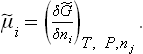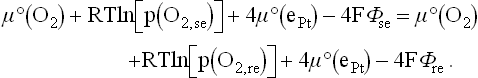Printed gas sensors based on electrolytes
Abstract:
In this chapter we present significant examples of solid electrolyte sensors. Lambda probes constitute one of the rare commercial success stories of gas sensors. The more recently developed proportional probes could still compete with them commercially with the development of lean-burn and diesel engines. Planar thick-film technology appears the most promising manufacturing process. It is cheaper and more versatile than traditional ceramic processes, with lower manufacturing costs and fewer ageing problems than planar thin film technologies. Although the measurement of oxygen partial pressure constitutes the leading application of solid electrolyte sensors, this chapter shows many possibilities for detecting other gases. Sensing such gases probably constitutes the most promising application to date. Some of these sensors are currently proposed commercially. They generally imply coupling catalytic with thermodynamic processes. Many studies must still be undertaken in order to address outstanding problems.
12.1 Introduction
Solid electrolytes play a special part in the field of gas sensors, for at least two reasons:
1. Studies on this type of sensor were among the earliest in the gas sensor field and a large amount of R&D has been devoted to solid electrolyte sensors since the early 1960s.
2. The λ-sensor based on zirconia for oxygen sensing in automotive exhausts is certainly the most successful story of a commercial sensor, with several hundreds of millions of units manufactured.
Section 12.2 will initially recall some basic notions on solid electrolytes. Since solid electrolytes are ion conductors, transport properties in such materials are primarily ensured by ions and not by electrons or holes, as in semiconductors. Solid electrolytes can be applied to the field of gas sensing in at least two types of devices: potentiometric and amperometric. The former can be based on O2 −, F−, H+, Li+, Na+, Ag+ … ion conductors, whereas the latter is practically restricted to O2 − ion conductors, namely zirconia.
Potentiometric sensors, dealt with in section 12.3, are classified by whether they are working at thermodynamic equilibrium or not. In case of thermodynamic equilibrium, potentiometric sensors are further classified according to the relationship between the gaseous species’ capability to sense and the mobile ion in the solid electrolyte. Potentiometric sensors can be assimilated to batteries that deliver at zero current, an electromotive force function of the partial pressure of the gas that is to be sensed (target gas).
Amperometric sensors, dealt with in section 12.4, are based on oxygen pumping by the application of a voltage across zirconia. Without a diffusion barrier at the cathode, the device does not operate as a sensor but as a simple ionic pump. Gaseous oxygen O2 or oxygen contained in oxygenated gases such as CO2, NOx, H2O, etc. is reduced to the cathode in O2 − ions. These ions are attracted towards the anode by the applied voltage and re-oxidized in gaseous oxygen O2 when they reach the anode. The presence of a diffusion barrier above the cathode limits this ionic current and ensures the proportionality between the latter (so-called limiting current) and the partial pressure of the target gas. More sophisticated devices are considered in section 12.5, associating several of the previous principles: oxygen pumps, potentiometric sensors and amperometric sensors. The best-known applications are detailed in corresponding sections, both on a theoretical basis and also from the point of view of the manufacturing technologies used. It will be demonstrated that printing technologies are of primary interest for the fabrication of many solid electrolyte sensors. Some possible future trends will be briefly mentioned in the conclusion.
12.2 Solid electrolytes
A solid electrolyte is, as its name indicates, a solid in which the transport of the electric charges is ensured by ions, as in liquids. However, whereas in a liquid, all the positive and negative charges are mobile under the action of an electric field, in a solid electrolyte generally only one species of ion can move within the crystal structure. To allow the movement of an ion, the crystal structure must necessarily comprise vacant sites, which will transitorily accommodate the mobile ions. Classically, there are two types of sites by which the ions can advance: vacancies characterized by the absence of ions in normal position in the crystal structure (Schottky defects) and interstitial sites, which are normally unoccupied (Frenkel defects). These defects can have two origins:
• A thermodynamic system at a temperature different from absolute zero always tends to evolve to a maximum entropy to minimize its Gibbs energy (G = H-TS). Consequently, any material in its purest state will always present pairs of defects (one negative and one positive defect per pair to respect electric neutrality). The higher the temperature is, the larger their number will be. These defects are known as intrinsic.
• The introduction of elements into the crystal structure with a valence different from that of the normal elements, will necessarily involve the presence of defects. For example, in the case of zirconia (ZrO2) doped with yttrium, which is the most well-known solid electrolyte (yttrium-stabilized zirconia, YSZ for), the substitution of tetravalent zirconium by trivalent yttrium must be compensated by an equivalent reduction of the negative charge, which leads to the presence of oxygen vacancies. The defects resulting from such a doping are known as extrinsic.
Experimentally, the ionic conductivity is thermally activated according to an Arrhenius relation:
At moderated temperatures, i.e. in the range where the extrinsic defects are prevalent, the activation energy EA reflects the energy required for an ion to jump from a vacant site to a nearby vacant site. At high temperatures, where the intrinsic defects dominate, the activation energy will include, in addition to the preceding term, a term relating to the energy formation of the intrinsic defects. The terms intrinsic and extrinsic, just as the various modes of conductivity, remind us of semiconductors. However, it is important to note that for the ionic conductivity, defined by the relation:
the mobility ui of the ions is generally 100 to 1000 times lower than that of the electrons in semiconductors (ci represents the voluminal concentration in mobile ions i and zi the charge number of these ions). To allow ionic conductivity to dominate the transport properties in a material, the concentration in electrons will have to be lower than the concentration in mobile ions by a factor of the same order of magnitude.
This is a first explanation for the relatively limited number of solid electrolytes. A second explanation arises from the nature of the mobile ions. To be able to move easily in the crystal structure, a mobile ion must conform to two conditions that one conceives rather well intuitively: firstly it must have a low valence as a high charge would indeed attract them too strongly towards the fixed ions of opposite charge of the crystal lattice; secondly it must be of small size. These two conditions produce the situation that the mobile cations are made up of almost only monovalent ions (H+, Li+, Na+, K+, Rb+, Ag+, Tl+) and the mobile anions of ions fluorine F− and oxygen O2 −, oxygen preceding very largely the other ions. The first true studies on solid electrolyte sensors were carried out for the proportioning of oxygen with zirconia (Peters and Mobius, 1961; Wagner, 1957; Weissbart and Ruka, 1961). The other principal coupled solid electrolyte/mobile ions studied are:
• alkaline carbonates and sulphates (Li+, Na+, K+)/Li+, Na+, K+,
• LiSICON and NaSICON (for Super Ionic CONductor)/Li+, Na+,
• HUP (hydrogen uranyl phosphatetetrahydrate: HUO2PO4, 4H2O/H+,
The only criterion common to the various solid electrolytes for a use as a functional sensor material is to have an electronic conductivity that is as low as possible and a notable ionic conductivity in the temperature range concerned with detection. The parameter of ionic conductivity increasing exponentially with temperature (Eq. [12.1]) is eminently critical in sensor operation. For example, stabilized zirconia is almost insulating at room temperature and sensors using this electrolyte cannot function properly below 500–600 °C. β-alumina and NaSICON can function at high temperatures, but also at temperatures hardly higher than the ambient one. Sulphates, on the other hand, break up beyond a few hundreds of degrees Celsius, and HUP can be generally used only at room temperature. More extensive lists of solid electrolytes with a potential use as sensor functional material are reported in Kleitz et al. (1991); Pasierb and Rekas (2009).
12.3 Potentiometric sensors
Potentiometric gas sensors may involve several mechanisms being used at the same time, and for this reason, an easy classification is not obvious. For our first step, we have tried to separate devices only controlled by thermodynamics from those controlled by both thermodynamics and kinetics.
