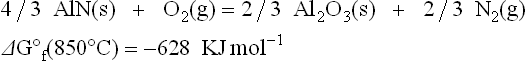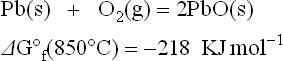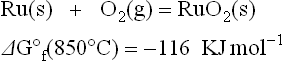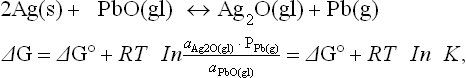Materials Science concepts for printed films
Abstract:
The chapter examines some illustrative cases of the complex interplay between the composition process and properties relations in simple printed film structures, such as resistors and interconnections. It is remarked that thermodynamics data can help, in some cases, in interpreting film phase stability, but richer information would be required to understand the complex phenomena that may occur in thick-printed films, which are typically systems far from thermal equilibrium. Examples of interactions that impact on the films’ electrical properties are reported. It is remarked that the microstructure and performance of thick-films start to evolve from temperatures as low as room temperature and continue to change during the whole course of processing or, when required, in post-process thermal annealing. It is shown that the presumed ideal picture of cermet systems consisting of single-phase conducting particles imbedded in vitreous phase is very far from the reality.
3.1 Introduction
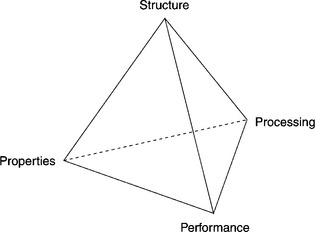
The paradigm for Materials Science is the concept of close inter-relation of principles–structure–processing–properties. Written like this, it may seem to be a ‘linear’ link instead of the very complex one depicted in Fig. 3.1.
The difficulty in realizing these complex relationships in thick-film (TF) technology is immediately apparent when one considers the myriad of ingredients in the paste compositions and the wide range of processing conditions. The ingredients encompass conducting phases such as precious metals or/and their alloys, glass powders, other inorganic materials, together with the organic vehicle and sometimes metal–organic compounds. Most precious metals, their alloys and their compounds are catalysts; therefore they can catalyze, even at room temperature, all sorts of reactions in the organic vehicle, based on the resins and solvents that provide the printability of the paste. Also other paste ingredients can react with the organic vehicle at temperatures encountered in the burn-out zone. Next, the printed films are exposed to a thermal process that spans a wide temperature range; in these conditions, TF glasses exhibit their corrosive properties (in the molten state, typically at T > ~ 500–600 °C) and dissolve the substrate materials, dissolved metals like Ag, interact with other inorganic materials and some glasses can crystallize.
The search for an immediate economic return and the complexity of these systems have not encouraged the systematic research of these composition–process–properties relationships.
In the first issue of Hybrid Circuits, the magazine of the International Society for Hybrid Microelectronics (ISHM), a contribution by P. L. Kirby (1982), then Vice President of the Society (now incorporated in IMAPS: International Microelectrics and Packaging Society), contains a heartfelt invitation to depart from the widespread attitude of beginners who design experiments ‘to see what happens’ without any consideration for a scientific approach or use of available knowledge.
This old attitude has been only partly overcome over the years thanks to very valuable educational efforts and research carried out in some universities in Europe (e.g. London and Southampton in the UK, the Technical University of Vienna, Wroclaw, Oulu, Italy and Switzerland) and in the USA (e.g. Purdue University). Investigations in these educational institutions, together with some multiannual research programs (e.g. in Sandia National Laboratories, Albuquerque and in Philips, Eindhoven) have greatly contributed to recall attention to Materials Science aspects for screen-printed films firstly, and more recently on films printed with the currently diversified methods to lay down functional films. However, another wide basket of knowledge in materials/process relationships have never been unfolded, because neither the scientific literature nor the patents disclose more than a small share of the results that have emerged during the development of new products by companies.
This chapter is an attempt to call the reader’s attention to a few, but nonetheless important, features of TF materials, where Materials Science concepts and experimental evidence can help in realizing not only the complexity of behavior, but also the fascinating and unique properties of TF technology. Of course, there is no claim of comprehensive coverage of the field, but topical examples will be presented. In addition, the order of arguments is not a systematic one but hopefully adequate to inspire further and deeper study.
3.2 Interactions of conducting materials with the organic vehicle at room temperature
Many ingredients of TF pastes are catalysts: Pd, Pt, RuO2, IrO2, very fine particles of Ag and Au, ruthenates and iridates. Catalytic activity increases with increases in the surface area. The RuO2, IrO2 and ruthenates used in TF composition have a wide range of surface areas, 10–60 m2/g, and they may interact with the organic vehicle. Interactions of RuO2 (similar interactions are expected for IrO2) with the organic vehicle span a broad range: from minor chemical changes of the organic vehicle, which affect the paste viscosity and lead to viscosity drift with time, to burning of the organic vehicle. To reduce its catalytic activity, RuO2 is usually washed by a solution of ammonium salts (Avtokratova, 1962; Gilchrist, 1929; Yi et al., 1997) after precipitation. Avtokratova (p. 39) reports ‘Precipitates, which have not been washed with ammonium sulfate might catch fire or even explode due to the dehydration of ruthenium hydroxide’.
RuO2 is usually precipitated from the ruthenium chloride (RuCl3) solution or by the reduction of sodium ruthenate solution (Na2RuO4) by alcohol (Seddon and Seddon, 1984). Both processes result in active RuO2 powders, but the sodium ruthenate route usually yields hydrous ruthenium dioxide (RuO2 · XH2O), which is contaminated with trace amounts of sodium or potassium ruthenates. These traces of Na2RuO4 can lead to interactions with the organic vehicle that include oxidation at room temperature and release of gas at higher temperatures. The latter, when the glass softens, may and usually does lead to the formation of blisters in resistor films (Hormadaly, unpublished).
3.3 Redox reactions
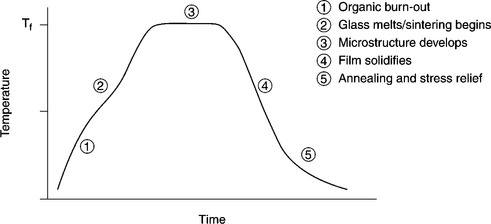
One of the most appreciated characteristics of the traditional TF technology (TFT) is the ability to process materials in air, most often in a belt furnace. The thermal processes are designed to allow a continuous production flow; therefore the so-called ‘firing profiles’ prescribe heating rate, peak temperature, Tf, and dwell time at the peak and cooling rate to ambient temperature. A typical firing profile for processing screen-printed films is shown in Fig. 3.2. Moreover, a frequent recommendation in processing schedules is to provide a controlled flow of ‘clean’ air in the furnace, free from humidity, dirt particles and unwanted gas.
The events allowed or prevented by these conditions include redox reactions, sometimes very far from the operator’s awareness; the reactions assume a ‘special’ character in the case of printed thick (and thin) films because of the large surface-to-volume ratio, which exposes a large fraction of the materials to the ambient and thus also provides easy access of oxygen or reducing gas to the material, with the consequent accelerated kinetics for oxidation and reduction.
One of the obvious and necessary reactions is the oxidation of the binder, i.e. the burn-out of the organic vehicle fraction left after the evaporation of the solvents (normally at 150–180 °C); this reaction must occur as long as the printed layer is porous enough to allow the reaction products (H2O, CO, CO2) to ‘rapidly’ leave the film, thus not charring the remains trapped inside the component. Yet, despite the excess of air in the firing furnace, in the burn-out temperature window there is a local redox disequilibrium, because the film is exposed to the reducing action of these gaseous byproducts. If and how much reduction of the material constituents in the film really takes place depends on the oxygen partial pressure (P O2) over the film, the rates of the reactions, the specific surface area of the particles and their surface preparation.
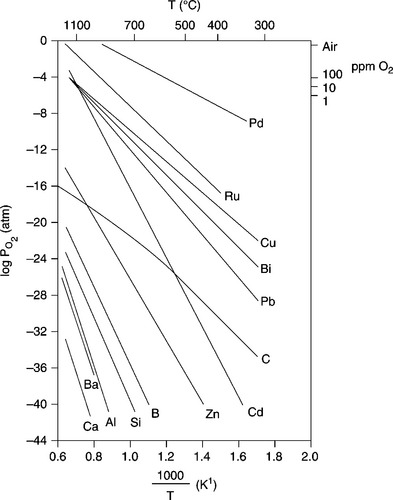
Figure 3.3 provides a phase stability diagram for metal–metal oxide systems of interest for TF compositions, i.e. the oxygen partial pressure in equilibrium with various metal–metal oxide couples vs. the temperature.
