Printed thick-film biosensors
Abstract:
The present review focuses on the thick-film electrochemical biosensors fabricated using screen-printing technology over the Past four years. Biosensors are composed of a biological recognition element acting as a receptor and a transducer, which converts the resulting biological activity into a measurable signal. A number of different transducers have been employed, but one of the most popular and successful is that manufactured by thick-film technologies such as screen-printing. These offer a number of advantages, such as economy, bio-compatibility and the possibility of miniaturisation, therefore allowing for the production of disposable one-shot devices. The review is divided by application area and specific emphasis is placed on sensor fabrication methods, operating details and performance characteristics for selected applications.
13.1 Introduction
13.1.1 Thick-film biosensors
Screen-printing is a proven technology used for the manufacture of devices in a wide range of industries, such as electronics and solar cells, through to the graphic arts. The technique of screen-printing is capable of producing electrodes in the range of a few μm to 100 μm in thickness; the sensors can hence be defined as thick-film electrodes. Various geometries can be easily made by simple alteration of the screen design, and other features, such as reference, counter electrodes and dielectric layers can be readily added in further printing steps. Carbon is a low-cost material and when coupled with screen-printing manufacturing can produce sensors very economically in large numbers, allowing these to be viewed as disposable, ‘one shot’ devices. This offers the possibility of decentralised testing, particularly in medical applications, exemplified by their application in the blood glucose monitoring market. Screen-printed electrodes (SPEs) offer comparable electrochemical behaviour to more expensive electrode materials, such glassy carbon, but can be used once and discarded, hence removing the need for regeneration or pre-treatment between each measurement. This also allows for their use by relatively untrained personnel, as no knowledge of the more in-depth aspects of electrochemistry and preparation is needed.
Reagents such as enzymes, antibodies, mediators, catalysts and chelating agents can be readily immobilised to the surface of the carbon or mixed with the ink itself. Because of this, and the economic manner in which SPEs can be manufactured, they offer an attractive approach for the construction of many different types of biosensors. These advantages have led to a large, active interest, both in research into and for the commercial application of these devices. A number of reviews have recently reported on the application of these sensors (Domínguez-Renedo et al., 2007; Hart et al., 2004, 2007; Kalcher et al., 2009; Mahbubur Rahman et al., 2010; Pohanka and Skládal, 2008; Privett et al., 2010; Reyes Plata et al., 2010).
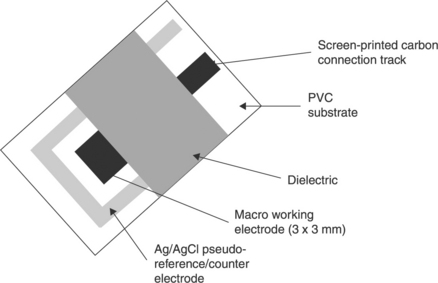
Figure 13.1 shows a representation of a screen-printed sensor, illustrating the general components that can be readily laid down by this technique. As can be seen, all the components required for the biosensor can be readily realised by screen-printing. Arrays of sensors can also be made, such as the ‘comb’ or array of screen-printed carbon electrodes (SPCEs), which have recently been developed for the determination of a number of organophosphate pesticides (OPs) at nM concentrations by Crew et al. (2011).
The following section gives a short overview of the underlying electrochemistry of these sensors and a brief history of the development of these devices. This is then followed by a review of the current applications of thick-film biosensors reported in the past four years. Emphasis is placed on sensor fabrication methods, operating details and performance characteristics for selected applications.
13.1.2 Definition of a biosensor
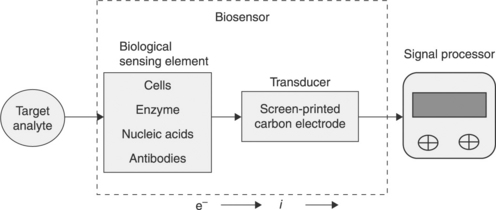
Biosensors can be defined in a number of ways (Ronkainen et al., 2010). Figure 13.2 shows a generalised scheme for the components of a thick-film or screen-printed electrode.
For the purposes of this review, biosensors have been defined using a version of the definition given by Théavenot et al. (1999). Hence, they are analytical devices that incorporate some form of biological material, such as tissue, microorganisms, organelles, cell receptors, enzymes, antibodies, nucleic acids, etc. These biological components are intimately associated with or integrated within a physicochemical transducer or transducing microsystem. Such devices usually produce a digital electronic signal that is proportional to the concentration of a specific analyte or group of analytes.
13.1.3 Practical experimental considerations
Reference electrodes
More detailed explanations regarding the underlying chemistry of commonly used reference electrodes (Bott, 1995; Compton and Banks, 2011) and their screen-printed counterparts have been already given (Shinwari et al., 2010). Reference electrodes are necessary to provide a stable, drift-free, accurate value of potential as a reference voltage. This can be used to monitor accurately the applied potential ‘sensed’ at the working electrode, so that, if necessary, adjustments can be made. Standard electrode potentials are written as reductions with respect to the standard hydrogen electrode (HE). The HE consists of a platinised platinum electrode immersed in a solution of HCl over which hydrogen gas bubbles at one atmosphere. It is extremely accurate, but is not convenient to use on a day-to-day basis. Alternative reference electrodes have been based upon metals that are in intimate contact with sparingly soluble salt of the corresponding cation, such as the Ag/AgCl (Eq. [13.1]) and calomel (Hg/Hg2Cl2) (Eq. [13.2]) reference electrodes.
It is important that the potential of the reference electrode be stable and not susceptible to change. To ensure this, the reference electrode is generally isolated from direct contact with the sample solution via a salt bridge.
Commonly, thick-film sensors employ reference electrodes screen-printed from various proprietary Ag/AgCl ink formulations. The choice of these materials is governed by their low solubility and toxicity. Strictly speaking, these thick-film reference electrodes should be referred to as quasi- or pseudo-reference electrodes, as they are not isolated from the sample and can be influenced by changes in chloride ion concentration or other sample components. However, generally this is not an issue, as a large proportion of thick-film sensors are designed as single-shot devices for samples with well-defined parameters.
The counter or auxiliary electrode
The counter or auxiliary electrode provides a means of applying input potential to the working electrode. The purpose of these electrodes is to complete the circuit and allow charge to flow. Consequently, they need to be manufactured from inert material such as carbon or platinum, and their size should be much larger than the working electrode to ensure no current limitations arise. The term ‘counter electrode’ is best used in connection with two-electrode experiments and auxiliary electrode is reversed for three-electrode experiments (Kissinger and Heineman, 1984).
Working electrodes
The working electrode can be defined as the electrode where the electrochemical reactions of interest occur. Ideally it should have a good signal-to-noise ratio, reproducible response, no interfering reactions over the potential of interest, high electrical stability, low cost, availability, low toxicity and long-term stability. Due to these factors, the working electrode has been made from noble metals such as Au, Ag, Pt, or Hg and C in the form of carbon paste or graphite.
Ink formulations for the production of these electrodes are generally commercially sensitive and hence not generally available for discussion or dissemination. However, these generally contain a number of components such as pH modifiers, humectants, resins, antifoaming agents, wetting agents, thickeners and preservatives (Wring et al., 1992). Nevertheless, generally, conductive inks used for screen-printing are comprised of three basic constituents (Gonzalez-Macia et al., 2010):
• A conductor. Powdered gold, platinum, silver or carbon.
• A binding agent. Glass powder, resins or cellulose acetate
• A solvent. Such as terpineol, 2-ethoxyethanol, cyclohexanone, ethylene glycol.
The solvent is necessary to provide suitable viscosity during the printing process and volatility for thermal curing. The binding agent is required to give sufficient mechanical strength and adhesion on the substrate. Once printed, the deposited ink is then cured by either UV irradiation or heating. Other sensor components, such as a dielectric insulator, can be printed using the same approach. The formulation of the ink and its subsequent printing can have a great effect on the performance and electrochemical properties of the biosensor (Kadara et al., 2009).
13.1.4 Electrochemical techniques
Common methods employed in the development of thick-film biosensors and their applications are amperometry, chronoamperometry, potentiometry, conductimetry and impedance spectroscopy. More recently, techniques such as stripping voltammetry have also been employed in conjugation with nanoparticle-based immunoassays, the application of which is described later. More detailed discussions dealing with the underlying mechanisms and parameters that can be probed and calculated by these techniques have been given (Adams, 1969; Compton and Banks, 2011; Galus, 1976; Grieshaber et al., 2008; Kissinger and Heineman, 1983, 1984; Mabbott, 1983; Pletcher, 1991; J. Wang, 2006). The following scheme gives a summary of the basic relationships:
• Chronoamperometry: current with voltage change.
• Amperometry: current at constant voltage.
• Potentiometry: potential difference at zero current.
• Conductimetry: inverse of solution resistance
Cyclic voltammetry
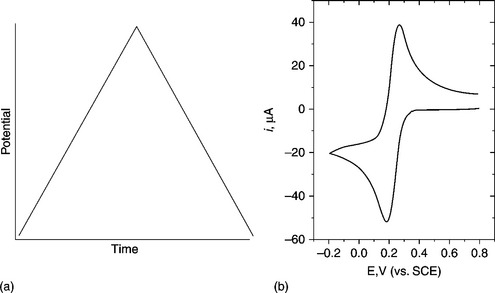
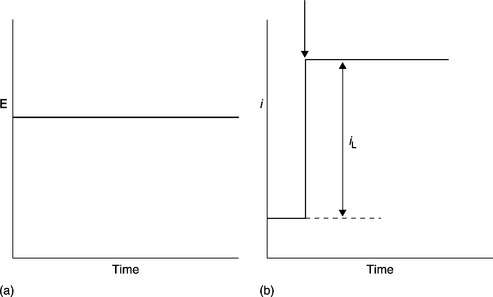
A very commonly employed electrochemical technique, however, generally employed for the development of biosensors, rather than in the actual application and determination of target analytes. The technique consists of cycling the applied potential from an initial starting potential to a switching potential where the potential is then reversed back to the starting potential using the triangular-shaped waveform shown in Fig. 13.3(a). This can be applied once or several times, depending on the information being sought. This is carried out as a function of time (change in applied potential/second) at the working electrode in a quiescent solution and then measuring the resulting current. This represents a very powerful technique for probing the redox behaviour of species present both in solution and sorbed to the electrode. A simple cyclic voltammogram representative of the resulting output for a quasi-reversible diffusion-controlled species is shown in Fig. 13.3(b). Cyclic voltammetry is a complicated time-dependent function of a large number of chemical and physical parameters. More detailed discussion and explanations of the underlying mechanisms and parameters that can be probed and calculated by this technique have been given (Compton and Banks, 2011; Galus, 1976; Kissinger and Heineman, 1983, 1984; Mabbott, 1983; Pletcher, 1991; J. Wang, 2006).
For a diffusion-controlled system, the peak currents (ip) can be explained by the Randles–Sevcik equation:
at 25 °C, where ip is the peak current, n is the number of electrons transferred, A is the electrode area (cm2), D is the diffusion coefficient of the species (cm2/s), v is the scan rate (V/s) and C* is the concentration of the species in the bulk solution (mol/cm3). For a diffusion-controlled system, ip will be directly proportional to the concentration of the bulk solution (C*) and to the square root of scan rate (v1/2).
Chronoamperometry
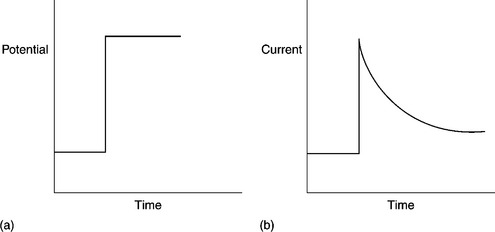
Due to its relative simplicity, chronoamperometry is one of the most commonly employed techniques utilised with blood glucose sensors. The waveform used in chronoamperometry is shown in Fig. 13.4(a). As with cyclic voltammetry, this technique is undertaken with a stationary electrode in quiescent solution, initially at open circuit or at a potential, where the target analyte does not undergo any electrochemical reactions. The potential is then stepped to a point beyond that required for the target analyte to be electrochemically oxidised or reduced. Here its surface concentration becomes effectively zero. The resulting current–time dependence is monitored and a typical profile is shown in Fig. 13.4(b).
The shape of this can be explained by the changes in concentration distance profiles of the target analyte in a similar manner as described for cyclic voltammetry. The characteristic shape of the resulting chronoamperogram can be represented by the Cottrell equation (Eq. [13.4]):
where n is the moles of electrons involved in the reaction, F is the Faraday constant, A is the area of the electrode (cm2), C* the concentration of the analyte in the bulk solution (mol./dm3), D is the diffusion coefficient (cm2/s) and t is time (s). Consequently, i will be proportional to t− 1/2.
Amperometry
This is one of the simpler electrochemical approaches requiring only the application of a fixed potential (Fig. 13.5(a)) and the reading of the subsequent current generated as a function of time (Fig. 13.5(b)). The general approach for this technique uses a constant potential applied in a solution under forced convection. The initial short-lived charging current is allowed to die before measurement is undertaken.
