Materials for printed films
Abstract:
The chapter provides a critical survey of the compositions of materials (pastes and substrates) for applications in thick-film microelectronics and sensors. The evolution of the technology is illustrated with wide references to publications and patents.
2.1 Introduction
Thick-film (TF) materials or TF compositions and the general term ‘thick-film technology’ have been the subject of reviews (Bouchard, 1999; Hoffman, 1984; Larry et al., 1980; Vest, 1986) and books (Borland, 1989; Holmes and Loasby, 1976, 1988; Prudenziati, 1994; Rikoski, 1973; Sergent, 2004; Vest, 1991). Traditionally, TF materials, which are also known as pastes, dispersions and inks, were classified into three material categories: conductive, resistive and dielectric. Nowadays, the term TF materials comprises a larger group of inorganic and organic materials and the previous classification needs revision to include new materials and new applications. TF materials have three major constituents:
1. the active phase, which was considered the phase that imparts the functional properties;
2. the glass/glass–ceramic binder;
3. the vehicle that controls the dispersion of the active phase, the glass/glass– ceramic binder and the viscosity of the paste.
This simple description of the paste does not include interactions of the active phase with the glassy binder, substrate, vehicle and the metallic terminations. The interactions of the paste ingredients with the substrate and the terminations are very important, and are the subject of Chapter 3.
This chapter is a critical review of the chemistry and material science of TF materials. While it is intended to be comprehensive treatment of TF materials, it makes no attempt to be historically complete, or to cover all the information available in the journals and patent literature.
2.2 Active phases
2.2.1 Classification of active phases
The TF literature refers to the functional, pigment or the active phase as the constituent that controls the electrical properties of fired paste composition. Thus, the metallic phase (Ag, Ag/Pd, Ag/Pt, Ag/Pt/Pd, Au, Au/Pd, Au/Pt, Au/Pd/Pt for air-fired conductors or Cu, Ni for nitrogen-fired conductors), which is the major constituent of the conductor composition, determines the electrical properties. For TF resistors the metallic oxide (RuO2, IrO2, Bi2 Ru2O7, Pb2Ru2O6 + δ, etc. for air-fired compositions) and the conducting phase (LaB6, borides, silicides, carbides, doped tin oxide, etc. for nitrogen-fired compositions) are considered as the active or the functional phases. In fact, the combination of materials, i.e. metallic oxide, glass and additives, determines the characteristic resistor properties, rather than the metallic phase alone. The same applies to the dielectric active phase: the combination of glass, high dielectric ceramic and additives determines the electrical properties.
In this chapter a different classification of the active phases is used. Metallic oxides and other electrically conducting materials are treated as a group of conducting materials for resistors. The conductors and dielectrics are treated traditionally.
Materials for nitrogen firing are treated as a separate group because the compositions and the thermodynamic properties required are different from air-fired compositions. Materials for AlN, SiC, glass and other substrates are different from standard TF composition and are treated as a separate group. All the above-listed subgroups are the subject of section 2.2. The organic vehicle for standard air-fired compositions and specialized compositions for inert atmosphere firing, photosensitive, water-based and other applications are the subject of section 2.3.
Glasses and glass ceramics are very important constituents of TF compositions and are treated as separate group in section 2.4. Glass compositions, mainly from the patent literature, are described for air-fired resistors, lead-free air-fired resistors, nitrogen-fired resistors, conductors, dielectrics overglazes and sensors.
2.2.2 Conducting materials for resistors
Resistive materials used in TF compositions are a subgroup of resistor materials that comprises metals, alloys, other elements, metal metal-oxides mixtures, inorganic oxide compositions, other inorganic compounds and organic composition. A review of resistor materials that was based on the patent literature of the period 1960 to 1971 was written by Conrad (1971), and the trends in TF resistor technology until 1971 were reviewed by Van Loan (1971). Prior to 1960, there are some resistor materials patents that facilitate the understanding of the evolution of TF resistive compositions; these are patents to Jira (1942, 1944, 1948a, 1948b), Rosenblatt (1939, 1943) and Soby (1951). One of the patents (which includes materials, processing, additives and concepts that are of importance to TF materials in general and specifically to resistor materials) is by Place and Place (1960a, 1960b). In one of the embodiments of the method of preparing the resistor material, the resistance material is prepared in large batches and stored indefinitely. The large batch is prepared by milling the glass binder, metals and metal oxides and the noble metals and oxidizable metals being present in the form of soluble metal compounds. The milled mixture is heated to about 700 °F to remove volatiles and organic materials from the mixture, to decompose the metal compounds and to oxidize the oxidizable metals. The resulting dry material is ground to fine powder and calcined at about 850 °F (454 °C). The resulting calcine is ground to fine powder and stored. The method described above may result in oxidation of Ru, Ir and Rh to the oxides RuO2, IrO2 and Rh2O3. Therefore, this patent (Place and Place, 1960a, 1960b) may be considered the first one that made use of the oxides RuO2 and IrO2.
Palladium – silver system
D’Andrea (1960) describes a vitreous enamel resistor composition that may be applied to and fired on a ceramic body to produce an electrical resistor. The composition consists of fine powders of Pd 4–50%; Ag 0–40% and flux (frit or frit plus Bi2O3 and PbO) 50–92%. The glass frits used were lead borosilicate and bismuthate glass (lead borosilicate plus Bi2O3). A related patent is Dumesnill (1962), which uses PdO plus RhO, metallic additives Ag, Au, Pt, enamel frit (lead borosilicate) and metal oxide taken from the group consisting of Zr, Al, Sr, Ca, Sn, Mg, Zn and the rare earth metals. Related to the Pd/Ag and PdO/Ag resistance compositions are the following variations of PdO/Rh2O3/Ag-based compositions: Mones and Neisser (1966) developed compositions containing PdO/Rh2O3/Ag and lead borosilicate glass. The semiconducting oxides PdO and Rh2O3 may be doped by univalent, divalent and multivalent cation selected from Sb, Cr, Mo, Cu, Li and Na. These compositions have a broader resistance range and better temperature coefficient of resistance (TCR). Mones (1967) used a resistance composition based on PdO, PdCl2, Pd and halides of silver like AgCl, AgBr and AgI and glass. In addition, the composition may contain Bi2O3, PbO and silica. Boyd et al. (1968) developed a process to control the crystallite size of PdO and they developed a method to prepare resistance compositions based on specified oxides of Pd and Rh. In addition to these oxides, the resistance compositions may contain Ag, Pt or Au and fine oxides of silicon and aluminium. Miller (1968a) uses a PdO/Ag composition, the synthesis of PdO and the roll milling process are described, and the correlation of roll milling with electrical properties is also discussed. This composition contains an organic vehicle based on an aromatic solvent, polystyrene resin and a nonylphenoxy polyoxyethylene ethanol surfactant. The glass used was multicomponent lead cadmium boroaluminosilicate with TiO2, ZrO2 and Na2O, a typical acid-resistant glass composition.
Properties of this system were reported by Coleman (1975), Melan and Mones (1967) and Okamoto and Aso (1967). The properties of this system were reviewed by Finch (1969) and discussed by Holmes and Loasby (1976).
The palladium–silver system is a reactive system: Pd can oxidize to PdO during firing, form an alloy with Ag, the alloy can oxidize too and the PdO formed can easily reduce back to the metal. These properties make the system very sensitive to firing conditions, glass composition and the surface area of the metallic ingredients. A vitreous enamel resistance material developed by Janakirama-Rao and Murphy (1964) consists of lead borosilicate glass or barium calcium borosilicate glass containing Ag, Cu and Au in the ionic state and finely divided metal selected from the group consisting of Pd, Pt and Rh.
The introduction of Ag, Cu or Au in the ionic state into the glass frit improves the melting of the frit so as to improve its wettability. In addition, it improves the overall performance of the electrical properties of the enamel resistance material in that it permits the obtaining of the desired conductivity of the material with smaller amount of the finely divided conductor – Rao and Murphy (1964).
The rationale behind the introduction of ionic Ag, Cu or Au into the glass was to improve wettability of the conductor material (Pd, Pt, Rh and Ag) by the glass frit so as to improve the conductive properties of the resistance material. The description of this patent regarding the conduction of electrical current and the role of the ionic Ag, Cu or Au in the glass is very interesting:
In an enamel type resistance material, the conductive metal particles exist as isolated islands in the glassy matrix. When an electrical potential difference is applied across such resistance film, conduction takes place between the conductive metal particles through the glassy matrix, which glassy matrix is normally an insulator. Also, the electronic configuration of Ag, Au, or Cu ions in the glass frit of the present invention is such that the ions are deficient in an electron so that the ions can momentarily act as electron acceptors. However, the electronic configuration of Pd, Pt, Rh metal of the conductive particles is such that they have a full quota of electrons and can act as electron donor. For this reason, it is felt that when an electrical potential difference is placed across the enamel resistance material of the present invention, the silver, gold or copper ions in the glassy matrix act as a bridge between the isolated islands of the conductive metal particles by accepting and passing on an electron, and thereby improve the conductivity of the enamel resistance material again quoting from the patent to Janakirama – Rao and Murphy (1964).
It seems that the concept of having a glassy film between the conducting particles was practised in the patent literature many years before it was republished in the open literature. The mediating role assigned to the ionic species in the glassy matrix may be applied to Ru-based materials to explain conductivity and to explain the role of TCR-drivers or modifiers.
The ruthenium and iridium system
Ruthenium and iridium have been used in resistors as metals, the dioxides RuO2 and IrO2, and as complex mixed oxides such as Bi2Ru2O7, Bi2Ir2O7 and Pb2 Ru2 O6 + δ where δ = 0 − 1. They are discussed together because both are members of the platinum metals – Group VIII, their dioxides are metallic and have the same crystal structure – rutile – and their ternary oxides are also metallic and have similar crystal structure – pyrochlore.
Most of their binary and ternary oxides are electrically conducting; the dioxides and ternary oxides of lead and bismuth Bi2Ru2O7, Bi2Ir2O7, Pb2Ru2O6 + δ are metallic oxides (Tsuda et al., 1991). RuO2 is not a stoichiometric compound: ‘RuO2 is seldom, if ever, obtained entirely pure but is usually defective in oxygen with corresponding amount of RuIII in place of RuIV’ (Cotton and Wilkinson, 1966).
The use of Ru as a resistor material prepared in a reducing atmosphere was described by Rosenblatt (1939, 1943) and in air atmosphere can be deduced from the explanation of Place and Place (1960a, 1960b). The patent to Daily et al. (1967) uses a resistance element consisting of minute crystals of metal (Ru) dispersed in a thin film of glass fired onto a base or substance of high temperature-resistant insulating material, such as ceramic. The composition of the resistor paste is: ground glass frit (lead–boro silicate) 8.7 wt%: ruthenium organosol (ruthenium resinate having 4% Ru by weight) 4.35 wt%, and screening and viscosifying agent 86.95 wt%. This patent explains that the composition of the glass used is not critical, nor the manner in which it is produced, and the glass becomes very corrosive at 800–850 °C firing and will attack the ceramic base. Although this patent describes minute crystals of Ru metals as the resistor material, it is more likely that the Ru resinate will decompose, as do all complexes of Ru when fired (Cotton and Wilkinson, 1966) the Ru will substantially oxidize to RuO2. Bruhl and Counts (1967) developed a process in which a cermet mixture based on an alloy of Au and Ir provides a resistance material with high resistivity and low TCR, and a second process (Counts and Bruhl, 1969) in which a cermet mixture formed of a glass and an alloy of Ir and at least one of the other noble metals, selected from the group consisting of Pt, Ag, Ru, Rh and Pd, provides a resistance material with high resistivity. During the firing of the alloys above, it is likely that some Ir, Ru and Rh will oxidize to IrO2, RuO2 and Rh2O3: the high resistance, not typical of metals, is due to the metallic oxides, IrO2 and RuO2.
A patent to Faber et al. (1967) describes a cermet-type resistor element with low TCR of about 0.01%/C and a broad resistance range from below 100Ω/![]() to about 180.000Ω/
to about 180.000Ω/![]() . The resistor composition is based on RuO2 and IrO2; substitution of IrO2 for RuO2 increases the resistance. The composition can be made with powders of RuO2 and IrO2, glass frit and an organic vehicle, or it can be made from resinates of Ir and Ru and organic precursor derivatives of the glass. The glass used is a simple lead borosilicate, PbO-63 wt%; B2O3-25 wt%; SiO2-12 wt%. To control the stability of the ceramic resistance elements, small percentages of cupric oxide and/or manganese oxide are preferably dissolved in the glass.