Each line descends from an equation of the type:
From Eq. [3.1], the mass action law and thermodynamics dictate that:
where K represents the reaction equilibrium constant, R the gas constant and ![]() the oxygen partial pressure where the metal and its oxide coexist at absolute temperature T. ∆G° is the standard free energy change for the formation of 1 mole of the oxide from the elements. ∆G° data are given in standard compilations of thermodynamic quantities (Turkdogan, 1980).
the oxygen partial pressure where the metal and its oxide coexist at absolute temperature T. ∆G° is the standard free energy change for the formation of 1 mole of the oxide from the elements. ∆G° data are given in standard compilations of thermodynamic quantities (Turkdogan, 1980).
For each chemical species, the element is the stable phase in the ![]() -T region below its curve (reducing conditions); conversely, the oxide is stable above the curve.
-T region below its curve (reducing conditions); conversely, the oxide is stable above the curve.
The lines of Fig. 3.3 are strictly valid only for the equilibrium between the pure metal and a pure oxide. Otherwise, for example when the metal is a constituent of a glass, its curve is shifted downward (Wang and Huebner, 1991). Note that the log ![]() in air equals − 0.675 atm.
in air equals − 0.675 atm.
The carbon curve is introduced in order to consider the C-CO-CO2 equilibrium for 1 total pressure of CO + CO2 (Vest, 1986); this serves for evaluation of the PO2-T region where the vehicle burn-out affects the redox reactions of the metal/ metal oxide systems.
Looking at Eqs. [3.1] to [3.4] and Fig. 3.3, a few examples of redox reactions become of obvious interest. Firstly, let us consider the stable phases of Pd, a common constituent of conductor inks also used for its catalytic property in gas sensors.
For the latter function, i.e. in enhancing the reaction rate of the specific target gas within the sensing material, PdO rather than Pd may be the more effective catalyst. This is the case for SnO2-based methane sensors (Debéda et al., 1997). Because PdO is thermodynamically stable only below ~ 820 °C in air, the firing process over this temperature (typically 850 °C–900 °C) to stabilize the microstructure results in a drastic decline of sensitivity in SnO2:5% Pd methane sensors. However, a long annealing (several hours at 600 °C) restores the oxidized form of PdO as well as the desired sensing properties.
When highly conductive films are the target, Pd is not used alone (because its resistivity is not suitable) but it is added to silver-based compositions. The benefit of adding Pd is mainly the increase in solder-leaching resistance (almost absent with pure silver). On heating at the peak firing temperature, Pd would be oxidized and the PdO would contrast the solid solution formation. The lower activity of Pd in the AgPd alloys, as compared to pure Pd, results in a decrease of the temperature for dissociating the remaining PdO, but the metal would be oxidized again on cooling down at room temperature. This would be a severe drawback: PdO on the surface of the film reduces the solderability. A proper design for firing dense and highly conducting AgPd films in air may be to heat up to Tf of 850 °C (or higher) and cooling very rapidly from the peak temperature at least down to 300 °C, where the reaction rate of oxidation becomes low enough (and there is still a chance to avoid too serious thermal stress on the printed films).
Let us now look at Eqs. [3.2] and [3.3]. The equilibrium reaction equations can be added, taking account of the number of moles involved, so that we have:
Based on the ΔG° f of reactions [3.2] and [3.3], the ΔG of reaction [3.7] is: − 628 − (218) = − 410 KJ mol−1 at 850 °C. The negative value of ∆G shows that reaction [3.7] is favorable toward the right, leading to a reduction of lead oxide and evolution of nitrogen gas (or NOx(g)); the latter accounts for the observed blistering of high-lead glassy films or conventional resistive films on aluminum nitride substrates (Norton, 1990).
Similar considerations allow us to realize why conventional lead–silicate glasses are unsuitable for systems compatible with AlN substrates: new ‘non-reducible’ glasses were developed either for ‘glass-bonded’ metallization and TF resistors (Burckhardt and Vavra 1989; Kretzschmar et al., 1993; Needes, 2003; Wang et al., 2003). The new compositions cannot be reduced by AlN, since they are PbO-free (and Bi2O3-free) or contain them only in very small amounts, but take as major components oxides (such as SiO2, B2O3, Li2O, CaO, BaO) whose ∆G°f is larger in absolute value than that of ∆G°f (AlN), at least for T < Tf.
Another effect of localized redox equilibria (Zdaniewski and Silverman, 1990) concerns gold-based metallization for dielectrics fired in nitrogen ambient conditions: the competition for oxygen (see Fig. 3.3) between the glass components of the dielectric material and decomposing organics results in a reduction of PbO to Pb metal; next, Pb and Au form an alloy, thus the wetting equilibrium between Au and ceramic is completely altered causing delamination of gold lines.
Turning back to the interactions in the burnout range (typically at T < 450 °C), we note that the general expression representing the interaction of the binder with the precious metal or the glass may be described by the following:
where M = Ru, Ir, Pb, Bi, Cd, CxHyOz represents the general formula of the binder (either an ethyl cellulose or acrylic resin) and MOw an oxide. For the reasons explained above, the thermodynamics shows that the enthalpy change ∆Hreaction of this type of reaction is negative, i.e. the reaction is likely to proceed from left to right. As far as char is present, the local atmosphere inside the film is on the carbon curve of Fig. 3.3, so that any temperature along this curve gives a sufficiently low PO2 to reduce the metal listed above. The reduction rate is quite different for the different metals, however. The reduction of precious metal oxides during TF processing was reported by Pierce et al. (1982). More detailed study of the reduction process of RuO2 powders and kinetics of their re-oxidation were reported by Prudenziati et al. (2003): the reduction is found to be an extremely fast (probably autocatalytic) phenomenon at temperatures as low as 100–150 °C, whereas on heating beyond the burnout stage – when the oxidizing conditions prevail – the reverse reaction, from Ru to RuO2 needs much longer times. Nakano and Yamaguchi (1995) reported the effect of O2, concentration in the firing atmosphere on the resistance of RuO2-based TF resistors. This report clearly shows a major difference between lead-free glasses and lead-containing glasses when fired at various O2 concentrations.
The reaction of polynary precious metal oxides with the organic binder during the burn out stage may be represented by:
where M' = Pb, Bi and a mixture of Pb, Bi with other metals; v is a number between 0 and 1.5.
Some of the pyrochlore constituents, PbO and Bi2O3, can also be reduced if sufficient organic binder remains. Interactions between ethylcellulose and TF resistors containing RuO2 and polinary Ru compounds (Bi2Ru2O7, Pb2Ru2O6 and Bi1.5Cu0.5Ru2O6.5) were studied with a combination of techniques by Krausse et al. (1991).
The areas most susceptible to the reduction reactions discussed above are the surfaces of conducting grains and very small conducting particles. According to the general scheme above, the conducting particles of precious metal oxides in the burnout stage are expected to be enveloped by a diffuse zone of metallic ‘shell’. Further heating in the temperature range that warrants oxidizing conditions can result in partial re-oxidation of these particles to the corresponding oxides and even to compounds’ formation between the very fine oxides and other paste ingredients.
The consequences of these reactions may be quite unexpected. Horowitz (1978) reported that:
when typical Pd/Ag conductor compositions have been used as terminations for certain ruthenium based resistors (such as low-ohm, less than 100 Ω/![]() /mil of thickness, resistors of pyrochlore-related oxides or RuO2), staining of the surface of the conductive termination adjacent to the resistor often occurs during resistor firing. Such surface stains are undesirable since they prevent complete soldering of the conductor in the region of the resistor; Bi2O3 is present in typical conductor compositions to enhance adhesion, but it seems to be responsible for such staining.
/mil of thickness, resistors of pyrochlore-related oxides or RuO2), staining of the surface of the conductive termination adjacent to the resistor often occurs during resistor firing. Such surface stains are undesirable since they prevent complete soldering of the conductor in the region of the resistor; Bi2O3 is present in typical conductor compositions to enhance adhesion, but it seems to be responsible for such staining.
Hormadaly (1984) studied this phenomenon and found that the addition of certain oxygen-containing materials such as Ag2O and CoCrO4, which gives off oxygen in the 200–400 °C temperature range, eliminated the staining. It seems that in the burnout zone volatile ruthenium compounds are formed, probably carbonyls of ruthenium, and they evaporate and oxidize to RuO2, which then reacts with the Bi2O3 in the terminations to form black Bi2Ru2O7. The burnout stage of TF compositions, which has not been the subject of detailed research, may reveal the formation of many volatile compounds of ruthenium and iridium.
The volatile compounds of ruthenium, RuO3 and RuO4, are known to coexist with RuO2 at high temperatures (Bell and Tagami, 1963).