12.4 Thermodynamically controlled sensors
A classification for this type of sensors was first given by Weppner (1987). For type I sensors, the target gas coincides with the mobile ion of the solid electrolyte (e.g. O2 with O2 − in zirconia, H2 with H+ in HUP). In type II, the target gas coincides with the non-mobile ion of a binary compound (e.g. I2 with I− in AgI, which is an Ag+ ion conductor). As for type III, the target gas does not match with any element of the ion conductor. One or several so-called auxiliary phases are required to achieve thermodynamic equilibrium between the gas phase, the electrode, the auxiliary phase(s) and the solid electrolyte.
What these various types of sensors all have in common is the ability to operate at thermodynamic equilibrium and to be able to deliver an e.m.f. depending on the partial pressure of the target gas. For this reason, the simplest theoretical approach to the operating principle of these sensors makes abundant use of electrochemical potentials. Electrochemical potential is of primary interest since it includes both chemical potential, which will take into account the partial pressure of the target gases, and electrical potential, which will determine the e.m.f. Before entering into more detail regarding the operating principles of the various sensor types, the following sub-section will briefly recall the definitions, properties and useful relationships in chemical and electrochemical potentials, especially in thermodynamic equilibriums.
12.4.1 Electrochemical potential properties at thermodynamic equilibrium
A simple approach consists in extending the well-known results obtained at thermodynamic equilibrium with chemical systems, containing only neutral species, to electrochemical systems containing at least one type of charged species (Menil, 2008).
Chemical systems without any charged species
In a chemical system without any charged species, only the caloric and mechanical energies are taken into account. The chemical potential μi of species i is defined as:
where G is the Gibbs energy of the system, i, j… the various types of species in the system, ni, nj… the corresponding number of moles, T and P the temperature and total pressure of the system. For a gaseous system, the previous definition of the chemical potential leads to:
where R is the perfect gas molar constant, μi0 the Gibbs energy of one mole of gas i, taken in its pure state (not in a mixture), xi the molar fraction of gas i in the mixture and pi its partial pressure, defined by:
If the system contains non-gaseous species, the chemical potential of such species has the same formulation, but the molar fraction should in principle be replaced by the activity.
Considering now a chemical system in equilibrium between two thermodynamic states (I) and (II):
where the αi, αj … are the stoichiometric coefficients of species i,j … in each member of the equilibrium, the Gibbs energy is the same for both states:
The development of this relationship leads to the mass action law. Another major consequence is that, if the same species is present in both states, its chemical potential is the same in both states:
One may take the example of water molecules in a system consisting of an aqueous liquid phase in equilibrium with its vapour. The chemical potential of water molecules has the same value in both states (which in the present case coincide with both liquid and gaseous phases). Because the chemical potential of water molecules is constant throughout the whole system, as well as that of all other species, no diffusion between liquid and gas phases occurs (on a macroscopic level), in agreement with the absence of spontaneous evolution of a system at thermodynamic equilibrium (ΔGI → II = 0).
Electrochemical systems with at least one type of species charged
For an electrochemical system in which at least one type of species is charged, not only the calorific and mechanical energies have to be taken into account, but also the electrical energy. One possible approach to dealing with such a system is to define electrochemical functions, which will play a part similar to the previous chemical functions, but which will include electrical energy. To distinguish an electrochemical function from a chemical one, a tilde is added above the symbol of the chemical function. The chemical Gibbs energy – G – thus becomes the electrochemical Gibbs energy – ![]() , and the chemical potential – μ – becomes the electrochemical potential
, and the chemical potential – μ – becomes the electrochemical potential ![]() . Transferring the results of the previous sub-section leads to:
. Transferring the results of the previous sub-section leads to:
The relationship for an electrochemical equilibrium becomes:
Combining [12.7] with the well-known relationship ∆GI → II = − zFE (as for example in batteries where E is the e.m.f., F the Faraday constant and z the number of charges implied in the equilibrium) leads to:
Intuitively, Eq. [12.8] suggests that the electrochemical potential of species i will have the form:
where zi and Φ are respectively the algebraic value of the charge and the electrical potential of species i.
Combining Eqs. [12.3] and [12.9] leads to:
Note that for uncharged species, the electrochemical potential identifies the chemical potential.
Considering now an electrochemical system in equilibrium between two thermodynamic states (I) and (II):
the electrochemical Gibbs energy is the same for both states (![]() I → II = 0), which leads to:
I → II = 0), which leads to:
The development of this relationship usually yields the Nernst law. As another consequence, when the same species is present in both states, its electrochemical potential has the same value in both states:
In other words, the electrochemical potential of a given species is constant in the whole system. Note that for conduction electrons, in metals and semiconductors, the electrochemical potential identifies to the Fermi level.
12.4.2 Type I sensors
Principle
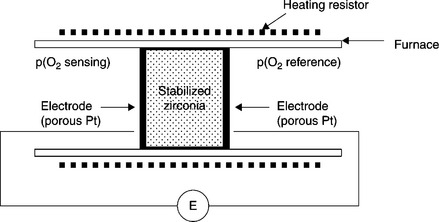
The best representative sensor of this type is of course the oxygen zirconia gauge. Figure 12.1 shows the principle of such a cell, based on dense oxygen ion-conducting YSZ.
This solid electrolyte covered with two identical porous electrodes made of platinum, for example, separates the sensing compartment from the reference one, in which the oxygen partial pressure is known. At sufficiently high temperatures (typically 800 °C), the gaseous oxygen, the mobile oxygen ions in zirconia and the conduction electrons of platinum electrodes in the electrodes are in thermodynamic equilibrium.
Let us take the following as given:
• ![]() and
and ![]() the electrochemical potential of electrons in the sensing (se) and reference (re) electrodes, respectively,
the electrochemical potential of electrons in the sensing (se) and reference (re) electrodes, respectively,
• μ(ese) and μ(ere) the chemical potential of electrons in the sensing and reference electrodes,
• ![]() and
and ![]() the electrochemical potential of O2 − ions in zirconia at the interface with the sensing and reference electrodes,
the electrochemical potential of O2 − ions in zirconia at the interface with the sensing and reference electrodes,
• μ(O2, se) and μ(O2, re) the chemical potential of gaseous oxygen in the sensing and reference compartments,
• Φse and Φre the electrical potentials of sensing and reference electrodes.
At each electrode, the following equilibrium occurs:
Applying Eq. [12.11] to this equilibrium at each interface leads to:
Moreover, since the system is in thermodynamic equilibrium, the electrochemical potential of O2 − ions is constant throughout the whole zirconia, and especially at the interfaces:
Combining Eqs. [12.14], [12.15] and [12.16] and applying the definition of chemical potential to gaseous oxygen and that of electrochemical potential to platinum electrons allows us to deduce:
The Nernst Law results from Eq. [12.17]:
The knowledge of p(O2, re) in the reference compartment and the measurement of the e.m.f. enable the determination of the unknown oxygen partial pressure p(O2, se) in the sensing compartment. This principle, already used in the 1960s for determining the oxygen content in molten metals, was transposed ten years later to the air/fuel ratio monitoring in internal combustion engines with so-called λ-sensors.
λ-sensors
The emission of large amounts of polluting gases by the old petrol engines without catalytic converters was the origin of serious pollution problems in California at the beginning of the 1960s. Research programs were launched for the reduction of these emissions and a regulation was initially defined in the USA in 1968, then in Europe in 1971. Since 1993, all vehicles using gasoline that are produced in Europe are equipped with catalytic converters, which eliminate the three principal pollutants from exhaust gases: NOx, HC and CO, by transforming them into nitrogen, oxygen, water and carbon dioxide. The catalysis is ensured inside the converter by a so-called three-way catalyst containing platinum, palladium and rhodium.