. The resistor composition is based on RuO2 and IrO2; substitution of IrO2 for RuO2 increases the resistance. The composition can be made with powders of RuO2 and IrO2, glass frit and an organic vehicle, or it can be made from resinates of Ir and Ru and organic precursor derivatives of the glass. The glass used is a simple lead borosilicate, PbO-63 wt%; B2O3-25 wt%; SiO2-12 wt%. To control the stability of the ceramic resistance elements, small percentages of cupric oxide and/or manganese oxide are preferably dissolved in the glass.
Herbst and Dawson (1971) reviewed some of the patent literature and explored a system based on oxides of ruthenium and iridium with metallic additives Ag, Au or Pt and oxides including ZrO2, HfO2, TiO2 and La2O3. These resistors have excellent noise, TCR and other properties at resistances up to 10 MΩ/![]() and in all formulations the frit content was less than 78%. The preferred metallic oxide is the hydrate RuO2*ZH2O, where Z = 0.1 to 3.5.
and in all formulations the frit content was less than 78%. The preferred metallic oxide is the hydrate RuO2*ZH2O, where Z = 0.1 to 3.5.
The conductor is a mixture of metals (M) and the ruthenium or iridium oxide (O), the mixture is defined by Mx + Oy and x and y define the weight ratio between M and O, both generally falling within the range of 0.2 to 0.8. The conducting mixture comprises from 20% to 70% of the composition and the remainder of the composition is the glass frit (lead borosilicate) and oxide (ZrO2, TiO2, HfO2, La2O3). At a given conducting mixture content the resistance increases with the increase in the silver content (M). The TCR of the resistors is a function of resistivity, and not of x and y. The resistance can be varied from 500Ω/![]() to 50 KΩ/
to 50 KΩ/![]() within a relatively narrow TCR range of 150 ppm/ºC. In a series of tests with conducting mixture Ag0.5 (RuO2*ZH2O)0.5 wherein the conductive fraction was 33.3% of the composition, and the value of Z varied over the 0.1 to 3.5 range, it was found that as the Ru content increases (i.e. Z decreases) the resistance decreases by about an order of magnitude and as the anhydrous state (RuO2) is approached resistance rises quickly to the megaohm range. The degree of RuO2 hydration affects resistivity. It is also clear that, by controlling the degree of hydration, higher resistivities can be obtained at higher metal concentrations.
within a relatively narrow TCR range of 150 ppm/ºC. In a series of tests with conducting mixture Ag0.5 (RuO2*ZH2O)0.5 wherein the conductive fraction was 33.3% of the composition, and the value of Z varied over the 0.1 to 3.5 range, it was found that as the Ru content increases (i.e. Z decreases) the resistance decreases by about an order of magnitude and as the anhydrous state (RuO2) is approached resistance rises quickly to the megaohm range. The degree of RuO2 hydration affects resistivity. It is also clear that, by controlling the degree of hydration, higher resistivities can be obtained at higher metal concentrations.
Angus and Gainsbury (1972) describe resistor compositions comprising ruthenium and/or iridium dioxides with fine crystallite size that does not exceed 50 nm. The resistors made from these compositions are characterized by low noise levels and low TCR. This patent shows that the TCR of an oxide–glass resistance film of a given composition depends largely upon the crystallite size of the metallic oxide, being more negative and thus less positive for smaller crystallite sizes. At the same time, the current noise of the resistance film is significantly lower than with oxides with larger crystallites. The size of the glass (lead borosilicate; 65% PbO, 25% SiO2, 10% B2O3) is not critical. The patent also describes the synthesis of RuO2*XH2O and IrO2, with small crystallite sizes, from the corresponding chlorides of Ru and Ir.
Iles and Casale (1967) reported RuO2-based glaze resistors for firing on mica and other low-temperature substrates. To stabilize the RuO2 the authors doped it with Nb2O5. The Nb2O5 could be introduced into the RuO2 lattice in quantities up to 50% molecular, i.e. up to the composition Ru0.5Nb0.5O2.
Casale et al. (1972) report a resistor composition in the form of an oxide containing niobium and ruthenium in which the atomic ratio of metal to oxygen is 1:2 and in which the atomic ratio of niobium to ruthenium is within the range of 1:2000 to 1:1. The resistance materials are prepared by heating RuO2 and Nb2O5 at 1400 °C for several hours in a closed ceramic container. It seems that a new compound is formed between the ingredients at 1400 °C, but the patent and the article do not disclose the composition.
Asada (1973) presents resistor compositions based on RuO2 coated by Nb2O5, sources of Nb2O5 are alkoxides such as Nb(OR)5 where R = − C2H5; − C4H9; − CH2CH2CH(CH3)2; C3H9;−C(CH3)3; Sec-C4H9; –CH(C2H5)CH2CH3; –CH(CH3) C3H7 – iso-C4H9; and NbO(OR′)3 where R′ = –C4H9; –C3H7.
Resistors based on these compositions have low TCR, a small spread of initial resistance values and excellent noise for resistance in the range of 2.7Ω/![]() to ~ 900Ω/
to ~ 900Ω/![]() . The glass used was borosilicate and the organic vehicle was a standard composition.
. The glass used was borosilicate and the organic vehicle was a standard composition.
Brady (1972) reports resistor compositions based on RuO2 and IrO2 (average particle size 0.7–1 μm), glass and a crystal growth controlling agent (submicron Al2O3). The composition is fired at 975–1025 °C for 45 to 60 minutes. These compositions have improved voltage stability and voltage withstanding ability characteristics. When the size of the alumina is about 20 μm the voltage characteristics are not improved, and when colloidal alumina is used the fired resistance element is nonconductive. This patent teaches that the alumina is not soluble to an appreciable extent in the glass. The glasses used are high-lead glasses: glass I PbO-65; SiO2-34; Al2O3-1 wt% and glass II PbO-72.15; SiO2-13.41; B2O3-9.04; ZnO-5.4 wt%. They are very corrosive and, at the firing range (975–1025 °C), they will dissolve all the added alumina to form glass with higher viscosity than the initial glass. The change in the glass viscosity with 0.3 μm alumina is gradual, while with colloidal alumina it is very likely that all the alumina dissolves in the early stages of firing. Brady (1975) reported an improved resistance composition and method of making electrical elements. The compositions were based on RuO2, iridium resinate, glass, an organic vehicle and additives such as Pd and copper resinate. The glasses used were lead borosilicates containing Bi2O3 and two leadless: barium borosilicate and strontium borosilicate. Three lead-free resistor compositions with resistances in the range of 3 kΩ/![]() to 250 kΩ/
to 250 kΩ/![]() and small TCR were described.
and small TCR were described.
Van Loan (1975) describes RuO2-based TF resistors, glass composition, TCR modifiers and materials that control bubble formation and viscosity. Example 1 of this patent shows that glass composition is very important in determining the resistance, TCR and noise of a given composition, i.e. the glass is not an inert binder. The glass compositions were: PbO, 55 to 75%; ZnO to 10%, MnO2 to 10%; B2O3, 5 to 20%; SiO2, 5 to 20% and ZrO2, 0 to 5%. Sb2O5 and V2O5 were used as TCR modifiers, CaF2 as a control material for bubble formation and CeO2 to raise viscosity without adversely affecting the electrical properties of the fired films. The roles of glass ingredients and paste additives are discussed.
Pukaite (1977) describes a low TCR cermet resistor based on RuO2 and/or IrO2. The low TCR is a result of the particular glass composition and vanadium oxide. The glasses used were: 44.9 wt% PbO; 20.1 wt% B2O3 and 35.0 wt% SiO2. The preferred glass compositions (in wt%) were: PbO 38–45; B2O3 17–21; SiO2 33–37; CaO 1–2 and Al2O3 1–2. Ruthenium, iridium and vanadium may be used as resinates or the oxides; in both cases the result is low TCR. These compositions also contain Bi2O3 (0.14 – 4.58 wt%, paste basis) and in some cases Al2O3 (0–7 wt%).
Larry (1978) describes resistor compositions based on RuO2, PbO containing glass, Nb2O5 and optimally CaF2. The glasses used were complex lead borosilicates containing MnO2, Al2O3, ZnO, ZrO2 and CuO. Resistor compositions with small TCR and a narrow spread of fired resistances in the range of about 50Ω/![]() to 100 kΩ/
to 100 kΩ/![]() , were disclosed.
, were disclosed.
In 1971, Bouchard patented oxides with cubic structures containing bismuth and at least one of ruthenium and iridium. The chemistry, the structure and the electrical properties of these new compounds were reported and reviewed (Bouchard and Gillson, 1971). Bi2Ru2O7 and Bi2Ir2O7 are stable in air to at least 1000 °C, are metallic oxides and their TCR is very small. These pyrochlores are much more stable than PdO, have remarkable stability under reducing conditions and are compatible with glass binders. Related oxygen deficient pyrochlore-type compounds Pb2M2O7-x (M = Ru, Ir, Re) were reported by Longo et al. (1969). The lead ruthenate pyrochlore is also a metallic oxide and has similar properties to bismuth ruthenate.
The new metallic oxides of the pyrochlore structure opened a new era in TF resistor development. A wider range of resistances, outstanding properties and ease of formulation and preparation became possible. Soon after the invention of metallic oxides of the pyrochlore family (Bouchard, 1971), patents on resistor compositions based on the new pyrochlores were issued, some of the first ones to Bouchard (1972), Hoffman (1971), Popowich (1971, 1974) and Schubert (1971).
Van Loan (1972) patented lead ruthenate (Pb2Ru2O6), its mixtures with RuO2 and a group of TCR modifiers (V2O3, Sb2O5, MnO2, Fe3O4) to control the TCR. The lead ruthenate pyrochlore, a metallic oxide, can be formed when RuO2 is mixed with a glass (PbO2) vehicle and fired. Soon after the lead ruthenate patent, Kasanami and Kano (1973) patented the use of PbO and RuO2 in the molar ratio of 3:1 to 1:3. The patent explains that some of the PbO may be substituted by Bi2O3 and 50% of the RuO2 may be replaced by IrO2. Thus, four systems may be designed for the conductive material:
To obtain high resistance, less than 50% of the RuO2 may be replaced molecularly by Ta2O5. The patent includes glass compositions and oxides TeO2, WO3, V2O5, TiO2, MnO2, SnO2, Sb2O3, Sb2O5, CuO, ZnO, Nd2O3, Nb2O5 and Ta2O5 to regulate the TCR. The next modifications of lead ruthenate-based TF resistors were: (1) Langley (1978), who teaches a method to make dispersed lead ruthenate or lead iridate in a glassy matrix. The glass constituents (lead oxide, silica) are mixed with RuO2 or IrO2 and heated to a temperature where a glass containing dispersed ruthenate is formed. After cooling the solid is comminuted and converted to a paste. This method facilitates the production of TF resistors exhibiting a low TCR, relative freedom from noise and drift, and high moisture resistance. (2) Fujimura (1979) describes a method to manufacture high resisting, low TCR and excellent voltage handling resistors. This method is similar to that of Langley but it includes other glass ingredients such as Al2O3 1–15%; ZrO2 2–10% and SnO2 0.5–20%. Other glass ingredients MgO, CaO, BaO, SrO, CdO, SnO and Bi2O3 are used in small quantities up to a sum of 5%. Hoffman and Horowitz (1981) in a very detailed patent described resistor compositions based on a group of pyrochlores, including 19 different glass compositions and low-expansion fillers such as zircon. In addition, the patent elucidates a new method of making the resistor compositions by presintering the solid ingredients in a temperature range of 650–850 °C for several hours, then milling and formulating the resistor pastes. Such TF resistor pastes afford resistors that have excellent stability to mechanical or thermal stresses.
Transition metal oxides, such as WO3, V2O5, TiO2, MnO2, Sb2O3, Sb2O5, CuO, CdO, Nb2O5 and TiO2 and resinates such as copper and vanadium resinates, reduce the TCR of Thick-film Resistors (TFRs) and also have other beneficial effects such as improvement of noise levels. These oxides, also known as TCR modifiers or TCR drivers, have been used as additives to the paste or as glass constituents. The mechanism of the TCR modification is not clear, but the data suggest that the TCR control is through the dissolution of the TCR modifier in the glass. Most of these oxides have one property in common: the metal ion can exist in at least two oxidation states in the glass. Cu2 +– Cu+; Fe2 +– Fe3 +, Mn2 +– Mn3 +– Mn4 +; V3 +– V4 +– V5 +; Mo5 +– Mo6 +; Sb3 +– Sb5 +; Nb4 +– Nb5 +; Ti3 +– Ti4 +. Divalent cadmium, Cd2 +, does not fit this trend; however, Cd+ was reported to exist in glasses.