Among the many studies of redox interactions concerning systems of interest for TF technology, it is worth mentioning those due to Palanisamy and Sarma (1987) on processing copper TFs in nitrogen and to Hong et al. (2009) on front-side Ag contacts of silicon solar cells, where a singularly rich variety of redox reactions results from the competition for oxygen by the Si (the cell), PbO (in the binding glass), Si3N4 (in the antireflection coating) and Ag (in the metal for bus bar and fingers).
3.4 Chemical diffusion-related interactions during the firing cycle
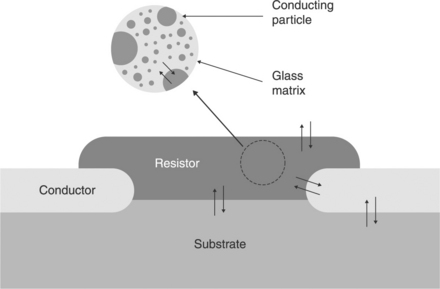
One topical example of looking at possible interactions via dissolution, interdiffusion and chemical interactions in TF materials is represented by TF resistors (Fig. 3.4). Here these phenomena affect the couples: (1) resistive film and substrate, (2) terminations and substrate, (3) resistive film and conductive terminations, (4) resistive film and environment, (5) glassy matrix of the resistor and various phases embedded in, including the main conductive oxide, the fillers, TCR modifiers and additives such as transition metal oxides or their compounds. The following sections cover aspects of these interactions in resistors or similar systems.
3.4.1 Interactions between substrates and silicate glasses
A wide range of substrates are used in TF technology; chemistry and properties of several substrates were discussed in Chapter 2. Alumina is the main substrate used in TF technology, more specifically 96%-Al2O3, the remaining 4% consisting of SiO2, CaO and MgO. The same oxides (SiO2, CaO, MgO) are common glass ingredients. Therefore, interactions between TF glasses and alumina are not surprising and were reported in the patent literature. In fact, substantial amounts of alumina are dissolved in TF glasses even during typical TF firing profiles. This modifies the properties of the glass: its viscosity increases, the expansion coefficient decreases, the wetting characteristics are affected and the dielectric constant and the ionic conductivity are also affected. The dissolved Al does not reach equilibrium during the firing cycle, thus concentration gradients exist in the fired films where alumina concentration is high near the substrate and decreases gradually toward the film surface. Glass/alumina interactions were reported by many authors in industry and academia, e.g. by Adachi and Kuno (2000), Angus and Gainsbury (1972), Bube (1972), Hoffman (1984), Hoffman and Jones (1983), Machin and Vest (1978), Moriwaki et al. (1993), Palanisamy et al. (1989) and Shah (1980).
Studies of seven compositions of high-lead glasses on 96%-Al2O3 (Prudenziati et al., 1989) have shown that interactions occur via correlated diffusion of Al from the substrate into the glassy films and counter-diffusion of Pb and B toward the substrate. The effective inter-diffusion coefficients at 850 °C are in the range from 10− 10 to 10− 6 cm2 s− 1, according to the glass composition. The higher the boron content, the higher the interdiffusion coefficient. In all the considered glasses, solubility of Al2O3 is in the range from 10 to 20% by weight, for peak temperatures of 850 °C. In some glasses this solubility is slightly dependent on Tf, whereas in other cases, and notably when high amounts of B2O3 are present, a relevant temperature dependence of the solubility is observed. Several consequences of alumina interaction with the glassy matrix of resistors were observed: the thickness-dependent composition of the glassy matrix results in the thickness-dependent sheet resistance of the resistor; the dissolution of Al2O3 affects the grain growth rate of RuO2 particles in RuO2-based resistors and the thermal stability of pyrochlores into ruthenate-based resistors (Prudenziati et al., 1989).
Also, beryllia is not ‘inert’ against high-lead glasses. Interactions between BeO substrates and several high-lead glasses annealed at the thermal conditions (temperatures and times) usually employed in the preparation of TF components were studied (Moro et al., 1992). Solubilities and diffusivities similar to those observed in the case of alumina were found. However, the interaction mechanisms seem quite different: no Al–Pb correlated diffusion process was observed in this case. This difference may account for the lack of relevant changes in the electrical properties of TF components prepared on beryllia, e.g. there is no evidence of thickness-dependent resistivity, or temperature-dependent phase transitions in Bi-ruthenate-based resistors.
3.4.2 Solubility of RuO2 and ruthenium compound in silicate glasses
The solubility of the conductive oxides in the glassy phase of TF resistors during firing plays a major role in determining the sintering and ripening kinetics of the conductive particles. In addition, the concentration of metal (or ion) in solution may contribute to the electronic transport. Motivated by the need to clarify these points, many investigations have been carried out on the solubility of Ru from RuO2 particles in lead borosilicate glass (Adachi et al., 1994; Palanisamy et al., 1989; Prabhu et al., 1974). The experiments were generally conducted in the range from 600 to 1000 °C. The results revealed very low solubility values, generally lower than 20–30 parts per million (ppm). Prabhu et al. observed that the average concentration Co of Ru in the glass (63 PbO, 25 B2O3, 12 SiO2,% wt) fired at 800 °C, 15 min (hence in typical conditions of firing in a tunnel kiln furnace) is Co = 4 ppm; even at 1000 °C, the solubility is less than 35 ppm. In addition, they noted that Co in the glass fired at 1000 °C is greater after 15 minutes (about 31 ppm) than after 13 hours (about 14 ppm). The proposed interpretation of this result was that during the initial heating, the smallest particles dissolve rapidly, creating a supersaturated solution in their vicinity. The Ru then diffuses through the glass and precipitates as RuO2, on the surfaces of larger particles eventually decreasing the total Ru dissolved in the glass to the equilibrium value.
Palanisamy et al. (1989) analyzed a glass with the same composition as above, but with addition of 2 to 10% wt Al2O3, The measured solubility in samples annealed from 700 to 1000 °C, 12 h exhibits an exponential dependence on temperature, and the dissolved alumina decreases the solubility at any given temperature, but in the same ratio whichever is the amount of Al2O3 added. Concentration of dissolved Ru never exceeds 25 ppm, being in the range 5 to 25 ppm at 700 to 1000 °C.
High lead borosilicate glasses in 26 different compositions were analyzed by Adachi et al. (1994) for Ru solubility after thermal treatment at 900 °C for 1 hour. The values were lower than the detection limit (10 ppm) for a dozen glasses, and generally lower than 100 ppm (only one exception found). The authors concluded, ‘it is evidently clear that the solubility is generally extremely low, as low as 10 ppm in weight in most glasses. When more additives are substituted for SiO2 the solubility tends to increase slightly’. This is partly attributed to the decrease in viscosity of glasses at 900 °C at decreasing SiO2 content. It was suggested that the additives Fe2O3 and TiO2 are more effective among others in increasing the Ru solubility.
Another wide category of investigations concerns RuO2 in lead-free glasses (Mukerji and Biswas, 1967; Pflieger et al., 2009; Pinet and Mure, 2009; Schreiber et al., 1986; Yamashita and Yamanaka, 2004) because of the interest in ruthenium–glass systems formed during the vetrification of nuclear wastes. In simple soda–silica glass (with 20–40 mol% Na2O) at temperatures above 1000 °C, Co > 100 ppm were measured (Mukerji and Biswas, 1967); in borosilicate and boroaluminosilicate glasses a much lower solubility was measured (or lower than the detection limit of the analytical methods used), even at the melting temperature of the glass (1200 to 1400 °C). Nevertheless, indirect evidence of dissolved Ru has been mentioned, such as modification of the glass density and diffusion-limited growth of precipitated RuO2 grains (Pflieger et al., 2009), and changes in optical absorption spectra (Herold et al., 1956; Mukerji, 1972; Yamashita and Yamanaka, 2004).
It is worth noting that most of the studies on ruthenium dissolution in glasses, especially in TF glasses, were concerned with the solubility of Ru4 +. No report deals with the solubility of other oxidation states of ruthenium, such as Ru3 + and Ru2 +, which are likely to form during the firing of TF resistors.