The resulting current (iL) can be described by the following equation [13.5]:
where FAD and C* are described and δ is the Nernst diffusion layer thickness.
Stripping voltammetry
Stripping voltammetry (Compton and Banks, 2011; J. Wang, 1985, 2006; Vydra et al., 1976) has been demonstrated to be a very sensitive and rapid electroanalytical technique. It has been successfully applied to the detection and quantitative determination of about 30 elements for some time (Vydra et al., 1976). Flameless atomic adsorption and inductively coupled spectroscopy are the only two common techniques that match it in terms of sensitivity. Stripping analysis is essentially a two-step technique. The first, deposition step or plating step, involves the electrolytic deposition of small portions of the target analyte ions in solution onto the electrode surface. This deposition step results in an effective pre-concentration step without the inherent problems of phase exchange or physical transfer of the sample. This is followed by the stripping step (measurement step), which involves the dissolution (stripping) of the deposit from the electrode. The deposited analyte is stripped from the electrode by a linear potential scan back into solution. The resulting current is proportional to the concentration of analyte deposited on the electrode and hence to the concentration in the original bulk sample solution. More detailed reviews on the application of SPEs have been given previously (Arduini et al., 2010a, 2010b; Honeychurch and Hart, 2003; Kokkinos and Economou, 2008; Stozhko et al., 2008; Svancara et al., 2010).
Potentiometry
Potentiometric sensors measure the accumulation of a charge potential at the working electrode compared to the reference electrode in an electrochemical cell with zero current flowing between them. The most commonly encountered example of this is the glass pH electrode and the ion-selective electrodes commonly utilised for the determination of ions such as Ca2 +, Cl−, K+ and NH4+. The relationship between the concentration and the potential is given from a variation of the Nernst equation (Eq. [13.6]):
where E0 is the standard potential for a1 = 1 M, R the universal gas constant, T the absolute temperature in degrees Kelvin, n the total number of charges on the ion, F the Faraday constant and a1 is analyte activity. More detailed discussions have previously been given (Evans, 1987; J. Wang, 2006). Biosensors can be readily made using this approach by modifying an ion-selective electrode with a suitable biological component, such as an enzyme, which produces a species that can then be detected at the ion-selective electrode potentiometrically (Mascini and Guilbault, 1986).
Conductometry
These generally consist of two noble-metal electrodes immersed in the sample solution where the conductance is measured. Some enzymatic reactions convert neutral substrates into charged products, which can be measured through the change arising in the conductance. Although this approach is relatively non-specific, the technique has been shown to be highly sensitive and rapid when used with whole cell-based biosensors (Lei et al., 2006).
Electrochemical impedance spectroscopy
Impedance spectroscopy measures the resistance and capacitance properties of a material via application of a sinusoidal AC excitation signal of c. 2–10 mV. An impedance spectrum is obtained by varying frequency over a defined range. The capacitance and resistance of the system can be then calculated by measurement of the in-phase and out-of-phase current responses. Impedance spectroscopy can be can be used to detect immunological binding events such as antibody binding occurring on the electrode surface. The technique has recently been applied to cell toxicology studies, monitoring changes in the cell motion and morphology (Z. Wang et al., 2009). Reportedly (Ronkainen et al., 2010), impedance can offer some advantages over amperometric-based biosensors, being able to directly monitor binding events that occur at the electrode overcoming issues with interference such as ascorbate and urate, which can interfere with amperometric-based biosensors, and there is no requirement for a mediator.
13.1.5 History of thick-film biosensors
Several very good reviews of the history and development of biosensor technology have already been given (D’Orazio, 2003; Newman and Turner, 2005; Vaddiraju et al., 2010; J. Wang, 2001, 2008). The first reported biosensor was made by Clark and Lyons in 1962 based on a modification of the laboratory-based oxygen electrode (Clark and Lyons, 1962). Their innovative idea was to modify working electrodes with glucose oxidase and measure the oxygen consumed by the enzyme as an indirect measurement of the glucose present in the sample. Utilising this approach, it was found possible to analyse a range of other biomedically important compounds by employing other oxidase enzymes (Chistiansen and Jakobsen, 1995; Guilbault, 1968; Mascini and Guilbault, 1986). In 1975, Yellow Springs Instrument Company put this concept on the market, making the first commercially available dedicated blood glucose sensor. However, these first-generation biosensors were found to suffer from variations in oxygen tension and stoichiometric limitations that can occur in the sample.
In the mid-1970s, non-physiological electron acceptors were introduced to overcome these issues (Schlapfer et al., 1974). Examples of such mediators include quinones and conductive organic salts, such as ferricyanide and ferrocene derivatives. A large volume of work occurred on these second-generation biosensors in the 1980s (Cass et al., 1984; Frew and Hill, 1987). This decade also saw the introduction of the first modified disposable thick-film screen-printed glucose biosensors (Hilditch and Green, 1991; Matthews et al., 1987), an important advance allowing for the decentralised testing and development of cheap single-shot devices, such as that used in the commercial ExacTech blood glucose meter (Cardosi and Turner, 1991), launched by Medisense Inc. in 1987. The introduction of this device led to a sea change in biosensors technology with the introduction of pen-sized (Cardosi and Turner, 1991) and mobile phone (Heller and Feldman, 2010) amperometric-based devices. The current operation of most commercial glucose biosensors does not differ significantly from the ExacTech meter.
A number of drawbacks have been highlighted with these second-generation biosensors. The potential toxicity of the mediator and its possible leaching from the electrode means that the possibility of in vivo usage is problematic. It is also possible that even when utilising a mediator the presence of oxygen can still result in the oxidation of the reduced enzyme, leading to errors in quantification. In the 1990s, a number of investigations into co-immobilising the enzyme and the mediator at the electrode surface were made to address some of these issues. This can be achieved by immobilising the mediator and enzyme in a conductive redox polymer on the electrode surface (Gregg and Heller, 1990; Ohara et al., 1993). This offers other advantages; surrounding the enzyme in this way helps to transport electrons from the redox centre of the enzyme to the electrode in an array of rapid electron relays, so giving faster responses and higher current densities. More recently, further advances have been obtained by the introduction of nanotechnology. Due to the similar size of these materials and the enzyme, it is possible to ‘wire’ the redox centre of the enzyme directly to the electrode (Gilardi and Fantuzzi, 2001). Reportedly, this can overcome problems associated with oxygen dependence. In these third-generation biosensors, the enzyme redox centre is bound to the electrode surface. This allows for electrons to flow to and from the electrode, directly monitoring the enzyme’s redox behaviour and its interaction with the substrate.
Commercially, the biosensor market is still dominated by glucose biosensors, accounting for approximately 85% of the world biosensors market in 2004, which was then estimated to be US$5 billion. According to one recent report, this will reach US$11.5 billion by 2012 (Yoo and Lee, 2010), and grow to an estimated US$14.42 billion by 2016 (Anon, 2010). Predictions show that security and bio-defence have exhibited the largest increase in annual revenue growth rates.
Nevertheless, market penetration can be difficult (Luong et al., 2008). Recent reviews highlight the problems and issues that commercialisation faces (Luong et al., 2008; Siontorou and Batzias, 2010). The reported cost of introducing the FreeStyle glucose biosensor to the commercial biosensor market was US$100 million, a sum beyond the means of most small companies (Heller and Feldman, 2010). At present, there are 18 companies in the USA producing 56 different glucose biosensors; however, only four major companies, Abbott, Bayer, LifeScan, and Roche, account for over 90% of the USA market (Yoo and Lee, 2010).
Future developments will properly focus on the further application of nanotechnology and techniques such as plastic electronics (Sokolov et al., 2009) and synthetic biology (Keasling, 2008). Other new markets will be identified, such as coronary heart disease, which at present represents the main cause of death throughout the world (World Health Organisation, 2008) and consequently presents an opportunity for the development of biosensors capable of its early diagnosis.
13.2 Pharmaceutical and medical applications of thick-film biosensors
13.2.1 Screen-printed glucose biosensors
Usiu and Ozkan (2007) have reviewed the application of SPEs and carbon electrodes for the determination of a number of pharmaceuticals. Arguably, the most successful both in terms of commercial successes and related research activity is the glucose biosensor (Hu, 2009; Lee, 2008; J. Wang, 2001, 2008). As can be seen from Table 13.1, the majority of these utilise glucose oxidise, EC 1.1.3.4 (GOx) with a suitable mediator for the measurement of the enzymatically generated hydrogen peroxide. GOx offers several advantages as it is a stable enzyme, allowing for the manufacture of sensors that are durable and reproducible. In the presence of oxygen, GOx catalyses via its redox centre flavin adenine dinucleotide (FAD) the formation of gluconolactone and hydrogen peroxide (Eq. [13.7]). Consequently, glucose concentrations can be determined by the consumption of oxygen or by the concentration of hydrogen peroxide produced, as described earlier. The former approach suffers from a number of problems, as the ambient level of oxygen must be controlled and needs to be constant, a situation that is not readily obtainable in real blood samples. Oxygen also exhibits a fairly high reduction potential in which other sample components may interfere, and its redox reaction is not readily reversible.
The latter approach, of monitoring the hydrogen peroxide generated, offers advantages over the measurement, allowing for simpler and more selective amperometric measurements to be utilised. The direct electrochemical determination of hydrogen peroxide requires high applied potentials, leaving the approach open to interference from a number of common blood components, such as uric acid and ascorbic acid. However, it is possible to employ some form of mediator or catalyst to lower the applied potentials required. This also has the added advantage of lowering the background currents and lessens the extent of interference of other sample components.
Screen-printed biosensors based on the cobalt phthalocyanine-mediated determination of hydrogen peroxide
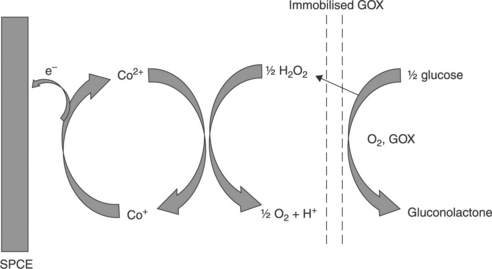
As depicted in Fig. 13.6, one such approach to determining the enzymatically generated hydrogen peroxide is the use of the electrocatalyst, cobalt phthalocyanine (CoPC), a compound that has been used for the determination of number compounds (Zagal et al., 2010).
The hydrogen peroxide generated by the enzymatic oxidation of glucose can be oxidised electrocatalytically via its interaction with the central Co2 + ion of the CoPC mediator, followed by electrochemical re-oxidation of Co+ at low overpotential giving the analytical signal. Cobalt phthalocyanine offers a number of other advantages for the development of screen-printed sensors, as it can be readily homogenised with the ink and used for printing. It is also insoluble in water and biological fluids, and hence will not leach from the printed electrode.
Recently, Pemberton et al. (2009a) have utilised such an approach with a screen-printed microband electrode for the determination of glucose in bovine serum. The use of such screen-printed working electrodes with dimensions in the μm range exhibit a number of advantages, including high mass transport, low ohmic drop and enhanced signal to noise ratios (Xie et al., 2005). Recent studies have shown the possibility of producing devices with features in the region of 100 μm × 85 μm by screen-printing coupled with a technique known as ‘print-n-shrink’ (Sollier et al., 2009). This approach utilised printing designs onto the child’s toy known as ‘Shrinky-Dinks’ (Chen et al., 2008; Grimes et al., 2008; Mandon et al., 2010). The material for this toy is formed from pre-stressed transparent polystyrene, which upon heating (3–5 min, 160 °C), shrinks isotropically in plane by approximately 63%, hence reducing the size of the printed sensor printed upon it. However, generally, the reproducible direct screen-printing of electrodes with dimensions in the μm is difficult. Nevertheless, microband electrodes could be readily fabricated by a simple procedure from the conventional-sized screen-printed glucose biosensors (Pemberton et al., 2009a). By cutting through the substrate, screen-printed track and dielectric, the thin 20 μm layer of screen-printed carbon track is exposed, hence in this case giving a 20 μm × 3 mm screen-printed carbon microband working electrode. Cyclic voltammetric investigations demonstrated sigmoidal-shaped voltammograms, indicative of microelectrode behaviour, and were found to be indicative of radial diffusion over the range of 1 to 50 mV/s; however, signals were not found to be completely independent of the scan rate, demonstrating mixed-mechanism mass transport involving both radial diffusion and planar diffusion. Further amperometric investigations in stirred and non-stirred solutions demonstrated no significant changes in maximum currents, confirming that the predominant mechanism was radial diffusion. In this study, a water-based screen-printing ink was utilised for the production of the working electrodes; this offers several advantages compared to conventional solvent-based SPCEs in terms of environmental considerations, and health and safety in production. However, most notably, utilising a biologically benign ink facilitates the direct incorporation of biological compounds, such GOx. This allows for a simpler manufacturing process, as the enzyme and ink can be printed in one step.
