2.3
Charge‐Selective Contact Materials for Perovskite Solar Cells (PSCs)
Peng Gao and Mohammad Khaja Nazeeruddin
Ecole Polytechnique Federale Lausanne, SCI‐SB‐MN, Rue de l'Industrie 17, Case postale 440, Sion, 1951, Switzerland
High‐efficiency perovskite solar cells (PSCs) are typically fabricated with an organometal halide perovskite–infiltrated mesostructure as light absorber and charge transporter, sandwiched between a p‐type electron‐blocking hole‐selective layer hole transporting materials (HTM) and an n‐type hole‐blocking electron‐selective layer electron transporting materials (ETM) [1–3]. During the early development stage of PSCs, researchers were puzzled by the situation that similar efficiencies can be obtained in devices employing completely different charge‐selective contact materials, such as mesoporous TiO2 [4], compact TiO2 [5], mesoporous ZnO [6], or fullerene derivatives [7] as electron‐selective materials; and spiro‐OMeTAD [8], polytriarylamine (PTAA) [9], other molecular [10, 11] and polymeric materials [12], or even inorganic CuSCN [13] and NiO [14] as hole‐selective materials. Although the organolead halide perovskites represented by methylammonium lead iodide (CH3NH3PbI3) have been proved to be ambipolar semiconductors [15], devices with either no electron‐selective materials [16] or hole‐selective materials [17] were produced to give much lower photoconversion efficiency (PCE). Nowadays, it is generally accepted that both the electron‐ and hole‐selective contact materials are critical to a high‐performance perovskite photovoltaic (PV) device and only the selective contacts that allow an efficient charge separation can lead to the highest device performance [18]. These materials served to (i) tune the work function of the electrode to promote Ohmic contact at the absorber layer and electrode interface; (ii) determine the polarity of the device; (iii) improve the selectivity toward holes or electrons while blocking the other and minimizing charge carrier recombination at the interface; (iv) enhance light harvesting; and (v) improve device stability (Figure 2.3.1). In this regard, it is important to exploit their best candidates; and in this chapter, we attempt to give a systematic introduction about the choices of both electron‐ and hole‐selective contact materials for efficient PSCs.

Figure 2.3.1 Working mechanism of a typical PSC with charge‐selective contact materials.
2.3.1 Hole‐Selective Electron‐Blocking Materials (HTMs)
An ideal HTM should meet some general requirements to work appropriately in PSCs, such as (i) good hole mobility, (ii) compatible HOMO (highest occupied molecular orbital) energy level, (iii) good solubility and film‐forming properties, (iv) excellent thermal and photochemical stability, as well as (v) high cost performance. A huge number of HTMs have been synthesized for PSCs, which can be generally divided into organic and inorganic HTMs. The organic HTMs can be further categorized into three types: molecular HTMs, polymeric HTMs, and organometallic complexes, while the inorganic HTMs are mainly transition metal oxides, halides, etc. According to Figure 2.3.1, the open circuit voltage (V OC) limit is imposed by the energetics level offset of the selective contact materials. Generally, most electron‐selective contact materials have an electron affinity or conduction band (CB) energy of ∼4 eV [19]. The ionization potential (IP) of the hole‐selective contact materials vary significantly, and this has a direct effect on V OC, providing all the rest conditions are the same. Figure 2.3.2b collates a large number (∼85) of different HTMs and plots the relationship between IP and V OC. Given the theoretical V OC maximum of CH3NH3PbI3 perovskite cells with optimized contacts is ∼1.3 V, there is still considerable scope for improving the V OC by optimizing the energetics of the contacts and transport layers.
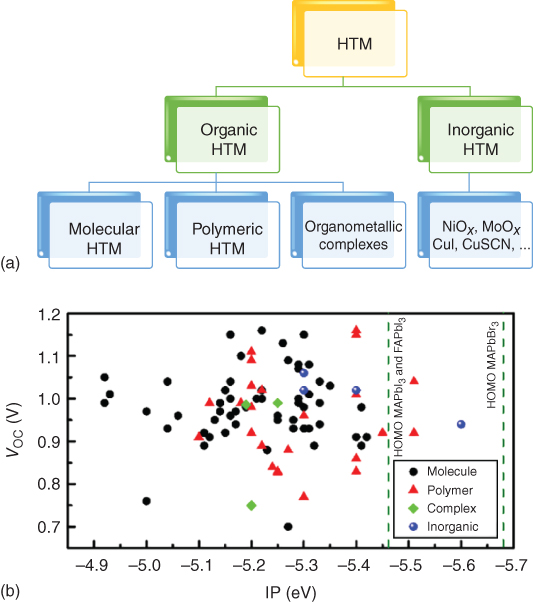
Figure 2.3.2 (a) Classification of hole‐selective electron‐blocking materials; (b) statistic distribution of V OC versus ionization potential (IP), a survey of 85 reported high‐efficiency perovskite solar cells [20].
2.3.1.1 Organic HTMs
2.3.1.1.1 Molecular HTMs
In a typical PSC, the most commonly used molecular hole‐selective contact material is spiro‐OMeTAD (1), which holds the record PCE (21.6%) of reported PSCs [21]. In the race toward achieving highly efficient and low‐cost devices, various molecular HTMs have been adopted, and some of them have a comparable PV performance to spiro‐OMeTAD [20] (Figure 2.3.3).

Figure 2.3.3 Chemical structures of selected molecular HTMs used in PSCs.
A large group of new HTMs are designed to mimic the spiro structure of spiro‐OMeTAD. Rakstys et al. reported a novel 9,9′‐bifluorenylidene‐based HTM (2, KR216), which has a pseudo spiro conformation and features straightforward synthesis from inexpensive starting materials [22]. The estimated price of 2 is around 50 times lower than that of commercial spiro‐OMeTAD. A remarkable PCE of 17.8% was obtained for PSCs using KR216. Ganesan et al. developed a new spiro‐type HTM, 4,4′,4″,4‴‐(2H,2′H,4H,4′H‐3,3′‐spiro‐bi[thieno[3,4‐b][1,4]dioxepine]‐6,6′,8,8′‐tetrayl)tetrakis‐(N,N‐bis(4‐methoxyphenyl)aniline) (3, PST1), via a facile synthetic route [10]. The PSC employing undoped 3 as the HTM layer exhibited a PCE of 12.7%, which is much higher than that of spiro‐OMeTAD under the same conditions. Recently, our group synthesized a highly hindered dispiro‐oxepine derivative as HTM (4), via a facile three‐step synthetic route [23]. PSCs that employed 4 as HTM showed one of the highest power conversion efficiencies (PCEs) of 19.4% reported to date. The solid structure study by single crystallography indicated again that the π–π interaction is not a prerequisite for designing effective HTMs. PSCs with high PCEs above 19% have also been realized from the two new HTMs (5 and 6), which have less crowded aryl amine substitution [24, 25]. Especially, the HTM 5–based devices compared favorably to spiro‐OMeTAD in all performance tests, yielding PCEs up to 20.2%, which is one of the highest (uncertified) reported values for molecular HTMs. The laboratory synthesis costs of fluorene–dithiophene (FDT) are estimated to be ∼60 US$ g−1. This is about a fifth of the costs of purified spiro‐OMeTAD (∼500 US$ g−1, high purity, Merck). Another spiro‐type HTM (7, 8) with silolothiophene‐linked methoxy triphenylamines was made by Paek and coworkers and found to have a half‐life of 6 K h, compared to 1 K h collected for the state‐of‐the‐art PSCs using spiro‐OMeTADs as HTMs [26].