Examples of TCR modifiers used are by: Hoffman (1971) V2O5, Cr2O3, Mn2O3, Fe3O4, Co3O4, NiO, CuO; Van Loan (1975, 1972) V2O5, V2O3, Sb2O5, MnO2, Fe3O4; Asada (1973), Casale (1972), Larry (1978), Nb2O5; Brady (1975) copper resinate; Holmes (1967) MnO2, CuO; Hoffman and Horowitz (1981), Popowich (1971) CdO glasses; Kuo and Angel (1973) CdO and TiO2 glass; Kasanami and Kano (1973) provide the list of oxides TeO2, WO3, V2O5, TiO2, MnO2, SnO2, Sb2O3, Sb2O5, CuO, ZnO, Nd2O3, Nb2O5 and Ta2O5 to regulate the TCR. Most TCR modifiers lower the TCR and raise the resistance, CuO and Cu2O increase the TCR. Hormadaly (1982, 2000) describes TCR modifiers MnV2O6, Mn2V2O7, which lower the TCR but do not raise the resistance like binary modifiers and a semiconducting inverse spinel Co2RuO4, which lowers the TCR, does not raise the resistance like binary modifiers and preserves laser trim stability. Yamaguchi and Kanai (1992) studied the role of Cu and Mn oxides TCR modifiers in RuO2-based resistors with emphasis on the effect of microstructure on their electrical properties. CuO addition resulted in the formation of a cell structure of Maragoni– Bernard convection, which modified the aggregation of conducting particles. The effect of Mn oxides has been explained by the large negative TCR of Mn2O3, which precipitates in the glass matrix. Weimann and Chong (2000) studied the effects of TiO2, GeO2 and TeO2 on the electrical properties of ruthenate TFRs. They argue that the addition of the oxides to the resistor glass increases the thickness of the glass barrier and reduces the impurity states in the glass by reducing the solubility of ruthenium ions.
Electrical and optical properties of RuO2 and IrO2 were reported by de Almeida and Ahuja (2006) and Ryden et al. (1968, 1970). Electrical properties, crystal structure, bonding trends and the crystal growth of ruthenium and iridium pyrochlores were discussed by Akazawa et al. (2004), Bouchard and Gillson (1971), Kennedy and Vogt (1996), Longo et al. (1969) and Tachibana et al. (2006). Dziedzic produced a bibliography on electrical conduction in TF resistors (1991).
Thick-film resistor materials have evolved during the last 40 years to a class of materials with wide resistance range and outstanding performances. These properties were achieved via innovation in conducting materials, glasses, dispersion techniques and proprietary additives. However, these unique properties were achieved with conducting materials and glasses that contain lead and cadmium. Cadmium compounds are carcinogens, and lead compounds are highly toxic. In recent years, the global trend has been to restrict and eventually eliminate the use of these compounds. After the RoHS (restriction of hazardous substances) directive was introduced, new conductor and solder compositions that are cadmium- and lead-free were introduced. The introduction of new lead-free TF resistor compositions (10Ω/![]() to 1 MΩ/
to 1 MΩ/![]() ) has not come to fruition yet, even though researchers and TF manufactures have been engaged in the development of Cd/Pb-free TFRs since the early 1980s.
) has not come to fruition yet, even though researchers and TF manufactures have been engaged in the development of Cd/Pb-free TFRs since the early 1980s.
Currently, TFRs are formulated with conducting materials such as RuO2 or ruthenates like Pb2Ru2O6 + δ and Bi2Ru2O7, and lead-containing glasses. The glasses are usually borosilicate modified with PbO, CdO, ZnO, Bi2O3, CaO, BaO, MnO, CuO, Al2O3, ZrO2; the most commonly used are high-lead glasses. The advantages of lead glasses and the problems of developing lead-free TF glasses have already been addressed in detail by Vest (1986).
Thick-film resistor systems for nitrogen atmosphere processing were developed in the late 1970s and early 1980s. These systems were based on cadmium-free and lead-free glasses and conducting materials such as LaB6 and Sn2–xTa2–ySny O7–x–y/2/SnO2 (Donohue et al., 1987, 1988a, 1988b). Other conducting materials such as nitrides (Mulligan, 1969), carbides (Murphy and Janakirama-Rao, 1965) and silicides (Kuo, 1987a) with lead-free glasses were also described in the patent literature as nitrogen-fireable TFRs. These nitrogen-fireable TFRs are not useful for air atmosphere processing.
A model system of lead-free TFRs was prepared (Morten et al., 1991) from lead-free glass and RuO2 or Bi2Ru2O7 as conducting phases. It was found that in the lead-free glass/RuO2 system, electrical properties are similar to leaded glass/RuO2, TFRs but TCR is large and positive at firing temperatures higher than 850 °C. The Bi2Ru2O7 lead-free glass system did not form resistors, and EDS analysis has shown that Bi from the conductive grains dissolved in the glass. Some properties of conducting materials RuO2, (Bi, Gd)2 Ru2O7 and Bi2Ru2O7 with lead-free glasses have been described previously in the patent literature (Hormadaly, 1996). Lead-free TF resistors were investigated by Busana et al. (2006), Gurunathan et al. (2005), Kshirsagar et al. (2007), Prudenziati et al. (2002) and Rane et al. (2006), and the stability of lead-free resistor compositions in RuO2-CaO-SiO2 and RuO2-Bi2O3–SiO2 systems were evaluated by Hrovat et al. (2008, 2010). Recent investigations on lead-free TF resistors are Jagtap et al. (2009) and Kielbasinski et al. (2010).
Patent activity in lead-free TFRs is based on ruthenium and iridium compounds. Fukaya et al. (2000, 2003) teach RuO2-based TFRs with a combination of two glasses, one calcium aluminoborosilicate and the second glass is sodium borosilicate. In a second patent the above inventors report RuO2-based TFRs and potassium borosilicate glass where K2 O content is 0.1% to 10%. Hormadaly (2006) describes lead-free TFRs based on Nd2–yCuy Ru2O7–y pyrochlore. Tanaka and Igarashi (2007) describe TFRs based on RuO2 or mixed oxide of Ru and lead-free alkaline earth borosilicates containing CuO and MnO. Tanaka and Igarashi (2009a, 2009b) describe a TFR composition based on alkaline earth borosilicate glass, which may contain ZrO2, Ta2O5 and Nb2O5 and conducting material selected from RuO2, Bi2Ru2O7, Tl2Ru2O7, MRuO3 (M = Ca, Sr, Ba) and LaRuO3; in the second patent, MTiO3 (M = Ca, Sr, Ba) additives, CuO and Cu2O are used with RuO2 and a Ru-composite oxide, as conducting materials. Endo et al. (2009) describe ruthenium-based lead-free TFR composition with alkaline earth aluminoborosilicate glasses modified with transition metal oxides. Although this patent provides a long list of Ru compounds, including those which are semiconducting, only RuO2 and CaRuO3 are used in the examples. Makuta and Maeda (2009) describe a high-resistance lead-free TFR composition based on IrO2 or IrO2 and RuO2 and borosilicate glass, 10 wt%-SrO, 43 wt%-SiO2, 16 wt%-B2O3, 4 wt%-Al2O3, 20 wt%-ZnO and 7 wt%-Na2O. VerNooy et al. (2009) report a lead-free TFR composition based on Li2RuO3 and compatible glass.
Hang et al. (2009) discuss surface-modified RuO2 and alkaline earth borosilicate glasses. Surface modification is done by wetting RuO2 by K2CO3 solution or other modifying materials, drying and calcining at ~ 900 °C. Resistor pastes formulated with surface-modified RuO2 have a broad resistance range of 10 kΩ/![]() to 10 MΩ/
to 10 MΩ/![]() and TCR of 100 ppm/ºC.
and TCR of 100 ppm/ºC.
Related to the recent patents above that are based on RuO2 and IrO2, are the works of Dziedzic (1989), Diezdzic and Golonka (1988) and Tankiewicz et al. (2002), which have important data and discussions for IrO2, RuO2, CaIrxTi1–xO3 and Bi2Ru2O7 conducting materials.
2.2.3 Conductors
Thick-film conductor compositions were reviewed by Finch (1969) and later by Holmes and Loasby (1976). These compositions are based on a metallic part, which is the major constituent, a binder and an organic vehicle. The metallic part is usually noble metal or a mixture of noble metals such as Ag, Ag/Pd, Ag/Pt, Ag/Pt/Pd, Au, Au/Pd, Au/Pt, Au/Pd/Pt for air-fired conductors, or a base metal such as Al, Cu or Ni. The binder phase may be divided into three groups: oxides, glass and oxides plus glass, also known as the mix-bonded phase. The oxides were mixtures of CdO, CuO, Cu2O and Bi2O3 and the glasses were based on high levels of lead and bismuth oxides. Although TF conductor compositions may be considered as a mature field, the patent literature activity of the recent decade shows that, on the contrary, the number of patents and the variety of applications are increasing at a fast rate. One of the reasons for new patents in this field is the requirement to remove CdO, lead and NiO from the compositions for toxicity and environmental considerations. Other reasons are the availability of nanoparticles of metals and the steep increase of activity in low-temperature co-fired ceramic (LTCC), solar energy and plasma display applications.
Examples of glass-bonded conductor compositions are: Christensen et al. (1949), Craig and Taylor (1987), Larry (1974), Larsen and Short (1958), Martin (1966), Miller (1968a), Minneman et al. (1974), Okamoto et al. (1997) and Soby (1951). Examples of oxide-bonded TF conductors are: Eustice (1994), Felten (1994), Frazee (1977) and Rellick (1982). Examples of mixed-bonded TF conductors are: Ballard and Hoffman (1969), Carrol and Kuno (1994), Gruber and Barringer (1991), Horowitz (1978, 1982), Saeki et al. (1991) and Taylor (1986).
Hormadaly (1995) reports lead-free glass compositions for Au conductors, conductor compositions and explanation of the role of CuOx, Bi2O3 and CdO in bonding of the metallization to the ceramic substrates. These oxides partially reduce during firing and alloy with the noble metal conductor, thus modifying its surface characteristics and mechanical properties. It is believed that these elements, which are more electropositive than gold, form the strong chemical bonds at the surface of the gold film with the ceramic dielectrics. Prunchak (2010) describes lead-free glasses that contain TeO2, SiO2 and Bi2O3 as their main constituents. Hang et al. (2010) teach lead-free glass compositions for TF pastes and LTCC.
The variety and types of applications of TF conductors as expressed in the patent activity of recent years are illustrated in the following examples: solar energy (Rose and Young, 2010; Young et al., 2010a, 2010b, 2010c); polymer-based conductors and TF conductors for use in biosensors (Chan 2000, 2003; Dorfman, 2010; Towlson 1997); low-temperature, very fast curing conductors (Kydd et al., 2002); new ink-jet printable TF conductors compositions (Yang, 2010). Previous work on ink-jet composition was Vest (1980), Vest et al. (1983); on processes for TF patterning, Keusseyan (2010); on conductor composition for plasma display, Matsuno and Barker (2010); on high conductivity Ag conductors based on very fine particles, Totokawa et al. (2010); on conductors for LTCC, Ollivier and Hang (2010); on conductors for Multi Layer capacitor (MLC) electrodes, Akimoto et al. (2010), Pepin (1990).
2.2.4 Dielectrics
The majority of current glasses for TF applications consist of lead borosilicate with minimal or no alkali oxides, therefore they are outstanding insulators. The insulating properties and moderate dielectric constants render them as candidates for TF applications such as insulators, capacitors, crossover dielectrics, multilayer materials and LTCC. For overglaze or encapsulant applications, low softening point glasses were used. Low softening point glasses typically have a high content of lead oxide, therefore they have a high linear coefficient of expansion and low durability. These glasses, which also contain CdO, were compounded with low expansion filler such as silica to lower their expansion, enhance their chemical durability and still maintain the low temperature processing characteristics. The overglazes also have a durable pigment such as Cr2O3. Typical composition is DuPont 9137. About 30 years ago, new compositions, which are CdO-free, were introduced by DuPont such as QQ550, which has similar properties to 9137. Environmental concerns have led to the development of lead-free and cadmium-free overglazes. Typical compositions and properties of TF overglazes are discussed by Hormadaly (1992a, 1992b) and Donohue (1990, 1998).
Dielectric compositions for crossover, capacitors and multilayers applications are described by Amin (1974), Barker et al. (1992), Bethke et al. (1998), Borland et al. (2010), Donohue et al. (1990), Hartmann (1991), Hu et al. (1993), Nair (1983), Shaikh et al. (1995), Stein et al. (1999), Tsuyuki (1995), Ulrich (1972) and Usui et al. (2002).