13.2.2 Glucose dehydrogenase-based screen-printed biosensors
Compared to GOx-based systems, glucose dehydrogenase (GDH) enzymes can offer certain advantages, such as oxygen independence, allowing for their use in low oxygen conditions such as fermentation. However, problems have been reported with the utilisation of the pyrroloquindine quinone (PQQ)-dependent form of GDH as this has a high relative activity in the presence of maltose, presenting a serious problem in the determination of blood glucose levels and consequent misdiagnoses. Again, as with H2O2, the direct determination of nicotinamide adenine dinucleotide (NADH) requires high applied potentials for its determination. However, it is possible to monitor this with the use of a suitable mediator.
Figure 13.7 illustrates that the underlying mechanism utilised for one such mediated approach is that made by Piano et al. (2010a) as part of a flow injection system for the determination of glucose in serum. The SPCE was modified with the electrochemical mediator Meldola’s Blue–Reinecke Salt (MBRS), coated with GDH (from Bacillussp.), and NAD+. A cellulose acetate layer was deposited on top of the device to act as a perm-selective membrane. The developed prototype biosensors were found to be stable for at least 240 days at a temperature of 37 °C. Using an applied potential of + 50 mV, the response was found to be linear over the range of 0.075–30 mM glucose, with the former representing the detection limit.
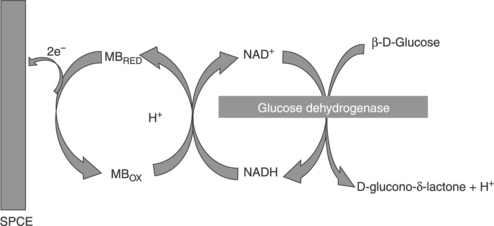
13.7 Sequence of reactions involved in the operation of the GDH-based glucose biosensor. (adapted from Piano et al., 2010a).
Screen-printing technology has focused on the production of two-dimensional sensors, planar sensors, which can be then integrated with devices produced by other production techniques, such as plastic injection moulding, if further functionality is required. Nevertheless, alternative approaches have been recently investigated, such as that reported recently by Santiago et al. (2010). They have utilised screen-printing to fabricate three-dimensional carbon micro-devices. These screen-printed microsystems consisted of a microchannel structure formed by two electrodes printed in parallel on a polyester film substrate; the sides of these act as the walls of the microchannel electrochemical cell. The working electrode was a graphite ink, and the reference/counter electrode was Ag/AgCl. The screen-printed carbon-exposed edge area was reported to be c. 0.1 cm2 with a final volume of the microchannel being in the region of 20 μl. The developed sensor was applied to the ultrasensitive electrochemical detection of alkaline phosphatase, obtaining a detection limit of below 10− 12 M for GOx-and of 10− 15 M for GDH-modified systems using a 15 minutes of incubation time. A potential pulse polypyrrole electropolymerised generated film was used to entrap either GOx or GDH to the working carbon electrode. This is reported to be superior to common methods of entrapment, as it is more reproducible and more stable.
13.2.3 Lactate
The monitoring of lactate is important for disciplines such as sport science, as it is produced in increasing amounts during physical exercise. Its major use is in the food and beverage industries, where it is used in cider and wine manufacture, and as an acidulant and for the manufacture of bread additives. It is also used as a chemical intermediate in textile finishing and in leather tanning. Recently, its determination has been utilised for monitoring toxic events in cell culture media at a screen-printed biosensor (Rawson et al., 2009).
Principally, two enzymes are used for the production of lactate biosensors; lactate oxidase (LOX) and lactate dehydrogenase (LDH). The combination of these with SPCEs has been shown to be a highly effective method and has been recently been reviewed (Hart et al., 2004, 2007; Kalcher et al., 2009; Nikolaus and Strehlitz, 2008; Privett et al., 2010; Zhou et al., 2009). A number of these devices are summarised in Table 13.2. LOX is one of the most commonly reported for biosensor construction; in the presence of oxygen it catalyses the production of pyruvate and hydrogen peroxide (EQ. [13.8]):
Table 13.2
Recent biosensors employed for the detection of lactate using either LOX or LDH as the biological agent incorporated into SPCEs
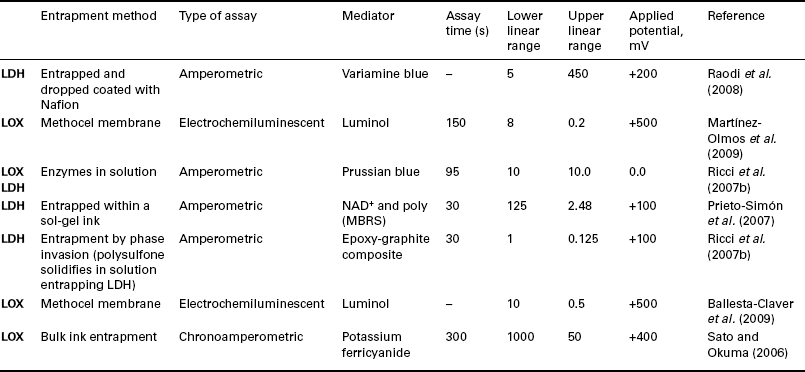
As with GDH, described in the previous section, alternatively LDH can be utilised (Eq. [13.9]). The enzyme does not require the presence of oxygen for the detection of lactate and so can be used in anaerobic conditions.
Piano et al. (2010b) have recently reported on the use of SPCEs modified with MBRS complex for the flow injection analysis of lactate with LDH. This was found to be an advantage over the previously reported MBRS-mediated electrodes, as the Reinecke salt was found to be insoluble and not leached from the SPCE, allowing for flow injection analysis to be successfully performed. The screen-printed biosensors were stable for at least 17 days when stored at 4 ° C.
13.2.4 Ethanol biosensors
Previous investigations on screen-printed alcohol biosensors have been reviewed (Hart et al., 2004; Kalcher et al., 2009). More recently, alcohol screen-print-based sensors have been developed for use in the field of forensic science investigations. Luo et al. (2008) have utilised such an approach for the forensic determination of blood alcohol utilising alcohol dehydrogenase (ADH) (Luo et al., 2008). This was fabricated by cross-linking ADH with glutaraldehyde and NAD+ on a screen-printed electrode previously modified with nafion. The electrode was modified with MBRS via repeated voltammetric scanning of a 1 mM solution in 0.1 M phosphate pH 8 buffer. The developed biosensor was able to determine serum alcohol concentrations over the range 1 to 5 mM. This could be used to determine alcohol concentrations in intoxicated drivers. However, post-mortem analysis of blood alcohol appears impossible, since the biosensor cannot differentiate between common alcohols and aldehydes found in these samples.
Mediator-free screen-printed alcohol and acetaldehyde biosensors have recently been reported for their determination in breath (Gessei et al., 2009). These ‘bio-sniffers’ were formed by screen-printing a carbon working electrode on one side of a filter paper substrate and on the reverse side, the Ag/AgCl electrode. The working electrodes were modified by either immobilizing alcohol oxidase (AOx) or aldehyde dehydrogenase (ALDH). AOx oxidises ethanol to acetaldehyde and hydrogen peroxide. The latter was detected directly by monitoring the amperometric oxidation peak as observed at + 0.9 V. In the presence of NAD+, acetaldehyde is oxidised to acetic acid, NADH and proton by ALDH. Hence by monitoring amperometric oxidation of NADH at + 0.65 V, the concentrations of acetaldehyde could be determined. Good responses to ethanol and acetaldehyde vapour were recorded over the ranges 1.0 to 100 ppm and 0.2 to 4.0 ppm, respectively, covering the ranges expected to be seen in human breath after consumption of alcoholic beverages.
13.2.5 Cholesterol
The term ‘total cholesterol’ refers to the total concentration of the two forms of cholesterol present in blood: the free form and the esterified form. To measure total cholesterol, two enzymatic reactions have been utilised. Cholesterol esterase (ChE) is employed to convert ester to cholesterol, which together with the original cholesterol can then be measured using cholesterol oxidase (ChOx), which in the presence of oxygen converts cholesterol to cholest-4-en-3-one and hydrogen peroxide. A number of previous applications of SPCEs for the determination of cholesterol have been reviewed by Hart et al. (2004, 2007).
Using a similar approach to that described for glucose and lactate above, the hydrogen peroxide generated can be monitored as an indirect way to quantify the concentration of cholesterol present in the sample. Shih et al. (2009) have used a similar approach to this, however, in utilising the catalytic reduction of hydrogen peroxide at a SPCE modified with Fe3O4, ChOx and ChE. Using a 10 μl sample volume, the screen-printed sensor was able to gain a detection limit of 19.4 mg/dl with a linear range from 100 to 400 mg/dl for total cholesterol.
Utilising electrodes fabricated from metals such as Au or Pt, it is possible to measure hydrogen peroxide at relatively low applied potentials. Shen and Lui (2007) have used an Au electrode with a self-assembled ChOx monolayer for the determination of cholesterol. The Au working was part of a screen-printed three-electrode cell, consisting of a screen-printed Ag/AgCl reference and Au counter electrode. Gold was found to be superior to Pt as a working electrode, giving better sensitivity.
Alternatives to ChOx have been also investigated at SPEs, such as a cholesterol biosensor based on cytochrome P450scc (Carrara et al., 2008). P450scc is highly specific towards cholesterol and its metabolites, catalysing the side-chain cleavage of C27 cholesterol to C21 pregnenolone in the presence of molecular oxygen and NADPH-ferri hemoprotein reductase. This approach has been investigated at a rhodium–graphite SPE modified with multi-walled carbon nanotubes (MWCNT) and cytochrome P450scc as catalytic enzyme. Recently its activity has also been shown to be enhanced at SPEs modified with gold nanoparticles (AuNP; Shumyantseva et al., 2005).
13.2.6 Miscellaneous proteins, peptides and amino acids
The amylases group of enzymes break down starches and glycogen to maltose and are widely distributed in both animal and plant tissues. The main biological function of salivary alpha-amylase (sAA) is the enzymatic digestion of carbohydrates, converting these to maltose and maltotriose. Consequently, during periods of energy expenditure and stress, elevated concentrations are seen and can be utilised as a method of evaluating and identifying such periods. Mahosenaho et al. (2010) have utilised a three-enzyme SPCE biosensor for its determination of sAA activity, but enzymatic products of this reaction are not readily determinable. However, utilising α-glucosidase, maltose, which can be hydrolysed to α-glucose, once generated, this can be covered by mutarotase into β-D-glucose, the substrate of GOx. The generated β-D-glucose is then hydrolysed to gluconic acid and hydrogen peroxide by GOx. The resulting hydrogen peroxide generated can then be measured via chronoamperometry utilising its Prussian blue-mediated reduction.
13.2.7 Nucleic acids and purines
SPCEs have recently been utilised for the monitoring of genotoxicity (Rawson et al., 2010). Investigations were made using differential pulse voltammetry (DPV) to identify the oxidation products generated from the interaction of Cr6 + with double-stranded DNA. Using such an approach, the reaction products could be readily screened in a single voltammetric scan. In a separate study, Ferancová et al. (2007) have investigated the effects of Sn2 + and As3 + at a DNA multi-walled nanotube modified SPCE using [Co(phen)3]3 + as an electrochemical DNA cleavage marker. Here, by utilising DPV, it was found possible to readily identify the DNA damage.
A number of drugs are known to interact with DNA and recently these interactions have been monitored voltammetrically at a SPCE. Ravera et al. (2007) has demonstrated that it possible to monitor covalent binding at N7 of guanine, electrostatic interactions, and intercalation resulting from interactions of antitumoral Pt-, Ru-, and Ti-based metallodrugs at DNA immobilised on screen-printed electrodes.
13.2.8 Food pathogens
Staphylococcus
The detection of micro-organisms capable of causing food poisoning is a highly important area. Escamilla-Gomez et al. (2008) have reported on the development of a biosensor for Staphylococcus aureus, one of the predominant food-borne pathogens. The amperometric immunosensor was formed by covalent immobilisation of antibodies at Au SPEs via hetero-bifunctional cross-linkers (3,3-dithiodipropionic acid di(N-succinimidyl ester), DTSP). The biosensor utilised tetrathiafulvalene as a redox mediator entrapped at the electrode surface. A limit of detection of 3.7 × 102 cells/mL was obtained with a dynamic range from 1.3 × 103 to 7.6 × 104 cells/mL.
Salmonella
Salmonella serotypes are one of the most common bacteria responsible for food-borne gastroenteritis and can be utilised as a potential micro-organism for bioterrorism. Salam and Tothill (2009) have recently reported a chronoamperometric screen-printed immunosensor capable of the rapid detection of Salmonella typhimurium. This was based on a screen-printed Au working electrode with co-printed carbon counter and Ag/AgCl pseudo-reference electrodes. Both physical and covalent immobilizations via amines were used to couple the S. typhimurium antibody to carboxymethyldextran on the surface of the gold working electrode. A direct sandwich enzyme-linked immunosorbent assay format was utilised using polyclonal anti-Salmonella antibodies conjugated to horseradish peroxidase (HRP) as the enzyme label and 3,3′,5,5′-tetramethylbenzidine dihydrochloride (TMB)/ H2O2 was utilised as the enzyme mediator/substrate the system. The determination of S. typhimurium concentrations of 5 × 103 cells/mL to 20 cells/mL was achieved by physical and covalent antibody immobilization, respectively.