Another type of HTMs features a C 4 or C 3 symmetric molecular geometry. Our group utilizing pyrene‐core arylamine (9) demonstrated a PCE of 12.4% under 1 sun [27]. Paek and coworkers synthesized a donor–acceptor‐type quinolizino acridine HTM (10), which possesses a well‐matched HOMO level with CH3NH3PbI3 (−5.23 versus −5.43 eV), and the highest PCE reported (12.8%) was achieved without any dopant and additive [28].
Rakstys et al. synthesized substituted triazatruxene and a remarkable PCE of 18.3% was obtained with the methoxyphenyl substituent (11), which is higher than that of spiro‐OMeTAD under the same conditions [29].
Molecular HTMs can also be synthesized with donor–π–donor (D‐π‐D) or donor–π–acceptor (D‐π‐A) electronic structures. Paek et al. synthesized three new D–π–D‐type HTMs (12,13,14), incorporating thiophene or thienothiophene with two electron‐rich triphenyl amine (TPA) units [30]. The optimized devices of 14 exhibited an impressive PCE of 16.9% under standard global AM 1.5 illumination with minimized hysteretic behavior, which is comparable to devices using a state‐of‐the‐art spiro‐OMeTAD hole transport layer under similar conditions. On the other hand, Bi and coworkers reported a novel D–π–A molecular HTM [31] (15) incorporating S,N‐heteropentacene as π‐spacer, triarylamin as donor, and dicyanovinylene as acceptor. PSCs using 15 achieved similar excellent PCEs of up to 16.9% [32]. However, a much prominent J–V hysteresis phenomenon was observed, possibly due to the asymmetric electronic structure of the HTM.
Acenes are intrinsic p‐type derivatives for semiconductors in optoelectrical devices. Their derivatives are potential HTM candidates as long as the energy level can be matched with that of perovskites. Rakstys and coworkers exploited TIPS‐pentacene (16) as the HTM in MAPbI3‐based PSCs [33]. The best PCE was obtained with the HTM in its pristine form, while the use of additives decreased the PV performance, which was attributed to the formation of trap sites or a disorder in chain packing.
2.3.1.1.2 Polymeric HTMs
The advantage of using polymer HTMs over molecular counterparts is that the material cost can be reduced, as the concentration used is much lower yet form very good films. Poly(triaryl amine) (17, PTAA) is the most popular polymer HTM and has been tested by Heo and coworkers, which demonstrated 12% efficiency using CH3NH3PbI3 perovskite light harvester (Figure 2.3.4) [34]. The highest PCE with this combination was just achieved by our group as 19.5% [35]. In 2014, Seok and coworkers recorded the highest certified PCE (16.2%) employing PTAA as the HTM [9]; an even higher certified PCE of 17.9% has also been demonstrated [36]. In the second year, the first certified 20.1% PCE was reported by the same group [1] (Figure 2.3.4).

Figure 2.3.4 Chemical structures of selected polymeric HTMs used in PSCs.
The exploitation of poly(3‐hexylthiophene‐2,5‐diyl) (18, P3HT) as the HTM was demonstrated by Abrusci et al. [37] and Heo et al. [34], where the PCEs of 3.8% and 6.7% were achieved, respectively. A remarkable improvement from 9.2% to 12.4% in the P3HT‐based PSCs was observed after doping P3HT with bis(trifluoromethane)sulfonamide lithium salt (Li‐TFSI) and 2,6‐di‐tert‐butylpyridine (D‐TBP) [38]. Another two TPA‐based polymer HTMs were reported by Yang and coworkers [39] and Nazeeruddin and coworkers [40] by either copolymerize polyfluorene with TPA or homopolymerize TPA derivatives (19 and 20). Devices based on HTM 19 in mesoscopic PSCs based on MAPbI3 absorber presented promising efficiencies (10.9–12.8%), which are comparable to the corresponding values for spiro‐OMeTAD (9.8–13.6%). Furthermore, Qin et al. used S197 (20) to replace PTAA and a PCE of 12% was measured, which was similar to the reference device made from PTAA.
Donor–acceptor‐conjugated polymers have the freedom of tuning their IP by varying the donor and acceptor moieties. Therefore, the prospect of having an even higher V OC seems possible with the combination of CH3NH3PbBr3 and HTMs with deeper HOMO levels. The first demonstration was realized by Cai et al. deploying diketopyrrolopyrrole (DPP)‐based copolymer (21, PCBTDPP) [41]. The developed PSCs exhibited high V OC of 1.16 V. This was supported with the work reported by Seok's group, where they demonstrated high V OC of 1.4 V using a combination of triarylamine‐based HTM similar to 19 and CH3NH3PbBr3 absorber [42]. Other DPP‐containing copolymer‐based HTMs were also studied in mesoscopic MAPbI3‐based PSCs. Qiao and coworkers [43] exploited (22, PDPP3T) as HTM in mesoporous devices attaining a PCE of 12.3%. They highlighted that by avoiding the use of t‐BP and lithium salts as p‐dopants in the HTM gave an enhancement in the stability of the device. A similar stability was observed in the device based on 23 reported by Kwon et al., where an excellent long‐term durability remained over 90% of their initial efficiencies after 1000 hours under a humidity of ≈20%, whereas spiro‐OMeTAD‐based devices only remained at ≈70% [44]. This excellent long‐term durability of 23 should be probably attributed to the hydrophobic properties of such polymers, which prevents water penetration into the perovskite surface. Benzothiadiazole was also used as the acceptor in the D–A‐conjugated polymer HTM. Seok and coworkers reported PCDTBT (24)‐based PSCs but the performance of the device was relatively low with a PCE of 4.2% for 24 [34]. PEDOT:PSS (poly(3,4‐ethylenedioxythiophene):polystyrene sulfonate, 25) were tested extensively in the p–i–n PSC devices as the hole‐selective contact devices. Recently, the PCE of such devices increased rapidly and was approaching 19% [45]. However, the acidity of such compounds poses a fundamental threat to the long‐term stability of the devices.