The correct operation of the catalytic converter supposes, however, that the admission gas mixture is regulated precisely by stoichiometry, i.e. the volume ratio of air to fuel (A/F) is equal to 14.6. This condition is fulfilled using a YSZ-based potentiometric sensor placed in the exhaust gas. Indeed, for A/F where the values are very slightly below the stoichiometric ratio of 14.6 (admission mixtures known as ‘rich’ in fuel), the composition of the exhaust gas (known as ‘rich-burn’) is excessively rich, with very low oxygen partial pressures. As soon as the A/F ratio of the admission mixture crosses the stoichiometric point (mixtures known as ‘lean’ in fuel), the oxygen partial pressure in the exhaust gas (known as ‘lean-burn’) increases by several orders of magnitude to possibly reach the afore-mentioned percentage. The logarithmic dependence of the sensor electromotive force with the oxygen partial pressure (Eq. [12.18]) makes it possible to measure this variation easily and to address a feedback signal to bring back the admission mixture to stoichiometry (Duecker et al., 1975; Eddy, 1971; Engh and Wallman, 1977; Fleming et al., 1973; Friese et al., 1973).
The left part of Fig. 12.2 represents a schematic cross view of a lambda probe like the type of those installed at the engine exit. The solid electrolyte, based on stabilized zirconia ceramic sintered at a high temperature, appears as a thimble of approximately 25 mm in length, 5 mm in internal diameter and 2 mm in thickness. Two porous platinum electrodes are deposited on the internal and external walls of the thimble, in the form of layers of a few microns in thickness. The internal electrode is linked by the sheath of the connexion lead, to the surrounding ‘clean’ air, which is used as a reference. In the internal cavity of the thimble, a cylindrical ceramic of approximately 3 mm in external diameter, equipped with a heating resistance deposited in a thick layer, ensures a minimal temperature of 700 °C for the operating of zirconia. The external electrode, covered with a protective coating of MgAl2O4 spinel is in direct contact with the exhaust gas through a metal envelope, which is used as mechanical sleeve for the unit. The latter electrode works as a sensing electrode. The right part of Fig. 12.2 shows the abrupt variation of the sensor f.e.m. when the admission mixture crosses the stoichiometric point.Various designs of planar lambda probes have been proposed. Figure 12.3 presents a sensor based on co-fired multilayer ceramics technology, which consists in assembling, before pressing and co-firing, raw ceramic sheets on which leads, electrodes and heating resistance (Ogasawara and Kurachi, 1988) are deposited by screen-printing. A cavity is arranged in one ceramic sheet so as to communicate with the surrounding air and to ensure the reference compartment for the sensor.
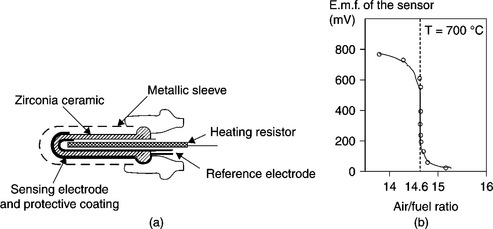
12.2 (a) Schematic view of λ-sensor and (b) typical response after Logothetis et al. (1992).
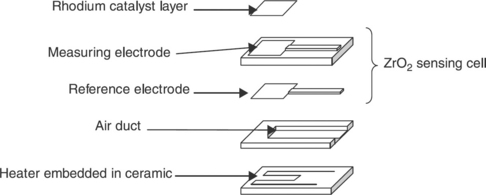
12.3 Perspective of a planar λ-sensor made with the co-fired multi-layer technology, after Ogasawara and Kurachi (1988).
Planar lambda probes, containing multi-layer ceramics, are now proposed by car equipment suppliers (Riegel et al., 2002).
Up to now, only dense ceramics have been considered in manufacturing the zirconia electrolyte used in type I oxygen sensors. In porous ceramics, diffusion of gaseous oxygen occurs between the sensing and reference compartments. This is the main reason why the use of printed zirconia paste as solid electrolyte in type I sensors is not straightforward. Sputtered thin films are comparatively denser. Figure 12.4 shows an oxygen sensor made of sputtered YSZ on a sapphire substrate (Velasco et al., 1982). Conversely to previous cases, for which ambient air fixes the oxygen concentration in the reference compartment, the reference oxygen concentration in the thin film device of Fig. 12.4 is fixed thanks to a so-called ‘oxygen buffer’ or solid-state internal reference made of Pd/PdO.

12.4 Schematic diagram of a thin film λ-sensor, after Velasco et al. (1982).
Hydrogen sensors
The use of a protonic conducting solid electrolyte for the detection of hydrogen gas, via the equilibrium:
constitutes another example of a type I sensor. Various studies were carried out on this topic in the 1980s with protonic conductors such as β-alumina, some zeolites but especially HUP (HUO2PO4, 4H2O) (Kumar and Fray, 1988; Lundsgaard et al., 1982; Schoonman et al., 1982, 1986; Velasco et al., 1982). Figure 12.5 represents two sensors of this type in the form of sintered pellet and thin layers for the measurement of the hydrogen partial pressure. One of the references likely to be used to fix the chemical potential of hydrogen is of course palladium, for its capacity to absorb this gas. The exchange of hydrogen with the HUP makes it possible to form at the interface the Pd/PdHx system, which is used then as an internal reference.
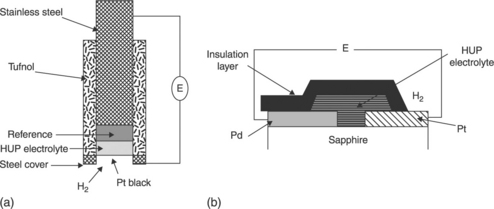
12.5 Hydrogen sensors based on HUP, in the form of (a) sintered ceramic (Kumar and Fray, 1988) and (b) thin film (Velasco et al., 1982).
12.4.3 Type II sensors
Type II sensors have not yet found any application and they mostly spark pedagogical interest. For example, an electrochemical cell, analogous to that of Fig. 12.1, in which O2 −-ion conducting YSZ is replaced by Ag+-ion conducting AgI, could be considered from a theoretical point of view for iodine vapour sensing. Conversely to type I sensors, the target gas (I2) does not correspond to the mobile ion (Ag+) of the binary solid electrolyte AgI, but to the other element. A theoretical treatment similar to that applied to zirconia again leads to Nernst’s Law, assuming at each interface the following equilibrium: AgI ↔ Ag+ + I.
In the case of symmetrical sensors (type I or type II), the only difference between the reference and sensing compartments is the partial pressure or the target gas. The e.m.f. does not depend on anything other than the logarithm of the ratio of the partial pressures. When the reference side consists of an internal solid reference, the e.m.f. still depends on the logarithm of the partial pressure of the target gas on the sensing side, but is shifted by a constant term corresponding to the standard Gibbs energy of formation of the internal reference. For example, let us consider a type II sensor based on the following galvanic cell:
The application of a formalism analogous to that of section 12.4.2 (see sub-heading Principle) to the following equilibriums:
leads to the following expression for the e.m.f.:
(neglecting the difference between the chemical potentials of electrons in platinum (ese−) and silver (ere−), which corresponds to the junction potential between both metals).
The previous theoretical treatment is in principle only valid for binary solid electrolytes. When the solid electrolyte consists of more than two elements, as in KAg4I5, which to our knowledge, has been used for the sole practical study of iodine vapour detection (Rolland, 1974), variations of the iodine partial pressure induce variations of the chemical potential not only of the mobile ion Ag+, but also of the K+ ion. No well-defined relationship between the chemical potentials of I− and Ag+ exists any longer. The treatment, however, remains valid with some modifications, when the element corresponding to the non-mobile ion is replaced by a molecular group, such as SO3, SO4, CO3, etc.
12.4.4 Type III sensors
As seen in section 12.2, mobile ions are mostly limited to F−, O2 −, H+, Li+, Na+, K+, Ag+, Tl+, which considerably restricts the possibilities of sensing chemical species with type I or type II sensors. In type III sensors, the addition of one or several auxiliary phases between the target gas and the solid electrolyte on the sensing electrode side enables us to circumvent the previous restrictions. Yamazoe and his co-workers have further subdivided the classification of Weppner for these type III sensors into a, b, c …, according to the relationship between the auxiliary phase with the target gas and the solid electrolyte (Miura et al., 1993a).