Escherichia coli
Bacteriophages are small viruses that have recently been postulated as promising recognition elements for the production of bacterial biosensors. Shabani et al. (2008) have recently shown the possibility of immobilising phage onto SPCEs in a similar manner to antibodies or DNA probes. In this approach, SPCEs were modified with 1-(3-dimethylaminopropyl)ethylcarbodiimide hydrochloride (EDC) by chronoamperometry using a potential of + 2.2 V to oxidise the carbon surface. The carboxyl groups generated react with EDC to produce an ester intermediate that can react with amines, resulting in their covalent attachment at the surface. Using this approach, the amino acids present on the outer membrane of a phage can bind to the SPCE-activated carboxylic groups, resulting in the attachment of phage to the surface. This approach was used in this work to covalently attach T4 bacteriophage (wild type). The SPCEs were rinsed with deionised water and then immersed and shaken in a solution of T4 bacteriophage (108 pfu/mL in SM buffer solution, pH 7.5). After washing, the modified SPCEs were dipped in a solution of borine serum albumin solution and rinsed with SM buffer. The biosensors were then exposed to a solution containing Escherichia coli K12 (108 cfu/mL) suspension in SM buffer pH 7.5 for 20 minutes. After rinsing with buffer, the SPCEs were covered with SM buffer to perform impedance measurements, allowing for a rapid, direct and label-free means of detecting specific bacteria. A detection limit of 104 cfu/mL for 50 μl samples was obtainable.
13.2.9 Viruses
Screen-printed biosensors have been developed for the detection of a number of important viruses and recently been reviewed (Yadav et al., 2010). Mahmoud and Luong (2010) have reported the development of a screen-printed gold electrode (SPGE) surface modified with either AuNPs or thiolated single-walled carbon nanotubes/gold nanoparticles (SWCNT/AuNPs) for the determination of human immunodeficiency virus 1 (HIV-1) in sera. DPV investigations showed a linear relationship between shifts in peak potential and increasing numbers of HIV-1 viruses over the range 6.8 × 102 to 1.11 × 106 copies/mL in HIV-infected serum samples.
13.3 Environmental applications of screen-printed electrodes
As a result of the possibility for decentralised testing that the use of screen-printed biosensors allows, a great deal of research has focused on this area (Badihi-Mossberg et al., 2007; Tudorache and Bala, 2007). The following section deals with this in greater detail.
13.3.1 Phosphate
The determination of inorganic phosphate (Pi) is important both environmentally and medicinally. It is an important parameter for both environmental and potable water quality. In the presence of inorganic phosphate, oxygen and cofactors, pyruvate can be decarboxylated by pyruvate oxidase to give H2O2, acetyl phosphate and CO2. It is possible to utilise the enzymatically generated H2O2 to quantify the corresponding concentration of Pi. Gilbert et al. (2009, 2010) have utilised this approach for the determination of Pi in human urine and water samples at a CoPC-modified SPCE. Utilising the CoPC-mediated electrocatalytic oxidation of the generated peroxide, a linear response for Pi was gained over the range 2.27 × 10− 5 to 1.81 × 10− 4 M, with a corresponding limit of detection of 4.27 × 10− 6 M. The principles underlying the mechanism of this biosensor are shown in Fig. 13.8.
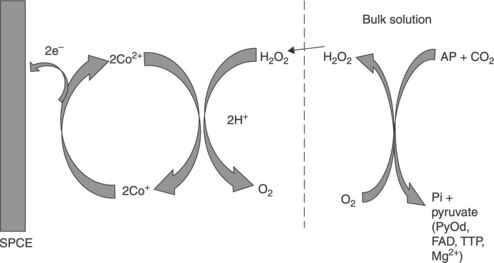
13.8 Schematic representation of the electrocatalytic oxidation of H2O2 at the cellulose acetate–CoPC–SPCE. The dashed line represents the 1.5% cellulose acetate membrane. (adapted from Gilbert et al., 2009, 2010).
13.3.2 Nitrates and nitrites
Nitrates are of both commercial and environmental importance, widely used in both explosives and fertilisers. One particular area of concern is pollution of the environment from agricultural run-off of nitrates to rivers, lakes and potentially to drinking water. Consequently, there is increasing interest in the development of sensors capable of determining nitrate levels in the environment and water-courses. Recently, Albanese et al. (2010) have utilised SPCEs modified with nitrate reductase obtained for Escherichia coli. The two mediators, Azure A or methyl viologen, were investigated. However, Azure A was found to be less effective, open to problems such as leaching into the sample solution. Using the optimised methyl viologen-based sensor as part of a flow injection system, detection limits of 0.1 mM with a linear to 10 mM nitrate were obtained.
Quan et al. (2005) have immobilised nitrate reductase derived from yeast using a polymer (poly(vinyl alcohol))-entrapment method to both a glassy carbon electrode and a SPCE. Utilising the electron-transfer mediator, methyl viologen, at an applied potential of − 0.90 V (vs. Ag/AgCl), a linear range of 15–250 μM and a detection limit of 5.5 μM was reported. The utility of the proposed sensor system was demonstrated by determining nitrate in Han River water samples, before and after treatment. Good agreement was obtained between the results gained by both ion chromatography and spectroscopic determination and the SPCE biosensor. The activity of nitrate reductase was affected by the presence of oxygen; however, this effect was readily overcome by the addition of a sulphite to the sample as an oxygen scavenger.
13.3.3 Pesticides and chemical warfare agents
A number of SPCE-based applications for the determination of OPs and chemical warfare agents have previously been reviewed (Amine et al., 2006; Andreescu and Marty, 2006; Arduini et al., 2010a; Hart et al., 2004, 2007; Kalcher et al., 2009; Miao et al., 2010; Wanekaya et al., 2008) and Table 13.3 summarises some recent developments made in this area. OPs and warfare agents act by inhibiting the enzyme acetylcholinesterase (AChE) required for normal nervous function, catalysing the hydrolysis of the neurotransmitter acetylcholine into acetate and choline. The inhibition of AChE results in the build-up of acetylcholine, which interferes with muscular responses, leading to a number of symptoms and eventually death due to respiratory failure (Miao et al., 2010).
Table 13.3
Summaries of some recent developments in the application of screen-printed biosensors for the determination of Ops
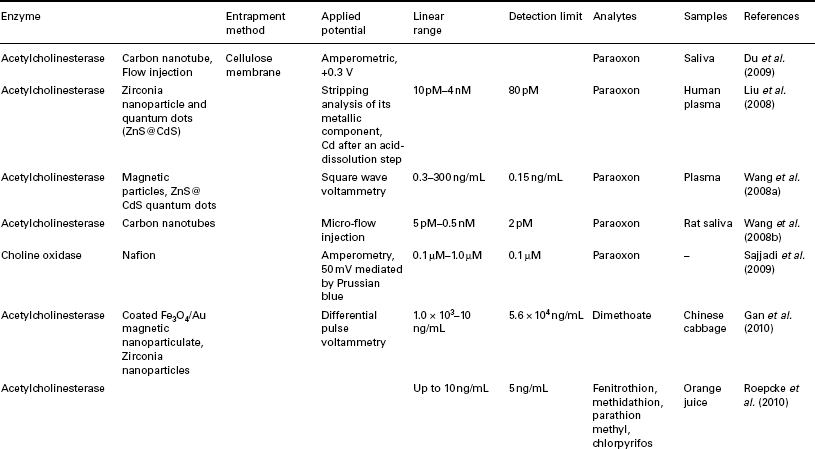

Amperometric measurement of enzyme inhibition can be made in a number of ways, such as by the measurement of thiocholine produced by the action of AChE on acetylthiocholine, or via the production of hydrogen peroxide formed by the oxidation of choline by choline oxidase (Wu et al., 2009). Similarly, p-aminophenol formed from the hydrolysis of p-aminophenyl acetate by AChE can also be readily determined. However, AChE activity can be inhibited by neurotoxins other than Ops, such as carbamates and heavy metals, and hence selectivity can suffer.
Organophosphate hydrolase (OPH) has also been utilised in such sensors (Joshi et al., 2006) exhibiting broad substrate specificity hydrolysing a number of OPs and chemical warfare agents such as sarin (O-isopropy lmethylphosphonofluoridate) and soman to p-nitrophenol, which can be detected electrochemically. Reportedly, this approach, utilising OPH, offers advantages for the biosensing of OPs as they act as the substrates for the enzyme rather than an inhibitor.
Arduini et al. (2007) have utilised a SPCE modified with an in-situ generated layer of Prussian blue formed from the addition of potassium ferricyanide in 10 mM HCl and 0.1 M ferric chloride in 10 mM HCl directly on the surface of the working electrode. Butyrylthiocholine (BChE) or AChE was then immobilised on the SPCE surface with glutaraldehyde and a nafion/BSA membrane was added across the surface. Sarin gas measurements were made by the addition of a 5 μl of a pH 7.4 phosphate buffer, the resulting depletion in enzyme activity was found to be dependent on sarin gas exposure and, for a 30 s incubation time, inhibitions of 43% and 34%, were obtained for 0.1 and 0.5 mg m− 3, respectively.
13.3.4 Whole cell photosynthetic pesticide biosensors
A contrasting approach is the application of whole cell photosynthetic-based biosensors for the determination of pesticides (Tibuzzi et al., 2008), recently reviewed by Scognamiglio et al. (2010). Several herbicides have been investigated in this manner utilising a screen-printed carbon transducer. Interestingly, screen-printing of actual algal cells has also been shown possible (Shitanda et al., 2009). The algal ink was prepared by mixing unicellular microalga Chlorella vulgaris cells, carbon nanotubes and a sodium alginate solution. The modified ink was screen-printed directly onto the surface of a SPCE. Photosynthetically generated oxygen was monitored amperometically as part of a flow injection system and exposures to atrazine (12 μM) and Duiron (DCMU) (1 μM) resulted in a 50% depletion in oxygen production.
13.3.5 Polychlorinated biphenyls
An important part of many assays is the isolation and separation of the target analyte from the sample and a wide number of procedures have been developed to achieve this goal. Recently, attention has focused on the development and application of magnetic separation techniques, which employ small magnetic particles (Šafařik et al., 1999, 2004; Saiyed et al., 2003). These consist mainly of nano-sized iron oxide particles (Fe3O4 or γ-Fe2O3), which are suspended in a carrier liquid. Frequently referred to as magnetic, the majority of the particles presently used are in fact superparamagnetic, consequently requiring an external magnetic field to be magnetic, and once this is removed they redisperse. This allows for a simple method for concentrating and isolating the particles from the sample matrix. It is possible to modify these particles with different ligands (streptavidin, Protein A, etc.) and chemically derivatised particles with specific recognition groups such as monoclonal and polyclonal antibodies. This allows for the possibility of using these modified particles to isolate analytes to the magnetic bead, which can then be easily isolated by the application of a magnetic field.
Magnetic bead-based screen-printed biosensors have been reported assays for the determination of several polychlorinated biphenyls (PCBs) (Centi et al., 2007). These are a group of highly stable and persistent industrial pollutants. They have been shown to undergo bioaccumulation and as a result are responsible for a number of environmental issues and poisonings.
Centi et al. (2006) have utilised magnetic beads modified with protein G for subsequent coating with the PCB antibodies, immunoglobalin G (IgG) anti-PCB28 or IgG anti-PCB77. A 50 mL suspension of these antibody-coated beads was then mixed with 940 mL of sample and 10 mL of alkaline phosphatase-labelled antibodies. After incubation for 20 minutes, the beads were magnetically separated down onto the SPCE surface and the supernatant removed.
Then the enzymatic substrate (α-naphthyl phosphate) in buffer was deposited on the SPCE to close the electric circuit. After five minutes, the enzymatic product was determined by DPV. Detection limits were found to be between 0.3 and 0.8 μg/l. The developed immunosensors were successfully applied for detection of PCBs in marine sediment extracts. Lin et al. (2008a) have utilised a similar approach with antibody-labelled magnetic beads. However, this approach utilised a HRP-labelled PCB (HRP–PCB) and an alkali phosphate-labelled PCB to compete with the native sample PCB. Once the competitive assay was complete, the beads were magnetically isolated and the enzyme substrate added for determination by square wave voltammetry at the SPCE.
13.3.6 Polyaromatic hydrocarbons
Polyaromatic hydrocarbons (PAHs) are known to interact with DNA producing a number of differing products, some of which result in strand cross-linking and eventual mutations. However, it is possible to utilise there phenomena as a means to determine their presence and concentration. Del Carlo et al. (2008) have used DNA electrochemical biosensors based on four different types of DNA (calf thymus ssDNA, calf thymus dsDNA, salmon testis ssDNA and salmon testis dsDNA). Using benzo(a)anthracene and phenanthrene as model compounds, they showed that variation in the guanine signal revealed the interaction of PAHs with the immobilised DNA. The salmon ssDNA modified device was found to be the most successful and was further evaluated for benzo(a)anthracene, fluorene, indeno(1,2,3-cd)pyrene, anthracene and phenanthrene in 5–40 ng/mL solutions, and for benzo(a)pyrene (5–50 ng/mL). A concentration-dependent variation of the DNA guanine oxidation peak was observed for all compounds. Using the salmon ssDNA-modified device, the PAH concentrations present in samples of contaminated mussels (Mytilus galloprovincialis) were successfully investigated.