2.3.1.1.3 Organometallic Complex HTMs
Phthalocyanines (Pcs) are mostly developed organometallic complex HTMs [46] (Figure 2.3.5). The tendency of Pcs to form aggregates on the metal oxide semiconductor surface is a blessing to use as HTMs to transport charges efficiently from the perovskite absorber [10, 31, 47]. In addition, Pcs are photochemically and electrochemically stable compounds, which add additional values for their use as HTMs. At the end of 2014, Kumar et al. reported the first non‐substituted Cu(II)‐phthalocyanine (26)‐based HTM deposited by vacuum deposition for solid‐state PSCs and a PCE of 5.0% was achieved [48]. Seok and coworkers used Cu(II) tert‐butyl phthalocyanine as HTM and demonstrated the fabrication of compositive perovskite‐based PSCs, which yielded an efficiency of 15.2% [49]. Zn(II)‐phthalocyanine is another Pc analog and features more shifted absorption compared with Cu(II)‐phthalocyanine in most common solvents [50]. Our group reported the first PSCs with easily soluble Zn(II) octa(2,6‐diphenylphenoxy) phthalocyanine (27) and Zn(II) 5‐hexyl‐2‐thiophene phthalocyanine (28) as the HTMs with a PCE of 6.7% and 12.3%, respectively [51, 52]. It was not until very recently that zinc porphyrin was used as HTMs in PSCs. The first report from Li et al. showed that zinc chlorophyll aggregates as efficient biocompatible dopant‐free HTM for PSCs with a PCE of 11.44% [53]. One month later, Chou et al. reported the best‐performing porphyrin‐based solar cell with symmetric ethynylaniline‐substituted porphyrins (29) as HTM giving a PCE of 16.60% [54].
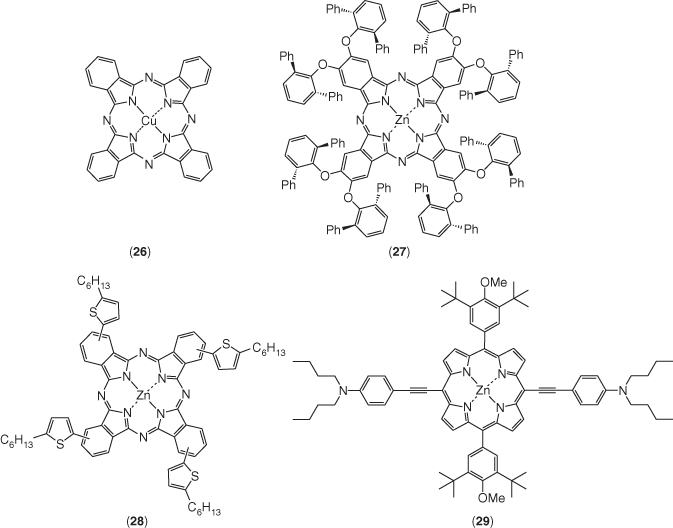
Figure 2.3.5 Chemical structures of selected organometallic complex HTMs used in PSCs.
2.3.1.2 Inorganic Hole‐Selective Electron‐Blocking Materials
Although the progress in inorganic HTMs is rather slow due to the limited selection of materials, their low cost and stability under ambient conditions verify why they are still competitive with organic HTMs. So far, inorganic semiconductor materials, such as CuI, CuSCN, NiO x , VO x , and MoO x , have also been explored as hole‐selective materials for use in perovskite‐based solar cells [55]. In 2013, Kamat and coworkers demonstrated the first example using inorganic p‐type copper(I) iodide (CuI)‐based HTMs in MAPbI3 PSCs, providing higher FF and better J SC stability compared with those devices based on spiro‐OMeTAD due to its higher electrical conductivity (Figure 2.3.6a) [57]. Nonetheless, the high recombination in the CuI layer limited the V OC. Following another copper‐based inorganic p‐type hole conductor, copper thiocyanate (CuSCN) has also been actively studied and the highest PCE (12.4%) achieved after optimization of the perovskite morphology (Figure 2.3.6b) [56].
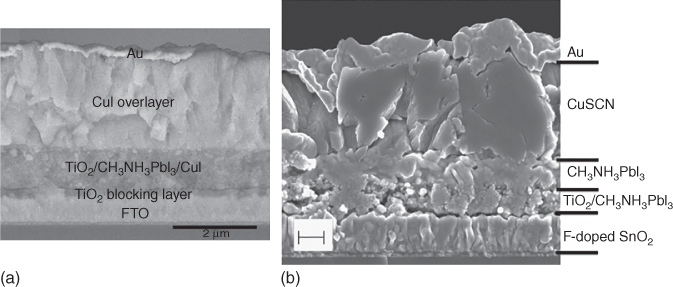
Figure 2.3.6 Scanning electron microscopic (SEM) cross‐section images of solar cells employing (a) CuI and (b) CuSCN.
Source: Qin et al. 2014 [56] and Christians et al. 2014 [57]. Reprinted with permission of Springer Nature.
In another interesting report by Yang's group employing nickel oxide (NiO), a PCE of 9.11% has been reported; and a slight improvement was achieved by Wang and coworkers, with a PCE of 9.51% utilizing NiO as a hole‐collecting electrode [58, 59]. Although the performance of the p‐type semiconductor is less promising, the highest V OC of about 1.05 V reported recently for NiO HTM is rather promising [60].
2.3.2 Electron‐Selective Hole‐Blocking Materials
In a typical PSCs, the electron‐selective contact material (ETM) is responsible for selective extraction of electrons at the anode contact, adopted from dye‐sensitized solar cells (DSSCs) and blocking holes from recombination with injected electrons. Similar to its counterpart HTM, the ETM can be divided into inorganic ETM, organic ETM, and composite ETM depending on the nature of the material. The lowest unoccupied molecular orbital (LUMO) or CB energy levels of some ETMs used in PSCs are shown in Figure 2.3.7b, which are crucial to the performance of the device.

Figure 2.3.7 (a) Classification of electron‐selective hole‐blocking materials; (b) energy level diagram showing conduction band minimum/LUMO levels of various ETLs.
Source: Yang et al. 2016 [19]. Reproduced with permission of Royal Society of Chemistry.
2.3.2.1 Inorganic Electron‐Selective Hole‐Blocking Materials
2.3.2.1.1 TiO2
In a classic n–i–p device configuration, the most commonly used electron‐selective contact materials is compact TiO2 [61]. Some device configurations also include a thin mesoporous layer of TiO2, which is infiltrated and capped with the perovskite absorber. This type of device structure holds so far the highest reported PCE of a PSC [21]. The metal oxide scaffold is believed to provide an effective n‐doping in this infiltrated layer, likely resulting in a favorable n‐type/intrinsic homojunction within the perovskite layer [62]. It was supposed that the effective n‐doping may be due to under‐coordinated halides acting as shallow electron donors on the crystal surface, or due to a surface charge effect of the mesoporous metal oxide. Our group recently found by focused ion beam energy dispersive X‐ray (FIB‐EDX) analysis in a cross‐section of PSCs the nonuniform distribution of iodine element from mesoporous layer to capping layer [35].