The dissolution of ruthenium pyrochlores (Bi2Ru2O7, Pb2Ru2O6 + δ, (Pb,Bi)2Ru2O6 + δ, (Bi,Gd)2Ru2O7, Bi1.5Cu0.5Ru2O7–δ, δ = 0–1) in the vitreous phase during the firing cycle is very different from that of RuO2. The majority of pyrochlores-based TF resistors make use of Bi2Ru2O7 and Pb 2Ru2O6 + δ. These pyrochlores have constituents (PbO, Bi2O3), which are known to form glasses easily with the main glass formers (SiO2, B2O3) and to dissolve readily in many glasses. Glass formation ranges of PbO and Bi2O3 in silicate and borate glasses are very broad and can extend to 90 mole%. Janakirama-Rao (1962) reported that Bi2O3 can form silicate and borate glasses where the Bi2O3 concentration exceeds 95 wt%. Therefore, Bi2Ru2O7 and Pb2Ru2O6 + δ are expected to interact with glasses during the firing cycle of TF resistors. The general scheme to describe these interactions may be written as:
In fact, several investigations (Morten et al., 1994; Nordstrom and Hills, 1980; Pike and Seager, 1977) have shown that in lead–silicate glasses the bismuth ruthenates dissolve Bi, while Pb from the glassy matrix replaces it in the conductive grains. Yet the newly formed Pb2Ru2O6 pyrochlore starts another phase transformation on heating, and eventually the pyrochlore may be converted completely into the rutile phase (RuO2). The key temperature for this final transformation decreases at increasing the Al content dissolved in the glass (Prudenziati et al., 1989).
Similarly, Ru-perovskite-based resistors (Kummer and Taitl, 1977) exhibit phase transformations during the firing process.
3.4.3 Dissolution of minor constituents in a vitreous matrix
In TF resistors, together with the main phases, i.e. the vitreous matrix and the conductive oxide, other inorganic materials are typically present such as fillers, e.g. Al2O3, ZrSiO4, SiO2, TCR modifiers and additives such as transition metal oxides or their compounds. The dissolution of these materials in the vitreous phase is commonly triggered during the firing cycle at temperatures higher than the glass transition temperature. The total or partial dissolution of the fillers modifies the thermal and mechanical properties of the glass: the dissolution of Al2O3, ZrSiO4 and SiO2 increases the glass viscosity, lowers its thermal expansion coefficient and affects the melting characteristics. Conversely, the dissolution of TCR modifiers and additives, which are used in very small quantities, does not have large influence on the thermal and mechanical properties but may greatly impact the wetting characteristics of the molten vitreous phase.
3.4.4 Interactions due to diffusion at resistor/terminations and dielectric/contacts interfaces
Most of the conductor terminations used in TF technology are based on precious metals (Pt, Pd, Au), silver, alloys of precious metals and alloys of silver and copper. Because of price and properties, silver and its alloys are the choice in the majority of applications. The platinum metal group (Pt, Pd, Rh, Ru, Ir) and Au exhibit very low solubility in lead-free glasses (Howes, 1957). The solubility in lead silicate glasses is slightly higher, but still in the range of several ppm and it rarely exceeds 50 ppm. Silver behaves in a different manner: its solubility in glasses and especially in lead-containing glasses is much higher than that of precious metals (Volf, 1984). Silver forms glasses with P2O5; in the binary Ag2O-P2O5 system, Ag2O content ranges from 34 mole% to 60 mole%, (Takahashi, 1962). Properties of silver phosphate glasses were reported by Bartholomew (1972).
Silver exists in silicate glass as neutral metal atom Ag° and as ions Ag+. The ionic radius of Ag+ in the glass is rAg +(VI) = 0.115 nm, thus slightly larger than that of Na+ and, like alkali ions, Ag+ will lower the viscosity and raise the expansion coefficient of the glass. Silver and precious metals are covered by a thin layer of oxide at room temperature. This oxide dissolves into the glass during firing or by contact at temperatures above the glass transition temperature (Tg). Thus one mode of silver dissolution into TF glasses is by the reaction of Ag2O with the glass to form Ag-containing glass. Another mode is by redox reactions. Redox reactions like the following were discussed by Nagesh et al. (1983):
where a is the activity and P is the vapor pressure. ∆G° for the above reaction is positive since the oxidation potential of Ag is lower than that of Pb. The reaction may proceed from left to right if the equilibrium constant K is very small. The dissolved silver can lower the TF glass viscosity, modify the expansion coefficient, change its wetting characteristics and increase the ionic conductivity of the glass (Nagesh et al., 1983).
Table 3.1 reports the solubility and diffusivity of Ag derived by the measured concentration profiles of silver diffused in a high-lead silicate glass (PbO/SiO2/Al2O3 = 68.2/30.5/1.3% wt, softening temperature T(s) = 606 °C) fired at various peak temperatures Tf, 8 minutes’ dwell time. The solubility, Co, remains nearly constant until the firing temperature Tf is ‘close’ to T(s), whereas it starts to increase at higher temperatures. Diffusivity values of the order of 10− 11 m2s−1 are observed at 850 °C (Prudenziati et al., 2004).
Table 3.1
Diffusion coefficients D and solubility values Co according to the best fits of measured diffusion profiles of silver in samples fired on alumina at various peak temperatures Tf
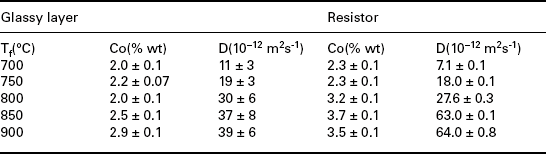
Note: Glassy layer prepared with the frit with composition PbO: SiO2: Al2O3 = 68.2: 30.5: 1.30% wt. Resistor prepared with 8%wt RuO2 fine particles.
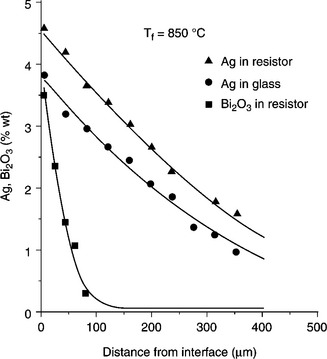
Both solubility and diffusivity are notably affected by changes in the glass composition, e.g. seemingly irrelevant changes due to change of the glass batch (Prudenziati et al., 1994) and substrate (Morten et al., 1981); moreover, both quantities increase in RuO2-based resistors, i.e. in resistors prepared with the same glass (same composition and batch) and RuO2 powders. Figure 3.5 compares the diffusion profiles for Ag in resistor and its ‘parent’ glass, both fired at Tf = 850 °C, 10 minutes. The interest in this complex behavior stems from the relevant changes in electrical properties exhibited by resistors fired on Ag-bearing terminations. These properties are referred to as ‘size effects’ and will be described in Chapter 4. Another chemical element that plays a relevant role in ‘size effects’ is bismuth, introduced in the form of Bi2O3 in many conductor compositions to promote wetting and bonding the film to the substrate. The Bi-solubility in Ru-based TF resistors was evaluated at between 2 and 3%, thus not dissimilar to that of Ag, but its diffusivity is considerably lower (of the order of 1.5 × 10− 12 m2s−1) in samples fired at 850 °C (Prudenziati et al., 1986), as immediately apparent in Fig. 3.5.
3.4.5 Reactions and exchange interactions in thick-film resistors
The reactions referred to in this section concern phase transformations such as compound formation and devitrifications. Devitrification is the term usually chosen to describe the (generally adverse) transition from the amorphous (glassy) state to the crystalline solid state; the same transition, when intentional, is more often mentioned as crystallization. Phenomena of partial devitrification are commonly observed in glassy printed films as well as in the matrix of TF resistors (Prudenziati et al., 2001, 2002); they are not a serious problem per se unless associated with the formation of crystalline phases which, because of their large mismatches in thermal expansion with the remaining glassy matrix, give rise to cracks in the film. The transformation may be reversible: on heating, the crystallites can be re-dissolved in the glass. However, the wetting properties, density, etc. of the system, temporarily modified, can modify the final resistor microstructure. Conversely, crystallization is intentionally designed in ‘crystallizable glasses’ (e.g. TF crossover and dielectrics for multilayer circuits) that are capable of flowing and that accomplish pin-hole free, dense amorphous layers in the first firing cycle but refire at the same or even higher temperatures without further softening during any subsequent firing steps. Nucleation and growth of the newly formed crystalline phase are to be completed at a temperature usually lower than 800 °C (Larry et al., 1980; Vest, 1986).
Interactions with compounds of interest in TF technology encompass:
• Reactions between oxides and dielectrics; one notable example is the reaction of copper oxide, or chromium oxide (formerly also CdO was used) added in the paste for ‘reaction bonded’ or ‘fritless’ conductors for alumina substrates. The oxide reacts at high temperature (950–1000 °C) with the alumina substrate to form spinel oxides like CuAl2O4. These compounds provide the required adhesion (Licari and Enlow 1998).
• Alloy formation from the elements, e.g. AgPd alloy in conductors.
• The redox reactions already described in section 3.3.
• Crystalline phases grown in dielectric materials for capacitors and multilayers.
Interest in the above-mentioned reactions derives from the awareness that understanding their source and mechanisms, as well as knowing their evolution, may guide the operator in the choice of pastes, in planning the deposition steps, in diagnosing possible failures and in evaluating the system’s thermal stability.