13.3.7 Biosensors for metal ion detection
A large volume of research has focused the determination of metal ions utilising stripping voltammetry at either at SPEs modified with Bi or Hg or at plain unmodified SPEs. These are not classed as biosensors and are consequently outside the scope of this review. However, a number of extensive reviews on these sensors have been recently reported (Arduini et al., 2010a; Honeychurch and Hart, 2003; Kokkinos and Economou, 2008; Stozhko et al., 2008; Svancara et al., 2010). Nevertheless, as mentioned earlier in the pesticide section above, other toxins, such as heavy metals, can also readily inhibit enzyme activity (Andreescu and Marty, 2006), an effect that can be harnessed to develop biosensors for their determination (Amine and Mohammadi, 2007). One such recent example utilises the inhibition effect that Hg2 + ions exhibit on the enzymatic activity of urease, which can be monitored at AuNP-modified SPCEs. (Domínguez-Renedo et al., 2009). Urease was immobilised to the SPCE surface by cross-linking with bovine serum albumin and glutaraldehyde. Metallic AuNPs were found to enhance the sensitivity of the sensor. These were electrochemically deposited on the modified carbon working electrode surface, using a 0.1 mM solution of HAuCl4 in 0.5 M H2SO4. Mercury measurements were undertaken once a steady-state current was obtained after the addition of urea. Additions of Hg2 + resulted in a current decrease proportional to the amount of Hg2 + added. Using this approach, a detection limit for Hg2 + of 4.2 μM was obtained. The sensor was found capable of successfully determining human plasma samples fortified with 1.0 μM Hg2 +.
A similar approach has been utilised by Sanllorente-Méndez et al. (2010) for determining arsenite (AsO33 −) as, in this case, an AchE-modified SPCE. Arsenite concentrations were measured utilising the inhibitory effect this species has on the activity of AChE on the conversion of acetylthiocholine iodide to thiocholine iodide and acetic acid. As thiocholine is electrochemically active, the product of this reaction can be readily monitored amperometically utilising the optimised applied potential of + 0.6 V. As additions of As3 + inhibit the enzymatic reaction, a corresponding drop in the amperometric signal is gained with increasing As3 + concentration. Using such an approach, a detection limit of 1.1 × 10− 8 M with a corresponding linear range up to 1 × 10− 7 M As3 + was obtained. The developed sensor was capable of determining 1.0 μM As3 + in tap water. Further investigation on a certified As5 + water sample was possible on adding sodium thiosulphate to facilitate the reduction of the AChE inert As5 + to As3 +.
The interaction of arsenate with L-cysteine has been successfully utilised as the basis of a biosensor for the determination of As in a number of environmental water samples (Sarkar et al., 2010). L-cysteine reduces arsenate to arsenite and is in the process oxidized to L-cystine. The reaction involves electron transfer at the working electrode and thus the response can be monitored amperometically. By immobilising L-cysteine to the surface of the SPCE, it was found possible to gain a limit of detection of between 1.2 and 4.6 ng/mL. Interferences for oxidising agents such as nitrate were investigated, and no effects were found from nitrate concentrations commonly present in drinking water.
SPCE biosensors based on the well understood glucose oxidase enzyme system have also been described for the determination of a number of metal ions, including; Hg2 +, Ag+, Cu2 +, Cd2 +, Co2 + and Ni2 + (Guascito et al., 2009). As part of a flow injection system the sensor was able to obtain detection limits in the low ppm with Ag+ detection limits in the ppb region.
Shewanellasp. is known for its role in biogeochemistry, as a metal-reducing bacterium involved in the cycling of iron, manganese, trace elements and phosphates. Prasad et al. (2009) have demonstrated the use of these bacteria as the electron transfer material for electrochemical sensors in the determination of arsenite, hydrogen peroxide and nitrite. Shewanellasp. CC-GIMA-1 bacterial suspension (prepared in 0.1 M, pH 7.4 phosphate-buffered saline solution) was drop-coated on the electrode surface and allowed to settle under room temperature for 1 hour. This simple approach was found better than growing bacterial biofilm on electrode material, which was laborious and time consuming. Cyclic voltammetric investigations showed the bacteria exhibit electron exchange with the SPE surface, behaving similarly to a bacterial biofilm. It is well known that graphite electrodes, containing a carbon–oxygen functional group similar to humates, provide a natural habitat for the bacteria. The bacteria films were found to be able to sustain themselves by using carbon–oxygen functionalities of SPCE as electron acceptors for respiration. The effects of arsenite (50–500 μM) hydrogen peroxide (50 μM–2.5 mM), and nitrite (100–500 μM) were studied by cyclic voltammetry and the reduction peak current of the Shewanellasp. modified electrode was found to increase in the case of As from c. 6 μA–10 μA for an addition of 500 μM arsenite. Additions of Fe3 + were also found to exhibit similar voltammetric behaviour.
13.3.8 Immunoassay based on stripping voltammetry of metal nanoparticle labels
Nanoparticles, owing to their similarity in sizes to a number of important biomolecules such as antibodies or DNA strands, make good candidates for labels in immunoassays. This is practically true if Au or other heavy metal nanoparticles are utilised, allowing for sensitive electrochemical techniques such as stripping voltammetry to be utilised. This approach has recently been reviewed by Escosura-Muniz and Merkoci (2010). One of the first reports using such an approach was made by Dequaire et al. (2000), who used AuNPs as labels for the determination of IgG. Quantification of the AuNPs was achieved by anodic stripping voltammetry (ASV) at a screen-printed electrode, gaining a detection limit of c. 450 pg/mL (3 pM). Similarly low detection limits (20 pg/mL) were reported using Cd nanoparticles labels for the determination of prostate-specific antigen (PSA) by Liu et al. (2007, 2008) and Lin et al. (2008b), again utilising ASV as the measurement step at a SPCE, a detection limit of 0.02 ng/mL being reported. Brainina et al. (2010) have utilised Fe nanoparticles for the determination of Salmonella typhimurium (strain SL 7207) utilising a magnetic-based separation, followed by digestion of the Fe nanoparticles in a mixture of 0.36 M H2SO4 and 0.28 M HNO3. The concentration of Fe3 + liberated was quantified by the cathodic adsorptive stripping voltammetry of its catechol complex in 0.1 M sodium acetate.
Silver nanoparticles have been used in a similar manner by Chikae et al. (2010). These have been utilised as part of a sandwich-type immunoassay for human chorionic gonadotropin (hCG). The biosensor comprises a primary antibody immobilised on the surface of a SPCE and an Ag nanoparticle-labelled secondary antibody. After the immunoreaction, the concentration of Ag captured on the SPCE surface was measured by ASV. To achieve this, the immobilised Ag metal nanoparticle was first electrochemically oxidised to form Ag+ ions by applying by a potential of + 1.6 V. The concentration of these Ag+ was then determined by a differential pulse voltammeter. Utilising this approach, a detection limit of 7.2 pg/mL hCG was obtained with a linear range from 10 to 1000 pg/mL.
13.3.9 Food
The application of SPCEs and biosensors for the determination of a wide range of analytes in food has recently been reviewed (Cock et al., 2009; Ricci et al., 2007a; Tudorache and Bala, 2007; Viswanathan et al., 2009). Romanazzo et al. (2009, 2010) have used an electrochemical competitive enzyme-linked immunomagnetic assay based on the use of magnetic beads as solid support for the immunochemical chain at a SPCE as a sensing platform. Their developed sensor was able to determine the total amount of the mycotoxins, HT-2 and T-2. The magnetic beads were first coated by immobilizing a HT2-KLH conjugate toxin, and after a blocking step to avoid non-specific absorption, a monoclonal antibody was then added allowing for competition between the immobilised HT-2 and free HT-2 or T-2 present in the sample. Hence at the end of the competition step, the amount of the monoclonal antibody linked to the immobilised HT-2 at the magnetic beads is inversely proportional to the amount of toxin that was present in the sample solution. A secondary antibody labelled with alkaline phosphatase is then added to bind with the immobilised HT-2. The final measurement step was performed by dropping an aliquot of magnetic bead suspension onto the surface of a screen-printed working electrode. The magnetic beads are immobilised and concentrated by means of a magnet placed precisely under the SPE. After two minutes of incubation between magnetic beads and a substrate for alkaline phosphatase, the enzymatic product is detected by DPV, giving a voltammetric signal proportional to the original mycotoxins’ sample concentration.
Recently, the inhibition of alkaline phosphatase by caffeine has been utilised for its determination in tea, coffee and cola at the Au SPE biosensor (Akyilmaz and Turemis, 2010). By measuring the decrease in conversion of the substrate, p-nitrophenol phosphate by alkaline phosphatase, a linear relationship with caffeine concentration was found from 0.1 μM to 10 μM, with a corresponding limit of detection of 8 nM.
Acetaldehyde is commonly found in alcoholic beverages, resulting from oxidation of ethanol by ADHs and from the fermentation of pyruvate by pyruvate decarboxylase. It is usually present in wine at concentrations between 60 μM and 300 μM. Its determination is important in such beverages as it interferes with the antioxidant and antiseptic function of sulphur dioxide. Noguer et al. (2004) have utilised a MBRS-modified SPE, modified with a sol-gel matrix containing AILDH and NAD+ for the determination of acetaldehyde in wine as part of a flow injection system. Acetaldehyde can be determined in beverages using enzymatic oxidation by an NAD+-dependant ALDH according to the reaction shown in equation (Eq. [13.10]).
The NADH formed is stoichiometric with the amount of acetaldehyde present and can be readily amperometically determined via its interaction with MBRS as described in the section describing glucose determination with GDH using an applied potential of − 150 mV.
The ability to monitor the fertility of animals is an important parameter in animal husbandry. Recently, Pemberton and Hart (2007) have developed a biosensor for the determination of progesterone in bovine milk. Oestrus onset is indicated by a rapid fall in the concentration of milk progesterone to below 2–5 ng/mL. The biosensor was based on competitive immunoassay either in batch mode (chronoamperometric measurement) or in stop–flow mode in a continuous flow system (amperometric). Utilising the chronoamperometric batch mode, a detection limit of around 5 ng/mL was obtained suitable for monitoring the onset of oestrus, giving a result within 40 minutes. However, this approach requires the use of a blank measurement step to cater for any variations due to electroactive interferents or surface-adsorbed species present in the milk sample. This problem can be overcome by utilising DPV instead of chronoamperometry. The stop–flow amperometric approach has the advantage of requiring no blank subtraction, but requires 60 minutes to complete and lends itself more to off-site rather than on-site analysis.
13.3.10 Aflatoxins
Aflatoxins produced by moulds Aspergillus flavus and Aspergillus parasiticus are carcinogenic to humans. Aflatoxin has an inhibitory effect on AchE, allowing for its measurement using a choline oxidase amperometric biosensor. Ben Rejeb et al. (2009) have utilised a choline oxidase enzyme-modified SPE for the determination of aflatoxin. The residual activity of the enzyme was calculated after application of the sample, hence giving an indirect detection of the aflatoxin that may be present. The AChE activity is highly pH-dependent with best results obtained at pH 7.4. The amperometric method allowed for the detection of low aflatoxin concentrations that cannot be detected by the classical spectrophotometry assay because of the omission of the dilution step commonly used for this latter approach.
13.3.11 Antibiotics
Sulphonamide antibiotics such as sulfamethoxazole (SMX) and sulfapyridine (SP) are widely used and have been employed for the control of diseases such as American foulbrood (Bacillus larvae) and European foulbrood (Streptococcus pluton) seen in the honey-bee. Consequently, it is necessary to monitor their residues in the honey produced. Centi et al. (2010) have utilised a screen-printed eight-electrode array for their determination. Each working electrode was printed with its own screen-printed silver pseudo-reference electrode and graphite counter electrode. An eight-hole methacrylate box was fixed onto the strip, so giving an eight-cell electrochemical array. Each array was then placed in a holding block with eight magnet bars. The assay was based on a competitive scheme, in which sulphonamides (competitors) compete with a fixed concentration of 2-(4-aminobenzensulfonylamino)ethanoic acid–alkaline phosphatase labelled (SA1-(CH2)5-AP), against antibodies immobilised on magnetic beads. Once the affinity reaction occurred, the electrochemical measurement was carried out by DPV to evaluate the extent of the immunochemical reaction. It was found necessary to utilise a sample clean-up step using solid phase extraction, as one of the sulphonamides was found to react with the reducing sugars in honey.