In general, the compact TiO2 blocking layer is deposited using a spin‐coating technique or spray pyrolysis method. The deposition of mesoporous TiO2 scaffold is now dominated by spin coating an organotitanium precursor, while the doctor blade technique derived from the DSSCs is used in rare cases. Optimal infiltration of the perovskite and HTM into the pores of the mesoporous TiO2 is desirable for highly efficient devices. Kim et al. investigated the thickness dependence of device performance and found that as the TiO2 thickness increases, the V OC and FF values decrease significantly [63]. Our group reported on a CH3NH3PbI3/TiO2 heterojunction solar cell using anatase nanosheets with domain (001) facets as the ETM, with a side length of 30 nm and a thickness of 7 nm [17]. The constructed device demonstrated a moderate performance with J SC of 16.1 mA cm−2, V OC of 0.631 V, and FF of 0.57 corresponding to 5.5% efficiency. The lower performance is arguably due to the absence of HTM in the device structure. Later, collaborative work between Park and Grätzel demonstrated high‐performance PSCs based on 0.6 μm rutile TiO2 nanorods along with CH3NH3PbI3 perovskite nanodots (Figure 2.3.8) [64]. The rutile nanorods were successfully hydrothermally grown and the lengths were controllable via the reaction time. They reported that shorter rutile nanorods were remarkably better for the infiltration of HTMs compared to those with longer length. The device produces 9.4% efficiency along with J SC of 15.6 mA cm−2, V OC of 0.955 V, and FF of 0.63.
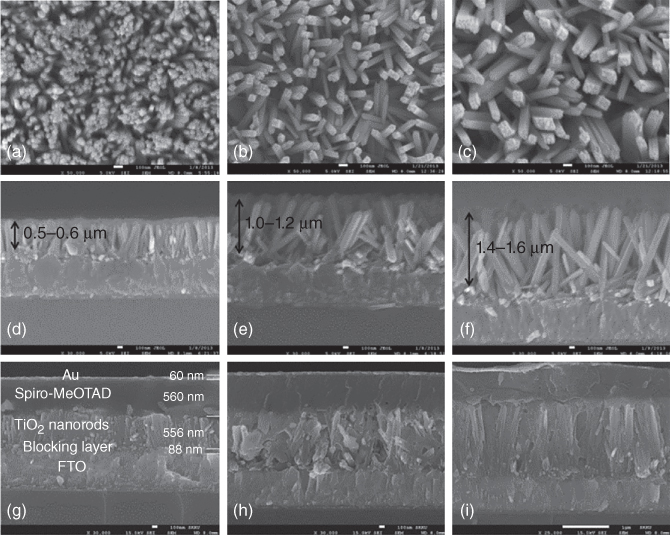
Figure 2.3.8 (a–c) Surface and (d–f) cross‐sectional field‐effect scanning electron microscopic (FESEM) images of rutile TiO2 nanorods grown on FTO substrate. (g–i) Cross‐sectional SEM images of solid‐state DSSCs based on perovskite CH3NH3PbI3‐sensitized rutile TiO2 nanorod photoanode, the spiro‐MeOTAD hole transporting layer, and the Au cathode.
Source: Kim et al. 2013 [64]. Reprinted with permission of American Chemical Society.
Gao et al. initiated a work on a freestanding TiO2 nanotube array film prepared by means of two‐step anodization process and detached from the substrate by in situ field‐assisted chemical dissolution and later transferred onto a fluorin‐doped tin oxide (FTO) substrate which was covered by a TiO2 blocking layer [65]. As comparison, the control device with TiO2 nanoparticles (similar thickness) was also developed and they found that their proposed TiO2 nanotube had a better absorption than with TiO2 nanoparticles, most likely due to the improved light‐trapping capability (Figure 2.3.9). Moreover, they also observed that the TiO2 nanotube array presented a higher charge collection efficiency compared to that of TiO2 nanoparticles. Their PSCs constructed with TiO2 nanotubes yielded 6.25% efficiency with a J SC of 17.9 mA cm−2. These observations show that the improvement of TiO2 nanotube PSCs was attributed to both the improved light harvesting and reduced carrier recombination.

Figure 2.3.9 Morphology characterization of TiO2 nanotubes before and after the perovskite dye deposition. (a–c) SEM images of the top view (a), the cross‐section image (b), and the transmission electron microscopic (TEM) image (c) of pristine TiO2; (d–f) SEM images of the top view (d), the cross‐section image (e), and the TEM image (f) of TiO2 nanotubes with CH3NH3PbI3 deposition.
Source: Gao et al. 2014 [65]. Reprinted with permission of Royal Society of Chemistry.
Despite being widely used as ETM, TiO2 also has some drawbacks which could possibly lead to Ohmic losses or nonideal space charge distribution with PSCs. Pathak et al. suggested that Al doping reduces the number of sub‐bandgap states of TiO2 (improving the V OC) and greatly improves electron conductivity [66]. PSCs with 0.3 mol% Al‐doping level produced 13.8% efficiency compared to those without doping of only 11.13%. Significant improvement in solar cells with doped TiO2 was mainly due to the improved quantum efficiency leading to J SC of 20 mA cm−2. Prior to this study (Ref. [67]), Yang and colleagues reported yttrium‐doped TiO2 in the PSCs which led to 19.3% efficiency. Doped TiO2 produced a better conductivity compared to undoped TiO2 film (2 × 10−5 versus 6 × 10−6 S cm−1), which was due to the improved carrier concentration as well as decreased series resistance in the device. It is worth noting that the conductivity of doped TiO2 was similar to our frequently used HTM, spiro‐OMeTAD, and 10 order of magnitudes higher compared with undoped TiO2.

Figure 2.3.10 TEM analysis of 0.5%Y–TiO2/CH3NH3PbI3 and TiO2/CH3NH3PbI3 electrodes. (a–e) 0.5%Y‐TiO2/CH3NH3PbI3 electrode and (f–j) TiO2/CH3NH3PbI3 electrode: (a, f) bright field transmission electron microscopy (BFTEM) micrographs; (b, g) high‐resolution transmission electron microscopy (HRTEM) micrographs of dark‐colored (encircled) individual nanoparticles deposited on TiO2; (c, h) fast Fourier transform (FFT) patterns obtained from the corresponding HRTEM; (d, i) zoomed HRTEM showing prominent lattice fringes; (e, j) histograms showing particle size distribution.
Source: Qin et al. 2014 [68]. Reprinted with permission of Royal Society of Chemistry.
Our group also successfully investigated the use of Y3+‐substituted TiO2 (0.5% Y‐TiO2) in PSCs comprising CH3NH3PbI3 and spiro‐OMeTAD (Figure 2.3.10) [68]. The doped devices produced 11.2% efficiency and observed 15% enhancement in the J SC compared with pristine TiO2. The J SC improvement was arguably because of the effect of Y3+ on the dimensions of perovskite nanoparticles formed on the semiconductor surface.
