder joints. Acta Mater. 2006; 54(3):613–621.
Burckbuchler, F.V., Reynolds, C.A. Anisotropy of the residual resistivity of tin with Sb, In, Zn, and Cd impurities, and the ideal resistivities and deviations from Matthiessen’s rule at 77 and 273 K. Phys Rev. 1968; 175(2):550–555.
Chen, C.M., Hung, Y.M., Lin, C.P., Su, W.C. Effect of temperature on microstructure changes of the Sn–9wt% Zn lead-free solder stripe under current stressing. Mater Chem Phys. 2009; 115:367–370.
Chen, C., Tong, H.M., Tu, K.N. Electromigration and thermomigration in Pb-free flip-chip solder joints. Ann Rev Mater Res. 2010; 40:531–555.
Chen, H.Y., Chen, C. Measurement of electromigration activation energy in eutectic SnPb and SnAg flip-chip solder joints with Cu and Ni under-bump metallization. J Mater Res. 2010; 25(9):1847–1853.
Chen, K., Tamura, N., Kunz, M., Tu, K.N., Lai, Y.S. In situ measurement of electromigration-induced transient stress in Pb-free Sn-Cu solder joints by synchrotron radiation based X-ray polychromatic microdiffraction. J Appl Phys. 2009; 106:023502.
Chen, S.W., Chen, C.M., Liu, W.C. Electric current effects upon the SnCu and SnNi interfacial reactions. J Electron Mater. 1998; 27(11):11931199.
Chiu, T.C., Lin, K.L. Electromigration behavior of the Cu/Au/SnAgCu/Cu solder combination. J Mater Res. 2008; 23(1):264–273.
Chiu, T.C., Lin, K.L. The growth of Sn whisker with dislocation inclusion upon electromigration through the Cu/Sn3.5Ag/Au Solder joint. Scripta Mater. 2009; 60(12):1121–1124.
Choi, W.J., Yeh, E.C.C., Tu, K.N. Mean-time-to-failure study of flip chip solder joints on Cu/Ni(V)/Al thin-film under-bump-metallization. J Appl Phys. 2003; 94(9):5665–5671.
Conrad, H. Effects of electric current on solid state phase transformations in metals. Mater Sci Eng. 2000; 287(2):227–237.
Dyson, B.F., Anthony, T.R., Turnbull, D. Interstitial diffusion of copper in tin. J Appl Phys. 1967; 38:3408.
Ebersberger, B., Bauer, R., Alexa, L. Reliability of lead-free SnAg solder bumps: influence of electromigration and temperature. Proc 55th ECTC, Lake Buena Vista, FL. 2005; 1407–1415.
Gan, H., Tu, K.N. Effect of electromigration on intermetallic compound formation in Pb-free solder–Cu interfaces. Proc 52nd ECTC, San Diego, CA. 2002; 1206–1212.
Guo, F., Xu, G., He, H., Zhao, M., Sun, J., Wang, H. Effect of electromigration and isothermal aging on the formation of metal whiskers and hillocks. J Electron Mater. 2009; 38(12):2647–2658.
Hu, Y.C., Lin, Y.H., Kao, C.R., Tu, K.N. Electromigration failure in flip chip solder joints due to rapid dissolution of copper. J Mater Res. 2003; 18(11):2544–2548.
Huntington, H.B. Electromigration in tin single crystals. J Phys Chem Solid. 1975; 36(5):395–399.
Jen, M.H.R., Liu, L.C., Lai, Y.S. Electromigration on void of Sn3Ag1.5Cu FCBGA solder joints. Microelectron Reliab. 2009; 49:734–745.
Kinney, C., Lee, T.K., Liu, K.C., Morris, J.W. The interaction between an imposed current and the creep of idealized Sn–Ag–Cu solder interconnects. J Electron Mater. 2009; 38(12):2585–2591.
Kuo, S.M., Lin, K.L. Electromigration-induced void formation at the Cu5Zn8/solder interface in a Cu/sn-9Zn/Cu sandwich. J Electron Mater. 2008; 27(10):1611–1617.
Kuo, S.M., Lin, K.L. Recrystallization under electromigration of a solder alloy. J Appl Phys. 2009; 106:123514.
Lee, T.Y., Tu, K.N., Frear, D.R. Electromigration of eutectic SnPb and SnAg3.8Cu0.7 flip chip solder bumps and under-bump metallization. J Appl Phys. 2001; 90(9):4502–4508.