Type IIIa sensors
In this configuration, the auxiliary phase contains both the mobile ion of the solid electrolyte and element(s) of the target gas. Various devices associating sodium ion conductors such as NaSICON or β-alumina with auxiliary phases such as sodium carbonate, sodium sulfate, sodium nitrate or sodium nitrite have successfully been tried for CO2, SO2, NO2 and NO sensing respectively. Figure 12.6 represents such a device with a tubular shape, for NO or NO2 sensing. If the oxygen partial pressure is the same on both the reference and sensing sides, the e.m.f. is again found to follow Nernst’s Law, with the partial pressure of nitrogen oxide independent of the oxygen partial pressure (Miura et al., 1993b; Yao et al., 1992a). NOx concentrations down to the ppm have been measured with such devices.
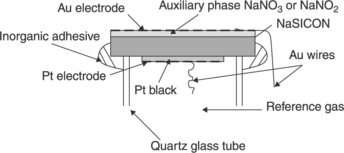
12.6 Structure of a tubular NOx-type IIIa sensor, according to Yao et al. (1992a).
Carbon dioxide sensors
CO2 sensors are the potentiometric sensors most studied after the oxygen sensors. The most obvious applications are ventilation control in houses, residences, schools, cinemas, tunnels, etc. and leak detection around the future sites of carbon dioxide storage. Review papers on these sensors were reported by various authors recently (Baliteau, 2005; Möbius, 2004; Pasierb and Rekas, 2009). NaSICON is the more used electrolyte.
In addition to good characteristics for detecting carbon dioxide, commercial development of a carbon dioxide sensor requires a low manufacturing cost, small overall dimensions and simplicity of use. If a device similar to that in Fig. 12.6, with a carbonate as an auxiliary phase, can attract interest at the research level for detecting CO2, it is clear that the use of a fixed and known gaseous reference, other than the ambient air, which generally constitutes the medium to be analysed, does not prove easy to utilize.
Planar devices represent an unquestionable projection towards the objectives of simplicity, miniaturization and low cost. These objectives obviously go hand in hand with the use of an ‘open’ reference, where both reference and sensing electrodes are exposed to the same analysed atmosphere (Holzinger et al, 1996). From a thermodynamic point of view, the best solution consists in using:
• a sensing electrode in equilibrium on one hand with the carbon dioxide and the oxygen of the target gas, and on the other with the auxiliary phase containing sodium carbonate, the latter being itself in equilibrium with the Na+ ions of NaSICON;
• a metal or metallic oxide electrode with solid reference, in equilibrium with the sole oxygen of the gaseous phase (thus not with carbon dioxide) and with the Na+ ions of NaSICON.
An assembly of this type was recently reported, with the electrodes, the auxiliary phase and the solid reference screen-printed on both sides of a sintered pellet of NaSICON (Baliteau 2005; Baliteau et al., 2005). Barium carbonate is added to the sodium carbonate for better resistance to moisture of the auxiliary phase. The reference electrode consists of mixed lanthanum strontium oxide (LSM) and the solid reference of a mixture of titanium oxides Na2Ti3O7-Na2Ti6O13. The electrochemical chain is then the following one:
The agreement between the experimental results and the thermodynamic Nernstian model is excellent, in particular for the 2 Na2Ti3O7/1 Na2Ti6O13 composition corresponding to the stoichiometric coefficients of the thermodynamic equilibrium between both compounds. Unfortunately, no solid reference studied until now seems to entirely give satisfaction with the level of long-term stability of the sensor.
Implementing NaSICON thick layers deposited with the electrodes, the auxiliary phase and, if necessary, the solid reference on the same side of a substrate (of alumina, for example), brings important advantages in comparison to the sintered pellets. Screen-printing technology makes it possible moreover to easily integrate a heating resistance, necessary for the operation of the sensor, by simply depositing it either on the same side, or on the back of the substrate. Initial work on this type of entirely screen-printed sensor was completed by Chu et al. (1991). Modifications of the initial device have since been made by various authors. Figure 12.7 represents a sensor of this type, studied by the authors of the present chapter, with major modifications compared to the initial sensor of Chu et al.:
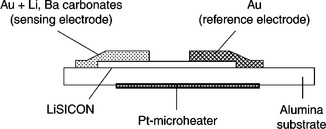
12.7 Screen-printed CO 2 sensor based on LiSICON, after Menil et al. (2005).
• the replacement of platinum by gold as the electrode metal, the former not being inert with respect to alkaline carbonates,
• the replacement of NaSiCON by the homologous Li+ conductor, LiSICON, being more resistant to moisture,
• the substitution of lithium carbonate with sodium carbonate, which is more resistant to moisture,
• the addition of alkaline-earth carbonate (Ca, Ba) to lithium carbonate, so as to minimize the influence of moisture on the sensor response,
• the realization of a composite sensing electrode by mixing carbonate and gold screen-printing inks.
The galvanic cell corresponding to Fig. 12.7 is:
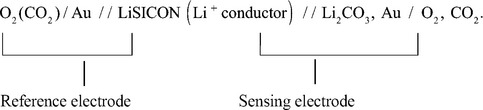
On the sensing electrode side:
Hence, by applying [12.11] to [12.21]:
On the reference electrode side, the lithium ions of LiSICON are supposed to react only with oxygen and not with carbon dioxide, which implies the presence of the Li2O phase (which is supposed to form in situ):
Because the system is in thermodynamic equilibrium, the electrochemical potential of the Li+ ions is constant throughout the whole electrolyte:
Combining [12.22], [12.24] and [12.25]:
Since the metal is the same on both the reference and sensing sides (μ(ere−) = μ(ese−))
∆G°f (Li2CO3) is the Gibbs standard energy for the formation of lithium carbonate from Li2O and CO2. Assuming that the Li2O activity in LiSICON constant leads to:
The sensor of Fig. 12.7 follows Nernst’s Law above 100 ppm CO2 in air. The sensor is barely sensitive to humidity and has a satisfying long-term stability. The critical point concerns the reality of Li2O, which is supposed to form in situ in a very small quantity, but which is known to be normally unstable.
Type IIIb
In the case of type IIIb sensors, the mobile ion in the solid electrolyte differs from the mobile ion in the auxiliary phase, but is of the same sign. For example, one can quote the CO2 sensors with NaSICON as the conductor of Na+ ions and lithium carbonate as the auxiliary phase (Yao et al., 1992b). The continuity of the electrochemical chain requires the presence of a junction phase at the interface ionic conductor/auxiliary phase, in which both mobile ions Li+ and Na+ coexist.
Type IIIc
In this type of sensor, the mobile ions of the solid electrolyte and of the auxiliary phase differ not only in kind as in the preceding case, but also in sign. A typical example is the CO2 sensor with stabilized zirconia as solid electrolyte (O2 − ions conductor) and lithium carbonate as auxiliary phase (Li+ ions conductor) (Miura et al., 1995). Again, the presence of a junction phase at the interface ionic conductor/auxiliary phase, in which the Li+ and O2 − ions coexist, proves to be necessary.
With zirconia, one can always also quote work on a NO sensor with barium nitrate Ba(NO3)2 doped with calcium carbonate, as an auxiliary phase (Kuroswawa et al., 1995). Figure 12.8 represents a studied planar configuration.
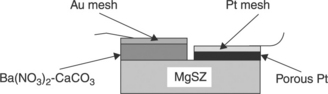
12.8 Structure of a planar NOx-type IIIc sensor, according to Kuroswawa et al. (1995).
The corresponding galvanic cell is as follows:
The experimental results and the theoretical treatment, which imply in situ formation of a second auxiliary phase BaZrO3 between zirconia and nitrate, show that the e.m.f. depends not only on the NO partial pressure, but also on the oxygen partial pressure, following the relation:
This device, which functions at 450 °C, is also sensitive to NO2.