2.3.2.1.2 ZnO
Apart from TiO2, there have been a lot of reports employing ZnO as a potential replacement to TiO2 for PSCs due to its comparable energy levels and good transport properties (bulk mobility: 205–300 cm2 V−1 s−1). Kumar et al. were among the first to employ the ZnO compact layer formed by electrodeposition technique and ZnO nanorods prepared by chemical bath deposition (Figure 2.3.11) [69]. The blocking‐ZnO/ZnO nanorod‐based PSCs documented 8.90% efficiency with astonishingly high J SC of 16.98 mA cm−2 compared to the planar ZnO‐based device with only 5.54% efficiency and a greatly lower J SC of 11.27 mA cm−2. In this study, they argued that the improved J SC was due to better charge generation and collection efficiency because of the enhanced light scattering and larger heterojunction interface.

Figure 2.3.11 FESEM images of (a) top view of the ZnO compact layer electrodeposited on FTO (inset shows a high‐resolution image); (b) cross‐sectional view of perovskite islands on the ZnO compact layer; (c) cross‐sectional view of the complete planar ZnO device; FTO, ZnO BL, perovskite + spiro, gold; (d) top view of ZnO nanorods grown on the ZnO compact layer on the FTO substrate; (e) cross‐sectional view of perovskite islands on ZnO nanorods; and (f) cross‐sectional view of the complete ZnO nanorod device; FTO, ZnO BL, ZnO nanorod + perovskite, spiro, gold.
Source: Kumar et al. 2013 [69]. Reprinted with permission of Royal Society of Chemistry.
Park's group also reported on ZnO nanorods grown on the ZnO seed layer from solution to fabricate their CH3NH3PbI3 PSCs (Figure 2.3.12) [70]. The lengths and diameters of ZnO nanorods were manipulated by precursor concentration as well as growth time. They were able to obtain 11.13% efficiency with J SC of 20.08 mA cm−2, V OC of 0.991 V, and FF of 0.56. Their control devices with TiO2 nanorods demonstrated a slightly less performance, with only 10.02% efficiency; and they attributed the decrease in device performance to a low V OC (0.869 V). The slightly lower V OC was due to the lower CB of TiO2 with respect to ZnO nanorods as well as slower recombination properties.

Figure 2.3.12 Surface SEM images of hexagonal ZnO nanorods grown at (a) 20 mM, (b) 25 mM, (c) 30 nM, and (d) 35 mM of the precursor solution containing equimolar zinc nitrate hexahydrate and hexamethylenetetramine. The ZnO seed layer–deposited FTO substrates were immersed in the precursor solution at 90 °C for 180 minutes. Insets represent distribution of diameters of ZnO nanorods.
Reprinted with permission [70].
Liu et al. independently demonstrated planar PSCs featuring ZnO nanoparticles [6]. They demonstrated the capability of ZnO nanoparticles under two different types of devices: rigid and flexible formats. A notable performance was demonstrated for the rigid format with 15.7% efficiency, while 10.2% efficiency was reported for flexible PSCs. The high performance of rigid solar cells was due to the unconstrained CH3NH3PbI3 perovskite crystallite growth that took place in the absence of a mesoporous scaffold. However, ZnO suffers from the issue of chemical instability [71].
2.3.2.1.3 SnO2
Recently, SnO2 has emerged as another promising ETM with a high transparency and electron mobility (bulk mobility: 240 cm2 V−1 s−1). Li et al. successfully utilized TiCl4‐treated SnO2 nanoparticles as ETMs in PSCs and the efficiency of the device with SnO2 films exceeded 10% [72]. Song et al. demonstrated that low‐temperature‐processed SnO2 compact layer‐based planar PSCs could achieve a high PCE of 13.0%, which is highly durable with exposure to the ambient air environment for 30 days [73]. Another low‐temperature sol–gel‐fabricated SnO2 ETM was reported by Yang et al. An average efficiency of 16.02% was obtained without hysteresis [74]. Recently, Hagfeldt and coworkers used a 15‐nm‐thick SnO2 as ETM processed by atomic layer deposition (ALD) technique [75]. They showed hysteresis‐free, high‐stabilized planar PSCs with a high voltage of 1.19 V.
2.3.2.2 Organic Electron‐Selective Hole‐Blocking Materials
In contrast to the inorganic ETMs, a smaller variety of organic materials has been checked as an alternative electron‐selective contact: 2,9‐dimethyl‐4,7‐diphenyl‐1,10‐phenanthroline (BCP), C60, indene‐C60 bisadduct (ICBA), phenyl‐C61‐butaric acid methyl ester (PCBM) are a few examples [19] (Figure 2.3.13). These ETMs are normally used in p–i–n structured PSC devices, where the perovskites are coated upon a p‐type HTM (for example, PEDOT:PSS or nickel oxide) before the deposition of an electron‐selective charge collection layer on top of the perovskite. It is worth mentioning that these so‐called inverted PSCs with all organic contacts generally exhibit little hysteresis. The use of fullerene (C60, 31) and its derivatives was proposed by Jeng et al. [76]. Their device consists of ITO/PEDOT:PSS/CH3NH3PbI3/C60/BCP/Al. A thin bathocuproine (BCP, 30) film functioned as a hole‐blocking layer and they obtained 3.0% efficiency. When C60 was substituted with PCBM (33) and ICBA (32), the efficiency improved to 3.9% and 3.4%, respectively. The results suggest the existence of donor–acceptor interface at the CH3NH3PbI3/fullerene heterojunction as well as the variation of device performance by acceptors of various LUMO levels. Snaith and collaborators constructed perovskite/PCBM flexible solar cells with a conversion efficiency of 6% and later the performance was slightly improved to 7% by Bolink's group adopting similar device architecture [77].

Figure 2.3.13 Chemical structures of selected organic ETMs used in PSCs.
2.3.2.3 Composite ETMs
In general, TiO2 surfaces possess deep mid‐gap states which enable non‐radiative recombination channels at the perovskite interface that will eventually influence the device performance [78]. Hence, voluminous reports have been put forward to passivate surface traps of TiO2 surface, for example, by introducing polar molecules [79], C60‐SAM (self‐assembled monolayer) [37, 80], graphene quantum‐dots [81–84], and antimony (III) sulfide (Sb2S3) [85]. It was found that the introduction of antimony (III) sulfide (Sb2S3) not only modified the TiO2/perovskite interface but also enhanced the lifetime of the device even under continuous light illumination. From this study, Ito and coworkers believe that the light degradation takes place at the TiO2/CH3NH3PbI3 interface.