The most intriguing research in the field of resistors concerns the phase changes from RuO2 to ternary compounds and vice versa. The effect of prolonged elevated temperatures on RuO2-based resistors prepared by lead borosilicate glass and the Pb2Ru2O6 + δ formation was first reported by Bube (1972). He described the interaction as a partial reduction of RuO2 at low temperature (burnout zone) and a successive reaction with the lead oxide in the glass. Conversely in other systems the Pb2Ru2O6.5 ruthenate is converted into the rutile phase (RuO2) (Adachi and Kuno, 1997; Morten et al., 1991; Prudenziati et al., 1989). Likewise, as already noted, several investigations (Hormadaly 1990; Morten et al., 1994; Nordstrom and Hills, 1980; Pike and Seager, 1977) have shown that Bi dissolves from the bismuth ruthenate grains in the glass and it is substituted for by Pb from the lead–silicate in the conductive ruthenate grains. The nucleation of the new phases and the reaction rates are apparently linked to several factors, the most important ones being: (1) the Pb activity gradient between the Pb-ruthenate and the surrounding glass (Adachi and Kuno, 1997), and (2) the glass viscosity. The latter is modified in the course of the thermal process, for instance by the interactions between glass and substrate. The key role of glass viscosity and the enhanced effect of Cu2O in these types of interactions were evidenced by Hormadaly (1990), who studied ruthenium-based pyrochlore and short borosilicate glasses or glass mixtures. Similarly Ru-perovskite-based resistors (Kummer and Taitl, 1977) exhibit phase transformations during the firing process.
Other phase changes have been noted studying the microstructure evolution of TFRs prepared by precipitation of RuCl3 on high-lead fritted-glass particles (Morten et al., 1988). It was shown that RuO2 (RuCl3 converts to RuO2 at temperatures below 400 °C; see Newkirk and McKee, 1968) reacts with lead borosilicate glass to form Pb2 Ru2 − x PbxO6 + δ intermediate phases in the course of firing, until Pb2Ru2O6 + δ is formed at 850 °C; the latter phase may eventually decompose to RuO2 at higher temperatures. Synthesis and properties of Pb2Ru2 − xPbxO6 + d and related compounds were previously reported (Horowitz et al., 1981).
No new phase has been detected in a study of IrO2-based resistors interacting with high lead glass (Tankiewicz et al., 2002). In investigations aimed at the development of new generations of lead-free TF resistors, the interactions between RuO2 and different lead-free glasses were addressed. Bismuthate glasses do not form any bismuth ruthenate on firing; conversely, a bismuth titanate pyrochlore is the result of interactions between the glass and TiO2, an additive in the matrix (Busana et al., 2006). No phase change was noted also in RuO2-based resistors in glass of the CaO-BaO-B2O3-Al2O3-SiO2 system (Kielbasinski et al., 2009; Morten et al., 1991), and in CaRuO3-based resistors with glass of the SiO2-CaO-BaO-SrO-K2O-B2O3 system (Rane et al., 2005). On the contrary, Bi2Ru2O7 is completely exhausted in lead-free glass-based resistors after a long firing process (Hrovat et al., 2008).
The thermodynamic aspects of these phase transformations have been made clearer from the studies by Hrovat et al. (2006a, 2008). The subsolidus phase equilibria in the PbO-poor part of RuO2-PbO-SiO2 system (Hrovat et al., 2006a) shows that lead ruthenate is not stable in the presence of the silica-rich glass phase. In the same conditions, RuO2-based resistors are expected to be stable after long-term high-temperature firing, a result supported by experiments. Similarly, from the phase equilibria in the RuO2-Bi2O3-SiO2 system, the instability of the bismuth ruthenate in the presence of silica-rich glass phase is apparent (Hrovat et al., 2008).
Interactions between pyrochlore-based resistors and Ag-Pd alloy composite contacts were reported by Needes et al. (1985). These authors also reported the effects of Al2O3 addition to paste composition and studied the infrared firing of pyrochlore-based pastes.
Manganese in amounts larger than 1%wt added to RuO2-based TF resistors with a lead–silicate glass reacts to form Pb2Si2Mn2O9 (kentrolite) (Morandi et al., 1991). At lower concentrations, Mn is soluble in glass and, especially when uniformly distributed, acts as an effective additive with benefits in TCR control and noise reduction in RuO2-based resistors.
The effect of glass composition on the electrical properties of TF resistors was reported by Adachi and Kuno (2000). A broad range of lead borosilicate glasses were studied with RuO2 and lead ruthenium as the conducting phases. Anomalously high resistivity was found for lead-depleted compositions.
The interactions between screen-printed and fired PbO layers and alumina occur via two main processes: (1) a reaction between PbO and Al2O 3 grains, which induces the formation of a crystalline phase, Pb2Al2O 5; and (2) an interdiffusion process involving Pb and the intergranular amorphous phase in the substrate, according to a diffusion limited process (diffusivity ranging from ~ 10− 9 to 2.5 × 10− 8 cm2s−1 for temperatures from 700 to 1000 °C, and activation energy of ~ 1.20 eV) (Bersani et al., 1997).
Notably different processes occur when Bi2O3 layers are fired on ceramic substrates (Immovilli et al., 1998) in terms of reaction products: they are invariably crystalline in nature in these systems. Several transitions of Bi2O3 in its polymorphic phases are found to occur on BeO substrates, while newly formed compounds grow up on alumina substrates, i.e. Al4Bi2O9 on 99.9%-Al2O3 and Bi12SiO20 on 96%-Al2O3. Bismuth deeply penetrates the ceramic interstices in all cases. Until Bi2O3 had not completely reacted, this penetration is a diffusion-limited process with values of the activation energy ranging from 3.7 ± 0.6 eV (on BeO) to 1.4 ± 0.06 eV (on 96%-Al2O3).
As a partial conclusion of this section, it is interesting to sum up the observations concerning ‘simple’ RuO2-based resistors to note that the conducting material (RuO2) undergoes a variety of changes during the processing: partial reduction to Ru in the burnout zone, reoxidation to variety of oxidation states, dissolution of Ru in several oxidation states in the vitreous phase, interaction with the PbO in the glass to form intermediate pyrochlore phase Pb2Ru2 − xPbxO6 + δ and possible decomposition of the pyrochlore to RuO2 at higher temperatures. The ideal picture of single-phase conducting particles imbedded in vitreous phase is very far from the real system, which has a variety of conducting phases (Ru, RuO2, Pb2, Ru2 − x PbxO6 + δ, Pb2Ru2O6 + δ) formed during firing and the dissolution of Ru ions in various oxidation states (Ru2 +, Ru+ 3, Ru+ 4) in the vitreous phase.
3.5 Sintering, grain growth and Ostwald ripening
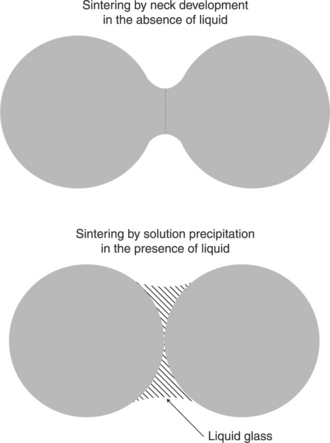
Sintering is a thermal process, below the melting temperature of the main constituent material, which transforms metallic or ceramic powders (or powder compacts) into bulk materials containing, in most cases, residual porosity. This is the final step in the mass production of complex-shaped components in both ceramic and powder metallurgy technologies (Kingery et al., 1976; Ohring, 1995; Rahaman, 2008). Figure 3.6 depicts the early stages of sintering in an idealized system, consisting in two solid spheres with the same radius, in contact without a liquid phase or in the presence of the liquid. The former case describes the sintering model in ‘fritless’ (chemically bonded) conductors, whereas the latter case is for glass-bonded conductors. In both cases, necks develop between the two particles when the temperature is raised because of transport of materials from the solid particles to the neck area.