Another example of the type IIIc sensor relates to the association of lanthanum fluoride (F− ion conductor) as a solid electrolyte with a carbonate (alkali-ion conductor) as an auxiliary phase, for carbon dioxide detection (Miura et al., 1993a).
12.5 Sensors controlled by both thermodynamics and kinetics
We therefore come back to the type I zirconia oxygen sensor. The Nernst’s law [12.18] is obtained by considering the sole equilibrium [12.13], i.e. the lattice oxygen ions at the three phase boundaries (zirconia, platinum, gas) are in equilibrium only with gaseous oxygen. This approximation is not valid under any circumstances. The literature reports many examples of a zirconia oxygen sensor that does not obey Nernst’s law in the presence of other gases, especially those resulting from engine combustion processes. Various mechanisms have been proposed to explain these interferences. We now present an experimental mechanism, based on proposals and results collected in the above-mentioned literature (Menil et al., 2000).
Schematically, the relevant reactions likely to occur at the electrode level in an engine exhaust gas can be divided into three sets:
The sense of equilibrium will depend on temperature and oxygen partial pressure. Moreover, it is important to note that reactions [12.30a–d] cannot take part directly in the electrochemical process, since no electron exchange is involved.
12.5.1 Oxygen electrode
This is the situation sought afterwards to measure the oxygen partial pressure in the exhaust gas. At relatively high temperatures, typically above 600 °C, the kinetics of reactions [12.31a–d] are low compared to that of reaction [12.29]. If, moreover, the electrode metal has a negligible catalytic activity with regard to reactions [12.30a–d], we practically come back to the ideal case of a type I sensor, which follows the Nernst’s law as a function of the sole oxygen partial pressure. Among metals exhibiting this property, silver, gold, and some alloys between platinum and bismuth may be quoted.
12.5.2 Equilibrium electrode
Conversely, but always in the same range of temperatures for which the kinetics of reactions [12.31] remain low compared to that of reaction [12.29], metals such as rhodium, palladium and sometimes platinum strongly catalyze reactions [12.30a–d]. Electrodes made of such metals are called equilibrium electrodes since they will promote the thermodynamic equilibrium of the exhaust gas.
At the electrode level, reactions [12.30a–d] induce a net production or consumption of oxygen. This quantity will either add or subtract to the oxygen already present in the exhaust gas. Nernst’s law will be ruled by this global oxygen partial pressure. The e.m.f. will no longer represent the free oxygen partial pressure in the exhaust gas, but the oxygen partial pressure that the exhaust gas would have if it were in thermodynamic equilibrium. This a priori drawback for oxygen sensing may, however, be useful for sensing other species.
12.5.3 Mixed potential electrode
At temperatures below 500–600 °C, the kinetics of reactions [12.31a–d] may become comparable to that of reaction [12.29]. Moreover, if the electrode has a weak catalytic activity regarding reactions [12.30a–d], reactions [12.31a–d] directly compete with reaction [12.29]. Thus, the chemical potential of the O2 − ions at the three phase boundaries on the sensing electrode side will not be fixed only by the partial pressure of oxygen at the electrode level, but also by that of other gases. This direct involvement of gases (such as CO, HxCy, and NO) in the electrochemical process, in the same way as oxygen, leads to the notion of mixed (Arias de Velasco et al., 1993; Baier et al., 1992; Okamoto et al., 1981) or non-Nernstian potential (Baier et al., 1993; Vogel et al., 1993).
This mechanism is especially attractive for sensing gases other than oxygen. The basic operating design of such sensors consists in using differential configurations. Two electrodes are exposed to the same gas to be detected, but only one, of the mixed potential type, exhibits a catalytic activity with regard to one of the reactions [12.31]. The other is either an oxygen or an equilibrium electrode. Over the past 20 years, various configurations based on this concept have been proposed (Menil et al., 2000; Pasierb and Rekas, 2009; Zhuiykov and Miura, 2007), even though the precise theoretical treatment of such sensors remains unknown.
Silver, gold, bismuth and lead, as well as alloys of these metals with platinum, are quoted as having a low catalytic activity with regard to reactions [12.30a–d]. Electrodes made with these metals or alloys, when operated at a low temperature, are quoted as mixed potential electrodes (Baier et al., 1993; Friese et al., 1995; Hamburg et al., 1993; Hoetzel et al., 1995; Lukacs et al., 1994; Vogel et al., 1993). Instead of using a metal or an alloy as a mixed potential electrode, it is also possible to use an inorganic oxide with metallic conductivity, such as perovskites La1–xSrxMO 3 (M = Cr, Mn, Fe, Co) (Brueser et al., 1994; Moebius et al., 1992). Asymmetry may also be introduced by coating or undercoating one electrode of a symmetrical sensor (sensor with two Pt electrodes, for example) with a catalyst. The catalyst may also simply be added by mixing it with the paste of the metallic electrode, before sintering.
CO sensing
Several papers deal with CO sensors based on zirconia. Both electrodes of a unique sensor immersed in the gas to sense (the exhaust of engines or of boilers) are dissymmetrical with regard to their catalytic activity. Working temperatures do not exceed 600 °C. In a similar device (Lalauze et al., 1993), the solid electrolyte is made of β-alumina, the mixed potential electrode of gold and the equilibrium electrode of platinum. The operating temperature is 500 °C.
Two regular platinum electrodes may also be used, but one of them is coated with a catalyst such as Pt-doped alumina (Okamoto et al., 1980) or CuO/ZnO (Li et al., 1993). Figure 12.9 represents the scheme of the latter device and the response to carbon monoxide. In both cases, the sensitivity vanishes above 500 °C. Because of the low operating temperature, the uncoated platinum electrode works as a mixed potential electrode (platinum at higher temperatures normally works as oxygen or equilibrium electrode). CO is oxidized in the catalyst, thus coating the other electrode, i.e. thermodynamic equilibrium is achieved upstream of the coated electrode, which then operates as an equilibrium electrode.

12.9 (a) Planar structure and (b) response curve of a catalytic CO sensor, according to Li et al. (1993).
NOx sensing
In order to decrease both fuel consumption and carbon dioxide production, and thus contribute to reducing the greenhouse effect, new engines with an excess of air versus stoichiometry have been developed. Such engines, operated above the A/F stochiometric value of 14.6 (see λ-sensors, section 12.4.2) are said to be ‘lean’ in fuel and the corresponding engines are called ‘lean-burn’ engines, in contrast to engines operated at stoichiometry. Diesel engines are also operated in the ‘lean-burn’ region. As mentioned above, the three-way catalyst does not operate properly when the admission mixture departs from stoichiometry. For this reason, NOx emissions, mostly eliminated with the three-way catalyst in stoichiometric engines, have again become a problem with lean-burn engines. NOx sensors have thus been mostly developed for this type of engine.
The principle for NO sensing is the same as for CO sensing: use a zirconia planar substrate with two (usually screen-printed) electrodes with a different catalytic activity. In Brueser et al. (1994), the equilibrium electrode is made of platinum and the mixed potential electrode of perovskite: La1-xSrxMO3 (M = Cr,Mn,Fe,Co). Various oxides such as WO3, Cr2O3, spinels, ZnO, NiO, etc. have been added to one platinum electrode to create the asymmetry (see Table 2 in Zhuiykov and Miura, 2007).
One of the problems in applying the previous simple design to NOx sensing in automotive exhaust is that NOx comprises both NO and NO 2, which often induce e.m.f. variations in opposite directions. Fig. 12.10 presents a more sophisticated device, taking into account the whole NOX (Ono et al., 2004).