2.3.3 Conclusion
Nowadays, both n–i–p and p–i–n device structures are reported to give high‐performance PSCs. In a p–i–n architecture, holes are extracted by the bottom electrode, transparent ITO or FTO in most cases, while electrons are extracted at the bottom electrode in the n–i–p architectures. The efficiency of the device is determined by the charge‐selective contact materials. Hence, by introducing proper contact materials with good charge selectivity, one could potentially reduce interfacial charge recombination as well as increase device performance. In order to efficiently extract hole from the anode electrode, hole‐selective materials should possess a suitable energy alignment with perovskite films. It is of importance that the CB or the LUMO of the HTMs should be high enough to efficiently block the back diffusion of electrons to the cathode electrode. Identical principles also apply to the electron‐selective layers. It is desirable that selective contact materials have larger bandgaps than that of perovskite films to avoid the excitons from recombining at the electrode. Also, it is crucial to design and synthesize selective contact materials with high conductivity to reduce the series resistance of the PSCs. In the past few years, copious selective contact materials have been proposed. It has been observed that both selective contacts contribute to enhance the cell FF, while the hole‐selective contact is mainly responsible for the high V OC [86].
References
- 1 Yang, W.S., Noh, J.H., Jeon, N.J. et al. (2015). High‐performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348 (6240): 1234–1237.
- 2 Bi, D., Tress, W., Dar, M.I. et al. (2016). Efficient luminescent solar cells based on tailored mixed‐cation perovskites. Sci. Adv. 2 (1): e1501170.
- 3 Gao, P., Grätzel, M., and Nazeeruddin, M.K. (2014). Organohalide lead perovskites for photovoltaic applications. Energy Environ. Sci. 7 (8): 2448.
- 4 Burschka, J., Pellet, N., Moon, S.‐J. et al. (2013). Sequential deposition as a route to high‐performance perovskite‐sensitized solar cells. Nature 499 (7458): 316–319.
- 5 Liu, M., Johnston, M.B., and Snaith, H.J. (2013). Efficient planar heterojunction perovskite solar cells by vapour deposition. Nature 501 (7467): 395–398.
- 6 Liu, D. and Kelly, T.L. (2013). Perovskite solar cells with a planar heterojunction structure prepared using room‐temperature solution processing techniques. Nat. Photonics 8 (2): 133–138.
- 7 Xiao, Z., Bi, C., Shao, Y. et al. (2014). Efficient, high yield perovskite photovoltaic devices grown by interdiffusion of solution‐processed precursor stacking layers. Energy Environ. Sci. 7 (8): 2619.
- 8 Ko, H.‐S., Lee, J.‐W., and Park, N.‐G. (2015). 15.76% efficiency perovskite solar cells prepared under high relative humidity: importance of PbI2 morphology in two‐step deposition of CH3NH3PbI3. J. Mater. Chem. A 3 (16): 8808–8815.
- 9 Jeon, N.J., Noh, J.H., Kim, Y.C. et al. (2014). Solvent engineering for high‐performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 13 (9): 897–903.
- 10 Ganesan, P., Fu, K., Gao, P. et al. (2015). A simple spiro‐type hole transporting material for efficient perovskite solar cells. Energy Environ. Sci. 8 (7): 1986–1991.
- 11 Li, H., Fu, K., Hagfeldt, A. et al. (2014). A simple 3,4‐ethylenedioxythiophene based hole‐transporting material for perovskite solar cells. Angew. Chem. Int. Ed. 53 (16): 4085–4088.
- 12 Lin, Q., Armin, A., Nagiri, R.C.R. et al. (2014). Electro‐optics of perovskite solar cells. Nat. Photonics 9 (2): 106–112.
- 13 Ye, S., Sun, W., Li, Y. et al. (2015). CuSCN‐based inverted planar perovskite solar cell with an average PCE of 15.6%. Nano Lett. 15 (6): 3723–3728.
- 14 Xu, X., Liu, Z., Zuo, Z. et al. (2015). Hole selective NiO contact for efficient perovskite solar cells with carbon electrode. Nano Lett. 15 (4): 2402–2408.
- 15 Ball, J.M., Lee, M.M., Hey, A., and Snaith, H.J. (2013). Low‐temperature processed meso‐superstructured to thin‐film perovskite solar cells. Energy Environ. Sci. 6 (6): 1739.
- 16 Liu, D., Yang, J., and Kelly, T.L. (2014). Compact layer free perovskite solar cells with 13.5% efficiency. J. Am. Chem. Soc. 136 (49): 17116–17122.
- 17 Etgar, L., Gao, P., Xue, Z. et al. (2012). Mesoscopic CH3NH3PbI3/TiO2 heterojunction solar cells. J. Am. Chem. Soc. 134 (42): 17396–17399.
- 18 Green, M.A. (2002). Photovoltaic principles. Physica E 14 (1–2): 11–17.
- 19 Yang, G., Tao, H., Qin, P. et al. (2016). Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 4 (11): 3970–3990.
- 20 Yu, Z. and Sun, L. (2015). Recent progress on hole‐transporting materials for emerging organometal halide perovskite solar cells. Adv. Energy Mater. 5 (12): 1500213.
- 21 Bi, D., Yi, C., Luo, J. et al. (2016). Polymer‐templated nucleation and crystal growth of perovskite films for solar cells with efficiency greater than 21%. Nat. Energy 1 (10): 16142.
- 22 Rakstys, K., Saliba, M., Gao, P. et al. (2016). Highly efficient perovskite solar cells employing an easily attainable bifluorenylidene‐based hole‐transporting material. Angew. Chem. Int. Ed. 55 (26): 7464–7468.
- 23 Rakstys, K., Paek, S., Sohail, M. et al. (2016). A highly hindered bithiophene‐functionalized dispiro‐oxepine derivative as an efficient hole transporting material for perovskite solar cells. J. Mater. Chem. A 4 (47): 18259–18264.
- 24 Saliba, M., Orlandi, S., Matsui, T. et al. (2016). A molecularly engineered hole‐transporting material for efficient perovskite solar cells. Nat. Energy 1 (2): 15017.
- 25 Bi, D., Xu, B., Gao, P. et al. (2016). Facile synthesized organic hole transporting material for perovskite solar cell with efficiency of 19.8%. Nano Energy 23: 138–144.
- 26 Abate, A., Paek, S., Giordano, F. et al. (2015). Silolothiophene‐linked triphenylamines as stable hole transporting materials for high efficiency perovskite solar cells. Energy Environ. Sci. 8 (10): 2946–2953.
- 27 Jeon, N.J., Lee, J., Noh, J.H. et al. (2013). Efficient inorganic–organic hybrid perovskite solar cells based on pyrene arylamine derivatives as hole‐transporting materials. J. Am. Chem. Soc. 135 (51): 19087–19090.
- 28 Qin, P., Paek, S., Dar, M.I. et al. (2014). Perovskite solar cells with 12.8% efficiency by using conjugated quinolizino acridine based hole transporting material. J. Am. Chem. Soc. 136 (24): 8516–8519.