The driving force for sintering and densification is the drop in total surface energy, which derives from the decrease of the surface area, when particles in contact mutually exchange constituent material and coalesce together into large aggregates or to a continuous solid. It is found that this driving force is more effective (has higher chemical potential) the smaller the size (e.g. the diameter of spherical grains) and the higher the surface energy of interacting particles. Hence the selection of the so-called ‘very active’ powders made of small particles in the ceramic technology, to start the sintering shrinkage of ceramic parts. With nanoparticles, both size and an increase in surface energy in the nanometer range are effective (Nanda et al., 2008), resulting in sintered films at temperatures as low as 100–200 °C (e.g. Girotto et al., 2009), therefore compatible with most of the flexible substrates, and depressed even more than 450 °C relative to the use of coarse powders (Tabellion et al., 2006). On the other hand, the transport of material may occur through various processes: bulk diffusion, surface and grain boundary diffusion, evaporation from the grains and condensation at negative radius surfaces (necks), and solution-precipitation in the presence of the liquid phase. The latter is the ‘sintering aid’, aimed at speeding up the kinetics of the transport phenomena. Hence, for example, the addition of a glassy phase in the sintering of Al2O3 for the production of dense alumina ceramics and in high-temperature co-fired ceramics (HTCC) technology or the various sintering aids used in low-temperature co-fired ceramics (LTCC) technology (see Chapter 6). The specific models for sintering of glass powders have been illustrated in the excellent review by Rabinovich (1985) and in Rahaman (2008). In screen-printed film technology, as in ceramic technology, the sintering process starts after the burnout of the vehicle and consists generally of two stages: 1) the neck growth between the particles and 2) the shrinking with the accompanying densification by pore annihilation and grain growth. In typical processes carried out on inks for microelectronic applications, the inks’ composition is normally tailored for a complete sintering at a temperature near 850 °C and dwell times of 5–20 minutes. However, different processes are demanded for various materials and structures. Often the target is to accomplish very dense components. In fact, densification is most often associated with better performances – such as high conductivity, small contact resistance, fine esthetic appearance of electronic conductors, low moisture absorption and negligible dielectric loss degradation in dielectrics, better piezoelectric and pyroelectric responses from ferroelectric/piezoelectric films, etc. (Debéda-Hickel et al., 2005).
However, there are applications such as gas sensors and fuel cells that require porous films, in spite of the processing at a temperature high enough to stabilize the structure, as well as to provide adhesion to the substrate and impart the functional desired properties. Many strategies have been envisaged for these applications. For example, in gas sensors based on nanosized semiconducting oxides, particle coarsening is prevented by adding a small amount of a further component to the main sensing oxide, namely the grain growth inhibitor (e.g. via a metal-organic, or a salt solution) aimed at retarding (shifts at higher temperatures and/or longer times) the initial stage of sintering of the original (‘pure’) composition (Martinelli et al., 1999; Xu et al., 1989). In this way, the large surface of sensing oxide is available for gas interaction after the firing process, preserving the sensor sensitivity, as well as keeping thermal stability after many hours of operation at temperatures of 300–500 °C.
In Ostwald ripening, larger particles grow at the expense of smaller ones; the latter dissolve in one solid or liquid medium and the dissolved atoms/molecules redeposit onto the surface of coarser crystalline aggregates. This is the mechanism (often confused with, or hastily mentioned as, sintering) that allows for the increase in size of RuO2 grains in RuO2-based resistors. This process was recognized by Hoffman and Jones (1983), Prabhu and Vest (1975), Prudenziati et al. (1989) and carefully studied by Nakano et al (1994). The latter authors analyzed the shape and size of RuO2 grains in glasses of different compositions, including high-lead and lead-free silicate glasses. It was remarked that while the particles rounded as the temperature increased – indicating that RuO2 dissolves in glass – the absence of necks between particles denies the possibility of material transport through inter-particle contact areas. Moreover, the theory for Ostwald kinetics was found in qualitative agreement with experimental data. Therefore, even if the solubility of RuO2 was not quantified (being lower than the detection limit of the authors’ equipment), its dependence on the glass composition was indirectly inferred and suggested to increase by increasing the PbO content and by SiO2 substitution for B2O3. Dissolution of Al2O3 in glass appeared to retard Ostwald ripening, an agreement with the results previously summarized in section 3.4.
The growth of silver grains was studied by Yata and Yamaguchi (1992a, 1992b, 1992c) in the presence of borosilicate glass with a composition common in the TF industry; they found that Ag particles grow in glass by the Ostwald ripening mechanism controlled by diffusion between 600 and 900 °C; addition of Pd retards the Ostwald ripening as a result of the decrease in solubility of Ag in glass due to the formation of Ag/Pd solid solutions.
3.6 Reactivity interactions in other systems
A lot of research has been recently focused on studies about the compatibility of films and pre-fired tapes, either buried inside, and co-fired with, laminated tapes for LTCC packages and microsystems. In terms of Materials Science, the most frequently observed interactions are due to materials transport, whose driving force is thermodynamic in nature: excess ion in a component flows to the component with deficient concentration to cancel the gradient. This is the well-known problem of TF resistors on/in LTCC tapes. To solve the problem, manufacturers of pastes for circuit components and LTCC tapes have developed systems containing the same (or very similar) glass compositions. However, this solution cannot be generalized, and many other strategies have been conceived by researchers. A possibility is to limit the interacting zone with a restriction of the glass transport, often taking glasses with relatively high transition and softening points, e.g. glasses with SiO2 and Al2O3 content greater than in the common pastes for alumina substrates. Another possibility for contrast the interdiffusion is the use of ‘diffusion barriers’. For example, Hrovat et al. (2006b) suggest interposing an alumina barrier layer between piezoelectric ceramic material (PZT) and LTCC substrates to avoid their mutual interactions and the consequent degradation of the PZT piezoelectric characteristics. A platinum layer between PZT and silicon for mechanical microsystems (MEMS) is taken to avoid strong interactions between the two materials (see Chapter 10).
3.7 The Kirkendall effect
A possible detrimental consequence of interdiffusion between two systems that mutually exchange elements moving through vacancies but with quite different diffusivity (at the operation temperature) is the coalescence of vacancies and thus the void formation on the side of the couple from which the most diffusing species come. This phenomenon, known as the Kirkendall effect, often occurs in microelectronics when soldering gold wires to aluminum pads (Ohring, 1995). In TF hybrid circuits, this phenomenon has been reported to affect the bond strength of aged Al wire bonds to Au conductors (Larry et al., 1980); to circumvent the problem, the alloy of Au with some percentage of Pd is recommended. Likewise, metallic interdiffusion at the printed electrode/wire junction has been recognized to be an issue for reliable application of semiconductor oxide gas sensors (Ménil et al., 2005); in this case, the phenomenon can appear evident after several months at the typical operating temperatures of such sensors (400–450 °C).
3.8 Conclusions and future trends
In conclusion, we have described a few examples of the wide variety of structure–properties relations experienced in TF materials. The microstructure and performance of TFs start to evolve from temperatures as low as room temperature and continue to change during the whole course of processing or, when required, in post-process thermal annealing. The flexibility of TF technology in terms of materials and processing parameters can mask the need for careful consideration and constant attention to design rules and performance evaluation guided by basic concepts typical in the approach of Materials Science to complex systems. Many other examples of interactions between the constituents of functional printed films and/or the films and other elements of the systems will be mentioned in other chapters of this book.
The lessons learned through TF materials research might form a solid basis for the development of the newer functional printed films promised by the most recently introduced printing technologies.
Some problems faced in connection with screen-printed and fired films will be exacerbated in thinner films, e.g. ink-jet printed or lithographically deposited films, or in films to be functionalized at low temperatures, due to the nature of the flexible substrates. Other intrinsically new problems might arise from the diversified new functions required by films for optoelectronics applications, sensors and flexible electronics. Thus, new approaches and new solutions should be conceived but close attention to both thermodynamics and the kinetic aspects of interactions and the structure/properties relations in the framework of Materials Science will always be a powerful resource in the continuous effort to improve our knowledge in this difficult but fascinating field.
3.9 Sources of further information
For a general view of concepts and methods of Materials Science, very ‘classical’ texts are recommended, for example:
Barsoum, M.W. Fundamentals of Ceramics. 2003:108. [Bristol].
Ohring, R.A. Engineering Materials Science. San Diego: Academic Press; 1995.
Swalin, M. Thermodynamics of Solids. New York: John Wiley and Sons; 1962.
3.10 References
Adachi, K., Kuno, H. Decomposition of ruthenium oxides in lead borosilicate glass. Journal of the American Ceramic Society. 1997; 80(5):1055–1064.
Adachi, K., Kuno, H. Effect of glass composition on the electrical properties of thick-film resistors. Journal of the American Ceramic Society. 2000; 83(10):2441–2448.
Adachi, K., Iida, S., Hayashi, K. Ruthenium clusters in lead–borosilicate glass in thick-film resistors. Journal of Materials Research. 1994; 9(7):1866–1878.
Angus, H.C., Gainsbury, P.E. Oxide resistor materials. 1972. [US Patent No. 3,679,607, July 25].
Avtokratova, T.D. Analytical chemistry of ruthenium. Jerusalem, Israel: S. Monson, 1962; . [1963 (transl. Analiticheskaya Khimya Rutheniya Isdatel’ stvo Akademii Nauk SSSR, Moskva)].
Barsoum, M.W. Fundamentals of Ceramics. Bristol: Institute of Physics; 2003.
Bartholomew, R.F. Structure and properties of silver phosphate system – infrared and visible spectra. Journal of Non-Crystalline Solids. 1972; 7(3):221–235.