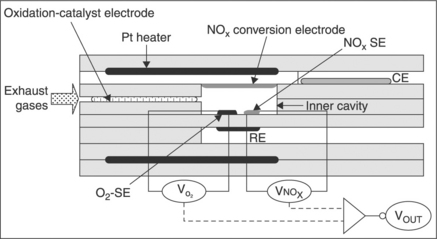
12.10 Cross-sectional view of the laminated-type total-NOx sensor attached with oxidation-catalyst. (reprinted from Ono et al., 2004, with permission from Elsevier Science)
The sensor is manufactured by pressing and sintering YSZ green sheets on which electrodes and a platinum heater are screen-printed. Thanks to the oxidation-catalyst, located upstream away from the inner cavity, and to the NOx conversion electrode located in the inner cavity, the gas composition in the inner cavity is likely to consist mostly of oxygen, nitrogen, carbon dioxide, water vapor and NO2, all reducing gases such as CO, NO and HC which have been oxidized. The oxygen sensing electrode and the reference electrode are made of platinum only. Thus, the electrode pair O2-SE + RE operates as a regular type I oxygen sensor. The NOx sensing electrode (actually NO2 sensing electrode since NO is supposed to have been completely converted), based on Cr2O3, is also sensitive to oxygen. Since both O2 and NOx sensing electrodes are mounted in differential with the same reference electrode, the NOx response yields the total NO + NO2 concentration, independently from the amount of the oxygen.
12.6 Amperometric sensors
12.6.1 Oxygen sensors
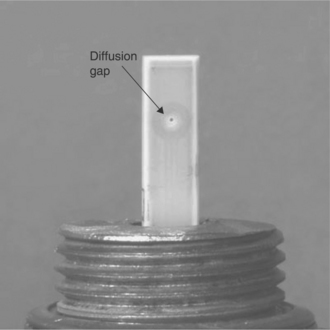
Another concept for sensing oxygen was introduced in 1982 (Dietz, 1982). The corresponding structure, schematized in Fig. 12.11(a), consists of a zirconia cell having one electrode confined in a restricted volume, which communicates with the gas to sense by a small aperture or diffusion hole.
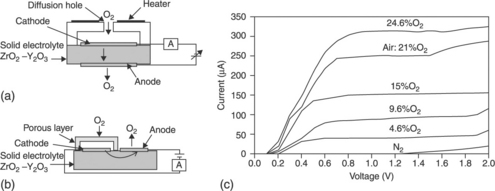
12.11 (a) Amperometric oxygen sensors with a diffusion hole and (b) with a porous layer, and (c) response curves at 600 °C of the latter, according to Ishibashi et al. (1993).
When the electrode inside the restricted volume is negatively biased, gaseous oxygen O2 is reduced to anions O2 − at this cathode, according to Eq. [12.13]. These anions are attracted inside zirconium towards the anode by the bias and re-oxidized to gaseous oxygen when they reach the anode. The current given by Faraday’s Law removes gaseous oxygen from the restricted volume through the solid electrolyte at a rate equal to I/4 F. Inside the restricted volume, the oxygen pumping will cause a decrease of the oxygen partial pressure from its initial value p(O2) to prv(O2). Simultaneously, an oxygen flux will leak through the aperture, according to Fick’s first diffusion law: D(O2)[p(O2)-prv(O2)], with D(O2) Signifying the leak conductance with respect to oxygen. At the steady state, the sum of both pumping and leaking fluxes cancels out, so that:
If the applied voltage is high enough (typically between 0.3 and 1.5 V), the oxygen partial pressure inside the restricted volume will decrease to nearly zero, because the diffusion hole limits the oxygen entry. With this limiting condition, the pumping current only depends on the oxygen diffusion rate through the hole. The corresponding so-called limiting current is given by:
An equivalent planar configuration is shown in Fig. 12.11(b). Both cathode and anode are on the same side of the solid electrolyte, and a porous layer coating the cathode acts as diffusion barrier (Ishibashi et al., 1993). Figure 12.11(c) represents the corresponding I(V) characteristics. The limiting currents, determined by the plateaus, are proportional to the oxygen concentration.
Proportional exhaust oxygen sensor
The relationship between the current and the oxygen partial pressure is linear in an amperometric sensor and not logarithmic, as in the potentiometric mode (Nernst’s Law). This is the reason why, in the field of exhaust sensors, this type of sensor is also called a proportional oxygen sensor, in order to distinguish it from the logarithmic λ-sensor.
The use of a λ-sensor is only satisfactory for stoichiometric engines, because the crossing of the stoichiometric admission ratio (A/F = 14.6 for gasoline) induces a change in the exhaust oxygen partial pressure of several orders of magnitude, resulting in a variation of the e.m.f. of several hundred mV. For ‘lean-burn’ engines (see NOx sensing, section 12.5.3) operated above stoichiometry, as well as for diesel engines, the curve in Fig. 12.2 shows that the e.m.f. variation of the λ-sensor becomes rather small, for example around A/F = 15, because the oxygen concentration does not vary much in this region. For such engines, the use of an amperometric sensor, which provides a response proportional to the oxygen concentration and no longer to the logarithm of the concentration, is highly preferable. Proportional exhaust oxygen sensors are now proposed by car equipment suppliers. Fig. 12.12 represents a planar proportional oxygen sensor, manufactured by the Robert Bosch Company. The structure again consists of pressed and sintered green sheets of YSZ.
12.6.2 Combustible sensors
Sensing combustibles (HxCy, CO, H2) with an amperometric cell a priori requires the presence of oxygen. The principle proposed by Logothetis et al. (1992) relies on the determination of the remaining oxygen in the restricted volume, after the combustion of the reducing gases on the platinum electrode at temperatures above 700 °C, according to reactions [12.30a], [12.30b]. The limiting current is shown to depend linearly on the concentration of the combustible.
At temperatures below 550 °C, another mechanism has been proposed for CO sensing (Zhang et al., 1995). It involves the direct participation of CO in the electrochemical process (reaction [12.31a]), which causes the current to decrease. The occurrence of this mechanism has been demonstrated in a paper where the oxidation of combustibles such as CO, CH4, C3H8 (Somov and Guth, 1998) at relatively low temperatures is proved to be only of electrochemical nature. The device shown in Fig. 12.13 is built with two amperometric cells mounted in series in the restricted volume, downstream of the diffusion hole. The first electrode, made of gold, works as cathode and carries out if necessary the nearly complete pumping of oxygen, without catalytic oxidation of the combustibles. The platinum electrode of the second cell works as an anode and ensures the electrocatalytic oxidation of the combustibles. Since the response of this double cells sensor to a few percent of methane is nearly the same, whether the nitrogen carrier gas contains oxygen or not (Fig. 12.14), the mechanism can only be explained on the basis of the electrochemical oxidation of methane, according to reaction [12.31b], excluding any chemical oxidation (reaction [12.30b]).
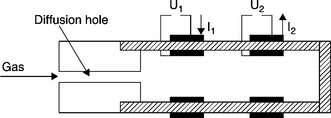
12.13 Amperometric combustible sensor with double cells in series, according to Somov and Guth (1998).
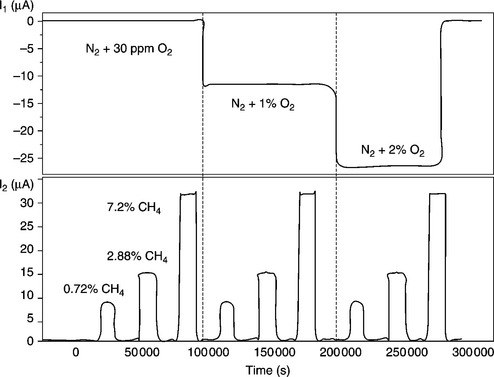
12.14 Simultaneous oxygen and methane responses of the sensor represented in Fig. 12.13, according to Somov and Guth (1998).