- 29 Rakstys, K., Abate, A., Dar, M.I. et al. (2015). Triazatruxene‐based hole transporting materials for highly efficient perovskite solar cells. J. Am. Chem. Soc. 137 (51): 16172–16178.
- 30 Paek, S., Zimmermann, I., Gao, P. et al. (2016). Donor–π–donor type hole transporting materials: marked π‐bridge effects on optoelectronic properties, solid‐state structure, and perovskite solar cell efficiency. Chem. Sci. 7 (9): 6068–6075.
- 31 Paek, S., Rub, M.A., Choi, H. et al. (2016). A dual‐functional asymmetric squaraine‐based low band gap hole transporting material for efficient perovskite solar cells. Nanoscale 8 (12): 6335–6340.
- 32 Bi, D., Mishra, A., Gao, P. et al. (2016). High‐efficiency perovskite solar cells employing a S,N‐heteropentacene‐based D–A hole‐transport material. ChemSusChem 9 (5): 433–438.
- 33 Kazim, S., Ramos, F.J., Gao, P. et al. (2015). A dopant free linear acene derivative as a hole transport material for perovskite pigmented solar cells. Energy Environ. Sci. 8 (6): 1816–1823.
- 34 Heo, J.H., Im, S.H., Noh, J.H. et al. (2013). Efficient inorganic–organic hybrid heterojunction solar cells containing perovskite compound and polymeric hole conductors. Nat. Photonics 7 (6): 486–491.
- 35 Zhang, Y., Gao, P., Oveisi, E. et al. (2016). PbI2‐HMPA complex pretreatment for highly reproducible and efficient CH3NH3PbI3 perovskite solar cells. J. Am. Chem. Soc. doi: 10.1021/jacs.6b08347.
- 36 Jeon, N.J., Noh, J.H., Yang, W.S. et al. (2015). Compositional engineering of perovskite materials for high‐performance solar cells. Nature 517 (7535): 476–480.
- 37 Abrusci, A., Stranks, S.D., Docampo, P. et al. (2013). High‐performance perovskite‐polymer hybrid solar cells via electronic coupling with fullerene monolayers. Nano Lett. 13 (7): 3124–3128.
- 38 Guo, Y., Liu, C., Inoue, K. et al. (2014). Enhancement in the efficiency of an organic–inorganic hybrid solar cell with a doped P3HT hole‐transporting layer on a void‐free perovskite active layer. J. Mater. Chem. A 2 (34): 13827.
- 39 Zhu, Z., Bai, Y., Lee, H.K.H. et al. (2014). Polyfluorene derivatives are high‐performance organic hole‐transporting materials for inorganic−organic hybrid perovskite solar cells. Adv. Funct. Mater. 24 (46): 7357–7365.
- 40 Qin, P., Tetreault, N., Dar, M.I. et al. (2015). A novel oligomer as a hole transporting material for efficient perovskite solar cells. Adv. Energy Mater. 5 (2): 1400980.
- 41 Cai, B., Xing, Y., Yang, Z. et al. (2013). High performance hybrid solar cells sensitized by organolead halide perovskites. Energy Environ. Sci. 6 (5): 1480.
- 42 Ryu, S., Noh, J.H., Jeon, N.J. et al. (2014). Voltage output of efficient perovskite solar cells with high open‐circuit voltage and fill factor. Energy Environ. Sci. 7 (8): 2614.
- 43 Dubey, A., Adhikari, N., Venkatesan, S. et al. (2016). Solution processed pristine PDPP3T polymer as hole transport layer for efficient perovskite solar cells with slower degradation. Sol. Energy Mater. Sol. Cells 145: 193–199.
- 44 Kwon, Y.S., Lim, J., Yun, H.‐J. et al. (2014). A diketopyrrolopyrrole‐containing hole transporting conjugated polymer for use in efficient stable organic–inorganic hybrid solar cells based on a perovskite. Energy Environ. Sci. 7 (4): 1454.
- 45 Nie, W., Tsai, H., Asadpour, R. et al. (2015). High‐efficiency solution‐processed perovskite solar cells with millimeter‐scale grains. Science 347 (6221): 522–525.
- 46 Cho, K.T., Rakstys, K., Cavazzini, M. et al. (2016). Perovskite solar cells employing molecularly engineered Zn(II) phthalocyanines as hole‐transporting materials. Nano Energy .
- 47 Liu, J., Wu, Y., Qin, C. et al. (2014). A dopant‐free hole‐transporting material for efficient and stable perovskite solar cells. Energy Environ. Sci. 7 (9): 2963.
- 48 Kumar, C.V., Sfyri, G., Raptis, D. et al. (2015). Perovskite solar cell with low cost Cu‐phthalocyanine as hole transporting material. RSC Adv. 5 (5): 3786–3791.
- 49 Seo, J., Jeon, N.J., Yang, W.S. et al. (2015). Effective electron blocking of CuPC‐doped spiro‐OMeTAD for highly efficient inorganic–organic hybrid perovskite solar cells. Adv. Energy Mater. 5 (20): 1501320.
- 50 Ghani, F., Kristen, J., and Riegler, H. (2012). Solubility properties of unsubstituted metal phthalocyanines in different types of solvents. J. Chem. Eng. Data 57 (2): 439–449.
- 51 Javier Ramos, F., Ince, M., Urbani, M. et al. (2015). Non‐aggregated Zn(ii)octa(2,6‐diphenylphenoxy) phthalocyanine as a hole transporting material for efficient perovskite solar cells. Dalton Trans. 44 (23): 10847–10851.
- 52 Gao, P., Cho, K.T., Abate, A. et al. (2016). An efficient perovskite solar cell with symmetrical Zn(ii) phthalocyanine infiltrated buffering porous Al2O3 as the hybrid interfacial hole‐transporting layer. Phys. Chem. Chem. Phys. 18 (39): 27083–27089.
- 53 Li, M., Li, Y., Sasaki, S. et al. (2016). Dopant‐free zinc chlorophyll aggregates as an efficient biocompatible hole transporter for perovskite solar cells. ChemSusChem 9 (19): 2862–2869.
- 54 Chou, H.‐H., Chiang, Y.‐H., Li, M.‐H. et al. (2016). Zinc porphyrin–ethynylaniline conjugates as novel hole‐transporting materials for perovskite solar cells with power conversion efficiency of 16.6%. ACS Energy Lett. 1 (5): 956–962.
- 55 Xiao, M., Gao, M., Huang, F. et al. (2016). Efficient perovskite solar cells employing inorganic interlayers. ChemNanoMat 2 (3): 182–188.
- 56 Qin, P., Tanaka, S., Ito, S. et al. (2014). Inorganic hole conductor‐based lead halide perovskite solar cells with 12.4% conversion efficiency. Nat. Commun. 5 (May): 1–6.