Bell, W.E., Tagami, M. High-temperature chemistry of the ruthenium–oxygen system. Journal of Physical Chemistry. 1963; 67(11):2432–2436.
Bersani, M., Morten, B., Prudenziati, M., Gualtieri, A. Interactions between lead oxide and ceramic substrates for thick-film technology. Journal of Materials Research. 1997; 12(2):501–508.
Bube, K.R. The effect of prolonged elevated temperature on thick-film resistors. Proceedings of ISHM. 1972. [2A6-1-2 A6-13, Park Ridge IL].
Burckhardt, H.-G., Vavra, H. Reactions and thick-film metallization on aluminium-nitride substrates. Proceedings of the 7th European ISHM Conference. 1989. [Hamburg, May, paper 4.2].
Busana, M.G., Prudenziati, M., Hormadaly, J. Microstructure development of RuO2-based lead-free thick-film resistors. Journal of Material Science: Materials in Electronics. 2006; 17(11):951–962.
Debéda, H., Massok, P., Lucat, C., Ménil, F., Aucouturier, J.L. Methane sensing: from sensitive thick-films to a reliable selective device. Measurement Science and Technology. 1997; 8(1):99–110.
Debéda-Hickel, H., Lucat, C., Menil, F. Influence of the densification parameters on screen-printed component properties. Journal of the European Ceramic Society. 2005; 25(12):2115–2119.
Gilchrist, R. Gravimetric method for the determination of ruthenium. Bureau of Standards Journal of Research. 1929; 3:993–1004.
Girotto, C., Rand, B.P., Steudel, S., Genoe, J., Heremans, P. Nanoparticle-based, spray-coated silver top contacts for efficient polymer solar cells. Organic Electronics. 2009; 10(4):735–740.
Herold, P.G., Planje, T.J., Williams, J.C., Ruthenium–containing glasses and stains, 1956. [US Patent 2-739-901; March 27].
Hoffman, L.C. An overview of thick-film hybrid materials. Ceramics Bulletin. 1984; 63(4):572–576.
Hoffman, L.C., Jones, A.W. Interaction of thick-film materials with roll compacted alumina ceramics. International Journal of Hybrid Microelectronics. 1983; 6(1):603–606.
Hong, K.-K., Cho, S.-B., You, J.S., Jeong, J.-W., Bea, S.-M., Huh, J.-Y. Mechanism for formation of Ag crystallites in the Ag thick-film contacts of crystalline Si solar cells. Solar Energy Materials and Solar Cells. 2009; 93(6–7):898–904.
Hormadaly, J., Stain-resistant ruthenium oxide-based resistors, 1984. [US Patent 4-476-039; October 9].
Hormadaly, J., Thermistor composition, 1990. [US Patent 4-906-406; March 6].
Horowitz, H.H., Longo, J.M., Lewandowski, J.T. New oxide pyrochlores: A2(B2−xAx) O7−x (A = Pb, Bi: B = Ru, Ir). Material Research Bulletin. 1981; 16:489–496.
Horowitz, S.J., Novel silver compositions, 1978. [US Patent 4-090-009; May 16].
Howes, M.G. The platinum metals in glass. Platinum Metals Review. 1957; 1(2):44–48.
Hrovat, M., Holc, J., Belavič, D., Bernard, J. Subsolidus phase equilibria in the PbO-poor part of RuO2-PbO-SiO2 system. Material Letters. 2006; 60(20):2501–2503.
Hrovat, M., Holc, J., Drnovsek, S., Belavic, D., Cilensek, J., Kosec, M. PZT thick-films on LTCC substrates with an interposed alumina barrier layer. Journal of European Ceramic Society. 2006; 26(6):897–900.
Hrovat, M., Meader, T., Holc, J., Belavič, D., Cilenšek, J., Bernard, J. Subsolidus phase equilibria in RuO2-Bi2O3-SiO2 system. Journal of European Ceramic Society. 2008; 28(11):2222–2224.
Immovilli, S., Morten, B., Prudenziati, M., Gualtieri, A., Bersani, M. Interactions between bismuth oxide and ceramic substrates for thick-film technology. Journal of Materials Research. 1998; 13(7):1865–1874.
Janakirama-Rao, B.V. Dielectric properties of glasses in the systems Bi2O3-CdO-SiO2, Bi2O3-CdO-B2O3 and Bi2O3-CdO-GeO2 and their relation to the structure of glass. Journal of the American Ceramics Society. 1962; 45(11):555–563.
Kingery, W.D., Bowen, H.K., Uhlmann, D.R. Introduction to Ceramics, 2nd ed. New York: John Wiley & Sons; 1976.
Kielbasinski, K., Jakubowska, M., Mlozniak, A., Hrovat, M., Holc, J., Belavic, D. Electrical and microstructure evolution of thick-film lead-free resistors after various temperature treatments. ISSE 2009, 32nd International Spring Seminar on Electronics Technology. 2009:1–5.
Kirby, P.L. EmpiricISHM – A noble art. Hybrid Circuits. 1982; 1:49–50.
Krausse, R., Behr, G., Schlafer, D., Krabbes, G. Interations between ethylcellulose and thick-film resistors containing ruthenium. Journal of Materials Science Letters. 1991; 10(23):1392–1393.
Kretzschmar, C., Otschik, P., Jaenicke-Rößler, K., Schläfer, D. The reaction between ruthenium dioxide and aluminium nitride in resistor pastes. Journal of Materials Science. 1993; 28(21):5713–5716.
Kummer, F., Taitl, I. Thermal expansion and laser trim stability of Ru-based thick-film resistors. Proceedings of the International Conference on Thin and Thick-film Technology. 1977:28–33. [Augsburg].
Larry, J.R., Rosenberg, R.M., Uhler, R.O. Thick-film technology: An introduction to the materials. IEEE Transactions of Components and Hybrids and Manufacturing Technology. 1980; CHMT-3(2):211–225.
Licari, J.J., Enlow, L.R. Hybrid Microcircuit Technology Handbook, 2nd ed. Westwood, New Jersey: Noyes Publications; 1998.
Machin, W.S., Vest, R.W. Reactivity of alumina substrates with high lead glasses. Materials Science Research. 1978; 11:243–251.
Martinelli, G., Carotta, M.C., Ghiotti, G., Traversa, E. Thick-film gas sensors based on nanosized semiconducting oxides powder. MRS Bulletin. 1999; 24(6):30–36.
Ménil, F., Debéda, H., Lucat, C. Screen-printed thick-films: From materials to functional devices. Journal of European Ceramic Society. 2005; 25:2105–2113.
Morandi, M., Morten, B., Prudenziati, M., Argentino, E., Ruffi, M.G. Manganese in ruthenium based thick-film resistors. Materials Engineering. 1991; 2(3):421–433.
Moriwaki H., Suzuki A., Watanabe Y., Ishiita M., Kamata T. and Adachi K., ‘Interactions between thick-film resistors and alumina substrate’, Japan International Electronics Manufacturing Technology Symposium, pp. 46–49
Moro, L., Lazzeri, P., Prudenziati, M., Morten, B., Savigni, P. Interactions between beryllia and high-lead glasses. Applied Physics Letters. 1992; 61(19):2299–2301.
Morten, B., Prudenziati, M., Sacchi, M., Sirotti, F. Phase transitions in Ru based thick-film (Cermet) resistors. Journal of Applied Physics. 1988; 63(7):2267–2271.
Morten, B., Prudenziati, M., Taroni, A. Metal migration from terminations in thick-film resistors: the effect of the substrate. International Journal of Hybrid Microelectronics. 1981; 4:341–346.
Morten, B., Ruffi, G., Sirotti, F., Tombesi, A., Moro, L., Akomolafe, T. Lead-free ruthenium-based thick-film resistors: a study of model systems. Journal of Materials Science: Materials in Electronics. 1991; 2(1):46–53.
Morten, B., Sirotti, F., Prudenziati, M., Manfredini, T. Evolution of ruthenate-based thick-film cermet resistors. Journal of Physics D: Applied Physics. 1994; 27(10):2227–2235.
Mukerji, J. Absorption spectra of ruthenium in borosilicate, phosphate and aluminoborophosphate glasses. Glass Technology. 1972; 13(5):135–137.
Mukerji, J., Biswas, S.R. Solubility of ruthenium in soda–silica glasses. Glass and Ceramic Bulletin. 1967; 14(2):30–34.
Nagesh, V.K., TomasiaA, P., Pask, J.A. Wetting and reactions in the lead borosilicate glass–precious metal systems. Journal of Materials Science. 1983; 18(7):2173–2180.