Tsekenis et al. (2008) have utilised a labelless immunosensor for the antibiotic ciprofloxacin in milk using AC impedance. Polyaniline was electrodeposited onto the SPE to immobilise a biotinylated antibody for ciprofloxacin using classical avidin–biotin interactions. The antibody-modified electrodes were exposed to solutions of antigen in milk and interrogated using AC impedance. The faradaic component of the impedance of the electrodes was found to increase with the growing concentration of antigen. Control sensors were fabricated using antibodies specific to species not found in milk. Calibration curves were obtained by subtraction of the responses for specific and control antibody-based sensors, so eliminating the effects of non-specific adsorption of antigen. Sensors exposed to ciprofloxacin in milk gave increases in impedance, whereas ciprofloxacin in phosphate buffer led to decreases, indicating the possibility of developing sensors that can both detect and differentiate between free and chelated antigen. A linear dependence of impedance current with log10 of concentration was obtained between 0.1 and 100 ng/mL ciprofloxacin.
13.4 Conclusions
The screen-printing of electrodes based on thick-film micro-fabrication allows for large-scale mass production of reproducible low-cost electrochemical biosensors. The number of applications utilising SPEs has increased greatly over the past four years. It would now seem that thick-film SPEs are now one of the common electrodes presently used. The biocompatible nature of carbon has been shown to permit easy modification, allowing for the production of a wide range of different biosensors. Whole cells, enzymes, DNA and antibodies have all been reported and successfully employed for the determination of a wide range of samples and analytes. However, glucose is still an important analyte, with a number of reports focused on both new research and new commercial developments for thick-film glucose biosensors.
Techniques such as genetic engineering techniques, such as protein engineering (Campàs et al., 2009) and synthetic biology will become more important in the future, providing improvements in the selectivity, sensitivity and range of the biological recognition element.
Miniaturisation of electrodes and use of nanomaterials and nanotechnology are becoming increasingly more important. There are an increasing number of reports utilising the highly sensitive method of stripping voltammetry for determining the metal nanoparticle labels utilised in immunoassay.
Recently there have been more reports made using more environmentally friendly and biocompatible inks and substrates, such as paper. As SPEs are principally seen as disposable and ‘one-shot’ devices, it is possible that more interest will develop in these areas.
The majority of the biosensors identified in this review are yet to be commercialised. Several reports have highlighted problems facing the introduction of new biosensors to the market, such as those recently identified by Siontorou and Batzias (2010). Issues such as limited stability of the biosensor biological component have been cited as a concern in their commercial adoption. However, recent reports (Choi, 2005; Piano et al., 2010a) have shown that shelf lives of up to year at 4 °C are obtainable.
13.6 Acknowledgements
I would like to thank all the researchers whose work has been described in this review.
13.5 Sources of further information
Brett, C.M.A., Brett, A.M.O. Electroanalysis. Oxford Chemistry Primers, 1998; . [no. 64].
Compton, R.G., Banks, C.E. Understanding Voltammetry, 2nd ed. Imperial College Press, 2011.
Cooper J., Cass A.E.G., eds. Biosensors. A Practical Approach, 2nd ed., The Practical Approach Series, Oxford University Press, 2004.
Eggins, B.R. Chemical Sensors and Biosensors (Analytical Techniques in the Sciences). John Wiley & Sons, 2007.
Fisher, A.C. Electrode Dynamics. Oxford Chemistry Primers, 1998; . [no. 34].
Hart, J.P.Electroanalysis of Biologically Important Compounds. London: Ellis Horwood, 1990.
Hobby, A. March, available from Gwent Electronic Material Ltd. Screen Printing for the Industrial User. DEK Printing Machines Ltd, 1997. http://www.gwent.org/Gem/ index.html [A good guide to the practice and technical aspects of screen-printing.].
Kissinger, P.T., Heineman, W.R. Laboratory Techniques in Electroanalytical Chemistry, 2nd ed. Inc: Marcel Dekker, 1996.
Pletcher, D.A First Course in Electrode Processes. The Electrochemical Consultancy, 1991.
Wang, J. Analytical Electrochemistry, 3rd ed. Wiley-VCH; 2006.
13.7 References
Adams, R.N. Electrochemistry at Solid Electrodes. New York: Dekker, 1969; . [Chapter 5].
Akyilmaz, E., Turemis, M. An inhibition type alkaline phosphatase biosensor for amperometric determination of caffeine. Electrochim Acta. 2010; 55:5195–5199.
Albanese, D., Di Matteo, M., Alessio, C., Screen-printed biosensors for detection of nitrates in drinking water. S. Piercucci, G. Buzzi. 20th European Symposium on Computer Aided Chemical Engineering, Vol. 28. 2010:283–288.
Amine, A., Mohammadi, H. Electrochemical Biosensors for Heavy Metal Based on Enzyme Inhibition. In: Alegret S., Merkoçi A., eds. Electrochemical Sensor Analysis. Amsterdam, The Netherlands: Elsevier; 2007:299–310. [Vol. 49].
Amine, A., Mohammadi, H., Bourais, I., Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens Bioelectron. 2006; 21:1405–1423.
Andreescu, S., Marty, J.-L. Twenty years research in cholinesterase biosensors: from basic research to practical applications. Biomol Eng. 2006; 23:1–15.
Anon, Analytical Review of World Biosensors Market. June, Frost & Sullivan, 2010.
Arduini, F., Amine, A., Moscone, D., Palleschi, G. Biosensors based on cholinesterase inhibition for insecticides, nerve agents and aflatoxin B1 detection (review). Microchim Acta. 2010; 170:193–214.
Arduini, F., Calvo, J.Q., Amine, A., Palleschi, G., Moscone, D. Bismuth-modified electrodes for lead detection. TrAC. 2010; 29:1295–1304.
Arduini, F., Amine, A., Moscone, D., Ricci, F., Palleschi, G. Fast, sensitive and cost-effective detection of nerve agents in the gas phase using a portable instrument and an electrochemical biosensor. Anal Bioanal Chem. 2007; 388:1049–1057.
Arduini, F., Ricci, F., Tuta, C.S., Mosconea, D., Amine, A., et al. Detection of carbamic and organophosphorous pesticides in water samples using a cholinesterase biosensor based on Prussian Blue-modified screen-printed electrode. Anal Chim Acta. 2006; 580:155–162.
Badihi-Mossberg, M., Buchner, V., Rishpon, J. Electrochemical Biosensors for Pollutants in the Environment. Electroanalysis. 2007; 19:2015–2028.
Ballesta-Claver, J., Valencia Mirón, M.C., Capitán-Vallvey, L.F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst. 2009; 134:1423–1432.
Ben, Rejeb I., Arduini, F., Arvinte, A., Amine, A., Gargouri, M., et al. Development of a bio-electrochemical assay for AFB1 detection in olive oil. Biosens Bioelectron. 2009; 24:1962–1968.
Bishop, D.K., La Belle, J.T., Vossler, S.R., Patel, D.R., Cook, C.B. A Disposable Tear Glucose Biosensor – and concept testing, Part 1: design. J Diabetes Sci Technol. 2010; 4:299–306.
Bishop, D.K., La Belle, J.T., Vossler, S.R., Patel, D.R., Cook, C.B. A disposable tear glucose biosensor – Part 2: system integration and model validation. J Diabetes Sci Technol. 2010; 4:307–311.
Bordonaba, J.G., Terry, L.A. Development of a glucose biosensor for rapid assessment of strawberry quality: relationship between biosensor response and fruit composition. J Agric Food Chem. 2009; 57:8220–8226.
Bott, A.W. Practical problems in voltammetry 3: reference electrodes for voltammetry. Curr Separations. 1995; 14:64–68.
Brainina, K.Z., Kozitsina, A.N., Glazyrina, Y.A. Hybrid electrochemical/ magnetic assay for Salmonella typhimurium detection. IEEE Sens J. 2010; 10:1699–1704.
Campàs, M., Prieto-Simón, B., Marty, J.-L. A review of the use of genetically engineered enzymes in electrochemical biosensors. Semin Cell Dev Biol. 2009; 20:3–9.
Cardosi, M.F., Turner, A.P.F. Mediated electrochemistry. A practical approach to biosensing. Advances in Biosensors. 1991; 1:125–169.
Carlo, M.D., Pepe, A., Sergi, M., Mascini, M., Tarentini, A., et al. Detection of coumaphos in honey using a screening method based on an electrochemical acetylcholinesterase bioassay. Talanta. 2010; 81:76–81.
Carrara, S., Shumyantseva, V.V., Archakov, A.I., Samorí, B. Screen-printed electrodes based on carbon nanotubes and cytochrome P450scc for highly sensitive cholesterol biosensors. Biosens Bioelectron. 2008; 24:148–150.
Cass, A.E.G., Davis, G., Francis, G.D., Hill, H.A.O., Aston, W.J., et al. Ferrocene-mediated enzyme electrode for amperometric determination of glucose. Anal Chem. 1984; 56:667–671.
Centi, S., Marrazza, G., Mascini, M. Coupling of Screen-printed Electrodes and Magnetic Beads for Rapid and Sensitive Immunodetection: polychlorinated Biphenyls analysis in environmental samples. In: Alegret S., Merkoçi A., eds. Electrochemical Sensor Analysis. Amsterdam, The Netherlands: Elsevier; 2007:585–602. [Vol. 49].
Centi, S., Rozum, B., Laschi, S., Palchetti, I., Mascini, M. Disposable electrochemical magnetic beads-based immunosensors for monitoring polychlorinated biphenyl (PCBs) pollutants. Chem Anal (Warsaw). 2006; 51:963–975.
Centi, S., Stoica, A.I., Laschi, S., Mascini, M. Development of an electrochemical immunoassay based on the use of an eight-electrodes screen-printed array coupled with magnetic beads for the detection of antimicrobial sulfonamides in honey. Electroanalysis. 2010; 22:1881–1888.
Chen, C.-S., Breslauer, D.N., Luna, J.I., Grimes, A., Chin, W.-C., et al. Shrinky-dink microfluidics: 3D polystyrene chips. Lab Chip. 2008; 8:622–624.
Chikae, M., Idegami, K., Nagatani, N., Tamiya, E., Takamura, Y. Highly sensitive method for electrochemical detection of silver nanoparticles labels in metalloimmunoassay with peroxidation/reduction signal enhancement. Electrochemistry. 2010; 78:748–753.
Chistiansen, T.F., Jakobsen, K.M. The slow penetration of enzyme-based biosensors into clinical chemistry analysis. Acta Anaesthesiol Scand. 1995; 39:31–35. [Sup 104].
Choi, M.M.F. Application of a long shelf-life biosensor for the analysis of L-lactate in dairy products and serum samples. Food Chem. 2005; 92:575–581.
Clark, L.C., Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann NY Acad Sci. 1962; 102:29–45.
Cock, L.S., Arenas, A.M.Z., Aponte, A.A. Use of enzymatic biosensors as quality indices: a synopsis of present and future trends in the food industry. Chil J Agr Res. 2009; 69:270–280.
Compton, R.G., Banks, C.E. Understanding Voltammetry, 2nd ed. London: Imperial College Press, 2011.
Crew, A., Lonsdale, D., Byrd, N., Pittson, R., Hart, J.P. A screen-printed, amperometric biosensor array incorporated into a novel automated system for the simultaneous determination of organophosphate pesticides. Biosens Bioelectron. 2011; 26:2847–2851.
D’Orazio, P. Biosensors for clinical chemistry. Clin Chim Acta. 2003; 334:41–69.
Del Carlo, M., Di Marcello, M., Perugini, M., Ponzielli, V., Sergi, M. Electrochemical DNA biosensor for polycyclic aromatic hydrocarbon detection. Microchim Acta. 2008; 163:163–169.
Dequaire, M., Degrand, C., Limoges, B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal Chem. 2000; 72:5521–5528.
Domínguez-Renedo, O., Alonso-Lomillo, M.A., Arcos Martınez, M.J. Recent developments in the field of screen-printed electrodes and their related applications. Talanta. 2007; 73:202–219.
Domínguez-Renedo, O., Alonso-Lomillo, M.A., Ferreira-Goncalves, L., Arcos-Martínez, M.J. Development of urease based amperometric biosensors for the inhibitive determination of Hg (II). Talanta. 2009; 79:1306–1310.
Dounin, V., Veloso, A.J., Schulze, H., Bachmann, T.T., Kerman, K. Disposable electrochemical printed gold chips for the analysis of acetylcholinesterase inhibition. Anal Chim Acta. 2010; 669:63–67.
Du, D., Wang, J., Smith, J.N., Timchalk, C., Lin, Y. Biomonitoring of organophosphorus agent exposure by reactivation of cholinesterase enzyme based on carbon nanotube-enhanced flow-injection amperometric detection. Anal Chem. 2009; 81:9314–9320.
Dungchai, W., Chailapakul, O., Henry, C.S. Electrochemical detection for paper-based microfluidics. Anal Chem. 2009; 81:5821–5826.
Escamilla-Gomez, V., Campuzano, S., Pedrero, M., Pingarron, J.M. Electrochemical immunosensor designs for the determination of Staphylococcus aureus using 3,3-dithiodipropionic acid di(N-succinimidyl ester)-modified gold electrodes. Talanta. 2008; 77:876–881.
Escosura-Muniz, A.D.L., Merkoci, A. Electrochemical detection of proteins using nanoparticles: applications to diagnostics. Expert Opin Med Diagn. 2010; 4:21–37.