12.6.3 NO differential sensor
Rhodium was used for the chemical dissociation of NO by NGK Insulators, Ltd. as early as 1988 (Noda et al., 1988). A unique zirconia substrate is used with two amperometric cells mounted in differential mode (Fig. 12.15). The diffusion barrier is a porous layer. All electrodes are made of platinum, but on the side of the diffusion barrier, one cathode is covered with a rhodium layer. The plot of the differential limiting current ∆I = IRh – IPt versus the NOx partial pressure is linear with a slope of about 10 μA/5000 ppm, for a fixed oxygen partial pressure (20% in N2). The proposed mechanism is the appearance of a larger oxygen partial pressure at the rhodium-covered electrode, resulting from the NO chemical rather than electrochemical decomposition (i.e. reaction [12.30c] rather than [12.31c], from left to right).
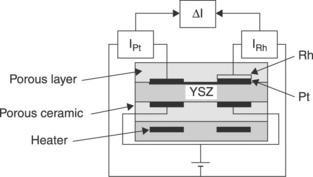
12.15 Differential potentiometric and amperometric sensor with a diffusion hole for NO sensing, according to Noda et al., 1988.
12.7 NOx sensing device, associating upstream oxygen pumping with potentiometric and amperometric operating principles
An extension of the concept presented in Fig. 12.15 has been proposed by NGK Insulators Ltd. (Kato and Nakagaki, 1996). The sensor consists of six superimposed zirconia layers (Fig. 12.16), using the same co-firing process. The operation principle is schematized in Fig. 12.17.
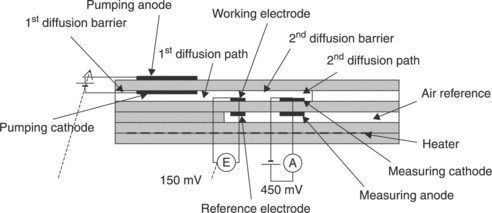
12.16 Cross view of a NO sensor with upstream oxygen pumping down to 1000 ppm, according to Kato and Nakagaki (1996).
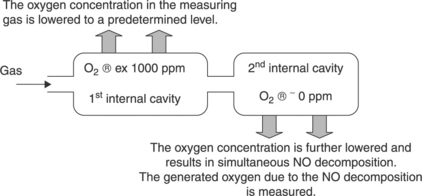
12.17 Principle of the NO sensor according to Kato and Nakagaki (1996).
The device consists of three cells:
• A first, pumping amperometric cell with its diffusion barrier and its restricted volume, from which oxygen is removed.
• A potentiometric cell, which measures the oxygen level in this first restricted volume and monitors the first pump so that the oxygen level is kept constant at 1000 ppm. This level has been chosen as a compromise for removing the maximum amount of oxygen without reducing NO.
• Downstream of the first restricted volume is a second diffusion barrier followed by a second restricted volume. The remaining 1000 ppm oxygen is totally removed by this second amperometric cell. Because of the total oxygen consumption in this second restricted volume, the NO dissociation reaction [12.30c] is completely shifted to the right, especially in presence of rhodium catalyst at the electrode. Consequently, the current of the second amperometric cell is proportional to the sum of the oxygen partial pressure issued from the first restricted volume (equal to the 1000 ppm pre-selected level) and of the oxygen partial pressure resulting from the complete NO dissociation.
The operating temperature is 700 °C. The current slope of the second amperometric cell as a function of NO concentration is about 0.6 μA/100 ppm NO.
12.8 Conclusion and possible future trends
In this chapter we tried to present the most significant examples of solid electrolyte sensors. The lambda probes constitute one of the rare success stories of gas sensors at a commercial level, with today one probe manufactured for each car using gasoline. The more recently developed proportional probes could nevertheless catch up with the former at the commercial level with the development of lean-burn and diesel engines.
Although the measurement of oxygen partial pressure certainly constitutes the leading application of solid electrolyte sensors, whether they work on a potentiometric or an amperometric principle, it was shown throughout this chapter that many possibilities exist for detecting other gases. The sensing of nitrogen oxides, carbon monoxide and unburnt hydrocarbons, in particular in exhaust gases, carbon dioxide in various mediums (house automation, storage, environment), probably constitute the most promising applications to date. Some of these sensors are currently proposed commercially (CO2, NOx). Nevertheless, the physical phenomena are not as simple as for oxygen. They generally imply the coupling of catalytic processes with thermodynamic processes. Many studies will have still to be undertaken from an empirical point of view as well as from a fundamental point of view, before being able to apprehend the problems not correctly solved to date. One of the major problems is certainly related to the lack of long-term reliability of most of these devices. Moreover, what about the influence, which is very seldom studied but undeniable, of the relative surfaces of the sensing and reference electrodes on the e.m.f. for some type III sensors with open reference, against all classically allowed theories to date (Guillet et al., 2002; Menil et al., 2005)?
As for manufacturing processes, the planar thick-film technology certainly appears as the most promising one. It is cheaper and more versatile than traditional ceramic processes, used for example to manufacture the thimble-shape λ-sensor. Manufacturing costs are also lower than for planar thin film technologies. Moreover, ageing problems linked to diffusion phenomena at high temperatures are comparatively worse with thin films.
For planar thick-film sensors requiring gas-tight electrolytes, green sheets will certainly remain favored. This is especially the case for zirconia sheets, which serve both as electrolyte and as substrate to screen-printed electrodes, auxiliary phases, catalyst, etc. When tightness to gas is not required, for example for sensors with both electrodes exposed to the same gas to sense (open reference), the electrolyte substrate may be replaced by an ion-conducting screen-printing paste deposited on an insulating substrate such as alumina.
12.9 References
Arias de Velasco, A.A., Moseley, P.T., Peat, R., Peleaz, J.G. Atmosphere-dependent potentials at oxide interfaces. Sensors and Actuators. 1993; B15(1–3):55–62.
Baier, G., Vogel, A., Schuele, V. Zirconia mixed potential sensors for control of combustion processes. VDI-Ber. 1992; 939:439–444. [(Sensoren)].
Baier, G., Schuele, V., Vogel, A. Non-nernstian zirconia sensors for combustion control. Appl Phys A. 1993; A57(1):51–56.
Baliteau, S. 2005 [Thesis, University of Grenoble].
Baliteau, S., Sauvet, A.-L., Lopez, C., Fabry, P. Characterization of a NaSICON-based CO2 sensor. J Eur Ceram Soc. 2005; 25:2965–2968.
Brueser, V., Lawrenz, U., Jacob, S., Moebius, H.H., Schoenauer, U., NOx determination with galvanic zirconia solid electrolyte cells. Diffus Defect Data, Pt B. 1994:39–40. [(System with fast ionic transport – IV), 269–272].
Chu, W.F., Leonhard, V., Erdmann, H., Ilgenstein, M. Thick-film chemical sensors. Sensors and Actuators. 1991; B4:321–324.
Dietz, H. Gas diffusion controlled solid electrolyte oxygen sensors. Solid State Ionics. 1982; 6(2):175–183.
Duecker, H., Friese, K.H., Haecker, W.D., Ceramic aspects of the Bosch Lambda-sensor SAE [Tech Pap], 750223. Robert Bosch, 1975. [GmbH, 18 pp.].
Eddy, D.S., Electrochemical apparatus for monitoring exhaust gas, 1971. [US 3616274, 26 October 1971, General Motors Corp, 6 pp.].
Engh, G.T., Wallman, S., Development of the Volvo Lambda-sond system SAE [Tech Pap], 770295, 1977:16. [AB Volvo].
Fleming, W.J., Howarth, D.S., Eddy, D.S., Sensor for on-vehicle detection of engine exhaust gas composition SAE [Tech Pap], 730575. General Motors Corp. 1973:16.
Friese, K.H., Geier, H., Pollner, R., Schallert, H., Electrochemical sensor for determination of oxygen in exhaust gases. Robert Bosch, GmbH, 1973.:12 Ger Offen DE, 2211585, 13 September
Friese, K.H., Wiedenmann, H.M., Stanglmeier, F., Electrochemical sensor with non catalytic measuring electrode for determining the oxygen concentration in rich exhaust gases, 1995:6 German patent DE, 4408361 A1 950928
Guillet, N., Lalauze, R., Viricelle, J.-P., Pijolat, C. The influence of the electrode size on the electrical response of a potentiometric gas sensor to the action of oxygen. IEEE Sensors J. 2002; 2:349–353.