Nakano, T., Yamaguchi, T. Effect of O2 concentration in firing atmosphere on resistance of RuO2-glass thick-film resistors. Journal of the American Ceramic Society. 1995; 78(6):1703–1704.
Nakano, T., Suzuki, K., Yamaguchi, T. Analysis of interaction between RuO2 and glass by growth of RuO2 particles in glasses. Journal of Adhesion. 1994; 46(1 and 4):131–144.
Nanda, K.K., Maisels, A., Kruis, F.E. Surface tension and sintering of free gold nanoparticles. Journal of Physical Chemistry C. 2008; 112(35):13488–13491.
Needes, C.R.S. Advanced thick-film system for AlN substrates. Microelectronics International. 2003; 20(1):48–51.
Needes, C.R.S., Pierce, J.W., Kuty, D.W., Ishibashi, S., Nishii, N. A new thick-film materials system for high volume microcircuits. International Journal for Hybrid Microelectronics. 1985; 8(1):24–30.
Newkirk, A.E., McKee, D.W. Thermal decomposition of rhodium, iridium and ruthenium chlorides. Journal of Catalysis. 1968; 11(4):370–377.
Nordstrom, T.V., Hills, C.R. Transmission electron microscopy studies of the microstructure of thick-film resistors. International Journal of Hybrid Microelectronics. 1980; 3(1):14–19.
Norton, M.G. Thermodynamic considerations in the thick-film metallization of aluminium nitride substrates. Journal of Material Science Letters. 1990; 9(1):91–93.
Ohring, M. Engineering Materials Science. San Diego: Academic Press; 1995.
Palanisamy, P., Sarma, D.H.R. Thermodynamics of processing copper thick-film systems in a reactive atmosphere. Microelectronic International. 1987; 4(2):13–20.
Palanisamy, P., Sarma, D.H.R., Vest, R.W. Solubility of ruthenium dioxide in lead borosilicate glasses. Journal of the American Ceramic Society. 1989; 72(9):1755–1756.
Pflieger, R., Lefebvre, L., Malki, M., Allix, M., Grandjean, A. Behavior of ruthenium dioxide particles in borosilicate glasses and melts. Journal of Nuclear Materials. 2009; 389(3):450–457.
Pierce, J.W., Kuty, D.W., Larry, J.R. The chemistry and stability of ruthenium based resistors. Solid State Technology. 1982; 25(10):85–93.
Pike, G.E., Seager, G.H. Electrical properties and conduction mechanisms of Ru-based thick-film (cermet) resistors. Journal of Applied Physics. 1977; 48(12):5152–5169.
Pinet, O., Mure, S. Redox behavior of platinum-group metals in nuclear glass. Journal of Non-Crystalline Solids. 2009; 355(3):221–227.
Prabhu, A.N., Vest, R.W. Investigation of microstructure development in ruthenium dioxide–lead borosilicate glass thick-films. Materials Science Research. 1975; 10(Sintering Catal):399–408.
Prabhu, A.N., Fuller, G.L., Vest, R.W. Solubility of ruthenium oxide in lead borate glass. Journal of the American Ceramic Society. 1974; 57(9):408–409.
Prudenziati, M., Morten, B., Cilloni, F., Ruffi, G., Sacchi, S. Interactions between alumina and high lead glasses for hybrid materials. Journal of Applied Physics. 1989; 65(1):146–153.
Prudenziati, M., Morten, B., Forti, B., Gualtieri, A.F., Mihai, Dilliway G. Devitrification kinetics of high lead glass for hybrid microelectronics. International Journal of Inorganic Materials. 2001; 3(7):667–674.
Prudenziati, M., Morten, B., Gualtieri, A.F., Leoni, M. Dissolution kinetics and diffusivity of silver in glassy layers for hybrid microelectronics. Journal of Materials Science: Materials in Electronics. 2004; 15(7):447–453.
Prudenziati, M., Morten, B., Moro, L., Olumekor, L., Tombesi, A. Interactions between terminations and thick-film (cermet) resistors: the role of bismuth. Journal of Physics D: Applied Physics. 1986; 19(2):275–282.
Prudenziati, M., Morten, B., Savigni, P., Guizzetti, G. Influence of the preparing conditions on the physico-chemical characteristics of glasses for thick-film hybrid microelectronics. Journal Materials Research. 1994; 9(9):2304–2313.
Prudenziati, M., Morten, B., Travan, E. Reduction process of RuO2 powders and kinetics of their re-oxidation. Materials Science and Engineering B. 2003; 98(2):167–176.
Prudenziati, M., Zanardi, F., Morten, B., Gualtieri, A.F. Lead-free thick-film resistors: an explorative investigation. Journal of Materials Science: Materials in Electronics. 2002; 13(1):31–37.
Rabinovich, E.M. Preparation of glass by sintering. Journal of Materials Science. 1985; 20(12):4259–4297.
Rahaman, M.N. Sintering of Ceramics. Boca Raton: CRC; 2008.
Rane, S., Prudenziati, M., Morten, B., Golonka, L.J., Dziedzic, A. Structural and electrical properties of perovskite ruthenate-based lead-free thick-film resistors on alumina and LTCC. Journal of Materials Science: Materials in Electronics. 2005; 16(10):687–691.
Schreiber, F.D., Settle, F.A., Jr., Jamison, P.L., Eckenrode, J.P., Headley, G.W. Ruthenium in glass-forming borosilicate melts. Journal of Less-Common Metals. 1986; 115(1):145–154.
Seddon, E.A., Seddon, K.R. The Chemistry of Ruthenium. Amsterdam and New York: Elsevier Science; 1984.
Shah, J. Strain sensitivity of thick-film resistors. IEEE Transactions of Components and Hybrids and Manufacturing Technology. 1980; CHMT-3(4):554–564.
Swalin, R.A. Thermodynamics of Solids. New York: John Wiley & Sons; 1962.
Tabellion, J., Zeiner, J., Clasen, R. Manufacturing of pure and doped silica and multicomponent glasses from SiO2 nanoparticles by reactive electrophoretic deposition. Journal of Materials Science. 2006; 41(24):8173–8180.
Takahashi, K., Proceedings of 6th International ‘Glass Congress, 1962:366. [Washington, D.C].
Tankiewicz, S., Morten, B., Prudenziati, M., Golonka, L.J. IrO2-based thick-film resistors. Journal of Applied Physics. 2002; 91(7):4261–4266.
Turkdogan, E.T. Physical Chemistry of High Temperature Technology. New York: Academic Press; 1980.
Vest, R.W. Materials science of thick-film technology. American Ceramic Society Bulletin. 1986; 65(4):631–636.
Volf, M.B. Chemical approach to glass. In: Glass Science and Technology. Elsevier; 1984. [transl. Chemie Skla, Vol. 7].
Wang, S.F., Huebner, W. Thermodynamic modeling of equilibrium subsolidus phase relations in the Ag–Pd–O2 system. Journal of the American Ceramic Society. 1991; 74(6):1349–1353.
Wang, Y.L., Carroll, A.F., Smith, J.D., Cho, Y., Bacher, R.J., et al. Oxynitride based glass bonding to AlN for thick-films. Proceedings of the International Symposium on Microelectronics. International Society for Hybrid Microelectronics, Reston, VA USA, 1991.:533–535.
Wang, Y.L., Carroll, A.F., Smith, J.D., Cho, Y., Bacher, R.J., et al. Advanced thick-film system for AlN substrates. Microelectronics International. 2003; 20(1):48–51.
Xu, C., Tamaki, J., Miura, N., Yamazoe, N. Promoting effects of additives on thermal stability of tin oxide (IV) fine particles. Journal of Materials Science Letters. 1989; 8(9):1092–1094.
Yamashita, M., Yamanaka, H. Dissolution and separation of ruthenium in borosilicate glass. Journal of the American Ceramic Society. 2004; 87(5):967–969.
Yata, K., Yamaguchi, T. Ostwald ripening of silver in glass. Journal of Materials Science. 1992; 27(1):101–106.
Yata, K., Yamaguchi, T. Effect of temperature on Ostwald ripering of silver in glass. Journal of the American Ceramic Society. 1992; 75(8):2071–2075.
Yata, K., Yamaguchi, T. Effect of additives on Ostwald ripening of silver in glass. Journal of the American Ceramic Society. 1992; 75(10):2910–2914.
Yi, K.M., Lee, K.W., Chung, K.W., Woo, W.S., Lee, H.S., et al. Conductive powder preparation and electrical properties of RuO2 thick-film resistors. Journal of Materials Science: Materials in Electronics. 1997; 8(4):247–251.
Zdaniewski, W.A., Silverman, L.D. Effect of localized redox equilibria on adhesion between gold and thick-film dielectrics. Journal of Materials Science. 1990; 25(7):3155–3158.