Evans, A., Potentiometry and Ion Selective ElectrodesAnalytical Chemistry by Open Learning. Chichester: John Wiley & Sons, 1987.
Ferancová, A., Adamovski, M., Gründler, P., Zima, J., Barek, J., et al. Interaction of tin(II) and arsenic(III) with DNA at the nanostructure film modified electrodes. Bioelectrochemistry. 2007; 71:33–37.
Frew, J.E., Hill, H.A.O. Electrochemical biosensors. Anal Chem. 1987; 59:933A–944A.
Galus, Z.Fundamentals of Electrochemical Analysis. NY: John Wiley & Sons, 1976.
Gan, N., Yang, X., Xie, D., Wu, Y., Wen, W. A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen-printed carbon electrode. Sensors. 2010; 10:625–638.
Gessei, T., Sato, H., Kazawa, E., Kudo, H., Saito, H., et al. Bio-sniffers for ethanol and acetaldehyde using carbon and Ag/AgCl coated electrodes. Microchim Acta. 2009; 165:179–186.
Gilardi, G., Fantuzzi, A. Manipulating redox systems: application to nanotechnology. Trends Biotechnol. 2001; 19:468–476.
Gilbert, L., Jenkins, A.T.A., Browning, S., Hart, J.P. Development of an amperometric assay for phosphate ions in urine based on a chemically modified screen-printed carbon electrode. Anal Biochem. 2009; 393:242–247.
Gilbert, L., Browning, S., Jenkins, A.T.A., Hart, J.P. Studies towards an amperometric phosphate ion biosensor for urine and water analysis. Microchim Acta. 2010; 170:331–336.
Gonzalez-Macia, L., Morrin, A., Smyth, M., Killard, A. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analyst. 2010; 135:845–867.
Gonzalo-Ruiz, J., Alonso-Lomillo, M.A., Munoz, F.J. Screen-printed biosensors for glucose determination in grape juice. Biosens Bioelectron. 2007; 22:1517–1521.
Gregg, B.A., Heller, A. Cross-linked redox gels containing glucose oxidase for amperometric biosensor applications. Anal Chem. 1990; 62:258–263.
Grieshaber, D., MacKenzie, R., Vörös, J., Reimhult, E. Electrochemical biosensors – sensor principles and architectures. Sensors. 2008; 8:1400–1458.
Grimes, A., Breslauer, D.N., Long, M., Pegan, J., Lee, L.P., et al. Shrinky-dink microfluidics: rapid generation of deep and rounded patterns. Lab Chip. 2008; 8:170–172.
Guascito, M.R., Malitesta, C., Mazzotta, E., Turco, A. Screen-printed glucose oxidase-based biosensor for inhibitive detection of heavy metal ions in a flow injection system. Sens Lett. 2009; 7:153–159.
Guilbault, G.G. Use of enzymes in analytical chemistry. Anal Chem. 1968; 40:459R–471R.
Hart, J.P., Crew, A., Crouch, E., Honeychurch, K.C., Pemberton, R.M. Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental, and industrial analyses. Anal Lett. 2004; 37:789–830.
Hart, J.P., Crew, A., Crouch, E., Honeychurch, K.C., Pemberton, R.M. Screen-printed electrochemical (bio)sensors in biomedical, environmental and industrial applications. In: Alegret S., Merkoçi A., eds. Electrochemical Sensor Analysis. Amsterdam, The Netherlands: Elsevier; 2007:497–557. [vol. 49].
Heller, A., Feldman, B. Electrochemistry in Diabetes Management. Acc Chem Res. 2010; 43:963–973.
Hildebrandt, A., Ribas, J., Bragos, R., Marty, J.-L., Tresanchez, M., et al. Development of a portable biosensor for screening neurotoxic agents in water samples. Talanta. 2008; 75:1208–1213.
Hilditch, P.M., Green, M. Disposable electrochemical biosensors. Analyst. 1991; 116:1217–1220.
Honeychurch, K.C., Hart, J.P. Screen-printed electrochemical sensors for monitoring metal pollutants. TrAC. 2003; 22:456–469.
Hu, J. The evolution of commercialized glucose sensors in China. Biosens Bioelectron. 2009; 24:1083–1089.
Iwamoto, M., Tokonami, S., Shiigi, H., Nagaoka, T. Activity enhancement of a screen-printed carbon electrode by modification with gold nanoparticles for glucose determination. Res Chem Intermed. 2009; 35:919–930.
Joshi, K.A., Prouza, M., Kum, M., Wang, J., Tang, J., et al. V-type nerve agent detection using a carbon nanotube-based amperometric enzyme electrode. Anal Chem. 2006; 78:331–336.
Kadara, R.O., Jenkinson, N., Banks, C.E. Characterisation of commercially available electrochemical sensing platforms. Sensor Actuat B-Chem. 2009; 138:556–562.
Kalcher, K., Svancara, I., Buzuk, M., Vytras, K., Walcarius, A. Electrochemical sensors and biosensors based on heterogeneous carbon materials. Monatsh Chem. 2009; 140:861–889.
Keasling, J.D. Synthetic biology for synthetic chemistry. ACS Chem Biol. 2008; 3:64–76.
Kissinger, P.T., Heineman, W.R. Cyclic voltammetry. J Chem Edu. 1983; 60:702–706.
Kissinger, P.T., Heineman, W.R.Techniques in Electroanalytical Chemistry. New York: Dekker, 1984.
Kitamura, T., Kaimori, S., Harada, A., Ishikawa, T., Fujimura, T., et al. Development of blood glucose monitoring sensor strip that uses small blood sample. SEI Technical Review. 2006; 63:19–22.
Kokkinos, C., Economou, A. Stripping at bismuth-based electrodes. Curr Anal Chem. 2008; 4:183–190.
Kotzian, P., Brazdilová, P., Rezková, S., Kalcher, K., Vytras, K. Amperometric glucose biosensor based on rhodium dioxide-modified carbon ink. Electroanalysis. 2006; 18:1499–1504.
Lee, S.-H., Fang, H.-Y., Chen, W.-C. Amperometric glucose biosensor based on screen-printed carbon electrodes mediated with hexacyanoferrate–chitosan oligomers mixture. Sensor Actuat B-Chem. 2006; 117:236–243.
Lee, T.M.-H. Over-the-counter biosensors: past, present, and future. Sensors. 2008; 8:5535–5559.
Lei, Y., Chen, W., Mulchandani, A. Microbial biosensors. Anal Chim Acta. 2006; 568:200–210.
Lin, Y.-Y., Liu, G., Wai, C.M., Lin, Y. Bioelectrochemical immunoassay of polychlorinated biphenyl. Anal Chim Acta. 2008; 612:23–28.
Lin, Y.-Y., Wang, J., Liu, G., Wu, H., Wai, C.M., Lin, Y. A nanoparticle label/immunochromatographic electrochemical biosensor for rapid and sensitive detection of prostate-specific antigen. Biosens Bioelectron. 2008; 23:1659–1665.
Liu, G., Lin, Y.-Y., Wang, J., Wu, H., Wai, C.M., et al. Disposable electrochemical immunosensor diagnosis device based on nanoparticle probe and immunochromatographic strip. Anal Chem. 2007; 79:7644–7653.
Liu, G., Wang, J., Barry, R., Petersen, C., Timchalk, C., et al. Nanoparticle-based electrochemical immunosensor for the detection of phosphorylated acetylcholinesterase: an exposure biomarker of organophosphate pesticides and nerve agents. Chem Eur J. 2008; 14:9951–9959.
Luo, P., Liu, Y., Xie, G., Xiong, X., Deng, S., et al. Determination of serum alcohol using a disposable biosensor. Forensic Sci Inter. 2008; 179:192–198.
Luong, J.H.T., Male, K.B., Glennon, J.D. Biosensor technology: technology push versus market pull. Biotechnol Adv. 2008; 26:492–500.
Mabbott, G.A. An introduction to cyclic voltammetry. J Chem Edu. 1983; 60:697–701.
Mahbubur Rahman, M., Saleh Ahammad, A.J., Jin, J.-H., Ahn, S.J., Lee, J.-J. A comprehensive review of glucose biosensors based on metal-oxides. Sensors. 2010; 10:4855–4886.
Mahmoud, K.A., Luong, J.H.T. A sensitive electrochemical assay for early detection of HIV-1 protease using ferrocene-peptide conjugate/Au nanoparticle/single walled carbon nanotube modified electrode. Anal Lett. 2010; 43:1680–1687.
Mahosenaho, M., Caprio, F., Micheli, L., Sesay, A.M., Palleschi, G., et al. A disposable biosensor for the determination of alpha-amylase in human saliva. Microchim Acta. 2010; 170:243–249.
Mandon, C.A., Heyries, K.A., Blum, L.J., Marquette, C.A. Polyshrink™ based microfluidic chips and protein microarrays. Biosens Bioelectron. 2010; 26:1218–1224.
Martínez-Olmos, A., Ballesta-Claver, J., Palma, A.J., Valencia-Mirón Mdel, C., Capitán-Vallvey, L.F. A portable luminometer with a disposable electrochemiluminescent biosensor for lactate determination. Sensors. 2009; 9:7694–7710.
Mascini, M., Guilbault, G.G. Clinical uses of enzyme electrode probes. Biosensors. 1986; 2:147–172.
Matthews, D., Holman, R., Bown, E., Streemson, J., Watson, A., et al. Pen sized digital 30-second blood glucose meter. Lancet. 1987; 2:778.
Miao, Y., He, N., Zhu, J.-J. History and new developments of assays for cholinesterase activity and inhibition. Chem Rev. 2010; 110:5216–5234.
Newman, J.D., Turner, A.P.F. Home blood glucose biosensors: a commercial perspective. Biosens Bioelectron. 2005; 20:2435–2453.
Nie, Z., Nijhuis, C.A., Gong, J., Chen, X., Kumachev, A., et al. Electrochemical sensing in paper-based microfluidic devices. Lab Chip. 2010; 10:477–483.
Nikolaus, N., Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim Acta. 2008; 160:15–55.
Noguer, T., Szydlowska, D., Marty, J.-L., Trojanowicz, M. Sol-gel immobilization of aldehyde dehydrogenase and NAD+ on screen-printed electrodes for designing of amperometric acetaldehyde biosensor. Polish J Chem. 2004; 78:1679–1689.
Ohara, T.J., Rajagopalan, R., Heller, A. Glucose electrodes based on cross-linked bis(2,2′-bipyridine)chloroosmium(+/2 +) complexed poly(1-vinylimidazole) films. Anal Chem. 1993; 65:3512.
Pemberton, R.M., Hart, J.P. Preparation of electrochemical screen-printed immunosensors for progesterone and their application in milk analysis. In: Alegret S., Merkoçi A., eds. Electrochemical Sensor Analysis. Amsterdam, The Netherlands: Elsevier; 2007:1207–1212. [vol. 49, Procedure 35].
Pemberton, R.M., Pittson, R., Biddle, N., Hart, J.P. Fabrication of microband glucose biosensors using a screen-printing water-based carbon ink and their application in serum analysis. Biosens Bioelectron. 2009; 24:1246–1252.
Pemberton, R.M., Xu, J., Pittson, R., Biddle, N., Drago, G.A., et al. Application of screen-printed microband biosensors to end-point measurements of glucose and cell numbers in HepG2 cell culture. Anal Biochem. 2009; 385:334–341.
Pemberton, R.M., Xu, J., Pittson, R., Drago, G.A., Griffiths, J., et al. A screen-printed microband glucose biosensor system for real-time monitoring of toxicity in cell culture. Biosens Bioelectron. 2011; 26:2448–2453.
Piano, M., Serban, S., Biddle, N., Pittson, R., Drago, G.A., et al. A flow injection system, comprising a biosensor based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt coated with glucose dehydrogenase, for the measurement of glucose. Anal Biochem. 2010; 396:269–274.
Piano, M., Serban, S., Pittson, R., Drago, G.A., Hart, J.P. Amperometric lactate biosensor for flow injection analysis based on a screen-printed carbon electrode containing Meldola’s Blue-Reinecke salt, coated with lactate dehydrogenase and NAD+. Talanta. 2010; 82:34–37.
Pletcher, D. A First Course in Electrode Processes. London: The Electrochemical Consultancy, Royal Society of Chemistry, 1991; .
Pohanka, M., Skládal, P. Electrochemical biosensors–principles and applications. J Appl Biomed. 2008; 6:57–64.
Prasad, K.S., Arun, A.B., Rekha, P.D., Young, C.C., Chang, J.L., et al. Microbial sensor based on direct electron transfer at Shewanella Sp. Drop-coated screen-printed carbon electrodes. Electroanalysis. 2009; 21:1646–1650.
Prieto-Simón, B., Fàbregas, E., Hart, A. Evaluation of different strategies for the development of amperometric biosensors for L-Lactate. Biosens Bioelectron. 2007; 22:2663–2668.
Privett, B.J., Shin, J.H., Schoenfisch, M.H. Electrochemical sensors. Anal Chem. 2010; 82:4723–4741.