Hamburg, D.R., Logothetis, E.M., Visser, J.H., Soltis, R.E., Oxygen sensor for exhaust gases, 1993:40 PCT Int Appl, WO 9303357 A1
Hoetzel, G., Neumann, H., Riegel, J., Stanglmeier, F., Exhaust gas sensors with catalytic electrodes, 1995:8 German patent DE, 4408504 A1 950921
Holzinger, M., Maier, J., Sitte, W. Fast CO2-selective potentiometric sensor with open reference electrode. Solid State Ionics. 1996; 86–88:1055–1062.
Ishibashi, K., Kashima, T., Asada, A. Planar type of limiting current oxygen sensor. Sensors and Actuators. 1993; B13(1–3):41–44.
Kato, N., Nakagaki, K., Thick-film ZrO2 sensor, 1996. SAE, 960334
Kleitz, M., Siebert, E., Fabry, P., Fouletier, J., Solid-state electrochemical sensors Vol. 2Gopel W., Hesse J., Zemel J.N., eds. Sensors: A Comprehensive Survey. Chemical and Biochemical Sensors. V.C.H. Weinheim:, 1991:341. [chapter 8].
Kumar, R.V., Fray, D.J. Development of solid-state hydrogen sensors. Sensors and Actuators. 1988; 15:185–191.
Kuroswawa, H., Yan, Y., Miura, N., Yamazoe, N. Stabilized zirconia-based NOx sensor operative at high temperature. Solid State Ionics. 1995; 79:338–343.
Lalauze, R., Visconte, E., Montanaro, L., Pijolat, C. A new type of mixed potential sensor using a thick-film of β-alumina. Sensors and Actuators. 1993; B13(1–3):241–243.
Li, N., Tan, T.C., Zeng, H.C. High temperature carbon monoxide potentiometric sensor. J Electrochem Soc. 1993; 140(4):1068–1073.
Logothetis, E.M., Visser, J.H., Soltis, R.E., Rimai, L. Chemical and physical sensors based on oxygen pumping with solid state electrochemical cells. Sensors and Actuators. 1992; B9(3):183–189.
Lukacs, Z., Sinz, M., Staikov, G., Lorenz, W.J., Baier, G., et al. Electrochemical investigations of a carbon monoxide – oxygen sensor. Solid State Ionics. 1994; 68:93–98.
Lundsgaard, J.S., Malling, J., Birchall, M.L.S. Solid State Ionics. 1982; 7:53–56.
Menil, F., Chemical sensors and electronic components: a common approach (in French). F. Menil. Microcapteurs de gaz, Lavoisier, 2008. [Chapter 2].
Menil, F., Coillard, V., Lucat, C. Critical review of nitrogen monoxyde sensors for exhaust gases of lean burn engines. Sensors and Actuators. 2000; B67:1–23.
Menil, F., Ould, Daddah B., Tardy, P., Debéda, H., Lucat, C. Planar LiSICON-based potentiometric CO2 sensors: influence of the working and reference electrodes’ relative size on the sensing properties. Sensors and Actuators. 2005; B107:695–707.
Miura, N., Yao, S., Sato, M., Shimizu, Y., Kuwata, S., et al. Carbon dioxide sensor using combination of fluoride ion conductor and metal carbonate. Chem Lett. 1993; 1993:1973–1976.
Miura, N., Yao, S., Shimizu, Y., Yamazoe, N. Development of high-performance solid-electrolyte sensors for NO and NO2. Sensors and Actuators. 1993; B13(1–3):387–390.
Miura, N., Yan, Y., Nonaka, S., Yamazoe, N. Sensing propreties and mechanism of a planar carbon dioxide sensor using magnesia-stabilized zirconia and lithium carbonate auxiliary phase. J Mater Chem. 1995; 5(9):1391–1394.
Möbius, H.-H. Galvanic solid electrolyte cells for the measurement of CO2 concentrations. J Solid Electrochem. 2004; 8:94–109.
Moebius, H., Sandow, H., Hartung, R., Jakobs, S., Guth, U., et al. Development of new sensor systems with galvanic high-temperature solid-state electrolyte cells. DECHEMA Monogr. 1992; 126:329–344. [(Elektrochem Sens: Neues Forsch Anwend)].
Noda, M., Kato, N., Kurachi, H. Electrochemical nitrogen oxide (NOx) sensor. Eur Pat Appl EP. 1988; 257842(A2):17.
Ogasawara, T., Kurachi, H. Multi-layered zirconia oxygen sensor with modified rhodium catalyst electrode. SAE. 1988; 880557:89–95.
Okamoto, H., Obayashi, H., Kudo, T. Carbon monoxide gas sensor made of stabilized zirconia. Solid State Ionics. 1980; 1:319–326.
Okamoto, H., Obayashi, H., Kudo, T. Non-ideal emf behavior of zirconia oxygen sensors. Solid State Ionics. 1981; 3–4:453–456.
Ono, T., Hasei, M., Kunimoto, A., Miura, N. Improving of sensing performance of zirconia-based total NOx sensor by attachment of oxidation-catalyst electrode. Solid State Ionics. 2004; 175:503–506.
Pasierb, P., Rekas, M. Solid-state potentiometric gas sensors – current status and future trends. J Solid State Electrochem. 2009; 13:3–25.
Peters, H., Mobius, H.H. Ger (East) Pat. 1961; 21673.
Riegel, J., Neumann, H., Wiedenmann, H.M. Exhaust gas sensors for automotive emission control. Solid State Ionics. 2002; 152–153:783–800.
Rolland, P. Thesis. University of Paris; 1974.
Schoonman, J., Franceschetti, D.R., Hannken, J.W. Electrochemical determination of local hydrogen concentration in a metal. Ber Bunsenges Phys Chim. 1982; 86:701–703.
Schoonman, J., de Roo, J.L., de Kreuk, C.W., Mackor, A., Electrochemical hydrogen sensor. J.L. Aucouturier, et al. Proceedings of International Meeting on Chemical Sensors, Bordeaux. 1986:319–322.
Somov, S.I., Guth, U. A parallel analysis of oxygen and combustibles in solid electrolyte amperometric cells. Sensors and Actuators. 1998; B47:131–138.
Velasco, G., Schnell, J.P., Croset, M. Thin solid state electrochemical gas sensors. Sensors and Actuators. 1982; 2:371–384.
Vogel, A., Baier, G., Schuele, V. Non-Nernstian potentiometric sensors: screening of potential working electrode materials. Sensors and Actuators. 1993; B15(1–3):147–150.
Wagner, C.. Proceedings of International Committee Electochemical Thermodynamics and Kinetics (CITCE). Butterworth Scientific Publisher, London, 1957.:361–377.
Weissbart, J., Ruka, R. Oxygen gage. Rev Sci Instrum. 1961; 32:593–595.
Weppner, W. Solid-state electrochemical gas sensor. Sensors and Actuators. 1987; B12(2):107–119.
Yao, S., Shimizu, Y., Miura, N., Yamazoe, N. Use of sodium nitrite auxiliary electrode for solid electrolyte sensor to detect nitrogen oxides. Chem Lett. 1992; 4:587–590.
Yao, S., Shimizu, Y., Miura, N., Yamazoe, N. Solid electrolyte carbon dioxide sensor using sodium ion conductor and Li2CO3-BaCO3 electrode. Jpn J Appl Phys. 1992; 31:L197–L199.
Zhang, Yi Can, Narita, H., Mizusaki, J., Tagawa, H. Detection of carbon monoxide by using zirconia oxygen sensor. Solid State Ionics. 1995; 79:344–348.
Zhuiykov, S., Miura, N. Development of zirconia-based potentiometric NOx sensors for automotive and energy industries in the early 21st century: what are the prospects for sensors? Sensors and Actuators. 2007; B121:639.