Rellick (1987, 1989) reports a casting composition for making green tapes that contain noncrystallizable glasses, refractory oxide, an organic vehicle for processing in nonoxidizing atmosphere and a method for fabricating multilayer circuits on rigid ceramic substrates using conventional dielectric green tape and TF-conductive pastes. Donohue (2000) describes a glass composition, a castable dielectric composition comprising the glass and refractory oxide, organic binder and vehicle. This composition is used in a method of forming a low-loss green tape. Related compositions for integrated circuits are shown by Cho and Hang (2004), Jean (1998), Jean and Gupta (1993), Kumar et al. (1999), Muralidhar et al. (1993), Senkalski and Hasenmayer (1990). Methods to control the planar shrinkage of green tapes and LTCC are described by Khadilkar et al. (2009) and Wang et al. (2004, 2006).
A non-photographic method for making patterns in organic polymer films, diffusion patterning (DP), is described by Felten (1991) and Felten et al. (1997).
2.2.5 Materials for nitrogen firing
Materials for nitrogen or inert atmosphere firing have to be stable at low concentrations of oxygen, about 5–10 ppm, and not be reduced by the organic vehicle. Materials like precious metal oxides, PbO and other oxides that reduce easily have to be replaced by materials that survive firing in harsh conditions. The replacement is easier for conductors and dielectrics because they contain one major ingredient, but much more complicated for resistors. The conducting phases RuO2, lead ruthenate, bismuth ruthente and the glass, which contains lead, have to be substituted with other materials. Several types of conducting materials were used with leadless glasses to make TFRs, which can be processed in an inert or reducing atmosphere. The first group is based on borides, nitrides and silicides. The second group is based on oxides such doped SnO2, In2O3 and ruthenates of the perovskite family, MRuO3; the third group is based on the interactions of materials during firing to form a conducting phase; an example of this group is the interaction between Mo and MoO3 to produce MoO2, which is metallic oxide.
Examples of conducting materials based on a mixture of refractory carbide and refractory metal are given by Murphy and Janakirama-Rao (1965), who describe W and WC with barium borosilicate glass consisting of 48% BaO, 8% CaO, 23% B2O3 and 21% SiO2. Mixtures of transition element nitrides and transition metals and carbide–metal mixtures, WC-W, are described by Mulligan (1969). Mackenzie (1974) describes a resistance material based on TaC and Ti and alkaline earth borosilicate glass. A vitreous enamel resistance material comprising of a glass frit and fine metal boride of the transition elements of groups IV, V and VI of the periodic table, where the metal boride may be CrB2, ZrB2, MoB2, TaB2 or TiB2, is discussed by Huang and Merz (1970). Merz and Shapiro (1980) describe a vitreous enamel resistance material comprising barium borosilicate glass, Ta and additives Ti, B, Ta2O5, TiO, BaO2, ZrO2, WO3, Ta2N, MoSi2 and MgSiO3. Shapiro and Merz (1980) describe a vitreous enamel resistance material comprising Ta2N, glass and additives selected from B, Ta, Si, ZrO2 and MgSiO3. Merz and Shapiro (1977) describe a resistance composition comprising glass and tantalum nitride or glass and W and WC. Nair (1987a, 1987b, 1987c) discusses resistor compositions based on a semiconducting material consisting essentially of a refractory metal carbide, oxycarbide or mixtures thereof and a non-reducing glass; an anion-deficient semiconducting material consisting essentially of a refractory metal nitride, oxynitride or mixture thereof and a non-reducing glass; and a semiconducting material consisting essentially of a cationic excess solid solution and a non-reducing glass. Kuo (1987a) describes resistor compositions based on silicides TiSi2, Ti5Si3, Al2O3 and alkaline borosilicate glasses.
Donohue (1985, 1986a, 1986b) and Donohue and Marcus (1980) describe nitrogen-fireable TFR compositions, TCR modifiers, suitable glass compositions, thermodynamic calculations regarding reducibility of glass ingredients, Ta2O5 containing glasses and review of the literature. Watanabe et al. (1991) describe resistive paste that contains metal hexaboride, a vitreous binder that contains a specified amount of Nb2O5 and in addition the paste may contain at least one nitride selected from the group consisting of AlN and BN.
Tin dixode, SnO2, was used to fabricate refractory material that is extremely resistant to the corrosive attack of molten glass (Hood, 1941). Later, Mochel (1949) discovered that when SnO2 is mixed with a shrinking agent comprising compounds of one of Cu, Ag, Au, Mn, Fe, Co, and Ni, and heated to 1200 °C or above, the resulting sintered body had high electrical conductivity. Doped SnO2 was used for high voltage resistors, conducting glazes and resistors. These uses and other properties of doped SnO2 were the subject of: Basu et al. (1974), Binns (1974), Burkett (1961), Dearden (1967), Gress et al. (1968) and Powell (1974). Whalers and Merz (1977, 1980, 1981, 1982a, 1982b, 1983a, 1983b) describe a vitreous enamel resistor material based on SnO2, glass, Ta2O5 and other additives. These patents also provide pretreatment of SnO2 and additive at reducing and nitrogen atmospheres. Hormadaly (1985a, 1985b, 1985c, 1985d, 1986a, 1987a, 1987b) describes nitrogen-fireable TFR compositions based on Sn(II)2–x Ta2–y
Sn(IV) yO7–x–y/2 pyrochlores, SnO2, lead-free glass compositions and TCR modifiers. Kuo (1987a, 1987b, 1987c, 1988) describes TFR compositions based on SnO2, resinate solutions such as transition metal resinates and heat treatment of the resinate-coated SnO2 in reducing atmosphere, Ta2O5 glass and additives. Asada (1991) describes resistor compositions based on SnO2, heat-treated powder of SnO2 and Ta2O5, glass and tantalates.
In2O3 is an n-type semiconductor that has found uses in electrical contacts (Richardson and Swinehart, 1951) and light transmitting electrodes (Amans, 1966). Block and Mones (1968) describe an indium oxide resistor composition, method and article. Compositions were formulated with lead-free glass: 9.5 wt% Al2O3, 49.4 wt% SiO2, 10.4 wt% B2O3, 30.3 wt% BaO and 0.4 wt% of non-alkali oxides. The pastes may be fired in the air or in inert atmosphere at 800 to 1200 °C. Firing in inert atmosphere lowers the resistance and raises the TCR. The conductivity of In2O3 can be modified by addition of dopants. Dopants that increase the conductivity are Sb, As, P, Nb, Ce, Si, Ta, Zr, Ti, Sn and Mo and dopants that lower the conductivity are Cu, Au, Ag, Pt, Pd and Li. The conductivity can also be increased by forming oxygen-deficient In2O3 and by substituting halogen for oxygen. Prabhu and Hang (1983a, 1984a) describe In2O3 resistor compositions. The dopants used were MgO, V2O3, V2O5, ferric oxide and the glass compositions were: (1) 20.21 wt% SiO2, 15.62 wt% B2O3, 51.59 wt% BaO, 12.58 wt% CaO, (2) 16.75 wt% SiO2, 19.42 wt% B2O3, 51.32 wt% BaO, 12.51 wt% CaO. Compositions were fired in nitrogen atmosphere at 850 to 950 °C.
Hankey (1985) describes substituted ruthenium perovskite A′1-xA′′xB′1-yB′′yO3, where A′-Sr, A′′-Ba, La, Y, Ca, and Na, B′-Ru and B′′-Ti, Cd, Zr, V, Co and alkaline earth borosilicate glass. Steinberg (1989) describes the same perovskite with additives such as Ni, Cu and CuO.
Pedder (1982) explains a method to make resistors compatible with a copper conductor. The resistor composition is a mixture of Mo and MoO3, lead zinc borosilicate glass and additives such as W and V2O5. During firing in nitrogen atmosphere, molybdic oxide is reduced, by Mo and the organic vehicle, to MoO2,which has high electrical conductivity and rutile structure. Prabhu and Hang (1983b, 1984b) describe low value resistor inks for porcelain-coated metal substrates, which are based on SnO-MoO3 or Mo-MoO3 mixtures, alkaline earth borosicate glass, organic vehicle and TCR modifiers CdO and V2O5. The molybdic oxide will be reduced by SnO to a mixture of two rutile structure oxides SnO2 and MoO2, which can form solid solution.
Some copper conductor compositions are described by Martin (1997), Prabhu et al. (1988, 1989a, 1989b) and Siuta (1986, 1987).
2.2.6 Materials for AlN, SiC, glass and other substrates
AlN substrates are manufactured from AlN powder, additives such as Y2O3, rare earth oxides, alkaline earth compounds and other compounds. The AlN and the additives are heated in nitrogen atmosphere to sinter the powders. The starting AlN powder contains oxygen as Al2O3, and at high temperature in nitrogen atmosphere, the alumina, AlN and the additives react to form AlON, yttrium aluminium garnet (YAG), and compounds that are products of the reaction of alkaline earth oxides with alumina. The synthesis of aluminum nitride substrates, the additives used and the process of preparation are described by Iwase et al. (1987), Nakahashi et al. (1986), Ohkawa et al. (1998), Sato et al. (1988) and Toyoda et al. (1998). Even with the oxygen-containing products listed above, the wettability of sintered AlN with metal is poor and the result is low bonding strength or no bonding. In addition, because of large and negative ∆G of Al2O3 formation, many ingredients of TF materials react with AlN; for example, PbO, a major TF ingredient (in glasses or lead ruthenate) is expected to react with aluminum nitride as follows:
The ∆G of this reaction is: ∆Gf (1123 K) = − 410 kJ mole− 1. Detailed information about thermodynamic considerations in the TF metallization of aluminium nitride substrates is given by Norton (1990). Similar reactions are expected with many TF materials, therefore most of the TF materials developed for alumina are not compatible with AlN. Since many TF compositions contain glass or glass ceramics, which soften or melt during the firing process, and nitrogen gas is released as discussed above, bubbles and blisters are formed when conventional TF materials are fired on AlN. One way to avoid the blister formation is to oxidize the surface of AlN substrates to form a layer of alumina, 3–5 μm thick. A second way is to use TF formulations that are not reduced by AlN and a third way is to use glasses that do not spread substantially on AlN during firing, thus they form a porous fired structure. Similar thermodynamic considerations can be applied to SiC substrates: here, the Si will oxidize to SiO2 and the carbon to CO or CO2, thus blister formation is likely with conventional TF materials. Oxidation of the surface of SiC substrates to form a thin coating of SiO2 will prevent blister formation and will increase the bonding strength of TF materials to the substrate.
AlN-compatible TF binder glasses and pastes were reported by Harster and Mattox (1993) and Yamaguchi and Kageyama (1989). Mattox (1997) describes AlN-compatible TF binder glasses and TF compositions. The glasses discussed are mixed alkaline earth boroaluminate glasses comprising BaO combined with at least one of CaO, SrO and MgO and up to 30 mole% SiO2. An example of an Ag conductor according to the patent is: 85 volume% of Ag and 15 volume% of glass, glass composition 54 mole% B2O3, 7.2 mole% Al2O3, 10 mole% SiO2, 14.4 mole% BaO and 14.4 mole% CaO. Composition was fired on AlN substrate in air; no blisters or bubbles formed.
Reactions between ruthenium dioxide and aluminium nitride in resistor pastes, the development of resistor and conductor pastes for AlN and the properties of AlN-compatible pastes were reported by Kretzschmar et al. (1993a, 1993b, 1997) and Otschik et al. (1998a, 1998b).
Frit-free conductor compositions are described by Okamoto (2000). The development of glass frit-free metallization systems for AlN was reported by Adlanig and Schuster (1998) and the bonding mechanism and stress distribution of a glass frit-free TF metallization for AlN ceramic by Reicher and Smetana (1998). Recent examples of materials for aluminium nitride are described by Bloom (2001) and Cho and Hang (2006).
2.3 Deposition medium – vehicle
The organic or the carrier vehicle for printing pastes consists of three major parts: solvents, a resin binder and additives. The solvents are selected based on their ability to dissolve the resin, dissolve or disperse the paste ingredients and on the type of application required. Typical solvents and other organic ingredients that were taught in the patent literature (Christensen et al., 1949; D’Andrea, 1960; Dumesnil, 1962; Faber et al., 1967; Felten, 1978; Miller, 1968a, 1968b, 1968c; Minneman et al., 1974; Place and Place, 1960b) are: higher-boiling paraffins, cycloparaffins and aromatic hydrocarbons or mixtures thereof; cellosolve acetate (ethylene glycol monoethyl ether acetate), carbitol acetate (diethylene glycol monoethyl ether acetate) and higher alcohols; di-alkyl ethers of diethylene glycol or their derivatines such as C4H9-O-CH2-CH2-O-CH2-CH2-OOCCH3 and C4H9-O-CH2-CH2-O-CH2-CH2-O-C4H9; terpenes; esters; ketones and alcohols. Examples of resin binders include cellulose derivatives, natural gums, polyterpenes, synthetic resins such as polymethylmethacrylate, polybutylmethacrylate, polystyrene, poly alphamethylstyrene and other polymers of alkyl esters of methacrylic acid (vinyl or substituted vinyl polymer), polyisobutylene and itaconic acid polymers.