Liang, S.W., Chen, C., Han, J.K., Xu, L., Tu, K.N., Lai, Y.S. Blocking hillock and whisker growth by intermetallic compound formation in Sn–0.7Cu flip chip solder joints under electromigration. J Appl Phys. 2010; 107:093715.
Lin, Y.H., Hu, Y.C., Tsai, C.M., Kao, C.R., Tu, K.N. In situ observation of the void formation-and-propagation mechanism in solder joints under current-stressing. Acta Mater. 2005; 53:2029–2035.
Lloyd, J.R. Electromigration induced resistance decrease in Sn conductors. J Appl Phys. 2003; 94(10):6483–6486.
Lu, M., Shih, D.Y., Kang, S.K., Goldsmith, C., Flaitz, P. Effect of Zn doping on SnAg solder microstructure and electromigration stability. J Appl Phys. 2009; 106:053509.
Lu, M., Shih, D.Y., Lauro, P., Goldsmith, C., Henderson, D.W. Effect of Sn grain orientation on electromigration degradation mechanism in high Sn-based Pb-free solders. Appl Phys Lett. 2008; 92:211909.
Lu, Y.D., He, X.Q., En, Y.F., Wang, X., Zhuang, Z.Q. Polarity effect of electromigration on intermetallic compound formation in SnPb solder joints. Acta Mater. 2009; 57:2560–2566.
Nah, J.W., Kim, J.H., Lee, H., M, H., Paik, K.W. Electromigration in flip chip solder bump of 97Pb–3Sn/37Pb–63Sn combination structure. Acta Mater. 2004; 52:129–136.
Song, H., Wang, Z.J. Microcrack healing and local recrystallization in pre-deformed sheet by high density electropulsing. Mater Sci Eng A. 2008; 490(1–2):1–6.
Tseng, H.W., Lu, C.T., Hsiao, Y.H., Laio, P.L., Chuang, Y.C., Chung, T.Y., Liu, C.Y. Electromigration-induced failures at Cu/Sn/Cu flip-chip joint interfaces. Microelectron Reliab. 2010; 50:1159–1162.
Tu, K.N. Irreversible processes of spontaneous whisker growth in bimetallic Cu-Sn thin-film reactions. Phys Rev B. 1994; 49(3):2030–2034.
Tu, K.N., Yeh, C.C., Liu, C.Y., Chen, C. Effect of current crowding on vacancy diffusion and void formation in electromigration. Appl Phys Lett. 2000; 76(8):988–990.
Wu, A.T., Gusak, A.M., Tu, K.N., Kao, C.R. Electromigration-induced grain rotation in anisotropic conducting beta tin. Appl Phys Lett. 2005; 86:241902.
Wu, A.T., Tu, K.N., Lloyd, J.R., Tamura, N., Valek, B.C., Kao, C.R. Electromigration-induced microstructure evolution in tin studied by synchrotron X-ray microdiffraction. Appl Phys Lett. 2004; 85(13):2490–2491.
Wu, A.T., Hsieh, Y.C. Direct observation and kinetic analysis of grain rotation in anisotropic tin under electromigration. Appl Phys Lett. 2008; 92:121921.
Wu, A.T., Sun, K.H. Determination of average failure time and microstructural analysis of Sn–Ag–Bi–In solder under electromigration. J Electron Mater. 2009; 38(12):2780–2785.
Wu, B.Y., Chan, Y.C. Electric current effect on microstructure of ball grid array solder joint. J Alloys Compounds. 2005; 392:237–246.
Yamanaka, K., Tsukada, Y., Suganuma, K. Studies on solder bump electromigration in Cu/Sn–3Ag–0.5Cu/Su system. Microelectron Reliab. 2007; 47:1280–1287.
Yeh, D.C., Huntington, H.B. Extreme fast-diffusion system: nickel in single-crystal tin. Phys Rev Lett. 1984; 53(15):1469–1472.
Yeh, E.C.C., Choi, W.J., Tu, K.N. Current-crowding-induced electromigration failure in flip chip solder joints. Appl Phys Lett. 2002; 80(4):580–582.
Zhang, J.S., Chan, Y.C., Wu, Y.P., Xi, H.J., Wu, F.S. Electromigration of Pb-free solder under a low level of current density. J Alloys Compounds. 2008; 458:492–499.