- 57 Christians, J.A., Fung, R.C.M., and Kamat, P.V. (2014). An inorganic hole conductor for organo‐lead halide perovskite solar cells. Improved hole conductivity with copper iodide. J. Am. Chem. Soc. 136 (2): 758–764.
- 58 Zhu, Z., Bai, Y., Zhang, T. et al. (2014). High‐performance hole‐extraction layer of sol–gel‐processed NiO nanocrystals for inverted planar perovskite solar cells. Angew. Chem. Int. Ed. 53 (46): 12571–12575.
- 59 Wang, K.‐C., Jeng, J.‐Y., Shen, P.‐S. et al. (2014). p‐Type mesoscopic nickel oxide/organometallic perovskite heterojunction solar cells. Sci. Rep. 4: 4756.
- 60 Hu, L., Peng, J., Wang, W. et al. (2014). Sequential deposition of CH3NH3PbI3 on planar NiO film for efficient planar perovskite solar cells. ACS Photonics 1 (7): 547–553.
- 61 Park, N.‐G. (2013). Organometal perovskite light absorbers toward a 20% efficiency low‐cost solid‐state mesoscopic solar cell. J. Phys. Chem. Lett. 4 (15): 2423–2429.
- 62 Leijtens, T., Stranks, S.D., Eperon, G.E. et al. (2014). Electronic properties of meso‐superstructured and planar organometal halide perovskite films: charge trapping, photodoping, and carrier mobility. ACS Nano 8 (7): 7147–7155.
- 63 Kim, H.‐S., Lee, C.‐R., Im, J.‐H. et al. (2012). Lead iodide perovskite sensitized all‐solid‐state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2: 591.
- 64 Kim, H.‐S., Lee, J.‐W., Yantara, N. et al. (2013). High efficiency solid‐state sensitized solar cell‐based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano Lett. 13 (6): 2412–2417.
- 65 Gao, X., Li, J., Baker, J. et al. (2014). Enhanced photovoltaic performance of perovskite CH3NH3PbI3 solar cells with freestanding TiO2 nanotube array films. Chem. Commun. 50 (48): 6368–6371.
- 66 Pathak, S.K., Abate, A., Ruckdeschel, P. et al. (2014). Performance and stability enhancement of dye‐sensitized and perovskite solar cells by Al doping of TiO2. Adv. Funct. Mater. 24 (38): 6046–6055.
- 67 Zhou, H., Chen, Q.Q., Li, G. et al. (2014). Interface engineering of highly efficient perovskite solar cells. Science 345 (6196): 542–546.
- 68 Qin, P., Domanski, A.L., Chandiran, A.K. et al. (2014). Yttrium‐substituted nanocrystalline TiO2 photoanodes for perovskite based heterojunction solar cells. Nanoscale 6 (3): 1508–1514.
- 69 Kumar, M.H., Yantara, N., Dharani, S. et al. (2013). Flexible, low‐temperature, solution processed ZnO‐based perovskite solid state solar cells. Chem. Commun. 49 (94): 11089.
- 70 Son, D.‐Y., Im, J.‐H., Kim, H.‐S., and Park, N.‐G. (2014). 11% efficient perovskite solar cell based on ZnO nanorods: an effective charge collection system. J. Phys. Chem. C 118 (30): 16567–16573.
- 71 Dong, X., Hu, H., Lin, B. et al. (2014). The effect of ALD‐Zno layers on the formation of CH3NH3PbI3 with different perovskite precursors and sintering temperatures. Chem. Commun. 50 (92): 14405–14408.
- 72 Li, Y., Zhu, J., Huang, Y. et al. (2015). Mesoporous SnO2 nanoparticle films as electron‐transporting material in perovskite solar cells. RSC Adv. 5 (36): 28424–28429.
- 73 Song, J., Zheng, E., Bian, J. et al. (2015). Low‐temperature SnO2‐based electron selective contact for efficient and stable perovskite solar cells. J. Mater. Chem. A 3 (20): 10837–10844.
- 74 Ke, W., Fang, G., Liu, Q. et al. (2015). Low‐temperature solution‐processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 137 (21): 6730–6733.
- 75 Correa Baena, J.P., Steier, L., Tress, W. et al. (2015). Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 8 (10): 2928–2934.
- 76 Jeng, J.‐Y., Chiang, Y.‐F., Lee, M.‐H. et al. (2013). CH3NH3PbI3 perovskite/fullerene planar‐heterojunction hybrid solar cells. Adv. Mater. 25 (27): 3727–3732.
- 77 Malinkiewicz, O., Yella, A., Lee, Y.H. et al. (2014). Perovskite solar cells employing organic charge‐transport layers. Nat. Photonics 8 (2): 128–132.
- 78 Nakamura, I., Negishi, N., Kutsuna, S. et al. (2000). Role of oxygen vacancy in the plasma‐treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A: Chem. 161 (1–2): 205–212.
- 79 Ogomi, Y., Morita, A., Tsukamoto, S. et al. (2014). All‐solid perovskite solar cells with HOCO‐R‐NH3 +I− anchor‐group inserted between porous titania and perovskite. J. Phys. Chem. C 118 (30): 16651–16659.
- 80 Wojciechowski, K., Stranks, S.D., Abate, A. et al. (2014). Heterojunction modification for highly efficient organic–inorganic perovskite solar cells. ACS Nano 8 (12): 12701–12709.
- 81 Zhu, Z., Ma, J., Wang, Z. et al. (2014). Efficiency enhancement of perovskite solar cells through fast electron extraction: the role of graphene quantum dots. J. Am. Chem. Soc. 136 (10): 3760–3763.
- 82 Liu, T., Kim, D., Han, H. et al. (2015). Fine‐tuning optical and electronic properties of graphene oxide for highly efficient perovskite solar cells. Nanoscale 7 (24): 10708–10718.
- 83 Yeo, J.‐S., Kang, R., Lee, S. et al. (2015). Highly efficient and stable planar perovskite solar cells with reduced graphene oxide nanosheets as electrode interlayer. Nano Energy 12: 96–104.
- 84 Wang, C., Tang, Y., Hu, Y. et al. (2015). Graphene/SrTiO3 nanocomposites used as an effective electron‐transporting layer for high‐performance perovskite solar cells. RSC Adv. 5 (64): 52041–52047.
- 85 Ito, S., Tanaka, S., Manabe, K., and Nishino, H. (2014). Effects of surface blocking layer of Sb2S3 on nanocrystalline TiO2 for CH3NH3PbI3 perovskite solar cells. J. Phys. Chem. C 118 (30): 16995–17000.
- 86 Juarez‐Perez, E.J., Wuβler, M., Fabregat‐Santiago, F. et al. (2014). Role of the selective contacts in the performance of lead halide perovskite solar cells. J. Phys. Chem. Lett. 5 (4): 680–685.