The additives include a long list of materials that modify the viscosity and impart special properties to the paste. Some examples are: (1) surfactants – this group includes many materials, organic and inorganic; examples of surfactants described in the literature are (Felten, 1978; Miller, 1968c) compounds like soya lecithin, Igepal CO 430 (an alkyl phenoxypoly (ethylene-oxy) ethanol and alkyl esters of phosphoric acid were used in resistor, conductor and dielectric paste compositions; (2) vaporizable solid – the vaporizable solid in the vehicle results in essential dimensional stability of the printed line. Examples of vaporizable solids (Miller, 1968b) are terphthalic acid, furoic acid and ammonium compounds such as (NH4)2CO3 and (NH4)2SO4; (3) antioxidants; examples of food antioxidants used in paste compositions are butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA).
The above description relates to materials used in conventional paste compositions and some pastes with improved printing characteristics (Miller, 1968c; Minneman et al., 1974). Specialized vehicle compositions were formulated for the following. (1) Nitrogen-fired compositions; here, the paste compositions are fired in nitrogen where the concentration of oxygen is very low, less than 10 ppm. Therefore, the organic vehicle consists of polymers that depolymerize and evaporate before the vitreous phase softens and seals the underlying materials. Examples of organic vehicles for nitrogen firing are given by Scheiber (1981). (2) Photosensitive vehicles were developed to facilitate the formation of fine lines and spaces by TF technology. Paste containing the photosensitive vehicle and the desired function (conductor, resistor, etc.) is deposited by screen printing on a substrate and exposed through the desired pattern to actinic radiation. The exposed parts of the print harden and the unexposed parts are washed by solvent to leave thin lines of material that are subsequently fired to form a conductor, resistor or other passive component. Photosensitive vehicles were revealed in many patents and reviewed by Felten (1975) and Scheiber and Weaver (1975). (3) Water-based printing vehicles were developed to reduce the pollution from volatile organic chemicals (VOC). Chan and Dorfman (1999) describe an improved water-based vehicle for TF paste and present a review of the prior art.
The use of organic precursors for the inorganic paste ingredients (glass, conductor and resistor materials) was suggested by Faber et al. (1967) for all paste ingredients and by Place and Place (1960b) for most paste ingredients. Precursor-derived printable conductor compositions are described by Vest (1980) and ink-jet printing of hybrid circuits by Vest et al. (1983). The recent trend is the preparation of precursor compositions for the deposition of electronic components such as resistors and dielectrics and new methods for the deposition of these compositions. These compositions have low viscosity, about 1000 centipoise, and can be deposited using a direct-write tool. In addition to the above, these materials have a low conversion temperature, enabling the formation of electronic features on a wide variety of substrates including low-temperature substrates. The above-mentioned trend is described and reviewed by Kodas et al. (2009).
2.4 Glasses and glass ceramics
Glass compositions for microelectronics and TF applications were the subject of two reports, Cox (1968) and Vest (1979), respectively. The role of the glass frit in TF composition was described by Holmes and Loasby (1976) as of holding, binding, sealing the fired composition and bonding to the ceramic substrate. To fulfill these functions the glass frit has to have a compatible linear coefficient of expansion with the substrate, its sintering temperature must be below the maximum firing temperature, the glass must form a ‘chemically and physically stable interface with the substrate and the conductive following firing’ (Holmes and Loasby, 1976) and the glass should be inert to the environment. Vest (1979) described and listed several properties that are important to TF glasses: viscosity, surface tension, linear coefficient of expansion. Previous authors (Cox, Vest, Holmes and Loasby) were aware of the interactions of the glass frit with the substrate and the functional phase. Vest (1979) reviewed the literature and correlated physical properties with glass composition, and concluded ‘that quantitative correlations between physical properties and chemical composition of glasses are only possible over very limited composition range at constant temperature’, and that ‘present theoretical models which describe the temperature dependence of thermal expansion, surface tension, or viscosity of glasses are woefully inadequate’. The TF industry’s approach to glass development was mostly empirical and the success of this approach is evident in the many glass compositions since developed. Nowadays, because of the availability of custom glass producers for TF applications and the new model for calculating the linear coefficient of expansion of glass from its chemical composition (Hormadaly 1985a, 1986b), which were not available at the time of the Vest report (1979), it is easier to develop TF glasses.
Because TF glass is a very reactive substance, especially when molten, it dissolves small quantities of the inert container material during the frit preparation. Therefore, all glass compositions melted in a platinum crucible may contain ionic or metallic platinum, typically less than 50 ppm. During the milling process, the milling medium and the container linings erode to some extent, and the erosion products contaminate the glass powder. These materials (Pt) and the major erosion products (Al2O3 or ZrO2) can modify the glass properties.
TF glasses, especially lead-containing ones, are very corrosive when molten and they interact with the active phase, substrate, terminations and additives. After the firing process the glass composition and its properties are modified. The interactions of TF glasses with TF ingredients are dealt with in Chapter 3.
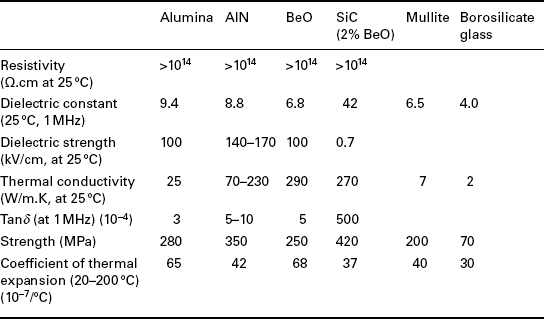
Typical glass compositions used in early development of TF resistors are collected in Table 2.1. Table 2.1 shows that most of the compositions are simple high lead borosilicate glasses, some contain other elements such as Zn, Ba, Ca, Ag, Bi, Al and Zr. The patent literature after 1972 contains a large number of glass compositions used in the development of TF resistors. For example, Hoffman and Horowitz (1981) present very detailed information on TF resistors based on pyrochlores and refractory fillers and describe 19 glass compositions that are mostly lead boroaluminosilicates modified with oxides of TiO2, ZrO2, CdO, Li2O, BaO, CaO, ZnO, Na2O and MnO2; two of these glasses are used as TCR drivers, the glass containing CdO and the glass containing MnO2.
Table 2.1
Glass compositions used in resistors (patent literature for 1948Ð1972 period), compositions in wt% oxides
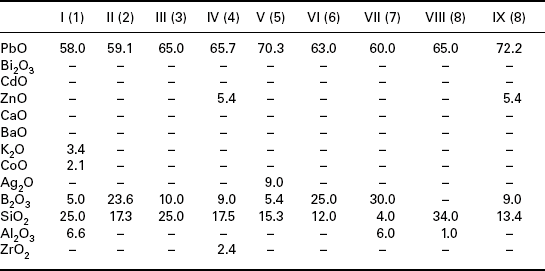
Note: Roman numerals denote glass number and number in parenthesis is the reference number.
Sources for Tables 2.1 and 2.2 (patent literature, 1948–1972): (1) Jira, 1948; (2) D′Andrea, 1960; (3) Dumesnil, 1962; (4) Place and Place, 1964; (5) Janakirama-Rao and Murphy, 1964; (6) Faber et al., 1969; (7) Van Loan, 1972; (8) Brady 1972; (9) Soby, 1951; (10) Martin, 1966; (11) Ballard, 1969; (12) Miller, 1968; (13) Larry, 1974; (14) Minneman et al., 1974.
Typical glass compositions used in conductors are collected in Table 2.2. Table 2.2 shows that glasses used in conductors are based on lead borosilicates, bismuth borosilicates or lead–bismuth borosilicates. In addition, Table 2.2 also shows that these glasses may contain CuO and CdO, which are easily reduced oxides.
Table 2.2
Typical glass compositions used in TF conductors
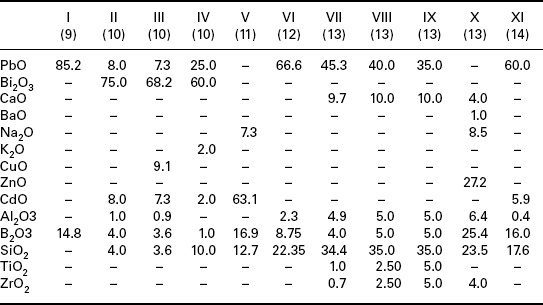
Note: Roman numerals denote glass number and number in parenthesis is the reference number.
Glasses for dielectrics (overglazes, crossover, capacitors, multilayers and LTCC) are a large group based on several glass systems: lead borosilicates, bismuth borosilicates, lead silicates, lead-germanates, lead-free glasses, which may be alkaline earth borosilicates, or silicates. Some of these glasses are crystallizable, a property that is used in crossover dielectrics: after crystallization, the composition is a glass ceramics and it does not soften on subsequent thermal process.
Many glass compositions are used in TF materials and the scope of this section does not allow detailed treatment, however the citations of this chapter were selected to include hundreds of glass compositions for TF applications.
2.5 Substrates
Substrates for TF applications provide the mechanical base on which TF materials are deposited and subsequently fired at 500–1000 °C in air or inert atmosphere.
The requirements are mechanical strength, high resistivity, chemical inertness, thermal conductivity, moderate expansion, stability at a broad temperature range and price. Alumina and alumina-based ceramics have been used because they have most of the properties listed above, therefore the majority of TF materials were developed for alumina. The materials developed have linear coefficients of expansion compatible with alumina, typically 96% alumina, and they were formulated with lead-containing glasses. For applications where high thermal conductivity was required, beryllia, BeO, was used. BeO has a higher thermal conductivity than alumina, it is more expansive, its mechanical strength is lower than that of alumina, and BeO particles and vapor are highly toxic. AlN has a high thermal conductivity, a low linear coefficient of expansion, high flexural strength, low hardness and stability at high temperatures, up to 900 °C in air and up to 1600 °C in reducing atmosphere. Typical properties of ceramics used for substrates and packages are collected in Table 2.3. The data in Table 2.3 are based on Miyashiro et al. (1990) and Tummala (1991).
Tummala et al. (1993) treat a large variety of materials for microelectronics, including substrates. A system for high temperatures of 500 °C, including SiC elements, was discussed by Chen et al. (2001). Specialized substrates such as enameled steel substrates for TF applications and comparison of properties with common substrates were treated by Stein et al. (1980). Novel substrates for TF technology, such as diamond, were discussed by Lux and Smetana (1991).
2.6 Conclusion
This chapter is an attempt to review the state of the art of TF materials. Most information sources are in English and from the patent literature. TF materials are commercially important and the majority of research has been done in industry. Therefore, most of the references are from the patent literature, where a basic understanding of the material is often not addressed. Furthermore, the patent literature discloses very important information regarding the materials and their interactions. Since the early 1960s, TF materials and technology have been the subject of intensive research in academic institutions. A simple search of TF materials in the literature shows well over 1000 papers. Research in universities advanced public understanding of TF materials, which were considered in the past as proprietary and very complex.
Many facets of TF materials are still not fully understood and it is hoped that this review will enhance interest in these materials, their properties and the interpretation of the conduction mechanism of TF resistors.
2.7 References
Adlanig, A., Schuster, J.C. Development of glass frit free metallization systems for AlN. Journal of Material Science. 1998; 33(20):4887–4892.
Akazawa, T., Inaguma, Y., Katsumata, T., Hiraki, K., Takahashi, T. Flux growth and physical properties of pyrochlore Pb2Ru2O6.5 single crystals. Journal of Crystal Growth. 2004; 271:445.
Akimoto, Y., Nagashima, K., Kimura, T., Kamahori, Y. Nickel–rhenium alloy powder and conductor paste containing the same, 2010. [US Patent 7-744-779; June 29].
Amans, R.L. Light transmitting electrode including N-type semiconductive In2O3, 1966. [US Patent 3-295-002; December 27].
Amin, R.B. CaTiO3 – Crystallizable glass dielectric compositions, 1974. [US Patent 3-787-219; January 22].
Angus, H.C., Gainsbury, P.E. Oxide resistor materials, 1972. [US Patent 3-679-607; July 25].
Asada, E. Electrical resistance composition and resistance element, 1973. [US Patent 3-776-772; December 4].
Asada, E. Resistor compositions, 1991. [US Patent 4-986-933; January 22].
Ballard, K.H., Hoffman, L.C. Noble metal metalizing compositions, 1969. [US Patent 3-450-545; June 17].
Barker, M.F., Craig, A., Donohue, P.C., Hang, K.W., Haun, M.J., Pickering, C.R. Dielectric composition containing kerf additive, 1992. [US Patent 5-137-848; August 11].
Basu, M.K., Lele, R.V. Effect of reducing atmosphere of SnO2-Sb2O5-based semiconducting glaze. American Ceramic Society Bulletin. 1974; 53(6):488–489.