Quan, D., Shim, J.H., Kim, J.D., Park, H.S., Cha, G.S., et al. Electrochemical determination of nitrate with nitrate reductase-immobilized electrodes under ambient air. Anal Chem. 2005; 77:4467–4473.
Raodi, A., Compagnone, D., Valcarcel, M.A., Placidi, P., Materazzi, S. Detection of NADH via electrocatalytic oxidation at single-walled carbon nanotubes modified with Variamine blue. Electrochim Acta. 2008; 53:2161–2169.
Ravera, M., Bagni, G., Mascini, M., Osella, D. DNA-metallodrugs interactions signaled by electrochemical biosensors: an overview. Bioinorg Chem Appl. 2007. [2007, Article ID 91078].
Rawson, F.J., Jackson, S.K., Hart, J.P. Voltammetric behaviour of DNA and its derivatives using screen-printed carbon electrodes and its possible application in genotoxicity screening. Anal Lett. 2010; 43:1790–1800.
Rawson, F.J., Purcell, W.M., Xu, J., Pemberton, R.M., Fielden, P.R., et al. A microband lactate biosensor fabricated using a water-based screen-printed carbon ink. Talanta. 2009; 77:1149–1154.
Reyes, Plata M., Contento, A.M., Ríos, A. State-of-the-art of (bio)chemical sensor developments in analytical Spanish groups. Sensors. 2010; 10:2511–2576.
Ricci, F., Volpe, G., Micheli, L., Palleschi, G. A review on novel developments and applications of immunosensors in food analysis. Anal Chim Acta. 2007; 605:111–129.
Ricci, F., Amine, A., Moscone, D., Palleschi, G. A probe for NADH and H2O2 at low applied potential for oxidase and dehydrogenase based biosensor application. Biosens Bioelectron. 2007; 22:854–862.
Roepcke, C.B.S., Muench, S.B., Schulze, H., Bachmann, T., Schmid, R.D., et al. Analysis of phosphorothionate pesticides using a chloroperoxidase pretreatment and acetylcholinesterase biosensor detection. J Agric Food Chem. 2010; 58:8748–8756.
Romanazzo, D., Ricci, F., Vesco, S., Piermarini, S., Volpe, G., et al, ELIME (enzyme linked immuno magnetic electrochemical) method for mycotoxin detection. J Vis Exp 2009; 32:e1588, doi: 10.3791/1588.
Romanazzo, D., Ricci, F., Volpe, G., Elliott, C.T., Vesco, S., et al. Development of a recombinant Fab-fragment based electrochemical immunosensor for deoxynivalenol detection in food samples. Biosens Bioelectron. 2010; 25:2615–2621.
Ronkainen, N.J., Halsall, H.B., Heineman, W.R. Electrochemical biosensors. Chem Soc Rev. 2010; 39:1747–1763.
Šafařık, I., Šafařková, M. Use of magnetic techniques for the isolation of cells. J Chromatograph B. 1999; 722:33–53.
Šafařık, I., Šafařková, M. Magnetic techniques for the isolation and purification of proteins and peptides. BioMagn Res Technol. 2004; 2:7.
Saiyed, Z.M., Telang, S.D., Ramchand, C.N. Application of magnetic techniques in the field of drug discovery and biomedicine. BioMagn Res Technol. 2003; 1(1):2.
Sajjadi, S., Ghourchiana, H., Tavakoli, H. Choline oxidase as a selective recognition element for determination of paraoxon. Biosens Bioelectron. 2009; 24:2509–2514.
Salam, F., Tothill, I.E. Detection of Salmonella typhimurium using an electrochemical immunosensor. Biosens Bioelectron. 2009; 24:2630–2636.
Sanllorente-Méndez, S., Domínguez-Renedo, O., Arcos-Martínez, M.J. Immobilization of acetylcholinesterase on screen-printed electrodes. Application to the determination of arsenic(III). Sensors. 2010; 10:2119–2128.
Santiago, L.M., Bejarano-Nosas, D., Lozano-Sanchez, P., Katakis, I. Screen-printed microsystems for the ultrasensitive electrochemical detection of alkaline phosphatase. Analyst. 2010; 135:1276–1281.
Sarkar, P., Banerjee, S., Bhattacharyay, D., Turner, A.P.F. Electrochemical sensing systems for arsenate estimation by oxidation of L-cysteine. Ecotox Environ Safe. 2010; 73:1495–1501.
Sato, N., Okuma, H. Amperometric simultaneous sensing system for D-Glucose and L-Lactate based on enzyme-modified bilayer electrodes. Anal Chim Acta. 2006; 565:250–254.
Schlapfer, P., Mindt, W., Racine, P. Electrochemical measurement of glucose using various electron acceptors. Clin Chim Acta. 1974; 57:283–289.
Scognamiglio, V., Pezzotti, G., Pezzotti, I., Cano, J., Buonasera, K., et al. Biosensors for effective environmental and agrifood protection and commercialization: from research to market. Microchim Acta. 2010; 170:215–225.
Shabani, A., Zourob, M., Allain, B., Marquette, C.A., Lawrence, M.F., et al. Bacteriophage-modified microarrays for the direct impedimetric detection of bacteria. Anal Chem. 2008; 80:9475–9482.
Shen, J., Liu, C.-C. Development of a screen-printed cholesterol biosensor: comparing the performance of gold and platinum as the working electrode material and fabrication using a self-assembly approach. Sensor Actuat B-Chem. 2007; 120:417–425.
Shih, W.-C., Yang, M.-C., Lin, M.S. Development of disposable lipid biosensor for the determination of total cholesterol. Biosens Bioelectron. 2009; 24:1679–1684.
Shinwari, M.W., Zhitomirsky, D., Deen, I.A., Selvaganapathy, P.R., Deen, M.J., et al. Microfabricated reference electrodes and their biosensing applications. Sensors. 2010; 10:1679–1715.
Shitanda, I., Takamatsu, S., Watanabe, K., Itagaki, M. Amperometric screen-printed algal biosensor with flow injection analysis system for detection of environmental toxic compounds. Electrochim Acta. 2009; 54:4933–4936.
Shumyantseva, V.V., Carrara, S., Bavastrello, V., Riley, J.D., Bulko, T.V., et al. Direct electron transfer between cytochrome P450scc and gold nanoparticles on screen-printed rhodium–graphite electrodes. Biosens Bioelectron. 2005; 21:217–222.
Siontorou, C.G., Batzias, F.A. Innovation in biotechnology: moving from academic research to product development – the case of biosensors. Crit Rev Biotechnol. 2010; 30:79–98.
Sokolov, A.N., Roberts, M.E., Bao, Z. Fabrication of low-cost electronic biosensors. Mater Today. 2009; 12:12–20.
Sollier, K., Mandon, C.A., Heyries, K.A., Blum, L.J., Marquette, C.A. ‘Print-n-Shrink’ technology for the rapid production of microfluidic chips and protein microarrays. Lab Chip. 2009; 9:3489–3494.
Stozhko, N.Y., Malakhova, N.A., Fyodorov, M.V., Brainina, K.Z. Modified carbon-containing electrodes in stripping voltammetry of metals. Part II. Composite and microelectrodes. J Solid State Electrochem. 2008; 12:1219–1230.
Sun, T.-P., Shieh, H.-L., Ching, C.T.-S., Yao, Y.-D., Huang, S.-H., et al. Carbon nanotube composites for glucose biosensor incorporated with reverse iontophoresis function for noninvasive glucose monitoring. Int J Nanomed. 2010; 5:343–349.
Svancara, I., Prior, C., Hocevar, S.B., Wang, J. A decade with bismuth-based electrodes in electroanalysis. Electroanalysis. 2010; 22:1405–1420.
Théavenot, D.R., Toth, K., Durst, R.A., Wilson, G.S. Electrochemical biosensors: recommended definitions and classification. Pure Appl Chem. 1999; 71:2333–2348.
Tibuzzi, A., Pezzotti, G., Lavecchia, T., Rea, G., Giardi, M.T. A portable light-excitation bio-amperometric for electrogenic biomaterials to support the technical development of most biosensors. Sensors and Transducers Journal. 2008; 88:9–20.
Tsekenis, G., Garifallou, G.-Z., Davis, F., Millner, P.A., Pinacho, D.G., et al. Detection of fluoroquinolone antibiotics in milk via a labeless immunoassay based upon an alternating current impedance protocol. Anal Chem. 2008; 80:9233–9239.
Tudorache, M., Bala, C. Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Anal Bioanal Chem. 2007; 388:565–578.
Usiu, B., Ozkan, S.A. Electroanalytical application of carbon based electrodes to the pharmaceuticals. Anal Lett. 2007; 40:817–853.
Vaddiraju, S., Tomazos, I., Burgess, D.J., Jain, F.C., Papadimitrakopoulos, F. Emerging synergy between nanotechnology and implantable biosensors: a review. Biosens Bioelectron. 2010; 25:1553–1565.
Vastarella, W., Della, Seta L., Masci, A., Maly, J., De Leo, M., et al. Biosensors based on gold nanoelectrode ensembles and screen-printed electrodes. Intern J Environ Anal Chem. 2007; 87:701–714.
Viswanathan, S., Radecka, H., Radecki, J. Electrochemical biosensors for food analysis. Monatsh Chem. 2009; 140:891–899.
Vydra, F., Stulik, K., Julakova, E.Electrochemical Stripping Analysis. Chichester: Ellis Horwood, 1976.
Waibel, M., Schulze, H., Huber, N., Bachmann, T.T. Screen-printed bienzymatic sensor based on sol–gel immobilized Nippostrongylus brasiliensis acetylcholinesterase and a cytochrome P450 BM-3 (CYP102-A1) mutant. Biosens Bioelectron. 2006; 21:1132–1140.
Wanekaya, A.K., Chen, W., Mulchandani, A. Recent biosensing developments in environmental security. J Environ Monit. 2008; 10:703–712.
Wang, J. Stripping Analysis: Principles, Instrumentation and Applications. Weinheim: VCH, 1985; .
Wang, J. Glucose biosensors: 40 years of advances and challenges. Electroanalysis. 2001; 13:983–988.
Wang, J. Analytical Electrochemistry, 3rd ed. Hoboken, NJ: Wiley-VCH, 2006.
Wang, J. Electrochemical glucose biosensors. Chem Rev. 2008; 108:814–825.
Wang, H., Wang, J., Timchalk, C., Lin, Y. Magnetic electrochemical immunoassays with quantum dot labels for detection of phosphorylated acetylcholinesterase in plasma. Anal Chem. 2008; 80:8477–8484.
Wang, J., Timchalk, C., Lin, Y. Carbon nanotube-based electrochemical sensor for assay of salivary cholinesterase enzyme activity: an exposure biomarker of organophosphate pesticides and nerve agents. Environ Sci Technol. 2008; 42:2688–2693.
Wang, Z., Zhu, Q., Kiely, J., Luxton, R. Hilbert Huang transform impedance measurement data for cellular toxicity monitoring. Proceedings of the 2009 IEEE International Conference on Networking, Sensing and Control,Okayama,Japan March 26–29. 2009:767–772.
World Health Organisation. The top ten causes of death, Fact sheet No 310. 2008. [November 2008, World Health Organisation].
Wring, S.A., Hart, J.P., Birch, B.J. Development of an amperometric assay for the determination of reduced glutathione, using glutathione peroxidase and screen-printed carbon electrodes chemically modified with cobalt phthalocyanine. Electroanalysis. 1992; 4:299–309.
Wu, H.-Z., Lee, Y.-C., Lin, T.-K., Shih, H.-C., Chang, F.-L., et al. Development of an amperometric micro-biodetector for pesticide monitoring and detection. J Taiwan Inst Chem E. 2009; 40:113–122.
Xie, X., Stueben, D., Berner, Z. The application of microelectrodes for the determination of trace metal in water. Anal Lett. 2005; 38:2281–2300.
Yadav, R., Dwivedi, S., Kumar, S., Chaudhury, A. Trends and perspectives of biosensors for food and environmental virology. Food Environ Virol. 2010; 2:53–63.
Yang, T.-H., Hung, C.-L., Ke, J.-H., Zen, J.-M. An electrochemically preanodized screen-printed carbon electrode for achieving direct electron transfer to glucose oxidase. Electrochem Comm. 2008; 10:1094–1097.
Yoo, E.-H., Lee, S.-Y. Glucose biosensors: an overview of use in clinical practice. Sensors. 2010; 10:4558–4576.
Zagal, J.H., Griveau, S., Silva, J.F., Nyokong, T., Bedioui, F. Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord Chem Rev. 2010; 254:2755–2791.
Zhou, J.-L., Nie, P.-P., Zheng, H.-T., Zhang, J.-M. Progress of electrochemical biosensors based on nicotinamide adenine dinucleotide (phosphate)-dependent. Chin J Anal Chem. 2009; 37:617–623.
Zuo, S., Teng, Y., Yuan, H., Lan, M. Development of novel silver nanoparticles-enhanced screen-printed amperometric glucose biosensor. Anal Lett. 2008; 41:1158–1172.