Bethke, S.J., Miesem, R.A., Chiou, W.W., Pastor, R.D.G. Ceramic composition, 1998. [US Patent 5-821-181; October 13].
Binns, D.B. Conducting glazes. Transactions of the Journal of the British Ceramic Society. 1974; 73:7–17.
Block, M.N., Mones, A.H., Indium oxide resistor composition, method, and article, 1968. [US Patent 3-411-947; November 19].
Bloom, T. Copper ink for aluminum nitride, 2001. [US Patent 6-207-288; March 27].
Borland, W., Thick-film Hybrids in Electronic Materials Handbook: Packaging. L. M. Merrill, ASM International Handbook Committee, 1989.
Borland, W.J., Jones, A.B., Renovales, O.L., Hang, K.W. Thick-film dielectric and conductive compositions, 2010. [US Patent 7-688-569; March 30].
Bouchard, R.J. Compositions for making electrical elements containing pyrochlore-related oxides, 1972. [US Patent 3-681-262; August 1].
Bouchard, R.J. Oxides of cubic crystal structure containing bismuth and at least one of ruthenium and iridium, 1971. [US Patent 3-583-931; June 8].
Bouchard, R.J. Thick-film technology: An historical perspective. Dielectric Ceramic Materials: Ceramic Transactions. 1999; 100:429–442.
Bouchard, R.J., Gillson, J.L. A new family of bismuth – precious metal pyrochlores. Material Research Bulletin. 1971; 6:669–679.
Boyd, J.R., Mones, A.H., Schottmiller, J.C. Electrical device, method and material, 1968. [US Patent 3-372-058; March 5].
Brady, L.J. Electrical resistance elements, their composition and method of manufacture, 1972. [US Patent 3-655-440; April 11].
Brady, L.J. Resistance composition and method of making electrical resistance element, 1975. [US Patent 3-916-037; October 28].
Bruhl, D.A., Jr., Counts, W.E. Cermet resistance composition and resistor, 1967. [US Patent 3-326-720; June 20].
Burkett, R.H.W. Tin oxide resistors. Journal of the British IRE. 1961; 301–304.
Busana, M.G., Prudenziati, M., Hormadaly, J. Microstructure development and electrical properties of RuO2-based lead-free thick-film resistors. Journal of Material Science: Materials in Electronics. 2006; 17(11):951–962.
Carroll, A.F., Kuno, H. Automotive glass thick-film conductor paste, 1994. [US Patent 5-296-413; March 22].
Casale, M.E.A., Collier, O.N., Iles, G.S. Resistor composition, 1972. [US Patent 3-637-530: January 25].
Chan, M.S. Thick-film conductor composition for use in biosensors, 2000. [US Patent 6-642-751; March 28].
Chan, M.S. Thick-film conductor composition for use in biosensors, 2003. [US Patent 6-627-058; September 30].
Chan, M.S., Dorfman, J.R., Water-based thick-film conductive compositions, 1999. [US Patent 5-855-829; January 5].
Chen, L.-Y.u., Okojie, R.S., Neudeck, P.G., Hunter, G.W., Lin, S-Tien T. Material system for packaging 500 °C microsystems. Materials Research Society Symposium Proceedings. 2001; 682:79–90.
Cho, Y., Hang, K.W. High thermal expansion glass and tape composition, 2004. [US Patent 6-835-682; December 28].
Cho, Y., Hang, K.W. Thick-film dielectric compositions for use on aluminum nitride substrates, 2006. [US Patent 7-087-293; August 8].
Christensen, C.J., Rigterink, M.D., Treptow, A.W. Metallizing composition, 1949. [US Patent 2-461-878; February 15].
Coleman, M.V. Evaluation methods for the examination of thick-film materials. Radio and Electronic Engineer. 1975; 45(3):121.
Conrad, P. Resistor materials. In: Electronics Materials Review. Park Ridge, NJ, USA: Nayes Data Corp; 1971:12.
Cotton, F.A., Wilkinson, G. Advanced Inorganic Chemistry, 2nd edn. New York: Interscience Publishers; 1966.
Counts, W.E., Bruhl, D.A., Jr. Cermet resistance element, 1969. [US Patent 3-479-216; November 18].
Cox, S.M., Survey of glass materials in microelectronics Report No. AD682914. Ministry of Technology, London, UK, 1968.
Craig, W.A., Taylor, B.E. Thick-film conductor composition, 1987. [US Patent 4-636-332; January 13].
Daily, A.M., Boykin, O.F., Rartman, C.W., Electrical resistance element and method of making the same, 1967. [US Patent 3-329-526; July 4].
D’Andrea, J.B. Ceramic composition and article, 1960. [US Patent 2-924-540; February 9].
de Almeida, J.S., Ahuja, R. Electronic and optical properties of RuO2 and IrO2. Physical Review B. 2006; 73:165102.
Dearden, J. High voltage resistors. Electronic Components. 259–262, 1967.
Donohue, P.C. Hexaboride resistor composition, 1985. [US Patent 4-512-917; April 23].
Donohue, P.C. Thick-film copper compatible based on hexaboride conductors and nonreducible glass, 1986. [US Patent 4-585-580; April 29].
Donohue, P.C. Hexaboride resistor composition, 1986. [US Patent 4-597-897; July 1].
Donohue, P.C. Encapsulant composition, 1990. [US Patent 4-966-926; October 30].
Donohue, P.C. Lead and cadmium-free encapsulant composition, 1998. [US Patent 5-753-571; May 19].
Donohue, P.C. Borate glass based ceramic tape, 2000. [US Patent 6-147-019; November 14].
Donohue, P.C., Marcus, S.M., Temperature coefficient of resistance modifiers for thick-film resistors. Temperature coefficient of resistance modifiers for thick-film resistors. 1980. [US Patent 4-225-468; September 30].
Donohue, P.C., Hang, K.W., Haun, M.J. Crystallizable glass and thick-film compositions thereof, 1990. [US Patent 4-959-330; September 25].
Donohue, P.C., Hormadaly, J., Needes, C.R.S., Horowitz, S.J., Knaak, J.F. Nitrogen fireable resistors: emerging technology for thick-film hybrids. IEEE Transactions on Components, Hybrids and Manufacturing Technology. 1987; 10(4):537–544.
Donohue, P.C., Hormadaly, J., Needes, S.C.R., Horowitz, S.J., Knaak, J.F. Nitrogen fireable resistors: emerging technology for thick-film hybrids – Part I. Hybrid Circuit Technology. 1988; 11–14. [February.].
Donohue, P.C., Hormadaly, J., Needes, C.R.S., Horowitz, S.J., Knaak, J.F. Nitrogen fireable resistors: emerging technology for thick-film hybrids – Part II. Hybrid Circuit Technology. 1988; 39–43. [March.].
Dorfman, J.R. High conductivity polymer thick-film silver conductor composition for use in RFID and other applications, 2010. [US Patent 7-857-998; December 28].
Dumesnil, M.E. Resistor and resistor composition, 1962. [US Patent 3-052-573; September 4].
Dziedzic, A. Thick-film resistors with IrO2 and CaIrxTi1-xO3 – Examples of chemically reactive and unreactive systems. Microelectronics Journal. 1989; 19(6):24–42.
Dziedzic, A. Bibliography on electrical conduction in thick-film resistors. Microelectronics Reliability. 1991; 31:549–558.
Dziedzic, A., Golonka, L. Electrical properties of conductive materials used in thick-film resistors. Journal of Materials Science. 1988; 23(9):3151–3155.
Endo, T., Mashima, H. Kanasaku T., Tanaka, T., Yamazoe, M. Resistor composition and thick-film resistor, 2009. [US Patent 7-476-342; January 13].
Eustice, A.I. Conductor compositions, 1994. [US Patent 4-446-059; May 1].
Faber, W.M., Sr., Francis, G.L., Holmes, C.L., Boykin, O.F. Electrical resistance element, 1967. [US Patent 3-304-199; February 14].
Felten, J.J. Photosensitive gold compositions, 1975. [US Patent 3-877-950; April 15].
Felten, J.J. Compositions containing diethylene glycol ether, 1978. [US Patent, 4-070-200; January 24].
Felten, J.J. Non-photographic method for patterning organic polymer films, 1991. [US Patent 5-032-216; July 16].
Felten, J.J. Palladium thick-film compositions, 1994. [US Patent 5-338-708; August 16].
Felten, J.J., Hertler, W.R., Ma, S.H., Compositions for diffusion patterning, 1997. [US Patent 5-654-354; August 5].
Finch, R.G. Thick-film materials. Thin Solid Films. 1969; 3:189–199.
Frazee, L.E. Humidity sensor, material therefore and method, 1977. [US Patent 4-016-308; April 5].
Fujimura, K. Method of manufacturing resistor paste, 1979. [US Patent 4-175-061; November 20].
Fukaya, M., Matsuo, T., Watanabe, Y., Higuchi, C. Thick-film resistor paste, 2000. [US Patent 6-123-874; September 26].
Fukaya, M., Shibata, K., Higuchi, C., Watanabe, Y. Thick-film resistor and ceramic circuit board’, 2003. [US Patent 6-544-654; April 8].
Gress, R.W., Murphy, J.A., Talwalkar, A.T. Characterizations of antimony-doped tin (IV) oxide films. Proceedings of Electronic Components. 1968; 108(3):277–283.
Gruber, W.C., Barringer, E.A. Metallic inks for co-sintering process, 1991. [US Patent 5-062-891; November 5].
Gurunathan, K., Vyawahare, N., Amalnerkar, D.P. Synthesis and characterization of CaRuO3 and SrRuO3 for resistor paste application. Journal of Material Science: Materials in Electronics. 2005; 16:47–53.
Hang, K.W., Labranche, M.H., Taylor, B.E., Vernooy, P.D. Surface-modified ruthenium oxide conductive material, lead-free glass(es), thick-film resistor paste(s), and devices made therefrom, 2009. [US Patent Application 20009/0261941; October 22].
Hang, K.W., Nair, K.M., McCombs, M.F. Lead free glass(es), thick-film paste(s), tape composition(s) and low temperature cofired ceramic devices made therefrom, 2010. [US Patent 7-687-417; March 30].
Hankey, D.L. Electrical resistance composition and methods of making the same, 1985. [US Patent 4-536-328; August 20].
Hrovat, M., Meader, T., Holc, J., Belavic, D., Cileniek, J., Bernard, J. Subsolidus phase equilibria in RuO2-Bi2O3-SiO2 system. Journal of European Ceramic Society. 2008; 28(11):2221–2224.
Harster, T.E., Mattox, D.M. AlN – Compatible thick-film binder glasses and pastes. Proceedings of ISHM 1993. 1993; 393–398.
Hartmann, H.S. Crystallizable, low dielectric constant, low dielectric loss composition, 1991. [US Patent 5-024-975; June 18].
Herbst, D.I., Dawson, W.L. Cermet resistor composition and method of making same, 1971. [US Patent 3-573-229; March 30].
Hoffman, L.C. Resistor composition containing pyrochlore-related oxides and noble metal’, 1971. [US Patent 3-553-109; January 5].
Hoffman, L.C. An overview of thick-film hybrid materials. American Ceramic Society Bulletin. 1984; 63(4):572–576.
Hoffman, L.C., Horowitz, S.J. Stable pyrochlore resistor compositions, 1981. [US Patent 4-302-362; November 24].
Holmes, C.L. Precision resistance element and method of making the same, 1967. [US Patent 3-324-049; June 6].
Holmes, P.J., Loasby, R.G. Handbook of Thick-film Technology. Ayr, Scotland: Electrochemical Publications Limited; 1976.
Holmes, P.J., Loasby, L.R. Hybrid Microcircuit Technology Handbook: Materials Processes. Noyes, Park Ridge, NJ: Testing and Production; 1988.
Hood, H.P. Refractory product and method of making the same, 1941. [US Patent 2-244-777; June 10].
Hormadaly, J. Thick-film resistor compositions, 1982. [US Patent 4-362-656; December 7].
Hormadaly, J., New model for estimating the expansion coefficient of glasses used in microelectronics. Proceedings of International Symposium on Microelectronics, ISHM 85, Anaheim, CA. 1985:77–82.
Hormadaly, J. Borosilicate glass compositions, 1985. [US Patent 4-536-329; August 20].
Hormadaly, J. Borosilicate glass compositions, 1985. [US Patent 4-537-703; August 27].
Hormadaly, J. Method of doping tin oxide, 1985. [US Patent 4-548-741; October 22].
Hormadaly, J. Resistor compositions, 1985. [US Patent 4-548-742; October 22].
Hormadaly, J. Method of doping tin oxide, 1986. [US Patent 4-613-539; September 23].
Hormadaly, J. Empirical methods for estimating the linear coefficient of expansion of oxide glasses from their composition. Journal of Non-Crystalline Solids. 1986; 79:311–324.
Hormadaly, J., Resistor compositions, 1987. [US Patent 4-654-166; March 31].
Hormadaly, J., Method of doping tin oxide, 1987. [US Patent 4-707-346; November 17].
Hormadaly, J., Encapsulant composition, 1992. [US Patent 5-114-885; May 19].
Hormadaly, J., Encapsulant composition, 1992. [US Patent 5-137-851; August 11].
Hormadaly, J., Cadmium-free and lead-free thick-film conductor composition, 1995. [US Patent 5-439-852; August 8].
Hormadaly, J., Cadmium-free and lead-free thick-film paste composition, 1996. [US Patent 5-491-118; February 13].
Hormadaly, J., Cobalt ruthenate thermistors, 2000. [US Patent 6-066-271; May 23].
Hormadaly, J., Thick-film compositions containing pyrochlore-related compounds’, 2006. [US Patent 6-989-111; January 24].
Horowitz, S.J., Novel silver compositions, 1978. [US Patent 4-090-009; May 16].
Horowitz, S.J., Thick-film conductors having improved aged adhesion, 1982. [US Patent 4-318- 830; March 9].
Hrovat, M., Holc, J., Jakubowska, M., Kielbasinski, K., Makarovic, K., Belavic, D. Subsolidus phase equilibria in the CaO-poor part of the RuO2-CaO-SiO2 system. Materials Research Bulletin. 2010; 45(12):2040–2043.
Hrovat, M., Maeder, T., Holc, J., Belavic, D., Cilensek, J., Bernard, J. Subsolidus phase equilibria in the RuO2-Bi203-system. Journal of the European Ceramic Society. 2008; 28(12):2221–2224.
Hu, Y.H., Saltzberg, M.A., Shannon, M.A., Metaphosphate glass composition, 1993. [US Patent 5-196-381; March 23].
Huang, C.Y.D., Merz, K.M., Vitreous enamel resistance material and resistor made therefrom, 1970. [US Patent 3-503-801; March 31].
Iles, G.S., Casale, M.E.A. Ruthenium oxide glaze resistors. Platinum Metals Review. 1967; 11:126–129.
Iwase, N., Anzai, K., Shinozaki, K., Tsuge, A., Kawasaki, K.S., et al, Circuit substrate having high thermal conductivity, 1987. [US Patent 4-659-611; April 21].
Jagtap, S., Rane, S., Amalnerkar, D. Environmentally sustainable composite resistors with low temperature coefficient of resistance. Microelectronic Engineering. 2009; 86:2026–2029.
Janakirama-Rao, B.V., Murphy, R.M., Resistance material and resistor made therefrom, 1964. [US Patent 3-154-503; October 27].
Jean, J.H., Low dielectric ceramic compositions for multilayer ceramic package, 1998. [US Patent 5-786-288; July 28].
Jean, J.H., Gupta, T.K., Dielectric composition containing cordierite and glass. Patent No. 5-206-190. 1993. [April 27].
Jira, J.W., Metal film resistor, 1942. [US Patent 2-281-843; May 5].
Jira, J.W., Protective coating for resistors, 1944. [US Patent 2-357-473; September 5].
Jira, J.W., Alloy metal film resistor, 1948. [US Patent 2-440-691; May 4].
Jira, J.W., Resistor and method of making, 1948. [US Patent 2-457-678; December 28].
Kasanami, T., Kano, O., Electro-conductive material containing PbO and RuO2’, 1973. [US Patent 3-778-389; December 11].
Kennedy, B.J., Vogt, T. Structural and bonding trends in ruthenium pyrochlores. Journal of Solid State Chemistry. 1996; 126:261–270.
Keusseyan, R.L., Process for thick-film circuit pattering, 2010. [US Patent 7-741-013; June 22].
Khadilkar, C.S., Sridharan, S., Shaikh, A.S., Method of making multilayer structures using tapes on non-densifying substrates, 2009. [US Patent 7-547-369; June 16].
Kielbasinski, K., Jakubowska, M., Mlozniak, A., Hrovat, M., Holc, J., Belavic, D. Investigation on electrical and microstructural properties of thick-film lead free resistor series under various firing conditions. Journal of Materials Science: Materials in Electronics. 2010; 21(10):1099–1105.
Kodas, T.T., Hampden-Smith, M.J., Vanheusden, K., Denham, H., Stump, A.D., et al, Precursor compositions and methods for the deposition of passive electrical components on a substrate, 2009. [US Patent 7-524-528; April 28.].
Kretzschmar, C., Otschik, P., Eichler, K., Influence of the solid particle size on the properties of RuO2-based thick-film resistors. International Conference on Materials by Powder Technology 1993, Dresden. 1993:713–718.
Kretzschmar, C., Otschik, P., Jaenicke-Röβler, K., Schlaefer, D. The reaction between ruthenium dioxide and aluminum nitride in resistor pastes. Journal of Materials Science. 1993; 28(21):5713–5716.
Kretzschmar, C., Thick layer resistor coatings on AlN ceramics for high operational temperature. Werkstoffe Fuer die Info, Symposium1, Werkstoffwoche ’96, Stuttgart. 1997:133–138.
Kshiragar, A., Rane, S., Mulik, U., Amalnerkar, D. Microstructure and electrical performance of eco-friendly thick-film resistor composition fired at different firing conditions. Materials Chemisty and Phyics. 2007; 101:492–498.
Kumar, A.H., Thaler, B.J., Prabhu, A.N., Tormey, E.S., Low dielectric loss glass ceramic compositions, 1999. [US Patent 5-958-807; September 28].
Kuo, C.Y., Thick-film resistive paints and resistors made therefrom, 1987. [US Patent 4-639-391; January 27].
Kuo, C.Y., Base metal resistive paints, 1987. [US Patent 4-655-965; April 7].
Kuo, C.Y., Base metal resistors, 1987. [US Patent 4-698-265; October 6].
Kuo, C.Y., Megaohm resistor paint and resistor made therefrom, 1987. [US Patent 4-711-803; December 8].
Kuo, C.Y., Pre-reacted resistor paint and resistors mode therefrom, 1988. [US Patent 4-720-418; January 19].
Kuo, C.Y., Angel, H.S., Method of preparing ruthenium- or iridium-containing components for resistors, 1973. [US Patent 3-769-382; October 30].
Kydd, P.H., Jablonski, G.A., Richard, D.L., Low temperature method and compositions for producing electrical conductors, 2002. [US Patent 6-379-745; April 30].
Langley, R.C., Electrical circuit element comprising thick-film resistor bonded to conductor, 1978. [US Patent 4-076-894; February 28].
Larry, J.R., High adhesion metallizing compositions, 1974. [US Patent 3-827-891; August 6].
Larry, J.R., Resistor compositions, 1978. [US Patent 4-101-708; July 18].
Larry, J.R., Rosenberg, R.M., Uhler, R.O. ‘Thick-film technology: an introduction to the materials’, IEEE Transactions on Components. Hybrids and Manufacturing Technology. 1980; 3(2):211.
Larsen, W.R., Short, O.A., Vitrifiable flux and silver compositions containing same, 1958. [US Patent 2-822-279; February 4].
Longo, J.M., Raccah, P.M., Goodenough, J.B. Pb2M2O7-x (M = Ru, Ir, Re) – Preparation and properties of oxygen. Materials Research Bulletin. 1969; 4:191–202.
Lux, H., Smetana, W. Diamond: a novel substrate for thick-film technology. Surface and Coating Technology. 1991; 47:553–558.
Mackenzie, G.D., Resistance material and electrical resistor made therefrom, 1974. [US Patent 3-788-997; January 29].
Makuta, F., Maeda, T., Resistance paste and resistor, 2009. [US Patent 7-591-965; September 22].
Martin, J.H., Ceramic with metal film via binder of copper oxide containing glass’, 1966. [US Patent 3-293-501; December 20].
Martin, T.O., Glass frit compositions and electrical conductor compositions made therefrom compatible with reducing materials’, 1997. [US Patent 5-608-373; March 4].
Matsuno, H., Barker, M.F., Conductive composition for black bus electrode and front panel of plasma display panel, 2010. [US Patent 7-781-971; August 24].
Mattox, D.M., Aluminum nitride-compatible thick-film binder glass and thick-film paste composition, 1997. [US Patent 5-637-261; June 10].
Melan, E.H., Mones, A.H. The glaze resistor – its structure and reliability. IEEE Transactions Components Parts CP-11. 1967; 76–85.
Merz, K.M., Shapiro, H.E., Electrical resistor with novel termination and method of making the same, 1977. [US Patent 4-053-866; October 11].
Merz, K.M., Shapiro, H.E., Resistor material, resistor made therefrom and method of making the same, 1980. [US Patent 4-209-764; June 24].
Miller, L.F., Method of fabricating resistor compositions, 1968. [US Patent 3-414-641; December 3].
Miller, L.F., Method of rendering noble metal conductive composition non-wettable by solder, 1968. [US Patent 3-401-126; September 10].
Miller, L.F., Electrical resistor compositions, elements and method of making same’, 1968. [US Patent 3-390-104; June 25].
Minneman, L.C., Trease, R.E., Wells, L.J., Dietz, R.L., Fine line electronic micro-circuitry printing pastes, 1974. [US Patent 3-830-651; August].
Miyashiro, F., Iwase, N., Tsuge, A., Ueno, F., Nakahashi, M., Takahashi, T. High thermal conductivity aluminum nitride ceramic substrates and packages. IEEE Transactions on Components, Hybrids, and Manufacturing Technology. 1990; 13(2):313–319.
Mochel, J.M., Electrically conducting refractory body, 1949. [US Patent 2-467-144; April 12].
Mones, A.H., Electrical resistance composition and method of using the same to form a resistor’, 1967. [US Patent 3-337-365; August 22].
Mones, A.H., Neisser, K.E., Jr., Electrical resistance compositions, elements and method of making the same, 1966. [US Patent 3-248-345; April 26].
Morten, B., Ruffi, G., Sirotti, F., Tombesi, A., Moro, L., Akomolafe, T. Lead-free ruthenium-based thick-film resistors: a study of model systems. Journal of Materials Science: Materials in Electronics. 1991; 2(1):46–53.
Mulligan, W.A., Vitreous enamel resistor composition and resistor made therefrom, 1969. [US Patent 3-441-516; April 29].
Muralidhar, S.K., Roberts, A.G., Shaikh, J.S., Leandri, D.J., Hankey, D.L., Vlach, T.J., Low dielectric, low temperature fired glass ceramics, 1993. [US Patent 5-258-335; November 2].
Murphy, R.M., Janakirama-Rao, B.V., Resistance material and resistor made therefrom’, 1965. [US Patent 3-180-841; April 27].
Nair, K.M., Screen-printable dielectric composition, 1983. [US Patent 4-392-180; July 5].
Nair, K.M., Resistor compositions, 1987. [US Patent 4-645-621; February 24].
Nair, K.M., Resistor compositions, 1987. [US Patent 4-652-397; March 24].
Nair, K.M., Resistor compositions, 1987. [US Patent 4-657-699; April 14].
Nakahashi, M., Shirokane, M., Yamazaki, T. Yoshino H., Kawasaki, A.H., Takeda, H., for preparing highly heat-conductive substrate and copper wiring sheet usable in the same’, 1986. [US Patent 4-611-745; September 16].
Norton, M.G. Thermodynamic considerations in the thick-film metallization of aluminium nitride substrates. Journal of Materials Science, Letters. 1990; 9:91–93.
Ohkawa, Y., Ikeda, M., Miyahara, K., Itoh, Y., Aluminum nitride substrate and process for preparation thereof, 1998. [US Patent 5-830-570; November 3].
Okamoto, H.O., Aso, T. Formation of thin films of PdO and their electrical properties. Japanese Journal Applied Physics. 1967; 6(6):779.
Okamoto, K., Kuno, H., Yaguchi, I., Koishikawa, J., Thick-film conductor paste for automotive glass, 1997. [US Patent 5-616-173; April 1].
Okamoto, K., Thick-film conductor paste compositions for aluminum nitride substrates, 2000. [US Patent 6-103-146; August 15].
Ollivier, P.J., Hang, K.W., Thick-film conductor paste composition for LTCC tape in microwave applications’, 2010. [US Patent 7-740-725; June 22].
Otschik, P., Kretzschmar, C., Lefranc, G. AlN properties of various products. Int Wissenschaftliches Kolloquium – Technische Unviersitaet Ilmenau. 1998; 43rd(Band 2):88–91.
Otschik, P., Kretzschmar, C., Reppe, G., Scheffel, A. Pastes for high temperature resistors in power hybrids and microwave circuits. DVS-Berichte. 1998; 191(EuPac 1998):147–150.
Pedder, D.T., Thick-film circuits, 1982. [US Patent 4-311-730; January 19].
Pepin, J.G., Thick-film conductor composition, 1990. [US Patent 4-954-926; September 4].
Place, T.M., Sr., Place, T.M., Jr., Electrical resistance element, 1960. [US Patent 2-950-995; August 30].
Place, T.M., Sr., Place, T.M., Jr., Electrical resistance material and method of making same, 1960. [US Patent 2-950-996; August 30].
Place, T.M. Sr, Place, T.M., Jr., Method of making electrical resistance element’, 1964. [US Patent 3-149-002; September 15].
Popowich, M.J., Resistor compositions containing pyrochlore-related oxides and platinum, 1971. [US Patent 3-630-969; December 28].
Popowich, M.J., Compositions for stable low resistivity resistors, 1974. [US Patent 3-816-348; June 11].
Powell, D.G. Semiconducting glazes for high voltage insulators. American Ceramics Socieety Bulleetin. 1974; 52(8):600–603.
Prabhu, A.N., Hang, K.W., Indium oxide resistor inks, 1983. [US Patent 4-380-750; April 19].
Prabhu, A.N., Hang, K.W., Indium oxide resistor inks, 1984. [US Patent 4-467-009; August 21].
Prabhu, A.N., Hang, K.W., Low value resistor inks’, 1983. [US Patent 4-379-195; April 5].
Prabhu, A.N., Hang, K.W., Low value resistor inks’, 1984. [US Patent 4-452-844; June 5].
Prabhu, A.N., Hang, K.W., Conlon, E.J., Thick-film copper conductor inks, 1988. [US Patent 4-733-018; March 22].
Prabhu, A.N., Hang, K.W., Conlon, E.J., Thick-film copper conductor inks, 1989. [US Patent 4-808,770; February 28].
Prabhu, A.N., Hang, K.W., Conlon, E.J., Thick-film copper conductor inks’, 1989. [US Patent 4-816-615; March 28].
Prudenziati M., ed. Thick-film Sensors. Amsterdam: Elsevier, 1994.
Prudenziati, M., Zanardi, F., Morten, B., Gualtieri, A.F. Lead-free thick-film resistors: an explorative investigation. Journal of Materials Science: Materials in Electronics. 2002; 13(1):31–37.
Prunchak, R., Glass frits, 2010. [US Patent 7-736-546; June 15].
Pukaite, C.J., Low temperature coefficient of resistivity cermet resistors, 1977. [US Patent 4-006-278; February 1].
Rane, S., Prudenziati, M., Morten, B., Golonka, L., Dziedzic, A. Structural and electrical properties of perovskite ruthenate-based lead-free thick-film resistors on aluminna and LTCC. Journal of Materials Science: Materials in Electronics. 2006; 15(10):687–691.
Reicher, R., Smetana, W. Bonding mechanism and stress distribution of a glass frit free thick-film metallization for AlN-Ceramic. Journal of Materials Science: Materials in Electronics. 1998; 9:429–434.
Rellick, J.R., Mixed oxide bonded copper conductor compositions, 1982. [US Patent 4-323-483; April 6].
Rellick, J.R., Dielectric compositions and method of forming a multilayer interconnection using same’, 1987. [US Patent 4-655-864; April 7].
Rellick, J.R., Method for fabricating multilayer circuits, 1989. [US Patent 4-799-984; January 24].
Richardson, L.T., Swinehart, M.R., Electrical contact, 1951. [US Patent 2-572-662; October 23].
Rikoski, R.A. Hybrid microelectronic circuits: the thick-film. New York: Wiley & Sons; 1973.
Rose, M., Young, R.J.S., Aluminum thick-film composition(s), electrode(s), semiconductor device(s) and methods of making thereof’, 2010. [US Patent 7-718-092; May 18].
Rosenblatt, E.F., Platinum metal compounds and methods of making them, 1939. [US Patent, 2-166-076; July 11].
Rosenblatt, E.F., Method of providing adherent metal coatings on surfaces’, 1943. [US Patent 2-328-101; August 31].
Ryden, W.D., Lowson, A.W., Sartain, C.C. Temperature dependence of the resistivity of ruthenium dioxide and iridium dioxide. Physics Letters A. 1968; 26(5):209–210.
Ryden, W.D., Lowson, A.W., Sartain, C.C. Electrical transport properties of iridium oxide and ruthenium oxide. Physics Review B: Solid State. 1970; 3(1(4)):1494–1500.
Saeki, S., Suehiro, M., Echigo, M., Okada, S., Sakuraba, M., Copper paste composition, 1991. [US Patent 5-035-837; July 30].
Sato, H., Mizunoya, N., Nagata, M., Aluminum nitride substrate, 1988. [US Patent 4-761-345; August 2].
Scheiber, D.H., Vehicle for thick-film resistors fireable in nonoxidizing atmosphere, 1981. [US Patent 4-251-397; February 17].
Scheiber, D.H., Weaver, R.V., Photohardenable paste compositions having high resolution’, 1975. [US Patent 3-914-128; October 21].
Schubert, K.E., Resistor compositions containing pyrochlore-related oxides and cadmium oxide, 1971. [US Patent 3-560-410; February 2].
Senkalski, R.E., Hasenmayer, D.L., Dielectric composition having controlled thermal expansion’, 1990. [US Patent 4-961-998; October 9].
Sergent, C.J.E. Materials and processes for hybrid micrelectronics and multichip modules. In Harper A., ed.: Electronic Materials and Processes Handbook, 3rd edn, McGraw Hill, 2004.
Shaikh, A.S., Roberts, G.J., Sarkar, G., Dielectric materials, 1995. [US Patent 5-397-830; March 14].
Shapiro, H.E., Merz, K.M., Resistor material, resistor made therefrom and method of making the same’, 1980. [US Patent 4-205-298; May 27].
Siuta, V.P., Copper conductor compositions, 1987. [US Patent 4-687-597; August 18].
Siuta, V.P., Metal oxide-coated copper powder’, 1986. [US Patent 4-600-604; July 15].
Soby, W., Process of metallizing surfaces, 1951. [US Patent 2-551-712; May 8].
Stein, S.J., Huang, C., Gelb, S. Comparison of enameled steeel substrate properties for thick-film use. Electroncomponent Science and Technology. 1980; 7:55–61.
Stein, S.J., Wahlers, R.L., Bless, P.W., Barclay, C., Tait, R.B., Dielectric pastes and tapes, and metal substrates coated therewith, 1999. [US Patent 5-998-036; December 7].
Steinberg, J., Nitrogen fireable resistor compositions, 1989. [US Patent 4-814-107; March 21].
Tachibana, M., Kohama, Y. Shimoyama T., Harada, A., Taniyama, T., et al. Electronic properties of metallic pyrochlore ruthenates Pb2Ru2O6.5 and Bi2Ru2O7. Physics Review B. 2006; 73:193107.
Tanaka, H., Igarashi, K., Resistor paste, resistor, and electronic device, 2007. [US Patent 7-282-163; October 16].
Tanaka, H., Igarashi, K., et al, Thick-film resistor paste and thick-film resistor, 2009. [US Patent 7-481-953; January 27].
Tanaka, H., Igarashi, K., Glass composition for thick-film resistor paste, thick-film resistor paste, thick-film resistor, and electronic device, 2009. [US Patent 7-544-314; June 9].
Tankiewicz, S., Morten, B., Prudenziati, M., Golonka, L.J. IrO2-based thick-film resistors. Journal of Applied Physics. 2002; 91(7):4261–4266.
Taylor, B.E., Binder glass of Bi2O3-SiO2-GeO2(-PbO optional) admixed with ZnO/ZnO and Bi2O3’, 1986. [US Patent 4-567-151; January 28].
Totokawa, M., Ootani, Y., Imal, H., Shintai, A., Conductor composition, a mounting substrate and a mounting structure utilizing the composition, 2010. [US Patent 7-807-073; October 5].
Towlson, S.M., Flexible thick-film conductor composition, 1997. [US Patent 5-653-918; August 5].
Toyoda, S., Kuromitsu, Y., Sugamure, K., Nakabayashi, A., Aluminum nitride substrate and method of producing the same, 1998. [US Patent 5-780-162; July 14].
Tsuda, N., Nasu, K., Yanase, A., Siratori, K. Electronic Conduction in Oxides. Heidelberg: Springer-Verlag; 1991.
Tsuyuki, H., Glass, dielectric composition, multilayer wiring substrate, and multilayer ceramic capacitor, 1995. [US Patent 5-378-662].
Tummala, R.R. Ceramic and glass-ceramic packaging in the 1990s. Journal of the American Ceramic Society. 1991; 74(5):895–908.
Tummala, R.R., Haley, M.R., Czornyj, G. Materials in Microelectronics. Ceramics International. 1993; 19:191–210.
Ulrich, D.R., Screened circuit capacitors, 1972. [US Patent 3-649-353; March 14].
Usui, H., Dotani, Y., Tanabe, R., Manabe, T., Low melting point glass and glass ceramic composition, 2002. [US Patent 6-355-586; March 12].
Van Loan, P.R. Current trends in thick-film resistor technology. Journal Canadian Ceramic Society. 1971; 40:49–54.
Van Loan, P.R., Electrical resistor containing lead ruthenate, 1972. [US Patent 3-682-840; August 8].
Van Loan, P.R., Resistive glaze and paste compositions, 1975. [US Patent 3-868-334; February 25].
VerNooy, P.D., Walker, A.T., Hang, K.W., Non-lead resistor composition, 2009. [US Patent 7-608-206; October 27].
Vest, R.V. Thick-film glasses. In: Final technical Report No. ADA062748. Naval Research Laboratory; 1979. [January 3.].
Vest, R.W. Metallo-organic materials for improved thick-film reliability. In: Final report, contract #N00163-79-C-0352. National Avionic Center; 1980. [November 1.].
Vest, R.W. Materials science of thick-film technology. American Ceramic Society Bulletin. 1986; 65(4):631–636.
Vest, R.W. ‘Materials aspect in thick-film technology’, Chapter 8. In Buchanan R.C., ed.: Ceramic Materials for Electronics: Processing, Properties and Applications, 2nd edn, New York: Marcel Dekker Inc, 1991.
Vest, R.W., Tweedell, E.P., Buchanan, B.C. Ink jet printing of hybrid circuits. International Journal of Hybrid Microelectronics. 1983; 6(2):261–267.
Wang, C.B., Hang, K.W., Needes, C.R.S., Tape composition and process for internally constrained sintering of low temperature co-fired ceramic, 2004. [US Patent 6-776-861; August 17].
Wang, C.B., Hang, K.W., Needes, C.R.S., Tape composition and process for internally constrained sintering of low temperature co-fired ceramic, 2006. [US Patent 7-147-736; December 12].
Watanabe, S., Tani, H., Kasanami, T., Resistive paste, 1991. [US Patent 4-985-176; January 15].
Weiβmann, R., Chong, W. Glasses for high-resistivity thick-film resistors. Advanced Eng Materials. 2000; 2(6):359–362.
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1977. [US Patent 4-065-743; December 27].
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1980. [US Patent 4-215-020; July 29].
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1981. [US Patent 4-293-838; October 6].
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1982. [US Patent 4-322-477; March 20].
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1982. [US Patent 4-340-508; July 20].
Whalers, R.L., Merz, K.M., Resistor material, resistor made therefrom and method of making the same, 1983. [US Patent 4-378-409; March 29].
Whalers, R.L., Merz, K.M., ‘Resistor material, resistor made therefrom and method of making the same’, US Patent 4-397-915; August 9, 1983.
Yamaguchi, T., Kageyama, M. ‘Oxidation behavior of AlN in the presence of oxide and glass for thick-film applications’, IEEE Transactions on Components. Hybrids and Manufacturing Technology. 1989; 12(3):402–405.
Yamaguchi, T., Kanai, K., Proceedings of the International Symposium on Microelectronics, 1992:463–467.
Yang, H., Ink jet printable thick-film compositions and processes, 2010. [US Patent 7-683-107; March 23].
Young, R.J.S., Rose, M., Raby, J.A., Hang, K.W., Aluminum thick-film composition(s), electrode(s), semiconductor device(s) and methods of making thereof’, 2010. [US Patent 7-771-623; August 10].
Young, R.J.S., Rose, M., Mikeska, K.R., Carroll, A.F., Hang, K.W., Prince, A.G., Lead-free conductive compositions and processes for use in the manufacture of semiconductor devices: mg-containing additive, 2010. [US Patent 7-780-878; August 24].
Young, R.J.S., Rose, M., Raby, J.A., Aluminum thick-film composition(s), electrode(s), semiconductor device(s) and methods of making thereof, 2010. [US Patent 7-824-579; November 2].